Abstract
Left ventricle (LV) pacing can be considered peculiar due to its different lead/tissue interface (epicardial pacing) and the small vein wedging lead locations with less reliable lead stability. The current technologies available for LV capture automatic confirmation adopt the evoked response (ER), as well as “LV pace to right ventricular (RV) sense” algorithms. The occurrence of anodal RV capture is today completely solved by the use of bipolar LV leads, while intriguing data are recently published regarding the unintentional LV anodal capture beside the cathodal one, which may enlarge the front wave of cardiac resynchronization therapy (CRT) delivery. The LV threshold behavior over time leading to ineffective CRT issues (subthreshold stimulation or concealed loss of capture), the extracardiac capture with phrenic nerve stimulation (PNS), the flexible electronic cathode reprogramming and the inadequate CRT delivery related to inadequate AV and VV pace timing (and its management by LV “dromotropic pace-conditioning”) are discussed.
Moreover, recently, His bundle pacing (HBP) and left bundle branch pacing (LBBP) have shown growing interest to prevent pacing-induced cardiomyopathy as well as for direct intentional CRT.
The purpose of the present review is to explore these new challenges regarding LV pacing starting from old concepts.
Keywords: Left Ventricle Pacing Challenges
Highlights
-
•
The left ventricle (LV) pacing can be considered peculiar due to its different lead/tissue interface (epicardial pacing) and the small vein wedging lead locations with less reliable lead stability.
-
•
The current technologies available for LV capture automatic confirmation adopt the evoked response (ER), as well as “LV pace to right ventricular (RV) sense” algorithms.
-
•
The occurrence of anodal RV or LV capture, the subthreshold stimulation or concealed loss of capture and finally, the inadequate pacing CRT delivery related to inadequate atrio-ventricular or RV to LV pace timing are discussed.
1. Introduction
Cardiac electrical resynchronization has completely changed the care of patients with reduced ejection fraction and electrical dyssynchrony. In late 90s, CRT became available and proved to ameliorate patients’ outcome; however, patient selection as well as post implant pacing CRT optimization are important variables, since one third of CRT patients are non-responder. The innovations of pacing algorithms may improve CRT delivery as well as increase technical challenges [1].
Automatic threshold test is also now available for LV pacing and includes the ER and other dedicated algorithms for capture confirmation [[2], [3], [4], [5], [6]]. With this regard, an increased device longevity and a fully pacing automaticity are associated with a better cost-effectiveness of cardiac resynchronization therapy-defibrillators (CRT-D) [[7], [8], [9], [10]].
Intriguing data regarding a useful though unintentional LV anodal capture beside the cathodal one have been recently published. Moreover, new peculiar issues have to be addressed regarding the LV threshold behavior over time (leading to intermittent ineffective CRT), and the extracardiac capture (which may be fixed by a flexible electronic cathode reprogramming). Inadequate CRT delivery may also be related to inadequate atrio-ventricular (A-V) and right ventricular-left ventricular (V–V) pace timing.
Moreover, recent data have highlighted the HBP and LBBP in the setting of preventing the pacing-induced cardiomyopathy, due to standard right ventricle pacing. On these basis, emerging data are now suggesting these pacing techniques for direct intentional CRT as well.
Our aim is to describe some pacing management features, including some technical and peculiar challenges.
2. The LV automatic capture confirmation
The technologies for the LV capture automatic confirmation are based on ER, available for Boston Scientific, Biotronik and Abbott Medical devices, or “LV pace to RV sense” analysis, available only for Medtronic devices. The Microport and the MedicoPace CRT devices are not yet equipped with ER based auto-threshold algorithm [[11], [12], [13], [14], [15]].
It should be underlined that, unlike most of the RV automatic threshold measurement algorithms, all the LV automatic threshold tests adopted by all CRT systems do not run on a “beat to beat” fashion, being a programmable safety pacing margin available. Moreover, the following algorithms have never been suggested neither they have been validated for alternative CRT techniques, such as His bundle or left bundle branch pacing (see section 5).
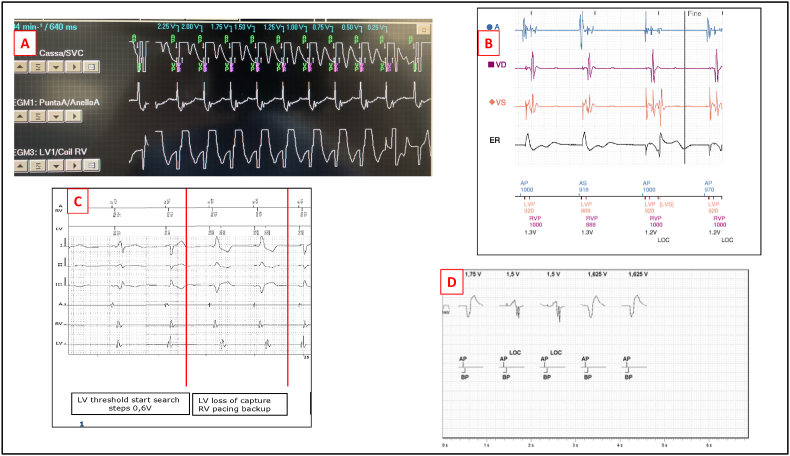
The main features are summarized in Table 1 and Fig. 1.
Table 1.
LV automatic algorithms according to manufacturers.
| LV lead auto-threshold features | ABBOTT (LVCapConfirm) | MEDTRONIC (LVCM) | BIOTRONIK (VCC) | BOSTON SCIENTIFIC (PaceSafe LVAT) |
|---|---|---|---|---|
| Default enabled at implant | no | yes | yes | no |
| Algorithm | ER | LV pace to RV sense | ER | ER |
| ER Analysis | Area under the curve | / | Peak timing | Peak timing and first peak morphology/amplitude |
| Capacitor special setup | no | / | no | No for the LV threshold |
| Mode of programming | ON/Monitor /OFF |
Adapted/Monitor/OFF | ON/Monitor /OFF |
ON/Daily Trend/OFF |
| LV Pacing polarity | All LV pacing vectors are programmable | All LV pacing vectors are programmable | All LV pacing vectors are programmable | All LV pacing vectors are programmable |
| Simultaneous pacing from multiple LV electrodes for quadripolar leads (only recent models) | yes | yes | yes | yes |
| Safety margin | Programmable form 0.25V to 2.5V (1 V nominal) | Programmable +0.5 to + 2.5 or “AUTO” |
Programmable: 1V, 1.2V (1V nominal) |
Programmable from 0.5 V to 2.5V (1V nominal) |
| Test Time Interval | Every 8 h o 24 h | Daily at 1:00 a.m. | Intervals (0,1; 0,3; 1; 3; 6; 12; 24 h) Time (00:00.23:50 hh:mm, nominal 00:30) |
Every 21 h |
| Back-up pulse during the test | 5V RV pulse | same amplitude but @1 ms |
5V@1 ms | 5V RV pulse |
| Pulse width | Programmable | 0.4 ms or 1.5 ms | max 0.4 ms | Programmable |
| Diagnostics | yes | yes | yes | yes |
| Available on models | All models | All models | All, but not for the models: Ilesto, Idova and the 3-Series | Only for the models: Resonate, Perciva, Charisma, Vigilant, Momentum, Autogen |
| VVT and DDT mode available | yes | no | no | no |
LV = left ventricle; RV = right ventricle, ER = evoked response; LVcapConfirm, LVCM, VCC and PaceSafe LVAT are the manufacturers’ algorithms for LV capture tests; MPP = multipoint pacing; V = volt.
Fig. 1.
LV lead threshold management according to manufacturer
Fig. 1 Legend:
Panel A: LV automatic threshold test for Medtronic devices (Vector Express), displaying loss of capture with intrinsic RV conduction. The channels displayed are: EGM 1 = atrial channel; ECG = the “large” RV dipole (coil to can) with A-V markers; EGM 3 = the “LV cathode–RV coil” dedicated channel (the same used for the “EffectivCRT” algorithm) displaying the “QR” morphology during LV capture and the “RS” morphology as loss of capture occurs at the ninth beat of the test (RV sense).
