Abstract
Objective:
We investigated if anti-tumor necrosis factor-α (anti-TNF-α) drugs used in the treatment of inflammatory bowel disease (IBD) alter the incidence of MS and if so, to understand the magnitude of such an effect.
Methods:
This is a retrospective cohort study of data from Truven Health Market Scan administrative claims database. The patients included in the study had to be ≥ 18 years of age. The presence of IBD was based on at least 2 claims of International Classification of Diseases (ICD-9 or 10) diagnosis codes. The IBD diagnosis index date had to precede the MS diagnosis index date for inclusion in the study. The diagnosis of multiple sclerosis (MS) was defined as having at least 2 claims for the disease (ICD 9, 340 and ICD 10 codes, G35) and at least one prescription claim for any of the drugs that were defined as MS therapy.
Results:
Patients with IBD had 1.32 times the risk of MS incidence compared to healthy controls (adjusted incidence rate ratio (IRR): 1.32; 95% CI: 1.03 – 1.71; p = .0312). Patients with IBD exposed to anti-TNF-α therapies had a 43% increase in the incidence of MS compared to those with IBD without exposure (adjusted incidence rate: 1.43; 95% CI: .062 – 3.32; p = .3989). Among CD patients treated anti-TNF-α medications an increase in the incidence of MS, compared to CD patients not exposed to such medications was observed (IRR = 2.62; 95% CI: 1.00 to 6.83; p = 0.049), statistically significant. After adjusting for age/gender, patients with CD using anti-TNF-α agents had an increase of incidence in MS (adjusted IRR: 2.24; 95% CI: 0.85 – 5.94; p = .1035) but it was not statistically significant.
Conclusions:
Use of anti-TNF-α drugs in CD was associated with a statistically significant increase in the incidence of MS but this effect was lost when controlled for age/gender.
Keywords: TNF alpha blockers, Crohn” disease, Multiple sclerosis, Inflammatory bowel disease, Retrospective data analysis
1. Introduction
IBD is characterized by alterations of intestinal perfusion and one of the cytokines underlying the pathogenesis of mucosal inflammation is TNF-α. It is therefore the target of biologic therapies (Fasano and Shea-Donohue, 2005; Yapali and over Hamzaoglu, 2007). Increased numbers of TNF-α producing cells are present in intestinal biopsy specimens, more frequently in CD than UC (Breese et al., 1994). Breakdown of the intestinal barrier is an essential aspect of the pathophysiology of IBD (Camara-Lemarroy et al., 2018).
Published literature indicates that IBD and MS patients have a fifty percent increased risk (bi-directional) of MS or IBD comorbidity, respectively, with no apparent differences between patients with CD or UC (Kosmidou et al., 2017). The association between MS and IBD is strengthened by observations of an increased prevalence of IBD among MS patients compared to the general population (Langer-Gould et al., 2010; Marrie et al., 2015). Another study published in 2005 noted increased prevalence of MS, demyelination, and optic neuritis in patients with IBD (for UC, IRR, 2.63, 95% CI 1.29 to 5.15 and CD, IRR 2.12, 95% CI 0.94–4.50) (Gupta et al., 2005).
Genome-wide association studies (GWAS) have confirmed the complexity of MS and uncovered immune-related gene variants linked to other autoimmune disorders, including IBD (Sawcer et al., 2011). The association between MS and IBD shows an increased incidence of IBD, including both CD and UC, among MS patients (Langer-Gould et al., 2010; Zephir et al., 2014). Indeed, a meta-analysis of 10 case-control studies including over 1 million patients found an incident risk ratio (IRR) of 1.54 for multiple sclerosis/ IBD co-morbidity, with no difference between CD and UC (Kosmidou et al., 2017).
Therapeutic options in IBD include anti-TNF-α therapies and have been associated with CNS demyelination (Katsanos and Katsanos, 2014). The effects of anti-TNF-α drugs are mediated through TNF-α receptors which are present ubiquitously (Brenner et al., 2015). However, blocking TNF-α receptors in MS was a failure, as seen in the lenercept trial (1999). Lenercept is a recombinant fusion protein with two p55 soluble TNF receptors fused to the Fc portion of IgG1 and is protective against experimental autoimmune encephalitis (EAE), the animal model for CNS demyelinating disease but failed in clinical study. Instead of alleviation of symptoms, TNF-α blocking drugs may cause demyelinating events with a clinical pattern typical for MS (Kosmidou et al., 2017).
Recent studies show that the intestinal microbiome could play a role in the pathophysiology of MS. In one study, a 3-fold increase of white matter hyperintensities in the MRIs of patients with IBD was found (Geissler et al., 1995). Other findings in IBD include decreased gray matter volume and decreased axial diffusivity in major white matter tracts (Zikou et al., 2014). Mechanistic studies using EAE have suggested Th17 cells may play an essential role in EAE as well as in MS and IBD (Kunz and Ibrahim, 2009). The intestinal inflammation characteristic of IBD and the effects of Th17 cells are perhaps mediated by overproduction of pro-inflammatory cytokines, such as TNF-α and IL-6; IL-6 acting together with TGF-beta mediates the differentiation of Th17 cells (Liu et al., 2009). Several studies have implicated Th17 cells in MS and they can efficiently cross the blood-brain barrier using alternate ways from Th1 cells and induce inflammation in the brain (Dos Passos et al., 2016). Additionally, in EAE, increased intestinal permeability, overexpression of the tight junction protein, zonulin, and alterations in intestinal morphology were shown to occur by adoptive transfer of autoreactive T cells (Nouri et al., 2014).
