Abstract
Myeloid-derived suppressor cells (MDSCs) have strong immunosuppressive activity and are morphologically similar to conventional monocytes and granulocytes. The development and classification of these cells have, however, been controversial. The activation network of MDSCs is relatively complex, and their mechanism of action is poorly understood, creating an avenue for further research. In recent years, MDSCs have been found to play an important role in immune regulation and in effectively inhibiting the activity of effector lymphocytes. Under certain conditions, particularly in the case of tissue damage or inflammation, MDSCs play a leading role in the immune response of the central nervous system. In cancer, however, this can lead to tumor immune evasion and the development of related diseases. Under cancerous conditions, tumors often alter bone marrow formation, thus affecting progenitor cell differentiation, and ultimately, MDSC accumulation. MDSCs are important contributors to tumor progression and play a key role in promoting tumor growth and metastasis, and even reduce the efficacy of immunotherapy. Currently, a number of studies have demonstrated that MDSCs play a key regulatory role in many clinical diseases. In light of these studies, this review discusses the origin of MDSCs, the mechanisms underlying their activation, their role in a variety of clinical diseases, and their function in immune response regulation.
Keywords: Myeloid-derived suppressor cells, Immunosuppression, Tumor disease, Neuroinflammatory
INTRODUCTION
Myeloid derived suppressor cells (MDSCs) are a group of heterogeneous bone marrow cells that play an immunosuppressive role in the body (1). MDSCs are composed of immature myeloid cells (IMCs) and can be divided into 2 cell subtypes: 1) mononuclear MDSCs (M-MDSCs), and 2) polymorphonuclear MDSCs (PMN-MDSCs), also described as granulocytic MDSCs (G-MDSCs) (2). Both types of MDSCs have been shown to have inhibitory effects in mouse tumor models and several human cancers (3). Mouse MDSCs are characterized by co-expression of CD11b and Gr-1, while human MDSCs are most characterized by CD11b+ and CD33+ and low level of HLA-DR, which is the MHC class II molecule (4,5). Moreover, in addition to the 2 major subtypes mentioned above, there is another subtype lacking macrophage and granulocyte markers, which is called early MDSCs (e-MDSCs) (6,7). The function of MDSCs is defined as its ability to inhibit the response of T cells, NK cells and B cells, so as to change the disease outcome under various pathological conditions (8).The immunosuppression mediated by MDSCs is related to arginase-I (Arg-I), inducible nitric oxide synthase (iNOS), TGF-β, IL-10, cyclooxygenase 2 (COX-2), indoleamine 2,3-dioxygenase (IDO) chelating cysteine, and other factors. Decreased expression of T cells and L-selectin are also involved in MDSC immunosuppression (9,10,11,12). MDSCs accumulate in tumor and inflammatory tissues, and block immune cell effector function (13). Although MDSCs are involved in immunosuppression related to many different cells, they play a key role in T cell tolerance (14). However, MDSCs also regulate other cell populations, including B cells, in the inflammatory response. In fact, B cells are essential for an Ab-mediated immune response. MDSCs regulate the immune response of B cells directly by expressing effector molecules and indirectly by controlling other immunomodulatory cells. As the B cell-mediated immune response is the main component of the overall immune response, MDSCs play a prominent role in its regulation (15). Human neutrophils, as effective effector cells, play a well-known role in killing pathogenic microorganisms. In addition to their role in innate immunity, neutrophils also have the ability to inhibit T-cell-mediated immune responses, known as G-MDSCs, affecting clinical outcomes in various diseases, such as cancer. These findings also suggest that MDSC activity does not completely overlap with the antimicrobial activity of human neutrophils and provide an opportunity to elucidate the unique characteristics of their T cell inhibitory activity (16). Leiber et al. (17) reported that the phagocytic activity of G-MDSCs towards Escherichia coli cells is similar to that of mature neutrophil, but apoptosis is reduced in case of the former, whereas the proliferation of T cells is strongly inhibited in the presence of G-MDSCs. Emerging research is revealing the role of MDSCs in clinical disease, which is of serious concern; however, it is yet to be extensively studied. This review summarizes the origin of MDSCs, the mechanism underlying their activation, their role in various clinical diseases, and their immune response-regulatory function.
ORIGIN AND CLUSTERING OF MDSCs
MDSCs are composed of myeloid progenitor cells and IMCs, which have the ability to inhibit immune response at different levels (18). The different cells forming heterogeneous MDSC populations originate and develop in the bone marrow. During MDSC formation, hematopoietic stem cells are differentiated into normal myeloid progenitor cells and IMCs, via complex molecular networks (4). In general, bone marrow stromal cells account for ≤20%–30% of the normal bone marrow. They migrate to different peripheral organs and rapidly differentiate into mature granulocytes, macrophages, and dendritic cells (DCs). Under pathological conditions, however, IMCs cannot differentiate into mature cells. Further, MDSCs can proliferate, activate, and accumulate as immature cells due to long-term pathological conditions, such as chronic inflammation or cancer (19). Immunosuppression caused by MDSCs plays an important role in addressing acute inflammation. However, in chronic inflammatory diseases, MDSC activation suppresses both inherent and adaptive immune responses, thereby aggravating disease processes associated with tumors, chronic infections, and many degenerative diseases (20). MDSCs have been reported to play a role in amplifying and suppressing host immune responses during chronic viral infection (21). Currently, MDSCs mainly include PMN-MDSCs and M-MDSCs, which are phenotypically and morphologically similar to neutrophils and monocytes, respectively (22,23). These MDSCs perform their immunosuppressive functions via different mechanisms: PMN-MDSCs act mainly through the induction of Ag-specific T cell tolerance, whereas M-MDSC activity is dependent on the blocking of T cell response in Ag-specific and non-specific ways by cytokines (24). Based on the above studies, we summarized the characteristics of the 2 main subtypes of MDSCs, so as to better distinguish them (Table 1). In addition, there is no unified standard for human surface molecular markers. MDSCs and many other markers have been reported gradually. The phenotypes of common reported specific markers are as follows: CD16low CD11b+ CD14− HLA-DR− CD15+ CD33+ (30), CD14+ HLA-DR−/low (31,32), CD11b+ CD14− HLA-DR− CD33+ CD15+ ILT3 high (33), Lin− CD14− CD11b+ CD39+ CD73+ (34), Lin− CD14− HLA-DR – (35), Lin− CD33+ CD14+ CD15− HLA-DR− (36), CD33+ CD11b+ CD14− and CD33+ CD11b+ CD14+ HLA-DR−/low (37). In humans, MDSCs can be isolated from neutrophils and monocytes based on phenotypic markers and density gradients (38). Human PMN-MDSCs have a gene expression profile that distinguishes them from neutrophils in cancer patients and healthy donors (39). In addition to gene and protein expression profiles, MDSCs differ from neutrophils and monocytes in the activity and expression of specific molecules. STAT3 upregulation is a marker of MDSCs because this transcription factor is directly involved in MDSC accumulation in humans and mice (40,41). The phenotypic and functional differences between MDSCs, neutrophils, and monocytes are described in Table 2.
Table 1. Characteristics of M-MDSCs and PMN-MDSCs.
| Characteristics | M-MDSCs | PMN-MDSCs | References |
|---|---|---|---|
| Phenotype | CD11b+Ly6G−Ly6Chigh | CD11b+Ly6G+Ly6Clo | (25) |
| Definition | CD11b+CD15−CD14+HLA-DR−/low | CD11b+CD14− CD15+/CD66b+ | (26) |
| Similarly | Monocytes | Neutrophils | (22) |
| Main mechanism of action | Ag-specific and nonspecific methods | Ag-specific T cell tolerance | (24) |
| In tumor tissue | In large proportion, strong inhibition | A relatively low percentage | (27) |
| Main expressed markers | PD-L1 | LOX-1 | (28) |
| Co-expressed markers | CD33 | (29) |
LOX-1, low-density lipoprotein receptor-1.
Table 2. Differences between human neutrophils, monocytes, and MDSCs.
| Type | Surface phenotype | Density | Immunosuppression | STAT-3 | ROS | NO | Arg-1 |
|---|---|---|---|---|---|---|---|
| Neutrophils | CD11b+CD14−CD15+CD66b+LOX-1− | High | − | −/+ | + | − | + |
| Monocytes | CD14+CD15−HLA-DR+ | Low | − | −/+ | −/+ | + | − |
| M-MDSCs | CD14+CD15−HLA-DR−/low | Low | ++ | ++ | −/+ | +++ | − |
| PMN-MDSCs | CD11b+CD14−CD15+CD66b+LOX-1+ | Low | + | ++ | +++ | + | ++ |
LOX-1, low-density lipoprotein receptor-1.
MECHANISM UNDERLYING THE ACTIVATION OF MDSCs
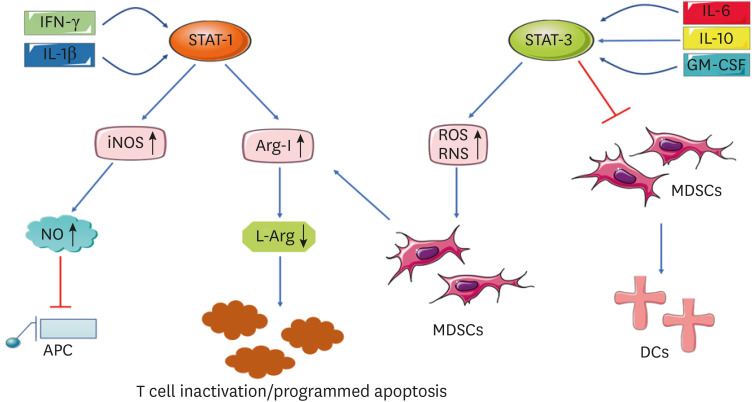
The activation of MDSCs is a very complex process, wherein the main signal pathways and transduction are closely related to the STATs. The classical activation of MDSCs occur in response to strong signals, which often appear in the form of pathogen-associated molecular patterns or damage associated molecular patterns. This activation is relatively short-lived. When the stimulation stops, the activation mode stops, showing strong phagocytosis, respiratory burst, and the release of pro-inflammatory cytokines (42,43,44). Therefore, MDSCs can play an inflammatory role (45). When MDSCs are recruited to inflammatory tissues, they inhibit the acute inflammatory response and trigger the regression of inflammation; however, if the pathogenic agents are not eliminated, long-existing MDSCs can suppress the host's immune defenses, increasing susceptibility to infection and tumorogenesis (46). Currently, the dual signal model is mainly used to describe the process. One group is majorly driven by tumor-derived growth factors, including STAT-3, interferon regulatory factor 8 (IRF8), CCAAT enhancer-binding protein β (C/EBPβ), notch, adenosine receptor A2B signal transduction, and nucleotide-binding oligomerization domain-like receptor protein 3 (47). Another group of signals are mainly mediated by related factors produced by the tumor matrix, including NF-κB pathway, STAT-1, STAT-6, prostaglandin E2 (PGE2), and COX-2 (48). In fact, STAT-3 regulates the proliferation and activation of MDSCs in different ways. First, the inhibition of STAT-3 signals can significantly inhibit MDSC proliferation. Second, STAT-3 is involved in regulating the immunosuppressive function of MDSCs. In vitro and in vivo studies have shown that certain factors induce MDSCs to produce IL-6, which leads to STAT-3 phosphorylation and enhances the immunosuppressive function of MDSCs (49). Here, we draw the Fig. 1 to more vividly depict the activation mechanism.
