Abstract
Due to the inconsistent fluctuation of blood supply for transfusion, much attention has been paid to the development of artificial blood using other animals. Although mini-pigs are candidate animals, contamination of mini-pig T cells in artificial blood may cause a major safety concern. Therefore, it is important to analyze the cross-reactivity of IL-7, the major survival factor for T lymphocytes, between human, mouse, and mini-pig. Thus, we compared the protein sequences of IL-7 and found that porcine IL-7 was evolutionarily different from human IL-7. We also observed that when porcine T cells were cultured with either human or mouse IL-7, these cells did not increase the survival or proliferation compared to negative controls. These results suggest that porcine T cells do not recognize human or mouse IL-7 as their survival factor.
Keywords: IL-7, Miniature swine, Graft-versus-host disease, T-lymphocytes
INTRODUCTION
Blood transfusion is the process of supplying blood cells and plasma components to patients lacking adequate volumes of blood in their system. It includes homologous transfusions and autologous transfusions, which use other people's blood and the patient's own blood, respectively. To date, if serious bleeding occurs or massive blood transfusion is needed, most transfusions depend on homologous blood transfusions (1). However, with societal factors including an aging population, it is becoming increasingly difficult to obtain the volumes of blood needed for transfusions only through donations (2). One possible solution to this problem is the use of artificial blood, produced from medium-large animals like mini-pigs with human blood stem cells through bone marrow transplantation (3,4,5).
A bone marrow transplant involves replacing unhealthy marrow with a healthy one (6,7,8). When the bone marrow is replaced, it grows and begins to produce red blood cells, white blood cells, and platelets. When human stem cells are transplanted into the bone marrow of another animal, it can get replaced with human bone marrow. The hematopoietic stem cells in the bone marrow can differentiate into many different types of blood cells; thus, it is possible that human blood can be produced after bone marrow transplantation. If this idea could work, bone marrow transplantation in animals can be used to generate artificial blood without the need for human blood donors. Mini-pigs are good candidates for this method because they are easy to manipulate genetically, have abundant amounts of blood, and can have many offspring (9,10,11).
Although artificial blood appears promising, the transfusion of porcine artificial blood into humans is expected to cause a variety of immune diseases such as graft-versus-host disease (GVHD) (12,13). Especially, porcine T cells, present as contaminants in the artificial blood, may develop into mature T cells in the human bone marrow following transfusion and could cause severe GVHD (12). In addition, porcine stem cells contaminated in the artificial blood may develop into mature porcine T cells in human bone marrow following transfusion, and these T cells are potent to cause GVHD in human bodies. Mature T cells can develop the disease in multiple pathways (14) While CD4 T cells respond to Ags by producing cytokines that carry out several actions like the recruitment and activation of other leukocytes (15,16,17), CD8 T cells respond by killing cells directly. Therefore, to avoid GVHD and other concerns, it is necessary to create conditions under which donor lymphocytes and stem cells cannot survive (18,19). In this study, we studied whether porcine T cells can develop and survive within human bodies.
The development and homeostasis of T cells in humans and mice depends on survival factors. Of particular interest is IL-7, because T lymphocytes are terminally differentiated and removed when they do not receive IL-7 (20-22). In addition, this survival factor plays a crucial role in the development of T cells in the thymus (23,24,25,26,27). Although IL-7 is known to be a survival factor for naïve and memory T lymphocytes in mice and humans (20,21,22,28), its role in the survival of porcine T lymphocytes is unknown. In addition, it is important to investigate if porcine T cells can respond to human or mouse IL-7 because the survival of porcine T cells, as contaminants in the artificial blood, is a crucial safety concern.
MATERIALS AND METHODS
Protein sequence comparison
The protein sequences of porcine, human, and mouse IL-7 were obtained in the FASTA format from the PubMed site. In order to confirm the evolutionary similarities, these sequences were expressed as phylogenetic tree using the Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets (MEGA7) program (29). We obtained the crystal data for human IL-7 with unglycosylated human IL-7Rα complex from PDB. The structure for mouse and porcine IL-7 were predicted by homology modeling using I-Tasser (30). We identified the main interactions between human IL-7 and human IL-7Rα and confirmed through superposition whether porcine or mouse IL-7 could bind to the human IL-7 receptor by using the PyMol molecular graphics program. All animal experimental designs and procedures were approved by Institutional Animal Care and Use Committee protocols of Korea Research Institute of Bioscience and Biotechnology (KRIBB) (KRIBB-AEC-16075).
Porcine PBMC isolation
Pigs were housed and maintained in good condition (Table 1) in the facility of Futuristic Animal Resource and Research Center of the KRIBB. We bled 2 different pigs of the same strain without euthanasia. Peripheral blood samples were collected from 2 KSP mini-pigs and diluted with 1× PBS (Hyclone®; Hyclone Laboratories Inc., Logan, UT, USA) at a 1:2 ratio in 50 ml tubes. We prepared 15 ml tubes filled with 3 ml of Biocoll (Biochrom, Cambridge, UK), added 10 ml of the diluted blood, and centrifuged the mixture under natural step conditions at 700 g for 20 min. After centrifugation, we were able to see the PBMC layer (buffy coat) between the plasma layer and Biocoll. The PMBC layers were transferred into a new tube, and then washed with 1× PBS. The collected cells were resuspended in RPMI 1640 medium containing 10% FBS (Hyclone®) and 1% penicillin/streptomycin (Hyclone®), and were stored at 4°C or frozen in liquid nitrogen.