The effect of anti-TNF-α drugs used in IBD patients on the incidence of MS using a large database has not been studied. Our study is an attempt to understand the relationship between the use of TNF-α blocking drugs among IBD patients and the incidence of MS using a large, commercial database and to ascertain the magnitude of such an effect, if present.
2. Methods
Data for our study was obtained from Truven Health MarketScan Commercial database which contains inpatient, outpatient, and outpatient prescription drug experience data of employees and their dependents from all US census regions who are covered under a variety of fee-for-service and capitated health plans, including exclusive provider organization (EPO), preferred provider organization (PPO), and point of service plans as well as indemnity plans and health maintenance organization (HMO) plans. The MarketScan data are fully de-identified prior to analysis in compliance with the Health Insurance Portability and Accountability Act regulations; thus, neither institutional review board approval nor informed patient consent was sought or required. The MarketScan Commercial data included in our study was from January 1, 2010, to March 31, 2018. The MarketScan Commercial database contained 96,523,179 distinct enroll ID values during this period. The data therefore do not belong to the authors and they are not permitted to share data, except in aggregate form.
The patients included in this study were 18 years of age or older. The presence of IBD was based on at least 2 claims of International Classification of Diseases (ICD-9 or 10) diagnosis codes. The diagnosis of IBD was classified as either CD, (K50 and all sub-groups and ICD 9 diagnosis of 555.9 and all sub-groups) or UC, (556.9 including all subgroups and K51 and all sub-codes). Enrollment of patients with IBD included medical and pharmacy benefits for at least 12 months before IBD index date (first date of diagnosis for IBD) and for at least 6 months after the index date was a prerequisite to be included in our study. The anti-TNF-α therapies for patients with IBD included in the study were adalimumab, infliximab, certolizumab, and golimumab. Etanercept was not included in this study as it is not an FDA-approved drug for the treatment of IBD. The anti-TNF-α therapy cohort data was collected from the prescription date to the last day of coverage, end of enrollment, or MS index date, which ever was earliest.
For inclusion in the study, the IBD diagnosis index date had to precede the MS diagnosis index date. The patients had to be on either anti-TNF-α therapy or a non-TNF-α drug therapy following the diagnosis of IBD. Patients with IBD were matched on age, gender and year of index date to 827,045 non-IBD controls. The non-IBD controls were required to have had enrollment with medical and pharmacy benefits for at least 12 months before assigned an index date and at least 6 months after the assigned index date and no MS-related claims on or before the assigned index dates. The ratio of IBD to unaffected controls cohort was set at 1:4.
MS diagnosis was defined as having at least 2 claims for the disease (ICD 9, 340 and ICD 10 codes, G35) and at least one prescription claim for any of the following drugs that we defined as MS therapy: interferon beta 1-a (intramuscular), interferon beta 1-b, glatiramer acetate, interferon beta-1a (subcutaneous), fingolimod, dimethyl fumarate, natalizumab, teriflunomide, peginterferon beta-1a, or alemtuzumab. Non-FDA drugs used in MS therapies were not included in this study. Participants were followed up until a MS diagnosis, the end of enrollment, or March 31, 2018, whichever was earliest.
The incidence of MS in each group was calculated as the number of individuals diagnosed with MS divided by the time at risk, as described below. The IRR of MS in the IBD vs control sample was estimated using Poisson regression. Both unadjusted and models adjusting for age and sex were used to estimate the IRRs. All models were offset using time at risk. Time at risk was calculated as the difference between the study enrollment (IBD index date among those with IBD) and either MS diagnosis date or the end of enrollment, whichever came first.
Additionally, within the IBD sample, the impact of anti-TNF-α blocking drug use on the IRR of MS was estimated using Poisson regression, offsetting for time at risk as described above, but instead using anti-TNF-α initiation as the index date. Similar to the previous analysis, both unadjusted and adjusted IRRs were estimated. Subgroup analyses were performed within the UC and CD samples, comparing those with and without anti-TNF exposure. Data management and analyses were performed using SAS 9.4 (SAS Institute Inc.), using two-sided tests with α = 0.05.
3. Data availability statement
De-identified data included for analysis in this study are available from the Truven Health MarketScan Commercial database, licensed to University of Kentucky.
4. Results
Between January 1, 2010 and March 31, 2018 a total of 208,681 individuals met criteria for inclusion in the IBD cohort (Table 1). Of these, 86,161 (41.3%) had a primary CD diagnosis and the remaining 122,520 (58.7%) had a primary UC diagnosis. The IBD patents were matched with 827,045 controls for a total sample of 1,035,726 individuals. Controls were slightly older than patients with IBD (mean age = 53.6 y and 49.4 y, respectively) and less likely to reside in the Northeast region of the U.S.
Table 1.
