Abstract
Purpose
As a ClinGen Expert Panel (EP) we set out to adapt the ACMG pathogenicity criteria for classification of RYR1 variants as related to autosomal dominantly-inherited malignant hyperthermia (MH).
Methods
We specified ACMG/AMP criteria for variant classification for RYR1 and MH. Proposed rules were piloted on 84 variants. We applied quantitative evidence calibration for several criteria using likelihood ratios based on the Bayesian framework.
Results
Seven ACMG/AMP criteria were adopted without changes, nine were adopted with RYR1-specific modifications, and ten were dropped. The in silico (PP3 and BP4) and hot spot criteria (PM1) were evaluated quantitatively. REVEL gave an odds ratio (OR) of 23:1 for PP3 and 14:1 for BP4 using trichotomized cut-offs of ≥0.85 (pathogenic) and ≤0.5 (benign). The PM1 hotspot criterion had an OR of 24:1. PP3 and PM1 were implemented at moderate strength. Applying the revised ACMG criteria to 44 recognized MH variants, 29 were classified as pathogenic, 13 as likely pathogenic, and two as variants of uncertain significance.
Conclusion
Curation of these variants will facilitate classification of RYR1/MH genomic testing results, which is especially important for secondary findings analyses. Our approach to quantitatively calibrating criteria is generalizable to other variant curation expert panels.
INTRODUCTION
Malignant hyperthermia susceptibility (MHS) is a potentially lethal inherited disorder of skeletal muscle calcium signaling, predisposing individuals to a hypermetabolic reaction triggered by exposure to inhalational anesthetics or depolarizing muscle relaxants such as succinylcholine.1,2 Inheritance of MHS is predominantly autosomal dominant, although autosomal recessive inheritance has been reported3 and non-Mendelian models proposed.4 Variants in RYR1 (MIM:180901; MHS1, MIM:145600) and CACNA1S (MIM:114208; MHS5, MIM:601887) have been associated with MH, and both genes are in the American College of Medical Genetics and Genomics (ACMG) return of secondary findings recommendations.5,6 RYR1 variants account for ~76% of MH events while ~1%7 are attributable to CACNA1S and <1% are attributable to STAC3 (MIM:615521; Bailey-Bloch myopathy, MIM:255995). Four additional loci have been mapped (MHS2, MIM:154275; MHS3, MIM:154276; MHS4, MIM:600467; MHS6, MIM:601888). RYR1 has a complex gene-to-phenotype relationship, being associated with several apparently distinct disorders and both autosomal dominant and autosomal recessive inheritance. Overlapping conditions include central core disease (CCD, MIM:117000) and King-Denborough syndrome (MIM:145600) and individuals with these disorders may be at risk for MH. Generally, these disorders result from monoallelic RYR1 variants while biallelic variants cause other myopathies, however, this correlation is evolving.8
Classification of RYR1 variants is complicated by variable expressivity, reduced penetrance, and high alleleic heterogeneity. While the European Malignant Hyperthermia Group (EMHG; http://www.emhg.org/home/) has assessed 48 RYR1 variants as diagnostic of MHS, over 165 additional variants have been reported as disease mutations/pathogenic/likely pathogenic for MH in the literature and databases including HGMD9 and ClinVar.10 While the ACMG/AMP guidelines provided general criteria that can be used to classify variants, many of the criteria require adaptation to be accurately applied. As part of ClinGen, we convened an RYR1-related Malignant Hyperthermia variant curation expert panel (https://clinicalgenome.org/affiliation/50038/) to adapt the general ACMG/AMP pathogenicity guidelines to autosomal dominantly inherited RYR1/MH, with gene-specific recommendations, to improve classification of RYR1 variant pathogenicity.
We first reviewed each ACMG/AMP criterion to determine applicability to autosomal dominantly inherited RYR1/MH and then adapted them with gene/disease specific guidelines, if appropriate. We piloted these guidelines on 84 variants, 44 variants from the EMHG list of “diagnostic mutations” and 40 variants with MH pathogenicity classifications in ClinVar.
METHODS
ClinGen’s RYR1/MH Expert Panel
The RYR1/MH expert panel (EP) is composed of clinical molecular geneticists, clinical geneticists, anesthesiologists, biochemists, and physiologists to provide a balance of expertise relevant to RYR1 variant classification. The RYR1/MH EP met monthly via conference calls over a two-year period.
Evaluation and Adaptation of the ACMG Pathogenicity Guidelines
The general ACMG/AMP pathogenicity guidelines were evaluated for relevance to autosomal dominantly-inherited RYR1/MH and non-relevant criteria were dropped. ClinGen-recommended amendments to the criteria were incorporated when applicable. Lastly, applicable criteria were further assessed to determine if gene-specific recommendations were warranted. Proposed changes were discussed amongst the full EP by emails and conference calls. Approval of revised rules required consensus of the full EP. Draft rules were piloted on a subset of RYR1 variants representing the EMHG “diagnostic mutation” list. Individual panel members scored variants using the draft guidelines and variant classifications were presented to the full panel. Areas of disagreement were used to refine the draft guidelines. Per the ClinGen FDA-approved process, rules were reviewed by the ClinGen Sequence Variant Interpretation (SVI) committee (LGB recused).
