Abstract
Chitinase-3-like-1 (CHI3L1) is known to induce inflammation in the progression of allergic diseases. Previous our studies revealed that 2-({3-[2-(1-cyclohexen-1-yl)ethyl]-6,7-dimethoxy-4-oxo-3,4-dihydro-2-quinazolinyl}sulfanyl)-N-(4-ethylphenyl)butanamide (K284-6111; K284), the CHI3L1 inhibiting compound, has the anti-inflammatory effect on neuroinflammation. In this study, we investigated that K284 treatment could inhibit the development of atopic dermatitis (AD). To identify the effect of K284, we used phthalic anhydride (5% PA)-induced AD animal model and in vitro reconstructed human skin model. We analyzed the expression of AD-related cytokine mediators and NF-κB signaling by Western blotting, ELISA and quantitative real-time PCR. Histological analysis showed that K284 treatment suppressed PA-induced epidermal thickening and infiltration of mast cells. K284 treatment also reduced PA-induced release of inflammatory cytokines. In addition, K284 treatment inhibited the expression of NF-κB activity in PA-treated skin tissues and TNF-α and IFN-γ-treated HaCaT cells. Protein-association network analysis indicated that CHI3L1 is associated with lactoferrin (LTF). LTF was elevated in PA-treated skin tissues and TNF-α and IFN-γ-induced HaCaT cells. However, this expression was reduced by K284 treatment. Knockdown of LTF decreased the expression of inflammatory cytokines in TNF-α and IFN-γ-induced HaCaT cells. Moreover, anti-LTF antibody treatment alleviated AD development in PA-induced AD model. Our data demonstrate that CHI3L1 targeting K284 reduces AD-like skin inflammation and K284 could be a promising therapeutic agent for AD by inhibition of LTF expression.
Keywords: CHI3L1, Lactoferrin, Atopic dermatitis, K284-6111
INTRODUCTION
Atopic dermatitis (AD), a common chronic inflammatory skin disease is characterized by an imbalance of Th1/Th2 cells and increase in inflammatory cells and IgE (1,2,3). The release of cytokines and infiltration of various inflammatory cells, including mast cells, Th2 cells, and lymphocytes, in the skin lesions causes the development of AD (4,5,6).
Keratinocytes form the skin barrier protecting the body from environmental damage due to allergens, scratches, or microbial toxins. In AD development, keratinocytes play a crucial role in the pathogenesis of inflammatory skin disease by secreting proinflammatory mediators as a cellular source of the danger signal (7). Moreover, activated keratinocytes stimulate mast cells and dendritic cells that trigger Th2 cell polarization, thus promoting AD development (8).
Numerous drugs that inhibit the activation of both immune cells and mast cells as well as the release of cytokines have been used. For example, Omalizumab is an anti-IgE antibody that mitigates AD-related cytokine release and the recruitment of immune cells by inactivating mast cells (9). Dupilumab is an anti-IL-4Rα receptor antibody that blocks IL-4Rα, thus preventing Th2 allergic inflammation due to IgE production (10,11,12). Crisaborole is a topical calcineurin inhibitor that suppresses the release of inflammatory cytokines by inhibiting both PDE4 and the NF-κB signaling pathway, thereby improving the symptoms of AD (13). Pimecrolimus is a non-steroidal anti-inflammatory drug that is safe and effective in the treatment of AD by selectively inhibiting T cell and mast cell activation (14,15).
CHI3L1 is secreted by macrophages, neutrophils, synoviocytes, chondrocytes, and keratinocytes, and exhibits properties of cytokines and growth factors (16,17). The potential importance of CHI3L1 has been reported for a large number of diseases (9,18). Several diseases such as diabetes, Alzheimer's disease, asthma, and many human neoplasias have demonstrated increased expression and serum levels of CHI3L1 (16,18,19). High genetic variation in the promoter region of CHI3L1 has been associated with atopy (20). A population-based study involving 6514 Danish adults showed an association between polymorphisms of the CHI3L1 gene and atopy (21). Another study found that CHI3L1 contributed to Th2 inflammation, M2 macrophage activation, and skin barrier function in ovalbumin-induced AD model CHI3L1 knockout (KO) mice (22). CHI3L1 serum levels were significantly higher in patients with AD compared with serum levels of the controls (23,24). Thus, blocking the expression of CHI3L1 could be critical for the treatment of AD (18,21).
Lactoferrin (LTF) is a member of the transferrin gene family, and its protein product is found in milk and secondary granules of neutrophils (25). LTF is an important component of the nonspecific immune system (26). It is noteworthy that CHI3L1 is colocalized with LTF in specific granules of neutrophils (27).
Human LTF administration reduced has been reported to reduce the cognitive decline resulting from oxidative stress and inflammation in an Alzheimer's disease mouse model (28). In a clinical study, oral administration of bovine LTF dpatient scores for AD symptoms and dermatology life quality index in patients with AD (29). Our web-based analysis showed that CHI3L1-related LTF expression was increased in patients with AD. However, the relationship between CHI3L1 and LTF in AD development has not been investigated.
Previously, using 3D chemistry database analysis with virtual screening, we found 11 candidate compounds with CHI3L1-inhibiting activity among 14 million chemicals. Among the 11 compounds, K284 particularly had the highest binding to CHI3L1. We also found that K284 ameliorated the development of Alzheimer's disease (30). Therefore, based on these observations, we investigated the anti-atopic and anti-inflammatory effects of K284 in a PA-induced AD animal model, an AD-like reconstructed human skin (RHS) model, and an in vitro model.
MATERIALS AND METHODS
Reagents
K284-6111 was obtained from ChemDiv, Inc. (San Diego, CA, USA). K284-6111 was dissolved in dimethyl sulfoxide (DMSO; final concentration of 100 mM) and stored at −20°C until use. Human TNF-α, IFN-γ, IL-4 and IL-13 were purchased from Peprotech Inc. (Rocky Hill, NJ, USA) and were dissolved according to manufacturer's instructions. Aliquots were stored at −20°C until use. Transfection agents, Lipofectamine® 3000 and RNAiMAX, and Opti-MEM were obtained from Thermofisher Scientific (Waltham, MA, USA).
Ethical approval and animal care
The experimental protocols were carried out according to the guidelines for animal experiments of the Institutional Animal Care and Use Committee of Laboratory Animal Research Center at Chungbuk National University, Korea (CBNUA-1344-20-01). All efforts were made to minimize animal suffering, and to reduce the number of animals used. All mice were housed in 3 mice per cage with automatic temperature control (21°C –25°C), relative humidity (45%–65%), and 12 hours light-dark cycle. Food and water were available ad libitum.
Animal treatment
C57BL/6J mice (8-wk-old, n=40) were randomly divided into one of 3 groups. In the first group (PA, n=10), 200 μl (10 μl/cm2) of 5% phthalic anhydride (PA) solution was spread on the back skin 3 times a week for 4 wk. The second group (K284; 1 mg/kg, n=10) and third group (K284; 2 mg/kg, n=10) were applied with PA, and 3 hours after 200 μL of 1 mg/ml and 2 mg/ml K284 solution (10 μg or 20 μg/cm2) were applied. Age-matched C57BL/6J were used as the control group (control, n=10) and treated AOO solution (vehicle; acetone:olive oil=4:1). By the end of the study period, mice were sacrificed, and blood samples were collected. K284 was diluted in AOO solution at indicated concentrations. C57BL/6J mice were purchased from Daehan Biolink (Eumseong, Korea).
