Abstract
Objective:
Identifying preoperative pulmonary venous obstruction in total anomalous pulmonary venous connection (TAPVC) is important to guide treatment-planning and risk prognostication. No standardized echocardiographic definition of obstruction exists in the literature. Definitions based on absolute velocities are affected by technical limitations and variations in pulmonary venous return. We developed a metric to quantify pulmonary venous blood flow variation: pulmonary venous variability index (PVVI). We aimed to demonstrate its accuracy in defining obstruction.
Methods:
All patients cared for with TAPVC at our institution were identified. Echocardiograms were reviewed, and maximum (Vmax), mean (Vmean), and minimum velocities (Vmin) along the pulmonary venous pathway were measured. PVVI was defined as (Vmax–Vmin)/Vmean. These metrics were compared to pressures measured by cardiac catheterization. Echocardiographic measures were then compared between the patients with and without clinical preoperative obstruction (defined as a need for preoperative intubation, catheter-based intervention, or surgery within one day of diagnosis), as well as pulmonary edema by chest X-ray and markers of lactic acidosis. 137 patients were included with 22 having catheterization pressure recordings.
Results:
Maximum and mean velocity were not different between patients with catheter gradients ≥4 mmHg and <4 mmHg, while PVVI was significantly lower and minimum velocity higher in those with gradients ≥4 mmHg. The composite outcome of preoperative obstruction occurred in 51 patients (37%). Absolute velocities were not different between patients with and without clinical obstruction, while PVVI was significantly lower in patients with obstruction. All metrics except maximum velocity were associated with pulmonary edema; none were associated with blood gas metrics.
Conclusions:
We developed a novel quantitative metric of pulmonary venous flow, which was superior to traditional echocardiographic metrics. Decreased PVVI was highly associated with elevated gradients measured by catheterization and clinical preoperative obstruction. These results should aid risk assessment and diagnosis preoperatively in patients with TAPVC.
Keywords: Congenital heart disease, Pediatric cardiology, Total anomalous pulmonary venous connection, Doppler echocardiography
Introduction
Preoperative pulmonary venous obstruction in total anomalous pulmonary venous connection (TAPVC) is a major cause of morbidity and mortality1-6. The detection of obstruction has important clinical implications; patients may be referred for cardiac catheterization or undergo urgent surgical repair. Pulmonary venous obstruction is especially relevant in patients with single-ventricle heart disease, a particularly vulnerable cohort6-7. Accurate echocardiographic diagnosis of preoperative obstruction would likely lead to improved prognosis and appropriate timing of treatment.
The most common location of preoperative pulmonary venous obstruction in TAPVC is along the course of the vertical vein or at its entrance into the systemic venous circulation. Echocardiographic diagnosis of vertical vein obstruction can be challenging. Most studies of obstruction have used a definition based on a maximum velocity threshold2-6. However, such thresholds vary significantly between studies and have not been standardized2-6. In clinical practice, echocardiographers often report the mean gradient, a strategy that has not been assessed in the literature. Furthermore, any threshold based on either the maximum or mean velocity is dependent on the validity of the assumptions of the simplified Bernoulli equation; thus, they may be poor predictors of obstruction due to the complex and varied nature of anatomy and physiology in TAPVC.
In this study, we hypothesized that quantification of the pulmonary venous Doppler flow pattern would allow detection of obstruction with increased sensitivity and specificity. Qualitative categorization of venous flow patterns has been reported previously8-9, but our goal was to develop a quantitative metric to improve measurement variability. Our first goal was to compare the performance of our metric, termed the “pulmonary venous variability index” (PVVI), against that of absolute measures of velocity to detect elevated gradients from the pulmonary veins to the systemic venous system as measured by cardiac catheterization. We also analyzed whether echocardiographic measures differed between patients with and without clinical, radiographic, and laboratory markers of obstruction.
Methods:
Study Population
We performed a retrospective review of all patients with a diagnosis of TAPVC who received care at the Children’s Hospital of Philadelphia (CHOP). The study was reviewed by our Institutional Review Board and was determined to be exempt from the need for informed consent. Patients were eligible for inclusion if (1) they had a date of birth between 1/1/06 and 12/17/19, (2) had a diagnosis of TAPVC (either isolated or in combination with other congenital heart disease), and (3) had at least one preoperative echocardiogram performed at CHOP. Patients were excluded if (1) they received surgical TAPVC repair at another institution prior to referral to CHOP, (2) no echocardiograms with spectral Doppler interrogation of the pulmonary venous connections were available, or (3) they had a cardiac-type anomalous pulmonary venous connection that did not necessitate repair.
Echocardiographic Measures
Echocardiographic imaging was performed on either a Philips Epiq, iE33, or Sonos 5500 machine (Phillips Medical Systems, Andover, MA) with a probe selected based on patient size and image quality. The images, including spectral Doppler tracings, were obtained during routine clinical care. All images were digitally stored using Syngo Dynamics (Siemens Healthcare, Ann Arbor, MI), which was used for all measurements. Images were retrospectively reviewed, and all calculations were made using off-line measurements made by the investigators (any tracings or measurements made during the original clinical read were ignored). Note was also made of whether the pulmonary venous return was termed obstructed in the original clinical read.
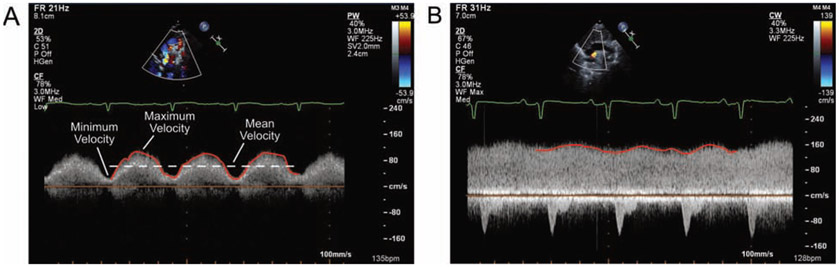
All calculations were performed on the spectral Doppler tracing obtained from the point along the pulmonary venous pathway (e.g., vertical vein, confluence, ductus venosus, or coronary sinus) that had the highest maximum velocity, as this tracing was assumed to have had an angle of interrogation that was most parallel with any possible obstruction. The pulmonary venous velocity was traced over three beats; and the maximum, minimum, and mean velocity over this interval were noted. The pulmonary venous variability index was defined as the maximum velocity minus the minimum velocity divided by the mean velocity: PVVI = (Vmax – Vmin) / Vmean (Fig. 1). The maximum and minimum velocities were chosen from the extreme velocities over the three-beat span.
Figure 1.
Examples of the calculation of maximum, mean, and minimum velocities along with venous variability index (PVVI) in two patients with supracardiac total anomalous pulmonary venous connection. Spectral Doppler tracings of the vertical vein obtained from suprasternal views are shown. (A) In this patient, the PVVI is approximately 1.15. (B) In this patient, the PVVI is about 0.20.
For the main outcomes analyses, all echocardiographic metrics (i.e., Vmax, Vmean, Vmin, and PVVI) were measured by one reader (BRW). In order to determine the intra- and inter-reader reliability, twenty echocardiograms were reviewed twice by the primary reader and once by a second reader (MSC). For intra-reader reliability, these measurements happened at least two weeks apart. In all cases, the readers were blinded to any velocities calculated during the original clinical read, the clinical outcomes, and any prior study measurements.
Study Outcomes
Our first analysis was a comparison of echocardiographic metrics to the gradient from the pulmonary veins to the systemic venous system as measured by cardiac catheterization. We determined which patients had a cardiac catheterization with relevant pressure measurements. One catheterization per patient was paired with an echocardiogram using the following rule. (1) For patients who did not undergo an intervention on their vertical vein, the measured pressure was compared to the most proximate prior echocardiogram if available, or the most proximate subsequent echocardiogram, if necessary. (2) For patients who underwent a vertical vein intervention, the pre-intervention pressure measurement was compared to the most proximate prior echocardiogram. (3) For patients who underwent a vertical vein intervention, but who did not have a pre-catheterization echocardiogram or a pre-intervention gradient measured, then the post-intervention gradient was compared to the most proximate subsequent echocardiogram. The first cardiac catheterization was used for each patient for which one of these conditions could be satisfied.
