Abstract
Despite advances in treatment, most patients with multiple myeloma (MM) will relapse, and long-term survival remains poor. B-cell maturation antigen (BCMA) is an ideal therapeutic target as it is expressed throughout the disease course with normal tissue expression limited to plasma and some B-cell lineages. This phase 1, multicenter, first-in-human study evaluated the safety and efficacy of KITE-585, an autologous anti-BCMA chimeric antigen receptor (CAR) T-cell therapy, in patients with relapsed/refractory MM (RRMM). Key eligibility criteria included measurable MM and progression, defined by the International Myeloma Working Group Consensus Criteria within 60 days of the last treatment. Patients underwent leukapheresis and subsequently received a 3-day conditioning therapy regimen (cyclophosphamide [300 mg/m2/day] and fludarabine [30 mg/m2/day]). Patients then received a flat dose of 3 × 107 to 1 × 109 KITE-585 CAR T cells in a 3+3 dose-escalation design. The primary endpoint was incidence of adverse events (AEs) defined as dose-limiting toxicities (DLTs). Key secondary and exploratory endpoints included efficacy outcomes, incidence of AEs, levels of KITE-585 in blood, serum cytokines, and incidence of anti-BCMA CAR antibodies. Seventeen patients were enrolled, and 14 received KITE-585 with a median follow-up of 12.0 months. The median age of patients was 56 years, 41.2% had an Eastern Cooperative Oncology Group performance status of 1, 92.9% had baseline BCMA expression on plasma cells, and median number of prior therapies was 5.5. No patients experienced a DLT, all patients experienced ≥ 1 grade ≥ 3 treatment-emergent AE (TEAE), and no grade 5 TEAEs were observed. There were no grade ≥ 3 events of cytokine release syndrome, neurologic events, or infections; all were grade 1 or 2, and each occurred in 21.4% of patients. Among all patients infused with KITE-585, 1 patient who received 3 × 107 anti-BCMA CAR T cells experienced a partial response. Median peak CAR T-cell expansion was low (0.98 cells/μL), as were median peak serum levels of CAR-associated cytokines, including interferon-γ (61.45 pg/mL) and interleukin-2 (0.9 pg/mL). KITE-585 demonstrated a manageable safety profile; however, the limited CAR T-cell expansion and associated lack of anti-tumor response in patients with RRMM treated with KITE-585 is consistent with the minimal CAR T-cell activity observed.
Keywords: Chimeric antigen receptor T cell, B-cell maturation antigen, relapsed/refractory multiple myeloma
Introduction
More than 32,000 new cases of multiple myeloma (MM) occur every year in the United States, with greater than 12,800 estimated deaths annually and a 5-year survival rate of 54% [1]. In recent decades, outcomes for patients with MM have greatly improved with the advent of new treatments; however, despite the increasing number of treatment options, the majority of patients will eventually relapse [2,3]. Additionally, with each subsequent line of treatment and progression, genetic aberrations can accumulate in association with more aggressive disease and shorter durations of response [4]. The unmet medical need for innovative therapies that improve outcomes in relapsed/refractory (RR) MM is considerable.
B-cell maturation antigen (BCMA) is the cell surface receptor for B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) [5]. BCMA expression is tightly regulated and is normally restricted to plasma cells and some B cell lineages with limited to no expression in other tissues [6,7], making it an ideal therapeutic target for the treatment of plasma cell disorders such as MM [6,8]. Gene and protein expression profiling has shown that BCMA is broadly expressed in myeloma cell lines as well as primary myeloma cells from patients [6-11]. BCMA expression on myeloma cells has been demonstrated to be maintained from initial diagnosis through relapse [8].
The efficacy of chimeric antigen receptor (CAR) T-cell therapies directed against hematologic malignancies has been demonstrated, particularly for CD19-expressing B-cell lymphomas and leukemias [12,13]. In addition, CAR T-cell therapies targeting BCMA have demonstrated impressive activity in preclinical and clinical studies of MM [7,14-19]. Here we describe results from a phase 1 study of KITE-585, an autologous, fully human, anti-BCMA CAR for the treatment of patients with RRMM, which has preclinically demonstrated the ability to specifically target BCMA-expressing MM lines [20,21]. KITE-585 CAR T cells are genetically engineered ex vivo by transduction with the lentiviral vector containing the anti-BCMA CAR construct that consists of a human, anti-BCMA single-chain variable fragment with high specific binding to BCMA and CD28 and CD3ζ domains that participate in T-cell activation. In this phase 1, multicenter, open-label, first-in-human study, the safety and efficacy of KITE-585 in patients with RRMM was evaluated.
