Abstract
TLR signaling is critical for broad scale immune recognition of pathogens and/or danger molecules. TLRs are particularly important for the activation and the maturation of cells comprising the innate immune response. In recent years it has become apparent that several different TLRs regulate the function of lymphocytes as well, albeit to a lesser degree compared to innate immunity. TLR2 heterodimerizes with either TLR1 or TLR6 to broadly recognize bacterial lipopeptides as well as several danger-associated molecular patterns. In general, TLR2 signaling promotes immune cell activation leading to tissue inflammation, which is advantageous for combating an infection. Conversely, inappropriate or dysfunctional TLR2 signaling leading to an overactive inflammatory response could be detrimental during sterile inflammation and autoimmune disease. This review will highlight and discuss recent research advances linking TLR2 engagement to autoimmune inflammation.
Keywords: Toll-like receptor; Toll-like receptor 2; Autoimmunity; Th17 Cells; T Lymphocytes, Regulatory; Experimental autoimmune encephalomyelitis
INTRODUCTION
Autoimmune diseases including rheumatoid arthritis (RA), multiple sclerosis (MS), psoriasis and psoriatic arthritis, systemic lupus erythematosus (SLE), and ankylosing spondylitis (AS) are typically debilitating, and are often progressive, incurable, and can be eventually life-threating (1,2,3,4,5,6). Among the many molecules identified to have roles in autoimmune disease initiation and pathogenesis, TLRs especially TLR2, have emerged as important regulators of autoimmune-associated inflammation. Despite recent advances in immunotherapies and other treatment strategies for several autoimmune diseases, a majority of patients still suffer from damaging inflammation and often require treatments that are both long-term and costly.
TLRs are a type of pattern recognition receptor that drive immune responses through broad scale detection of pathogens. TLRs are particularly advantageous to the cells comprising innate immunity because receptor rearrangement is not a prerequisite compared to the antigen receptor expressed by lymphocytic cells that encompass adaptive immunity. This allows a macrophage, for example, to rapidly respond to a threat with a pathogen detection system encoded strictly through the germline. While TLRs are typically known for their ability to detect pathogens, certain TLRs are also very important for recognizing endogenous danger signals (7). TLRs accomplish both feats by binding to either pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs). Thus, by playing a central role in the non-lymphocyte pathogen recognition response, TLRs represent a very important mechanism for the detection of microbes. Consequentially, TLRs crucially function in raising the alarm for the rest of the immune response when danger is present.
There are 10 TLRs expressed in humans and 12 are expressed in mice. Many of these TLRs overlap between species in terms of PAMP and DAMP specificity, which helps validate the use of certain mouse models to study the influence of TLR signaling on autoimmune diseases. TLR molecules reside on the surface of a cell (cell membranes) or internally within cytosolic endosomal membranes. Structurally, TLRs can be grouped into subfamilies based on homology, but all contain a transmembrane domain, a C-terminal signaling domain, and an antigen recognition domain (8,9).
The expression of various TLRs has been linked to several inflammatory autoimmune diseases. For example, TLR4, a molecule that recognizes bacterial LPS, was shown to promote the development of a mouse model of type I diabetes (10). Our group has demonstrated that TLR4 signaling also drives the proliferation of autoreactive Th17 cells in a murine model of MS (11). Additionally, the nucleic acid sensors TLR9 and TLR7 along with TLR2 and TLR4 have been shown to contribute to the pathogenesis of SLE as well as RA, which is a research topic subject that has been reviewed several times (12,13,14,15).
TLR2 contributes to inflammation directed against self-tissues in a variety of cell types. While many studies have focused on TLR2 expression in innate immune cells, such as macrophages and dendritic cells, it is also important to note that TLRs are expressed by lymphocytes and activation of these TLRs can have dramatic effects on both T and B cell functions during autoimmune diseases. This review will focus on the roles and functions of TLR2 in driving autoimmune disease with a specific emphasis on the impact to T and B cell function. Additionally, this review will highlight the importance of TLR2 signaling in human autoimmune diseases as well as murine models.
TLR2 EXPRESSION AND LIGATION
TLR2 is anchored to the cell membrane and resides on the cell surface to recognize pathogen-associated molecules (16). TLR2 heterodimerizes with either TLR1 or TLR6 to form the functional receptor. One of the best characterized functions of TLR2 is to recognize different types of lipopeptides derived from bacteria as well as parasites. However, several publications have demonstrated that TLR2 is also capable of recognizing endogenous (host-derived) danger signals such as HMGB1 and biglycan (7). DNA binding high mobility group box 1 protein (HMGB1) is a DAMP recognized by both receptor for advanced glycation end products and TLR2, whereas HMGB1 accumulation has been observed in inflammatory brain lesions of people with MS. Paralytic disease severity also correlates with the levels of HMGB1 accumulating in damaged central nervous system (CNS) regions of mice in the MS model experimental autoimmune encephalomyelitis (EAE) (17,18). Additionally, the adaptor protein SNAPIN as well as certain heat shock proteins have been shown to trigger TLR2-dependent responses and are similarly correlated to downstream levels of inflammation (19,20). Thus, in addition to pathogen-derived signals, endogenous triggering of TLR2 by host-derived danger signals is a plausible contributor to the propagation of inflammation that is characteristic to a wide variety of autoimmune diseases.
