Abstract
Preservatives play an essentially role in ensuring that cosmetic formulations remain safe for use via control of microbial contamination. Commonly used preservatives include organic acids, alcohols and phenols and these play an essential role in controlling the growth of bacteria, fungi and moulds in substrates that can potentially act as a rich food source for microbial contaminants. Whilst the activity of these compounds is clear, both in vitro and in formulation, little information exists on the potential impact that common preservative systems, in full formulation, have on the skin’s resident microbiome. Dysbiosis of the skin’s microbiome has been associated with a number of cosmetic conditions but there currently are no in vivo studies investigating the potential for preservative ingredients, when included in personal care formulations under normal use conditions, to impact the cutaneous microbiome. Here we present an analysis of four in vivo studies that examine the impact of different preservation systems in full formulation, in different products formats, with varying durations of application. This work demonstrates that despite the antimicrobial efficacy of the preservatives in vitro, the skin microbiome is not impacted by preservative containing products in vivo.
Introduction
The human skin microbiome, the collection of bacteria, viruses and fungi that inhabit the human skin surface and invaginations has become a research topic of fundamental interest. Skin is the largest epithelial surface that is colonised by microbes [1–3], and its microbial inhabitants are believed to play an important role in the maintenance of healthy skin [4,5]. Microbial composition of the skin varies across body sites driven by the nutrients available in characteristic dry (arm), moist (axilla) and sebaceous (scalp) sites across the body [6]. This site-specific community composition means that in reality, the skin microbiome can more appropriately be considered a consortium of distinct microbiomes [7].
It is well documented that numerous members of the skin microbiome play a crucial role in the production of various metabolites that have an impact on skin health including free fatty acids from Cutibacterium acnes [8,9], antimicrobial peptides and phenol soluble modulins from Staphylococcus epidermidis [10,11] and other staphylococcal species [12,13]. An abundance of these organisms is commonly associated with healthy skin. Conversely, the presence of skin disease is commonly accompanied by a shift in microbiome composition to one taxonomically or functionally distinct from an individual’s baseline healthy state. Common conditions associated with microbiome community alterations include acne [14], atopic skin [15], dandruff [16] and axillary odour [17]. In some instances, a causal microbiome variation has been unequivocally proven [18] whereas in others conditions a complete understanding of the complex multifaceted interactions between microbes and human skin has yet to be completely characterised [19].
To control cosmetic conditions the use of personal care products has become common place. These products attempt to alleviate symptoms through the use of ingredients including skin moisturisers and topical antimicrobials [20,21]. However, despite the use of these products, in the absence of disease flares, variations in seasons, environmental conditions or perturbations in the structure or integrity of the skin barrier, the temporal stability of the skin microbiome has been shown to be robust [22,23]. This suggests a degree of community resilience to the skin microbiome, an essential ability of the community to respond to disturbance and return to its previous state, both structurally and functionally [24]. Ensuring community resilience is not compromised by the application of cosmetic products has been identified as a key consumer safety parameter [25].
Cosmetics are produced in a non-sterile but hygienically controlled environment however inadvertent contamination may occur. In addition, microbial challenge can be common place during consumer use [26]. Cosmetic ingredients can act as nutrient sources to facilitate the growth of contaminating microorganism under the appropriate physiochemical conditions. This contamination can range from Gram-negative and Gram-positive bacteria, yeasts and fungi, many of which are opportunistic pathogens which can cause serious infection and illness [26,27]. Therefore, every manufacturer of cosmetics has a responsibility to ensure the microbiological safety of its products for the intended use lifespan [28]. Under EU cosmetics regulations, each cosmetic product placed on the market should have its own Product Information File (PIF) which captures details on the microbiological quality of the raw materials and the finished product [29].
Cosmetic manufactures use approved preservative systems to maintain product quality and protect against the growth of spoilage and pathogenic microorganisms [30]. The preservatives system must have a broad spectrum of activity and be compatible with the product ingredients as well as being stable over the shelf-life and intended usage time [31]. To achieve this, a combination of preservatives and formulation hurdles are used to gain a broad spectrum of activity and reduce the necessary concentration of single actives [32]. For example, the use of certain ingredients and formulation hurdle benefits such as pH control and reduced water activity can be used to improve the innate robustness of the product or to potentiate antimicrobial activity [27].
The most common antimicrobial preservatives can be divided into a number of groups according to their chemical structure and functional groups. These include, organic acids, alcohols, phenols, aldehydes, and formaldehyde donors, isothiazolinones, biguanides, quaternary ammonium compounds, nitrogen compounds, heavy metal derivatives, and inorganic compounds [27]. Each of these preservative groups will have a different mode of action and spectrum of activity under the correct concentration and formulation properties. The use of certain preservatives may be limited, or the concentration restricted depending on the type of product and the area for application. For example, rinse-off products such as shampoo will typically have fewer restrictions compared to leave-on products such as skin moisturiser, which have prolonged skin contact [33].
