Abstract
A 65-year-old man with valvular disorder presented to his physician because of widespread purpura in both lower extremities. Blood tests showed elevated serum creatinine levels and proteinase 3-anti-neutrophil cytoplasmic antibody (ANCA) with hematuria, suggesting ANCA-related rapidly progressive glomerulonephritis (RPGN). Although multiple blood cultures were negative, transthoracic echocardiography revealed warts in the valves, and a renal biopsy also showed findings of glomerular infiltration by mononuclear leukocytes and C3 deposition in the glomeruli, suggesting infection-related glomerulonephritis. Later, Bartonella antibody turned positive. Antimicrobial treatment improved the purpura and renal function without any recurrence. ANCA-positive RPGN requires the exclusion of infective endocarditis, especially that induced by Bartonella spp.
Keywords: ANCA, rapidly progressive glomerulonephritis, Bartonella, infective endocarditis
Introduction
Infective endocarditis is a fatal systemic septic disease characterized by the formation of warts in the valvular or large-vessel endocardium that contain bacterial, fungal, viral, or other infectious microorganisms. It presents with a variety of clinical symptoms.
At least three sets of blood cultures are recommended for the diagnosis of infective endocarditis before the administration of antimicrobial agents. However, it has been reported that culture-negative infective endocarditis accounts for 12-25% of total cases (1,2). Patients with infective endocarditis caused by Bartonella spp. often have negative blood cultures (1). In addition, infective endocarditis presents with symptoms similar to vasculitis with anti-neutrophil cytoplasmic antibodies (ANCAs) (3). When we treat cases of ANCA-positive infective endocarditis with renal impairment, it is essential to differentiate between infection-related glomerulonephritis (IRGN) due to infective endocarditis and ANCA-related nephritis to determine the appropriate treatment.
We treated a case of ANCA-positive glomerulonephritis caused by Bartonella spp. infective endocarditis. An early renal biopsy helped to differentiate IRGN due to infective endocarditis from ANCA-associated nephritis with a favorable prognosis following treatment with antibacterial agents.
Case Report
A 65-year-old man with a history of alcoholism and aortic and mitral regurgitation who was living in a lodging house without any contact with cats presented to his physician because of a fever (38.2℃), weakness in both lower extremities that had started 1 month before the appointment, and purpura on the extensor surfaces of both lower extremities that started 1 week before the appointment. He was referred to our hospital because he was suspected of having rapidly progressive glomerulonephritis based on elevated creatinine levels (3.07 mg/dL) with hematuria, although there were no abnormalities in the blood analysis, including creatinine levels (0.85 mg/dL), or according to a urinalysis performed 6 months earlier. A physical examination revealed no apparent petechial hemorrhaging in his conjunctiva, Osler's nodes, or Janeway lesions. However, chest auscultation revealed a systolic ejection murmur (Levine III/VI) at the right margin of the second intercostal sternum. In addition, the patient had pitting edema and numerous sites of purpura several millimeters across on the bilateral lower extremities (Fig. 1A). However, he lacked lymphadenopathy.
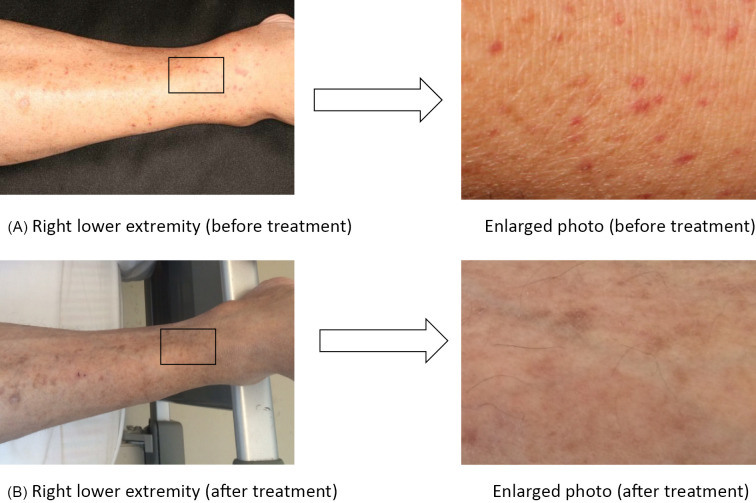
Figure 1.
