Abstract
Ischemia is a common pathological condition present in many neurodegenerative diseases, including ischemic stroke, retinal vascular occlusion, diabetic retinopathy, and glaucoma, threatening the sight and lives of millions of people globally. Ischemia can trigger excessive oxidative stress, inflammation, and vascular dysfunction, leading to the disruption of tissue homeostasis and, ultimately, cell death. Current therapies are very limited and have a narrow time window for effective treatment. Thus, there is an urgent need to develop more effective therapeutic options for ischemia-induced neural injuries. With emerging reports on the pharmacological properties of natural flavonoids, these compounds present potent antioxidative, anti-inflammatory, and antiapoptotic agents for the treatment of ischemic insults. Three major active flavonoids, baicalein, baicalin, and wogonin, have been extracted from Scutellaria baicalensis Georgi (S. baicalensis); all of which are reported to have low cytotoxicity. They have been demonstrated to exert promising pharmacological capabilities in preventing cell and tissue damage. This review focuses on the therapeutic potentials of these flavonoids against ischemia-induced neurotoxicity and damage in the brain and retina. The bioactivity and bioavailability of baicalein, baicalin, and wogonin are also discussed. It is with hope that the therapeutic potential of these flavonoids can be utilized and developed as natural treatments for ischemia-induced injuries of the central nervous system (CNS).
1. Introduction
Ischemia is a common pathological or traumatic condition accompanied by the reduction of blood supply to the major organs, such as the heart, kidney, intestine, brain, and eye [1]. This leads to an insufficient supply of oxygen and nutrients and an accumulation of metabolic wastes, causing organ damage or failure and resulting in death in severe cases [1]. Neurons in the brain are the most sensitive and vulnerable cells to ischemia. Only a short period of ischemia can elicit irreversible damage to brain tissue, leading to paralysis or death [2, 3]. Stroke was defined by the World Health Organization (WHO) in the 1970s as “rapidly developing clinical signs of focal disturbance of cerebral function, lasting more than 24 hours or leading to death with no apparent cause other than that of vascular origin” [4]. Around 87% of stroke cases are ischemic stroke, which is triggered by a lack of blood supply to focal brain areas, leading to subsequent damage and neurodegeneration [5–7]. Stroke is a leading cause of disability and death worldwide [7, 8]. Thrombolytic medication, such as alteplase (t-PA), is the only FDA-approved therapeutic agent for treating acute ischemic stroke within a few hours after its onset [9]. Given the narrow time window of treatment and high risk of complications, such as hemorrhagic transformation, cerebral edema, and other adverse effects [10], the development of novel neuroprotective therapies against ischemia is paramount.
The visual system is comprised of the sensory organ (eyes) and connecting axon fibers to the visual targets of the brain [11]. Light, as a stimulus, is captured by photoreceptors in the retina, initiating a cascade of chemical and electrical events. The signal is then transferred to the visual center of the brain via the ganglion cell axons of the optic nerve [12–14]. The visual centers process and transform these signals into visual images. Retinal ischemia is frequently involved in various forms of retinal neuropathies, such as age-related macular degeneration (AMD), diabetic retinopathy (DR), glaucoma, and central/branch retinal artery/vein occlusion [15–19]. Following ischemic injuries, a series of events are triggered, including oxidative stress, neovascular and apoptotic changes, and, ultimately, the death of retinal neurons and vision loss [5, 20]. The retina is an extension of the brain in terms of anatomical and embryonic development [21, 22]. The retina also displays similarities to the brain regarding its neuronal and immune responses to injury [22]. The latter is possibly contributed by the structural similarity between the blood-retinal barrier (BRB) and blood-brain barrier (BBB), to which the retina sustains an immune privilege site and shares a similar pattern of immune surveillance and immunoregulatory processes [23, 24]. In response to perturbations in the retina and the brain, innate immunity can be rapidly activated through transcriptional and phenotypic alterations of immune glial cells and the release of inflammatory cytokines [25, 26]. However, excessive activation of innate immune reactivity under injury or traumatic stress can promote further activation of adaptive immunity by antigen-presenting cells that attract and guide peripheral immune cells, such as T cells to the injury sites [27–29]. Neuroinflammation has been well documented as a pathological factor in neuronal death in the brain and in retinal disorders. Because of these similarities, the retina has been commonly considered as an easily accessible indicator of brain disorders, such as Alzheimer's disease (AD), Parkinson's disease (PD), and stroke.
Accumulating evidence suggests that natural herbs exhibit therapeutic potential for the treatment of ischemic stroke [30]. Active ingredients extracted from herbs, including salvianolic acid B and tanshinone from Salvia miltiorrhiza, scutellarin from Scutellaria baicalensis Georgi (S. baicalensis), and honokiol and magnolol from the bark of Magnolia officinalis, have been found to have therapeutic potential, because of their antioxidative and anti-inflammatory properties, as well as their ability to maintain BBB permeability [30–36]. The neuroprotective capabilities of other natural extracts, including resveratrol, curcumin, vitamins C and E, and Gingko biloba, have also been reported in various CNS disorders [37]. Flavonoids, which are easily accessible by daily consumption of fruits and vegetables, have been found to have high therapeutic efficacy and fewer side effects in both in vitro and in vivo studies [38, 39]. Because of their various pharmacological effects, many flavonoids have demonstrated promising protective effects in the prevention or treatment of various diseases [40–46]. This review mainly focuses on three flavonoids: baicalein (5,6,7-trihydroxyflavone; C15H10O5), baicalin (5,6-dihydroxy-7-O-glucuronide), and wogonin (5,7-dihydroxy-8-methoxy-flavone) (Figure 1), all of which are isolated from the roots of S. baicalensis, a widely used herbal medicine in Asian countries [47–51]. Previous studies have demonstrated that baicalein, baicalin, and wogonin have a broad spectrum of biological functions, including antioxidation, anti-inflammation, antiapoptosis, and antiexcitotoxicity [51, 52]. Because of these bioactivities, many published reports have suggested the possibility of developing flavonoids for the treatment of various diseases, including hepatitis, breast cancer, virus infection, and neurodegenerative diseases [43–46]. These pharmacological activities also provide a solid basis for their neuroprotective properties in different models of neuropathies and cognitive impairments. Because of their easy accessibility and low toxicity, baicalein, baicalin, and wogonin may be effective alternatives for the treatment of stroke and other neurodegenerative diseases affecting CNS.
Figure 1.
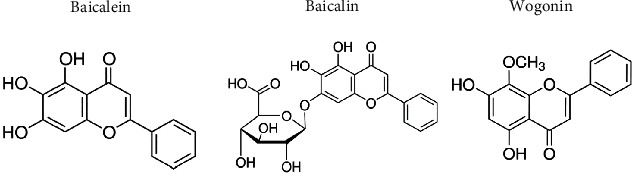
Molecular structures of baicalein, baicalin, and wogonin.
2. Bioactivity and Safety of Baicalein, Baicalin, and Wogonin
As the major active flavonoids extracted from S. baicalensis, baicalein, baicalin, and wogonin share some common pharmacological properties against inflammation, oxidation, and apoptosis. A comparison of baicalein, baicalin, and wogonin is shown in Table 1, based on the parameters collected from the database of the traditional Chinese medicine lab of systems pharmacology (TCMSP), a database integrating systems biology and pharmacology for drug discovery, development, and understanding of therapeutic mechanisms [53]. The database can be found in the following link (https://old.tcmsp-e.com/tcmsp.php).
Table 1.
Summary of physical parameters of baicalein, baicalin, and wogonin. MW: molecular weight (Dalton (Da)); TPSA: topological polar surface area (angstroms squared (A2)) indicates the membrane permeability; Caco-2: Caco-2 cell monolayer permeability (10−6 cm·s−1); and BBB: blood-brain barrier permeability (10−6 cm·s−1) were derived based on TPSA; half-time (HL) (hours (h)), oral bioavailability (OB) (%), the logarithm of 1-octanol/water partition coefficient (AlogP), and drug-likeness (DL) represent the pharmacological properties for each molecule.
| Molecule | MW (Da) | TPSA (A2) | Caco-2 (10−6 cm·s−1) | BBB (10−6 cm·s−1) | HL (h) | OB (%) | AlogP | DL |
|---|---|---|---|---|---|---|---|---|
| Baicalein | 270.25 | 90.9 | 0.63 | −0.05 | 16.25 | 33.52 | 2.33 | 0.21 |
| Baicalin | 460.42 | 187.12 | −1.1 | −1.97 | — | 29.53 | 0.84 | 0.77 |
| Wogonin | 284.28 | 79.9 | 0.79 | 0.04 | 17.75 | 30.68 | 2.56 | 0.23 |
Baicalein and wogonin have a lower molecular weight (MW) and a lower value of the topological polar surface area (TPSA) than baicalin, indicating a higher cell membrane permeability of baicalein and wogonin [54]. Furthermore, the permeability of Caco-2 monolayers (intestinal epithelial cells) and the BBB, calculated based on the values of TPSA [55, 56], was found to be higher for baicalein and wogonin, compared to baicalin. In addition, baicalein and wogonin display slower elimination half-time (HL) and higher oral bioavailability (OB) compared to baicalin, suggesting a longer duration of these two flavonoids in systemic circulation. Based on multiparametric guidelines, also known as rules and ligand efficiency (LE) metrics, which determine the extent of druglikeness (DL) [57], all three flavonoids meet the criteria of “drug-like” compounds. This demonstrates the potential of baicalein, baicalin, and wogonin to be easily accessible agents for future clinical use. However, the relatively high hydrophobicity of baicalein and wogonin is reflected by their lipophilicity (AlogP, a logarithm of 1-octanol/water partition coefficient) values [58], compared to that of baicalin. Due to this low water solubility, solvents or carriers may be necessary to enhance the solubility of these flavonoids for therapeutic purposes [50, 59–61].
