Supplemental Digital Content is available in the text.
Keywords: adult/therapy, fluid therapy/methods, hemodynamics, intensive care units/statistics and numerical data, positive-pressure respiration, respiratory distress syndrome, water-electrolyte balance
Abstract
OBJECTIVES:
Acute respiratory distress syndrome is underrecognized in the ICU, but it remains uncertain if acute respiratory distress syndrome recognition affects evidence-based acute respiratory distress syndrome care in the modern era. We sought to determine the rate of clinician-recognized acute respiratory distress syndrome in an academic medical ICU and understand how clinician-recognized-acute respiratory distress syndrome affects clinical care and patient-centered outcomes.
DESIGN:
Observational cohort study.
SETTING:
Single medical ICU at an academic tertiary-care hospital.
PATIENTS:
Nine hundred seventy-seven critically ill adults (381 with expert-adjudicated acute respiratory distress syndrome) enrolled from 2006 to 2015.
INTERVENTIONS:
Clinician-recognized-acute respiratory distress syndrome was identified using an electronic keyword search of clinical notes in the electronic health record. We assessed the classification performance of clinician-recognized acute respiratory distress syndrome for identifying expert-adjudicated acute respiratory distress syndrome. We also compared differences in ventilator settings, diuretic prescriptions, and cumulative fluid balance between clinician-recognized acute respiratory distress syndrome and unrecognized acute respiratory distress syndrome.
MEASUREMENTS AND MAIN RESULTS:
Overall, clinician-recognized-acute respiratory distress syndrome had a sensitivity of 47.5%, specificity 91.1%, positive predictive value 77.4%, and negative predictive value 73.1% for expert-adjudicated acute respiratory distress syndrome. Among the 381 expert-adjudicated acute respiratory distress syndrome cases, we did not observe any differences in ventilator tidal volumes between clinician-recognized-acute respiratory distress syndrome and unrecognized acute respiratory distress syndrome, but clinician-recognized-acute respiratory distress syndrome patients had a more negative cumulative fluid balance (mean difference, –781 mL; 95% CI, [–1,846 to +283]) and were more likely to receive diuretics (49.3% vs 35.7%, p = 0.02). There were no differences in mortality, ICU length of stay, or ventilator-free days.
CONCLUSIONS:
Acute respiratory distress syndrome recognition was low in this single-center study. Although acute respiratory distress syndrome recognition was not associated with lower ventilator volumes, it was associated with differences in behaviors related to fluid management. These findings have implications for the design of future studies promoting evidence-based acute respiratory distress syndrome interventions in the ICU.
The acute respiratory distress syndrome (ARDS) is an inflammatory lung disorder characterized by disruption of the alveolar epithelial barrier and development of noncardiogenic pulmonary edema, leading to hypoxemic respiratory failure (1). Although ARDS is common in the ICU and carries a high mortality risk (1–5), it remains underdiagnosed and undertreated even after the consensus definition of ARDS was revised to the current Berlin definition (1, 4, 6–11).
Recognition of ARDS is poor in clinical practice (4, 8, 9, 11), and deviation from recommended management of ARDS is common (12–15). Thus, there is substantial interest in developing decision support tools to increase ARDS recognition (16–19). The utility of an ARDS recognition tool depends on the premise that increased recognition would change clinician behaviors, but the impact of ARDS recognition on evidence-based ARDS management practices remains uncertain (4, 11, 20). A recent single-center study found that recognized ARDS patients had a higher probability of receiving lower tidal volumes (11), whereas a large multinational study reported no difference in ventilator tidal volumes among recognized or unrecognized (UR) ARDS patients (4). Additionally, the impact of ARDS recognition on other evidence-based practices remains unknown.
In this study, we sought to quantify ARDS recognition in a well-phenotyped longitudinal cohort of adult medical ICU (MICU) patients at a tertiary academic medical center and determine whether ARDS recognition was associated with provision of evidenced-based ARDS care or affected clinical outcomes.
MATERIALS AND METHODS
Study Population
We analyzed patients enrolled in the Validating Acute Lung Injury biomarkers for Diagnosis (VALID) study, a prospective observational cohort of critically ill adults at risk for ARDS (21), admitted to our MICU from January 2006 to August 2015. All patients were enrolled on the morning of the second ICU day. Other inclusion criteria, enrollment and consent procedures for VALID have been previously described (21). We excluded patients under 18 years old, died or were discharged from the ICU within 48 hours of admission, expected to transfer out of the ICU on the day of enrollment, admitted to another hospital for greater than or equal to 3 days, or experienced a cardiopulmonary arrest prior to enrollment. The Vanderbilt Institutional Review Board (IRB; Nashville, TN) reviewed and approved the study protocol (IRB #051065). Study personnel obtained informed consent from patients or a surrogate decision maker whenever possible, and the IRB approved a waiver of informed consent when no surrogate decision maker was available.
ARDS Case Definition
Two expert physician investigators manually reviewed clinical records and chest radiographs to determine adjudicated ARDS status (21). Any patient with expert-adjudicated ARDS during the first 4 ICU days was considered an ARDS case. As the study period overlapped with an update to the consensus definition of ARDS, for patients enrolled prior to June 2012, we used the American-European Consensus Conference (AECC) definition (22), and for patients enrolled after June 2012, we used the Berlin definition (1). Controls were patients who never had expert-adjudicated ARDS during the first 4 ICU days. We excluded patients who were not mechanically ventilated but met all other criteria for ARDS (Fig. 1) to better homogenize patients identified by the two definitions, allow uniform assessment of ventilation practices, and maintain consistency with a prior ARDS recognition study (4).
