Abstract
Background:
Although many patients with Kaposi’s sarcoma (KS) in sub-Saharan Africa are diagnosed with ACTG T1 disease, T1 staging insufficiently captures clinical heterogeneity of advanced KS. Using a representative community-based sample, we detail disease severity at diagnosis to inform KS staging and treatment in sub-Saharan Africa.
Methods:
We performed rapid case ascertainment on people living with HIV ≥18 years newly diagnosed with KS from 2016–2019 at three clinic sites in Kenya and Uganda to ascertain disease stage as close as possible to diagnosis. We reported KS severity using ACTG and WHO staging criteria, as well as detailed measurements not captured in current staging systems.
Results:
We performed rapid case ascertainment within 1 month for 241 adults newly diagnosed with KS out of 389 adult patients with suspected KS. Patients were 68% male, median age 35 years and median CD4 count 239. The majority had advanced disease, with 82% qualifying as ACTG T1 and 64% as WHO Severe/Symptomatic KS. The most common ACTG T1 qualifiers were edema (79%), tumor associated ulceration (24%), extensive oral KS (9%), pulmonary KS (7%), and gastrointestinal KS (4%). There was marked heterogeneity within T1 KS, with 25% of patients having two T1 qualifying symptoms and 3% having three or more.
Conclusion:
The majority of patients newly diagnosed with KS had advanced stage disease, even in the current ART “Treat All” era. We observed great clinical heterogeneity among advanced stage patients, leading to questions about whether all patients with advanced KS require the same treatment strategy.
Introduction
Despite increasing availability of antiretroviral therapy (ART) in the “treat all” era, HIV-associated Kaposi’s sarcoma (KS) remains a common cancer in sub-Saharan Africa.1,2 One-year mortality is estimated between 21 to 35%.3–5 There are likely many reasons for this high mortality, but one that is potentially modifiable is the extent, or stage, of KS disease at time of diagnosis.6,7 Stage of disease at time of KS diagnosis is a critical parameter in population-level cancer control and individual-level management. Prognosis and treatment also depend on stage of disease at diagnosis. In addition, stage at diagnosis is the culmination of multiple factors that together provide insight on a region’s ability to implement early cancer detection: patient awareness of disease, access to care, and capacity of the healthcare system to promptly make a diagnosis.7
Unlike in resource-rich settings, where government funded surveillance routinely collects stage of cancer at time of diagnosis, these data are very limited in sub-Saharan Africa.6,8–10 Where information on KS stage is available, it is frequently from research studies in tertiary settings, and often from clinical trials.3,4 Reports like these often stage patients far removed in time from initial diagnosis and mostly include highly selected patients either based on eligibility for clinical trials or those able to access tertiary care. How well the distribution of stage in these reports represents all KS that arises from a community — the parameter of interest to document cancer control in the region — is unknown. In addition, most reports on KS stage in Africa use the AIDS Clinical Trials Group (ACTG) system, which was developed in the pre-ART era in a resource-rich setting.11,12 Within ACTG staging, patients are classified with advanced KS (“T1” stage) if they have raised oral lesions, visceral KS, or tumor-associated edema.11 However, because T1 lumps a number of manifestations together, ranging from disease limited to certain regions of the mouth only all the way to fulminant pulmonary KS, gradations of severity are not expressed. Furthermore, in the “treat all” era of antiretroviral therapy, there are increasing numbers of patients who develop KS while on therapy. How these patients affect distribution of stage at KS diagnosis is unknown.3,13 Finally, another KS staging system was recently developed by the WHO,14 based on functional disability, but it has not been applied in published reports or compared with ACTG staging.
To better describe the extent of HIV-related KS at the time of diagnosis in a contemporary primary care representative sample of patients, we used a rapid case ascertainment approach to identify patients from three clinical care facilities in Kenya and Uganda. We sought to identify patients with newly diagnosed KS and perform detailed history and physical examination to measure stage of KS as close as possible to the time of healthcare system diagnosis.
Methods
Overall Design
We used rapid case ascertainment15–17 to identify all patients newly diagnosed with KS from 2016 to 2019 at three healthcare centers in Kenya and Uganda. Upon confirming the new diagnosis of KS, we sought to perform a detailed history and physical examination to document extent of KS as close as possible to time of diagnosis, as detailed below.
Study Population
Our target population was all HIV-infected adults (≥ 18 years old) with newly diagnosed KS in East Africa. To attempt to achieve this, we sought all HIV-infected adults receiving care at one of three healthcare facilities in Kenya and Uganda who were newly diagnosed with KS, either pathologically or on clinical grounds. Enrollment began in June 2016 in western Kenya at the Academic Model Providing Access to Healthcare (AMPATH) network, which at the time was caring for approximately 160,000 people living with HIV at over 30 sites. We added two sites in January 2018 in Uganda: The Immune Suppression Syndrome (ISS) Clinic in Mbarara and the AIDS Healthcare Foundation (AHF) Uganda Cares Clinic in Masaka. At the time of study initiation, there were 10,889 adults with HIV actively receiving care at the ISS Clinic in southwestern Uganda, while AHF Uganda Cares Clinic in southcentral Uganda had 13,317 adults. All sites provided ART free of charge and skin biopsies for KS diagnosis18 and have electronic databases to document HIV ambulatory care which are supported by the East Africa International Epidemiologic Databases to Evaluate AIDS (IeDEA) Consortium. All sites are affiliated with regional hospitals, and AMPATH and ISS-Uganda have affiliated oncology and dermatology clinics.