Panel B LV auto-threshold for Boston Scientific devices, with the ER dedicated channel showing the pacing artifact changes corresponding to the loss of capture (LOC)
Panel C: LV auto-capture test for Biotronik devices, with RV back up pacing occurring at loss of capture during the 0.6 V step threshold search. The red arrow in the left side of the tracing indicates RV and LV synchronous pacing; the red arrow in the right side of the tracing indicates the sequential RV to LV during back up RV pacing, as loss of LV capture occurs.
Panel D: the algorithm LV ACap Confirm (Abbot Medical devices), adopts the ER by comparing the polarization artifact. to confirm capture. Tracings available in the patients’ data by remote monitoring.
2.1. Capture confirmation by ER
The ER is currently the most used parameter adopted by the majority of CIED manufacturers to confirm myocardium capture in both ventricles, with the exception of the RV sensed LV capture confirmation adopted by Medtronic systems (Fig. 1, panels B–C -D).
In 2007, Biffi et al. published their data about the feasibility of transvenous LV pacing in patients requiring ‘conventional’ dual chamber pacing, without class I CRT indication. The study relates to the possibility of using the same auto-capture technology currently employed in RV pacing, for this peculiar left epicardial pacing site and, even though the number of the recruited patients was quite low, the authors demonstrated that a reliable LV ER might allow the use of the same algorithm as for RV automatic capture management. Their data showed that no special requirements were needed to reliably detect LV ER as well as for RV ER. These results had been confirmed both at the end of implant and over long term. In their study, the pacing algorithm provided beat-to-beat capture verification outputs only 0.5 V above threshold. ER needed to be reliably distinguished by the polarization artifact at the tissue-electrode interface: the authors stated that the reduced (2.2 μF) coupling capacitor technology of the pacemaker used (Insignia DDD - Boston Scientific), might speed the slope of decay time of the post-pacing artifact, thus allowing reliable ER detection regardless of pacing configuration and, importantly, of the pacing lead technology [[15], [16], [17]]. Alternatively, a technology based on independent vectors for LV pacing and ER detection (other than a specific capacitor set-up), proved to be superior, but a bipolar LV lead was mandatory for this purpose.
Thus, the major technical obstacle for the conventional auto-threshold testing using the ER detection is the magnitude of pacing induced artifact, which is linearly related with the capacitor coupling technology.
Early in the past decade, a milestone paper by Sperzel et al. focused on this issue, using a custom-made external pacing system equipped with a 10- μF pacing storage capacitor and a 2.2- μF coupling capacitor, in series, which is equivalent to an output capacitance of 1.8 μF. They evaluated the ER response in both bipolar and unipolar leads; both acutely and chronically implanted leads were included. Capture verification was based on peak amplitudes measured within a time window from 10 to 64 ms after the pacing stimulus. The polarization artifact decay is determined by the time constant formed by the product of the coupling capacitor and the load (combination impedance of lead, electrode to tissue interface, and myocardium). If the capacitance is reduced, the artifact initially larger, dissipates faster and, consequently a further capacitance reducing will lead to a much faster return to the baseline of the intracardiac signals, that will even advance the onset of ER. They demonstrated that pacing output capacitance can be safely reduced to 1.8 μF allowing an effective ER sensing with minimal changes in the pacing threshold [18].
2.2. Capture confirmation by RV sensing
The Left Ventricular Capture Management™ is an automatic algorithm, adopted by Medtronic devices, which monitors the pacing amplitude threshold and adjusts LV outputs, by means of RV sensing (Fig. 1, panel A).
During the test pace, the ventricular pacing configuration switches to LV-only. The test looks for an RV sense to determine capture in the following pacing mode:
-
1)
Capture is confirmed when LV-only pacing is delivered, and RV sensing is detected shortly after the LV pace (to check for a V–V conduction), in case of sinus rhythm as well as atrial fibrillation.
-
2)
Loss of capture is confirmed when LV-only pacing is delivered, and an RV sense is detected at the intrinsic AV interval (conduction from atrial pace or sense) or when an RV sense is not detected (pacemaker dependent patients).
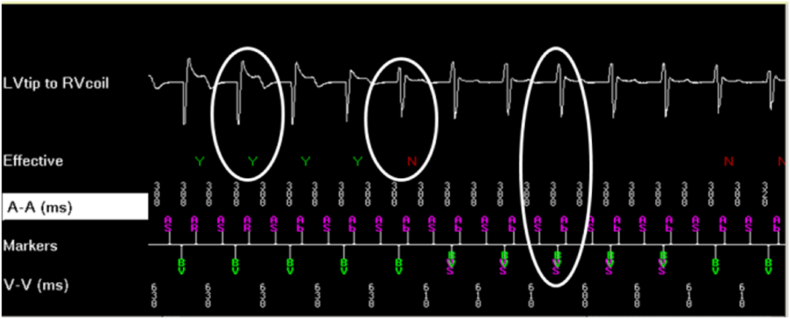
A recent algorithm, which evaluates the morphology of the LV EGM to determine, beat to beat, an effective LV capture, has been also assessed. The algorithm analyzes the morphology of a dedicated unipolar LV EGM (for CRTD LV cathode–RV coil EGM, for CRTP LV cathode-can) during biventricular or LV pacing. The algorithm was validated as compared to a 12-lead surface ECG with 98% sensitivity for effectiveness [5,11]. (Fig. 2)
Fig. 2.
Left ventricle capture confirmation by “EffectivCRT Diagnostic” algorithm
Fig. 2 legend: dedicated unipolar LV EGM (LV cathode–RV coil EGM) during biventricular or LV pacing; until the third paced beat a “Qr” morphology indicate the effective LV capture; the fourth beat represent a kind of fusion and the further beats are represented by an “rS” morphology indicating the loss of LV capture. (with permission by Medtronic).LV = left ventricle; RV = right ventricle; Y = LV effective OK; N = loss of LV effective capture; AS = atrial sense; Ab = atrial blanking; VS = ventricular.
The EffectivCRT™ Diagnostic algorithm is now available in some Medtronic CRT-D systems; it analyzes 100 consecutive ventricular events every hour and determines the percentage of effective CRT pacing. All encountered ventricular safety pacing (back up paced events) and/or ventricular sensing events noted in the 100 consecutive ventricular events, are reject. During data collection, the device transiently switches to the dedicated LV to RV coil unipolar EGM [19].
3. The cathodal-anodal coupling
In the early era of unipolar LV lead, the anodal part of the pacing circuit was set in the shocking coil or the RV lead ring. In the last decade, there were only few papers addressing the problem of an anodal myocardium capture for CRT-P systems (with LV tip to RV ring configuration) as well as CRT-D systems, a rare phenomenon which could be under-recognized and therefore under-reported. The RV leads used in these latter systems are different from standard pacemaker leads due to different design, for the presence of shocking coils and for the diameter; moreover, an active fixation system is mostly used. Thibault et al., found an evidence of the anodal capture during LV stimulation in 11 of 11 patients (100%) with a RV defibrillation lead with true bipolar design and in none of the 15 patients with a system using an integrated design for RV pacing. The most likely explanation for this discrepancy may be the higher current density associated with the smaller surface of the proximal RV ring with the first design, compared to the much larger surface of the distal RV shocking coil used with the second design. The authors also state that, because of the small number of patients included in the study, anodal capture cannot be excluded for integrated lead technology as well. The anodal occurrence might be under-recognized also for the current bi or quadripolar lead technology and ignoring it could lead to inadequate device programming. In inexperienced hands, loss of anodal capture could be mistaken with the loss of LV cathodal capture and, therefore, LV “pure" threshold could be overestimated. The ECG changes associated with anodal capture are more easily noticed in lead V1, even though a12-lead recordings are more helpful.