Figure 1.
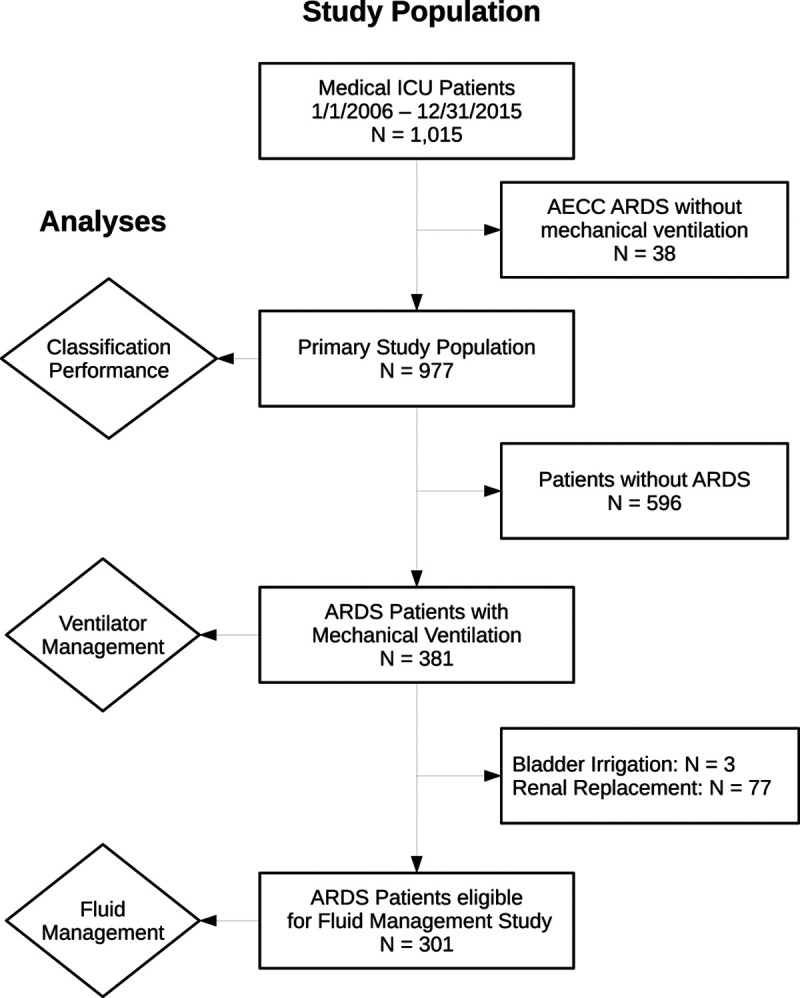
Flow diagram of study population and analyses. Patients were excluded from the acute respiratory distress syndrome (ARDS) recognition analysis if they met the American-European Consensus Conference (AECC) ARDS definition but did not receive mechanical ventilation (n = 38) to allow a more homogeneous analysis of clinician-recognition of ARDS (CR-ARDS) across time and different ARDS definitions (AECC and Berlin). In the analysis of CR-ARDS on ventilator management, we excluded patients without ARDS (n = 596). Finally, in the fluid management analyses, we excluded patients who received either continuous bladder irrigation (n = 3) or renal replacement therapy (n = 77) while in the ICU. Further details and rationale are provided in the Supplementary Methods (http://links.lww.com/CCX/A687).
Clinician-Recognized ARDS
Clinician-recognized ARDS (CR-ARDS) was determined by systematically searching the admission histories, daily progress notes, and discharge summaries using an electronic keyword search function available in our internally developed electronic health record (EHR) (23, 24). The keyword phrases indicating ARDS was identified by clinicians included “acute lung injury,” “acute respiratory distress syndrome,” “ALI,” and “ARDS.” Study personnel performing the electronic search were blinded to the expert-adjudicated ARDS classifications. We manually reviewed all positive keyword matches to exclude negations and confirm suspicion or certainty of the clinicians’ ARDS diagnosis. UR-ARDS was defined as expert-adjudicated ARDS without any keywords in the notes. The accuracy of this EHR-based search algorithm has been previously reported (25).
Data Collection
As previously described, trained study nurses collected demographic, historical, laboratory, and physiologic data from ICU admission through ICU day 4 (21). Ventilator tidal volumes and pressures were obtained from respiratory flow sheets. Predicted body weight was calculated from the respiratory management in acute lung injury/ARDS Trial equations using the mode of each patient’s height measurements (5). Diuretic doses were collected from the EHR medication administration record and converted into IV furosemide equivalents (Supplementary Table 1, http://links.lww.com/CCX/A687). Fluid administration, urine output, and central venous pressure (CVP) were collected from the EHR nursing flow sheets.
Outcomes and Statistical Analysis
The study analyses are outlined in Figure 1. We first assessed the classification performance of CR-ARDS among all patients in the primary study population. We estimated sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and Cohen kappa of CR-ARDS for identifying expert-adjudicated ARDS. We also compared classification performance of CR-ARDS among patients with mild (Pao2:Fio2 greater than or equal to 201), moderate (Pao2:Fio2 101–200), or severe (Pao2:Fio2 less than or equal to 100) oxygenation impairment, and between patients admitted prior to or after publication of the Berlin definition (Supplementary Methods, http://links.lww.com/CCX/A687).