Measurement of body and lymph node weight
Alterations of body weight during the experimental procedure were measured with an electronic balance (Mettler Toledo, Greifensee, Switzerland) once a week for 4 wk. Weights of lymph nodes collected from the sacrificed mice were also measured by the same method.
AD-like RHS model
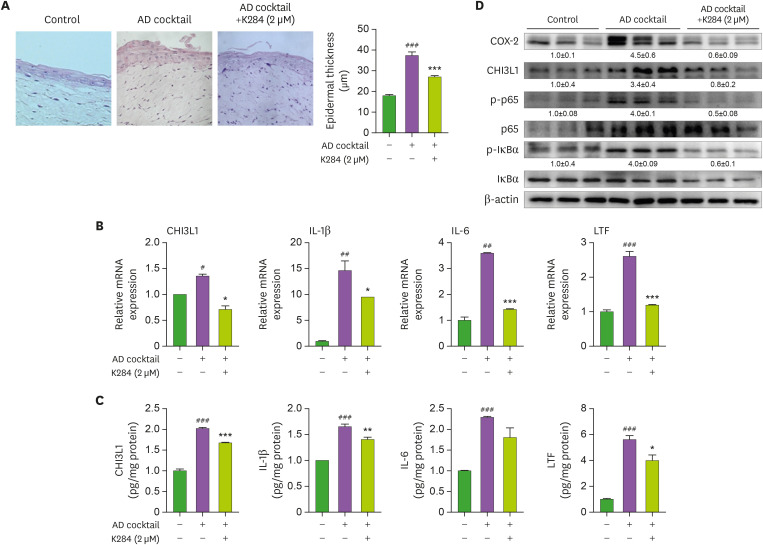
A RHS model (Neoderm®-ED) was purchased from TEGO Science Inc (Seoul, Korea). The RHS model contained epidermis and dermis. The RHS model generated AD-like inflammation via use of an inflammatory cocktail according to the previously described method with some modifications (31). In brief, an inflammatory cocktail (an AD cocktail consisting of 30 ng/ml of IL-4, 30 ng/ml of IL-13, and 3.5 ng/ml of TNF-α) was supplemented with or without K284 (2 μM) to the culture medium for 6 days. The culture medium was changed every 48 h.
Cell culture and transfection
The HaCaT keratinocyte cell line was kindly provided from Prof. Do-Young Yoon (Konkuk University, Seoul, Korea). These cells were grown at 37 °C in DMEM medium supplemented with 10% FBS, penicillin (100 units/ml) and streptomycin sulfate (100 μg/ml) in humidified atmosphere of 5% CO2. Cells were incubated with K284 at various concentrations (0.5, 1 and 2 μM; dissolved in DMSO) and then stimulated with the TNF-α and IFN-γ (20 ng/ml) combination for the indicated time points in figure legends. The final concentration of DMSO used was less than 0.05%. Cells were treated with 0.05% DMSO as vehicle control. For transfection, HaCaT cells were transiently transfected with CHI3L1 plasmid vector, CHI3L1 siRNA or LTF siRNA, using Lipofectamine 3000 (for plasmid vector) and RNAiMAX (for siRNA) reagent in Opti-MEM, according to the manufacturer's specifications. CHI3L1 siRNA and LTF siRNA were purchased from Origene (Rockville, MA, USA).
Immunohistochemistry (IHC)
Back skins removed from mice and RHS tissues were fixed with 4% formalin, embedded in paraffin wax, routinely processed, and then sectioned into 5 μm thick slices. The skin sections were then stained with H&E. The thickness of the epidermis was also measured using a light microscope (Olympus, Tokyo, Japan). The slides were incubated with specific primary antibodies: p-p65 and LTF (Abcam). Mast cells were stained with toluidine blue solution according to manufacturer's instruction (IHC World, Ellicott City, MD, USA).
ELISA analysis
The serum IgE and cytokines in skin tissues were measured by ELISA kits obtained from KOMA Biotech (Seoul, Korea) and R&D systems (Minneapolis, MN, USA) according to the manufacturer's instruction. Human serum LTF levels were analyzed by LTF ELISA kit (myBioSource, San Diego, CA, USA).
Western blot analysis
100 mg skin tissues or about 1 × 106 cells were harvested and homogenized with lysis buffer (Pro-prep protein extraction buffer, iNtRON, Seongnam, Korea). Nuclear extraction of samples was performed using a nuclear extraction kit (Abcam, Cambridge, MA, USA).The membrane was incubated for 4 hours at room temperature with specific antibodies: rabbit polyclonal antibodies against CHI3L1, LTF, p-p65 and COX-2 (1:1,000, Abcam), and rabbit polyclonal antibodies against myc-tag, p65, p50, histone H1, p-IκB-α and IκB-α (1:1,000, Cell Signaling) and mouse monoclonal antibody against β-actin (1:1,000, Sigma Aldrich). The intensity of the bands was measured using the Fusion SOLO S image acquisition system (Vilber Lourmat, Eberhardzell, Germany). Band intensity was measured by ImageJ software (NIH, Bethesda, MA, USA).
Quantitative real-time PCR
Total RNA of skin tissues, RHS tissues or cells was extracted by a RiboEX RNA Extraction Kit (GeneAll Biotechnology, Seoul, Korea) and cDNA was synthesized using a High Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA, USA). Quantitative real-time PCR was performed using specific primers with SYBR Green master mix (ELPIS Biotech, Daejeon, Korea) in a StepOnePlusTM PCR System (Applied Biosystems, Foster City, CA, USA) (Supplementary Table 1). The values obtained for the target gene expression were normalized to 18S and quantified relative to the expression in control samples.
Protein-protein interaction network and AD patient analysis
The protein-protein interaction network of CHI3L1 was analyzed by STRING database (http://string-db.org), which is publicly available sources of protein-protein interaction information (32). Expression of CHI3L1-associated genes in AD patients was analyzed using ArrayExpress (http://ebi.ac.uk/arrayexpress), archive of functional genomics data from high-throughput functional genomics experiments (Accession No. E-GEOD-32924).
Human samples
Human serum samples from patients with AD and healthy controls (20 samples, respectively) were obtained from Chungbuk National University Hospital Biobank and Korea Institute of Radiological & Medical Sciences Radiation Biobank. All studies using human samples were conducted in accordance with the Declaration of Helsinki and were approved by the Ethics Committee of Chungbuk National University Medical Center (IRB No. CBNU-201902-BR-786-01).
