Abstract
Aortic graft infection is a rare complication after endovascular aneurysm repair that is usually caused by gram-positive organisms such as Staphylococcus spp or gram-negative organisms such as Enterobacteriaceae or Salmonella spp. We have presented a unique case of a patient with acute graft infection secondary to Burkholderia pseudomallei. Because treatment of B. pseudomallei infections is challenging owing to its inherent resistance to multiple antibiotics, we have proposed an approach for managing similar cases in the future. Lifestyle advice on avoiding soil exposure in the postoperative period after endovascular aneurysm repair might be an important preventative measure in endemic regions.
Keywords: Abdominal Aortic Aneurysm, Aortic graft infection, Endograft infection, Melioidosis
Aortic graft infection (AGI) is a rare complication after endovascular aneurysm repair (EVAR), with an incidence of 0.5% to 1.4%.1 We have reported a case of Burkholderia pseudomallei AGI, which was managed successfully with explantation of the aortic stent and revascularization with in situ reconstruction.
Case report
Our patient was a 64-year-old man with no chronic medical conditions. He had presented to the urology clinic at another institution for asymptomatic microscopic hematuria. Urine microscopy showed one red blood cell and one white blood cell (WBC)/high-power field. The findings from the corresponding urine cultures were negative. Computed tomography (CT) intravenous (IV) pyelography revealed an incidental saccular, infrarenal abdominal aorta aneurysm (AAA) measuring 3.3 × 1.7 × 2.1 cm with perianeurysmal fat stranding, which was concerning for a contained leak (Fig 1, A). No radiographic features were seen of urinary tract infection or a fistulous connection between the urinary tract and aorta. Owing to the CT findings, he was promptly advised for admission. He was afebrile and clinically asymptomatic. His admission WBC count, C-reactive protein (CRP), and procalcitonin were within normal limits. The blood and urine culture findings were negative. We performed a dedicated CT aortogram, which confirmed the presence of a 3.3-cm saccular AAA with no fat stranding or contrast extravasation. In view of the saccular nature of the AAA, he was offered repair via an open or endovascular approach. He choose EVAR and underwent stenting of the aorta with a 14- × 8-mm Begraft stent (Bentley InnoMed, Hechingen, Germany) via a percutaneous approach with cefazolin administered preoperatively as prophylaxis. He recovered uneventfully and was discharged on postoperative day 3. He had resumed his usual activities, including gardening, at 2 weeks after his EVAR.
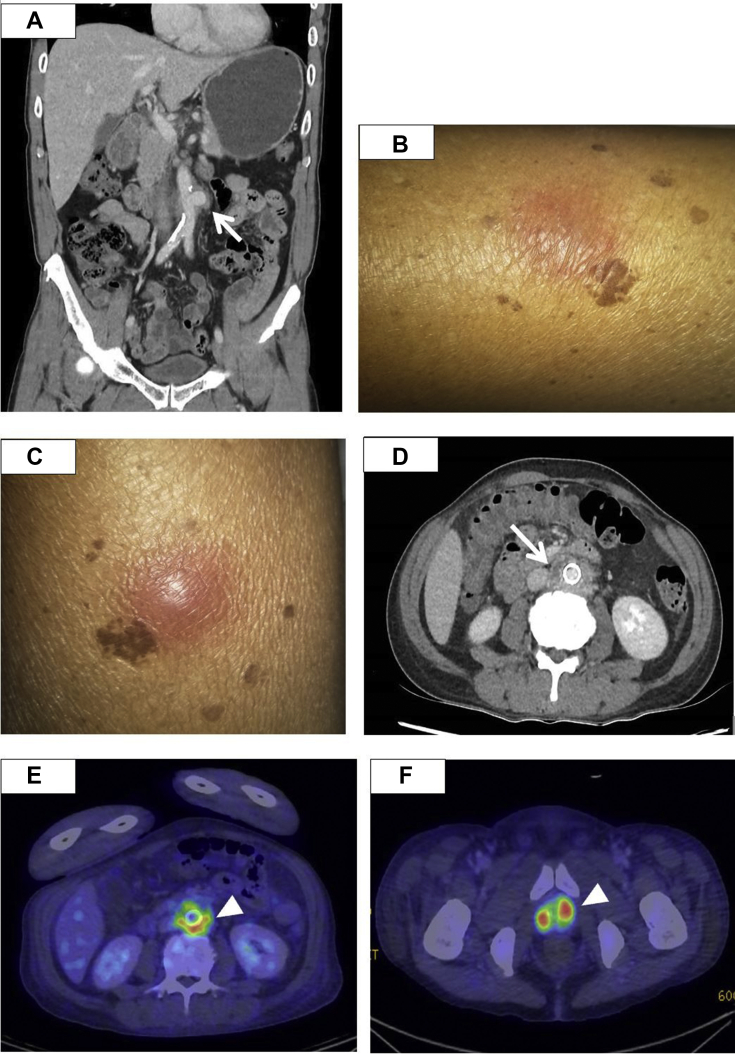
Fig 1.