The activity of preservatives has been examined in vitro using standard minimum inhibitory concentration tests [34,35], which can provide insights into antimicrobial performance in the neat product. However, there is limited information on the impact of different preservative systems on the skin microbiome under in vivo conditions. Only through the use of in vivo analysis are realistic effects of product usage, dilution, cutaneous substantivity and the ability of the microbiome to respond to perturbations by seeding the skin from protected invaginations faithfully represented [23]. Where previous analysis sought to investigate the impact of preservative ingredients on skin bacteria ex vivo [36], this analysis sought to investigate the impact of cosmetic formulations containing four different commonly used preservative systems on the skin microbiome of healthy adult females in vivo.
Leg skin microbiome samples were collected from each subject at baseline and after final product application and assessment on the impact of the preservative containing products was carried out using standard microbiome analysis including taxonomic and diversity analysis.
Materials and methods
Study populations and test materials
Key inclusion and exclusion criteria for each study population (A-D, corresponding to preservative system A-D) included females between 18–55 years of age in general good health with intact skin free of disease. A complete list of inclusion and exclusion criteria is outlined in S1 Table. The compositions of the preservative systems examined are outlined in Table 1 and a list of the associated formulation ingredients can be found in S2 Table.
Table 1. Summary information on the different preservation systems, product formats, and product application regime used in the subsequent analysis.
| Preservation System Name | Preservation System Composition | Format | Timepoints Assessed | Application |
|---|---|---|---|---|
| A | • Glydant Plus (IPBC/DMDM) [0.26%] • Tetrasodium EDTA [0.05%] |
Wash off shower gel | Baseline (pre use) and 24 hours post use. | Once |
| B | • Phenoxyethanol [0.6%] • Tetrasodium EDTA [0.05%] • IPBC [0.007%] |
Wash off shower gel | Baseline (pre use) and 24 hours post use. | Once |
| C | • Glydant Plus (IPBC/DMDM) [0.26%] • Tetrasodium EDTA [0.05%] |
Wash off shower gel | Baseline (pre use) and following 2 weeks of use. | Once daily for 2 weeks |
| D | • Methylparaben (0.2%) • Propylparaben (0.1%) • Phenoxyethanol (0.4%) |
Leave on lotion | Baseline (pre use) and following 5 weeks of use. | Twice daily for 5 weeks |
Each preservative containing formulation was applied to 15 subjects per study with varying frequency of application and study duration. The study location and relevant population metadata can be seen in Table 2.
Table 2. Summary information for cohorts of subject used for subsequent analysis.
| Preservation System | Study Location | Sex | Age Range | Average Age |
|---|---|---|---|---|
| A | Canada | Female | 21–63 | 51.7 |
| B | USA | Female | 19–65 | 39.5 |
| C | USA | Female | 21–50 | 33.2 |
| D | UK | Female | 21–51 | 37.6 |
Subjects enrolled in the studies were not subjected to a conditioning phase and were permitted to continue using their standard cleansers in advance of the study. This was to retain the natural variation seen in a subject’s skin microbiome as a result of their current hygiene routine and environment. All subjects were required not to bathe or apply cosmetics for a minimum of 12 hours before sampling. For studies where products C and D were applied post application samples were taken 12–18 hours after last product application.
Sample selection
Samples were selected for this cohort analysis from 4 separate intervention studies carried out to examine the impact of cosmetic formulations on the adult leg skin microbiome. Written informed consent for all studies was obtained from all enrolled individuals. The study protocols were reviewed and approved by the appropriate independent ethics committees, Study A: Institutional Review Board Services, Study B and C: Gallatin Institutional Review Board, Study D: Reading Independent Ethics Committee. The methods were carried out in accordance with the principles of the Declaration of Helsinki and principles of Good Clinical Practice as applicable to clinical studies on cosmetics. Sample collection, shipping and processing of samples for all studies were carried out in an identical fashion minimising any potential bias as a result of sample collection methodologies or data processing variations. No adverse events were reported for any of the studies in question and all subjects enrolled in the study maintained good skin condition throughout the study.
Microbiome sample collection and processing
Buffer washes were collected using a sterile Teflon sampling ring with a 3.5cm internal diameter (total diameter 5cm and height 3.5cm) using the method previously described [37]. The ring was placed in the sampling site and held firmly in place by a second operator. Using a digital pipette and barrier (filter) pipette tip, 2.0ml of buffer wash solution (sterile phosphate-buffered saline pH7.9 containing 0.1% Triton X-100) was placed into the sampling ring and the skin surface gently agitated for one minute with a sterile Teflon rod (with rounded, smooth ends). The sampling fluid was collected using a sterile disposable pipette and placed into a sterile centrifuge tube. The sampling procedure was repeated with a further 2.0ml aliquot of buffer wash material and both aliquots pooled. Samples were placed on ice during the collection process and then stored at -80°C prior to DNA extraction. Shipment of samples from all studies prior to extraction was carried out on dry ice with appropriate temperature logging.