Statistical analysis
The experiments were conducted in triplicate, and all experiments were repeated at least 3 times with similar results. All statistical analysis was performed with GraphPad Prism 5 software (Version 5.03; GraphPad software, Inc., San Diego, CA). Group differences were analyzed by one-way ANOVA followed by Tukey's multiple comparison test. All values are presented as mean ± SD. Significance was set at p < 0.05 for all tests
RESULTS
Effects of K284 treatment on the AD development
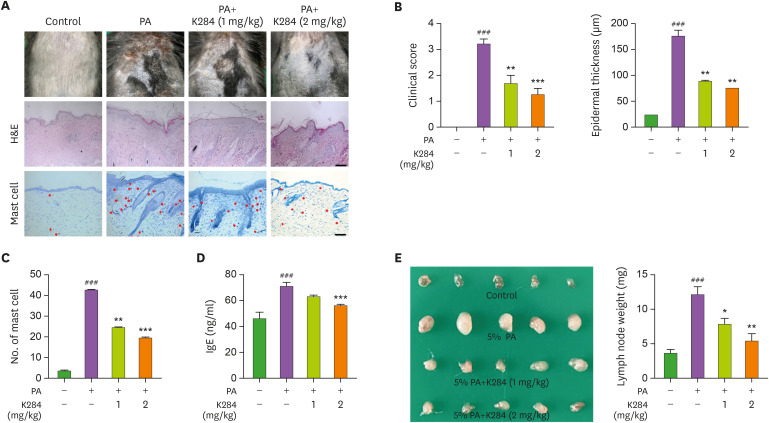
We measured changes in body weight during the experimental period and found no significant differences after any of the treatments (Supplementary Fig. 1). To investigate whether K284 treatment could suppress PA-induced AD development, we observed epidermal thickness and histological changes. AD symptoms, consisting of erythema, edema, and erosion (clinical score), were increased compared with those of the control group. Significant increases in epidermal thickness were also detected after PA treatments; these changes were dramatically reduced upon K284 treatment (Fig. 1A and B). Next, we checked the infiltration of mast cells in the AD skin lesion. K284 treatment reduced the number of infiltrating mast cells in the skin compared with that of the PA-induced group (Fig. 1C). The hyperproduction of IgE is also one of the characteristic features of AD; therefore, we measured serum IgE concentration in PA-induced mice. The PA-induced serum IgE concentration was decreased in the K284-treated group in a dose-dependent manner (Fig. 1D). In addition, we evaluated the weight of the skin’s draining lymph node as an indicator of skin inflammation. PA treatment increased lymph node weight in treated mice compared with the weight of control mice. In contrast, lymph node weight was significantly reduced in the K284-treated mice in a dose-dependent manner (Fig. 1E).
Figure 1. K284 inhibits PA-induced AD. (A) PA solution was applied topically to the dorsal skin of mice in each group 3 times a week for 4 wk. K284 was topically administrated to the dorsal skin 2 h before PA solution application with indicated doses. (B) The photographs are representative of each group of mice. Bar graphs represent clinical scores, epidermal thickness and the number of mast cells. Scale bar=100 μm. (C) The morphologic, histologic and the number of mast cell changes in mice. (D) Serum IgE concentration was measured by ELISA (n=5). (E) Changes of skin draining lymph node weight in each group (n=5).
#Control vs. PA and *PA vs. PA+K284. *p<0.05, **p<0.01 and ###,***p<0.001.
Effect of K284 treatment on the release of cytokines
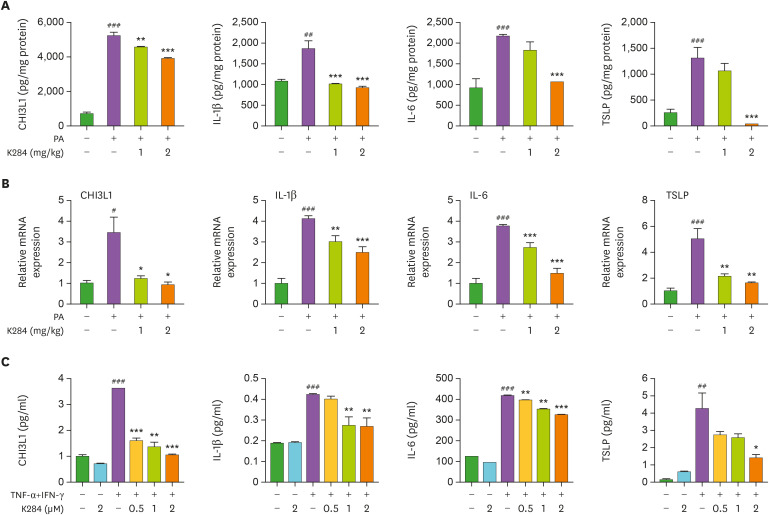
To determine whether K284 treatment could reduce the release of inflammatory cytokines in PA-treated skin tissue, we measured the levels of CHI3L1, IL-1β, IL-4, IL-6, and din skin tissues of the control, PA-, and PA with K284-treated groups. The levels of these inflammatory mediators were generally higher in the PA-treated group than in the control group. However, these levels were dramatically and dose-dependently lower in the K284-treated group compared with those of the control group (Fig. 2A). Moreover, mRNA expression of AD-related genes such as TNF-α, IL-1β, IL-4, IL-5, IL-6, IL-13, IL-31, IL-33, and CCL17 was reduced in the K284-treated PA-induced group compared with that of the PA-induced group (Fig. 2B and Supplementary Fig. 2). Next, we examined cytokine release in HaCaT cells treated with TNF-α/IFN-γ combination. This combination treatment significantly increased the levels of cytokine release; however, these elevated cytokine levels were inhibited by treatment with K284 in a concentration-dependent manner as determined by ELISA (protein levels) (Fig. 2C) and qPCR (mRNA levels) (Supplementary Fig. 2B).
Figure 2. K284 inhibits AD-related cytokine release. (A) Levels of AD-related cytokines CHI3L1, IL-1β, IL-6 and TSLP in PA-treated skin tissues measured by ELISA (n=5). (B) Levels of AD-related cytokines CHI3L1, IL-1β, IL-6 and TSLP in PA-treated skin tissues measured by qPCR (n=5). (C) HaCaT cells were pre-treated with K284 at indicated concentrations. After 2 h, cells were treated with the TNF-α and IFN-γ combination (20 ng/ml) for another 4 h. The mRNA expression of CHI3L1, IL-1β, IL-6 and TSLP was analyzed by qPCR (n=3).
#Control vs. PA and *PA vs. PA+K284. #,*p<0.05, ##,**p<0.01 and ###,***p<0.001.
Effect of K284 treatment on NF-κB signaling
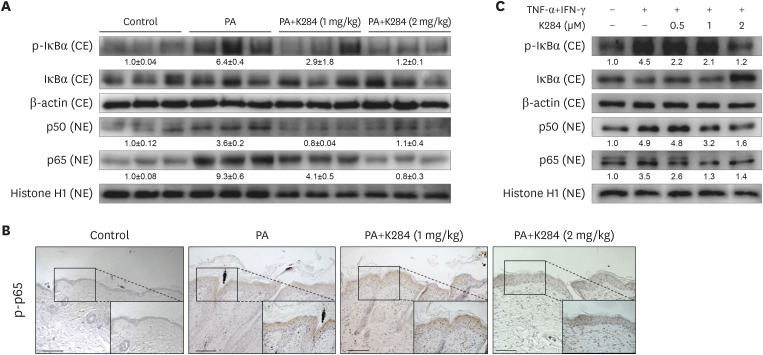
NF-κB has been implicated in the inflammatory response of the AD model. Furthermore, a previous study revealed that CHI3L1 was involved in NF-κB activation (30). To investigate whether K284 could inhibit NF-κB activation in the PA-induced AD model, we prepared and assayed nuclear extracts from skin tissues by Western blot analysis. PA-treated mice showed an increase in the expression of p50 and p65 in the nucleus; whereas, K284 inhibited the nuclear translocation of p50 and p65 in a dose-dependent manner (Fig. 3A and B). In addition, PA-treated skin tissues showed significantly increased phosphorylation of IκBα (p-IκBα) in the cytosolic fraction compared with that of the control group. In contrast, p-IκBα expression in K284-treated skin tissues was significantly reduced in comparison with that of the PA-treated skin tissues (Fig. 3A). Additionally, increased phosphorylation of p65 in PA-induced skin tissues was inhibited. Next, we determined NF-κB activation in TNF-α/IFN-γ-activated HaCaT cells. HaCaT cells were pre-treated with K284 for 2 h and then treated with the TNF-α/IFN-γ combination for 4 h, which is the time necessary to activate NF-κB maximally using this combination (data not shown). We investigated the inhibitory effects of K284 on the translocation of the NF-κB subunits p50 and p65 and IκB phosphorylation. Consistent with the inhibitory effects on the in vivo sample, both the nuclear translocation of p50 and p65 and the TNF-α/IFN-γ-combination-induced phosphorylation of IκBα were inhibited by K284 in a concentration-dependent manner (Fig. 3C).