Materials and methods
Patients aged ≥ 18 years with measurable RRMM and progression defined by the International Myeloma Working Group (IMWG) Consensus Criteria [22] were enrolled. Progression must have occurred within 60 days after 1) the last dose of the last line of therapy and following treatment with ≥ 3 prior lines of therapy including both a proteasome inhibitor [PI] and an immunomodulatory drug [IMiD] or 2) the last dose of a regimen containing both a PI and an IMiD, regardless of number of prior lines of therapy. Patients must also have had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 and adequate bone marrow, renal, hepatic, pulmonary, and cardiac function. Key exclusion criteria included plasma cell leukemia, non-secretory MM, active or prior history of central nervous system or meningeal involvement by malignant plasma cells, prior BCMA-targeted therapy, and prior CAR therapy or other genetically modified T cells. Each study site’s Institutional Review Board reviewed and approved the study protocol and amendments, and all patients provided written informed consent.
After enrollment and leukapheresis, patients could receive optional bridging chemotherapy at the investigator’s discretion up to 7 days prior to initiation of lymphodepleting conditioning therapy (cyclophosphamide [300 mg/m2/day] and fludarabine [30 mg/m2/day]). Patients then received a single dose of KITE-585 CAR T cells with doses ranging from 3 × 107 to 1 × 109 KITE-585 CAR T cells.
This study followed a 3+3 dose-escalation design with the option to expand enrollment, including an expansion cohort composed of patients with moderate renal impairment (ie, creatinine clearance 30 to 59 mL/min), at doses that passed dose-limiting toxicity (DLT) criteria. A DLT was any KITE-585-related event with onset within 28 days of KITE-585 infusion, defined as any grade 5 adverse event (AE), grade 3 cytokine release syndrome (CRS) or non-hematologic AE ongoing ≥ 72 hours, grade 4 hematologic AEs ongoing ≥ 30 days, or grade 4 CRS or non-hematologic AE of any duration, unless otherwise specified by the protocol (Supplementary Appendix). For details on the schedule of assessments, please refer to the protocol.
The primary endpoint was the incidence of DLTs. Key secondary endpoints included objective response rate, progression-free survival (PFS), overall survival (OS), and incidence of AEs and clinically significant changes in laboratory values. Key exploratory endpoints included levels of KITE-585 in blood, serum cytokines, and the incidence of anti-BCMA CAR antibodies. BCMA espression was detected via quantitative flow cytometry assay and immunohistochemistry (Neogenomics Laboratories, Inc.; Supplementary Appendix). All statistical analyses were descriptive. A safety review team reviewed data at the completion of each dose cohort to make recommendations on dose levels based on DLTs and find the maximum tolerated dose.
This study is registered with ClinicalTrials.gov, number NCT03318861.
Results
Between October 31, 2017 and November 19, 2018, 17 patients were enrolled and underwent leukapheresis, and 14 patients were treated with KITE-585 (Table 1). Three patients did not receive KITE-585: one patient had a serious AE (grade 3 febrile neutropenia) considered related to conditioning chemotherapy, one patient had a low platelet count, and for the third patient, the product was not dosed due to a low CAR T-cell count during product manufacturing. Among the 14 treated patients, 3 patients were included in each of the 4 dose-escalation cohorts, and 2 patients were included in the renal impairment cohort and received 3 × 107 KITE-585 CAR T cells. The median time from leukapheresis to receipt of manufactured KITE-585 at the site was 17.0 days (range, 15 to 28).
Table 1.