Table 1. Health Monitoring List.
| Species: porcine | METHODS | RESULTS | |
|---|---|---|---|
| VIRAL INFECTIONS | |||
| Classical swine fever virus (hog cholera) | ELISA (blood) | Negative | |
| Aujeszky's disease (pseudorabies) | ELISA (blood) | Negative | |
| Porcine reproductive respiratory syndrome virus | ELISA (blood) | Negative | |
| Porcine circovirus type 2 | ELISA (blood) | Negative | |
| Swine influenza virus (H1N1) | ELISA (blood) | Negative | |
| BACTERIAL INFECTIONS | |||
| Salmonella spp. | ELISA (blood), PCR (fecal) | Negative | |
| Erysipelothrix rhusiopathiae | PCR (skin swap) | Negative | |
| Bordetella bronchiseptica | PCR (nasal swab) | Negative | |
| Actinobacillus pleuropneumoniae | ELISA (blood), PCR (nasal swab) | Negative | |
| Streptococcus suis | PCR (nasal swab) | Negative | |
| Haemophilus parasuis | PCR (nasal swab) | Negative | |
| Actinobacillus suis | PCR (nasal swab) | Negative | |
| Mycoplasma hyopneumoniae | ELISA (blood) | Negative | |
Mouse splenocyte isolation
C57BL/6 (B6) mice were purchased from the Orient Bio. Mice were sacrificed, and the spleens were placed in a sterile petri dish containing RPMI 1640 (Hyclone®) medium supplemented with 0.05mM beta-mercaptoethanol, 1% FBS and 1% penicillin/streptomycin. Spleens were gently mashed using the plunger from a syringe and the suspensions were centrifuged at 1,500 rpm for 5 min. The red blood cells were removed using 0.83% ammonium chloride for 2 min and then diluted by 1% RPMI 1640 medium. The aggregating cells were readily dissociated into single cells by gentle pipetting. The collected cells were then passed through a piece of nylon filter to remove debris and to yield a crude splenocyte preparation.
Cell culture
Porcine PBMCs were seeded in 48-well plates and treated with either human IL-7 (R&D system, Minneapolis, MN, USA) or mouse IL-7 (R&D system) at a concentration of 1.6 ng/ml per well. On day 7, the cell culture media was changed and treated with fresh IL-7 at the same concentration, if necessary. The cells were maintained in RPMI 1640 medium containing 10% FBS (Hyclone®) and 1% penicillin/streptomycin (Hyclone®) at 37°C under 5% CO2/95% air.
In vitro carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling
Porcine PBMCs were washed twice with PBS and resuspended in PBS at 1×107 cells/ml without protein components. The 5- and 6-CFSE (Invitrogen, Waltham, MA, USA) was added to a final concentration of 1 μM and incubated at room temperature (RT) for 10 min. After incubation, 1/10 volume of the tubes were filled with FBS and topped up with 1% RPMI 1640. The cells were washed twice with cold RPMI 1640 containing 1% FBS.
Flow cytometry
Cells were stained with Abs specific for anti-CD172α (clone 74-22-15A), anti-CD8α (clone 76-2-11), and anti-CD4α (clone 74-12-4) from BD, anti-CD16 (clone G7) and anti-CD45RA (clone MIL13) from Bio-Rad, and anti-CD4 (clone 74-12-4) and anti-CD3 (clone C363.29B) from Abcam in U-bottom 96-well plates for 30 min on ice without light. Flow cytometry analysis was performed using a BD Biosciences LSRFortessa™ cell Analyzer according to standard procedures. Data were analyzed with FlowJo® software (version 9.7.3; Tree star Inc., Ashland, OR, USA).
In vitro survival assay with propidium iodide (PI)
Porcine PBMCs were plated at a density of 1.68×105 cells per well in 96-well plates and cultured in RPMI 1640 medium containing 10% FBS and 1% penicillin/streptomycin at 37°C under 5% CO2/95% air. On day 5 of integral viable cell, the cells were harvested, counted, and transferred into a U-bottom 96-well plate. The plate was centrifuged at 1,650 rpm, at 4°C for 2 min. After the supernatant was removed, the cells were stained with PI (BD pharmingen™; BD Biosciences, Franklin Lakes, NJ, USA) and Abs at RT for 20 min in the dark. After 20 min, PI and Abs were diluted with flow cytometry buffer and analyzed within 1 h.
Statistical analysis
All comparisons were carried out using a Student's 2-tailed unpaired t-test where p<0.05 was considered statistically significant.