Cutaneous findings of the lower extremities. A) Right lower extremity, enlarged photo (before treatment). B) Right lower extremity, enlarged photo (after treatment). Red to purplish-red purpura a few millimeters in size were observed in both lower extremities on admission, but they disappeared after the antimicrobial treatment.
Blood and urine analyses showed elevated blood urea nitrogen levels (46 mg/dL) and serum creatinine levels (2.69 md/dL), occult hematuria, and proteinuria, which suggested glomerulonephritis. They also showed a normal range of leukocytes (7,500 cells/μL) but mildly elevated C-reactive protein (CRP; 3.36 mg/dL) and normocytic hypochromic anemia with 8.2 g/dL hemoglobin. We observed no decreases in complement, but we did observe elevated rheumatoid factor (2,880 IU/mL), proteinase 3 (PR3)-ANCA (186 U/mL), and myeloperoxidase (MPO)-ANCA (7.0 U/mL). Three sets of blood cultures were all negative (Table 1).
Table 1.
Blood Analysis at Admission.
| WBC | 7,500 | /μL | PT | 12.1 | s | |
| Neutrophil | 86.0 | % | PT-INR | 0.98 | ||
| Eosinophil | 0.0 | % | APTT | 38.0 | s | |
| Basophil | 3.0 | % | D-Dimer | 3.6 | μg/dL | |
| Monocyte | 7.0 | % | ||||
| Lymphocyte | 4.0 | % | IgG | 3,320 | mg/dL | |
| RBC | 2.83×104 | /μL | IgA | 288 | mg/dL | |
| Hb | 8.2 | g/dL | IgM | 966 | mg/dL | |
| MCV | 93 | fL | IgE | 937 | IU/mL | |
| HCT | 24.6 | % | C3 | 91 | mg/dL | |
| PLT | 22.2×104 | /μL | C4 | 70 | mg/dL | |
| ASO | 32 | IU/mL | ||||
| TP | 8.3 | g/dL | Antinuclear antibody | <40 | times | |
| Alb | 3.0 | g/dL | RF | 2,880 | IU/mL | |
| T-Bil | 0.1 | mg/dL | PR3-ANCA | 186 | U/mL | |
| AST | 21 | IU/L | MPO-ANCA | 7.0 | U/mL | |
| ALT | 12 | IU/L | Anti GBM antibody | <2.0 | U/mL | |
| LDH | 343 | IU/L | Cryoglobulin | (-) | ||
| γGTP | 29 | IU/L | FT3 | 1.96 | pg/mL | |
| BUN | 46 | mg/dL | FT4 | 0.95 | ng/mL | |
| Cre | 2.69 | mg/dL | TSH | 7.35 | μU/mL | |
| Na | 137 | mEq/L | BNP | 113.3 | pg/mL | |
| K | 5.1 | mEq/L | ||||
| Cl | 104 | mEq/L | HBS-Ag | (-) | ||
| Ca | 8.0 | mg/dL | HCV-Ab | (-) | ||
| IP | 3.9 | mg/dL | HIV | (-) | ||
| TC | 131 | mg/dL | ||||
| HDL-C | 23 | mg/dL | T-SPOT | (-) | ||
| LDL-C | 82 | mg/dL | ||||
| CRP | 3.36 | mg/dL | Blood culture | 3/3set | negative | |
| Glu | 126 | mg/dL | ||||
| HbA1c | 5.6 | |||||
| <Urineanalysis> | ||||||