Patient disposition and baseline characteristics
| Characteristic/outcome | Dose levels (total anti-BCMA CAR T cells) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Dose escalation cohorts | RI cohort | |||||
|
|
|
|||||
| 3 × 107 (N=3) | 1 × 108 (N=4) | 3 × 108 (N=3) | 1 × 109 (N=4) | 3 × 107 (N=2) | Overall (N=17) | |
| Patient disposition | ||||||
| Enrolled and leukapheresed, n (%) | 3 (100) | 4 (100) | 3 (100) | 4 (100) | 3 (100) | 17 100) |
| Received bridging therapy, n (%) | 1 (33) | 2 (50) | 0 | 0 | 2 (67) | 5 (29) |
| Received conditioning chemotherapy, n (%) | 3 (100) | 4 (100) | 3 (100) | 3 (75) | 2 (67) | 15 (88) |
| Patients who did not receive KITE-585, n (%) | 0 | 1 (25) | 0 | 1 (25) | 1 (33) | 3 (18) |
| AE | 0 | 1 (25) | 0 | 0 | 0 | 1 (6) |
| Other | 0 | 0 | 0 | 0 | 1 (33) | 1 (6) |
| Product not available | 0 | 0 | 0 | 1 (25) | 0 | 1 (6) |
| Patients who received KITE-585, n (%) | 3 (100) | 3 (75) | 3 (100) | 3 (75) | 2 (67) | 14 (82) |
| Median KITE-585 follow-up time (range), mo | 17.0 (16.0-17.6) | 13.9 (11.0-14.0) | 8.7 (8.7-9.2) | 6.8 (6.0-7.3) | 13.5 (13.0-13.9) | 12.0 (8.7-14.0) |
| Baseline characteristics | ||||||
| Median age (range), y | 51 (50-56) | 59.5 (49-71) | 62 (49-67) | 56 (47-58) | 57 (47-65) | 56 (47-71) |
| Male, n (%) | 3 (100) | 3 (75) | 1 (33) | 4 (100) | 1 (33) | 10 (59) |
| ECOG performance status 1, n (%) | 1 (33) | 3 (75) | 0 | 2 (50) | 1 (33) | 7 (41) |
| Prior ASCT, n (%) | 3 (100) | 4 (100) | 2 (67) | 4 (100) | 3 (100) | 16 (94) |
| Median time from initial diagnosis (range), mo | 50.0 (40.0-85.0) | 80.5 (36.0-134.0) | 27.0 (27.0-30.0) | 95.5 (27.0-119.0) | 61.0 (32.0-76.0) | 61.0 (27.0-134.0) |
| International Staging System, n (%)* | ||||||
| Stage I/II | 2 (67) | 1 (25) | 2 (67) | 2 (50) | 0 | 7 (41) |
| Stage III | 1 (33) | 0 | 1 (33) | 1 (25) | 1 (33) | 4 (24) |
| High-risk cytogenetics, n (%) | 0 | 0 | 0 | 1 (25) | 1 (33) | 2 (12) |
| Creatinine clearance 30-59 mL/min, n (%) | 0 | 0 | 0 | 0 | 3 (100) | 3 (18) |
| Median prior therapy (range), n† | 6 (3-7) | 7 (6-9) | 4 (4-5) | 4 (4-5) | 7 (6-8) | 5.5 (3-8) |
| No. of lines of prior therapy, n (%)† | ||||||
| 3 | 1 (33) | 0 | 0 | 0 | 0 | 1 (7) |
| 4 | 0 | 0 | 2 (67) | 2 (67) | 0 | 4 (29) |
| 5 | 0 | 0 | 1 (33) | 1 (33) | 0 | 2 (14) |
| > 5 | 2 (67) | 3 (100) | 0 | 0 | 2 (100) | 7 (50) |
| Refractory to, n (%)† | ||||||
| Both PI and IMiD | 3 (100) | 3 (100) | 3 (100) | 2 (67) | 2 (100) | 13 (93) |
| Last line of previous therapy | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) |
| Positive plasma cell BCMA expression, n/N‡ (%) | 3/3 (100) | 3/3 (100) | 3/3 (100) | 2/2 (100) | 2/2 (100) | 13/13 (100) |
| Median plasma cell BCMA expression (range), % | 90.2 (51.9-99.9) | 100 (100-100) | 92.9 (86.9-100) | 86.0 (73.0-99.0) | 97.8 (96.3-99.3) | 99.0 (51.9-100) |
AE, adverse event; ASCT, autologous stem cell transplantation; BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor; ECOG, Eastern Cooperative Oncology Group; RI, renal impairment; TEAE, treatment-emergent adverse event.
International Staging System score was unavailable for 6 patients.
Calculated out of patients who received KITE-585.
N refers to the total number of patients with evaluable baseline bone marrow aspirate samples available for central BCMA assessment.
Across all 17 enrolled patients, the median time since initial myeloma diagnosis to leukapheresis was 61 months, the median age was 56 years, 59% of patients were male, 41% had an ECOG performance status of 1, and 94% had undergone a prior autologous stem cell transplant. Patients had a median of 5.5 lines (range, 3 to 8) of prior therapy, and over half of patients (64%) had ≥ 5 lines of prior therapy. All patients had disease that was refractory to their last line of therapy, with 93% being refractory to both a PI and IMiD, and 85.7% of patients had received prior anti-CD38 antibody therapy. Among the 14 patients who were treated with KITE-585, 13 patients had local and central bone marrow aspirates at baseline that were evaluable for BCMA expression on plasma cells. Samples from all 13 patients showed BCMA-expressing plasma cells in bone marrow aspirate, with a median frequency of 99.0% (range, 51.9 to 100; Table 1).