Figure 1. Activation mechanism of MDSCs. Several factors are involved in the activation and immunosuppressive function of MDSCs. STAT, such as STAT-1 and STAT-3, are involved in MDSC regulation. IFN-γ and IL-1 β can trigger STAT-1 signals, leading to high levels of iNOS and Arg-I. IL-6, IL-10, and GM-CSF can activate the STAT-3 signaling pathway, thereby promoting the high expression of ROS in MDSCs, inhibiting the differentiation of MDSCs into DCs, and enhancing the immunosuppressive function of MDSCs. When ROS and RNS increase, MDSCs are activated. MDSCs can induce Arg-I, which leads to non-production and depletion of L-arginine, and finally, T cell inactivation or programmed apoptosis, thus promoting the immunosuppressive function of MDSCs.
RNS, reactive nitrogen species.
THE MECHANISMS OF ACTION OF MDSCs IN TUMOR DISEASES
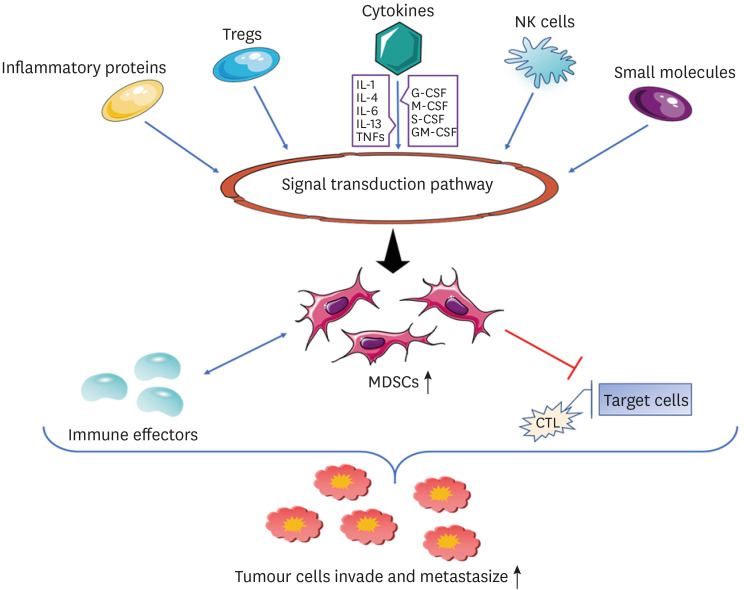
Tumor microenvironment (TME) is a complex network of epithelial cells and stromal cells, in which stromal components support tumor cells at all stages of tumorigenesis. These stromal cell populations include myeloid cells, which are mainly composed of tumor-associated macrophages (TAMs), DCs, MDSCs, and tumor-associated neutrophils (TANs), among which MDSCs play a major role in tumor growth (50). The pathophysiological characteristics of tumors are influenced by the interaction of tumor cells, T cells, and myeloid cells in the TME. The study of the interaction between them by researchers has become critical in the study of tumor immunology (51). During the differentiation and development of MDSCs, their functions are also affected by a series of regulatory factors in the TME, such as metabolic reprogramming, epigenetic modification, and cell signaling pathways, and there is crosstalk among these regulatory factors (52). There is a strong correlation between TME and MDSCs, We used Fig. 2 to describe the mechanism of action between related cytokines and MDSCs in the background of tumor microenvironment, which ultimately promoted the invasion and metastasis of tumor cells. With developments in oncology, there is enough evidence that MDSCs and neutrophils play a key role in the occurrence and development of tumors (53,54). At present, there is also growing evidence that MDSCs are a key factor in immunosuppression in cancer patients (55). MDSCs were first observed in patients with advanced cancer, and in a variety of tumor entities, a high number of circulating MDSCs is associated with advanced tumor stage, poorer prognosis, and a weaker response to treatment (56). The main inhibitory immune cells in tumor sites are MDSCs, TAMs, and Treg cells. The main roles of these inhibitory immune cells include blocking T cell activity and supporting tumor progression and survival (57). In addition, it has been reported that M-MDSCs have stronger inhibitory effect than PMN-MDSCs, and have become an important mediator of tumor-induced immunosuppression (58). MDSCs play a crucial role in the immunosuppression of tumor hosts. MDSCs express Arg-I and indoleamine 2, 3-dioxygenase. They inhibit T cell function by decreasing L-arginine and L-tryptophan levels, respectively (59). MDSCs can inhibit the activation of T cells in TME, cause immune response inhibition, reduce the anti-tumor activity of the body, and promote tumor invasion and metastasis, thus playing an important role in promoting tumor growth (60). The growing tumor has many mechanisms that can resist the recognition of the immune system, one of which includes MDSCs, that provide a tolerant environment for tumor cells by inhibiting the immune specificity of T cells. This mechanism leads to a disorder in the immune function of the body, which is conducive to tumor occurrence, development, and escape (61). The immunosuppressive effect of MDSCs is mainly related to some small molecules, NK cells, cytokines, inflammatory proteins, Tregs, and the signal transduction pathway. For example, cytokines are needed for proliferation; colony-stimulating factors (CSFs) such as G-CSF, M-CSF, GM-CSF, S-CSF, and those related to functional maturity (IL-1 family cytokines, IL-4, IL-6, IL-13, TNF, etc.) can induce MDSCs (38,62). Among them, the cytokine IL-6 has been found to be a key regulator of MDSC accumulation and activation, as well as a factor that stimulates the proliferation, survival, invasion, and metastasis of tumor cells (63). The host immune response is the basic mechanism for slowing cancer progression. Studies have revealed the tumor suppressor mediated the IL-6/G-MDSC S/CD8+ T cell immune cascade, which protects the host's adaptive anti-tumor immunity (64). Currently, many reports have revealed the critical role of MDSCs in tumor progression. Zhang et al. (65) found that intestinal microbiome can control the formation of immunosuppressive environment in liver cells by increasing PMN-MDSC, thus promoting the occurrence of liver cancer. Sun et al. (66) demonstrated that MDSCs and Th17 are closely associated with the progression of cell-dependent lymphoma. Tavukçuoğlu et al. (67) reported significantly higher levels of low-density PMN-MDSCs in the spleens of cancer patients compared to peripheral blood, but low levels of e-MDSCs and M-MDSCs. Low-density polynucleated (PMN) cells were enriched in IMCs and showed higher levels of CD10, CD16, and ROS than blood-derived cells. Both low-density and normal-density PMN cells from the human spleen inhibited T cell proliferation and IFN-γ production (67). Recent studies have shown the value of MDSCs in predicting the treatment response for various cancers: M-MDSC levels are inversely correlated with chemotherapy response in breast, cervical, prostate, and colorectal cancers (CRCs), and likewise, in CRC, the PMN-MDSC value is inversely correlated with chemotherapy response (68,69,70). Recent studies have also shown that MDSCs can be used as prognostic biomarkers and targets for cancer immunotherapy. Preclinical and clinical studies have identified novel approaches to combined immunoregulatory therapy that deplete MDSC populations and inhibit MDSC function, including chemotherapeutic agents combined with immune checkpoint-guided therapy (71). In addition, the motor capacity of MDSCs is affected by cancer cells and tumor cell secretory bodies. Epithelial dedifferentiation may be the mechanism by which cancer cells respond to changes in the movement of MDSCs. These results highlight the biochemical and biological structural conditions that MDSCs support cancer cell migration, thus providing a new avenue for research and treatment to inhibit cancer progression (72). The immunosuppressive nature of the TME is a major factor hindering the success of many cancer therapies. Therefore, the deletion or reprogramming of MDSCs and Tregs to restore tumor immunosuppression is urgently needed clinically (73). Therefore, it is of great significance to study the relevant mechanism of action of MDSCs.
Figure 2. Regulation mechanism of MDSC in TME. In TME, some small molecules, NK cells, cytokines, inflammatory proteins and Tregs promote the immunosuppressive effect of MDSCs through signal transduction pathways. Among them, cytokines mainly include two categories of cytokines required for proliferation (G-CSF, M-CSF, GM-CSF and S-CSF, etc.) and cytokines related to functional maturation (IL-1 family cytokines, IL-4, IL-6, IL-13 and TNFs, etc.), which can induce and activate MDSCs. MDSCs have bidirectional activation effect with the immune effectors with anti-tumor activity. After activation, MDSCs can inhibit the response of CTLs to target cells and reduce the anti-tumor activity of the body, thus providing a tolerant environment for tumor cells and promoting tumor invasion and metastasis.
CRC and MDSCs
CRC has a relatively high incidence and is one of the more common malignant tumors in the world (74). CRC occurrence and development involve many pathological factors. Several studies have shown a close relationship between the increased risk of CRC and the immune escape microenvironment formed by chronic inflammation or autoimmune diseases (75). Karakasheva et al. (76) used flow cytometry to quantitatively detect diseased and normal groups, and showed that CD38+ M-MDSCs and CD38+ PMN-MDSCs were significantly increased in CRC patients (accompanied by increased CD38 expression in M-MDSCs and PMN-MDSCs). M-MDSCs mainly produce nitric oxide as the precursor of TAMs, while G-MDSCs can produce living oxygen and differentiate into TANs (77). Wang et al. (78) demonstrated that G-MDSCs promote stem cell formation and growth of CRC cells through S100A9 exosomes. Some studies have shown that STAT-3 plays a key role in CRC expression; the interaction between sphingosine-1-phosphate receptor 1 (S1PR1) and STAT-3 can induce MDSCs to form a pre-metastasis niche in CRC cells and promote organ specific metastasis (79,80). Recent studies have further revealed that intestinal flora play a role in CRC. Long et al. (81) analyzed the tumor infiltrated immune cell population of Apc(Min/+) mice treated with Peptostreptococcus anaerobius and found that MDSCs, TAMs, and TANs increased significantly. The selective enrichment of these immunosuppressive cells revealed another mechanism of anoxic Plasmodium-promoting CRC (81). Pro-inflammatory cytokine expression is widely induced by anaerobic Plasmodium in vivo, which may be mediated by NF-κB activation. Increase in MDSCs can promote IL-6 and IL-10 production, thereby directly inhibiting CD4+ and CD8+ T cell activity, whereas TAMs block the anti-tumor immune response of T cells and contribute to angiogenesis and tumor cell metastasis (82). Furthermore, Ibrahim et al. (83) found that IL-6 activates STAT3 to up-regulate the expression of DNMT1 and DNMT3b in colon cancer cells, thus revealing an epigenetic mechanism that mediates the IL-6–STAT3 signaling pathway in colon cancer. In conclusion, MDSCs promote the inhibition of autologous T cell proliferation and CRC cell development in vitro and in vivo by inducing the increase of the S1PR1-STAT3-IL-6 axis and the S100A9 exosome.