We considered the pressure gradient as measured by cardiac catheterization as both a continuous variable and as a dichotomized variable. Prior literature has suggested a catheterization-based definition of pulmonary venous obstruction in TAPVC of ≥ 4 mmHg3-4. So, for the dichotomized analysis, patients were divided into two populations, one with gradients ≥ 4 mmHg (obstructed) and those < 4 mmHg (unobstructed). However, in our cohort, multiple patients had gradients by cardiac catheterization of 4-6 mmHg (i.e., obstructed based on this prior definition) but did not receive a vertical vein intervention. The minimum gradient for which a catheter intervention was performed was 7 mmHg. Thus, we performed a second analysis dichotomizing patients based on a new, post hoc clinically-informed definition of > 6 mmHg being obstructed and ≤ 6 mmHg being unobstructed.
We then analyzed whether echocardiographic metrics difference between patients with and without clinical markers of obstruction. We assessed three clinical surrogates for significant pulmonary venous pathway obstruction determined through retrospective chart review: (1) preoperative intubation, (2) catheter-based vertical vein intervention, or (3) surgical repair within one day of diagnosis. Patients were not deemed to have required preoperative intubation if they were intubated solely for stability during transport or if they were intubated in the delivery room for cyanosis and extubated after congenital heart disease was confirmed. We also assessed the composite outcome consisting of patients having at least one of the above markers. For this analysis, the last echocardiogram prior to any catheter or surgical intervention from which Doppler measurements could be made was used for all patients.
Finally, we assessed whether echocardiographic metrics were associated with radiographic and laboratory markers of pulmonary venous obstruction. We considered pulmonary edema, as determined by the clinical read, on chest X-ray (CXR) as a marker of pulmonary venous obstruction. We also extracted markers of hypoperfusion (pH and lactate) from arterial blood gases. For these outcomes, the CXR and blood gas at presentation and prior to surgery were examined, and the worse result of these two was used. Lactate and pH were also dichotomized using thresholds of a lactate ≥ 2 mmol/L and pH < 7.35 as abnormal. The last echocardiogram prior to any catheter or surgical intervention was used for comparison.
Statistical Analysis
Patient information is reported as the number and percentage for categorical variables and as a median and interquartile range (IQR) for continuous variables. All statistical tests were 2-sided and performed at the alpha=0.05 significance level using SAS version 9.4 statistical software or MATLAB version 2019a.
PVVI, Vmax, Vmean and Vmin as well as the calculated pressures (Pmax = 4Vmax2 and Pmean = 4Vmean2) were compared to the pressures by catheterization using Pearson correlation analysis. Then, we compared the patients dichotomized based on cardiac catheterization gradients into obstructed and unobstructed cohorts. For each echocardiographic metric, the distribution of the echocardiographic metric in patients deemed obstructed by catheterization was compared against the distribution in patients deemed unobstructed using the Wilcoxon rank sum test (repeated for both dichotomized catheter definitions of obstruction). In order to calculate sensitivity and specificity for each echocardiographic variable’s ability to discriminate patients deemed obstructed or unobstructed by catheterization, a threshold was chosen to divide patients into obstructed and unobstructed patients based on each echocardiography definition (for Vmax, Vmean, and Vmin, values above the threshold were considered obstructed, and for PVVI, values below the threshold were considered obstructed). Each threshold was manually varied, and sensitivity and specificity were calculated for each possible threshold in order to create an ROC curve from which an area under the curve (AUC) was calculated. The original clinical reads of the echocardiograms (obstructed vs. unobstructed) were compared to the dichotomized catheter definitions of obstruction using Fisher’s exact test (for this analysis, we only considered echocardiograms performed prior to catheterization).
Then, echocardiographic measures were compared to the three clinical outcomes (preoperative intubation, vertical vein intervention, and urgent surgery) as well as the composite outcome. The distributions of echocardiographic variables in the two populations were again compared with Wilcoxon rank sum tests. Using a method analogous to that above, a manual ROC curve analysis was performed to identify sensitivity and specificity for each echocardiographic variable’s ability to discriminate the composite outcome of clinical obstruction. The qualitative reads were compared to clinical markers of obstruction again using Fisher’s exact test. For the analysis of clinical obstruction, we also examined the performance of the different metrics in two subgroups: patients with supracardiac or cardiac TAPVC and patients with infracardiac or mixed TAPVC.
The distributions of echocardiographic variables for patients with and without pulmonary edema on CXR were compared with Wilcoxon rank sum tests. Echocardiographic metrics were compared to arterial lactate and pH by Pearson correlation coefficients; and Wilcoxon rank sum tests were performed using the dichotomized outcomes. The qualitative reads were again compared to the dichotomized outcomes using Fisher’s exact test.
Measurement reliability for all metrics was calculated using the intra-class correlation coefficient (ICC) and the coefficient of variation (using the root-mean-square method). Additionally, we examined whether PVVI was less dependent on angle of insonation than absolute velocity measurements. For thirty echocardiograms, all metrics were calculated from at least two standard views (subcostal, apical, parasternal, and suprasternal). The view with the highest Vmax (i.e., the most aligned with the venous flow) was compared with the view with the second highest Vmax, and agreement was again determined with and ICC and coefficient of variation.
Results:
Demographics
During the study interval, 137 patients satisfied the inclusion and exclusion criteria (Table 1). Forty patients (29%) had heterotaxy syndrome and 55 (40%) had single-ventricle congenital heart disease (38 patients, 28%, had both). Twenty nine patients underwent a preoperative cardiac catheterization, with 22 of these patients having pressure recordings and applicable echocardiograms from comparison. Of these catheterizations, 4 (14%) were performed immediately postpartum due to concerns for vertical vein or atrial septal obstruction on fetal echocardiography, 9 (31%) were performed due a clinical concern (e.g., desaturation or pulmonary edema), and 16 (55%) were performed due to a diagnostic question (e.g., to further define pulmonary venous anatomy or routine pre-Glenn catheterization). Note that in some patients the diagnostic question for the catheterization may have been related to other aspects of their congenital heart disease and not the pulmonary venous return.
Table 1.
Baseline characteristics of the study population.
| N (%) or median (interquartile range) |
||
|---|---|---|
| “Sex | Male | 94 (68.6%) |
| Female | 43 (31.4%) | |
| Gestational age | Term | 106 (77.4%) |
| 35-37 weeks | 14 (10.2%) | |
| <35 weeks | 17 (12.4%) | |
| Birth weight (kg) | 3.09 (2.68, 3.46) | |
| Age at diagnosis (days) | 0 (0, 2) | |
| Type | Cardiac | 29 (20.2%) |
| Supracardiac | 66 (48.1 %) | |
| Infracardiac | 27 (19.7%) | |
| Mixed | 15 (11.0%) | |
| Heterotaxy | 40 (29.2%) | |
| Single-ventricle | 55 (40.1%) | |
| Known genetic anomaly | 20 (14.6%) | |
| Preoperative intubation | 42 (30.7%) | |
| Preoperative catheterization | 29 (21.1%) | |
| Catheter intervention | 7 (5.1%) | |
| Disposition | Surgery | 129 (94.1%) |
| Preoperative Death | 6 (4.4%) | |
| Awaiting Surgery | 2 (1.6%) | |
| Age at surgery (days) | 7 (3, 38) | |
| Days from diagnosis to surgery | 4 (1, 15) | |
| Surgery within 1 day of diagnosis | 36 (26.3%) | |
| Composite outcome | 51 (37.2%) | |
Forty-two patients (31%) were intubated preoperatively. A catheter-based intervention on the vertical vein was performed in 7 patients (5%). Surgery was performed within one day of diagnosis in 36 patients (26%). The composite outcome of the above three events occurred in 51 patients (37%). Eight patients (6%) met the composite outcome solely due to preoperative intubation. Similarly, eight patients (6%) met the composite outcome solely due to surgery within one day of diagnosis. One patient (0.8%) had a vertical vein intervention without being intubated preoperatively.
