Abstract
Human papilloma virus (HPV) is the main causative agent in cervical cancers. High-risk HPV cancers, including cervical cancer, are driven by major HPV oncogene, E6 and E7, which promote uncontrolled cell growth and genomic instability. We have previously shown that the presence of HPV E7 sensitizes cells to inhibition of aurora kinases (AURKs), which regulates the control of cell entry into and through mitosis. Such treatment is highly effective at eliminating early tumors and reducing large, late tumors. In addition, the presence of HPV oncogenes also sensitizes cells to inhibition of phosphoinositide 3-kinases (PI3Ks), a family of enzymes involved in cellular functions such as cell growth and proliferation. Using MLN8237 (Alisertib), an oral, selective inhibitor of AURKs, we investigated whether Alisertib treatment can improve tumor response when combined with either radiotherapy (RT) treatment or with a PI3K inhibitor, BYL719 (Alpelisib). Indeed, both RT and Alpelisib significantly improved Alisertib-mediated tumor killing, and the promising achieved results warrant further development of these combinations, and potentially translating them to the clinics.
Keywords: Radiation, Alisertib, Alpelisib, aurora kinase, PI3K, cervical cancer, HPV, E7
Introduction
Human papilloma virus (HPV) infection is the cause of cancers of the cervix, penis, anus and head and neck. Cervical cancer makes up a large portion of HPV-driven cancers, and was estimated to be the fourth most common female malignancy worldwide in 2019 [1]. High risk HPV promotes cancer via the actions of its major oncogenes, E6 and E7. Importantly, HPV-driven cervical tumors have an absolute requirement for these oncogenes for its survival [2]. Although the HPV vaccine is now available, the slow and gradual process by which HPV cancers form means little change in cervix cancer induction and patient survival for the next 25 years [3]. In Australia, the incidence of cervical cancer is not estimated to reach less than 1/100,000 until 2066 [4]. This warrants the development of new therapeutic strategies for both newly diagnosed and recurrent patients. Radio-chemotherapy, the current treatment modality for cervical cancer, is curative in more than 50% of locally advance cervical cancer cases. Despite this, the 5-year overall survival for advanced stage III-IVa patients (<20%) remains markedly lower than that for stage II patients (>60%) [5,6]. Recurrent cervical cancer is refractory to currently available treatments and survival is usually short, resulting in a need to develop more effective treatments for patients and salvage treatments for those who progress after standard management.
We have previously performed a synthetic lethality screen to identify new selective targets in HPV-transformed tumors and identified inhibition of key regulators of mitosis, the aurora kinase (AURK) family as the strongest hits [7,8]. Indeed, we have shown that E7 expression strongly sensitized cells to killing by the AURK A and B dual selective inhibitor, MLN8237 (Alisertib) [7,8]. Alisertib is currently in phase II/III clinical trials for a range of cancer types [9,10], and we have reported on its delivery and use in treating HPV-driven cancers as a single agent with promising results [7,11]. In the current study, we tested novel approaches to enhance the anti-tumor effects of Alisertib. We recently showed enhanced cervical cancer cell killing when Alisertib is combined with a range of Bcl-2 family anti-apoptotic protein pharmacological inhibitors [12]. In our current study, we extend our pharmacological screen to test the effect of combining Alisertib with a phosphoinositide 3-kinase (PI3K) inhibitor, BYL719 (Alpelisib), as PI3K was also one of the strongest hits that came up in the synthetic lethality screen [7]. Alpelisib is an α-specific PI3K inhibitor which has been studied as an adjuvant therapy for various cancer types and showed particularly promising activity in combination with fulvestrant for breast cancer in phase III clinical trials [13-18]. Here, we assess the effect of combining Alisertib either with the current gold standard of treatment, radiotherapy (RT), or the PI3K inhibitor (Alpelisib) on HPV-driven cervical cancer tumors.
Materials and methods
Cell culture
HeLa cells were obtained from the ATCC and maintained in complete media; DMEM (Gibco-Invitrogen, Waltham, MA) supplemented with 10% heat inactivated fetal bovine serum (FBS) (Gibco-Invitrogen, Waltham, MA) and 1% of antibiotic/glutamine preparation (100 U/ml penicillin G, 100 U/ml streptomycin sulphate, and 2.9 mg/ml of L-glutamine) (Gibco-Invitrogen, Waltham, MA).
Chemicals
MLN8237 (Alisertib) and BYL719 (Alpelisib) were purchased from Selleck Chemicals (Houston, TX) and powder dissolved in sterile-grade DMSO (Sigma-Aldrich, St Louis, MI).
Cell viability and IC50 assessment
Cell viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. MTT reagent was added at a final concentration of 0.5 mg/ml for an additional 1 h. MTT crystals were dissolved in 100% DMSO before reading the colorimetric absorbance at 544 nm on a FLUOstar OPTIMA microplate reader (BMG LabTech, Germany). Cells were treated with Alisertib or Alpelisib with concentrations ranging from 40 mM to 40 nM for 72 h before performing the MTT assay to determine the IC50.
Drug synergism determination
Drug interactions between Alisertib and Alpelisib were assessed using CompuSyn software version 1.0 (ComboSyn, Inc., Paramus, NJ), and determined by isobologram analysis at fraction response 0.9 (90% killing). Combination index (CI) analysis was based on the median-effect principle and computed using the following formula: CI = D1/(Dx)1 + D2/(Dx)2. D1 and (Dx)1 are concentrations of Alisertib and Alpelisib, respectively, that inhibit cell growth by 90% of control when used alone. D2 and (Dx)2 are concentrations of Alisertib and Alpelisib, respectively, that inhibit cell growth by 90% of control when used in combination. The combined effects of various concentrations at a ratio of 1:1 of Alisertib and Alpelisib were assessed, and the CI was calculated according to the Chou-Talalay method [19]. In brief, a CI value which was <1 = synergistic effect, 1 = additive effect and >1 = antagonistic effect.
Time-lapse microscopy
Treated cells were followed by time-lapse microscopy using Holometer®, a cell stain-free phase holographic imager (PHI AB, Lund, Sweden) at 37°C and 5% CO2 and data analyzed in Hstudio 2.7.5 ™ (PHI AB, Lund, Sweden) on 24-well plate (STARSTED, Nümbrecht, Germany). Images were captured at 10 min intervals.