Lung cancer (LC) and MDSCs
LC is the second most common cancer worldwide. Despite advances in cancer treatment, it remains the leading cause of cancer mortality, with a 5-year survival rate of 18%, the lowest of all malignancies (84,85). The occurrence of LC can cause local and systemic immunosuppression, promote tumor occurrence and development, and do great harm to human beings. Immunosuppressive cells, such as MDSCs, TAMs, and Tregs, act as inhibitory time components to weaken the immune response. In these cells, the role of MDSCs in the prognosis, development, and treatment of LC has attracted more and more attention (86,87,88). As found in other types of tumors, there is increasing evidence that MDSCs play multiple roles in the promotion and progression of LC. This includes inhibition of tumor growth and progression mediated by anti-tumor immunity, and the relationship between MDSCs and poor prognosis and increased resistance to chemotherapy and immunotherapy (89). Li et al. (90) collected the peripheral blood of patients with metastatic brain tumor and LC before metastasis, and quantitatively detected immunosuppressive monocytes, MDSCs, and Tregs via flow cytometry. T cell activity analysis via ELISPOT assay showed that compared with the patients before early metastasis and the healthy control (HC) group, the PD-L1 and MDSC abundance and percentage of Tregs in peripheral blood mononuclear cells of patients having LC with brain metastasis increased (90). Related data showed that upregulation of K-ras gene expression and activation of the JAK-STAT signaling pathway were closely related to disease progression (91). Lee et al. (92) found that using Trp53FloxFlox; KrasG12D/+; Rosa26LSL-Luciferase/LSL-Luciferase (PKL) gene-engineered mice cultured with autologous lung tumors, PD-L1 was highly expressed in both tumor-containing lungs and MDSCs, thus confirming that MDSCs played an important role in promoting LC development. It has been reported that MDSCs in patients with non-small cell LC can inhibit T cell activity, enhance immunosuppression, and accelerate tumor progression through arginase, ROS, and the IL-13/IL-4R axis (31). Li et al. (34) showed that phosphorylation of the mammalian rapamycin target protein induced by TGF-β could activate hypoxia inducible factor-1α (HIF-1α), and then induce MDSCs to express CD39/CD73. CD39 and CD73 can produce adenosine, and then inhibit the antitumor activity of NK cells, effector T cells, and other effector cells according to paracrine signals, so as to further promote the escape of tumor cells from cytotoxic T cell responses (34). Based on a large number of studies, we conclude that exposure of MDSCs to hypoxia in TME leads to the increase of arg1 and iNOS mediated by HIF-1 α and the up-regulation of PD-L1, which is the surface inhibitor of MDSCs. MDSCs can also produce cytokines such as IL-10 and TGF-β, which can attract Treg cells to tumor sites, enhance their immunosuppressive function, and inhibit B cells, NK cells, D cells, and the function of C. Adenosine from CD39-high/CD73-high MDSCs is another major NK suppressor. At the same time, due to the effect of hypoxia, the activity of STAT3 in MDSCs was greatly reduced, which led to the rapid differentiation of M-MDSCs into TAMs. PMN MDSCs die rapidly due to endoplasmic reticulum stress, and the factors released by the dead cells can promote the immunosuppressive mechanism. MDSCs can promote tumor angiogenesis and metastasis by producing VEGF, MMPs and exons. Tumor-derived exons can also affect the recruitment and immunosuppression of MDSCs (89). In conclusion, we can understand that the mTOR-HIF-1α-CD39/CD73-adenosine-MDSCs-PDL1 pathway is the main mechanism of MDSCs involved in LC.
Breast cancer (BC) and MDSCs
BC is one of the most common malignant tumors in the world. Although progress has been made in diagnosis and treatment, it remains a major cause of cancer-related deaths (93,94). Tumor and immune analysis revealed the potential mechanism of immune evasion in BC, as well as the unique aspects of the TME. These elements include those related to Ag processing and presentation, as well as immunosuppressive elements (95). MDSCs play a key role in malignant BC differentiation. BC cells can recruit tumor infiltrating leukocytes, such as Tregs, MDSCs, and type II macrophages, to form the TME, which aids tumor development and plays a "downregulatory" role in anti-tumor immunity (96). Invasive MDSCs can induce epithelial mesenchymal transition of tumor cells and increase the metastasis of BC by up-regulating the levels of TGF-β1, VEGF, and IL-10 (97). Hoffmann et al. (98) found that in BC models containing the polyoma virus middle T Ag, overall recruitment of MDSCs to promote tumor immunosuppression increased. Safarzadeh et al. (99) co-cultured purified HLA-DR−CD33+ MDSCs with CD3+ T cells and showed that MDSCs in the BC group inhibit T cell proliferation more effectively than those in the healthy group. MDSCs are more abundant and are effective as T cell inhibitors with double immunosuppressive effect (99). Hsu et al. (100) showed that the secretion of CXCL17 by BC cells increases the accumulation of CD11b+Gr-1+ MDSCs in the lung. Metastatic lung infiltration of CD11b+Gr-1+ MDSCs can play a role in inducing pulmonary angiogenesis, promoting tumor extravasation and survival, BC proliferation, and ultimately promoting lung metastasis (100). Additionally, MDSCs can directly react with BC cells. The STAT3-NF-κB-IDO, STAT3/IRF-8, and PTEN/Akt pathways play a decisive role in MDSC recruitment from tumor cells (101). The direct effect of MDSCs and BC cells is also evident in the activation of IL-6 produced by MDSCs, which simultaneously express IL-6 and soluble IL-6Rα. The trans signal of IL-6 then stimulates STAT-3 phosphorylation in BC cells, which is helpful for BC invasion and metastasis (102). Triple-negative BC (TNBC) accounts for 20% of all BC patients. Compared with estrogen receptor positive BC, which can be effectively controlled by endocrine therapy, TNBC is more invasive and has a worse prognosis (103). Kumar et al. (104) found that MDSCs promote TNBC stem cell function by secreting MMP9 and chitinase 3-like-1 protein, which confirmed the non-immune effect of MDSCs in promoting TNBC progression and metastasis. Further, some studies have shown that glycolysis is closely related to tumor development (105,106). Through the AMP-activated protein kinase-Unc-like kinase 1, autophagy, and C/EBPβ pathways, tumor glycolysis restrictively inhibits the expression of the tumor G-CSF and GM-CSF, thereby inhibiting the development of MDSCs and maintaining tumor immunosuppression (107). Therefore, aerobic glycolysis regulates the development of MDSCs through a unique molecular mechanism, thus affecting tumor progression and outcomes. Based on the above studies, MDSCs mainly affect the BC stage through the STAT3-NF-κB-IDO pathway and directly promote the occurrence of BC by increasing IL-6. MDSCs also act on T and NK cells to inhibit immune-induced tolerance of the body and promote BC progression and metastasis. Therefore, in BC, MDSCs do not only weaken anti-tumor immunity and promote BC occurrence and progression, but also reduce the effect of other immunotherapies.
MDSCs IN NEUROINFLAMMATORY
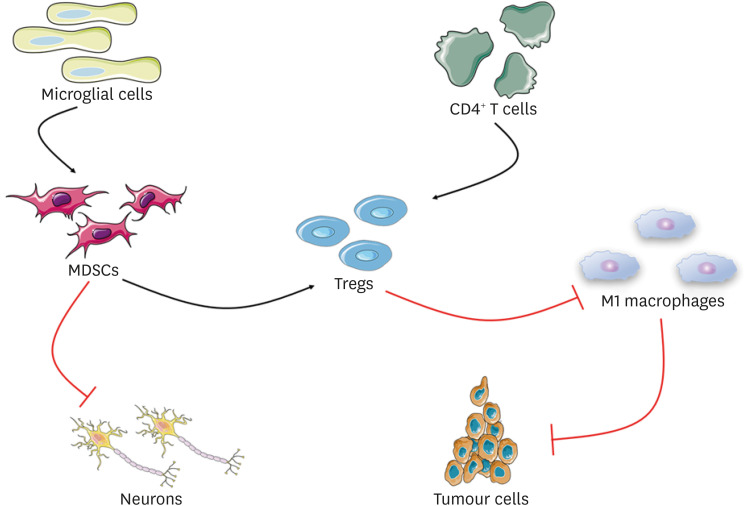
Neuroinflammatory and neurodegenerative diseases are characterized by the interaction of several molecular pathways that can be evaluated through biological fluids, especially cerebrospinal fluid and blood (108). In recent years, the concept of immune system regulation has received extensive attention in the field of neuroimmunology (109). The central nervous system (CNS) is an important system in the human body and has certain immune functions. Immune response in the CNS is relatively complex, and microglia play a leading role. MDSCs can play a corresponding role when the phagocytic activity of M2-polarized microglia decreases (110). Under the condition of focal brain injury, MDSC infiltration can inhibit neuronal inflammation and interact with microglia and other immune cells (111,112). However, MDSCs can increase Tregs, that have immunosuppressive effects and inhibit M1 macrophages, that in turn have tumor inhibitory effects (112). The role of Tregs in the immune system is closely related to the activation of CD4+ T cells and the differentiation of related effector subsets. The initial activation of CD4+ T cells requires intercellular adhesion molecule (ICAM)-1. ICAM-1 and ICAM-2 mediate the migration of Th1 and Th17 cells in the blood-brain barrier (BBB). They travel through the BBB to the CNS to induce related neuroinflammation (113). Th1 is related to the production of IFN-γ and TNF-α, and Th1 type T cells also play a role in tissue damage (114). T cells provide important immune monitoring for the CNS, and CSF is considered to be the main entry pathway of T cells (115). All these activities are specialized in producing different mediators and recruiting different immune cells, thus causing certain inflammatory reactions. For the mechanism of MDSCs in neuroinflammation, we simply drew Fig. 3 to describe it. Some studies have shown that Ly6G+ cells are recruited into the CNS in experimental autoimmune encephalomyelitis (EAE), interact with B cells to produce the cytokines, GM-CSF and IL-6, and rely on the signal transducer, STAT-3, to obtain the characteristics of PMN-MDSCs in CNS. Conditional ablation of STAT-3 leads to the depletion or dysfunction of Ly6G+ cells, which further leads to selective accumulation of GM-CSF-producing B cells in CNS chambers, thus promoting phenotype activation of microglia and making it difficult to recover from EAE (116). In the context of neuroimmunology, MDSCs are not only a powerful controller of T cell activity, but also an important regulator of immune recovery (117). With the above information, we can conclude that MDSCs play an important role in neuroinflammatory diseases.
