Abstract
Loss of patient-specific HLA after haploidentical hematopoietic stem cell transplantation (haplo-HSCT) is considered as a relapse mechanism for lacking the incompatible molecule to elicit alloreactivity, which extensively diminishing graft-versus-leukemia (GVL) effects. Blinatumomab, as a CD3/CD19 bispecific antibody, can yield a profound response via redirecting T cells towards malignant lymphoblasts in B-cell acute lymphoblastic leukemia (B-ALL). We aimed to assess the feasibility of blinatumomab in treating patients with HLA loss relapse after haplo-HSCT. Four eligible patients undergoing HLA loss relapse after haplo-HSCT were enrolled in the study. Four patients achieved a complete remission/complete remission with partial he-matologic recovery (CR/CRh) with three minimal residual disease (MRD)-negative response within the first cycle of treatment. Three of the four met a primary endpoint with CR/CRh and MRD-negative response within 2 cycles of treatment. One patient developed new extramedullary sites of skin after the first cycle. Cytokine release syndrome was observed in one patient. Cytopenias, as well as elevated alanine aminotransferase and aspartate aminotransferase, were two common adverse effects during treatment. By redirecting lysis of CD19-positive lymphoblast who losing the incompatible HLA, blinatumomab is a potential strategy to eradicate malignant cells via restoring GVL effects. A randomized clinical trial assessing blinatumomab in patients with HLA loss relapse after HSCT is warranted.
Keywords: B-cell acute lymphoblastic leukemia, HLA loss relapse, blinatumomab
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) can durably control and even cure B-cell acute lymphoblastic leukemia (B-ALL) in the long-term owing to the graft-versus-leukemia (GVL) effect of donor T cells [1]. In the case of HLA (human leukocyte antigen) haploidentical HSCT (haplo-HSCT), which provides an accessible option for nearly all patients with allo-HSCT indications lacking fully compatible hematopoietic stem cell resources, the incompatible HLA is an additional target for donor T cells exerting GVL effects compared with HLA identical HSCT, however, relapse is still an unsolved problem [2,3]. A retrospective study examined patients of B-ALL with recurrence after the first allo-HSCT showed 1- and 2-year overall survival rates of 17% and 10%, respectively, although receiving salvage therapy including a second HSCT, donor lymphocyte infusion (DLI), radiation therapy, mild chemotherapy [4]. Besides, the median survival for relapsed patients is less than a year [4,5]. Given the relatively high relapse rate and its dismal prognosis following allo-HSCT, further exploration of the underlying mechanism of relapse is warranted as the basis of treatment with pertinence.
Occurring in a proportion of patients who relapse after haplo-HSCT, there is a kind of relapse characterizing in loss of patient-specific HLA genomes in leukemic cells, which facilitates the malignant cells escaping from the GVL effect [6]. A study examined patients with myeloid malignancies found that HLA loss accounted for 33% of relapse in patients undergoing haplo-HSCT [7]. Because of the loss of patient-specific HLA, it is not difficult to explain why DLI as an extension of the GVL effect is redundant in some relapsed patients.
Blinatumomab (Blincyto, Amgen) is the first bispecific T-cell engager (BiTE) consisting of two single-chain variable fragments joined by a linker, bypassing the HLA pathway and dragging T cells closer to malignant B cells by its dual-specificity for CD19 and CD3 [8,9]. CD3 of T-cell receptor and CD19 expressed by nearly all B-cell linage are connected by blinatumomab, which allows the patient’s endogenous T cells to recognize and eliminate CD19-positive B-ALL blasts through several antileukemia processes such as perforin and granzyme release and interferon-γ release [10-13]. Because of its HLA-independent feature, it holds a therapeutic promise in HLA loss recurrence. Currently, as there are no clinical data on blinatumomab for posttransplantation HLA loss relapse, we herein report the preliminary results of blinatumomab in treating B-ALL with HLA loss recurrence after haplo-HSCT.
Method
Patients
From January 2017 to July 2019, patients relapsed after haplo-HSCT fulling the criteria were enrolled in the study as part of a larger registered study (CTR20170176/NCT03476239). Eligible patients met the criteria: 1) diagnosed with B cell precursor ALL undergoing haplo-HSCT; 2) achieved complete remission (CR) and full donor engraftment posttransplantation before relapse; 3) had HLA loss relapse after haplo-HSCT; 4) confirmed CD19 expression on blast cells by flow cytometry (FCM). Relapse was defined as ≥5% morphologic lymphoblasts counts in bone marrow (BM) and the reappearance of previously detected clonal cytogenetic abnormalities. The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics review committee of the First Affiliated Hospital of Zhejiang University School of Medicine. Informed consent was obtained from all recruited patients in accordance with the Declaration of Helsinki.
HLA typing and HLA loss
HLA typing of patients and donors on peripheral blood lymphocytes (PBLs) was performed at diagnosis by the Blood Center of Zhejiang Province or Shanghai Tissuebank Diagnositics Co., Ltd. evaluating 5 loci HLA-A, -B, -C, -DRB1, -DQB1. HLA loss was defined as the no detection of the genome of patient-specific HLA loci in purified CD19+/CD34+ leukemic cells harvested in BM. HLA-KMR, a methodology to detect HLA loss, was based on quantitative polymerase chain reaction (qPCR) by targeting patient-specific HLA markers and non-HLA makers with gene polymorphisms [14]. After haplo-HSCT, the HLA loss relapse or classic relapse (non-HLA loss relapse) pattern was monitored by detection of patient-specific HLA markers and patient-specific non-HLA markers. HLA loss confirmed diagnosis was drawn when patient-specific HLA markers were negative (<3%) and patient-specific non-HLA markers were positive (>3%). For those without ad hoc covered allele groups, next-generation sequencing was applied for chimerism.