Flow cytometry
The percentage of cells undergoing apoptosis was determined by flow cytometry using the Annexin V-FITC Apoptosis Kit (#K101) (BioVision, Milpitas, CA) as per manufacturer’s protocol. Samples were analyzed on a BD LSR FORTESSA cell analyzer (BD bioscience, San Jose, CA).
Drug oral gavage formulation
Alisertib was formulated in 10% 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich, St Louis, MI)/1% sodium bicarbonate (Sigma-Aldrich, St Louis, MI) and was dosed orally (10 mg/kg) by gavage as previously described [20]. The final concentration of DMSO per dose was less than 1% (~0.75%). For RT + Alisertib treatment, Alisertib was administered 1.5-2 h post-RT treatment. Alpelisib was formulated as a suspension in 0.5% Tween 80 (Sigma-Aldrich, St Louis, MI)/1% carboxymethyl cellulose (Sigma-Aldrich, St Louis, MI) in water and was dosed orally (65 mg/kg) by gavage as previously described [21].
CT imaging and radiation treatment
CT tumor imaging and targeted radiation treatments were done as previously described [19]. The X-Rad 225Cx (Precision X-ray) X-ray tube was calibrated at 225 kVp, 13 mA (HVL: 0.93 mm CU, added filtration: 0.3 mm Cu). CT imaging was local to the tumor and not a whole-body scan. Anesthetized mice were immobilized in a Lucite jig for radiation treatment and cone-beam CT (CBCT) imaging (X-Rad 225Cx, Precision X-ray). CBCT imaging was used before treatment and at weekly intervals afterwards to evaluate primary tumor volume, and immediately prior to each radiation treatment fraction to assure reproducible tumor targeting. Imaging dose was <0.01 Gy per CBCT. Over the entire study time, the dose cumulated is approximately 0.5 Gy. Radiation treatment was delivered to the primary tumor at a dose rate of 3 Gy/minute using eight coplanar, circular (8-15 mm in diameter depending on tumor size) beams with equal 45° angular spacing around the tumor (SmART Plan).
HeLa cell xenografts and treatment protocol
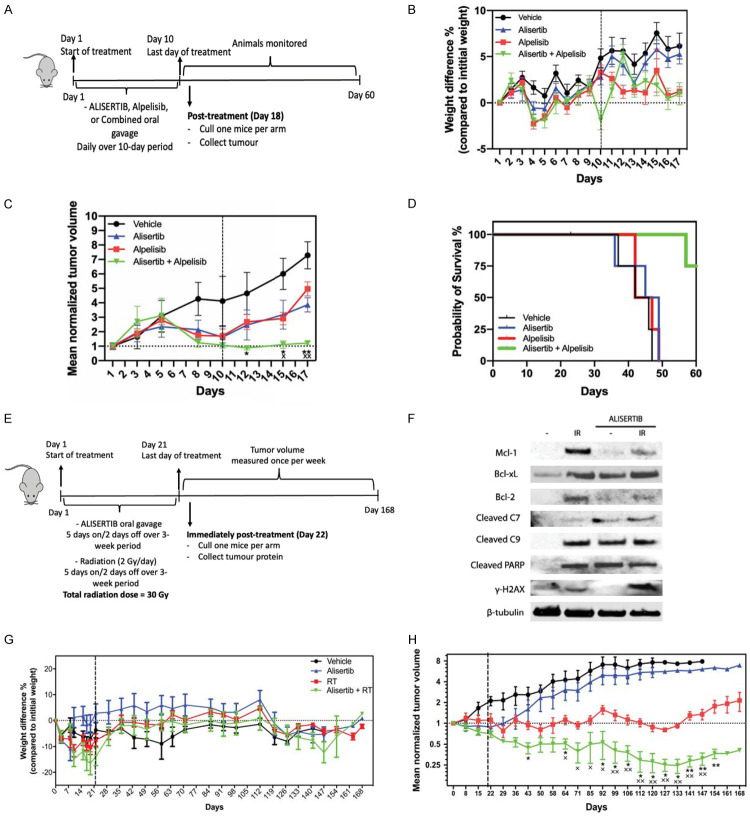
Female Rag mice (6-8 weeks old) were purchased from ARC, Perth, Australia. All animal experimentations were performed according to the guidelines of the Australian and New Zealand Council for the Care and Use of Animals in Research and was approved by the Griffith University Animal Ethics committee (Animal ethics approval # MSC/01/20/AEC). The treatment protocol is summarized schematically (Figure 2A). Tumors were established by subcutaneously injecting 1×106 Hela cells/100 µl/injection (50% PBS: 50% Matrigel) in the right flank of mice. Mice were monitored for tumor growth and when the tumors reached a size of approximately 25-50 mm3 (6 days after transplantation), mice were orally treated with 100 µl of either vehicle control, Alisertib at 10 mg/kg, Alpelisib at 65 mg/kg, or combination of both drugs, once per day for 10 consecutive days (n = 5/treatment). Tumors were visualized and their volumes were measured using digital calipers and tumor volume calculated. Tumor size was measured 3 times/week and mice health were monitored daily. Mice were euthanized and culled at the end of designated monitoring period or when tumor mass reached 1000 mm3.
Figure 2.