Figure 3. Schematic diagram of the mechanism of MDSCs in neuroinflammatory. In the case of local brain injury, microglia cell activity was reduced, and the infiltration of MDSCs could directly inhibit the inflammatory process of nerve cells. Initial CD4+ T cells via the activation of ICAM-1 and ICAM-2 mediated into Tregs, MDSCs can play a role of immunosuppression by increasing Tregs, Tregs inhibits the M1 macrophages, and macrophages can exert the function of inhibiting tumor cell, eventually forming an inhibition ring, have the effect of immunosuppression, inhibiting the inflammatory process.
Multiple sclerosis (MS) and MDSCs
MS, which involves an imbalance of brain-derived T cells in the CNS, is a difficult disease to cure. MS is the second major cause of paraplegia in young people, second only to various types of CNS injury (118). MDSCs are recognized for their important role in regulating T cell responses (119). MS is an autoimmune demyelinating disease that mainly occurs in young people. In addition, it is a chronic autoimmune disease that cannot be cured (120,121). Available data show that MDSCs may be a clinical target for the diagnosis and treatment of several neuroimmunologic diseases, including MS (117). The "inside out" model proposed by neuroscientists indicates that MS is a primary degenerative disease with secondary host abnormal inflammatory response (122). However, EAE is the most commonly used animal model for studying the inflammatory components of MS. Several cell types are involved in the regulation of EAE immune response. MDSCs can be an important factor in EAE immune regulation because of their role in inducing T cell apoptosis and inhibiting inflammatory responses (123). MDSCs are cells in the innate immune system that regulate the activity of T cells in EAE (120). In fact, some researchers have observed that the number and maturity of M-MDSCs are negatively correlated with the clinical results of EAE (123,124). In addition, the increase in the number and activity of M-MDSCs paralleled the improvement of clinical course in different MS animal models (125,126). Cantoni et al. (127) demonstrated that bone marrow derived cells play an important regulatory role in MS and EAE models. MDSCs can inhibit T cell activity in EAE, thus playing an immunosuppressive role (127). It has been shown that the number of CD138+ B cells is negatively correlated with PMN-MDSCs in the examination of CSF from MS patients. Cytokines and chemokines in EAE models promote Th1 and Th17 to regulate the migration of leukocytes to the CNS during disease (116). Pathogenic T lymphocyte subsets, such as Th1 and Th17 cells, play an important role in the pathogenesis, development, and subsequent autoimmune cascade of tissue damage in MS. Hence, DCs, MDSCs, γ δ T cells, and NK subsets regulate the autoimmune response of the CNS under certain conditions. MDSCs can induce T cell apoptosis by preventing the proliferation of CD4+ T cells and related inflammatory cytokines (128). In MS, T cells abnormally recognize myelin autopeptides and attack the CNS (129). Based on the above information and other studies, we further summarize the mechanism of MDSCs in MS as follows: under MS conditions, MDSCs rapidly proliferate and are activated, showing a strong inhibitory and potential pro-inflammatory phenotype, which can produce cytokines, such as IL-6, accelerate the differentiation of Th17, and induce T cell inactivation. To provide protection against MS, MDSCs eventually inhibit the proliferation of CD4+ T cells and the secretion of related inflammatory cytokines by Arg-I-mediated cell contact (122,127,128,129,130).
Glioblastoma (GBM) and MDSCs
Glioma is the most common and fatal primary brain malignant tumor. A large amount of evidence supports the important contribution of MDSCs to the tumor immunosuppressive microenvironment, which is the key factor to stimulate the progression of glioma (131). GBM accounts for 56% of all newly diagnosed gliomas and has a high incidence rate and aggressive characteristics (132). One of the mechanisms of GBM induced immunosuppression is the accumulation of Tregs and MDSCs (133). In vivo, GBM particularly exhibits a large number of immune cells, such as microglia and tumor infiltrating macrophages, in which MDSCs account for a large proportion, and play a variety of roles in the development of tumors, including promoting tumor cell proliferation, survival, migration, and immunosuppression of the organism (134). Two subgroups of MDSCs have been found in the blood and tumor tissues of glioma (including GBM multiforme) patients (135). It can be concluded that the glioma microenvironment may contribute to the immunosuppressive function of MDSCs and negatively regulate immune system response (136). Hence, it is believed that the interaction between MDSCs and glioma cells can lead to the inhibition of effective antitumor immune response. However, based on in vitro studies, researchers have proposed that MDSCs play an important role in promoting the growth, invasion, and angiogenesis of glioma and the systematic expansion of Tregs cells (137). LOX-1+ PMN-MDSCs inhibit T cell proliferation and enhance immunosuppression, which may play a key role in driving the progression of GBM (138). Alban et al. (139) analyzed 259 patients with primary and metastatic brain tumors from benign to malignant by flow cytometry. They found that the MDSCs in the peripheral blood of GBM patients increased significantly, whereas the Tregs of immunosuppression were not found to be significantly increased. The increase in MDSCs in the recurrent GBM indicates a poor prognosis (139). Another study showed that glioma cells expressed many factors (IL-6, IL-10, VEGF, PGE-2, GM-CSF, and TGF-β2) related to MDSC proliferation. On the contrary, blocking the CCL2 signaling pathway of chemokines in glioma cells reduced recruitment of MDSCs, indicating that MDSCs need to play a unique role in the corresponding TME produced by glioma cells in GBM (140). Gielen et al. (141) found that the intracellular S100A8/9 level of M-MDSCs in GBM patients was higher than that in HCs, which was related to the increase in serum arginase activity. PMN-MDSCs highly express arginase in blood and tumor tissue, and PMN-MDSCs from blood strongly inhibit T-cells in vitro, suggesting that PMN-MDSCs play a role in GBM by inhibiting T cell function (141). According to relevant research data, glioma-associated microglia/macrophages are negatively correlated with survival time of patients with malignant gliomas microglia/macrophages (GAMs), MDSCs have the highest intratumoral density, and both GAMs and MDSCs have the ability to attract Tregs to tumors. The presence of Tregs may further lead to a lack of effective immune activation in gliomas (142). The above results suggest that MDSCs can promote the growth and deterioration of GBM by inhibiting NK cell-mediated cytotoxicity, thus releasing a variety of cytokines and chemokines and hindering the activation of CD4+ and CD8+ T cells.
Uveitis and MDSCs
Uveitis is a group of diseases characterized by intraocular inflammation, including inflammation of adjacent intraocular structures, such as the retina, vitreous body, and optic nerve. It can be caused by some autoimmune factors and is a major cause of blindness worldwide (143,144). Systemic diseases are often associated with uveitis (145). The experimental autoimmune uveoretinitis (EAU) model, which is used to study human endophthalmitis, is a common laboratory animal model for studying uveitis (146). Jeong et al. (147) proved that the number of HLA-DR−CD11b+CD33+CD14+ human MDSCs and CD11b+Ly6G-Ly6C+ mouse MDSCs increased significantly before and after the regression period of EAU. CD11b+Ly6C+ monocytes can be isolated from the EAU model; they can block T cell proliferation during culture, and the adoptive transfer of cells can accelerate the remission of EAU in mice (147). Therefore, mononuclear MDSCs are the key regulatory cells that mediate the regression of EAU. Tu et al. (148) found that there may be another mechanism for the control of retinal immune response, that is, the indirect control mechanism of MDSCs through the induction of the retinal pigment epithelium (RPE). However, IL-6 also plays a key role in the differentiation of MDSCs induced by RPE cells (148). The above studies indicate that MDSCs were recruited to the inflammatory site to inhibit the autoimmunity, thus playing a protective role in the development of EAU (149). In general, although there are studies that prove that MDSCs play an important role in the protection against uveitis, the specific mechanism underlying the involvement of MDSCs in uveitis is not clear; this needs to be further explored by researchers.
SUMMARY AND PROSPECT
In conclusion, MDSCs have a unique immunosuppressive function in tumor and nervous system-related diseases as well as other clinical diseases. They also play an important role in autoimmune diseases. Therefore, MDSC research has become an important aspect in the field of tumor immunology. Several studies have shown that MDSCs can be used as a therapeutic target to enhance the efficacy of checkpoint inhibitors by reducing their pre-tumorigenic function and immunosuppressive activity. MDSCs are not only involved in cancer, but also in chronic inflammation, bacterial/viral infections, autoimmune diseases, trauma, and graft-versus-host disease. However, compared with cancer and infectious diseases, MDSC expansion in these other conditions is not significant, which leads to greater heterogeneity in the myeloid population, and the variable frequency of MDSCs among myeloid cells may lead to conflicting results. This heterogeneity is obviously due to the different severity of autoimmune diseases and the particularity of the microenvironment. The related mechanism can be used to study the treatment and prognosis of related diseases and improve its clinical utilization. At present, we can make good use of existing resources and conditions to better understand the biological characteristics of MDSCs and provide a more reliable theoretical basis for targeted therapy. Notably, the absence of MDSCs in homeostasis conditions provides a unique opportunity to target these cells without side effects. Understanding the molecular mechanisms that regulate the accumulation and function of these cells offers the possibility of more precise targeted therapies. The clinical significance of MDSCs in cancer and some infectious diseases is now well established. In future studies, we need to focus on whether targeting MDSCs can provide real clinical benefits. If we can make good use of these research findings, it will be of great benefit to many clinical diseases. We hope that our research can go deep, in order to seek more accurate targeted treatment, find the right target, symptomatic in-depth, so as to benefit more patients.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (No. 81801557, 81671632, 81874169), Shandong Provincial Natural Science Foundation Key Project (No. ZR2020KH033), National Training Program of Innovation and Entrepreneurship for Undergraduates (No. 201910443013), Jining Medical University Training Program of Innovation and Entrepreneurship for Undergraduates (No. cx2019002).
Abbreviations
- Arg-I
arginase-I
- BBB
blood-brain barrier
- BC
breast cancer
- C/EBPβ
CCAAT enhancer-binding protein β
- CNS
central nervous system
- COX-2
cyclooxygenase 2
- CRC
colorectal cancer
- CSF
colony-stimulating factor
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- EAU
experimental autoimmune uveoretinitis
- e-MDSC
early myeloid-derived suppressor cell
- GAM
gliomas microglia/macrophage
- GBM
glioblastoma
- G-MDSC
granulocytic myeloid-derived suppressor cell
- HC
healthy control
- HIF-1α
hypoxia inducible factor-1α
- ICAM
intercellular adhesion molecule
- IDO
indoleamine 2,3-dioxygenase
- IMC
immature myeloid cell
- iNOS
inducible nitric oxide synthase
- IRF8
interferon regulatory factor 8
- LC
lung cancer
- MDSC
myeloid-derived suppressor cell
- M-MDSC
mononuclear myeloid-derived suppressor cell
- MS
multiple sclerosis
- PGE2
prostaglandin E2
- PMN
polynucleated
- PMN-MDSC
polymorphonuclear myeloid-derived suppressor cell
- RPE
retinal pigment epithelium
- S1PR1
sphingosine-1-phosphate receptor 1
- TAM
tumor-associated macrophage
- TAN
tumor associated neutrophils
- TME
tumor microenvironment
- TNBC
triple-negative breast cancer
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Zhang J.