LOSS OF TLR2 SIGNALING IN MODELS OF AUTOIMMUNE INFLAMMATION AND HUMAN CORRELATIONS
Like most TLRs, TLR2 activation events require the MyD88 adaptor for signal transduction leading to the eventual activation of NF-κb and AP-1, among others. Autoimmune studies in MyD88-deficient animals, which effectively hampers signaling of most TLRs, have proven to be controversial. For example, MyD88 was found to be dispensable for the generation of autoreactive T cells (21). Conversely, MyD88 deficiency protects mice from disease development in models of MS and lupus (22,23). Certainly, one of the major caveats with using the total cell MyD88-deficiency approach is the potential impact to both inflammatory and anti-inflammatory pathways that can be differentially regulated by TLR signaling depending on the cell type. When MyD88 inhibition was focused directly on antigen-presenting cells or T cells, both human and mouse T cells exhibit reduced encephalitogenic cytokine production (24). Another caveat with MyD88 inhibition or deletion strategies is that one cannot assume that just TLR signaling is being targeted. Both IL-1 and IL-18 also utilize the MyD88 signaling adaptor (25). This becomes particularly important for EAE models because IL-1 has been shown to be critical for the development of inflammatory Th17 cells and that IL-1R1 knockout protects animals from EAE development in a T cell-intrinsic fashion (26).
For TLR2 specifically, loss of expression by germline knockout in mice often results in lack of or attenuated autoimmune inflammation. For example, TLR2-deficient animals exhibited reduced dsDNA IgG in a pristine-induced lupus model (27). TLR2 deficiency also affects the B6-Faslpr/lpr murine lupus model where reduced ANA, ameliorated glomerulonephritis, and reduced marginal zone B cells were observed (28,29). Another study, however, did not observe a difference between TLR2 deficient and sufficient mice littermates in the MRL/Mp-Tnfrsf6lpr/lpr SLE model (30). In the EAE model, TLR2-/- C57BL/6 mice exhibit an almost complete lack of clinical symptoms (31). When TLR2 deletion is strictly relegated to the hematopoietic compartment, mice are similarly protected against EAE development (32). Furthermore, TLR2 deletion is protective for models of diabetes (33) and autoimmune arthritis (34). Taken together, these studies demonstrate the potential for TLR2 in regulating autoimmune inflammatory responses in a variety of immunological populations.
In addition to loss of function studies, several reports have demonstrated that TLR2 expression in certain tissues can potentially impact the development and progression of autoimmune inflammation. For example, the expression of TLR2 was found increased on synovial fibroblasts following exposure to IL-1β or TNFα, both of which are inflammatory stimuli relevant to autoimmune inflammation (35). A similar result was observed with cultured synovial fluid cells (35). Furthermore, inhibition of TLR2 blocks cytokine release from synovial tissue explants from RA patients (36). For human patients with MS, increased responsiveness to TLR2 stimuli was observed in a large fraction of the subjects tested (37). Likewise, peripheral blood neutrophils and lymphocytes exhibit higher TLR2 expression in patients with MS and serum levels of soluble TLR2 molecules are increased in MS patients with the relapsing-remitting pattern of disease over healthy control patients (38,39). In people with RA, TLR2 expression was also upregulated on synovial monocytes, where these cells demonstrated an increased sensitivity to TLR2 ligation (40). Thus, regardless of the source of TLR ligands (microbial or host-derived), enhanced TLR2 expression and hyperactivity may be a common characteristic of several autoimmune disorders.
INNATE ROLES OF TLR2 IN DRIVING AUTOIMMUNE INFLAMMATION
Not only is TLR2 expressed at a very high frequency on macrophages, but it is also very important for macrophage function, especially for pathogen-induced activation and maturation (41,42). Macrophages play an immense role in the inflammation and destruction characteristic of several autoimmune diseases, especially RA. Macrophages polarized in vitro as M2 macrophages and stimulated with the synthetic TLR2 agonist, PAM3CKS4, lose their anti-inflammatory activity. Furthermore, these macrophages exhibit a cytokine production profile similar to M1 macrophages despite maintaining surface molecule expression (CD14 and CD163) that is typical of M2 macrophages (43). In the K/BxN serum transfer mouse model of RA, which induces joint inflammation through the formation of immune complexes, germline loss of TLR2 actually worsened symptoms of inflammation (44,45). This effect was directly a result of macrophage activity where these cells produced less anti-inflammatory IL-10 in the absence of TLR2 signaling (45). Studies of this nature highlight the importance of considering both inflammatory and anti-inflammatory outcomes of TLR signaling, which may be also dictated by previous epigenetic programming events (46). In addition to TLR2, several excellent reviews have highlighted the roles of TLRs in the pathogenesis of both RA and SLE (13,47).
TLR2 signaling can readily increase the inflammatory capability in macrophages, but this outcome remains a normal immunological process involved in host defense and tissue homeostasis in addition to autoimmune pathology. So, what may be just as important is whether TLR2 signaling tolerance or sensitivity changes over the course of a given autoimmune disease. Such changes in macrophages have been well-documented in autoimmunity, especially in those with MS. Macrophages obtained from MS patients exhibited enhanced responses to TLR2 signals compared to those isolated from healthy counterparts (37), which implies that TLR2 expression may be enhanced when there is less tolerance. Conversely, other studies have demonstrated that increased TLR2 tolerance or a reduction of TLR2 signaling actually resulted in remyelination in mouse CNS (48,49). Thus, while the idea of inhibiting TLRs in general may be promising for certain autoimmune diseases, a more productive therapeutic avenue could be through inducing TLR2 tolerance for treatment of human MS similar to what has been demonstrated in animal studies (48,49,50).