S. baicalensis is a major ingredient in many prescriptions of traditional Chinese medicine (TCM). Numerous studies have been conducted to evaluate its pharmacokinetic profile and bioavailability for its safety and efficacy in clinical applications. It has been reported that baicalein and its metabolite baicalein 6-O-sulfate exist in blood plasma for up to 36 hours after a single oral administration of Xiaochaihu Tang (Sho-Saiko-To) [62], a popular TCM treatment containing extract of S. baicalensis. The bioavailability of wogonin, baicalein, and baicalin has also been evaluated in healthy human urine. After a single administration of S. baicalensis decoction (equal to 9 g of crude drug), wogonin, baicalein, and baicalin were still detectable in the urine 36 hours postdosing [63]. A similar time profile has also been demonstrated in monkey plasma after three doses of baicalein [64]. The presence of baicalin has been detected in human plasma after administration [65, 66]. Additionally, the safety profile of single or multiple administrations of chewable baicalein tablets has been assessed in healthy subjects. In addition to detecting sustained levels of baicalein and its metabolite baicalin in vivo, these studies revealed that single or multiple doses of baicalein (100–800 mg) were safe and well tolerated with no sign of toxicity in the kidney or liver [67, 68]. These findings implicate the feasibility of developing baicalein, baicalin, and wogonin as safe and long-lasting agents for clinical application.
3. Neuroprotective Effects of Baicalein, Baicalin, and Wogonin on the Brain and Retina Ischemia
Neurodegeneration often occurs through the progressive loss of the structure or functions of neurons [69]. Neurodegenerative diseases, such as Parkinson's disease (PD), Alzheimer's disease (AD), and stroke, affect millions of people worldwide, especially in aging populations. The pathogenesis of neurodegeneration is complicated and has been associated with genetics, protein misfolding, intracellular mechanisms, and programmed cell death [70–74]. In ischemic stroke patients, typical symptoms are characterized by the sudden loss of mobility, speaking, or vision unilaterally [75]. Ischemia in the brain can be caused by cardioembolic vessel occlusion, artery to artery embolism, or in-situ small-vessel disease [75]. In the retina, ischemia can result from occlusion of the central or branch retinal vessels or DR [76]. Retinal ischemia may lead to visual impairment and blindness [19].
Ischemia induces a cascade of neuropathological activities, including oxygen and energy depletion, disruption of ion homoeostasis, glutamate and free radical release, Ca2+ channel dysfunction, BBB or BRB disruption, and changes to the inflammatory microenvironment, ultimately leading to cell death and irreversible functional loss [5, 77, 78]. The cellular changes after ischemic stroke are illustrated in Figure 2. There are three major types of stress. First, oxidative stress is induced by rapidly increased reactive oxygen species (ROS) postischemia [79]. This stress can subsequently lead to the peroxidation of membrane lipid, mitochondrial dysfunction, and DNA damage, eventually causing apoptosis and irreversible neuron loss [80, 81]. Second, neuroinflammatory stress, initiated by the activation of innate and adaptive immunity, is a well-known pathological factor in CNS disorders [82, 83]. The resident immune cells (microglia and astrocytes) can be rapidly activated upon sensing damage-associated molecular patterns (DAMPs) released by apoptotic cells [83, 84]. Subsequently, adaptive immune cells can be recruited even with minimized disturbances or disruptions of BBB or BRB postischemia insults, ultimately causing neuron death [29, 69, 85]. Lastly, stress of energy deprivation triggers cytotoxicity at the lesion site. Apart from the excessive free radicals, the disruption of ion homeostasis postischemia/reperfusion injury can lead to overload of Na+ and Ca2+, which aggravates mitochondrial dysfunction and initiates apoptosis and inflammatory cascades [86]. Furthermore, the release and accumulation of glutamate can cause excitotoxicity of neurons and ultimately leads to cell death [87, 88]. Based on the understanding of neurochemical events under ischemic stress, many neuroprotective therapies are focused on targeting the upstream pathways to reduce damage to the neurons induced by downstream cascades. The protective effects of natural herbs have been demonstrated by many research groups [89]. In the following discussion, the neuroprotective effects of baicalein, baicalin, and wogonin, the major bioactive molecules in S. baicalensis, are described based on the sites (i.e., the brain and retina) of postischemic injury.
Figure 2.
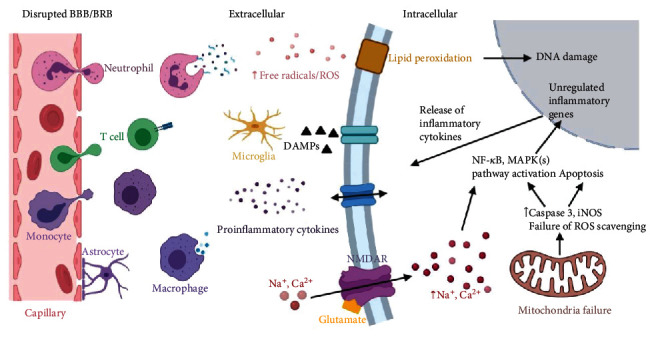
Schematic representation of the main pathological events subsequent to brain ischemia. Created with http://BioRender.com.
3.1. Ischemia in the Brain
Following ischemic injury in the brain, a cascade of changes is elicited, including oxygen and energy deprivation, increased expression of free radicals and inflammatory cytokines, ion overload, and activation of immune responses [90, 91]. The upstream triggers of these changes and maintenance of tissue homeostasis are recognized as potential therapeutic targets for ischemic stroke treatment. Multiple pharmacological properties of flavonoids as reported in the literature include antioxidant, anti-inflammation, antiapoptosis, and antiexcitotoxicity characteristics that are neuroprotective [92, 93]. The neuroprotective effects of baicalein, baicalin, and wogonin on both in vitro and in vivo ischemic models are discussed (also see Tables 2 and 3 for details).
Table 2.
In vitro findings of baicalein, baicalin, and wogonin on different brain cell types.
| Cell types | Stimulating molecule(s) | Baicalein | Baicalin | Wogonin | Reference(s) | |||
|---|---|---|---|---|---|---|---|---|
| Conc. | Effects | Conc. | Effects | Conc. | Effects | |||
| Mouse hippocampal HT22 cell line | 20 μM IAA | 2 μM | Antioxidant 12/15-lipoxygenase inhibitor | [93] | ||||
| Primary cortical neurons | Glutamate, OGD, H2O2, or xanthine/xanthine oxidase | 10 μM; 3.5 μM | Promote cell survival through inhibiting 12/15-LOX and removing intracellular ROS and nitrotyrosine reactivities by regulating the PI3/AKT pathway | 9.0 μg/ml; 23.7 μg/ml | Improve neuron survival by radical scavenging activity and inhibiting the initiation of LOX-induced apoptosis | [92, 94, 98] | ||
| PC-12 | OGD | 1 μg/ml; 10 μg/ml | Increase survival rate and suppress pro-inflammatory cytokine expression | 0.01 mg/ml, 0.1 mg/ml, and 1 mg/ml | Increase survival rate and suppress proinflammatory cytokine expression | 0.01 mg/ml, 0.1 mg/ml, and 1 mg/ml | Increase survival rate and suppress proinflammatory cytokine expression | [99] |
| Primary hippocampal neurons | OGD | 1 μM | Protect neurons from apoptosis by suppressing phosphorylation of CaMKII | 50 μM | Improve neuron survival on hippocampal slice culture | [97, 100] | ||
Table 3.
Baicalein, baicalin, and wogonin performance on different brain ischemia models.
| Models | Species | Baicalein | Baicalin | Wogonin | Reference(s) | |||
|---|---|---|---|---|---|---|---|---|
| Conc. | Effects | Conc. | Effects | Conc. | Effects | |||
| SCEM | Rabbit | 100 mg/kg | Improve behavioral performance | [93] | ||||
| MCAO | Rat/mouse | 20 mg/kg; 200 mg/kg | Decrease the infarct volume and neurological score; inhibiting 12/15-LOX-induced oxidative toxicity; regulate M1/M2 transformation of microglia/macrophages and block NF-κB nuclear translocation; suppress PARP-1/AIF pathway-induced neuroinflammation; antioxidant 12/15-LOX inhibitor | 5, 100, and 200 mg/kg | Reduced the neurological deficit scores, cerebral infarct volume by inhibiting TLR2/4-mediated NF-κB pathway, and expression of iNOS, COX2, and caspase3 | 20 mg/kg; 50 μM | Reduce infarct area and improve behavior performance through upregulating TGF-β1 | [92, 94, 106–109, 112–116, 118, 119] |
| GCI/R | Gerbil/rats | 100 mg/kg; 200 mg/kg | Improve learning and memory ability post-I/R injury via anti-inflammatory and antiapoptosis; perform neuroprotection by upregulating HSP70 expression and influencing MAPK cascades in the gerbil hippocampus | 0.5, 1, and 10 mg/kg | Increase neuron survival in the hippocampus area by suppressing inflammation (iNOS, TNF-α) | [100, 101, 103, 117, 118] | ||
3.1.1. In Vitro Studies
Free radicals and ROS are known as inducers of cell death postischemic injury. Baicalein has been shown to exert neuroprotective effects and attenuate apoptosis by acting as a scavenger of ROS and nitric oxide (NO) [94]. These baicalein-induced protective effects have been found to be mediated by upregulating the phosphatase and tensin homolog gene (PTEN) and the phosphoinositide 3-kinase (PI3K/AKT) pathway in primary cultures of cortical neurons after oxygen and glucose deprivation (OGD) treatment [94]. Additionally, it has been reported that baicalein improves the survival of HT22 cells after iodoacetic acid- (IAA-) induced oxidative stress, by inhibiting 12/15-lipoxygenase (12/15-LOX) [93], an enzyme which oxidizes polyunsaturated fatty acids to generate bioactive lipid metabolites [95] and is toxic to neurons in neurological disorders [96].