- Software: Jia Q.
- Visualization: Ge Y, Cheng D, Xiong H.
- Writing - original draft: Ge Y.
- Writing - review & editing: Xiong H, Zhang J.
References
- 1.Bruger AM, Dorhoi A, Esendagli G, Barczyk-Kahlert K, van der Bruggen P, Lipoldova M, Perecko T, Santibanez J, Saraiva M, Van Ginderachter JA, et al. How to measure the immunosuppressive activity of MDSC: assays, problems and potential solutions. Cancer Immunol Immunother. 2019;68:631–644. doi: 10.1007/s00262-018-2170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llitjos JF, Auffray C, Alby-Laurent F, Rousseau C, Merdji H, Bonilla N, Toubiana J, Belaïdouni N, Mira JP, Lucas B, et al. Sepsis-induced expansion of granulocytic myeloid-derived suppressor cells promotes tumour growth through Toll-like receptor 4. J Pathol. 2016;239:473–483. doi: 10.1002/path.4744. [DOI] [PubMed] [Google Scholar]
- 3.Luker AJ, Graham LJ, Smith TM, Jr, Camarena C, Zellner MP, Gilmer JS, Damle SR, Conrad DH, Bear HD, Martin RK. The DNA methyltransferase inhibitor, guadecitabine, targets tumor-induced myelopoiesis and recovers T cell activity to slow tumor growth in combination with adoptive immunotherapy in a mouse model of breast cancer. BMC Immunol. 2020;21:8. doi: 10.1186/s12865-020-0337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poschke I, Kiessling R. On the armament and appearances of human myeloid-derived suppressor cells. Clin Immunol. 2012;144:250–268. doi: 10.1016/j.clim.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Cassetta L, Baekkevold ES, Brandau S, Bujko A, Cassatella MA, Dorhoi A, Krieg C, Lin A, Loré K, Marini O, et al. Deciphering myeloid-derived suppressor cells: isolation and markers in humans, mice and non-human primates. Cancer Immunol Immunother. 2019;68:687–697. doi: 10.1007/s00262-019-02302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruderek K, Schirrmann R, Brandau S. Immunophenotyping of circulating myeloid-derived suppressor cells (MDSC) in the peripheral blood of cancer patients. Mol Biol. 2021;2236:1–7. doi: 10.1007/978-1-0716-1060-2_1. [DOI] [PubMed] [Google Scholar]
- 8.Singh A, Rieber N. In vitro generation of human neutrophilic myeloid-derived suppressor cells. Methods Mol Biol. 2021;2236:77–83. doi: 10.1007/978-1-0716-1060-2_8. [DOI] [PubMed] [Google Scholar]
- 9.Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183:937–944. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi H, Dong G, Yan F, Zhang H, Li C, Ma Q, Zhang J, Ning Z, Li Z, Dai J, et al. Arctigenin ameliorates inflammation by regulating accumulation and functional activity of MDSCs in endotoxin shock. Inflammation. 2018;41:2090–2100. doi: 10.1007/s10753-018-0852-1. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Sun W, Shang W, Li Y, Zhang D, Wang T, Zhang X, Zhang S, Zhang Y, Yang R. Lnc-C/EBPβ negatively regulates the suppressive function of myeloid-derived suppressor cells. Cancer Immunol Res. 2018;6:1352–1363. doi: 10.1158/2326-6066.CIR-18-0108. [DOI] [PubMed] [Google Scholar]
- 12.Pinton L, Solito S, Damuzzo V, Francescato S, Pozzuoli A, Berizzi A, Mocellin S, Rossi CR, Bronte V, Mandruzzato S. Activated T cells sustain myeloid-derived suppressor cell-mediated immune suppression. Oncotarget. 2016;7:1168–1184. doi: 10.18632/oncotarget.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bird L. MDSC metabolite stuns T cells. Nat Rev Immunol. 2020;20:352–353. doi: 10.1038/s41577-020-0336-z. [DOI] [PubMed] [Google Scholar]
- 14.Xu Z, Ji J, Xu J, Li D, Shi G, Liu F, Ding L, Ren J, Dou H, Wang T, et al. MiR-30a increases MDSC differentiation and immunosuppressive function by targeting SOCS3 in mice with B-cell lymphoma. FEBS J. 2017;284:2410–2424. doi: 10.1111/febs.14133. [DOI] [PubMed] [Google Scholar]
- 15.Özkan B, Lim H, Park SG. Immunomodulatory function of myeloid-derived suppressor cells during B cell-mediated immune responses. Int J Mol Sci. 2018;19:1468. doi: 10.3390/ijms19051468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aarts CEM, Hiemstra IH, Furumaya C, van Bruggen R, Kuijpers TW. Different MDSC activity of G-CSF/dexamethasone mobilized neutrophils: benefits to the patient? Front Oncol. 2020;10:1110. doi: 10.3389/fonc.2020.01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leiber A, Schwarz J, Köstlin N, Spring B, Fehrenbach B, Katava N, Poets CF, Gille C. Neonatal myeloid derived suppressor cells show reduced apoptosis and immunosuppressive activity upon infection with Escherichia coli. Eur J Immunol. 2017;47:1009–1021. doi: 10.1002/eji.201646621. [DOI] [PubMed] [Google Scholar]
- 18.Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, Umansky V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120:16–25. doi: 10.1038/s41416-018-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aarts CEM, Kuijpers TW. Neutrophils as myeloid-derived suppressor cells. Eur J Clin Invest. 2018;48(Suppl 2):e12989. doi: 10.1111/eci.12989. [DOI] [PubMed] [Google Scholar]
- 20.Salminen A, Kaarniranta K, Kauppinen A. Phytochemicals inhibit the immunosuppressive functions of myeloid-derived suppressor cells (MDSC): Impact on cancer and age-related chronic inflammatory disorders. Int Immunopharmacol. 2018;61:231–240. doi: 10.1016/j.intimp.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Thakuri BKC, Zhang J, Zhao J, Nguyen LN, Nguyen LN, Schank M, Khanal S, Dang X, Cao D, Lu Z, et al. HCV-associated exosomes upregulate RUNXOR and RUNX1 expressions to promote MDSC expansion and suppressive functions through STAT3-miR124 axis. Cells. 2020;9:2715. doi: 10.3390/cells9122715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tcyganov E, Mastio J, Chen E, Gabrilovich DI. Plasticity of myeloid-derived suppressor cells in cancer. Curr Opin Immunol. 2018;51:76–82. doi: 10.1016/j.coi.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koehn BH, Apostolova P, Haverkamp JM, Miller JS, McCullar V, Tolar J, Munn DH, Murphy WJ, Brickey WJ, Serody JS, et al. GVHD-associated, inflammasome-mediated loss of function in adoptively transferred myeloid-derived suppressor cells. Blood. 2015;126:1621–1628. doi: 10.1182/blood-2015-03-634691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandruzzato S, Brandau S, Britten CM, Bronte V, Damuzzo V, Gouttefangeas C, Maurer D, Ottensmeier C, van der Burg SH, Welters MJ, et al. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: results from an interim study. Cancer Immunol Immunother. 2016;65:161–169. doi: 10.1007/s00262-015-1782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliott LA, Doherty GA, Sheahan K, Ryan EJ. Human tumor-infiltrating myeloid cells: phenotypic and functional diversity. Front Immunol. 2017;8:86. doi: 10.3389/fimmu.2017.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Safarzadeh E, Orangi M, Mohammadi H, Babaie F, Baradaran B. Myeloid-derived suppressor cells: important contributors to tumor progression and metastasis. J Cell Physiol. 2018;233:3024–3036. doi: 10.1002/jcp.26075. [DOI] [PubMed] [Google Scholar]
- 28.Cassetta L, Bruderek K, Skrzeczynska-Moncznik J, Osiecka O, Hu X, Rundgren IM, Lin A, Santegoets K, Horzum U, Godinho-Santos A, et al. Differential expansion of circulating human MDSC subsets in patients with cancer, infection and inflammation. J Immunother Cancer. 2020;8:e001223. doi: 10.1136/jitc-2020-001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother. 2012;61:1155–1167. doi: 10.1007/s00262-012-1294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heuvers ME, Muskens F, Bezemer K, Lambers M, Dingemans AC, Groen HJ, Smit EF, Hoogsteden HC, Hegmans JP, Aerts JG. Arginase-1 mRNA expression correlates with myeloid-derived suppressor cell levels in peripheral blood of NSCLC patients. Lung Cancer. 2013;81:468–474. doi: 10.1016/j.lungcan.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Huang A, Zhang B, Wang B, Zhang F, Fan KX, Guo YJ. Increased CD14(+)HLA-DR (-/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol Immunother. 2013;62:1439–1451. doi: 10.1007/s00262-013-1450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian T, Gu X, Zhang B, Liu Y, Yuan C, Shao L, Guo Y, Fan K. Increased circulating CD14(+)HLA-DR-/low myeloid-derived suppressor cells are associated with poor prognosis in patients with small-cell lung cancer. Cancer Biomark. 2015;15:425–432. doi: 10.3233/CBM-150473. [DOI] [PubMed] [Google Scholar]
- 33.de Goeje PL, Bezemer K, Heuvers ME, Dingemans AC, Groen HJ, Smit EF, Hoogsteden HC, Hendriks RW, Aerts JG, Hegmans JP. Immunoglobulin-like transcript 3 is expressed by myeloid-derived suppressor cells and correlates with survival in patients with non-small cell lung cancer. OncoImmunology. 2015;4:e1014242. doi: 10.1080/2162402X.2015.1014242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Wang L, Chen X, Li L, Li Y, Ping Y, Huang L, Yue D, Zhang Z, Wang F, et al. CD39/CD73 upregulation on myeloid-derived suppressor cells via TGF-β-mTOR-HIF-1 signaling in patients with non-small cell lung cancer. OncoImmunology. 2017;6:e1320011. doi: 10.1080/2162402X.2017.1320011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vetsika EK, Koinis F, Gioulbasani M, Aggouraki D, Koutoulaki A, Skalidaki E, Mavroudis D, Georgoulias V, Kotsakis A. A circulating subpopulation of monocytic myeloid-derived suppressor cells as an independent prognostic/predictive factor in untreated non-small lung cancer patients. J Immunol Res. 2014;2014:659294. doi: 10.1155/2014/659294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Limagne E, Richard C, Thibaudin M, Fumet JD, Truntzer C, Lagrange A, Favier L, Coudert B, Ghiringhelli F. Tim-3/galectin-9 pathway and mMDSC control primary and secondary resistances to PD-1 blockade in lung cancer patients. OncoImmunology. 2019;8:e1564505. doi: 10.1080/2162402X.2018.1564505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Passaro A, Mancuso P, Gandini S, Spitaleri G, Labanca V, Guerini-Rocco E, Barberis M, Catania C, Del Signore E, de Marinis F, et al. Gr-MDSC-linked asset as a potential immune biomarker in pretreated NSCLC receiving nivolumab as second-line therapy. Clin Transl Oncol. 2020;22:603–611. doi: 10.1007/s12094-019-02166-z. [DOI] [PubMed] [Google Scholar]