While macrophages and other inflammatory myeloid cells get a lot of attention due to their central effector roles in autoimmune inflammation, dendritic cells also play a critical role in these responses by driving the activation of inflammatory T cells. Indeed, psoriatic arthritis patients were shown to exhibit increased TLR2 expression in their immature dendritic cell fraction (51). TLR2 is also critical for the ability of dendritic cells to drive Th1 activation during certain infections (52), where Th1 cells can be highly pathogenic for several autoimmune disorders. TLR2, along with TLR3, 4, and 7, are also associated with increased T cell-polarizing cytokine production by dendritic cells following ligation (53). Therefore, dendritic cells are strategically positioned to influence the autoimmune response through differential regulation of T cell subset generation. TLR2 signaling could impact these processes in several different ways and is an area of very active research.
INTRINSIC TLR2 SIGNALING IN B CELL AND T CELLS CONTRIBUTES TO AUTOIMMUNE DISEASE PATHOGENESIS
B cells
Targeting autoantibody production from the B lymphocytes, through drugs such as rituximab or even intravenous immunoglobulin, has at least been somewhat successful in treating several autoimmune diseases especially MS, RA and SLE (54). TLR2 and TLR4 as well as TLR7 and TLR9 signaling in B cells leads to increased cytokine and growth factor production with described implications for autoimmune diseases (55,56). Furthermore, people with MS who are infected with helminths have higher expression of TLR2 on the surface of their B cells as well as their dendritic cells (57). TLR2 as well as TLR4 deficiency exerts a protective role for the B6lpr/lpr strain of lupus-prone mice, which are characterized by reduced intensity of glomerulonephritis and reduction of autoantibody production compared to TLR4 and TLR2 sufficient B6lpr/lpr mice (28). This phenotype in TLR4-deficient mice was accompanied by a significant decrease of 2 cytokines implicated in autoantibody production: IL-6 and IFNγ (28). Moreover, similar to the phenomenon described for macrophages above, TLR2 or TLR4 signaling through MyD88 contributes to IL-10 production by regulatory B cells during EAE and, therefore, the ability to suppress inflammatory T cells (58). TLR2 activation can then impact many facets of the autoimmune inflammation process and targeting such outcomes may represent a more focused therapeutic strategy compared to treatments based on broadly disrupting B lymphocytes.
CD4+ T cells
T cells are key mediators of autoimmune inflammatory processes, both in the initiation and the maintenance of autoinflammation. CD4+ T cells express various TLRs including TLR2, TLR4, TLR1, TLR6, TLR7, and TLR9 (32,59,60). Likewise, TLR2, TLR4, and TLR9 expression is significantly higher in both CD4+ and CD8+ T cells from MS patients than those isolated from healthy controls (61). While TLR expression in CD4+ T cells is generally on a smaller scale compared to cells of the innate immune response, utilizing these receptors in addition to the TCR allows for TLRs to perform very specific functions in this cell type. Indeed, several studies have indicated that TLR2 acts as a costimulatory receptor to induce T cell proliferation, survival, and cytokine production (62,63,64,65,66). A more recent study demonstrated that TCR activation induces the TLR adaptor molecule TIRAP through mTOR signaling, resulting in TLR2-induced IFNγ production by CD4+ T cells (62). Therefore, the use of TLRs by T lymphocytes may be restricted to very specific cellular functions. The next sections will discuss recent findings for TLR2 and autoimmunity in the context of CD4+ Treg and Th17 cells.
Regulatory T cells
Several studies have investigated the role of TLR2 activation in autoimmune inflammation specifically in CD4+ T cells. In the case of regulatory T cells, activation of TLR2 resulted in increased IL-17 production and a loss of Treg cell suppressive capabilities (61,67,68). However, TLR2 does still increase Treg cell proliferation, similar to the effect of TLR2 on other CD4+ T cells subsets. For this reason, the activation of TLR2 in Treg cells may eventually result in greater numbers of Treg cells, although their plasticity could still remain questionable. Our recent study has also demonstrated that TLR2 activation on in vivo-generated Tregs substantially increases their migratory potential towards CCL1 and CCL19 (69). Therefore, TLR2 appears to influence Treg proliferation, persistence, and suppressive abilities.
Other groups have demonstrated that Tregs isolated from MS patients are more susceptible to TLR2-induced modulation of function. For example, TLR2 stimulation was found to tip the Treg and Th17 balance in the favor of enhanced Th17 responses in samples obtained from relapsing-remitting MS patients. This effect was also more pronounced in MS patients compared to healthy controls (70). This study in particular supports the hypothesis that infections may also influence the Treg/Th17 cell balance (through direct TLR2 stimulation) and result in favoring proinflammatory Th17 cell dominance (70). Similar imbalances have been reported for other autoimmune diseases, such as RA and MS (71), illustrating a clear need to further elucidate the outcomes for TLR2 ligation in the inflammatory microenvironment.
Th17 cells
Under certain conditions, Th17 cells can become highly inflammatory and help orchestrate the autoimmune response. Indeed, uncontrolled Th17-driven inflammation has been linked to many inflammatory autoimmune diseases especially MS and psoriasis (4). In this context, Th17 cells are often considered pathogenic due to their ability to direct autoreactive inflammation in tissues. This has led to the development of several new therapeutics aimed at blocking inflammatory cytokines traditionally produced by Th17 cells or targeting cytokines (e.g., IL-23) with known roles in amplifying the inflammatory potential of Th17 cells for the treatment of autoimmune diseases. Such treatments have proven to be most effective for the management of inflammation in spondyloarthropathies, psoriasis, and AS, but have been only marginally successful for the treatment of MS or RA (72,73). This further emphasizes the necessity of better understanding the regulation of Th17 cells in the context of autoimmunity, including the identification of undescribed pathological mechanisms that support such inflammatory potential. These mechanisms, of course, would be outside of just inflammatory cytokine production.