RESULTS
Genetic comparison of human, porcine, and mouse IL-7 protein sequences
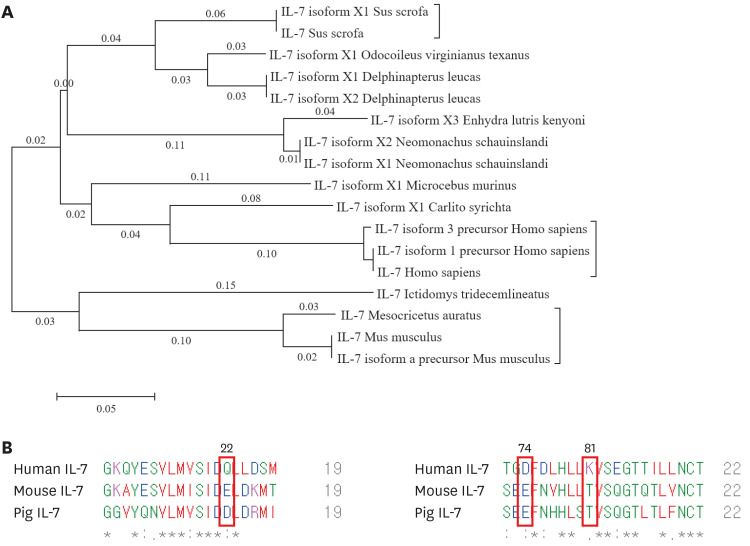
The development of porcine stem cells into mature T cells when transferred into humans can cause serious concerns for artificial blood production. Since IL-7 is known to be the most important factor for the development and maintenance of T cells, we first analyzed the homology of human, mouse, and porcine IL-7, using the MEGA7 program (29,31,32). As shown in Fig. 1A, we constructed a phylogenetic tree and found that the evolutionary distance between porcine and human IL-7 was greater than the porcine, Delphinapterus, and Odocoileus virginianus IL-7. These data suggest that porcine IL-7 is not structurally similar to human IL-7.
Figure 1. Phylogenetic tree analysis and structural comparison of human, porcine, and mouse IL-7. (A) The phylogenetic tree shows the relationship between human, porcine, and mouse IL-7 protein sequences. The evolutionary history was inferred using the neighbor-joining method. Evolutionary analyses were conducted in MEGA7. (B) The protein sequence homology among human, mouse, and mini-pig IL-7. The protein sequences of porcine, human, and mouse IL-7 were obtained in the FASTA format from the PubMed site. The sequences of the α-helix of human or mouse or porcine IL-7, which constitutes the binding site with IL-7 receptor, was examined by homologous analysis using Clustal Omega.
Next, we examined the similarity of the IL-7 protein sequences between species that constitute the binding site for the IL-7 receptor by homologous analysis of crystal omega (Fig. 1B). The key human IL-7 residues that were suggested to bind to human IL-7Rα were glutamine (Q), aspartic acid (D), and lysine (K). While glutamine (Q) at position 22 was replaced by aspartic acid (D) in pig IL-7, glutamic acid (E) was found in mouse IL-7. In addition, aspartic acid (D) at position 74 of human IL-7 is replaced by glutamic acid (E) in mouse and porcine IL-7. Lysine (K) at position 81 in human IL-7 is changed to threonine (T) in mouse and porcine IL-7. These results suggest that neither mouse nor porcine IL-7 have cross-activity for binding to the human IL-7 receptor.
Lastly, we compared the interactions between human IL-7 and human IL-7Rα and confirmed through superposition whether porcine or mouse IL-7 could bind to the human IL-7 receptor. Using the PyMOL molecular graphics program, we found that the interaction of porcine or mouse IL-7 with the human IL-7 receptor disappeared when human IL-7 was replaced (Supplementary Fig. 1). These results indicated that human and pig IL-7 are evolutionarily apart and show structurally different characteristics based on protein sequences.
Human or mouse IL-7 is not critical for the homeostasis of porcine T cells
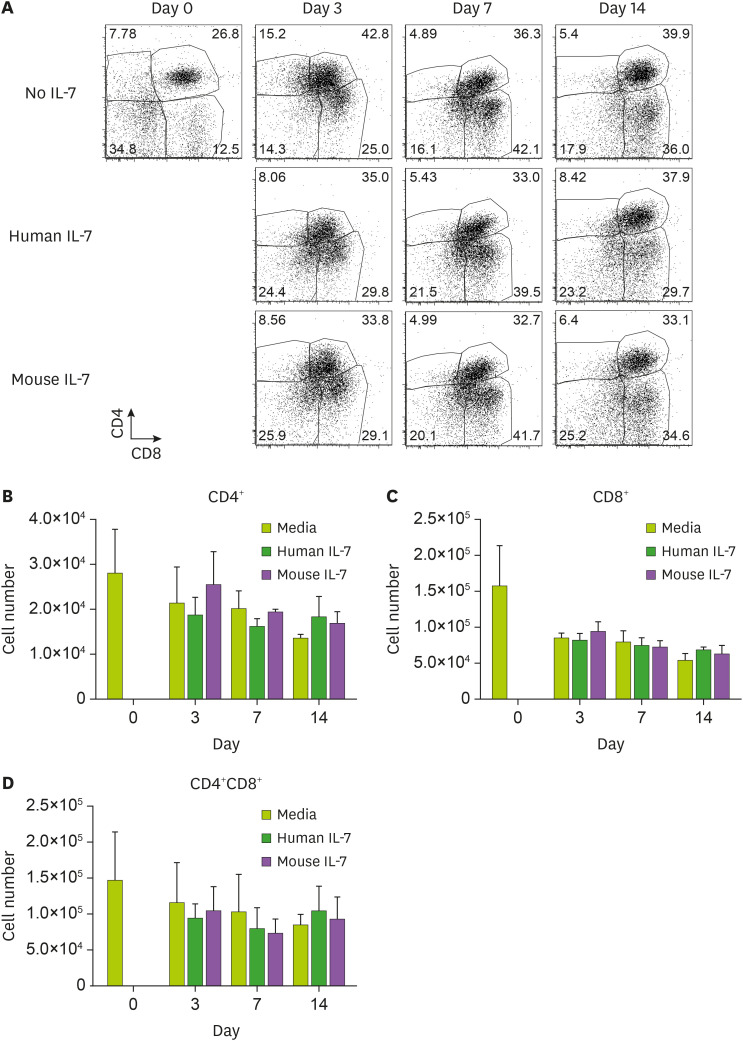
Since human and porcine IL-7 are evolutionarily distant from each other and the amino acids that constitute their binding sites are distinct (Fig. 1), we further investigated whether human IL-7 has cross-activity in porcine T cells in vitro. To test this, porcine PBMCs were treated with either media alone, human IL-7, or mouse IL-7, and the numbers and frequencies of CD4+, CD8+, or CD4+CD8+T cells were compared by flow cytometry. The frequencies of porcine CD4+, CD8+, or CD4+CD8+ T cells were not found to be significantly different after IL-7 treatment at every time point examined, and were comparable to the negative controls (Fig. 2A). Next, we calculated and plotted the numbers of porcine CD4+, CD8+, or CD4+CD8+ T cells in response to human or mouse IL-7 (Fig. 2B-D). The CD4+ T cells treated with IL-7 showed a tendency to increase in viable cell count compared to the non-stimulated group on day 14 in both the porcine samples analyzed (Fig. 2B). In addition, CD8+ T cells treated with IL-7 appeared to have a higher survival rate than the non-stimulated group on day 14 (Fig. 2C). However, there was no statistically significant difference in the number of CD4+ or CD8+ T cells with or without human or mouse IL-7 treatment. CD4 and CD8 double positive T cells also showed similar results (Fig. 2D). By the contrary, the number of mouse CD8+ T cells substantially increased on day 14 of culture with mouse IL-7 (Supplementary Fig. 2). These results led us to conclude that human or mouse IL-7 does not have a critical effect on the homeostasis of porcine T lymphocytes.