Demographic characteristics.
| Characteristic | Persons with IBD (n=208,681) | Persons with CD (n=86,161) | Persons with UC (n=122,520) | Persons without IBD (n=827,045) |
|---|---|---|---|---|
| Index age (years) | ||||
| 18–39 | 61,361 (29.4%) | 28,547 (33.1%) | 32,814 (26.8%) | 161,886 (19.6%) |
| 40–49 | 40,657 (19.5%) | 17,017 (19.8%) | 23,640 (19.3%) | 139,117 (16.8%) |
| 50–64 | 72,231 (34.6%) | 28,536 (33.1%) | 43,695 (35.7%) | 334,999 (40.5%) |
| ≥65 | 34,432 (16.5%) | 12,061 (14.0%) | 22,371 (18.3%) | 191,043 (23.1%) |
| Mean Age (SD) | 49.40 (16.75) | 47.67 (16.69) | 50.62 (16.69) | 53.56 (17.65) |
| Gender | ||||
| Male | 91,930 (44.1%) | 37,248 (43.2%) | 54,682 (44.6%) | 381,227 (46.1%) |
| Female | 116,751 (56.0%) | 48,913 (56.8%) | 67,838 (55.4%) | 445,818 (53.9%) |
| Index Year | ||||
| 2009 | 0 (0%) | 0 (0%) | 0 (0%) | 23,849 (2.9%) |
| 2010 | 45,567 (21.8%) | 18,207 (21.1%) | 27,360 (22.3%) | 182,474 (22.1%) |
| 2011 | 38,691 (18.5%) | 15,738 (18.3%) | 22,953 (18.7%) | 171,472 (20.7%) |
| 2012 | 34,147 (16.4%) | 13,830 (16.1%) | 20,317 (16.6%) | 151,547 (18.3%) |
| 2013 | 24,767 (11.9%) | 10,091 (11.7%) | 14,676 (12.0%) | 103,246 (12.5%) |
| 2014 | 20,870 (10.0%) | 8,742 (10.2%) | 12,128 (9.9%) | 75,329 (9.1%) |
| 2015 | 14,692 (7.0%) | 6,219 (7.2%) | 8,473 (6.9%) | 50,337 (6.1%) |
| 2016 | 12,413 (6.0%) | 5,628 (6.5%) | 6,785 (5.5%) | 38,905 (4.7%) |
| 2017 | 10,427 (5.0%) | 4,582 (5.3%) | 5,845 (4.8%) | 24,856 (3.0%) |
| 2018 | 7,107 (3.4%) | 3,124 (3.6%) | 3,983 (3.3%) | 5,030 (0.6%) |
| US Geographic Region | ||||
| Northeast | 51,988 (24.9%) | 21,336 (24.8%) | 30,652 (25.0%) | 73,296 (8.9%) |
| North Central | 48,202 (23.1%) | 20,445 (23.7%) | 27,757 (22.7%) | 276,197 (33.4%) |
| South | 71,955 (34.5%) | 30,243 (35.1%) | 41,712 (34.1%) | 369,291 (44.7%) |
| West | 33,458 (16.0%) | 12,754 (14.8%) | 20,704 (16.9%) | 107,793 (13%) |
| Unknown | 3,078 (1.5%) | 1,383 (1.6%) | 1,695 (1.4%) | 468 (0.1%) |
IBD = inflammatory bowel disease. CD = Crohn’s disease. UC = ulcerative colitis. Percentage in parentheses depicts the proportion of the original number of cases as categorized by age, gender, disease or controls and geographical region.
A total of 80 IBD patients (0.038%) and 235 controls (0.002 %) were subsequently diagnosed with MS. This equates to incidence rates per 1000 person-years of 0.062 and 0.043 for IBD and controls, respectively (Table 2). For both unadjusted and adjusted analyses, IBD patients had a significant increase in MS incidence rates (adjusted IRR = 1.32; 95% CI: 1.03 to 1.71; p = 0.031) compared to controls. Women had significantly higher incidence rates than men (adjusted IRR = 2.40; 95% CI: 1.87 to 3.09; p < 0.001), and age had a negative relationship with MS incidence (adjusted IRR = 0.98; 95% CI: 0.97 to 0.98; p < .001).
Table 2.
Incidence of MS in persons with IBD and non-IBD matched controls
| Univariate Poisson Model | Multivariate Poisson Modelb | ||||||
|---|---|---|---|---|---|---|---|
| Group | MS Event | Person-years | Ratea | Crude IR | P value | Adjusted IR | P value |
| IBD | 80 | 1,287,242 | 0.062 | 1.45 (1.12 −1.87) | 0.0042 | 1.32 (1.03 −1.71) | 0.0312 |
| Control | 235 | 5,478,412 | 0.043 | 1 (Reference) | 1 (Reference) | ||
Abbreviations: MS = multiple sclerosis. IBD = inflammatory bowel disease.
Indicates significance, p-value <0.05
Incidence rate per 1,000 person-years.
