Abstract
Introduction:
Cryptococcosis remains a leading cause of meningitis and mortality among people living with HIV (PLHIV) worldwide. We sought to evaluate laboratory-based cryptococcal antigen (CrAg) reflex testing and a clinic-based point-of-care (POC) CrAg screening intervention for preventing meningitis and mortality among PLHIV in South Africa.
Methods:
We conducted a prospective pre-post intervention study of adults presenting for HIV testing in Umlazi Township, South Africa over a six-year period (2013–2019). Participants were enrolled during three phases of CrAg testing – CrAg testing ordered by a clinician (“Clinician-directed testing”; 2013–2015); routine lab-based CrAg reflex testing for blood samples with CD4 ≤100 cells/mm3 (“Lab reflex testing”; 2015–2017), and a clinic-based intervention with POC CD4 testing and POC CrAg testing for PLHIV with CD4 ≤200 cells/mm3 with continued standard-of-care routine lab-reflex testing among those with CD4 ≤100 cells/mm3 (“Clinic-based testing”; 2017–2019). The laboratory and clinical teams performed serum CrAg by enzyme immunoassay and lateral flow assay (Immy Diagnostics, Norman, USA). We followed participants for up to 14 months to compare associations between baseline CrAg positivity, ART and fluconazole treatment initiation, and outcomes of cryptococcal meningitis, hospitalization and mortality.
Results:
3,105 (39.4%) of 7,877 people screened were HIV-positive, of whom 908 had CD4 ≤200 cells/mm3 and were included in analyses. Lab reflex and clinic-based testing increased CrAg screening (p<0.001) and diagnosis of CrAg-positive PLHIV (p=0.011). As compared to clinician-directed testing, clinic-based CrAg testing increased the number of PLHIV diagnosed with cryptococcal meningitis (4.5% compared to 1.5%; p=0.059), initiation of fluconazole pre-emptive therapy (7.2% compared to 2.5%; p=0.010), and initiation of ART (96.8% compared to 91.3%; p=0.012). Comparing clinic-based testing to lab reflex testing, there was no significant difference in the cumulative incidence of cryptococcal meningitis (4.5% compared to 4.1%; p=0.836) or mortality (8.1% compared to 9.9%; p=0.557).
Conclusions:
Lab reflex and clinic-based CrAg testing facilitated diagnosis of HIV-associated cryptococcosis and fluconazole initiation, but did not reduce cryptococcal meningitis or mortality. In this non-randomized cohort, clinical outcomes were similar between lab reflex testing and clinic-based point-of-care CrAg testing.
Keywords: HIV/AIDS, Cryptococcus, cryptococcal meningitis, screening, point-of-care testing, mortality
Introduction
Cryptococcosis is an opportunistic fungal infection that causes approximately 15% of AIDS-related deaths worldwide, the majority of which occur in sub-Saharan Africa [1]. In South Africa, cryptococcal infections account for approximately 63% of meningitis cases, due in part to the high prevalence of HIV/AIDS [2]. Cryptococcal capsular antigens (CrAg) can be detected before the onset of symptomatic cryptococcal meningitis [3–5], and oral fluconazole significantly reduces the risk of cryptococcal meningitis and mortality [6–9]. The World Health Organization (WHO) recommends CrAg screening for all antiretroviral therapy (ART)-naïve people living with HIV (PLHIV) with CD4 T-cell count <100 cells/mm3 [10], and consideration for CrAg screening for those with CD4 100–199 cells/mm3 [11].
Several sub-Saharan African countries have incorporated CrAg screening into guidelines, and some are evaluating laboratory reflex CrAg testing for blood samples with CD4 <100 cells/mm3 to improve screening coverage [1]. Since survival benefit is related to prompt initiation of fluconazole therapy, minimizing the time to complete CrAg screening and treatment initiation is critical [12]. A rapid lateral flow assay (LFA) was developed to simplify CrAg testing for clinic-based point-of-care (POC) screening, and has demonstrated good accuracy on serum and cerebrospinal fluid specimens [13–16]. A possible implementation strategy may be integrating clinic-based POC CrAg screening with fluconazole pre-emptive therapy when fungal burden is relatively low prior to the onset of symptomatic meningitis [7,9,12].
In the Umlazi Township of KwaZulu-Natal, South Africa, the prevalence of cryptococcal antigenemia is 1.2% among PLHIV initiating ART [17]. In 2015, routine lab-based reflex CrAg testing was implemented for people with CD4 ≤100 cells/mm3. In this study, we sought to determine if lab reflex CrAg testing improved diagnosis of CrAg-positive PLHIV and initiation of fluconazole, and reduced incidence of cryptococcal meningitis or mortality. In addition, we sought to determine if clinic-based POC CD4 and CrAg screening offered additional benefits for diagnosis, treatment, and clinical outcomes in comparison to standard-of-care lab testing.
Methods
Study design and participants
Following the WHO and South African recommendations for CrAg screening among PLHIV with immunodeficiency, we conducted a prospective pre-post intervention study from September 12th 2013 to February 28th 2019. During the initial study phase (September 12th 2013 to June 4th 2015), the clinical standard-of-care (SoC) was to conduct CrAg testing when ordered by a clinician, called “clinician-directed testing,” for PLHIV with CD4 <100 cells/mm3, according to South African guidelines. During the second study phase (June 5th 2015 to November 8th 2017), the National Health Laboratory Services (NHLS) laboratory implemented routine CrAg reflex testing, whereby blood samples with CD4 <100 cells/mm3 were reflexively tested for CrAg by a lateral flow assay (LFA), called “lab reflex testing.” During the first two study phases, the study was observational and there was no intervention. During the third study phase (November 9th 2017 to February 28th 2019), the clinical research team performed POC CD4 testing for all study participants, and those with CD4 <200 cells/mm3 were tested for CrAg by a rapid lateral flow assay (LFA) at the point of care during routine clinic visits. The lab reflex CrAg testing continued during the third study phase and lasted until the end of the study period.