Neuroprotective abilities of wogonin and baicalin have also been reported. In OGD-induced toxicity to rat hippocampal slice culture, pretreatment and posttreatment of hippocampal slices with 50 μM baicalin could significantly prevent cell death, especially in the pyramidal cell layer [97]. Wogonin has been demonstrated to act as an antioxidant and 12/15-LOX inhibitor to improve the survival of primary cortical neurons after H2O2 or xanthine/xanthine oxidase challenge [98].
It is worth noting that not only oxidative stress but also the dysregulated immune microenvironment can lead to the degeneration of brain cells. In OGD-induced ischemia of PC12 cells, which were derived from a pheochromocytoma of the rat adrenal medulla, baicalein, baicalin, and wogonin all exhibited significant neuroprotective effects by inhibiting oxidation and suppressing inflammation [99]. These protective effects were shown to be mediated by the reduced expression of Toll-like receptor 2 (TLR2), tumor necrosis factor alpha (TNF-α), and caspase-3 [99]. Baicalin acts through suppressing OGD-induced phosphorylation of calmodulin- (CaM-) dependent protein kinase II (CaMKII) in primary hippocampal neurons and SH-SY5Y cells [100]. The antiapoptotic effects of baicalin have also been shown to suppress caspase-3 and Bax expression and to promote antiapoptotic factor Bcl-2 expression in hippocampal neurons [100]. The effect of baicalin on rescuing neurons from OGD was comparable with that of CaMKII siRNA knockdown in SH-SY5Y cells, suggesting that baicalin may function as a potent CaMKII inhibitor in neuroprotection [100].
3.1.2. In Vivo Studies
Neuroprotective effects of baicalein, baicalin, and wogonin against ischemic injuries have been reported in various animal models. In the global cerebral ischemia/reperfusion (GCI/R) rat model, Cheng et al. reported that oral administration of 100 mg/kg baicalin for 7 d consecutively can rescue the spatial learning and memory abilities of gerbil significantly, as assessed by the water maze test [101]. Subsequently, Wang et al. showed that baicalin, given at the same dose by intraperitoneal injection for one week immediately after GCI/R injury, can improve the learning and memory abilities in gerbil [100]. These neuroprotective effects exerted by baicalin have been found to be related to the inhibition of CaMKII-mediated downstream biochemical events [100]. CaMKII is an important protein involved in Ca2+/glutamate-mediated excitotoxicity under the stress of ischemia [87, 102]. These findings imply that neuroprotective effects arising from baicalin are possibly related to its antiexcitotoxicity capacity. In addition to the CaMKII pathway, Dai et al. found that neuroprotective effects of baicalin can be achieved by its mediation of heat shock protein 70 (HSP70) and mitogen-activated protein kinase (MAPKs) cascades [103]. HSP70 is a critical cytoprotective factor, which is responsible for proper protein folding [104]. And MAPKs are important pathways regulating cell survival and death, including the subgroups of phosphorylated extracellular signal-regulated kinase (pho-ERK), phosphorylated c-Jun N-terminal kinase (pho-JNK), and phosphorylated p38 (pho-p38) [105]. Upregulation of HSP70 and mediation of MAPK subgroups by intraperitoneal administration of baicalin could effectively rescue neurons in the hippocampus after GCI/R injuries [103]. In addition, other studies have shown that baicalin can inhibit the activation of TLR signaling and the relevant downstream inflammatory pathway (i.e., nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway) [106, 107]. Following middle cerebral artery occlusion (MCAO), intravenous or intraperitoneal administration of baicalin has been shown to effectively attenuate cerebral infarction by regulating inflammation, including the expression of proinflammatory cytokines TNF-α and interleukin-1β (IL-1β), via TLR2/4 and NF-κB pathway signaling cascades [106, 107]. Baicalin's antioxidative and anti-inflammatory properties are also indicated by decreased levels of both mRNA and iNOS and COX2 protein levels [106, 108].
Similar neuroprotective and antineuroinflammatory effects have been reported with baicalein. For instance, baicalein was found to ameliorate the neurobehavioral deficits and infarct volume caused by small clot embolic stokes (SCEM) or MCAO [93, 94]. Modulating microglia/macrophage M1/M2 polarization and suppressing the NF-κB signaling are suggested to be responsible for the antineuroinflammation and neuroprotection [109]. As a potent antioxidant inhibitor of 12/15-LOX, baicalein was shown to effectively reduce infarct size, edema formation, and 12/15-LOX-induced neuron death in various brain ischemia animal models [92, 110–112]. The inhibitory effect of baicalein is comparable to the protective effects observed in 12/15-LOX knockout mice ALOX15−/− [92]. Pallast et al. found that the protective effects of baicalein are mediated by the suppression of apoptosis-inducing factor (AIF) nuclear translocation in an MCAO model [113]. Later, in addition to confirming the antiapoptotic effect through AIF regulation, Li et al. reported the involvement of the poly (ADP-ribose) polymerase-1 (PARP-1)/AIF pathway in baicalein-induced neuroprotection [114]. Furthermore, inflammation-related factors, such as cytosolic phospholipase A2 (cPLA2) and p38 MAPK, have been reported to be downregulated after baicalein administration in the MCAO rat model [112, 115]. The alteration of peroxisome proliferator-activated receptor γ (PPARγ) and nuclear translocation induced by brain ischemia/reperfusion injury have also been reported to be returned to the balanced stage by baicalein pretreatment [116].
Wogonin is also found to exert neuroprotective effects on both GCI/R and MCAO rat models. In the GCI/R rat model, wogonin was shown to effectively attenuate neuron loss and histological changes in the hippocampal CA1 region [117]. The expression of inflammatory mediators (e.g., iNOS and TNF-α) at the injury site was significantly suppressed by intraperitoneal administration of wogonin in rats after ischemia injury [117]. In the MCAO rat model, wogonin pre- and posttreatment was shown to both alleviate the infarct volume and behavioral deficits and promote angiogenesis in the peri-ischemia area [118, 119]. Upregulation of transforming growth factor beta (TGF-β1) expression in the ischemic brain tissue was observed in wogonin-treated rats 2 weeks after ischemic injury [119]. TGF-β1 has previously been reported as an important regulator of angiogenesis in hypoxic tissue [120]. This finding indicates that wogonin protects neurons by promoting microvascular formation and subsequently restoring blood supply via the TGF-β1 pathway. The vasodilatory effect of wogonin mediated by inhibition of both intracellular Ca2+ release and extracellular Ca2+ influx could be a potential treatment paradigm for ischemia [121]. This evidence strongly supports the feasibility of developing baicalein, baicalein, and wogonin as candidates for neuroprotection following ischemic stroke.
3.2. In Retina Ischemia
Injuries caused by retinal ischemia are common in many ocular disorders, such as central/branch retinal artery/vein occlusion, DR, glaucoma, and AMD [15–19]. Similar to ischemic brain injuries, retinal ischemia triggers oxidative stress, inflammation, neovascularization, and, ultimately, the death of retinal neurons [5, 20]. Figure 3 illustrates the morphological changes to the retina and neuron death after ischemia-induced stress. In the following, the antioxidative, anti-inflammatory, antiapoptotic effects of baicalein, baicalin, and wogonin after retinal ischemia for in vitro and in vivo studies are summarized (Tables 4 and 5).
Figure 3.
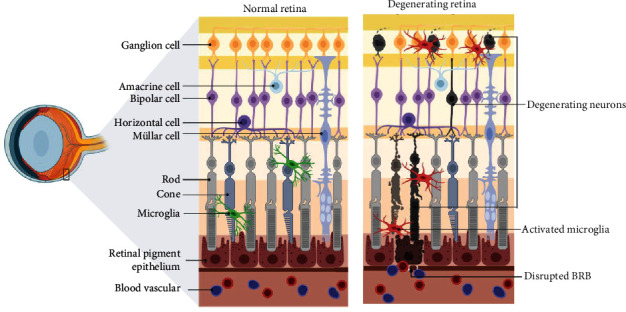
Schematic diagram showing the normal retina and degenerating retina resulting from ischemia. Created with http://BioRender.com.
Table 4.
In vitro findings of baicalein, baicalin, and wogonin on different brain cell types.
| Cell types | Baicalein | Baicalin | Wogonin | Reference(s) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stimulator | Conc. | Effects | Stimulator | Conc. | Effects | Stimulator | Conc. | Effects | ||
| Primary rat retinal cells | Ascorbate/FeSO4 | 100 μM | Attenuate oxidative stress-induced ROS | [123] | ||||||
| ARPE-19 cells | H2O2; IL-1b | 50 μM; 40 μM | Antioxidation by scavenging ROS; inhibit the expression of MMP-9 and VEGF; suppress IL-6 and IL-8 proinflammatory cytokines | High glucose | 50 μM | Protect ARPE-19 cells from apoptosis through upregulating the release of microRNA-145; anti-inflammation by initiating the regulation of NF-κB and p38MAPK signaling pathways | LPS; IL-1 β | 10 μM or 50 μM; 1–10 μM | Suppress LPS-induced inflammatory responses via the TLR4/NF-κB pathway and protect the tight junctions; inhibit IL-6 and IL-8 via NF-κB pathway suppression; upregulated claudin-1 and ZO-1 | [122, 124, 125, 127] |
| HRMEC | High glucose | High glucose | 50 μM | Protect ARPE-19 cells from apoptosis through upregulating the release of microRNA-145; anti-inflammation by initiating the regulation of NF-κB and p38MAPK signaling pathways | [127] | |||||
Table 5.