A, Computed tomography (CT) urogram at initial presentation showing a saccular infrarenal abdominal aortic aneurysm (AAA). The arrow points to the area suspicious for perianeurysmal fat stranding, for which the radiologist was concerned contained a leak of the aneurysm (arrow). B and C, Physical examination on postoperative day (POD) 54 revealed the presence of new subcutaneous raised nodules over the bilateral lower limbs, which was associated with a febrile illness, highly suggestive of a disseminated infection. D, Abdominal CT on POD 54 showed a new rim-enhancing collection adjacent to the endograft (arrow), most likely due to the endograft infection. Position emission tomography scans from POD 60 confirmed the diagnosis of aortic endograft infection, with fluorodeoxyglucose-avid uptake around the endograft (E) and prostate (F; arrowhead).
At 2 months after EVAR, he had presented to the emergency department with a 2-day history of fever and painful nodules on his right lower limb. He was febrile with a temperature of 38.4°C, heart rate of 91 bpm, and blood pressure of 135/74 mm Hg. His physical examination revealed tender erythematous nodules on his bilateral thighs (Fig 1, B and C). No cardiac murmurs were found, and his bilateral pedal pulses were palpated well with a capillary refill time of <2 seconds. The laboratory test results showed a WBC count 20.2 × 109/L, CRP of 166.7 mg/L, and procalcitonin of 0.52 μg/L. CT of the abdomen and pelvis revealed a new rim-enhancing collection adjacent to the aortic stent and bilateral pyelonephritis (Fig 1, D). Positron emission tomography/CT showed fluorodeoxyglucose-avid uptake at the stent, suggestive of AGI, and multiple abscesses at the bilateral kidneys and prostate (Fig 1, E and F). No vegetations were found on transesophageal echocardiography. Blood cultures grew Burkholderia pseudomallei, which was sensitive to ceftazidime, trimethoprim-sulfamethoxazole, imipenem, and meropenem. Despite the administration of meropenem for 7 days, the bacteremia persisted. He was then transferred to our institution for further treatment. In consultation with our infectious diseases team, he received IV meropenem and trimethoprim-sulfamethoxazole. A combined decision was made for the patient to undergo surgery to remove the infected stent for source control.