Data Collection Methods
Population data was ascertained from gnomAD v2.1.1.11 REVEL scores (v0.19.1) were used for bioinformatic predictions for single nucleotide variants (SNVs).12 The literature was searched for relevant data including case information and functional data. For case information, the number of unrelated probands with either a personal or family history of an MH event was recorded (see supplemental information). Care was taken to avoid double counting cases reported multiple times. Reports were examined for instances of de novo inheritance and/or segregation.
Pathogenicity Assessment
Revised ACMG/AMP criteria were used to assess 44 EMHG MH “diagnostic mutations”. Four of 48 EMHG variants were excluded because they were associated with RYR1-related myopathies and not MH. An additional 40 ClinVar RYR1 variants were also classified. Individual criteria were weighted based on available evidence and weighted criteria were combined using the Bayesian framework for variant scoring.13
RESULTS AND DISCUSSION
The ACMG/AMP guidelines are generic and broadly useful for all Mendelian genes and disorders. These generic rules may over- or under-estimate evidence for any specific gene and must be adapted for specific implementations. As an EP, we suggest guidelines to be used/dropped, guidelines to be refined, and weight adjustments where appropriate. A summary of revised guidelines is in Table 1 and a full description is in Table S1 with gene/disease specific adaptations highlighted below (updated versions will be maintained at clinicalgenome.org).
Table 1.
Modified ACMG criteria suggested for autosomal dominantly inherited RYR1/MH.a
| Criteria | Criteria Description | Specification | Specifying Group |
|---|---|---|---|
| Pathogenic Criteria | |||
| VERY STRONG CRITERIA | |||
| PS2/PM6_Very Strong | Each proven de novo occurrence, 2 points, each assumed de novo occurrence, 1 point, ≥8 points | Strengthb | SVId |
| STRONG CRITERIA | |||
| PS1 | Same amino acid change as a previously established pathogenic variant regardless of nucleotide change
|
None | |
| PS2/PM6_Strong | Each proven de novo occurrence, 2 points, each assumed de novo occurrence, 1 point, a total of 4-7 points | Strengthb | SVId |
| PS3 | Well-established functional studies supportive of a damaging effect on protein function
|
Strengthb, Disease-Specific | RYR1/MHS EP |
| PS4 | The prevalence of the variant in affected individuals significantly increased compared with the prevalence in controls
|
Strengthb, Disease-Specific | RYR1/MHS EP |
| PP1_Strong |
|
Strengthb | CMP EPe |
| MODERATE CRITERIA | |||
| PM1 | Located in a mutational hot spot and/or critical and well established functional domain
|
Disease-Specific | RYR1/MHS EP |
| PM5 | Missense change at an amino acid residue where a different missense varaint previously determined to be pathogenic
|
None | RYR1/MHS EP |
| PS2/PM6_Moderate | Each proven de novo occurrence, 2 points, each assumed de novo occurrence, 1 point, a total of 2-3 points | Strengthb | SVId |
| PS3_Moderate | Well-established functional studies supportive of a damaging effect on protein function
|
Strengthb, Disease-Specific | RYR1/MHS EP |
| PS4_Moderate | The prevalence of the variant in affected individuals is significantly increased compared with the prevalence in controls
|
Strengthb, Disease-Specific | RYR1/MHS EP |
| PP1_Moderate |
|
Strengthb | CMP EPe |
| PP3_Moderate | Multiple lines of computational evidence support a deleterious effect on the gene or gene product
|
Strengthb | RYR1/MHS EP |
| SUPPORTING CRITERIA | |||
| PP1 | Co-segregation with disease in 3-4 reported meioses | Strengthb | CMP EPe |
| PS2/PM6_Supporting | Each proven de novo occurrence, 2 points, each assumed de novo occurrence, 1 point, a total of 1 point | Strengthb | SVId |
| PS3_Supporting | Well-established functional studies studies supportive of a damaging effect on protein function
|
Strengthb, Disease-Specific | RYR1/MHS EP |
| PS4_Supporting | The prevalence of the variant in affected individuals is significantly increased compared with the prevalence in controls
For variants with popmax MAF in gnomAD >0.00006, an odds ratio of ≥2.08 when comparing MH case points to allele count in gnomAD can qualify. Popmax in gnomAD must be <0.0038 |
Strengthb, Disease-Specific | RYR1/MHS EP |
| PM1_Supporting | Located in a mutational hot spot and/or critical and well established functional domain
|
Strengthb, Disease-Specific | RYR1/MHS EP |
| BENIGN CRITERIA | |||
| STAND ALONE CRITERION | |||
| BA1 | Popmax allele frequency >0.0038 (0.38%) | Disease-Specific | RYR1/MHS EP |
| STRONG CRITERIA | |||
| BS1 | Popmax allele frequency >0.0008 (0.08%) | Disease-Specific | RYR1/MHS EP |
| BS2 | Observed in a healthy adult individual for a recessive (homozygous), dominant (heterozygous), or X-linked (hemizygous) disorder with full penetrance expected at an early age.
|
Disease-Specific | RYR1/MHS EP |
| MODERATE CRITERIA | |||
| BS2_Moderate | Observed in a healthy adult individual for a recessive (homozygous), dominant (heterozygous), or X-linked (hemizygous) disorder with full penetrance expected at an early age.
|
Strengthb, Disease-Specific | RYR1/MHS EP |
| BS3_Moderate | Well-established functional studies show no damaging effect on protein function
|
Strengthb, Disease-Specific | RYR1/MHS EP |
| SUPPORTING CRITERIA | |||
| BP2 | Observed in cis with a pathogenic variant in any inheritance pattern | None | RYR1/MHS EP |
| BP4 | Computational evidence suggest no impact on gene or gene product, REVEL score of ≤0.5 | Disease-Specific | RYR1/MHS EP |
| BP7 | A synonymous (silent) variant for which splicing prediction algorithms predict no impact to the splice consensus sequence nor the creation of a new splice site AND the nucleotide is not highly conserved | None | |
| BS3_Supporting | Well-established functional studies studies show no damaging effect on protein function
|
Strengthb, Disease-Specific | RYR1/MHS EP |
Table S6 presents this information grouped by criteria rather than by strength, this supplemental table may be more useful in laboratory practice.