Comparison with Catheter Gradients
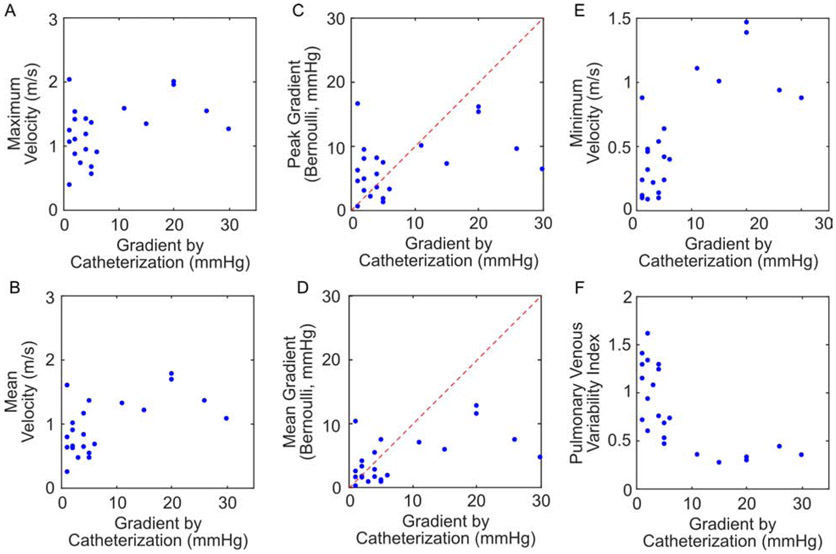
We first compared echocardiographic Doppler measures with gradients measured by cardiac catheterization (N=22). Echocardiograms were performed a median of 5 days prior to the catheterization (IQR: 0 – 15 days). These patients showed a range of gradients through their vertical veins (median: 4 mmHg, IQR: 2-11 mmHg, range: 1-30 mmHg). By echocardiography, maximum velocity ranged from 0.40-2.04 m/s (median: 1.26 m/s, IQR: 0.91-1.54 m/s), corresponding to peak gradients by the simplified Bernoulli equation (ΔP = 4v2) of 0.6-16.6 mmHg (median: 6.4 mmHg, IQR: 3.3-9.5 mmHg). Mean echocardiographic velocities measured 0.26-1.79 m/s (median: 0.88 m/s, IQR: 0.64-1.33 m/s), corresponding to mean gradients of 0.3-12.8 mmHg (median: 3.1 mmHg, IQR: 1.6-7.1 mmHg). Minimum velocities ranged 0.09-1.47 m/s (median: 0.44 m/s, IQR: 0.22-0.88 m/s).
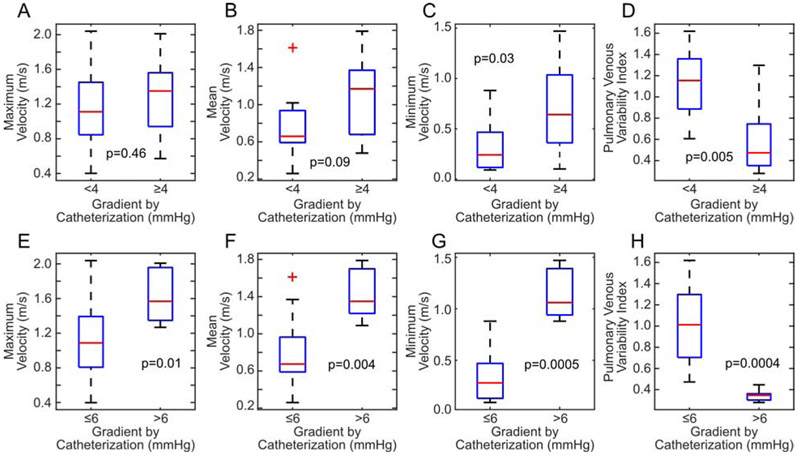
The maximum velocity did show a modest positive correlation with increasing gradient (r = 0.42), however, this failed to attain statistical significance (p = 0.053, Fig. 2A). Increasing mean velocity was significantly correlated with increasing catheter gradients (r = 0.57, p = 0.006, Fig. 2B). When using the simplified Bernoulli equation, we observed that the use of maximum or mean pressures poorly estimated the gradient by catheterization; particularly, echocardiography underestimated catheter gradients over 10 mmHg (Fig. 2C-D). Minimum velocity had the highest correlation with catheter gradients (r = 0.74, p = 0.0001, Fig 2E). Using the threshold published in prior literature, we divided patients into those deemed obstructed by cardiac catheterization (gradient ≥ 4 mmHg) and those deemed unobstructed (< 4 mmHg). Maximum velocity was not different between those patients below (median: 1.11 m/s, IQR: 0.95 – 1.45 m/s) and above (median: 1.35 m/s, IQR: 0.94 – 1.56 m/s) this threshold (p = 0.46, Fig. 3A). Mean velocity was higher in obstructed patients (median: 0.66 m/s, IQR: 0.59 – 0.94 m/s vs. median: 1.17 m/s, IQR: 0.68 – 1.37 m/s), but this did not reach statistical significance (p = 0.088, Fig. 3B). Minimum velocity was the only absolute velocity measure that was significantly higher in obstructed patients (median: 0.24 m/s, IQR: 0.12 – 0.47 m/s vs. median: 0.64 m/s, IQR: 0.36 – 1.04 m/s, p = 0.03, Fig. 3C).
Figure 2.
Comparison of Doppler echocardiographic measures with catheter gradients (N=22). (A) Maximum velocity. (B) Mean velocity. (C) Peak gradient calculated from the maximum velocity and the simplified Bernoulli equation. (D) Mean gradient calculated from the mean velocity and the simplified Bernoulli equation. In (C) and (D), the red dashed line shows the line of unity. (E) Minimum velocity. (F) Pulmonary venous variability index.
Figure 3.
Assessment of the ability of echocardiographic measures to discriminate patients with and without elevated catheterization gradients. (A-D, first row) Using the traditional threshold of < 4 mmHg or ≥ 4 mmHg, minimum velocity and pulmonary venous variability index (PVVI) were able to predict elevated catheterization gradients with statistical significance. (E-H, second row). With a slightly modified threshold of > 6 mmHg or ≤ 6 mmHg, all metrics were able to discriminate the obstructed patients, although PVVI does so with the best sensitivity and specificity.
Decreasing PVVI was significantly correlated with increasing catheter gradients (r = −0.68. p = 0.0004, Fig. 2F). Note that PVVI appears to depend nonlinearly on catheter gradient; however, to maintain a consistent model across all echocardiographic metrics, a Pearson correlation coefficient was still used. Patients below the traditional catheter-measured threshold of ≥4 mmHg had a median PVVI of 1.15 (IQR: 0.87 – 1.36), while those above the threshold had a median PVVI of 0.47 (IQR: 0.35 – 0.74). This difference was statistically significant (p = 0.005, Fig. 3C). A threshold of PVVI ≤ 0.77 was able to discriminate obstructed patients with a sensitivity of 85% and a specificity of 78% (AUC = 0.87).
We repeated the above analysis with a clinically-informed threshold of >6 mmHg defining obstruction. Using this alternative threshold definition, all Doppler echocardiographic metrics were significantly different in obstructed patients (Vmax: p = 0.01, Vmean: p = 0.004, Vmin: p = 0.005, PVVI: p = 0.0004, Fig. 3E-H). Now, a threshold of PVVI of ≤ 0.45 was able to differentiate obstructed and unobstructed patients with 100% sensitivity and specificity. A threshold of Vmax ≥ 1.27 m/s resulted in 100% sensitivity and 69% specificity, a threshold of Vmean ≥ 1.09 m/s resulted in 100% sensitivity and 81% specificity, and a threshold of Vmin > 0.75 m/s had 100% sensitivity and 94% specificity.
Of the patients receiving cardiac catheterizations, the echocardiogram was read as obstructed in six patients (30%), all with measured catheterization gradients of ≥ 5 mmHg. The qualitative opinion of the original clinical reader was associated with catheter gradients ≥ 4 mmHg and > 6 mmHg (p = 0.04 and p = 0.0004, respectively). As no echocardiograms with a gradient of 4 mmHg were read as obstructed, the sensitivity of the qualitative read was highly dependent on the threshold chosen; the qualitative opinion of the echocardiographer had a sensitivity of only 50% for gradients ≥ 4 mmHg, but the sensitivity was 100% for gradients > 6 mmHg.