We then assessed the association of CR-ARDS with ventilator and fluid management practices among patients with expert-adjudicated ARDS (Fig. 1). Outcomes included daily set tidal volume, set positive end-expiratory pressure (PEEP), cumulative fluid balance, diuretic prescriptions, and CVP. We examined the association between CR-ARDS and cumulative fluid balance using a multivariable linear mixed-effects regression model to account for repeat observations over each patient’s ICU stay and used a directed acyclic graph to identify relevant clinical confounders for inclusion in the regression model (Supplementary Fig. 1 and Supplementary Table 2, http://links.lww.com/CCX/A687) (26). Further details are provided in the Supplementary Methods (http://links.lww.com/CCX/A687). We focused our analyses on days when expert-adjudicated ARDS status was assessed (ICU days 1–4) and report results for longer periods when such outcome data were available.
Continuous variables are presented as mean and sd or median and interquartile range, categorical variables as frequency and proportion, and differences between groups as mean difference and 95% CIs. We assessed groupwise differences using the Mann-Whitney U test for continuous outcomes and Pearson chi-square test for categorical outcomes. A two-sided p value of less than 0.05 was considered statistically significant. No adjustments were made for multiple comparisons as all analyses were considered exploratory. Statistical analyses were performed using R version 3.3.0 (R Foundation for Statistical Computing, Vienna, Austria), and data visualization using the Python packages “Plotly” (Montreal, Quebec, Canada) (27) and “seaborn” (28). Code is available upon request. The reporting of this study conforms to the STROBE statement (http://links.lww.com/CCX/A688).
RESULTS
Study Population
We identified 1,015 critically ill adults enrolled in VALID from the MICU between January 2006 and August 2015. We excluded 38 patients (3.7%) who met AECC criteria but were not mechanically ventilated leaving 977 patients for the primary study population, of which 381 had ARDS by expert adjudication (39.0%) (Fig. 1). Characteristics of expert-adjudicated ARDS patients are shown in Table 1 and for the entire study population in Supplementary Table 3 (http://links.lww.com/CCX/A687).
TABLE 1.
Patient Characteristics of Acute Respiratory Distress Syndrome Population
| Characteristic | Clinician-Recognized ARDS (n = 181) | Unrecognized ARDS (n = 200) | pa |
|---|---|---|---|
| Demographics | |||
| Age (yr) | 51 (36–62) | 56 (46–68) | <0.001 |
| Gender (female) | 107 (59.1) | 85 (42.5) | 0.001 |
| Race (Caucasian) | 162 (89.5) | 163 (81.5) | 0.05 |
| Comorbid medical disease | |||
| Pulmonary | 34 (21.1) | 39 (22.9) | 0.86 |
| Diabetes | 45 (24.9) | 67 (33.5) | 0.07 |
| Immunodeficiency | 60 (33.1) | 58 (29.0) | 0.38 |
| Congestive heart failure | 14 (7.7) | 28 (14.0) | 0.05 |
| Chronic kidney disease | 22 (12.2) | 50 (25.0) | 0.001 |
| Maintenance hemodialysis | 1 (0.6) | 15 (7.5) | <0.001 |
| Chronic liver disease | 24 (13.2) | 30 (15.0) | 0.63 |
| Solid tumor malignancy | 19 (11.8) | 28 (16.5) | 0.30 |
| Hematological malignancy | 29 (18.0) | 17 (10.0) | 0.02 |
| ICU admission characteristicsb | |||
| Source of admissionc | |||
| Emergency department | 50 (27.9) | 72 (36.5) | 0.13d |
| Hospital ward | 66 (36.9) | 70 (35.5) | |
| Transfer from another hospital | 58 (32.4) | 51 (25.9) | |
| Operating room | 5 (2.8) | 2 (1.0) | |
| Other | 0 (0.0) | 2 (1.0) | |
| ARDS risk factore | |||
| Extrapulmonary sepsis | 66 (36.5) | 62 (31.2) | 0.14d |
| Pneumonia | 69 (38.1) | 57 (28.6) | |
| Aspiration | 32 (17.7) | 59 (29.6) | |
| Multiple transfusions | 3 (1.7) | 6 (3.0) | |
| Otherf | 11 (6.1) | 15 (7.5) | |
| Severe sepsis | 157 (86.7) | 156 (78.0) | 0.03 |
| Shock | 123 (68.0) | 141 (70.5) | 0.59 |
| Renal replacement therapy in ICU | 35 (19.3) | 42 (21.0) | 0.69 |
| Acute Physiology and Chronic Health Evaluation II score | 30 (25–36) | 30 (24–36) | 0.99 |
| Respiratory characteristicsb | |||
| Respiratory rate (breaths/min) | 33 (28–39) | 29 (25–35) | < 0.001 |
| Lowest Pao2/FiO2g | 100 (68–158) | 152 (104–223) | < 0.001 |
| Static complianceh (mL/cm H2O) | 25.0 (16.7–34.9) | 24.9 (18.9–35.8) | 0.56 |
| Clinical outcomes | |||
| ICU length of stay (d) | 8 (5–13) | 7 (4–12) | 0.13 |
| Ventilator-free days | 16 (0–23) | 14 (1–24) | 0.38 |
| Inhospital mortality | 66 (36.5) | 67 (33.5) | 0.67 |
ARDS = acute respiratory distress syndrome.