DNA extraction
Samples were defrosted and concentrated by centrifugation (10mins/13,000rpm, Eppendorf 5810R, Germany), supernatant removed, and the cells resuspended in 500μl of sterile TE buffer (10 mM Tris-HCl; 1 mM EDTA, pH 7.4). The cell suspension was transferred to a 96-well Lysing Matrix Plate B (MP Biomedicals). Addition of 3ul of Ready-Lyse lysozyme (Epicentre, 250U/ul) was followed by incubation with agitation at 300rpm, 37°C for 18 hours. Following incubation, a bead-beating step was performed using a Tissue Lyser (Qiagen, Germany) for 3 minutes at 20 Hz. An off-board lysis was performed by incubating the samples at 68°C for 15 minutes in the presence of Proteinase K, Carrier RNA, ATL and ACL buffer in a Qiagen S-plate following manufacturer guidelines. Post-incubation, the samples were processed using the QIAamp UCP DNA Micro kit according to manufacturer instructions (56204, Qiagen). Extracted DNA was frozen prior to 16S rRNA gene library preparation.
16S rRNA library preparation and sequencing
Oligonucleotide primers targeting the V1-V2 hypervariable region of the 16S rRNA gene were selected. PCR was carried out using the following primers,
U28F: 5’-ACACTCTTTCCCTACACGACGCTCTTCCGATCTNNNNNAGAGTTTGATCMTGGCTCA G-3’
U338R: 5’-GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTTGCTGCCTCCCGTAGGAGT-3’
PCR primers were modified version of the standard 28F and 338R primers which contain additional recognition sequences to facilitate nested PCR to add Illumina sequencing adapters and index sequences to resulting amplicons using methods described previously [38].
A second round PCR incorporated Illumina adapters containing indexes (i5 and i7) for sample identification utilising eight forward primers and twelve reverse primers each of which contained a separate barcode allowing up to 96 different combinations. General sequences of the primers are illustrated below with the variable 8 bp barcode underlined and amplification carried out as previously described [38].
N501f 5′AATGATACGGCGACCACCGAGATCTACACTAGATCGCACACTCTTTCCCTACACGACGCT3′
N701r 5′CAAGCAGAAGACGGCATACGAGATTCGCCTTAGTGACTGGAGTTCAGACGTGTGCTC3′.
Informatics processing
All steps were performed using the QIIME2 microbiome analysis tool suite [39] version 2019.1. The paired end sequences were imported into QIIME2 format, then denoised using DADA2 [40]. The primer sequence regions were removed during denoising by setting DADA2’s forward and reverse read trim parameters to the length of the forward and reverse primers, respectively. A complete list of software parameters and versions can be found in S3 and S4 Tables. Rooted and unrooted phylogenetic trees were generated for the ASVs (Amplicon Sequence Variants) using the QIIME2 phylogeny align-to-tree-mafft-fasttree workflow. Taxonomic assignments were generated by comparing ASVs against a BLAST database composed of the HOMD, HOMD extended and Greengenes sequences (HOMDEXTGG version 14.51) described in [41]. Taxonomic classification was performed as previously described [42] at 99% identity across 98% of the read length. Visualization and plotting of resulting data was carried out using the QIIME2 suite and JMP v 14.1 [43].
Statistical analysis
All statistical analysis were carried out using the QIIME2 microbiome analysis tool suite [39] version 2019.1. Within sample group diversity (alpha) changes were estimated and tested using non-parametric approaches. A signed rank test for changes across time-points for each treatment that accounts for paired differences within subjects. Kruskal Wallis tests were used for pairwise treatment comparisons. Between group diversity (beta) was assessed visually using principal co-ordinate ordination plots for key metric distance matrices, Bray-Curtis(semi-metric), Jaccard, weighted and unweighted Unifrac [44]. Statistical inference was achieved using permutation analysis of variance (PERMANOVA). Taxonomic differences in mean relative abundance were assessed using ANCOM (analysis of compositions) [45] to access differences within treatments across timepoints.
Results
Taxonomy
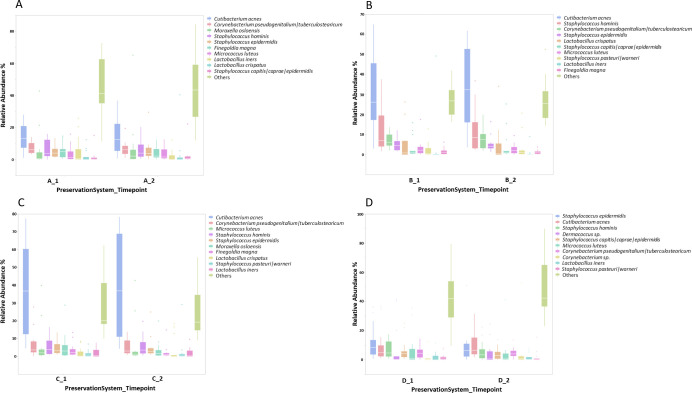
Results of taxonomic analysis can be seen in Fig 1A–1D showing the top 10 most abundant species in each study. Figures A-D correspond to products/preservation systems A-D. In all studies the leg skin microbiome was dominated by species from the genera Staphylococcus, Cutibacterium and Corynebacterium with species from the genera Moraxella, Micrococcus, Lactobacillus and Dermacoccus present in different study subject populations.