Comparison with Clinical Variables
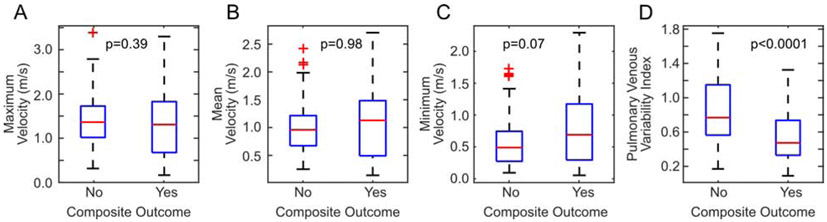
Next, we analyzed how well Doppler echocardiographic measures corresponded to preoperative clinical outcomes consistent with preoperative obstruction. PVVI was lower in patients who were intubated preoperatively, in patients who had surgery within one day of diagnosis, and in patients who received a catheter intervention (Table 2). No absolute velocity metrics were statistically different between any of these populations (Table 2). A similar pattern held for the composite outcome of preoperative obstruction. While PVVI was lower in patients who met the composite outcome compared to those patients who did not (p < 0.0001, Table 2, Fig. 4), maximum (p = 0.39) and mean velocities (p = 0.98) were not different between these groups; minimum velocity was higher in patients who met the composite outcome, but not statistically significant (p = 0.07).
Table 2.
Comparison of echocardiographic metrics between those patients who satisfied clinical outcomes of preoperative obstruction and those who did not. Data are presented as medians and interquartile ranges (IQR).
| Metric | No Preoperative Intubation (N=95) |
Preoperative Intubation (N=42) |
p-value |
|---|---|---|---|
| Pulmonary venous variability index | 0. 76 (0.56 – 1.12) | 0. 42 (0.32 - 0.70) | < 0.0001 |
| Maximum Velocity (m/s) | 1.34 (0.99 – 1.70) | 1.35 (0.66 – 1.83) | 0.66 |
| Mean Velocity (m/s) | 0.95 (0.67 – 1.25) | 1.16 (0.51 – 1.49) | 0.72 |
| Minimum Velocity (m/s) | 0.49 (0.28 – 0.79) | 0.68 (0.30 – 1.19) | 0.06 |
| No Urgent Surgery (N=101) |
Urgent Surgery (N=36) | p-value | |
| Pulmonary venous variability index | 0.72 (0.52 – 1.12) | 0.48 (0.32 – 0.78) | 0.0002 |
| Maximum Velocity (m/s) | 1.35 (1.00 – 1.65) | 1.34 (0.65 – 1.87) | 0.67 |
| Mean Velocity (m/s) | 0.97 (0.67 – 1.24) | 1.10 (0.44 – 1.60) | 0.96 |
| Minimum Velocity (m/s) | 0.52 (0.29 – 0.86) | 0.69 (0.27 – 1.20) | 0.08 |
| No Catheter Intervention (N=130) |
Catheter Intervention (N=7) |
p-value | |
| Venous variability index | 0.67 (0.45 – 1.05) | 0.35 (0.30 – 0.55) | 0.046 |
| Maximum Velocity (m/s) | 1.35 (0.93 – 1.73) | 1.26 (1.13 – 1.60) | 0.80 |
| Mean Velocity (m/s) | 0.97 (0.65 −1.35) | 1.16 (0.89 – 1.41) | 0.58 |
| Minimum Velocity (m/s) | 0.53 (0.27 – 0.98) | 0.86 (0.64 – 1.18) | 0.27 |
| No Composite Outcome (N=86) |
Composite Outcome (N=51) |
p-value | |
| Venous variability index | 0.77 (0.57 – 1.15) | 0.48 (0.33 – 0.74) | < 0.0001 |
| Maximum Velocity (m/s) | 1.36 (1.01 – 1.72) | 1.30 (0.67 – 1.82) | 0.39 |
| Mean Velocity (m/s) | 0.96 (0.67 −1.21) | 1.13 (0.49 – 1.48) | 0.98 |
| Minimum Velocity (m/s) | 0.49 (0.27 – 0.74) | 0.69 (0.29 – 1.17) | 0.07 |
Figure 4.
Differences in echocardiographic metrics between patients with and without the composite outcome of clinical preoperative obstruction. Maximum (A), mean (B), and minimum (C) velocities were unable to discriminate patients with and without the outcome. Patients with the composite outcome had statistically significantly lower pulmonary venous variability indices (D).
In the entire cohort, the echocardiogram was read as obstructed in 60 patients (44%). The qualitative read of the echocardiographer was not associated with vertical vein intervention (p > 0.99). However, the qualitative read was associated with preoperative intubation (p = 0.002), with the need for surgery within one day of diagnosis (p = 0.0004), and with the composite outcome (p = 0.0007).
Examining patients with supracardiac or cardiac TAPVC, the same relationships held. PVVI (p = 0.01) and Vmin (p = 0.048) were statistically associated with the composite outcome (p = 0.002). Vmax (p = 0.90) and Vmean (p = 0.41) were not associated with the composite outcome. Additionally, the qualitative opinion of the echocardiographer was associated with the outcome in supracardiac or cardiac TAPVC patients (p = 0.03). In patients with infracardiac or mixed TAPVC, PVVI was the only metric associated with the composite outcome (p = 0.01); Vmax (p = 0.09), Vmean (p = 0.32), Vmin (p = 0.97), and the qualitative opinion of the echocardiographer (p = 0.34) were not.
When assessing the composite outcome using ROC analysis, PVVI was able to yield reasonable area under the curve of 0.71 (Fig. 5). Choosing a threshold with the goal of prioritizing sensitivity, resulted in a choice of defining obstruction as a PVVI ≤ 0.92 yielding a sensitivity of 88.3% and a specificity of 43.0%. Maximum, mean, and minimum velocities were not sensitive or specific in discriminating whether patients would meet the composite outcome with AUCs not significantly different than 0.5 (Fig. 5). The opinion of the echocardiographer as to whether there was obstruction was moderately sensitive (63%) and specific (67%).
Figure 5.
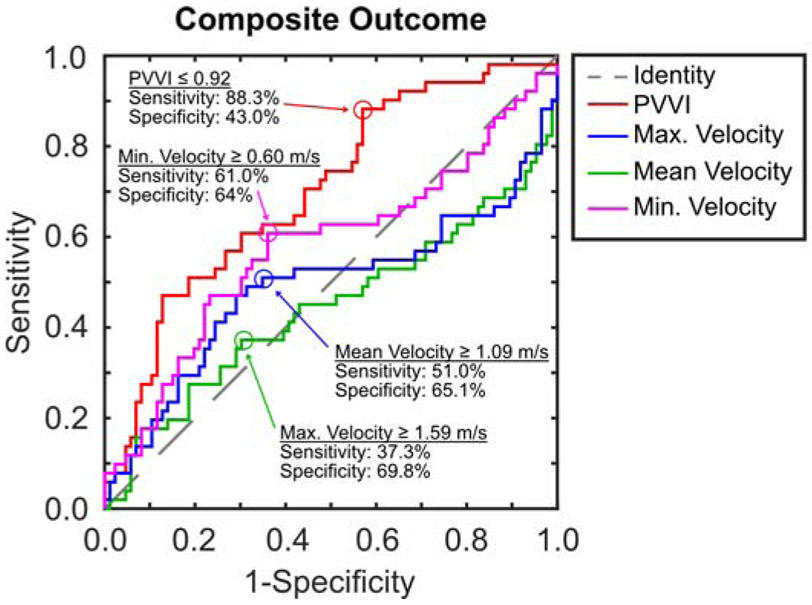
ROC curves for all four echocardiographic variables showing the effect of changing the threshold on sensitivity and specificity for discriminating the composite outcome of clinical preoperative obstruction. Possible cut-points prioritizing sensitivity are also shown.
