Abstract
This study aimed to characterize peak exercise cardiac function and thigh muscle fatty infiltration and their relationships with VO2peak among anthracycline-treated breast cancer survivors (BCS). BCS who received anthracycline chemotherapy ~ 1 year earlier (n = 16) and matched controls (matched-CON, n = 16) were enrolled. Resting and peak exercise cardiac function, myocardial T1 mapping (marker of fibrosis), and thigh muscle fat infiltration were assessed by magnetic resonance imaging, and VO2peak by cycle test. Compared to matched-CON, BCS had lower peak SV (64 ± 9 vs 57 ± 10 mL/m2, p = 0.038), GLS (− 30.4 ± 2.2 vs − 28.0 ± 2.5%, p = 0.008), and arteriovenous oxygen difference (16.4 ± 3.6 vs 15.2 ± 3.9 mL/100 mL, p = 0.054). Mediation analysis showed: (1) greater myocardial T1 time (fibrosis) is inversely related to cardiac output and end-systolic volume exercise reserve; (2) greater thigh muscle fatty infiltration is inversely related to arteriovenous oxygen difference; both of which negatively influence VO2peak. Peak SV (R2 = 65%) and thigh muscle fat fraction (R2 = 68%) were similarly strong independent predictors of VO2peak in BCS and matched-CON combined. Post-anthracyclines, myocardial fibrosis is associated with impaired cardiac reserve, and thigh muscle fatty infiltration is associated with impaired oxygen extraction, which both contribute to VO2peak.
Subject terms: Breast cancer, Chemotherapy
Introduction
Elevated cardiovascular risk and impaired cardiorespiratory fitness (VO2peak) are now considered hallmarks of early stage breast cancer survivorship1–4. Understanding the mechanisms of impaired VO2peak is required to develop effective prevention and treatment strategies to reduce cardiovascular risk. Reduced VO2peak may be due to a lower peak exercise cardiac output (CO, product of stroke volume, SV, and heart rate, HR) and/or a lower arteriovenous oxygen difference (avO2diff), which indicates lower oxygen extraction by the exercising muscles. The direct cardiotoxic effects from treatment for breast cancer, especially anthracycline-based chemotherapy, is assumed to be the primary factor underlying these impairments4. Two prior studies, using indirect estimates or submaximal measures of SV during exercise, suggest that anthracycline chemotherapy has a detrimental effect on exercise SV in BCS2,5. One study has also reported that calculated avO2diff with submaximal exercise is reduced by anthracycline chemotherapy5.
Magnetic resonance imaging (MRI) is the gold standard for quantification of resting and exercise cardiac function and enables acquisition of complementary central and peripheral metrics with implications for VO2peak, including global longitudinal strain (GLS), and tissue characterization of the myocardium and the exercising thigh muscles (such as T1 mapping metric of fibrosis and fatty infiltration, respectively). The longitudinal shortening of the left ventricle (LV) is a primary determinant of SV6, and while resting GLS is a sensitive marker of anthracycline-related myocardial injury7, it is unknown whether exercise GLS is related to VO2peak. Diffuse myocardial fibrosis as measured by T1 mapping is another reported manifestation of anthracycline-related myocardial injury8. We previously reported strong associations between elevated myocardial T1 values with reduced VO2peak in anthracycline-treated BCS9,10, but etiology of the associations is not understood. Recently, we and others have also reported associations between non-cardiac parameters, specifically, fatty infiltration of the thigh muscles, with VO2peak following anthracycline therapy10,11.
We performed a comprehensive MRI study with an overarching goal of characterizing peak exercise cardiac function, including GLS, myocardial T1 time, and thigh muscle fatty infiltration (measured by fat fraction) and their relationships with VO2peak among anthracycline-treated BCS. The first objective was to compare peak exercise cardiac function among anthracycline-treated BCS, age- and body mass index (BMI)-matched non-cancer controls (matched-CON), and normative values for young, normal BMI, healthy controls (young-CON). We hypothesized that peak exercise cardiac function would be impaired in BCS compared to both matched-CON and young-CON. The second objective was to determine the relative contributions of central and peripheral factors to VO2peak with the hypothesis that both would be important predictors of VO2peak in BCS. The final objective was to determine whether exercise cardiac function and avO2diff mediate the relationship between cardiac and skeletal muscle tissue characteristics and VO2peak.
Methods
Design
This was a cross-sectional comparison of the three groups outlined below.
Participants
We have previously reported skeletal muscle composition, bioenergetics, and oxygenation and gated-segmented cardiac MRI assessment of resting LV function and structure of the participants in this study9,10. In this manuscript we report exercise and reserve cardiac function and uncover underlying mechanisms for reduced VO2peak.
BCS
We mailed letters to a random selection of 75 women from 240 who received anthracycline-based chemotherapy for breast cancer at the Cross Cancer Institute (Edmonton, Canada) in the previous calendar year (2017). Exclusion criteria were: diagnosed cardiovascular disease, diabetes, lung disease, MRI contraindications. Twenty-two (29%) women responded and six were excluded (MRI contraindication, diabetes, not interested, n = 2 each); 16 participants were enrolled. One participant received doxorubicin (250 mg/m2) and the rest received epirubicin (median dose: 300 mg/m2) 12.8 ± 4.7 months prior; none received trastuzumab.
