Abstract
Vaping is the process of inhaling and exhaling an aerosol produced by an e-cigarette, vape pen, or personal aerosolizer. When the device contains nicotine, the Food and Drug Administration (FDA) lists the product as an electronic nicotine delivery system or ENDS device. Similar electronic devices can be used to vape cannabis extracts. Over the past decade, the vaping market has increased exponentially, raising health concerns over the number of people exposed and a nationwide outbreak of cases of severe, sometimes fatal, lung dysfunction that arose suddenly in otherwise healthy individuals. In this review, we discuss the various vaping technologies, which are remarkably diverse, and summarize the use prevalence in the U.S. over time by youths and adults. We examine the complex chemistry of vape carrier solvents, flavoring chemicals, and transformation products. We review the health effects from epidemiological and laboratory studies and, finally, discuss the proposed mechanisms underlying some of these health effects. We conclude that since much of the research in this area is recent and vaping technologies are dynamic, our understanding of the health effects is insufficient. With the rapid growth of ENDS use, consumers and regulatory bodies need a better understanding of constituent-dependent toxicity to guide product use and regulatory decisions.
Keywords: Electronic cigarettes, nicotine, cannabinoids, electronic nicotine delivery system or ENDS, flavorings, oxidative stress, DNA damage, lung injury
Introduction
Two distinct yet related epidemics are currently facing the nation: the outbreak of e-cigarette or vaping product use-associated lung injury (EVALI) and the increased use of electronic nicotine delivery system (ENDS) devices in young people (CDC, 2016; Fedt, Bhattarai, & Oelstrom, 2020; B. A. King, Jones, Baldwin, & Briss, 2020; NIDA, 2020). Intertwined among these is the assumed safety of using ENDS compared to traditional combustible cigarettes. Regulators walk a tight rope juggling the potential health benefit of these devices for adult smokers trying to quit, reducing the appeal and addictive potential for youth, and limiting the assumed culprit for EVALI cases. The recent surge in EVALI cases has prompted federal and local health organizations to issue health advisories aimed at curbing both the youth vaping epidemic and the number of EVALI cases (NIDA, 2020). Regulatory agencies at the local, state, and national levels have issued restrictions on the sale of certain types of vaping products, aimed at reducing the prevalence of teen smoking and access to ENDS products (Baker & Campbell, 2020; CDC, 2016). To help combat EVALI, some states with legal cannabis markets have established, or are considering, laws to limit the use of additives in tetrahydrocannabinol (THC)-containing vaping products (B. A. King, et al., 2020). Concurrently, research on ENDS use has increased rapidly since 2010. A PubMed database search shows a dramatic increase in the number of publications containing the words “vaping” or “e-cigarette” over the past decade (Figure 1). Publications containing the term “e-cigarette” have increased the greatest, peaking at 1,193 publications in 2020. The term “vaping” produced 1,108 publications in 2020. This increase in relevant research is consistent with the increased use of ENDS products over the past decade and rising public concern over their potential health impacts. While several excellent review articles have been published on trends in ENDS use and its effects on human health, this review takes a novel and inclusive approach, discussing many key aspects of ENDS use, from trends in use over time to chemical transformations and possible mechanisms by which ENDS use induces toxicity.
Figure 1.
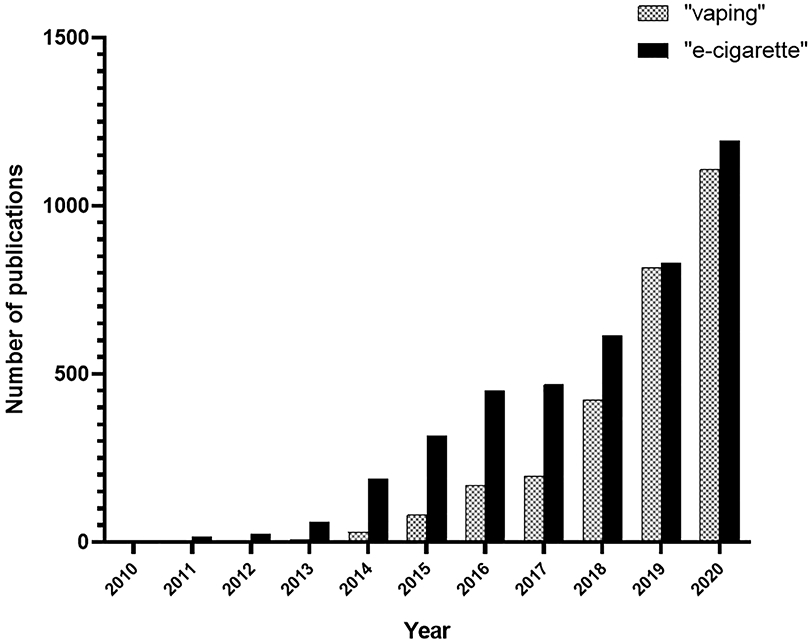
Number of publications in PubMed database produced by the search terms “vaping” or “e-cigarette”, or from 2010 to 2020.
Section 1. Commercially Available Vaping Platforms and Use Prevalence
Section 1.1. Vaping Platforms
A large diversity of available vaping devices, spanning several generations of designs and produced by a variety of manufacturers, includes e-cigarettes, vape pens, tanks, and ‘mod’-style devices. Customizability of device components and settings makes these devices even more diverse. Despite this variability, vaping platforms generally consist of three standard components: battery, atomizer, and fluid reservoir. When the user inhales from a mouthpiece, either an airflow sensor or button is used to heat a filament in the atomizer, which then generates the e-liquid aerosol. The e-liquid stored in the fluid reservoir is delivered to the filament by capillary action through wicks, usually made of silica or cotton. Fluid reservoirs are replaceable or refillable tanks built into the devices. The most recent fourth-generation devices all have a prefilled or refillable pod cartridge and a modifiable system (pod mod). These devices provide users with the highest degree of modification freedom, such as a mode for automated temperature control (Protano, et al., 2018).
Section 1.2. Vaping Market and Marketing
Since its inception in 2007, the vaping market has grown exponentially. While it is difficult to know exactly how much is currently spent on vaping marketing, because there is no requirement to report marketing expenditures to any regulatory agency, some estimations have been made. Estimations for the value of the global market ranged from USD 12 to 14 billion in 2018 and 2019 (Grand-View-Research, 2020; PRNewswire, 2019). Market research reports predict that increased growth will continue, with estimated valuations of USD 29.39 billion by 2022 (PRNewswire, 2019) and USD 67.31 billion by 2027 (Al Rifai, et al., 2020). The U.S. currently has the largest vaping market in the world (Jones, 2019), valued at USD 2.5 billion in 2014, and USD 3.5 billion in 2015 (CDC, 2016). The industry grew from USD 19 million in retail sales in the first quarter of 2011 to up to USD 409 million in the last quarter of 2017 (Huang, et al., 2019). In 2014, there were an estimated 460 ENDS brands available selling thousands of flavor options (Mackey, Miner, & Cuomo, 2015), and as many as 35,000 vape shops in the U.S. alone (CDC, 2016). The online marketplace has also played an important role; in early 2015, online sales comprised approximately 30% of the vaping market (Mackey, et al., 2015). One of the most significant factors in rapid growth and product recognition has been marketing campaigns. In 2012, ENDS companies began spending millions on marketing, celebrity endorsements, and event sponsorships targeted to younger audiences (e.g., music festivals) (Hess, et al., 2017). Data from Kantar Media, a media measurement company, shows that advertisement spending for vaping jumped from just under USD 3 million in 2010, to over USD 20 million in 2012, to an estimated USD 125 million in 2014 (Elliott, 2013; Kornfield, Huang, Vera, & Emery, 2015).
Section 1.3. Prevalence of ENDS and Electronic Cannabis Systems
Since their introduction in 2007, and especially since 2011, the use of ENDS devices has ballooned among young users (CDC, 2011). The number of high school students in the U.S. using ENDS products increased from approximately 660,000 to 2 million in just one year (2013–2014) (CDC, 2015). Similarly, use among young adults (18–24 years old) more than doubled from 2013–2014 (CDC, 2016). In fact, vaping had such a surge in popularity that the term “vape” was selected as the Oxford Dictionary’s 2014 word of the year (Oxford, 2014), and the FDA commissioner declared youth vaping an “epidemic” in 2018 (Gottlieb, 2018). Among adults, the most common motivators for use include curiosity, smoking cessation, and perception of lower risk compared to traditional tobacco products (Boyle, Richter, & Helgertz, 2019; Pepper, Ribisl, Emery, & Brewer, 2014). A study published in 2017 assessed reasons for ENDS usage based on a content analysis of all English language public Twitter posts in 2012 and 2015 (Ayers, et al., 2017). The authors found that mentions of “social image and ENDS use” increased from 21% of tweets in 2012 to 37% in 2015. While ENDS use-related posts increased rapidly from 2009 to 2015, posts related to health concerns and smoking cessation decreased from 2013 to 2014, indicating shifting motivations for use over time.
Section 1.3.1. ENDS Use by Youth
Studies tracking use of ENDS by youth began around 2010. At two large suburban high schools in 2010 and 2011, the results of a self-administered written survey indicated that the percentage of students who had used ENDS products in the previous 30 days more than doubled across sixteen months, and that current cigarette use strongly predicted ENDS use by adolescents (Camenga, et al., 2014). On a larger scale, the U.S. FDA and The Centers for Disease Control (CDC) report prevalence and trends of youth tobacco use through the annual National Youth Tobacco Survey (NYTS) (CDC, 2019). Between 2014 and 2019, the percent of youth who had ever used ENDS products, but no other tobacco products, steadily increased from 3.7% to 19.5% for high school students and from 2.9% to 9.7% for middle school students (Tam, 2021). In the same study, for those who used both ENDS and other tobacco products, prevalence increased for both age groups between 2014 and 2019, but not consistently across individual years. Most recently between 2019 and 2020, the NYTS showed a decrease in ENDS current use from 27.5% to 19.6% among high school students, and from 10.5% to 4.7% among middle school students (T. W. Wang, et al., 2020). While this decrease in use is significant, over 3.5 million middle and high school students still used ENDS products in 2020.
There is evidence that ENDS use differs among youth demographics including race/ethnicity and concurrent use of other tobacco products. The U.S. Department of Health and Human Services compiled data from nationally representative datasets and peer-reviewed literature of subnational and international surveillance studies of ENDS use from 2011 through 2015 (CDC, 2016). These data showed a higher percentage of students who had used ENDS in the previous 30 days among high school students compared to middle school students. In middle school, use was highest among Hispanic students. In high school, use was higher among both White and Hispanic students compared to Black students. For high school seniors who reported e-cigarette use in the past 30 days, the frequency of use was almost twice as high for regular smokers of conventional cigarettes versus those who had never smoked before. Newer studies continue to support these initial observations. One report demonstrated more e-cigarette use among high school students than 8th-grade students from 2017 to 2019 (Miech, Johnston, O'Malley, Bachman, & Patrick, 2019). A second 2019 report demonstrated higher use among White and Hispanic students, and a higher likelihood among White students to use flavored products (T. W. Wang, et al., 2019).
Section 1.3.2. ENDS Use by Adults
Studies tracking ENDS use among adults also began in approximately 2010. Responses from a consumer survey from 2010–2013 showed different patterns of ENDS use across demographics and time (Brian A King, Patel, Nguyen, & Dube, 2015). Across all adults surveyed, prevalence of those who had ever used ENDS products increased from 3.3% (2010) to 8.5% (2013). When broken down by demographics, significant increases were seen across all socio-demographics studied except young adults aged 18-24, Hispanic users, those living in the Midwest, and those who reported never smoking traditional cigarettes. This study also indicated higher recent use (in previous 30 days) among older adults, those with high school education or less, those located in the South or Midwest U.S. regions, current smokers, and those with lower incomes. Across all demographics, current or former cigarette use was the greatest predictor of ENDS use (Brian A King, et al., 2015). Similar trends of higher use among smokers and former smokers were observed in a 2013 study of young adults in metropolitan areas (Biener, Song, Sutfin, Spangler, & Wolfson, 2015). As of 2019, 4.5% of U.S. adults reported current ENDS use, with a skew toward males, young adults 18-24 years old, those with annual household income <$35,000, and lesbian, gay, or bisexual adults (Cornelius, Wang, Jamal, Loretan, & Linda J. Neff, 2020; McMillen, Gottlieb, Shaefer, Winickoff, & Klein, 2014; T. W. Wang, et al., 2019).
Section 1.3.3. Second- and Thirdhand Exposure to ENDS
If the prevalence of vaping increases over time and across demographics, so too will unintentional exposures. Vaping aerosols often produces pleasant smells which may make emissions seem benign. Several studies have concluded that secondhand exposure, or “passive vaping” is to be expected from nearby ENDS use (Czogala, et al., 2014; Schober, et al., 2014; Schripp, Markewitz, Uhde, & Salthammer, 2013; Sleiman, et al., 2016). An additional potential exposure posed by ENDS use is “thirdhand vaping,” or exposure to residues deposited on surfaces after ENDS use. This residue is sometimes referred to as e-cigarette exhaled aerosol residue, or ECEAR, and may contain tobacco-specific nitrosamines, nicotine, and other alkaloids (Khachatoorian, Jacob Iii, Benowitz, & Talbot, 2019). ECEAR has been detected in homes of users, in vape shops, and even in buildings near vape shops (Khachatoorian, Jacob, et al., 2019). While the body of evidence on these unintended exposures is still evolving, the potential for second- and thirdhand exposure to ENDS aerosols and aerosol residues should be considered when evaluating the health effects of ENDS use. Physicians, non-users, and those living or working near areas with heavy ENDS use (e.g., vape shops) should be aware of their potential exposure.
Section 1.3.4. Electronic Cannabis Use by Youth and Adults
There is little information on youth use of electronic devices to vaporize cannabis derivatives including THC e-liquids, hash oil, THC wax, etc. Data collected from the 2016 NYTS indicated that prevalence of use of these products was significantly higher among male students, high school students, Hispanic students, current users of tobacco products (including ENDS), youth with a high frequency of e-cigarette use (20–30 days in the past 30 days), and those who lived with a user of tobacco products (Trivers, Phillips, Gentzke, Tynan, & Neff, 2018). Researchers also found increased use prevalence among older high school students (Kowitt, et al., 2019). A study of high school e-cigarette users in Connecticut found that aerosolized cannabis intake was common among e-cigarette users (18%), lifetime cannabis users (18.4%), and highest among lifetime dual users (26.5%) (Morean, Kong, Camenga, Cavallo, & Krishnan-Sarin, 2015). Statistical analysis of survey responses determined that males and younger high school students, lifetime e-cigarette users, and lifetime cannabis users had higher odds of aerosolizing cannabis products.
When investigating trends of aerosolized cannabis use in adults, Schauer, King, Bunnell, Promoff, and McAfee (2016) reported similar usages across races and education. An online survey via Facebook advertisements found no significant differences in use based on education (McMillen, et al., 2014). Cranford et al. examined the role of vaping as a route of cannabis administration in medical cannabis patients and found that while 39% of participants reported vaping cannabis in the previous month, only 2.3% chose vaping as the sole route of cannabis administration (Cranford, Bohnert, Perron, Bourque, & Ilgen, 2016). As of 2016, aerosolizing cannabis did not appear to be a primary method of use among adults, with studies reporting only 2.2% (Earleywine & Barnwell, 2007), 7.6% (Schauer, et al., 2016), 11.2% (Hindocha, Freeman, Ferris, Lynskey, & Winstock, 2016), and 12% (D. C. Lee, Crosier, Borodovsky, Sargent, & Budney, 2016) of adult cannabis users preferring aerosolizers.
Section 1.4. ENDS Risk Perceptions
The first commercially viable e-cigarette was developed as a safer alternative to combustible tobacco products. Now, over 15 years later, a primary motivator for ENDS usage is still the perception that vaping comes with a lower risk of adverse health effects than traditional tobacco smoking (Etter & Bullen, 2011; Farsalinos, Romagna, Tsiapras, Kyrzopoulos, & Voudris, 2014). However, recent trends suggest that perception is changing. A study published in 2019 reviewed trends in risk perception of vaping versus smoking cigarettes from 2012 to 2017 across two surveys: the Tobacco Products and Risk Perceptions Survey (TPRPS) and the Health Information National Trends Survey (HINTS). The percentage of adults who perceived e-cigarettes as equally harmful to traditional cigarettes increased from 46.4% to 55.6% (HINTS) and 11.5% to 36.4% (TPRPS), while the percentage who considered e-cigarettes as more harmful than traditional cigarettes more than tripled from 2.8% to 9.9% (HINTS) and 1.3% to 4.3% (TPRPS) from 2012 to 2017 (Huang, et al., 2019).
As perceptions of the risks associated with vaping shift, it is relevant to draw comparisons between ENDS usage and traditional tobacco smoking, and the associated health effects. One survey of ENDS users conducted at vape shops across Louisville, Kentucky indicated that ENDS users who had decreased traditional cigarette use by greater than two cartons/month since beginning ENDS use were significantly more likely to report experiencing health improvements than those who had reduced cigarette consumption by 1.5 cartons or less (Hart, et al., 2018). A clinical study of traditional cigarette smokers who switched to ENDS for 12 weeks showed no significant improvements in vital signs, lung function, or electrocardiogram, but no additional adverse long-term effects of ENDS were observed. Adverse acute effects such as headache, sore throat, and cough were highest in the first week after switching, and then reduced throughout the rest of the study period. Nicotine withdrawal symptoms increased during the first two weeks and then subsided, suggesting ENDS use offers smokers a functional, if not a substantially safer, alternative to traditional cigarettes (Cravo, et al., 2016), though the long-term health effects are still unknown.
Section 2. Regulatory Actions
Recent federal and local health regulatory action has been aimed at the outbreak of EVALI and the increased use of ENDS devices among young people (FDA, 2016; Fedt, et al., 2020; B. A. King, et al., 2020; NIDA, 2020). Such regulation often assumes benefit of ENDS devices over traditional combustible cigarettes. Regulators juggle the potential health benefit of these devices for adult smokers trying to quit, with reducing the appeal and addictive potential to youth, and limiting the culprit of EVALI (NIDA, 2020). Agencies at local, state, and national levels have issued restrictions on the sale of certain vaping products to youths (Baker & Campbell, 2020; FDA, 2016). To help combat EVALI, some states with legal cannabis markets have established, or are considering, laws to limit the use of additives in THC-containing vape products (CDC, 2020b; B. A. King, et al., 2020). The need for regulation of ENDS devices and vape products has become a key public health concern.
Section 2.1. Federal Regulations
In 2008, the FDA attempted to regulate ENDS devices under the Federal Food, Drug, and Cosmetic Act as a drug delivery device. Under this classification ENDS devices would be required to have “pre-approval, registration, and listing with the FDA” (Cox, Barry, & Glantz, 2016; FDA, 2020a). This was challenged in court by Sottera, an ENDS product company (PHLC, 2009). The court ruled that the FDA could only regulate ENDS products as tobacco products under the 2009 Family Smoking and Prevention Tobacco Control Act (FSPTCA) (Congress, 2009; Cox, et al., 2016). When it was enacted, the FSPTCA covered “cigarettes, cigarette tobacco, roll-your-own tobacco, and smokeless tobacco, and required that any tobacco product that appeared on the market after February 15, 2007… get approval from the FDA” (Congress, 2009). After a lengthy review in 2016, the FDA extended its authority to cover “all products that meet the statutory definition of ‘tobacco products… including electronic nicotine delivery systems (ENDS: e-cigarettes, e-hookah, e-cigars, vape pens, advanced refillable personal vaporizers, and electronic pipes) and other products such as dissolvable, gels, water pipe tobacco, cigars, and pipe tobacco’ ” (FDA, 2016). The FDA process for reviewing premarket tobacco products includes consideration of: (1) the product’s components, ingredients, additives, and properties; (2) manufacturing practices; and (3) any studies or investigations into the health risks of the tobacco product (FDA, 2020c). The FDA stated in a Federal Register notice that its enforcement of premarket approval is motivated by a “dramatic increase in youth use of these products” (FDA, 2020c).
The FDA continued to extend the period in which it delayed enforcement action for premarket review requirements under this final Deeming Rule (FDA, 2020c), instead issuing numerous warning letters and civil money penalty (CMP) complaints to manufacturers and retailers (Gottlieb, 2018). This delay of enforcement was challenged in federal court by the American Academy of Pediatrics in 2019 with the court directing the FDA to require premarket authorization applications for all new deemed tobacco products be submitted to the Agency by May 12, 2020. This deadline was extended to September 9, 2020 due to the novel Coronavirus outbreak. To date, no ENDS products have been authorized by the FDA (FDA, 2020a). In December 2019, The White House signed legislation amending the Food, Drug, and Cosmetic Act which raised the federal minimum age of sale of tobacco products, including ENDS, from 18 to 21 years old (FDA, 2019b).
Following a string of vaping-related illnesses that emerged in 2019, the FDA issued updated guidance on unauthorized flavored cartridge-based ENDS devices. Under this new policy, the manufacture, distribution, and sale of these products must cease. It is important to note that this guidance does not apply to tobacco-, menthol-, or non-flavored ENDS products and any non-cartridge based, flavored ENDS products (FDA, 2020a; Gottlieb, 2018). Additionally, the use of the word “unauthorized” in the guidance leaves room for manufacturers to apply for premarket approval for flavored cartridge-based ENDS devices. After receiving FDA approval, such products could then be legally sold. The FDA has not stated whether they intend to give approval for such products. The guidance does not constitute a ban of any particular product or flavoring.
Little federal regulation exists regarding the manufacture and packaging of vaping products. Manufacturers are subject to FDA premarket approval requirements. Furthermore, vape shops that modify tobacco products can be considered manufacturers and would then be subject to premarket approval for any modified product they sell. Modification can take many forms and includes, but is not limited to, refilling closed ENDS products, repairing or modifying the atomizer, replacing the coil, or assembling a custom final product. Modifying the e-liquid (i.e. mixing) is considered modification as well (FDA, 2020a). Again, FDA’s enforcement of premarket approval on manufacturers will focus efforts on two areas, (1) flavored cartridge-based e-cigarettes, and (2) other ENDS products when the manufacturer has not taken adequate measures to prevent minors’ access (FDA, 2020c). Packaging regulation is the same for tobacco products: one cannot sell, distribute, or advertise e-cigarettes without warning statements (FDA, 2020a, 2020c).
There is no current federal taxation specific to vaping products. However, the House Ways and Means Committee passed a bill, “Protecting American Lungs Act of 2019” (H.R.4742), that, if passed by Congress, would make federal tobacco taxes apply to vaping products that contain nicotine (H.R.4742, 2019). Additionally, no federal regulations prohibit vaping in particular spaces or around specific groups of individuals.
Section 2.2. State/Territory Regulations
Currently, 33 states and territories require some form of a license to sell ENDS products and e-liquids. Kansas became the first state to require such a license in July of 2012. The overwhelming majority of these states require a license to sell ENDS products in a store; however, some states such as Texas only require a sales license if the product is sold via delivery. Other states, including California, have more specific licenses depending on whether or not a business is a retailer or wholesaler/distributer of ENDS products (PHLC, 2020).
As mentioned above, the federal government has recently enacted a ban on the sale of nicotine products to anyone under the age to 21 (FDA, 2019b). However, it is important to note that this does not apply to non-nicotine containing e-liquids or to the delivery systems, and these restrictions still fall to states’ discretion.
Many states also regulate the occurrence and placement of vending machines or other self-servicing sales of ENDS devices. For example, Oregon prohibits the placement of ENDS vending machines on any property or premise that are not permanently inaccessible to persons under the age of 21 (PHLC, 2020). In comparison, Nevada has no laws in place restricting placement or access to ENDS vending machines (PHLC, 2020). Meanwhile, only New York, Massachusetts, Michigan, Montana, New Jersey, and Rhode Island prohibit the sale of flavored ENDS devices (PHLC, 2020).
Thirty-one U.S. states and territories regulate the packaging of ENDS products. South Dakota was the first of four states to introduce packaging regulation in 2014. Since then, 27 more states and territories have followed suit. Requirements for child-resistant packaging are by far the most abundant regulations at the state/territory level. However, many states such as Michigan exempt non-refillable devices from this requirement and some, such as North Dakota, specify that this regulation only applies to nicotine containing products, not specifically to the devices (PHLC, 2020). Utah and Washington provide the most extensive set of regulations for ENDS product packaging, including declaration of certain additives, maximum nicotine levels, warnings to keep products away from children, and warnings that vaping is illegal for minors. The state of Oregon is the only state to specifically require packaging to be “not attractive to persons under age 18” (PHLC, 2020).