- 38.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, Tcyganov E, Hashimoto A, Nefedova Y, Lin C, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016;1:aaf8943. doi: 10.1126/sciimmunol.aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rébé C, Végran F, Berger H, Ghiringhelli F. STAT3 activation: a key factor in tumor immunoescape. JAKSTAT. 2013;2:e23010. doi: 10.4161/jkst.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Brajer-Luftmann B, Nowicka A, Kaczmarek M, Wyrzykiewicz M, Yasar S, Piorunek T, Grabicki M, Kostrzewska M, Sikora J, Batura-Gabryel H. Molecules of damage-associated patterns in bronchoalveolar lavage fluid and serum in chronic obstructive pulmonary disease. Adv Exp Med Biol. 2019;1113:27–35. doi: 10.1007/5584_2018_165. [DOI] [PubMed] [Google Scholar]
- 43.Mei Y, Zhao B, Basiorka AA, Yang J, Cao L, Zhang J, List A, Ji P. Age-related inflammatory bone marrow microenvironment induces ineffective erythropoiesis mimicking del(5q) MDS. Leukemia. 2018;32:1023–1033. doi: 10.1038/leu.2017.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakamura K, Smyth MJ. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol Immunol. 2020;17:1–12. doi: 10.1038/s41423-019-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koehn BH, Saha A, McDonald-Hyman C, Loschi M, Thangavelu G, Ma L, Zaiken M, Dysthe J, Krepps W, Panthera J, et al. Danger-associated extracellular ATP counters MDSC therapeutic efficacy in acute GVHD. Blood. 2019;134:1670–1682. doi: 10.1182/blood.2019001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salminen A, Kaarniranta K, Kauppinen A. The role of myeloid-derived suppressor cells (MDSC) in the inflammaging process. Ageing Res Rev. 2018;48:1–10. doi: 10.1016/j.arr.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Condamine T, Mastio J, Gabrilovich DI. Transcriptional regulation of myeloid-derived suppressor cells. J Leukoc Biol. 2015;98:913–922. doi: 10.1189/jlb.4RI0515-204R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaib M, Chauhan SC, Makowski L. Friend or foe? Recent strategies to target myeloid cells in cancer. Front Cell Dev Biol. 2020;8:351. doi: 10.3389/fcell.2020.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kondratova M, Czerwinska U, Sompairac N, Amigorena SD, Soumelis V, Barillot E, Zinovyev A, Kuperstein I. A multiscale signalling network map of innate immune response in cancer reveals cell heterogeneity signatures. Nat Commun. 2019;10:4808. doi: 10.1038/s41467-019-12270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai H, Xu H, Wang S, Ma J. Connections between metabolism and epigenetic modification in MDSCs. Int J Mol Sci. 2020;21:7356. doi: 10.3390/ijms21197356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 54.Treffers LW, Hiemstra IH, Kuijpers TW, van den Berg TK, Matlung HL. Neutrophils in cancer. Immunol Rev. 2016;273:312–328. doi: 10.1111/imr.12444. [DOI] [PubMed] [Google Scholar]
- 55.Xie Z, Ago Y, Okada N, Tachibana M. Valproic acid attenuates immunosuppressive function of myeloid-derived suppressor cells. J Pharmacol Sci. 2018;137:359–365. doi: 10.1016/j.jphs.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 56.Bobetsis YA, Barros SP, Offenbacher S. Exploring the relationship between periodontal disease and pregnancy complications. J Am Dent Assoc. 2006;137(Suppl):7S–13S. doi: 10.14219/jada.archive.2006.0403. [DOI] [PubMed] [Google Scholar]
- 57.Koh J, Kim Y, Lee KY, Hur JY, Kim MS, Kim B, Cho HJ, Lee YC, Bae YH, Ku BM, et al. MDSC subtypes and CD39 expression on CD8+ T cells predict the efficacy of anti-PD-1 immunotherapy in patients with advanced NSCLC. Eur J Immunol. 2020;50:1810–1819. doi: 10.1002/eji.202048534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mengos AE, Gastineau DA, Gustafson MP. The CD14+ HLA-DRlo/neg monocyte: an immunosuppressive phenotype that restrains responses to cancer immunotherapy. Front Immunol. 2019;10:1147. doi: 10.3389/fimmu.2019.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Satoh Y, Kotani H, Iida Y, Taniura T, Notsu Y, Harada M. Supplementation of l-arginine boosts the therapeutic efficacy of anticancer chemoimmunotherapy. Cancer Sci. 2020;111:2248–2258. doi: 10.1111/cas.14490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ostrand-Rosenberg S. Myeloid derived-suppressor cells: their role in cancer and obesity. Curr Opin Immunol. 2018;51:68–75. doi: 10.1016/j.coi.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Law AMK, Valdes-Mora F, Gallego-Ortega D. Myeloid-derived suppressor cells as a therapeutic target for cancer. Cells. 2020;9:9. doi: 10.3390/cells9030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37:208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weber R, Groth C, Lasser S, Arkhypov I, Petrova V, Altevogt P, Utikal J, Umansky V. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cell Immunol. 2021;359:104254. doi: 10.1016/j.cellimm.2020.104254. [DOI] [PubMed] [Google Scholar]
- 64.Li S, Feng J, Wu F, Cai J, Zhang X, Wang H, Fetahu IS, Iwanicki I, Ma D, Hu T, et al. TET2 promotes anti-tumor immunity by governing G-MDSCs and CD8+ T-cell numbers. EMBO Rep. 2020;21:e49425. doi: 10.15252/embr.201949425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Q, Ma C, Duan Y, Heinrich B, Rosato U, Diggs LP, Ma L, Roy S, Fu Q, Brown ZJ, et al. Gut microbiome directs hepatocytes to recruit MDSCs and promote cholangiocarcinoma. Cancer Discov. 2021;11:1248–1267. doi: 10.1158/2159-8290.CD-20-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun R, Zheng Z, Wang L, Cheng S, Shi Q, Qu B, Fu D, Leboeuf C, Zhao Y, Ye J, et al. A novel prognostic model based on four circulating miRNA in diffuse large B-cell lymphoma: implications for the roles of MDSC and Th17 cells in lymphoma progression. Mol Oncol. 2021;15:246–261. doi: 10.1002/1878-0261.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tavukçuoğlu E, Horzum U, Yanik H, Uner A, Yoyen-Ermis D, Nural SK, Aydin B, Sokmensuer C, Karakoc D, Yilmaz KB, et al. Human splenic polymorphonuclear myeloid-derived suppressor cells (PMN-MDSC) are strategically located immune regulatory cells in cancer. Eur J Immunol. 2020;50:2067–2074. doi: 10.1002/eji.202048666. [DOI] [PubMed] [Google Scholar]
- 68.Kawano M, Mabuchi S, Matsumoto Y, Sasano T, Takahashi R, Kuroda H, Kozasa K, Hashimoto K, Isobe A, Sawada K, et al. The significance of G-CSF expression and myeloid-derived suppressor cells in the chemoresistance of uterine cervical cancer. Sci Rep. 2015;5:18217. doi: 10.1038/srep18217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J, Yang J. Identification of CD4+CD25+CD127- regulatory T cells and CD14+HLA-DR-/low myeloid-derived suppressor cells and their roles in the prognosis of breast cancer. Biomed Rep. 2016;5:208–212. doi: 10.3892/br.2016.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tada K, Kitano S, Shoji H, Nishimura T, Shimada Y, Nagashima K, Aoki K, Hiraoka N, Honma Y, Iwasa S, et al. Pretreatment immune status correlates with progression-free survival in chemotherapy-treated metastatic colorectal cancer patients. Cancer Immunol Res. 2016;4:592–599. doi: 10.1158/2326-6066.CIR-15-0298. [DOI] [PubMed] [Google Scholar]
- 71.Gunes EG, Rosen ST, Querfeld C. The role of myeloid-derived suppressor cells in hematologic malignancies. Curr Opin Oncol. 2020;32:518–526. doi: 10.1097/CCO.0000000000000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shukla VC, Duarte-Sanmiguel S, Panic A, Senthilvelan A, Moore J, Bobba C, Benner B, Carson WE. Ghadiali SN, Gallego-Perez D. Reciprocal signaling between myeloid derived suppressor and tumor cells enhances cellular motility and is mediated by structural cues in the microenvironment. Adv Biosyst. 2020;4:e2000049. doi: 10.1002/adbi.202000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feng PH, Lam B, Tseng SH, Kung YJ, Farmer E, Cheng MA, Hung CF. NKG2D-Fc fusion protein promotes antitumor immunity through the depletion of immunosuppressive cells. Cancer Immunol Immunother. 2020;69:2147–2155. doi: 10.1007/s00262-020-02615-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 75.Liu Z, Xie Y, Xiong Y, Liu S, Qiu C, Zhu Z, Mao H, Yu M, Wang X. TLR 7/8 agonist reverses oxaliplatin resistance in colorectal cancer via directing the myeloid-derived suppressor cells to tumoricidal M1-macrophages. Cancer Lett. 2020;469:173–185. doi: 10.1016/j.canlet.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 76.Karakasheva TA, Dominguez GA, Hashimoto A, Lin EW, Chiu C, Sasser K, Lee JW, Beatty GL, Gabrilovich DI, Rustgi AK. CD38+ M-MDSC expansion characterizes a subset of advanced colorectal cancer patients. JCI Insight. 2018;3:e97022. doi: 10.1172/jci.insight.97022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. 2016;2016:6058147. doi: 10.1155/2016/6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Yin K, Tian J, Xia X, Ma J, Tang X, Xu H, Wang S. Granulocytic myeloid-derived suppressor cells promote the stemness of colorectal cancer cells through exosomal S100A9. Adv Sci (Weinh) 2019;6:1901278. doi: 10.1002/advs.201901278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ye TH, Yang FF, Zhu YX, Li YL, Lei Q, Song XJ, Xia Y, Xiong Y, Zhang LD, Wang NY, et al. Inhibition of Stat3 signaling pathway by nifuroxazide improves antitumor immunity and impairs colorectal carcinoma metastasis. Cell Death Dis. 2017;8:e2534. doi: 10.1038/cddis.2016.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin Q, Ren L, Jian M, Xu P, Li J, Zheng P, Feng Q, Yang L, Ji M, Wei Y, et al. The mechanism of the premetastatic niche facilitating colorectal cancer liver metastasis generated from myeloid-derived suppressor cells induced by the S1PR1-STAT3 signaling pathway. Cell Death Dis. 2019;10:693. doi: 10.1038/s41419-019-1922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Long X, Wong CC, Tong L, Chu ESH, Ho Szeto C, Go MYY, Coker OO, Chan AWH, Chan FKL, Sung JJY, et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat Microbiol. 2019;4:2319–2330. doi: 10.1038/s41564-019-0541-3. [DOI] [PubMed] [Google Scholar]