| pH | 5.5 | |||||
| Urine specific gravity | 1.011 | |||||
| glycosuria | (-) | |||||
| proteinuria | (2+) | |||||
| hematuria | (3+) | |||||
| Urobilinogen | (-) | |||||
| Acetone body | (-) | |||||
| RBC | 100< | /field | ||||
| WBC | 1~4 | /field | ||||
| hyaline cast | 10~19 | /field | ||||
| granular cast | (-) | |||||
| Urinary NAG | 17.4 | IU/L | ||||
| Urinary β-2MG | 23.89 | mg/L | ||||
| Urinary TP/Cr | 1.12 | g/gCr | ||||
Transthoracic and transesophageal echocardiography revealed warts on the aortic and mitral valves with moderate to severe aortic regurgitation (Fig. 2). Magnetic resonance imaging of the head showed a 6-mm cerebral aneurysm at the bifurcation of the right middle cerebral artery (Fig. 3). In addition, a skin biopsy of the purpura demonstrated lymphocyte and neutrophil infiltration around and within the arteriole of the dermal reticular layer, consistent with vasculitis (Fig. 4A, B). A renal biopsy performed one week after antimicrobial treatment showed a fibrous to fibrocellular crescent in one glomerulus (Fig. 5A). Glomerular infiltration by mononuclear leukocytes was observed, although no capillary occlusion was identified. Neither mesangial expansion nor hypercellularity were observed. Immunofluorescent staining revealed IgM and complement component 3c (C3) in the mesangial region (Fig. 5B), and electron microscopy revealed high-electron-density deposits in the mesangial region (Fig. 5C). These findings were consistent with the resolution of IRGN.
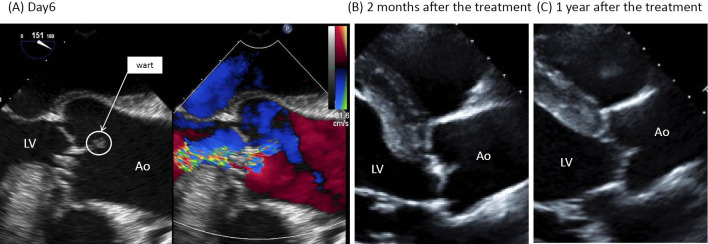
Figure 2.
Transesophageal echocardiography findings. A) Day 6. Warts 10×6 mm in size were found at the aortic valve (total valve apex) and mitral valve. Moderate-to-severe aortic regurgitation and mitral regurgitation were also detected. B) Two months after treatment. The warts at the aortic valve (total valve apex) and mitral valve had remarkably regressed. Moderate aortic regurgitation and mitral regurgitation were still detected. C) One year after treatment. Regression was maintained without treatment. Moderate aortic regurgitation and mitral regurgitation were still detected. LV: left ventricle, Ao: aorta
Figure 3.
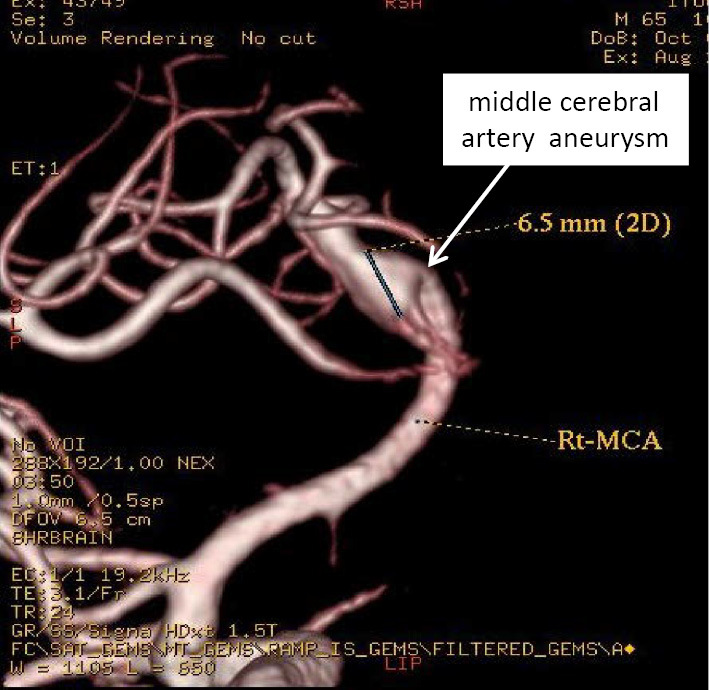
Head MRI. A 6-mm aneurysm was detected at the bifurcation of the right middle cerebral artery.