Continuous data presented as median (25th–75th percentile). Categorical data are presented as number and percentage (%).
aStatistical testing performed using Pearson χ2 test for categorical variables unless otherwise noted and Mann-Whitney U test for ordinal and continuous variables.
bValues for day of study enrollment (ICU day 1).
cSource of ICU admission available for 376 patients (179 clinician-recognized [CR]-ARDS, 197 unrecognized [UR]-ARDS).
dStatistical testing by two-tailed Fisher exact test.
eARDS risk factor available for 380 patients (181 CR-ARDS, 199 UR-ARDS).
f“Other” category includes pancreatitis (one CR-ARDS, one UR-ARDS), severe trauma (one CR-ARDS, one UR-ARDS), drug overdose (one CR-ARDS, two UR-ARDS), no identifiable risk factor (one CR-ARDS, one UR-ARDS), and other rare risk factors (seven CR-ARDS, 10 UR-ARDS) including tumor lysis syndrome, sickle cell crisis, pulmonary graft-vs-host disease, eosinophilic pneumonia, alveolar hemorrhage, and acute pulmonary drug toxicity.
gPao2/Fio2 ratio available in 267 patients (137 CR-ARDS, 129 UR-ARDS).
hStatic compliance of respiratory system available for 293 patients (139 CR-ARDS, 154 UR-ARDS).
Classification Performance of CR-ARDS
In the primary study population (381 with ARDS, 596 without ARDS), CR-ARDS had a sensitivity of 47.5%, specificity 91.1%, PPV 77.4%, and NPV 73.1% (Table 2). Recognition increased with more severe oxygenation impairment, with highest sensitivity in patients with Pao2:Fio2 less than or equal to 100 (70.0%, p < 0.001, Supplementary Table 4, http://links.lww.com/CCX/A687). For patients enrolled after publication of the Berlin definition, CR-ARDS had modestly lower sensitivity (41.2% vs 49.2%, p = 0.21) and substantially lower PPV (60.0% vs 82.7%, p < 0.001) compared with those enrolled before the Berlin definition (Supplementary Table 5, http://links.lww.com/CCX/A687).
TABLE 2.
Classification Performance of Clinician-Recognized Acute Respiratory Distress Syndrome
| Group | Patientsa | Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | Cohen Kappa |
|---|---|---|---|---|---|---|
| All patients | 977 (381) | 47.5 | 91.1 | 77.4 | 73.1 | 0.42 |
| Lowest Pao2/FiO2b | ||||||
| Pao2:Fio2 ≤ 100 | 165 (100) | 70.0 | 81.5 | 85.4 | 63.9 | 0.49 |
| Pao2:Fio2 101–200 | 273 (112) | 45.5 | 86.4 | 75.0 | 63.9 | 0.33 |
| Pao2:Fio2 ≥ 201 | 233 (55) | 30.9 | 96.1 | 70.8 | 81.8 | 0.34 |
| Acute respiratory distress syndrome diagnostic era | ||||||
| American-European Consensus Conference (before June 2012) | 714 (301) | 49.2 | 92.5 | 82.7 | 71.4 | 0.44 |
| Berlin (after June 2012) | 263 (80) | 41.2 | 88.0 | 60.0 | 77.4 | 0.32 |
aTotal number of patients (number with acute respiratory distress syndrome [ARDS]).
bPao2:Fio2 ratio was available for 671 patients (267 with ARDS).
Clinical Features Associated With CR-ARDS
Among the 381 patients with expert-adjudicated ARDS, treating clinicians documented CR-ARDS in 181 (47.5%), whereas 200 ARDS cases (52.5%) went UR. Baseline variables most strongly associated with CR-ARDS (vs UR-ARDS) included younger age, female gender, absence of chronic kidney disease (CKD), more severe oxygenation impairment, increased respiratory rate, and severe sepsis at admission (Table 1). We did not observe any differences between the groups in ARDS risk factors, source of ICU admission, or presence of shock. Static compliance of the respiratory system was also not different between the groups. There was no difference in timing of ARDS onset between CR-ARDS and UR-ARDS (p = 0.93), and greater than 90% of patients met ARDS criteria on ICU day 1 or 2 (Supplementary Table 6 and Supplementary Fig. 2, http://links.lww.com/CCX/A687). Patients with CR-ARDS also had similar inhospital mortality, ICU length of stay, and ventilator-free days compared with UR-ARDS (Table 1).
Ventilator Management
Set tidal volumes were not different between CR-ARDS and UR-ARDS. The mean (±sd) tidal volume on ICU day 1 was 6.60 (±1.10) mL/kg for CR-ARDS and 6.40 (±0.90) mL/kg for UR-ARDS (Supplementary Table 7, http://links.lww.com/CCX/A687). We observed a small difference in mean daily PEEP between the groups: CR-ARDS patients received approximately 2 cm H2O higher PEEP during the first 2 ICU days and approximately 1 cm H2O higher PEEP thereafter (Supplementary Table 8, http://links.lww.com/CCX/A687).