Figure 3. K284 inhibits the NF-κB activity. (A) Expression of phosphorylated IκBα in cytosolic fractions and p50 and p65 in nuclear fractions in PA-treated skin tissues using Western blot analysis. (B) Expression of phosphorylated p65 in PA-treated skin tissues analyzed by IHC. Scale bar=50 μm. (C) HaCaT cells were pre-treated with K284 at indicated concentrations. After 2 h, cells were treated with TNF-α and IFN-γ (20 ng/ml) for another 4 h. Expression of phosphorylated IκBα in cytosolic fractions and p50 and p65 in nuclear fractions was detected by Western blot analysis.
CE: cytosolic faction / NE: nuclear fraction.
LTF was associated with CHI3L1-mediated AD development
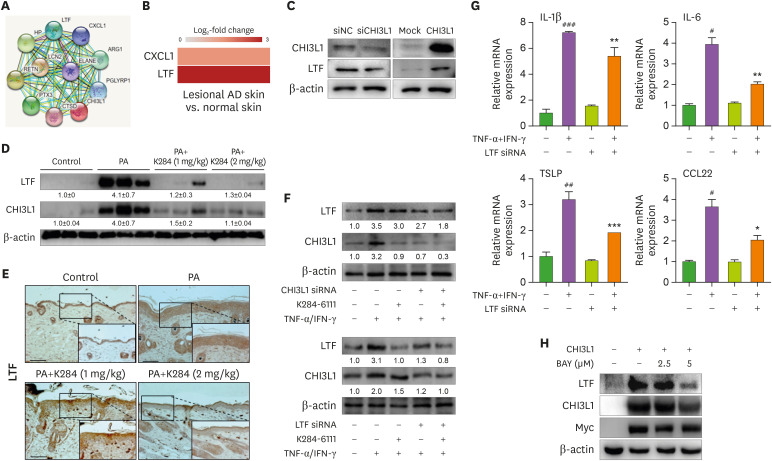
To determine which factors were involved in CHI3L1-induced inflammation, we used protein-protein interaction network analysis, and the results showed that CHI3L1 was associated with several proteins (Fig. 4A). Among these proteins, ArrayExpress analysis showed that CXCL1 and LTF were elevated in the AD skin tissues compared with those of healthy controls (Fig. 4B). Next, we checked whether CHI3L1 could regulate the expression of CXCL1 and LTF in HaCaT cells. The results showed that LTF expression was regulated by CHI3L1 (Supplementary Figs. 3A and 4C), but CHI3L1 knockdown and overexpression did not change the expression of CXCL1 (Supplementary Fig. 3B). However, K284 treatment reduced LTF expression in PA-induced skin tissues (Fig. 4D and E). We also analyzed the serum level of LTF in patients with AD. Serum LTF was significantly increased in patients with AD compared with that of the controls (Supplementary Fig. 4). Receiver operating characteristic (ROC) analysis of LTF showed that sensitivity, specificity, cut-off value, and area under the curve were 75%, 75%, 1186 ng/ml, and 0.8425, respectively (Supplementary Fig. 4). Regarding inflammatory status, our analysis showed that CHI3L1 could mediate LTF expression. Furthermore, K284 treatment reduced TNF-α/IFN-γ-induced LTF expression, while the knockdown of CHI3L1 or LTF by siRNA synergistically reduced K284-inhibited LTF expression (Fig. 4F). To identify the role of LTF in inflammation, we inhibited LTF expression by siRNA and confirmed the expression of inflammatory cytokines in TNF-α/IFN-γ-treated HaCaT cells. The mRNA expression of IL-1β, IL-6, thymic stromal lymphopoietin (TSLP), and CCL22 was reduced in LTF knockdown HaCaT cells compared with the levels in the control cells in response to TNF-α/IFN-γ stimulation (Fig. 4G). To identify whether CHI3L1-mediated NF-κB activation could mediate LTF expression, we treated the NF-κB inhibitor BAY 11-7082 (BAY) in CHI3L1-overexpressed HaCaT cells. The results showed that CHI3L1-induced LTF expression was reduced by the inhibition of NF-κB (Fig. 4H). Thus, LTF is critical for CHI3L1-mediated inflammatory responses in AD development.
Figure 4. K284 reduces LTF expression. (A) Protein-association network analysis of Chi3L1 (Human). (B) The changes of CHI3L1-related proteins in the patients of AD obtained from ArrayExpress. (C) HaCaT cells were transfected with CHI3L1 plasmid vector or siRNA (20 nM) for 24 h. The levels of CHI3L1 and LTF were measured by Western blot analysis. (D) The protein expression levels of CHI3L1 and LTF in PA-treated skin tissues were measured by Western blot. (E) Expression of LTF in in PA-treated skin tissues analyzed by IHC. Scale bar=50 μm. (F) HaCaT cells were transfected with CHI3L1 of LTF siRNA for 24 h. Cell were pre-treated with K284 for 2 h. Then, cells were treated with TNF-α and IFN-γ (20 ng/ml) 4 h. The levels of CHI3L1 and LTF were determined by Western blot analysis. (G) HaCaT cells were transfected with LTF siRNA (20 nM). After 24 h, cells were treated with TNF-α and IFN-γ combination for 4 h. The mRNA expression of IL-1β, IL-6, TSLP and CCL22 was determined by qPCR (n=3).
#Control vs. TNF-α+IFN-γ and *TNF-α+IFN-γ vs. TNF-α+IFN-γ with LTF siRNA. *p<0.05, **p<0.01 and ***p<0.001.
K284 attenuates the inflammation in the AD-like RHS model
To confirm the anti-inflammatory effects of K284 on AD development, we used an AD-like RHS model. In this model, we found that K284 treatment significantly inhibited epidermal thickness (Fig. 5A). Increased levels of expression of AD-related cytokines (i.e., IL-1β, IL-6, TSLP, and CHI3L1) were also suppressed by K284 treatment, as detected using qPCR and ELISA (Fig. 5B and C). The AD cocktail-induced expression of COX-2, CHI3L1, and NF-κB activity was also reduced in K284-treated RHS tissues (Fig. 5D).