Study design
Blinatumomab was administered via continuous intravenous infusion (CIVI). One cycle of blinatumomab treatment lasted for 6 weeks, including a 4-week continuous i.v. infusion period and a 2-week treatment-free interval. During the first induction cycle, an initial dose of 9 μg/day was used for the first 7 days (to reduce the likelihood of cytokine release syndrome [CRS] and immune effector cell-associated neurotoxicity syndrome [ICANS] adverse events associated with blinatumomab), and the dose was increased to 28 μg/day starting on day 8 (week 2) and continuing through day 28 (week 4). The 28 μg/day dose was used for all subsequent cycles. Patients received up to 5 cycles of blinatumomab, with efficacy follow-up at 3, 6, 9, 12, 18, and 24 months after treatment initiation. If a patient experiences a grade 3 central nervous system (CNS)-related adverse event or other clinically relevant grades 3 or 4 adverse event, blinatumomab therapy is discontinued and treatment is restarted when the adverse event is reduced to grade 1 or baseline.
Outcomes evaluation
The primary endpoint observed complete remission/complete remission with partial hematologic recovery (CR/CRh) rates in the 2 cycles of blinatumomab treatment. The secondary endpoints included minimal residual disease (MRD) response, relapse-free survival (RFS) rates, overall survival (OS) rates, and adverse events (AEs) during the course of blinatumomab treatment. CR was defined as BM lymphoblasts ≤5%, no evidence of active disease, and complete recovery of peripheral blood counts (platelet count >100×109/L, absolute neutrophil count >1×109/L); CRh was defined as BM lymphoblasts ≤5%, no evidence of active disease, and partial recovery of peripheral blood counts (platelet count>50×109/L and absolute neutrophil count >0.5×109/L). A BM examination was performed on the first 2 cycles after blinatumomab administration assessing the response. RFS and OS were calculated from the start of blinatumomab treatment. AEs with clinical relevance were based on the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) v5.0; ICANS and CRS were graded according to American Society for Transplantation and Cellular Therapy (ASTCT) consensus [15].
Statistical analysis
The patients’ characteristics were presented in descriptive form. Survival curves were described using the Kaplan-Meier method. Data were all analyzed using SPSS statistical software version 22.0.01 (IBM, NY, USA).
Results
Patients characteristics and transplantation algorithm
Between January 2017 and July 2019, four patients (two females, two males; age range: 22-52 years) with Philadelphia (Ph) chromosome-negative B-ALL receiving haploidentical grafts from their relatives and having posttransplant incipient relapse were enrolled in this study (baseline characteristics and HSCT details were presented in Table 1). Four patients all had CR1 status before HSCT, received cytarabine, busulfan, cyclophosphamide, Me-CCNU and antithymocyte globulin as the conditioning regimen, and peripheral blood stem cell as graft resource. The graft-versus-host disease (GVHD) prophylaxis algorithm consisted of cyclosporine, mycophenolate mofetil, and methotrexate [16]. No patient was weighted less than 45 kg.
Table 1.
Characteristics of patients and baseline data
| Patient | Gender | Gene abnormities | Age (year) | Status at HSCT | Donor age (year) type, gender, HLA match | KIR/KIR ligand mismatch | Conditioning regimen | CD34+ (×106/kg recipient weight) | Neutrophil engraftment post HSCT | platelet engraftment post HSCT | aGVHD | cGVHD | Time to relapsed after HSCT (months) | Extramedullary disease when relapse | BM blasts at relapse (%) | Salvage therapies before blinatumomab | Time from relapse to the first blinatumomab (days) | BM blasts at blinatumomab | Weight at blinatumomab (kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | F | None | 27 | MRD-negative CR1 | 23, F, 5/10 sister | C2 | MAC | 8.5 | 11 | 12 | Liver, GI tract | Mild | 8.8 | No | 38.5 | No | 13 | 38.5% | 46 |
| #2 | F | E2A-PBX1 | 52 | MRD-negative CR1 | 25, F, 5/10 daughter | No | MAC | 6.0 | 13 | 13 | Skin | Mild | 10.7 | No | 5.5 | No | 21 | 37.5% | 59 |
| #3 | M | None | 22 | MRD-negative CR1 | 54, M, 5/10 father | C2 | MAC | 3.1 | 11 | 11 | Skin | Mild | 19.0 | No | 69.0 | VICP | 35 | 61.5% | 62 |
| #4 | M | None | 32 | MRD-negative CR1 | 31, M, 5/10 brother | No | MAC | 10.6 | 12 | 15 | No | No | 9.5 | Testicles | 18.9 | VMCP | 18 | 6.0% | 58 |
aGVHD, acute graft-versus-host disease; BM, bone marrow; cGVHD, chronic graft-versus-host disease; CR, complete remission; F, female; GI, gastrointestinal; HSCT, hematopoietic stem cell transplantation; KIR, killer-cell immunoglobulin-like receptor; M, male; MAC, myeloablative conditioning; MRD, minimal residual disease; VICP, vincristine, idarubicin, cyclophosphamide and prednisone; VMCP, vincristine, melphalan, cyclophosphamide and prednisone.