In vivo findings of baicalein, baicalin, and wogonin on different animal models.
| Models | Species | Baicalein | Baicalin | Wogonin | References | |||
|---|---|---|---|---|---|---|---|---|
| Conc. | Effects | Conc. | Effects | Conc. | Effects | |||
| I/R | Rat | 0.5 nmol | Effectively protect retinal cells and electrical functions from oxidation and apoptosis; upregulation of HO-1 and downregulation of HIF-1α, VEGF, and MMP-1 | 12.5 mg/kg | Protect RGCs from retinal ischemia injury and suppress glial cell activity | [123, 128] | ||
| DR | Rat; mice | 150 mg/kg in rat; 75 mg/kg in mice | Significantly suppressed the inflammatory processes of retinal microglia and Muller cells; enhanced vascular permeability and blood-retina barrier; protect BRB permeability as antioxidant 12/15-LOX inhibitor, anti-inflammation, and antiangiogenesis | 150 mg/ml in rat | Protect retinal cells from apoptosis by promoting Bcl-2; perform as an inhibitor of ARA and delay the progression of diabetic retinopathy | [130, 131] | ||
3.2.1. In Vitro Studies
The antioxidative properties of baicalein against retinal ischemia were first reported by Liu et al. [122]. Baicalein was shown to significantly increase cell viability of human retinal pigment epithelium cells (hRPEs) against H2O2-induced oxidative stress by scavenging ROS and suppressing the production of matrix metalloproteinase-9 (MMP-9) and vascular endothelial growth factor (VEGF) [122]. Subsequently, the antioxidative effects of baicalein on dissociated primary rat retinal cells were demonstrated to be subjected to ascorbate- and FeSO4-induced oxidative stress [123]. Baicalein pretreatment not only suppresses the expression of hypoxia-inducible factor-1α (HIF-1α), VEGF, and MMP-9 but also increases the level of heme oxygenase-1 (HO-1) in ascorbate- and FeSO4-stimulated retinal cells [123]. These findings indicate that baicalein has strong antioxidative capabilities in retinal cells, which are comparable to the antioxidative effects exerted by Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) [123]. In addition, baicalein and wogonin have been found to demonstrate anti-inflammatory effects by suppressing IL-6 and IL-8 expression in IL-1β-challenged ARPE-19 cells, while NF-κB binding activity is suppressed by wogonin [124]. Similar anti-inflammatory effects have been reported by other recent studies. Chen et al. found that wogonin effectively suppressed LPS-induced activation of the TLR4/NF-κB pathway and subsequently increased expression of inflammatory cytokines IL-1β, IL-6, IL-8, cyclooxygenase-2 (COX-2), TNF-α, and iNOS in ARPE-19 cells [125]. It was also reported that administration of wogonin to endoplasmic reticulum- (ER-) challenged ARPE-19 cells increased the expression of tight junction proteins claudin-1 and ZO-1 [126]. In another study which mimicked DR in vitro by treating ARPE-19 cells and human retinal microvascular endothelial cells (HRMECs) with high glucose, baicalin was shown to exert antiapoptotic effects by inhibiting the release of proinflammatory cytokines and ROS [127]. These protective effects of baicalin are likely mediated by the suppression of NF-κB and p38 MAPK pathways by upregulating miRNA-145 expression [127].
3.2.2. In Vivo Studies
In the rat retinal ischemia/reperfusion model, baicalein pretreatment effectively regulated the expression of apoptotic factors, including Bax and Bcl-2, subsequently reducing retinal cell apoptosis [123]. In addition, baicalein pretreatment significantly improved the inner retinal functions in electroretinogram (ERG) assessments [123]. Similar neuroprotective effects were also observed in baicalin-treated rats with ischemia/reperfusion injury. A reduced loss of Thy-1+ neuron cells and reduced expression of apoptosis markers, including caspase-3 and 8 and poly (ADP-ribose) polymerase-1 (PARP-1), have been found in the retina after ischemic insults [128]. In addition, the downregulated expression of GFAP after baicalin treatment indicates the involvement of baicalin in regulating glial responses and neuroinflammation [128], in addition to its antioxidant and antiapoptosis properties.
The anti-inflammatory effects of baicalein and baicalin have been demonstrated in rats with DR. The excessive activation of resident retinal immune cells is widely known as a pathogenic factor of neurodegeneration in retinal disorders [84, 129]. Yang et al. reported the occurrence of vascular abnormality and RGC loss in the DR rat retina in combination with activation of microglia and Müller cell dysfunction [130]. Oral administration of baicalein has been shown to significantly protect retinal vessels and neurons from DR-induced dysfunction and apoptosis through suppressing the activation of retinal inflammatory processes modulated by microglia and Müller cells and by reducing the release of proinflammatory cytokines, including IL-18, TNF-α, and IL-1β [130]. The protective effects of baicalin are believed to function through inhibiting the expression of apoptosis regulators, including Bax and Bcl-2, on the RGC layer [131]. Intraperitoneal application of baicalin was found to inhibit the otherwise-elevated aldose reductase activity (ARA) in diabetes. This suggests that baicalin acts as an aldose reductase inhibitor, potentially retarding the progression of apoptosis induced by diabetes [131].
In addition to oxidative stress and inflammation, vascular hyperpermeability of retinal blood vessels has been suggested to be a pathogenic factor in retinal ischemia, DR, and AMD. Othman et al. reported that 12/15-LOX activation leads to vascular hyperpermeability in DR by inhibiting protein tyrosine phosphatase and activating VEGF-R2 signaling pathways [132]. As a potent inhibitor of 12/15-LOX, baicalein significantly reduces the lipid metabolites of 12- and 15-hydroxyeicosatetreanoic acids (HETE) expressed during 12/15-LOX activation in DR [132]. The HETE-induced upregulation of NOX2 and ROS is also reported to be downregulated by baicalein treatment in Ins2Akita diabetic mouse retina. Likewise, baicalein has been shown to protect against HETE-induced vascular hyperpermeability by acting as a VEGF-R2 inhibitor, restoring phosphoserine phosphatase-1 (pSHP1) levels in DR [132]. The inflammatory cytokine IL-6 and intracellular adhesion molecules (ICAMs) ICAM-1 and vascular cell adhesion molecule-1 (VCAM-1) were remarkably inhibited in the diabetic retina [132]. Taken together, baicalein functions as a 12/15-LOX inhibitor, mediating its effects primarily on vascular and retinal barriers.
4. Pathways Targeted by Baicalein, Baicalin, and Wogonin during Neuroprotective Processes
As described above, the neuroprotective effects triggered by baicalein, baicalin, and wogonin are possibly related to their anti-inflammatory, antioxidative, and antiapoptotic capabilities (Figure 4).
Figure 4.
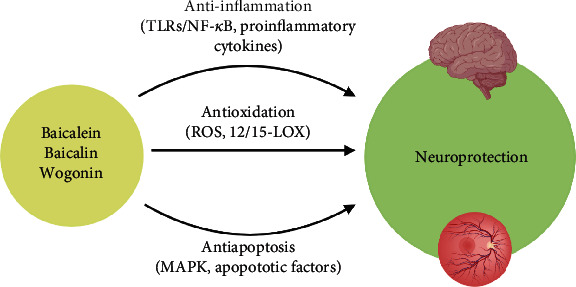
Targeted pathways initiated by baicalein, baicalin, and wogonin demonstrating the neuroprotective effects in the brain and retina. Created with http://BioRender.com.
Firstly, the antioxidative effects of baicalein, baicalin, and wogonin are recognized by their ROS scavenging properties. Under normal circumstance, ROS play a pivotal role in many biological processes, such as redox balance in cells. However, a dramatic increase of ROS production may disturb this homeostatic balance under oxidative stress conditions (e.g., ischemia), eventually leading to cell death. Typically, scavenging excessive ROS is a neuroprotective target in neurodegenerative disorders [133]. Baicalein and baicalin serve as potent 12/15-LOX inhibitors with high antioxidative efficiencies. 12/15-LOX is found to be upregulated after stroke, resulting in neuronal death and leakage of BBB and BRB [134]. Inhibition of 12/15-LOX was also shown to reduce infarct volume and edema in the stroke area, suggesting its potential role as an effective and viable therapeutic option for ischemia [92, 135, 136]. Furthermore, the protection of BBB and BRB help preserves the microenvironment at the injured site from disruption by systemic immunity and secondary inflammatory responses caused by degeneration.
Secondly, the anti-inflammatory effect initiated by these flavonoids after ischemia is revealed by the suppression of proinflammatory cytokine release. Excessive activation of microglia has been emergingly reported as a pathogenesis for the development of neurodegenerative diseases [137]. In addition, baicalein and baicalin exhibit the capability in regulating microglia homeostasis after ischemic stress [109]. These properties have been demonstrated to be mediated through interactions with TLR/NF-κB and PARP-1/AIF pathways [99, 106, 107, 124, 125, 127].
Lastly, the antiapoptotic capacity of baicalein, baicalin, and wogonin has been reported in ischemia-injured brain and retina. Through modulating the MAPK pathway and the production of apoptotic factors, these flavonoids effectively rescue neurons in the brain and retina [100, 112, 113, 115, 123, 127, 128, 131]. In addition, the blockage of ion channel dysfunction after ischemic stress remarkably ameliorates the excitotoxicity caused by ion overload and subsequently decreases neuronal death [86, 121]. Taken together, all the evidence strongly supports the feasibility of developing these flavonoids as natural neuroprotectants.