Fluid Balance, Diuretic Usage, and Central Venous Pressure
Among 301 ARDS patients eligible for the fluid management analysis (Fig. 1, and Supplementary Methods, http://links.lww.com/CCX/A687), CR-ARDS had progressively lower cumulative fluid balance than UR-ARDS after ICU day 2 (Fig. 2; and Supplementary Table 9, http://links.lww.com/CCX/A687). The mean difference in cumulative fluid balance between the groups on ICU day 4 was –862 mL (95% CI, [–2,131 to +408], p = 0.28), with even greater differences by ICU day 7 (–1737 mL [–3,403 to –69], p = 0.04) (Fig. 2; and Supplementary Table 9, http://links.lww.com/CCX/A687). This difference was predominantly driven by significantly higher fluid output among CR-ARDS starting on ICU day 3, whereas fluid intake remained similar between the groups (Supplementary Fig. 3, http://links.lww.com/CCX/A687). When controlling for clinical confounders, multivariable mixed-effects regression demonstrated a modest effect of CR-ARDS on cumulative fluid balance (–781 mL [–1,846 to +283 mL]) (Table 3). This was smaller than the effect of hemodynamic instability, but comparable with having underlying congestive heart failure or CKD (Table 3). Additionally, more CR-ARDS patients received at least one loop diuretic over the first 4 ICU days (42.4% vs 30.5%, p = 0.04) and the first 7 days (49.3% vs 35.7%, p = 0.02). Cumulative doses of diuretics administered in the ICU also tended to be higher for CR-ARDS starting on ICU day 2 (Supplementary Table 10, http://links.lww.com/CCX/A687). Lowest CVP did not differ between groups on any day (Supplementary Table 11, http://links.lww.com/CCX/A687), but these comparisons were limited by smaller samples sizes as many patients did not have central venous catheters.
Figure 2.
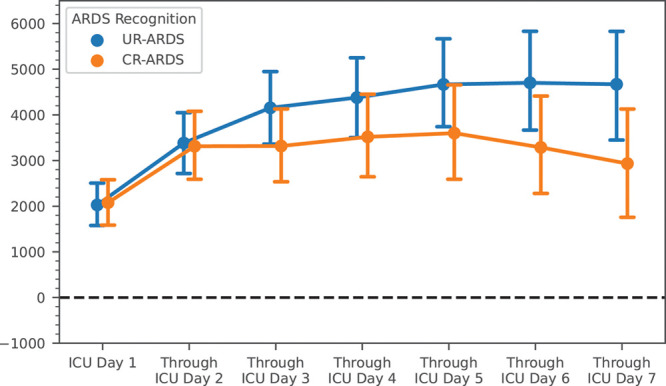
Cumulative net fluid balance by study day. Cumulative net fluid balance over the first 7 ICU days. Dots represent group means by day and error bars represent 95% CIs for the group means. Statistical comparisons between the groups were performed using the Mann-Whitney U test. ARDS = acute respiratory distress syndrome, CR-ARDS = clinician-recognized ARDS, UR-ARDS = unrecognized ARDS.
TABLE 3.
Regression Analysis of Cumulative Net Fluid Balance
| Regression Variable | Effect Estimate (mL) | se (95% CIs) | p |
|---|---|---|---|
| Unadjusted analysis | |||
| Study observation day | +297 | 32 (+234 to +360) | <0.001 |
| Clinician-recognized ARDS | +525 | 536 (–525 to +1,574) | 0.33 |
| Adjusted analysis | |||
| Study observation day | +297 | 32 (+234 to +360) | <0.001 |
| Presence of shock at admission | +2,133 | 559 (+1,053 to +3,229) | <0.001 |
| Oxygenation impairmenta | +424 | 359 (–279 to +1,128) | 0.24 |
| Presence of congestive heart failure or chronic kidney disease | –1,010 | 652 (–2,289 to +268) | 0.12 |
| Clinician-recognized ARDS | –781 | 543 (–1,846 to +283) | 0.15 |
ARDS = acute respiratory distress syndrome.
aOxygenation impairment was categorized into four ordinal groups using the lowest Pao2:Fio2 and/or Spo2:Fio2 ratio during the first 4 ICU days: severe ARDS (Pao2:Fio2 ≤ 100), moderate ARDS (Pao2:Fio2 ≥ 101 and ≤ 200), mild ARDS (Pao2:Fio2 ≥ 201 and ≤ 300), or ARDS met by Spo2:Fio2 criteria only (lowest Pao2:Fio2 > 300, but lowest Spo2:Fio2 ≤ 315 while Spo2 > 96%) (29).
DISCUSSION
This study confirms that ARDS is underrecognized in our MICU, with over half of expert-adjudicated ARDS cases not recognized as such by treating clinicians. Similar to previous reports, ARDS recognition by clinicians was more common for younger patients and those with more severe respiratory impairment (4, 7, 8). ARDS recognition was lower for patients enrolled after publication of the Berlin definition, but the smaller sample size in this subgroup precludes any firm conclusions as to whether clinicians were less able to identify ARDS under the Berlin framework.
CR-ARDS was not associated with lower tidal volumes in our study. These observations contrast those of Schwede et al (11), who reported lower tidal volumes for CR-ARDS in a single-center cohort of 141 ARDS patients but support the findings of the multinational LUNG-SAFE study, which found no difference in daily tidal volumes between CR-ARDS and UR-ARDS among 2,377 ARDS patients in 459 ICUs (4). Mean tidal volumes were lower in our study population (UR-ARDS: 6.4 cc/kg, CR-ARDS: 6.6 cc/kg) than both Schwede et al (11) (UR-ARDS: 7.8 cc/kg, CR-ARDS: 6.9 cc/kg) and LUNG-SAFE (UR-ARDS: 7.7 cc/kg, CR-ARDS: 7.5 cc/kg). Our MICU routinely implements a respiratory therapist-driven protocol favoring lower tidal volumes and plateau pressures (tidal volume less than 8 cc/kg and plateau pressure less than 30 cm H2O) for essentially all ventilated patients regardless of diagnosis (B Lloyd, RRT-ACCS, oral communication, April 2020), so it is possible that the protocolized approach to ventilator tidal volume settings overcame any possible effect of ARDS recognition on tidal volume selection. Also similar to LUNG-SAFE, patients with CR-ARDS received consistently higher PEEP on each ICU day (4), which likely reflected the more severe oxygenation impairment in the CR-ARDS group.