HLA loss relapse
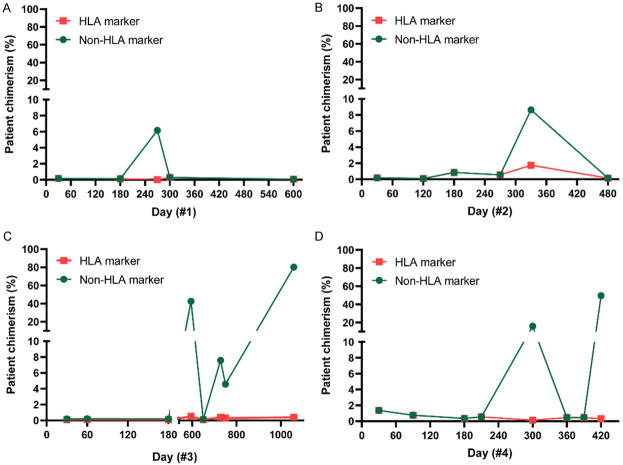
Only Patient #4 relapsed in both EM sites of testicles and BM, while other patients had isolated BM relapse. Of note, patient #4 showed an early relapse within 6 months after HSCT. Patients had confirmed HLA loss relapse when enrollment. Patients’ and donors’ HLA typing outcomes before haplo-HSCT and patients’ specific HLA markers showing in red were summarized in Table 2. As shown in Table 2 and Figure 1, when HLA makers and non-HLA markers were discordant, indicating an HLA loss relapse.
Table 2.
HLA typing performed on blood samples diagnosis and specific HLA marker used for loss detection
| HLA-A | HLA-B | HLA-C | HLA-DRB1 | HLA-DQB1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | A*02:01 | A*33:03 | B*15:18 | B*58:01 | C*03:02 | C*07:04 | DRB1*03:01 | DRB1*04:01 | DQB1*02:01 | DQB1*03:01 |
| #1 Donor | A*02:01 | A*30:01 | B*15:18 | B*13:02 | C*06:02 | C*07:04 | DRB1*07:01 | DRB1*04:01 | DQB1*02:02 | DQB1*03:01 |
| #2 | A*11:01 | A*24:02 | B*15:02 | B*40:06 | C*08:01 | C*08:01 | DRB1*04:05 | DRB1*08:03 | DQB1*06:01 | DQB1*04:01 |
| #2 Donor | A*02:01 | A*11:01 | B*15:02 | B*40:01 | C*03:04 | C*08:01 | DRB1*04:05 | DRB1*11:01 | DQB1*03:01 | DQB1*04:01 |
| #3 | A*02:01 | A*02:06 | B*07:02 | B*13:01 | C*03:04 | C*07:02 | DRB1*12:02 | DRB1*15:01 | DQB1*06:02 | DQB1*03:01 |
| #3 Donor | A*02:06 | A*02:07 | B*13:01 | B*40:01 | C*03:04 | C*04:82 | DRB1*04:03 | DRB1*12:02 | DQB1*03:01 | DQB1*03:02 |
| #4 | A*11:01 | A*24:02 | B*27:04 | B*40:01 | C*03:04 | C*12:02 | DRB1*09:01 | DRB1*12:02 | DQB1*03:01 | DQB1*03:03 |
| #4 Donor | A*11:01 | A*11:01 | B*27:04 | B*55:12 | C*01:02 | C*12:02 | DRB1*04:05 | DRB1*12:02 | DQB1*03:01 | DQB1*04:01 |
Red label indicates the patient-specific HLA loci for HLA-KMR detection.
Figure 1.
Detection of patient-specific HLA marker (C*03 in patient #1 [A], A*24 in patient #2 [B], C*07 in patient #3 [C], A*24 and C*03 in patient #4 [D]) and a patient-specific non-HLA marker after haplo-HSCT.
Response and outcomes
Response and outcome data were summarized in Figure 2. Two male patients had one course of salvage chemotherapy before blinatumomab treatment. No patient received blinatumomab in remission status. CD19 expression was detected and confirmed by FCM during the course of blinatumomab.
Figure 2.
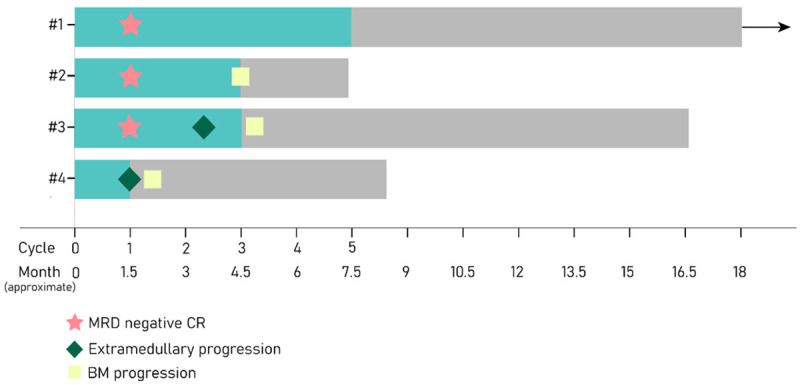
Survival span after blinatumomab. The green bar indicates time treated with blinatumomab.
Blinatumomab can exert a potent and prompt efficacy. Four patients (#1, #2, #3, #4) achieved a CR/CRh with three MRD-negative response (#1, #2, #3) within the first 1 cycle of treatment. Three of the four (#1, #2, #3) met a primary endpoint with CR/CRh and MRD-negative response within 2 cycles of treatment. Patient #4 developed new EM sites of skin after the first cycle, for which disrupted the further course of blinatumomab despite remained BM morphological remission and FCM MRD-positive of 0.644%.
Patient #1 completed 5 cycles of blinatumomab, remaining CR since the first cycle. Patient #2 experienced relapse with 19.5% of lymphoblasts in BM after finishing the third cycle. Patient #3 achieved morphological and molecular remission after the first cycle of blinatumomab and kept the leukemia-free for 2 cycles. He developed EM involvement for the first time during the course of the third cycle, manifesting by subcutaneous nodules, while BM kept in remission.