Section 2.3. Regulation of Cannabis E-liquids
Since ENDS devices were introduced, their use has evolved to include aerosol delivery of cannabidiol (CBD) and THC. Simultaneously, an increasing number of states have legalized cannabis for recreational and medical use, which has been associated with decreased public perception of risk for these products (National Academies of Sciences & Medicine, 2017). This has resulted in increased use of cannabis-related products, particularly among youth (Hasin, 2018; National Academies of Sciences & Medicine, 2017). Since THC products are legalized on a statewide level, there are no federal regulations and all regulatory policies regarding THC e-liquid cartridges are specific to state cannabis laws. Theoretically, if a THC e-liquid also contained nicotine, it could fall under the FSPTCA and be subject to the same federal regulation as other nicotine devices. The CDC currently recommends against using any vaping products containing THC (CDC, 2020a; Ind, 2020) and the FDA has issued a warning to consumers to stop vaping products containing THC (FDA, 2019c); however, the FDA has approved one cannabis-derived drug product (FDA, 2018a) and three synthetic cannabis-related drug products for medical purposes under the Federal Food, Drug, and Cosmetic Act (FDA, 2020b).
To date, it is illegal to market CBD by adding it to a food or labeling it as a dietary supplement (FDA, 2021), but no regulations currently exist for e-liquids. The FDA has yet to issue regulations for these products as they sit in a legal grey area (Kosecki, 2019), neither explicitly legal nor prohibited under federal law as these products are both tobacco- and nicotine-free, and therefore do not fall under the FSPTCA.
Section 3. Complex Chemical Composition and Transformations
Section 3.1. Carrier Solvents
The largest components of e-liquids are solvents, occasionally referred to as humectants or stabilizing agents. The purpose of the solvents in e-liquid formulas is to keep the other chemical components such as nicotine and flavorants in suspension (P. Wang, et al., 2017), to enhance absorption of the wicking material in the devices, and to generate aerosolized plumes of the e-liquid (Clapp & Jaspers, 2017). The two most common solvents used in e-liquids are propylene glycol (PG) and vegetable glycerin (VG), synonymous with glycerol or glycerin. The FDA, under subchapter B for food and human consumption classifies propylene glycol as “generally recognized as safe” (GRAS) for ingestion, but not aerosolization (FDA, 2019a). It has defined maximum levels in consumable products such as 2.5% in frozen dairy products, up to 97% in seasonings and flavorings, and 2% in all other food categories (FDA, 2019a). Glycerol is also classified as GRAS under the same subchapter, although maximum levels are not defined. Rather, the substance is GRAS when used in accordance with good manufacturing practice (FDA, 2007).
Section 3.1.1. Solvent Ratios
Although present in varying ratios, it is commonly accepted that propylene glycol and glycerol comprise 80% - 94.9% of the volume of e-liquids (Dai, et al., 2018; Y. J. Lee, Na, Botao, Kim, & Son, 2020). Propylene glycol and glycerol are three-carbon compounds with two and three alcohol groups, respectively. The additional alcohol group on glycerol contributes to a higher boiling point, viscosity, aerosol density, and lower vapor pressure than propylene glycol. Rather than drawing comparisons by these measures, many vaping blogs and merchants compare propylene glycol and glycerol for criteria relevant to the consumer, such as sensation in the throat (“throat hit”), residue build-up, and flavor intensity. When over 17,000 reviews were collected from JuiceDB (a consumer driven web site) over two years, it was noted that both glycerol and propylene glycol were frequently discussed in the reviews. Glycerol was reported to increase the flavor of an e-liquid and create a larger amount of aerosol, while propylene glycol produced a greater “throat hit” (Z. Chen & Zeng, 2017). These solvents are typically both present as a mixture in e-liquid formulas, with common propylene glycol:glycerol ratios of 10:90, 20:80, 50:50, and 80:20 (Ooi, Dutta, Kazipeta, & Chong, 2019), although many other ratios exist on the market. The ratio of propylene glycol to glycerol influences many aspects of the vaping experience, such as nicotine yield (Kosmider, et al., 2014), e-liquid consumption (Y. J. Lee, et al., 2020), and generation of toxic thermal decomposition products (Bitzer, et al., 2018; Ooi, et al., 2019; P. Wang, et al., 2017).
Section 3.1.2. Carrier Solvent toxicity
Propylene glycol and glycerol are both airway irritants (Callahan-Lyon, 2014; Chun, Moazed, Calfee, Matthay, & Gotts, 2017). Occupational exposure to aerosolized propylene glycol has been documented in theatrical workers (Fowles, Barreau, & Wu, 2020; Fowles & DiBartolomeis, 2017). Individuals exposed to propylene glycol reported experiencing work-related chest tightness and wheezing in proportion to estimated cumulative exposure. Propylene glycol also causes eye, throat, mucous membrane, and respiratory irritation and constriction of peripheral airways (Chun, et al., 2017). The International Agency for Research on Cancer (IARC) concluded the transformation product propylene oxide has sufficient evidence in experimental animals for carcinogenicity (Schulte, Hemminiki, & Hopf, 1994).
Section 3.2. Nicotine
Nicotine is a natural alkaloid component of the nightshade (Solanaceae) family. It is found at the highest concentrations in the leaves of the tobacco plant, Nicotiana tabacum L (Qayyum, Fazal-ur-Rehman, & Ibrahim, 2018). For vaping, the nicotine must be extracted from the tobacco leaf prior to solubilization in the e-liquid solvent. Alternatively, nicotine can be synthesized in a lab for use in e-liquids. Synthesis has historically been more expensive than extracting nicotine and can generate a racemic mixture of nicotine enantiomers (Zettler, Hemmerich, & Berman, 2019). The speciation of nicotine is important to the chemical composition of e-liquids. Naturally, nicotine exists only as the (S)-nicotine enantiomer, which has two major sites for protonation to occur, with pKa values of approximately 3.1 and 8.0. The mono-protonated form is most prevalent in tobacco leaves due to their pH; however, the addition of nicotine extracts into more basic e-liquid solvents results in higher proportions of the deprotonated species. From a consumer standpoint, this difference is noticeable. The deprotonated form tends to cause sensations in the throat described as “harsh” or “intense” when inhaled, unlike the monoprotonated form. Yet some consumers still prefer deprotonated nicotine because it behaves differently in the body. The neutrality of the species makes it more bioavailable, allowing higher concentrations to reach active sites in the brain (El-Hellani, et al., 2015).
E-liquid producers have noticed consumer-preferences related to nicotine speciation and have altered the pH of their e-liquids accordingly. The addition of benzoic acid or citric acid to the e-liquid lowers the pH to the point where there is primarily mono-protonated nicotine (El-Hellani, et al., 2015; Jackler & Ramamurthi, 2019). As a result, there are now both “freebase nicotine” (deprotonated) and “nic salts” (mono-protonated) labeled e-liquids on the market, although the abundance of nicotine species is variable (Jackler & Ramamurthi, 2019). El-Hellani, et al. (2015) concluded that freebase nicotine (as opposed to other species) accounted for 18–95% of the total nicotine content depending on the product. Marketed nicotine concentrations also change with speciation. The lower bioavailability and potency of protonated nicotine has led to major increases in nicotine concentrations in products. For traditionally deprotonated nicotine products, 0 mg/mL, 3 mg/mL (0.3%), 6 mg/mL (0.6%), and 12 mg/mL (1.2%) are common commercially available nicotine contents in e-liquids, with concentrations of 3% marketed as ‘super high nicotine content’ (Stratton, Kwan, & Eaton, 2018). In contrast, some suppliers sell “nic salts” products with a minimum of 5% nicotine content (Jackler & Ramamurthi, 2019).
Section 3.2.1. Nicotine Toxicity
E-liquids can contain high concentrations of nicotine that can be toxic to children and adults upon inhalation, dermal or ingestion routes of exposure. Short term effects from exposure to nicotine include tremors, increased heart and respiratory rate, increased blood pressure, and increased level of alertness (Callahan-Lyon, 2014; Kim, Kabir, & Jahan, 2016; Schober, et al., 2014). The other effects of nicotine are reviewed elsewhere (Scarpino, et al., 2020), thus, we focus on the other chemicals resulting specifically from ENDS use.
Section 3.3. Cannabinoids
Vaping cannabinoids encompasses everything from heating dried cannabis to solvent extracting cannabis and blending the extract with carrier solvents and additives. The resulting exposures and implications for toxicity differ dramatically between these activities, highlighting the need for specific terminology. Even within extracts there are many different techniques and solvents that can be applied, which result in different chemical mixtures and leave behind varying amounts of residual solvents (Pegoraro, Nutter, Thevenon, & Ramirez, 2019; Ramirez, Fanovich, & Churio, 2019). To further complicate matters, synthetic cannabinoids or “K2, Spice, Black Mamba” differ in functionality from naturally occurring cannabinoids and are not regulated as they are often marketed as “not for human consumption”(Spaderna, Addy, & D'Souza, 2013). In addition to e-cigarette use, synthetic cannabinoids have been found as adulterants in CBD vaping products (Horth, et al., 2018). Epidemiologists and medical professionals have recognized the challenge posed by the diverse group of exposures that all fall under the guise of ‘not for human consumption’. Many surveys seeking to understand the use of ENDS devices have used vague terminology that could apply equally to cannabis or nicotine, resulting in increased uncertainty about the specific products to which respondents were referring. Researchers have acknowledged that to study nicotine vaping, we need a better understanding of cannabis vaping.
All EVALI patients have used e-cigarette, or vaping, products, and >85% have reported using products containing THC, the principal psychoactive component of cannabis (Ghinai, et al., 2019; Moritz, et al., 2019). The Illinois Department of Public Health (IDPH) conducted an online public survey of 4,631 e-cigarette, or vaping, product users in Illinois and 94% reported using any nicotine-containing e-cigarette, or vaping, products in the past 3 months; 21% used any THC-containing products; and 11% used both THC-containing products and nicotine-containing products. When e-cigarette behaviors of 66 EVALI patients aged 18–44 interviewed as part of the 2019 outbreak investigation were compared with a subset of 519 IDPH survey respondents aged 18–44 who reported use of THC-containing e-cigarette, or vaping, products, the EVALI patients had higher odds of reporting exclusive use of THC-containing products and more frequent use (more than five times per day) of these products (Navon, et al., 2019). IDPH concluded that their study reinforced recommendations not to use e-cigarette, or vaping, products that contain THC.
A recent observational study of cannabis use and EVALI data from U.S. states' health departments produced unexpected results. The study concluded that states with higher rates of ENDS and cannabis use prior to the 2019 EVALI outbreak had lower EVALI prevalence. This suggested that EVALI cases did not arise from ENDS or cannabis use per se, but rather from locally distributed e-liquids or additives most prevalent in the affected areas (Friedman, 2020). Studies indicate faster absorption and higher plasma THC concentrations for vaping THC compared to smoking cannabis (Spindle, et al., 2018). There is also growing evidence that vaping solvent extracts of cannabis may be associated with more behavioral and psychological issues than smoking cannabis (Chan, et al., 2017; Keller, Chen, Brodsky, & Yoon, 2016; Pierre, Gandal, & Son, 2016). Beyond understanding how vaping affects people directly, a growing body of research suggests that vaping instead of smoking cannabinoids can change an individual’s co-consumption of other drugs, such as nicotine (Apollonio, et al., 2019; Hindocha, et al., 2016). Poly-substance use is an important factor in mediating individuals’ exposure to recreational drugs, and more work is needed to understand how vaping cannabinoids changes use patterns of other drugs. Addressing these knowledge gaps can inform policy decisions and improve our understanding of the hazards presented by these compounds and routes of exposure.
To date, approximately 120 different phytocannabinoids have been documented, although there are likely others (Radwan, Wanas, Chandra, & ElSohly, 2017). These terpenophenolic compounds tend to share a standard mode of action, interacting with cannabinoid receptors to repress neurotransmitter release in the brain. The most well recognized (and most heavily marketed) cannabinoids are cannabidiol (CBD) and tetrahydrocannabinol (Δ9-THC, 1) (Radwan, et al., 2017). Growth in popularity of vaping cannabis products has led people to extract cannabinoids from the plant for use in vaping e-liquids (Giroud, et al., 2015). This process involves the drying and pulverization of the flower buds, heat “activation” of cannabinoid acid precursors (no-160°C) to decarboxylated neutral forms, gas or solvent extraction, and purification. In combustion, the cannabinoids are naturally decarboxylated (e.g., tetrahydrocannabinolic acid A [THC-A] to psychoactive THC), but vaping avoids combustion, so this transformation occurs before the extract ends up in the e-liquid.
Section 3.3.1. Cannabinoid Toxicity
Synthetic cannabinoids are associated with much more morbidity and mortality than the phytocannabinoids (Kelly & Nappe, 2020). Synthetic cannabinoids are the most abused synthetic drug and second most abused drug among adolescents (Cordeiro, Daro, Seung, Klein-Schwartz, & Kim, 2018). The legalization of cannabis within the U.S. has led to increased pediatric exposures to cannabinoid products including e-liquids. Emergency room visits and poison control calls for unintentional pediatric exposure to cannabinoids have increased considerably in the last 2 years (Wong & Baum, 2019). Outcomes from exploratory or unintentional ingestion of cannabis-containing products include respiration depression, encephalopathy, and coma (Blohm, Sell, & Neavyn, 2019). Adolescent cannabis use has been linked to short-term and long-term neurocognitive, academic, and health consequences, though these studies are often inconclusive. Importantly despite evidence suggesting cannabis use is harmful to adolescents, youth in the U.S. perceive it as safe (Hasin, 2018). Reports of abuse and toxicity are steadily growing as the number of synthetic cannabinoids produced increases. Geographic clusters of high use, toxicity, and death have been reported. Despite known toxicity and increased availability of cannabis, many people continue to abuse synthetic cannabinoids for various reasons including lower cost as compared to cannabis and lack of detection on routine drug screening (Kelly & Nappe, 2020).
Section 3.4. Metals
Various components of ENDS devices are sources of metal contamination in e-liquids. Examples include the nicotine extract, as well as physical components of ENDS devices including filaments, wicks and sheath, solder joints, outer fibers, thick wire, brass clamps, and filters (Gaur & Agnihotri, 2019). Metal concentrations in e-liquids from ENDS device components are reportedly associated with specific vaping practices, such as increased voltages, resistance, and temperature, which can contribute to the degradation of heating coils and other metal elements. High variability in metal concentrations exists between brands of vaping devices, potentially due to the heating element (Hess, et al., 2017; Olmedo, et al., 2018) and/or poor manufacturing practices which results in fraying at the soldered joints of the heating coils (Williams, To, Bozhilov, & Talbot, 2015). Comparison of metal concentrations found in e-liquids across 13 different studies identified nickel (1.94 x 107 ppm), manganese (5.94 x 106 ppm), zinc (3.25 x 106 ppm), copper (1.99 x 106 ppm), and iron (1.88 x 106 ppm) as the 5 most highly detected metals (J. Zhao, et al., 2018). E-liquids may also contain arsenic (As) and other metals/metalloids (Beauval, et al., 2016; Olmedo, et al., 2018; D. Zhao, et al., 2020; D. Zhao, et al., 2019).
Metals/metalloids may originate from the coil (Farsalinos, Voudris, & Poulas, 2015; Olmedo, et al., 2018) and from soldered joints and other parts of the device (Williams, Bozhilov, Ghai, & Talbot, 2017). Commonly used coils are made of alloys such as kanthal [iron (Fe), chromium (Cr), and aluminum (Al)], nichrome [nickel (Ni) and Cr], or high-purity metals (e.g., Ni or titanium) (Farsalinos, Voudris, et al., 2015; Olmedo, et al., 2018). Tin (Sn) and other metals are used in soldered joints (Williams, et al., 2017; Williams & Talbot, 2019; Williams, et al., 2015). Metal/metalloid contamination in e-liquids also depends on the storage device of the e-liquid as it may come from a tank or a refill cartridge (Olmedo, et al., 2018; J. Zhao, et al., 2018). Comparison of metal/metalloid detection in tanks vs. refills found that both the number and concentrations were higher in the tank e-liquids than in the refill e-liquids (Beauval, et al., 2016; Hess, et al., 2017; Kamilari, Farsalinos, Poulas, Kontoyannis, & Orkoula, 2018; Olmedo, et al., 2018; J. Zhao, et al., 2018), indicating that metal contamination is primarily from the metal components of ENDS devices, and not from the added e-liquids.
Section 3.4.1. Metal toxicity
The presence of metals and metalloids (e.g., arsenic, chromium, lead, nickel) in ENDS aerosols is a major concern, given their serious health effects, including cancer (Garcia-Esquinas, et al., 2014; Kuo, Moon, Wang, Silbergeld, & Navas-Acien, 2017), cardiovascular disease (Chowdhury, et al., 2018; Moon, Guallar, & Navas-Acien, 2012), renal damage (Suwazono, et al., 2006), and neurotoxicity (Caito & Aschner, 2015). For most of the detected metals/metalloids associated with ENDS use, D. Zhao, et al. (2020) found that levels were heterogeneous according to sample (e-liquid, aerosol), source of the sample (bottle, cartridge, open wick tank), and device type (cig-a-likes and tank). Most metal/metalloid levels in the biosamples of ENDS users, with the exception of Cd, were similar or even higher in ENDS users compared to conventional cigarette users. Comparison of metal/metalloid aerosol levels to human biosample levels (Aherrera, et al., 2017) supported the hypothesis that aerosol metals/metalloids are inhaled and absorbed by ENDS users.
Section 3.5. Flavoring Chemicals
E-liquids come in many different flavors. Evaluation of nine different studies that investigated the chemical composition of over 670 flavored e-liquids identified ethyl maltol, ethyl vanillin, vanillin, cinnamaldehyde, and menthol as the most common flavoring chemicals (Aszyk, et al., 2018; Behar, Luo, McWhirter, Pankow, & Talbot, 2018; Bitzer, et al., 2018; Czoli, et al., 2019; Hua, et al., 2019; Hutzler, et al., 2014; Lisko, Tran, Stanfill, Blount, & Watson, 2015; Omaiye, et al., 2019; Tierney, Karpinski, Brown, Luo, & Pankow, 2016). In these nine studies, there were between 1 and 47 different flavoring chemicals detected in a single e-liquid. Comparison of flavor chemical concentrations of 476 e-liquids from seven individual studies identified concentrations from < 5 to 1.55 x 105 ppm (Aszyk, et al., 2018; Behar, et al., 2018; Bitzer, et al., 2018; Hua, et al., 2019; Lisko, et al., 2015; Omaiye, et al., 2019; Tierney, et al., 2016). Cinnamaldehyde, menthol, ethyl maltol, benzyl alcohol, and vanillin had the highest average reported concentrations which typically exceeded reported concentrations found in food or consumer products (Good-Scents-Company, 2021).
Section 3.5.1. Flavoring Chemical Toxicity
While the flavoring compounds in vape products are generally considered safe for oral ingestion (Adams, et al., 2005), there is no evidence to indicate they are safe to inhale as aerosols. On the contrary, the limited evidence available would suggest that they pose significant inhalation hazard. For instance, Erythropel, et al. (2019) recently reported that flavor aldehydes including benzaldehyde, cinnamaldehyde, citral, ethylvanillin, and vanillin rapidly reacted with the e-liquid solvent PG after mixing, with > 40% of flavor aldehyde content converted to flavor aldehyde PG acetals which in turn activated aldehyde-sensitive TRPA1 irritant receptors and aldehyde-insensitive TRPV1 irritant receptors in vitro. When heated at high temperatures using ENDS devices, aerosolized flavorants can form ultrafine particles that penetrate deeply into the lung (Kim, et al., 2016). The Flavor and Extract Manufacturers Association (FEMA) of the United States released a report in 2012 identifying a list of 27 priority flavoring agents that may pose a risk to workers' respiratory health when inhaled including acetaldehyde, acetoin, benzaldehyde, diacetyl, cinnamaldehyde, and ethyl acetate (FEMA, 2012). Nearly all aldehydes are respiratory irritants when inhaled at significant concentrations (Fowles & DiBartolomeis, 2017).
Diacetyl and its structural analogs (2,3-pentadione, 2,3-hexanedione, and 2,3-heptanedione) are commonly used in buttery or caramellic flavored e-liquids (Ind, 2020). Diacetyl is associated with the severe respiratory disease bronchiolitis obliterans observed in popcorn factory workers (Barrington-Trimis, Samet, & McConnell, 2014; Fowles & DiBartolomeis, 2017). Additionally, research by Potera (2012) suggests that diacetyl substitutes may be just as toxic to the lung as diacetyl. Diacetyl substitutes have also been associated with adverse respiratory outcomes (Stratton, et al., 2018). Another study by Farsalinos, Kistler, Gillman, and Voudris (2015) concluded that daily inhaled exposures of diacetyl and 2,3-pentadione in e-liquids exceeded NIOSH recommended standards.
Pulegone, a component of mint oil extract, has been identified at substantial concentrations in mint and menthol flavored e-liquids (Lisko, et al., 2015). In 2018, the FDA banned synthetic pulegone as a food additive due to its association with several cancers in rodent models (FDA, 2018b; NTP, 2011). A recent study calculated the margin of exposure (MOE) for pulegone using FDA no-observed adverse effect levels and average human exposure for five different e-liquids in three different brands at variable levels of ENDS use. The pulegone MOE for e-liquids in this study suggested an increased cancer risk associated with exposure (Jabba & Jordt, 2019).
Section 3.6. Transformation Products
Section 3.6.1. Device Impacts on Transformation Products
Multiple device characteristics affect the rate of volatilization and transformation of e-liquid into an aerosol, including wicking efficiency, heating coil material and design, battery voltage, and temperature. Higher wicking efficiency (how easily the e-liquid is transferred to the heating coil) leads to aerosolization occurring faster than the liquid transfer, solvent over-heating, and increased chemical transformations (Gillman, Kistler, Stewart, & Paolantonio, 2016). The stability of the wick temperature has been correlated with the degradation of propylene glycol and glycerol (Vreeke, et al., 2018). Heating coils contribute to variability in the produced chemical profile and concentrations of transformation products, even in devices with similar components (Jensen, Strongin, & Peyton, 2017). One study used an infrared camera to monitor the temperature of heating coils, and found that temperature was not evenly distributed amongst the coils, resulting in hot spots and variability of formaldehyde formation (Z. Chen & Zeng, 2017). Talih, et al. (2017) studied the formation of volatile aldehydes in ENDS devices and found that while no correlation was observed with power, there was a correlation after power normalization by coil surface area.
Operational settings used in third and fourth generation vaping devices influence chemical composition of vape aerosols. Variable power devices allow the user to adjust wattage settings at preset increments ranging from 5–25 W (Geiss, Bianchi, & Barrero-Moreno, 2016). At lower wattage, the user experiences cooler, less dense aerosols. At a higher wattage, the user experiences warmer, more voluminous clouds but also higher levels of carbonyl inhalation (Geiss, et al., 2016). A more recent study showed that higher wattage was positively correlated with e-liquid and glycerol consumption, but was inversely related to consumption of propylene glycol and nicotine, suggesting chemical transformations of the latter (Y. J. Lee, et al., 2020). Epoxides have been observed as transformation products at lower wattage and enols are observed at higher wattage (Jensen, et al., 2017).
Section 3.6.2. Carrier Solvents
In one of the first studies aimed at investigating the chemical composition and transformation of carrier solvents in aerosol, the composition of the collected aerosol was found to be similar to that of the e-liquid (Tayyarah & Long, 2014). A separate analysis identified transformation products of the carrier solvents including formaldehyde, acetaldehyde, acrolein, methyl glyoxal, and glyoxal (Margham, et al., 2016). Another study of three different e-liquids and two different aerosolizers found that seven of the most abundant products found in ENDS aerosol were carrier solvent transformation products (Sleiman, et al., 2016). Glycidol and acrolein were primarily produced by glycerol degradation, while acetol and 2-propen-1-ol were produced mostly from propylene glycol. Formaldehyde originated from both solvents. Based on detected concentrations of formaldehyde, researchers calculated that the estimated daily exposure to formaldehyde at 215°C exceeded the U.S. Environmental Protection Agency (EPA) and California Office of Environmental Health Hazard Assessment acceptable cancer risk estimates for ENDS users (P. Wang, et al., 2017). A third study identified fifteen breakdown products associated with both carrier solvents, many of them being reported for the first time (Jensen, et al., 2017).
Section 3.6.3. Vitamin E Acetate
Some of the most recent studies of aerosols of concern have focused on vitamin E acetate (VEA) related to cannabinoid use (Duffy, et al., 2020; D. Wu & O'Shea, 2020). VEA can be used as a thickening agent or diluent in THC and CBD products due to its oily consistency. Researchers discovered that VEA has the potential to produce toxic ketene gas, as well as carcinogenic benzene and alkenes (D. Wu & O'Shea, 2020). Other researchers discovered that aerosols containing both THC and VEA mixtures form a hydrogen-bonded complex of both compounds (Lanzarotta, Falconer, Flurer, & Wilson, 2020). More research is needed to assess the formation of products related to VEA. We discuss their relation to toxic effects observed in users of cannabinoid vaping mixtures in a later section of this review.