Cumulative fluid balance was modestly lower among CR-ARDS compared with UR-ARDS. This was driven nearly entirely by increased fluid output among CR-ARDS patients starting on ICU day 3, which aligns with the increased administration of loop diuretics to this group starting the day prior. Furthermore, when controlling for other clinical factors that could independently affect fluid balance, ARDS recognition had an effect comparable with that of having a medical diagnosis such as CHF and CKD, which are associated with chronic volume overload and diuretic use. Although we did not examine individual medical records to capture why clinicians diuresed CR-ARDS more frequently than UR-ARDS, it is reasonable to infer from these data that ARDS recognition may prompt clinicians to target a more conservative fluid balance through increased diuretic administration. Notably, both groups still had a mean cumulative fluid balance of over 3 L positive by ICU day 4, which is substantially more positive than the fluid balance observed in both the conservative arm of the Fluids and Catheter Treatment Trial (FACTT) Study (30) and the “FACTT Lite” protocol used by subsequent NHLBI ARDS Network trials (31). These findings are similar to Seitz et al (32) who also reported that volume overload was common during ARDS and occurred within 24–72 hours, suggesting that overall adherence to conservative fluid management is low in routine clinical practice even at academic tertiary-care centers and even when ARDS is appropriately recognized.
Our findings do raise questions about the importance of ARDS recognition in current clinical practice, particularly as ventilator tidal volumes—a key metric of the quality of ARDS management—were similar between CR-ARDS and UR-ARDS, as were rates of inhospital mortality and ICU length of stay. Clinicians may have relied upon ventilator protocols or incorporated other clinical information (such as static compliance of the respiratory system) that resulted in selection of similar tidal volume irrespective of ARDS recognition. Notably, the CR-ARDS group had a less positive fluid balance and a numerically higher number of ventilator-free days (VFDs), but the difference in VFDs did not reach statistical significance. As our study had fewer patients and the difference in fluid balance between CR-ARDS and UR-ARDS (1,737 mL at ICU day 7) was substantially less than the separation achieved in the FACTT trial (greater than 7,000 mL at ICU day 7), we were likely underpowered to determine if ARDS recognition would translate to earlier liberation from mechanical ventilation.
With the increased use of EHR data for decision support (19, 33, 34) and to facilitate research (35, 36), there is increasing interest in identifying ARDS patients using the EHR (17, 25, 34, 36). Our findings suggest that clinical note text alone may not reliably identify even the most severe ARDS cases. Clinicians and researchers interested in identifying ARDS patients from the EHR will need to consider additional data features to achieve satisfactory classification performance. Our findings also highlight the importance of selecting appropriate outcomes and evaluation methods when designing support systems to improve processes and outcomes in ARDS (37). Changing ventilation practices may not be an optimal intervention for an ARDS recognition support tool, as clinicians can safely implement lower tidal volumes regardless of a patient’s ARDS status using a protocolized approach (38–40). In contrast, fluid management could be a more appropriate process outcome for future studies aimed at improving ARDS recognition. Alternatively, fluid management during mechanical ventilation for any cause of acute respiratory failure could be amenable to a protocolized, diagnosis-independent approach. However, as many clinicians perceive that fluid balance strongly interacts with blood pressure and renal function (41, 42), more data may be required to demonstrate that conservative fluid management is safe, beneficial, and practical outside of the context of ARDS. A small pilot trial of protocolized fluid management in 30 patients with sepsis in our MICU was unable to achieve a prespecified target of at least 500-mL difference between the protocolized care and usual care groups (43), illustrating that implementing a fluid management protocol can face substantial challenges even in a highly motivated academic ICU.
This study has several limitations. First, this observational study was performed at a single MICU within a single center. Despite this, our findings were consistent with other studies reporting poor ARDS recognition among ICU clinicians. Second, our definition of CR-ARDS relied upon EHR-based text searches of clinical documents. Although documentation may not capture the full range of the clinician’s thought process, we feel that documentation of an ARDS diagnosis in a clinical note strongly indicates that ARDS was suspected by the treating clinician. Other studies have used investigator review of the medical record to determine CR-ARDS (7, 8), whereas LUNG-SAFE prospectively used a provider questionnaire once a patient developed hypoxemic respiratory failure (4). Although our methodology may be less sensitive, questionnaires can induce response bias by prompting clinicians to consider a diagnosis that they would not have considered otherwise (44). We also did not consider other EHR data elements such as diagnostic billing codes, but the accuracy of billing codes for identifying ARDS patients is also poor (45). We did not examine how clinician-specific factors such as experience or training level influenced CR-ARDS. Dozens of critical care faculty and over 100 trainees rotate through our MICU every year, so assessing clinician-specific factors was impractical. Expert-adjudicated ARDS status was only available for the first 4 ICU days, but since greater than 90% of ARDS patients in our study met criteria before ICU day 3, the number of patients with later-onset ARDS is likely low. Finally, we did not examine the association of CR-ARDS with rescue therapies such as inhaled pulmonary vasodilators, prone positioning, or venovenous extracorporeal membrane oxygenation, which other studies have reported (4). These rescue therapies were infrequently used in our MICU during the study period; therefore, our study was underpowered for these outcomes.