Key: Disease-Specific, Disease-specific modifications based on what is known about MHS; Strength, Increasing or decreasing strength of criteria based on the amount of evidence; N/A: not applicable for MHS; None, no changes made to existing criteria definitions; IVCT, in vitro contracture test; CHCT, caffeine-halothane contracture test; MH, Malignant hyperthermia; MHS, MH susceptibility.
For criteria that can be assigned different levels of strength based on evidence, only the highest applicable strength level should be used. For example, if PS4 is met, then PS4_Moderate and PS4_Supporting are not used.
Positive family history defined by variant positive family member with MH reaction and/or positive IVCT/CHCT.
Sequence Variant Interpretation committee, ClinGen.
Cardiomyopathy Expert Panel.14
Criteria Dropped for MH/RYR1: PVS1/PM2/PM3/PM4/PP2/PP4/BS4/BP1/BP3/BP5
These criteria were dropped based on the biology of MH/RYR1. See supplemental information for details.
Criteria Used According to General Guidelines: PS1/PS2/PM5/PM6/PP1/BP2/BP7
These criteria were retained in the RYR1/MH-specific guidelines including adaptations as recommended by the ClinGen SVI committee (PS2/PM6, weighting of de novo observations, https://clinicalgenome.org/site/assets/files/3461/svi_proposal_for_de_novo_criteria_v1_0.pdf) and the Cardiomyopathy EP (PP1, weighting segregation events).14 We made further modifications to the ACMG/AMP criteria, which may not be specific to RYR1/MH. The PS1 (same amino acid change, different nucleotide change) and PM5 (different amino acid change, same codon) criteria were modified such that in order to use either of them, a previously classified variant must have been classified as pathogenic without the use of PS1 or PM5. Furthermore, for PM5, we added a requirement that the Grantham score difference compared to reference of the new variant must be greater than that for the previously identified pathogenic variant compared to reference. For criterion BP2 (evidence against pathogenicity based on presence of known pathogenic variant) it is suggested that only variants identified in cis with the variant under review be considered. Because the occurrence of biallelic pathogenic RYR1 variants has been described in MHS,3,15 two variants in trans is not considered evidence against pathogenicity. Finally, as RYR1/MH primarily results from missense alterations, BP7 (synonymous variant without splicing effect) is used as recommended.
Criteria Specified for RYR1/MH: BA1/BS1/PS4/BS2/PS3/BS3/PM1/PP3/BP4
Allele Frequency Specificiations: BA1/BS1/PS4
BA1 and BS1 use minor allele frequencies (MAF) in population datasets to support benign classification for common variants. The BA1 criterion is considered stand alone and was originally set to 0.05 (5%) MAF.16 It has been suggested that BA1 can be defined as the combined MAF for all pathogenic variants in the population for the gene/disease dyad with the understanding that any one variant should have a lower MAF than the combined total. To determine a gene/disease-specific cutoff for BA1, disease prevalence, penetrance, and gene contribution need to be considered. This can be estimated by the formula: .14 The prevalence of MH (defining the disorder as MH, not MHS) in the population can be estimated using the frequency of MH events in individuals exposed to triggering agents. The frequency of events is as high as 1/10,000 pediatric anesthesias.2 The rate of adult MH events seems lower than that of children17 but the underlying genetic risk is assumed to be the same. The gene contribution of RYR1 to MH is ~76% depending on ethnicity.7 Calculating thresholds for BA1 relies on an accurate estimate of penetrance, which is difficult to determine for MHS.18 In lieu of using an estimate for MHS penetrance, we instead substituted a value of 1%, as it is a reasonable boundary between the penetrance of a Mendelian disorder variant and that of a risk allele. This value is nearly certain to be lower than the actual penetrance of MHS, but underestimating this value is conservative with respect to the outcome in that it will numerically raise BA1, which would lead to fewer variants being classified as benign based on this single criterion. Using 0.01 to adjust our calculated BA1 allows for a BA1 MAF of 0.0038 (0.38%).
In addition to a stand alone MAF (BA1), BS1 defines the MAF at which a variant is considered to have strong evidence against pathogenicity. The field has been moving to define BS1 based on the contribution of the most common pathogenic allele for a disorder. For RYR1/MH, we calculated BS1 considering the frequency of MH reactions in children (1/10,000) a value of 0.01 substituted for penetrance (as explained above), and a maximum individual allele contribution of 16% (variant c.7300G>A was identified in 118/722 MH families, 16.3%).7 Correcting for alleles/person gives a BS1 value of 0.0008 (0.08%).