Another important observation relates to the differential effects of pulse width variation on capture thresholds of the RV anode and LV cathode. In the previously mentioned study by Thibault, it was found that decreasing the pulse duration had more significant effects on the anodal threshold compared to the LV cathodal threshold; this difference should always be searched and measured and could be used in some patients to overcome the narrow safety margin between LV and anodal threshold [20,21].
In a recent paper Dell’Era et al. showed that a “pure” anodal capture from multiple electrodes of the LV lead might occur in quadripolar LV leads. The authors stated that the myocardium located below the epicardium of the left ventricle could also be captured by the anode of the stimulating dipole, provided that the programmed energy was above the anodal threshold. With this regard, indeed they found a higher LV threshold of the latter, for each specific dipole [22].
Thus, even though, in the era of unipolar LV leads, anodal capture has been regarded as an undesirable CRT, determining “double capture” from RV lead, several methods have been proposed in order to avoid it (coil-integrated RV ICD lead dipole, as first). Some authors have also suggested that adding an intentional RV anodal capture to CRT pacing could improve resynchronization and narrow the QRS complex [[16], [17], [18], [19], [20], [21], [22]].
Interestingly it has also been speculated about the shortening of the local mechanical systole (probably by a larger tissue recruitment), arguing whether, the newer commercially available multipoint LV CRT systems may give the same clinical and electrical advantage as an unintentional concomitant cathodal and anodal capture do in traditional CRT leads. Moreover, in some cases, the cathodal/anodal capture inevitably occurs for a given LV dipole; indeed, when the anodal threshold of the programmed anode is lower than the output programmed for the cathode, unintentional “multipoint” stimulation is obtained [[22], [23], [24]].
Regarding anodal stimulation, another potential field of interest may be the direct effect of the new cathode-anode configuration on myocardial contractility. Some authors have observed an increase in ventricular function and contraction in animal models during unipolar anodal stimulation, when mediated by tissue hyperpolarization before depolarization [[25], [26], [27], [28]].
4. LV pacing counter vs CRT effectiveness
4.1. - Ineffective CRT due to pacing-timing
The causes of ineffective CRT can be related to pacing timing other than pacing capture issues.
The former can occur by fusion or pseudo-fusion paced complexes, in case of sensed atrio - ventricular interval, with preserved or with frequent changes of dromotropic properties of nodal or infranodal His Purkinje conduction. Both conditions, can be corrected by A-V reprogramming to obtain an AV “dromotropic pace conditioning”.
In other cases, during simultaneous biventricular pacing, the dominant contribution of RV pacing front wave, may render the LV tissue somehow refractory (regionally or temporally) with transient loss or just local capture. This can be fixed by selecting a different LV pacing electrode in multipolar leads, or, more intriguingly, by enhance a LV pacing pre-excitation by 10 up to 80 msec (advance the LV capture, thus reducing the LV recruitment by RV sensed or paced activation, in a kind of “dromotropic RV to LV pace conditioning”).
Finally, a rate competitive atrial fibrillation (AF) or a high daily burden of premature ventricular contractions (PVC) may also affect the CRT, by means of fusion or pseudo-fusion captures as well. In this case little can be done by re-programming. In atrial fibrillation patients, with adequate dromotropic drug control, but highly variable R-R cycles, it may be discussed whether a triggered VVT pacing mode, including the “LV pre-excitation” may improve a LV capture by increasing the fusion captures instead of pseudo-fusion complexes. Based on this concept, most modern devices have “VVT-like” (V sensed-triggered) features designed to increase effective pacing during AF and to respond to PVCs; however, few of these devices have been shown to substantially increase CRT pacing percentage, and none has been evaluated for effective CRT pacing. Anyway, in AF patients with difficult rate control, on top of rate-controlling drugs, the AV node ablation remains the first choice, to ensure effective CRT delivery [29,30].
The timely delivery of LV pacing and eventual LV-RV preexcitation, should be addressed by direct electrogram interventricular delay, during the CRT implant. Patients with stricter LBBB criteria have shown to have a better outcome, as compared with RBBB and nonspecific intraventricular conduction delay patterns. This underlines that the QRS absolute width itself may not reflect the real pattern of conduction delay to the LV, which may be measured by the Q-LV interval. The Q-LV interval is defined as the interval from the onset of the intrinsic QRS on the 12-lead surface ECG to the first large positive or negative peak of the LV EGM [31,32].
In a recent paper by Pastore et al., it has been reported that the Q-LV measure is able to detect a highly prolonged LV conduction delay in patient presenting stricter criteria for LBBB; such delay is just limited to the RV in RBBB patients. More important and quite unexpected, patients with nonspecific intraventricular conduction delay showed a very high variability in Q-LV interval, from a poor to a very long, being the latter better CRT candidate. Moreover, patients with an ECG pattern resembling RBBB in lead V1, but without the terminal S waves in the lateral limb ECG leads (I and aVL), presented an unexpectedly long Q-LV interval [31].
The above discussed ECG and echocardiographic outcomes represent the routine periprocedural target in the daily practice, easily guided by the Q-LV delay; this interval has shown to be a strong predictor also for the acute hemodynamic response. In a recent and interesting study by Van Gelder et al., a direct LV dP/dt max measure has been performed at CRT implant. An acute 15% increase in LV dP/dt max corresponded to a better hemodynamic response and correlated with the longest Q-LV measured interval, leading to LV lead repositioning into different coronary sinus tributaries, guided by that hemodynamic index. Interestingly, the LV endocardial pacing opposite to those epicardial sites did not show acute hemodynamic improvement; this led the authors to state that the epicardial pacing at the optimal site may be a good alternative, avoiding the risk of an endocardial LV lead placement, even though, a validated LV dP/dt cut off still remain uncertain [32].
In summary, the pacing timing may affect the effective CRT delivery in several ways, this should be addressed at implant (inter-ventricular EGM delay) as well as along the follow up by ECG changes. In patients with stricter LBBB criteria, the search for LV fusion with intrinsic right bundle branch conduction, may need a LV offset (preexcitation) which may be empirical (QRS width guided) or speculatively set based on the intrinsic RV activation via right bundle branch (therefore considering the HV interval). Over time, some LBBB patients may develop complete AV block, by losing the right bundle branch intrinsic conduction, becoming pace dependent; in such cases, it may be discussed whether an “extensive” LV offset may offer a better resynchronization compared with simultaneous biventricular pacing.
4.2. - LV pacing threshold fluctuation over time
Beside some accepted clinical characteristics associated with improved CRT response, such as nonischemic etiology of the cardiomyopathy or a stricter definition of left bundle branch block ECG criteria, there is still a need for additional markers to identify non responders as early as possible [28,29]. Among potential determinants, LV lead position away from myocardial scar areas has been shown to play an important role. With this regard, a high LV pacing threshold may be a marker of inadequate lead positioning and thus a risk for future lead dislodgement, as well as a manifestation of diseased myocardium both resulting in loss or even suboptimal LV pacing [33].
In a recent paper, Pires et al. reported a sub-analysis of data from a MADIT CRT randomized trial, concerning a significant relationship between LV lower threshold and echocardiographic and clinical outcome in CRT-D patients. Higher LV threshold was associated with substantially lower left atrium and LV long term reverse remodeling [34].
In the daily practice, the placement of LV lead at the latest activated segment or away from scar areas seems to be associated to better clinical outcome, but scar localization requires specialized imaging techniques that may not be preoperatively available [[35], [36], [37], [38]]. Moreover, the lead positioning at a pre-specified site is not always feasible due to the lack of suitable coronary sinus anatomy, lead stability or PNS.
The further rise in LV threshold, in patients with an already elevated threshold, may lead to loss of LV effective pacing; in these cases, a traditional threshold safety margin of two-to three-fold of output during “in office” device programming, may not be enough [[39], [40], [41]].
