Abstract
Background
Eculizumab is approved for the treatment of atypical hemolytic uremic syndrome (aHUS). Its use off-label is frequently reported. The aim of this study was to describe the broader use and outcomes of a cohort of pediatric patients exposed to eculizumab.
Methods
A retrospective, cohort analysis was performed on the clinical and biomarker characteristics of eculizumab-exposed patients ≤ 25 years of age seen across 21 centers of the Pediatric Nephrology Research Consortium. Patients were included if they received at least one dose of eculizumab between 2008 and 2015. Traditional summary statistics were applied to demographic and clinical data.
Results
A total of 152 patients were identified, mean age 9.1 (+/−6.8) years. Eculizumab was used “off-label” in 44% of cases. The most common diagnoses were aHUS (47.4%), Shiga toxin-producing Escherichia coli HUS (12%), unspecified thrombotic microangiopathies (9%), and glomerulonephritis (9%). Genetic testing was available for 60% of patients; 20% had gene variants. Dosing regimens were variable. Kidney outcomes tended to vary according to diagnosis. Infectious adverse events were the most common adverse event (33.5%). No cases of meningitis were reported. Nine patients died of noninfectious causes while on therapy.
Conclusions
This multi-center retrospective cohort analysis indicates that a significant number of children and young adults are being exposed to C5 blockade for off-label indications. Dosing schedules were highly variable, limiting outcome conclusions. Attributable adverse events appeared to be low. Cohort mortality (6.6%) was not insignificant. Prospective studies in homogenous disease cohorts are needed to support the role of C5 blockade in kidney outcomes.
Keywords: Eculizumab, Pediatric, Hemolytic uremic syndrome, Atypical hemolytic uremic syndrome
Introduction
Eculizumab (Soliris®, Alexion Pharmaceuticals, Cheshire, CT, USA) is a recombinant, humanized monoclonal antibody directed against human complement component C5. It inhibits the function of the terminal complement pathway by binding C5 and preventing the cleavage of C5 into C5a (a potent anaphylatoxin) and C5b, the first protein of the membrane attack complex (MAC) [1–3]. The efficacy of eculizumab for the treatment of atypical hemolytic syndrome (aHUS) was first described in 2009 [4–8]. Subsequent data from both uncontrolled (case reports) and controlled (clinical trials) further established the safety and efficacy of eculizumab in this setting [9–12]. Eculizumab received approval for the treatment of aHUS by the European Medicines Agency (EMA) in July 2009 and by the US Food and Drug Administration (FDA) in September 2011 [13]. Where available, eculizumab has become the standard of care for treating aHUS as well as other rare diseases: paroxysmal nocturnal hemoglobinuria (PNH), neuromyelitis optica spectrum disorder (NMOSD), and refractory myasthenia gravis [14].
Eculizumab has appeared in a number of case reports describing off-label use, particularly in diseases that are presumed to have dysregulated complement activity as a part of their underlying pathology. Single-center publications exist for the off-label use of eculizumab in (1) Shiga toxin-producing Escherichia coli hemolytic uremic syndrome (STEC HUS) [15–19], (2) hematopoietic stem cell transplantation-associated thrombotic microangiopathy (TMA) [20, 21], (3) complement-mediated glomerulonephritis (GN) [22–31], and (4) antibody-mediated kidney allograft rejection (AMR) [32–44]. Until results become available from prospective, randomized control trials in these presumed abnormal complement activity disorders, practitioners will continue to rely on small case series, single-center studies, and case reports to guide their use of eculizumab for off-label indications. Circumstantial data reporting such as this is heavily influenced by publication bias—particularly as it applies to the over-reporting of positive outcomes. We conducted a multi-center retrospective chart review to describe the spectrum of eculizumab use in children and young adults in the USA from 2008 to 2015. Our goal was to understand the contemporary use of terminal complement blockade, including indications, dosing practices, kidney outcomes, and adverse events.
Methods
Twenty-one (of 60) Pediatric Nephrology Research Consortium (PNRC) centers volunteered to participate in this study. All centers were governed by local IRB. Patients were identified from the medical records of participating centers. All patients ≤ 25 years of age who received at least one dose of eculizumab between August 1, 2008 and July 31, 2015 were included in the study. Data collection ended on July 31, 2015.
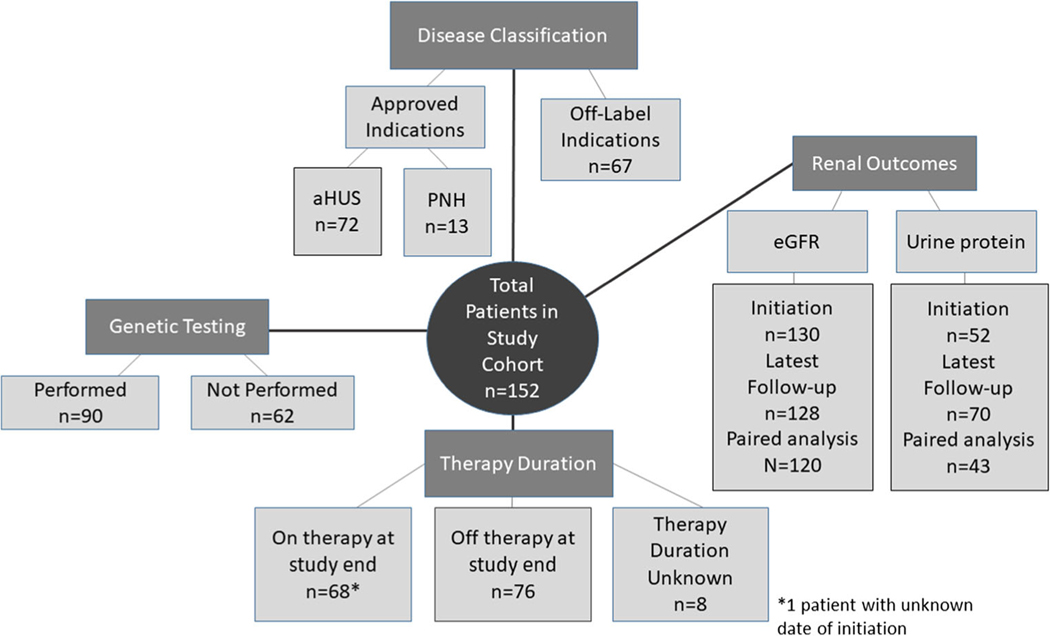
Six hundred and eleven data points were collected on each patient (REDCap ©- University of Iowa) including demographics, diagnoses and disease characteristics, reported indication and prescribing information for eculizumab, genetic testing results, adverse events during therapy, and estimated glomerular filtration rate (eGFR) and urine protein. See Fig. 1 for the schematic of the disease classification, genetic testing, treatment duration, and kidney outcomes in the 152 patients. Diagnoses were defined per institution and included atypical hemolytic uremic syndrome (aHUS), Shiga toxin-producing Escherichia coli HUS (STEC HUS), non-STEC infection-related TMA, GN, other TMA, paroxysmal nocturnal hemoglobinuria (PNH), AMR, and other diagnoses. Non-STEC infection-related TMA included patients with clinical TMA associated with other or unknown infections. “Patients with other TMA” captures those with thrombotic microangiopathy that did not fit into the other available categories.
Fig. 1.