Th17 cells express several TLRs including TLR4, TLR7, and TLR2, as well as TLR1 and TLR6 which, as highlighted above, heterodimerize with TLR2 (32). Stimulation of TLR7 in Th17 cells induces SOCS1, 3, and 5, which subsequently inhibit Th17 cell differentiation and reduce EAE disease severity (74). Ligation of TLR4 in Th17 cells, however, promotes Th17 cell proliferation and migration. Additionally, EAE disease development is substantially dampened in Rag1-/- mice following transfer of TLR4-/- CD4+ T cells compared to transfer of wild-type CD4+ T cells (11). TLR4, however, is dispensable for Th17 lineage differentiation and does not promote IL-17 production form Tregs (11,75,76).
TLR2 is particularly enriched in Th17 cells compared to other CD4+ Th cell subsets (11,32). In fact, Th17 cells, made more encephalitogenic through polarization with IL-1β and IL-23, appear to express the highest levels of TLR2 (69). Furthermore, our group demonstrated that TLR2 signaling induces substantial proliferation by Th17 cells in addition to other Th cell subsets. TLR2 can enhance IL-17 production when stimuli is added during in vitro Th17 differentiation. In vivo, TLR2 expression on CD4+ T cells was necessary to induce EAE (32). TLR2 activation in human CD4+ T cells was likewise shown to promote the generation of Th17 cells and the production of IL-17 (68,74,77). Our group has also recently demonstrated that activation of TLR2 on antigen-specific Th17 cells is sufficient to drive pathogenicity and EAE disease (69). Moreover, TLR2 engagement resulted in increased fatty acid synthesis and improved migration towards CCL20, both of which contribute to pathogenic Th17 cell differentiation and the development of EAE disease in mice (69,78). Another relevant molecule for autoimmune inflammation is the highly inflammatory cytokine IL-1, which amplifies the inflammatory capacity of Th17 cells. As outlined above, both TLR2 and IL-1R signal through the MyD88 adaptor molecule, which may indicate potential functional overlap between these 2 inflammatory stimuli in Th17 cells and both could impact their ability to promote autoimmune disease (69).
The expression of TLR2 on Th17 cells from patients with MS was found to be much higher than that of Th17 cells obtained from healthy controls (61). The same study demonstrated that the percentage of IL-6, IL-17, and TLR subsets were positively correlated with disease using measures of radiological activity and the Expanded Disability Status Scale score. Furthermore, CD4+ T cell production of both IFNγ and IL-17 in response to the TLR2 agonist Pam3CSK4 was directly correlated with the same clinical parameters (61). A similar observation was found in Th17 cells from people with neuromyelitis optica spectrum disorder (NMOSD) as well. Both IL-17 and IL-21 production by purified CD4+ T cells from NMOSD patients was increased (compared to control samples) following stimulation through TLR2 in the absence or presence of TCR engagement (76). Thus, the influence of TLR2 activation on CD4+ T cells is not just simply a murine phenomenon; studies such as these highlight the importance of the TLR2 pathway in the context of human autoimmune disease.
CONCLUDING REMARKS
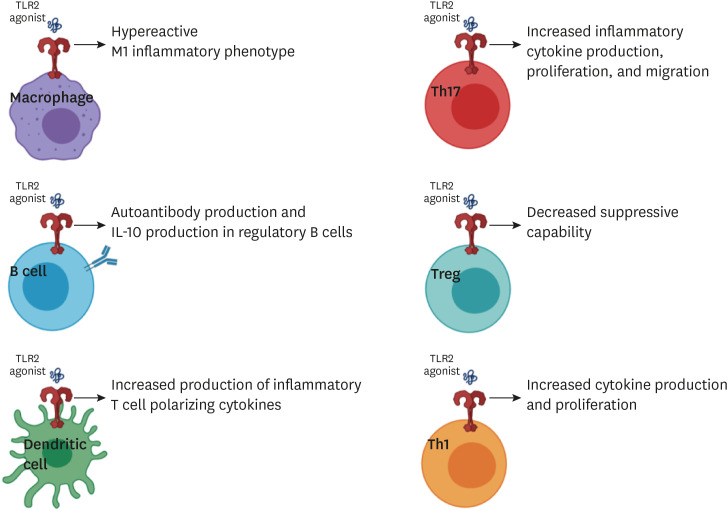
The research presented and discussed herein demonstrates the importance of TLR2 in the pathogenesis of autoimmune diseases. Due to the ubiquitous nature of TLR2 expression, this pathway has the potential to impact a wide variety of cell types and inflammatory conditions (summarized in Figure 1). TLR2 is more often highly expressed in immune populations of people with autoimmune diseases compared to control populations. In addition, patients with autoimmune diseases may have less tolerance for TLR2 signaling. The consequences of both innate and adaptive immunity utilizing TLR2 to promote inflammation may be even more severe when the inflammation is directed against self-antigens. While more work is needed to determine the physiological relevance of DAMPs driving inflammation through TLR pathways, research has demonstrated that both exogenous and endogenous ligands may contribute to the activation of TLR2. Moreover, in the case of host-derived danger signals, auto inflammation could potentially create a positive feedback loop where tissue destruction results in the release of TLR ligands that continuously amplify the inflammatory response. Future research should be focused on teasing out such contributions.
Figure 1. Graphical summary of the variety of roles of TLR2 on immunological populations.