- 82.Sica A, Porta C, Morlacchi S, Banfi S, Strauss L, Rimoldi M, Totaro MG, Riboldi E. Origin and functions of tumor-associated myeloid cells (TAMCs) Cancer Microenviron. 2012;5:133–149. doi: 10.1007/s12307-011-0091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ibrahim ML, Lu C, Klement JD, Redd PS, Yang D, Smith AD, Liu K. Expression profiles and function of IL6 in polymorphonuclear myeloid-derived suppressor cells. Cancer Immunol Immunother. 2020;69:2233–2245. doi: 10.1007/s00262-020-02620-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 85.Conway EM, Pikor LA, Kung SH, Hamilton MJ, Lam S, Lam WL, Bennewith KL. Macrophages, inflammation, and lung cancer. Am J Respir Crit Care Med. 2016;193:116–130. doi: 10.1164/rccm.201508-1545CI. [DOI] [PubMed] [Google Scholar]
- 86.Milette S, Fiset PO, Walsh LA, Spicer JD, Quail DF. The innate immune architecture of lung tumors and its implication in disease progression. J Pathol. 2019;247:589–605. doi: 10.1002/path.5241. [DOI] [PubMed] [Google Scholar]
- 87.Adah D, Hussain M, Qin L, Qin L, Zhang J, Chen X. Implications of MDSCs-targeting in lung cancer chemo-immunotherapeutics. Pharmacol Res. 2016;110:25–34. doi: 10.1016/j.phrs.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 88.Tavakkoli M, Wilkins CR, Mones JV, Mauro MJ. A novel paradigm between leukocytosis, G-CSF secretion, neutrophil-to-lymphocyte ratio, myeloid-derived suppressor cells, and prognosis in non-small cell lung cancer. Front Oncol. 2019;9:295. doi: 10.3389/fonc.2019.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang Z, Guo J, Weng L, Tang W, Jin S, Ma W. Myeloid-derived suppressor cells-new and exciting players in lung cancer. J Hematol Oncol. 2020;13:10. doi: 10.1186/s13045-020-0843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li YD, Lamano JB, Lamano JB, Quaggin-Smith J, Veliceasa D, Kaur G, Biyashev D, Unruh D, Bloch O. Tumor-induced peripheral immunosuppression promotes brain metastasis in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2019;68:1501–1513. doi: 10.1007/s00262-019-02384-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mohrherr J, Haber M, Breitenecker K, Aigner P, Moritsch S, Voronin V, Eferl R, Moriggl R, Stoiber D, Győrffy B, et al. JAK-STAT inhibition impairs K-RAS-driven lung adenocarcinoma progression. Int J Cancer. 2019;145:3376–3388. doi: 10.1002/ijc.32624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee JW, Zhang Y, Eoh KJ, Sharma R, Sanmamed MF, Wu J, Choi J, Park HS, Iwasaki A, Kaftan E, et al. The combination of MEK inhibitor with immunomodulatory antibodies targeting programmed death 1 and programmed death ligand 1 results in prolonged survival in Kras/p53-driven lung cancer. J Thorac Oncol. 2019;14:1046–1060. doi: 10.1016/j.jtho.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 94.Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H Japan Cancer Surveillance Research Group. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45:884–891. doi: 10.1093/jjco/hyv088. [DOI] [PubMed] [Google Scholar]
- 95.Gatti-Mays ME, Balko JM, Gameiro SR, Bear HD, Prabhakaran S, Fukui J, Disis ML, Nanda R, Gulley JL, Kalinsky K, et al. If we build it they will come: targeting the immune response to breast cancer. NPJ Breast Cancer. 2019;5:37. doi: 10.1038/s41523-019-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duechler M, Peczek L, Zuk K, Zalesna I, Jeziorski A, Czyz M. The heterogeneous immune microenvironment in breast cancer is affected by hypoxia-related genes. Immunobiology. 2014;219:158–165. doi: 10.1016/j.imbio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 97.Ma X, Wang M, Yin T, Zhao Y, Wei X. Myeloid-derived suppressor cells promote metastasis in breast cancer after the stress of operative removal of the primary cancer. Front Oncol. 2019;9:855. doi: 10.3389/fonc.2019.00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoffmann SHL, Reck DI, Maurer A, Fehrenbacher B, Sceneay JE, Poxleitner M, Öz HH, Ehrlichmann W, Reischl G, Fuchs K, et al. Visualization and quantification of in vivo homing kinetics of myeloid-derived suppressor cells in primary and metastatic cancer. Theranostics. 2019;9:5869–5885. doi: 10.7150/thno.33275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Safarzadeh E, Hashemzadeh S, Duijf PHG, Mansoori B, Khaze V, Mohammadi A, Kazemi T, Yousefi M, Asadi M, Mohammadi H, et al. Circulating myeloid-derived suppressor cells: an independent prognostic factor in patients with breast cancer. J Cell Physiol. 2019;234:3515–3525. doi: 10.1002/jcp.26896. [DOI] [PubMed] [Google Scholar]
- 100.Hsu YL, Yen MC, Chang WA, Tsai PH, Pan YC, Liao SH, Kuo PL. CXCL17-derived CD11b+Gr-1+ myeloid-derived suppressor cells contribute to lung metastasis of breast cancer through platelet-derived growth factor-BB. Breast Cancer Res. 2019;21:23. doi: 10.1186/s13058-019-1114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shou D, Wen L, Song Z, Yin J, Sun Q, Gong W. Suppressive role of myeloid-derived suppressor cells (MDSCs) in the microenvironment of breast cancer and targeted immunotherapies. Oncotarget. 2016;7:64505–64511. doi: 10.18632/oncotarget.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oh K, Lee OY, Shon SY, Nam O, Ryu PM, Seo MW, Lee DS. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res. 2013;15:R79. doi: 10.1186/bcr3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Z, Kong Q, Su P, Duan M, Xue M, Li X, Tang J, Gao Z, Wang B, Li Z, et al. Regulation of Hippo signaling and triple negative breast cancer progression by an ubiquitin ligase RNF187. Oncogenesis. 2020;9:36. doi: 10.1038/s41389-020-0220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kumar S, Wilkes DW, Samuel N, Blanco MA, Nayak A, Alicea-Torres K, Gluck C, Sinha S, Gabrilovich D, Chakrabarti R. ΔNp63-driven recruitment of myeloid-derived suppressor cells promotes metastasis in triple-negative breast cancer. J Clin Invest. 2018;128:5095–5109. doi: 10.1172/JCI99673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24:657–671. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 106.Chang CH, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li W, Tanikawa T, Kryczek I, Xia H, Li G, Wu K, Wei S, Zhao L, Vatan L, Wen B, et al. Aerobic glycolysis controls myeloid-derived suppressor cells and tumor immunity via a specific CEBPB isoform in triple-negative breast cancer. Cell Metab. 2018;28:87–103.e6. doi: 10.1016/j.cmet.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gaetani L, Paolini Paoletti F, Bellomo G, Mancini A, Simoni S, Di Filippo M, Parnetti L. CSF and blood biomarkers in neuroinflammatory and neurodegenerative diseases: implications for treatment. Trends Pharmacol Sci. 2020;41:1023–1037. doi: 10.1016/j.tips.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 109.González H, Elgueta D, Montoya A, Pacheco R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J Neuroimmunol. 2014;274:1–13. doi: 10.1016/j.jneuroim.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 110.Borisov KE, Sakaeva DD. The immunosuppressive microenvironment of malignant gliomas. Arkh Patol. 2015;77:54–63. doi: 10.17116/patol201577654-63. [DOI] [PubMed] [Google Scholar]
- 111.Hosomi S, Koyama Y, Watabe T, Ohnishi M, Ogura H, Yamashita T, Shimazu T. Myeloid-derived suppressor cells infiltrate the brain and suppress neuroinflammation in a mouse model of focal traumatic brain injury. Neuroscience. 2019;406:457–466. doi: 10.1016/j.neuroscience.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 112.Ranjan A, Wright S, Srivastava SK. Immune consequences of penfluridol treatment associated with inhibition of glioblastoma tumor growth. Oncotarget. 2017;8:47632–47641. doi: 10.18632/oncotarget.17425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haghayegh Jahromi N, Marchetti L, Moalli F, Duc D, Basso C, Tardent H, Kaba E, Deutsch U, Pot C, Sallusto F, et al. Intercellular adhesion molecule-1 (ICAM-1) and ICAM-2 differentially contribute to peripheral activation and CNS entry of autoaggressive Th1 and Th17 cells in experimental autoimmune encephalomyelitis. Front Immunol. 2020;10:3056. doi: 10.3389/fimmu.2019.03056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jin P, Zhao Y, Liu H, Chen J, Ren J, Jin J, Bedognetti D, Liu S, Wang E, Marincola F, et al. Interferon-γ and tumor necrosis factor-α polarize bone marrow stromal cells uniformly to a Th1 phenotype. Sci Rep. 2016;6:26345. doi: 10.1038/srep26345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pappalardo JL, Zhang L, Pecsok MK, Perlman K, Zografou C, Raddassi K, Abulaban A, Krishnaswamy S, Antel J, van Dijk D, et al. Transcriptomic and clonal characterization of T cells in the human central nervous system. Sci Immunol. 2020;5:eabb8786. doi: 10.1126/sciimmunol.abb8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Knier B, Hiltensperger M, Sie C, Aly L, Lepennetier G, Engleitner T, Garg G, Muschaweckh A, Mitsdörffer M, Koedel U, et al. Myeloid-derived suppressor cells control B cell accumulation in the central nervous system during autoimmunity. Nat Immunol. 2018;19:1341–1351. doi: 10.1038/s41590-018-0237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Melero-Jerez C, Ortega MC, Moliné-Velázquez V, Clemente D. Myeloid derived suppressor cells in inflammatory conditions of the central nervous system. Biochim Biophys Acta. 2016;1862:368–380. doi: 10.1016/j.bbadis.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 118.Melero-Jerez C, Alonso-Gómez A, Moñivas E, Lebrón-Galán R, Machín-Díaz I, de Castro F, Clemente D. The proportion of myeloid-derived suppressor cells in the spleen is related to the severity of the clinical course and tissue damage extent in a murine model of multiple sclerosis. Neurobiol Dis. 2020;140:104869. doi: 10.1016/j.nbd.2020.104869. [DOI] [PubMed] [Google Scholar]