We recruited persons who presented for voluntary HIV testing at the iThembalabantu Clinic in the Umlazi township of KwaZulu-Natal, South Africa. The clinic provides free clinic- and community-based HIV care and treatment for over 15,000 PLHIV. Before conducting HIV testing, we enrolled English or Zulu speaking adults ≥18 years of age, who were not pregnant, and had not taken anti-fungal therapy in the preceding three months. All study participants provided written informed consent, and received routine medical care, including ART, CD4 T-cell testing and CrAg screening and treatment, according to local and national guidelines [18]. Throughout the study, all CrAg-positive participants were referred to a clinician for consideration of fluconazole therapy, lumbar puncture, and/or referral for hospitalization. Lumbar puncture was indicated for patients who were CrAg-positive and had headache for >24 hours, fever, neck stiffness, blurry or double vision, or confusion. The study was approved by the University of Washington’s Institutional Review Board (IRB #49563), Partners Human Research Committee (#2013P002513), and the University of KwaZulu-Natal’s Medical Research Ethics Committee (Protocol #BF052/13).
Data collection
At enrollment, research assistants completed a sociodemographic questionnaire and HIV counselors performed serial rapid HIV testing according to South African guidelines.18 Among HIV-positive participants, research nurses obtained a medical history and administered a clinical symptom questionnaire. The research team contacted participants by phone and reviewed medical charts to record their treatment course and clinical outcomes. The procedures for recruitment, informed consent, administering a sociodemographic questionnaire, conducting HIV testing, and phlebotomy for CrAg testing were the same across the three study periods.
At the study end, we reviewed each participant’s medical chart and attempted up to three phone calls to participants and/or next of kin. We assessed local hospital records at Prince Mshiyeni Memorial Hospital, which is a single designated hospital serving the Umlazi catchment area, and all hospitalized participants had a hospital chart review to determine the cause of hospitalization. For individuals whose vital status could not be ascertained through direct contact or medical records, we searched the South African national death registry. All participants were followed for up to 14 months after enrollment to assess vital status and other study outcomes.
Clinic-based CD4 T-cell and CrAg Testing
In the intervention phase, all participants received rapid clinic-based CD4 testing using an m-PIMA (Abbott). Individuals with a POC CD4 ≤200 cells/mm3 had a venous blood draw for clinic-based CrAg testing. Trained research nurses conducted clinic-based screening on serum samples using a CrAg LFA (Immy Diagnostics, Norman, Oklahoma, USA), according to the manufacturer’s instructions. In brief, a research nurse placed each CrAg LFA test strip in a 1.5-mL Eppendorf tube containing 2 drops of “specimen diluent”, which was provided by the test manufacturer. All tests rested upright at room temperature for 10 minutes before interpretation. All tests were independently read by two trained readers. We also performed positive control testing with a CrAg-spiked solution provided by the test manufacturer and according to their instructions, which were consistently positive.
Study Outcomes
The primary study outcomes were diagnosis of cryptococcal meningitis, all-cause hospitalization, or all-cause death within 14 months of enrollment. Cryptococcal meningitis was defined as either CrAg-positive in cerebrospinal fluid or a recorded clinical diagnosis of cryptococcal meningitis by the treating medical team in hospital records. The secondary endpoints included hospitalization due to known cryptococcal meningitis diagnosis, mortality due to known cryptococcal meningitis diagnosis, initiation of antiretroviral therapy (ART), initiation of fluconazole prophylaxis, and treatment with intravenous amphotericin-B. After 14 months since enrollment, all participants were categorized and censored as either retained in care at the study clinic, transferred to another HIV clinic, lost to clinical follow-up, or deceased.
This study investigated process outcomes including standard of care CrAg testing and positivity to quantify background Cryptococcus diagnosis and treatment and to determine the delivery of Cryptococcus guidelines and the study intervention. Process outcomes included documented CrAg reflex testing, CrAg positivity among those tested or among the entire analysis set, and conducting the intervention POC PIMA CD4 and POC serum CrAg LFA testing.
Statistical Analyses
The analysis set included individuals who tested HIV-positive and who had a baseline CD4 count ≤200 cells/mm3 as measured by the standard of care NHLS testing within six months of study enrollment. The demographic profile and clinical profile of each study group was quantified to assess how the study population changed over the study period and may have impacted participant outcomes. Demographics of interest included age, gender, employment status, income, marital status, number of children and education level. Clinical history included in the analysis included symptoms that may be indicative of cryptococcal infection (headache, confusion, fever, neck stiffness, blurry or double vision and seizure within seven days of the baseline visit), baseline CD4 testing by NHLS blood testing and PIMA testing in the intervention group, and HIV and Cryptococcus diagnosis and treatment.
To assess how background SoC Cryptococcus testing services may have impacted study outcomes, the association between study group and receipt of services was compared using a Fisher’s exact test to determine if participants who enrolled during each study phase reported clinical symptoms. Additionally, we conducted separate analyses for participants with CD4 <100 cells/mm3, since this group is recommended for CrAg screening under current guidelines [10,11].
Analyses comparing the percentage of study participants who experienced study outcomes between study groups were conducted in duplicate with one comparison between the intervention group and the clinician-directed treatment and another between the intervention group and lab reflex testing to isolate the effect of POC CrAg testing alone. Kaplan-Meier curves were conducted to compare time to hospitalization, cryptococcal meningitis, death, and fluconazole prophylaxis across study groups. Finally, we explored the association between CrAg positivity and primary study outcomes among participants who received laboratory-based reflex testing during all study phases as well as POC LFA CrAg testing during the intervention period. All analyses were conducted in SAS 9.4 (Cary, USA).
Results
Overall, we screened and tested 7,877 people for HIV, and enrolled 3,105 (39%) adults who were HIV-positive (Figure 1). Among those, 908 (29%) participants had a lab-based CD4 ≤200 cells/mm3, and were included in the analyses. During the clinician-directed testing period (phase 1), we enrolled 1,031 participants, among whom 323 (31.3%) had a CD4 ≤200 cells/mm3. During the lab reflex testing period (phase 2), we enrolled 1,354 PLHIV, among whom 363 (26.8%) had a CD4 ≤200 cells/mm3. During the clinic-based testing period (phase 3), we enrolled 720 PLHIV, among whom 222 (30.8%) had a CD4 ≤200 cells/mm3.
Figure 1. Participant enrollment and flowchart.