Section 3.6.4. Flavoring Chemical Transformation Products
Our understanding of the formation of flavoring chemical transformation products and their potential toxicity is improving. Free radical formation during aerosolization was assessed in 49 e-liquid flavors, of which 20 increased and one decreased radical production (Bitzer, et al., 2018). Nearly 300 compounds were identified in the aerosols of the 49 flavors, and the 10 compounds most highly correlated with radical production were selected for further investigation (Bitzer, et al., 2018). Results indicated that increased and decreased radical production was concentration-dependent and identified ethyl vanillin as the only flavoring compound that significantly decreased radical production. This result correlated with decreased radical production associated with other vanilla flavor mixtures (Bitzer, et al., 2018). Degradation of flavoring compounds to aldehydes and those containing aldehyde functional groups was touched on in Section 3.5.1. Flavoring Chemical Toxicity. Flavoring compounds are the primary source of aldehyde formation, including acetaldehyde and formaldehyde, during aerosolization (Khlystov & Samburova, 2016). Benzene formation has been observed from the carboxylation and subsequent decarboxylation of benzaldehyde (a cherry flavor compound) during aerosolization (Pankow, et al., 2017). Researchers noted that this transformation pathway is likely to occur for other flavor compounds with aldehyde functional groups. Formation of benzene ranged from 100–5,000 μg/m3, was temperature-dependent, and was observed only in the variable power aerosolizers tested (Pankow, et al., 2017).
Section 3.6.5. Transformation Product Toxicity
Toxic transformation products result predominantly from the transformation of carrier solvents or flavoring agents. As mentioned above, one such transformation product, formaldehyde, is classified as a carcinogen to humans by IARC and a probable human carcinogen by the EPA (ATSDR, 2020). Formaldehyde affects the gastrointestinal, immunological, and respiratory systems (ATSDR, 2020). Exposure to formaldehyde can irritate the skin, throat, lungs, and eyes, while repeated exposure to formaldehyde can lead to certain types of cancers (NIOSH, 2014). Benzene is also a classified carcinogen by the EPA and IARC (ATSDR, 2020). Health effects associated with exposure to benzene include effects on the hematological, immunological, and neurological systems. Exposure to benzene affects the eyes, skin, airway, nervous system, and lungs, and can result in blood cancers such as leukemia (Health, 2019). Acrolein is not a classifiable carcinogen by IARC or the EPA due to the lack of available evidence to assess potential carcinogenicity (ATSDR, 2020); however, health effects associated with exposure to acrolein include effects on the cardiovascular, hematological, ocular, and respiratory systems (ATSDR, 2020). Additionally, inhalation exposure to acrolein can result in severe respiratory irritation, including irritation of the nasal cavity, and is known to damage the lining of the lungs (Clapp & Jaspers, 2017; Fowles & DiBartolomeis, 2017).
Section 4. Clinical & Epidemiological Findings for ENDS Adverse Health Outcomes
ENDS use has been widely touted as less harmful than traditional tobacco cigarette use due to the formation of fewer carcinogenic byproducts of combustion from ENDS use (Bhalerao, Sivandzade, Archie, & Cucullo, 2019; Cooke, Fergeson, Bulkhi, & Casale, 2015; Meo & Al Asiri, 2014; Mravec, Tibensky, Horvathova, & Babal, 2020). A lower degree of harm is almost certainly real, but not easily quantified. ENDS use affects the cardiovascular system, causing increased rates of myocardial infarction (Lippi, et al., 2014) and is clearly associated with EVALI in human adolescents (D. R. Rao, et al., 2020) and with impaired endothelial function in rats (Rao, Liu, & Springer, 2020). Burns and addictive behaviors are also associated with ENDS use (Dinardo & Rome, 2019). The association between ENDS use and pulmonary difficulties is more complicated. Before the emergence of EVALI as a public health threat, pulmonary complaints including respiratory distress syndrome and various types of pneumonias following ENDS use were reported infrequently and with varying levels of severity, currently making it impossible to draw a definitive tie between ENDS use and damage to the pulmonary system (Cooke, et al., 2015; Meo & Al Asiri, 2014).
Other challenges in associating ENDS use to characteristic human health effects include variability in device configuration, frequency and duration of use, and confounding lifestyle habits of users, such as co-use of combustible tobacco. Much of the data on the health effects of vaping comes from self-reported responses to surveys that have inconsistent language or insufficient data on participant lifestyles and patterns of use to account for potential confounders. Frequency and duration of ENDS use have the potential to alter the presentation and severity of toxic effects, as demonstrated by a variety of case studies (Fonseca Fuentes, et al., 2019). Additionally, results of epidemiological studies can be confounded by a current or previous smoking habit, use of cannabis products, and simultaneous use of multiple ENDS products, all of which may not be disclosed by study participants on questionnaires (Polosa, et al., 2017; Staudt, Salit, Kaner, Hollmann, & Crystal, 2018). These issues, along with rapidly changing product designs and a lack of long-term follow-up with test subjects, contribute to the difficulty associated with characterizing the effects of acute and chronic ENDS use on human health (Agustin, Yamamoto, Cabrera, & Eusebio, 2018; Pisinger & Dossing, 2014; Polosa, et al., 2017).
Section 4.1. EVALI
An emerging and alarming health effect associated with vaping is EVALI (sometimes called vaping-related acute lung injury, or VpALI) (Fonseca Fuentes, et al., 2019). EVALI is characterized by 1) respiratory symptoms including cough, chest pain, and shortness of breath (experienced by 95% of patients); 2) constitutional symptoms including fever, chills, and weight loss (85% of patients); and 3) gastrointestinal symptoms including abdominal pain, nausea, vomiting, and diarrhea (77% of patients) (Salzman, Alqawasma, & Asad, 2019). Patients do not need to exhibit all or most of the symptoms to be diagnosed and it is typically diagnosed by the elimination of other illnesses in patients who use vaping devices. EVALI has been associated with both nicotine and cannabis products, though it has been more commonly associated with cannabinoid vaping. While vaping results in lower exposure to known toxic combustion products than smoking, vaping presents additional exposures to carrier solvents and additives as discussed above, and the hazards of these compounds as aerosols are just starting to be defined. Ongoing research strongly suggests additives, including VEA and their transformation products, are responsible for EVALI (Blount, et al., 2020; D. Wu & O'Shea, 2020).
As of February 2020, the CDC had identified over 2800 cases of EVALI in the U.S., 15% of which were under 18 years of age. Symptoms of EVALI in adolescents were similar to those in adults (Cherian, Kumar, & Estrada, 2020; Messina, Levin, Conrad, & Bidiwala, 2020; Thakrar, Boyd, Swanson, Wideburg, & Kumbhar, 2020). In a series of case reports presented by Kass, Overbeek, Chiel, Boyer, and Casey (2020), a spectrum of clinical findings was reported, from a cough that resolved with discontinuation of ENDS use to respiratory failure with hypoxia requiring prolonged intensive care. Carroll, et al. (2020) reported a series of adolescent case studies with confirmed EVALI. Half of the patients required intensive care, presenting with gross bronchio-pathologic abnormalities. Despite improvement with treatment, residual airway reactivity or diffusion abnormalities persisted when patients were re-evaluated in the short-term (mean of 4.5 weeks). This outbreak highlights the urgent need to better understand and safeguard against the hazards of vaping (Blount, et al., 2020; D. Wu & O'Shea, 2020), especially in the young.
Section 4.2. Acute & Chronic Health Effects of ENDS Use
Section 4.2.1. Acute Effects
Characterizing acute toxic effects of ENDS use in adults is an additional concern. Staudt, et al. (2018) conducted a prospective observational epidemiological study that analyzed health outcomes from acute exposures to ENDS. To avoid potential confounding variables, such as a previous or current smoking habit, ten never smokers were instructed to take ten puffs on an ENDS device, wait 30 minutes, and take another ten puffs. Endpoints consisted of patients’ perceived symptoms, vital signs, lung function, and oxygen saturation. Researchers did not find a consistent change in any of the endpoints; however, multiple case studies have demonstrated the variety of toxic effects that can occur because of acute exposures to ENDS (Attis, King, Hardison, & Bridges, 2018; Fonseca Fuentes, et al., 2019; Moore, Young, & Ryan, 2015; Thota & Latham, 2014). In a separate study evaluating pulmonary effects of acute exposure, reductions in exhaled fractional nitric oxide (FeNO), a common measure of airway inflammation, were measured after five minutes of ENDS use. The authors also noted greater observed respiratory resistance, respiratory impedance, and overall peripheral airway resistance, similar to effects seen immediately after cigarette smoking (Vardavas, et al., 2012). Additional studies with well-defined exposure regimens are necessary to further explore the acute effects of ENDS use.
Section 4.2.2. Chronic Effects
While arguably more challenging to study, identifying the chronic effects of ENDS use in adults is essential if we are to understand the range of toxic effects. Polosa, et al. (2017) conducted a prospective observational epidemiological study that analyzed the health outcomes of nine daily ENDS users over 3.5 years. Patients’ vital signs, lung function, and respiratory symptoms were analyzed throughout the study after 12, 24, and 48 months of daily ENDS use. While no consistent changes in endpoints were observed, the authors suggested that these endpoints might not be sensitive enough to show chronic effects. Due to the small study size, additional studies with a larger sample size are necessary to improve our understanding of the toxic effects of chronic ENDS use in adults.
When studying the toxic effects of ENDS use in adults, it is essential to recognize how the duration of exposure can alter the severity of effects. For example, a prospective observational study conducted by Blagev, et al. (2019) explored the range of sub-chronic effects from ENDS use among hospital patients presenting with EVALI symptoms, with a median exposure of 225 days and a maximum exposure of five years. Because the chronic exposure regimen was not well-defined within the study and inconsistent across patients, the frequency of ENDS use ranged from one or two times per week to over 50 times per day for varying durations. The varied frequency and duration of ENDS use led to a range in severity of symptoms. While some individuals were treated at hospitals as outpatients, the majority were admitted to the Intensive Care Unit, and a small percentage of individuals died. The deaths were of patients with underlying illness or patients who continued using ENDS after they became sick. Patients experienced respiratory and gastrointestinal symptoms consistent with EVALI, and hospital data described high heart rates, high respiratory rates, and low oxygen saturations, as well as abnormal chest radiographs. Prescribed therapy included antibiotics, steroids, and supplemental oxygen. Two-week follow-up appointments with the patients showed substantially improved radiographic findings (Blagev, et al., 2019). The results of this study suggested that toxic effects from chronic ENDS use can be reversed, assuming the patients cease ENDS use upon experiencing symptoms.
Section 4.3. Co-use of ENDS and traditional tobacco
A year-long study invited cigarette smokers to switch, either partially or entirely, to ENDS products. Less fractional exhaled nitric oxide and carbon monoxide were observed in exhaled breath from those who had completely quit traditional cigarettes, but not from participants who continued to smoke cigarettes (Campagna, et al., 2016). Similarly, in a separate year-long study observing spirometric data, participants who completely stopped smoking cigarettes showed improvements in mid-expiratory flow rate (FEF25-75%) at week 52 compared to week 1 (Cibella, et al., 2016). Each of these measurements is associated with improved lung function and may indicate long-term health improvements of switching from traditional cigarettes to ENDS, but only with complete tobacco cigarette cessation. Observed health improvements of switching from cigarettes to ENDS may largely be driven by the cessation of tobacco cigarette smoking. Co-use of combustible tobacco with ENDS may eliminate the benefits and compound the health concerns of the two methods. One longitudinal study on associations between ENDS use and respiratory disease identified both traditional cigarette and ENDS use as independent risk factors for respiratory disease, but dual use of both products increased risk of respiratory disease greater than either product alone (Bhatta & Glantz, 2020). This result is especially troubling because higher nicotine dependence has been observed among dual users over those using only ENDS or tobacco cigarettes. Dependence is even greater with the use of three or more nicotine-containing products (Ali, Gray, Martinez, Curry, & Horn, 2016; Stanton & Halenar, 2018).
Cigarette smoking is also strongly associated with reduced immune function. Studies comparing immune effects between tobacco cigarette smokers and ENDS users, or before and after switching from tobacco cigarettes to ENDS, are largely biomarker-based. A short-term controlled study on immediate effects of ENDS aerosol vs. cigarette smoke on blood cell counts showed that exposure to ENDS aerosol (from both active and passive use) did not significantly affect complete blood counts, while exposure to cigarette smoke increased white blood cell, lymphocyte, and granulocyte counts (Flouris, et al., 2012). Another study found that genes repressed in nasal mucosa samples from tobacco cigarette smokers were similarly repressed in samples from ENDS users, though these genes were more highly repressed among ENDS users. Samples from ENDS users also showed a greater number of repressed genes than samples from cigarette smokers (Martin, et al., 2016). In a separate proteomics study, sputum samples were collected from cigarette smokers, ENDS users, and non-users. Increases in proteins associated with oxidative stress and aldehyde-detoxification were observed in samples from ENDS users as well as innate defense proteins associated with COPD. ENDS use and tobacco cigarette use altered some similar and some unique proteomic profiles compared to non-users indicating similar biological responses between traditional cigarette smoking and ENDS use (Reidel, et al., 2018).
Section 4.4. Considerations for Vulnerable Populations
Section 4.4.1. Adolescent Health Effects of ENDS Use
As the prevalence of adolescent ENDS use increases, so does the urgency to understand the hazards of ENDS use in this population. Toxicant exposure from ENDS products may result in greater harm in adolescents than in adults (Wild & Kleinjans, 2003). Rubinstein, Delucchi, Benowitz, and Ramo (2018) indicated that while adolescent exposure to toxic volatile organic compounds (VOCs) was decreased in e-cigarette-only users versus dual users of e-cigarettes and traditional cigarettes, adolescent e-cigarette-only users still had levels of five different VOC toxicants detected in their urine in quantities up to three times greater than in matched controls. In addition to EVALI, multiple adverse health outcomes have also been associated with ENDS use, including bronchitic symptoms (chronic cough, phlegm, and bronchitis) (McConnel, et al., 2016), seizures, and acute esophageal injury (Bozzella, Magyar, DeBiasi, & Ferrer, 2020).
Along with health effects associated with novel e-liquid constituents, nicotine itself is an extremely addictive substance known to affect the developing adolescent brain. Seizures are a known potential side effect of nicotine toxicity and have been reported after vaping. Between April 3 and June 30, 2019, the FDA, received reports of 117 cases of seizures related to vaping, the majority of which were in adolescents or young adults (Weidner, Rudy, & Faulcon, 2020). Adolescents who smoke combustible cigarettes have more difficulty with working memory and attention than non-smokers (Jacobsen, et al., 2005). The human brain continues to develop until approximately age 25, so exposure to nicotine during adolescence can impact brain development and affect memory, attention, learning, and impulse control (Yuan, Cross, Loughlin, & Leslie, 2015), making ENDS use among adolescents particularly troubling.
Section 4.4.2. Effects of ENDS Use during Pregnancy
Exposure to e-liquid components and transformation products is especially concerning during fetal and early childhood development. A period of development in which nicotine exposure can be particularly detrimental is in utero. ENDS use during pregnancy has been steadily increasing due to the notion that it is safer than tobacco cigarette use while pregnant (Whittington, et al., 2018). Whittington, et al. (2018) reviewed 40 articles and found the prevalence of ENDS use during pregnancy was between 0.6% and 15%. Nicotine use during pregnancy can have adverse health outcomes on the offspring’s immune system, neural development, lung function, and cardiac function (England, et al., 2017).
Section 4.4.3. Accidental Ingestion of e-liquids in Children
There has also been an increase in the number of unintentional exposures to e-liquids, primarily among small children. A retrospective analysis using the National Poison Data System published in 2016 found that the monthly number of exposures associated with ENDS products increased by about 1500% from 2012 to 2015, including ingestion, dermal, inhalation/nasal, ocular, other, and unknown routes of exposure. Children below two years old accounted for 44% of unintentional exposures, and children accidentally exposed to ENDS as opposed to traditional cigarettes had 5.2 times higher odds of a healthcare facility admission and 2.6 times higher odds of having a severe outcome (Kamboj, Spiller, Casavant, Chounthirath, & Smith, 2016; Quail, 2020). A prospective observational study was conducted from 2014 to 2017, in which 265 calls to the Oregon Poison Center were evaluated for characteristics of exposure to ENDS devices and e-liquids among children and adults. The majority of these cases were associated with mild symptoms and rare nicotine toxicity. Most children who ingested e-liquid were exposed to only a small amount. The onset of symptoms occurred almost immediately after ingestion in the majority of these cases. The most commonly reported symptoms were vomiting, tachycardia, diarrhea, lethargy, jitteriness, agitation, nausea, and oral mucosal irritation (Hughes & Hendrickson, 2019). Additional case studies have reported symptoms including constitutional and gastrointestinal discomfort, respiratory distress, and a decline of neurocognitive functioning after ingestion of e-cigarette liquid (Bassett, Osterhoudt, & Brabazon, 2014; Eggleston, Nacca, Stork, & Marraffa, 2016; Gill, Sangha, Poonai, & Lim, 2015; Noble, Longstreet, Hendrickson, & Gerona, 2017; Seo, Kim, Yu, & Kang, 2016).
Section 5. Animal Models of ENDS Use Hazards
The latest research on effects of ENDS use suggests acute and chronic toxicities from inhaling aerosols of glycerol, propylene glycol, nicotine, and flavoring agents (Pulvers, et al., 2018; J. Wang, et al., 2020). The complex nature of e-liquids makes it difficult to parse out which substances, or their potential synergistic effects, are harmful (Fowles & DiBartolomeis, 2017). Most flavoring agents and carrier solvents in e-liquids are considered “food safe” for ingestion as minor ingredients (Barrington-Trimis, et al., 2014; Chun, et al., 2017; Clapp, Peden, & Jaspers, 2020) or by dermal exposure; however, these safety assessments for ingestion and dermal exposure to not reflect the hazard of inhalation, which is inherent with ENDS use. Exposure of different animal models to ENDS products via various routes of exposure has informed the potential for adverse health outcomes resulting from ENDS usage.
Animal evidence suggests that vaping of nicotine-containing e-liquid mixtures affects numerous organ systems throughout the body, including respiratory, cardiovascular, immune, and nervous systems. Damage to the various body systems likely results from a combinatorial effect of disrupting numerous molecular pathways and vital cellular processes, as discussed in a later section of this review. Chamber exposures to ENDS products are typically studied in mice as this method most closely represents human vaping exposures. Nebulizers and nose-only inhalation chambers are less commonly used (Garcia-Arcos, et al., 2016; Husari, et al., 2016), and several studies have employed intraperitoneal injection of unheated e-liquids (El Golli, Jrad-Lamine, et al., 2016; El Golli, Rahali, et al., 2016; Golli, et al., 2016; Rahali, et al., 2018).
Acute and chronic studies have used mice and rats ranging in age from 6 (Crotty Alexander, et al., 2018) to 14 (Olfert, et al., 2018; Szostak, et al., 2020) and 16 weeks (Schweitzer, et al., 2015), with varying exposure frequencies, durations, and dosages of nicotine e-liquid aerosols. Exposure durations vary across studies, with acute exposures ranging from two individual doses (Schweitzer, et al., 2015) up to 4 weeks of nearly daily exposure regimens (Canistro, et al., 2017; Cardenia, et al., 2018; Glynos, et al., 2018; Hwang, et al., 2016). Chronic exposures have ranged from a few weeks (Laube, et al., 2017), up to 3-8 months, depending on the outcome measured (Crotty Alexander, et al., 2018; Espinoza-Derout, et al., 2019; Olfert, et al., 2018; Szostak, et al., 2020). For this review, acute exposures were considered single or repeated dosages up to 4 weeks in exposure period, and anything longer than 4 weeks was considered chronic, generally following Organization for Economic Co-operation and Development (OECD) guidelines (OECD, 2018).
Rodent pre- and post-natal studies have also helped researchers understand how maternal ENDS use affects offspring health, a growing concern as ENDS use during human pregnancy has become more prevalent, as discussed above. Animal studies have involved exposures that are 1) preconception, where just the dams (Wetendorf, et al., 2019) or both the dams and males (Noel, et al., 2020) were exposed before mating; 2) prenatal, where dams were exposed only during gestation (Church, et al., 2020; Noel, et al., 2020; Orzabal, et al., 2019); 3) pre- and postnatal, where dams were exposed through gestation and lactation (H. Chen, et al., 2018; T. Nguyen, et al., 2018, 2019; Wetendorf, et al., 2019) or dams were exposed through lactation and pups were also exposed postnatal (Lauterstein, et al., 2016; Smith, et al., 2015; Zelikoff, et al., 2018); or 4) postnatal (McGrath-Morrow, et al., 2015), during which only the pups were exposed.
Section 5.1. Effects of Acute Exposure to ENDS Aerosols
Section 5.1.1. Pulmonary System
Several recent studies reported redox imbalance and increased inflammatory response in the lungs of mice and rats exposed to e-cigarette aerosols. 3-day exposures resulted in diminished lung glutathione levels (Lerner, et al., 2015), protein thiol oxidation and perturbations in protein quality control (J. Wang, et al., 2020), and increased expression of pro-inflammatory cytokines (Lerner, et al., 2015). Glynos, et al. (2018) reported that 3-day acute exposure, but not 4-week subchronic exposure, significantly increased oxidative stress in lung tissue. Cirillo, et al. (2019) found exposure to e-cigarette aerosol for 28 days altered lung structure, with large areas of airflow collapse, and caused modulation of antioxidant and phase II enzymes, suggesting perturbation of the lung redox status. It would seem more than plausible that e-cigarette aerosols present an oxidative stress and an inflammatory response hazard to human lungs.
Rodent studies evaluating the effects of ENDS use on the pulmonary system have observed mixed effects on baseline lung mechanics and airway responsiveness depending on the specific e-liquid treatment, flavoring, and duration of exposure. After 18 days of exposure, Chapman, et al. (2019) found that e-cigarette treatments did not affect baseline lung function or airway hyper-responsiveness, except for ENDS aerosol containing cinnacide flavoring without nicotine, which increased tissue elastance. Glynos, et al. (2018) did observe a dose-dependent increase in airway hyper-responsiveness after three days of exposure to cigarette smoke and tobacco blend-flavored e-cigarette treatment with nicotine, separately. No gross histopathological effects were observed after any ENDS aerosol treatments tested (Glynos, et al., 2018; Laube, et al., 2017); however, exposure to “banana pudding” flavored e-cigarette aerosol without nicotine was shown to increase soluble collagen in the lungs of house dust mite-treated mice (Chapman, et al., 2019). Carrier solvent aerosols alone have been reported to alter lung function following acute 3-day exposure (Glynos, et al., 2018) and to increase mucociliary clearance following chronic exposure (Laube, et al., 2017). Despite the lack of histological effects and mixed effects on lung mechanics, different ENDS exposures clearly alter the pulmonary system in various ways.
Section 5.1.2. Cardiovascular System
Animal studies examining ENDS aerosol effects on the cardiovascular system have addressed multiple endpoints such as cardiac function, histology, thrombosis, and angiogenesis; however, there are insufficient studies from which to draw a clear consensus. Acute ENDS exposure in mice significantly decreased heart rate and slightly decreased heart weight without changes in aortic dimensions and left ventricle size in one study (Shi, et al., 2019), but increased heart weight in another (Y. M. Chen, Huang, Sung, Lee, & Hsiao, 2019). Cardiac histological assessment after exposure to various e-liquid aerosols with multiple nicotine concentrations revealed no significant effects (Y. M. Chen, et al., 2019). Shi, et al. (2019) concluded that short-term e-cigarette exposure did not affect cardiac fibrosis, but slightly increased the expression of collagen I protein with no effect on alpha smooth muscle.
While little overlap exists between studies focused on thrombosis and blood content, available studies showed that e-cigarette treatment affected cardiovascular variables. Qasim, et al. (2018) observed that exposure of mice to ENDS aerosol caused a cessation of bleeding in significantly less time than for control mice. In a ferric chloride carotid artery injury-induced thrombosis model, ENDS aerosol-exposed mice had shortened occlusion times, attributed to hyperactive platelets (Qasim, et al., 2018). While e-cigarette exposure did not affect platelet count, it did enhance agonist-induced platelet aggregation and secretion and led to elevated integrin GPIIb-IIIa activation, required for platelet aggregation. Exposure also increased phosphatidylserine expression on platelet surfaces, which plays a role in the assembly of coagulation factor complexes, and enhanced Akt and ERK phosphorylation, critical to platelet function (Qasim, et al., 2018). After analyzing the blood of rats exposed to e-cigarettes, Canistro, et al. (2017) found significantly increased esterified cholesterol, total cholesterol, triglycerides, and saturated fatty acids, decreased polyunsaturated fatty acids, and DNA damage in leukocytes and reticulocytes (Canistro, et al., 2017). Shi, et al. (2019) found that e-cigarette exposure significantly increased cardiac angiogenesis with no change in vascular endothelial growth factor levels. Nicotine has stimulatory effects on angiogenesis, while cigarette smoke can cause angiogenesis dysfunction (Ejaz & Lim, 2005; Shi, et al., 2019). Impacts to a broad range of endpoints suggest that acute ENDS exposure causes cardiovascular effects.
Section 5.1.3. Nervous System
Kaisar, et al. (2017) found evidence of integrity impairment of the blood-brain barrier in mice acutely exposed to ENDS aerosol, which could further harm the functioning of the cerebral vasculature. Interestingly, the study did not identify significant differences between mice exposed to tobacco smoke and mice exposed to ENDS aerosol, providing preliminary evidence that tobacco smoking and vaping are associated with similar nervous system toxicities. In a separate study, both ENDS aerosol and cigarette smoke exposure altered the cognitive functioning of mice, likely from an olfactory deficit typically seen in smokers (Prasedya, et al., 2020). When observed the following day, cigarette-exposed mice had greatly improved cognitive memory functioning, while e-cigarette aerosol-exposed mice had not yet recovered, indicating the potential for ENDS exposure to reduce short-term memory functions.