Figure 5. K284 reduces AD-like skin inflammation in AD-like RHS models. RHS model inserts were cultured for AD cocktail for 6 days. (A) H&E staining was performed to confirm the histological changes of AD-RHS. The bar graph indicates the thickness of the epidermal thickness (n=3). (B and C) The levels of IL-1β, IL-6, CHI3L1 and LTF in AD-RHS tissues were measured by qPCR and ELISA (n=3). (D) Expressions of COX-2, CHI3L1, p-p65, p65, p-IκBα and IκBα were observed by Western blot analysis in AD-RHS tissues.
#Control vs. AD cocktail and *AD cocktail vs. AD cocktail+K284. #,*p<0.05, ##,**p<0.01 and ###,***p<0.001.
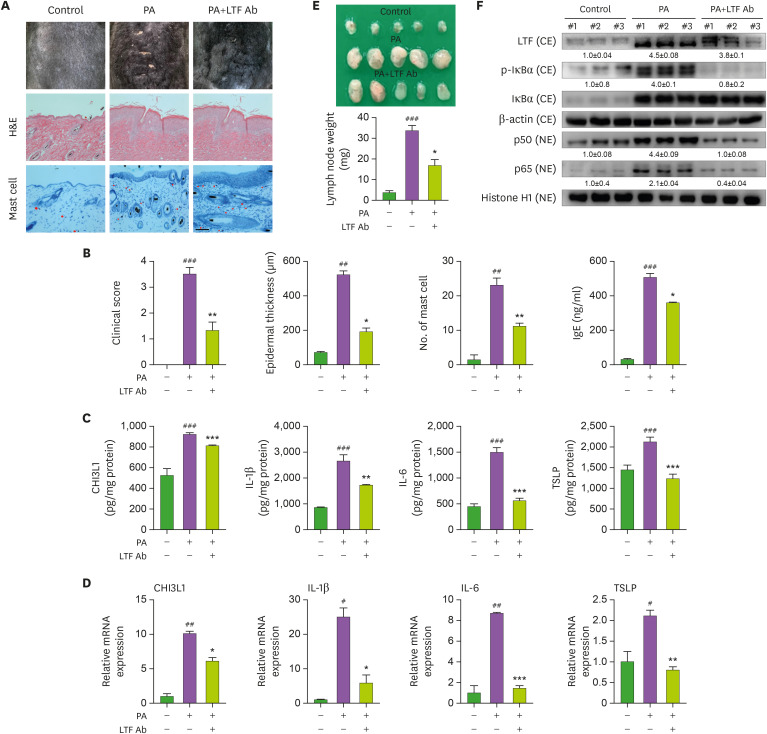
Blockade of LTF suppresses AD development in PA-induced model
We further examined the inflammatory effects of LTF in the PA-induced AD model. The administration of an anti-LTF antibody (LTF Ab) reduced PA-induced AD development (Fig. 6A). Histologic analysis showed that LTF Ab administration reduced epidermal thickening, mast cell infiltration, and serum IgE levels (Fig. 6A and B). The protein and mRNA levels of AD-related cytokines, including CHI3L1, IL-1β, IL-6, and TSLP, in skin tissue samples were significantly reduced by blocking LTF (Fig. 6C and D). The draining lymph node weight was significantly reduced in the LTF Ab-administered PA-induced group compared with that of the PA-induced group (Fig. 6E). Moreover, anti-LTF antibody treatment reduced NF-κB activation in the PA-induced mice (Fig. 6F).
Figure 6. Blockade of LTF reduced PA-induced AD development. (A) PA solution was applied topically to the dorsal skin of mice in each group 3 times a week for 4 wk. From the third week, LTF Ab (1 mg/kg; diluted in PBS) was subcutaneously injected 2 h after PA solution treatment. The photographs are representative of each group of mice (n=5). (B) Bar graphs represent clinical scores, epidermal thickness and the number of mast cells. Scale bar=100 μm. Serum IgE concentration was measured by ELISA. (C and D) The levels of CHI3L1, IL-1β, IL-6 and TSLP in PA-induced skin tissues measured by ELISA (n=5). (E) Expressions of LTF as well as p-IκBα, p50 and p65 were observed by Western blot analysis in PA-induced AD tissues.
CE: cytosolic faction / NE: nuclear fraction.
#Control vs. PA and *PA vs. PA+LTF Ab. #,*p<0.05, ##,**p<0.01 and ###,***p<0.001.
DISCUSSION
AD is a chronic inflammatory skin disease characterized by eczematous skin lesions. Despite their irreversible side effects, oral and topical immunosuppressive drugs have been used because of their remarkable anti-inflammatory and anti-allergic activities (33). However, their effectiveness is limited as their exact mechanism-based targets in the treatment of AD are not clear.
The chronic inflammation and skin damage that occur in AD involve a complex interplay between environmental, genetic, immunological, and biochemical factors (34). For instance, mast cells, T cells, and keratinocytes interact with one another in the uppermost dermis of inflamed skin. In addition, keratinocytes stimulated by proinflammatory cytokines are important cellular sources of chemokines that affect T lymphocyte differentiation and leukocyte recruitment to skin lesions.
Several studies have demonstrated that CHI3L1 may be a critical target for the treatment of various inflammatory diseases such as rheumatoid arthritis, osteoarthritis, inflammatory bowel disease, chronic obstructive pulmonary disease, atherosclerosis, and cancer (3,27). Our previous results likewise revealed that CHI3L1 could be a significant target of AD because ROC analysis of several AD biomarkers in serum from affected patients indicated that CHI3L1 had the highest score. Moreover, CHI3L1 KO mice showed a significantly reduced occurrence of AD (35). In our other study, K284 was found to be the best potential candidate compound for CHI3L1 inhibition because it ameliorated the onset and development of Alzheimer's disease through its anti-inflammatory effects (30). Based on these results, therefore, we investigated the anti-atopic and anti-inflammatory effects of K284 in a PA-induced AD model, an AD-like RHS model, and an in vitro model. We found that the topical application of K284 ameliorated dermatitis severity, epidermal hyperplasia, and the infiltration of inflammatory cells. We also found that K284 effectively reduced PA-induced inflammatory cytokine release and allergic responses. Similarly, IgE levels, as well as the number of mast cells that infiltrated the dermis, were also significantly reduced in the K284-treated mice. These data suggest that K284 could be an appropriate treatment for AD-like inflammatory diseases.