Alpelisib (A-D) and RT (E-H) enhances Alisertib-mediated tumor killing. (A) The experimental design for Alpelisib treatment testing is shown in the schematic, indicating the treatment window and monitoring of tumor regrowth. (B) Mice were weighed daily during the experiment as indicated. Dotted vertical line indicates treatment window up to day 10. Mean percentage weight loss is plotted (grams) with error bars representing SEM. (C) Tumor volume loss showing enhanced tumor response to combined Alpelisib and Alisertib treatment (concurrent). Each point represents mean of n = 5 mice/group. Mean tumor volumes were normalized to 1 cm3 against the initial tumor start size (25-50 mm3) with error bars representing SEM. Dotted vertical line indicates treatment window up to day 10. *P<0.05, **P<0.01, Mann-Whitney test (Alpelisib + Alisertib vs Alpelisib alone), ×P<0.05, ××P<0.01, Mann-Whitney test (Alpelisib + Alisertib vs Alisertib alone). (D) Endpoint was reached in all mice in the control and individual treatment within 49 days. The average survival time was 43 d, 44,75 d, 45 d for control, Alisertib, and Alpelisib respectively. In the combination group, 25% of the mice (n = 1) reached endpoint on day 57, and 75% (n = 3) did not reach the endpoint for the duration of the study (60 d). (E) The experimental design for RT testing is shown in the schematic, indicating the treatment window and monitoring of tumor regrowth. (F) Tumors were either irradiated with (IR) or without (-) Alisertib. Proteins were extracted from tumors at the end of treatment and immunoblotted for indicated proteins. ß-tubulin was used as a loading control. (G) Mice were weighed daily during treatment and weekly post-treatment as indicated. Dotted vertical line indicates treatment window up to Day 21. Mean weights are plotted (grams) with error bars representing SEM. (H) Tumor volume loss showing enhanced tumor response to combined radiation and Alisertib treatment (concurrent). Each point represents mean of n = 4-6 mice/group. Mean tumor volumes were normalized to 1 cm3 against the initial tumor start size (5-6 mm3) with error bars representing SEM. Dotted vertical line indicates treatment window up to Day 21. *P<0.05, **P<0.01, Mann-Whitney test (RT + Alisertib vs RT alone), ×P<0.05, ××P<0.01, Mann-Whitney test (RT + Alisertib vs Alisertib alone).
Patient-derived orthotopic cervical cancer xenografts (OCICx) and treatment protocol
OCICx models were developed from a HPV+ clinical cervical cancer biopsy sample at the Princess Margaret Cancer Centre (PMCC)/University Health Network, Toronto and grown orthotopically in the cervices of 6 to 8-week-old female NOD-SCID mice as previously described [22]. The patient tumor model (OCICx1) used in the current study was derived from a HPV16+ squamous cell carcinoma. Mice were bred in-house at the Ontario Cancer Institute (OCI) small animal facility accredited by the Canadian Council on Animal Care and treated in accordance with approved animal care protocols (Animal ethics approval #AUP762.29). The treatment protocol is summarized schematically (Figure 2E). Mice were randomly assigned to one of four experimental groups containing 5-6 mice each when the cervical PDXs reached a size of 5-6 mm3 as determined using biweekly CBCT imaging. Tumor volume was calculated from the largest diameter in each dimensional plane (x, y, z) based on an ellipsoid model of volume (4/3*π*(x/2)*(y/2)*(z/2)). All of the CT scan measurements were made by the same observer, and a second observer confirmed these measurements. The groups were: Vehicle (DMSO in 10% 2-hydroxypropyl-β-cyclodextrin and 1% sodium bicarbonate, 5 mice); Alisertib alone (30 mg/kg oral administration 5 days on/2 days off over 3 weeks, 6 mice), Radiation (RT) alone (5 days on/2 days off, 2 Gy fractions per day over 3 weeks-Total 30 Gy, 5 mice); Alisertib + Radiation (RT + concurrent 30 mg/kg oral administration 5 days on/2 days off over 3 weeks, 5 mice). Animals were weighed and examined for signs of illness weekly after treatment. Primary tumor volume was also assessed weekly using CBCT. Mice were euthanized at endpoint when the maximal primary diameter reached 1 to 1.5 cm or they became ill due to developing thymic lymphomas (lost >20% body weight). NOD-SCID mice are prone to thymic lymphomas, which develop over time with age. Mice deaths: Vehicle (1 culled for tumor analysis (Day 22), 3 died based on endpoint criteria (Day 65, 99 and 127)), Alisertib alone (1 culled for tumor analysis (Day 22), 4 died based on endpoint criteria (Day 106, 120, 127, 154)), RT alone (1 culled for tumor analysis (Day 22)) and Alisertib + RT (1 culled for tumor analysis (Day 22), died based on endpoint criteria (Day 99, 120, 154)).
Immunoblotting and antibodies
Protein from cell lines (Alpelisib combination) or day 22 tumors (radiotherapy combination) was extracted using the RNA/DNA/Protein Purification Plus Kit (Norgen Biotek Corp, Thorold, ON, Canada) and protein separated on a 4-12% Bis-Tris gradient gel (BioRad, Hercules, CA). Immunoblots were probed with antibodies against Mcl-1 (Cell Signaling Technologies (CST), Danvers, MA, #39224), Bcl-XL (CST #2764), Bcl-2 (CST, #15071), cleaved PARP (cPARP) (CST, #9524), cleaved caspase 7 (CST, #8438S), cleaved caspase 9 (CST, #20750S), phosphorylated γH2AX (Sigma-Aldrich, St Louis, MI, #H5912), and β-actin (CST, #4967) were used using manufacturers’ recommended dilutions. Rabbit (CST, #7074) and mouse (CST, #7076) secondary antibodies and ECL were used to detect the signals on a Chemidoc XRS Visualizer (BioRad, Hercules, CA).
Statistical analysis
Mann-Whitney test was done to compare the tumor volumes between different treatment groups as each time point. In addition, the Kruskal-Wallis test (with Dunn’s postdoc multiple testing correction) was done to compare the cumulative tumor volumes between each treatment group. Statistical analysis was done on GraphPad Prism v9.
Results and discussion
Alisertib and Alpelisib work synergistically and results in enhanced cervical tumour killing
Alisertib is a potent Aurora kinase inhibitor that has been shown to be effective against HPV-driven cancers. Its efficacy on these cancers is driven by the expression of E7, which sensitizes cervical cancer tumors to Alisertib [7,8]. Our work to date has been limited to the use of Alisertib as a single therapeutic agent. While treatment with this molecule has shown promising effects in early-stage cancers [7], a more effective approach is still warranted for later stages. In the current study, we evaluated the possibility of enhancing the anti-tumor activity of Alisertib by either combining it with Alpelisib or RT. Alpelisib pharmacologically inhibits PI3K. The gene encoding for PI3K (PIK3CA) is one of the most upregulated genes found in cancer [23], and high mutational frequencies have been reported in tumours of the vagina, colon, breast, uterus, and cervix [24,25]. Our work has also identified the inhibition of this gene as one of the top hits on HPV-transformed tumors [7], suggesting that it has a role in the oncogenesis of these cancers. PI3K activation leads to the phosphorylation of serine-threonine kinase Protein kinase B (AKT), culminating in the activation of mechanistic target of rapamycin (mTOR). The PI3K/AKT/mTOR pathway regulates the cell cycle and has a role in proliferation, senescence and apoptosis. Hence, over activation of this pathway is strongly linked to cancer development and resistance to treatment [26]. Indeed, Alpelisib tested as a co-adjuvant therapy to enhance the effect of various other anti-cancer treatments [15,17,18,27-29]. Notably, Alpelisib has been granted FDA approval to be used as a coadjuvant therapy for metastatic breast cancer [30].