Section 5.1.4. Immune System
Increased immune cell recruitment and cytokine levels are widely observed effects of acute ENDS exposure. Studies commonly investigate bronchoalveolar lavage fluid (BALF) and lung homogenate after ENDS exposure and have found mixed results regarding the recruitment of immune cells and cytokine levels. Blount, et al. (2019) noted an increase in polymorphonuclear cells in BALF after 24 hours of exposure. After three days of exposure, Glynos, et al. (2018) found an increase in BALF total cell counts for all tested e-cigarette and cigarette smoke treatments, primarily a result of macrophage influx; however, cell counts were only elevated in the cigarette smoke and flavored e-liquid groups after four weeks. These results suggested that immune cell recruitment effects only persisted during longer acute ENDS exposures because of flavoring constituents. Elevated levels of oxidative stress in the lungs, and a modest inflammatory response in the airways were also observed (Sussan, et al., 2015), but with no difference in neutrophil, eosinophil, or lymphocyte counts in mice exposed to e-cigarette aerosol (Hwang, et al., 2016; Sussan, et al., 2015). In contrast, a mouse allergic airways disease model revealed that the tested nicotine containing e-liquids and the propylene glycol/glycerol control, dampened airway inflammation. The total number of leukocytes, eosinophils, and macrophages in the BALF were reduced upon exposure to e-liquid aerosol containing cinnacide without nicotine but was unchanged by propylene glycol/glycerol alone and other flavored e-liquid aerosols. While the nicotine e-cigarette treatments universally suppressed airway inflammation, the effects of aerosolized e-cigarettes without nicotine were flavor-specific (Chapman, et al., 2019).
Several studies have investigated the susceptibility to infection after ENDS exposure. In a recent study, ENDS-exposed mice infected with viruses or bacteria had reduced antiviral defenses with increased virus-induced morbidity and mortality, or significantly increased pulmonary bacterial burden due to suppression of bacterial clearance by alveolar macrophages (Sussan, et al., 2015). Defective phagocytic function was observed, however, surface expression of CD36 and MARCO macrophage scavenger receptors were not altered by e-cigarette aerosol exposure. In another study, S. pneumoniae strain TIGR4 was suspended in either control, 25% cigarette smoke extract, or 100% e-cigarette extracts (with and without nicotine) and then inoculated into mice (Bagale, Paudel, Cagle, Sigel, & Kulkarni, 2020). Similar levels of total protein in the BALF, and of cytokines IL-6 and IFN-γ in lung tissue, were observed from all treatment groups and no differences were observed for transcript levels of TNF-α, IL-6, IFN-γ, CCL2, or CSCL10. These results led the authors to conclude that the exposure of TIGR4 to both cigarette and e-cigarette extracts did not significantly affect its virulence in a mouse acute pneumonia model. However, in a similar study, mice infected with methicillin-resistant S. aureus (MRSA) grown in medium with e-cigarette aerosol extract exhibited higher mortality and ten-fold higher bacterial burdens in the lungs compared to mice infected with MRSA grown in control medium (Hwang, et al., 2016). Effects ranging from cytokine and immune cell recruitment to adverse immune defense mechanisms at the organismal level were noted after ENDS exposure in this study.
Section 5.1.5. Whole Organism Effects
Several recent studies have observed that mice exposed to some ENDS aerosols exhibit a significant decrease in weight gain, especially among males (Chapman, et al., 2019; Prasedya, et al., 2020; Shi, et al., 2019). In one study, female mice exposed to aerosolized e-liquid (0.5 or 5 mg/mL) exhibited no significant change in body weight or food and water consumption compared to controls after 14 days, though heart weight was significantly increased with exposure (Y. M. Chen, et al., 2019). Investigation of the effects of acute nicotine vaping on the strength and energy of mice exposed to 5 mg/mL nicotine in this study demonstrated decreased forelimb strength and a slight decrease in time to fatigue during swimming compared to controls. The decrease in energy was attributed to dose-dependent decreases in liver and muscle glycogen stores upon exposure.
Section 5.2. Effects of Chronic Exposure to ENDS Aerosol
Chronic chemical exposures differ in their resulting health effects from acute, high dose exposures. In terms of vaping e-liquid mixtures, persistent exposure to lower doses of chemical toxicants can produce both obvious manifestations of disease (i.e., respiratory disruption/coughing) along with more subtle adverse health effects that may only culminate in a poor health outcome later in life.
Section 5.2.1. Pulmonary System
Chronic exposure studies in rodents have identified several pulmonary effects of ENDS products, including the development of lung adenocarcinomas, suggesting carcinogenic potential (Tang, et al., 2019). In a study evaluating the relative impacts of ENDS versus traditional tobacco cigarette smoke exposure on pulmonary outcomes, female mice exposed to ENDS aerosol had fewer emphysema-associated changes measured by histological scoring and respiratory function (Olfert, et al., 2018). A separate study on ENDS-induced COPD-related endpoints evaluated in a chronic exposure mouse model found that nicotine-containing ENDS aerosol increased symptoms of COPD pathology, including airway hyper-reactivity, distal airspace enlargement, increased inflammatory cytokine and protease expression, and production of mucin, while non-nicotine containing aerosol did not (Garcia-Arcos, et al., 2016). Additionally, nicotine-exposed mice exhibited lung tissue destruction consistent with a COPD phenotype and increased phosphorylation of MAP kinase pathway components PKCa and ERK. The authors proposed a mechanistic model whereby this axis drives the increased protease and cytokine expression observed in vitro. Rigorous work performed by Madison, et al. (2019) provided strong evidence that glycerol/propylene glycol solvent, independent from nicotine, has a profound impact on alveolar macrophage function. Glycerol/propylene glycol inhibited the ability of macrophages to maintain proper surfactant and lipid metabolism in metabolomics and functional assays and demonstrated that this compromised metabolism led to attenuated macrophage function and reduced ability to fight off an infectious challenge.
Increased oxidative stress in the lungs and BALF has been a common observation across studies (Blount, et al., 2019; Glynos, et al., 2018; Sussan, et al., 2015; J. Wang, et al., 2020), and one study noted a decrease in mucociliary clearance, an essential mechanism for lung defense and health (Laube, et al., 2017). Sprague Dawley rats exposed to ENDS aerosol experienced dysfunction of lung redox homeostasis as measured by a reduction in antioxidant enzymes (catalase, DT-diaphorase, and superoxide dismutase), reduced systemic antioxidant capacity, increased reactive oxygen species (ROS), and increased 8-OHdG, a marker of oxidative stress. Additionally, exposure increased expression of cytochrome P450 enzymes, CYP1A1/2, CYP2B1/2, 2C11, and CYP3A, which may further alter redox homeostasis (Canistro, et al., 2017). After three days of exposure, propylene glycol/glycerol and nicotine-containing e-liquids with flavoring caused increased oxidative stress in the BALF and lung tissue, which persisted after four weeks of the same flavored e-liquid treatment. The authors suggested that nicotine seemed to mitigate solvent effects, but that the addition of flavoring superseded this mitigation (Glynos, et al., 2018). Lastly, after C57BL/6 mice were exposed to e-cigarette aerosol, mucociliary clearance was significantly lower in the 2.4% compared to the 0% nicotine e-cigarette group after a three-week exposure regimen, with no significant difference after one week (Laube, et al., 2017). While no gross histological effects were observed, altered pulmonary mechanics at the whole organ scale, increased oxidative stress, and decreased mucociliary clearance were caused by acute ENDS exposure, with notable variation caused by the duration of exposure and addition of flavoring.
Section 5.2.2. Other Health Effects
Effects of chronic nicotine vaping exposures on non-pulmonary systems have been documented less extensively. Crotty Alexander, et al. (2018) characterized the impact of inflammatory cytokines, produced presumably in the lung, on other organ systems and tissues peripheral to the lung. Chronic exposures of three and six months caused increased fibrosis in the liver, kidney, and cardiovascular system, reduced renal function, and gene expression signatures consistent with a pro-fibrotic phenotype. Studies on the effects of chronic vaping exposure on the nervous system found that mice exposed to a flavored e-liquid formulation containing nicotine exhibited reduced food intake and increased locomotor activity (Shao, et al., 2019). In another study, exposure to nicotine-containing e-cigarette aerosol drove a pro-inflammatory phenotype in mouse ventricular tissue as measured by transcriptomic profiling. Further investigation demonstrated decreased cardiac fractional shortening and ejection fractions, and increased atherosclerosis in the aortic root of exposed mice (Espinoza-Derout, et al., 2019). In summary, studies using rodent models to evaluate chronic vaping mixture exposures point to both nicotine and carrier solvent components (glycerol/propylene glycol) disrupting proper lung function. Vaping exposures in these models negatively affect peripheral organ systems, either through increased circulating inflammatory factors or through other pathological mechanisms.
Section 5.3. Effects of Pre- and Post-Natal Exposure to ENDS Aerosol
Section 5.3.1. Pulmonary System
Rodent maternal exposure to ENDS aerosols causes detrimental health effects on the offspring's pulmonary system. Preconception and prenatal exposures to cinnamon-flavored e-cigarettes caused structural changes to the lung which were associated with the downregulation of wnt and shh, involved in lung organogenesis (Noel, et al., 2020). Neonates exposed to e-cigarette aerosol from 24 hours of life had diminished alveolar proliferation and impaired lung growth (McGrath-Morrow, et al., 2015). Additionally, maternal exposure to e-cigarette aerosol increased proinflammatory cytokines (IL-1B, IL-6, TNF-a) in the lungs of both mothers and offspring, and induced epigenetic changes in the offspring’s lungs (H. Chen, et al., 2018). The pulmonary health effects appear to be driven, at least in part, by components other than nicotine (H. Chen, et al., 2018; Noel, et al., 2020).
Section 5.3.2. Cardiovascular and Reproductive Systems
Few studies have investigated the cardiovascular and reproductive health effects of rodent maternal exposure to e-cigarette aerosol. Dams exposed to nicotine-containing e-cigarette aerosol had significantly reduced blood flow in both maternal uterine artery and the fetal umbilical artery, signs of early-onset intrauterine growth restriction (IUGR) (Orzabal, et al., 2019). Maternal e-cigarette exposure also significantly delayed the first litter, possibly due to the lack of implantation sites compared to control mice (Wetendorf, et al., 2019). There was also a non-significant reduction in pup numbers and a decrease in fertility of male offspring, corroborating evidence from other studies that e-cigarette aerosol exposure causes a variety of potentially long-term detrimental health effects.
Section 5.3.3. Nervous System
In general, developmental exposures of nicotine-containing e-cigarette aerosol caused hyperactive behaviors in both male and female mice in adulthood (Church, et al., 2020; T. Nguyen, et al., 2018, 2019; Smith, et al., 2015). Offspring of dams switched from cigarette to e-cigarette exposure had more severe behavior deficits than offspring of dams exposed to only e-cigarette aerosol, though the latter offspring were not without significant behavior deficits (T. Nguyen, et al., 2019). In addition to hyperactivity, T. Nguyen, et al. (2018) showed that the offspring of dams exposed to nicotine-containing e-cigarette aerosol during and after pregnancy had short-term memory deficits attributed to the nicotine exposure. Additionally, upon maternal exposure, the other constituents of the e-cigarette aerosol were associated with reduced anxiety-like behavior in the offspring. Studies have identified significant epigenetic changes including global DNA hypomethylation and alterations to chromatin modifiers in offspring brains (T. Nguyen, et al., 2018, 2019), and several global gene expression changes associated with adverse neurobiological and behavioral outcomes (Lauterstein, et al.,2016). Increased levels of IL-6 and Iba-1 (markers for microglia) were detected in the brains of adults and juveniles, respectively (Church, et al., 2020; Zelikoff, et al., 2018). These studies highlight the long-term neuroinflammatory consequences of developmental e-cigarette exposure.
Section 5.3.4. Whole Organism Effects
Studies on pre- and post-natal effects of exposure to ENDS aerosols show a general lack of consensus on whether maternal ENDS exposure alters the body length and weight of the offspring. Some studies have found that exposure to ENDS aerosols does not alter maternal weight in mice (Church, et al., 2020) or rats (Orzabal, et al., 2019) but it may alter offspring weight. Some studies have identified no change in mouse birth length and weight (H. Chen, et al., 2018; Lauterstein, et al., 2016; T. Nguyen, et al., 2018), while others found that maternal mouse exposure to e-cigarette aerosol, with or without nicotine, reduced offspring weight and length at birth and for up to four weeks after (McGrath-Morrow, et al., 2015; T. Nguyen, et al., 2019; Noel, et al., 2020; Smith, et al., 2015). One of these studies showed that the weight and length returned to control levels by week 13 (T. Nguyen, et al., 2019), however, another demonstrated that maternal e-cigarette induction of underweight female offspring persisted until 8.5 months of age (Wetendorf, et al., 2019). The reduction in offspring length and weight may be driven by nicotine in the e-cigarette aerosol (T. Nguyen, et al., 2019; Orzabal, et al., 2019); however, other constituents may also be driving the reduction (McGrath-Morrow, et al., 2015).
Section 5.4. Non-Mammalian models
While rodents are the most commonly used animal models to study effects of ENDS exposure, other model organisms have been leveraged and can offer unique advantages.
5.4.1. Zebrafish Model
Fish obviously cannot model the specifics of aerosolized insult, but they can serve as a whole animal biosensor of e-cigarette ingredient hazard in a model that is both faster and less expensive than rodents (Holden, Truong, Simonich, & Tanguay, 2020; Truong, et al., 2014). Palpant, Hofsteen, Pabon, Reinecke, and Murry (2015) compared toxicities of tobacco cigarette smoke extract to e-cigarette aerosol extracts with equal nicotine concentrations of 6.8, 13.7, and 34 μM. Zebrafish (Danio rerio) embryos were exposed in individual wells until 72 hours post-fertilization (hpf) when evidence of cardiotoxicity was measured. Although both exposures produced significant cardiac developmental deficiencies measured by phenotypic abnormalities and heart rate, zebrafish exposed to tobacco smoke extracts had a higher percent occurrence and severity of heart defects, as well as several cardiac gene expression changes at 24 hpf compared to e-cigarette extract-exposed zebrafish (Palpant, et al., 2015).
Studies have also utilized zebrafish to understand the developmental toxicity of individual components of e-cigarette liquid. The carrier solvent, propylene glycol, caused reduced body size and morphological, cardiovascular, and neurological effects at concentrations 20–40 times lower than what is found in e-cigarette liquids (Lahnsteiner, 2008; Massarsky, Abdel, Glazer, Levin, & Di Giulio, 2017). Propylene glycol was also capable of causing long-term persistent neurological defects in adult zebrafish (Massarsky, Abdel, Glazer, Levin, & Di Giulio, 2018). Developing zebrafish appeared to tolerate propylene glycol, but this might have been due to lower exposure concentrations (Holden, et al., 2020; Maes, et al., 2012). Holden, et al. (2020) investigated the toxicity of several individual nicotine-containing, flavored e-liquid mixtures in developing zebrafish. Developmental toxicity effects were driven by the flavorings, not by nicotine, and cinnamaldehyde was the most bioactive flavor in the model.
Section 5.4.2. Other Non-Mammalian Models
Only a limited number of studies have used other animal models to understand the effects of chronic exposure to nicotine-containing e-liquids. Kennedy, Kandalam, Olivares-Navarrete, and Dickinson (2017) found that a variety of e-cigarette aerosol mixtures induced craniofacial malformations and reduction of blood supply to the face of African clawed frog (Xenopus laevis) embryos. The authors noted that nicotine was not the primary chemical driving the malformations, but rather nicotine was exacerbating the effects caused by other e-liquid components (Kennedy, et al., 2017). Studies have also used the nematode Caenorhabditis elegans as a model to understand the toxic effects of both e-liquids and aerosol extracts. Cai, et al. (2020) discovered that both exposures altered the head thrashing behavior and progeny number, while only the aerosol extract increased nematode body bend behavior. Panitz, Swamy, and Nehrke (2015) found that e-cigarette liquids and their aerosols are capable of causing increased oxidative stress in nematodes, with propylene glycol being the main driver (Panitz, et al., 2015). These studies demonstrate the cross-phyla craniofacial and neurological effects of e-cigarette ingredients.
Section 5.6. Effects of Cannabis-Containing e-Cigarette Aerosol
Use of animal models to study the effects of cannabinoid vaping has placed a greater emphasis on behavioral responses than on endpoints such as histology and respiratory function. A common approach is to expose mice or rats to cannabinoids and observe behavioral changes dependent on endogenous cannabinoid receptor CB-1. Rodents exposed to cannabinoids generally exhibit decreased locomotor activity, body temperature, and sensitivity to pain. This approach, known as the tetrad test, assesses hypomotility, catalepsy, hypothermia, and analgesia, characteristic responses to CB-1 agonists (Metna-Laurent, Mondesir, Grel, Vallee, & Piazza, 2017). Studies that compared effects from traditional rodent intraperitoneal injection to aerosol inhalation demonstrated different pharmacokinetics, underscoring the importance of inhalation testing (Javadi-Paydar, Creehan, Kerr, & Taffe, 2019; Wiley, Lefever, Glass, & Thomas, 2019). These studies found faster onset and shorter duration for some effects when cannabinoids were vaped compared to intraperitoneal injection, and that the potency of some compounds differed by route of administration (Lefever, et al., 2017; J. D. Nguyen, et al., 2016; Wiley, et al., 2019). Another area of active research is how cannabinoids and other constituents of inhaled aerosols interact. It is known that vaping exposures represent complex mixtures, but animal studies are only beginning to explore binary mixtures such as THC and CBD (Boggs, Nguyen, Morgenson, Taffe, & Ranganathan, 2018; Javadi-Paydar, et al., 2018; E. Russo & Guy, 2006; E. B. Russo, 2011). Because real vaping exposures are inherently mixtures, modeling the hazard is challenging and important.
Section 6. In vitro Mechanisms of ENDS Toxicity
Mechanisms of e-cigarette toxicity have been investigated by exposing monocultures of different cell types directly to e-cigarette liquids. Recent high-throughput technology improvements have enabled screens of large e-liquid libraries (Sassano, et al., 2018; G. Wang, Liu, & Song, 2019). Although these studies are informative, they do not recapitulate in vivo aerosol exposures which include particles and transformation products. Some studies overcome this limitation by exposing cells to aerosol extracts (C. Anderson, Majeste, Hanus, & Wang, 2016; Farsalinos, et al., 2013), and with recent technological advancements, it is now possible to conduct exposures to both cigarette smoke and e-cigarette aerosols at the air–liquid interface (Bathrinarayanan, Brown, Marshall, & Leslie, 2018; Leigh, Lawton, Hershberger, & Goniewicz, 2016; Neilson, et al., 2015). In vitro studies are beginning to use more physiologically relevant co-culture models for e-cigarette mechanistic studies where the aerosol is delivered directly to the co-culture (Bathrinarayanan, et al., 2018).
The toxic mechanism in vitro is a function of exposure type (aerosol, aerosol extract, or the e-liquid itself), and cell line (Leslie, et al., 2017; Scheffler, Dieken, Krischenowski, & Aufderheide, 2015), the product type, voltage, aerosol output (Farsalinos, et al., 2013; Leigh, et al., 2016; Leslie, et al., 2017; Rowell, et al., 2017), duration of exposure (Bathrinarayanan, et al., 2018), nicotine concentration (Cervellati, et al., 2014; Cudazzo, Smart, McHugh, & Vanscheeuwijck, 2019; Lerner, et al., 2015; Schweitzer, et al., 2015), and the flavoring chemicals (Al-Saleh, et al., 2020; Bahl, et al., 2012; Farsalinos, et al., 2013; Leigh, et al., 2016; Leslie, et al., 2017; Muthumalage, Lamb, Friedman, & Rahman, 2019; Romagna, et al., 2013). There is plenty of evidence that in vitro cellular effects are driven by components in the aerosols other than nicotine (Al-Saleh, et al., 2020; Farsalinos, et al., 2013; Leigh, et al., 2016; Leslie, et al., 2017; Rowell, et al., 2020; Ween, et al., 2020). The predominant mechanisms identified include cell viability and cytotoxicity, oxidative stress and inflammation, barrier and membrane dysfunction, and genotoxicity and DNA damage.
Section 6.1. ENDS Mixtures - Liquids and Aerosols
Section 6.1.1. Cell Viability and Cytotoxicity
E-liquids
Numerous studies have found that e-liquid exposures cause cytotoxicity, noting both nicotine- and flavor-driven effects (Al-Saleh, et al., 2020; Bahl, et al., 2012; Cudazzo, et al., 2019; Lerner, et al., 2015; Rowell, et al., 2017; Sassano, et al., 2018). Both e-liquids and cigarette smoke extracts induced cytotoxicity in human lung fibroblasts causing reduced cell count and increased morphological abnormalities (Lerner, et al., 2015). Exposure to some e-liquids without nicotine resulted in effects similar to propylene glycol exposure, whereas those with nicotine caused changes more closely resembling those of cigarette smoke extract (Lerner, et al., 2015). Upon exposing BALB/c 3T3 murine cells to e-liquids, Cudazzo, et al. (2019) observed a nicotine-driven cytotoxicity decrease at low concentrations and an increase at high concentrations, suggested to be the result of localized effects on acidic organelles. E-liquids with nicotine decreased Cell Counting Kit-8 (CCK8) activity in rat lung endothelial cells, an indicator of endothelial proliferation (Schweitzer, et al., 2015). On the other hand, several studies found that cytotoxicity was positively correlated with increasing number and height of extract HPLC chromatographic peaks (Bahl, et al., 2012; Sassano, et al., 2018). Notably in one study, cytotoxicity profiles were similar when e-liquids were tested directly or first aerosolized, suggesting that even after heating and chemical transformation, similar toxic effects persisted (Rowell, et al., 2017). Screening e-liquids for cytotoxicity in multiple cell lines, Bahl, et al. (2012) and Otero, et al. (2019) concluded that flavoring was the driving toxic factor, and high nicotine concentration was not correlated with high toxicity. E-liquid cytotoxicity was driven by both apoptosis and autophagy in human middle ear epithelial cells (HMEECs), with observations of higher BAX and caspase 3 expression, lower expression of Bcl-2 and other anti-apoptotic genes, and accumulation of LC3, an endogenous sign of autophagy (Go, Mun, Chae, Chang, & Song, 2020). A higher degree of cytotoxic effects was observed after the addition of S9 metabolic activating agent to e-liquid exposed TK6 human lymphoblast cells, indicating bioactivation of e-liquids to more cytotoxic metabolites (Al-Saleh, et al., 2020).
ENDS Aerosols and Extracts
ENDS aerosols and aerosol extracts in vitro exert cytotoxicity and reduced viability, altered metabolic activity, and altered cellular morphology in various cell lines, but effects are generally less severe than those of tobacco cigarette smoke (C. Anderson, et al., 2016; Cervellati, et al., 2014; Farsalinos, et al., 2013; Leigh, et al., 2016; Romagna, et al., 2013); however, there are exceptions to ENDS aerosol hazard. Several studies have reported ENDS aerosols that neither reduced cell viability nor cell junction integrity in tracheobronchial, human alveolar basal epithelial, and human bronchial epithelial (HBEC) cells (Antherieu, et al., 2017; Husari, et al., 2016; Neilson, et al., 2015). In the larger picture of evidence, these results appear exceptional and were ascribed to interstudy differences in cell line and exposure paradigm, such as the number of puffs and duration of exposure used.
Cytotoxicity in many cell lines exposed to ENDS aerosol occurs via apoptosis (Romagna, et al., 2013; Rouabhia, et al., 2017; Sancilio, Gallorini, Cataldi, & di Giacomo, 2016; Ween, et al., 2020; Yu, et al., 2016), and some studies have identified programmed necrosis in alveolar macrophage and human umbilical vein endothelial cells (HUVECs) (C. Anderson, et al., 2016; Scott, et al., 2018). Treatment with anti-oxidants prevented necrosis and partially rescued apoptosis, demonstrating possible cell-line specific response profiles and mechanisms of ENDS-induced cytotoxicity (C. Anderson, et al., 2016). Adult mice exposed to ENDS aerosols had increased levels of oxidative stress as well as inflammatory responses in the cerebral vasculature (Kaisar, et al., 2017; Kuntic, et al., 2020), but due to the differences in experimental design, conclusions could not be drawn regarding the role of nicotine. Another study in a co-culture of HBECs and pulmonary fibroblasts identified a caspase-independent mechanism for cytotoxicity induced by e-cigarettes while tobacco cigarette smoke-induced cell death is caspase-dependent (Bathrinarayanan, et al., 2018). While both ENDS aerosol and tobacco cigarette smoke can cause cell toxicity and have probable mechanistic overlap, their mechanisms may also differ substantially.