Patient #2 and patient #3 achieved CR/CRh had an RFS of 5.1 and 3.5 months, respectively. The RFS of patient #1 has already sustained for 34.3 months until the latest follow-up (Figure 3A). The median overall survival was 12.8 months (range, 7.2-34.3), and patient #2, #3, #4 died of disease relapse (Figure 3B).
Figure 3.
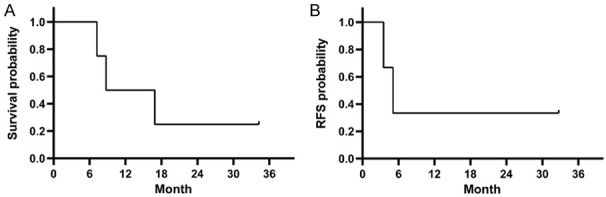
Overall survival (OS) (A) and the incidence of relapse-free survival (RFS) (B) after blinatumomab.
Safety
CRS was observed in patients #1 who had a high fever with a maximum temperature of 39.6°C, requiring medications of temporary dexamethasone, which then pacified in four days.
There were no ICNAS observed. Cytopenia, as well as elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST), were two major AEs during blinatumomab treatment. Cytopenia of grades 1-2 was reported in all patients and no transfusions of blood products required. Patient #1 experienced grade 2 neutropenia; patient #2 experienced grade 2 neutropenia; patient #3 experienced grade 2 pancytopenia; patient #4 experienced grade 4 elevated ALT (max 699 U/L) and AST (max 595 U/L), thus the study was discontinued for 11 days, thereafter reinitiated when AEs reduced to grade 1 after medication interventions. Chronic GVHD was observed in patient #2 involving mouth and skin (mild) and controlled with prednisone 0.2 mg/kg/d.
Discussion
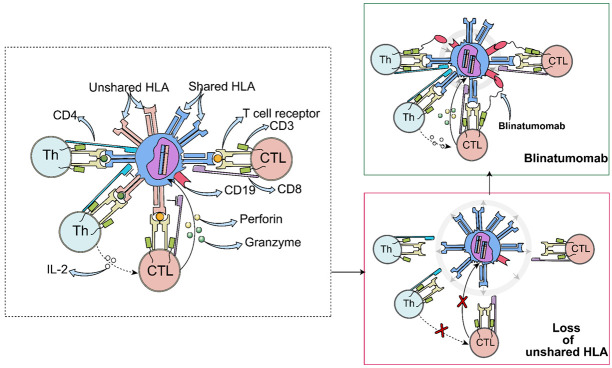
Loss of mismatched HLA has been considered as an underlying mechanism for leukemia recurrence after haploidentical HSCT [17]. Because the entire HLA haplotype mismatch between patient and donor, effective alloreactivity could be induced by donor T cells which directly contributes to the GVL effect [18]. Since the relapse after HSCT is characterized by a subset of leukemic cells losing unshared HLA haplotype, it is not difficult to speculate how the relapse occurred: the GVL effect is greatly diminished (Figure 4). For those with HLA loss relapse, selection of accessibly salvage therapeutic options were endowed with more prudent and sensible manner. Since donor T cells acting as an immunological pressure of selection, unlike pan-killing effects of chemotherapies, it may trigger leukemic cells displaying great initiative in removing detectable HLA haplotype [19]. Lost patient-specific HLA haplotype partly explains the nonresponsiveness to DLI in HLA-loss patients, for who lacks incompatible HLA to elicit furious alloreactivity, though DLI intends to bring additional donor T cells to magnify GVL effects. Early detection of HLA loss on blasts rather than radically introducing DLI in relapsed patients after allo-HSCT could be more beneficial for saving a lot of troubles to balance the pros and cons of DLI. However, it is crucial to clarify that the seemingly useless DLI might be feasible in some circumstances, which will be discussed later.
Figure 4.
A proposed mechanism for the escape of malignant lymphoblasts and mode of blinatumomab. The figure illustrates how cytotoxic T cell (CTLs) and T helper (Th) of donors cooperate to kill lymphoblasts via the incompatible HLA molecules, that is alloreactivity. Under the immunological pressure of donor T cells, leukemic cells escape from immunosurveillance by loss of the genome of patient-specific HLA. Blinatumomab can redirect donor T cells to malignant lymphoblasts.
Based on the rationale that loss of unshared HLA disguises leukemic cells for escaping T-cell surveillance, to retain antileukemia effects, donor cytotoxic T cells must redirect against those without patient-specific HLA expression once inducing alloreactivity [20]. Hence, as an antibody could bind together CD3 of T cells and CD19 of leukemic B cell, blinatumomab is apropos to restore T-cell antileukemia ability by forming a cytolytic synapse.
A profound and rapid response can be induced by blinatumomab. Previous clinical studies showed the CR/CRh rate in relapsed/refractory (r/r) B-ALL was approximately 50% after two cycles [21,22]. In this study, three patients (75%) achieved CR/CRh after the first cycle, showing a comparable response in the allo-HSCT context, which could be somehow postulated that T cells derived from donors are powerful enough to smash leukemic cells. Patient #1 has been keeping CR after full treatment course and it is vital to note she was the one developed CRS, indicating the furious T cell activation [23].
A study indicated that concomitant or prior EM disease of B-ALL might vitiate blinatumomab’s efficiency, and EM relapse or progression was frequently observed in blinatumomab responders [24]. Several cases showed the poor response of blinatumomab in EM disease treatment [25,26]. In this study, patient (#4) with testicle relapse did show an inferior response than others. One patient (#3) developed EM disease for the first-time during treatment.