Overall schematic of the disease classification, genetic testing, treatment duration, and renal outcomes in the 152 children from 21 different centers within the Pediatric Nephrology Research Consortium study who had received eculizumab therapy from 2008 to 2015
Genetic variants described in association with aHUS and included in the study survey were complement factor H (CFH), membrane cofactor protein (MCP), complement component 3 (C3), diacylglycerol kinase-ε (DGKE), complement factor I ( CFI ), complement factor B ( CFB ), and thrombomodulin (THBD).
Estimated GFR was calculated by modified Schwartz equation (0.413 × Ht(cm)/serum creatinine (mg/dL)) for patients < 18 years of age and by the CKD-EPI equation for those > 18 years of age (eGFR = 141 × min (SCr/κ or 1)α × max (SCr/κ or 1)−1.209 × 0.993Age × 1.018 [if female] × 1.159 [if Black]) [45, 46]. The eGFR was calculated for patients at initiation and at the patient’s latest follow-up if available, regardless of whether they remained on eculizumab. For all patients on dialysis, eGFR was corrected to 10 mL/min/1.73 m2 and all eGFR values greater than 150 mL/min/1.73 m2 were corrected to 150 mL/min/1.73 m2 based upon previously published data [47]. The paired difference for eGFR was calculated by subtracting the eGFR at initiation from the eGFR at latest follow-up.
Proteinuria was quantified by urine protein-to-creatinine ratio (UPC) (mg/mg), from convenience samples or timed urine collections, at initiation and at the latest follow-up visit. A UPC value of 10 mg/mg was substituted for all UPC values greater than 10 mg/mg based upon the median and range data and the lack of clinical significance of a UPC far above nephrotic range proteinuria (2 mg/mg). The paired difference for UPC was calculated by subtracting the UPC at initiation from the UPC at latest follow-up. Medians were calculated for both eGFR and UPC paired difference given skewed distribution. Eculizumab-exposed patients were excluded from eGFR and UPC analysis if they received the agent prophylactically in preparation for kidney transplant or if they received a kidney transplant between initiation of eculizumab and latest follow-up.
Categorical variables were summarized using frequencies and percentages. Continuous variables were summarized by measures of central tendency. Signed ranked tests were used to calculate p-values for kidney outcome data. Statistical significance was defined as a p-value of < 0.05, but our main goal was to evaluate direction and potential impacts for guiding future research. All analyses were conducted using the SAS software version 8.
Results
Patient characteristics
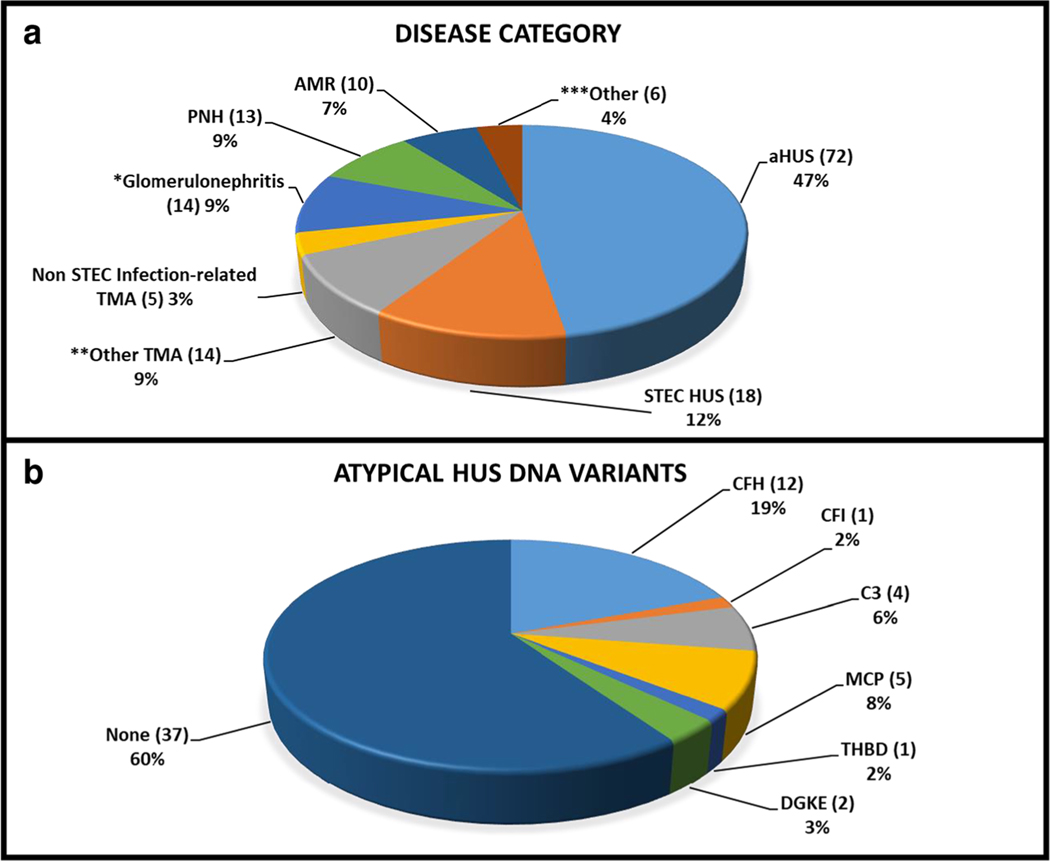
We identified 152 patients from 21 centers within the PNRC who were exposed to eculizumab during the designated period. The mean age at eculizumab initiation was 9.1 ± 6.8 years (range 0.1–25 years). Fourteen of the patients (9%) were between the ages of 18 and 25 at the time of eculizumab initiation. Fifty-three percent of patients were male, 67.1% were Caucasian, 13.2% African-American, and 19.7% other. The most frequent diagnosis associated with eculizumab exposure was aHUS (47.4%) (Fig. 2, panel a). Forty-four percent of the patients in our cohort received eculizumab for off-label indications including STEC HUS (11.8%), non-STEC infection-related TMA (3.3%), other TMA (9.2%), GN (9.2%), AMR (6.6%), and other (3.9%). The most common indication for eculizumab use in patients with STEC HUS and non-STEC infection-related TMA was neurologic impairment (43.4%) and risk of death (13%). The identified indications for initiation of eculizumab use in GN were progressive kidney failure (67%), proteinuria (53%), hypertension (20%), concern for TMA (13%), and disease recurrence after transplantation (7%). The non-STEC infection-related TMA category included one patient with non-Shiga toxin-producing E. coli 0157, one with Clostridium septicum bacteremia, and three with unknown infections. Within the other TMA group, the majority of the patients (9) who received eculizumab were designated as hematopoietic stem cell transplant TMA-related patients (64.3%) with three thrombotic thrombocytopenic purpura (TTP) patients (21.4%), one patient with TMA secondary to systemic lupus erythematosus, and one patient with TMA from an unknown etiology. GN diagnoses, as reported by the local provider, included seven patients with C3GN (50%), five with dense deposit disease (35.7%), and two with membranoproliferative GN (14.3%). In this cohort, ten patients with various forms of solid organ transplantation (3 kidney, 1 combined liver/kidney, 4 heart, and 1 lung) received eculizumab for AMR.
Fig. 2.