5. Conclusion and Future Directions
Ischemia often results in physical disabilities, including paralysis and blindness. This is particularly common in the aging population. Currently, effective clinical intervention for ischemia-induced damage is limited. Ample evidence has demonstrated the potent neuroprotective properties of baicalein, baicalin, and wogonin in both in vitro and in vivo models of ischemia. Flavonoids exert their anti-inflammatory, antioxidative, and antiapoptotic effects on CNS postischemia insults by initiating various potential signaling pathways. Flavonoids are herb extracts, suggesting their potential to be developed as natural neuroprotective agents.
Additional studies are required to elucidate and characterize the pharmacological properties and bioactivities of these flavonoids in combating neuropathies, in order to facilitate the future development of neuroprotectants that are safe and effective. As an extension of the brain, the retina may serve as an easily accessible model and potential therapeutic target site for various neuropathies and other neurodegenerative diseases. Additionally, pharmacodynamic and pharmacokinetic studies, including time- and dose-dependent responses, cytotoxicity, and drug metabolism after systemic and topical administration of these three flavonoids, need to be established. One recent study has reported that the metabolic abilities of flavonoids in the liver and intestines are markedly different among different species, including mice, rats, dogs, monkeys, and humans [138]. In addition, potential targets or receptors through which baicalein, baicalin, and wogonin act on may need to be further characterized and studied. Studies on the combined effects of baicalein, baicalin, and wogonin are limited, raising the possibility that combined flavonoids can achieve longer and stronger protective effects. Lastly, due to the limited water solubility and liposolubility of baicalein, baicalin, and wogonin, formulations and optimizations of these flavonoids, possibly including nanoparticles or other newly developed carriers, may be needed to achieve higher bioactivity and clinical efficacy. It is envisaged that these natural flavonoids can eventually offer new therapeutic therapies for patients with ischemia-induced neural disorders.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH)/National Eye Institute (NEI) (Grants EY025913 and EY025259 to D.F.C.), Bright Focus Foundation grant and The Glaucoma Foundation grant to K.C., the Core Grant for Vision Research from NIH/NEI to the Schepens Eye Research Institute (P30EY03790), Health Medical Research Fund (16172571 to C.W.D), PolyU Postgraduate Studentship (L.P.), Henry G. Leong Endowed Professorship in Elderly Vision Health and Dean Reserve (8-8475 and 1-ZVN2 to C.H.T), and PolyU internal grants (UAGF and UAHG to C.W.D).
Contributor Information
Dong Feng Chen, Email: dongfeng_chen@meei.harvard.edu.
Chi-Wai Do, Email: chi-wai.do@polyu.edu.hk.
Data Availability
All data mentioned in this review article are published findings. They have been properly cited in the article.
Conflicts of Interest
D.F.C. is a consultant for Boston Pharma and Pri-Med. Other authors have no conflicts of interest.
References
- 1.Eltzschig H. K., Eckle T. Ischemia and reperfusion-from mechanism to translation. Nature Medicine. 2011;17(11):1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amani H., Mostafavi E., Alebouyeh M. R., et al. Would colloidal gold nanocarriers present an effective diagnosis or treatment for ischemic stroke? International Journal of Nanomedicine. 2019;14:8013–8031. doi: 10.2147/IJN.S210035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amani H., Habibey R., Shokri F., et al. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Scientific Reports. 2019;9(1, article 6044) doi: 10.1038/s41598-019-42633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capildeo R., Haberman S., Rose F. C. The definition and classification of stroke: a new approach. The Quarterly Journal of Medicine. 1978;47(2):177–196. doi: 10.1093/oxfordjournals.qjmed.a067535. [DOI] [PubMed] [Google Scholar]
- 5.Deb P., Sharma S., Hassan K. M. Pathophysiologic mechanisms of acute ischemic stroke: an overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. 2010;17(3):197–218. doi: 10.1016/j.pathophys.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Writing Group Members, Thom T., Haase N., et al. Heart disease and stroke statistics—2006 Update. Circulation. 2006;113(6):e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 7.Boling B., Keinath K. Acute ischemic stroke. AACN Advanced Critical Care. 2018;29(2):152–162. doi: 10.4037/aacnacc2018483. [DOI] [PubMed] [Google Scholar]
- 8.Yang Q., Tong X., Schieb L., et al. Vital signs: recent trends in stroke death rates - United States, 2000-2015. MMWR Morbidity and Mortality Weekly Report. 2017;66(35):933–939. doi: 10.15585/mmwr.mm6635e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J. S. tPA helpers in the treatment of acute ischemic stroke: are they ready for clinical use? Journal of Stroke. 2019;21(2):160–174. doi: 10.5853/jos.2019.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma V. K., Teoh H. L., Wong L. Y. H., Su J., Ong B. K. C., Chan B. P. L. Recanalization therapies in acute ischemic stroke: pharmacological agents, devices, and combinations. Stroke Research and Treatment. 2010;2010:8. doi: 10.4061/2010/672064.672064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mai J. K., Paxinos G. The Human Nervous System. Cambridge, MA, USA: Academic Press; 2011. [Google Scholar]
- 12.Pang J. J., Gao F., Wu S. M. Light-evoked excitatory and inhibitory synaptic inputs to ON and OFF α ganglion cells in the mouse retina. The Journal of Neuroscience. 2003;23(14):6063–6073. doi: 10.1523/JNEUROSCI.23-14-06063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tessier-Lavigne M. Principles of Neural Science. Elsevier; 2000. Visual processing by the retina; pp. 507–522. [Google Scholar]
- 14.Prasad S., Galetta S. L. Chapter 1 - Anatomy and physiology of the afferent visual system. In: Kennard C., Leigh R. J., editors. Handbook of Clinical Neurology. Elsevier; 2011. pp. 3–19. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y. Q., Pan W. H. T., Liu J. H., et al. The effects and underlying mechanisms ofS-Allyll-Cysteine treatment of the retina after ischemia/reperfusion. Journal of Ocular Pharmacology and Therapeutics. 2012;28(2):110–117. doi: 10.1089/jop.2011.0099. [DOI] [PubMed] [Google Scholar]
- 16.Peng P. H., Chao H. M., Juan S. H., Chen C. F., Liu J. H., Ko M. L. Pharmacological preconditioning by low dose cobalt protoporphyrin induces heme oxygenase-1 overexpression and alleviates retinal ischemia-reperfusion injury in rats. Current Eye Research. 2011;36(3):238–246. doi: 10.3109/02713683.2010.539760. [DOI] [PubMed] [Google Scholar]
- 17.Wessel M. M., Nair N., Aaker G. D., Ehrlich J. R., D'Amico D. J., Kiss S. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. The British Journal of Ophthalmology. 2012;96(5):694–698. doi: 10.1136/bjophthalmol-2011-300774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osborne N. N., Ugarte M., Chao M., et al. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Survey of Ophthalmology. 1999;43:S102–S128. doi: 10.1016/S0039-6257(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 19.Osborne N. N., Casson R. J., Wood J. P. M., Chidlow G., Graham M., Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Progress in Retinal and Eye Research. 2004;23(1):91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Lam T. T., Abler A. S., Tso M. Apoptosis and caspases after ischemia-reperfusion injury in rat retina. Investigative Ophthalmology & Visual Science. 1999;40(5):967–975. [PubMed] [Google Scholar]
- 21.MacCormick I. J., Czanner G., Faragher B. Developing retinal biomarkers of neurological disease: an analytical perspective. Biomarkers in Medicine. 2015;9(7):691–701. doi: 10.2217/bmm.15.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.London A., Benhar I., Schwartz M. The retina as a window to the brain--from eye research to CNS disorders. Nature Reviews Neurology. 2013;9(1):44–53. doi: 10.1038/nrneurol.2012.227. [DOI] [PubMed] [Google Scholar]
- 23.Madeira M. H., Ambrósio A. F., Santiago A. R. Glia-mediated retinal neuroinflammation as a biomarker in Alzheimer’s disease. Ophthalmic Research. 2015;54(4):204–211. doi: 10.1159/000440887. [DOI] [PubMed] [Google Scholar]