Figure 2. Effect of human or mouse IL-7 on the survival of porcine T cells. Porcine PBMCs were seeded in 48-well plates and treated with either human or mouse IL-7. On days 3, 7, and 14, cells were harvested, counted, and stained with anti-CD4 and anti-CD8 Abs and analyzed by flow cytometry. (A) The frequency of porcine CD4+ or CD8+ or CD4+CD8+T cells are shown by dot plots. (B-D) The numbers of CD4+ or CD8+ T cells were calculated based on flow cytometry data. We obtained similar results from other pigs of the same strain. Each experiment involved 7 independent experiments. Error bars represent SD. The statistical significance was measured by t-test.
Homeostatic proliferation of porcine CD4 or CD8 T cells by human or mouse IL-7 in vitro
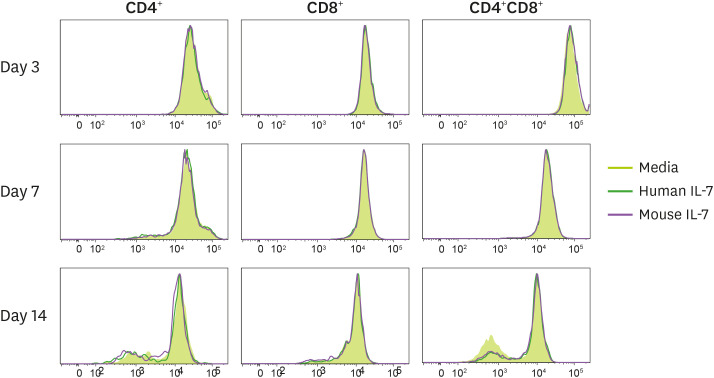
As shown above, porcine T lymphocytes treated with IL-7 did not show a significant difference in cell number compared to the media treated group. Since IL-7 can contribute to homeostatic proliferation in mice and humans, we investigated whether human IL-7 contributes to the homeostatic proliferation of porcine T cell. The porcine PBMCs were labeled with CFSE, cultured with either media alone, human IL-7, or mouse IL-7, and analyzed for the proliferation of porcine T cells (Fig. 3). We also treated mouse splenocytes with mouse IL-7 to serve as control.
Figure 3. Homeostatic proliferation of porcine T cells induced by either human or mouse IL-7 in vitro. The CFSE-labeled porcine PBMCs were seeded in 48-well plates and treated with either human or mouse IL-7. These cells were cultured and harvested on days 3, 7, and 14. The CFSE profiles were gated on CD4+ or CD8+ T cell staining. Histograms of CFSE-labeled CD4+ or CD8+ or CD4+CD8+T cells are shown. We obtained similar results from other pigs of the same strain. Each experiment involved 3 independent experiments.
In the first 7 days, no proliferation of the porcine T cells occurred. On day 14, a small division peak was found in porcine CD4+ and CD4+CD8+T cells, but there was no proliferation of porcine CD8+ T cells. However, the media treated group also showed the same peak, possibly indicating that the homeostatic proliferation of CD4+ and CD4+CD8+T cells were independent of IL-7. By the contrary, the majority of mouse CD8+ T cells underwent a division when cultured with mouse or human IL-7 for 14 days, while CD4+ T cell did not divide (Supplementary Fig. 3). Through these experiments, we concluded that human IL-7 does not affect the homeostatic proliferation of porcine T lymphocytes. However, questions remained as to whether the small division peak of porcine CD4+ T cells was due to homeostatic proliferation.