Fig 1. Taxonomic analysis of leg skin microbiome before and after formulation application.
Species level taxonomic analysis of leg skin microbiome at baseline (T1) and following product application (T2). Panels A-D correspond to preservation systems A-D and show the top 10 most abundant species in each study.
Differential abundance assessment was carried out using ANCOM using a centred log ratio approach on all genera/species in the dataset. No differentially abundant species were identified between timepoints for each individual product application group.
Alpha diversity
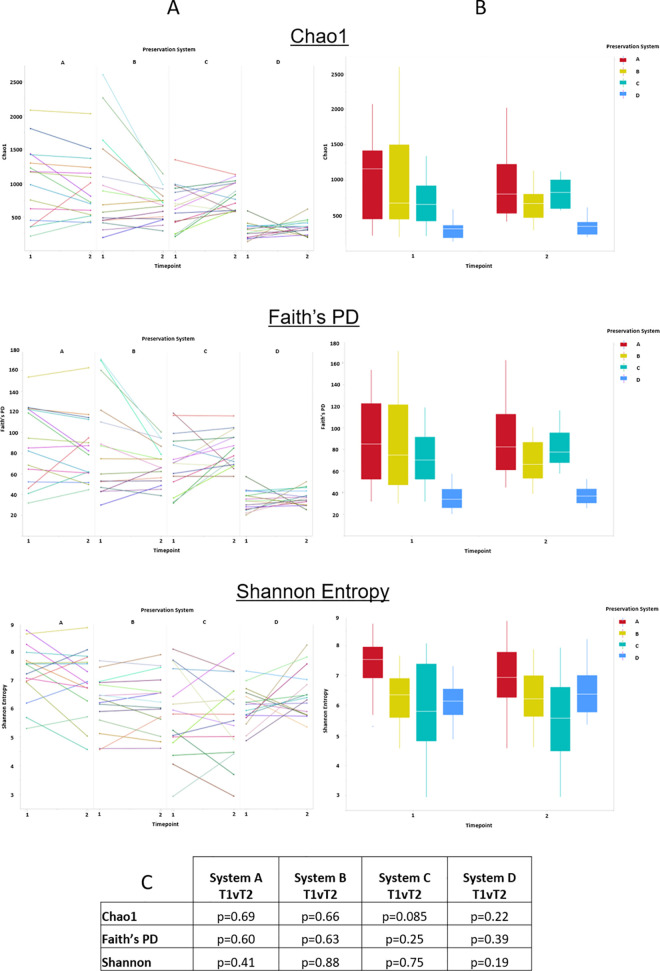
Alpha diversity analysis using 3 commonly used metrics, Chao1, Faith’s Phylogenetic Distance and Shannon Entropy were carried out to assess the impact of the different preservative systems on the leg skin microbiome following treatment. All samples were rarefied at a read count of 8000 as determined from analysis of appropriate rarefaction curves. A summary of alpha diversity analysis can be seen in Fig 2A (per subject analysis) and Fig 2B (per group analysis). For all metrics examined no significant differences were seen in alpha diversity of the leg skin microbiome following product application, Fig 2C.
Fig 2. Alpha diversity analysis of leg skin microbiome before and after formulation application.
A, Per sample alpha diversity assessment of the impact of the different preservation systems on the skin microbiome. B, Group alpha diversity assessment of the impact of the different preservation systems on the skin microbiome. C, Statistical analysis of alpha diversity changes between timepoints for each preservation system.
Beta diversity
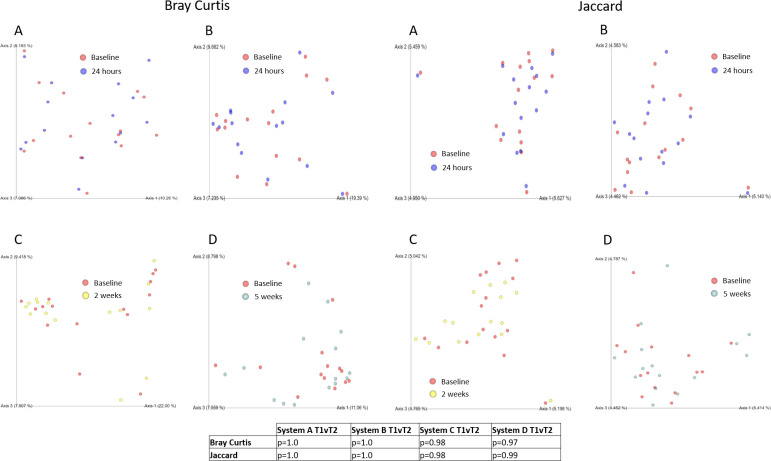
Potential changes in microbiome community structure were examined using beta diversity for all product groups. Beta diversity metrics Bray Curtis [46] and Jaccard [47] were used to determine if significant community shifts were occurring following product use. PcoA analysis of beta diversity analysis can be seen in Fig 3A–3D.
Fig 3. Beta diversity analysis of leg skin microbiome before and after formulation application.