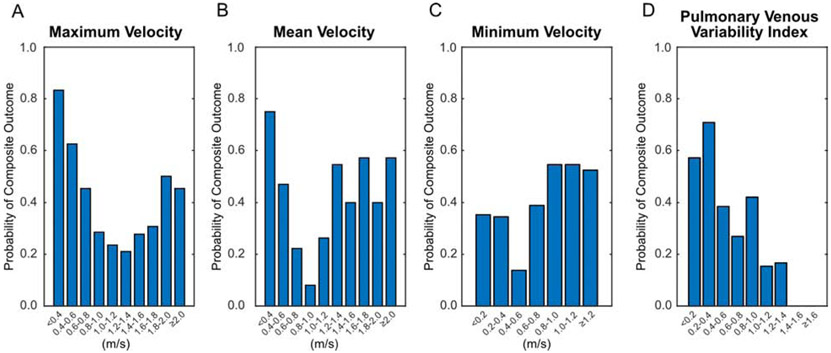
We then calculated the probability of satisfying the composite outcome for patients within each interval of velocity or PVVI (Fig. 6). For absolute velocity measurements, clinical preoperative obstruction was frequently seen in patients with maximum and mean velocities towards both the high and low extremes, while the likelihood of obstruction was lowest for velocities in the middle of the range (Vmax 1.0-1.4 m/s and Vmean 0.6-1.2 m/s). Minimum velocity did not show a clear trend that would indicate any measurement was particularly high risk for the outcome. Alternatively, as PVVI increased, the probability of preoperative obstruction nearly monotonically decreased. No patient with PVVI ≥ 1.4 met the outcome of obstruction.
Figure 6.
Probability of satisfying the composite outcome of clinical preoperative obstruction for patients with different ranges of the four echocardiographic metrics. For both maximum (A) and mean (B) velocities, both high and low velocities result in higher risk of preoperative obstruction. Thus, no single threshold is able to discriminate patients. Minimum velocity (C) shows minimal overall trend with slightly higher rates at faster velocities. Conversely, decreasing pulmonary venous variability index (D) results in higher risks of obstruction.
Comparison with Laboratory Variables
A CXR was available for 131 patients with 80 (61%) having pulmonary edema. PVVI was lower (p < 0.0001), and Vmean (p = 0.006) and Vmin (p < 0.0001) were higher in patients with pulmonary edema, while Vmax (p = 0.06) was higher in patients with edema but failed to meet criteria for statistical significance (Table 3). Arterial pH was available in 67 patients (median 7.38, IQR: 7.34-7.41), and arterial lactate was available in 42 (median 2.4 mmol/L, IQR: 1.9-3.4 mmol/L). No echocardiographic metrics were correlated with pH (PVVI: p = 0.73; Vmax: p = 0.73; Vmean: p = 0.46; Vmin: p = 0.68); similarly echocardiographic metrics were not different when pH was dichotomized based on a threshold of pH < 7.35 being abnormal (Table 3). No echocardiographic metrics were correlated with lactate (PVVI: p = 0.17; Vmax: p = 0.07; Vmean: p = 0.11; Vmin: p = 0.16); and, echocardiographic metrics were not different when lactate was dichotomized based on a threshold of ≥ 2 mmol/L being abnormal (Table 3). The qualitative opinion of the echocardiographer as to whether there was pulmonary venous obstruction was associated with pulmonary edema on CXR (p = 0.0004) but not pH (p = 0.81) or lactate (p = 0.84).
Table 3.
Comparison of echocardiographic metrics between those patients who satisfied radiographic and laboratory outcomes of preoperative obstruction and those who did not. Data are presented as medians and interquartile ranges (IQR).
| Metric | No Pulmonary Edema on Chest X-Ray (N=51) |
Pulmonary Edema on Chest X-Ray (N=80) |
p-value |
|---|---|---|---|
| Pulmonary venous variability index | 0.92 (0.64 – 1.27) | 0.59 (0.37 - 0.86) | < 0.0001 |
| Maximum Velocity (m/s) | 1.12 (0.88 – 1.58) | 1.39 (1.01 – 1.84) | 0.06 |
| Mean Velocity (m/s) | 0.82 (0.59 – 1.04) | 1.09 (0.71 – 1.49) | 0.006 |
| Minimum Velocity (m/s) | 0.33 (0.23 – 0.57) | 0.67 (0.45 – 1.09) | < 0.0001 |
| Lactate < 2 mmol/L (N=11) |
Lactate ≥ 2 mmol/L (N=31) |
p-value | |
| Pulmonary venous variability index | 0.64 (0.48 – 0.78) | 0.52 (0.35 – 0.94) | 0.12 |
| Maximum Velocity (m/s) | 1.00 (0.66 – 1.51) | 1.43 (0.73 – 1.70) | > 0.99 |
| Mean Velocity (m/s) | 0.71 (0.45 – 1.14) | 1.09 (0.53 – 1.39) | 0.72 |
| Minimum Velocity (m/s) | 0.41 (0.26 – 0.77) | 0.66 (0.33 – 0.98) | 0.47 |
| pH ≥ 7.35 (N=47) | pH < 7.35 (N=20) | p-value | |
| Venous variability index | 0.65 (0.40 – 0.95) | 0.55 (0.37 – 0.82) | 0.14 |
| Maximum Velocity (m/s) | 1.22 (0.91 – 1.59) | 1.30 (0.59 – 1.58) | 0.15 |
| Mean Velocity (m/s) | 0.90 (0.56 −1.30) | 1.02 (0.47 – 1.35) | 0.41 |
| Minimum Velocity (m/s) | 0.53 (0.32 – 0.89) | 0.67 (0.21 – 1.01) | 0.93 |
Assessment of Measurement Reliability
Intra-rater reliability as measured by ICCs was excellent with values above 0.93 for all measures (Table 4). Inter-rater reliability was slightly lower, but good with ICCs above 0.73 for all measures. When comparing absolute velocities calculated from different echocardiographic views, agreement was poor (ICCs<0.60), as expected due to differences in the angle of insonation. PVVI was relatively invariant (ICC>0.85) when calculated from different views (Table 4). Variation as assessed by the coefficient of variation was higher for PVVI than for absolute velocities, except when considering variation across echocardiographic views, where PVVI was superior (Table 4).
Table 4.
Intra- and inter-rater reliability of echocardiographic metrics by intraclass correlation coefficient with 95% confidence intervals (CI) as well as coefficients of variation.
| Metric | Intra-rater reliability (95% CI) |
Inter-rater reliability (95% CI) |
Across echocardiographic views (95% CI) |
|---|---|---|---|
| Pulmonary venous variability index | 0.935 (0.848, 0.973) | 0.739 (0.466, 0.884) | 0.858 (0.725. 0.929) |
| Maximum Velocity | 0.940 (0.860, 0.975) | 0.780 (0.539, 0.904) | 0.424 (0.086, 0.676) |
| Mean Velocity | 0.975 (0.940, 0.990) | 0.879 (0.664, 0.934) | 0.408 (0.067, 0.666) |
| Minimum Velocity | 0.986 (0.967, 0.994) | 0.846 (0.664, 0.934) | 0.586 (0.301, 0.775) |
| Metric | Intra-Reader Coefficient of Variation |
Inter-Reader Coefficient of Variation |
Coefficient of Variation across echocardiographic views |
| Pulmonary venous variability index | 11.6% | 30.6% | 23.3% |
| Maximum Velocity | 6.5% | 13.1% | 43.7% |
| Mean Velocity | 5.1% | 10.8% | 44.4% |
| Minimum Velocity | 13.1% | 21.1% | 47.1% |
Discussion:
We evaluated a novel quantitative metric, termed the pulmonary venous variability index, with the goal of better predicting preoperative pulmonary venous obstruction in patients with TAPVC in a reproducible fashion. Our results demonstrate that across all analyses, PVVI had superior performance to more typical Doppler echocardiographic measures including maximum, mean, and minimum velocity. PVVI was better able to discriminate patients with elevated pulmonary venous gradients measured by cardiac catheterization. PVVI was also more associated with clinical markers that may represent preoperative obstruction than absolute velocity measures.