While a high MAF of a variant in controls can be used to refute pathogenicity, criterion PM2 gives weight for absence or very low frequency in control populations. Based on observations that the majority of possible RYR1 missense variants (~30,000 variants) are not represented in gnomAD v2.1.1 (2,800 RYR1 missense variants) and many known pathogenic variants (classified without the use of PM2) are present in gnomAD, it is unlikely that the absence of a variant in gnomAD is support for pathogenicity. While the absence or low frequency of a variant in gnomAD has little value alone, it is important in weighting PS4. PS4 takes into consideration the prevalence of the variant in affected individuals compared to controls. For RYR1/MH, we modified the PS4 criterion using a point system, awarding 0.5 case points for each unrelated proband reported to have undergone an MH event and awarding an additional 0.5 case points for a positive in vitro contracture test (IVCT) or caffeine-halothane contracture test (CHCT) in either the proband or a variant-positive family member. The strength level of PS4 is based on odds ratios comparing total case points, an approximation of the total number of cases of MH investigated in the literature (3,000) and the number of alleles in the gnomAD continental population with the highest MAF (popmax). When the popmax MAF is ≤0.00006 (~7/113,000 alleles), strength levels are awarded according to the following system: PS4 for ≥7 MH case points; PS4_Mod for 2-6 MH case points; and PS4_Sup for one MH case point. When gnomAD popmax MAF is >0.00006, case points can be counted and compared to alleles in the gnomAD population with the highest MAF by calculating an odds ratio (OR, MedCalcs online calculator (https://www.medcalc.org/calc/odds_ratio.php), awarding PS4 for an OR ≥18.7; PS4_Mod for an OR ≥4.33; and PS4_Sup for an OR ≥2.08. Every effort needs to be made to avoid double counting of cases reported in multiple studies. The Bayesian framework for the classification of variants using the ACMG/AMP criteria was used to set the OR value for each strength level.13
Disease-Specific Phenotype: BS2
The IVCT/CHCT diagnostic tests have low false negative rates19,20 and can be used to determine MHS status in individuals who carry RYR1 variants. A negative IVCT or CHCT result supports benign status. Two or more unrelated individuals with a negative result allow BS2 to be applied. One individual with a negative result allows BS2_Mod.
Functional Assay Specifications: PS3/BS3
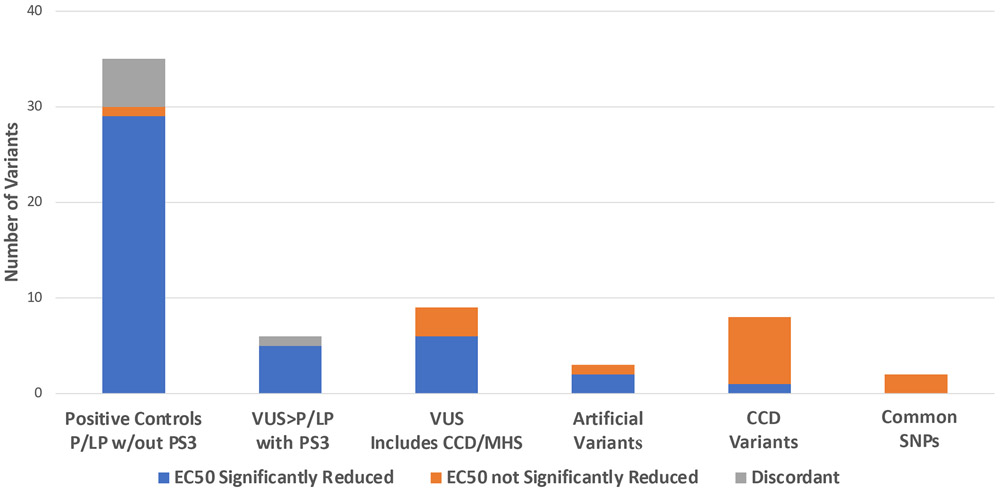
Functional characterization is considered a crucial determinant of the pathogenicity of RYR1 variants.21 Within the ACMG/AMP guidelines, functional assay results are used for PS3 (well-established in vitro or in vivo functional studies supportive of a damaging effect) and BS3 (well-established in vitro or in vivo functional studies show no damaging effect on protein function or splicing). RYR1 is a homotetrameric calcium channel in the sarcoplasmic reticulum (SR) of skeletal muscle important in excitation-contraction coupling. Volatile anesthetics and depolarizing muscle relaxants can cause increased release of SR calcium in a dysfunctional RYR1 channel resulting in MH. When considering functional assays for variant assessment it is desirable to identify assays that are closely related to the physiologic defect causative of disease. For RYR1/MH, assays that measure release of calcium in response to pharmacologic agents are considered good representations of the disease mechanism. Well-recognized assays include transfection of RYR1 cDNA into HEK293 cells, CHO cells, or RYR1 knockout myotubes followed by SR calcium release measurements in response to caffeine, halothane, voltage/potassium, or 4-chloro-m-cresol. A significant decrease in the EC50 for the sensitivity of calcium release compared to wildtype RYR1, is considered evidence for pathogenicity. Multiple replicates for each variant within a single instance of the assay are necessary to determine significance of these values. Positive (pathogenic) and negative (benign) controls support that the assay categorizes the variants accurately. For the purpose of assessing RYR1 transfection studies to weight PS3, results were dichotomized into pathogenic EC50 values that are significantly decreased as compared to WT versus benign EC50 values that are not significantly decreased. For RYR1 pathogenicity assessment, the whole of prior published work (Figure 1, Table S2)22 allows us to consider transfection assays in HEK293 cells using photometry/imaging to measure calcium release a well defined functional test. However, recommendations for increased stringency in analyses of functional data have recently been suggested.23 To determine the appropriate PS3 weight based on HEK293 transfection assays we have considered published results including results for 35 variants assessed to be likely pathogenic or pathogenic (LP/P) without the use of functional data, and ten control variants including eight variants associated with CCD and two common variants. Of the 35 LP/P variants, 29 have been shown to reduce the calcium release EC50 in response to RYR1 agonsits. Five variants have shown discordant results across assays, and one variant has shown an EC50 increase. Of the ten control variants, one variant has shown an EC50 reduction in response to agonist and nine variants have either shown no response to agonist (6) or a response similar to WT (3). This set of variants suggests a likelihood ratio for an EC50 reduction of 9.11:1 with a 95% confidence interval of 1.4:1 to 59:1. This level of support is above the threshold for moderate evidence (4.33:1 odds). We suggest that functional evidence supporting pathogenicity from HEK293 cells be used at the level of moderate. When the field generates additional data for control variants the weighting of PS3 for this assay should be reconsidered.