Analysis of the impact of cosmetic products containing different preservation systems (A-D) using Bray Curtis and Jaccard Diversity metrics. Panels A-D correspond to preservation systems A-D.
No statistical differences were identified in either beta diversity metric following product application. Additional analysis (not shown) was carried out using both weighted UniFrac and unweighted UniFrac [44] diversity analysis. Neither of these metric show statistically significant shifts in community composition following product application.
Discussion
Antimicrobial preservation systems are widely used across a range of personal care products. They provide an essential function of ensuring that bacterial and fungal growth in cosmetic formulations is controlled to enable safe use of products by consumers. With the growing realisation of the importance of the human microbiome it has been hypothesized that the impact of preservatives may extend beyond the confines of the product formulation and may have a potentially detrimental impact on the skin microbiome. While preservative compounds have indeed been shown to be active against skin relevant bacteria in vitro these analyses ignore three crucial elements. Firstly, the in-use concentration of cosmetic preservatives can be drastically reduced following dilution. In the case of skin wash products this can be by up to a factor of 5-10x. At these diluted concentrations, and the limited contact time, the likelihood of antimicrobial preservatives remaining active are greatly diminished. Secondly, current in vitro antimicrobial tests neglect a key facet of the human microbiome namely its ability to respond to external insult and re-seed its composition from skin invaginations and glands that are protected from the formulation. The “microbiome resilience” of the community is essential in the restoration of microbiome structure and function following multiple external insults that our skin is exposed to on a daily basis. Finally, cutaneous substantivity, the persistent activity of an antimicrobial agent following application, is a key consideration [48]. While in vitro activity of preservative ingredients and other antimicrobial agents is obvious, their ability to bind to and remain active on skin varies considerably resulting in differences between in formulation and in use activity [49].
This work set out to examine the in vivo effects of cosmetic formulations containing different preservation systems on the skin microbiome in full formulation. Two different product formats (wash off and leave on) and three different use durations (1 day, 2 weeks, 5 weeks) were utilised and their impact on standard microbiome metrics was examined.
Species level taxonomic assessment revealed no statistical differences in community profile following product application. Fig 1 outlines major species identified however both these, and minor community members, remained consistent following application. Where between study comparisons was not a goal in this study it is worth noting that while leg skin microbiome samples were taken from all study populations some in-between study variation exists. Two study populations (B and C) had highly abundant levels of C. acnes where studies A and D had more balanced levels of dominant community members. No significant differences exist in the age of the study populations so it is unlikely that this variation can be explained by the varying ages of the study population as previously described [50]. Currently this variation between studies remains unexplained but it is consistent at both timepoints in both studies. It is additionally noteworthy that the UK cohort (Study D) showed less inter-subject variation in taxonomic diversity in comparison to the North American cohorts potentially worthy of further investigation focussing on skin microbiome variations based on geographical location aligned to previous analysis [51]. This reduced variation may also be explained by study D having additional exclusion criteria on subjects including exclusion of smokers and subjects who were peri- or post-menopausal, both elements that have recently been shown to impact the skin microbiome [52].
Alpha diversity analysis was used to determine the impact of the formulations on skin biodiversity. Using Chao1 (richness), Faith’s Phylogenetic Distance (phylogeny-based diversity) and Shannon (richness and abundance) diversity metrics it was shown that group alpha diversity metrics remained unchanged following product use. As outlined in Fig 2, visulisation of alpha diversity changes on a per subject basis shows that a subset of individuals in each of the product groups demonstrated a reduction in diversity there were also a number of subjects that showed an increase in diversity. In general, those subjects that started with higher than average diversity reduce, where those with lower than average diversity, increased.
Finally, beta diversity analysis was used to examine the overall impact on community structure of product application. Bray Curtis diversity and Jaccard diversity were used to examine community structure weighted towards dominant and sparce community members respectively. For both metrics, no significant changes were seen in community structure as a result of product application.
Taken together these data suggest that the different preservation systems in full formulation have minimal impact on the skin microbiome. Indeed, these results are in line with recent analyses examining the potential impact of soaps and antiseptic agents following cutaneous application, which only elicited a short-term microbiome alteration [53,54]. While additional analysis may be needed to assess the short-term impact of product application this analysis shows that the leg skin microbiome is not perturbed to a point where it is unable to recover to its baseline state following product use. This was the case for wash off products that are diluted before/during use but also in the case of a leave on lotion where dilution does not occur, and contact time is extended. Future investigations should examine the impact of preservative systems using methods including shotgun metagenomics, across multiple body sites, to facilitate strain level analysis of the skin microbiome and, if possible, include no-preservative controls, not possible here due to ethics board restrictions.
Conclusion
Preservative systems remain an essential component of current cosmetic formulations. They provide a vital means to ensure product stability and shelf life and play a key role in consumer safety. Work presented here suggests that fully formulated cosmetics products that contain a variety of preservative systems do not have any detrimental impact on the structure or diversity of the skin microbiome for both wash off and leave on product formats.
Supporting information
(PDF)
(PDF)
Software parameters for QIIME2 used to process and analyse metataxonomic data.
(PDF)
Software versions utilised to process and analyse metataxonomic data.