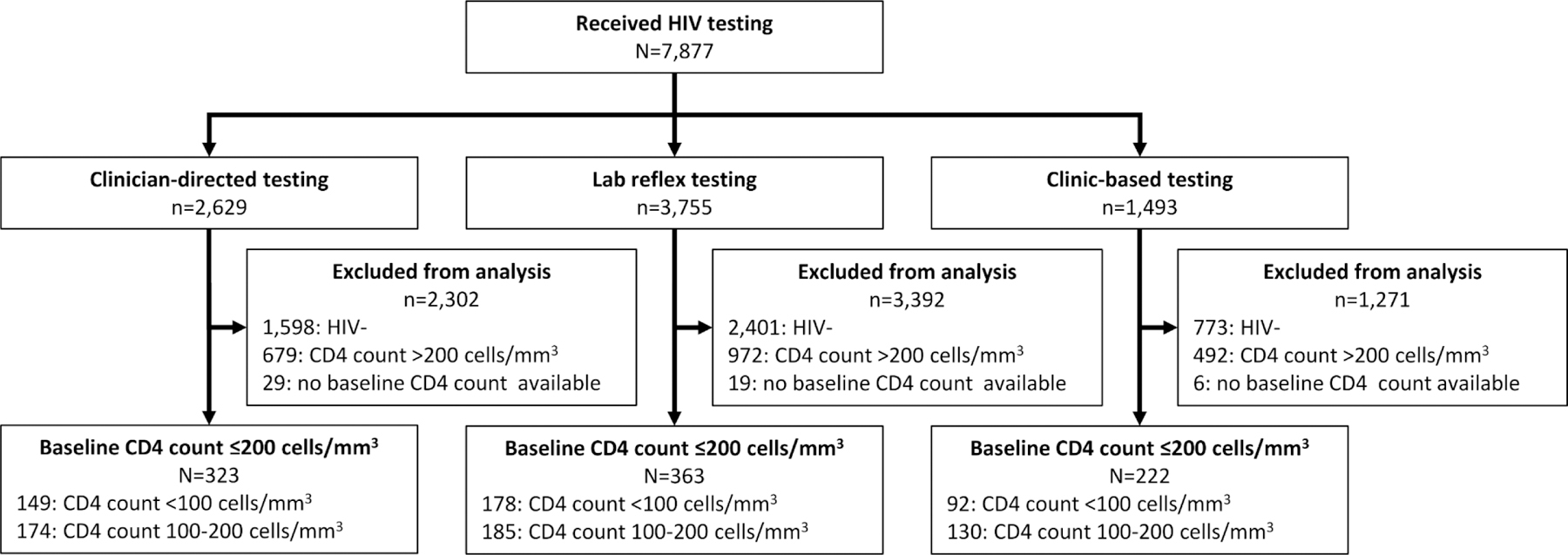
“Clinician-directed testing” refers to the time period (September 12th, 2013 to June 4th, 2015) before implementation of routine lab-based reflex testing where the standard of care was testing among individuals suspected for cryptococcosis. “Lab reflex testing” refers to the time period (June 5th, 2015 to November 8th, 2017) in which the standard of care was laboratory-based reflex cryptococcal antigen (CrAg) testing among all HIV+ patients with CD4 count<100 cells/mm3 in addition to suspected cryptococcosis cases. “Clinic-based testing” refers to the implementation of the study intervention that included clinic-based, point of care serum lateral flow assay (LFA) CrAg testing among all HIV+ patients with CD4 count≤200 in the context of background standard of care laboratory-based reflex testing.
Among the complete cohort of 3,105 PLHIV, mean age was 33.2 (standard deviation [SD] ±9.3) years and 1,331 (43%) were female (Table 1). Six people reported a prior positive test for Cryptococcus infection, and four of those people reported receiving treatment for Cryptococcus. Participants commonly reported clinical symptoms that have been associated with cryptococcal infection or meningitis, including headache for >24 hours (23%), fever (23%), neck stiffness (16%), blurry or double vision (11%), confusion (7%), and having a seizure within the last seven days (1%). The median CD4 count was 313 cells/mm3 (interquartile range [IQR]: 173–486 cells/mm3), and the analyses were restricted to PLHIV with CD4 ≤200 cells/mm3. Among this subset, the median CD4 was 107 cells/mm3 (IQR: 52–153 cells/mm3). The baseline demographics and clinical presentations were not substantially different between the three enrollment periods.
Table 1.
Baseline sociodemographic and clinical characteristics of study participants included in the analysis.