CONCLUSIONS
In a cohort of adult MICU patients, ARDS recognition was low, with clinicians documenting ARDS in less than half of expert-adjudicated ARDS cases. Clinicians were more likely to recognize ARDS in younger patients and those with more severe respiratory impairment. CR-ARDS was not associated with differences in ventilator tidal volumes, nor was it associated with mortality, ICU length of stay, or ventilator-free days. However, CR-ARDS was associated with more negative fluid balance and greater use of loop diuretics compared with patients with UR-ARDS. Thus, improved ARDS recognition could still be useful for implementing evidence-based interventions that are not as easily amenable to diagnosis-independent protocolization, such as fluid management. These findings have implications for design of future decision support systems and EHR-based ARDS
studies.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Drs. Kerchberger and Brown contributed equally as co-first authors.
Dr. Kerchberger, Mr. Zhao, Drs. Koyama, and Ware had full access to all of the data in the study and take responsibility for the integrity of the data. Drs. Kerchberger, Brown, and Ware designed the study. Drs. Brown, Janz, and Semler contributed to data acquisition. Drs. Kerchberger, Brown, Mr. Zhao, and Dr. Koyama performed data analysis. Drs. Kerchberger, Brown, Semler, Bastarache, and Ware contributed to data interpretation. Drs. Kerchberger, Brown, and Ware contributed to the initial draft of the article. All authors participating in critical revision of the article and important intellectual content; and all authors approved the final article.
Research reported in this publication was supported, in part, by the National Institutes of Health (NIH) under award numbers: NIH T15LM007450 (to Dr. Kerchberger), NIH K01HL157755 (to Dr. Kerchberger), NIH K23HL143053 (to Dr. Semler), NIH R01HL135849 (to Drs. Bastarache and Ware), NIH R01 HL126671 (to Dr. Brown), Department of Defense W81XWH-18-1-0683 (to Dr. Bastarache), and NIH K24HL103836 (to Dr. Ware). The project publication described was also supported by Clinical and Translational Science award number UL1TR002243 from the National Center for Advancing Translational Sciences. This research was also supported by Courtney’s Race for the Acute Respiratory Distress Syndrome Cure and the Courtney Charneco Family.
The authors have disclosed that they do not have any potential conflicts of interest.
The contents are solely the responsibility of the authors and do not necessarily represent official views of the Department of Defense, National Center for Advancing Translational Sciences, or the National Institutes of Health.
All work was performed at the Vanderbilt University Medical Center, Nashville, TN.
REFERENCES
- 1.The ARDS Definition Task Force. Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012; 307:2526–2533 [DOI] [PubMed] [Google Scholar]
- 2.Goss CH, Brower RG, Hudson LD, et al. ; ARDS Network. Incidence of acute lung injury in the United States. Crit Care Med. 2003; 31:1607–1611 [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest. 2007; 131:554–562 [DOI] [PubMed] [Google Scholar]
- 4.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016; 315:788–800 [DOI] [PubMed] [Google Scholar]
- 5.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000; 342:1301–1308 [DOI] [PubMed] [Google Scholar]
- 6.Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med. 2012; 38:1573–1582 [DOI] [PubMed] [Google Scholar]
- 7.Fröhlich S, Murphy N, Doolan A, et al. Acute respiratory distress syndrome: Underrecognition by clinicians. J Crit Care. 2013; 28:663–668 [DOI] [PubMed] [Google Scholar]
- 8.Ferguson ND, Frutos-Vivar F, Esteban A, et al. Acute respiratory distress syndrome: Underrecognition by clinicians and diagnostic accuracy of three clinical definitions. Crit Care Med. 2005; 33:2228–2234 [DOI] [PubMed] [Google Scholar]
- 9.Laffey JG, Pham T, Bellani G. Continued under-recognition of acute respiratory distress syndrome after the Berlin definition: What is the solution? Curr Opin Crit Care. 2017; 23:10–17 [DOI] [PubMed] [Google Scholar]
- 10.Ware LB, Zhao Z, Koyama T, et al. Derivation and validation of a two-biomarker panel for diagnosis of ARDS in patients with severe traumatic injuries. Trauma Surg Acute Care Open. 2017; 2:e000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwede M, Lee RY, Zhuo H, et al. Clinician recognition of the acute respiratory distress syndrome: Risk factors for under-recognition and trends over time. Crit Care Med. 2020; 48:830–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubenfeld GD, Cooper C, Carter G, et al. Barriers to providing lung-protective ventilation to patients with acute lung injury. Crit Care Med. 2004; 32:1289–1293 [DOI] [PubMed] [Google Scholar]
- 13.Weinert CR, Gross CR, Marinelli WA. Impact of randomized trial results on acute lung injury ventilator therapy in teaching hospitals. Am J Respir Crit Care Med. 2003; 167:1304–1309 [DOI] [PubMed] [Google Scholar]