Section 6.1.2. Oxidative Stress and Inflammation
E-liquids
From in vitro e-liquid exposures, it is still unclear whether oxidative stress is the primary mechanism of toxicity (Aug, et al., 2015; Bharadwaj, Mitchell, Qureshi, & Niazi, 2017). Bharadwaj, et al. (2017) used whole-cell biosensors in the form of bioluminescent recombinant E. coli designed to respond to oxidative stress, and noted repression of sodA and a shift in the intracellular metabolome of HBECs after e-liquid exposure; however, the addition of antioxidants ameliorated the effect, suggesting that e-liquid toxicity is at least in part a result of oxidant-driven mechanisms. The observed metabolome shift partially overlapped the shift observed after exposure to tobacco cigarette smoke condensate, indicating that ENDS and cigarette toxicity mechanisms may share some modes of action (Aug, et al., 2015).
E-liquid exposure alters pro-inflammatory cytokines and gene expression related to immune system defense. Nicotine concentration and e-liquid flavor can drive e-liquid induced inflammatory responses in human lung fibroblasts (Lerner, et al., 2015). For instance, IL-8 and IL-6 levels showed dose-dependent increases after exposure of human tracheobronchial epithelial cells to non-nicotine e-liquid, and the addition of nicotine only slightly enhanced IL-6 response (Q. Wu, Jiang, Minor, & Chu, 2014). Exposure of HMEECs to propylene glycol/glycerol and flavored e-liquids similarly increased expression of inflammatory cytokines (COX-2 and TNF-α) and mucin production (Go, et al., 2020). Further, e-liquid exposures with and without nicotine promoted human rhinovirus (HRV) infection, enhanced HRV-induced IL-6 production, and inhibited expression of SPLUNC1, an antimicrobial protein in the airways that contributes to lung immune defense. Inflammatory and immune-response effects are important ENDS related stressors, especially when compounded with additional stressors such as infection (Q. Wu, et al., 2014).
ENDS Aerosols and Aerosol Extracts
In vitro exposures to ENDS aerosol produces ROS in multiple cell lines (Bathrinarayanan, et al., 2018; Lerner, et al., 2015; Schweitzer, et al., 2015), though a clear picture of the amount of ROS and its ultimate effect on cells is lacking. Oxidative stress as a function of ENDS aerosol toxicity is further evidenced by significantly altered glutathione levels in the BALF of mice exposed to ENDS aerosol (Lerner, et al., 2015), and induction of various Nrf2 associated oxidative stress genes in normal human bronchial epithelial cells (NHBEs) (Solleti, et al., 2017). But there are exceptions. One study in human coronary artery endothelial cells showed that tobacco cigarette smoke activated Nrf2 and led to its nuclear translocation, while ENDS aerosol did not (Teasdale, Newby, Timpson, Munafo, & White, 2016). ENDS aerosols containing nicotine were not cytotoxic to HBECs and did not increase glutathione levels, but induced IL-6, indicating an inflammatory response (Antherieu, et al., 2017). Several studies detected induction of both IL-6 and IL-8 (Bathrinarayanan, et al., 2018; Lerner, et al., 2015; Muthumalage, et al., 2019; Q. Wu, et al., 2014), and another study showed decreased IL-6 in human keratinocyte and lung cancer cell lines following ENDS aerosol exposure. It is difficult to gauge the significance of reductions in inflammatory cytokines following exposure to some ENDS aerosols without further study in animals, or at least careful comparison of ingredients among those that induce cytokines versus those that do not. On the surface, inflammatory cytokine reduction would seem a good thing, but it could also indicate an inadequate immune response to foreign agents (Cervellati, et al., 2014). In addition to IL-8 and IL-6, several other pro-inflammatory cytokines were altered in both cell lines as well as in mouse BALF upon exposure to e-cigarette nicotine aerosols and aerosol extracts, demonstrating a critical role of inflammation in overall ENDS toxicity (Cervellati, et al., 2014; Leigh, et al., 2016; Muthumalage, et al., 2019; Ween, et al., 2020; Q. Wu, et al., 2014).
There is evidence that ENDS use adversely affects host defenses, though the mechanistic basis is unclear. Exposure of isolated human neutrophils to e-cigarette aerosol extract altered membrane fluidity, inhibited chemotaxis, NET production, and ROS production (a key driver of antibacterial activity), and reduced neutrophilic phagocytosis by 40% (Corriden, et al., 2020). The results were confirmed in vivo: in the peritoneal lavage fluid of mice exposed to ENDS aerosol; the neutrophils demonstrated a decreased ability to travel to infection sites. Similarly, other studies demonstrated suppression of macrophage bacterial recognition receptors (SR-A1 and TLR-2) and apoptotic cell recognition receptors (CD33 and CD36) (Ween, et al., 2020; Ween, Whittall, Hamon, Reynolds, & Hodge, 2017) and diminished phagocytosis of macrophages following ENDS aerosol extract. These responses effectively increased bacterial virulence (Hwang, et al., 2016; Scott, et al., 2018; Ween, et al., 2020). An increase in cell permeability was observed in NHBEs following infection with Pseudomonas aeruginosa, which suggested that both immune system and barrier dysfunction were responsible for lowering host defenses (Crotty Alexander, et al., 2018). ENDS aerosol and aerosol extract exposures lead to diminished barrier function in other epithelial cell lines against pollutants which could induce an inflammatory response (Crotty Alexander, et al., 2018; Gerloff, et al., 2017; Muthumalage, et al., 2019).
Section 6.1.3. Barrier and Membrane Dysfunction
Studies have found that e-liquid exposure affects cellular membrane integrity, specifically through calcium signaling pathways and transport protein-related differential gene expression changes. Exposure to e-liquid with and without nicotine caused general barrier dysfunction in rat lung endothelial cells, a more potent effect than those seen from aerosol condensate exposure (Schweitzer, et al., 2015). One study demonstrated that only the e-cigarette aerosol, but not the e-liquid, altered ion transport by alternative chloride channels in CALU3 cells as well as in HBECs (Lin, et al., 2019). The effect was attributed to the production of acrolein, which plays a role in acquired dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR). Both e-liquid and aerosolized exposures induced cytosolic calcium (Ca2+) signaling response in CALU3 cells, which suggests that this response was primarily due to initial e-liquid constituents, not metabolites or thermal degradants (Rowell, et al., 2020). Ca2+ replacement studies have demonstrated that the underlying mechanism is an endoplasmic reticulum-dependent store-operated Ca2+ entry (SOCE) response.
Section 6.1.4. Genotoxicity and DNA Damage
There is some evidence indicating that e-liquids are genotoxic. Human lymphoblastoid TK6 cells exposed to select e-liquids elicited an increase in the median tail moment (TM), indicative of DNA fragmentation, with even higher measured DNA damage after the addition of S9 metabolic agent (Al-Saleh, et al., 2020). Using recA recombinant E. coli that respond to single-stranded DNA breaks, e-liquid exposure produced measurable DNA damage (Bharadwaj, et al., 2017). Bioluminescence of e-liquid-treated E. coli showed a dose-dependent decrease, potentially from inhibited DNA repair. Interestingly, while e-liquid exposure repressed recA, exposure to solubilized e-liquid aerosol had the opposite effect and induced recA in a dose-dependent manner, suggestive of more DNA damage and corresponding induction of repair mechanisms in response to aerosol (Bharadwaj, et al., 2017).
Double-stranded breaks in DNA are produced in a variety of cell lines exposed to ENDS mixtures (C. Anderson, et al., 2016; Muthumalage, et al., 2019; Ween, et al., 2020; Yu, et al., 2016). DNA double-strand breaks in leucocytes and micronuclei formation in reticulocytes have been observed in vivo in mice exposed to ENDS aerosol, providing further evidence for the genotoxic potential of ENDS products (Canistro, et al., 2017). Additional mechanisms may lead to DNA damage, such as increased cellular ROS and decreased total antioxidant capacity (Ganapathy, et al., 2017). DNA damage in HUVECs has been associated with increased ROS (C. Anderson, et al., 2016). This result is supported by Moses, et al. (2017), who identified several transcriptomic changes associated with DNA damage as well as oxidative stress and apoptosis in HBECs exposed to ENDS aerosol. Notably, one study demonstrated oxidative DNA damage by measuring significantly increased levels of 8-oxo-dG, a significant ROS-induced DNA damage lesion that is highly mutagenic. Interestingly, another study assessed genotoxicity of total particulate matter from both e-cigarette aerosol and e-liquids and found both to be non-genotoxic in a mouse lymphoma model (Thorne, et al., 2019). Variation in study results may be a product of the different cell-types used or may simply be attributed to variation between individual e-liquids with different constituents, such as flavorings. Overall, DNA damage seems to be a player in ENDS toxicity by not only leading to increased cytotoxicity but also by increasing the release of inflammatory mediators (Muthumalage, et al., 2019).
Section 6.2. Component Toxicity Mechanisms
Section 6.2.1. Carrier Solvents
Liquids
E-liquid carrier solvents have been shown to be non-cytotoxic across the different humectant ratios (Leslie, et al., 2017) and in multiple cell lines (Bahl, et al., 2012). Carrier solvents generally caused little to no cytotoxicity in these studies; however, mixed results have been found regarding effects on specific cellular mechanisms. While one study found that 1% pure humectants did not increase IL-8 release in fetal human lung fibroblasts (Lerner, et al., 2015), another found that 50:50 propylene glycol:glycerol increased expression of the inflammatory cytokines COX-2 and TNF-α, and also increased expression of MUC5B mRNA, a mucin-related gene in HMEECs (Go, et al., 2020). Rowell, et al. (2020) concluded that while e-liquid mixtures induced calcium-signaling effects in CALU3 cells, 3% propylene glycol/glycerol (55:45) control exposure did not. Since ratios of humectants vary between ENDS products, a better understanding of how toxic effects can change based on the humectant ratio is still needed.
Aerosols
Studies have demonstrated mixed results regarding the mechanisms of toxicity of carrier solvents in various cell lines. Transcriptomic analysis revealed aerosolized solvent exposures affected predominantly inflammatory processes in buccal cultures, but cell fate, cell stress, and inflammatory processes were similarly affected in small airway cultures (Iskandar, et al., 2019). Viability of cultured human keratinocytes and lung epithelial cells did not change following exposure to aerosolized humectants, but exposure to ENDS aerosol initiated the release of cytokines and proinflammatory mediators (Cervellati, et al., 2014). HBECs from two different donors exposed to propylene glycol or glycerol exhibited reduced viability, while only the cells exposed to glycerol aerosols had increased oxidative stress (Scheffler, Dieken, Krischenowski, Forster, et al., 2015). This suggested that propylene glycol aerosols elicit different toxic effects compared to glycerol aerosols. Cultured HBECs had altered cellular metabolic activity following exposures to propylene glycol/glycerol and glycerol-only aerosols, but not propylene glycol-only aerosols (Leigh, et al., 2016). However, in this study, propylene glycol-only exposures led to an increase in cytokine release compared with the other humectant treatment groups, further demonstrating differences in propylene glycol and glycerol toxicities.
Collecting lung cells from individuals that have inhaled ENDS aerosols has the potential to provide the most accurate and representative insight into in vivo effects of ENDS use. A recent study of the effects of ENDS use on inflammatory processes in the human lung analyzed serial bronchoscopies before and after four weeks of vaping a mixture of 50:50 propylene glycol:glycerol at least twice per day. Unexpectedly, the results showed no change in inflammatory cell counts, cytokines, or gene expression (Song, et al., 2020). More research is necessary to determine if and how inflammatory processes are affected by aerosolized carrier solvents in ENDS mixtures.
Section 6.2.2. Flavoring Chemicals
Flavoring compounds have been identified as sources of toxicity from ENDS devices; however, compared to the hundreds of flavoring constituents found in e-liquids, there are relatively few studies addressing the specific flavoring chemicals driving ENDS toxicity. Fewer still have addressed the hazard potential when these compounds are in mixtures (Marescotti, et al., 2020). What we know to date is that cinnamaldehyde and diacetyl have emerged as some of the most hazardous flavoring chemicals.
In one study, upon e-liquid exposure, AQP4, a water channel-related gene, was significantly increased by 20-fold for propylene glycol/glycerol, 40-fold for tobacco-flavored e-liquid, and 60-fold for menthol-flavored e-liquid in HMEECs (Go, et al., 2020). The epithelial sodium channel family of genes had significantly decreased expression after only menthol-flavored e-liquid exposure, suggesting that the e-liquid effect on membrane function may be exacerbated by flavoring constituents.
Cell Viability and Cytotoxicity
Certain flavors inhibit cell proliferation while others induce cytotoxicity, indicating that flavoring constituents may perturb different cellular processes (Rowell, et al., 2017). Cinnamon flavored e-liquids were consistently ranked among the most cytotoxic to multiple cell types (Bahl, et al., 2012; Otero, et al., 2019). Aerosolized cinnamaldehyde resulted in increased cytotoxicity in human pulmonary fibroblasts, human lung epithelial cells, and human embryonic stem cells. While the lung cells were unable to recover, the embryonic cells did recover after exposure (Behar, et al., 2016). In a separate study, HBECs exposed to 2,5-dimethylpyrazine, damascenone, linalool, α-ionone, and ethyl maltol, but not furancol and vanillin, resulted in decreased cell index and increased cytotoxicity (Sherwood & Boitano, 2016). 2,5-dimethylpyrazine also altered cellular physiology and ion channel regulation, which increased ion conductance.
Flavoring compounds also disrupt other cellular functions. Cinnamaldehyde exposures decreased signaling amplitude, beat rate, and cell index in human stem cell-derived cardiac myocytes, though when cinnamaldehyde was heated, the effects of exposure decreased, suggesting chemical transformation (Nystoriak, et al., 2019). In another study, HBECs exposed to aerosolized cinnamaldehyde temporarily decreased ATP production, mitochondrial function, and ciliary activity; however, all endpoints returned to control levels after 120 minutes of recovery with no long-lasting effects on cell viability (Clapp, et al., 2019). In comparison, Park, et al. (Park, et al., 2019) found that diacetyl and 2,3-pentanedione significantly downregulated genes involved in cilia processes and significantly decreased the number of ciliated cells, suggesting that both these compounds negatively affected cilia biogenesis.
Oxidative Stress and Inflammation
Multiple studies have reported that flavoring compounds increase pro-inflammatory cytokines. Of the commercially available e-liquids tested by Lerner, et al. (2015), Vape Dudes cinnamon roll flavor significantly increased IL-8 release. Exposure of monocyte-macrophage cells to the flavoring chemicals acetoin, diacetyl, pentanedione, maltol, ortho-vanillin, coumarin, and cinnamaldehyde revealed that all compounds induced a dose-dependent increase in ROS production. All the tested compounds resulted in an increase in the pro-inflammatory cytokines IL-6 and IL-8, except for acetoin, which resulted in a statistically significant dose-dependent decrease in IL-8. Cinnamaldehyde only resulted in an increase in IL-8 at a high concentration of 100 μM (Muthumalage, et al., 2017). A separate study that tested the same suite of chemicals found that maltol, pentanedione, and o-vanillin increased IL-8 levels in human lung fibroblasts (HFL), and acetoin, diacetyl, o-vanillin, and maltol increased IL-8 levels in HBECs. None of the tested chemicals increased IL-8 in human transformed lung epithelial cells (H292) (Gerloff, et al., 2017). Similarly, Hickman, Herrera, and Jaspers (2019) found acetoin, pentanedione, maltol, and ortho-vanillin induced IL-8 in HFL-1 cells but not in H292 cells. Clapp, et al. (2017) found that cinnamaldehyde was one of the main flavoring compounds found in e-liquid whole mixtures that resulted in decreased immune function and increased IL-8 expression. Further investigation into the effects of cinnamaldehyde alone showed that exposure in human alveolar macrophages, neutrophils, and natural killer cells reduced phagocytosis and cell viability, suggesting that cinnamaldehyde negatively affects immune function.
Barrier Dysfunction
HBECs exposed to diacetyl, coumarin, acetoin, maltol, pentanedione, ortho-vanillin, or cinnamaldehyde resulted in varying degrees of decreased epithelial barrier function. For example, in one study, 20 minutes after exposure, diacetyl and coumarin caused a decrease in trans-epithelial electrical resistance (TEER) and two hours after exposure, acetoin, maltol, and cinnamaldehyde was associated with decreased epithelial barrier function (Gerloff, et al., 2017). McGraw, et al. (2020) also found that diacetyl aerosol decreases barrier function in HBECs as measured by decreased TEER, even after a 23-hour recovery period for the highest exposure concentration (50 mM). Additionally, up to five days after the initial one-hour exposure to diacetyl aerosol, the ubiquitin-proteasome system was activated, causing a decrease in keratin 5 (Krt5) and ΔNp63 expression. Inhibition of proteolysis with MG132 prolonged Krt5 protein degradation and prevented recovery, suggesting that the ubiquitin-proteasome pathway was responsible for the decrease in Krt 5 protein degradation and basal cell injury (McGraw, et al., 2020).
The studies to date suggest that flavoring compounds affect barrier function, which is important for maintaining tight junctions and intercellular contacts (J. M. Anderson & Van Itallie, 2009). More studies are necessary to further understand the extent and the mechanisms by which flavoring compounds alter barrier integrity.
Genotoxicity and DNA Damage
Investigations into ENDS flavoring chemicals causing DNA damage, or genotoxicity, are lacking. One study screened flavored e-liquids representing 33 different brands and found that loaded glazed donut flavor e-liquid induced a higher median tail moment than the H2O2 positive control, and four other e-liquid flavors induced a higher median tail moment than pure nicotine. A micronucleus (MN) assay revealed that “of the 63 flavored e-liquids, 26 showed high %MN frequencies (≥3-fold that of the untreated cells)” (Al-Saleh, et al., 2020). Ultimately, more research is needed to understand how flavoring compounds interact with DNA.
Section 6.3. Potential Mechanisms of EVALI
Use of cannabinoid vaping mixtures is closely associated with EVALI, as 80–95% of clinical cases detected THC or its metabolites in the BALF of patients (Blount, et al., 2020; Boudi, Patel, Boudi, & Chan, 2019). A majority of EVALI cases present with characteristic exogenous lipoid pneumonia due to the observation of foamy and vacuolated macrophages in the BALF (Gay, et al., 2020; Ind, 2020). Exogenous lipoid pneumonia is caused by the accumulation of exogenous lipid molecules in the alveoli, and the subsequent adverse reaction in lung tissue (Marchiori, Zanetti, Mano, & Hochhegger, 2011). Vitamin E acetate (VEA), coconut oil, and limonene are used as fatty diluents in cannabinoid-vaping mixtures. VEA is ubiquitous in the BALF of EVALI patients (Blount, et al., 2020; Ind, 2020). Despite the co-occurrence of VEA and THC in BALF from EVALI patients, we still do not understand exactly how habitual cannabinoid vaping results in EVALI.
The presence of foamy, lipid-laden macrophages in the first reports of EVALI led to the characterization of the event as an exogenous lipoid pneumonia (Layden, et al., 2020; Maddock, et al., 2019). Mechanistically, it is possible that VEA accumulation in the lung disrupts alveolar macrophage function and impairs proper homeostasis of surfactant lining the alveoli, maintained by macrophages. This mechanistic phenomenon was proposed for propylene glycol/glycerol, where these fatty solvents caused severely attenuated macrophage function and increased susceptibility to infection (Madison, et al., 2019). Another possible mechanism is that the long aliphatic tail of the vitamin E molecule disrupts surfactant composition through the transition from a gel-like to crystalline state as increasing concentrations of vitamin E accumulate in the lung (Blount, et al., 2020). A third proposed mechanism is that pyrolysis of VEA can lead to the generation of highly toxic ketene gas, in addition to carcinogenic benzene and alkene compounds (D. Wu & O'Shea, 2020). The authors demonstrated that significant amounts of ketene gas could be formed upon thermal decomposition of VEA at temperatures achieved by vaping devices. At this stage, no single mechanism can be conclusively distilled from the recent studies, and VEA, despite strong correlation and a reasonable mechanistic basis, cannot be unambiguously declared the sole agent of EVALI.
Summary
Vaping technology continues to expand and its use will grow without strong regulation and public education that mirrors our history of success in curbing tobacco use. Given that our understanding of the health effects of vaping lags substantially behind consumer usage, it would seem premature at best to take solace in the somewhat better safety profile that e-cigarettes have over tobacco cigarettes. Unlike many other toxicological challenges, vaping is an intentional exposure driven by complex aspects of human adolescent and adult behavior. As the literature overwhelmingly indicates, e-cigarette use can be profoundly hazardous. Consumers deserve, and regulatory bodies require, a more complete understanding of constituent-dependent toxicity to their decisions.