| Cohort of PLHIV | PLHIV with CD4 ≤200 cells/mm3 | |||
|---|---|---|---|---|
| Clinician-directed testing | Lab reflex testing | Clinic-based testing | ||
| (N=3,105) | (N=323) | (N=363) | (N=222) | |
| Baseline characteristics | n (%) | n (%) | n (%) | n (%) |
| Sociodemographics | ||||
| Age (years): mean (±SD) | 33.2 (9.3) | 35.1 (8.9) | 35.7 (9.8) | 36.0 (9.4) |
| Female gender | 1,331 (42.9) | 165 (51.1) | 198 (54.5) | 114 (51.4) |
| Completed high school or higher degree | 1,525 (49.1) | 112 (34.7) | 162 (44.6) | 136 (61.3) |
| Marital status | ||||
| Married | 193 (6.2) | 21 (6.5) | 22 (6.1) | 13 (5.8) |
| Single (never married) | 2,879 (92.7) | 298 (92.3) | 338 (93.1) | 203 (91.4) |
| Widowed/Divorced | 33 (1.1) | 4 (1.2) | 3 (0.8) | 6 (2.7) |
| Number of children (N=3,086)* | ||||
| No children | 585 (19.0) | 58 (17.9) | 68 (18.9) | 45 (20.5) |
| ≥1 children | 2,501 (81.0) | 264 (82.0) | 292 (81.1) | 174 (79.5) |
| Employed | 1,312 (42.3) | 149 (46.1) | 156 (43.0) | 97 (43.7) |
| Income level ZAR <2,000 Rand (~USD $150) (N=3,078)* | 2,226 (72.3) | 246 (77.1) | 262 (72.4) | 127 (57.5) |
| HIV and Medical History | ||||
| Previously received HIV testing | 2,390 (77.0) | 220 (68.1) | 228 (62.8) | 209 (94.1) |
| Previously tested HIV positive, among those tested (N=2,380)* | 791 (33.2) | 41 (18.9) | 77 (33.9) | 98 (46.7) |
| Partner HIV status (N=3,087)* | ||||
| HIV-positive | 847 (27.4) | 88 (27.4) | 91 (25.2) | 53 (24.8) |
| HIV-negative | 508 (16.5) | 40 (12.5) | 48 (13.3) | 18 (8.4) |
| Unknown | 1,732 (56.1) | 193 (60.1) | 222 (61.5) | 143 (66.8) |
| Ever tested Cryptococcus positive | 6 (0.2) | 2 (0.6) | 2 (0.6) | 0 (0.0) |
| Ever received Cryptococcus treatment, among those positive | 4 (0.1) | 1 (0.3) | 2 (0.6) | - |
| Clinical Symptoms and Signs | ||||
| Headache for >24 hours | 703 (22.6) | 113 (35.0) | 68 (18.7) | 33 (14.9) |
| Fever | 698 (22.5) | 122 (37.8) | 116 (32.0) | 45 (20.3) |
| Neck stiffness | 503 (16.2) | 71 (22.0) | 72 (19.8) | 42 (18.9) |
| Blurry or double vision | 325 (10.5) | 39 (12.1) | 52 (14.3) | 46 (20.7) |
| Confusion | 216 (7.0) | 20 (6.2) | 32 (8.8) | 18 (8.1) |
| Seizure with the prior 7 days | 26 (0.8) | 6 (1.9) | 3 (0.8) | 3 (1.4) |
| CD4 T-cell Count (cells/mm3) | ||||
| Baseline CD4 count using lab-based test (N=3,057): median (IQR) | 313 (173 – 486) | 108 (51 – 151) | 101 (45 – 147) | 121 (65 – 161) |
| Baseline CD4 count using POC test (N=720): median (IQR)** | 361 (214 – 563) | -- | -- | 147 (95 – 214) |
IQR: interquartile range, SD: standard deviation.
Does not sum to column total due to missing data.
Intervention period was clinic-based testing period only.
CrAg Testing
During the clinician-directed testing period, only 10 of 149 (6.7%) PLHIV with CD4 <100 cells/mm3 were screened for CrAg, whereas 100% were screened during the lab reflex and clinic-based testing periods (Table 2). Among PLHIV with CD4 ≤200 cells/mm3, 51.8% (188/363) and 45.9% (102/222) participants had CrAg screening during the lab reflex and clinic-based testing periods, respectively.
Table 2.
Cryptococcus diagnosis and treatment delivery by study phase and baseline CD4 count.
| Clinician-directed testing | Lab reflex testing | Clinic-based testing | ||
|---|---|---|---|---|
| n/N (%) | n/N (%) | n/N (%) | p | |
| Standard of care diagnosis and treatment | ||||
| Received laboratory-based SoC cryptococcal antigen (CrAg) testing | ||||
| Lab CD4 <100 cells/mm3 | 10/149 (6.7) | 178/178 (100.0) | 92/92 (100.0) | <0.001 |
| Lab CD4 ≤200 cells/mm3 | 11/323 (3.4) | 188/363 (51.8) | 102/222 (45.9) | <0.001 |
| CrAg+ by laboratory-based SoC CrAg testing | ||||
| Lab CD4 <100 cells/mm3 | 3/149 (2.0) | 12/178 (6.7) | 6/92 (6.5) | 0.096 |
| Lab CD4 ≤200 cells/mm3 | 3/323 (0.9) | 16/363 (4.4) | 9/222 (4.1) | 0.011 |
| CrAg+ from laboratory-based SoC CrAg testing, among those who received testing | ||||
| Lab CD4 <100 cells/mm3 | 3/10 (30.0) | 12/178 (6.7) | 6/92 (6.5) | 0.053 |
| Lab CD4 ≤200 cells/mm3 | 3/11 (27.3) | 16/188 (8.5) | 9/102 (8.8) | 0.131 |
| Study intervention Clinic-based CD4 and CrAg testing* | ||||
| Received point-of-care (POC) CD4 test | ||||
| Lab CD4 <100 cells/mm3 | -- | -- | 92/92 (100.0) | -- |
| Lab CD4 ≤200 cells/mm3 | -- | -- | 222/222 (100.0) | -- |
| POC CD4 count ≤200 cells/mm3 | ||||
| Lab CD4 <100 cells/mm3 | -- | -- | 90/92 (97.8) | -- |
| Lab CD4 ≤200 cells/mm3 | -- | -- | 155/222 (69.8)** | -- |
| Received POC serum CrAg lateral flow assay (LFA), if POC CD4 count ≤200 cells/mm3 | ||||
| Lab CD4 <100 cells/mm3 | -- | -- | 90/90 (100.0) | -- |
| Lab CD4 ≤200 cells/mm3 | -- | -- | 155/155 (100.0) | -- |
| CrAg+ by POC serum CrAg LFA | ||||
| Lab CD4 <100 cells/mm3 | -- | -- | 6/90 (6.7) | -- |
| Lab CD4 ≤200 cells/mm3 | -- | -- | 10/155 (6.5) | -- |
ART: antiretroviral therapy, CrAg: Cryptococcal antigen, LFA: lateral flow assay, POC: point-of-care.
Intervention period was clinic-based testing period only. Denominators reflect the results from lab-based CD4 testing.
Results indicate that 67 people had a POC CD4 >200 cells/mm3, whose had a lab CD4 ≤200 cells/mm3
During the clinician-directed testing period, 3 of 149 (2.0%) PLHIV with CD4 <100 cells/mm3 were CrAg positive by lab-based CrAg testing, whereas 12 of 178 (6.7%) and 6 of 92 (6.5%) were CrAg positive among those receiving lab reflex testing and clinic-based testing, respectively. CrAg positivity was 1.4% (7/489) among people with CD4 100–200 cells/mm3.