Potential cases of KS were identified via rapid case ascertainment, a process which refers to the expeditious and detailed research-level evaluation of a condition as close as possible after diagnosis.15–17 Long used in resource-rich settings to facilitate research on cancer,19,20 rapid case ascertainment has been used for infectious diseases in sub-Saharan Africa, where it has been useful to inform contact tracing.17,21,22 To our knowledge, it has heretofore not been performed for the study of cancer in Africa. We sought all new diagnoses of KS by serial i) automated querying of outpatient electronic medical records for KS diagnosis or symptoms possibly attributable to KS; ii) manual querying of reports of KS at pathology departments; iii) manual review of patient registers in medical wards, oncology clinics, and dermatology clinics; and iv) sporadic clinician notification. Upon identification of a patient with possible new KS diagnosis, the study team contacted the patient either in-person or by telephone to determine eligibility and plan a rapid case ascertainment encounter. After patients were confirmed to have newly diagnosed KS and provided written informed consent, they underwent a structured interview, physical examination, and had biologic samples collected.
Measurements
Pathology.
KS diagnosis was made with histopathology, with anti-LANA staining when needed. Pathology was performed and read locally, and was additionally reviewed by a dermatopathologist based in the United States. A patient was considered to have KS if the biopsy was positive, or if it was indeterminate with positive clinical findings. In rare cases that biopsy was not possible due to location of lesions, diagnosis was made on clinical findings alone.
Questionnaire-based self-reported manifestations of KS.
Participants were asked about the presence of symptoms suggestive of gastrointestinal or pulmonary KS as well as the impact of these symptoms on function. Similarly, for oral lesions and edema which were compatible with KS, participants were asked about the impact on function. For each symptom category, the research assistants (who were clinical officers) made an overall clinical determination whether reported symptoms were “definitely (>95%)” or “probably (60–94%)” related to KS.
Physical examination.
A full oral, skin and lymph node exam, with removal of clothing, was performed with documentation of lesions suspected to be KS as well as edema. Additionally, the research assistant assessed Karnofsky performance status.23
Laboratory.
Blood samples were collected to determine hemoglobin, HIV plasma RNA levels, and CD4+ T cell count.
Statistical Analysis
The analysis herein is limited to patients where we were able to perform RCA within 1 month of KS diagnosis. Raw measurements were used to stage patients based on ACTG KS Staging system and WHO KS Treatment guidelines classification (Figure 1).11,14 Measurements for ACTG T1 were derived entirely from clinician-documented physical examination, while WHO classification included a mixture of patient self-reported manifestations of KS and physical examination. For binary variables, we estimated proportions and confidence intervals. For continuous variables, we summarized median and interquartile range (with confidence intervals). All analyses were performed using Stata (Version 16.0).
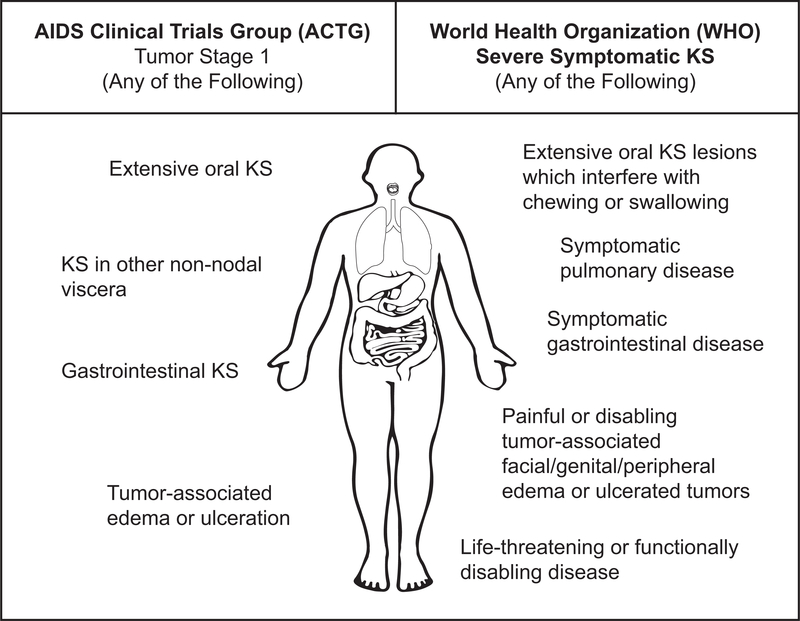
Figure 1. A comparison of AIDS Clinical Trials Group (ACTG) and World Health Organization (WHO) KS Treatment Guidelines staging systems for extent of KS tumor burden.
The ACTG staging system was developed in 1989 to facilitate therapeutic clinical trials for KS and is primarily based on exam, while the WHO staging system was developed in 2014 to guide treatment of KS in resource-limited settings and includes patient experience of symptoms and functional impairment.
Footnotes for Figure 1:
*Staging of visceral disease in this analysis: Patients in this study were asked about the presence of the following symptoms by the local clinician. Pulmonary KS: presence of shortness of breath, cough, and/or coughing up blood. GI KS: abdominal pain, vomiting, hematemesis, early satiety, and/or blood in stool. For all possible pulmonary and GI symptoms, the clinical team assessing the patient was then asked whether in their clinical judgement the reported pulmonary or GI symptoms were related to KS, and if they answered “probably” or “definitely” (≥60% chance that the symptoms were related to KS), then these symptoms were considered attributable to KS in the analysis. Our patients were then further characterized according to i) ACTG T1 definitions (GI KS and disease in non-nodal viscera) and ii) WHO severe stage disease (definition slightly narrower, including symptomatic visceral disease specifically defined by the WHO as shortness of breath, hemoptysis, or moderate/severe cough not attributable to other causes (for pulmonary KS), and bleeding from mouth or rectum not attributable to other causes (for GI KS)).