Figure 1.
Cumulative HEK293 transfection assay data for RYR1 variants from the literature. Variants are grouped according to pathogenicity assessment without consideration of PS3/BS3 (functional data).
While positive evidence (reduced EC50) is considered moderate support for pathogenicity, reduced penetrance and the limitations of expression systems,24 suggest a non-significant change in EC50 values may not support benign status at a moderate level. It was decided that lack of response to agonists be weighted as supporting evidence, BS3_Sup. Regarding other in vitro assays that test calcium release in response to agonists, where historical data were limited, we suggest that multiple controls be run in parallel and statistical analyses be used to determine the level of strength for PS3 according to the Bayesian framework.
In addition to in vitro assays, the RYR1/MH field has established ex vivo assays measuring calcium release in patient cells. These assays do not isolate the RYR1 variant from other potential variants (in RYR1, CACNA1S, or other MHS-associated genes), which may affect calcium release. Rather, these assays are a measure of the cellular phenotype in the patient. Although we recognize this limitation of ex vivo studies, we also recognize that they have utility. As the main concern for such assays is the potential presence of other variants, this concern is mitigated if multiple unrelated individuals with the same primary variant are shown to exhibit enhanced ex vivo sensitivity to agonist. Two unrelated individuals with ex vivo tests showing increased sensitivity of calcium release in response to agonist allow PS3_Sup. For variants where ≥3 unrelated individuals had ex vivo tests showing increased sensitivity of calcium release, PS3_Mod can be applied. Ex vivo tests that do not show increased sensitivity of calcium release in response to agonist (negative result) support a benign classification of the variant. BS3_Sup can be applied if one or two unrelated individuals are tested with negative results, when ≥3 unrelated individuals are tested and all results are negative BS3_Mod can be applied.
Knock-in mouse models created to date to test RYR1 variants have shown MH reactions in response to volatile anesthetic and ex vivo studies of muscle samples from these mice show increased ligand sensitivity of calcium release as compared to WT.25-28 When knock-in mice have an MH reaction in response to agonist, and where ex vivo studies show increased calcium release as compared to WT in response to agonist, PS3 can be awarded. For mouse models where either an MH crisis can be triggered by agonist or ex vivo assays show increased calcium release, but both conditions are not met, PS3_Mod can be awarded. For mouse models that do not exhibit an MH reaction when exposed to agonist and ex vivo studies do not show increased release of calcium, BS3_Sup can be awarded.
Hotspot Specifications: PM1
The ACMG/AMP criteria includes moderate weight for variation in critical protein domains or mutational hotspots, PM1. While critical domains may be well-defined for a protein, the concept of mutational hotspots is less clearly defined. A general rule for consideration of a mutational hotspot would be an excess of pathogenic variation as compared to benign variation. In MH, variants have been noted to cluster in three regions of RYR1 identified as “hotspots” historically: the N-terminal region (residues 1-552), the central region (residues 2,101-2,458) and the C-terminal region (4,631-4,991).29 Rather than defining clear functional domains, these regions are defined by an increase in variation identified in individuals with MH. We assessed this criterion using a test set of 19 variants (Table S3) assessed to be pathogenic for MH without the use of PM1 and 27 benign variants (Table S4) that met criterion BA1. This set of variants suggests a likelihood ratio for hotspots of 24:1 with a 95% confidence interval of 3.4:1 to 163:1 (Table 2). This level of support is above the threshold for strong evidence (18.7:1 odds) and the lower bound of that confidence interval is above supporting (2.1:1). This would suggest that PM1 could be modified to PM1_strong. However, because there is a significant bias in the literature toward identifying pathogenic variants in the hotspots, and to avoid the possibility of overestimating pathogenicity, we suggest using PM1 at its default level of moderate for variants in the N-terminal and central regions. As variants in the C-terminal region may be associated with CCD and not cause MH, we suggest using PM1_supporting for variants in this region. Future studies that interrogate the gene without these biases should provide additional data on the positional skewing of pathogenic variants, which could allow upgrading PM1 to strong in the future.
Table 2.