Disease characteristics of patients treated with eculizumab within the Pediatric Nephrology Research Consortium from 2008 to 2015. a Provider reported diagnoses in children treated with terminal complement blockade. *The glomerulonephritis category includes patients with C3 GN, dense deposit disease, and membranoproliferative GN. **The other TMA category includes patients with hematopoietic stem cell transplant TMA, thrombotic thrombocytopenic purpura (TTP), TMA secondary to systemic lupus erythematosus, and TMA from an unknown etiology. ***The other category captures all remaining indications. b DNA variants reported in those patients diagnosed with atypical hemolytic uremic syndrome. Sixty-two atypical hemolytic uremic syndrome patients underwent genetic testing. Twenty-five DNA variants were identified, across 6 different genes and 37 patients did not have a reported abnormality in these gene variants. The authors made no attempt to confirm the relative pathogenicity of reported DNA variants
Genetic testing was performed per local practice preference and was available for 91 (59.8%) of the 152 patients. Thirty-one patients of 91 tested (20.4%) possessed DNA variants in CFH, CFI, C3, MCP, THBD, and/or DGKE. The authors made no attempt to confirm the relative pathogenicity of these reported DNA variants. CFH variants (45.2%) were the most commonly reported of the 31 positively identified variants, followed by C3 (19.3%), MCP (19.3%), THBD (6.5%), DGKE (6.5%), and CFI (3.2%) gene variants. Of the 62 aHUS patients who had genetic testing, 37 patients did not have a reported abnormality in these gene variants (Fig. 2 panel b). However, four patients with GN (12.9%) were found to have gene variants (two with CFH and two with C3 gene variants) and two patients with other TMAs (6.5%) were found to have gene variants (one with MCP and one with THBD gene variants). No DNA variants in the aforementioned genes were reported in STEC HUS or PNH patients.
Treatment characteristics
Therapy duration was available for 143 of the 152 patients. The date of initiation was unknown for one child and the date of the latest eculizumab dose was unknown in eight. The median length of therapy for known patients was 5.25 months (IQR 1.1, 22.2), 14.7 months for aHUS patients (IQR 4.7, 29.1), and 1.8 months (IQR 0.2, 1.8) for STEC HUS patients. At the time of data collection, 67 patients were still on eculizumab therapy with a median length of eculizumab therapy of 21.1 months (IQR 6.1, 32.4). Of the patients on eculizumab at study end, 83% (56/67) were for approved indications (aHUS and PNH).
Seventy-six patients were exposed to eculizumab but were no longer on the agent at the time of data collection. Median length of therapy for this group was 1.6 months (IQR 0.5, 5.1). Seven patients received only one dose of eculizumab (1 aHUS, 3 STEC HUS, 1 non-STEC infection-related HUS, 1 AMR, and 1 other). Therapy was discontinued in 16/72 aHUS patients for the following reasons: disease improved (75%), kidney function did not recover (19%), physician preference (31%), patient or parent preference (12.5%), negative genetic testing (6%), and one patient was treated per protocol for 1 year following kidney transplantation. For those receiving eculizumab for off-label indications, therapy was discontinued for disease improved (33%), kidney function not recoverable (19%), patient preference (14.3%), per AMR protocol (14.3%), physician preference (4.7%), adverse events of therapy (9.5%), and no response to therapy (4.7%). Five patients were restarted on eculizumab after discontinuation; two patients were off therapy for 1 month or less and three patients were off for 12 or more months. These five particular patients were taken off eculizumab therapy due to disease improvement (80%), physician preference (40%), or kidney function was non-recoverable (20%). Indications for restarting eculizumab therapy for the five patients who underwent stop and restart therapy were worsened kidney function (40%), disease recurrence (40%), and genetic mutation later identified (40%).
One hundred and two patients (67.1%) were dosed as per the aHUS dosing recommendations [14], 46 patients (30.2%) followed an alternative dosing regimen, and in 4 patients (2.6%), the dosing was unclear from the medical record. The explanations for alternative dosing regimens included physician preference (17%), critical illness (13%), re-dosing after plasmapheresis (15%), PNH regimen (12.8%), hematopoietic stem cell transplant TMA regimen (8.5%), transplant protocol (10.6%), STEC HUS (4.2%), frequent relapses (2%), dense deposit disease (2%), NMOSD (2%), and unknown (13%). Nine out of ten patients with AMR were not dosed per guidelines. Eighty-six percent of aHUS patients were reported to be dosed according to current aHUS guidelines but in the remainder of the aHUS patients (9/72), the guidelines were modified for alternative dosing for physician preference (33.3%), peri-transplant protocol (22.2%), critical illness (11.1%), relapse requiring higher/more frequent dosing (11.1%), and unknown (22.2%).
Kidney outcomes with eculizumab therapy
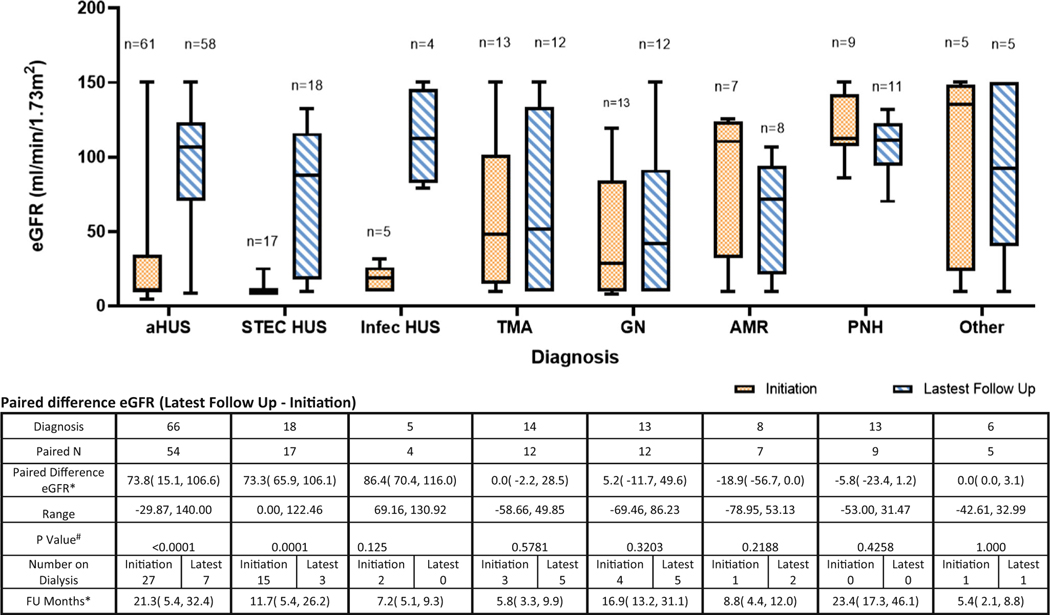
Estimated GFR (eGFR) was available in 130/152 patients at initiation, and 128/152 patients at the latest follow-up visit. Thirteen patients had no eGFR data available at initiation, 15 had no eGFR data available at latest follow-up, and nine patients were excluded as they received kidney transplants in association with eculizumab initiation. Paired data analysis was performed in 120 patients (Fig. 3). The median eGFR of the paired analysis was 18.8 mL/min/1.73 m2 (IQR 10, 67.8) for all diagnoses at initiation and 94.4 mL/min/1.73 m2 (IQR 55.9, 119.8) at the latest follow-up visit whether or not they remained on eculizumab therapy. The median eGFR for aHUS patients increased by 73.8 mL/min/1.73 m2 (IQR 15.1, 106.6) from eculizumab initiation to the latest follow-up (p < 0.05) over a median follow-up time of 21.3 months (IQR 5.4, 32.4). Median eGFR improved by 68.44 mL/min/1.73 m2 in STEC HUS (p < 0.05) over a median follow-up time of 11.7 months (IQR 5.4, 26.2). There were no statistically significant differences in eGFR at initiation and latest follow-up in patients with all other diagnoses. The number of patients on dialysis for all diagnoses improved from 53 at initiation of eculizumab therapy to 23 at the latest follow-up (7 aHUS, 3 STEC HUS, 5 other TMA, 5 GN, 2 AMR, and 1 other). However, there were a greater number of patients receiving dialysis at latest follow-up as compared to the time of initiation of eculizumab therapy for the diagnoses of other TMA (5), GN (5), and AMR (2).