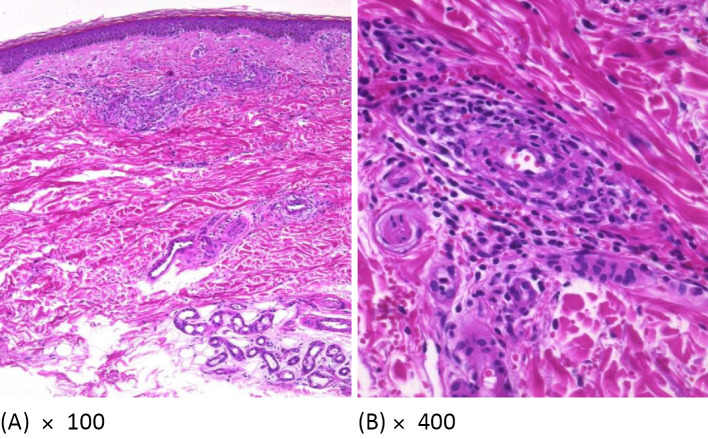
Figure 4.
Skin biopsy findings. A) 100×. B) 400×. A skin biopsy of the purpura was performed on Day 8. Hematoxylin and Eosin staining revealed the infiltration of lymphocytes and neutrophils with nuclear dust from the dermal papillary layer to the dermal reticular layer, and red blood cell leakage was observed around the capillary lumen with swollen endothelial cells and arterioles where inflammatory cells had infiltrated into the vessel wall. These findings were consistent with ANCA-associated vasculitis.
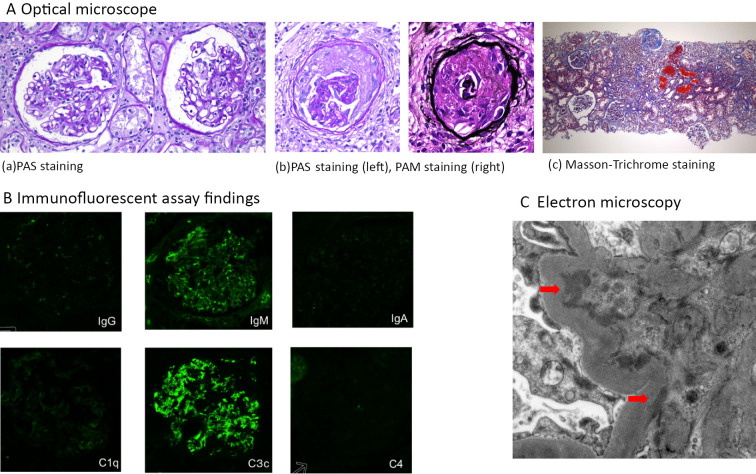
Figure 5.
Kidney biopsy findings. A) Optical microscopy. a: PAS staining, b: PAS staining (left) and PAM staining (right), c: Masson-Trichrome staining (×100). Cortical:medulla=6:4. A total of 25 glomeruli, 8 global sclerosis, 1 fibrous crescent, tubular atrophy, and interstitial fibrosis, hyalinosis of small arteries, and erythrocytic casts were detected. B) Immunofluorescent assay findings. Positive IgM and C3c findings in the mesangial region were detected. C) Electron microscopy findings. High-electron-density deposits were found in the mesangial region (arrows).
The patient was diagnosed with infective endocarditis according to the modified Duke's criteria (4) (one major criterion, warts by echocardiography; and four minor criteria, predisposing cardiac disease, fever of 38.2℃, cerebral aneurysm, and positive for rheumatoid factor). He was then treated with ampicillin/sulbactam (7 weeks) and gentamicin (2 weeks) for blood culture-negative infective endocarditis. After 7 weeks of antimicrobial treatment, his serum creatinine levels decreased from 2.69 mg/dL (on admission) to 1.95 mg/dL, and his CRP level improved from 3.4 mg/dL (on admission) to 0.5 mg/dL. In addition, the purpura that had been observed at the time of admission disappeared (Fig. 1B).