Survival of porcine T cells does not depend on the concentration of human IL-7
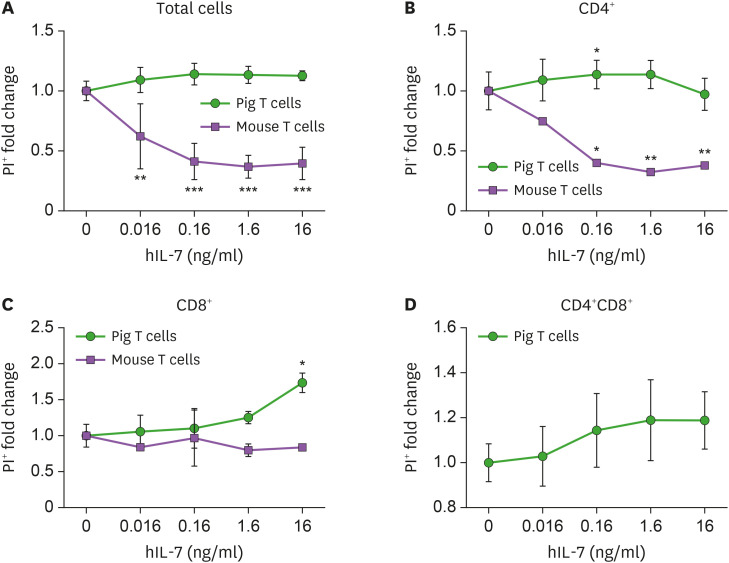
The survival rate of the mouse or human T lymphocytes was shown to be dependent on the dose of human IL-7 (33). To investigate the difference in the survival rate of porcine T lymphocytes with various concentrations of IL-7, porcine PBMCs were cultured with either media alone, or human IL-7 at different concentrations. Mouse splenocytes were used as a positive control in this experiment. Cultured porcine PBMCs were counted and stained with specific Abs, and cell death was observed by flow cytometry.
Unlike mouse T lymphocytes, fold changes of PI+ porcine total PBMCs and CD4+ T cells were not affected by IL-7, regardless of dosage (Fig. 4A and B). The fold change of percentage of PI+ porcine CD8+ and CD4+CD8+ T cells increased proportionally to human IL-7 concentration (Fig. 4C and D). Some samples of CD4+ and CD8+ T cells showed a significant increase in the fold change of percentage at specific concentrations. We also analyzed the changes in cell numbers of pig T lymphocytes. As shown in Supplementary Fig. 4, the numbers of PI+ cells in porcine CD4+ or CD8+ or CD4+CD8+ T cells treated with human IL-7 at various concentrations were not significantly different from that in the unstimulated group. Based on the experiments, we concluded that changes in human IL-7 concentrations do not positively affect porcine T cell survival.
Figure 4. Influence of human IL-7 on the survival of porcine T cells. Porcine PBMCs and mouse splenocytes were cultured in 48-well plates for 5 days. The frequencies and numbers of PI+ cells were measured by flow cytometry. (A) The fold change of percentage of PI+ lymphocytes are plotted at the indicated concentration of human IL-7. The percentage of PI+ porcine CD4+ (B) or CD8+ (C) or CD4+CD8+ (D) T cells is shown. Mouse splenocytes were used as a positive control in this experiment. These experiments were done in quadruplicates, in 3 independent experiments. Error bars represent SD. The statistical significance was measured by t-test.
*p<0.05, **p<0.01, ***p<0.005.
Porcine T cell survival did not depend on the concentration of mouse IL-7
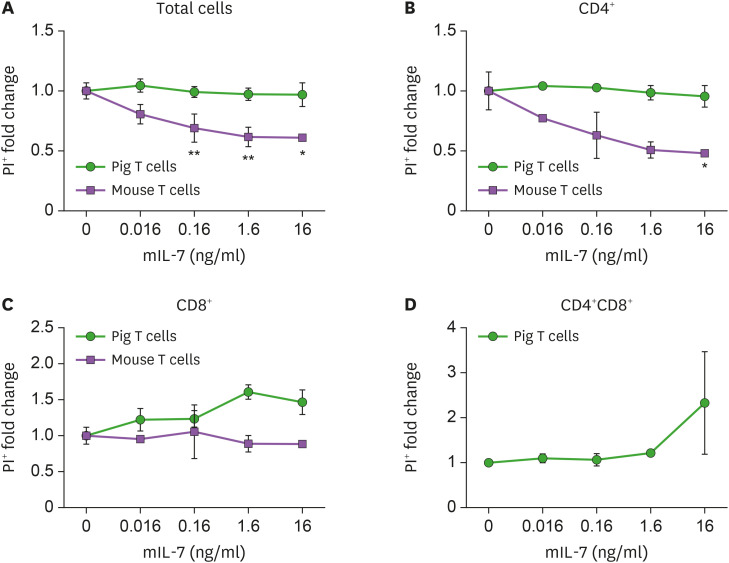
We investigated whether there are any differences in the survival of porcine T lymphocytes in the presence of mouse IL-7. The fold change in the PI+ of the porcine total cell and CD4+ T cells decreased, but was not significantly affected by IL-7, regardless of concentration (Fig. 5A and B).
Figure 5. Influence of mouse IL-7 on the survival of porcine T cells. Porcine PBMCs and mouse splenocytes were cultured and treated with either media alone or different concentrations of mouse IL-7 for 5 days. The frequency and number of PI+ or PI− cells were measured by flow cytometry. (A) The fold change of percentage of propidium iodide positive (PI+) lymphocytes are plotted at the indicated concentration of mouse IL-7. The percentage of PI+ porcine CD4+ (B) or CD8+ (C) or CD4+CD8+ (D) T cells is shown. Mouse splenocytes were used as a positive control in this experiment. These experiments were done in quadruplicates, in 3 independent experiments. Error bars represent SD. The statistical significance was measured by t-test.
*p<0.05, **p<0.01.