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams TB, Cohen SM, Doull J, Feron VJ, Goodman JI, Marnett LJ, Munro IC, Portoghese PS, Smith RL, Waddell WJ, Wagner BM, Expert Panel of the, F., & Extract Manufacturers, A. (2005). The FEMA GRAS assessment of benzyl derivatives used as flavor ingredients. Food Chem Toxicol, 43, 1207–1240. [DOI] [PubMed] [Google Scholar]
- Agustin M, Yamamoto M, Cabrera F, & Eusebio R (2018). Diffuse Alveolar Hemorrhage Induced by Vaping. Case Rep Pulmonol, 2018, 9724530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aherrera A, Olmedo P, Grau-Perez M, Tanda S, Goessler W, Jarmul S, Chen R, Cohen JE, Rule AM, & Navas-Acien A (2017). The association of e-cigarette use with exposure to nickel and chromium: A preliminary study of non-invasive biomarkers. Environ Res, 159, 313–320. [DOI] [PubMed] [Google Scholar]
- Al-Saleh I, Elkhatib R, Al-Rajoudi T, Al-Qudaihi G, Manogarannogaran P, Eltabache C, Alotaibi A, Mummer AB, & Almugbel S (2020). Cytotoxic and genotoxic effects of e-liquids and their potential associations with nicotine, menthol and phthalate esters. Chemosphere, 249, 126153. [DOI] [PubMed] [Google Scholar]
- Al Rifai M, Merchant AT, Nambi V, Jia X, Gulati M, Valero-Elizondo J, Nasir K, Ballantyne CM, & Virani SS (2020). Temporal Trends in E-Cigarette Use Among U.S. Adults: Behavioral Risk Factor Surveillance System, 2016 to 2018. Am J Med. [DOI] [PubMed] [Google Scholar]
- Ali M, Gray TR, Martinez DJ, Curry LE, & Horn KA (2016). Risk Profiles of Youth Single, Dual, and Poly Tobacco Users. Nicotine Tob Res, 18, 1614–1621. [DOI] [PubMed] [Google Scholar]
- Anderson C, Majeste A, Hanus J, & Wang S (2016). E-Cigarette Aerosol Exposure Induces Reactive Oxygen Species, DNA Damage, and Cell Death in Vascular Endothelial Cells. Toxicol Sci, 154, 332–340. [DOI] [PubMed] [Google Scholar]
- Anderson JM, & Van Itallie CM (2009). Physiology and function of the tight junction. Cold Spring Harb Perspect Biol, 1, a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antherieu S, Garat A, Beauval N, Soyez M, Allorge D, Garcon G, & Lo-Guidice JM (2017). Comparison of cellular and transcriptomic effects between electronic cigarette vapor and cigarette smoke in human bronchial epithelial cells. Toxicol In Vitro, 45, 417–425. [DOI] [PubMed] [Google Scholar]
- Apollonio DE, Spetz J, Schmidt L, Jacobs L, Kaur M, & Ramo D (2019). Prevalence and Correlates of Simultaneous and Separate 30-Day Use of Tobacco and Cannabis: Results from the California Adult Tobacco Survey. Subst Use Misuse, 54, 1627–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aszyk J, Kubica P, Wozniak MK, Namiesnik J, Wasik A, & Kot-Wasik A (2018). Evaluation of flavour profiles in e-cigarette refill solutions using gas chromatography-tandem mass spectrometry. J Chromatogr A, 1547, 86–98. [DOI] [PubMed] [Google Scholar]
- ATSDR, A. f. T. S. a. D. R. (2020). Toxic Substances Portal. In (Vol. 2020): USDHHS. [Google Scholar]
- Attis M, King J, Hardison D, & Bridges B (2018). The journey to ECMO could start with a single vape: a case of severe hypersensitivity pneumonitis in a pediatric patient. ASAIO J, 64. [Google Scholar]
- Aug A, Altraja S, Kilk K, Porosk R, Soomets U, & Altraja A (2015). E-Cigarette Affects the Metabolome of Primary Normal Human Bronchial Epithelial Cells. PLoS One, 10, e0142053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers JW, Leas EC, Allem JP, Benton A, Dredze M, Althouse BM, Cruz TB, & Unger JB (2017). Why do people use electronic nicotine delivery systems (electronic cigarettes)? A content analysis of Twitter, 2012-2015. PLoS One, 12, e0170702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagale K, Paudel S, Cagle H, Sigel E, & Kulkarni R (2020). Electronic Cigarette (E-Cigarette) Vapor Exposure Alters the Streptococcus pneumoniae Transcriptome in a Nicotine-Dependent Manner without Affecting Pneumococcal Virulence. Appl Environ Microbiol, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl V, Lin S, Xu N, Davis B, Wang YH, & Talbot P (2012). Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol, 34, 529–537. [DOI] [PubMed] [Google Scholar]
- Baker KA, & Campbell NJ (2020). Combatting Teen Vaping in School Settings. J Addict Nurs, 31, 73–76. [DOI] [PubMed] [Google Scholar]
- Barrington-Trimis JL, Samet JM, & McConnell R (2014). Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA, 312, 2493–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett RA, Osterhoudt K, & Brabazon T (2014). Nicotine poisoning in an infant. N Engl J Med, 370, 2249–2250. [DOI] [PubMed] [Google Scholar]
- Bathrinarayanan PV, Brown JEP, Marshall LJ, & Leslie LJ (2018). An investigation into E-cigarette cytotoxicity in-vitro using a novel 3D differentiated co-culture model of human airways. Toxicology in Vitro, 52, 255–264. [DOI] [PubMed] [Google Scholar]
- Beauval N, Howsam M, Antherieu S, Allorge D, Soyez M, Garcon G, Goossens JF, Lo-Guidice JM, & Garat A (2016). Trace elements in e-liquids - Development and validation of an ICP-MS method for the analysis of electronic cigarette refills. Regul Toxicol Pharmacol, 79, 144–148. [DOI] [PubMed] [Google Scholar]
- Behar RZ, Luo W, Lin SC, Wang Y, Valle J, Pankow JF, & Talbot P (2016). Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tob Control, 25, ii94–ii102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar RZ, Luo W, McWhirter KJ, Pankow JF, & Talbot P (2018). Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci Rep, 8, 8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalerao A, Sivandzade F, Archie SR, & Cucullo L (2019). Public Health Policies on E-Cigarettes. Curr Cardiol Rep, 21, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj S, Mitchell RJ, Qureshi A, & Niazi JH (2017). Toxicity evaluation of e-juice and its soluble aerosols generated by electronic cigarettes using recombinant bioluminescent bacteria responsive to specific cellular damages. Biosens Bioelectron, 90, 53–60. [DOI] [PubMed] [Google Scholar]
- Bhatta DN, & Glantz SA (2020). Association of E-Cigarette Use With Respiratory Disease Among Adults: A Longitudinal Analysis. Am J Prev Med, 58, 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, Song E, Sutfin EL, Spangler J, & Wolfson M (2015). Electronic Cigarette Trial and Use among Young Adults: Reasons for Trial and Cessation of Vaping. Int J Environ Res Public Health, 12, 16019–16026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzer ZT, Goel R, Reilly SM, Elias RJ, Silakov A, Foulds J, Muscat J, & Richie JP Jr. (2018). Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radic Biol Med, 120, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagev DP, Harris D, Dunn AC, Guidry DW, Grissom CK, & Lanspa MJ (2019). Clinical presentation, treatment, and short-term outcomes of lung injury associated with e-cigarettes or vaping: a prospective observational cohort study. Lancet, 394, 2073–2083. [DOI] [PubMed] [Google Scholar]
- Blohm E, Sell P, & Neavyn M (2019). Cannabinoid toxicity in pediatrics. Curr Opin Pediatr, 31, 256–261. [DOI] [PubMed] [Google Scholar]
- Blount BC, Karwowski MP, Morel-Espinosa M, Rees J, Sosnoff C, Cowan E, Gardner M, Wang L, Valentin-Blasini L, Silva L, De Jesus VR, Kuklenyik Z, Watson C, Seyler T, Xia B, Chambers D , Briss P, King BA, Delaney L, Jones CM, Baldwin GT, Barr JR, Thomas J, & Pirkle JL (2019). Evaluation of Bronchoalveolar Lavage Fluid from Patients in an Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injury - 10 States, August-October 2019. MMWR Morb Mortal Wkly Rep, 68, 1040–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount BC, Karwowski MP, Shields PG, Morel-Espinosa M, Valentin-Blasini L, Gardner M, Braselton M, Brosius CR, Caron KT, Chambers D, Corstvet J, Cowan E, De Jesus VR, Espinosa P, Fernandez C, Holder C, Kuklenyik Z, Kusovschi JD, Newman C, Reis GB, Rees J, Reese C, Silva L, Seyler T, Song MA, Sosnoff C, Spitzer CR, Tevis D, Wang L, Watson C, Wewers MD, Xia B, Heitkemper DT, Ghinai I, Layden J, Briss P, King BA, Delaney LJ, Jones CM, Baldwin GT, Patel A, Meaney-Delman D, Rose D, Krishnasamy V, Barr JR, Thomas J, Pirkle JL, & Lung Injury Response Laboratory Working, G. (2020). Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N Engl J Med, 382, 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs DL, Nguyen JD, Morgenson D, Taffe MA, & Ranganathan M (2018). Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and Delta(9)-Tetrahydrocannabinol. Neuropsychopharmacology, 43, 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudi FB, Patel S, Boudi A, & Chan C (2019). Vitamin E Acetate as a Plausible Cause of Acute Vaping-related Illness. Cureus, 11, e6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle RG, Richter S, & Helgertz S (2019). Who is using and why: Prevalence and perceptions of using and not using electronic cigarettes in a statewide survey of adults. Addict Behav Rep, 10, 100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzella MJ, Magyar M, DeBiasi RL, & Ferrer K (2020). Epiglottitis Associated With Intermittent E-cigarette Use: The Vagaries of Vaping Toxicity. Pediatrics, 145. [DOI] [PubMed] [Google Scholar]
- Cai H, Xu Y, Tang S, Yang X, Zou Y, Wang X, Mo L, Wu B, Liang Z, Li Y, Wei X, Ao Q, Meng L, Zhang N, Chen M, Lan C, Li X, & Lu C (2020). Systemic toxicity evaluation of novel tobacco products in Caenorhabditis elegans. Toxicol In Vitro, 62, 104671. [DOI] [PubMed] [Google Scholar]
- Caito S, & Aschner M (2015). Neurotoxicity of metals. Handb Clin Neurol, 131, 169–189. [DOI] [PubMed] [Google Scholar]
- Callahan-Lyon P (2014). Electronic cigarettes: human health effects. Tob Control, 23 Suppl 2, ii36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenga DR, Delmerico J, Kong G, Cavallo D, Hyland A, Cummings KM, & Krishnan-Sarin S (2014). Trends in use of electronic nicotine delivery systems by adolescents. Addict Behav, 39, 338–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna D, Cibella F, Caponnetto P, Amaradio MD, Caruso M, Morjaria JB, Malerba M, & Polosa R (2016). Changes in breathomics from a 1-year randomized smoking cessation trial of electronic cigarettes. Eur J Clin Invest, 46, 698–706. [DOI] [PubMed] [Google Scholar]
- Canistro D, Vivarelli F, Cirillo S, Babot Marquillas C, Buschini A, Lazzaretti M, Marchi L, Cardenia V, Rodriguez-Estrada MT, Lodovici M, Cipriani C, Lorenzini A, Croco E, Marchionni S, Franchi P, Lucarini M, Longo V, Della Croce CM, Vornoli A, Colacci A, Vaccari M, Sapone A, & Paolini M (2017). E-cigarettes induce toxicological effects that can raise the cancer risk. Sci Rep, 7, 2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenia V, Vivarelli F, Cirillo S, Paolini M, Canistro D, & Rodriguez-Estrada MT (2018). The effect of electronic-cigarettes aerosol on rat brain lipid profile. Biochimie, 153, 99–108. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Kim M, Hemyari A, Thakrar P, Kump TE, Wade T, De Vela G, Hall J, Diaz CD, & D'Andrea LA (2020). Impaired lung function following e-cigarette or vaping product use associated lung injury in the first cohort of hospitalized adolescents. Pediatr Pulmonol, 55, 1712–1718. [DOI] [PubMed] [Google Scholar]
- CDC. (2011). National Youth Tobacco Survey (NYTS) 2011 Questionnaire. In.
- CDC. (2015). E-Cigarette Use Triples among Middle and High School Students in Just One Year. In.
- CDC. (2016). E-cigarette use among youth and young adults. In N. C. f. C. D. P. a. H. P. U. O. o. S. a. Health. (Ed.), A report of the Surgeon General. [Google Scholar]
- CDC. (2019). National Youth Tobacco Survey (NYTS). In.
- CDC. (2020a). Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. In Smoking and Tobacco Use. [Google Scholar]
- CDC. (2020b). Smoking & Tobacco Use-Fast Facts. In.
- Cervellati F, Muresan XM, Sticozzi C, Gambari R, Montagner G, Forman HJ, Torricelli C, Maioli E, & Valacchi G (2014). Comparative effects between electronic and cigarette smoke in human keratinocytes and epithelial lung cells. Toxicol In Vitro, 28, 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan GCK, Hall W, Freeman TP, Ferris J, Kelly AB, & Winstock A (2017). User characteristics and effect profile of Butane Hash Oil: An extremely high-potency cannabis concentrate. Drug Alcohol Depend, 178, 32–38. [DOI] [PubMed] [Google Scholar]
- Chapman DG, Casey DT, Ather JL, Aliyeva M, Daphtary N, Lahue KG, van der Velden JL, Janssen-Heininger YMW, & Irvin CG (2019). The Effect of Flavored E-cigarettes on Murine Allergic Airways Disease. Sci Rep, 9, 13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Li G, Chan YL, Chapman DG, Sukjamnong S, Nguyen T, Annissa T, McGrath KC, Sharma P, & Oliver BG (2018). Maternal E-Cigarette Exposure in Mice Alters DNA Methylation and Lung Cytokine Expression in Offspring. Am J Respir Cell Mol Biol, 58, 366–377. [DOI] [PubMed] [Google Scholar]
- Chen YM, Huang CC, Sung HC, Lee MC, & Hsiao CY (2019). Electronic cigarette exposure reduces exercise performance and changes the biochemical profile of female mice. Biosci Biotechnol Biochem, 83, 2318–2326. [DOI] [PubMed] [Google Scholar]
- Chen Z, & Zeng DD (2017). Mining online e-liquid reviews for opinion polarities about e-liquid features. BMC Public Health, 17, 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian SV, Kumar A, & Estrada YMRM (2020). E-Cigarette or Vaping Product-Associated Lung Injury: A Review. Am J Med, 133, 657–663. [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Ramond A, O'Keeffe LM, Shahzad S, Kunutsor SK, Muka T, Gregson J, Willeit P, Warnakula S, Khan H, Chowdhury S, Gobin R, Franco OH, & Di Angelantonio E (2018). Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ, 362, k3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun LF, Moazed F, Calfee CS, Matthay MA, & Gotts JE (2017). Pulmonary toxicity of e-cigarettes. Am J Physiol Lung Cell Mol Physiol, 313, L193–L206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JS, Chace-Donahue F, Blum JL, Ratner JR, Zelikoff JT, & Schwartzer JJ (2020). Neuroinflammatory and Behavioral Outcomes Measured in Adult Offspring of Mice Exposed Prenatally to E-Cigarette Aerosols. Environ Health Perspect, 128, 47006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibella F, Campagna D, Caponnetto P, Amaradio MD, Caruso M, Russo C, Cockcroft DW, & Polosa R (2016). Lung function and respiratory symptoms in a randomized smoking cessation trial of electronic cigarettes. Clin Sci (Lond), 130,1929–1937. [DOI] [PubMed] [Google Scholar]
- Cirillo S, Vivarelli F, Turrini E, Fimognari C, Burattini S, Falcieri E, Rocchi MBL, Cardenia V, Rodriguez-Estrada MT, Paolini M, & Canistro D (2019). The customizable e-cigarette resistance influences toxicological outcomes: lung degeneration, inflammation and oxidative stress-induced in a rat model. Toxicol Sci. [DOI] [PubMed] [Google Scholar]
- Clapp PW, & Jaspers I (2017). Electronic Cigarettes: Their Constituents and Potential Links to Asthma. Curr Allergy Asthma Rep, 17, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp PW, Lavrich KS, van Heusden CA, Lazarowski ER, Carson JL, & Jaspers I (2019). Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am J Physiol Lung Cell Mol Physiol, 316, L470–L486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp PW, Pawlak EA, Lackey JT, Keating JE, Reeber SL, Glish GL, & Jaspers I (2017). Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am J Physiol Lung Cell Mol Physiol, 313, L278–L292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp PW, Peden DB, & Jaspers I (2020). E-cigarettes, vaping-related pulmonary illnesses, and asthma: A perspective from inhalation toxicologists. J Allergy Clin Immunol, 145, 97–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congress, U. S. (2009). Family Smoking and Prevention Tobacco Control Act. In U. S. Congress (Ed.), Public Law 111–31 (Vol. HR 1256). Washington D.C.: U.S. Federal Government. [Google Scholar]
- Cooke A, Fergeson J, Bulkhi A, & Casale TB (2015). The Electronic Cigarette: The Good, the Bad, and the Ugly. J Allergy Clin Immunol Pract, 3, 498–505. [DOI] [PubMed] [Google Scholar]
- Cordeiro SK, Daro RC, Seung H, Klein-Schwartz W, & Kim HK (2018). Evolution of clinical characteristics and outcomes of synthetic cannabinoid receptor agonist exposure in the United States: analysis of National Poison Data System data from 2010 to 2015. Addiction, 113, 1850–1861. [DOI] [PubMed] [Google Scholar]
- Cornelius ME, Wang TW, Jamal A, Loretan CG, & Linda J Neff LJ (2020). Tobacco Product Use Among Adults — United States, 2019. CDC Morbidity and Mortality Weekly Report (MMWR), 69, 1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriden R, Moshensky A, Bojanowski CM, Meier A, Chien J, Nelson RK, & Crotty Alexander LE (2020). E-cigarette use increases susceptibility to bacterial infection by impairment of human neutrophil chemotaxis, phagocytosis, and NET formation. Am J Physiol Cell Physiol, 318, C205–C214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E, Barry RA, & Glantz S (2016). E-cigarette Policymaking by Local and State Governments: 2009-2014. Milbank Q, 94, 520–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranford JA, Bohnert KM, Perron BE, Bourque C, & Ilgen M (2016). Prevalence and correlates of “Vaping” as a route of cannabis administration in medical cannabis patients. Drug and alcohol dependence, 169, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravo AS, Bush J, Sharma G, Savioz R, Martin C, Craige S, & Walele T (2016). A randomised, parallel group study to evaluate the safety profile of an electronic vapour product over 12 weeks. Regul Toxicol Pharmacol, 81 Suppl 1, S1–S14. [DOI] [PubMed] [Google Scholar]
- Crotty Alexander LE, Drummond CA, Hepokoski M, Mathew D, Moshensky A, Willeford A, Das S, Singh P, Yong Z, Lee JH, Vega K, Du A, Shin J, Javier C, Tian J, Brown JH, & Breen EC (2018). Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am J Physiol Regul Integr Comp Physiol, 314, R834–R847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudazzo G, Smart DJ, McHugh D, & Vanscheeuwijck P (2019). Lysosomotropic-related limitations of the BALB/c 3T3 cell-based neutral red uptake assay and an alternative testing approach for assessing e-liquid cytotoxicity. Toxicol In Vitro, 61, 104647. [DOI] [PubMed] [Google Scholar]
- Czogala J, Goniewicz ML, Fidelus B, Zielinska-Danch W, Travers MJ, & Sobczak A (2014). Secondhand exposure to vapors from electronic cigarettes. Nicotine Tob Res, 16, 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoli CD, Goniewicz ML, Palumbo M, Leigh N, White CM, & Hammond D (2019). Identification of flavouring chemicals and potential toxicants in e-cigarette products in Ontario, Canada. Can J Public Health, 110, 542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Kim KH, Szulejko JE, Jo SH, Kwon K, & Choi DW (2018). Quantification of nicotine and major solvents in retail electronic cigarette fluids and vaped aerosols. Microchemical Journal, 140, 262–268. [Google Scholar]
- Dinardo P, & Rome ES (2019). Vaping: The new wave of nicotine addiction. Cleve Clin J Med, 86, 789–798. [DOI] [PubMed] [Google Scholar]
- Duffy B, Li L, Lu S, Durocher L, Dittmar M, Delaney-Baldwin E, Panawennage D, LeMaster D, Navarette K, & Spink D (2020). Analysis of Cannabinoid-Containing Fluids in Illicit Vaping Cartridges Recovered from Pulmonary Injury Patients: Identification of Vitamin E Acetate as a Major Diluent. Toxics, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earleywine M, & Barnwell SS (2007). Decreased respiratory symptoms in cannabis users who vaporize. Harm Reduct J, 4, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggleston W, Nacca N, Stork CM, & Marraffa JM (2016). Pediatric death after unintentional exposure to liquid nicotine for an electronic cigarette. Clin Toxicol (Phila), 54, 890–891. [DOI] [PubMed] [Google Scholar]
- Ejaz S, & Lim CW (2005). Toxicological overview of cigarette smoking on angiogenesis. Environ Toxicol Pharmacol, 20, 335–344. [DOI] [PubMed] [Google Scholar]
- El-Hellani A, El-Hage R, Baalbaki R, Salman R, Talih S, Shihadeh A, & Saliba NA (2015). Free-Base and Protonated Nicotine in Electronic Cigarette Liquids and Aerosols. Chem Res Toxicol, 28, 1532–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Golli N, Jrad-Lamine A, Neffati H, Rahali D, Dallagi Y, Dkhili H, Ba N, El May MV, & El Fazaa S (2016). Impact of e-cigarette refill liquid with or without nicotine on liver function in adult rats. Toxicol Mech Methods, 26, 419–426. [DOI] [PubMed] [Google Scholar]
- El Golli N, Rahali D, Jrad-Lamine A, Dallagi Y, Jallouli M, Bdiri Y, Ba N, Lebret M, Rosa JP, El May M, & El Fazaa S (2016). Impact of electronic-cigarette refill liquid on rat testis. Toxicol Mech Methods, 26, 427–434. [DOI] [PubMed] [Google Scholar]
- Elliott S (2013). E-cigarette makers’ ads echo tobacco’s heyday. New York Times. [Google Scholar]
- England LJ, Aagaard K, Bloch M, Conway K, Cosgrove K, Grana R, Gould TJ, Hatsukami D, Jensen F, Kandel D, Lanphear B, Leslie F, Pauly JR, Neiderhiser J, Rubinstein M, Slotkin T , Spindel E, Stroud L, & Wakschlag L (2017). Developmental toxicity of nicotine: A transdisciplinary synthesis and implications for emerging tobacco products. Neurosci Biobehav Rev, 72, 176–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erythropel HC, Jabba SV, DeWinter TM, Mendizabal M, Anastas PT, Jordt SE, & Zimmerman JB (2019). Formation of flavorant-propylene Glycol Adducts With Novel Toxicological Properties in Chemically Unstable E-Cigarette Liquids. Nicotine Tob Res, 21, 1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza-Derout J, Hasan KM, Shao XM, Jordan MC, Sims C, Lee DL, Sinha S, Simmons Z, Mtume N, Liu Y, Roos KP, Sinha-Hikim AP, & Friedman TC (2019). Chronic intermittent electronic cigarette exposure induces cardiac dysfunction and atherosclerosis in apolipoprotein-E knockout mice. Am J Physiol Heart Circ Physiol, 317, H445–H459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF, & Bullen C (2011). Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction, 106, 2017–2028. [DOI] [PubMed] [Google Scholar]
- Farsalinos KE, Kistler KA, Gillman G, & Voudris V (2015). Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine Tob Res, 17, 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Allifranchini E, Ripamonti E, Bocchietto E, Todeschi S, Tsiapras D, Kyrzopoulos S, & Voudris V (2013). Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int J Environ Res Public Health, 10, 5146–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, & Voudris V (2014). Characteristics, perceived side effects and benefits of electronic cigarette use: a worldwide survey of more than 19,000 consumers. Int J Environ Res Public Health, 11, 4356–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Voudris V, & Poulas K (2015). Are metals emitted from electronic cigarettes a reason for health concern? A risk-assessment analysis of currently available literature. Int J Environ Res Public Health, 12, 5215–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. (2007). Guidance for Industry Testing of Glycerin for Diethylene Glycol. In.
- FDA. (2016). Deeming tobacco products to be subject to the Federal Food, Drug, and Cosmetic Act, as amended by the Family Smoking Prevention and Tobacco Control Act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Final rule. In Federal register (Vol. 81, pp. 28973). [PubMed] [Google Scholar]
- FDA. (2018a). FDA Approves First Drug Comprised of an Active Ingredient Derived from Marijuana to Treat Rare, Severe Forms of Epilepsy. In Press Announcement. [Google Scholar]
- FDA. (2018b). Food Additive Regulations; Synthetic Flavoring Agents and Adjuvants. In.
