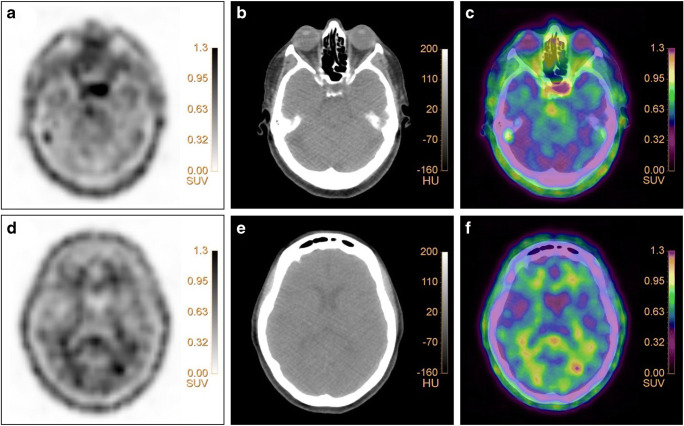
16α-[18F]-fluoro-17β-estradiol ([18F]FES) is a positron emission tomography (PET) tracer, developed for in vivo visualization of the estrogen receptor (ER). It is widely used in oncology, mostly in patients with breast cancer, to identify ER positive lesions. However, besides its application in oncology, [18F]FES has also been evaluated for assessment of ER expression in the brain [1]. This has been investigated in rats where high specific [18F]FES uptake could be observed in regions with high ER density in the brain, i.e., the pituitary gland and hypothalamus [1]. Hattersley et al. confirmed this clinically in healthy post-menopausal women [2]. Strikingly, this study showed that [18F]FES also accumulates in white matter [2], as was also found in a case of our recent study (NCT03726931) and depicted in the Figure. A 71-year-old post-menopausal woman with ER-positive breast cancer underwent [18F]FES PET/CT imaging (Figure: pituitary gland and white matter on PET (a/d), low-dose CT (b/e), and fused PET/CT (c/f) images). The Figure shows the characteristic uptake of the radiotracer in the pituitary gland (SUVmax: 1.88, SUVmean: 1.44) and in white matter (SUVmax:1.36, SUVmean: 1.16). Interestingly, in the study performed by Hattersley et al., [18F]FES uptake in the pituitary gland could be blocked with an ER-antagonist, while uptake in the white matter could not, suggesting that [18F]FES uptake in white matter reflects non-specific uptake [1, 2]. The present finding requires awareness in clinical practice as it suggests that [18F]FES uptake in the pituitary gland and white matter occurs physiologically and that it does not necessarily indicate pathological uptake of the tracer.
Authors’ contributions
R. Iqbal: concept, design, data acquisition, data-analysis and interpretation, drafting, and revision of the manuscript
C. W. Menke-van der Houven van Oordt: concept, data interpretation, and critical review of the manuscript
D.E. Oprea-Lager concept, data interpretation, and critical review of the manuscript
J. Booij: concept, data interpretation, and critical review of the manuscript
Funding
Open access funding provided by Amsterdam UMC (Vrije Universiteit Amsterdam). The scan is performed as part of the FORESIGHT study (NCT03726931), financially supported by the Cancer Center Amsterdam (grant number: CCA2017-1-23).
Data availability
The data supporting the conclusions of this article is included within the article.
Declarations
Ethics approval and consent to participate
This study has been performed in accordance with the Declaration of Helsinki, and it has been approved by the Medical Ethics Review Committee of the Amsterdam UMC – location VUmc. Informed consent was obtained from all individual participants included in the study.
Consent for publication
All participants included in this study provided consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Image of the month
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morago-Amaro R, van Waarde A, Doorduin J, de Vries EFJ. Sex steroid hormones and brain function: PET imaging as a tool for research. J Neuroendocrinol. 2018;30:e12565. doi: 10.1111/jne.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hattersley G, David F, Harris A, et al. A phase 1 dose escalation study of RAD1901, an oral selective estrogen receptor degrader, in healthy postmenopausal women. Cancer Res. 2016;76:abstract nr. 6–13-02. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusions of this article is included within the article.