Targeting TLR2 for the treatment of several diseases has been proposed (49,79,80). In fact, to our knowledge, pharmaceutical inhibitors of TLR2 have been designed but have yet to make it past phase II of development (81,82). One issue is that blocking TLR2 could carry the risk of increased susceptibility to infection. Additionally, broad targeting of TLR2 signaling may not just reduce inflammatory potential of T cells; instead, the loss of this pathway may also deleteriously impact TLR2-dependent anti-inflammatory activities of other populations, such as regulatory T and B cells (58). Instead, we believe that research should be focused on the outcome of TLR2 signaling in both regulatory and inflammatory populations. Understanding more about these downstream pathways will give us the ability to think about therapeutics based on more specific functional outcomes rather general targeting of a ubiquitous signaling pathway. Overall further study is required to understand the many aspects of TLR2 signaling and its contribution to immune regulation during inflammatory autoimmune diseases.
ACKNOWLEDGEMENTS
This review and some of the publications cited were supported in part by U.S. National Institutes of Health (5K22AI104941 and 1R01AI141596 to J.M.R.). The summary image (Figure 1) was created with Biorender.com.
Abbreviations
- AS
ankylosing spondylitis
- CNS
central nervous system
- DAMP
danger-associated molecular patterns
- EAE
experimental autoimmune encephalomyelitis
- EDSS
expanded Disability Status Scale
- MS
multiple sclerosis
- NMOSD
neuromyelitis optica spectrum disorder
- PAMP
pathogen-associated molecular patterns
- PRR
pattern recognition receptor
- RA
rheumatoid arthritis
- SLE
systemic lupus erythematosus
- TCR
T cell receptor
- TLR
Toll-like receptor
- Treg
regulatory T
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Marks KE, Reynolds JM.
- Investigation: Marks KE, Cho K, Stickling C, Reynolds JM.
- Writing - original draft: Marks KE, Reynolds JM.
- Writing - review & editing: Marks KE, Reynolds JM.
References
- 1.Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 2.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2:119–125. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 3.Jadidi-Niaragh F, Mirshafiey A. Th17 cell, the new player of neuroinflammatory process in multiple sclerosis. Scand J Immunol. 2011;74:1–13. doi: 10.1111/j.1365-3083.2011.02536.x. [DOI] [PubMed] [Google Scholar]
- 4.Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol. 2019;41:283–297. doi: 10.1007/s00281-019-00733-8. [DOI] [PubMed] [Google Scholar]
- 5.Thomas SL, Griffiths C, Smeeth L, Rooney C, Hall AJ. Burden of mortality associated with autoimmune diseases among females in the United Kingdom. Am J Public Health. 2010;100:2279–2287. doi: 10.2105/AJPH.2009.180273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houge IS, Hoff M, Thomas R, Videm V. Mortality is increased in patients with rheumatoid arthritis or diabetes compared to the general population - the Nord-Trøndelag Health Study. Sci Rep. 2020;10:3593. doi: 10.1038/s41598-020-60621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 8.Botos I, Segal DM, Davies DR. The structural biology of Toll-like receptors. Structure. 2011;19:447–459. doi: 10.1016/j.str.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell JK, Mullen GE, Leifer CA, Mazzoni A, Davies DR, Segal DM. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 2003;24:528–533. doi: 10.1016/s1471-4906(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 10.Alibashe-Ahmed M, Brioudes E, Reith W, Bosco D, Berney T. Toll-like receptor 4 inhibition prevents autoimmune diabetes in NOD mice. Sci Rep. 2019;9:19350. doi: 10.1038/s41598-019-55521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds JM, Martinez GJ, Chung Y, Dong C. Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc Natl Acad Sci U S A. 2012;109:13064–13069. doi: 10.1073/pnas.1120585109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devarapu SK, Anders HJ. Toll-like receptors in lupus nephritis. J Biomed Sci. 2018;25:35. doi: 10.1186/s12929-018-0436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horton CG, Farris AD. Toll-like receptors in systemic lupus erythematosus: potential targets for therapeutic intervention. Curr Allergy Asthma Rep. 2012;12:1–7. doi: 10.1007/s11882-011-0234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fillatreau S, Manfroi B, Dörner T. Toll-like receptor signalling in B cells during systemic lupus erythematosus. Nat Rev Rheumatol. 2021;17:98–108. doi: 10.1038/s41584-020-00544-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 17.Andersson A, Covacu R, Sunnemark D, Danilov AI, Dal Bianco A, Khademi M, Wallström E, Lobell A, Brundin L, Lassmann H, et al. Pivotal advance: HMGB1 expression in active lesions of human and experimental multiple sclerosis. J Leukoc Biol. 2008;84:1248–1255. doi: 10.1189/jlb.1207844. [DOI] [PubMed] [Google Scholar]
- 18.Jafarzadeh A, Nemati M, Khorramdelazad H, Mirshafiey A. The Toll-like receptor 2 (TLR2)-related immunopathological responses in the multiple sclerosis and experimental autoimmune encephalomyelitis. Iran J Allergy Asthma Immunol. 2019;18:230–250. doi: 10.18502/ijaai.v18i3.1117. [DOI] [PubMed] [Google Scholar]