** Staging of oral disease: Patients in the study were assessed for the presence of oral disease during physical exam, and oral lesions were characterized by location within the oropharynx and whether they were nodular or non-nodular. In addition, patients were asked about symptoms related to their oral disease, including difficulty swallowing, eating, drinking, or speaking, bleeding in the mouth, or pain in the mouth. For oral symptoms, the clinical team assessing the patient was then asked whether in their clinical judgement the reported symptoms were related to oral KS, and if they answered “probably” or “definitely” (≥60% chance that the symptoms were related to KS), then these symptoms were considered attributable to KS in the analysis. ACTG T1 extensive oral disease was defined as anything other than non-nodular KS confined to the palate, regardless of symptoms. WHO severe stage disease was defined as extensive oral KS, thought to be attributable to KS by the clinical team, that interferes with chewing or swallowing.
Results
Characteristics of the Study Population
Out of 389 adults patients identified with suspected KS, we performed rapid case ascertainment within one month for 241 (62%) of participants living with HIV diagnosed with KS from 2016 to 2019 at three health centers in East Africa. Sixty-six percent of these were identified at AMPATH-Kenya, 9% at ISS-Uganda, and 25% at AHF-Uganda; 97% were confirmed pathologically and the remainder were diagnosed based on clinical criteria. The median participant age was 35 years (IQR 30–42) and 68% were men (Table 1). A high fraction (77%) could read at grade 5 level, but only one-third achieved more than primary school education. Median monthly household income was approximately $270 USD, 34% had electricity in their homes, and 3.7% had a flush toilet.
Table 1.
Characteristics at the time of rapid case ascertainment among participants living with HIV diagnosed with KS within 1 month from 2016 to 2019 at three community-based health centers in East Africa.
| Characteristic | Median (IQR) or percentage (N=241) |
|---|---|
| Age | 35 (30–42) |
| Male sex | 68% |
| Site | |
| Kenya – AMPATH | 66% |
| Uganda – Uganda Cares | 25% |
| Uganda – ISS | 9% |
| Education, highest level achieved | |
| None/Primary | 67% |
| Secondary | 27% |
| Tertiary/University | 5.0% |
| Literate* | 77% |
| Average monthly household income (USD) | $270 (100–1200) |
| Electricity in home | 34% |
| Toilet type in home | |
| Uncovered pit latrine | 12% |
| Covered pit latrine | 84% |
| Flush toilet | 3.7% |
| Cost of one-way travel to care facility (USD) | $7 (2–100) |
| Weight (kg) | 58 (52–67) |
| Karnofsky Performance Status | 70 (60–80) |
| Antiretroviral therapy being used at time of rapid case ascertainment | 89% |
| Hemoglobin, g/dl | 11 (9.0–13) |
| CD4+ T-cells, cells/μl | |
| <150 cells/μl | 34% |
| ≥150 cells/μl | 61% |
| Unknown | 5.8% |
| Viral load categories, copies/ml | |
| < 40 | 37% |
| 40 – 1,000 | 29% |
| 1,001 – 10,000 | 10% |
| > 10,000 | 3.7% |
| Unknown | 20% |
Self-reported Symptoms
In structured interviews with clinicians, 75% of patients reported extremity edema (Table 2). For all patients, 30% reported persistent edema that did not improve with limb elevation, while 43.5% had at least partial improvement with limb elevation. A total of 27% of patients reported severe pain associated with extremity edema. Thirteen percent of patients reported periorbital edema, and overall 3.3% reported there was pain associated with this edema. Additionally, 14% of males reported scrotal edema and 2.1% of females reported genital edema. Across all patients, 7.5% reported occasional genital edema and 3.3% reported constant genital edema which impaired function. Overall patients had both moderate (3.7%) and severe (2.9%) genital edema.
Table 2.
Self-reported symptoms compatible with Kaposi sarcoma (KS) at the time of rapid case ascertainment among participants living with HIV diagnosed within 1 month with KS from 2016 to 2019.
| Symptom | Percent (N=241) |
|---|---|
| Oral | |
| Difficulty swallowing, eating, drinking or speaking | 17% |
| Bleeding in the mouth | 8.3% |
| Pain in the mouth | 12% |
| One oral symptom only | 12% |
| Two oral symptoms | 7.5% |
| Three oral symptoms | 3.7% |
| Gastrointestinal | |
| Abdominal Pain | 21% |
| Vomiting | 12% |
| Hematemesis | 1.2% |
| Blood in stool | 5.8% |
| Early satiety | 27% |
| One gastrointestinal symptom only | 16% |
| Two gastrointestinal symptoms | 12% |
| Three gastrointestinal symptoms | 7.9% |
| Four gastrointestinal symptoms | 1.7% |
| Pulmonary | |
| Shortness of breath | 22% |
| Cough | 28% |
| Coughing up blood | 3.7% |
| One pulmonary symptom only | 22% |
| Two pulmonary symptoms | 12% |
| Three pulmonary symptoms | 2.5% |
| Extremity edema | |
| Present | 75% |
| Severity of edema | |
| Resolves with limb elevation | 9.5% |
| Does not completely resolve with limb elevation | 34% |
| No improvement limb elevation | 30% |
| Significantly reduced function | 1.7% |
| Pain severity | |
| Mild | 5.8% |
| Moderate | 27% |
| Severe | 27% |
| Periorbital edema | |
| Present | 13% |
| Severity of edema | |
| Occasional edema | 6.2% |
| Sometimes edema | 2.9% |
| Always edema but doesn’t impair function | 2.5% |
| Always edema which impairs function | 1.2% |
| Pain severity | |
| Mild | 2.5% |
| Moderate | 3.3% |
| Severe | - |
| Scrotal or genital edema | |
| Scrotal edema (men only) | 14% |
| Genital edema (women only) | 2.1% |
| Severity of edema | |
| Occasional edema | 7.5% |
| Sometimes edema | 2.5% |
| Always edema but doesn’t impair function | 1.7% |
| Always edema which impairs function | 3.3% |
| Pain severity | |
| Mild | - |
| Moderate | 3.7% |
| Severe | 2.9% |
In total, 17% of patients reported difficulty with swallowing, eating, drinking or speaking, with 8.3% noting bleeding in the mouth and 12% noting pain in the mouth. Overall 21% of patients experienced abdominal pain, with 12% noting vomiting, 1.2% hematemesis, 5.8% hematochezia, and 27% early satiety. Finally, 22% of patients experienced shortness of breath and 28% cough, with fewer (3.7%) reporting hemoptysis.