What is already known is that the LV pacing percentage relates linearly with outcome.
Koplan et al. compared patients’ outcomes and LV pacing percentage and reported a 44% reduction in the risk of death or HF hospitalizations among those paced >92% [39]. However the worst outcome of patients with higher LV threshold (1.8 V) could be better explained by the progression of the disease which causes a change in LV threshold, rather than by the of loss of biventricular pacing, as suggested by Pires et al. [34].
In this view, the automatic threshold measurement beside assuring an effective LV capture, could be a marker of electrical viability of the paced region, providing information on the progression of the disease, thus leading to a close follow up.
In summary, the efficacy of CRT and its overall percentage over time, may be reduced due to pacing inhibition (by sensed LV activity) or inadequate capture due to subthreshold current or pacing into refractory tissue. These considerations may weaken the predictive power of traditional LV pacing percentage counter and, it may be discussed if an RV sensed (V to V interval counter) based LV - auto threshold algorithm could be superior to a traditional ER technology to detect effective LV capture. An intermittent loss of LV capture may also represent an important contributor to ineffective pacing, and a mere LV pacing percentage counter may overestimate this data.
The EffectivCRT™ algorithm, above described, may be helpful to fix such a pacing/capture mismatch.
5. Alternative resynchronization
5.1. His bundle/left bundle branch pacing using the device LV connector port
Nowadays, HBP is a field of growing interest to prevent pacing-induced cardiomyopathy and it has also been proposed to be applied in the setting of CRT. In spite of its newly clinical applications, there has been minimal evolution in technologies or pacing systems dedicated to His bundle anatomy. To date, there are no HBP specific commercially available pacemakers or algorithms. New unmet pacing challenges encountered during HBP are mainly related to sensing issues (proximity atrial and His bundle EGM magnitude) and to His EGM signal amplitudes, which are lower because of the surrounding interventricular membranous septum. Moreover, depending on the pacing lead fixation site, multiple tissues may be recruited each with a distinct pacing threshold, including atrial and ventricular myocardium and His bundle itself. Briefly, at least 3 anatomical variants of the His bundle have been described by Kawashima et al.: Type I, which includes the His bundle course just along the lower border of the membranous septum, but covered with a thin layer of common myocardium fibers; Type II, which includes a course apart from the lower border of the membranous septum running within the interventricular septum muscle and, Type III with the His bundle course immediately beneath the endocardium onto the interventricular septum (so called naked His) [42].
At present, the lumen-less Medtronic 3830 lead model with an IS-1 ring and pin arrangement (combined with a dedicated delivery sheath, pre-shaped for HBP only) is the main lead model suggested for HBP, with a 1.8 mm-long active screw, the length of which may be more than what is necessary for a type III variant, but possibly not long enough for a type II. Even though a solid literature, have been collected with regard to this lead model, recently, data regarding an alternative use of standard stylet-driven lead (Solia S60–S52 Biotronik model), combined with dedicated delivery sheaths, have been reported, showing comparable acute bio-electric performance and safety profile. The latter systems introduce the standard active fixation with extendable helix lead feature in the field of HBP procedure, otherwise traditionally attempted by means of the 3830 lumen-less leads. These lead models are the same suggested also in the setting of LBBP [43,44].
On the basis of the above discussed anatomical issues, concerns are now arising regarding higher pacing thresholds, lower R-wave signals, and the possibility to develop distal conduction block have limited the clinical application of HBP in certain subgroups of patients. This is stimulating a development of the LBBP, as an alternative method for delivering physiological pacing which require a deeper lead screwing in the muscular part of the interventricular septum to reach the endocardial surface of its left side along the course of the left bundle. This technique is being developed fast, assuring a better threshold, more stable over time [[45], [46], [47]].
The current choices to get a HBP include the insertion of the Medtronic 3830 lead model pin in the atrial connector port for patients with atrial fibrillation (by using a DDD device), and the LV connector port for patient in sinus rhythm (CRT device), maintaining the RV connection for the back-up RV lead, when used. With regard to the former choice, it should be considered the possibility of gaining a sinus rhythm over time, which may require an upgrading procedure.
The following considerations on the choice between unipolar and bipolar sensing configurations became soon crucial, since standard pacemakers are widely used for HBP: myopotential oversensing in the unipolar configuration can result in inhibition, and hence should be avoided in pacemaker dependent patients, as well as the unipolar pacing configuration, which may lead to pectoralis muscle capture. On the other hand, the bipolar sensing polarity might often result in a typical smaller R-wave amplitude as compared with standard RV positions, resulting in ventricular under-sensing. On these bases, a tailored device programming is necessary for HBP, as it is mandatory to turn off the automatic threshold management algorithm with every manufacturer’s device used. Moreover, in patients with nonselective HBP, where the RV capture threshold is lower than His bundle capture, the algorithm may inappropriately program an output resulting in RV septum-only capture, while in selective HBP, the absence of an ER may result in unnecessarily high pacing outputs.
In selected patients with a selective HBP and a small “margin” to nonselective capture with higher output, a LV threshold management algorithm based on RV sensing other than ER based one, could help to maintain a safe and effective His selective pacing capture, with an RV back up lead sensing, by means of adequate programming a LV (His) pre-excitation offset. Apart from this, anyway, is suggested to turn off all the sensing capabilities for the HBP lead.
The newer interest gained by the LBBP is related to the easier lead implant, which may require less operator’s skills, as well as better intraprocedural capture threshold and sensing issues, when compared with the HBP; this may offer a more suitable condition for an eventual switch-on of automatic capture algorithms and sensing set up. Moreover, as compared with HBP, the LBBP seems to have a more reliable behavior over time which may warrant a standard device programmability optimization and generator lifetime saving.
5.2. LV endocardial pacing
Up to 30%–50% of patients do not show improvement with conventional CRT by an epicardial coronary sinus pacing.
In addition, an epicardial LV lead implantation, into the coronary sinus, is not always possible due to unavailable suitable vein tributaries or in patients undergoing an upgrade from a pre-existing device because, of central venous stenosis or occlusion. The LV endocardial pacing may represent a potential alternative for patient with failed lead delivery as well as for non-responders.
A novel commercially available wireless pacing system (WiSE-CRT system; EBR Systems, Sunnyvale, CA) delivers electrical stimulation to the LV endocardium by transducing acoustic energy from an ultrasound pulse generator implanted subcutaneously in an intercostal space. The ultrasound waves are converted into electrical stimulation energy by a small receiver electrode deployed percutaneously into the LV cavity, by means of aortic retrograde approach or even a transeptal catheterization.
In a recent post marketing Registry, the WiSE-CRT system achieved good procedural success, with endocardial pacing confirmed in 94% of patients; however, a significant device and procedural-related adverse events rate occurred. Among the 90 study patients, three procedural deaths (3.3%) have been reported; one fifth of study patients had a complication in the first month after the procedure. At 6 months, the system was associated with a favorable clinical response rate of 70%. In particular, the risk of cardiac tamponade was comparable with other left-sided vascular procedures such as left atrial appendage occlusion. The operators’ learning curve mainly affected the safety results as stated by the authors, who conclude to suggest such a difficult procedure to centers with available cardiac surgery on-site [[48], [49], [50]].