During the clinic-based testing phase, all 222 PLHIV with a lab-based CD4 ≤200 cells/mm3 received a POC CD4 test. Among those, 155 of 222 (69.8%) had a POC CD4 test result ≤200 cells/mm3 and therefore received clinic-based POC serum CrAg testing. Among those who received clinic-based POC serum CrAg testing, the CrAg test positivity was 6.7% (6/90) and 6.5% (10/155) among PLHIV with CD4 <100 cells/mm3 and ≤200 cells/mm3, respectively. Clinic-based testing identified significantly more people and a higher percentage of CrAg-positive PLHIV who would have been missed by lab reflex testing (p=0.011).
Clinical Outcomes
During the follow-up period for 908 participants with a lab-based CD4 ≤200 cells/mm3, 30 (3.3%) PLHIV were diagnosed with cryptococcal meningitis confirmed by medical or hospital records, 98 (10.8%) participants had been hospitalized, and 85 (9.4%) participants had died (Table 3). As compared to clinician-directed testing, an intervention of clinic-based testing increased the number of PLHIV diagnosed with cryptococcal meningitis (p=0.059), but did not alter hospitalization or mortality rates. Comparing the clinic-based testing intervention against lab reflex testing, there was no significant difference in the cumulative incidence of cryptococcal meningitis (4.5% compared to 4.1%; p=0.836) or mortality (8.1% compared to 9.9%; p=0.557).
Table 3.
Clinical outcomes by study phase.
| Intervention group | Standard-of-Care groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (N=908) |
Clinic-based testing (N=222) | Clinician-directed testing (N=323) | Lab reflex testing (N=363) | |||||||
| Post-baseline clinical outcomes | n | % (CI) | n | % (CI) | n | % (CI) | p* | n | % (CI) | p* |
| Primary outcomes | ||||||||||
| Cryptococcal meningitis diagnosis | 30 | 3.3 (2.3–4.7) | 10 | 4.5 (2.4–8.2) | 5 | 1.5 (0.6–3.7) | 0.059 | 15 | 4.1 (2.5–6.8) | 0.836 |
| All-cause hospitalization | 98 | 10.8 (8.9–13.0) | 21 | 9.5 (9.5–14.1) | 28 | 8.7 (6.0–12.3) | 0.762 | 49 | 13.5 (10.3–17.4) | 0.151 |
| All-cause mortality | 85 | 9.4 (7.6–11.4) | 18 | 8.1 (5.1–12.5) | 31 | 9.6 (6.8–13.3) | 0.648 | 36 | 9.9 (7.2–13.5) | 0.557 |
| Secondary outcomes | ||||||||||
| Hospitalization due to known cryptococcal infection | 11 | 1.2 (0.7–2.2) | 4 | 1.8 (0.5–4.7) | 4 | 1.2 (0.4–3.3) | 0.721 | 3 | 0.8 (0.2–2.5) | 0.436 |
| Mortality due to known cryptococcal infection | 9 | 1.0 (0.5–1.9) | 4 | 1.8 (0.5–4.7) | 1 | 0.3 (0.0–1.9) | 0.164 | 4 | 1.1 (0.3–2.9) | 0.486 |
| Initiation of antiretroviral therapy | 850 | 93.6 (91.8–95.0) | 215 | 96.8 (93.5–98.6) | 295 | 91.3 (87.7–94.0) | 0.012 | 340 | 93.7 (90.6–95.8) | 0.121 |
| Received fluconazole preventative therapy** | 46 | 5.1 (3.8–6.7) | 16 | 7.2 (4.4–11.5) | 8 | 2.5 (1.2–4.9) | 0.010 | 22 | 6.1 (4.0–9.1) | 0.607 |
| Received intravenous amphotericin-B | 8 | 0.9 (0.9–3.1) | 3 | 1.4 (0.3–4.1) | 4 | 1.2 (0.4–3.3) | 1.000 | 1 | 0.3 (0.0–1.7) | 0.156 |
| Lost to follow-up | 113 | 12.4 (10.5–14.8) | 16 | 7.2 (4.4–11.5) | 56 | 17.3 (13.6–21.9) | 0.001 | 41 | 11.3 (8.4–15.0) | 0.116 |
CI: 95% confidence interval.
p-value represents a Fisher’s exact test of the comparison between each standard of care group with the Intervention group.
Oral fluconazole was indicated for people with serum cryptococcal antigenemia, but without cryptococcal meningitis.
For secondary outcomes during the clinical follow-up period, 850 (93.6%) PLHIV initiated ART, 46 (5.1%) PLHIV were started on fluconazole preventative therapy, eight (0.9%) received intravenous amphotericin-B treatment, and 113 (12.4%) were lost to follow-up. Clinic-based testing led to more initiation of antiretroviral therapy (96.8% vs. 91.3%, p=0.012), fluconazole pre-emptive therapy (7.2% vs. 2.5%, p=0.010), and reduced lost to follow-up (7.2% vs. 17.3%, p=0.001), as compared to the clinic-directed testing period. There were no significant differences in secondary outcomes between the clinic-based testing and the lab reflex testing groups (Table 3).
Overall, when comparing the three time periods, clinic-based CrAg testing significantly accelerated the time to initiation of fluconazole pre-emptive therapy (p=0.027), and had a trend for accelerated diagnosis of cryptococcal meningitis (p=0.093), but had no significant impact on time to hospitalization (p=0.105) or time to mortality (0.685) (Figure 2). The median follow-up time ranged from 364 to 367 days depending on the outcome of interest.
Figure 2. Time to (A) cryptococcal meningitis diagnosis, (B) hospitalization, (C) death and (D) fluconazole prophylaxis by study group.