- 24.Fernández-Albarral J. A., Salobrar-García E., Martínez-Páramo R., et al. Cambios de las celulas gliales de la retina en la enfermedad de Alzheimer - Revision bibliografica. Journal of Optometry. 2019;12(3):198–207. doi: 10.1016/j.optom.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niederkorn J. Y. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nature Immunology. 2006;7(4):354–359. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- 26.Arcuri C., Mecca C., Bianchi R., Giambanco I., Donato R. The pathophysiological role of microglia in dynamic surveillance, phagocytosis and structural remodeling of the developing CNS. Frontiers in Molecular Neuroscience. 2017;10(191) doi: 10.3389/fnmol.2017.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khanh Vu T. H., Chen H., Pan L., et al. CD4+ T-Cell Responses Mediate Progressive Neurodegeneration in Experimental Ischemic Retinopathy. The American Journal of Pathology. 2020;190(8):1723–1734. doi: 10.1016/j.ajpath.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murshid A., Gong J., Calderwood S. K. The role of heat shock proteins in antigen cross presentation. Frontiers in Immunology. 2012;3:p. 63. doi: 10.3389/fimmu.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang S., Kametani M., Chen D. F. Adaptive immunity: new aspects of pathogenesis underlying neurodegeneration in glaucoma and optic neuropathy. Frontiers in Immunology. 2020;11:p. 65. doi: 10.3389/fimmu.2020.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng T., Jiang Y., Farhan M., Lazarovici P., Chen L., Zheng W. Anti-inflammatory effects of traditional chinese medicines on preclinical in vivo models of brain ischemia-reperfusion-injury: prospects for neuroprotective drug discovery and therapy. Frontiers in Pharmacology. 2019;10(204) doi: 10.3389/fphar.2019.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park J. H., Park O. ., Cho J. H., et al. Anti-inflammatory effect of tanshinone I in neuroprotection against cerebral ischemia–reperfusion injury in the gerbil hippocampus. Neurochemical Research. 2014;39(7):1300–1312. doi: 10.1007/s11064-014-1312-4. [DOI] [PubMed] [Google Scholar]
- 32.Dong K., Xu W., Yang J., Qiao H., Wu L. Neuroprotective effects of Tanshinone IIA on permanent focal cerebral ischemia in mice. Phytotherapy Research. 2009;23(5):608–613. doi: 10.1002/ptr.2615. [DOI] [PubMed] [Google Scholar]
- 33.Ling C., Liang J., Zhang C., et al. Synergistic effects of salvianolic acid B and puerarin on cerebral ischemia reperfusion injury. Molecules. 2018;23(3):p. 564. doi: 10.3390/molecules23030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang X., Li Y., Qiao J., Guo Y., Miao M. Neuroprotective effect of total flavonoids from Ilex pubescens against focal cerebral ischemia/reperfusion injury in rats. Molecular Medicine Reports. 2017;16(5):7439–7449. doi: 10.3892/mmr.2017.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan Y., Fang M., Wu C. Y., Ling E. A. Scutellarin as a potential therapeutic agent for microglia-mediated neuroinflammation in cerebral ischemia. Neuromolecular Medicine. 2016;18(3):264–273. doi: 10.1007/s12017-016-8394-x. [DOI] [PubMed] [Google Scholar]
- 36.Wu C. Y., Fang M., Karthikeyan A., Yuan Y., Ling E. A. Scutellarin attenuates microglia-mediated neuroinflammation and promotes astrogliosis in cerebral ischemia-a therapeutic consideration. Current Medicinal Chemistry. 2017;24(7):718–727. doi: 10.2174/0929867324666161118142045. [DOI] [PubMed] [Google Scholar]
- 37.Wąsik A., Antkiewicz-Michaluk L. The mechanism of neuroprotective action of natural compounds. Pharmacological Reports. 2017;69(5):851–860. doi: 10.1016/j.pharep.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Daglia M., Lorenzo A., Nabavi S., Talas Z., Nabavi S. Polyphenols: well beyond the antioxidant capacity: gallic acid and related compounds as neuroprotective agents: you are what you eat! Current Pharmaceutical Biotechnology. 2014;15(4):362–372. doi: 10.2174/138920101504140825120737. [DOI] [PubMed] [Google Scholar]
- 39.Orhan I. E., Daglia M., Nabavi S. F., Loizzo M. R., Sobarzo-Sanchez E., Nabavi S. M. Flavonoids and dementia: an update. Current Medicinal Chemistry. 2015;22(8):1004–1015. doi: 10.2174/0929867322666141212122352. [DOI] [PubMed] [Google Scholar]
- 40.Chen A. Y., Chen Y. C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chemistry. 2013;138(4):2099–2107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alrawaiq N. S., Abdullah A. A review of flavonoid quercetin: metabolism, bioactivity and antioxidant properties. International Journal of PharmTech Research. 2014;6(3):933–941. [Google Scholar]
- 42.Hosseinzadeh H., Nassiri-Asl M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. Journal of Endocrinological Investigation. 2014;37(9):783–788. doi: 10.1007/s40618-014-0096-3. [DOI] [PubMed] [Google Scholar]
- 43.Liu A., Wang W., Fang H., et al. Baicalein protects against polymicrobial sepsis-induced liver injury via inhibition of inflammation and apoptosis in mice. European Journal of Pharmacology. 2015;748:45–53. doi: 10.1016/j.ejphar.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Ma X., Yan W., Dai Z., et al. Baicalein suppresses metastasis of breast cancer cells by inhibiting EMT via downregulation of SATB1 and Wnt/β-catenin pathway. Drug Design, Development and Therapy. 2016;10, article 1419 doi: 10.2147/dddt.s102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Z., Pan X., Zhou J., Leung W. T., Li C., Wang L. Chinese herbal medicine for acute upper respiratory tract infections and reproductive safety: a systematic review. Bioscience Trends. 2019;13(2):117–129. doi: 10.5582/bst.2018.01298. [DOI] [PubMed] [Google Scholar]
- 46.Bae M. J., Shin H. S., See H. J., Jung S. Y., Kwon D. A., Shon D. H. Baicalein induces CD4+Foxp3+ T cells and enhances intestinal barrier function in a mouse model of food allergy. Scientific Reports. 2016;6(1):1–11. doi: 10.1038/srep32225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amakura Y., Yoshimura A., Yoshimura M., Yoshida T. Isolation and characterization of phenolic antioxidants from Plantago herb. Molecules. 2012;17(5):5459–5466. doi: 10.3390/molecules17055459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cong Q., Shang M., Dong Q., Liao W., Xiao F., Ding K. Structure and activities of a novel heteroxylan from Cassia obtusifolia seeds and its sulfated derivative. Carbohydrate Research. 2014;393:43–50. doi: 10.1016/j.carres.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 49.Ming J., Zhuoneng L., Guangxun Z. Protective role of flavonoid baicalin from Scutellaria baicalensis in periodontal disease pathogenesis: A literature review. Complementary Therapies in Medicine. 2018;38:11–18. doi: 10.1016/j.ctim.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Gong W. Y., Zhao Z. X., Liu B. J., Lu L. W., Dong J. C. Exploring the chemopreventive properties and perspectives of baicalin and its aglycone baicalein in solid tumors. European Journal of Medicinal Chemistry. 2017;126:844–852. doi: 10.1016/j.ejmech.2016.11.058. [DOI] [PubMed] [Google Scholar]
- 51.Xiao J. R., Do C. W., To C. H. Potential therapeutic effects of baicalein, baicalin, and wogonin in ocular disorders. Journal of Ocular Pharmacology and Therapeutics. 2014;30(8):605–614. doi: 10.1089/jop.2014.0074. [DOI] [PubMed] [Google Scholar]
- 52.Dinda B., Dinda S., DasSharma S., Banik R., Chakraborty A., Dinda M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. European Journal of Medicinal Chemistry. 2017;131:68–80. doi: 10.1016/j.ejmech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Ru J., Li P., Wang J., et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics. 2014;6(1):1–6. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsson P., Kihlberg J. How big is too big for cell permeability? Journal of Medicinal Chemistry. 2017;60(5):1662–1664. doi: 10.1021/acs.jmedchem.7b00237. [DOI] [PubMed] [Google Scholar]
- 55.Ertl P., Rohde B., Selzer P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. Journal of Medicinal Chemistry. 2000;43(20):3714–3717. doi: 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]
- 56.Tattersall M. H. N., Sodergren J. E., Sengupta S. K., Trites D. H., Modest E. J., Frei E., III Pharmacokinetics of actinomycin 0 in patients with malignant melanoma. Clinical Pharmacology and Therapeutics. 1975;17(6):701–708. doi: 10.1002/cpt1975176701. [DOI] [PubMed] [Google Scholar]
- 57.Tao W., Xu X., Wang X., et al. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. Journal of Ethnopharmacology. 2013;145(1):1–10. doi: 10.1016/j.jep.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 58.Viswanadhan V. N., Ghose A. K., Revankar G. R., Robins R. K. Atomic physicochemical parameters for three dimensional structure directed quantitative structure-activity relationships. 4. Additional parameters for hydrophobic and dispersive interactions and their application for an automated superposition of certain naturally occurring nucleoside antibiotics. Journal of Chemical Information and Modeling. 1989;29(3):163–172. doi: 10.1021/ci00063a006. [DOI] [Google Scholar]
- 59.Luo J., Kong H., Zhang M., et al. Novel carbon dots-derived fromRadix PuerariaeCarbonisata significantly improve the solubility and bioavailability of baicalin. Journal of Biomedical Nanotechnology. 2019;15(1):151–161. doi: 10.1166/jbn.2019.2675. [DOI] [PubMed] [Google Scholar]
- 60.Zahra G., Khadijeh B., Mortaza K., Ali S. Potential therapeutic effects and bioavailability of wogonin, the flavone of Baikal skullcap. Journal of Nutritional Medicine and Diet Care. 2019;5(2):p. 39. doi: 10.23937/2572-3278.1510039. [DOI] [Google Scholar]
- 61.Gao Y., Snyder S. A., Smith J. N., Chen Y. C. Anticancer properties of baicalein: a review. Medicinal Chemistry Research. 2016;25(8):1515–1523. doi: 10.1007/s00044-016-1607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muto R., Motozuka T., Nakano M., Tatsumi Y., Sakamoto F., Kosaka N. The chemical structure of new substance as the metabolite of baicalin and time profiles for the plasma concentration after oral administration of sho-saiko-to in human. Yakugaku Zasshi. 1998;118(3):79–87. doi: 10.1248/yakushi1947.118.3_79. [DOI] [PubMed] [Google Scholar]
- 63.Lai M. Y., Hou Y. C., Hsiu S. L., Chen C. C., Chao P. D. L. Relative flavone bioavailability of Scutellariae Radix between traditional decoction and commercial powder preparation in humans. Journal of Food and Drug Analysis. 2002;10(2) doi: 10.38212/2224-6614.2762. [DOI] [Google Scholar]