Distribution of 19 pathogenic and 27 benign variants in relation to position of defined RYR1/MH hotspots. Likelihood ratios calculated based on distribution.
| Presence in HotSpot |
Pathogenic | Benign | Likelihood ratio (LR) | Inverse LR | 95% CI (inverse) |
|---|---|---|---|---|---|
| HotSpot (1-552; 2101-2458; 4631-4991) | 16 | 1a | 23.58 | 3.41-163.18 | |
| Non-HotSpot | 3 | 27 | 0.164 | 6.10 | 0.06-0.46 (2.17-16.7) |
No benign variants were identified in the hotspot regions, for calculation of LR we used a value of 1.
Computational Evidence: PP3/BP4
The PP3 and BP4 criteria consider computational evidence estimating the impact of a variant on protein function. REVEL is an ensemble method based on a number of individual tools and precomputed scores are available for all missense variants (https://omictools.com/revel-tool).12 Importantly, REVEL does not consider population frequency, which reduces double counting of evidence. Using a set of 20 pathogenic variants determined to be pathogenic without the use of PP3 and 27 benign variants described above, we tested the likelihood ratios of the predictive power of REVEL in several iterations. We settled on a trichotomization of scores with PP3, (computational evidence supporting pathogenicity), requiring a REVEL score of ≥0.85 and BP4, (computational evidence against pathogenicity), requiring a REVEL score of ≤0.5 (Table 3). Based on the Bayesian model for weighting criteria, these results suggest that PP3 and BP4 could be employed at the strong level. However, based on wide confidence intervals of the likelihood ratios for this conditional probability, we chose to weight PP3 as moderate and BP4 as supporting.13 Based on piloting these criteria it was determined that BP4 should only be implemented with other criteria. Using the Bayesian framework, BP4 in isolation results in an assessment of likely benign (LB) and it was determined that additional evidence should be available for a LB classification. For a fuller explanation of deriving such likelihood ratios, see Supplemental information.
Table 3.
REVEL score distribution for 20 pathogenic and 27 benign variants for RYR1/MH. Likelihood ratio for separation of pathogenic and benign variants based on REVEL scores using cutoff values of ≥0.85 and ≤0.5.
| REVEL score | Pathogenic | Benign | Likelihood ratio (LR) |
Inverse LR | 95% CI |
|---|---|---|---|---|---|
| ≥0.85 | 17 | 1a | 22.68 | 3.27-157.08 | |
| >0.5-<0.85 | 3 | 8 | 0.50 | 2.00 | 0.15-1.66 |
| ≤0.5 | 1a | 19 | 0.07 | 14.29 | 0.01-0.48 |
No benign variants were identified with a REVEL score ≥0.85 and no pathogenic variants were identified with a REVEL score ≤0.5, for calculation of LR we used a value of 1.
Piloting RYR1/MH Classification Criteria
We applied these modified criteria to 44 variants EMHG determined to be “diagnostic mutations” and 40 RYR1 variants with pathogenicity classifications for MH in ClinVar. The classification of each of the variants is shown in Table S3 and Table S5. Of the 44 EMHG variants, we classified 29 as P, 13 as LP, and two as variants of uncertain significance (VUS). Variant c.1589G>A p.(Arg530His) was classified as VUS and had limited functional data including a single ex vivo sample30, which did not meet PS3_Sup based on the requirement for a minimum of two unrelated individuals. Variant c.1598G>A p.(Arg533His) was classified as VUS based on functional data (PS3_Mod) and presence in a hotspot (PM1). PS4 was not met by this variant based on a high allele count (32 alleles) in gnomAD.
The revised criteria were applied to 40 additional variants with pathogenicity classifications for MH in ClinVar. Ten variants had conflicting pathogenicity classifications for MH (pathogenicity classifications for disorders other than MH were not considered), nine B/LB/VUS and one P/LP/VUS. Five variants with B/LB/VUS classifications in ClinVar were determined to be B/LB based on BA1/BS1. The remaining five discordant variants were classified as VUS. Of the remaining 30 variants, 14 were classified as P/LP, 11 as B/LB and five as VUS. Applying the revised ACMG criteria 12/14 variants with a classification of P/LP in ClinVar and 3/11 variants with an classification of B/LB in ClinVar were classified as VUS. All variants classified as B/LB (13) using our criteria had ether BA1 or BS1 applied. The 19/24 variants classified as VUS had limited data, only five VUS variants had data that refuted pathogenicity (5/24, 21%).
CONCLUSIONS
As a ClinGen expert panel, we set out to adapt the ACMG pathogenicity criteria for classification of RYR1 variants as related to autosomal dominanty inherited MH. Combining expertise of anesthesiologists, physiologists, biochemists, and geneticists allowed for a thorough evaluation of factors that should be considered. It is also important to recognize that we successfully unified the efforts of the American-based ACMG/AMP criteria with the extensive expertise and experience of the European Malignant Hyperthermia Group, benefiting from both. In revising these guidelines, we have considered the statistical evidence weight as it relates to the Bayesian adaptation of the ACMG scoring system. Weighting of evidence using statistical measures should allow for a more robust and consistent pathogenicity classification framework and is broadly applicable to other disease/gene systems. The revised RYR1/MHS specific criteria should allow clinical laboratories to more consistently classify these variants based on expert guidelines and should increase the consistency of classifications, as has been demonstrated for the generic ACMG/AMP pathogenicity recommendations.31 These recommendations should be especially useful to laboratories that classify RYR1 variants as secondary findings. That MH is a pharmacogenetic trait with relatively low penetrance makes it especially challenging to classify for laboratories that do not peform a high volume of diagnostic RYR1 testing. The availability of three star ClinGen classifications in ClinVar should significantly reduce the amount of time that secondary findings evaluations consume. As well, the RYR1/MH expert panel will continue to curate variants and deposit classifications into ClinVar. Moving forward, the field should strive to increase relevant data through functional studies and shared case documentation allowing variants to move from a classification of VUS to either LB/B or LP/P. Beyond secondary findings, ClinGen classifications of RYR1 variant pathogenicity will allow the field to consider pre-surgicial screening of patients toward elimination of MH morbidity and mortality.32
Supplementary Material
Figure 2.