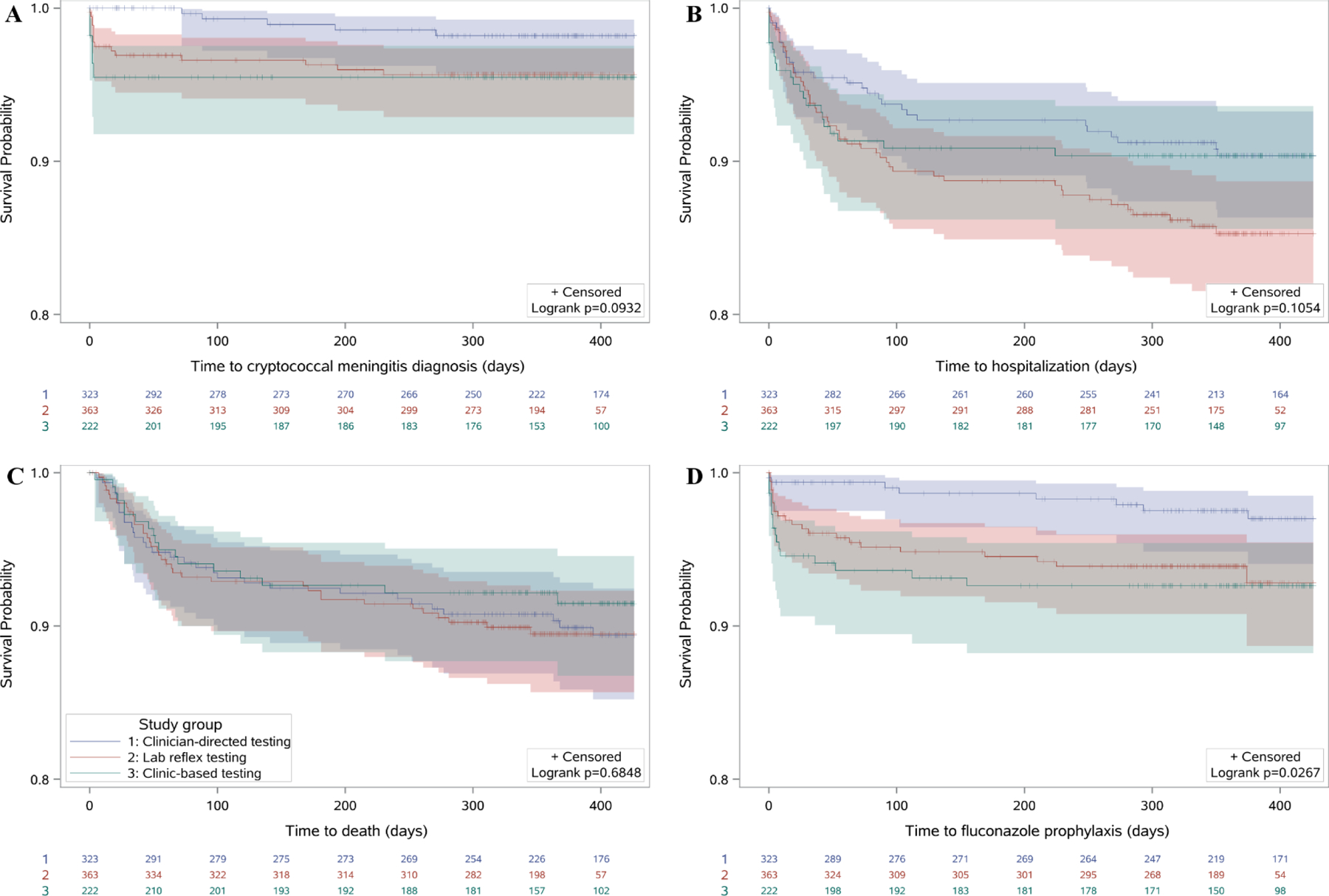
Participants were followed for one year and all events censored at 14 months. Y-axes range from a survival probability of 0.8 to 1.0. Lines in blue represent clinician-directed testing (phase 1), red represent lab reflex testing (phase 2), and green represent clinic-based testing (phase 3) and bands around lines indicate 95% confidence intervals.
Association between CrAg positivity and clinical outcomes
During the lab-based CrAg testing period, CrAg positivity remained strongly associated with diagnosis of cryptococcal meningitis, all-cause hospitalization, and all-cause mortality (Table 4). These strong associations persisted for the intervention period of clinic-based CrAg testing.
Table 4.
Association between CrAg positivity and study outcomes among patients.
| CrAg− | CrAg+ | ||||
|---|---|---|---|---|---|
| Post-baseline clinical outcomes | n/N | % (CI) | n/N | % (CI) | p |
| Standard of care lab testing* | |||||
| Cryptococcal meningitis diagnosis | |||||
| <100 cells/mm3 | 3/259 | 1.2 (0.2–3.5) | 19/21 | 90.5 (69.9–98.6) | <0.001 |
| ≤200 cells/mm3 | 3/273 | 1.1 (0.2–3.3) | 25/28 | 89.3 (72.0–97.1) | <0.001 |
| All-cause hospitalization | |||||
| <100 cells/mm3 | 43/259 | 16.6 (12.5–21.6) | 8/21 | 38.1 (20.7–59.2) | 0.033 |
| ≤200 cells/mm3 | 47/273 | 17.2 (13.2–22.2) | 11/28 | 39.3 (23.5–57.6) | 0.010 |
| All-cause mortality | |||||
| <100 cells/mm3 | 30/259 | 11.6 (8.2–16.1) | 7/21 | 33.3 (17.1–54.8) | 0.012 |
| ≤200 cells/mm3 | 35/273 | 12.8 (9.3–17.3) | 10/28 | 35.7 (20.6–54.3) | 0.004 |
| Intervention clinic-based testing** | |||||
| Cryptococcal meningitis diagnosis | |||||
| <100 cells/mm3 | 1/84 | 1.2 (0.0–7.1) | 6/6 | 100.0 (55.7–100.0) | <0.001 |
| ≤200 cells/mm3 | 1/145 | 0.7 (0.0–4.2) | 9/10 | 90.0 (57.4–100.0) | <0.001 |
| All-cause hospitalization | |||||
| <100 cells/mm3 | 13/84 | 15.5 (9.1–24.8) | 3/6 | 50.0 (18.8–81.2) | 0.067 |
| ≤200 cells/mm3 | 16/145 | 11.0 (6.8–17.3) | 4/10 | 40.0 (16.7–68.8) | 0.026 |
| All-cause mortality | |||||
| <100 cells/mm3 | 11/84 | 13.1 (7.3–22.1) | 4/6 | 66.7 (29.6–90.8) | 0.007 |
| ≤200 cells/mm3 | 12/145 | 8.3 (4.7–14.0) | 5/10 | 50.0 (23.7–76.3) | 0.002 |
Among patients who were documented receiving a standard of care laboratory-based CrAg test. Includes participants from all three study periods.