As of the data cutoff (May 19, 2019), the median follow-up from KITE-585 infusion was 12.0 months (range, 8.7 to 14.0). Adverse events are summarized in Table 2. No patient who received KITE-585 experienced a DLT. All patients experienced ≥ 1 grade ≥ 3 treatment-emergent AEs (TEAE), with the most common being decreased neutrophil count (50%), decreased white blood cell count (42.9%), neutropenia (35.7%), anemia (35.7%), and thrombocytopenia (28.6%). There were no grade ≥ 3 events of CRS, neurologic events (NEs), or infections attributed to KITE-585; all were grade 1 or 2 in severity, and each occurred in 21.4% of patients. There were no grade 5 TEAEs. While no induction of anti-Kite-585 antibodies was observed, at baseline, 4 patients (28.6%) tested positive for serum anti-Kite-585 CAR-reactive antibodies (Table 2), which remained positive, turned negative, or some patients had no samples collected post-baseline. Seven patients (50%) had died more than 30 days after KITE-585 infusion: 6 patients (43%) due to progressive disease and 1 patient (7%) due to infection.
Table 2.
Safety and efficacy outcomes
| Characteristic/outcome | Dose levels (total anti-BCMA CAR T cells) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Dose escalation cohorts | RI cohort | |||||
|
|
|
|||||
| 3 × 107 (N=3) | 1 × 108 (N=3) | 3 × 108 (N=3) | 1 × 109 (N=3) | 3 × 107 (N=2) | Overall (N=14) | |
| Safety outcomes | ||||||
| Grade ≥ 3 TEAE, n (%)* | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 2 (100) | 14 (100) |
| Neutrophil count decreased | 2 (67) | 1 (33) | 1 (33) | 3 (100) | 0 | 7 (50) |
| White blood cell count decreased | 2 (67) | 2 (67) | 1 (33) | 1 (33) | 0 | 6 (43) |
| Neutropenia† | 0 | 2 (67) | 2 (67) | 0 | 1 (50) | 5 (36) |
| Anemia | 0 | 2 (67) | 2 (67) | 0 | 1 (50) | 5 (36) |
| Thrombocytopenia‡ | 0 | 1 (33) | 2 (67) | 0 | 1 (50) | 4 (29) |
| Infection, n (%)§ | 0 | 1 (33) | 2 (67) | 0 | 0 | 3 (21) |
| Upper respiratory tract infection | 0 | 1 (33) | 1 (33) | 0 | 0 | 2 (14) |
| Oral candidiasis | 0 | 0 | 1 (33) | 0 | 0 | 1 (7) |
| CRS, n (%)§ | 0 | 0 | 2 (67) | 1 (33) | 0 | 3 (21) |
| Hypotension | 0 | 0 | 2 (67) | 0 | 0 | 2 (14) |
| Pyrexia | 0 | 0 | 1 (33) | 1 (33) | 0 | 2 (14) |
| Chills | 0 | 0 | 0 | 1 (33) | 0 | 1 (7) |
| NE, n (%)§ | 1 (33) | 1 (33) | 0 | 1 (33) | 0 | 3 (21) |
| Nystagmus | 0 | 1 (33) | 0 | 1 (33) | 0 | 2 (14) |
| Cognitive disorder | 1 (33) | 0 | 0 | 0 | 0 | 1 (7) |
| Anti-KITE-585 CAR antibodies any time on study | 2 (67) | 1 (33) | 1 (33) | 0 | 0 | 4 (29) |
| Patients who died after enrollment | 1 (33) | 2 (67) | 2 (67) | 1 (33) | 1 (50) | 7 (50) |
| Progressive disease | 0 | 2 (67) | 2 (67) | 1 (33) | 1 (50) | 6 (43) |
| Infection | 1 (33) | 0 | 0 | 0 | 0 | 1 (7) |
| Efficacy outcomes | ||||||
| Response category | ||||||
| Investigator-assessed ORR, n (%) | 1 (33) | 0 | 0 | 0 | 0 | 1 (7) |
| Partial response | 1 (33) | 0 | 0 | 0 | 0 | 1 (7) |
| Stable disease | 1 (33) | 0 | 0 | 0 | 2 (100) | 3 (21) |
| Progressive disease | 1 (33) | 3 (100) | 2 (67) | 3 (100) | 0 | 9 (64) |
| Not done | 0 | 0 | 1 (33) | 0 | 0 | 1 (7) |
| Median KM PFS (95% CI), mo | 1.1 (0.5-3.2) | 1.0 (0.9-1.0) | 0.8 (0.5-1.0) | 1.0 (0.9-1.0) | NE (2.0-NE) | 1.0 (0.9-1.1) |
| Median KM OS (95% CI), mo | 0 (11.1-NE) | 5.1 (3.0-NE) | 6.9 (3.2-NE) | 0 (5.5-NE) | 12.2 (NE-NE) | 12.2 (5.1-NE) |
AE, adverse event; BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor; CRS, cytokine release syndrome; KM, Kaplan Meier; NE, neurological event; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; RI, renal impairment; TEAE, treatment-emergent adverse event.