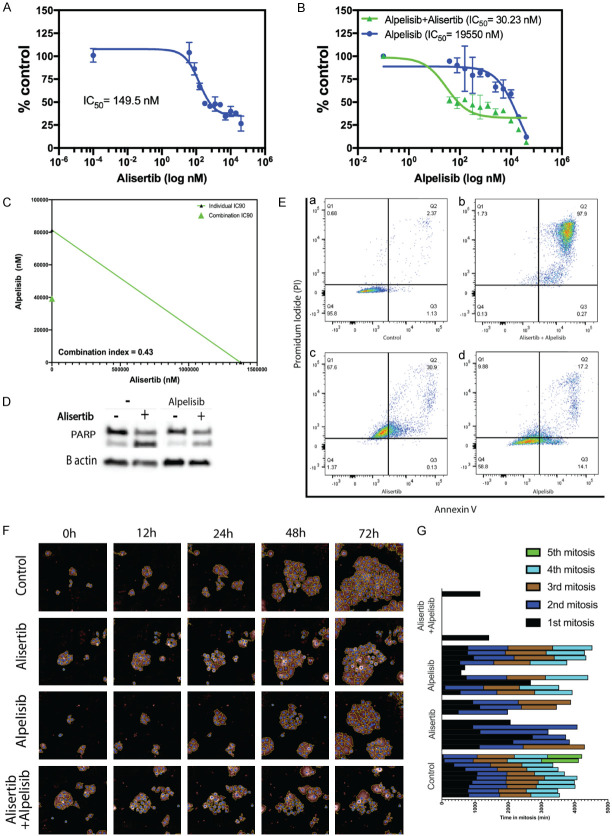
Here, we evaluated Alpelisib as a co-adjuvant therapy alongside Alisertib for the treatment of cervical cancer. We investigated whether dual inhibition of the PI3K and AURK pathways with Alpelisib and Alisertib, respectively, were synergistic. Consistent with our previous observation [7], HPV +ve HeLa cells were sensitive to the treatment by Alisertib (IC50 = 146.5 nM) (Figure 1A). Importantly, dual treatment with Alisertib and Alpelisib resulted in a significant decrease in IC50 (from 19550 nM to 30.3 nM) (Figure 1B) and this effect is synergistic (combination index (CI) = 0.43) (Figure 1C). Indeed, enhanced killing with combined treatment correlated with elevated PARP cleavage (Figure 1D) consistent with increased apoptosis seen in the vast majority of cells (97.9%) (Figure 1E). While Alisertib treatment alone lengthened average cell division time (15.63 h ± 0.55 to 35.2 h ± 3.29), dual treatment resulted in failed cell division (Figure 1F, 1G). Collectively, this data suggests that dual treatment exacerbates cell killing. Although Alpelisib showed minimal potency when used alone, it significantly improved the efficacy of Alisertib and showed synergistic effect. This suggests that the inhibition of PI3K improves sensitivity to AURKA inhibition. Similar effect was previously described, where the activation of the PI3K/AKT/mTOR pathway was found essential for AURKA induced carcinogenesis [31]. Hence, inhibition of this pathway may explain the synergy seen with Alisertib co-treatment. AURKA is known to limit PI3K-pathway inhibitor efficacy [32]. Therefore, the inhibition of AURKA exhibited by Alisertib in our study may have potentiated the effect of Alpelisib. While HPV-driven cancers that have mutations in PI3K are expected to be the top candidates for this combination treatment, it should be pointed out that the cells used in this study (HeLa cells) do not have such mutations in this gene. The synergy between the two drugs was evident on the time it took cells to undergo cell cycle. Indeed, dual therapy resulted in apoptosis/necrosis in the vast majority of cells (97.9%) within 48 h of being exposed to treatment. It is important to note that we have only tested Alisertib and Alpelisib combinations on one cell type in this study. Future work is also focused on testing Alpelisib and Alisertib combinations on a range of other HPV+ cervical cancer cell lines.
Figure 1.
Alpelisib enhanced Alisertib-mediated toxicity in cervical cancer cells. A. Hela cells were treated with ALISERTIB at doses ranging from 40 uM to 40 nM in a 96 well plate. Cell viability was measured by the MTT assay at 3-days post-treatment. A dose dependent curve was generated using GraphPad Prism v9 to determine IC50 values. Data points are representative of the mean ± SEM (n = 3). B. Cells were treated with Alpelisib at increasing doses either in the presence or absence of Alisertib (concentration used: 0.5 Alisertib IC50 = 73.25 nM) before measuring cell viability at 3-days post-treatment by the MTT assay. Data is representative of one out of three independent experiments. Data points represent the mean ± SEM. C. Standard isobologram analysis of cell killing by the drug combinations. IC90 values of each drug are plotted on the axes; the solid line represents the additive effect, while the point represents concentrations of each drug resulting in 90% inhibition of growth. The point falls below the line indicating synergism between the drugs. Combination index (CI) at fractional response 0.9 (90% killing) is also shown. CI value is <1, indicative of a synergistic effect. Data is representative of three independent experiments. D. Proteins from cells collected at 24 h were immunoblotted for cleaved PARP. β-actin was used as a loading control. Individual blots shown are representative of three independent experiments. E. HeLa cells exposed to Alisertib, Alpelisib, or a combination of both were stained with annexin V-FITC and PI for FACS analysis after 48 h. Cell populations shown in the lower left quadrant represent living cells; lower right quadrant represents apoptotic cells, upper right quadrant represents necrotic cells and upper left quadrant represents pre-necrotic cells. Data is representative of 3 experiments. F. HeLa cells were treated either alone, with Alisertib (500 nM) or in combination with Alpelisib (20 uM). Images were captured by time-lapse microscopy over 72 h. Still images shown were captured at times indicated using the Hstudio 2.7.5™ live imaging. Data presented are representative of one out of three independent experiments. G. 10 individual cells were tracked from time-lapse microscopy videos using the Hstudio 2.7.5™ live imaging software and the time in and number of cell divisions were plotted on the graph over a 72 h period.