Intervention period was clinic-based testing period only.
ART: antiretroviral therapy, CI: 95 % confidence interval, CrAg: Cryptococcal antigen.
Discussion
In this cohort of ambulatory adults in Umlazi Township, South Africa, both lab reflex and clinic-based CrAg testing facilitated diagnosis of HIV-associated cryptococcosis and fluconazole initiation, as compared to the prior practice of clinician-directed CrAg testing. As compared to clinician-directed testing, clinic-based CrAg testing increased the number of PLHIV diagnosed with cryptococcal meningitis, but did not alter hospitalization or mortality rates. When comparing the lab reflex testing and clinic-based testing, there were not significant differences in diagnosis of cryptococcal meningitis, hospitalization, mortality, or any secondary outcomes. The results of this non-randomized study support lab reflex or clinic-based CrAg testing to facilitate diagnosis of HIV-associated cryptococcosis and early initiation of fluconazole pre-emptive therapy for those CrAg-positive.
The estimated prevalence of cryptococcal antigenemia in our cohort was consistent with other studies of PLHIV in sub-Saharan Africa [1,20]. An earlier study in Cape Town reported a higher CrAg prevalence of 12% (42/336) among pre-ART PLHIV with CD4 ≤100 cells/mm3 [5]; the overall incidence of HIV-associated cryptococcosis has been declining throughout South Africa since 2006 [21]. In our cohort, the vast majority (95%) of participants who developed cryptococcal meningitis had a baseline CD4 ≤200 cells/mm3 [11]. While these data also support increasing the CrAg screening threshold to CD4 ≤200 cells/mm3 among newly-diagnosed PLHIV, the study was not designed to address this important research question. Either clinic-based or lab-reflex CrAg screening remains an important component of clinical care for PLHIV with advanced disease.
In multiple studies, CrAg LFA testing has proven accurate when compared to CrAg EIA and latex agglutination testing in serum and/or cerebrospinal specimens [13–16,22,23]. However, one study has reported false negative results from CrAg LFA testing on serum due to a prozone effect [24]. We have previously reported on the diagnostic accuracy of clinic-based POC CrAg LFA testing, when compared to lab-based serum CrAg EIA testing [17]. In this study, POC CrAg LFA testing was feasible, and may be an alternative to lab-based reflex CrAg testing for clinics that have the capacity to conduct POC CD4 testing. In addition, CrAg screening for HIV-positive adults with CD4 100–200 cells/mm3 identified additional people with cryptococcal antigenemia at risk for poor clinical outcomes.
While our study showed improvements in clinical diagnosis of cryptococcal meningitis and proportion who initiated pre-emptive fluconazole therapy, there was no observed impact on mortality. Another report from South Africa found no reduction in the annual case fatality ratio for cryptococcal meningitis, which was attributed to delays in diagnosing HIV-associated cryptococcal infections [28]. In the REALITY trial, the provision of fluconazole improved outcomes for PLHIV with low CD4 count, regardless of CrAg screening results [9]. The absolute benefits were still greater for CrAg-positive patients, and the authors concluded that CrAg screening should be routine for PLHIV with CD4 <100 cells/mm3 [9]. In our cohort, many people who were serum CrAg positive during clinic-based testing already had evidence of cryptococcal meningitis. Therefore, while clinic-based testing may have accelerated time to initiation of fluconazole and diagnosis of cryptococcal meningitis, the accelerated treatment may have already been too late to significantly reduce mortality rates.
This study had several limitations and strengths. The study was conducted over a six-year time period and included a significant number of immunosuppressed PLHIV at substantial risk for cryptococcal meningitis or mortality. The study was implemented as a pre-post study design, which does not provide a direct comparison between study arms. However, conducting a randomized trial in which one arm does not adequately provide CrAg testing for immunosuppressed PLHIV would be considered unethical. Despite the large sample size, the relatively fewer participants with cryptococcal antigenemia and/or CD4 immunosuppression reflected real-world practice in South Africa [28], but was a limitation for assessing differences between study periods with progression to cryptococcal meningitis, hospitalization, and death as the primary outcome measures. The average CD4 count among PLHIV presenting for initiation of care and treatment may have changed during the six-year study period, but analyses focused on PLHIV with CD4 ≤200 cells/mm3 for the entire study duration.
In conclusion, lab reflex and clinic-based CrAg testing facilitated diagnosis of HIV-associated cryptococcosis and fluconazole initiation, but did not reduce cryptococcal meningitis or mortality. As programs move towards same day ART initiation, implementing either clinic-based or lab reflex CrAg testing may be helpful in identifying those with cryptococcal antigenemia for fluconazole therapy, since clinician-directed CrAg testing will likely miss many more cases. Overall, CrAg testing was feasible when performed by trained nurses at the clinical point of care, and led to more people being diagnosed with cryptococcosis and initiated on fluconazole therapy. While programs should emphasize early ART initiation to prevent severe immunosuppression, CrAg testing will help accelerate diagnosis and treatment of HIV-associated cryptococcal infections in order to reduce HIV-associated cryptococcal mortality.
Acknowledgements
We thank the women and men who participated in this study, the clinical sites for sharing their space, and our research staff and nurses who conducted the study. We thank Dr. William Powderly for his advice on study design.
Funding: Funded by the National Institute of Allergy and Infectious Diseases (AI108293 to PKD and K24AI141036 to IVB).
Funding
This work was supported by the Infectious Disease Society of America Education & Research Foundation and National Foundation for Infectious Diseases (PKD); Massachusetts General Hospital Executive Committee on Research (PKD); the Harvard University Center for AIDS Research [P30 AI060354] (PKD); and the National Institute of Allergy and Infectious Diseases [K23 AI108293] (PKD) and [K24AI141036] (IVB). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or other funding agencies.
Role of the Funding Source
The funder of the study (US National Institutes of Health) had no role in the study design, data collection, data analyses, results interpretation, or writing of the report.
Footnotes
Declaration of interests
We declare that we have no competing interests.