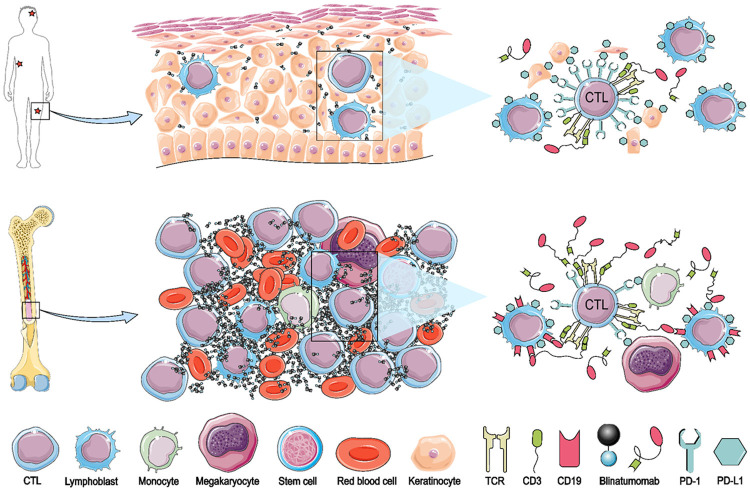
This response differentiation between BM and EM sites might lay in the concentration of blinatumomab, quality and capacity of donor T cells, and the level of target antigen expression on lymphoblasts (Figure 5). The traffic incompetence of blinatumomab resulting in inadequate focal concentration, presenting an attachment with BM, is consistent with the clinical evidence that the inferior efficacy in non-Hodgkin lymphomas compared with B-ALL at the same dose while meeting a better response at a higher dose level [27]. Shortcoming of insufficient concentration of blinatumomab outside BM might be overcome by combining with chemotherapy (NCT03023878). Total body irradiation or focal radiation therapy represents an approach to EM sites, for example, a patient with leukemic optic nerve infiltration recovered by combining radiotherapy with blinatumomab [28].
Figure 5.
A possible mechanism of response differentiation between BM and EM sites. Compared to EM sites (for example, skin), BM might have a higher concentration of blinatumomab, more cytotoxic T cell (CTLs), and less inhibitory signals (PD-L1/PD-1), as well as a higher level of CD19 in leukemic cells.
Meanwhile, prior publications indicate that GVHD has a positive effect on preventing BM relapse rather than EM relapse [29-31], which can be assumed that the shortage and inefficiency of cytotoxic T cells in EM compartment and these EM sites play as sanctuaries escaping for immunosurveillance. When privileged niches lacking sufficient donor T cells, or in other terms, the spatial heterogeneity of GVL effects in patient’s body parts [32-34], for example, T cells with high killing potential accumulating in BM, blinatumomab might increase the anatomic compartmentalization, favoring EM relapse or progression. To date, the mechanisms behind the segregation remain enigmatic, however, interestingly, one of the relevant reasons can be imposed by the varied level of PD-L1 expression in the microenvironment [35,36]. Furthermore, this mechanism decreases the antineoplastic capacity of donor T cells such as reducing effector cytokines and curtailing proliferation, resulting in exhausted T cells [37,38]. By artificially fusing extracellular domain of PD-1 to CD3, ex vivo experiment generated highly cytotoxic and targeted T cells [39]. Given the established rationale, inhibiting PD-L1/PD-1 pathway such as bifunctional checkpoint-inhibitory T cell engagers (CITE), nivolumab (NCT04546399) and pembrolizumab (NCT03512405), might facilitate blinatumomab against EM disease and rescue effector T cells of the donor.
The downregulation or loss of CD19 expression on lymphoblast can explain the EM relapse after blinatumomab to some extent, which is observed in about 40% responders [24]. Conversions of cell lineage such as myeloid shift after blinatumomab blunting treatment efficiency has been reported [40-42]. Combination of immunotherapy with other targets provides a feasible option for eradicating CD19-negative variant (NCT03739814).
On the other hand, in patients with HLA loss relapse, DLI seems to be ineffective since leukemic cells have lost the key incompatible molecular to elicit GVL effects [20]. But DLI provides additional T cells in the patient’s body as arsenal despite the possibly disproportionate T-cell distributions, and when combined with blinatumomab, it may arouse robust antileukemia efficacy and overcomes T-cell exhaustion, which is being investigated in a clinical trial (NCT03982992).
The AEs observed in these four patients with HLA loss relapse after allo-HSCT were consistent with previous clinical reports in r/r B-ALL, suggesting a well-tolerant feature of blinatumomab. Nealy no AEs greater than grade 3 were reported except patient #4 who developed grade 4 hepatic toxicity showing an elevated ALT and AST after the first dose and then recovered to baseline after herbal medicines treatment. The incidences of GVHD and CRS were reported in prior study approximately 11% and 3%, respectively [43]. No fatal infection event was observed relating to blinatumomab. From the aspect of safety profile, blinatumomab appears safer compared with anti-CD19 chimeric antigen receptor T-cell therapy which has an incidence of CRS approximately 37%-93% and ICANS about 12%-30% [44]. Although the aforementioned idea of combining DLI with blinatumomab in patients with HLA loss is theoretically feasible, it should be interpreted and practiced with caution because the increased risk of severe GVHD and GVHD-related mortality, since GVHD incidence after DLI being about 30-70% [45-47].
In summary, the early detection of patient-specific HLA loss on leukemic cells is of the great importance of selecting salvage treatment and our study was the first one reported the clinical efficacy of blinatumomab in HLA loss occurrence. Blinatumomab can redirect allogeneic T cells to leukemic cells, restoring GVL effects in patients of HLA loss relapse after allo-HSCT. It can exert quick resolution with controllable AEs. Further investigations concerning combination with blockage of PD-1/PD-L1 pathway or DLI are therefore warranted, aiming the goal of sustained remission and EMD clearance.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81730008) and the Key Research and Development Program of Zhejiang Province (Grant Nos. 2019C03016).