The fold change of the percentage of PI+ porcine CD8+ and CD4+CD8+ T cells seemed to increase in proportion to mouse IL-7 concentration (Fig. 5C and D). However, there was no significant difference in the cell death of all pig T lymphocytes according to the concentration of mouse IL-7 (Supplementary Fig. 5). Based on these results, we concluded that IL-7 does not positively affect the survival of porcine T cell survival regardless of concentration.
DISCUSSION
In this study, we investigated whether IL-7 is a survival factor for porcine T lymphocytes, and if human or mouse IL-7 cross-react to mini-pig T lymphocytes. By comparing the protein sequences of human, porcine, and mouse IL-7 in conjunction with the human IL-7 receptor using virtual modeling, we found that porcine IL-7 could not bind to human IL-7 receptor. We also observed that human or mouse IL-7 did not affect the survival or homeostatic proliferation of porcine T cells in vitro. These results suggest that porcine T lymphocytes do not recognize human or mouse IL-7 as a survival factor. Therefore, we conclude that porcine T lymphocytes cannot maintain homeostasis in the human body after artificial blood transfusion, which adds another dimension to the safety considerations for the use of artificial blood.
We first compared the IL-7 protein sequences to examine the influence of human IL-7 on porcine T cells and found that the porcine IL-7 was evolutionarily different from human IL-7; also, it would not fit the human IL-7 receptor-binding site. When porcine T cells were cultured with either human or mouse IL-7 in vitro, they showed neither enhanced survival nor proliferation compared to media control, suggesting that porcine T cells do not recognize human or mouse IL-7 as their survival factor.
Blood transfusion is a necessary procedure for patients undergoing surgery due to severe bleeding or disease. However, it is becoming increasingly difficult to obtain sufficient amount of blood through blood donations alone (1,2,5). The availability of human-like red blood cells has helped to produce human artificial blood from the other animals that increases the amount of blood available for transfusions. Porcine is one of the candidates because they are easy to manipulate genetically, have abundant amounts of blood, and can have many offspring. However, a solution is needed for the immunological problems like GVHD, caused by porcine immune cells after the transfusion of this artificial blood into humans. Since the immune cells that induce GVHD are T lymphocytes (13,18,34), blocking the survival factor of T lymphocyte is suggested to induce T lymphocyte depletion, which could help solve this problem.
IL-7 is known to be a naïve T lymphocyte survival factor in mice and humans and is also documented as a survival factor and homeostatic proliferation cytokine in memory T lymphocytes (20,21,22,28,35,36,37,38). However, the importance of IL-7 in the survival of porcine T lymphocytes is unclear. In the present study, we found that IL-7, as a survival factor, does not cross-react in porcine T lymphocytes as well as in humans. Since the porcine T cells are expected to respond to porcine IL-7, it may be a good approach to generate IL-7R conditional knock-out mini-pigs. These pigs may harbor T lymphocytes to protect against pathogens until they are used to generate artificial blood. During the generation of artificial blood, contaminating porcine T cells in the blood can be eliminated by the inhibition of IL-7R. Therefore, transgenic mini-pigs generated by conditional knocking-out of IL-7R gene would ensure the survival and protection of these animals against life-threatening pathogens until they grow into adults; the porcine T cells from these adult animals are eliminated during the preparation of artificial blood. Further studies regarding the homeostasis of porcine T cells may shed more light on the safety information for this approach.
Based on these observations, we concluded that neither human nor mouse IL-7 contributes to the survival of porcine T lymphocytes. These results also suggest that the survival factors of porcine and human T lymphocytes do not have cross-activity and T lymphocytes that infiltrate into the human body during artificial blood transfusion cannot survive. Finally, these data suggest that pigs could be used as a safe organism for the production of artificial blood.
ACKNOWLEDGEMENTS
We thank Kyong Hoon Kim, Seung-min Yeon and Aryeong Choi for their critical comments.
This study was supported by grant from the KRIBB Research Initiative Program (KGM4252122) and by Basic Science Research Program (grant NRF-2020R1F1A1048531, NRF-2019R1A6A1A03031807, and NRF-2021R1A2C2004279) of the National Research Foundation of Korea.
Abbreviations
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- GVHD
graft-versus-host disease
- KRIBB
Korea Research Institute of Bioscience and Biotechnology
- MEGA7
Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets
- PI
propidium iodide
- RT
room temperature
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Hong JH, Kim SH, Park YH, Kim SU, Jung YW.
- Data curation: Hong JH, Kim SH, Kim HG, Jang JH, Son RG.
- Formal analysis: Hong JH, Kim SH, Kim HG, Pack SP, Park YH, Choi H, Kim SU, Jung YW.
- Funding acquisition: Jung YW.
- Investigation: Kim SU, Jung YW.
- Methodology: Kang P.
- Resources: Jeong KJ, Kim JS, Kim SU.
- Writing - original draft: Hong JH, Kim SH, Kim SU, Jung YW.
- Writing - review & editing: Choi H.
SUPPLEMENTARY MATERIALS
Comparison of IL-7 structures. Estimated molecular structures of IL-7 (blue: human IL-7, gray: mouse IL-7, pink: pig IL-7).
Influence of mouse IL-7 on the survival of mouse T cells. The mouse splenocytes were cultured with mouse IL-7. On days 3, 7, and 14, the numbers of CD4+ or CD8+ T cells were calculated by flow cytometry. Similar data were obtained 2 independent experiments. Error bars represent SD. The statistical significance was measured by t-test.