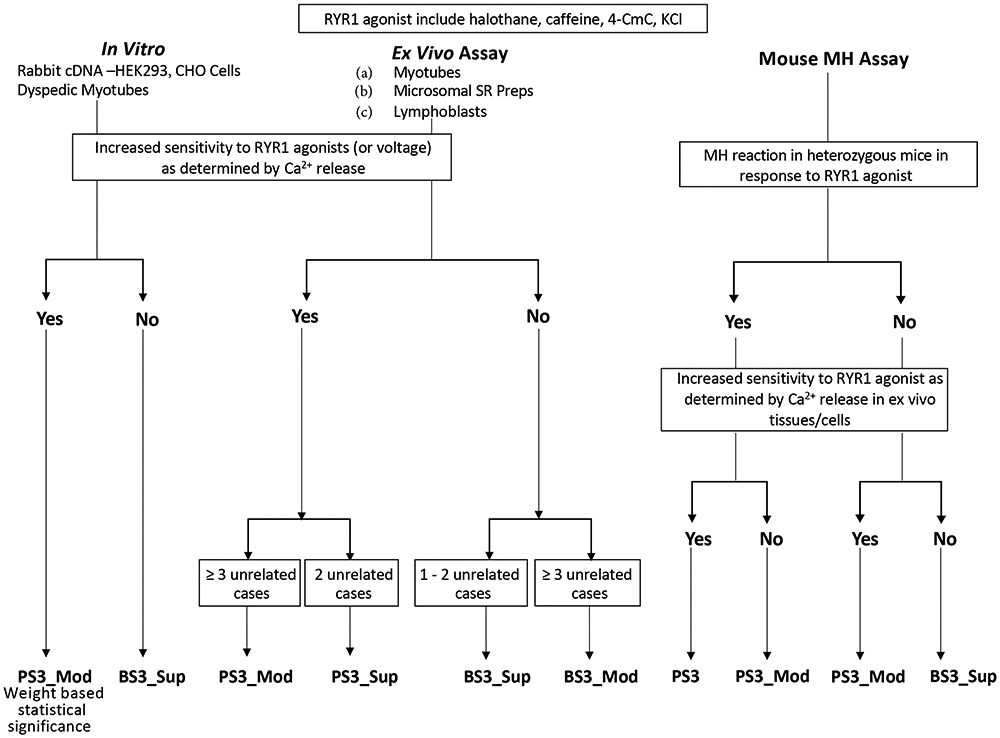
Decision tree for weighting functional evidence PS3/BS3.
ACKNOWLEDGEMENTS
ClinGen is funded by the National Human Genome Research Institute: U41HG006834, U41HG009649, U41HG009650. ClinGen receives support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development: U24HD093483, U24HD093486, U24HD093487. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. JJJ and LGB were supported by NIH grant HG200359-12. RTD is supported by grant R01 AR053349. PH is supported by the National Institute of Arthritis, Musculoskeletal and Skin Diseases: 2P01 AR-05235, 1R01AR068897-01A1. SR is funded by merit award from the Department of Anesthesia and Pain Medicine, University of Toronto, Canada.
Footnotes
Conflict of Interest:
LGB has received in kind research support from ArQule, Inc (now wholly owned by Merck, Inc) and honoraria from Cold Spring Harbor Press.
DATA AVAILABILITY
Any variant classification described herein that has not been posted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/?term=ryr1%5Bgene%5D) at the time of publication will be made available upon request.