- 19.Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, Vartanian T. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci U S A. 2010;107:11555–11560. doi: 10.1073/pnas.1006496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi B, Huang Q, Tak PP, Vervoordeldonk MJ, Huang CC, Dorfleutner A, Stehlik C, Pope RM. SNAPIN: an endogenous Toll-like receptor ligand in rheumatoid arthritis. Ann Rheum Dis. 2012;71:1411–1417. doi: 10.1136/annrheumdis-2011-200899. [DOI] [PubMed] [Google Scholar]
- 21.Gray DH, Gavanescu I, Benoist C, Mathis D. Danger-free autoimmune disease in Aire-deficient mice. Proc Natl Acad Sci U S A. 2007;104:18193–18198. doi: 10.1073/pnas.0709160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen SJ, Cohen IR, Nussbaum G. IL-10 mediates resistance to adoptive transfer experimental autoimmune encephalomyelitis in MyD88(-/-) mice. J Immunol. 2010;184:212–221. doi: 10.4049/jimmunol.0900296. [DOI] [PubMed] [Google Scholar]
- 23.Sadanaga A, Nakashima H, Akahoshi M, Masutani K, Miyake K, Igawa T, Sugiyama N, Niiro H, Harada M. Protection against autoimmune nephritis in MyD88-deficient MRL/lpr mice. Arthritis Rheum. 2007;56:1618–1628. doi: 10.1002/art.22571. [DOI] [PubMed] [Google Scholar]
- 24.Dishon S, Cohen SJ, Cohen IR, Nussbaum G. Inhibition of myeloid differentiation factor 88 reduces human and mouse T-cell interleukin-17 and IFNγ production and ameliorates experimental autoimmune encephalomyelitis induced in mice. Front Immunol. 2017;8:615. doi: 10.3389/fimmu.2017.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 26.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urbonaviciute V, Starke C, Pirschel W, Pohle S, Frey S, Daniel C, Amann K, Schett G, Herrmann M, Voll RE. Toll-like receptor 2 is required for autoantibody production and development of renal disease in pristane-induced lupus. Arthritis Rheum. 2013;65:1612–1623. doi: 10.1002/art.37914. [DOI] [PubMed] [Google Scholar]
- 28.Lartigue A, Colliou N, Calbo S, François A, Jacquot S, Arnoult C, Tron F, Gilbert D, Musette P. Critical role of TLR2 and TLR4 in autoantibody production and glomerulonephritis in lpr mutation-induced mouse lupus. J Immunol. 2009;183:6207–6216. doi: 10.4049/jimmunol.0803219. [DOI] [PubMed] [Google Scholar]
- 29.Ma K, Li J, Fang Y, Lu L. Roles of B Cell-Intrinsic TLR Signals in Systemic Lupus Erythematosus. Int J Mol Sci. 2015;16:13084–13105. doi: 10.3390/ijms160613084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freeley SJ, Giorgini A, Tulone C, Popat RJ, Horsfield C, Robson MG. Toll-like receptor 2 or toll-like receptor 4 deficiency does not modify lupus in MRLlpr mice. PLoS One. 2013;8:e74112. doi: 10.1371/journal.pone.0074112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds JM, Angkasekwinai P, Dong C. IL-17 family member cytokines: regulation and function in innate immunity. Cytokine Growth Factor Rev. 2010;21:413–423. doi: 10.1016/j.cytogfr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds JM, Pappu BP, Peng J, Martinez GJ, Zhang Y, Chung Y, Ma L, Yang XO, Nurieva RI, Tian Q, et al. Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity. 2010;32:692–702. doi: 10.1016/j.immuni.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devaraj S, Tobias P, Kasinath BS, Ramsamooj R, Afify A, Jialal I. Knockout of toll-like receptor-2 attenuates both the proinflammatory state of diabetes and incipient diabetic nephropathy. Arterioscler Thromb Vasc Biol. 2011;31:1796–1804. doi: 10.1161/ATVBAHA.111.228924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frasnelli ME, Tarussio D, Chobaz-Péclat V, Busso N, So A. TLR2 modulates inflammation in zymosan-induced arthritis in mice. Arthritis Res Ther. 2005;7:R370–RR379. doi: 10.1186/ar1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seibl R, Birchler T, Loeliger S, Hossle JP, Gay RE, Saurenmann T, Michel BA, Seger RA, Gay S, Lauener RP. Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol. 2003;162:1221–1227. doi: 10.1016/S0002-9440(10)63918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ultaigh SN, Saber TP, McCormick J, Connolly M, Dellacasagrande J, Keogh B, McCormack W, Reilly M, O'Neill LA, McGuirk P, et al. Blockade of Toll-like receptor 2 prevents spontaneous cytokine release from rheumatoid arthritis ex vivo synovial explant cultures. Arthritis Res Ther. 2011;13:R33. doi: 10.1186/ar3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujiwara M, Anstadt EJ, Flynn B, Morse K, Ng C, Paczkowski P, Zhou J, Mackay S, Wasko N, Nichols F, et al. Enhanced TLR2 responses in multiple sclerosis. Clin Exp Immunol. 2018;193:313–326. doi: 10.1111/cei.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Labib DA, Ashmawy I, Elmazny A, Helmy H, Ismail RS. Toll-like receptors 2 and 4 expression on peripheral blood lymphocytes and neutrophils of Egyptian multiple sclerosis patients. Int J Neurosci. 2020 doi: 10.1080/00207454.2020.1812601. [DOI] [PubMed] [Google Scholar]