(PDF)
Acknowledgments
DNA extractions were carried out by the QIAGEN Services Laboratory, Hilden, Germany. DNA sequencing was performed by the Centre for Genomic Research, Liverpool, UK. The bioinformatics processing was completed by Eagle Genomics, Cambridge UK. The authors gratefully acknowledge Aline Metris for her critical review of the manuscript.
Data Availability
All sequencing data and appropriate metadata have been deposited in the SRA (Accession number PRJNA702440).
Funding Statement
I confirm that this work was funded by Unilever Research and Development. This does not alter our adherence to PLOS ONE policies on sharing data and materials. Barry Murphy, Michael Hoptroff, David Arnold, and Stuart Campbell-Lee contributed to or conducted the study while employed by Unilever. The funder provided support in the form of salaries for authors (BM, MH, DA, SCL), but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the Author Contributions section.
References
- 1.Grice EA, Segre JA. The skin microbiome. Nature Reviews Microbiology. 2011;9(4):244–53. doi: 10.1038/nrmicro2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakatsuji T, Chiang HI, Jiang SB, Nagarajan H, Zengler K, Gallo RL. The microbiome extends to subepidermal compartments of normal skin. Nat Commun. 2013;4. doi: 10.1038/ncomms2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallo RL. Human Skin Is the Largest Epithelial Surface for Interaction with Microbes. Journal of Investigative Dermatology. 2017;137(6):1213–4. doi: 10.1016/j.jid.2016.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stacy A, Belkaid Y. Microbial guardians of skin health. Science. 2019;363(6424):227–8. doi: 10.1126/science.aat4326 [DOI] [PubMed] [Google Scholar]
- 5.Eisenstein M. The skin microbiome and its relationship with the human body explained. Nature. 2020;588(7838):S210–s1. Epub 2020/12/18. doi: 10.1038/d41586-020-03524-6 . [DOI] [PubMed] [Google Scholar]
- 6.Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nature Reviews Microbiology. 2018;16:143. doi: 10.1038/nrmicro.2017.157 [DOI] [PubMed] [Google Scholar]
- 7.Samaras S, Hoptroff M. Book Chapter—The Microbiome of Healthy Skin in Skin Microbiome Handbook: From Basic Research to Product Development 2020. [Google Scholar]
- 8.Prouty SM, Pappas A. Sapienic Acid: Species-Specific Fatty Acid Metabolism of the Human Sebaceous Gland. In: Pappas A, editor. Lipids and Skin Health. Cham: Springer International Publishing; 2015. p. 139–57. [Google Scholar]
- 9.Wille JJ, Kydonieus A. Palmitoleic acid isomer (C16:1delta6) in human skin sebum is effective against gram-positive bacteria. Skin pharmacology and applied skin physiology. 2003;16(3):176–87. Epub 2003/04/05. doi: 10.1159/000069757 . [DOI] [PubMed] [Google Scholar]
- 10.Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature. 2010;465(7296):346–9. Epub 2010/05/21. doi: 10.1038/nature09074 . [DOI] [PubMed] [Google Scholar]
- 11.Cogen AL, Yamasaki K, Muto J, Sanchez KM, Crotty Alexander L, Tanios J, et al. Staphylococcus epidermidis antimicrobial delta-toxin (phenol-soluble modulin-gamma) cooperates with host antimicrobial peptides to kill group A Streptococcus. PLoS One. 2010;5(1):e8557. Epub 2010/01/07. doi: 10.1371/journal.pone.0008557 ; PubMed Central PMCID: PMC2796718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Science Translational Medicine. 2017;9(378):eaah4680. doi: 10.1126/scitranslmed.aah4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bitschar K, Sauer B, Focken J, Dehmer H, Moos S, Konnerth M, et al. Lugdunin amplifies innate immune responses in the skin in synergy with host- and microbiota-derived factors. Nature Communications. 2019;10(1):2730. doi: 10.1038/s41467-019-10646-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitz-Gibbon S, Tomida S, Chiu B-H, Nguyen L, Du C, Liu M, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. Journal of Investigative Dermatology. 2013;133(9):2152–60. doi: 10.1038/jid.2013.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakatsuji T, Gallo RL. The role of the skin microbiome in atopic dermatitis. Annals of Allergy, Asthma & Immunology. 2019;122(3):263–9. doi: 10.1016/j.anai.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimshaw SG, Smith AM, Arnold DS, Xu E, Hoptroff M, Murphy B. The diversity and abundance of fungi and bacteria on the healthy and dandruff affected human scalp. PLOS ONE. 2019;14(12):e0225796. doi: 10.1371/journal.pone.0225796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James AG. Book Chapter—The Axillary Microbiome and its Relationship with Underarm Odor in Skin Microbiome Handbook: From Basic Research to Product Development 2020. [Google Scholar]