REFERENCES
- 1.Gonsalves SG, Dirksen RT, Sangkuhl K et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for the use of potent volatile anesthetic agents and succinylcholine in the context of RYR1 or CACNA1S genotypes. Clin Pharmacol Ther (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg H, Sambuughin N, Riazi S & Dirksen R Malignant Hyperthermia Susceptibility. in GeneReviews((R)) (eds. Adam MP et al. ) (University of Washington, Seattle (WA), 1993). [Google Scholar]
- 3.Monnier N, Krivosic-Horber R, Payen JF et al. Presence of two different genetic traits in malignant hyperthermia families: implication for genetic analysis, diagnosis, and incidence of malignant hyperthermia susceptibility. Anesthesiology 97, 1067–74 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Carpenter D, Robinson RL, Quinnell RJ et al. Genetic variation in RYR1 and malignant hyperthermia phenotypes. Br J Anaesth 103, 538–48 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Green RC, Berg JS, Grody WW et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med 15, 565–74 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalia SS, Adelman K, Bale SJ et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet Med 19, 249–255 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Miller DM, Daly C, Aboelsaod EM et al. Genetic epidemiology of malignant hyperthermia in the UK. Br J Anaesth 121, 944–952 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jungbluth H, Treves S, Zorzato F et al. Congenital myopathies: disorders of excitation-contraction coupling and muscle contraction. Nat Rev Neurol 14, 151–167 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Stenson PD, Mort M, Ball EV, Shaw K, Phillips A & Cooper DN The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet 133, 1–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landrum MJ, Lee JM, Benson M et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res 46, D1062–D1067 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karczewski KJ, Francioli LC, Tiao G et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ioannidis NM, Rothstein JH, Pejaver V et al. REVEL: An Ensemble Method for Predicting the Pathogenicity of Rare Missense Variants. Am J Hum Genet 99, 877–885 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tavtigian SV, Greenblatt MS, Harrison SM et al. Modeling the ACMG/AMP variant classification guidelines as a Bayesian classification framework. Genet Med 20, 1054–1060 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly MA, Caleshu C, Morales A et al. Adaptation and validation of the ACMG/AMP variant classification framework for MYH7-associated inherited cardiomyopathies: recommendations by ClinGen's Inherited Cardiomyopathy Expert Panel. Genet Med 20, 351–359 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraeva N, Riazi S, Loke J et al. Ryanodine receptor type 1 gene mutations found in the Canadian malignant hyperthermia population. Can J Anaesth 58, 504–13 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Richards S, Aziz N, Bale S et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17, 405–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halliday NJ Malignant hyperthermia. J Craniofac Surg 14, 800–2 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Ibarra Moreno CA, Hu S, Kraeva N et al. An Assessment of Penetrance and Clinical Expression of Malignant Hyperthermia in Individuals Carrying Diagnostic Ryanodine Receptor 1 Gene Mutations. Anesthesiology 131, 983–991 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ording H, Brancadoro V, Cozzolino S et al. In vitro contracture test for diagnosis of malignant hyperthermia following the protocol of the European MH Group: results of testing patients surviving fulminant MH and unrelated low-risk subjects. The European Malignant Hyperthermia Group. Acta Anaesthesiol Scand 41, 955–66 (1997). [DOI] [PubMed] [Google Scholar]
- 20.Allen GC, Larach MG & Kunselman AR The sensitivity and specificity of the caffeine-halothane contracture test: a report from the North American Malignant Hyperthermia Registry. The North American Malignant Hyperthermia Registry of MHAUS. Anesthesiology 88, 579–88 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Hopkins PM, Ruffert H, Snoeck MM et al. European Malignant Hyperthermia Group guidelines for investigation of malignant hyperthermia susceptibility. Br J Anaesth 115, 531–9 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Lawal TA, Wires ES, Terry NL, Dowling JJ & Todd JJ Preclinical model systems of ryanodine receptor 1-related myopathies and malignant hyperthermia: a comprehensive scoping review of works published 1990-2019. Orphanet J Rare Dis 15, 113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brnich SE, Abou Tayoun AN, Couch FJ et al. Recommendations for application of the functional evidence PS3/BS3 criterion using the ACMG/AMP sequence variant interpretation framework. Genome Med 12, 3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato K, Roesl C, Pollock N & Stowell KM Skeletal muscle ryanodine receptor mutations associated with malignant hyperthermia showed enhanced intensity and sensitivity to triggering drugs when expressed in human embryonic kidney cells. Anesthesiology 119, 111–8 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Feng W, Barrientos GC, Cherednichenko G et al. Functional and biochemical properties of ryanodine receptor type 1 channels from heterozygous R163C malignant hyperthermia-susceptible mice. Mol Pharmacol 79, 420–31 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andronache Z, Hamilton SL, Dirksen RT & Melzer W A retrograde signal from RyR1 alters DHP receptor inactivation and limits window Ca2+ release in muscle fibers of Y522S RyR1 knock-in mice. Proc Natl Acad Sci U S A 106, 4531–6 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez JR, Kaura V, Diggle CP, Hopkins PM & Allen PD Malignant hyperthermia, environmental heat stress, and intracellular calcium dysregulation in a mouse model expressing the p.G2435R variant of RYR1. Br J Anaesth 121, 953–961 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuen B, Boncompagni S, Feng W et al. Mice expressing T4826I-RYR1 are viable but exhibit sex- and genotype-dependent susceptibility to malignant hyperthermia and muscle damage. FASEB J 26, 1311–22 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maclennan DH & Zvaritch E Mechanistic models for muscle diseases and disorders originating in the sarcoplasmic reticulum. Biochim Biophys Acta 1813, 948–64 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Zullo A, Klingler W, De Sarno C et al. Functional characterization of ryanodine receptor (RYR1) sequence variants using a metabolic assay in immortalized B-lymphocytes. Hum Mutat 30, E575–90 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Amendola LM, Jarvik GP, Leo MC et al. Performance of ACMG-AMP Variant-Interpretation Guidelines among Nine Laboratories in the Clinical Sequencing Exploratory Research Consortium. Am J Hum Genet 99, 247 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biesecker LG, Dirksen RT, Girard T et al. Genomic Screening for Malignant Hyperthermia Susceptibility. Anesthesiology (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any variant classification described herein that has not been posted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/?term=ryr1%5Bgene%5D) at the time of publication will be made available upon request.