Disclosure of conflict of interest
None.
References
- 1.Medd PG, Peniket AJ, Littlewood TJ, Pearce R, Perry J, Kirkland KE, Shaw BE, Potter MN, Craddock CF, Milligan DW, Fielding AK, Marks DI, Cook G British Society of Blood and Marrow Transplantation. Evidence for a GVL effect following reduced-intensity allo-SCT in ALL: a British Society of Blood and Marrow Transplantation study. Bone Marrow Transplantation. 2013;48:982–987. doi: 10.1038/bmt.2012.261. [DOI] [PubMed] [Google Scholar]
- 2.Bethge WA, Storer BE, Maris MB, Flowers ME, Maloney DG, Chauncey TR, Woolfrey AE, Storb R, Sandmaier BM. Relapse or progression after hematopoietic cell transplantation using nonmyeloablative conditioning: effect of interventions on outcome. Exp Hematol. 2003;31:974–980. doi: 10.1016/s0301-472x(03)00225-x. [DOI] [PubMed] [Google Scholar]
- 3.Kurosawa S, Fukuda T, Tajima K, Saito B, Fuji S, Yokoyama H, Kim SW, Mori S, Tanosaki R, Heike Y, Takaue Y. Outcome of 93 patients with relapse or progression following allogeneic hematopoietic cell transplantation. Am J Hematol. 2009;84:815–820. doi: 10.1002/ajh.21555. [DOI] [PubMed] [Google Scholar]
- 4.Poon LM, Hamdi A, Saliba R, Rondon G, Ledesma C, Kendrick M, Qazilbash M, Hosing C, Jones RB, Popat UR, Nieto Y, Alousi A, Ciurea S, Shpall EJ, Champlin RE, Kebriaei P. Outcomes of adults with acute lymphoblastic leukemia relapsing after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1059–1064. doi: 10.1016/j.bbmt.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crotta A, Zhang J, Keir C. Survival after stem-cell transplant in pediatric and young-adult patients with relapsed and refractory B-cell acute lymphoblastic leukemia. Curr Med Res Opin. 2018;34:435–440. doi: 10.1080/03007995.2017.1384373. [DOI] [PubMed] [Google Scholar]
- 6.Vago L, Toffalori C, Ciceri F, Fleischhauer K. Genomic loss of mismatched human leukocyte antigen and leukemia immune escape from haploidentical graft-versus-leukemia. Semin Oncol. 2012;39:707–715. doi: 10.1053/j.seminoncol.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Crucitti L, Crocchiolo R, Toffalori C, Lupo-Stanghellini MT, Assanelli A, Carrabba M, Mazzi B, Zino E, Marktel S, Marcatti M, Bernardi M, Peccatori J, Bordignon C, Bonini C, Fleischhauer K, Ciceri F, Vago L. Incidence, risk factors and clinical outcome of leukemia relapses due to loss of the mismatched HLA haplotype after partially-incompatible hematopoietic stem cell transplantation. Blood. 2013;122:918–918. doi: 10.1038/leu.2014.314. [DOI] [PubMed] [Google Scholar]
- 8.Löffler A, Kufer P, Lutterbüse R, Zettl F, Daniel PT, Schwenkenbecher JM, Riethmüller G, Dorken B, Bargou RC. A recombinant bispecific single-chain antibody, CD19×CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95:2098–2103. [PubMed] [Google Scholar]
- 9.Goebeler ME, Bargou R. Blinatumomab: a CD19/CD3 bispecific T cell engager (BiTE) with unique anti-tumor efficacy. Leuk Lymphoma. 2016;57:1021–1032. doi: 10.3109/10428194.2016.1161185. [DOI] [PubMed] [Google Scholar]
- 10.Kong Y, Yoshida S, Saito Y, Doi T, Nagatoshi Y, Fukata M, Saito N, Yang SM, Iwamoto C, Okamura J, Liu KY, Huang XJ, Lu DP, Shultz LD, Harada M, Ishikawa F. CD34+CD38+CD19+ as well as CD34+CD38-CD19+ cells are leukemia-initiating cells with self-renewal capacity in human B-precursor ALL. Leukemia. 2008;22:1207–1213. doi: 10.1038/leu.2008.83. [DOI] [PubMed] [Google Scholar]
- 11.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas C, Krinner E, Brischwein K, Hoffmann P, Lutterbüse R, Schlereth B, Kufer P, Baeuerle P. Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiology. 2009;214:441–453. doi: 10.1016/j.imbio.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Stein A, Franklin JL, Chia VM, Arrindell D, Kormany W, Wright J, Parson M, Amouzadeh HR, Choudhry J, Joseph G. Benefit-risk assessment of blinatumomab in the treatment of relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Drug Saf. 2019;42:587–601. doi: 10.1007/s40264-018-0760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahci M, Toffalori C, Bouwmans E, Crivello P, Brambati C, Pultrone C, Stempelmann K, Bost D, Mazzi B, Beelen DW, Ciceri F, Mulder W, Fleischhauer K, Vago L. A new tool for rapid and reliable diagnosis of HLA loss relapses after HSCT. Blood. 2017;130:1270–1273. doi: 10.1182/blood-2017-05-784306. [DOI] [PubMed] [Google Scholar]
- 15.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, Maus MV, Park JH, Mead E, Pavletic S, Go WY, Eldjerou L, Gardner RA, Frey N, Curran KJ, Peggs K, Pasquini M, DiPersio JF, van den Brink MRM, Komanduri KV, Grupp SA, Neelapu SS. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Wu H, Shi J, Luo Y, Li X, Lan J, Ni W, Lu Y, Chen L, Tan Y, Lai X, Yu J, Huang H. Ruxolitinib combined with etanercept induce a rapid response to corticosteroid-refractory severe acute graft vs host disease after allogeneic stem cell transplantation: results of a multi-center prospective study. Am J Hematol. 2020;95:1075–1084. doi: 10.1002/ajh.25898. [DOI] [PubMed] [Google Scholar]