Physical examination
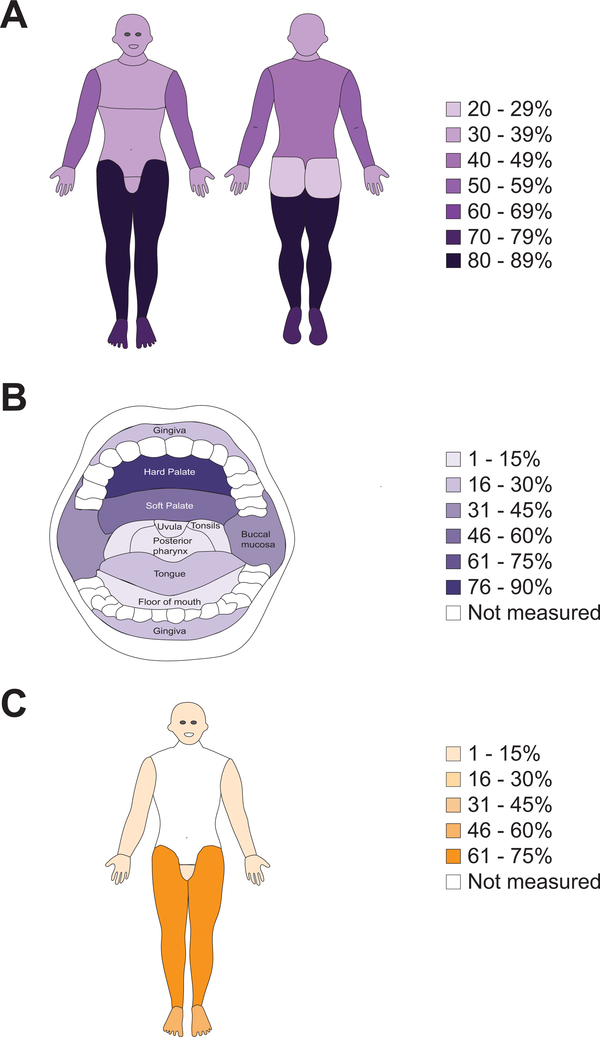
On clinician-documented physical exam, all patients had cutaneous lesions suggestive of KS, with 36% demonstrating ≥ 50 lesions (Table 3). The most common cutaneous sites were the legs (87%), feet (71%), arms (56%), back (39%), head (41%), chest (37%) and oral cavity (34%) (Figure 2); patients often had lesions in more than one location. Of the 34% of patients with oral KS, the most common sites were the hard palate (81%), soft palate (47%) and buccal mucosa (33%).
Table 3.
Physical examination findings compatible with Kaposi sarcoma (KS) at the time of rapid case ascertainment among participants living with HIV diagnosed within 1 month with KS from 2016 to 2019.
| Characteristic | Median (IQR) or Percentage (N=241) |
|---|---|
| Lesions compatible with KS | |
| ≥ 50 skin lesions* | 36% |
| Percentage of lesions that are raised† | 55% (35%–75%) |
| ≥ 1 lesion of tumor (≥ 20 mm) morphology | 37% |
| ≥ 1 lesion with ulceration | 24% |
| Largest diameter of ulceration (mm) | 20 (14–45) |
| ≥ 1 lesion with superinfection | 19% |
| Largest diameter of superinfection | 30 (15–50) |
| Median number of anatomic regions with KS** | 4 (2–7) |
| Lesion with the largest diameter (mm) | 28 (15–50) |
| Edema | |
| Any edema present | 77% |
| Facial (including periorbital) | 7.3% |
| Upper extremity | 0.4% |
| Genital | 21% |
| Lower extremity | 68% |
| No. of anatomic regions with edema*** | 2 (1 to 2) |
The presence of ≥ 50 skin lesions, as compared to <50 skin lesions, was not associated with ACTG T1 stage, presence of visceral disease, or CD4 count ≥200.
Anatomic regions for KS include the head, oral cavity, neck, chest, abdomen, back, arms, legs, hands, feet, genitals and gluteals
Anatomic regions for edema include the face, genitals, arms, legs, hands and feet.
Measured as number of raised compared to flat lesions averaged across three representative areas
Figure 2. Physical examination findings at the time of rapid case ascertainment among participants diagnosed within 1 month with KS.
A) Prevalence of at least one skin lesion suspected to be KS in various anatomical regions; B) Prevalence of at least one oral lesion suspected to be KS in various anatomical regions of the oral cavity; C) Prevalence of at least some edema in various anatomical regions.
When comparing the number of raised to flat lesions averaged across three representative areas of KS, the median percentage of raised lesions was 55% (IQR 35% to 75%). The median of the largest diameter of lesions was 28 mm (IQR 15 to 50). An ulcerated lesion was documented in 24% of patients and superinfection was documented in 19%.