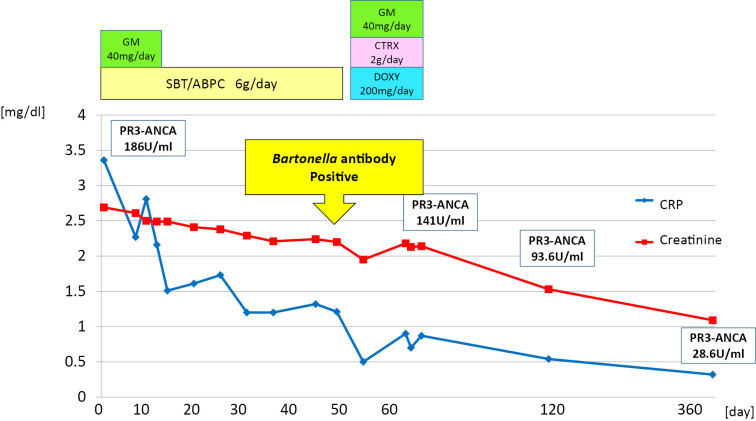
The antibodies against Bartonella spp. used to identify the causative organism were markedly elevated to more than 1,024× the typical level for anti-Bartonella henselae IgG and 512× for anti-Bartonella quintana IgG, which led to the diagnosis of infective endocarditis caused by Bartonella spp. After two weeks of antimicrobial treatment with ceftriaxone, gentamicin, and doxycycline, the patient self-discharged from the hospital and refused further treatment. Judging by the numerous reports that infective endocarditis caused by Bartonella spp. is difficult to treat with only antimicrobial agents and based on his moderate to severe valve insufficiency, we proposed a valve replacement operation, which the patient unfortunately refused. At the time of discharge, his serum creatinine had decreased to 2.14 mg/dL, CRP to 0.87 mg/dL, and PR3-ANCA to 141 U/mL. Thereafter, given the possibility of relapse, the patient continued to be followed in our outpatient clinic. Two months after his discharge, the warts had remarkably regressed (Fig. 2B). His creatinine level had further decreased to 1.53 mg/dL, CRP to 0.54 mg/dL, and PR3-ANCA to 93.6 U/mL, and urine sediment showed improvement in hematuria (Table 2). Furthermore, at 1 year later, his regression was maintained without treatment (Fig. 2C), and his creatinine level had decreased to 1.09 mg/dL and PR3-ANCA to 28.6 U/mL without any signs of relapse. His urine sediment showed no hematuria (Table 2). Clinical course was shown in Fig. 6.
Table 2.
Urinalysis.
| 2 months after the treatment | 1 year after the treatment | ||||
| pH | 5.0 | pH | 6.5 | ||
| Urine specific gravity | 1.013 | Urine specific gravity | 1.015 | ||
| Glycosuria | (-) | Glycosuria | (-) | ||
| Proteinuria | (1+) | Proteinuria | (±) | ||
| Hematuria | (2+) | Hematuria | (-) | ||
| Urobilinogen | (±) | Urobilinogen | (±) | ||
| Acetone body | (-) | Acetone body | (-) | ||
| RBC | 10-19 | /field | RBC | <1 | /field |
| WBC | 1~4 | /field | WBC | <1 | /field |
| hyaline cast | 5~9 | /field | hyaline cast | (-) | |
| granular cast | (-) | granular cast | (-) | ||
Figure 6.
Clinical course. The clinical course is shown along with the kind, amount, and duration of antimicrobial agents. Creatinine and CRP levels were used as indicators of the disease severity. GM: Gentamicin, SBT/ABPC: Sulbactam/Ampicillin, CTRX: Ceftriaxone, DOXY: Doxycycline
Discussion
We experienced a case of ANCA-positive glomerulonephritis caused by Bartonella spp. infective endocarditis. An early renal biopsy helped to differentiate IRGN due to infective endocarditis from ANCA-associated nephritis, with the patient showing a favorable prognosis following treatment with antibacterial agents.