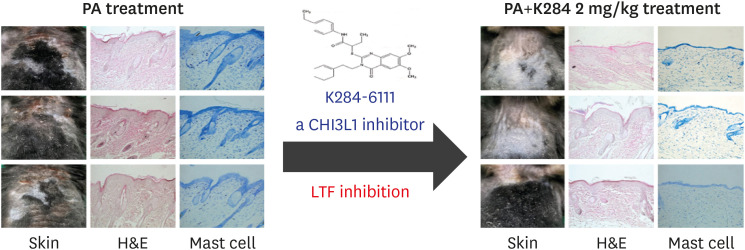
LTF, a member of the transferrin gene family, is an important component of the nonspecific immune system and exhibits anti-inflammatory activity (36). LTF has been known to has anti-microbial activities, modulates the immune responses, and protects against viral infections. It was found that CHI3L1 is colocalized with lactoferrin in neutrophils of the arthritis rheumatic inflamed joint (27), and is a close relation between serum CHI3L1 and lactoferrin levels in persons with acute bacterial pneumonia (37) as well as inflammatory bowel disease and hydrometra (38,39). However, contrary results have been reported. LTF was found to reduce the production of inflammatory mediators and reactive oxygen species and inhibit immune cell recruitment in various diseases, including Alzheimer's disease, inflammatory bowel disease, and asthma. However, a recent study has associated LTF allergenicity. LTF sensitization increased hypersensitivity through the production of IgG and IgE (40). It is noteworthy that LTF administration induced allergic airway inflammation in an asthma mouse model (41). LTF release has been reported to also correlate with serum IgE levels and asthma severity in patients with asthma (42). Our web-based protein-association network and serum analyses showed that CHI3L1 and LTF significantly correlated with AD patients. We also found that the mRNA and protein levels of CHI3L1 and LTF were concomitantly increased in AD-like skin inflammation. Another result is that the overexpression of CHI3L1 elevated the expression of LTF, but the knockdown of CHI3L1 suppressed this expression. However, the K284 treatment suppressed the expression of LTF. In addition, the combination treatment of K284 with the siRNA of CHI3L1 further suppressed LTF expression. Thus, these 2 factors could be associated with each other in inflammatory atopy, just as the anti-inflammatory and anti-atopic effect of K284, a CHI3L1 inhibitor, could be associated with the inhibition of LTF expression. NF-κB is responsible for the up-regulation of proinflammatory cytokines—an important factor to be considered in the treatment of AD (43). IgE-induced TNF-α and IL-6 stimulate the activation of NF-κB in mast cells (44). Many studies have shown that compounds that suppress NF-κB activation could act as therapeutic agents for AD (45,46,47,48,49). In the present study, we found that the K284 treatment inhibited the expression of NF-κB subunits p50 and p65, which could contribute to reduced levels of proinflammatory cytokines (e.g., TNF-α, IL-1β, IL-6), the expression of COX-2, as well as mast cell activation and production of IgE (50,51). CHI3L1 activates the NF-κB signaling pathway and enhances the secretion of TNF-α and IL-8. In our previous study, we found that K284-6111 directly binds to CHI3L1, showing the strongest protein-binding affinity (−9.7 kcal/mol) in a docking model. K284-6111 also exhibits an anti-inflammatory effect in LPS-induced microglial BV-2 cells and primary astrocytes via the inhibition of NF-κB activation (30). Similar to these previous findings, our K284 treatment inhibited CHI3L1-dependent NF-κB activity. This inhibition suppressed the levels of AD-related cytokines and serum IgE and suppressed the infiltration of mast cells, thus leading to reduced AD-like skin inflammation. These data suggest that the inhibitory effects of K284 on NF-κB could contribute to its anti-inflammatory and anti-atopic properties. K284-6111 exhibited significantly advantageous drug-likeness in the diseases evaluated by computational ADME QSAR models using preADMET (http://preadmet.bmdrc.org) and StarDrop (http://www.optibrium.com). Furthermore, K284-6111 showed no toxic effects with an oral intake of 5 mg/kg/day for 4 wk (data not shown) and showed a bioavailability of 73.6% (Tmax: 309 min; Cp Max: 0.1 μg/ml). Thus, K284-6111 could be a candidate compound to be developed as a drug for treating AD. These results suggest that CHI3L1 modulates LTF to exacerbate atopic dermatitis through the NF-κB pathway and is a new inflammatory target for AD (Fig. 7).
Figure 7. Graphic illustration of the inhibition of atopic kin inflammation by CHI3L1 via repressing lactoferrin.
These results suggest that CHI3L1 modulates LTF to exacerbate atopic dermatitis through the NF-κB pathway and is a new inflammatory target for AD. Therefore, the CHI3L1 inhibitor K284-6111 is a potential candidate as a therapeutic agent that can relieve allergic dermatitis and improve AD.
ACKNOWLEDGEMENTS
This work is financially supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIP) (No. MRC, 2017R1A5A2015541).
Abbreviations
- AD
atopic dermatitis
- AUC
area under the curve
- CHI3L1
Chitinase-3-like 1
- KO
knockout
- LTF
lactoferrin
- PA
phthalic anhydride
- RHS
reconstructed human skin
- ROC
receiver operating characteristic
- TSLP
thymic stromal lymphopoietin
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Hong JT.
- Formal analysis: Jeon SH, Lee YS, Yeo IJ, Lee HP, Yoon JS, Son DJ, Han SB, Hong JT.
- Funding acquisition: Hong JT.
- Investigation: Jeon SH, Lee YS, Hong JT.
- Supervision: Hong JT.
- Writing - original draft: Jeon SH, Lee YS, Yeo IJ, Lee HP, Yoon JS, Son DJ, Han SB, Hong JT.
- Writing - review & editing: Hong JT.
SUPPLEMENTARY MATERIALS
Primer sequence information
Changes of body weight during the 4-wk experiment. The average weight of each group was checked by measuring twice a week for 4 wk with an electronic scale during the experiment.
K284 inhibits inflammatory cytokine release. (A) Levels of AD-related cytokines IL-4, IL-5, IL-13, IL-31, IL-33 and CCL17 in PA-treated skin tissues measured by qPCR. (B) HaCaT cells were pre-treated with K284 at indicated concentrations. After 2 h, cells were treated with the TNF-α and IFN-γ combination (20 ng/ml) for another 4 h. The mRNA expression of IL-1β, IL-6, TSLP, CHI3L1 and LTF was analyzed by qPCR.
CHI3L1 regulated LTF expression. (A and B) HaCaT cells were transfected with CHI3L1 plasmid vector or human CHI3L1 siRNA (20 nM) for 24 h. The mRNA expression of LTF (A) and CXCL1 (B) was determined by qPCR (n=3).
Serum analysis of LTF in AD patients. The serum level and ROC curve of LTF in patients with AD and healthy controls (n=20).