- 119.Iacobaeus E, Douagi I, Jitschin R, Marcusson-Ståhl M, Andrén AT, Gavin C, Lefsihane K, Davies LC, Mougiakakos D, Kadri N, et al. Phenotypic and functional alterations of myeloid-derived suppressor cells during the disease course of multiple sclerosis. Immunol Cell Biol. 2018;96:820–830. doi: 10.1111/imcb.12042. [DOI] [PubMed] [Google Scholar]
- 120.Melero-Jerez C, Suardíaz M, Lebrón-Galán R, Marín-Bañasco C, Oliver-Martos B, Machín-Díaz I, Fernández Ó, de Castro F, Clemente D. The presence and suppressive activity of myeloid-derived suppressor cells are potentiated after interferon-β treatment in a murine model of multiple sclerosis. Neurobiol Dis. 2019;127:13–31. doi: 10.1016/j.nbd.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 121.Elliott DM, Singh N, Nagarkatti M, Nagarkatti PS. Cannabidiol attenuates experimental autoimmune encephalomyelitis model of multiple sclerosis through induction of myeloid-derived suppressor cells. Front Immunol. 2018;9:1782. doi: 10.3389/fimmu.2018.01782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mastorodemos V, Ioannou M, Verginis P. Cell-based modulation of autoimmune responses in multiple sclerosis and experimental autoimmmune encephalomyelitis: therapeutic implications. Neuroimmunomodulation. 2015;22:181–195. doi: 10.1159/000362370. [DOI] [PubMed] [Google Scholar]
- 123.Moliné-Velázquez V, Ortega MC, Vila del Sol V, Melero-Jerez C, de Castro F, Clemente D. The synthetic retinoid Am80 delays recovery in a model of multiple sclerosis by modulating myeloid-derived suppressor cell fate and viability. Neurobiol Dis. 2014;67:149–164. doi: 10.1016/j.nbd.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 124.Moliné-Velázquez V, Cuervo H, Vila-Del Sol V, Ortega MC, Clemente D, de Castro F. Myeloid-derived suppressor cells limit the inflammation by promoting T lymphocyte apoptosis in the spinal cord of a murine model of multiple sclerosis. Brain Pathol. 2011;21:678–691. doi: 10.1111/j.1750-3639.2011.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alabanza LM, Esmon NL, Esmon CT, Bynoe MS. Inhibition of endogenous activated protein C attenuates experimental autoimmune encephalomyelitis by inducing myeloid-derived suppressor cells. J Immunol. 2013;191:3764–3777. doi: 10.4049/jimmunol.1202556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mecha M, Feliú A, Machín I, Cordero C, Carrillo-Salinas F, Mestre L, Hernández-Torres G, Ortega-Gutiérrez S, López-Rodríguez ML, de Castro F, et al. 2-AG limits Theiler's virus induced acute neuroinflammation by modulating microglia and promoting MDSCs. Glia. 2018;66:1447–1463. doi: 10.1002/glia.23317. [DOI] [PubMed] [Google Scholar]
- 127.Cantoni C, Cignarella F, Ghezzi L, Mikesell B, Bollman B, Berrien-Elliott MM, Ireland AR, Fehniger TA, Wu GF, Piccio L. Mir-223 regulates the number and function of myeloid-derived suppressor cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. 2017;133:61–77. doi: 10.1007/s00401-016-1621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ioannou M, Alissafi T, Lazaridis I, Deraos G, Matsoukas J, Gravanis A, Mastorodemos V, Plaitakis A, Sharpe A, Boumpas D, et al. Crucial role of granulocytic myeloid-derived suppressor cells in the regulation of central nervous system autoimmune disease. J Immunol. 2012;188:1136–1146. doi: 10.4049/jimmunol.1101816. [DOI] [PubMed] [Google Scholar]
- 129.Wegner A, Verhagen J, Wraith DC. Myeloid-derived suppressor cells mediate tolerance induction in autoimmune disease. Immunology. 2017;151:26–42. doi: 10.1111/imm.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Glenn JD, Liu C, Whartenby KA. Frontline Science: Induction of experimental autoimmune encephalomyelitis mobilizes Th17-promoting myeloid derived suppressor cells to the lung. J Leukoc Biol. 2019;105:829–841. doi: 10.1002/JLB.4HI0818-335R. [DOI] [PubMed] [Google Scholar]
- 131.Mi Y, Guo N, Luan J, Cheng J, Hu Z, Jiang P, Jin W, Gao X. The emerging role of myeloid-derived suppressor cells in the glioma immune suppressive microenvironment. Front Immunol. 2020;11:737. doi: 10.3389/fimmu.2020.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vidyarthi A, Agnihotri T, Khan N, Singh S, Tewari MK, Radotra BD, Chatterjee D, Agrewala JN. Predominance of M2 macrophages in gliomas leads to the suppression of local and systemic immunity. Cancer Immunol Immunother. 2019;68:1995–2004. doi: 10.1007/s00262-019-02423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kamran N, Chandran M, Lowenstein PR, Castro MG. Immature myeloid cells in the tumor microenvironment: implications for immunotherapy. Clin Immunol. 2018;189:34–42. doi: 10.1016/j.clim.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Arcuri C, Fioretti B, Bianchi R, Mecca C, Tubaro C, Beccari T, Franciolini F, Giambanco I, Donato R. Microglia-glioma cross-talk: a two way approach to new strategies against glioma. Front Biosci (Landmark Ed) 2017;22:268–309. doi: 10.2741/4486. [DOI] [PubMed] [Google Scholar]
- 135.Gielen PR, Schulte BM, Kers-Rebel ED, Verrijp K, Petersen-Baltussen HM, ter Laan M, Wesseling P, Adema GJ. Increase in both CD14-positive and CD15-positive myeloid-derived suppressor cell subpopulations in the blood of patients with glioma but predominance of CD15-positive myeloid-derived suppressor cells in glioma tissue. J Neuropathol Exp Neurol. 2015;74:390–400. doi: 10.1097/NEN.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 136.Parney IF. Basic concepts in glioma immunology. Adv Exp Med Biol. 2012;746:42–52. doi: 10.1007/978-1-4614-3146-6_4. [DOI] [PubMed] [Google Scholar]
- 137.Wurdinger T, Deumelandt K, van der Vliet HJ, Wesseling P, de Gruijl TD. Mechanisms of intimate and long-distance cross-talk between glioma and myeloid cells: how to break a vicious cycle. Biochim Biophys Acta. 2014;1846:560–575. doi: 10.1016/j.bbcan.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 138.Chai E, Zhang L, Li C. LOX-1+ PMN-MDSC enhances immune suppression which promotes glioblastoma multiforme progression. Cancer Manag Res. 2019;11:7307–7315. doi: 10.2147/CMAR.S210545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alban TJ, Alvarado AG, Sorensen MD, Bayik D, Volovetz J, Serbinowski E, Mulkearns-Hubert EE, Sinyuk M, Hale JS, Onzi GR, et al. Global immune fingerprinting in glioblastoma patient peripheral blood reveals immune-suppression signatures associated with prognosis. JCI Insight. 2018;3:e122264. doi: 10.1172/jci.insight.122264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kohanbash G, McKaveney K, Sakaki M, Ueda R, Mintz AH, Amankulor N, Fujita M, Ohlfest JR, Okada H. GM-CSF promotes the immunosuppressive activity of glioma-infiltrating myeloid cells through interleukin-4 receptor-α. Cancer Res. 2013;73:6413–6423. doi: 10.1158/0008-5472.CAN-12-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gielen PR, Schulte BM, Kers-Rebel ED, Verrijp K, Bossman SA, Ter Laan M, Wesseling P, Adema GJ. Elevated levels of polymorphonuclear myeloid-derived suppressor cells in patients with glioblastoma highly express S100A8/9 and arginase and suppress T cell function. Neuro-oncol. 2016;18:1253–1264. doi: 10.1093/neuonc/now034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gieryng A, Pszczolkowska D, Walentynowicz KA, Rajan WD, Kaminska B. Immune microenvironment of gliomas. Lab Invest. 2017;97:498–518. doi: 10.1038/labinvest.2017.19. [DOI] [PubMed] [Google Scholar]
- 143.Krishna U, Ajanaku D, Denniston AK, Gkika T. Uveitis: a sight-threatening disease which can impact all systems. Postgrad Med J. 2017;93:766–773. doi: 10.1136/postgradmedj-2017-134891. [DOI] [PubMed] [Google Scholar]
- 144.Mochizuki M, Sugita S, Kamoi K, Takase H. A new era of uveitis: impact of polymerase chain reaction in intraocular inflammatory diseases. Jpn J Ophthalmol. 2017;61:1–20. doi: 10.1007/s10384-016-0474-9. [DOI] [PubMed] [Google Scholar]
- 145.Yang P, Zhong Z, Du L, Li F, Chen Z, Zhu Y, Zhang W, Huang F, Ye X, Su G, et al. Prevalence and clinical features of systemic diseases in Chinese patients with uveitis. Br J Ophthalmol. 2021;105:75–82. doi: 10.1136/bjophthalmol-2020-315960. [DOI] [PubMed] [Google Scholar]
- 146.Kerr EC, Raveney BJ, Copland DA, Dick AD, Nicholson LB. Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J Autoimmun. 2008;31:354–361. doi: 10.1016/j.jaut.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 147.Jeong HJ, Lee HJ, Ko JH, Cho BJ, Park SY, Park JW, Choi SR, Heo JW, Yoon SO, Oh JY. Myeloid-derived suppressor cells mediate inflammation resolution in humans and mice with autoimmune uveoretinitis. J Immunol. 2018;200:1306–1315. doi: 10.4049/jimmunol.1700617. [DOI] [PubMed] [Google Scholar]
- 148.Tu Z, Li Y, Smith D, Doller C, Sugita S, Chan CC, Qian S, Fung J, Caspi RR, Lu L, et al. Myeloid suppressor cells induced by retinal pigment epithelial cells inhibit autoreactive T-cell responses that lead to experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2012;53:959–966. doi: 10.1167/iovs.11-8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee HJ, Ko JH, Jeong HJ, Ko AY, Kim MK, Wee WR, Yoon SO, Oh JY. Mesenchymal stem/stromal cells protect against autoimmunity via CCL2-dependent recruitment of myeloid-derived suppressor cells. J Immunol. 2015;194:3634–3645. doi: 10.4049/jimmunol.1402139. [DOI] [PubMed] [Google Scholar]