Multiple blood cultures from the patient were negative. In addition to prior administration of antimicrobials, difficulty in detecting microbes can be caused by slow-growing pathogens, such as Haemophilus spp., Actinobacillus spp., Cardiobacterium hominis, Eikenella corrodens, and Kingella spp.; intracellular parasites, such as Coxiella, Bartonella, Brucella, Mycoplasma, Legionella, or Chlamydia spp.; and pathogens that are difficult to culture, such as fungi and acid-fast bacteria (5). In particular, Bartonella spp. infection reportedly accounts for 28% of blood culture-negative endocarditis in Europe and the United States (1).
However, as few as 25% of Bartonella spp. are detected in blood cultures. Detection requires a long incubation period of at least 21 days in a special environment: culture in 5% CO2 in chocolate agar or heart infusion medium with 5% rabbit serum at 35-37℃ (6). Other known diagnostic methods include serum antibody testing, serum polymerase chain reaction (PCR) testing, PCR testing of heart valves, and serum Western blotting, but these are not covered by insurance. In serological tests, anti-B. henselae and anti-B. quintana IgG levels are considered positive if they are greater than 800× those in healthy individuals. However, the differentiation of these two bacteria is difficult because the antibodies show cross-reactivity (7). In contrast, serum PCR testing reportedly has a sensitivity of 58% and a specificity of 100%. PCR testing of heart valves has a sensitivity of >95%; even after antimicrobial treatment, it still has a sensitivity >60% (8). Serum Western blotting has a sensitivity of 97% for infective endocarditis caused by B. quintana and 100% for infective endocarditis caused by B. henselae (7). In our case, we requested that research institutions perform serum PCR and Western blotting tests, but they failed to detect any bacteria, partly because the patient had been treated with antimicrobials for four weeks prior to testing. Furthermore, we were unable to perform PCR on the heart valve because the patient declined surgery. However, the serum antibody test showed 1,024× higher levels of anti-B. henselae IgG than in healthy serum, which enabled us to diagnose infective endocarditis caused by this bacterium. Unfortunately, we failed to freeze the serum before antimicrobial administration because infective endocarditis caused by Bartonella spp. was not listed as a differential diagnosis at the time of admission.
Infective endocarditis caused by Bartonella spp. typically occurs in adults, with more than 70% of cases occurring in men (9). Individuals are particularly susceptible if they reside in an unsanitary environment where lice are present, as is often the case for homeless individuals, or if they have a history of alcoholism or cardiac valvular disease (6,8). Our patient was considered at a high risk for Bartonella spp. infection because he was living in temporary accommodations, bathed once a month, and had a medical history of cardiac valvular disease. Furthermore, although B. henselae is also known to cause cat scratch disease, the patient had had no contact with cats nor any symptoms of lymphadenopathy.
In our case, we detected strong PR3-ANCA positivity and weak MPO-ANCA positivity. Among infective endocarditis cases, the rate of ANCA positivity has been reported to range from 18% to 33% (10). Langlois et al. reported that 63% of cases were positive for PR3-ANCA alone, 17% were positive for MPO-ANCA alone, and 10% were positive for both PR3- and MPO-ANCA (3). Streptococcus spp. (38%), Bartonella spp. (18%), Staphylococcus spp. (12%), and Enterococcus spp. (12%) are listed as the causative organisms of infective endocarditis that is likely to be positive for ANCA (3).
Conversely, it has been reported that approximately 60% of infective endocarditis cases caused by Bartonella spp. are positive for ANCA (11). Although the mechanism by which infective endocarditis generates a positive ANCA result is unclear, it has been speculated that the bacterium possesses a peptide homologous to PR3 with autoantibodies produced during the antibacterial immune response, or that hypomethylated bacterial DNA acts as a ligand for toll-like receptor 9 and triggers ANCA production by B cells (12,13).