5.3. HBP-optimized resynchronization (HOT CRT)
As discussed above, HBP can narrow and sometimes correct the LBBB; this is more often observed in patients with stricter LBBB criteria, which may speculatively imply a proximal conduction system block. However, in patients with advanced cardiomyopathy a typical LBBB and intraventricular conduction defect, may coexist. In these cases, it has been reported that resynchronization may be gained when conduction system pacing (such as HBP) is delivered in conjunction with sequential LV pacing by traditional epicardial coronary sinus pacing. In a recent paper by Vijayaraman, observational data from 27 patients have been reported. The study addresses the intraprocedural Q-LV during native LBBB or RV pacing and during HBP. By ECG, the HBP resulted in significant bundle branch correction in 70% (19/27 pts) of cases, while no significant QRS narrowing was achieved in 8 patients (4 with typical LBBB). The device programming for His optimized CRT is peculiar: in patients with chronic AF, the HBP lead was connected to the atrial port, the LV lead to the LV port and the RV lead to the RV port. The pacing mode was DDD or DDI with an AV delay (His-LV delay) equal to HV or stimulus to ventricular interval. The LV offset up to 80 ms (with subthreshold RV output in nondependent patients to avoid RV apical pacing or even fusion. The atrial (HBP) sensitivity was programmed to the least sensitive setting to avoid sensing in the His lead. In patients with normal sinus rhythm undergoing CRT-D implant, the His lead was connected to the LV port and a bipolar LV lead in the pace/sense portion of RV DF-1 port. The pace-sense portion of the spliced ICD lead (DF-1) was capped. The device was programmed to DDD with LV (His)–RV (LV) delay set at HV or stimulus to ventricular interval. In patients with normal sinus rhythm undergoing CRT- P implant, the His lead was connected to the RV port and the LV lead to the LV port. The device was programmed to DDD with RV (His)-LV delay equal to HV or stimulus to ventricular interval. As discussed above, to engage LV-RV fusion with intrinsic AV and right bundle conduction, the tailoring of His to LV offset interval needs to be corrected for the HV interval, as well. In this study patients’ cohort, where the advanced heart failure begets a merged conduction system impairment (LBBB and nonspecific conduction block), a sequential CRT His optimized, has shown significant echocardiographic and clinical favorable outcomes. The technique aims to improve or achieve an intra-ventricular LV synchrony, by delivering a sequential His-LV pacing immediately after a completed RV activation (HV based) [51].
The physiologic conduction system pacing is being recently engaged also in less conventional subset of paced patients, such as the grown-up congenital heart disease. In particular, the congenitally corrected transposition of the great arteries (CCTGA), is a condition associated with cardiomyopathy occurring in up to 67% of patients due to RV maladaptation and failure (as systemic ventricle). The frequent occurrence of spontaneous atrioventricular block and need of permanent pacing accelerate the disease progression with a pacing-induced trigger. In this context, the unique anatomy of the disease may need an alternative resynchronization, other than traditional CRT, otherwise indicated. In a recent paper by Moore et al. reported data form 15 patients collected by 10 international centers. The authors give data with regard of feasibility and clinical and ECG outcomes. They also discuss as the unique congenital characteristics of CCTGA may favor an HBP approach, over a conventional CRT, given the superficial location of the distal His bundle and left bundle branches, both accessible from a venous approach [52].
6. Extracardiac capture and multiple cathode flexibility
6.1. - PNS
Due to the left phrenic nerve course, crossing the left obtuse marginal vein in almost 80% of cases it is not unlikely that phrenic stimulation is elicited when aiming at a posterior-lateral LV placement. The PNS may occur at the site of optimal LV lead in up to one-fifth of patients. Although reprogramming bipolar leads may solve it, there is often a need to revise the LV lead position and, though infrequently, a refractory PNS may lead to turned off the CRT delivery [53,54].
Early in this decade the quadripolar lead technology has been introduced allowing to increase the procedure success and reduce the lead dislodgement and the PNS [55,56]. In a recent multicenter study, Behar et al. compared quadripolar vs bipolar LV lead and found that the PNS, was entirely eliminated by re-programming in the quadripolar group, while 40% of those from the bipolar lead group required LV lead revision [46]. Moreover, in the quadripolar LV leads patients’ group, the LV threshold and radiation exposition were significantly lower. They found also a lower LV lead displacement and need of revision in the quadripolar LV lead patients, compared with the bipolar ones. The frequency of PNS over the follow-up period was also surprisingly slightly higher among the quadripolar cohort, although this did not reach statistical significance; the authors state that this may be related to physicians’ feeling that have “greater freedom” to place leads with the knowledge that more proximal pacing vectors are available if necessary. Therefore, a logical approach would be to wedge the lead as distal as possible.
To accomplish that, an LV lead with two or more electrodes with programming capability to choose cathode in multiple pacing vectors are needed [57,58]. This strategy, termed ‘electronic repositioning’ by Gurevitz et al. has proved to be highly successful [59].
At implantation PNS is also influenced by body position, with false negatives being discovered after implantation in positions other than supine in 10–20% of cases [60].
In a pivotal paper by Biffi et al., the body position and cathode programmability had been addressed prospectively in a case series of 197 CRT patients. PNS at follow-up was investigated during respiratory changes in some body positions, occurring in supine position in 59%, standing position in 13%, left lateral in 72%, right lateral in 19%; and sitting in 36%; most of them of course not identified at implant. The authors address this as the main reason why the PNS occurrence may increase along the follow up. In their case series, PNS occurred in 37% (73 patients) but it was clinically relevant in 22%; among them PNS was corrected with cathode re-programming when possible by capable devices, while 10 patients underwent lead repositioning and 4 had CRT turned off. At implant, by cathode reprogramming, they found the configuration “LV ring-can” in 8% of patients, the “LV ring-RV coil” in 43% of patients, “LV tip-RV coil” in 36% of patients, and “LV tip-ring “in 13% of patients, respectively as the best performing configuration, defined as those with the largest PNS-LV threshold safety margin [54]. These data were mostly stable in the follow up. Of note, in 14 patients it was not possible to preserve a 100% of safety margin between the LV and PNS thresholds; thanks to the cathode programmability and with the use of quadripolar lead capable of several cathode configurations, the automatic threshold algorithms to avoid PNS remain useful, probably just in a minority of patients [54].
6.2. - Quadripolar leads for synchronous multiple pacing
After CRT implant, the need for reoperation is variably reported, mainly due to LV lead dislodgment with loss of capture, PNS, or increased LV pacing threshold without obvious lead dislocation. The first quadripolar LV lead released in the market (Quartet 1458Q, St Jude Medical, Sylmar, CA) have been investigated for the first time, early in this decade, by Forleo et al., who compared it with bipolar LV leads [54]. Because of the relatively small diameter and electrode choices of the Quartet lead tested (4 Fr at the tip) capable of 10 cathode configurations, the author emphasized the concept that with a quadripolar technology, now available for all manufacturers CRT systems, it is possible to advance the distal tip more toward the apex to ensure lead stability, while retaining the ability to program the lead to pace more proximally if needed [[59], [60], [61], [62], [63]].
In another multicenter but similar study, the same LV lead technology was tested with mostly the same positive result regarding, safety, lead stability and PNS avoidance by multiple lead configurations; even though the shorth (one month) follow up may weaken the data reported when considering the percentage of LV lead performance changes in CRT systems over time [64].
In another recent study by Forleo et al., data about the multipoint pacing on board of CRT systems via quadripolar lead, were assessed. The magnitude of QRS shortening was significantly reached compared with bipolar LV pacing; the multipoint early activation during the follow up was found as an independent predictor of better reverse remodeling.
Not all the clinical contributions given by multi-point pacing are free of caveat or limitations. The avoidance of PNS and the availability of flexible electronic repositioning should be counterbalanced with the battery drainage when using contemporary multisite pacing [59]. On the other hand, for CRT non responder patients, activation of multipoint pacing may present an option to increase clinical response.
For the above reasons some may adopt a watch and wait strategy while managing CRT response along the follow up, and enable this triple site pacing only in non-responder patients; with this regards data about the MORE CRT MPP phase II trial will give some more information [[65], [66], [67], [68]].
7. Limitations
The present work is intended to explore the available technology and the modern challenges regarding the capture issues adopted for LV pacing, which presents some peculiarities such as, a different lead/tissue interface (epicardial pacing), vein wedging lead locations, and less reliable lead stability.
The CRT systems manufacturers which have not yet the auto-threshold analysis technology implemented have not been included in the tracing examples’ figures.