Based on exam, edema was present in 77% of patients, with the most common locations including the feet (52%), legs (68%), genitals (21%), face including eyes (7.3%), hands (2.5%) and arms (0.4%). Median Karnofsky Performance Scale score was 70 (IQR 60 to 80).
Laboratory Measurements
At time of performing rapid case ascertainment the median Hemoglobin was 11 (IQR 9.0 to 13) g/dl. Overall 89% of patients were on ART at time of study enrollment. Median CD4 cell count was 239 (IQR 87–408) cells/μl. Overall 61% of patients had a CD4 count of >150 cells/μl at time of RCA and 37% had an undetectable viral load.
KS Staging at Time of Diagnosis
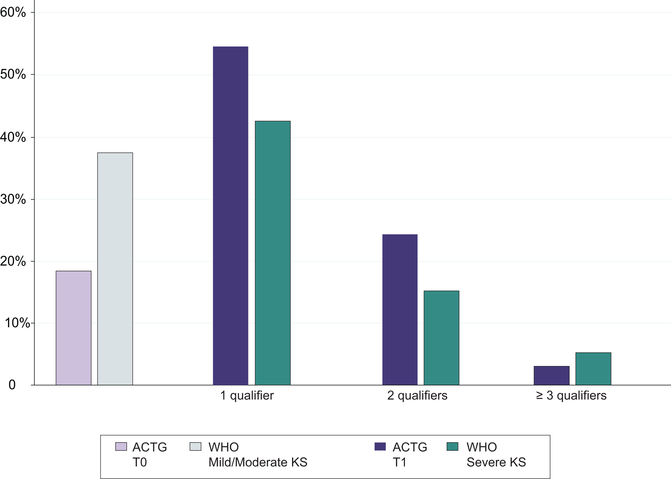
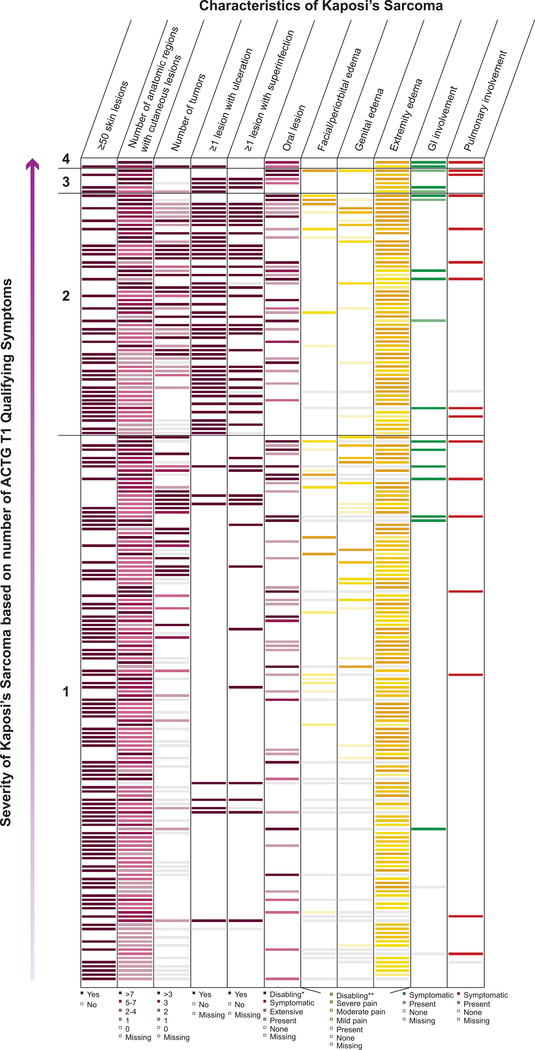
The majority of patients had advanced stage of disease, with 82% qualifying as ACTG T1 and 64% as WHO Severe/Symptomatic KS. Of all patients, 54% had one qualifier for ACTG T1, while 25% had two qualifiers and 3% had three or more qualifiers (Figure 3). In addition to variability based on the number of qualifying symptoms for ACTG T1 disease, there was variability of type of symptoms and clinical findings within each subgroup, e.g. within the subgroup that had just one qualifier for T1 disease (Figure 4, where each row represents one patient). For patients with one qualifier, the most common symptoms were edema (79%), tumor associated ulceration (24%), extensive oral KS (9%), pulmonary KS (7%), and gastrointestinal KS (4%). For patients with two or more ACTG T1 qualifiers, the most common symptom combinations included edema plus ulceration and edema plus oral KS.
Figure 3.
Distribution of conditions qualifying for ACTG T1 stage or WHO “severe” classification at the time of rapid case ascertainment among participants living with HIV newly diagnosed with Kaposi sarcoma from 2016 to 2019 at three community-based health centers in East Africa.
Figure 4:
Heterogeneity of KS characteristics in patients with ACTG T1 disease, grouped by severity of KS (defined by number of ACTG qualifying signs and symptoms). 1= one symptom qualifying the patient for ACTG T1, and 4= four symptoms qualifying the patient for ACTG T1.
Footnote: Each row represents one patient. Patients are grouped by number of ACTG qualifying symptoms, with the patients with the most qualifying symptoms for ACTG T1 at the top, and the patients with the least qualifying symptoms for ACTG T1 at the bottom.