Increased proliferation of mouse T cells in response to mouse IL-7. The CFSE-labeled mouse lymphocytes were treated with mouse IL-7. On day 7, cells were harvested and analyzed by flow cytometry. Histograms of CFSE-labeled CD4+ or CD8+ T cells are shown. Each experiment involved 2 independent experiments.
Influence of human IL-7 on the survival of porcine T cells. Porcine PBMCs and mouse splenocytes were cultured in 48-well plates for 5 days. The frequencies and numbers of PI+ cells were measured by flow cytometry. (A) The number of PI+ lymphocytes are plotted at the indicated concentration of human IL-7. The number of PI+ porcine CD4+ (B) or CD8+ (C) or CD4+CD8+ (D) T cells is shown. Mouse splenocytes were used as a positive control in this experiment. These experiments were done in quadruplicates, in 3 independent experiments. Error bars represent SD. The statistical significance was measured by t-test.
Influence of mouse IL-7 on the survival of porcine T cells. Porcine PBMCs and mouse splenocytes were cultured and treated with either media alone or different concentrations of mouse IL-7 for 5 days. The frequency and number of PI+ or PI− cells were measured by flow cytometry. (A) The number of propidium iodide positive (PI+) lymphocytes are plotted at the indicated concentration of mouse IL-7. The number of PI+ porcine CD4+ (B) or CD8+ (C) or CD4+CD8+ (D) T cells is shown. Mouse splenocytes were used as a positive control in this experiment. These experiments were done in quadruplicates, in 3 independent experiments. Error bars represent SD. The statistical significance was measured by t-test.
References
- 1.Park CG, Kim JS, Shin JS, Kim YH, Kim SJ. Current status and future perspectives of xenotransplantation. J Korean Soc Transplant. 2009;23:203–213. [Google Scholar]
- 2.Kim JH. Recent review on blood transfusion therapy. J Korean Med Assoc. 2013;56:496–503. [Google Scholar]
- 3.Kresie L. Artificial blood: an update on current red cell and platelet substitutes. Proc Bayl Univ Med Cent. 2001;14:158–161. doi: 10.1080/08998280.2001.11927754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper DK. A brief history of cross-species organ transplantation. Proc Bayl Univ Med Cent. 2012;25:49–57. doi: 10.1080/08998280.2012.11928783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moradi S, Jahanian-Najafabadi A, Roudkenar MH. Artificial blood substitutes: first steps on the long route to clinical utility. Clin Med Insights Blood Disord. 2016;9:33–41. doi: 10.4137/CMBD.S38461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wakelin D, Donachie AM. Genetic control of immunity to Trichinella spiralis. Donor bone marrow cells determine responses to infection in mouse radiation chimaeras. Immunology. 1981;43:787–792. [PMC free article] [PubMed] [Google Scholar]
- 7.Wiener LS, Steffen-Smith E, Fry T, Wayne AS. Hematopoietic stem cell donation in children: a review of the sibling donor experience. J Psychosoc Oncol. 2007;25:45–66. doi: 10.1300/J077v25n01_03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao E, Xu H, Wang L, Kryczek I, Wu K, Hu Y, Wang G, Zou W. Bone marrow and the control of immunity. Cell Mol Immunol. 2012;9:11–19. doi: 10.1038/cmi.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hara H, Long C, Pawlikowski Z, Koike N, Ezzelarab M, Yeh P, Ayares D, Yazer M, Cooper DK. Abstract #657. Genetically-engineered pigs as a potential source of red blood cells for clinical blood transfusion. Am J Transplant. 2009;9:381. doi: 10.1111/j.1537-2995.2009.02306.x. [DOI] [PubMed] [Google Scholar]
- 10.Taylor MJ, Yomtovian R. Optimizing red blood cell transfusion therapy in the 21st century: the power of data analysis for past understanding and future guidance. Transfusion. 2013;53:470–475. doi: 10.1111/trf.12093. [DOI] [PubMed] [Google Scholar]
- 11.Yum SY, Yoon KY, Lee CI, Lee BC, Jang G. Transgenesis for pig models. J Vet Sci. 2016;17:261–268. doi: 10.4142/jvs.2016.17.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dasararaju R, Marques MB. Adverse effects of transfusion. Cancer Contr. 2015;22:16–25. doi: 10.1177/107327481502200104. [DOI] [PubMed] [Google Scholar]
- 13.Markey KA, MacDonald KP, Hill GR. The biology of graft-versus-host disease: experimental systems instructing clinical practice. Blood. 2014;124:354–362. doi: 10.1182/blood-2014-02-514745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burrows SR, Khanna R, Burrows JM, Moss DJ. An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross-reactive with a single Epstein-Barr virus CTL epitope: implications for graft-versus-host disease. J Exp Med. 1994;179:1155–1161. doi: 10.1084/jem.179.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okoye IS, Wilson MS. CD4+ T helper 2 cells--microbial triggers, differentiation requirements and effector functions. Immunology. 2011;134:368–377. doi: 10.1111/j.1365-2567.2011.03497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosmann TR. Cytokines, differentiation and functions of subsets of CD4 and CD8 T cells. Behring Inst Mitt. 1995:1–6. [PubMed] [Google Scholar]