Moreover, some CIED features that involve the LV pacing and CRT optimization algorithms are also not included since they relate to CRT management aside of the present work purposes.
8. Conclusions
Modern CRT device-based threshold detection enables capture management to combine an efficient LV pacing delivery with acceptable battery drainage. The current technologies available for the LV capture automatic confirmation are based on ER or “LV pace to RV sense” analysis. The automatic detection of effective LV myocardium capture, the cathodal/anodal “coupling” issues, the cathodal flexible programmability (to solve threshold problems or extracardiac capture), and the LV “pace-conditioning”, are concepts becoming crucial in modern knowledge, for CRT management.
Declaration of competing interest
None.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Normand C., Linde C., Singh J., Dickstein K. Indications for cardiac resynchronization therapy: a comparison of the major international guidelines. JACC Heart Fail. 2018;6(4):308–316. doi: 10.1016/j.jchf.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Brignole M., Auricchio A., Baron-Esquivias G., Bordachar P., Boriani G., Breithardt O., Cleland J., Deharo J.C., Delgado V., Elliott P.M. ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA) Eur Heart J. 2013;34:2281–2329. doi: 10.1093/eurheartj/eht150. 2013. [DOI] [PubMed] [Google Scholar]
- 3.Sperzel J., Neuzner J., Schwarz T., Qingsheng Z., Konig A., Kay N. Reduction of pacing output coupling capacitance for sensing the evoked response. Pacing Clin Electrophysiol. 2001;24:1377–1382. doi: 10.1046/j.1460-9592.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 4.Clarke M., Liu B., Schuller H., Binner L., Kennergren C., Guerola M., Weinmann P., Ohm O.J. Automatic adjustment of pacemaker stimulation output correlated with continuously monitored capture thresholds: a multicenter study. Pacing Clin Electrophysiol. 1998;21:1567–1575. doi: 10.1111/j.1540-8159.1998.tb00244.x. [DOI] [PubMed] [Google Scholar]
- 5.O’Hara G., Kirstensson B., Lundstrom R., Kempen K., Soucy B., Lynn T. First clinical experience with a new pacemaker with ventricular capture management (Abstract) Pacing Clin Electrophysiol. 1998;21:892. [Google Scholar]
- 6.Neuzner J., Schwarz T., Sperzel J. Pacemaker automaticity. Am J Cardiol. 2000;86(suppl):104K–110K. doi: 10.1016/s0002-9149(00)01191-7. [DOI] [PubMed] [Google Scholar]
- 7.Crossley G., Mead H., Kleckner K., Sheldon T., Davenport L., Harsch M.R., Parikh P., Brian B., Robert Fishel, Bailey J.R. LVCM study investigators. Automated left ventricular capture management. Pacing Clin Electrophysiol. 2007;30(10):1190–1200. doi: 10.1111/j.1540-8159.2007.00840.x. [DOI] [PubMed] [Google Scholar]
- 8.Boriani G., Braunschweig F., Deharo J.C., Leyva F., Lubinski A., Lazzaro C. Impact of extending device longevity on the long-term costs of implantable cardioverter-defibrillator therapy: a modelling study with a 15-year time horizon. Europace. 2013;15:1453e–1462. doi: 10.1093/europace/eut133. [DOI] [PubMed] [Google Scholar]
- 9.Sanders G.D., Hlatky M.A., Owens D.K. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353:1471e–1480. doi: 10.1056/NEJMsa051989. [DOI] [PubMed] [Google Scholar]
- 10.Giammaria M., Quirino G., Alberio M., Parravicini U., Cipolla E., Rossetti G., Ruocco A., Senatore G., Rametta F., Pistelli P. Automatic atrial capture device control in real-life practice: a multicenter experience. Journal of Arrhythmia. 2017;33:139–143. doi: 10.1016/j.joa.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murgatroyd F., Helmling E., Lemke B., Eber B., Mewis C., van der Meer-Hensgens J., Chang T., Khalameizer V., Katz A. Manual vs. automatic capture management in implantable cardioverter defibrillators and cardiac resynchronization therapy defibrillators. Europace. 2010;12:811–816. doi: 10.1093/europace/euq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.www.global.medtronic.com/xg-en/healthcare-professionals/products/cardiac-rhythm/crt-systems.html
- 13.www.bostonscientific.com/en-US/products/crt-d/momentum-x4.html
- 14.www.biotronik.com/en-gb/products/cardiac-resynchronization
- 15.www.cardiovascular.abbott/us/en/hcp/products/cardiac-rhythm-management.html?filter=cardiac-resynchronization-therapy-crt-devices-mpp
- 16.Biffi M., Bertini M., Ziacchi M., Boriani G. Left ventricular pacing by automatic capture verification. Europace. 2007;9:1177–1181. doi: 10.1093/europace/eum225. [DOI] [PubMed] [Google Scholar]
- 17.Thakral A., Stein L.H., Shenai M., Gramatikov B.I., Thakor N.V. Effects of anodal vs. cathodal pacing on the mechanical performance of the isolated rabbit heart. J Appl Physiol. 2000;89:1159–1164. doi: 10.1152/jappl.2000.89.3.1159. [DOI] [PubMed] [Google Scholar]
- 18.Mower M.M., Hepp D., Hall R. Comparison of chronic biphasic pacing versus cathodal pacing of the right ventricle on left ventricular function in sheep after myocardial infarction. Ann Noninvasive Electrocardiol. 2011;16:111–116. doi: 10.1111/j.1542-474X.2011.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sperzel J., Nowak B., Himmrich E., Zhang G., Konig A., Willems R., Reister C., Sathaye A., Frohlig G. Acute performance evaluation of a new ventricular automatic capture algorithm. Europace. 2006;8:65–69. doi: 10.1093/europace/euj008. Tamborero D, Mont L, Alanis R, Berruezo A, Tolosana JM, Sitges M, Vidal B and Brugada J. Anodal capture in cardiac resynchronization therapy implications for device programming, Pacing Clin Electrophysiol. 9, 940-945(2006) [DOI] [PubMed] [Google Scholar]
- 20.Hernández-Madrid A., Facchin D., Nicholson Klepfer R., Ghosh S., Matía R., Moreno L., Locatelli A. Device pacing diagnostics overestimate effective cardiac resynchronization therapy pacing results of the hOLter for Efficacy analysis of CRT (OLÉ CRT) study. Heart Rhythm. 2017;14:541–547. doi: 10.1016/j.hrthm.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Thibault B., Roy D., Guerra P.G., Macle L., Dubuc M., Gagné P., Greiss I., Novac P., Furlani A., Talajic M. Anodal right ventricular capture during left ventricular stimulation in CRT-implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2005;28:613–619. doi: 10.1111/j.1540-8159.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 22.Dell’Era G., De Vecchi F., Prenna E., Devecchi C., Degiovanni A., Malacrida M., Magnani A., Occhetta E., Marino P. Feasibility of cathodic-anodal left ventricular stimulation for alternative multi-site pacing. Pacing Clin Electrophysiol. 2018;6:597–602. doi: 10.1111/pace.13344. [DOI] [PubMed] [Google Scholar]
- 23.Occhetta E., Bortnik M., Marino P. Ventricular capture by anodal pacemaker stimulation. Europace. 2006;8:385–387. doi: 10.1093/europace/eul013. [DOI] [PubMed] [Google Scholar]
- 24.Dendy K.F., Powell B.D., Cha Y.M., Espinosa R.E., Friedman P.A., Rea R.F., Hayes D.L. Anodal stimulation: an underrecognized cause of nonresponders to cardiac resynchronization therapy. Indian Pacing Electrophysiol J. 2011;11(3):64–72. [PMC free article] [PubMed] [Google Scholar]