- FDA. (2019a). Code of Federal Regulations: 21CFR184.1666. In (Vol. 21). [Google Scholar]
- FDA. (2019b). Newly Signed Legislation Raises Federal Minimum Age of Sale of Tobacco Products to 21. In Center for Tobacco Products Newsroom (pp. Press Announcement; ). [Google Scholar]
- FDA. (2019c). Vaping Illness Update: FDA Warns Public to Stop Using Tetrahydrocannabinol (THC)-Containing Vaping Products and Any Vaping Products Obtained Off the Street. In (Vol. 2019, pp. Press Announcement; ). [Google Scholar]
- FDA. (2020a). Enforcement Priorities for Electronic Nicotine Delivery Systems and Other Deemed Products on the Market Without Premarket Authorization (Revised); Guidance for Industry; Availability. In (Vol. 85 FR 23973, pp. 23973–23974 ). [Google Scholar]
- FDA. (2020b). FDA and Cannabis: Research and Drug Approval Process. In Public Health Focus. [Google Scholar]
- FDA. (2020c). Federal Register Volume 85, Issue 4 (January 7, 2020) In (Vol. 85, pp. 720–722). [Google Scholar]
- FDA. (2021). FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD). In Public Health Focus. [Google Scholar]
- Fedt A, Bhattarai S, & Oelstrom MJ (2020). Vaping-Associated Lung Injury: A New Cause of Acute Respiratory Failure. J Adolesc Health, 66, 754–757. [DOI] [PubMed] [Google Scholar]
- FEMA, T. F. a. E. M. A. o. t. U. S. (2012). Respiratory Health and Safety in the Flavor Manufacturing Workplace In. 1620 I Street, N.W., Suite 925 Washington, D.C., 20006: The Flavor and Extract Manufacturers Association of the United States. [Google Scholar]
- Flouris AD, Poulianiti KP, Chorti MS, Jamurtas AZ, Kouretas D, Owolabi EO, Tzatzarakis MN, Tsatsakis AM, & Koutedakis Y (2012). Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem Toxicol, 50, 3600–3603. [DOI] [PubMed] [Google Scholar]
- Fonseca Fuentes X, Kashyap R, Hays JT, Chalmers S, Lama von Buchwald C, Gajic O, & Gallo de Moraes A (2019). VpALI-Vaping-related Acute Lung Injury: A New Killer Around the Block. Mayo Clin Proc, 94, 2534–2545. [DOI] [PubMed] [Google Scholar]
- Fowles J, Barreau T, & Wu N (2020). Cancer and Non-Cancer Risk Concerns from Metals in Electronic Cigarette Liquids and Aerosols. Int J Environ Res Public Health, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles J, & DiBartolomeis M (2017). Toxicological Concerns from Inhaled Food Flavorings Found in Electronic (E-) Cigarette Aerosols: A Report from the Environmental Health Investigations Branch. In C. D. o. P. Health (Ed.), (pp. 1–36). Richmond, CA: California Department of Public Health. [Google Scholar]
- Friedman AS (2020). Association of vaping-related lung injuries with rates of e-cigarette and cannabis use across US states. Addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy V, Manyanga J, Brame L, McGuire D, Sadhasivam B, Floyd E, Rubenstein DA, Ramachandran I, Wagener T, & Queimado L (2017). Electronic cigarette aerosols suppress cellular antioxidant defenses and induce significant oxidative DNA damage. PLoS One, 12, e0177780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Arcos I, Geraghty P, Baumlin N, Campos M, Dabo AJ, Jundi B, Cummins N, Eden E, Grosche A, Salathe M, & Foronjy R (2016). Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax, 71, 1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Esquinas E, Pollan M, Tellez-Plaza M, Francesconi KA, Goessler W, Guallar E, Umans JG, Yeh J, Best LG, & Navas-Acien A (2014). Cadmium exposure and cancer mortality in a prospective cohort: the strong heart study. Environ Health Perspect, 122, 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur S, & Agnihotri R (2019). Health Effects of Trace Metals in Electronic Cigarette Aerosols-a Systematic Review. Biol Trace Elem Res, 188, 295–315. [DOI] [PubMed] [Google Scholar]
- Gay B, Field Z, Patel S, Alvarez RM, Nasser W, Madruga M, & Carlan SJ (2020). Vaping-Induced Lung Injury: A Case of Lipoid Pneumonia Associated with E-Cigarettes Containing Cannabis. Case Rep Pulmonol, 2020, 7151834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss O, Bianchi I, & Barrero-Moreno J (2016). Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int J Hyg Environ Health, 219, 268–277. [DOI] [PubMed] [Google Scholar]
- Gerloff J, Sundar IK, Freter R, Sekera ER, Friedman AE, Robinson R, Pagano T, & Rahman I (2017). Inflammatory Response and Barrier Dysfunction by Different e-Cigarette Flavoring Chemicals Identified by Gas Chromatography-Mass Spectrometry in e-Liquids and e-Vapors on Human Lung Epithelial Cells and Fibroblasts. Appl In Vitro Toxicol, 3, 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghinai I, Pray IW, Navon L, O'Laughlin K, Saathoff-Huber L, Hoots B, Kimball A, Tenforde MW, Chevinsky JR, Layer M, Ezike N, Meiman J, & Layden JE (2019). E-cigarette Product Use, or Vaping, Among Persons with Associated Lung Injury - Illinois and Wisconsin, April-September 2019. MMWR Morb Mortal Wkly Rep, 68, 865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill N, Sangha G, Poonai N, & Lim R (2015). E-Cigarette Liquid Nicotine Ingestion in a Child: Case Report and Discussion. CJEM, 17, 699–703. [DOI] [PubMed] [Google Scholar]
- Gillman IG, Kistler KA, Stewart EW, & Paolantonio AR (2016). Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul Toxicol Pharmacol, 75, 58–65. [DOI] [PubMed] [Google Scholar]
- Giroud C, de Cesare M, Berthet A, Varlet V, Concha-Lozano N, & Favrat B (2015). E-Cigarettes: A Review of New Trends in Cannabis Use. Int J Environ Res Public Health, 12, 9988–10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynos C, Bibli SI, Katsaounou P, Pavlidou A, Magkou C, Karavana V, Topouzis S, Kalomenidis I, Zakynthinos S, & Papapetropoulos A (2018). Comparison of the effects of e-cigarette vapor with cigarette smoke on lung function and inflammation in mice. Am J Physiol Lung Cell Mol Physiol, 315, L662–L672. [DOI] [PubMed] [Google Scholar]
- Go YY, Mun JY, Chae SW, Chang J, & Song JJ (2020). Comparison between in vitro toxicities of tobacco- and menthol-flavored electronic cigarette liquids on human middle ear epithelial cells. Sci Rep, 10, 2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golli NE, Jrad-Lamine A, Neffati H, Dkhili H, Rahali D, Dallagi Y, El May MV, & El Fazaa S (2016). Impact of e-cigarette refill liquid exposure on rat kidney. Regul Toxicol Pharmacol, 77, 109–116. [DOI] [PubMed] [Google Scholar]
- Good-Scents-Company. (2021). The Good Scents Company Information System. In. Oak Creek, WI: The Good Scents Company. [Google Scholar]
- Gottlieb S (2018). Statement from FDA Commissioner Scott Gottlieb, M.D., on New Steps to Address Epidemic of Youth e-Cigarette Use. In (Vol. 2018, pp. Press Announcement). Silver Spring, MD: U.S. Food and Drug Administration. [Google Scholar]
- Grand-View-Research. (2020). E-cigarette & Vape Market Worth $67.31 Billion By 2027 ∣ CAGR: 23.8%. In. [Google Scholar]
- H.R.4742. (2019). Protecting American Lungs Act of 2019. In. Washington, D.C.: Congress. [Google Scholar]
- Hart JL, Walker KL, Sears CG, Lee AS, Ridner SL, & Keith RJ (2018). E-cigarette use and perceived health change: Better health through vaping? Tob Induc Dis, 16, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS (2018). US Epidemiology of Cannabis Use and Associated Problems. Neuropsychopharmacology, 43, 195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health, T. N. I. f. O. S. a. (2019). Benzene. In (Vol. 2020). [Google Scholar]
- Hess CA, Olmedo P, Navas-Acien A, Goessler W, Cohen JE, & Rule AM (2017). E-cigarettes as a source of toxic and potentially carcinogenic metals. Environ Res, 152, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman E, Herrera CA, & Jaspers I (2019). Common E-Cigarette Flavoring Chemicals Impair Neutrophil Phagocytosis and Oxidative Burst. Chem Res Toxicol, 32, 982–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C, Freeman TP, Ferris JA, Lynskey MT, & Winstock AR (2016). No Smoke without Tobacco: A Global Overview of Cannabis and Tobacco Routes of Administration and Their Association with Intention to Quit. Frontiers in Psychiatry, 7, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden LL, Truong L, Simonich MT, & Tanguay RL (2020). Assessing the hazard of E-Cigarette flavor mixtures using zebrafish. Food Chem Toxicol, 136, 110945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horth RZ, Crouch B, Horowitz BZ, Prebish A, Slawson M, McNair J, Elsholz C, Gilley S, Robertson J, Risk I, Hill M, Fletcher L, Hou W, Peterson D, Adams K, Vitek D, Nakashima A, & Dunn A (2018). Notes from the Field: Acute Poisonings from a Synthetic Cannabinoid Sold as Cannabidiol — Utah, 2017–2018. In C. f. D. C. a. Prevention; (Ed.), (Vol. 67, pp. 587–588): MMWR Morb Mortal Wkly Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua M, Omaiye EE, Luo W, McWhirter KJ, Pankow JF, & Talbot P (2019). Identification of Cytotoxic Flavor Chemicals in Top-Selling Electronic Cigarette Refill Fluids. Sci Rep, 9, 2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Feng B, Weaver SR, Pechacek TF, Slovic P, & Eriksen MP (2019). Changing Perceptions of Harm of e-Cigarette vs Cigarette Use Among Adults in 2 US National Surveys From 2012 to 2017. JAMA Netw Open, 2, e191047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A, & Hendrickson RG (2019). An epidemiologic and clinical description of e-cigarette toxicity. Clin Toxicol (Phila), 57, 287–293. [DOI] [PubMed] [Google Scholar]
- Husari A, Shihadeh A, Talih S, Hashem Y, El Sabban M, & Zaatari G (2016). Acute Exposure to Electronic and Combustible Cigarette Aerosols: Effects in an Animal Model and in Human Alveolar Cells. Nicotine Tob Res, 18, 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzler C, Paschke M, Kruschinski S, Henkler F, Hahn J, & Luch A (2014). Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol, 88, 1295–1308. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Lyes M, Sladewski K, Enany S, McEachern E, Mathew DP, Das S, Moshensky A, Bapat S, Pride DT, Ongkeko WM, & Crotty Alexander LE (2016). Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl), 94, 667–679. [DOI] [PubMed] [Google Scholar]
- Ind PW (2020). E-cigarette or vaping product use-associated lung injury. Br J Hosp Med (Lond), 81, 1–9. [DOI] [PubMed] [Google Scholar]
- Iskandar AR, Zanetti F, Marescotti D, Titz B, Sewer A, Kondylis A, Leroy P, Belcastro V, Torres LO, Acali S, Majeed S, Steiner S, Trivedi K, Guedj E, Merg C, Schneider T, Frentzel S, Martin F, Ivanov NV, Peitsch MC, & Hoeng J (2019). Application of a multi-layer systems toxicology framework for in vitro assessment of the biological effects of Classic Tobacco e-liquid and its corresponding aerosol using an e-cigarette device with MESH technology. Arch Toxicol, 93, 3229–3247. [DOI] [PubMed] [Google Scholar]
- Jabba SV, & Jordt SE (2019). Risk Analysis for the Carcinogen Pulegone in Mint- and Menthol-Flavored e-Cigarettes and Smokeless Tobacco Products. JAMA Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackler RK, & Ramamurthi D (2019). Nicotine arms race: JUUL and the high-nicotine product market. Tob Control, 28, 623–628. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, & Pugh KR (2005). Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry, 57, 56–66. [DOI] [PubMed] [Google Scholar]
- Javadi-Paydar M, Creehan KM, Kerr TM, & Taffe MA (2019). Vapor inhalation of cannabidiol (CBD) in rats. Pharmacol Biochem Behav, 184, 172741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi-Paydar M, Nguyen JD, Kerr TM, Grant Y, Vandewater SA, Cole M, & Taffe MA (2018). Effects of Delta9-THC and cannabidiol vapor inhalation in male and female rats. Psychopharmacology (Berl), 235, 2541–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RP, Strongin RM, & Peyton DH (2017). Solvent Chemistry in the Electronic Cigarette Reaction Vessel. Sci Rep, 7, 42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L (2019). Vaping: How popular are e-cigarettes? BBC News. September, 15. [Google Scholar]
- Kaisar MA, Villalba H, Prasad S, Liles T, Sifat AE, Sajja RK, Abbruscato TJ, & Cucullo L (2017). Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: Is Metformin a viable countermeasure? Redox Biol, 13, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboj A, Spiller HA, Casavant MJ, Chounthirath T, & Smith GA (2016). Pediatric Exposure to E-Cigarettes, Nicotine, and Tobacco Products in the United States. Pediatrics, 137. [DOI] [PubMed] [Google Scholar]
- Kamilari E, Farsalinos K, Poulas K, Kontoyannis CG, & Orkoula MG (2018). Detection and quantitative determination of heavy metals in electronic cigarette refill liquids using Total Reflection X-ray Fluorescence Spectrometry. Food Chem Toxicol, 116, 233–237. [DOI] [PubMed] [Google Scholar]
- Kass AP, Overbeek DL, Chiel LE, Boyer EW, & Casey AMH (2020). Case series: Adolescent victims of the vaping public health crisis with pulmonary complications. Pediatr Pulmonol, 55, 1224–1236. [DOI] [PubMed] [Google Scholar]
- Keller CJ, Chen EC, Brodsky K, & Yoon JH (2016). A case of butane hash oil (marijuana wax)-induced psychosis. Subst Abus, 37, 384–386. [DOI] [PubMed] [Google Scholar]
- Kelly BF, & Nappe TM (2020). Cannabinoid Toxicity. Treasure Island, FL: StatPearls Publishing. [PubMed] [Google Scholar]
- Kennedy AE, Kandalam S, Olivares-Navarrete R, & Dickinson AJG (2017). E-cigarette aerosol exposure can cause craniofacial defects in Xenopus laevis embryos and mammalian neural crest cells. PLoS One, 12, e0185729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachatoorian C, Jacob Iii P, Benowitz NL, & Talbot P (2019). Electronic cigarette chemicals transfer from a vape shop to a nearby business in a multiple-tenant retail building. Tob Control, 28, 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachatoorian C, Jacob P 3rd, Sen A, Zhu Y, Benowitz NL, & Talbot P (2019). Identification and quantification of electronic cigarette exhaled aerosol residue chemicals in field sites. Environ Res, 170, 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khlystov A, & Samburova V (2016). Flavoring Compounds Dominate Toxic Aldehyde Production during E-Cigarette Vaping. Environ Sci Technol, 50, 13080–13085. [DOI] [PubMed] [Google Scholar]
- Kim KH, Kabir E, & Jahan SA (2016). Review of electronic cigarettes as tobacco cigarette substitutes: Their potential human health impact. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev, 34, 262–275. [DOI] [PubMed] [Google Scholar]
- King BA, Jones CM, Baldwin GT, & Briss PA (2020). The EVALI and Youth Vaping Epidemics - Implications for Public Health. N Engl J Med, 382, 689–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BA, Patel R, Nguyen KH, & Dube SR (2015). Trends in awareness and use of electronic cigarettes among US adults, 2010–2013. Nicotine & Tobacco Research, 17, 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfield R, Huang J, Vera L, & Emery SL (2015). Rapidly increasing promotional expenditures for e-cigarettes. Tob Control, 24, 110–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosecki D (2019). Is CBD legal? In many parts of the country, CBD exists in a legal gray zone. In CNET. [Google Scholar]
- Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, & Goniewicz ML (2014). Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res, 16, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowitt SD, Osman A, Meernik C, Zarkin GA, Ranney LM, Martin J, Heck C, & Goldstein AO (2019). Vaping cannabis among adolescents: prevalence and associations with tobacco use from a cross-sectional study in the USA. BMJ Open, 9, e028535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntic M, Oelze M, Steven S, Kroller-Schon S, Stamm P, Kalinovic S, Frenis K, Vujacic-Mirski K, Bayo Jimenez MT, Kvandova M, Filippou K, Al Zuabi A, Bruckl V, Hahad O, Daub S, Varveri F, Gori T, Huesmann R, Hoffmann T, Schmidt FP, Keaney JF, Daiber A, & Munzel T (2020). Short-term e-cigarette vapour exposure causes vascular oxidative stress and dysfunction: evidence for a close connection to brain damage and a key role of the phagocytic NADPH oxidase (NOX-2). Eur Heart J, 41, 2472–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Moon KA, Wang SL, Silbergeld E, & Navas-Acien A (2017). The Association of Arsenic Metabolism with Cancer, Cardiovascular Disease, and Diabetes: A Systematic Review of the Epidemiological Evidence. Environ Health Perspect, 125, 087001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahnsteiner F (2008). The effect of internal and external cryoprotectants on zebrafish (Danio rerio) embryos. Theriogenology, 69, 384–396. [DOI] [PubMed] [Google Scholar]
- Lanzarotta A, Falconer TM, Flurer R, & Wilson RA (2020). Hydrogen Bonding between Tetrahydrocannabinol and Vitamin E Acetate in Unvaped, Aerosolized, and Condensed Aerosol e-Liquids. Anal Chem, 92, 2374–2378. [DOI] [PubMed] [Google Scholar]
- Laube BL, Afshar-Mohajer N, Koehler K, Chen G, Lazarus P, Collaco JM, & McGrath-Morrow SA (2017). Acute and chronic in vivo effects of exposure to nicotine and propylene glycol from an E-cigarette on mucociliary clearance in a murine model. Inhal Toxicol, 29, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterstein DE, Tijerina PB, Corbett K, Akgol Oksuz B, Shen SS, Gordon T, Klein CB, & Zelikoff JT (2016). Frontal Cortex Transcriptome Analysis of Mice Exposed to Electronic Cigarettes During Early Life Stages. Int J Environ Res Public Health, 13, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde MW, Navon L, Hoots B, Salvatore PP, Elderbrook M, Haupt T, Kanne J, Patel MT, Saathoff-Huber L, King BA, Schier JG, Mikosz CA, & Meiman J (2020). Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin - Final Report. N Engl J Med, 382, 903–916. [DOI] [PubMed] [Google Scholar]
- Lee DC, Crosier BS, Borodovsky JT, Sargent JD, & Budney AJ (2016). Online survey characterizing vaporizer use among cannabis users. Drug and Alcohol Dependence, 159, 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Na CJ, Botao L, Kim KH, & Son YS (2020). Quantitative insights into major constituents contained in or released by electronic cigarettes: Propylene glycol, vegetable glycerin, and nicotine. Sci Total Environ, 703, 134567. [DOI] [PubMed] [Google Scholar]
- Lefever TW, Marusich JA, Thomas BF, Barrus DG, Peiper NC, Kevin RC, & Wiley JL (2017). Vaping Synthetic Cannabinoids: A Novel Preclinical Model of E-Cigarette Use in Mice. Subst Abuse, 11, 1178221817701739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh NJ, Lawton RI, Hershberger PA, & Goniewicz ML (2016). Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS). Tob Control, 25, ii81–ii87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, & Rahman I (2015). Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One, 10, e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie LJ, Vasanthi Bathrinarayanan P, Jackson P, Mabiala Ma Muanda JA, Pallett R, Stillman CJP, & Marshall LJ (2017). A comparative study of electronic cigarette vapor extracts on airway-related cell lines in vitro. Inhal Toxicol, 29, 126–136. [DOI] [PubMed] [Google Scholar]
- Lin VY, Fain MD, Jackson PL, Berryhill TF, Wilson LS, Mazur M, Barnes SJ, Blalock JE, Raju SV, & Rowe SM (2019). Vaporized E-Cigarette Liquids Induce Ion Transport Dysfunction in Airway Epithelia. Am J Respir Cell Mol Biol, 61, 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G, Favaloro EJ, Meschi T, Mattiuzzi C, Borghi L, & Cervellin G (2014). E-cigarettes and cardiovascular risk: beyond science and mysticism. Semin Thromb Hemost, 40, 60–65. [DOI] [PubMed] [Google Scholar]
- Lisko JG, Tran H, Stanfill SB, Blount BC, & Watson CH (2015). Chemical Composition and Evaluation of Nicotine, Tobacco Alkaloids, pH, and Selected Flavors in E-Cigarette Cartridges and Refill Solutions. Nicotine Tob Res, 17, 1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey TK, Miner A, & Cuomo RE (2015). Exploring the e-cigarette e-commerce marketplace: Identifying Internet e-cigarette marketing characteristics and regulatory gaps. Drug Alcohol Depend, 156, 97–103. [DOI] [PubMed] [Google Scholar]
- Maddock SD, Cirulis MM, Callahan SJ, Keenan LM, Pirozzi CS, Raman SM, & Aberegg SK (2019). Pulmonary Lipid-Laden Macrophages and Vaping. N Engl J Med, 381, 1488–1489. [DOI] [PubMed] [Google Scholar]
- Madison MC, Landers CT, Gu BH, Chang CY, Tung HY, You R, Hong MJ, Baghaei N, Song LZ, Porter P, Putluri N, Salas R, Gilbert BE, Levental I, Campen MJ, Corry DB, & Kheradmand F (2019). Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest, 129, 4290–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes J, Verlooy L, Buenafe OE, de Witte PA, Esguerra CV, & Crawford AD (2012). Evaluation of 14 organic solvents and carriers for screening applications in zebrafish embryos and larvae. PLoS One, 7, e43850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiori E, Zanetti G, Mano CM, & Hochhegger B (2011). Exogenous lipoid pneumonia. Clinical and radiological manifestations. Respir Med, 105, 659–666. [DOI] [PubMed] [Google Scholar]
- Marescotti D, Mathis C, Belcastro V, Leroy P, Acali S, Martin F, Dulize R, Bornand D, Peric D, Guedj E, Ortega Torres L, Biasioli M, Fuhrimann M, Fernandes E, Frauendorfer F, Gonzalez Suarez I, Sciuscio D, Ivanov NV, Peitsch MC, & Hoeng J (2020). Systems toxicology assessment of a representative e-liquid formulation using human primary bronchial epithelial cells. Toxicol Rep, 7, 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margham J, McAdam K, Forster M, Liu C, Wright C, Mariner D, & Proctor C (2016). Chemical Composition of Aerosol from an E-Cigarette: A Quantitative Comparison with Cigarette Smoke. Chem Res Toxicol, 29, 1662–1678. [DOI] [PubMed] [Google Scholar]
- Martin EM, Clapp PW, Rebuli ME, Pawlak EA, Glista-Baker E, Benowitz NL, Fry RC, & Jaspers I (2016). E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol, 311, L135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarsky A, Abdel A, Glazer L, Levin ED, & Di Giulio RT (2017). Exposure to 1,2-Propanediol Impacts Early Development of Zebrafish (Danio rerio) and Induces Hyperactivity. Zebrafish, 14, 216–222. [DOI] [PubMed] [Google Scholar]
- Massarsky A, Abdel A, Glazer L, Levin ED, & Di Giulio RT (2018). Neurobehavioral effects of 1,2-propanediol in zebrafish (Danio rerio). Neurotoxicology, 65, 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath-Morrow SA, Hayashi M, Aherrera A, Lopez A, Malinina A, Collaco JM, Neptune E, Klein JD, Winickoff JP, Breysse P, Lazarus P, & Chen G (2015). The effects of electronic cigarette emissions on systemic cotinine levels, weight and postnatal lung growth in neonatal mice. PLoS One, 10, e0118344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw MD, Kim SY, Reed C, Hernady E, Rahman I, Mariani TJ, & Finkelstein JN (2020). Airway basal cell injury after acute diacetyl (2,3-butanedione) vapor exposure. Toxicol Lett, 325, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen RC, Gottlieb MA, Shaefer RMW, Winickoff JP, & Klein JD (2014). Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine & Tobacco Research, 17, 1195–1202. [DOI] [PubMed] [Google Scholar]
- Meo SA, & Al Asiri SA (2014). Effects of electronic cigarette smoking on human health. Eur Rev Med Pharmacol Sci, 18, 3315–3319. [PubMed] [Google Scholar]
- Messina MD, Levin TL, Conrad LA, & Bidiwala A (2020). Vaping associated lung injury: A potentially life-threatening epidemic in US youth. Pediatr Pulmonol, 55, 1705–1711. [DOI] [PubMed] [Google Scholar]
- Metna-Laurent M, Mondesir M, Grel A, Vallee M, & Piazza PV (2017). Cannabinoid-Induced Tetrad in Mice. Curr Protoc Neurosci, 80, 9 59 51–59 59 10. [DOI] [PubMed] [Google Scholar]
- Miech R, Johnston L, O'Malley PM, Bachman JG, & Patrick ME (2019). Trends in Adolescent Vaping, 2017-2019. N Engl J Med, 381, 1490–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon K, Guallar E, & Navas-Acien A (2012). Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep, 14, 542–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K, Young HI, & Ryan M (2015). Bilateral Pneumonia and Pleural Effusions Subsequent to Electronic Cigarette Use. Scientific Research Publishing, 3, 18–22. [Google Scholar]
- Morean ME, Kong G, Camenga DR, Cavallo DA, & Krishnan-Sarin S (2015). High school students’ use of electronic cigarettes to vaporize cannabis. Pediatrics, 136, 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz ED, Zapata LB, Lekiachvili A, Glidden E, Annor FB, Werner AK, Ussery EN, Hughes MM, Kimball A, DeSisto CL, Kenemer B, Shamout M, Garcia MC, Reagan-Steiner S, Petersen EE, Koumans EH, Ritchey MD, King BA, Jones CM, Briss PA, Delaney L, Patel A, Polen KD, Sives K, Meaney-Delman D, Chatham-Stephens K, Lung Injury Response Epidemiology/Surveillance, G., & Lung Injury Response Epidemiology/Surveillance Task, F. (2019). Update: Characteristics of Patients in a National Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injuries - United States, October 2019. MMWR Morb Mortal Wkly Rep, 68, 985–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses E, Wang T, Corbett S, Jackson GR, Drizik E, Perdomo C, Perdomo C, Kleerup E, Brooks D, O'Connor G, Dubinett S, Hayden P, Lenburg ME, & Spira A (2017). Molecular Impact of Electronic Cigarette Aerosol Exposure in Human Bronchial Epithelium. Toxicol Sci, 155, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravee B, Tibensky M, Horvathova L, & Babal P (2020). E-Cigarettes and Cancer Risk. Cancer Prev Res (Phila), 13, 137–144. [DOI] [PubMed] [Google Scholar]
- Muthumalage T, Lamb T, Friedman MR, & Rahman I (2019). E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci Rep, 9, 19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumalage T, Prinz M, Ansah KO, Gerloff J, Sundar IK, & Rahman I (2017). Inflammatory and Oxidative Responses Induced by Exposure to Commonly Used e-Cigarette Flavoring Chemicals and Flavored e-Liquids without Nicotine. Front Physiol, 8, 1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, E., & Medicine. (2017). The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Navon L, Jones CM, Ghinai I, King BA, Briss PA, Hacker KA, & Layden JE (2019). Risk Factors for E-Cigarette, or Vaping, Product Use-Associated Lung Injury (EVALI) Among Adults Who Use E-Cigarette, or Vaping, Products - Illinois, July-October 2019. MMWR Morb Mortal Wkly Rep, 68, 1034–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson L, Mankus C, Thorne D, Jackson G, DeBay J, & Meredith C (2015). Development of an in vitro cytotoxicity model for aerosol exposure using 3D reconstructed human airway tissue; application for assessment of e-cigarette aerosol. Toxicol In Vitro, 29, 1952–1962. [DOI] [PubMed] [Google Scholar]