Shown are most common grade ≥ 3 AEs that occurred in ≥ 25% of all patients.
Neutropenia included the terms neutropenia, febrile neutropenia, and neutrophil count decreases.
Thrombocytopenia included the terms thrombocytopenia and platelet count decreased.
All grade 1 or 2 in severity.
Among all patients infused with KITE-585, 1 patient in the 3 × 107 KITE-585 CAR T-cell dose group had a best response of partial response (PR) 2 weeks after infusion, which was maintained at the week 4 and month 2 follow-ups, and was followed by disease progression at month 3. Further, 3 patients (21.4%) had a best response of stable disease, with a duration ranging from 2 weeks to 2 months, 9 patients (64.3%) had a best response of progressive disease. The median PFS was 1.0 month, and the median overall survival was 12.2 months. One patient (7.1%) received subsequent anticancer therapy due to disease progression prior to first disease assessment and was therefore not evaluated for response.
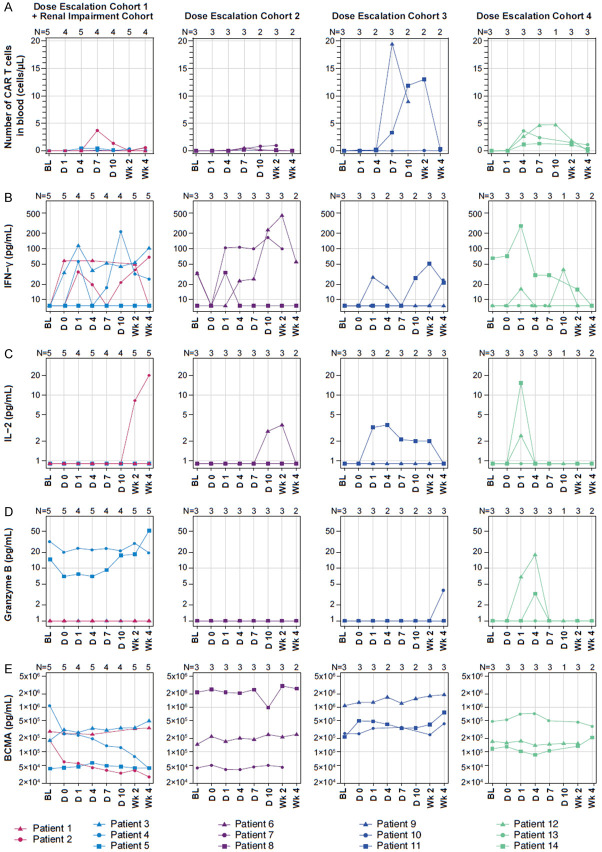
Among all patients infused with KITE-585, the median peak CAR T-cell expansion was 0.98 cells/μL (range, 0.06 to 19.54), and the median area under the curve within the first 28 days (AUC0-28) after treatment was 5.44 cells/μL × days (range, 0.0 to 171.75; Figure 1A), indicating poor expansion relative to published data [7,16]. Assessment of cytokine levels revealed limited cytokine responses after CAR T-cell infusion (Figure 1B-D). There was a minimal increase in serum interferon gamma with a median baseline value of 7.5 pg/mL (range, 7.5 to 65.0), a median peak value of 61.45 pg/mL (range, 7.5 to 451.6), and a median AUC0-28 of 945.2 pg/mL × days (range, 247.5 to 5531.0). Serum interleukin-2 values also remained largely unchanged over time with a median baseline value of 0.9 pg/mL (range, 0.9 to 0.9), a median peak value of 0.9 pg/mL (range, 0.9 to 19.9), and a median AUC0-28 of 29.7 pg/mL × days (range, 17.1 to 228.4), supporting observations of a lack of functional CAR T-cell activity. Similarly, serum granzyme B remained static over time with a median baseline value of 1.0 pg/mL (range, 1.0 to 32.0), a median peak value of 1.0 pg/mL (range, 1.0 to 51.4), and a median AUC0-28 of 33 pg/mL × days (range, 19 to 804.6). Lastly, serum BCMA levels remained largely unchanged for most patients; however, a reduction in serum BCMA was observed in the one patient who achieved PR (Figure 1E).