For WHO staging, 36% of patients had WHO Mild/Moderate KS while 64% of patients had WHO Severe KS. Of all patients, 42% had one qualifier for WHO Severe KS, 15% had two qualifiers, and 7% had three or more qualifiers. The most common qualifying symptoms for WHO Severe were painful or disabling edema or ulcerated tumors (46%), life threatening or functionally disabling disease (22%), extensive oral KS interfering with chewing or swallowing (13%), symptomatic gastrointestinal disease (8%) and symptomatic pulmonary disease (7%). For those with two or more WHO Severe KS qualifiers, the most common symptom combination was edema plus life-threatening/disabling symptoms (n=34). Of patients who were staged ACTG T1, 70% were staged with WHO Severe KS. Of those with ACTG T0, 34% were staged with WHO Severe KS. Of the patients staged with WHO Severe KS, 90% were staged with ACTG T1 disease. Of those staged with WHO Mild/Moderate KS, 67% were staged with ACTG T1 disease. The association between ACTG and WHO staging was statistically significant (p<0.001).
Discussion
This study provides a comprehensive assessment of disease stage among a primary care facility-based sample of HIV-infected adults newly diagnosed with KS in East Africa. Rapid case ascertainment was used to obtain detailed measurements on 62% of people living with HIV within 1 month of KS diagnosis, making this study to our knowledge the most thorough and proximal assessment of KS stage at diagnosis in sub-Saharan Africa. By using detailed patient interviews, physical examinations, and laboratory evaluations, we were able to demonstrate substantial clinical heterogeneity among patients with advanced KS, particularly within ACTG T1 stage of disease.
The majority of patients in our study were diagnosed with advanced disease, with 82% of patients having ACTG T1 disease and 64% having WHO Severe/Symptomatic KS. These findings are similar to prior studies in sub-Saharan Africa reporting that 69 to 89% of KS patients present with T1 disease.4,5,24–26 This finding contrasts with high income countries where there is much less T1 disease, with recent estimates of 34–35%.27,28 Compared to prior studies, our documentation of advanced stage of disease is likely closer in time and more representative in a multi-site sub-Saharan African setting. In contrast, the majority of prior studies on KS stage in sub-Saharan Africa relied on chart review data, which often has missing records and incomplete documentation to determine staging.3,5,24–26 Other studies used clinical trial data or prospective cohort data to report stage, which contained rigid inclusion criteria and often excluded the sickest patients.4,29,30
A highlight of this study was our ability to document the clinical variability within the 82% of patients with ACTG T1 stage, which until now has not been reported in the literature. A smaller percentage of patients qualified for WHO Severe/Symptomatic KS compared to ACTG T1 KS, an effect mostly mediated by the classification of tumor-associated edema. While 70% of patients qualified for ACTG T1 due to “tumor associated edema,”, only 46% qualified for WHO Severe/Symptomatic KS due to “painful or disabling edema or ulcerated tumors.”
The differences we found between ACTG and WHO staging are important, as the diagnosis of advanced KS currently delineates which patients are prescribed chemotherapy. The WHO staging criteria focuses on the impact of KS symptoms on function and quality of life, while ACTG staging focuses on the presence or absence of KS symptoms. Classifying tumor burden in KS is challenging as there is no “primary” site.31 These uncertainties about staging have direct implications for KS treatment, as there continues to be ambiguity regarding which subtype of patients would benefit from chemotherapy in addition to ART. For example, in one study from South Africa although only 15% of KS patients were T0, 39% of patients responded to ART alone.4 Given the immense heterogeneity described among T1 patients in our study, we remain skeptical that all patients classified as “T1” require the same treatment approach. For example, some patients with T0 KS may have extensive skin disease requiring chemotherapy, and some patients with T1 KS may have mild edema not requiring chemotherapy. Especially in resource-limited areas with higher rates of opportunistic infection, loss to follow-up, and decreased capacity for supportive care, both under treatment with ART alone and overtreatment with chemotherapy are potentially perilous.
The reason for the large burden of T1 KS in sub-Saharan Africa is at least partially due to diagnostic delays, both during the time interval between patients noticing symptoms and reaching a clinician (“primary delay”), as well as between seeing a clinician and obtaining a diagnosis (“secondary delay”).29 Indeed, of the patients in our study where we were unable to complete rapid case ascertainment within one month of diagnosis, the most common reason was death very rapidly after diagnosis. However, we did not quantify the time between patients first noticing symptoms of KS and receiving a formal diagnosis in this analysis. A study from Uganda showed that 45% of KS patients had a primary delay of greater than 3 months due to lack of pain, lack of money for transportation, and distance to the facility. Patients who had a primary delay of three or more months were three times as likely to present with ACTG poor risk stage.29 This study further found that KS patients already enrolled in a HIV primary care clinic had the same diagnostic delay interval as those not accessing care, suggesting a lack of screening and knowledge of KS among HIV healthcare workers.29 This underscores the need to improve knowledge of HIV-associated malignancies among frontline HIV cadres, as well as incorporate cancer screening with routine HIV services. Especially in settings with limited ability to provide first line chemotherapy, early diagnosis of KS is critical.
The proximity of our assessment of stage to time of diagnosis additionally provided unique insights about the natural course of KS. The lower limbs were the most common sites of cutaneous KS.5,24 Similar to one prior study we found that only 34% of patients presented with oral KS5, which is lower than the 58%−65% reported in other studies.24,32 Comparable to prior studies we found a large number of lesions that were either ulcerated or superinfected.3 We demonstrated that 77% of patients presented with edema with the most common location being the lower limbs and genitals, consistent with prior literature.3,32 We demonstrated that 7% of patients presented with pulmonary KS and 8% presented with GI KS, which is similar to reports from prior retrospectives studies.5,24
Designing a staging system for HIV-associated KS presents unique challenges in the era of ART, where many patients are already on ART at time of diagnosis. In this cohort, 72% of patients were on ART greater than 60 days prior to KS diagnosis and 48% had an undetectable viral load, underscoring the burden of KS despite adequate HIV treatment. We surmise that the magnitude of disease burden has heightened importance in this setting, and that immune status (I) and systemic symptoms (S), which are greatly modified by ART, 3,4,29,19 may be less important now than they were during the development of the ACTG staging system in the 1980s.