- Nguyen JD, Aarde SM, Vandewater SA, Grant Y, Stouffer DG, Parsons LH, Cole M, & Taffe MA (2016). Inhaled delivery of Delta(9)-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropharmacology, 109, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Li GE, Chen H, Cranfield CG, McGrath KC, & Gorrie CA (2018). Maternal E-Cigarette Exposure Results in Cognitive and Epigenetic Alterations in Offspring in a Mouse Model. Chem Res Toxicol, 31, 601–611. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Li GE, Chen H, Cranfield CG, McGrath KC, & Gorrie CA (2019). Neurological effects in the offspring after switching from tobacco cigarettes to e-cigarettes during pregnancy in a mouse model. Toxicol Sci. [DOI] [PubMed] [Google Scholar]
- NIDA. (2020). Vaping Devices (Electronic Cigarettes) Drug Facts. In. Bethesda, MD: USDHHS. [Google Scholar]
- NIOSH. (2014). Formaldehyde: Evidence of Carcionogenicity. In USDHHS (Ed.), (Vol. 34). Bethesda, MD: U.S. Government Printing Office. [Google Scholar]
- Noble MJ, Longstreet B, Hendrickson RG, & Gerona R (2017). Unintentional Pediatric Ingestion of Electronic Cigarette Nicotine Refill Liquid Necessitating Intubation. Ann Emerg Med, 69, 94–97. [DOI] [PubMed] [Google Scholar]
- Noel A, Hansen S, Zaman A, Perveen Z, Pinkston R, Hossain E, Xiao R, & Penn A (2020). In utero exposures to electronic-cigarette aerosols impair the Wnt signaling during mouse lung development. Am J Physiol Lung Cell Mol Physiol, 318, L705–L722. [DOI] [PubMed] [Google Scholar]
- NTP. (2011). Toxicology and carcinogenesis studies of pulegone (CAS No. 89-82-7) in F344/N rats and B6C3F1 mice (gavage studies). In N. T. Program (Ed.), (2011/September/17 ed., pp. 1–201). Research Triangle Park, NC: USDHHS. [PubMed] [Google Scholar]
- Nystoriak MA, Kilfoil PJ, Lorkiewicz PK, Ramesh B, Kuehl PJ, McDonald J, Bhatnagar A, & Conklin DJ (2019). Comparative effects of parent and heated cinnamaldehyde on the function of human iPSC-derived cardiac myocytes. Toxicol In Vitro, 61, 104648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD. (2018). Test No. 412: Subacute Inhalation Toxicity: 28-Day Study. In. Paris, FRA: OECD publishing. [Google Scholar]
- Olfert IM, DeVallance E, Hoskinson H, Branyan KW, Clayton S, Pitzer CR, Sullivan DP, Breit MJ, Wu Z, Klinkhachorn P, Mandler WK, Erdreich BH, Ducatman BS, Bryner RW, Dasgupta P, & Chantler PD (2018). Chronic exposure to electronic cigarettes results in impaired cardiovascular function in mice. J Appl Physiol (1985), 124, 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmedo P, Goessler W, Tanda S, Grau-Perez M, Jarmul S, Aherrera A, Chen R, Hilpert M, Cohen JE, Navas-Acien A, & Rule AM (2018). Metal Concentrations in e-Cigarette Liquid and Aerosol Samples: The Contribution of Metallic Coils. Environ Health Perspect, 126, 027010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omaiye EE, McWhirter KJ, Luo W, Tierney PA, Pankow JF, & Talbot P (2019). High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci Rep, 9, 2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi BG, Dutta D, Kazipeta K, & Chong NS (2019). Influence of the E-Cigarette Emission Profile by the Ratio of Glycerol to Propylene Glycol in E-Liquid Composition. ACS Omega, 4, 13338–13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzabal MR, Lunde-Young ER, Ramirez JI, Howe SYF, Naik VD, Lee J, Heaps CL, Threadgill DW, & Ramadoss J (2019). Chronic exposure to e-cig aerosols during early development causes vascular dysfunction and offspring growth deficits. Transl Res, 207, 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero CE, Noeker JA, Brown MM, Wavreil FDM, Harvey WA, Mitchell KA, & Heggland SJ (2019). Electronic cigarette liquid exposure induces flavor-dependent osteotoxicity and increases expression of a key bone marker, collagen type I. J Appl Toxicol, 39, 888–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford U (2014). Oxford's 2014 Word of the Year Is Vape. Time. [Google Scholar]
- Palpant NJ, Hofsteen P, Pabon L, Reinecke H, & Murry CE (2015). Cardiac development in zebrafish and human embryonic stem cells is inhibited by exposure to tobacco cigarettes and e-cigarettes. PLoS One, 10, e0126259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panitz D, Swamy H, & Nehrke K (2015). A C. elegans model of electronic cigarette use: Physiological effects of e-liquids in nematodes. BMC Pharmacol Toxicol, 16, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow JF, Kim K, McWhirter KJ, Luo W, Escobedo JO, Strongin RM, Duell AK, & Peyton DH (2017). Benzene formation in electronic cigarettes. PLoS One, 12, e0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HR, O'Sullivan M, Vallarino J, Shumyatcher M, Himes BE, Park JA, Christiani DC, Allen J, & Lu Q (2019). Transcriptomic response of primary human airway epithelial cells to flavoring chemicals in electronic cigarettes. Sci Rep, 9, 1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegoraro CN, Nutter D, Thevenon M, & Ramirez CL (2019). Chemical profiles of cannabis sativa medicinal oil using different extraction and concentration methods. Nat Prod Res, 1–4. [DOI] [PubMed] [Google Scholar]
- Pepper JK, Ribisl KM, Emery SL, & Brewer NT (2014). Reasons for starting and stopping electronic cigarette use. Int J Environ Res Public Health, 11, 10345–10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHLC, P. H. L. C. (2009). Sottera Inc. v. U.S. Food and Drug Administration/Smoking Everywhere, Inc. v. U.S. Food and Drug Administration (2009). In. Saint Paul, MN: Mitchell Hamline School of Law. [Google Scholar]
- PHLC, P. H. L. C. (2020). U.S. E-Cigarette Regulations - 50 State Review (2020). In. Saint Paul, MN: Mitchell Hamline School of Law. [Google Scholar]
- Pierre JM, Gandal M, & Son M (2016). Cannabis-induced psychosis associated with high potency "wax dabs". Schizophr Res, 172, 211–212. [DOI] [PubMed] [Google Scholar]
- Pisinger C, & Dossing M (2014). A systematic review of health effects of electronic cigarettes. Prev Med, 69, 248–260. [DOI] [PubMed] [Google Scholar]
- Polosa R, Cibella F, Caponnetto P, Maglia M, Prosperini U, Russo C, & Tashkin D (2017). Health impact of E-cigarettes: a prospective 3.5-year study of regular daily users who have never smoked. Scientific Reports, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potera C (2012). Still searching for better butter flavoring. Environ Health Perspect, 120, A457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasedya ES, Ambana Y, Martyasari NWR, Aprizal Y, Nurrijawati, & Sunarpi. (2020). Short-term E-cigarette toxicity effects on brain cognitive memory functions and inflammatory responses in mice. Toxicol Res, 36, 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRNewswire. (2019). Vaping Market to Reach a Value $29.39 Billion at a CAGR of 20.3% From 2018-2022 ∣ a Report From TBRC. In. [Google Scholar]
- Protano C, Avino P, Manigrasso M, Vivaldi V, Perna F, Valeriani F, & Vitali M (2018). Environmental Electronic Vape Exposure from Four Different Generations of Electronic Cigarettes: Airborne Particulate Matter Levels. Int J Environ Res Public Health, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvers K, Emami AS, Nollen NL, Romero DR, Strong DR, Benowitz NL, & Ahluwalia JS (2018). Tobacco Consumption and Toxicant Exposure of Cigarette Smokers Using Electronic Cigarettes. Nicotine Tob Res, 20, 206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasim H, Karim ZA, Silva-Espinoza JC, Khasawneh FT, Rivera JO, Ellis CC, Bauer SL, Almeida IC, & Alshbool FZ (2018). Short-Term E-Cigarette Exposure Increases the Risk of Thrombogenesis and Enhances Platelet Function in Mice. J Am Heart Assoc, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qayyum I, Fazal-ur-Rehman M, & Ibrahim MS (2018). Extraction of Nicotine (3-(1-methyl-2-pyrrolidinyl) pyridine) from Tobacco Leaves Separated from Gold Live Classic Brand™ Cigarettes by Solvent Extraction Approach and Characterization via IR Analysis. Biosciences Biotechnology Research Asia, 15, 799–804. [Google Scholar]
- Quail MT (2020). Nicotine toxicity: Protecting children from e-cigarette exposure. Nursing, 50, 44–48. [DOI] [PubMed] [Google Scholar]
- Radwan M, Wanas A, Chandra S, & ElSohly M (2017). Natural Cannabinoids of Cannabis and Methods of Analysis. In Cannabis sativa L. - Botany and Biotechnology (pp. 161–182). [Google Scholar]
- Rahali D, Jrad-Lamine A, Dallagi Y, Bdiri Y, Ba N, El May M, El Fazaa S, & El Golli N (2018). Semen Parameter Alteration, Histological Changes and Role of Oxidative Stress in Adult Rat Epididymis on Exposure to Electronic Cigarette Refill Liquid. Chin J Physiol, 61, 75–84. [DOI] [PubMed] [Google Scholar]
- Ramirez CL, Fanovich MA, & Churio MS (2019). Cannabinoids: Extraction Methods, Analysis, and Physicochemical Characterization. In Atta-ur-Rahman (Ed.), Studies in Natural Products Chemistry (Vol. 61, pp. 143–173): Elsevier. [Google Scholar]
- Rao DR, Maple KL, Dettori A, Afolabi F, Francis JKR, Artunduaga M, Lieu TJ, Aldy K, Cao DJ , Hsu S, Feng SY, & Mittal V (2020). Clinical Features of E-cigarette, or Vaping, Product Use-Associated Lung Injury in Teenagers. Pediatrics, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P, Liu J, & Springer ML (2020). JUUL and Combusted Cigarettes Comparably Impair Endothelial Function. Tob Regul Sci, 6, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidel B, Radicioni G, Clapp PW, Ford AA, Abdelwahab S, Rebuli ME, Haridass P, Alexis NE, Jaspers I, & Kesimer M (2018). E-Cigarette Use Causes a Unique Innate Immune Response in the Lung, Involving Increased Neutrophilic Activation and Altered Mucin Secretion. Am J Respir Crit Care Med, 197, 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagna G, Allifranchini E, Bocchietto E, Todeschi S, Esposito M, & Farsalinos KE (2013). Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): comparison with tobacco cigarette smoke extract. Inhal Toxicol, 25, 354–361. [DOI] [PubMed] [Google Scholar]
- Rouabhia M, Park HJ, Semlali A, Zakrzewski A, Chmielewski W, & Chakir J (2017). E-Cigarette Vapor Induces an Apoptotic Response in Human Gingival Epithelial Cells Through the Caspase-3 Pathway. J Cell Physiol, 232, 1539–1547. [DOI] [PubMed] [Google Scholar]
- Rowell TR, Keating JE, Zorn BT, Glish GL, Shears SB, & Tarran R (2020). Flavored e-liquids increase cytoplasmic Ca(2+) levels in airway epithelia. Am J Physiol Lung Cell Mol Physiol, 318, L226–L241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell TR, Reeber SL, Lee SL, Harris RA, Nethery RC, Herring AH, Glish GL, & Tarran R (2017). Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol, 313, L52–L66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein ML, Delucchi K, Benowitz NL, & Ramo DE (2018). Adolescent Exposure to Toxic Volatile Organic Chemicals From E-Cigarettes. Pediatrics, 141, e20173557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E, & Guy GW (2006). A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses, 66, 234–246. [DOI] [PubMed] [Google Scholar]
- Russo EB (2011). Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol, 163, 1344–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman GA, Alqawasma M, & Asad H (2019). Vaping Associated Lung Injury (EVALI): An Explosive United States Epidemic. Mo Med, 116, 492–496. [PMC free article] [PubMed] [Google Scholar]
- Sancilio S, Gallorini M, Cataldi A, & di Giacomo V (2016). Cytotoxicity and apoptosis induction by e-cigarette fluids in human gingival fibroblasts. Clin Oral Investig, 20, 477–483. [DOI] [PubMed] [Google Scholar]
- Sassano MF, Davis ES, Keating JE, Zorn BT, Kochar TK, Wolfgang MC, Glish GL, & Tarran R (2018). Evaluation of e-liquid toxicity using an open-source high-throughput screening assay. PLoS Biol, 16, e2003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpino M, Rosso T, Lanzo G, Lolli F, Bonizzoli M, Lazzeri C, Mannaioni G, Baronti R, Fattapposta F , & Grippo A (2020). Severe neurological nicotine intoxication by e-cigarette liquids: Systematic literature review. Acta Neurol Scand. [DOI] [PubMed] [Google Scholar]
- Schauer GL, King BA, Bunnell RE, Promoff G, & McAfee TA (2016). Toking, Vaping, and Eating for Health or Fun: Marijuana Use Patterns in Adults, U.S., 2014. Am J Prev Med, 50, 1–8. [DOI] [PubMed] [Google Scholar]
- Scheffler S, Dieken H, Krischenowski O, & Aufderheide M (2015). Cytotoxic Evaluation of e-Liquid Aerosol using Different Lung-Derived Cell Models. Int J Environ Res Public Health, 12, 12466–12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler S, Dieken H, Krischenowski O, Forster C, Branscheid D, & Aufderheide M (2015). Evaluation of E-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells. Int J Environ Res Public Health, 12, 3915–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober W, Szendrei K, Matzen W, Osiander-Fuchs H, Heitmann D, Schettgen T, Jorres RA, & Fromme H (2014). Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health, 217, 628–637. [DOI] [PubMed] [Google Scholar]
- Schripp T, Markewitz D, Uhde E, & Salthammer T (2013). Does e-cigarette consumption cause passive vaping? Indoor Air, 23, 25–31. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Hemminiki K, & Hopf NB (1994). Propylene Oxide (PO), IARC Monographs. In I. A. f. R. o. Cancer (Ed.), (Vol. 60): World Health Agency. [Google Scholar]
- Schweitzer KS, Chen SX, Law S, Van Demark M, Poirier C, Justice MJ, Hubbard WC, Kim ES, Lai X, Wang M, Kranz WD, Carroll CJ, Ray BD, Bittman R, Goodpaster J, & Petrache I (2015). Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am J Physiol Lung Cell Mol Physiol, 309, L175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A, Lugg ST, Aldridge K, Lewis KE, Bowden A, Mahida RY, Grudzinska FS, Dosanjh D, Parekh D, Foronjy R, Sapey E, Naidu B, & Thickett DR (2018). Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax, 73, 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo AD, Kim DC, Yu HJ, & Kang MJ (2016). Accidental ingestion of E-cigarette liquid nicotine in a 15-month-old child: an infant mortality case of nicotine intoxication. Korean J Pediatr, 59, 490–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM, Lopez B, Nathan D, Wilson J, Bankole E, Tumoyan H, Munoz A, Espinoza-Derout J, Hasan KM, Chang S, Du C, Sinha-Hikim AP, Lutfy K, & Friedman TC (2019). A mouse model for chronic intermittent electronic cigarette exposure exhibits nicotine pharmacokinetics resembling human vapers. J Neurosci Methods, 326, 108376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood CL, & Boitano S (2016). Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respir Res, 17, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Fan X, Horton A, Haller ST, Kennedy DJ, Schiefer IT, Dworkin L, Cooper CJ, & Tian J (2019). The Effect of Electronic-Cigarette Vaping on Cardiac Function and Angiogenesis in Mice. Sci Rep, 9, 4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiman M, Logue JM, Montesinos VN, Russell ML, Litter MI, Gundel LA, & Destaillats H (2016). Emissions from Electronic Cigarettes: Key Parameters Affecting the Release of Harmful Chemicals. Environ Sci Technol, 50, 9644–9651. [DOI] [PubMed] [Google Scholar]
- Smith D, Aherrera A, Lopez A, Neptune E, Winickoff JP, Klein JD, Chen G, Lazarus P, Collaco JM, & McGrath-Morrow SA (2015). Adult Behavior in Male Mice Exposed to E-Cigarette Nicotine Vapors during Late Prenatal and Early Postnatal Life. PLoS One, 10, e0137953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solleti SK, Bhattacharya S, Ahmad A, Wang Q, Mereness J, Rangasamy T, & Mariani TJ (2017). MicroRNA expression profiling defines the impact of electronic cigarettes on human airway epithelial cells. Sci Rep, 7, 1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MA, Reisinger SA, Freudenheim JL, Brasky TM, Mathe EA, McElroy JP, Nickerson QA, Weng DY, Wewers MD, & Shields PG (2020). Effects of Electronic Cigarette Constituents on the Human Lung: A Pilot Clinical Trial. Cancer Prev Res (Phila), 13, 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaderna M, Addy PH, & D'Souza DC (2013). Spicing things up: synthetic cannabinoids. Psychopharmacology (Berl), 228, 525–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, Hayes E, & Vandrey R (2018). Acute Effects of Smoked and Vaporized Cannabis in Healthy Adults Who Infrequently Use Cannabis: A Crossover Trial. JAMA Netw Open, 1, e184841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton CA, & Halenar MJ (2018). Patterns and Correlates of Multiple Tobacco Product Use in the United States. Nicotine Tob Res, 20, S1–S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt MR, Salit J, Kaner RJ, Hollmann C, & Crystal RG (2018). Altered lung biology of healthy never smokers following acute inhalation of E-cigarettes. Respir Res, 19, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton K, Kwan LY, & Eaton DL (2018). Public Health Consequences of E-Cigarettes. [PubMed]
- Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim JH, Sudini K, Consolini N, Cormier SA, Lomnicki S, Hasan F, Pekosz A, & Biswal S (2015). Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One, 10, e0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwazono Y, Sand S, Vahter M, Filipsson AF, Skerfving S, Lidfeldt J, & Akesson A (2006). Benchmark dose for cadmium-induced renal effects in humans. Environ Health Perspect, 114, 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J, Wong ET, Titz B, Lee T, Wong SK, Low T, Lee KM, Zhang J, Kumar A, Schlage WK, Guedj E, Phillips B, Leroy P, Buettner A, Xiang Y, Martin F, Sewer A, Kuczaj A, Ivanov NV, Luettich K, Vanscheeuwijck P, Peitsch MC, & Hoeng J (2020). A 6-month systems toxicology inhalation study in ApoE(−/−) mice demonstrates reduced cardiovascular effects of E-vapor aerosols compared with cigarette smoke. Am J Physiol Heart Circ Physiol, 318, H604–H631. [DOI] [PubMed] [Google Scholar]
- Talih S, Salman R, Karaoghlanian N, El-Hellani A, Saliba N, Eissenberg T, & Shihadeh A (2017). "Juice Monsters": Sub-Ohm Vaping and Toxic Volatile Aldehyde Emissions. Chem Res Toxicol, 30, 1791–1793. [DOI] [PubMed] [Google Scholar]
- Tam J (2021). E-cigarette, combustible, and smokeless tobacco product use combinations among youth in the united states, 2014-2019. Addict Behav, 112, 106636. [DOI] [PubMed] [Google Scholar]
- Tang MS, Wu XR, Lee HW, Xia Y, Deng FM, Moreira AL, Chen LC, Huang WC, & Lepor H (2019). Electronic-cigarette smoke induces lung adenocarcinoma and bladder urothelial hyperplasia in mice. Proc Natl Acad Sci U S A, 116, 21727–21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayyarah R, & Long GA (2014). Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regul Toxicol Pharmacol, 70, 704–710. [DOI] [PubMed] [Google Scholar]
- Teasdale JE, Newby AC, Timpson NJ, Munafo MR, & White SJ (2016). Cigarette smoke but not electronic cigarette aerosol activates a stress response in human coronary artery endothelial cells in culture. Drug Alcohol Depend, 163, 256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakrar PD, Boyd KP, Swanson CP, Wideburg E, & Kumbhar SS (2020). E-cigarette, or vaping, product use-associated lung injury in adolescents: a review of imaging features. Pediatr Radiol, 50, 338–344 [DOI] [PubMed] [Google Scholar]
- Thorne D, Leverette R, Breheny D, Lloyd M, McEnaney S, Whitwell J, Clements J, Bombick B, & Gaca M (2019). Genotoxicity evaluation of tobacco and nicotine delivery products: Part One. Mouse lymphoma assay. Food Chem Toxicol, 132, 110584. [DOI] [PubMed] [Google Scholar]
- Thota D, & Latham E (2014). Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J Emerg Med, 47, 15–17. [DOI] [PubMed] [Google Scholar]
- Tierney PA, Karpinski CD, Brown JE, Luo W, & Pankow JF (2016). Flavour chemicals in electronic cigarette fluids. Tob Control, 25, e10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers KF, Phillips E, Gentzke AS, Tynan MA, & Neff LJ (2018). Prevalence of Cannabis Use in Electronic Cigarettes Among US Youth. JAMA Pediatr, 172, 1097–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L, Reif DM, St Mary L, Geier MC, Truong HD, & Tanguay RL (2014). Multidimensional in vivo hazard assessment using zebrafish. Toxicol Sci, 137, 212–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, & Behrakis PK (2012). Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest, 141, 1400–1406. [DOI] [PubMed] [Google Scholar]
- Vreeke S, Korzun T, Luo W, Jensen RP, Peyton DH, & Strongin RM (2018). Dihydroxyacetone levels in electronic cigarettes: Wick temperature and toxin formation. Aerosol Sci Technol, 52, 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Liu W, & Song W (2019). Toxicity assessment of electronic cigarettes. Inhal Toxicol, 31, 259–273. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang T, Johnston CJ, Kim SY, Gaffrey MJ, Chalupa D, Feng G, Qian WJ, McGraw MD, & Ansong C (2020). Protein thiol oxidation in the rat lung following e-cigarette exposure. Redox Biol, 37, 101758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Chen W, Liao J, Matsuo T, Ito K, Fowles J, Shusterman D, Mendell M, & Kumagai K (2017). A Device-Independent Evaluation of Carbonyl Emissions from Heated Electronic Cigarette Solvents. PLoS One, 12, e0169811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TW, Gentzke AS, Creamer MR, Cullen KA, Holder-Hayes E, Sawdey MD, Anic GM, Portnoy DB, Hu S, & Homa DM (2019). Tobacco product use and associated factors among middle and high school students—United States, 2019. MMWR Surveillance Summaries, 68, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TW, Neff LJ, Park-Lee E, Ren C, Cullen KA, & King BA (2020). E-cigarette Use Among Middle and High School Students — United States, 2020. CDC Morbidity and Mortality Weekly Report (MMWR), 69, 1310–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ween MP, Hamon R, Macowan MG, Thredgold L, Reynolds PN, & Hodge SJ (2020). Effects of E-cigarette E-liquid components on bronchial epithelial cells: Demonstration of dysfunctional efferocytosis. Respirology, 25, 620–628. [DOI] [PubMed] [Google Scholar]
- Ween MP, Whittall JJ, Hamon R, Reynolds PN, & Hodge SJ (2017). Phagocytosis and Inflammation: Exploring the effects of the components of E-cigarette vapor on macrophages. Physiol Rep, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner AS, Rudy SF, & Faulcon LM (2020). Comment on "Does vaping cause seizures? The need for comprehensive drug testing". Clin Toxicol (Phila), 58, 435–436. [DOI] [PubMed] [Google Scholar]
- Wetendorf M, Randall LT, Lemma MT, Hurr SH, Pawlak JB, Tarran R, Doerschuk CM, & Caron KM (2019). E-Cigarette Exposure Delays Implantation and Causes Reduced Weight Gain in Female Offspring Exposed In Utero. J Endocr Soc, 3, 1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington JR, Simmons PM, Phillips AM, Gammill SK, Cen R, Magann EF, & Cardenas VM (2018). The Use of Electronic Cigarettes in Pregnancy: A Review of the Literature. Obstet Gynecol Surv, 73, 544–549. [DOI] [PubMed] [Google Scholar]
- Wild CP, & Kleinjans J (2003). Children and increased susceptibility to environmental carcinogens: evidence or empathy? Cancer Epidemiol Biomarkers Prev, 12, 1389–1394. [PubMed] [Google Scholar]
- Wiley JL, Lefever TW, Glass M, & Thomas BF (2019). Do you feel it now? Route of administration and Delta(9)-tetrahydrocannabinol-like discriminative stimulus effects of synthetic cannabinoids in mice. Neurotoxicology, 73, 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Bozhilov K, Ghai S, & Talbot P (2017). Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS One, 12, e0175430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, & Talbot P (2019). Design Features in Multiple Generations of Electronic Cigarette Atomizers. Int J Environ Res Public Health, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, To A, Bozhilov K, & Talbot P (2015). Strategies to Reduce Tin and Other Metals in Electronic Cigarette Aerosol. PLoS One, 10, e0138933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KU, & Baum CR (2019). Acute Cannabis Toxicity. Pediatr Emerg Care, 35, 799–804. [DOI] [PubMed] [Google Scholar]
- Wu D, & O'Shea DF (2020). Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate. Proc Natl Acad Sci U S A, 117, 6349–6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Jiang D, Minor M, & Chu HW (2014). Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One, 9, e108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V, Rahimy M, Korrapati A, Xuan Y, Zou AE, Krishnan AR, Tsui T, Aguilera JA, Advani S, Crotty Alexander LE, Brumund KT, Wang-Rodriguez J, & Ongkeko WM (2016). Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol, 52, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Cross SJ, Loughlin SE, & Leslie FM (2015). Nicotine and the adolescent brain. J Physiol, 593, 3397–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikoff JT, Parmalee NL, Corbett K, Gordon T, Klein CB, & Aschner M (2018). Microglia Activation and Gene Expression Alteration of Neurotrophins in the Hippocampus Following Early-Life Exposure to E-Cigarette Aerosols in a Murine Model. Toxicol Sci, 162, 276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettler, Hemmerich, & Berman. (2019). Closing the Regulatory Gap for Synthetic Nicotine Products. Boston College Law Review. [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Aravindakshan A, Hilpert M, Olmedo P, Rule AM, Navas-Acien A, & Aherrera A (2020). Metal/Metalloid Levels in Electronic Cigarette Liquids, Aerosols, and Human Biosamples: A Systematic Review. Environ Health Perspect, 128, 36001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Navas-Acien A, Ilievski V, Slavkovich V, Olmedo P, Adria-Mora B, Domingo-Relloso A, Aherrera A, Kleiman NJ, Rule AM, & Hilpert M (2019). Metal concentrations in electronic cigarette aerosol: Effect of open-system and closed-system devices and power settings. Environ Res, 174, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Nelson J, Dada O, Pyrgiotakis G, Kavouras IG, & Demokritou P (2018). Assessing electronic cigarette emissions: linking physico-chemical properties to product brand, e-liquid flavoring additives, operational voltage and user puffing patterns. Inhal Toxicol, 30, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]