- 25.Morishima I., Tomomatsu T., Morita Y., Tsuboi H. Intentional anodal capture of a left ventricular quadripolar lead enhances resynchronization equally with multipoint pacing. Heart Rhythm Case Reports. 2015;1(5):386–388. doi: 10.1016/j.hrcr.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Gelder B.M., Bracke F.A., Pilmeyer A., Meijer A. Triple site ventricular pacing in a biventricular pacing system. Pacing Clin Electrophysiol. 2001;24:1165–1167. doi: 10.1046/j.1460-9592.2001.01165.x. [DOI] [PubMed] [Google Scholar]
- 27.Tamborero D., Mont L., Alanis R., Berruezo A., Tolosana J.M., Sitges M., Vidal B., Brugada J. Anodal capture in cardiac resynchronization therapy implications for device programming. Pacing Clin Electrophysiol. 2006;9:940–945. doi: 10.1111/j.1540-8159.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 28.Occhetta E., Dell’Era G., Giubertoni A., Magnani A., Rametta F., Blandino A., Magnano V., Malacrida M., Marino P. Occurrence of simultaneous cathodal-anodal capture with left ventricular quadripolar leads for cardiac resynchronization therapy: an electrocardiogram evaluation. Europace. 2017;4:596–601. doi: 10.1093/europace/euw046. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh S., Stadler R.W., Mittal S. Automated detection of effective left-ventricular pacing: going beyond percentage pacing counters. Europace. 2015;17:1555–1562. doi: 10.1093/europace/euv062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Bommel R.J., Bax J.J., Abraham W.T., Chung E.S., Pires L.A., Tavazzi L., Zimetbaum P.J., Gerritse B., Kristiansen N., Ghio S. Characteristics of heart failure patients associated with good and poor response to cardiac resynchronization therapy: a PROSPECT (Predictors of Response to CRT) sub-analysis. Eur Heart J. 2009;30:2470–2477. doi: 10.1093/eurheartj/ehp368. [DOI] [PubMed] [Google Scholar]
- 31.Pastore G., Maines M., Marcantoni L., Zanon F., Noventa F., Corbucci G., Baracca E., Aggio S., Picariello C., Lanza D., Rigatelli G., Carraro M., Roncon L., Barold S.S. ECG parameters predict left ventricular conduction delay in patients with left ventricular dysfunction. Heart Rhythm. 2016 Dec;13(12):2289–2296. doi: 10.1016/j.hrthm.2016.07.010. Epub 2016 Jul 14. [DOI] [PubMed] [Google Scholar]
- 32.van Gelder B.M., Nathoe R., Bracke F.A. Haemodynamic evaluation of alternative left ventricular endocardial pacing sites in clinical non-responders to cardiac resynchronisation therapy. Neth Heart J. 2016 Jan;24(1):85–92. doi: 10.1007/s12471-015-0773-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldenberg I., Moss A.J., Hall W.J., Foster E., Goldberger J.J., Santucci P., Shinn T., Solomon S., Steinberg J.S., Wilber D. Predictors of response to cardiac resynchronization therapy in the multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy (MADIT-CRT) Circulation. 2011;124:1527–1536. doi: 10.1161/CIRCULATIONAHA.110.014324. [DOI] [PubMed] [Google Scholar]
- 34.Pires L., Mcnitt S., Solomon S., Goldenberg I., Zareba W., Moss A.J. Left ventricular pacing threshold and outcome in MADIT-CRT. J cardiovasc Electrophysiology. 2014;25:1005–1011. doi: 10.1111/jce.12448. [DOI] [PubMed] [Google Scholar]
- 35.Hsu J.C., Solomon S.D., Bourgoun M., McNitt S., Goldenberg I., Klein H., Moss A.J., Foster E. Predictors of super-response to cardiac resynchro- nization therapy and associated improvement in clinical outcome. The MADIT-CRT (multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy) study. J Am Coll Cardiol. 2012;59:2366–2373. doi: 10.1016/j.jacc.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 36.Delgado V., van Bommel R.J., Bertini M., Borleffs C.J.W., Marsan N.A., Ng A.C.T., Nucifora G., van de Veire N.R.L., Ypenburg C., Boersma E. Relative merits of left ventricular dyssynchrony, left ventricular lead position, and myocardial scar to predict long- term survival of ischemic heart failure patients undergoing cardiac resynchronization therapy. Circulation. 2011;123:70–78. doi: 10.1161/CIRCULATIONAHA.110.945345. [DOI] [PubMed] [Google Scholar]
- 37.Burri H., Gerritse B., Davenport L., Demas M., Sticherling C. Fluctuation of left ventricular thresholds and required safety margin for left ventricular pacing with cardiac resynchronization therapy. Europace. 2009;11:931–936. doi: 10.1093/europace/eup105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ypenburg C., Van Bommel R.J., Delgado V., Mollema S.A., Bleeker G.B., Boersma E., Schalij M.J., Bax J.J. Optimal left ventricular lead position predicts response and prognosis to cardiac resynchronization therapy. J Am Coll Cardiol. 2008;52:1402–1409. doi: 10.1016/j.jacc.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 39.Ansalone G., Giannantoni P., Ricci R., Trambaiolo P., Fedele F., Santini M. Doppler myocardial imaging to evaluate the effectiveness of pacing sites in patients receiving biventricular pacing. J Am Coll Cardiol. 2002;39:489–499. doi: 10.1016/s0735-1097(01)01772-7. [DOI] [PubMed] [Google Scholar]
- 40.Adelstein E.C., Saba S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J. 2007;153:105–112. doi: 10.1016/j.ahj.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Koplan B.A., Kaplan A.J., Weiner S., Jones P.W., Seth M., Christman S.A. Heart failure decompensation and all-cause mortality in relation to percent biventricular pacing in patients with heart failure. J Am Coll Cardiol. 2009;53:355–360. doi: 10.1016/j.jacc.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 42.Kawashima T., Sasaki H. A macroscopic anatomical investigation of atrioven- tricular bundle locational variation relative to the membranous part of the ventricular septum in elderly human hearts. Surg Radiol Anat. 2005;27:206–213. doi: 10.1007/s00276-004-0302-7. [DOI] [PubMed] [Google Scholar]
- 43.De Pooter J., Calle S., Timmermans F., Van Heuverswyn F. Left bundle branch area pacing using stylet-driven pacing leads with a new delivery sheath: a comparison with lumen-less leads. J Cardiovasc Electrophysiol. 2021 Feb;32(2):439–448. doi: 10.1111/jce.14851. Epub 2021 Jan 4. PMID: 33355969. [DOI] [PubMed] [Google Scholar]
- 44.Zingarini G., Notaristefano F., Spighi L., Bagliani G., Cavallini C. Permanent His bundle pacing using a new tridimensional delivery sheath and a standard active fixation pacing lead: the telescopic technique. J Cardiovasc Electrophysiol. 2021 Feb;32(2):449–457. doi: 10.1111/jce.14869. Epub 2021 Jan 19. PMID: 33410557. [DOI] [PubMed] [Google Scholar]
- 45.Lustgarten D., Sharma P.S., Vijayaraman P. Troubleshooting and programming considerations for His bundle pacing. Heart Rhythm. 2019;16:654–662. doi: 10.1016/j.hrthm.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 46.Huang W., Su L., Wu S. Long-term outcomes of His bundle pacing in patients with heart failure with left bundle branch block. Heart. 2019;105:137–143. doi: 10.1136/heartjnl-2018-313415. [DOI] [PubMed] [Google Scholar]