However, the question remains which signs or symptoms of KS are predictive of KS mortality. In addition to TIS staging predictors, previous studies from both high and low income settings have found that pulmonary involvement,13 woody edema,26 low BMI,33 low hemoglobin,33 low albumin,32 older age,28 decreased performance status,28,32 and positive HHV-8 DNA to be predictive of mortality.30,34 The linkage of staging criteria with outcomes has recently been performed in the pediatric KS literature in Malawi, but has not yet been performed for the adult KS population in sub-Saharan Africa during the treat-all era.35 Our next steps will be to link the detailed measurements from this study on stage of disease in the ART era with mortality of these patients.
Our study has several limitations. The majority of eligible patients were enrolled within 1 month of diagnosis (62%), however some patients were enrolled up to six months after diagnosis, and some eligible patients were not captured. Of the eligible patients not captured, 63 died prior to obtaining study measurements. Therefore, our study likely underrepresents disease burden at time of diagnosis and could overestimate prevalence of ART use at time of diagnosis, though we did not capture whether patients who died were currently taking ART. For patients where other logistical reasons such as incorrect patient contact information prevented us from enrolling the patient within 1 month of diagnosis, KS severity was likely not different. Additionally, there were unique facilitators at our sites that may not be generalizable to other locations. All of the sites had affiliated oncology and dermatology departments and were additionally part of the IeDEA network, which provided KS biopsy services free of charge, allowing for a more rapid diagnosis. Additionally, all participating sites were part of a HIV-primary care network, thus a larger percentage of our patients may have been on ART compared to other areas. Because this study was conducted across multiple sites, there is likely some variability in how measurements were taken. Furthermore, this study relied on both self-reported measures as well as physical exam-documented measures, which were not always concordant. This was especially problematic for measuring visceral KS involvement, as we were unable to endoscopically confirm GI or pulmonary KS. Chest X-Rays, CT scans, and endoscopies were not routinely collected in our patients as part of standard of care in this setting, and thus extent of visceral KS is likely under-reported.36
In conclusion, a large fraction of patients with KS in this study had advanced stage disease at time of diagnosis, underscoring the need to target interventions to improve earlier diagnosis of KS. Additionally, within this group of advanced stage patients there is substantial heterogeneity, leading to questions about whether all patients with advanced KS require the same treatment strategy. Further studies are needed to confirm this clinical heterogeneity, and determine whether this heterogeneity is predictive of outcomes and should be included in a modified KS staging system.
Acknowledgments
Funding:
National Institutes of Health (U01 AI069911, K23 AI136579, K24 AI141036, U54 CA190153, and P30 AI027763) and the Dermatology Foundation.
Footnotes
Ethics: In Kenya the study was approved by the Institutional Research Ethics Committee (IREC) at Moi University in Eldoret, (Protocol No. 0001827) and in Uganda by the Makerere University College of Health Sciences School of Biomedical Sciences Higher Degrees Research and Ethics Committee (Protocol No. SBS-HD-REC-495) and the Uganda National Council of Science and Technology (UNCST) (Protocol No. HS157ES). Secondary data analysis was approved by the Mass General Brigham IRB (USA).
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018. [DOI] [PubMed] [Google Scholar]
- 2.Semeere A, Wenger M, Busakhala N, et al. A prospective ascertainment of cancer incidence in sub-Saharan Africa: The case of Kaposi sarcoma. Cancer Med. 2016;5(5):914–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okuku F, Krantz EM, Kafeero J, et al. Evaluation of a Predictive Staging Model for HIV-Associated Kaposi Sarcoma in Uganda. J Acquir Immune Defic Syndr. 2017;74(5):548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosam A, Shaik F, Uldrick TS, et al. A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa. J Acquir Immune Defic Syndr. 2012;60(2):150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sengayi MM, Kielkowski D, Egger M, Dreosti L, Bohlius J. Survival of patients with Kaposi’s sarcoma in the South African antiretroviral treatment era: A retrospective cohort study. S Afr Med J. 2017;107(10):871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kingham TP, Alatise OI, Vanderpuye V, et al. Treatment of cancer in sub-Saharan Africa. Lancet Oncol. 2013;14(4):e158–167. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Guide to cancer early diagnosis. 2017; https://apps.who.int/iris/bitstream/handle/10665/254500/9789241511940-eng.pdf;jsessionid=AF50425D982F68D3A8164560CE0908C7?sequence=1 (accessed February 26, 2020).