- 17.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, Shlomchik MJ, Emerson SG. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 19.Hauschild J, Petersen B, Santiago Y, Queisser AL, Carnwath JW, Lucas-Hahn A, Zhang L, Meng X, Gregory PD, Schwinzer R, et al. Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proc Natl Acad Sci U S A. 2011;108:12013–12017. doi: 10.1073/pnas.1106422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 23.Hong C, Luckey MA, Park JH. Intrathymic IL-7: the where, when, and why of IL-7 signaling during T cell development. Semin Immunol. 2012;24:151–158. doi: 10.1016/j.smim.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hare KJ, Jenkinson EJ, Anderson G. An essential role for the IL-7 receptor during intrathymic expansion of the positively selected neonatal T cell repertoire. J Immunol. 2000;165:2410–2414. doi: 10.4049/jimmunol.165.5.2410. [DOI] [PubMed] [Google Scholar]
- 25.Offner F, Plum J. The role of interleukin-7 in early T-cell development. Leuk Lymphoma. 1998;30:87–99. doi: 10.3109/10428199809050932. [DOI] [PubMed] [Google Scholar]
- 26.Krenger W, Blazar BR, Holländer GA. Thymic T-cell development in allogeneic stem cell transplantation. Blood. 2011;117:6768–6776. doi: 10.1182/blood-2011-02-334623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma D, Wei Y, Liu F. Regulatory mechanisms of thymus and T cell development. Dev Comp Immunol. 2013;39:91–102. doi: 10.1016/j.dci.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Kieper WC, Tan JT, Bondi-Boyd B, Gapin L, Sprent J, Ceredig R, Surh CD. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J Exp Med. 2002;195:1533–1539. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 30.Kaur G, Iyer LM, Subramanian S, Aravind L. Evolutionary convergence and divergence in archaeal chromosomal proteins and Chromo-like domains from bacteria and eukaryotes. Sci Rep. 2018;8:6196. doi: 10.1038/s41598-018-24467-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendoza A, Fang V, Chen C, Serasinghe M, Verma A, Muller J, Chaluvadi VS, Dustin ML, Hla T, Elemento O, et al. Lymphatic endothelial S1P promotes mitochondrial function and survival in naive T cells. Nature. 2017;546:158–161. doi: 10.1038/nature22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanabe T, Watanabe H, Shah JA, Sahara H, Shimizu A, Nomura S, Asfour A, Danton M, Boyd L, Dardenne Meyers A, et al. Role of intrinsic (graft) versus extrinsic (host) factors in the growth of transplanted organs following allogeneic and xenogeneic transplantation. Am J Transplant. 2017;17:1778–1790. doi: 10.1111/ajt.14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892–3904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- 35.Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barata JT, Silva A, Abecasis M, Carlesso N, Cumano A, Cardoso AA. Molecular and functional evidence for activity of murine IL-7 on human lymphocytes. Exp Hematol. 2006;34:1133–1142. doi: 10.1016/j.exphem.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Francois B, Jeannet R, Daix T, Walton AH, Shotwell MS, Unsinger J, Monneret G, Rimmele T, Blood T, Morre M, et al. Interleukin-7 restores lymphocytes in septic shock: the iris-7 randomized clinical trial. JCI Insight. 2018;3:e98960. doi: 10.1172/jci.insight.98960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Lent AU, Dontje W, Nagasawa M, Siamari R, Bakker AQ, Pouw SM, Maijoor KA, Weijer K, Cornelissen JJ, Blom B, et al. IL-7 enhances thymic human T cell development in “human immune system” Rag2-/-IL-2Rgammac-/- mice without affecting peripheral T cell homeostasis. J Immunol. 2009;183:7645–7655. doi: 10.4049/jimmunol.0902019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of IL-7 structures. Estimated molecular structures of IL-7 (blue: human IL-7, gray: mouse IL-7, pink: pig IL-7).
Influence of mouse IL-7 on the survival of mouse T cells. The mouse splenocytes were cultured with mouse IL-7. On days 3, 7, and 14, the numbers of CD4+ or CD8+ T cells were calculated by flow cytometry. Similar data were obtained 2 independent experiments. Error bars represent SD. The statistical significance was measured by t-test.
Increased proliferation of mouse T cells in response to mouse IL-7. The CFSE-labeled mouse lymphocytes were treated with mouse IL-7. On day 7, cells were harvested and analyzed by flow cytometry. Histograms of CFSE-labeled CD4+ or CD8+ T cells are shown. Each experiment involved 2 independent experiments.
Influence of human IL-7 on the survival of porcine T cells. Porcine PBMCs and mouse splenocytes were cultured in 48-well plates for 5 days. The frequencies and numbers of PI+ cells were measured by flow cytometry. (A) The number of PI+ lymphocytes are plotted at the indicated concentration of human IL-7. The number of PI+ porcine CD4+ (B) or CD8+ (C) or CD4+CD8+ (D) T cells is shown. Mouse splenocytes were used as a positive control in this experiment. These experiments were done in quadruplicates, in 3 independent experiments. Error bars represent SD. The statistical significance was measured by t-test.
Influence of mouse IL-7 on the survival of porcine T cells. Porcine PBMCs and mouse splenocytes were cultured and treated with either media alone or different concentrations of mouse IL-7 for 5 days. The frequency and number of PI+ or PI− cells were measured by flow cytometry. (A) The number of propidium iodide positive (PI+) lymphocytes are plotted at the indicated concentration of mouse IL-7. The number of PI+ porcine CD4+ (B) or CD8+ (C) or CD4+CD8+ (D) T cells is shown. Mouse splenocytes were used as a positive control in this experiment. These experiments were done in quadruplicates, in 3 independent experiments. Error bars represent SD. The statistical significance was measured by t-test.