Fig. 3.
Median estimated glomerular filtration (eGFR) at initiation and at follow-up by diagnosis for pediatric patients receiving eculizumab from 2008 to 2015. *Median with interquartile range. #p values were calculated using signed rank test. aHUS: atypical hemolytic uremic syndrome, STEC HUS: Shiga toxin-producing Escherichia coli hemolytic uremic syndrome, non-STEC Inf: non-Shiga toxin-producing Escherichia coli infection-related thrombotic microangiopathy, TMA: other thrombotic microangiopathy, GN: glomerulonephritis, AMR: antibody-mediated rejection, PNH: paroxysmal nocturnal hemoglobinuria, other
Proteinuria data were available in 58/152 patients at initiation and in 64/152 patients at latest follow-up. Paired data analysis was performed in 39 patients (Fig. 4). The overall median UPC ratio from eculizumab initiation was 6.1 mg/g (IQR 1.7, 10) and at latest follow-up 0.4 mg/g (IQR 0.2, 1.1). The UPC ratio demonstrated statistically significant improvement from initiation to latest follow-up among patients with aHUS (paired difference UPC of −5 mg/g (IQR −9.6, −0.4)) and other TMA (paired difference UPC of −9 mg/g (IQR −9.5, −1.5)) (p < 0.05). Patients with STEC HUS, other non-STEC infection-related HUS, GN, AMR, and other diagnoses demonstrated improvements in UPC ratios from initiation to latest follow-up, but the differences were not statistically significant.
Fig. 4.
Median urine protein-to-creatinine (UPC) at initiation of eculizumab and at follow-up by diagnosis for pediatric patients receiving eculizumab from 2008 to 2015. *Median with interquartile range. #p values were calculated using signed rank test. aHUS: atypical hemolytic uremic syndrome, STEC HUS: Shiga toxin-producing Escherichia coli hemolytic uremic syndrome, non-STEC Inf: non-Shiga toxin-producing Escherichia coli infection-related thrombotic microangiopathy, TMA: other thrombotic microangiopathy, GN: glomerulonephritis, AMR: antibody-mediated rejection, PNH: paroxysmal nocturnal hemoglobinuria, other
Adverse events reported after eculizumab therapy
Overall 34.9% (53/152) of the cohort experienced at least one infectious adverse event following eculizumab initiation and 17.8% experienced a noninfectious adverse event following initiation of eculizumab (Table 1). There were 46 bacterial infection events among 40 persons (26.3%) during the follow-up period. Central line infections were the most frequent bacterial infections (26.1% of all bacterial infections). The other bacterial infections were blood stream infection, pneumonia, urinary tract infections, endocarditis, tracheitis, pharyngitis, groin abscess, cellulitis, and peritonitis. Two patients (1.3%) experienced an infection caused by an encapsulated organism, and this included a Streptococcus pneumoniae central line infection and a Klebsiella pneumoniae urinary tract infection and central line infection in a single patient. No patient was diagnosed with an infection due to Neisseria meningitides. Nineteen (12.5%) patients received meningococcal polysaccharide vaccination and 111 (73%) received meningococcal conjugate vaccine prior to eculizumab therapy initiation. There were 24 viral infection events in 22 (14.5%) patients during the follow-up period. The most frequent viral infection that patients experienced was influenza A (20.8% of all viral infections). Other viral infections observed were cytomegalovirus, BK virus, Epstein-Barr virus, human herpesvirus-6, norovirus, herpes simplex virus, varicella, and unknown viral upper respiratory tract infections. Twenty-seven (17.8%) patients in the entire cohort were reported to experience 44 noninfectious adverse events after initiation of eculizumab. The most commonly reported adverse events after eculizumab were nausea and abdominal pain (13.6% each). The other noninfectious adverse events reported were infusion reactions (6.8%), hypertension (6.8%), headache (6.8%), vomiting (6.8%), back pain (4.5%), diarrhea (4.5%), and anemia (4.5%). There were 31.8% other noninfectious adverse events reported after eculizumab initiation and included one patient each reporting fever, fatigue, constipation, body aches, dizziness, chest pain, and edema.
Table 1.
Infectious and non-infectious adverse events reported in children while receiving eculizumab between 2008–2015 in the Pediatric Nephrology Research Consortium study
| Total n=152 | aHUS n=72 | STEC HUS n=18 | Non STEC Infec HUS n=5 | Other TMA n=14 | GN n=14 | AMR n=10 | PNH n=13 | Other n=6 | |
|---|---|---|---|---|---|---|---|---|---|
| Infectious Adverse Events | |||||||||
| Persons with Bacterial Infections, n(%) | 40(26.3) | 18(25) | 3(16.7) | 1(20) | 6(42.9) | 6(42.9) | 1(10) | 5(38.5) | - |
| Total # of Bacterial Infections, n | 46 | 20 | 4 | 1 | 6 | 8 | 2 | 6 | - |
| # of Central Line Infections, n(%) | 12(26.1) | 5(25) | 1(25) | - | 3(50) | 2(25) | - | 1(16.7) | - |
| # of Bacteremia Infections, n(%) | 6(13) | 1(5) | 2(50) | - | 2(33.3) | 1(12.5) | - | - | - |
| # of Pulmonary Infections, n(%) | 3(6.5) | 2(10) | - | - | - | 1(12.5) | - | - | - |
| # of Abdominal Infections, n(%) | 4(8.7) | 3(15) | - | - | - | 1(12.5) | - | - | - |
| # of Urinary Tract Infections, n(%) | 9(19.6) | 5(25) | - | 1(100) | - | 1(12.5) | 1(50) | 2(33.3) | - |
| # of Other Infections1, n(%) | 12(26.1) | 4(20) | 1(25) | - | 1(1.7) | 2(25) | 1(50) | 3(50) | - |
| Persons with Viral Infections, n(%) | 22(14.5) | 9(12.5) | 1(5.6) | - | 7(50) | 1(7.1) | - | 4(3.1) | - |
| Total # of Viral Infections, n | 24 | 9 | 1 | - | 9 | 1 | - | 4 | |
| # of Influenza A Infections, n(%) | 5(20.8) | 1(11.1) | - | - | 2(22.2) | 1(100) | - | 1(25) | - |
| # of Influenza B Infections, n(%) | 1(4.2) | 1(11.1) | - | - | - | - | - | - | - |
| # of RSV Infections, n(%) | 3(12.5) | - | - | - | 3(33.3) | - | - | - | - |
| # of Parvovirus Infections, n(%) | 1(4.2) | 1(11.1) | - | - | - | - | - | - | - |
| # of Rhinovirus, n(%) | 3(12.5) | 1(11.1) | - | - | 2(22.2) | - | - | - | - |
| # of Other Infections2, n(%) | 11(45.8) | 5(55.6) | 1(100) | - | 2(22.2) | - | - | 3(75) | - |
| Non-Infectious Adverse Events | |||||||||
| Persons with Non-Infectious Adverse Events, n(%) | 27(17.8) | 15(20.8) | 1(5.6) | - | 2(14.3) | 4(2.9) | 1(10) | 4(3.1) | - |
| Total # of Non-Infectious Complications, n | 44 | 26 | 1 | - | 2 | 6 | 1 | 8 | - |
| # of Infusion Reactions, n(%) | 3(6.8) | 2(7.7) | - | - | - | 1(16.7) | - | - | - |
| # of Hypertension, n(%) | 3(6.8) | 3(11.5) | - | - | - | - | - | - | - |
| # of Headaches, n(%) | 3(6.8) | 1(3.8) | - | - | - | - | 1(100) | 1(12.5) | - |
| # of Back Pain, n(%) | 2(4.5) | 1(3.8) | - | - | - | 1(16.7) | - | - | - |
| # with Nausea, n(%) | 6(13.6) | 4(15.4) | - | - | - | 1(16.7) | - | 1(12.5) | - |
| # with Abdominal Pain, n(%) | 6(13.6) | 4(15.4) | - | - | 1(50) | - | - | 1(12.5) | - |
| # with Vomiting, n(%) | 3(6.8) | 2(7.7) | - | - | - | - | - | 1(12.5) | - |
| # with Diarrhea, n(%) | 2(4.5) | 2(7.7) | - | - | - | - | - | - | - |
| # with Anemia, n(%) | 2(4.5) | 1(3.8) | - | - | - | 1(16.7) | - | - | - |
| # with Other3, n(%) | 14(31.8) | 6(23.1) | 1(100) | - | 1(50) | 2(33.3) | - | 4(50) | - |
| Deaths | |||||||||
| Deaths on Therapy, n(%)4 | 9(5.9) | 1(1.4) | 2(11.1) | - | 5(35.7) | - | - | - | 1(16.7) |
Other bacterial infections included: endocarditis, tracheitis, pharyngitis, groin abscess, cellulitis, peritonitis.