Adjusted for Age and Sex
Of the of 208,681 IBD patients, 13,527 (6.48%) who had exposure to anti-TNF-α blocking drugs, 6 (0.04%) were diagnosed with MS whereas 195,154 (93.48%) no exposure to the anti-TNF-α drugs and of these, 74 (0.04%) were diagnosed with MS. This resulted in incidence rates of 0.099 and 0.061 per 1000 person-years in the exposed and unexposed groups, respectively. The incidence of MS among IBD patients exposed to anti-TNF-α drugs was 43% higher than those who were not (adjusted IRR = 1.43; 95% CI: 0.62 to 3.32; p = 0.399) (Table 3), but not statistically significant. In this sample, women had a significantly higher incidence rate compared to men (adjusted IRR = 2.12; 95% CI: 1.29 to 3.46; p = 0.003) and age had a negative relationship with MS incidence (adjusted IRR = 0.98; 95% CI: 0.97 to 0.99; p = 0.012).
Table 3.
Incidence of MS among patients with IBD by anti-TNF exposure
| Univariate Poisson Model | Multivariate Poisson Modelb | ||||||
|---|---|---|---|---|---|---|---|
| Anti-TNF Exposure | MS Event | Person-years | Ratea | Crude IR | p-value | Adjusted IR | p-value |
| All IBD | |||||||
| Yes | 6 | 60,713 | 0.099 | 1.62 (0.70 – 3.72) | 0.2561 | 1.43 (0.62 – 3.32) | 0.3989 |
| No | 74 | 1,212,610 | 0.061 | 1 (Reference) | 1 (Reference) | ||
| UC | |||||||
| Yes | 1 | 23,899 | 0.042 | 0.62 (0.09 – 4.52) | 0.6408 | 0.57 (0.08 – 4.15) | 0.5804 |
| No | 49 | 730,999 | 0.067 | 1 (Reference) | 1 (Reference) | ||
| CD | |||||||
| Yes | 5 | 36,814 | 0.136 | 2.62 (1.00 – 6.83) | 0.0496 | 2.24 (0.85 – 5.94) | 0.1035 |
| No | 25 | 481,611 | 0.052 | 1 (Reference) | 1 (Reference) | ||
Abbreviations: MS = multiple sclerosis. IBD = inflammatory bowel disease. CD = Crohn’s disease. UC = ulcerative colitis. TNF = tumor necrosis factor.
indicates significance, p-value <0.05
Incidence rate per 1,000 person-years.
Adjusted for Age and Sex
When separated by IBD type, UC patients with and without anti-TNF-α exposure had similar incidence rates (adjusted IRR = 0.57; 95% CI: 0.08 to 4.15; p = .580) (Table 3). Among CD patients, those treated anti-TNF-α medications showed an increase in the incidence of MS compared to those without anti-TNF-α exposure (IRR = 2.62; 95% CI: 1.00 to 6.83; p = 0.049), which was statistically significant. However, this increased risk was no longer statistically significant after adjusting for age/gender in CD patients exposed to anti-TNF-α drugs (adjusted IRR = 2.24; 95% CI: 0.85 to 5.94; p = 0.104). Fig. 1 presents a graphic representation of the cumulative incidence of MS for IBD, UC and CD patients, categorized by anti-TNF drug exposure.
Fig. 1.
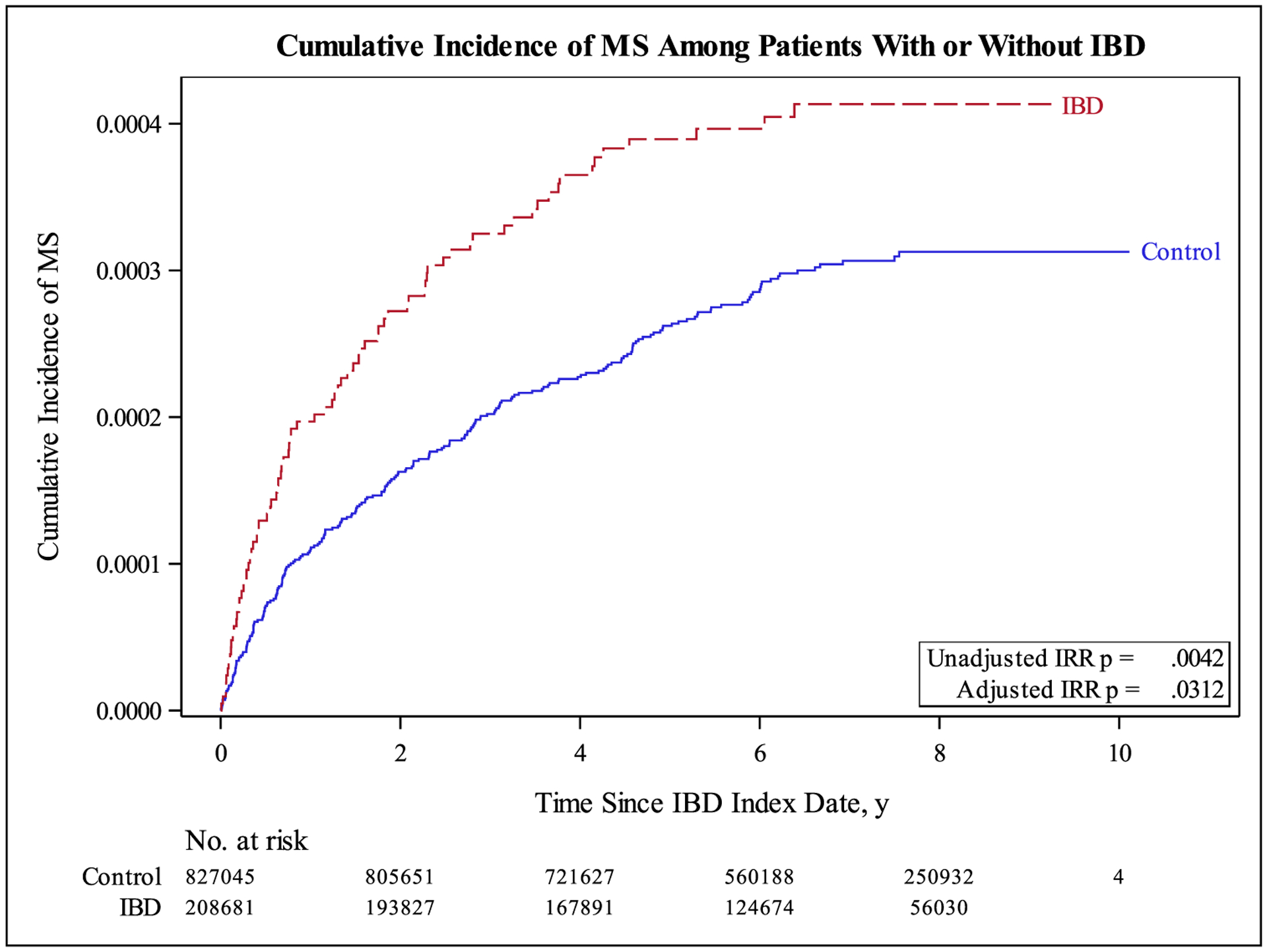
Cumulative Incidence of MS Among Patients With or Without IBD
5. Discussion
Our study included a total of 96,523,179 distinct enrollees included in the Truven Health MarketScan database from January 1, 2010 through March 31, 2018 of whom 208,681 patients with IBD were matched to 827,045 non-IBD controls. We observed a significantly higher incidence of MS in the IBD cohort, with a 1.32-fold increase in incidence rate compared to age/gender-matched non-IBD controls.
The effect of TNF-α therapies in the CD cohort was associated with an increase in the probability of developing MS, which was statistically significant when not matched for age/gender. It is possible that the spike found in the CD cohort alone is secondary to increased TNF-α producing cells present in intestinal biopsy specimens in CD compared to UC (Breese et al., 1994).