- 39.Hossain MJ, Morandi E, Tanasescu R, Frakich N, Caldano M, Onion D, Faraj TA, Erridge C, Gran B. The soluble form of Toll-like receptor 2 is elevated in serum of multiple sclerosis patients: a novel potential disease biomarker. Front Immunol. 2018;9:457. doi: 10.3389/fimmu.2018.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lacerte P, Brunet A, Egarnes B, Duchêne B, Brown JP, Gosselin J. Overexpression of TLR2 and TLR9 on monocyte subsets of active rheumatoid arthritis patients contributes to enhance responsiveness to TLR agonists. Arthritis Res Ther. 2016;18:10–10. doi: 10.1186/s13075-015-0901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flo TH, Halaas O, Lien E, Ryan L, Teti G, Golenbock DT, Sundan A, Espevik T. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J Immunol. 2000;164:2064–2069. doi: 10.4049/jimmunol.164.4.2064. [DOI] [PubMed] [Google Scholar]
- 42.Huang Q, Ma Y, Adebayo A, Pope RM. Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis Rheum. 2007;56:2192–2201. doi: 10.1002/art.22707. [DOI] [PubMed] [Google Scholar]
- 43.Quero L, Hanser E, Manigold T, Tiaden AN, Kyburz D. TLR2 stimulation impairs anti-inflammatory activity of M2-like macrophages, generating a chimeric M1/M2 phenotype. Arthritis Res Ther. 2017;19:245–245. doi: 10.1186/s13075-017-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali JL, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 45.Huang QQ, Koessler RE, Birkett R, Perlman H, Xing L, Pope RM. TLR2 deletion promotes arthritis through reduction of IL-10. J Leukoc Biol. 2013;93:751–759. doi: 10.1189/jlb.0912473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 47.Huang QQ, Pope RM. The role of Toll-like receptors in rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:357–364. doi: 10.1007/s11926-009-0051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wasko NJ, Kulak MH, Paul D, Nicaise AM, Yeung ST, Nichols FC, Khanna KM, Crocker S, Pachter JS, Clark RB. Systemic TLR2 tolerance enhances central nervous system remyelination. J Neuroinflammation. 2019;16:158. doi: 10.1186/s12974-019-1540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anstadt EJ, Fujiwara M, Wasko N, Nichols F, Clark RB. TLR tolerance as a treatment for central nervous system autoimmunity. J Immunol. 2016;197:2110–2118. doi: 10.4049/jimmunol.1600876. [DOI] [PubMed] [Google Scholar]
- 50.Kim DH, Lee JC, Kim S, Oh SH, Lee MK, Kim KW, Lee MS. Inhibition of autoimmune diabetes by TLR2 tolerance. J Immunol. 2011;187:5211–5220. doi: 10.4049/jimmunol.1001388. [DOI] [PubMed] [Google Scholar]
- 51.Candia L, Marquez J, Hernandez C, Zea AH, Espinoza LR. Toll-like receptor-2 expression is upregulated in antigen-presenting cells from patients with psoriatic arthritis: a pathogenic role for innate immunity? J Rheumatol. 2007;34:374–379. [PubMed] [Google Scholar]
- 52.Wang X, Yuan T, Yuan J, Su Y, Sun X, Wu J, Zhang H, Min X, Zhang X, Yin Y. Expression of Toll-like receptor 2 by dendritic cells is essential for the DnaJ-ΔA146Ply-mediated Th1 immune response against Streptococcus pneumoniae . Infect Immun. 2018;86:e00651–e00e17. doi: 10.1128/IAI.00651-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roelofs MF, Joosten LA, Abdollahi-Roodsaz S, van Lieshout AW, Radstake TR, van den Berg WB. Expression of Toll-like receptor (TLR) 2, TLR3, TLR4 and TLR7 is increased in rheumatoid arthritis synovium and regulates cytokine production by dendritic cells upon stimulation of TLR specific pathways. Arthritis Res Ther. 2005;7(Suppl 1):P70. [Google Scholar]
- 54.Lee DS, Rojas OL, Gommerman JL. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat Rev Drug Discov. 2021;20:179–199. doi: 10.1038/s41573-020-00092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agrawal S, Gupta S. TLR1/2, TLR7, and TLR9 signals directly activate human peripheral blood naive and memory B cell subsets to produce cytokines, chemokines, and hematopoietic growth factors. J Clin Immunol. 2011;31:89–98. doi: 10.1007/s10875-010-9456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green NM, Marshak-Rothstein A. Toll-like receptor driven B cell activation in the induction of systemic autoimmunity. Semin Immunol. 2011;23:106–112. doi: 10.1016/j.smim.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Correale J, Farez M. Helminth antigens modulate immune responses in cells from multiple sclerosis patients through TLR2-dependent mechanisms. J Immunol. 2009;183:5999–6012. doi: 10.4049/jimmunol.0900897. [DOI] [PubMed] [Google Scholar]
- 58.Lampropoulou V, Hoehlig K, Roch T, Neves P, Calderón Gómez E, Sweenie CH, Hao Y, Freitas AA, Steinhoff U, Anderton SM, et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 59.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Ye J, Wang Y, Liu X, Li L, Opejin A, Hsueh EC, Luo H, Wang T, Hawiger D, Peng G. TLR7 signaling regulates Th17 cells and autoimmunity: novel potential for autoimmune therapy. J Immunol. 2017;199:941–954. doi: 10.4049/jimmunol.1601890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferreira TB, Hygino J, Wing AC, Kasahara TM, Sacramento PM, Camargo S, Rueda F, Alves-Leon SV, Alvarenga R, Vasconcelos CC, et al. Different interleukin-17-secreting Toll-like receptor+ T-cell subsets are associated with disease activity in multiple sclerosis. Immunology. 2018;154:239–252. doi: 10.1111/imm.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Imanishi T, Unno M, Kobayashi W, Yoneda N, Akira S, Saito T. mTORC1 signaling controls TLR2-mediated T-cell activation by inducing TIRAP expression. Cell Reports. 2020;32:107911. doi: 10.1016/j.celrep.2020.107911. [DOI] [PubMed] [Google Scholar]