Our results highlight that the use of absolute velocity parameters alone is likely inadequate to assess for preoperative obstruction and to determine patient risk. This limitation of Doppler echocardiographic velocities is expected due to the limitations of the simplified Bernoulli equation. The use of an increased velocity as a proxy for stenosis relies on the following assumptions: (1) the stenotic region has no length, (2) the upstream velocity is negligible compared to the measured velocity, (3) the angle of insonation is close to parallel, and (4) the amount of blood flow through the measured region is independent of any obstruction. All of these assumptions are violated when assessing a vertical vein by echocardiography. The amount of pulmonary blood flow may differ significantly between patients or over time in the same patient (e.g., due to the normal neonatal transition or iatrogenic factors such as the degree of respiratory support). As TAPVC is a complete-mixing lesion, severe pulmonary venous obstruction could lead to pulmonary arterial hypertension and decreased pulmonary blood flow. Many TAPVC patients also have single-ventricle heart disease and undergo palliative procedures that alter pulmonary blood flow; thus these limitations are even more relevant.
Decreases in flow variation (as assessed by PVVI) are likely relatively insensitive to the exact anatomic nature of the stenosis or overall amount of pulmonary venous return. Additionally, note that since all of the measured absolute velocities are linearly dependent on the cosine of the angle of insonation, PVVI is independent of the angle of insonation, which accounts for its better performance across difference echocardiographic views. As the pulmonary venous return in TAPVC may be tortuous and difficult to visualize fully from any one angle, a metric able to be calculated robustly despite differences in technique may aid longitudinal monitoring. These benefits of PVVI indicate why it may be more clinically-applicable to the changing dynamics of congenital heart disease that absolute velocity measures.
Interestingly, the qualitative opinion of the echocardiographer performed reasonably well in detecting severe obstruction. However, qualitative judgements may be biased, impacting reliability and repeatability. As we used the original clinical reads for this analysis, we could not assess for inter- or intra-reader reliability. PVVI provides a quantitative metric that parallels what many experienced sonographers use to define obstruction qualitatively. In this manner, PVVI is analogous to ejection fraction as a mechanism for quantifying ventricular function. While generally accurate, qualitative assessments of function are prone to variability10-11, and the use of quantitative metrics increases reliability in both echocardiography10-12 and cardiac magnetic resonance imaging13. While the coefficient of variation between readers for PVVI was relatively high (30%), we expect that agreement could be improved in the future through standardization of technique.
Others have assessed the pulmonary venous Doppler pattern using qualitative and quantitative metrics. Our index was inspired by the definition of pulsatility index (PI), often used in fetal echocardiography14 to assess arterial flow. A similar index, termed the “pulsatility index for veins” (PIV) has been proposed for fetal echocardiography, particularly in the setting of left heart obstruction15-17. Our definition differs from PI and PIV in that we have removed the necessity to measure the velocity at specific times such as ventricular systole, atrial systole, or early diastole (as the pulmonary venous return in TAPVC rarely has a typical pattern with S-, D-, and A-waves). To our knowledge, ours is the first quantitative metric of pulmonary venous flow patterns in TAPVC.
Unfortunately, it is relatively common for studies of TAPVC to use obstruction as predictor of outcomes without mention of how obstruction was defined1,7,18-26. Such studies likely rely on mention of obstruction in the medical record, which lacks quantitation and reliability. Some previous studies have explicitly mentioned that their composite echocardiographic definition of pulmonary venous obstruction included the presence of continuous non-pulsatile flow without giving specifics as to their definitions27-30. Some fetal studies have been more explicit with their qualitative assessment by dividing Doppler tracings into categories such as “biphasic”, “biphasic with decreased pulsatility”, “monophasic”, and “continuous”9,31-32. While monophasic or continuous waveforms in these studies appeared to correspond to pulmonary venous obstruction, the cohort sizes were too small for statistical analysis.
The most common quantitative method to echocardiographically define preoperative obstruction for research studies is the maximum measured Doppler velocity2-6,28-30,33-36. However, there is no consistent threshold, with 1.2 m/s6, 1.3 m/s37, 1.5 m/s5,35-36, 1.6 m/s38, 1.7 m/s27, 1.8 m/s28,30, and 2.0 m/s2-4,29,33-34 all having been reported. In our experience, maximum velocity is rarely used clinically, with the mean gradient being more common. The mean echocardiographic gradient has been used in some studies of TAPVC5,35-36, but interestingly only as part of a composite variable along with the maximum velocity. Using the mean gradient as the sole echocardiographic method to define obstruction has been more common in the setting of postoperative pulmonary venous obstruction or in primary pulmonary venous obstruction (i.e., in patients without TAPVC)39-40.
While in some studies2,5,29-30, echocardiography alone was used to define obstruction, echocardiographic measures are commonly combined with gradients measured by cardiac catheterization or clinical variables into a composite definition3-4,6,28,34. Usually a catheterization definition of obstruction as greater than or equal to 4 mmHg is used3-4,6,33-34, although occasionally the criteria used have not been clearly defined28.
As this brief review demonstrates, the clinical literature on pulmonary venous obstruction in TAPVC has been beset by substantial heterogeneity. This variation in study design limits the adoption of standardized methods into clinical practice, and it also likely complicates meta-analyses41 and large registries42. How variability in definitions affects prognostication of outcomes has been rarely studied. We are unaware of any prior works that have directly compared echocardiography to catheterization markers of obstruction in this population. Similarly, we know of no studies that have examined how data from echocardiographic, catheterizations, and clinical markers should be integrated to assess the status of a patient. Our study is a first step in more rigorously analyzing such definitions to better standardize practice.
Although consideration of any echocardiographic metric in the setting of longitudinal data and the clinical status of the patient is ideal, it is natural to define obstruction via a threshold. Should one continue to use absolute velocity thresholds, our comparison with catheterization data suggests a Vmax threshold of greater than about 1.2 m/s as defining obstruction, which is substantially lower than that used in most prior studies, although similar to that found in our prior work6. Using PVVI, a threshold of ≤ 0.5 appeared to define severe obstruction (i.e., patients with elevated gradients by catheterization). Patients with a PVVI of less than about 0.9 were at risk for the clinical outcome.
The test characteristics of PVVI (as well as those for absolute velocities) should be validated in another sample. Specifically, the sensitivity and specificity values reported in this relatively small sample represent a best case scenario (especially as regards the catheter gradient comparison). Additionally, there is the potential for bias if clinical decisions for the patients in our cohort were made based on judgements about the same pulmonary venous flow characteristics that we studied. Echocardiographers may have read studies as obstructed based off of velocity or pressure calculations or based on a qualitative flow pattern. However, such bias would likely affect all measures in this study and would not account for the results seen.
Our study is limited by its retrospective nature. Cardiac catheterization remains the gold standard for pressure measurements, although such data were only available for a minority of the cohort as preoperative catheterization of TAPVC patients is not typically standard of care. However, in our cohort, patients were referred for catheterization for a variety of reasons, often not for clinical suspicion of pulmonary venous obstruction. Thus, we feel that these results are generalizable to the TAPVC population as whole. Additionally, the physiologic state of the patient (including the amount of pulmonary blood flow) may have differed between the echocardiogram and the catheterization.
When examining the entire cohort, we assessed clinical, radiographic, and laboratory variables that we felt were associated with preoperative obstruction. Unfortunately, it is difficult to determine retrospectively who was felt by the clinical team to have obstructed pulmonary venous return. The documentation in the medical record was often incomplete, and in patients with additional congenital heart diseases or prematurity, symptoms, pulmonary edema, or lactic acidosis may have been due to a variety of conditions. Furthermore, the clinical suspicion of obstruction by the providers may have been incorrect or been subject to variability. Thus, patients may have satisfied an outcome due to reasons unrelated to obstruction. For example, some patients may have been intubated for non-cardiac reasons. While patients intubated solely for transport were not considered to satisfy the outcome, we could not determine all patients that may have been intubated for “convenience”. As granular laboratory and clinical data were not available for all patients (especially those at the beginning of the study window), it was not possible to determine the clinical indication for every intubation or surgical date. That PVVI outperforms all other echocardiographic markers, as well as the subjective opinion on the echocardiographer, on both the composite outcome and all subset outcomes gives reassurance that PVVI is able to capture underlying pulmonary venous obstruction despite these limitations.