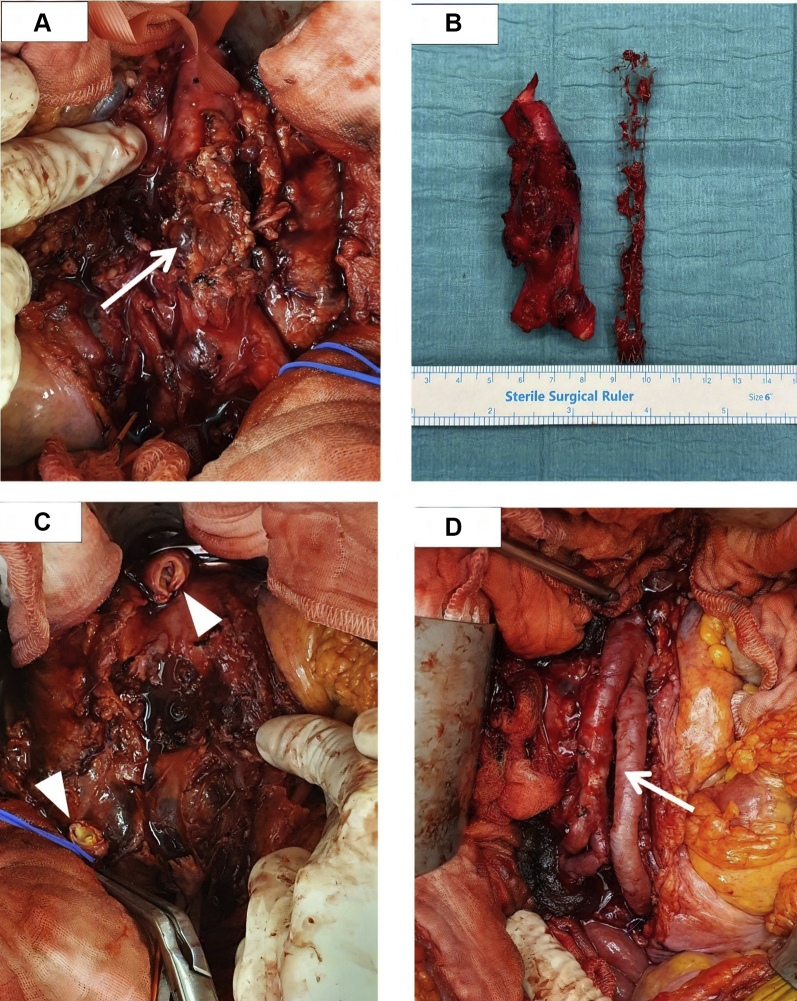
The patient underwent laparotomy, explantation of the infected stent, and creation of a neo-aorta iliac system. The intraoperative findings showed an inflammatory mass containing the infected stent, with pus tracking posteriorly (Fig 2). The infected aorta was divided and excised, and the stent was explanted. The periaortic tissue was debrided and washed out. The aorta was reconstructed with an autologous right femoral vein, which was spatulated on one end and sutured in a V fashion to form a “pantaloon” graft. This was anastomosed to the aorta in an end-to-end fashion and tagged over with a tongue of omental flap. Histologic examination showed inflammatory destruction of the aortic wall. The periaortic tissue and explanted stent cultures grew B. pseudomallei. His blood cultures cleared after stent removal. The antibiotics were switched to IV ceftazidime for 6 weeks, followed by oral trimethoprim-sulfamethoxazole for 3 months thereafter. He had an uneventful recovery, and the right lower limb nodules had also resolved before discharge. He was last seen in the clinic on February 1, 2021 and remained clinically well. He had completed the antibiotic regimens, and his latest CRP level was within normal limits. The patient provided written informed consent for the report of his case and imaging studies.
Fig 2.
A, Intraoperative photograph showing an inflammatory mass with extensive necrotic perigraft tissue, an anterior saccular aneurysm with a severely damaged wall, and intramural thrombus (white arrow). B, The unhealthy aorta was divided and excised. The infected aortic stent was also identified and explanted. C, Intraoperative photograph after division and excision of the infected aorta and endograft and debridement of necrotic tissue. White arrowheads demonstrate the proximal and distal ends of the excised aorta. D, Intraoperative photograph showing arterial reconstruction using neo-aorta iliac system method with autologous right superficial femoral vein graft (white arrow).
Discussion
Our patient had presented with fever and cutaneous lesions suggestive of septic emboli. These findings alluded to the possibility of an AGI, especially in the context of his recent EVAR. The CT imaging findings of perigraft fluid with corresponding fluorodeoxyglucose avidity on positron emission tomography/CT supported the diagnosis of an AGI. AGI is an uncommon complication of EVAR and occurs more often after emergency or repeat operations owing to intraoperative bacterial contamination.2 This was not the case for our patient. He had developed an early AGI that had occurred within 2 months of EVAR and presented with disseminated B. pseudomallei infection. This was unusual in a few aspects. First, our patient had undergone an uncomplicated EVAR with no evidence of infection during stent placement. The guidelines have typically recommended repair of symptomatic AAA and those >5.0 cm. For saccular aneurysms, no clear size cutoff has been established at which repair should be recommended, although repair has usually been recommended at a smaller diameter. Second, the AGI of our patient involved an uncommon pathogen, B. pseudomallei, the causative pathogen of melioidosis. B. pseudomallei is an environmental gram-negative bacillus found in the soil and surface water.3 Melioidosis will usually present with respiratory or genitourinary infection, with an incidence of mycotic aneurysm of <1%.4 We postulated that our patient's early exposure to soil after EVAR had resulted in his infection. It was unlikely that an indolent infection had caused his initial AAA, because the surveillance blood cultures collected before EVAR were negative and his inflammatory markers had not been elevated preoperatively. Contamination of the procedural field during stent placement was unlikely because no other patients with melioidosis were present in the unit, and an alternative exposure (his early return to gardening) had been identified.