- 47.Huang W., Chen X., Su L.L., Wu S., Xia X., Vijayaraman P. A beginner’s guide to permanent left bundle branch pacing Heart Rhythm. 2019;16:1791–1796. doi: 10.1016/j.hrthm.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 48.Sieniewicz B.J., Betts T.R., James S., Turley A., Butter C., Seifert M., Boersma L.V.A., Riahi S., Neuzil P., Biffi M., Diemberger I., Vergara P., Arnold M., Keane D.T., Defaye P., Deharo J.C., Chow A., Schilling R., Behar J., Rinaldi C.A. Real-world experience of leadless left ventricular endocardial cardiac resynchronization therapy: a multicenter international registry of the WiSE-CRT pacing system. Heart Rhythm. 2020 Aug;17(8):1291–1297. doi: 10.1016/j.hrthm.2020.03.002. Epub 2020 Mar 9. PMID: 32165181; PMCID: PMC7397503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Auricchio A., Delnoy P.P., Regoli F., Seifert M., Markou T., Butter C. First-in-man implantation of leadless ultrasound-based cardiac stimulation pacing system: novel endocardial left ventricular resynchronization therapy in heart failure pa- tients. Europace. 2013;15:1191–1197. doi: 10.1093/europace/eut124. [DOI] [PubMed] [Google Scholar]
- 50.Morgan J.M., Biffi M., Gellér L. ALternate Site Cardiac ResYNChronization (ALSYNC): a prospective and multicentre study of left ventricular endocardial pacing for cardiac resynchronization therapy. Eur Heart J. 2016;37:2118–2127. doi: 10.1093/eurheartj/ehv723. [DOI] [PubMed] [Google Scholar]
- 51.Vijayaraman P., Herweg B., Ellenbogen K.A., Gajek J. His-optimized cardiac resynchronization therapy to maximize electrical resynchronization: a feasibility study. Circ Arrhythm Electrophysiol. 2019 Feb;12(2) doi: 10.1161/CIRCEP.118.006934. PMID: 30681348. [DOI] [PubMed] [Google Scholar]
- 52.Moore J.P., Gallotti R., Shannon K.M., Pilcher T., Vinocur J.M., Cano Ó Kean A., Mondesert B., Nürnberg J.H., Schaller R.D., Sharma P.S., Nishimura T., Tung R. Permanent conduction system pacing for congenitally corrected transposition of the great arteries: a pediatric and congenital electrophysiology society (PACES)/International society for adult congenital heart disease (ISACHD) collaborative study. Heart Rhythm. 2020 Mar 13;(20):S1547–S5271. doi: 10.1016/j.hrthm.2020.01.033. 30088-6, Epub ahead of print. PMID: 32243875. [DOI] [PubMed] [Google Scholar]
- 53.Behar J.M., Bostock J., Zhu Li A.P., Chin H.M., Jubb S., Lent E., Gamble J., Foley P.W., Betts T.R., Rinaldi C.A., Herring N. Cardiac resynchronization therapy delivered via a multipolar left ventricular lead is associated with reduced mortality and elimination of phrenic nerve stimulation: long-term follow-up from a multicenter registry. J Cardiovasc Electrophysiol. 2015;26(5):540–546. doi: 10.1111/jce.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biffi M., Moschini C., Bertini M., Saporito D., Ziacchi M., Diemberger I., Valzania C., Domenichini G., Cervi E., Martignani C. Phrenic stimulation: a challenge for cardiac resynchronization therapy. Circ Arrhythm Electrophysiol. 2009;2:402–410. doi: 10.1161/CIRCEP.108.836254. [DOI] [PubMed] [Google Scholar]
- 55.Shetty A.K., Mehta P., Bostock J., Rinaldi C.A. Quad-site pacing using a quadripolar left ventricular pacing lead. Pacing Clin Electrophysiol. 2013;36:e48–e50. doi: 10.1111/j.1540-8159.2011.03267.x. [DOI] [PubMed] [Google Scholar]
- 56.Forleo G.B., Mantica M., Di Biase L., Panattoni G., Rocca Della D.G., Papavasileiou L.P., Santamaria M., Santangeli P., Avella A. Clinical and procedural outcome of patients implanted with a quadripolar left ventricular lead: early results of a prospective multicenter study. Heart Rhythm. 2012;9:1822–1828. doi: 10.1016/j.hrthm.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 57.Sperzel J., Danschel W., Gutleben K.-J., Kranig W., Mortensen P., Connelly D., Trappe H.-J., Seidl K., Duray G., Pieske B. First prospective, multi-centre clinical experience with a novel left ventricular quadripolar lead. Europace. 2012;14:365–372. doi: 10.1093/europace/eur322. [DOI] [PubMed] [Google Scholar]
- 58.Biffi M., Boriani G. Phrenic stimulation management in CRT patients: are we there yet? Curr Opin Cardiol. 2011;26:12–16. doi: 10.1097/HCO.0b013e3283413838. [DOI] [PubMed] [Google Scholar]
- 59.Gurevitz O., Nof E., Carasso S. Programmable multiple pacing configurations help to overcome high left ventricular pacing thresholds and avoid phrenic nerve stimulation. Pacing Clin Electrophysiol. 2005;28:1255–1259. doi: 10.1111/j.1540-8159.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 60.Seifert M., Schau T., Moeller V., Neuss M., Meyhoefer J., Butter C. Influence of pacing configurations, body mass index, and position of coronary sinus lead on frequency of phrenic nerve stimulation and pacing thresholds under cardiac resynchronization therapy. Europace. 2010;12:961–967. doi: 10.1093/europace/euq119. [DOI] [PubMed] [Google Scholar]
- 61.Burri H., Gerritse B., Davenport L. Fluctuation of left ventricular thresholds and required safety margin for left ventricular pacing with cardiac resynchronization therapy. Europace. 2009;11:931–936. doi: 10.1093/europace/eup105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leon A.R., Abraham W.T., Curtis A.B. Safety of transvenous cardiac resynchronization system implantation in patients with chronic heart failure: combined results of over 2,000 patients from a multicenter study program. J Am Coll Cardiol. 2005;46:2348–2356. doi: 10.1016/j.jacc.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 63.Forleo G.B., Della Rocca D.G., Papavasileiou L.P., Di Molfetta A., Santini L., Romeo F. Left ventricular pacing with a new quadripolar transvenous lead for CRT: early results of a prospective comparison with conventional implant outcomes. Heart Rhythm. 2011;8:31–37. doi: 10.1016/j.hrthm.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 64.Sperzel J., Dänschel W., Gutleben K.J., Kranig W., Mortensen P., Connelly D., Trappe H.J., Seidl K., Duray G., Pieske B. First prospective, multi-centre clinical experience with a novel left ventricular quadripolar lead. Europace. 2012;14:365–372. doi: 10.1093/europace/eur322. [DOI] [PubMed] [Google Scholar]
- 65.Antoniadis A.P., Behar J.M., Claridge S., Jackson T. Sohal M,rinaldi CA. Multisite pacing for cardiac resynchronization therapy: promise and pitfalls. Curr Cardiol Rep. 2016 Jul;18(7):64. doi: 10.1007/s11886-016-0741-x. [DOI] [PubMed] [Google Scholar]
- 66.Müller-Leisse J., Zormpas C., König T., Duncker D., Veltmann C. Multipoint pacing-more CRT or a waste of battery power? Herz. 2018;43:596–604. doi: 10.1007/s00059-018-4751-x. [DOI] [PubMed] [Google Scholar]
- 67.Forleo G.B., Gasperetti A., Ricciardi D., Curnis A., Bertaglia E., Calò L., Pignalberi C., Calzolari V., Ribatti V., Lavalle C. Impact of multipoint pacing on projected battery longevity in cardiac resynchronization therapy. An IRON-MPP Study Sub-Analysis Europace. 2017;19:1170–1177. doi: 10.1111/jce.14254. [DOI] [PubMed] [Google Scholar]
- 68.Leclercq C., Burri H., Curnis A., Delnoy P.P., Rinaldi C.A., Sperzel J., Lee K., Cohorn C., Thibault B. MORE-CRT MPP investigators. Rationale and design of a randomized clinical trial to assess the safety and efficacy of multipoint pacing therapy: MOre REsponse on cardiac resynchronization therapy with MultiPoint pacing (MORE-CRT MPP-PHASE II) Am Heart J. 2019;209:1–8. doi: 10.1016/j.ahj.2018.12.004. [DOI] [PubMed] [Google Scholar]