- 64.Tian S., He G., Song J., et al. Pharmacokinetic study of baicalein after oral administration in monkeys. Fitoterapia. 2012;83(3):532–540. doi: 10.1016/j.fitote.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 65.Huang T., Liu Y., Zhang C. Pharmacokinetics and bioavailability enhancement of baicalin: a review. European Journal of Drug Metabolism and Pharmacokinetics. 2019;44(2):159–168. doi: 10.1007/s13318-018-0509-3. [DOI] [PubMed] [Google Scholar]
- 66.Tang Y., Zhu H., Zhang Y., Huang C. Determination of human plasma protein binding of baicalin by ultrafiltration and high-performance liquid chromatography. Biomedical Chromatography. 2006;20(10):1116–1119. doi: 10.1002/bmc.655. [DOI] [PubMed] [Google Scholar]
- 67.Pang H., Xue W., Shi A., et al. Multiple-ascending-dose pharmacokinetics and safety evaluation of baicalein chewable tablets in healthy Chinese volunteers. Clinical Drug Investigation. 2016;36(9):713–724. doi: 10.1007/s40261-016-0418-7. [DOI] [PubMed] [Google Scholar]
- 68.Li M., Shi A., Pang H., et al. Safety, tolerability, and pharmacokinetics of a single ascending dose of baicalein chewable tablets in healthy subjects. Journal of Ethnopharmacology. 2014;156:210–215. doi: 10.1016/j.jep.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 69.Chen W. W., Zhang X., Huang W. J. Role of neuroinflammation in neurodegenerative diseases (Review) Molecular Medicine Reports. 2016;13(4):3391–3396. doi: 10.3892/mmr.2016.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DiMauro S., Schon E. A. Mitochondrial disorders in the nervous system. Annual Review of Neuroscience. 2008;31(1):91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- 71.Lardenoije R., Iatrou A., Kenis G., et al. The epigenetics of aging and neurodegeneration. Progress in Neurobiology. 2015;131:21–64. doi: 10.1016/j.pneurobio.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ciechanover A., Kwon Y. T. Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Experimental & Molecular Medicine. 2015;47(3, article e147) doi: 10.1038/emm.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Z., Zhou T., Ziegler A. C., Dimitrion P., Zuo L. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxidative Medicine and Cellular Longevity. 2017;2017:11. doi: 10.1155/2017/2525967.2525967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vila M., Przedborski S. Targeting programmed cell death in neurodegenerative diseases. Nature Reviews Neuroscience. 2003;4(5):365–375. doi: 10.1038/nrn1100. [DOI] [PubMed] [Google Scholar]
- 75.Donnan G. A., Fisher M., Macleod M., Davis S. M. Stroke. Lancet. 2008;371(9624):1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 76.Xu H. Z., Le Y. Z. Significance of outer blood–retina barrier breakdown in diabetes and ischemia. Investigative Ophthalmology & Visual Science. 2011;52(5):2160–2164. doi: 10.1167/iovs.10-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dirnagl U., Iadecola C., Moskowitz M. A. Pathobiology of ischaemic stroke: an integrated view. Trends in Neurosciences. 1999;22(9):391–397. doi: 10.1016/S0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 78.Gaire B. P. Herbal medicine in ischemic stroke: challenges and prospective. Chinese Journal of Integrative Medicine. 2018;24(4):243–246. doi: 10.1007/s11655-018-2828-2. [DOI] [PubMed] [Google Scholar]
- 79.Chen H., Yoshioka H., Kim G. S., et al. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxidants & Redox Signaling. 2011;14(8):1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Honda H. M., Korge P., Weiss J. N. Mitochondria and ischemia/reperfusion injury. Annals of the New York Academy of Sciences. 2005;1047(1):248–258. doi: 10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- 81.Woodruff T. M., Thundyil J., Tang S.-C., Sobey C. G., Taylor S. M., Arumugam T. V. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Molecular Neurodegeneration. 2011;6(1):p. 11. doi: 10.1186/1750-1326-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guzman-Martinez L., Maccioni R. B., Andrade V., Navarrete L. P., Pastor M. G., Ramos-Escobar N. Neuroinflammation as a common feature of neurodegenerative disorders. Frontiers in Pharmacology. 2019;10, article 1008 doi: 10.3389/fphar.2019.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jayaraj R. L., Azimullah S., Beiram R., Jalal F. Y., Rosenberg G. A. Neuroinflammation: friend and foe for ischemic stroke. Journal of Neuroinflammation. 2019;16(1):p. 142. doi: 10.1186/s12974-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kwon H. S., Koh S. H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Translational Neurodegeneration. 2020;9(1):p. 42. doi: 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang H., Xiong X., Gu L., Xie W., Zhao H. CD4 T cell deficiency attenuates ischemic stroke, inhibits oxidative stress, and enhances Akt/mTOR survival signaling pathways in mice. Chinese Neurosurgical Journal. 2018;4(1):p. 32. doi: 10.1186/s41016-018-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khanna A., Kahle K. T., Walcott B. P., Gerzanich V., Simard J. M. Disruption of ion homeostasis in the neurogliovascular unit underlies the pathogenesis of ischemic cerebral edema. Translational Stroke Research. 2014;5(1):3–16. doi: 10.1007/s12975-013-0307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coultrap S. J., Vest R. S., Ashpole N. M., Hudmon A., Bayer K. U. CaMKII in cerebral ischemia. Acta Pharmacologica Sinica. 2011;32(7):861–872. doi: 10.1038/aps.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Siu A. W., Lau M. K., Cheng J. S., et al. Glutamate-induced retinal lipid and protein damage: the protective effects of catechin. Neuroscience Letters. 2008;432(3):193–197. doi: 10.1016/j.neulet.2007.12.046. [DOI] [PubMed] [Google Scholar]
- 89.Tao T., Liu M., Chen M., et al. Natural medicine in neuroprotection for ischemic stroke: challenges and prospective. Pharmacology & Therapeutics. 2020;216, article 107695 doi: 10.1016/j.pharmthera.2020.107695. [DOI] [PubMed] [Google Scholar]
- 90.Radak D., Katsiki N., Resanovic I., et al. Apoptosis and acute brain ischemia in ischemic stroke. Current Vascular Pharmacology. 2017;15(2):115–122. doi: 10.2174/1570161115666161104095522. [DOI] [PubMed] [Google Scholar]
- 91.Yousufuddin M., Young N. Aging and ischemic stroke. Aging. 2019;11(9):2542–2544. doi: 10.18632/aging.101931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Leyen K., Kim H. Y., Lee S. R., Jin G., Arai K., Lo E. H. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke. 2006;37(12):3014–3018. doi: 10.1161/01.STR.0000249004.25444.a5. [DOI] [PubMed] [Google Scholar]
- 93.Lapchak P. A., Maher P., Schubert D., Zivin J. A. Baicalein, an antioxidant 12/15-lipoxygenase inhibitor improves clinical rating scores following multiple infarct embolic strokes. Neuroscience. 2007;150(3):585–591. doi: 10.1016/j.neuroscience.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 94.Liu C., Wu J., Xu K., et al. Neuroprotection by baicalein in ischemic brain injury involves PTEN/AKT pathway. Journal of Neurochemistry. 2010;112(6):1500–1512. doi: 10.1111/j.1471-4159.2009.06561.x. [DOI] [PubMed] [Google Scholar]
- 95.Kuhn H., Banthiya S., van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2015;1851(4):308–330. doi: 10.1016/j.bbalip.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh N. K., Rao G. N. Emerging role of 12/15-lipoxygenase (ALOX15) in human pathologies. Progress in Lipid Research. 2019;73:28–45. doi: 10.1016/j.plipres.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Son D., Lee P., Lee J., Kim H., Kim S. Y. Neuroprotective effect of wogonin in hippocampal slice culture exposed to oxygen and glucose deprivation. European Journal of Pharmacology. 2004;493(1-3):99–102. doi: 10.1016/j.ejphar.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 98.Cho J., Lee H. K. Wogonin inhibits excitotoxic and oxidative neuronal damage in primary cultured rat cortical cells. European Journal of Pharmacology. 2004;485(1-3):105–110. doi: 10.1016/j.ejphar.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 99.Li H. Y., Hu J., Zhao S., et al. Comparative Study of the Effect of Baicalin and Its Natural Analogs on Neurons with Oxygen and Glucose Deprivation Involving Innate Immune Reaction of TLR2/TNFα. Journal of Biomedicine and Biotechnology. 2012;2012:9. doi: 10.1155/2012/267890.267890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang P., Cao Y., Yu J., et al. Baicalin alleviates ischemia-induced memory impairment by inhibiting the phosphorylation of CaMKII in hippocampus. Brain Research. 2016;1642:95–103. doi: 10.1016/j.brainres.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 101.Cheng O., Li Z., Han Y., Jiang Q., Yan Y., Cheng K. Baicalin improved the spatial learning ability of global ischemia/reperfusion rats by reducing hippocampal apoptosis. Brain Research. 2012;1470:111–118. doi: 10.1016/j.brainres.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 102.Lu H. T., Feng R. Q., Tang J. K., Zhou J. J., Gao F., Ren J. CaMKII/calpain interaction mediates ischemia/reperfusion injury in isolated rat hearts. Cell Death & Disease. 2020;11(5):p. 388. doi: 10.1038/s41419-020-2605-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dai J., Chen L., Qiu Y. M., et al. Activations of GABAergic signaling, HSP70 and MAPK cascades are involved in baicalin's neuroprotection against gerbil global ischemia/reperfusion injury. Brain Research Bulletin. 2013;90:1–9. doi: 10.1016/j.brainresbull.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 104.Demyanenko S., Nikul V., Rodkin S., Davletshin A., Evgen’ev M. B., Garbuz D. G. Exogenous recombinant Hsp70 mediates neuroprotection after photothrombotic stroke. Cell Stress & Chaperones. 2021;26(1):103–114. doi: 10.1007/s12192-020-01159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nozaki K., Nishimura M., Hashimoto N. Mitogen-activated protein kinases and cerebral ischemia. Molecular Neurobiology. 2001;23(1):01–20. doi: 10.1385/MN:23:1:01. [DOI] [PubMed] [Google Scholar]