- 17.Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MTL, Perrelli NF, Cosentino C, Torri F, Angius A, Forno B, Casucci M, Bernardi M, Peccatori J, Corti C, Bondanza A, Ferrari M, Rossini S, Roncarolo MG, Bordignon C, Bonini C, Ciceri F, Fleischhauer K. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361:478–488. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 18.Casucci M, Perna SK, Falcone L, Camisa B, Magnani Z, Bernardi M, Crotta A, Tresoldi C, Fleischhauer K, Ponzoni M, Gregori S, Caligaris Cappio F, Ciceri F, Bordignon C, Cignetti A, Bondanza A, Bonini C. Graft-versus-leukemia effect of HLA-haploidentical central-memory T-cells expanded with leukemic APCs and modified with a suicide gene. Mol Ther. 2013;21:466–475. doi: 10.1038/mt.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toffalori C, Zito L, Gambacorta V, Riba M, Oliveira G, Bucci G, Barcella M, Spinelli O, Greco R, Crucitti L, Cieri N, Noviello M, Manfredi F, Montaldo E, Ostuni R, Naldini MM, Gentner B, Waterhouse M, Zeiser R, Finke J, Hanoun M, Beelen DW, Gojo I, Luznik L, Onozawa M, Teshima T, Devillier R, Blaise D, Halkes CJM, Griffioen M, Carrabba MG, Bernardi M, Peccatori J, Barlassina C, Stupka E, Lazarevic D, Tonon G, Rambaldi A, Cittaro D, Bonini C, Fleischhauer K, Ciceri F, Vago L. Immune signature drives leukemia escape and relapse after hematopoietic cell transplantation. Nat Med. 2019;25:603–611. doi: 10.1038/s41591-019-0400-z. [DOI] [PubMed] [Google Scholar]
- 20.Rovatti PE, Gambacorta V, Lorentino F, Ciceri F, Vago L. Mechanisms of leukemia immune evasion and their role in relapse after haploidentical hematopoietic cell transplantation. Front Immunol. 2020;11:147–147. doi: 10.3389/fimmu.2020.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topp MS, Gökbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, Dombret H, Fielding AK, Heffner L, Larson RA, Neumann S, Foà R, Litzow M, Ribera JM, Rambaldi A, Schiller G, Brüggemann M, Horst HA, Holland C, Jia C, Maniar T, Huber B, Nagorsen D, Forman SJ, Kantarjian HM. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 22.Aldoss I, Song J, Stiller T, Nguyen T, Palmer J, O’Donnell M, Stein AS, Marcucci G, Forman S, Pullarkat V. Correlates of resistance and relapse during blinatumomab therapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2017;92:858–865. doi: 10.1002/ajh.24783. [DOI] [PubMed] [Google Scholar]
- 23.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–122. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldoss I, Song J, Stiller T, Nguyen T, Palmer J, O’Donnell M, Stein AS, Marcucci G, Forman S, Pullarkat V. Correlates of resistance and relapse during blinatumomab therapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2017;92:858–865. doi: 10.1002/ajh.24783. [DOI] [PubMed] [Google Scholar]
- 25.Yoon SY, Yoon JH, Min GJ, Park SS, Park S, Lee SE, Cho BS, Eom KS, Kim YJ, Kim HJ, Min CK, Cho SG, Lee JW, Lee S. Experience of blinatumomab salvage for patients with acute lymphoblastic leukemia presenting with isolated extramedullary relapse after previous allogeneic hematopoietic cell transplantation. Bone Marrow Transplantation. 2020;55:1469–1472. doi: 10.1038/s41409-019-0708-9. [DOI] [PubMed] [Google Scholar]
- 26.Demosthenous C, Lalayanni C, Iskas M, Douka V, Pastelli N, Anagnostopoulos A. Extramedullary relapse and discordant CD19 expression between bone marrow and extramedullary sites in relapsed acute lymphoblastic leukemia after blinatumomab treatment. Curr Probl Cancer. 2019;43:222–227. doi: 10.1016/j.currproblcancer.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Goebeler ME, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, Noppeney R, Hess G, Kallert S, Mackensen A, Rupertus K, Kanz L, Libicher M, Nagorsen D, Zugmaier G, Klinger M, Wolf A, Dorsch B, Quednau BD, Schmidt M, Scheele J, Baeuerle PA, Leo E, Bargou RC. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-hodgkin lymphoma: final results from a phase i study. J Clinical Oncol. 2016;34:1104–1111. doi: 10.1200/JCO.2014.59.1586. [DOI] [PubMed] [Google Scholar]
- 28.Verter E, Yang A, Lim RP. Leukemic optic nerve infiltration responds to radiation and blinatumomab. Ophthalmology. 2018;125:746. doi: 10.1016/j.ophtha.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Lee JH, Choi SJ, Lee JH, Seol M, Lee YS, Ryu SG, Park CJ, Chi HS, Lee MS, Yun S, Lee JS, Lee KH. Anti-leukemic effect of graft-versus-host disease on bone marrow and extramedullary relapses in acute leukemia. Haematologica. 2005;90:1380–1388. [PubMed] [Google Scholar]