Figure 1.
KITE-585 anti-BCMA CAR T cells and select cytokines over time by patient and cohort. A. The number of anti-BCMA CAR T-cells/µL in blood by patient and cohort (n=14). B-E. Levels of serum cytokines over time by patient and cohort (n=14). BL, baseline; BCMA; B-cell maturation antigen; CAR, chimeric antigen receptor; D, day; IFN, interferon; IL, interleukin; Wk, week.
Discussion
In this phase 1 study investigating KITE-585 in patients with RRMM, no DLTs were observed in any dose group. No patients experienced AEs typically observed with CAR T-cell therapies, including grade ≥ 3 CRS or NEs or grade ≥ 4 neutropenia. Despite nearly all patients having BCMA target expression on plasma cells, only 1 patient experienced a PR, and 3 patients experienced SD. Treatment effects in these patients may not have been due to CAR T-cell activity, but instead, were potentially related to bendamustine bridging therapy, conditioning chemotherapy, or chemotherapy prior to enrollment. Although efforts were made to optimize treatment with increasing numbers of CAR T cells, expansion observed across dosing cohorts was 1 to 2 orders of magnitude lower than has been previously reported with another CD28-containing, anti-BCMA CAR T-cell therapy that exhibited anti-myeloma activity, despite similar or higher doses of CAR T cells [16]. Retrospective assessment of preclinical findings revealed potential areas of limitation that may have impeded interpretation of the data.
In previous preclinical experiments utilizing KITE-585 CAR T cells, assays were augmented with homeostatic cytokines such as IL-2, which may have boosted the efficacy of the KITE-585 CAR T cells, leading to positive preclinical results and highlighting the need for more standardized preclinical assessment in the field [7]. The preclinical models also had high levels of alloreactivity, which may have made results difficult to interpret. In addition, cell lines used for preclinical models tended to have high BCMA expression and did not necessarily recapitulate target heterogeneity in patients with MM observed here and previously described [7,11]. Further, methodological differences were present between the CAR T-cell production used in the preclinical models and in the present study. Future development activities will include more robust animal models evaluating target expression relevant to physiological levels, benchmarking where possible against clinically validated CAR designs, and also incorporating manufacturing processes that more closely mirror those used for clinical grade products.
Overall, while KITE-585 demonstrated a manageable safety profile, the limited anti-tumor response in patients with RRMM treated with KITE-585 is consistent with the minimal CAR T-cell expansion, cytokine production, and CAR T-cell function observed.
Acknowledgements
The authors thank the patients who participated in this study, their families, friends, and caregivers, and all study staff and healthcare providers. The authors would also like to acknowledge Chloe Slichter for her preclinical contributions, Myrna Nahas for her clinical perspective, and Ellie Huang of Kite, a Gilead Company, for statistical programming. This study was funded by Kite, a Gilead Company. Medical writing support was provided by Jared Hoffman, PhD, of Nexus Global Group Science, with funding from Kite, a Gilead Company.