Other viral infections included: CMV, BK virus, EBV, HHV-6, norovirus, HSV, varicella, and unknown viral URIs.
Other non-infectious complications included in the text.
One patient was taken off therapy and later died
Ten patients (6.6%) died during the follow-up period. Nine died while still on therapy. One patient with atypical HUS died several months after discontinuation of therapy. Death was most common in the other TMA group (5/14, 35.7%); there were two deaths in those with STEC HUS (2/18, 11.1%), and one death in aHUS patients (1/72, 1.4%). Of nine patients who died while receiving eculizumab therapy, the causes of death were reported as cardiac arrest (n = 5), central nervous system hemorrhage (n = 3), and viral infection (n = 1). No patient deaths were attributed to bacterial infections.
Discussion
Our study characterizes the use of eculizumab within the North American pediatric population and demonstrates that although the majority of pediatric patients receiving eculizumab are diagnosed with aHUS, a significant proportion of pediatric eculizumab use is also for off-label indications (44%). Dosing practices varied widely based upon the indication for eculizumab and reasons for deviation from the recommended aHUS dosing guidelines were widely variable. Though kidney outcomes appeared to improve for some patients receiving eculizumab by specific diagnosis, given the wide variation in follow-up times, and lack of power to study this, conclusions regarding kidney outcomes cannot be fully drawn from this study. Participants were reported to have few adverse events and a low number of infections in this cohort.
Similar to the French national hospitalization database report which revealed that 50% of eculizumab use was for non-EMA-approved indications [48], our study indicates that 44% of eculizumab use in our pediatric cohort was for off-label indications [14]. The four most common non-FDA-approved indications for eculizumab use in our cohort were STEC HUS, AMR, GN, and other TMA. This study did not address if the use of eculizumab was clinically indicated or how the clinician was able to get authorization for the off-label use; we only have the diagnosis and in some cases, a brief response on why the local clinician felt complement inhibition was indicated.
This data collected in 2015 likely represents contemporary use of eculizumab, as the current approved indications have not significantly changed during the period of data collection with the exception of approval of eculizumab for myasthenia gravis in 2017 [14]. The data from this study was insufficient to fully define the efficacy of the terminal complement blockade in off-label indications or define a clear dosing strategy in these disorders.
Given the heterogeneity of the patient diagnoses and the variability of therapy duration in this cohort, it is difficult to offer a summary comment on the significance of the reported kidney outcomes. Several patients (7) received only one dose of eculizumab, this occurred most commonly in patients with STEC HUS, and the most common reason for eculizumab therapy discontinuation was for disease improvement. Our aHUS population showed a significant improvement in eGFR similar to other reported studies [ 49–52]. Interestingly, eculizumab was discontinued in approximately 1 in 5 aHUS patients (22%) in this cohort, which may be secondary to the higher frequency of aHUS patients without identified genetic variants (47/72, 65.2%). Prior literature suggests a low risk of disease recurrence in aHUS patients without identified genetic variants [53]. STEC HUS was the only off-label diagnosis with a significant increase in the eGFR; however, this study was not designed to assess kidney function during the acute phase of this illness. Our data likely represents improvement in eGFR at latest follow-up as a natural progression of STEC HUS and this is not necessarily indicative of response to eculizumab. The reported rates of stage 5 chronic kidney disease and need for transplantation are low for STEC HUS (1.4–7.3%) [54–57], but 17% of patients remained on dialysis in our cohort. This leads us to believe that our cohort of STEC HUS patients had more severe disease courses. It is likely that the disease severity and increased risk of mortality drove most clinicians to consider eculizumab therapy. No significant trend in eGFR was noted in the other off-label indications, likely due to the heterogeneity of diagnoses, eculizumab dosing strategies, and the variable follow-up period. Proteinuria improved from the time of therapy initiation to the time of the most recent urinalysis for all diagnoses but this difference was only significant for aHUS. The number of urine protein measurements was very low or absent for several diagnoses. Whether the kidney outcomes, strictly speaking, reflect the general natural history of pediatric patients with acute disease or a terminal pathway targeted aspect of the individual diseases (anti-complement or anti-inflammation) is unknown.
Genetic testing was performed in 60% of the cohort per local practice preference. Not all patients with aHUS received genetic testing and this may have reflected availability of testing in early 2000s when some patients of this cohort were diagnosed. Lower rates of genetic testing by other diagnoses likely reflect practice variation in off-label use of eculizumab.
What is perhaps more salient is the fact that our data recapitulate formerly published safety data, suggesting that eculizumab is well tolerated [49–52]. Infectious and noninfectious adverse event rates in this cohort were similar to published safety data and no serious safety events were noted. Though we assessed adverse events listed as potential adverse events from eculizumab’s manufacturer, we are unable to determine if the reported adverse events in this cohort were specifically related to medication exposure or underlying disease-related morbidity (i.e., central venous catheters, dialysis). While the mortality rate in this study was 6.6% for all diagnoses, the mortality rate in this cohort was 1.4% in aHUS patients, and this is similar to the previously reported mortality for patients with aHUS on eculizumab therapy of 0–2% [49, 58]. The mortality of the STEC HUS patients was 11.1% which is higher than previously reported rates in the literature which range from 1.3 to 5% [54–57]. This may reflect that patients in this cohort had more significant disease severity as evidence by 43% of patients having severe neurologic impairment at time of eculizumab initiation.