Our findings cannot be extrapolated to those of another study that reported prevalence rates of MS in IBD (IRR of 1.54, 95% CI 1.40–1.67; p < 0.0001) and not incidence rates (Kosmidou et al., 2017). Another study suggested an association between IBD and demyelinating disorders but was limited by a small sample size and not specific to MS diagnosis (Gupta et al., 2005).
The strengths of our study include patients drawn from a well-defined ICD code-driven population, inclusion of a control group, a large sample size, cohort design and the ability to examine subgroups of patients such as those with CD and UC. We reverse engineered a published study which showed that the incidence of Parkinson’s disease (PD) is decreased by 78% in IBD patients using anti-TNF-α medications (Peter et al., 2018). Given the history of anti-TNF-α drug effects in MS, we hypothesized that the treatment of IBD with TNF-α blocking drugs could have an increase in the incidence of MS, contrasting with the decrease in incidence of PD (1999; Brenner et al., 2015).
The association between IBD and MS was recognized in the 1980s in a study of 2661 women who underwent total colectomy for UC in the UK which described an annual incidence of 18 per 100 000 persons compared the expected incidence of 5 per 100 000 persons in British women without UC.(Rang et al., 1982) Similarly, in a retrospective analysis of medical records from 1950 through 1995, the observed prevalence of MS at onset of IBD was 3.7 times the expected number [95% CI: 0.8–10.8] (Kimura et al., 2000). A previous study by Khalili and colleagues suggests that people living in the Northern regions had a higher incidence of IBD but our database analyses cannot make any such associations since our findings could reflect ascertainment bias (Khalili et al., 2012). In two large prospective cohorts of US women, the incidence of UC and CD was significantly lower among women who resided in the southern latitudes, particularly in later life (age 30 years), than in those in the northern latitudes.(Khalili et al., 2012). Both IBD and MS affect predominantly young adults, occur more frequently in those living in Northern latitudes and presumably have polygenic, hereditary and environmental susceptibility factors (Kurtzke, 1980). Additionally, an increased risk of infections in patients with IBD using anti-TNF-α agents has been described (Andersen et al., 2015).
The link between anti-TNF-α therapy and MS has been mainly explored through the failure of TNF-α blockade in clinical trials (1999; Bosch et al., 2011; Hare et al., 2014; Titelbaum et al., 2005). In clinical studies, anti-TNF-α therapy (Lenercept) in 168 patients with MS demonstrated a significantly increased risk of disease exacerbation (1999). In an open label, phase 1 trial with infliximab, two MS patients developed gadolinium enhancing lesions, increased CSF IgG index suggesting that non-selective inhibition of TNF-α could be harmful in MS (van Oosten et al., 1996). Expanding beyond TNF-α blockade and MS, others reported more generally on exposure to these drugs used for IBD and rheumatoid arthritis and the occurrence of unspecified demyelinating events. Among adalimumab-treated CD patients in placebo-controlled trials and open-label extension studies, the incidence of demyelination/optic neuritis is two events per 1000 patient-years (Simsek et al., 2007).