- 18.Minhas GS, Bawdon D, Herman R, Rudden M, Stone AP, James AG, et al. Structural basis of malodour precursor transport in the human axilla. eLife. 2018;7:e34995. doi: 10.7554/eLife.34995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grice EA, Dawson TL. Host–microbe interactions: Malassezia and human skin. Current Opinion in Microbiology. 2017;40:81–7. doi: 10.1016/j.mib.2017.10.024 [DOI] [PubMed] [Google Scholar]
- 20.Park M, Cho Y-J, Lee YW, Jung WH. Understanding the Mechanism of Action of the Anti-Dandruff Agent Zinc Pyrithione against Malassezia restricta. Scientific reports. 2018;8(1):12086–. doi: 10.1038/s41598-018-30588-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traupe B, Fölster H, Max H, Schulz J. Effective axillary malodour reduction by polyquaternium-16-containing deodorants. Int J Cosmet Sci. 2017;39(2):141–8. Epub 2016/08/11. doi: 10.1111/ics.12358 . [DOI] [PubMed] [Google Scholar]
- 22.Oh J, Byrd AL, Park M, Program NCS, Kong HH, Segre JA. Temporal Stability of the Human Skin Microbiome. Cell. 2016;165(4):854–66. doi: 10.1016/j.cell.2016.04.008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillebrand GG, Dimitriu P, Malik K, Park Y, Qu D, Mohn WW, et al. Temporal Variation of the Facial Skin Microbiome: A 2-Year Longitudinal Study in Healthy Adults. Plastic and reconstructive surgery. 2021;147(1s-2):50s–61s. Epub 2020/12/22. doi: 10.1097/PRS.0000000000007621 . [DOI] [PubMed] [Google Scholar]
- 24.Relman DA. The human microbiome: ecosystem resilience and health. Nutr Rev. 2012;70 Suppl 1(Suppl 1):S2–S9. doi: 10.1111/j.1753-4887.2012.00489.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McBain AJ, O’Neill CA, Amezquita A, Price LJ, Faust K, Tett A, et al. Consumer Safety Considerations of Skin and Oral Microbiome Perturbation. Clinical microbiology reviews. 2019;32(4). Epub 2019/08/02. doi: 10.1128/cmr.00051-19 ; PubMed Central PMCID: PMC6750131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neza E, Centini M. Microbiologically Contaminated and Over-Preserved Cosmetic Products According Rapex 2008–2014. Cosmetics. 2016;3(1):3. doi: 10.3390/cosmetics3010003 [DOI] [Google Scholar]
- 27.Halla N, Fernandes IP, Heleno SA, Costa P, Boucherit-Otmani Z, Boucherit K, et al. Cosmetics Preservation: A Review on Present Strategies. Molecules (Basel, Switzerland). 2018;23(7). Epub 2018/07/01. doi: 10.3390/molecules23071571 ; PubMed Central PMCID: PMC6099538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ISO. Microbiology—Guidelines for the risk assessment and identification of microbiologically low-risk products. 2017. https://www.iso.org/obp/ui/#iso:std:iso:29621:ed-2:v1:en.
- 29.March GA, Garcia-Loygorri MC, Eiros JM, Bratos MA, Ortiz de Lejarazu R. Chapter 18—Microbiological Quality in Cosmetics. In: Salvador A, Chisvert A, editors. Analysis of Cosmetic Products (Second Edition). Boston: Elsevier; 2018. p. 585–97. [Google Scholar]
- 30.Smith CN, Alexander BR. The relative cytotoxicity of personal care preservative systems in Balb/C 3T3 clone A31 embryonic mouse cells and the effect of selected preservative systems upon the toxicity of a standard rinse-off formulation. Toxicology in vitro: an international journal published in association with BIBRA. 2005;19(7):963–9. Epub 2005/08/02. doi: 10.1016/j.tiv.2005.06.014 . [DOI] [PubMed] [Google Scholar]
- 31.Kerdudo A, Fontaine-Vive F, Dingas A, Faure C, Fernandez X. Optimization of cosmetic preservation: water activity reduction. Int J Cosmet Sci. 2015;37(1):31–40. Epub 2014/09/27. doi: 10.1111/ics.12164 . [DOI] [PubMed] [Google Scholar]
- 32.Lundov MD, Moesby L, Zachariae C, Johansen JD. Contamination versus preservation of cosmetics: a review on legislation, usage, infections, and contact allergy. Contact dermatitis. 2009;60(2):70–8. Epub 2009/02/12. doi: 10.1111/j.1600-0536.2008.01501.x . [DOI] [PubMed] [Google Scholar]
- 33.EC. Regulation (EC) No 1223/2009 of the European Parliament and of the Council. 2009. https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32009R1223.