A strength of this study is the number of centers involved in data collection. This combined with the fact that all exposed patients at the enrolled PNRC centers were included lends toward reduced reporting bias. We ultimately decided to include young adult patients along with pediatric patients so as to increase the number of patients enrolled in this study and to include more variety in diagnoses, including C3GN which typically presents at a later age. It is our impression that this study provides a realistic description of practices and outcomes.
The primary weakness of this report is the retrospective nature of the data, particularly as it applies to the fact that local practice styles heavily influenced use of the agent. The lack of homogeneous use patterns and the variable time period until most recent follow-up in addition to the lack of a control population makes the interpretation of the findings difficult. Additionally, the number of patients for each off-label diagnosis was small.
In conclusion, we found that a significant number of pediatric patients are being exposed to C5 blockade for off-label indications. Dosing schedules are highly variable. Kidney outcomes are favorable in most. Although this study was not designed to show efficacy of eculizumab for off-label indications, it was able to show that the attributable adverse events and mortality with eculizumab in pediatric patients appear to be low.
Until such time when we fully understand the role of the terminal complement pathway (or associated anaphylatoxins) in the underlying pathogenesis of the various diseases, it will be difficult to create homogenous test populations. The number of publications involving off-label use of eculizumab continues to grow. These studies continue to consist mainly of single-center case reports or case series, which have an inherent positive bias; thus, the overall strength of evidence for eculizumab use in these indications remains low. More information is needed to determine the efficacy and effective dosing of eculizumab for these kidney diagnoses where terminal complement blockade is not yet the standard of therapy. Without homogenous disease cohorts (at least in some aspect of their disease), it may be difficult to reliably detect targeted outcomes. Medical practice patterns that allow varied patient characteristics and varied exposure protocols will continue to seriously limit the true scientific understanding of the efficacy of terminal complement blockade. Thus, we anxiously await the results of two ongoing prospective randomized trials of eculizumab in pediatric STEC HUS (NCT02205541 in France; ECUSTEC in the UK) and similar trials for other complement-medicated diseases. Additionally, randomized controlled trials are currently being conducted for longacting eculizumab (ravulizumab, ALXN1210) and other newly developed complement inhibitors with proposed use in C3GN, DDD, immune complex membranoproliferative GN, lupus nephritis, IgA nephropathy, and membranous nephropathy which we hope will guide therapy with complement inhibition in the future.
Acknowledgements
We would like to thank the following additional centers for contributing patients to this study: Seattle Children’s Hospital, Children’s Healthcare of Atlanta, Children’s Hospital of Richmond at Virginia Commonwealth University, UT Southwestern Children’s Hospital, and Buffalo Children’s Hospital.
Funding Dr. Sanderson is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR002490 and Grant 2015213 from the Doris Duke Charitable Foundation. Dr. Nester is supported by the National Institutes of Health, through Grant 1R01DK110023-01A1.
Footnotes
Data Availability Not applicable.
Code availability Not applicable.
Declarations
Ethics approval IRB approval was obtained at all participating centers.
Consent to participate This was a retrospective study, and no participant consent was required.
Consent for publication Not applicable.
Competing interests Carla Nester is a member of the Alexion advisory board.
References
- 1.Kaplan M (2002) Eculizumab (Alexion). Curr Opin Investig Drugs 3:1017–1023 [PubMed] [Google Scholar]
- 2.Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L (2007) Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol 25:1256–1264. 10.1038/nbt1344 [DOI] [PubMed] [Google Scholar]
- 3.Jodele S, Dandoy CE, Lane A, Laskin BL, Teusink-Cross A, Myers KC, Wallace GH, Nelson A, Bleesing J, Chima RS, Hirsch R, Ryan TD, Benoit SW, Mizuno K, Warren M, Davies SM (2020) Complement blockade for TA-TMA: lessons learned from large pediatric cohort treated with eculizumab. Blood. 10.1182/blood.2019004218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davin JC, Gracchi V, Bouts A, Groothoff J, Strain L, Goodship T (2010) Maintenance of kidney function following treatment with eculizumab and discontinuation of plasma exchange after a third kidney transplant for atypical hemolytic uremic syndrome associated with a CFH mutation. Am J Kidney Dis 55:708–711. 10.1053/j.ajkd.2009.08.011 [DOI] [PubMed] [Google Scholar]
- 5.Chatelet V, Fremeaux-Bacchi V, Lobbedez T, Ficheux M, Hurault de Ligny B (2009) Safety and long-term efficacy of eculizumab in a renal transplant patient with recurrent atypical hemolytic-uremic syndrome. Am J Transplant 9:2644–2645. 10.1111/j.1600-6143.2009.02817.x [DOI] [PubMed] [Google Scholar]
- 6.Gruppo RA, Rother RP (2009) Eculizumab for congenital atypical hemolytic-uremic syndrome. N Engl J Med 360:544–546. 10.1056/NEJMc0809959 [DOI] [PubMed] [Google Scholar]
- 7.Nurnberger J, Philipp T, Witzke O, Opazo Saez A, Vester U, Baba HA, Kribben A, Zimmerhackl LB, Janecke AR, Nagel M, Kirschfink M (2009) Eculizumab for atypical hemolytic-uremic syndrome. N Engl J Med 360:542–544. 10.1056/NEJMc0808527 [DOI] [PubMed] [Google Scholar]
- 8.Mache CJ, Acham-Roschitz B, Fremeaux-Bacchi V, Kirschfink M, Zipfel PF, Roedl S, Vester U, Ring E (2009) Complement inhibitor eculizumab in atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol 4:1312–1316. 10.2215/CJN.01090209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rathbone J, Kaltenthaler E, Richards A, Tappenden P, Bessey A, Cantrell A (2013) A systematic review of eculizumab for atypical haemolytic uraemic syndrome (aHUS). BMJ Open 3:e003573. 10.1136/bmjopen-2013-003573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cofiell R, Kukreja A, Bedard K, Yan Y, Mickle AP, Ogawa M, Bedrosian CL, Faas SJ (2015) Eculizumab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood 125:3253–3262. 10.1182/blood-2014-09-600411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tschumi S, Gugger M, Bucher BS, Riedl M, Simonetti GD (2011) Eculizumab in atypical hemolytic uremic syndrome: long-term clinical course and histological findings. Pediatr Nephrol 26:2085–2088. 10.1007/s00467-011-1989-4 [DOI] [PubMed] [Google Scholar]