In the United Kingdom, 36 cases of central demyelination and 1040 other neurological events complicating TNF-α therapy had been reported (Hare et al., 2014). Insight into drug-specific demyelinating events is provided by the BIOGEAS registry (Spanish study group of Biological agents in autoimmune diseases), a Spanish prospective cohort study that has been investigating the long-term safety and efficacy of biologics in off-label use among patients with systemic autoimmune disease since 2002 (Diaz-Lagares et al., 2011). In the BIOGEAS registry, IBD (n=845), demyelinating CNS disease (n=803) were among the most frequently reported immunological phenomena and anti-TNF-α agents (n=9133) were the ‘culprit drugs’ including adalimumab (n=4154), infliximab (n=3078) and etanercept (n=1681) (Perez-De-Lis et al., 2017). Demyelination following TNF-α therapy has been reported in IBD and most showed improvement of their neurological symptoms after discontinuation of anti-TNF-α drugs (Mohan et al., 2001).
Limitations of our study include the following: we could not examine the effect of race and ethnicity as they are unavailable in the Truven database. In Hispanic Americans and African-Americans, MS is often severe and IBD diagnosis over the years has increased but our study did not account for any categorization based on racial background or ethnicity (Aniwan et al., 2019; Ventura et al., 2017). Additionally, data on disease severity of either MS or IBD could not be ascertained given the lack of such data in the database. Our dataset does not also specify which specific drugs/frequency/dose contributed to the incidence of MS. We did not opt to test other drugs approved for IBD treatment such as Natalizumab or Vedolizumab (anti-integrins) as we sought to evaluate the association between a single class of drugs (anti-TNF-α) used in IBD and their relationship to the incidence of MS. No potential risk factors associated with MS such as smoking, vitamin D status, BMI, or family history were considered since such risk factors would confer risks to IBD as well and the design of this study was such that a diagnosis of IBD came first, followed by an MS diagnosis. Additionally, IBD patients were not randomly assigned to receive or not receive anti-TNF therapies, precluding the ability to draw causal conclusions. It is possible that anti-- TNF-α agents were prescribed to IBD patients who had a more severe disease phenotype, contributing to increased incidence of MS. The percentage of IBD patients under treatment with anti-TNF-α drugs was not ascertained as such data are unavailable and hence, ascertainment bias for cases and controls could represent another source of bias. Lastly, we did not include monophasic demyelinating events such as optic neuritis, transverse myelitis or central demyelinating lesions of the brain which are perhaps more common than a diagnosis of MS following the use of anti-TNF-α drugs. It has been reported that among patients with various autoimmune diseases treated with TNF-α blockers that developed CNS demyelination, only 26% were diagnosed with MS. In the majority of patients, a monophasic demyelinating event was confirmed. Moreover, discontinuation of TNF-α blockers usually results in remission and/or recovery of the affected patients. (Cruz Fernández-Espartero M et al. 2011) although this phenomenon has been observed in rheuma-tological disorders. With the widespread use of TNF-α blockers, a growing number of demyelinating events have been reported, including CNS demyelinating disorders including MS, optic neuritis (ON), acute transverse myelitis (TM), as well as peripheral nervous system disorders (Guillain-Barré syndrome, Miller Fisher syndrome, chronic inflammatory demyelinating polyneuropathy, multifocal motor neuropathy with conduction block, mononeuropathy multiplex, and axonal sensorimotor polyneuropathies have been described (Bosch X, et al., 2011) and what is unclear is if the side-effects are epigenetic phenomena or caused by side-effects of the anti-TNF-α drugs (Kemanetzoglou and Andreadou, 2017).
Given the low incidence of MS, even among IBD patients exposed to anti-TNF therapies, designing randomized clinical trials could make it expensive. While no substitute for a randomized trial, propensity score matching methods could also be used to strengthen causal claims in lieu of randomization. This would require the use of a larger datasets unavailable to us. Finally, administrative data claims datasets cannot be cross-checked with individual patient medical records to assess for the accuracy of the ICD or MS codes as reported since cases are de-identified and do not allow for this line of investigation. Prospective studies are needed to ascertain if anti-TNF-α drugs used in IBD treatment confer an additional risk of developing MS. An association with diametrically opposite effects among IBD patients treated with TNF-α drugs showing a decrease in the incidence of PD and an increase in the incidence of MS has never been demonstrated (Fig. 2).
Fig. 2.