- 14.Weiss CH, Baker DW, Weiner S, et al. Low tidal volume ventilation use in acute respiratory distress syndrome. Crit Care Med. 2016; 44:1515–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss CH, Baker DW, Tulas K, et al. A critical care clinician survey comparing attitudes and perceived barriers to low tidal volume ventilation with actual practice. Ann Am Thorac Soc. 2017; 14:1682–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herasevich V, Yilmaz M, Khan H, et al. Validation of an electronic surveillance system for acute lung injury. Intensive Care Med. 2009; 35:1018–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azzam HC, Khalsa SS, Urbani R, et al. Validation study of an automated electronic acute lung injury screening tool. J Am Med Inform Assoc. 2009; 16:503–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gajic O, Dabbagh O, Park PK, et al. ; U.S. Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS). Early identification of patients at risk of acute lung injury: Evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011; 183:462–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sjoding MW. Translating evidence into practice in acute respiratory distress syndrome: Teamwork, clinical decision support, and behavioral economic interventions. Curr Opin Crit Care. 2017; 23:406–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sjoding MW, Hyzy RC. Recognition and appropriate treatment of the acute respiratory distress syndrome remains unacceptably low. Crit Care Med. 2016; 44:1611–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Neal HR, Jr, Koyama T, Koehler EA, et al. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011; 39:1343–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994; 149:818–824 [DOI] [PubMed] [Google Scholar]
- 23.Giuse DA. Supporting communication in an integrated patient record system. AMIA Annu Symp Proc 2003; 2003:1065. [PMC free article] [PubMed] [Google Scholar]
- 24.Denny JC, Giuse DA, Jirjis JN. The Vanderbilt experience with electronic health records. Semin Colon Rectal Surg 2005; 16:59–68 [Google Scholar]
- 25.McKown AC, Brown RM, Ware LB, et al. External validity of electronic sniffers for automated recognition of acute respiratory distress syndrome. J Intensive Care Med. 2019; 34:946–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lederer DJ, Bell SC, Branson RD, et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019; 16:22–28 [DOI] [PubMed] [Google Scholar]
- 27.Plotly Technologies Inc: Collaborative Data Science. Montreal, QC, 2015. Available at: https://plot.ly. Accessed July 17, 2019 [Google Scholar]
- 28.Waskom ML. seaborn: Statistical data visualization. J Open Source Softw 2021; 6:3021 [Google Scholar]
- 29.Rice TW, Wheeler AP, Bernard GR, et al. ; National Institutes of Health, National Heart, Lung, and Blood Institute ARDS Network. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007; 132:410–417 [DOI] [PubMed] [Google Scholar]
- 30.The Acute Respiratory Distress Syndrome Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006; 354:2564–2575 [DOI] [PubMed] [Google Scholar]
- 31.Grissom CK, Hirshberg EL, Dickerson JB, et al. ; National Heart Lung and Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network. Fluid management with a simplified conservative protocol for the acute respiratory distress syndrome. Crit Care Med. 2015; 43:288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seitz KP, Caldwell ES, Hough CL. Fluid management in ARDS: An evaluation of current practice and the association between early diuretic use and hospital mortality. J Intensive Care. 2020; 8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varghese J, Kleine M, Gessner SI, et al. Effects of computerized decision support system implementations on patient outcomes in inpatient care: A systematic review. J Am Med Inform Assoc. 2018; 25:593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Afshar M, Joyce C, Oakey A, et al. A computable phenotype for acute respiratory distress syndrome using natural language processing and machine learning. AMIA Annu Symp Proc. 2018; 2018:157–165 [PMC free article] [PubMed] [Google Scholar]
- 35.Denny JC, Bastarache L, Ritchie MD, et al. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat Biotechnol. 2013; 31:1102–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeiberg D, Prahlad T, Nallamothu BK, et al. Machine learning for patient risk stratification for acute respiratory distress syndrome. PLoS One. 2019; 14:e0214465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ancker JS, Kern LM, Abramson E, et al. The Triangle Model for evaluating the effect of health information technology on healthcare quality and safety. J Am Med Inform Assoc. 2012; 19:61–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Futier E, Constantin JM, Paugam-Burtz C, et al. ; IMPROVE Study Group. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013; 369:428–437 [DOI] [PubMed] [Google Scholar]
- 39.Lanspa MJ, Gong MN, Schoenfeld DA, et al. Prospective assessment of the feasibility of a trial of low–tidal volume ventilation for patients with acute respiratory failure. Annals ATS 2018; 16:356–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simonis FD, Neto AS, Binnekade JM, et al. Effect of a low vs intermediate tidal volume strategy on ventilator-free days in intensive care unit patients without ARDS: A randomized clinical trial. JAMA 2018; 320:1872, 1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Self WH, Semler MW, Bellomo R, et al. ; CLOVERS Protocol Committee and NHLBI Prevention and Early Treatment of Acute Lung Injury (PETAL) Network Investigators. Liberal versus restrictive intravenous fluid therapy for early septic shock: Rationale for a randomized trial. Ann Emerg Med. 2018; 72:457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu KD, Thompson BT, Ancukiewicz M, et al. ; National Institutes of Health National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Acute kidney injury in patients with acute lung injury: Impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med. 2011; 39:2665–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semler MW, Janz DR, Casey JD, et al. Conservative fluid management after sepsis resuscitation: A pilot randomized trial. J Intensive Care Med. 2020; 35:1374–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furnham A. Response bias, social desirability and dissimulation. Pers Individ Dif. 1986; 7:385–400 [Google Scholar]
- 45.Howard AE, Courtney-Shapiro C, Kelso LA, et al. Comparison of 3 methods of detecting acute respiratory distress syndrome: Clinical screening, chart review, and diagnostic coding. Am J Crit Care. 2004; 13:59–64 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