- 12.Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nurnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C (2013) Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 368:2169–2181. 10.1056/NEJMoa1208981 [DOI] [PubMed] [Google Scholar]
- 13.Soliris (eculizumab) full prescribing information (2011) Alexion Pharmaceuticals, Cheshire, CT [Google Scholar]
- 14.Soliris (eculizumab) full prescribing information (2017) Boston, MA [Google Scholar]
- 15.Mahat U, Matar RB, Rotz SJ (2019) Use of complement monoclonal antibody eculizumab in Shiga toxin producing Escherichia coli associated hemolytic uremic syndrome: a review of current evidence. Pediatr Blood Cancer 66:e27913. 10.1002/pbc.27913 [DOI] [PubMed] [Google Scholar]
- 16.Trachtman H, Austin C, Lewinski M, Stahl RA (2012) Renal and neurological involvement in typical Shiga toxin-associated HUS. Nat Rev Nephrol 8:658–669. 10.1038/nrneph.2012.196 [DOI] [PubMed] [Google Scholar]
- 17.Delmas Y, Vendrely B, Clouzeau B, Bachir H, Bui HN, Lacraz A, Helou S, Bordes C, Reffet A, Llanas B, Skopinski S, Rolland P, Gruson D, Combe C (2014) Outbreak of Escherichia coli O104:H4 haemolytic uraemic syndrome in France: outcome with eculizumab. Nephrol Dial Transplant 29:565–572. 10.1093/ndt/gft470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pape L, Hartmann H, Bange FC, Suerbaum S, Bueltmann E, Ahlenstiel-Grunow T (2015) Eculizumab in typical hemolytic uremic syndrome (HUS) with neurological involvement. Medicine (Baltimore) 94:e1000. 10.1097/MD.0000000000001000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Percheron L, Gramada R, Tellier S, Salomon R, Harambat J, Llanas B, Fila M, Allain-Launay E, Lapeyraque AL, Leroy V, Adra AL, Berard E, Bourdat-Michel G, Chehade H, Eckart P, Merieau E, Pietrement C, Sellier-Leclerc AL, Fremeaux-Bacchi V, Dimeglio C, Garnier A (2018) Eculizumab treatment in severe pediatric STEC-HUS: a multicenter retrospective study. Pediatr Nephrol 33:1385–1394. 10.1007/s00467-018-3903-9 [DOI] [PubMed] [Google Scholar]
- 20.Jodele S, Fukuda T, Vinks A, Mizuno K, Laskin BL, Goebel J, Dixon BP, Teusink A, Pluthero FG, Lu L, Licht C, Davies SM (2014) Eculizumab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Biol Blood Marrow Transplant 20:518–525. 10.1016/j.bbmt.2013.12.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez C, Lario A, Fores R, Cabrera R (2015) Eculizumab treatment in a patient with hematopoietic stem cell transplantation-associated thrombotic microangiopathy and steroid-refractory acute graft versus host disease. Hematol Rep 7: 6107. 10.4081/hr.2015.6107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bomback AS, Smith RJ, Barile GR, Zhang Y, Heher EC, Herlitz L, Stokes MB, Markowitz GS, D’Agati VD, Canetta PA, Radhakrishnan J, Appel GB (2012) Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am Soc Nephrol 7:748–756. 10.2215/CJN.12901211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daina E, Noris M, Remuzzi G (2012) Eculizumab in a patient with dense-deposit disease. N Engl J Med 366:1161–1163. 10.1056/NEJMc1112273 [DOI] [PubMed] [Google Scholar]
- 24.Vivarelli M, Pasini A, Emma F (2012) Eculizumab for the treatment of dense-deposit disease. N Engl J Med 366:1163–1165. 10.1056/NEJMc1111953 [DOI] [PubMed] [Google Scholar]
- 25.Gurkan S, Fyfe B, Weiss L, Xiao X, Zhang Y, Smith RJ (2013) Eculizumab and recurrent C3 glomerulonephritis. Pediatr Nephrol 28:1975–1981. 10.1007/s00467-013-2503-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez-Moreno A, De la Cerda F, Cabrera R, Fijo J, Lopez-Trascasa M, Bedoya R, Rodriguez de Cordoba S, Ybot-Gonzalez P (2014) Eculizumab in dense-deposit disease after renal transplantation. Pediatr Nephrol 29:2055–2059. 10.1007/s00467-014-2839-y [DOI] [PubMed] [Google Scholar]
- 27.Inman M, Prater G, Fatima H, Wallace E (2015) Eculizumab-induced reversal of dialysis-dependent kidney failure from C3 glomerulonephritis. Clin Kidney J 8:445–448. 10.1093/ckj/sfv044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Quintrec M, Lionet A, Kandel C, Bourdon F, Gnemmi V, Colombat M, Goujon JM, Fremeaux-Bacchi V, Fakhouri F (2015) Eculizumab for treatment of rapidly progressive C3 glomerulopathy. Am J Kidney Dis 65:484–489. 10.1053/j.ajkd.2014.09.025 [DOI] [PubMed] [Google Scholar]
- 29.Oosterveld MJ, Garrelfs MR, Hoppe B, Florquin S, Roelofs JJ, van den Heuvel LP, Amann K, Davin JC, Bouts AH, Schriemer PJ, Groothoff JW (2015) Eculizumab in pediatric dense deposit disease. Clin J Am Soc Nephrol 10:1773–1782. 10.2215/CJN.01360215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebreton C, Bacchetta J, Dijoud F, Bessenay L, Fremeaux-Bacchi V, Sellier-Leclerc AL (2017) C3 glomerulopathy and eculizumab: a report on four paediatric cases. Pediatr Nephrol 32:1023–1028. 10.1007/s00467-017-3619-2 [DOI] [PubMed] [Google Scholar]
- 31.Ozkaya O, Nalcacioglu H, Tekcan D, Genc G, Meydan BC, Ozdemir BH, Baysal MK, Keceligil HT (2014) Eculizumab therapy in a patient with dense-deposit disease associated with partial lipodystropy. Pediatr Nephrol 29:1283–1287. 10.1007/s00467-013-2748-5 [DOI] [PubMed] [Google Scholar]
- 32.Locke JE, Magro CM, Singer AL, Segev DL, Haas M, Hillel AT, King KE, Kraus E, Lees LM, Melancon JK, Stewart ZA, Warren DS, Zachary AA, Montgomery RA (2009) The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant 9:231–235. 10.1111/j.1600-6143.2008.02451.x [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Roncero F, Suner M, Bernal G, Cabello V, Toro M, Pereira P, Angel Gentil M (2012) Eculizumab treatment of acute antibody-mediated rejection in renal transplantation: case reports. Transplant Proc 44:2690–2694. 10.1016/j.transproceed.2012.09.038 [DOI] [PubMed] [Google Scholar]
- 34.Stewart ZA, Collins TE, Schlueter AJ, Raife TI, Holanda DG, Nair R, Reed AI, Thomas CP (2012) Case report: eculizumab rescue of severe accelerated antibody-mediated rejection after ABO-incompatible kidney transplant. Transplant Proc 44:3033–3036. 10.1016/j.transproceed.2012.03.053 [DOI] [PubMed] [Google Scholar]
- 35.Kocak B, Arpali E, Demiralp E, Yelken B, Karatas C, Gorcin S, Gorgulu N, Uzunalan M, Turkmen A, Kalayoglu M (2013) Eculizumab for salvage treatment of refractory antibody-mediated rejection in kidney transplant patients: case reports. Transplant Proc 45:1022–1025. 10.1016/j.transproceed.2013.02.062 [DOI] [PubMed] [Google Scholar]