Our patient did not have Osler's nodes or Janeway lesions, but he did present with purpura in his lower extremities, which was reminiscent of ANCA-related vasculitis. Lin et al. reported on the cutaneous manifestations of bartonellosis (14). In their review, they cited the paper from Teoh et al., reporting that vasculitis can be induced by bartonellosis and even simulate systemic vasculitis with ANCA positivity (15). Infective endocarditis caused by Bartonella spp. is also a difficult disease in terms of differentiation of eruption.
ANCA-positive rapidly progressive glomerulonephritis is difficult to differentiate from pauci-immune type glomerulonephritis and IRGN due to infective endocarditis. It has been reported that the renal impairment caused by infective endocarditis also accompanies crescentic glomerulonephritis (53%), diffuse proliferative glomerulonephritis (33%), mild mesangial hypercellularity (10%), and focal proliferative glomerulonephritis (4%). Furthermore, in immunofluorescence assays, C3 deposition was observed in 94% of cases, and 37% were IgM-positive, 29% were IgA-positive, and 27% were IgG-positive (16). Heijmeijer et al. described 11 cases of glomerulonephritis complicated by infective endocarditis caused by Bartonella spp. infection (17). According to their report, 7 of 11 cases were ANCA-positive: 6 cases were PR3-ANCA-positive, and only 1 case was positive for both PR3 and MPO. Morphologically, the disease was mainly characterized by segmental necrosis crescents with IgM and C3 deposition, as observed on immunofluorescence staining. Furthermore, 7 of 11 patients were diagnosed with infective endocarditis after immunosuppressive therapy. Therefore, it is important to distinguish IRGN due to infective endocarditis as the cause of ANCA-positive glomerulonephritis. Although immune-related markers, rheumatoid factor positivity, antinuclear antibody positivity, and low complement levels have been suggested to be effective in distinguishing the disease from ANCA-associated nephritis (18), they are not conclusive. Furthermore, hypocomplementemia has been identified in 35% to 80% of IRGN patients (19). Therefore, a kidney biopsy is considered essential prior to deciding to implement immunosuppressive therapy. In our case, the early kidney biopsy helped differentiate IRGN due to infective endocarditis from ANCA-associated nephritis.
Finally, concerning the appropriate treatment for infective endocarditis caused by Bartonella spp., if Bartonella spp. infection is suspected in culture-negative infective endocarditis, the recommended treatment is ceftriaxone, doxycycline, and gentamicin (20,21). After Bartonella spp. infection has been confirmed, treatment with doxycycline and gentamicin is recommended. The usual indication for surgery is continuous positive blood cultures. However, surgery is required in about 60% of cases, as Bartonella spp. are rarely positive in blood cultures, and the diagnosis can only be made after disease progression (20). There is no clear rationale for the duration of treatment, but given the severity of infective endocarditis caused by Bartonella spp., long-term oral doxycycline is recommended for six weeks in the case of surgery and for three months with conservative treatment (20,21). The present case involved moderate to severe aortic and mitral valve insufficiency, which was considered an indication for surgery, but the patient refused this option. As ampicillin/sulbactam and gentamicin were administered at the start of the treatment, the actual effective treatment period was only four weeks: two weeks with gentamicin and two weeks with doxycycline and gentamicin. Therefore, recurrence was a serious concern.
In addition, this case was characterized by long-term persistence of PR3-ANCA positivity after treatment. Kawamorita et al. explained that high ANCA levels persist for months after treatment due to the involvement of the immune system (22).
Conclusion
We treated a patient with glomerulonephritis due to infective endocarditis caused by Bartonella spp. that required differentiation from ANCA-associated nephritis. Our experience demonstrates that it is critical to exclude infective endocarditis prior to starting treatment.
The authors state that they have no Conflict of Interest (COI).
Ayumi Yoshifuji and Yuuka Hibino contributed equally to this work.