- 63.Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci U S A. 2004;101:3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jun JC, Jones MB, Oswald DM, Sim ES, Jonnalagadda AR, Kreisman LS, Cobb BA. T cell-intrinsic TLR2 stimulation promotes IL-10 expression and suppressive activity by CD45RbHi T cells. PLoS One. 2017;12:e0180688. doi: 10.1371/journal.pone.0180688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cottalorda A, Verschelde C, Marçais A, Tomkowiak M, Musette P, Uematsu S, Akira S, Marvel J, Bonnefoy-Berard N. TLR2 engagement on CD8 T cells lowers the threshold for optimal antigen-induced T cell activation. Eur J Immunol. 2006;36:1684–1693. doi: 10.1002/eji.200636181. [DOI] [PubMed] [Google Scholar]
- 66.Liew FY, Komai-Koma M, Xu D. A toll for T cell costimulation. Ann Rheum Dis. 2004;63(Suppl 2):ii76–ii78. doi: 10.1136/ard.2004.028308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nyirenda MH, Morandi E, Vinkemeier U, Constantin-Teodosiu D, Drinkwater S, Mee M, King L, Podda G, Zhang GX, Ghaemmaghami A, et al. TLR2 stimulation regulates the balance between regulatory T cell and Th17 function: a novel mechanism of reduced regulatory T cell function in multiple sclerosis. J Immunol. 2015;194:5761–5774. doi: 10.4049/jimmunol.1400472. [DOI] [PubMed] [Google Scholar]
- 68.Nyirenda MH, Sanvito L, Darlington PJ, O'Brien K, Zhang GX, Constantinescu CS, Bar-Or A, Gran B. TLR2 stimulation drives human naive and effector regulatory T cells into a Th17-like phenotype with reduced suppressive function. J Immunol. 2011;187:2278–2290. doi: 10.4049/jimmunol.1003715. [DOI] [PubMed] [Google Scholar]
- 69.Marks K, et al. Toll-like receptor 2 induces pathogenicity in Th17 cells and reveals a role for interactor protein for cytohesin exchange factors 1 (IPCEF) in regulating Th17 cell migration. Cell Rep. 2021 doi: 10.2139/ssrn.3701221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nyirenda MH, Morandi E, Vinkemeier U, Constantin-Teodosiu D, Drinkwater S, Mee M, King L, Podda G, Zhang GX, Ghaemmaghami A, et al. TLR2 stimulation regulates the balance between regulatory T cell and Th17 function: a novel mechanism of reduced regulatory T cell function in multiple sclerosis. J Immunol. 2015;194:5761–5774. doi: 10.4049/jimmunol.1400472. [DOI] [PubMed] [Google Scholar]
- 71.Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117:289–300. doi: 10.1111/j.1365-2567.2005.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Havrdová E, Belova A, Goloborodko A, Tisserant A, Wright A, Wallstroem E, Garren H, Maguire RP, Johns DR. Activity of secukinumab, an anti-IL-17A antibody, on brain lesions in RRMS: results from a randomized, proof-of-concept study. J Neurol. 2016;263:1287–1295. doi: 10.1007/s00415-016-8128-x. [DOI] [PubMed] [Google Scholar]
- 73.Taams LS. Interleukin-17 in rheumatoid arthritis: trials and tribulations. J Exp Med. 2020;217:e20192048. doi: 10.1084/jem.20192048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ye J, Wang Y, Liu X, Li L, Opejin A, Hsueh EC, Luo H, Wang T, Hawiger D, Peng G. TLR7 signaling regulates Th17 cells and autoimmunity: novel potential for autoimmune therapy. J Immunol. 2017;199:941–954. doi: 10.4049/jimmunol.1601890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McAleer JP, Liu B, Li Z, Ngoi SM, Dai J, Oft M, Vella AT. Potent intestinal Th17 priming through peripheral lipopolysaccharide-based immunization. J Leukoc Biol. 2010;88:21–31. doi: 10.1189/jlb.0909631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dias AS, Sacramento PM, Lopes LM, Sales MC, Castro C, Araújo AC, Ornelas AM, Aguiar RS, Silva-Filho RG, Alvarenga R, et al. TLR-2 and TLR-4 agonists favor expansion of CD4+ T cell subsets implicated in the severity of neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. 2019;34:66–76. doi: 10.1016/j.msard.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 77.Zhao RR, Yang XF, Dong J, Zhao YY, Wei X, Huang CX, Lian JQ, Zhang Y. Toll-like receptor 2 promotes T helper 17 cells response in hepatitis B virus infection. Int J Clin Exp Med. 2015;8:7315–7323. [PMC free article] [PubMed] [Google Scholar]
- 78.Young KE, Flaherty S, Woodman KM, Sharma-Walia N, Reynolds JM. Fatty acid synthase regulates the pathogenicity of Th17 cells. J Leukoc Biol. 2017;102:1229–1235. doi: 10.1189/jlb.3AB0417-159RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elshabrawy HA, Essani AE, Szekanecz Z, Fox DA, Shahrara S. TLRs, future potential therapeutic targets for RA. Autoimmun Rev. 2017;16:103–113. doi: 10.1016/j.autrev.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mullen LM, Chamberlain G, Sacre S. Pattern recognition receptors as potential therapeutic targets in inflammatory rheumatic disease. Arthritis Res Ther. 2015;17:122. doi: 10.1186/s13075-015-0645-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mistry P, Laird MH, Schwarz RS, Greene S, Dyson T, Snyder GA, Xiao TS, Chauhan J, Fletcher S, Toshchakov VY, et al. Inhibition of TLR2 signaling by small molecule inhibitors targeting a pocket within the TLR2 TIR domain. Proc Natl Acad Sci U S A. 2015;112:5455–5460. doi: 10.1073/pnas.1422576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anwar MA, Shah M, Kim J, Choi S. Recent clinical trends in Toll-like receptor targeting therapeutics. Med Res Rev. 2019;39:1053–1090. doi: 10.1002/med.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]