We then explored the effect of combining Alisertib and Alpelisib in vivo in a HeLa xenograft model (Figure 2A). Drug treatments had some effect on mice weights at day 4 and 5 before recovering at day 6 and at day 10 for the dual drug treatment group before recovering the day after (Figure 2B). Compared to the control group, all treatment groups reduced tumor volume significantly (Figure 2C). Concurrent treatment with Alpelisib and Alisertib resulted in significantly greater tumor volume loss compared with either treatment alone (Kruskal-Wallis test, Alpelisib vs Alpelisib+Alisertib P = 0.009, Alisertib vs Alpelisib+Alisertib P = 0.009). The average survival time was 43 d, 45 d, 45 d for control, Alisertib and Alpelisib treatment alone groups, respectively. In the dual combination group, 25% of the mice reached endpoint on day 57, whereas 75% did not reach the endpoint for the duration of the study (60 d) (Figure 2D). Importantly, dual therapy significantly enhanced tumor killing and improved the survival rate of tumor bearing mice. Alisertib treatment alone resulted in a noticeable delay in the progression of the tumors albeit not improving the survivability of the mice. Combining with Alpelisib significantly reduced tumor progression and improved the survival rate of the animals. These findings align with the synergistic effect we observed in vitro. It is important to note here that our study assessed the synergy when both drugs were administered simultaneously, assessing the timing and sequence of administering each drug may further potentiate the outcomes [33,34]. Assessment of repeated cycles of treatment may be beneficial in the future in addition to testing drug combinations in another HPV+ cervical cancer xenograft model (e.g., CaSki cell xenografts).
Radiotherapy enhances the anti-tumor effect of Alisertib in a cervical PDX mouse model
To examine the effect of combining Alisertib with RT, we utilized the OCICx model [22]. Here, we administered either vehicle alone or Alisertib via oral gavage with or without RT over a 3-week period to mice bearing orthotopic cervical cancer implants as per our experimental design (Figure 2E). RT (Kruskal-Wallis test, P<0.0001) and Alisertib alone (Kruskal-Wallis test, P = 0.0003) groups had the most impact on mice body weights when compared to the vehicle alone group alone (Figure 2G). Compared to the control group, Alisertib treatment alone decreased tumor volume (Figure 2H) albeit not significantly (Kruskal-Wallis test, P>0.9999). Concurrent treatment with RT and Alisertib resulted in significantly greater tumor volume loss compared with either treatment alone (Figure 2H) (Kruskal-Wallis test, RT vs RT+Alisertib P = 0.0018, Alisertib alone vs RT+Alisertib P<0.0001). Immunoblotting analysis of the tumors revealed several biochemical changes in apoptotic pathways (Figure 2F). Indeed, tumors irradiated alone resulted in H2A histone family member X phosphorylation (γ-H2AX), a marker of DNA damage, whereas combined treatment with Alisertib enhanced this effect. Other than the vehicle control, all treatments induced Poly (ADP-ribose) polymerase (PARP) and caspase cleavage, consistent with apoptotic cell death occurring in these tumors. Overall, our findings showed that Alpelisib treatment and RT enhanced the anti-tumor effect of Alisertib.
Alisertib has been shown to enhance RT sensitivity in atypical teratoid/rhabdoid tumor [35], glioblastoma [36,37] and lung cancer [38] cell lines and xenografts of cell lines [38]. Indeed, a recent Phase I trial revealed that concurrent fractionated stereotactic RT with Alisertib is well tolerated in patients with recurrent high-grade gliomas [39]. The mode of action of Alisertib requires cells to undergo cell division. Though ionizing radiation could induce cell cycle progression blockade (G2 phase checkpoint arrest) thus abrogating the mode of action of Alisertib, at the RT dose used here (2 Gy), this delay would be transient with cells continuing through into mitosis after a few hours. Indeed, 5 Gy RT treatment in vitro would only delay HeLa cell growth for 5 h [40]. In our treatment model, the long duration of Alisertib being present in the blood circulation would mean that dividing tumor cells would still be present as cells enters mitosis. Ionizing radiation is known to induce DNA damage by phosphorylation of γ-H2AX [41]. Though tumors irradiated alone had γ-H2AX phosphorylation, combined treatment with Alisertib enhanced this effect suggesting that Alisertib treatment reduces repair of DNA damage induced by RT. This is consistent with the expected action of Alisertib [20]. Our immunoblotting analysis suggest apoptotic cell death occurring with Alisertib and RT in cervical PDX tumors, possibly via the intrinsic mitochondrial pathway based on caspase 9 cleavage. It is important to note that the lack of enhancement of caspase and PARP cleavage seen in tumors treated together with RT and Alisertib. It is possible that the accelerated apoptotic progression in these tumors resulted in high levels of caspase-independent cell death (e.g. secondary necrosis) and subsequent degradation of apoptosis-dependent caspase cleavage products, which occurs in a number of cell type models [42]. RT alone elevated anti-apoptotic protein Mcl-1, Bcl-xL and Bcl-2 expression. This observation is parallel to that seen in other cancer types treated with RT [43-45]. Alisertib treatment resulted in the loss of radiation-induced Mcl-1 and Bcl-2, not Bcl-XL expression. Unlike Bcl-2, Alisertib treatment was able to decrease Mcl-1 expression as we previously reported [7,8]. Whether these events are directly indicative of apoptotic changes seen in the tumors (i.e. caspase and PARP cleavage) requires further investigation.