References
- 1.Thomsen SF. Atopic dermatitis: natural history, diagnosis, and treatment. ISRN Allergy. 2014;2014:354250. doi: 10.1155/2014/354250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone SP, Gleich GJ, Muller SA. Atopic dermatitis and IgE. Relationship between changes in IgE levels and severity of disease. Arch Dermatol. 1976;112:1254–1255. doi: 10.1001/archderm.112.9.1254. [DOI] [PubMed] [Google Scholar]
- 4.Kwak MH, Kim JE, Hwang IS, Lee YJ, An BS, Hong JT, Lee SH, Hwang DY. Quantitative evaluation of therapeutic effect of Liriope platyphylla on phthalic anhydride-induced atopic dermatitis in IL-4/Luc/CNS-1 Tg mice. J Ethnopharmacol. 2013;148:880–889. doi: 10.1016/j.jep.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 5.Sung JE, Lee HA, Kim JE, Go J, Seo EJ, Yun WB, Kim DS, Son HJ, Lee CY, Lee HS, et al. Therapeutic effect of ethyl acetate extract from Asparagus cochinchinensis on phthalic anhydride-induced skin inflammation. Lab Anim Res. 2016;32:34–45. doi: 10.5625/lar.2016.32.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang YY, Wang AX, Xu L, Shen N, Zhu J, Tu CX. Characteristics of peripheral blood CD4+CD25+ regulatory T cells and related cytokines in severe atopic dermatitis. Eur J Dermatol. 2016;26:240–246. doi: 10.1684/ejd.2015.2709. [DOI] [PubMed] [Google Scholar]
- 7.Park HJ, Jang YJ, Yim JH, Lee HK, Pyo S. Ramalin isolated from ramalina terebrata attenuates atopic dermatitis‐like skin lesions in balb/c mice and cutaneous immune responses in keratinocytes and mast cells. Phytother Res. 2016;30:1978–1987. doi: 10.1002/ptr.5703. [DOI] [PubMed] [Google Scholar]
- 8.Brandt EB, Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Immunol. 2011;2:110. doi: 10.4172/2155-9899.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coffman FD. Chitinase 3-Like-1 (CHI3L1): a putative disease marker at the interface of proteomics and glycomics. Crit Rev Clin Lab Sci. 2008;45:531–562. doi: 10.1080/10408360802334743. [DOI] [PubMed] [Google Scholar]
- 10.Blakely K, Gooderham M, Papp K. Dupilumab, a monoclonal antibody for atopic dermatitis: A review of current literature. Skin Therapy Lett. 2016;21:1–5. [PubMed] [Google Scholar]
- 11.Deleanu D, Nedelea I. Biological therapies for atopic dermatitis: an update. Exp Ther Med. 2019;17:1061–1067. doi: 10.3892/etm.2018.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeziorkowska R, Sysa-Jędrzejowska A, Samochocki Z. Topical steroid therapy in atopic dermatitis in theory and practice. Postepy Dermatol Alergol. 2015;32:162–166. doi: 10.5114/pdia.2014.40962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarnagin K, Chanda S, Coronado D, Ciaravino V, Zane LT, Guttman-Yassky E, Lebwohl MG. Crisaborole topical ointment, 2%: a nonsteroidal, topical, anti‐inflammatory phosphodiesterase 4 inhibitor in clinical development for the treatment of atopic dermatitis. J Drugs Dermatol. 2016;15:390–396. [PubMed] [Google Scholar]
- 14.Kapp A, Papp K, Bingham A, Fölster-Holst R, Ortonne JP, Potter PC, Gulliver W, Paul C, Molloy S, Barbier N, et al. Long-term management of atopic dermatitis in infants with topical pimecrolimus, a nonsteroid anti-inflammatory drug. J Allergy Clin Immunol. 2002;110:277–284. doi: 10.1067/mai.2002.126500. [DOI] [PubMed] [Google Scholar]
- 15.Meng Y, Liu Z, Zhai C, Di T, Zhang L, Zhang L, Xie X, Lin Y, Wang N, Zhao J, et al. Paeonol inhibits the development of 1‑chloro‑2,4‑dinitrobenzene‑induced atopic dermatitis via mast and T cells in BALB/c mice. Mol Med Rep. 2019;19:3217–3229. doi: 10.3892/mmr.2019.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kzhyshkowska J, Yin S, Liu T, Riabov V, Mitrofanova I. Role of chitinase-like proteins in cancer. Biol Chem. 2016;397:231–247. doi: 10.1515/hsz-2015-0269. [DOI] [PubMed] [Google Scholar]
- 17.Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, He CH, Takyar S, Elias JA. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeo IJ, Lee CK, Han SB, Yun J, Hong JT. Roles of chitinase 3-like 1 in the development of cancer, neurodegenerative diseases, and inflammatory diseases. Pharmacol Ther. 2019;203:107394. doi: 10.1016/j.pharmthera.2019.107394. [DOI] [PubMed] [Google Scholar]
- 19.Schimpl M, Rush CL, Betou M, Eggleston IM, Recklies AD, van Aalten DM. Human YKL-39 is a pseudo-chitinase with retained chitooligosaccharide-binding properties. Biochem J. 2012;446:149–157. doi: 10.1042/BJ20120377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohn MH, Lee JH, Kim KW, Kim SW, Lee SH, Kim KE, Kim KH, Lee CG, Elias JA, Lee MG. Genetic variation in the promoter region of chitinase 3-like 1 is associated with atopy. Am J Respir Crit Care Med. 2009;179:449–456. doi: 10.1164/rccm.200809-1422OC. [DOI] [PubMed] [Google Scholar]
- 21.Rathcke CN, Holmkvist J, Husmoen LL, Hansen T, Pedersen O, Vestergaard H, Linneberg A. Association of polymorphisms of the CHI3L1 gene with asthma and atopy: a populations-based study of 6514 Danish adults. PLoS One. 2009;4:e6106. doi: 10.1371/journal.pone.0006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwak EJ, Hong JY, Kim MN, Kim SY, Kim SH, Park CO, Kim KW, Lee CG, Elias JA, Jee HM, et al. Chitinase 3-like 1 drives allergic skin inflammation via Th2 immunity and M2 macrophage activation. Clin Exp Allergy. 2019;49:1464–1474. doi: 10.1111/cea.13478. [DOI] [PubMed] [Google Scholar]
- 23.Salomon J, Matusiak Ł, Nowicka-Suszko D, Szepietowski JC. Chitinase-3-like protein 1 (ykl-40) reflects the severity of symptoms in atopic dermatitis. J Immunol Res. 2017;2017:5746031. doi: 10.1155/2017/5746031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki H, Boki H, Kamijo H, Nakajima R, Oka T, Shishido-Takahashi N, Suga H, Sugaya M, Sato S, Miyagaki T. Ykl-40 promotes proliferation of cutaneous t-cell lymphoma tumor cells through extracellular signal–regulated kinase pathways. J Invest Dermatol. 2019;140:860–868.e3. doi: 10.1016/j.jid.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Semak I, Budzevich A, Maliushkova E, Kuzniatsova V, Popkov N, Zalutsky I, Ivashkevich O. Development of dairy herd of transgenic goats as biofactory for large-scale production of biologically active recombinant human lactoferrin. Transgenic Res. 2019;28:465–478. doi: 10.1007/s11248-019-00165-y. [DOI] [PubMed] [Google Scholar]
- 26.Leung DY, Soter NA. Cellular and immunologic mechanisms in atopic dermatitis. J Am Acad Dermatol. 2001;44:S1–S12. doi: 10.1067/mjd.2001.109815. [DOI] [PubMed] [Google Scholar]
- 27.Volck B, Price PA, Johansen JS, Sørensen O, Benfield TL, Nielsen HJ, Calafat J, Borregaard N. YKL-40, a mammalian member of the chitinase family, is a matrix protein of specific granules in human neutrophils. Proc Assoc Am Physicians. 1998;110:351–360. [PubMed] [Google Scholar]