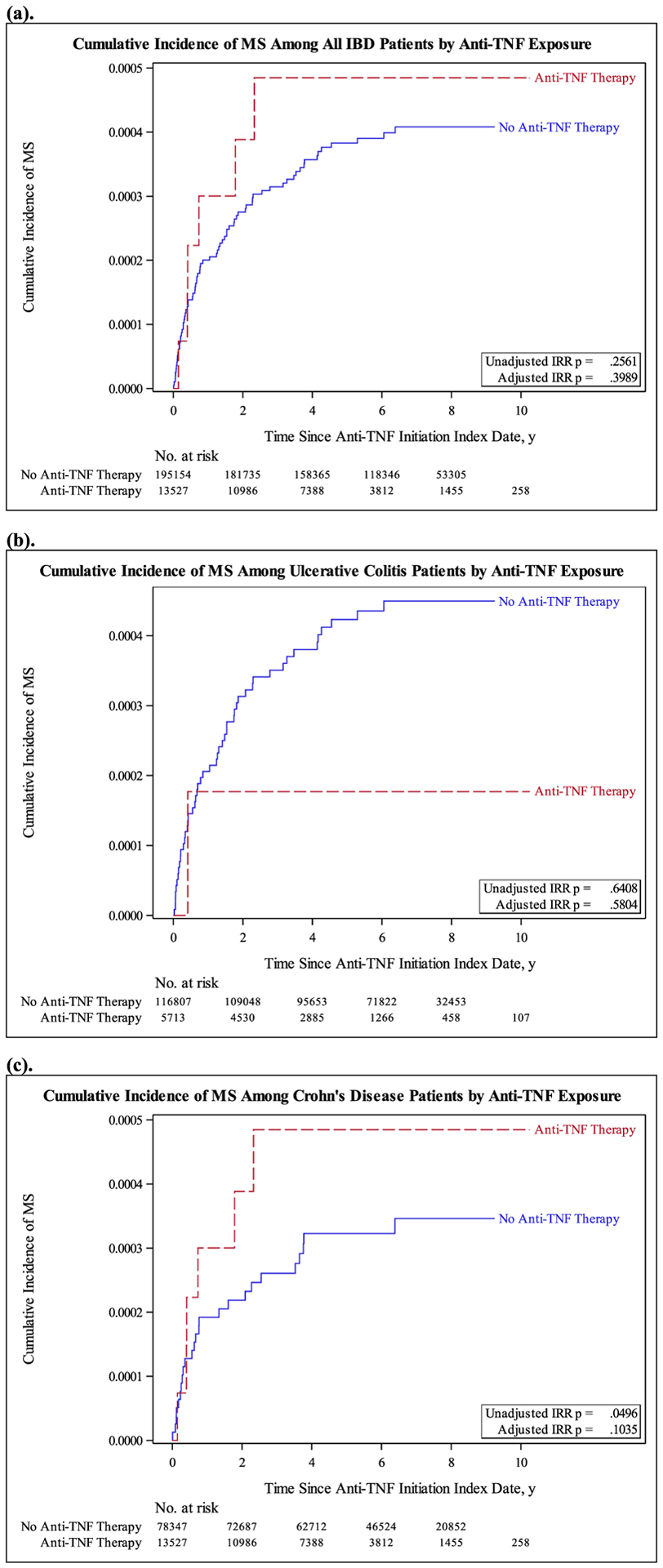
Cumulative Incidence of MS Among Patients by Anti-TNF-α Exposure for All IBD Patients (a), Ulcerative Colitis Patients (b), and Crohn’s Disease patients (c).
Primary funding source
The database used in this study was supported by the NIH National Center for Advancing Translational Sciences and Center for Clinical and Translational Science through grant number UL1TR001998. Our study was not supported by any funding.
Funding
NIH National Center for Advancing Translational Sciences and Center for Clinical and Translational Science (UL1TR001998) supported the database used in this study.
Footnotes
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- 1999 TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The lenercept multiple sclerosis study group and The University of British Columbia MS/MRI analysis group. Neurology 53 (3), 1999, 457–465. [PubMed] [Google Scholar]
- Aniwan S, et al. , 2019. Incidence of inflammatory bowel disease by race and ethnicity in a population-based inception cohort from 1970 through 2010. Therap. Adv. Gastroenterol 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch X, et al. , 2011. Monoclonal antibody therapy-associated neurological disorders. Nat. Rev. Neurol 7 (3), 165–172. [DOI] [PubMed] [Google Scholar]
- Breese EJ, et al. , 1994. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology 106 (6), 1455–1466. [DOI] [PubMed] [Google Scholar]
- Brenner D, et al. , 2015. Regulation of tumour necrosis factor signalling: live or let die. Nat. Rev. Immunol 15 (6), 362–374. [DOI] [PubMed] [Google Scholar]
- Camara-Lemarroy CR, et al. , 2018. The intestinal barrier in multiple sclerosis: implications for pathophysiology and therapeutics. Brain 141 (7), 1900–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Lagares C, et al. , 2011. Rates of, and risk factors for, severe infections in patients with systemic autoimmune diseases receiving biological agents off-label. Arthr. Res. Ther 13 (4), R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Passos GR, et al. , 2016. Th17 cells pathways in multiple sclerosis and neuromyelitis optica spectrum disorders: pathophysiological and therapeutic implications. Mediat. Inflamm 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Shea-Donohue T, 2005. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nature clinical practice. Gastroenterol. Hepatol 2 (9), 416–422. [DOI] [PubMed] [Google Scholar]
- Geissler A, et al. , 1995. Focal white-matter lesions in brain of patients with inflammatory bowel disease. Lancet (Lond. Engl.) 345 (8954), 897–898. [DOI] [PubMed] [Google Scholar]
- Gupta G, et al. , 2005. Increased risk for demyelinating diseases in patients with inflammatory bowel disease. Gastroenterology 129 (3), 819–826. [DOI] [PubMed] [Google Scholar]
- Hare NC, et al. , 2014. Multiple sclerosis in the context of TNF blockade and inflammatory bowel disease. QJM : Month. J. Asso. Phys 107 (1), 51–55. [DOI] [PubMed] [Google Scholar]
- Katsanos AH, Katsanos KH, 2014. Inflammatory bowel disease and demyelination: more than just a coincidence? Expert Rev. Clin. Immunol 10 (3), 363–373. [DOI] [PubMed] [Google Scholar]
- Khalili H, et al. , 2012. Geographical variation and incidence of inflammatory bowel disease among US women. Gut 61 (12), 1686–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, et al. , 2000. Concurrence of inflammatory bowel disease and multiple sclerosis. Mayo Clin. Proceed 75 (8), 802–806. [DOI] [PubMed] [Google Scholar]
- Kosmidou M, et al. , 2017. Multiple sclerosis and inflammatory bowel diseases: a systematic review and meta-analysis. J. Neurol 264 (2), 254–259. [DOI] [PubMed] [Google Scholar]
- Kunz M, Ibrahim SM, 2009. Cytokines and cytokine profiles in human autoimmune diseases and animal models of autoimmunity. Mediat. Inflamm, 979258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzke JF, 1980. Epidemiologic contributions to multiple sclerosis: an overview. Neurology 30 (7 Pt 2), 61–79. [DOI] [PubMed] [Google Scholar]