- 8.Bray F, Ferlay J, Laversanne M, et al. Cancer Incidence in Five Continents: Inclusion criteria, highlights from Volume X and the global status of cancer registration. Int J Cancer. 2015;137(9):2060–2071. [DOI] [PubMed] [Google Scholar]
- 9.Stefan DC. Cancer Care in Africa: An Overview of Resources. J Glob Oncol. 2015;1(1):30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howlader NNA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review. 1975–2016; https://seer.cancer.gov/csr/1975_2016/ (accessed February 27, 2020). [Google Scholar]
- 11.Krown SE, Metroka C, Wernz JC. Kaposi’s sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol. 1989;7(9):1201–1207. [DOI] [PubMed] [Google Scholar]
- 12.Krown SE, Testa MA, Huang J. AIDS-related Kaposi’s sarcoma: prospective validation of the AIDS Clinical Trials Group staging classification. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol. 1997;15(9):3085–3092. [DOI] [PubMed] [Google Scholar]
- 13.Nasti G, Talamini R, Antinori A, et al. AIDS-related Kaposi’s Sarcoma: evaluation of potential new prognostic factors and assessment of the AIDS Clinical Trial Group Staging System in the Haart Era--the Italian Cooperative Group on AIDS and Tumors and the Italian Cohort of Patients Naive From Antiretrovirals. J Clin Oncol. 2003;21(15):2876–2882. [DOI] [PubMed] [Google Scholar]
- 14.Guidelines on the treatment of skin and oral HIV-associated conditions in children and adults. WHO, Geneva, Switzerland. : World Health Organization (WHO);2014. [PubMed] [Google Scholar]
- 15.Tucker TC, Durbin EB, McDowell JK, Huang B. Unlocking the potential of population-based cancer registries. Cancer. 2019;125(21):3729–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schildkraut JM, Alberg AJ, Bandera EV, et al. A multi-center population-based case-control study of ovarian cancer in African-American women: the African American Cancer Epidemiology Study (AACES). BMC cancer. 2014;14:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biellik R, Madema S, Taole A, et al. First 5 years of measles elimination in southern Africa: 1996–2000. Lancet. 2002;359(9317):1564–1568. [DOI] [PubMed] [Google Scholar]
- 18.International Epdemiology Database to Evaluate AIDS (IeDEA). www.iedea.org (accessed January 24, 2020). accessed January 24, 2020.
- 19.Chen YT, Dubrow R, Zheng T, Barnhill RL, Fine J, Berwick M. Sunlamp use and the risk of cutaneous malignant melanoma: a population-based case-control study in Connecticut, USA. Int J Epidemiol. 1998;27(5):758–765. [DOI] [PubMed] [Google Scholar]
- 20.Berwick M, Armstrong BK, Ben-Porat L, et al. Sun exposure and mortality from melanoma. J Natl Cancer Inst. 2005;97(3):195–199. [DOI] [PubMed] [Google Scholar]
- 21.Fisman D, Tuite AR. Ebola: no time to waste. Lancet Infect Dis. 2014;14(12):1164–1165. [DOI] [PubMed] [Google Scholar]
- 22.Senga M, Koi A, Moses L, et al. Contact tracing performance during the Ebola virus disease outbreak in Kenema district, Sierra Leone. Philos Trans R Soc Lond B Biol Sci. 2017;372(1721). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2(3):187–193. [DOI] [PubMed] [Google Scholar]
- 24.Chu KM, Mahlangeni G, Swannet S, Ford NP, Boulle A, Van Cutsem G. AIDS-associated Kaposi’s sarcoma is linked to advanced disease and high mortality in a primary care HIV programme in South Africa. Journal of the International AIDS Society. 2010;13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fardhdiani V, Molfino L, Zamudio AG, et al. HIV-associated Kaposi’s sarcoma in Maputo, Mozambique: outcomes in a specialized treatment center, 2010–2015. Infect Agent Cancer. 2018;13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bekolo CE, Soumah MM, Tiemtore OW, et al. Assessing the outcomes of HIV-infected persons receiving treatment for Kaposi sarcoma in Conakry-Guinea. BMC cancer. 2017;17(1):806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bower M, Dalla Pria A, Coyle C, et al. Prospective stage-stratified approach to AIDS-related Kaposi’s sarcoma. J Clin Oncol. 2014;32(5):409–414. [DOI] [PubMed] [Google Scholar]
- 28.Stebbing J, Sanitt A, Nelson M, Powles T, Gazzard B, Bower M. A prognostic index for AIDS-associated Kaposi’s sarcoma in the era of highly active antiretroviral therapy. Lancet. 2006;367(9521):1495–1502. [DOI] [PubMed] [Google Scholar]
- 29.De Boer C, Niyonzima N, Orem J, Bartlett J, Zafar SY. Prognosis and delay of diagnosis among Kaposi’s sarcoma patients in Uganda: a cross-sectional study. Infect Agent Cancer. 2014;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borok M, Fiorillo S, Gudza I, et al. Evaluation of plasma human herpesvirus 8 DNA as a marker of clinical outcomes during antiretroviral therapy for AIDS-related Kaposi sarcoma in Zimbabwe. Clin Infect Dis. 2010;51(3):342–349. [DOI] [PubMed] [Google Scholar]
- 31.Cesarman E, Damania B, Krown SE, Martin J, Bower M, Whitby D. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseinipour MC, Kang M, Krown SE, et al. As-Needed Vs Immediate Etoposide Chemotherapy in Combination With Antiretroviral Therapy for Mild-to-Moderate AIDS-Associated Kaposi Sarcoma in Resource-Limited Settings: A5264/AMC-067 Randomized Clinical Trial. Clin Infect Dis. 2018;67(2):251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herce ME, Kalanga N, Wroe EB, et al. Excellent clinical outcomes and retention in care for adults with HIV-associated Kaposi sarcoma treated with systemic chemotherapy and integrated antiretroviral therapy in rural Malawi. Journal of the International AIDS Society. 2015;18:19929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Amari EB, Toutous-Trellu L, Gayet-Ageron A, et al. Predicting the evolution of Kaposi sarcoma, in the highly active antiretroviral therapy era. AIDS. 2008;22(9):1019–1028. [DOI] [PubMed] [Google Scholar]
- 35.El-Mallawany NK, Kamiyango W, Villiera J, et al. Proposal of a Risk-Stratification Platform to Address Distinct Clinical Features of Pediatric Kaposi Sarcoma in Lilongwe, Malawi. J Glob Oncol. 2018;4:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagata N, Shimbo T, Yazaki H, et al. Predictive clinical factors in the diagnosis of gastrointestinal Kaposi’s sarcoma and its endoscopic severity. PLoS One. 2012;7(11):e46967. [DOI] [PMC free article] [PubMed] [Google Scholar]