- 28.Guo C, Yang ZH, Zhang S, Chai R, Xue H, Zhang YH, Li JY, Wang ZY. Intranasal lactoferrin enhances α-secretase-dependent amyloid precursor protein processing via the ERK1/2-CREB and HIF-1α pathways in an Alzheimer's disease mouse model. Neuropsychopharmacology. 2017;42:2504–2515. doi: 10.1038/npp.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong PL, West NP, Cox AJ, Gebski VJ, Watts AM, Dodds A, de St Groth BF, Cripps AW, Shumack S. Oral supplementation with bovine whey-derived Ig-rich fraction and lactoferrin improves SCORAD and DLQI in atopic dermatitis. J Dermatol Sci. 2017;85:143–146. doi: 10.1016/j.jdermsci.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Choi JY, Yeo IJ, Kim KC, Choi WR, Jung JK, Han SB, Hong JT. K284-6111 prevents the amyloid beta-induced neuroinflammation and impairment of recognition memory through inhibition of NF-κB-mediated CHI3L1 expression. J Neuroinflammation. 2018;15:224–224. doi: 10.1186/s12974-018-1269-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Lee YS, Han SB, Ham HJ, Park JH, Lee JS, Hwang DY, Jung YS, Hong JT. Interleukin-32γ suppressed atopic dermatitis through inhibition of miR-205 expression via inactivation of nuclear factor-kappa B. J Allergy Clin Immunol. 2020;146:156–168. doi: 10.1016/j.jaci.2019.12.905. [DOI] [PubMed] [Google Scholar]
- 32.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen DE, Heidary N. Treatment of irritant and allergic contact dermatitis. Dermatol Ther. 2004;17:334–340. doi: 10.1111/j.1396-0296.2004.04031.x. [DOI] [PubMed] [Google Scholar]
- 34.Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437–446. doi: 10.1097/ACI.0b013e32832e7d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwak EJ, Hong JY, Kim MN, Kim SY, Kim SH, Park CO, Kim KW, Lee CG, Elias JA, Jee HM, et al. Chitinase 3-like 1 drives allergic skin inflammation via Th2 immunity and M2 macrophage activation. Clin Exp Allergy. 2019;49:1464–1474. doi: 10.1111/cea.13478. [DOI] [PubMed] [Google Scholar]
- 36.Farid AS, El Shemy MA, Nafie E, Hegazy AM, Abdelhiee EY. Anti-inflammatory, anti-oxidant and hepatoprotective effects of lactoferrin in rats. Drug Chem Toxicol. 2021;44:286–293. doi: 10.1080/01480545.2019.1585868. [DOI] [PubMed] [Google Scholar]
- 37.Nordenbaek C, Johansen JS, Junker P, Borregaard N, Sørensen O, Price PA. YKL-40, a matrix protein of specific granules in neutrophils, is elevated in serum of patients with community-acquired pneumonia requiring hospitalization. J Infect Dis. 1999;180:1722–1726. doi: 10.1086/315050. [DOI] [PubMed] [Google Scholar]
- 38.Franzago M, Di Ruscio D, Malavolta I, Muccini H. Collaborative model-driven software engineering: a classification framework and a research map. IEEE Trans Softw Eng. 2018;44:1146–1175. [Google Scholar]
- 39.Antonson P, Nalvarte I, Varshney M, Xu L, Windahl SH, Humire P, Ohlsson C, Gustafsson JÅ, Dahlman-Wright K. Identification of proteins highly expressed in uterine fluid from mice with hydrometra. Biochem Biophys Res Commun. 2015;466:650–655. doi: 10.1016/j.bbrc.2015.09.099. [DOI] [PubMed] [Google Scholar]
- 40.Negaoui H, El Mecherfi KE, Tadjer SA, Grar H, Kheroua O, Saidi D. Bovine lactoferrin allergenicity as studied in murine model of allergy. Food Agric Immunol. 2016;27:711–723. [Google Scholar]
- 41.Nagaoka K, Ito T, Ogino K, Eguchi E, Fujikura Y. Human lactoferrin induces asthmatic symptoms in NC/Nga mice. Physiol Rep. 2017;5:e13365. doi: 10.14814/phy2.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernández-Delgado L, Vega-Rioja A, Ventura I, Chamorro C, Aroca R, Prados M, Bobadilla P, Rodríguez D, Palacios R, Monteseirín J. Allergens induce the release of lactoferrin by neutrophils from asthmatic patients. PLoS One. 2015;10:e0141278. doi: 10.1371/journal.pone.0141278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim JM, Lee B, Min JH, Kim EY, Kim JH, Hong S, Kim JJ, Sohn Y, Jung HS. Effect of peiminine on DNCB-induced atopic dermatitis by inhibiting inflammatory cytokine expression in vivo and in vitro . Int Immunopharmacol. 2018;56:135–142. doi: 10.1016/j.intimp.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 44.Li L, Jin G, Jiang J, Zheng M, Jin Y, Lin Z, Li G, Choi Y, Yan G. Cornuside inhibits mast cell-mediated allergic response by down-regulating MAPK and NF-κB signaling pathways. Biochem Biophys Res Commun. 2016;473:408–414. doi: 10.1016/j.bbrc.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Leung DY. Atopic dermatitis: new insights and opportunities for therapeutic intervention. J Allergy Clin Immunol. 2000;105:860–876. doi: 10.1067/mai.2000.106484. [DOI] [PubMed] [Google Scholar]
- 46.Wullaert A, Bonnet MC, Pasparakis M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21:146–158. doi: 10.1038/cr.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim MS, Lee DY. Insulin-like growth factor binding protein-3 enhances etoposide-induced cell growth inhibition by suppressing the NF-κB activity in gastric cancer cells. Mol Cell Biochem. 2015;403:107–113. doi: 10.1007/s11010-015-2341-2. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka A, Konno M, Muto S, Kambe N, Morii E, Nakahata T, Itai A, Matsuda H. A novel NF-kappaB inhibitor, IMD-0354, suppresses neoplastic proliferation of human mast cells with constitutively activated c-kit receptors. Blood. 2005;105:2324–2331. doi: 10.1182/blood-2004-08-3247. [DOI] [PubMed] [Google Scholar]
- 49.Park JH, Kim MS, Jeong GS, Yoon J. Xanthii fructus extract inhibits TNF-α/IFN-γ-induced Th2-chemokines production via blockade of NF-κB, STAT1 and p38-MAPK activation in human epidermal keratinocytes. J Ethnopharmacol. 2015;171:85–93. doi: 10.1016/j.jep.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 50.Dajee M, Muchamuel T, Schryver B, Oo A, Alleman-Sposeto J, De Vry CG, Prasad S, Ruhrmund D, Shyamsundar R, Mutnick D, et al. Blockade of experimental atopic dermatitis via topical NF-kappaB decoy oligonucleotide. J Invest Dermatol. 2006;126:1792–1803. doi: 10.1038/sj.jid.5700307. [DOI] [PubMed] [Google Scholar]
- 51.Homey B, Steinhoff M, Ruzicka T, Leung DY. Cytokines and chemokines orchestrate atopic skin inflammation. J Allergy Clin Immunol. 2006;118:178–189. doi: 10.1016/j.jaci.2006.03.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequence information
Changes of body weight during the 4-wk experiment. The average weight of each group was checked by measuring twice a week for 4 wk with an electronic scale during the experiment.
K284 inhibits inflammatory cytokine release. (A) Levels of AD-related cytokines IL-4, IL-5, IL-13, IL-31, IL-33 and CCL17 in PA-treated skin tissues measured by qPCR. (B) HaCaT cells were pre-treated with K284 at indicated concentrations. After 2 h, cells were treated with the TNF-α and IFN-γ combination (20 ng/ml) for another 4 h. The mRNA expression of IL-1β, IL-6, TSLP, CHI3L1 and LTF was analyzed by qPCR.
CHI3L1 regulated LTF expression. (A and B) HaCaT cells were transfected with CHI3L1 plasmid vector or human CHI3L1 siRNA (20 nM) for 24 h. The mRNA expression of LTF (A) and CXCL1 (B) was determined by qPCR (n=3).
Serum analysis of LTF in AD patients. The serum level and ROC curve of LTF in patients with AD and healthy controls (n=20).