- Andersen NN, et al. , 2015. Association between tumor necrosis factor-α inhibitors and risk of serious infections in people with inflammatory bowel disease: nationwide Danish cohort study. BMJ 350, h2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer-Gould A, et al. , 2010. Autoimmune diseases prior to the diagnosis of multiple sclerosis: a population-based case-control study. Mult. Scler. (Houndmills, Basingstoke, England) 16 (7), 855–861. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, et al. , 2009. Potential role of Th17 cells in the pathogenesis of inflammatory bowel disease. World J. Gastroenterol 15 (46), 5784–5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie RA, et al. , 2015. A systematic review of the incidence and prevalence of autoimmune disease in multiple sclerosis. Mult. Scler. (Houndmills, Basingstoke, Engl.) 21 (3), 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan N, et al. , 2001. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthr. Rheum 44 (12), 2862–2869. [DOI] [PubMed] [Google Scholar]
- Nouri M, et al. , 2014. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PloS one 9 (9), e106335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-De-Lis M, et al. , 2017. Autoimmune diseases induced by biological agents. A review of 12,731 cases (BIOGEAS Registry). Expert Opin. Drug Saf 16 (11), 1255–1271. [DOI] [PubMed] [Google Scholar]
- Peter I, et al. , 2018. Anti-Tumor necrosis factor therapy and incidence of parkinson disease among patients with inflammatory bowel disease. JAMA Neurol 75 (8), 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang EH, et al. , 1982. Association of ulcerative colitis with multiple sclerosis. Lancet (Lond., Engl.) 2 (8297), 555. [DOI] [PubMed] [Google Scholar]
- Sawcer S, et al. , 2011. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476 (7359), 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek I, et al. , 2007. Optic neuritis occurring with anti-tumour necrosis factor alpha therapy. Ann. Rheumatic Dis 66 (9), 1255–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titelbaum DS, et al. , 2005. Anti-tumor necrosis factor alpha-associated multiple sclerosis. AJNR. Am. J. Neuroradiol 26 (6), 1548–1550. [PMC free article] [PubMed] [Google Scholar]
- van Oosten BW, et al. , 1996. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology 47 (6), 1531–1534. [DOI] [PubMed] [Google Scholar]
- Ventura RE, et al. , 2017. Hispanic Americans and African Americans with multiple sclerosis have more severe disease course than Caucasian Americans. Mult. Scler. (Houndmills, Basingstoke, England) 23 (11), 1554–1557. [DOI] [PubMed] [Google Scholar]
- Yapali S, over Hamzaoglu H.J.A.o.g., 2007. Anti-TNF treatment in inflammatory bowel disease
- Zephir H, et al. , 2014. Milder multiple sclerosis course in patients with concomitant inflammatory bowel disease. Mult. Scler. (Houndmills, Basingstoke, England) 20 (8), 1135–1139. [DOI] [PubMed] [Google Scholar]
- Zikou AK, et al. , 2014. Brain involvement in patients with inflammatory bowel disease: a voxel-based morphometry and diffusion tensor imaging study. Eur. Radiol 24 (10), 2499–2506. [DOI] [PubMed] [Google Scholar]
- Fernandez-Espartero MC, et al. , 2011. Demyelinating disease in patients treated with with TNF antagonists in rheumatology: data from BIOBADASER, a pharmacovigilance database, and a systematic review. Seminars Arthr. Rheumatism 41 (3), 524–533. [DOI] [PubMed] [Google Scholar]
- Bosch X, et al. , 2011. Monoclonal antibody therapy-associated neurologic disorders. Nat. Rev. Neurol 7 (3), 165–172, 2011. [DOI] [PubMed] [Google Scholar]
- Kemanetzoglou E, Andreadou E, 2017. CNS demyelination with TNF-α Blockers. Curr. Neurol. Neurosci. Rep 17 (4), 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified data included for analysis in this study are available from the Truven Health MarketScan Commercial database, licensed to University of Kentucky.