Disclosure of conflict of interest
R.F.C. has employment with AbbVie; honoraria from Karyopharm, Takeda, and GlaxoSmithKline; and consultancy or advisory role for Karyopharm, Takeda, and GlaxoSmithKline. M.R.B: honoraria from Kite, a Gilead Company, Incyte, Celgene, Sanofi, Novartis, Bristol Myers Squibb, and Agios; consultancy or advisory role for Novartis, Kite, a Gilead Company, CRISPR Therapeutics, Agios, Iovance, Bluebird Bio, WindMIL Therapeutics, and Arcellx; speakers’ bureau participation for Kite, a Gilead Company, Agios, Incyte, Sanofi, and Bristol Myers Squibb; research funding from Kite, a Gilead Company, Novartis, CRISPR Therapeutics, Arcellx, Autolus, Immatics, Triumvira, and Tmunity; and travel support from Kite, a Gilead Company, Novartis, Bristol Myers Squibb, Agios, and Incyte. S.K. has a consulting or advisory role for AbbVie, Celgene, Janssen, Takeda, Adaptive Biotechnologies, Kite, a Gilead Company, and Medimmune/AstraZeneca; research funding from AbbVie, Celgene, Janssen, Takeda, Adaptive Biotechnologies, Kite, a Gilead Company, Medimmune/AstraZeneca, Merck, Novartis, Roche, and Sanofi; other relationship with Oncopeptides Independent Review Committee. S.A.G. has a consulting or advisory role with Kite, a Gilead Company, Novartis, Amgen, Celgene, Sanofi, Pfizer, Miltenyi, Bristol Myers Squibb, GlaxoSmithKline, and Actinium; and research funding from Amgen, Celgene, Sanofi, Pfizer, Actinium, Bristol Myers Squibb, and Miltenyi. A.K.N. has a consulting or advisory role with Takeda, Amgen, Janssen, Bristol Myers Squibb, GlaxoSmithKline, Sanofi, Karyopharm, Adaptive, and Oncopeptides. S.M.L. has a consulting or advisory role for Bristol Myers Squibb; speakers’ bureau participation for Takeda; research funding from Bristol Myers Squibb, Takeda, Janssen, BioLineRx, and Novartis; and travel support from Bristol Myers Squibb. F.L.L. has a consulting or advisory role for Kite, a Gilead Company, Novartis, Amgen, Celgene/Bristol Myers Squibb, GammaDelta Therapeutics, Iovance, Bluebird Bio, Wugen Inc., Calibr, Cellular Biomedicine Group Inc., and Allogen; and research support from Kite, a Gilead Company. N.S.R. has a consulting or advisory role for Bristol Myers Squibb, Bluebird Bio, Takeda, and Amgen; and research funding from Bluebird Bio. L.L. has employment with Lei Lei LLC; leadership role with Lei Lei LLC, stock or other ownership in Amgen; and consulting or advisory role with Kite, a Gilead Company, and BeiGene. J.D. has employment with Kite, a Gilead Company; consultancy or advisory role for GliaCure/Tufts; and patents, royalties, or other intellectual property from Patent US8598141 (Dec 03, 2013). J.B.L. has employment with Kite, a Gilead Company, and stock or other ownership in Gilead and Allogene. J.M.R. has employment with Kite, a Gilead Company. R.Z.O. has stock or other ownership in Asylia Therapeutics; patents, royalties, other intellectual property from MD Anderson and Asylia Therapeutics; research funding from BioTheryX, CARsgen Therapeutics, Celgene, Exelixis, Janssen, Sanofi, and Takeda; advisory board membership for Amgen, Bristol Myers Squibb, Celgene, EcoR1 Capital, Forma, Genzyme, GlaxoSmithKline Biologicals, Ionis Pharmaceuticals, Janssen, Juno, Kite, a Gilead Company, Legend, Molecular Partners, Regeneron, Sanofi, Servier, and Takeda; and consultancy or advisory role for STATinMED Research.
Supplementary Appendix
References
- 1.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975-2017. Bethesda, MD: National Cancer Institute; 2020. [Google Scholar]
- 2.Chim CS, Kumar SK, Orlowski RZ, Cook G, Richardson PG, Gertz MA, Giralt S, Mateos MV, Leleu X, Anderson KC. Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond. Leukemia. 2018;32:252–262. doi: 10.1038/leu.2017.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 4.Egan JB, Shi CX, Tembe W, Christoforides A, Kurdoglu A, Sinari S, Middha S, Asmann Y, Schmidt J, Braggio E, Keats JJ, Fonseca R, Bergsagel PL, Craig DW, Carpten JD, Stewart AK. Whole-genome sequencing of multiple myeloma from diagnosis to plasma cell leukemia reveals genomic initiating events, evolution, and clonal tides. Blood. 2012;120:1060–1066. doi: 10.1182/blood-2012-01-405977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2:465–475. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- 6.Tai YT, Anderson KC. Targeting B-cell maturation antigen in multiple myeloma. Immunotherapy. 2015;7:1187–1199. doi: 10.2217/imt.15.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, Gress RE, Hakim FT, Kochenderfer JN. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19:2048–2060. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee L, Bounds D, Paterson J, Herledan G, Sully K, Seestaller-Wehr LM, Fieles WE, Tunstead J, McCahon L, Germaschewski FM, Mayes PA, Craigen JL, Rodriguez-Justo M, Yong KL. Evaluation of B cell maturation antigen as a target for antibody drug conjugate mediated cytotoxicity in multiple myeloma. Br J Haematol. 2016;174:911–922. doi: 10.1111/bjh.14145. [DOI] [PubMed] [Google Scholar]