In our cohort, we also did not attempt to differentiate patients with single-ventricle heart disease or heterotaxy syndrome (including any possible effects due to the presence or absence of antegrade pulmonary blood flow). Different metrics may perform better when limited to those patients with isolated TAPVC. Additionally, the presence of other congenital heart disease may affect the timing of repair as, in these patients, TAPVC repair was often timed to coincide with other surgical procedures (e.g., Blalock-Taussig shunt or Glenn). In our cohort, the majority of patients were repaired at less than one month of age, even when the pulmonary veins were deemed unobstructed. The optimal timing of TAPVC repair remains an open question, but is an important issue as routine pulse oximetry screening may lead to earlier diagnosis of TAPVC in neonates without obstruction. As PVVI outperforms the subjective determination of obstruction, the use of this metric may improve risk prognostication at diagnosis to help decide who is safe for outpatient management and who needs more urgent repair. Obstruction is not dichotomous; obstruction may worsen over time, and some patients may tolerate a small pulmonary venous gradient. A reliable quantitative metric might allow improved monitoring of trends and earlier detection of worsening obstruction.
In conclusion, our proposed metric of pulmonary venous flow variation, PVVI, performed superiorly to commonly-used echocardiographic velocity definitions in determining clinically meaningful degrees of preoperative obstruction in patients with total anomalous pulmonary venous connection. The further development of quantitative guidelines should improve diagnosis and clinical decision-making in this challenging group of patients.
Highlights.
Pulmonary venous variability index (PVVI) is a new metric of pulmonary venous obstruction in TAPVC.
Absolute echocardiographic velocities poorly predict catheter gradients.
Lower PVVI accurately predicts elevated gradients by catheterization.
PVVI better predicts clinical markers of venous obstruction.
Acknowledgments
Funding
This work was supported by the National Institutes of Health [Grant numbers T32HL007915 and K08NS117897], the Cardiac Center Clinical Research Core at the Children’s Hospital of Philadelphia, and the Children’s Hospital of Philadelphia Research Institute.
Abbreviations
- AUC
Area under the curve
- CXR
Chest X-Ray
- ICC
Intraclass correlation coefficient
- IQR
Interquartile range
- PI
Pulsatility index
- PIV
Pulsatility index for veins
- Pmax
Maximum pressure gradient
- Pmean
Mean pressure gradient
- TAPVC
Total anomalous pulmonary venous connection
- Vmax
Maximum velocity
- Vmean
Mean velocity
- Vmin
Minimum velocity
- PVVI
Pulmonary venous variability index
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kirshbom PM, Myung RJ, Gaynor JW, Ittenbach RF, Paridon SM, DeCampli WM, et al. Preoperative pulmonary venous obstruction affects long-term outcome for survivors of total anomalous pulmonary venous connection repair. Ann Thorac Surg 2002;74:1616–20. DOI: 10.1016/S0003-4975(02)03935-8 [DOI] [PubMed] [Google Scholar]
- 2.Karamlou T, Gurofsky R, Al Sukhni E, Coles JG, Williams WG, Caldarone CA, et al. Factors associated with mortality and reoperation in 377 children with total anomalous pulmonary venous connection. Circulation 2007;115:1591–8. DOI: 10.1161/CIRCULATIONAHA.106.635441 [DOI] [PubMed] [Google Scholar]
- 3.Seale AN, Uemura H, Webber SA, Partridge J, Roughton M, Ho SY, et al. Total anomalous pulmonary venous connection: morphology and outcome from an international population-based study. Circulation 2010;122:2718–26. DOI: 10.1161/CIRCULATIONAHA.110.940825 [DOI] [PubMed] [Google Scholar]
- 4.Nakayama Y, Hiranmatsu T, Iwata Y, Okamura T, Konuma T, Matusmura G, et al. Surgical results for functional univentricular heart with total anomalous pulmonary venous connection over a 25-year experience. Ann Thorac Surg 2012;93:606–13. DOI: 10.1016/j.athoracsur.2011.09.038 [DOI] [PubMed] [Google Scholar]
- 5.Hoashi T, Kagisaki K, Kurosaki K, Kitano M, Shiraishi I, Ichikawa H. Instrinsic obstruction in pulmonary venous drainage pathway is associated with poor surgical outcomes in patients with total anomalous pulmonary venous connection. Pediatr Cardiol 2015;36:432–7. DOI: 10.1007/s00246-014-1031-2 [DOI] [PubMed] [Google Scholar]
- 6.White BR, Ho DY, Faerber JA, Katcoff H, Glatz AC, Mascio CE, et al. Repair of total anomalous pulmonary venous connection: risk factors for postoperative obstruction. Ann Thorac Surg 2019;108:122–9. DOI: 10.1016/j.athoracsur.2019.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaynor JW, Collins MH, Rychik J, Gaughan JP, Spray TL. Long-term outcomes of infants with single ventricle and total anomalous pulmonary venous connection. J Thorac Cardiovasc Surg 1999;117:506–14. DOI: 10.1016/S0022-5223(99)70330-2 [DOI] [PubMed] [Google Scholar]
- 8.Files MD and Morray B. Total anomalous pulmonary venous connection: preoperative anatomy, imaging, and interventional management of postoperative pulmonary venous obstruction. Semin Cardiothroac Vasc Anesth 2017;21:123–31. DOI: 10.1177/1089253216672442 [DOI] [PubMed] [Google Scholar]
- 9.Ganesan S, Brook MM, Silverman NH, Moon-Grady AJ. Prenatal findings in total anomalous pulmonary venous return: a diagnostic road map startes with obstetric screening views. J Ultrasound Med 2014;33:1193–207. DOI: 10.7863/ultra.33.7.1193 [DOI] [PubMed] [Google Scholar]
- 10.Johri A, Picard MH, Newell J, Marshall JE, King MEE, Hung J. Can a teaching observation reduce interobserver variabilty in LVEF assessment. J Am Coll Cardiol Img 2011;4:821–9. DOI: 10.1016/j.jcmg.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 11.Ling LF, Obuchowski NA, Rodriguez L, Popovic Z, Kwon D, Marwick TH. Accuracy and interobserver concordance of echocardiographic assessment of right ventricular size and systolic function: a quality control exercise. J Am Soc Echocardiogr 2012;25:709–13. DOI: 10.1016/j.echo.2012.03.018 [DOI] [PubMed] [Google Scholar]
- 12.Thavendiranathan P, Popovic ZB, Flamm SD, Dahiya A, Grimm RA, Marwick TH. Improved interobserver variability and accuracy of echocardiographic visual left ventricular ejection fraction assessment through a self-directed learning program using cardiac magnetic resonance images. J Am Soc Echocardiogr 2013;26:1267–73. DOI: 10.1016/j.echo.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 13.Sievers B, Kirchberg S, Franken U, Puthenveettil BJ, Bakan A, Trappe HJ. Visual estimation versus quantitative assessmen of left ventricular ejection fraction: a comparison by cardiovascular magnetic resonance imaging. Am Heart J 2005;150:737–42. DOI: 10.1016/j.ahj.2004.11.017 [DOI] [PubMed] [Google Scholar]
- 14.Rychik J “The fetal cardiovascular examination,” Fetal Cardiovascular Imaging. Ed. Rychik J and Tian Z. Philadelphia: Elsevier; 2012. [Google Scholar]
- 15.Hecher K, Campbell S, Snijders R, and Nicolaides K. Reference ranges for fetal venous and atrioventricular blood flow parameters. Ultrasound Obstet Gynecol 1994;4:381–90. DOI: 10.1046/j.1469-0705.1994.04050381.x [DOI] [PubMed] [Google Scholar]
- 16.Lenz F and Chaoui R. References ranges for Doppler-assessed pulmonary venous blood flow velocity and pulsatility indices in normal human fetuses. Prenat Diagn 2002;22:786–91. DOI: 10.1002/pd.410 [DOI] [PubMed] [Google Scholar]