In established AGI, removal of the infected material is recommended. However, the approach for each patient must be individualized. Our patient had no comorbidities and had been well before his diagnosis of the AGI. Thus, the early removal of the infected graft with autogenous vein reconstruction would offer him the best outcome. Other surgical options for establishing arterial continuity include reconstruction with an extra-anatomic bypass, cryopreserved allografts, xenopericardial grafts,5 or antibiotic-impregnated grafts. These options are not ideal because the placement of prosthetic material in an infected field carries the risk of reinfection or thrombosis. The use of in situ reconstruction has increased in popularity owing to the lower reinfection rates, superior patency, and eliminated risk of aortic stump blowout. Conservative approaches such as long-term antibiotics, graft lavage, or drainage are less ideal owing to greater mortality and higher relapse rates.6
For B. pseudomallei AGI, the reported data have recommended ≥6 weeks of IV antibiotics, followed by a prolonged course of eradication therapy with oral antibiotics.7 The recommendations to prevent AGIs include perioperative prophylaxis with antistaphylococcal agents, increased vigilance for periprocedural infection, and providing thorough wound care education to patients to prevent postoperative wound infections. At present, the emphasis on lifestyle measures to prevent exposure to infectious agents has been insufficient.8 For patients living in regions endemic for melioidosis who will undergo aortic stenting, we suggest they should avoid activities with soil exposure for ≥4 to 6 months after the procedure to lower their risk of developing B. pseudomallei AGI.
Conclusions
B. pseudomallei is a rare causative agent of AGI but should be suspected in patients from endemic regions who report exposure to soil. A multidisciplinary approach involving the infectious diseases and vascular teams is crucial. Successful management requires a prompt diagnosis, directed antimicrobial therapy, and early surgery, if indicated. The advice to avoid soil exposure after stent placement could be important for the prevention of B. pseudomallei AGI.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Laser A., Baker N., Rectenwald J., Eliason J.L., Criado-Pallares E., Upchurch G.R., Jr. Graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2011;54:58–63. doi: 10.1016/j.jvs.2010.11.111. [DOI] [PubMed] [Google Scholar]
- 2.Wilson W.R., Bower T.C., Creager M.A., Amin-Hanjani S., O’Gara P.T., Lockhart P.B. Vascular graft infections, mycotic aneurysms, and endovascular infections: a scientific statement from the American Heart Association. Circulation. 2016;134:e412–e460. doi: 10.1161/CIR.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 3.Wiersinga W.J., Peacock S.J. Melioidosis. N Engl J Med. 2012;367:1035–1044. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 4.Currie B.J., Ward L., Cheng A.C. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4:e900. doi: 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czerny M., von Allmen R., Opfermann P., Sodeck G., Dick F., Stellmes A. Self-made pericardial tube graft: a new surgical concept for treatment of graft infections after thoracic and abdominal aortic procedures. Ann Thorac Surg. 2011;92:1657.e62. doi: 10.1016/j.athoracsur.2011.06.073. [DOI] [PubMed] [Google Scholar]
- 6.Akhtar M., Meecham L., Birkett R., Pherwani A.D., Fairhead J.F. Conservative treatment of an infected aortic graft with antibiotic irrigation. Int J Angiol. 2016;25:e118. doi: 10.1055/s-0035-1548738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemarajata P., Baghdadi J.D., Hoffman R., Humphries R.M. Burkholderia pseudomallei: challenges for the clinical microbiology laboratory. J Clin Microbiol. 2016;54:2866–2873. doi: 10.1128/JCM.01636-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaikof E.L., Dalman R.L., Eskandari M.K., Jackson B.M., Lee W.A., Mansour M.A. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77. doi: 10.1016/j.jvs.2017.10.044. [DOI] [PubMed] [Google Scholar]