- 36.Yelken B, Arpali E, Gorcin S, Kocak B, Karatas C, Demiralp E, Turkmen A (2015) Eculizumab for treatment of refractory antibody-mediated rejection in kidney transplant patients: a single-center experience. Transplant Proc 47:1754–1759. 10.1016/j.transproceed.2015.06.029 [DOI] [PubMed] [Google Scholar]
- 37.Burbach M, Suberbielle C, Brocheriou I, Ridel C, Mesnard L, Dahan K, Rondeau E, Hertig A (2014) Report of the inefficacy of eculizumab in two cases of severe antibody-mediated rejection of renal grafts. Transplantation 98:1056–1059. 10.1097/TP.0000000000000184 [DOI] [PubMed] [Google Scholar]
- 38.Ghirardo G, Benetti E, Poli F, Vidal E, Della Vella M, Cozzi E, Murer L (2014) Plasmapheresis-resistant acute humoral rejection successfully treated with anti-C5 antibody. Pediatr Transplant 18: E1–E5. 10.1111/petr.12187 [DOI] [PubMed] [Google Scholar]
- 39.Orandi BJ, Zachary AA, Dagher NN, Bagnasco SM, Garonzik-Wang JM, Van Arendonk KJ, Gupta N, Lonze BE, Alachkar N, Kraus ES, Desai NM, Locke JE, Racusen LC, Segev DL, Montgomery RA (2014) Eculizumab and splenectomy as salvage therapy for severe antibody-mediated rejection after HLA-incompatible kidney transplantation. Transplantation 98:857–863. 10.1097/TP.0000000000000298 [DOI] [PubMed] [Google Scholar]
- 40.Rousset-Rouviere C, Cailliez M, Garaix F, Bruno D, Laurent D, Tsimaratos M (2014) Rituximab fails where eculizumab restores renal function in C3nef-related DDD. Pediatr Nephrol 29:1107–1111. 10.1007/s00467-013-2711-5 [DOI] [PubMed] [Google Scholar]
- 41.Chehade H, Rotman S, Matter M, Girardin E, Aubert V, Pascual M (2015) Eculizumab to treat antibody-mediated rejection in a 7-year-old kidney transplant recipient. Pediatrics 135:e551–e555. 10.1542/peds.2014-2275 [DOI] [PubMed] [Google Scholar]
- 42.Smith B, Kumar V, Mompoint-Williams D, Reed RD, MacLennan PA, Stegner K, Locke JE (2016) Dosing eculizumab for antibody-mediated rejection in kidney transplantation: a case report. Transplant Proc 48:3099–3105. 10.1016/j.transproceed.2016.03.028 [DOI] [PubMed] [Google Scholar]
- 43.Tran CL, Sethi S, Murray D, Cramer CH, Sas DJ, Willrich M, Smith RJ, Fervenza FC (2016) Discontinuation of dialysis with eculizumab therapy in a pediatric patient with dense deposit disease. Pediatr Nephrol 31:683–687. 10.1007/s00467-015-3306-0 [DOI] [PubMed] [Google Scholar]
- 44.Stegall MD, Chedid MF, Cornell LD (2012) The role of complement in antibody-mediated rejection in kidney transplantation. Nat Rev Nephrol 8:670–678. 10.1038/nrneph.2012.212 [DOI] [PubMed] [Google Scholar]
- 45.Schwartz GJ, Work DF (2009) Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 4: 1832–1843. 10.2215/CJN.01640309 [DOI] [PubMed] [Google Scholar]
- 46.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, (CKD-EPI Chronic Kidney Disease Epidemiology Collaboration) (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saydah SH, Xie H, Imperatore G, Burrows NR, Pavkov ME (2018) Trends in albuminuria and GFR among adolescents in the United States, 1988–2014. Am J Kidney Dis 72:644–652. 10.1053/j.ajkd.2018.04.021 [DOI] [PubMed] [Google Scholar]
- 48.Castaneda-Sanabria J, Hajage D, Le Jouan M, Perozziello A, Tubach F (2016) Off-label use of the expensive orphan drug eculizumab in France 2009–2013 and the impact of literature: focus on the transplantation field. Eur J Clin Pharmacol 72:737–746. 10.1007/s00228-016-2027-z [DOI] [PubMed] [Google Scholar]
- 49.Greenbaum LA, Fila M, Ardissino G, Al-Akash SI, Evans J, Henning P, Lieberman KV, Maringhini S, Pape L, Rees L, van de Kar NC, Vande Walle J, Ogawa M, Bedrosian CL, Licht C (2016) Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int 89:701–711. 10.1016/j.kint.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 50.Menne J, Delmas Y, Fakhouri F, Licht C, Lommele A, Minetti EE, Provot F, Rondeau E, Sheerin NS, Wang J, Weekers LE, Greenbaum LA (2019) Outcomes in patients with atypical hemolytic uremic syndrome treated with eculizumab in a long-term observational study. BMC Nephrol 20:125. 10.1186/s12882-019-1314-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Licht C, Greenbaum LA, Muus P, Babu S, Bedrosian CL, Cohen DJ, Delmas Y, Douglas K, Furman RR, Gaber OA, Goodship T, Herthelius M, Hourmant M, Legendre CM, Remuzzi G, Sheerin N, Trivelli A, Loirat C (2015) Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int 87:1061–1073. 10.1038/ki.2014.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ito S, Hidaka Y, Inoue N, Kaname S, Kato H, Matsumoto M, Miyakawa Y, Mizuno M, Okada H, Shimono A, Matsuda T, Maruyama S, Fujimura Y, Nangaku M, Kagami S (2019) Safety and effectiveness of eculizumab for pediatric patients with atypical hemolytic-uremic syndrome in Japan: interim analysis of post-marketing surveillance. Clin Exp Nephrol 23:112–121. 10.1007/s10157-018-1610-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fakhouri F, Fila M, Provot F, Delmas Y, Barbet C, Chatelet V, Rafat C, Cailliez M, Hogan J, Servais A, Karras A, Makdassi R, Louillet F, Coindre JP, Rondeau E, Loirat C, Fremeaux-Bacchi V (2017) Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizumab discontinuation. Clin J Am Soc Nephrol 12:50–59. 10.2215/CJN.06440616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jenssen GR, Vold L, Hovland E, Bangstad HJ, Nygard K, Bjerre A (2016) Clinical features, therapeutic interventions and long-term aspects of hemolytic-uremic syndrome in Norwegian children: a nationwide retrospective study from 1999 to 2008. BMC Infect Dis 16:285. 10.1186/s12879-016-1627-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaterodt L, Holle J, Huseman D, Muller D, Thumfart J (2018) Short- and long-term renal outcome of hemolytic-uremic syndrome in childhood. Front Pediatr 6:220. 10.3389/fped.2018.00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mody RK, Gu W, Griffin PM, Jones TF, Rounds J, Shiferaw B, Tobin-D’Angelo M, Smith G, Spina N, Hurd S, Lathrop S, Palmer A, Boothe E, Luna-Gierke RE, Hoekstra RM (2015) Postdiarrheal hemolytic uremic syndrome in United States children: clinical spectrum and predictors of in-hospital death. J Pediatr 166:1022–1029. 10.1016/j.jpeds.2014.12.064 [DOI] [PubMed] [Google Scholar]
- 57.Rosales A, Hofer J, Zimmerhackl LB, Jungraithmayr TC, Riedl M, Giner T, Strasak A, Orth-Holler D, Wurzner R, Karch H, German-Austrian HUS Study Group (2012) Need for long-term follow-up in enterohemorrhagic Escherichia coli-associated hemolytic uremic syndrome due to late-emerging sequelae. Clin Infect Dis 54: 1413–1421. 10.1093/cid/cis196 [DOI] [PubMed] [Google Scholar]
- 58.Rondeau E, Cataland SR, Al-Dakkak I, Miller B, Webb NJA, Landau D (2019) eculizumab safety: five-year experience from the global atypical hemolytic uremic syndrome registry. Kidney Int Rep 4:1568–1576. 10.1016/j.ekir.2019.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]