Pre-clinical data presented here is limited to one OCICx model but is consistent with our previous cell line studies [7,46]. Compared to the other models studied, the OCICx model used in this study has a low E7 oncogene expression profile (data not shown). We have previously found that sensitivity to Alisertib correlated with higher E7 expression in HPV+ cancer cells [7,46]. This could explain the modest tumor volume loss seen in the Alisertib alone group in the OCICx model, in contrast to that seen in the xenograft model, where the E7 oncogene expression profile is higher (data not shown). To confirm this, we plan to extend our pre-clinical observations to other OCICx models with tumors with higher E7 expression. HPV E6 and E7 are also known to affect the G2 phase checkpoint response by destabilizing Claspin and other checkpoint components [47]. This further contributes to reducing the efficacy of the G2 checkpoint control. Indeed, higher expression of E6 and E7 oncogenes appears to be a clinically significant predictor of likely disease recurrence for HPV-driven cancers [48] and may have the potential to enhance tumor response to treatment. Despite significant progress in treatment for cervical cancers there is still a striking number of patients that fail treatment and ultimately develop metastasis. Identifying high E7, as a prognostic biomarker of response using Alisertib would represent an important step for personalized cancer care for patients with advanced HPV-driven cancers. Despite the advances in testing for a number of cancers types at preclinical and clinical trial stages, there is limited work done testing Alisertib in combination with RT for cervical cancer. To our knowledge, this is the first study that explored to test this combination for cervical cancer. We also plan to test Alpelisib and Alisertib drug combinations in the OCICx model used here in future work.
Acknowledgements
This work was supported by the National Health and Medical Research Council (APP1104186). Adi Idris was supported by the 2018 Australian Award Endeavour Research Fellowship. I would like to thank Professor Brian Gabrielli from Mater Research, University of Queensland for his intellectual input in this work. We would also like to thank Dr Naz Chaudary, Mrs Prathiba Thapap, Professor Richard Hill and Professor Michael Milosevic from the Radiation Medicine Program, University Health Network and Princess Margaret Cancer Centre, Toronto, Canada for providing materials, technical and intellectual input for the patient-derived orthotopic cervical cancer xenograft (OCICx) animal model and radiation work.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Gu W, Cochrane M, Leggatt GR, Payne E, Choyce A, Zhou F, Tindle R, McMillan NA. Both treated and untreated tumors are eliminated by short hairpin RNA-based induction of target-specific immune responses. Proc Natl Acad Sci U S A. 2009;106:8314–8319. doi: 10.1073/pnas.0812085106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodin D, Burger EA, Atun R, Barton M, Gospodarowicz M, Grover S, Hanna TP, Jaffray DA, Knaul FM, Lievens Y, Zubizarreta E, Milosevic M. Scale-up of radiotherapy for cervical cancer in the era of human papillomavirus vaccination in low-income and middle-income countries: a model-based analysis of need and economic impact. Lancet Oncol. 2019;20:915–923. doi: 10.1016/S1470-2045(19)30308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall MT, Simms KT, Lew JB, Smith MA, Brotherton JM, Saville M, Frazer IH, Canfell K. The projected timeframe until cervical cancer elimination in Australia: a modelling study. The Lancet Public Health. 2019;4:e19–e27. doi: 10.1016/S2468-2667(18)30183-X. [DOI] [PubMed] [Google Scholar]
- 5.Monk BJ, Tewari KS, Koh WJ. Multimodality therapy for locally advanced cervical carcinoma: state of the art and future directions. J. Clin. Oncol. 2007;25:2952–2965. doi: 10.1200/JCO.2007.10.8324. [DOI] [PubMed] [Google Scholar]
- 6.Tian T, Gong X, Gao X, Li Y, Ju W, Ai Y. Comparison of survival outcomes of locally advanced cervical cancer by histopathological types in the surveillance, epidemiology, and end results (SEER) database: a propensity score matching study. Infect Agent Cancer. 2020;15:33. doi: 10.1186/s13027-020-00299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabrielli B, Bokhari F, Ranall MV, Oo ZY, Stevenson AJ, Wang W, Murrell M, Shaikh M, Fallaha S, Clarke D, Kelly M, Sedelies K, Christensen M, McKee S, Leggatt G, Leo P, Skalamera D, Soyer HP, Gonda TJ, McMillan NA. Aurora A is critical for survival in HPV-transformed cervical cancer. Mol Cancer Ther. 2015;14:2753–2761. doi: 10.1158/1535-7163.MCT-15-0506. [DOI] [PubMed] [Google Scholar]
- 8.Shaikh MH, Idris A, Johnson NW, Fallaha S, Clarke DTW, Martin D, Morgan IM, Gabrielli B, McMillan NAJ. Aurora kinases are a novel therapeutic target for HPV-positive head and neck cancers. Oral Oncol. 2018;86:105–112. doi: 10.1016/j.oraloncology.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Liewer S, Huddleston A. Alisertib: a review of pharmacokinetics, efficacy and toxicity in patients with hematologic malignancies and solid tumors. Expert Opin Investig Drugs. 2018;27:105–112. doi: 10.1080/13543784.2018.1417382. [DOI] [PubMed] [Google Scholar]
- 10.Tayyar Y, Jubair L, Fallaha S, McMillan NAJ. Critical risk-benefit assessment of the novel anti-cancer aurora a kinase inhibitor alisertib (MLN8237): a comprehensive review of the clinical data. Crit Rev Oncol Hematol. 2017;119:59–65. doi: 10.1016/j.critrevonc.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Tayyar Y, Shiels R, Bulmer AC, Lam AK, Clarke D, Idris A, McMillan NA. Development of an intravaginal ring for the topical delivery of Aurora kinase A inhibitor, MLN8237. PLoS One. 2019;14:e0225774. doi: 10.1371/journal.pone.0225774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yumol J, Gabrielli B, Tayyar Y, McMillan NA, Idris A. Smart drug combinations for cervical cancer: dual targeting of Bcl-2 family of proteins and aurora kinases. Am J Cancer Res. 2020;10:3406–3414. [PMC free article] [PubMed] [Google Scholar]