- 30.Solh M, DeFor TE, Weisdorf DJ, Kaufman DS. Extramedullary relapse of acute myelogenous leukemia after allogeneic hematopoietic stem cell transplantation: better prognosis than systemic relapse. Biol Blood Marrow Transplant. 2012;18:106–112. doi: 10.1016/j.bbmt.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 31.Harris AC, Kitko CL, Couriel DR, Braun TM, Choi SW, Magenau J, Mineishi S, Pawarode A, Yanik G, Levine JE. Extramedullary relapse of acute myeloid leukemia following allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcomes. Haematologica. 2013;98:179–184. doi: 10.3324/haematol.2012.073189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg SL, Mangan KF, Klumpp TR, Cropper TM, Schnall SF, Macdonald JS. Lack of a graft-versus-leukemia effect in an immunologically privileged sanctuary site. Bone Marrow Transplant. 1994;14:180–181. [PubMed] [Google Scholar]
- 33.Lee KH, Lee JH, Kim S, Lee JS, Kim SH, Kim WK. High frequency of extramedullary relapse of acute leukemia after allogeneic bone marrow transplantation. Bone Marrow Transplantation. 2000;26:147–152. doi: 10.1038/sj.bmt.1702488. [DOI] [PubMed] [Google Scholar]
- 34.Au WY, Kwong YL, Lie AK, Ma SK, Liang R. Extra-medullary relapse of leukemia following allogeneic bone marrow transplantation. Hematol Oncol. 1999;17:45–52. doi: 10.1002/(sici)1099-1069(199906)17:2<45::aid-hon641>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Michonneau D, Sagoo P, Breart B, Garcia Z, Celli S, Bousso P. The PD-1 axis enforces an anatomical segregation of CTL activity that creates tumor niches after allogeneic hematopoietic stem cell transplantation. Immunity. 2016;44:143–154. doi: 10.1016/j.immuni.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Köhnke T, Krupka C, Tischer J, Knösel T, Subklewe M. Increase of PD-L1 expressing B-precursor ALL cells in a patient resistant to the CD19/CD3-bispecific T cell engager antibody blinatumomab. J Hematol Oncol. 2015;8:111. doi: 10.1186/s13045-015-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizuno R, Sugiura D, Shimizu K, Maruhashi T, Watada M, Okazaki IM, Okazaki T. PD-1 primarily targets TCR signal in the inhibition of functional T cell activation. Front Immunol. 2019;10:630. doi: 10.3389/fimmu.2019.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herrmann M, Krupka C, Deiser K, Brauchle B, Marcinek A, Ogrinc Wagner A, Rataj F, Mocikat R, Metzeler KH, Spiekermann K, Kobold S, Fenn NC, Hopfner KP, Subklewe M. Bifunctional PD-1×αCD3×αCD33 fusion protein reverses adaptive immune escape in acute myeloid leukemia. Blood. 2018;132:2484–2494. doi: 10.1182/blood-2018-05-849802. [DOI] [PubMed] [Google Scholar]
- 40.Duffner U, Abdel-Mageed A, Younge J, Tornga C, Scott K, Staddon J, Elliott K, Stumph J, Kidd P. The possible perils of targeted therapy. Leukemia. 2016;30:1619–1621. doi: 10.1038/leu.2016.18. [DOI] [PubMed] [Google Scholar]
- 41.Gardner R, Wu D, Cherian S, Fang M, Hanafi LA, Finney O, Smithers H, Jensen MC, Riddell SR, Maloney DG, Turtle CJ. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127:2406–2410. doi: 10.1182/blood-2015-08-665547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braig F, Brandt A, Goebeler M, Tony HP, Kurze AK, Nollau P, Bumm T, Böttcher S, Bargou RC, Binder M. Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood. 2017;129:100–104. doi: 10.1182/blood-2016-05-718395. [DOI] [PubMed] [Google Scholar]
- 43.Stein AS, Kantarjian H, Gökbuget N, Bargou R, Litzow MR, Rambaldi A, Ribera JM, Zhang A, Zimmerman Z, Zugmaier G, Topp MS. Blinatumomab for acute lymphoblastic leukemia relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019;25:1498–1504. doi: 10.1016/j.bbmt.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Santomasso B, Bachier C, Westin J, Rezvani K, Shpall EJ. The other side of CAR T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. Am Soc Clin Oncol Educ Book. 2019;39:433–444. doi: 10.1200/EDBK_238691. [DOI] [PubMed] [Google Scholar]
- 45.Scarisbrick JJ, Dignan FL, Tulpule S, Gupta ED, Kolade S, Shaw B, Evison F, Shah G, Tholouli E, Mufti G, Pagliuca A, Malladi R, Raj K. A multicentre UK study of GVHD following DLI: rates of GVHD are high but mortality from GVHD is infrequent. Bone Marrow Transplant. 2015;50:62–67. doi: 10.1038/bmt.2014.227. [DOI] [PubMed] [Google Scholar]
- 46.Yan CH, Liu DH, Xu LP, Liu KY, Zhao T, Wang Y, Chen H, Chen YH, Han W, Huang XJ. Modified donor lymphocyte infusion-associated acute graft-versus-host disease after haploidentical T-cell-replete hematopoietic stem cell transplantation: incidence and risk factors. Clin Transplant. 2012;26:868–876. doi: 10.1111/j.1399-0012.2012.01618.x. [DOI] [PubMed] [Google Scholar]
- 47.He FC, Weisdorf DJ, Warlick ED, Miller JS, Holtan SG, Verneris MR, Cao Q, MacMillan ML. Acute graft versus host disease after donor lymphocyte infusion: a single center analysis. Blood. 2014;124:5807–5807. [Google Scholar]