- 106.Tu X. K., Yang W. Z., Shi S. S., et al. Baicalin inhibits TLR2/4 signaling pathway in rat brain following permanent cerebral ischemia. Inflammation. 2011;34(5):463–470. doi: 10.1007/s10753-010-9254-8. [DOI] [PubMed] [Google Scholar]
- 107.Xue X., Qu X. J., Yang Y., et al. Baicalin attenuates focal cerebral ischemic reperfusion injury through inhibition of nuclear factor κB p65 activation. Biochemical and Biophysical Research Communications. 2010;403(3-4):398–404. doi: 10.1016/j.bbrc.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 108.Tu X. K., Yang W. Z., Shi S. S., Wang C. H., Chen C. M. Neuroprotective effect of baicalin in a rat model of permanent focal cerebral ischemia. Neurochemical Research. 2009;34(9):1626–1634. doi: 10.1007/s11064-009-9953-4. [DOI] [PubMed] [Google Scholar]
- 109.Yang S., Wang H., Yang Y., et al. Baicalein administered in the subacute phase ameliorates ischemia-reperfusion- induced brain injury by reducing neuroinflammation and neuronal damage. Biomedicine & Pharmacotherapy. 2019;117, article 109102 doi: 10.1016/j.biopha.2019.109102. [DOI] [PubMed] [Google Scholar]
- 110.Yigitkanli K., Pekcec A., Karatas H., et al. Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Annals of Neurology. 2013;73(1):129–135. doi: 10.1002/ana.23734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jin G., Arai K., Murata Y., et al. Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke. 2008;39(9):2538–2543. doi: 10.1161/STROKEAHA.108.514927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cui L., Zhang X., Yang R., et al. Baicalein is neuroprotective in rat MCAO model: role of 12/15-lipoxygenase, mitogen-activated protein kinase and cytosolic phospholipase A2. Pharmacology, Biochemistry, and Behavior. 2010;96(4):469–475. doi: 10.1016/j.pbb.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 113.Pallast S., Arai K., Pekcec A., et al. Increased nuclear apoptosis-inducing factor after transient focal ischemia: a 12/15-lipoxygenase-dependent organelle damage pathway. Journal of Cerebral Blood Flow and Metabolism. 2010;30(6):1157–1167. doi: 10.1038/jcbfm.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li W. H., Yang Y. L., Cheng X., et al. Baicalein attenuates caspase-independent cells death via inhibiting PARP-1 activation and AIF nuclear translocation in cerebral ischemia/reperfusion rats. Apoptosis. 2020;25(5-6):354–369. doi: 10.1007/s10495-020-01600-w. [DOI] [PubMed] [Google Scholar]
- 115.Stephenson D., Rash K., Smalstig B., et al. Cytosolic phospholipase A2 is induced in reactive glia following different forms of neurodegeneration. Glia. 1999;27(2):110–128. doi: 10.1002/(SICI)1098-1136(199908)27:2<110::AID-GLIA2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 116.Xu Y. W., Sun L., Liang H., Sun G. M., Cheng Y. 12/15-Lipoxygenase inhibitor baicalein suppresses PPARγ expression and nuclear translocation induced by cerebral ischemia/reperfusion. Brain Research. 2010;1307:149–157. doi: 10.1016/j.brainres.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 117.Lee H., Kim Y. O., Kim H., et al. Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. The FASEB Journal. 2003;17(13):1–21. doi: 10.1096/fj.03-0057fje. [DOI] [PubMed] [Google Scholar]
- 118.Cho J., Lee H. K. Wogonin inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Biological & Pharmaceutical Bulletin. 2004;27(10):1561–1564. doi: 10.1248/bpb.27.1561. [DOI] [PubMed] [Google Scholar]
- 119.Kong Z., Shen Q., Jiang J., Deng M., Zhang Z., Wang G. Wogonin improves functional neuroprotection for acute cerebral ischemia in rats by promoting angiogenesis via TGF-β1. Annals of Translational Medicine. 2019;7(22):639–639. doi: 10.21037/atm.2019.10.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guerrero P. A., McCarty J. H. TGF-β Activation and Signaling in Angiogenesis. IntechOpen; 2017. [DOI] [Google Scholar]
- 121.Qu J. T., Zhang D. X., Liu F., et al. Vasodilatory effect of wogonin on the rat aorta and its mechanism study. Biological & Pharmaceutical Bulletin. 2015;38(12):1873–1878. doi: 10.1248/bpb.b15-00444. [DOI] [PubMed] [Google Scholar]
- 122.Liu J. H., Wann H., Chen M. M., et al. Baicalein significantly protects human retinal pigment epithelium cells against H2O2-Induced oxidative stress by scavenging reactive oxygen species and downregulating the expression of matrix metalloproteinase-9 and vascular endothelial growth factor. Journal of Ocular Pharmacology and Therapeutics. 2010;26(5):421–429. doi: 10.1089/jop.2010.0063. [DOI] [PubMed] [Google Scholar]
- 123.Chao H. M., Chuang M. J., Liu J. H., et al. Baicalein protects against retinal ischemia by antioxidation, antiapoptosis, downregulation of HIF-1α, VEGF, and MMP-9 and upregulation of HO-1. Journal of Ocular Pharmacology and Therapeutics. 2013;29(6):539–549. doi: 10.1089/jop.2012.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nakamura N., Hayasaka S., Zhang X. Y., et al. Effects of baicalin, baicalein, and wogonin on interleukin-6 and interleukin-8 expression, and nuclear factor-κb binding activities induced by interleukin-1β in human retinal pigment epithelial cell line. Experimental Eye Research. 2003;77(2):195–202. doi: 10.1016/S0014-4835(03)00116-7. [DOI] [PubMed] [Google Scholar]
- 125.Chen C., Guo D., Lu G. Wogonin protects human retinal pigment epithelium cells from LPS-induced barrier dysfunction and inflammatory responses by regulating the TLR4/NF-κB signaling pathway. Molecular Medicine Reports. 2017;15(4):2289–2295. doi: 10.3892/mmr.2017.6252. [DOI] [PubMed] [Google Scholar]
- 126.Yoshikawa T., Ogata N., Izuta H., Shimazawa M., Hara H., Takahashi K. Increased expression of tight junctions in ARPE-19 cells under endoplasmic reticulum stress. Current Eye Research. 2011;36(12):1153–1163. doi: 10.3109/02713683.2011.606592. [DOI] [PubMed] [Google Scholar]
- 127.Dai C., Jiang S., Chu C., Xin M., Song X., Zhao B. Baicalin protects human retinal pigment epithelial cell lines against high glucose-induced cell injury by up-regulation of microRNA-145. Experimental and Molecular Pathology. 2019;106:123–130. doi: 10.1016/j.yexmp.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 128.Jung S. H., Kang K. D., Ji D., et al. The flavonoid baicalin counteracts ischemic and oxidative insults to retinal cells and lipid peroxidation to brain membranes. Neurochemistry International. 2008;53(6-8):325–337. doi: 10.1016/j.neuint.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 129.Kaur D., Sharma V., Deshmukh R. Activation of microglia and astrocytes: a roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology. 2019;27(4):663–677. doi: 10.1007/s10787-019-00580-x. [DOI] [PubMed] [Google Scholar]
- 130.Yang L. P., Sun H. L., Wu L. M., et al. Baicalein reduces inflammatory process in a rodent model of diabetic retinopathy. Investigative Ophthalmology & Visual Science. 2009;50(5):2319–2327. doi: 10.1167/iovs.08-2642. [DOI] [PubMed] [Google Scholar]
- 131.Wang X. J., Liu C. S., Li Z. X. The effect of baicalin on tissue aldose reductase activity and retinal apoptosis in diabetic rats. Chinese Journal of Diabetes. 2008;16(8):p. 26. [Google Scholar]
- 132.Othman A., Ahmad S., Megyerdi S., et al. 12/15-Lipoxygenase-derived lipid metabolites induce retinal endothelial cell barrier dysfunction: contribution of NADPH oxidase. PLoS One. 2013;8(2, article e57254) doi: 10.1371/journal.pone.0057254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Uttara B., Singh A., Zamboni P., Mahajan R. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Current Neuropharmacology. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van Leyen K., Holman T. R., Maloney D. J. The potential of 12/15-lipoxygenase inhibitors in stroke therapy. Future Medicinal Chemistry. 2014;6(17):1853–1855. doi: 10.4155/fmc.14.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu Y., Zheng Y., Karatas H., et al. 12/15-lipoxygenase inhibition or knockout reduces warfarin-associated hemorrhagic transformation after experimental stroke. Stroke. 2017;48(2):445–451. doi: 10.1161/STROKEAHA.116.014790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Karatas H., Cakir-Aktas C. 12/15 lipoxygenase as a therapeutic target in brain disorders. Noro Psikiyatri Arsivi. 2019;56(4):288–291. doi: 10.29399/npa.23646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tang Y., Xiao Z., Pan L., et al. Therapeutic targeting of retinal immune microenvironment with CSF-1 receptor antibody promotes visual function recovery after ischemic optic neuropathy. Frontiers in Immunology. 2020;11, article 585918 doi: 10.3389/fimmu.2020.585918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hanioka N., Isobe T., Tanaka-Kagawa T., Ohkawara S. Wogonin glucuronidation in liver and intestinal microsomes of humans, monkeys, dogs, rats, and mice. Xenobiotica. 2020;50(8):906–912. doi: 10.1080/00498254.2020.1725180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data mentioned in this review article are published findings. They have been properly cited in the article.