- 13.Fritsch C, Huang A, Chatenay-Rivauday C, Schnell C, Reddy A, Liu M, Kauffmann A, Guthy D, Erdmann D, De Pover A, Furet P, Gao H, Ferretti S, Wang Y, Trappe J, Brachmann SM, Maira SM, Wilson C, Boehm M, Garcia-Echeverria C, Chene P, Wiesmann M, Cozens R, Lehar J, Schlegel R, Caravatti G, Hofmann F, Sellers WR. Characterization of the novel and specific PI3Kα inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Molecular Cancer Therapeutics. 2014;13:1117. doi: 10.1158/1535-7163.MCT-13-0865. [DOI] [PubMed] [Google Scholar]
- 14.André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, Yamashita T, Lu YS, Inoue K, Takahashi M, Pápai Z, Longin AS, Mills D, Wilke C, Hirawat S, Juric D SOLAR-1 Study Group. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 15.van Geel RMJM, Tabernero J, Elez E, Bendell JC, Spreafico A, Schuler M, Yoshino T, Delord JP, Yamada Y, Lolkema MP, Faris JE, Eskens FALM, Sharma S, Yaeger R, Lenz HJ, Wainberg ZA, Avsar E, Chatterjee A, Jaeger S, Tan E, Maharry K, Demuth T, Schellens JHM. A phase Ib dose-escalation study of encorafenib and cetuximab with or without alpelisib in metastatic BRAF-mutant colorectal cancer. Cancer Discov. 2017;7:610–619. doi: 10.1158/2159-8290.CD-16-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer IA, Prat A, Egle D, Blau S, Fidalgo JAP, Gnant M, Fasching PA, Colleoni M, Wolff AC, Winer EP, Singer CF, Hurvitz S, Estévez LG, van Dam PA, Kümmel S, Mundhenke C, Holmes F, Babbar N, Charbonnier L, Diaz-Padilla I, Vogl FD, Sellami D, Arteaga CL. A phase II randomized study of neoadjuvant letrozole plus alpelisib for hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer (NEO-ORB) Clin Cancer Res. 2019;25:2975–2987. doi: 10.1158/1078-0432.CCR-18-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain S, Shah AN, Santa-Maria CA, Siziopikou K, Rademaker A, Helenowski I, Cristofanilli M, Gradishar WJ. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res Treat. 2018;171:371–381. doi: 10.1007/s10549-018-4792-0. [DOI] [PubMed] [Google Scholar]
- 18.Dunn LA, Riaz N, Fury MG, McBride SM, Michel L, Lee NY, Sherman EJ, Baxi SS, Haque SS, Katabi N, Wong RJ, Xiao H, Ho AL, Pfister DG. A phase 1b study of cetuximab and BYL719 (alpelisib) concurrent with intensity modulated radiation therapy in stage III-IVB head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2020;106:564–570. doi: 10.1016/j.ijrobp.2019.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudary N, Pintilie M, Jelveh S, Lindsay P, Hill RP, Milosevic M. Plerixafor improves primary tumor response and reduces metastases in cervical cancer treated with radio-chemotherapy. Clin Cancer Res. 2017;23:1242–1249. doi: 10.1158/1078-0432.CCR-16-1730. [DOI] [PubMed] [Google Scholar]
- 20.Manfredi MG, Ecsedy JA, Chakravarty A, Silverman L, Zhang M, Hoar KM, Stroud SG, Chen W, Shinde V, Huck JJ, Wysong DR, Janowick DA, Hyer ML, Leroy PJ, Gershman RE, Silva MD, Germanos MS, Bolen JB, Claiborne CF, Sells TB. Characterization of Alisertib (MLN8237), an investigational small-molecule inhibitor of aurora A kinase using novel in vivo pharmacodynamic assays. Clin Cancer Res. 2011;17:7614–7624. doi: 10.1158/1078-0432.CCR-11-1536. [DOI] [PubMed] [Google Scholar]
- 21.Bosbach B, Rossi F, Yozgat Y, Loo J, Zhang JQ, Berrozpe G, Warpinski K, Ehlers I, Veach D, Kwok A, Manova K, Antonescu CR, DeMatteo RP, Besmer P. Direct engagement of the PI3K pathway by mutant KIT dominates oncogenic signaling in gastrointestinal stromal tumor. Proc Natl Acad Sci U S A. 2017;114:E8448–E8457. doi: 10.1073/pnas.1711449114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaudary N, Pintilie M, Schwock J, Dhani N, Clarke B, Milosevic M, Fyles A, Hill RP. Characterization of the tumor-microenvironment in patient-derived cervix xenografts (OCICx) Cancers (Basel) 2012;4:821–845. doi: 10.3390/cancers4030821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustin JP, Cosgrove DP, Park BH. The PIK3CA gene as a mutated target for cancer therapy. Curr Cancer Drug Targets. 2008;8:733–740. doi: 10.2174/156800908786733504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tate JG, Bamford S, Jubb HC, Sondka Z, Beare DM, Bindal N, Boutselakis H, Cole CG, Creatore C, Dawson E, Fish P, Harsha B, Hathaway C, Jupe SC, Kok CY, Noble K, Ponting L, Ramshaw CC, Rye CE, Speedy HE, Stefancsik R, Thompson SL, Wang S, Ward S, Campbell PJ, Forbes SA. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Research. 2018;47:D941–D947. doi: 10.1093/nar/gky1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samuels Y, Waldman T. Oncogenic mutations of PIK3CA in human cancers. Curr Top Microbiol Immunol. 2010;347:21–41. doi: 10.1007/82_2010_68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46:372–383. doi: 10.3109/07853890.2014.912836. [DOI] [PubMed] [Google Scholar]
- 27.Mayer IA, Prat A, Egle D, Blau S, Fidalgo JAP, Gnant M, Fasching PA, Colleoni M, Wolff AC, Winer EP, Singer CF, Hurvitz S, Estevez LG, van Dam PA, Kummel S, Mundhenke C, Holmes F, Babbar N, Charbonnier L, Diaz-Padilla I, Vogl FD, Sellami D, Arteaga CL. A phase II randomized study of neoadjuvant letrozole plus alpelisib for hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer (NEO-ORB) Clin Cancer Res. 2019;25:2975–2987. doi: 10.1158/1078-0432.CCR-18-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nct. A Phase Ib dose escalation/randomized phase II, multicenter, open-label study of BYL719 in combination with cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma. Clinicaltrials.gov [www.clinicaltrials.gov] 2012
- 29.Nct. Study assessing the efficacy and safety of Alpelisib plus fulvestrant in men and postmenopausal women with advanced breast cancer which progressed on or after Aromatase inhibitor treatment. https://clinicaltrials.gov/show/ NCT02437318 2015.