- 34.Lundov MD, Johansen JD, Zachariae C, Moesby L. Low-level efficacy of cosmetic preservatives. Int J Cosmet Sci. 2011;33(2):190–6. Epub 2011/01/29. doi: 10.1111/j.1468-2494.2010.00619.x . [DOI] [PubMed] [Google Scholar]
- 35.Lambert RJW, Pearson J. Susceptibility testing: accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. Journal of Applied Microbiology. 2000;88(5):784–90. doi: 10.1046/j.1365-2672.2000.01017.x [DOI] [PubMed] [Google Scholar]
- 36.Wang Q, Cui S, Zhou L, He K, Song L, Liang H, et al. Effect of cosmetic chemical preservatives on resident flora isolated from healthy facial skin. Journal of cosmetic dermatology. 2019;18(2):652–8. Epub 2018/12/15. doi: 10.1111/jocd.12822 . [DOI] [PubMed] [Google Scholar]
- 37.Williamson P, Kligman AM. A new method for the quantitative investigation of cutaneous bacteria. J Invest Dermatol. 1965;45(6):498–503. Epub 1965/12/01. doi: 10.1038/jid.1965.164 . [DOI] [PubMed] [Google Scholar]
- 38.Adams SE, Arnold D, Murphy B, Carroll P, Green AK, Smith AM, et al. A randomised clinical study to determine the effect of a toothpaste containing enzymes and proteins on plaque oral microbiome ecology. Sci Rep. 2017;7:43344. Epub 2017/02/28. doi: 10.1038/srep43344 ; PubMed Central PMCID: PMC5327414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet C, Al-Ghalith GA, et al. QIIME 2: Reproducible, interactive, scalable, and extensible microbiome data science. PeerJ Preprints. 2018;6:e27295v2. doi: 10.7287/peerj.preprints.27295v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–3. Epub 2016/05/24. doi: 10.1038/nmeth.3869 ; PubMed Central PMCID: PMC4927377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Hebshi NN, Nasher AT, Idris AM, Chen T. Robust species taxonomy assignment algorithm for 16S rRNA NGS reads: application to oral carcinoma samples. Journal of Oral Microbiology. 2015;7:10.3402/jom.v7.28934. doi: 10.3402/jom.v7.28934 PMC4590409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen T, Yu W-H, Izard J, Baranova OV, Lakshmanan A, Dewhirst FE. The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database: the journal of biological databases and curation. 2010;2010:baq013. MEDLINE:20624719. doi: 10.1093/database/baq013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sas Institute Inc CNC. JMP. 14.1 ed.
- 44.Lozupone C, Knight R. UniFrac: a New Phylogenetic Method for Comparing Microbial Communities. Applied and Environmental Microbiology. 2005;71(12):8228. doi: 10.1128/AEM.71.12.8228-8235.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandal S, Van Treuren W, White RA, Eggesbo M, Knight R, Peddada SD. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microbial ecology in health and disease. 2015;26:27663. Epub 2015/06/02. doi: 10.3402/mehd.v26.27663 ; PubMed Central PMCID: PMC4450248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sørensen TJ. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. København: I kommission hos E. Munksgaard; 1948. [Google Scholar]
- 47.Jaccard P. Nouvelles recherches sur la distribution florale. Lausanne: Rouge; 1908. [Google Scholar]
- 48.Nishioka H, Nagahama A, Inoue Y, Hagi A. Evaluation of fast-acting bactericidal activity and substantivity of an antiseptic agent, olanexidine gluconate, using an ex vivo skin model. Journal of Medical Microbiology. 2018;67(12):1796–803. doi: 10.1099/jmm.0.000870 [DOI] [PubMed] [Google Scholar]
- 49.Li BS, Cary JH, Maibach HI. Stratum corneum substantivity: drug development implications. Archives of dermatological research. 2018;310(7):537–49. doi: 10.1007/s00403-018-1841-9 [DOI] [PubMed] [Google Scholar]
- 50.Shibagaki N, Suda W, Clavaud C, Bastien P, Takayasu L, Iioka E, et al. Aging-related changes in the diversity of women’s skin microbiomes associated with oral bacteria. Scientific reports. 2017;7(1):10567–. doi: 10.1038/s41598-017-10834-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta VK, Paul S, Dutta C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Frontiers in Microbiology. 2017;8(1162). doi: 10.3389/fmicb.2017.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carrieri AP, Haiminen N, Maudsley-Barton S, Gardiner LJ, Murphy B, Mayes AE, et al. Explainable AI reveals changes in skin microbiome composition linked to phenotypic differences. Sci Rep. 2021;11(1):4565. Epub 2021/02/27. doi: 10.1038/s41598-021-83922-6 ; PubMed Central PMCID: PMC7907326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.SanMiguel AJ, Meisel JS, Horwinski J, Zheng Q, Bradley CW, Grice EA. Antiseptic Agents Elicit Short-Term, Personalized, and Body Site–Specific Shifts in Resident Skin Bacterial Communities. Journal of Investigative Dermatology. 2018;138(10):2234–43. 10.1016/j.jid.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Two AM, Nakatsuji T, Kotol PF, Arvanitidou E, Du-Thumm L, Hata TR, et al. The Cutaneous Microbiome and Aspects of Skin Antimicrobial Defense System Resist Acute Treatment with Topical Skin Cleansers. Journal of Investigative Dermatology. 2016;136(10):1950–4. doi: 10.1016/j.jid.2016.06.612 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Software parameters for QIIME2 used to process and analyse metataxonomic data.
(PDF)
Software versions utilised to process and analyse metataxonomic data.
(PDF)
Data Availability Statement
All sequencing data and appropriate metadata have been deposited in the SRA (Accession number PRJNA702440).