- 9.Moreaux J, Legouffe E, Jourdan E, Quittet P, Reme T, Lugagne C, Moine P, Rossi JF, Klein B, Tarte K. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–3157. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, Gross JA, Greipp PR, Jelinek DF. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. 2004;103:689–694. doi: 10.1182/blood-2003-06-2043. [DOI] [PubMed] [Google Scholar]
- 11.Bellucci R, Alyea EP, Chiaretti S, Wu CJ, Zorn E, Weller E, Wu B, Canning C, Schlossman R, Munshi NC, Anderson KC, Ritz J. Graft-versus-tumor response in patients with multiple myeloma is associated with antibody response to BCMA, a plasma-cell membrane receptor. Blood. 2005;105:3945–3950. doi: 10.1182/blood-2004-11-4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, Reagan P, Bot A, Rossi J, Navale L, Jiang Y, Aycock J, Elias M, Chang D, Wiezorek J, Go WY. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA, Grupp SA. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith EL, Staehr M, Masakayan R, Tatake IJ, Purdon TJ, Wang X, Wang P, Liu H, Xu Y, Garrett-Thomson SC, Almo SC, Riviere I, Liu C, Brentjens RJ. Development and evaluation of an optimal human single-chain variable fragment-derived BCMA-targeted CAR T cell vector. Mol Ther. 2018;26:1447–1456. doi: 10.1016/j.ymthe.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman KM, Garrett TE, Evans JW, Horton HM, Latimer HJ, Seidel SL, Horvath CJ, Morgan RA. Effective targeting of multiple B-cell maturation antigen-expressing hematological malignances by anti-B-cell maturation antigen chimeric antigen receptor T cells. Hum Gene Ther. 2018;29:585–601. doi: 10.1089/hum.2018.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, Brudno JN, Stetler-Stevenson M, Feldman SA, Hansen BG, Fellowes VS, Hakim FT, Gress RE, Kochenderfer JN. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sohail A, Mushtaq A, Iftikhar A, Warraich Z, Kurtin SE, Tenneti P, McBride A, Anwer F. Emerging immune targets for the treatment of multiple myeloma. Immunotherapy. 2018;10:265–282. doi: 10.2217/imt-2017-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao WH, Liu J, Wang BY, Chen YX, Cao XM, Yang Y, Zhang YL, Wang FX, Zhang PY, Lei B, Gu LF, Wang JL, Yang N, Zhang R, Zhang H, Shen Y, Bai J, Xu Y, Wang XG, Zhang RL, Wei LL, Li ZF, Li ZZ, Geng Y, He Q, Zhuang QC, Fan XH, He AL, Zhang WG. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol. 2018;11:141. doi: 10.1186/s13045-018-0681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, Vogl DT, Weiss BM, Dengel K, Nelson A, Plesa G, Chen F, Davis MM, Hwang WT, Young RM, Brogdon JL, Isaacs R, Pruteanu-Malinici I, Siegel DL, Levine BL, June CH, Milone MC. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. 2019;129:2210–2221. doi: 10.1172/JCI126397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams GB, Feng J, Ghogha A, Mardiros A, Murakami J, Phung T, Rodriguez R, Sievers S, Spindler TJ, Wiltzius J, Yarka C, Yoder SC, Polverino T. Development of KITE-585: a fully human BCMA CAR T cell therapy for the treatment of multiple myeloma. Cancer Res. 2017;77 Abstract 4979. [Google Scholar]
- 21.Adams GB, Feng J, Ghogha A, Mardiros A, Rodriguez A, Spindler TJ, Wiltzius J, Polverino T. Selectivity and specificity of engineered T cells expressing KITE585, a chimeric antigen receptor targeting B cell maturation antigen (BCMA) Cancer Res. 2017;77 Abstract 2135. [Google Scholar]
- 22.Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, Blade J, Richardson P, Orlowski R, Siegel D, Jagannath S, Facon T, Avet-Loiseau H, Lonial S, Palumbo A, Zonder J, Ludwig H, Vesole D, Sezer O, Munshi NC, San Miguel J International Myeloma Workshop Consensus Panel 1. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.