- 30.SERVICES” USDOHAH. Approved Drug Products with Therapeutic Equivalence Evaluations (The Orange Book) 2021 [Google Scholar]
- 31.Yang L, Zhou Q, Chen X, Su L, Liu B, Zhang H. Activation of the FAK/PI3K pathway is crucial for AURKA-induced epithelial-mesenchymal transition in laryngeal cancer. Oncol Rep. 2016;36:819–826. doi: 10.3892/or.2016.4872. [DOI] [PubMed] [Google Scholar]
- 32.Donnella HJ, Webber JT, Levin RS, Camarda R, Momcilovic O, Bayani N, Shah KN, Korkola JE, Shokat KM, Goga A, Gordan JD, Bandyopadhyay S. Kinome rewiring reveals AURKA limits PI3K-pathway inhibitor efficacy in breast cancer. Nat Chem Biol. 2018;14:768–777. doi: 10.1038/s41589-018-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen D, Liu X, Yang Y, Yang H, Lu P. Systematic synergy modeling: understanding drug synergy from a systems biology perspective. BMC Syst Biol. 2015;9:56–56. doi: 10.1186/s12918-015-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in Enzyme Regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 35.Venkataraman S, Alimova I, Tello T, Harris PS, Knipstein JA, Donson AM, Foreman NK, Liu AK, Vibhakar R. Targeting Aurora Kinase A enhances radiation sensitivity of atypical teratoid rhabdoid tumor cells. J Neurooncol. 2012;107:517–526. doi: 10.1007/s11060-011-0795-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong X, O’Donnell JP, Salazar CR, Van Brocklyn JR, Barnett KD, Pearl DK, deCarvalho AC, Ecsedy JA, Brown SL, Mikkelsen T, Lehman NL. The selective Aurora-A kinase inhibitor MLN8237 (alisertib) potently inhibits proliferation of glioblastoma neurosphere tumor stem-like cells and potentiates the effects of temozolomide and ionizing radiation. Cancer Chemother Pharmacol. 2014;73:983–990. doi: 10.1007/s00280-014-2430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehman NL, O’Donnell JP, Whiteley LJ, Stapp RT, Lehman TD, Roszka KM, Schultz LR, Williams CJ, Mikkelsen T, Brown SL, Ecsedy JA, Poisson LM. Aurora A is differentially expressed in gliomas, is associated with patient survival in glioblastoma and is a potential chemotherapeutic target in gliomas. Cell Cycle. 2012;11:489–502. doi: 10.4161/cc.11.3.18996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu N, Wang YA, Sun Y, Ecsedy J, Sun J, Li X, Wang P. Inhibition of Aurora A enhances radiosensitivity in selected lung cancer cell lines. Respir Res. 2019;20:230. doi: 10.1186/s12931-019-1194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song A, Andrews DW, Werner-Wasik M, Kim L, Glass J, Bar-Ad V, Evans JJ, Farrell CJ, Judy KD, Daskalakis C, Zhan T, Shi W. Phase I trial of alisertib with concurrent fractionated stereotactic re-irradiation for recurrent high grade gliomas. Radiother Oncol. 2019;132:135–141. doi: 10.1016/j.radonc.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 40.Tsuchida E, Kaida A, Pratama E, Ikeda MA, Suzuki K, Harada K, Miura M. Effect of X-irradiation at different stages in the cell cycle on individual cell-based kinetics in an asynchronous cell population. PLoS One. 2015;10:e0128090. doi: 10.1371/journal.pone.0128090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Redon CE, Dickey JS, Bonner WM, Sedelnikova OA. γ-H2AX as a biomarker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin. Adv Space Res. 2009;43:1171–1178. doi: 10.1016/j.asr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicotera P, Melino G. Regulation of the apoptosis-necrosis switch. Oncogene. 2004;23:2757–2765. doi: 10.1038/sj.onc.1207559. [DOI] [PubMed] [Google Scholar]
- 43.Loriot Y, Mordant P, Dugue D, Geneste O, Gombos A, Opolon P, Guegan J, Perfettini JL, Pierre A, Berthier LK, Kroemer G, Soria JC, Depil S, Deutsch E. Radiosensitization by a novel Bcl-2 and Bcl-XL inhibitor S44563 in small-cell lung cancer. Cell Death Dis. 2014;5:e1423–e1423. doi: 10.1038/cddis.2014.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yasser M, Shaikh R, Chilakapati MK, Teni T. Raman spectroscopic study of radioresistant oral cancer sublines established by fractionated ionizing radiation. PLoS One. 2014;9:e97777. doi: 10.1371/journal.pone.0097777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yi FT, Lu QP. Mucin 1 promotes radioresistance in hepatocellular carcinoma cells through activation of JAK2/STAT3 signaling. Oncol Lett. 2017;14:7571–7576. doi: 10.3892/ol.2017.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin D, Fallaha S, Proctor M, Stevenson A, Perrin L, McMillan N, Gabrielli B. Inhibition of aurora A and aurora B is required for the sensitivity of HPV-driven cervical cancers to aurora kinase inhibitors. Mol Cancer Ther. 2017;16:1934–1941. doi: 10.1158/1535-7163.MCT-17-0159. [DOI] [PubMed] [Google Scholar]
- 47.Spardy N, Covella K, Cha E, Hoskins EE, Wells SI, Duensing A, Duensing S. Human papillomavirus 16 E7 oncoprotein attenuates DNA damage checkpoint control by increasing the proteolytic turnover of claspin. Cancer Res. 2009;69:7022–7029. doi: 10.1158/0008-5472.CAN-09-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spector ME, Sacco AG, Bellile E, Taylor JMG, Jones T, Sun K, Brown WC, Birkeland AC, Bradford CR, Wolf GT, Prince ME, Moyer JS, Malloy K, Swiecicki P, Eisbruch A, McHugh JB, Chepeha DB, Rozek L, Worden FP. E6 and E7 antibody levels are potential biomarkers of recurrence in patients with advanced-stage human papillomavirus-positive oropharyngeal squamous cell carcinoma. Clin Cancer Res. 2017;23:2723–2729. doi: 10.1158/1078-0432.CCR-16-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]