References
- 1.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis 2010; 10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.French N, Gray K, Watera C, Nakiyingi J, Lugada E, Moore M, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS 2002; 16:1031–8. [DOI] [PubMed] [Google Scholar]
- 4.Liechty CA, Solberg P, Were W, Ekwaru JP, Ransom RL, Weidle PJ, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health 2007; 12:929–35. [DOI] [PubMed] [Google Scholar]
- 5.Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, Harrison TS. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment clinic in South Africa. Clin Infect Dis 2009; 48:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mfinanga S, Chanda D, Kivuyo SL, Guinness L, Bottomley C, Simms V, et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet 2015: 385:2173–82. [DOI] [PubMed] [Google Scholar]
- 7.Kapoor SW, Magambo KA, Kalluvya SE, Fitzgerald DW, Peck RN, Downs JA. Six-month outcomes of HIV-infected patients given short-course fluconazole therapy for asymptomatic cryptococcal antigenemia. AIDS 2015; 29:2473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letang E, Muller MC, Ntamatungiro AJ, Kimera N, Faini D, Furrer H, et al. Cryptococcal antigenemia in immunocompromised HIV patients in rural Tanzania: a preventable cause of early mortality. Open Forum Infect Dis 2015; 2:ofv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pett SL, Haddow L, Nhema R, Spyer M, Benjamin L, Najjuka G. CrAg status and effects on benefits from enhanced prophylaxis in the REALITY trial. Conference on Retroviruses and Opportunistic Infections, 2018. Poster #784. [Google Scholar]
- 10.World Health Organization. Rapid Advice: Diagnosis, prevention and management of Cryptococcal disease in HIV-infected adults, adolescents and children. Geneva: WHO; 2011. [PubMed] [Google Scholar]
- 11.World Health Organization. Guidelines for the diagnosis, prevention, and management of cryptococcal disease in HIV-infected adults, adolescents and children, March 2018. Geneva: World Health Organization. License: CC BY-NC-SA 3.0 IGO. [PubMed] [Google Scholar]
- 12.Rajasingham R, Meya DB, Boulware DR. Integrating cryptococcal antigen screening and pre-emptive treatment into routine HIV care. J of Acquir Immune Defic Syndr 2012; 59:e85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarvis JN, Percival A, Bauman S, Pelfrey J, Meintjes G, Ntombomzi Williams G, et al. Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis. Clin Infect Dis 2011; 53:1019–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rugemalila J, Maro VP, Kapanda G, Ndaro AJ, Jarvis JN. Cryptococcal antigen prevalence in HIV-infected Tanzanians: a cross sectional study and evaluation of a point-of-care lateral flow assay. Trop Med Int Health 2013; 18:1075–9. [DOI] [PubMed] [Google Scholar]
- 15.Klausner JD, Chiller TV. Sensitivity and specificity of a new cryptococcal antigen lateral flow assay in serum and cerebrospinal fluid. MLO Med Lab Obs 2013; 45:16–20. [PMC free article] [PubMed] [Google Scholar]
- 16.McMullan BJ, Halliday C, Sorrell TC, Judd D, Sleiman S, Marriott D, et al. Clinical utility of the cryptococcal antigen lateral flow assay in a diagnostic mycology laboratory. PLoS ONE 2012; 7:e49541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drain PK, Hong T, Krows M, Govere S, Thulare H, Wallis CL, et al. Validation of clinic-based cryptococcal antigen lateral flow assay screening in HIV-infected adults in South Africa. Sci Rep 2019; 9:2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Republic of South Africa Department of Health. The South African antiretroviral treatment guidelines. 2017.
- 19.Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 2016; 6:e012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford N, Shubber Z, Jarvis JN, Chiller T, Greene G, Migone C, et al. CD4 cell count threshold for cryptococcal antigen screening of HIV-infected individuals: a systematic review and meta-analysis. Clin Infect Dis 2018; 66 (Suppl 2):S152–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Govender NP. Declining incidence of HIV-associated cryptococcosis in South Africa, 2005–2015. Conference on Retroviruses and Opportunistic Infections, 2018. Poster #789. [Google Scholar]
- 22.Beyene T, Woldeamanuel Y, Asrat D, Ayana G, Boulware DR. Comparison of cryptococcal antigenemia between antiretroviral naïve and antiretroviral experienced HIV positive patients at two hospitals in Ethiopia. PLoS ONE 2013; 8:e75585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen J, Slechta ES, Gates-Hollingsworth MA, Neary B, Barker AP, Bauman S, et al. Large-scale evaluation of the immune-mycologics lateral flow and enzyme-linked immunoassay for detection of cryptococcal antigen in serum and cerebrospinal fluid. Clin and Vaccine Immunology 2013; 20:52–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams DA, Kiiza T, Kwizera R, Kiggundu R, Velamakanni S, Meya DB, et al. Evaluation of fingerstick cryptococcal antigen lateral flow assay in HIV-infected persons: a diagnostic accuracy study. Clin Infect Dis 2015; 61:464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee GH, Arthur I, Leung M. False negative serum cryptococcal lateral flow assay due to prozone. J Clin Microbiol 2018; 56:e01878–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longley N, Jarvis JN, Meintjes G. Cryptococcal antigen screening in patients initiating ART in South Africa: A prospective cohort study. Clin Infect Dis 2016; 62:581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magambo KA, Kalluvya SE, Kapoor SW, Seni J, Chofle AA, Fitzgerald DW, et al. Utility of urine and serum lateral flow assays to determine the prevalence and predictors of cryptococcal antigenemia in HIV-positive outpatients beginning antiretroviral therapy in Mwanza, Tanzania. J Int AIDS Soc 2014; 17:19040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Temfack E, Kouanfack C, Mossiang L, Loyse A, Fonkoua MC, Molloy SF, et al. Cryptococcal antigen screening in asymptomatic HIV-infected antiretroviral naïve patients in Cameroon and evaluation of the new semi-quantitative Biosynex CryptoPS Test. Front Microbio 2018; 9:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wake RM, Britz E, Sriruttan C, Rukasha I, Omar T, Spencer DC, et al. High Cryptococcal Antigen Titers in Blood Are Predictive of Subclinical Cryptococcal Meningitis Among Human Immunodeficiency Virus-Infected Patients. Clin Infect Dis 2018; 66:686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]