References
- 1.Werner M, Andersson R, Olaison L, Hogevik H. A clinical study of culture-negative endocarditis. Medicine (Baltimore) 82: 263-273, 2003. [DOI] [PubMed] [Google Scholar]
- 2. Cecchi E, Forno D, Imazio M, et al. ; Piemonte Infective Endocarditis Study Group. New trends in the epidemiological and clinical features of infective endocarditis: results of a multicenter prospective study. Ital Heart J 5: 249-256, 2004. [PubMed] [Google Scholar]
- 3.Langlois V, Lesourd A, Girszyn N, et al. Antineutrophil cytoplasmic antibodies associated with infective endocarditis. Medicine (Baltimore) 95: e2564, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30: 633-638, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Khalighi MA, Nguyen S, Wiedeman JA, Palma Diaz MF. Bartonella endocarditis-associated glomerulonephritis: a case report and review of the literature. Am J Kidney Dis 63: 1060-1065, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Larson AM, Dougherty MJ, Nowowiejski DJ, et al. Detection of Bartonella (Rochalimaea) quintana by routine acridine orange staining of broth blood cultures. J Clin Microbiol 32: 1492-1496, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houpikian P, Raoult D. Western immunoblotting for Bartonella endocarditis. Clin Diagn Lab Immunol 10: 95-102, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fournier PE, Lelievre H, Eykyn SJ, et al. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine (Baltimore) 80: 245-251, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Baorto E, Payne RM, Slater LN, et al. Culture-negative endocarditis caused by Bartonella henselae. J Pediatr 132: 1051-1054, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Mahr A, Batteux F, Tubiana S, et al. Prevalence of anti-neutrophil cytoplasmic antibodies in infective endocarditis. Arthritis Rheumatol 66: 1672-1677, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Aslangul E, Goulvestre C, Mallat Z, et al. Human Bartonella infective endocarditis is associated with high frequency of anti-proteinase 3 antibodies. J Rheumatol 41: 408-410, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Wagner J, Andrassy K, Ritz E. Is vasculitis in subacute bacterial endocarditis associated with ANCA? Lancet 337: 799-800, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Matera G, Liberto MC, Quirino A, et al. Bartonella quintana lipopolysaccharide effects on leukocytes, CXC chemokines and apoptosis: a study on the human whole blood and a rat model. Int Immunopharmacol 3: 853-864, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Lins KA, Drummond MR, Velho PENF. Cutaneous manifestations of bartonellosis. An Bras Dermatol 94: 594-602, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teoh LSG, Hart HH, Soh MC, et al. Bartonella henselae aortic valve endocarditis mimicking systemic vasculitis. BMJ Case Rep 2010: bcr0420102945, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boils CL, Nasr SH, Walker PD, Couser WG, Larsen CP. Update on endocarditis-associated glomerulonephritis. Kidney Int 87: 1241-1249, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Haare Heijmeijer S, Wilmes D, Aydin S, Clerckx C, Labriola L. Necrotizing ANCA-positive glomerulonephritis secondary to culture-negative endocarditis. Case Rep Nephrol 2015: 649763, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh GC, Sharma B, Katageri B, Bhardwaj M. ANCA positivity in a patient with infective endocarditis-associated glomerulonephritis: a diagnostic dilemma. Yale J Biol Med 87: 373-377, 2014. [PMC free article] [PubMed] [Google Scholar]
- 19.Nasr SH, Radhakrishnan J, D'Agati VD. Bacterial infection-related glomerulonephritis in adults. Kidney Int 83: 792-803, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Raoult D, Fournier PE, Vandenesch F, et al. Outcome and treatment of Bartonella endocarditis. Arch Intern Med 163: 226-230, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Rolain JM, Brouqui P, Koehler JE, Maguina C, Dolan MJ, Raoult D. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother 48: 1921-1933, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamorita Y, Fujigaki Y, Imase A, et al. Successful treatment of infectious endocarditis associated glomerulonephritis mimicking C3 glomerulonephritis in a case with no previous cardiac disease. Case Rep Nephrol 2014: 569047, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]