- 17.Lenz F and Chaoui R. Changes in pulmonary venous Doppler parameters in fetal cardiac defects. Ultrasound Obstet Gynecol 2006;28:63–70. DOI: 10.1002/uog.2796 [DOI] [PubMed] [Google Scholar]
- 18.Yun TJ, Coles JG, Konstantinov IE, Al-Radi OO, Wald RM, Guerra V, et al. Conventional and sutureless techniques for management of the pulmonary veins: Evolution of indications from postrepair pulmonary vein stenosis to primary pulmonary vein anomalies. J Thorac Cardiovasc Surg 2005;129:167–74. DOI: 10.1016/j.jtcvs.2004.08.043 [DOI] [PubMed] [Google Scholar]
- 19.Kelle AM, Backer CL, Gossett JG, Kaushal S, Mavroudis C. Total anomalous pulmonary venous connection: results of surgical repair of 100 patients at a single institution. J Thorac Cardiovasc Surg 2010;139:1387–94. DOI: 10.1016/j.jtcvs.2010.02.024 [DOI] [PubMed] [Google Scholar]
- 20.Yong MS, d’Udekem Y, Robertson T, Horton S, Dronavalli M, Brizard C, et al. Outcomes of surgery for simple total anomalous pulmonary venous drainage in neonates. Ann Thorac Surg 2011;91:1921–7. DOI: 10.1016/j.athoracsur.2010.12.069 [DOI] [PubMed] [Google Scholar]
- 21.Karaci AR, Harmandar B, Aydemir NA, Sasmazel A, Balci AY, Saritas T, et al. Early and intermediate term resutls for surgical correction of total anomalous pulmonary venous connection. J Card Surg 2012;27:376–80. DOI: 10.1111/j.1540-8191.2012.01435.x [DOI] [PubMed] [Google Scholar]
- 22.Husain SA, Maldonado E, Rasch D, Michalek J, Taylor R, Curzon C, et al. Total anomalous pulmonary venous connection: factors associated with mortality and recurrent pulmonary venous obstruction. Ann Thorac Surg 2012;94:825–32. DOI: 10.1016/j.athoracsur.2012.04.026 [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Zhang L, Xie F, Wang B, Sun Z, Kong S, et al. Echocardiographic diagnosis of anomalous pulmonary venous connections: Experience of 84 cases from 1 medical center. Medicine 2016;95:44. DOI: 10.1097/md.0000000000005389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemaire A, DiFilippo S, Parienti JJ, Metton O, Mitchell J, Hénaine R, et al. Total anomalous pulmonary venous connection: a 40 years’ experience analysis. Thorac Cardiovasc Surg 2017;65:9–17. DOI: 10.1055/s-0036-1588007 [DOI] [PubMed] [Google Scholar]
- 25.Hancock HS, Romano JC, Armstrong A, Yu S, Lowery R, Gelehrter S. Single Ventricle and Total Anomalous Pulmonary Venous Connection: Implications of Prenatal Diagnosis. World J Pediatr Congenit Heart Surg 2018;9:434–9. DOI: 10.1177/2150135118771344 [DOI] [PubMed] [Google Scholar]
- 26.Dawary M, Alshamdin F, Alkhalaf L, Khouqeer F. Pulmonary venous obstruction in patients who underwent surgical repair of total anomalous pulmonary venous connection: A retrospective study from Saudi Arabia. [Google Scholar]
- 27.Ricci M, Elliott M, Cohen GA, Catalan G, Stark J, de Laval MR, et al. Management of pulmonary venous obstruction after correction of TAPVC: risk factors for adverse outcome, Europ J Cardiothorac Surg 2003;24:28–36. DOI: 10.1016/s1010-7940(03)00180-5 [DOI] [PubMed] [Google Scholar]
- 28.Shi G, Zhu Z, Chen J, Ou Y, Hong H, Nie Z, et al. Total anomalous pulmonary venous connection: the current management strategies in a pediatric cohort of 768 patients. Circulation 2016;135:48–58. DOI: 10.1161/circulationaha.116.023889 [DOI] [PubMed] [Google Scholar]
- 29.Zhang C, Ou Y, Zhuang J, Chen J, Nie Z, Ding Y. Comparison of Sutureless and Conventional Techniques to Repair Total Anomalous Pulmonary Venous Connection. Semin Thoracic Surg 2016;28:473–84. DOI: 10.1053/j.semtcvs.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 30.Harada T, Nakano T, Oda S, Kado H. Surgical results of total anomalous pulmonary venous connection repair in 256 patients. Interact CardioVasc Thorac Surg 2018; DOI: 10.1093/icvts/ivy267 [DOI] [PubMed] [Google Scholar]
- 31.Valsangiacomo ER, Hornberger LK, Barrea C, Smallhorn JF, Yoo SJ. Partial and total anomalous pulmonary venous connection in the fetus: two-dimensional and Doppler echocardiographic findings. Ultrasound Obstet Gynecol 2003;22: 257–63. DOI: 10.1002/uog.214 [DOI] [PubMed] [Google Scholar]
- 32.Tongsong T, Luewan S, Jatavan P, Tongprasert F, Sukpan K. A Simple Rule for Prenatal Diagnosis of Total Anomalous Pulmonary Venous Return. J Ultrasound Med 2016;35:1601–7. DOI: 10.7863/ultra.15.08016 [DOI] [PubMed] [Google Scholar]
- 33.Hancock Friesen CL, Zurakowski D, Thiagarajan RR, Forbess JM, del Nido P, Mayer JE et al. Total anomalous pulmonary venous connection: an analysis of current management strategies in a single institution, Ann Thorac Surg 2005;79:596–606. DOI: 10.1016/j.athoracsur.2004.07.005 [DOI] [PubMed] [Google Scholar]
- 34.Chowdhury UK, Airan B, Malhotra A, Bisoi AK, Saxena A, Kothari SS, et al. Mixed total anomalous pulmonary venous connection: Anatomic variations, surgical approach, techniques, and results. J Thorac Cardiovasc Surg 2008;135:106–16. DOI: 10.1016/j.jtcvs.2007.08.028 [DOI] [PubMed] [Google Scholar]
- 35.Domadia S, Kumar SR, Votava-Smith JK, Pruetz JD. Neonatal Outcomes in Total Anomalous Pulmonary Venous Return: The Role of Prenatal Diagnosis and Pulmonary Venous Obstruction. Pediatr Cardiol 2018;39:1346–54. DOI: 10.1007/s00246-018-1901-0 [DOI] [PubMed] [Google Scholar]
- 36.Choi EY, Lee CH, Park SJ, Jang SI, Kim ES. Assessing the recently noted surgical outcome of isolated total anomalous pulmonary venous connection repair: A single-secondary center experience. J Card Surg. 2019;34:1526–32. DOI: 10.1111/jocs.14284 [DOI] [PubMed] [Google Scholar]
- 37.Jiang L, Xie LJ, Yang ZG, Shi K, Xu HY, Li R, et al. Preoperative evaluation of anomalous pulmonary venous connection using dual-source computed tomography: Comparison with echocardiography. Eur J Radiol 2017;94:107–14. DOI: 10.1016/j.ejrad.2017.06.015 [DOI] [PubMed] [Google Scholar]
- 38.Seale AM, Webber SA, Uemura H, Partridge J, Roughton M, Ho SY, et al. Pulmonary vein stenosis: the UK, Ireland and Sweden collaborative study. Heart 2009;95:1944–9. DOI: 10.1136/hrt.2008.161356 [DOI] [PubMed] [Google Scholar]
- 39.Lo Rito M, Gazzaz T, Wilder T, Saedi A, Chetan D, Van Arsdell GS, et al. Repair Type Influences Mode of Pulmonary Vein Stenosis in Total Anomalous Pulmonary Venous Drainage. Ann Thorac Surg 2015;100:654–62. DOI: 10.1016/j.athoracsur.2015.04.121 [DOI] [PubMed] [Google Scholar]
- 40.Kalfa D, Belli E, Bacha E, Lambert V, di Carlo D, Kostolny M, et al. Outcomes and prognostic factors for postsurgical pulmonary vein stenosis in the current era. J Thorac Cardiovasc Surg 2018;156:278–86. DOI: 10.1016/j.jtcvs.2018.02.038 [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, Xin L, Zhou Y, Kuang H, Jin X, Li Y, et al. Is sutureless technique beneficial in the primary repair of total anomalous pulmonary venous connection? A systematic review and meta-analysis. Pediatr Cardiol 2019;40:881–91. DOI: 10.1007/s00246-018-1948-y [DOI] [PubMed] [Google Scholar]
- 42.St. Louis JD, Harvey BA, Menk JS, Raghuveer G, O’Brien JE, Bryant R, et al. Repair of “simple” total anomalous pulmonary venous connection: a review from the pediatric critical care consortium. Ann Thorac Surg 2012;94:133–8. DOI: 10.1016/j.athoracsur.2012.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]