Matched controls
We used word-of-mouth and posters to recruit a woman without a history of cancer to match each BCS within two years of age and whenever possible, to within 3.0 kg/m2 of BMI. The same exclusion criteria were applied.
Normative data
Normative female peak exercise cardiac function is not well quantified. Therefore, we recruited a convenience sample of 12 young (22–32 years), healthy (BMI = 19–24.9 kg/m2, no cardiovascular risk factors) women to perform the resting and peak exercise cardiac MRI only to disentangle the influence of cardiovascular risk factors beyond cardiotoxic treatment. Cardiovascular risk factors were defined as sedentary behaviour, hypertension, diabetes, dyslipidemia, overweight/obese, and smoking status.
MRI examination
MRI examinations were performed on a 3 T Siemens Prisma system with a 36-element chest/back array for signal reception (Siemens Healthcare, Erlangen, Germany). We used the SAturation recovery single SHot Acquisition (SASHA) pulse sequence to acquire native (non-contrast) T1 mapping for a single mid-basal short-axis slice at end-expiration12. SASHA acquisition parameters were: 0.96 ms echo time, 2.18 ms repetition time, 70° flip angle, 8 mm slice thickness, field of view 360 × 294 mm, acquisition matrix 208 × 128, 1415 Hz/pixel, rate 2 parallel imaging (GRAPPA) with acquisition during diastasis, and 10 saturation recovery images with a fixed recovery time of 630 ms. Real-time, free-breathing resting and exercise cine acquisitions of the left ventricle included six contiguous slices each of the two- and four-chamber long-axis views (typical parameters: FOV = 440 × 260 mm, matrix = 224 × 90, flip angle = 30°, GRAPPA = 3, TE = 1.0 ms, TR = 2.12 ms, 38 ms per image, 60 images/slice, 8 mm slice thickness, 2 mm gap). Exercise was performed with an MRI-compatible stepping device that enables real-time cardiac imaging during in-magnet exercise (Cardiostep, Ergospect; Innsbruck, Austria). After resting images were acquired, exercise was initiated at 20 watts, with an increase of 5 watts every 20 s with a 40 steps/minute frequency13. A study team member provided verbal encouragement from inside the scanner room and collected a rating of perceived exertion (Borg 0–10 scale) at the end of each minute of exercise and after the termination of the exercise test. Peak HR was acquired by finger pulse oximetry. Exercise tests ended when the participant reached volitional exhaustion or could no longer maintain the stepping frequency, followed immediately by acquisition of peak exercise images. A multi-slice, multi-echo acquisition of the right thigh was used to reconstruct water and fat separated images using the modified Dixon approach14. Image parameters include 50 axial slices with 4 mm slice thickness (no gap), and 1.0–1.3 mm in-plane resolution.
Image analysis
T1 maps were reconstructed on the scanner and the reported values are the average of all segments from the middle 2 mm layer of the myocardium. We used in-house software (MATLAB, MathWorks, Natick, USA) to measure LV end-diastolic and end-systolic volumes (EDV, ESV) using the bi-plane method of disks15 with tracings completed on a single two-chamber and a single four-chamber long-axis image. This method has been previously described in detail and shown to be reliable and reproducible for exercise cardiac function assessment13. A complete cardiac cycle was selected for each slice at end-expiration, with manual tracing of the LV endocardium and epicardium on end-diastolic and end-systolic images. SV was measured as the difference between EDV and ESV. Ejection fraction was calculated as SV/EDV. All volumes were normalized to body surface area (BSA)16. GLS was calculated as the fractional change in length of the endocardial contour from end-diastole to end-systole relative to end-diastolic length, averaged across the two- and four-chamber long-axis images. For all parameters, reserve was calculated as the difference between peak exercise and resting values.
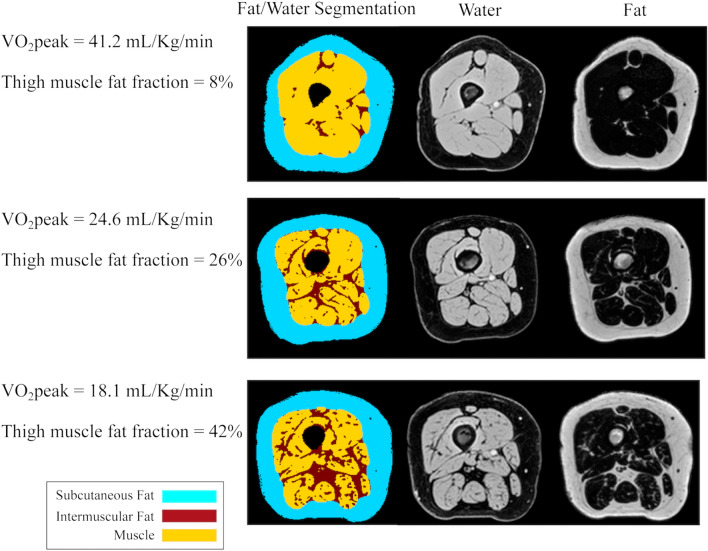
For the thigh images, we used custom semi-automated MATLAB software and the disk summation method for 10 consecutive slices starting 110 mm from the distal femur to measure absolute volumes of muscle and intermuscular fat (IMF), with the femur removed. We calculated the muscle fat fraction as: IMF/(IMF + muscle)*100% (Fig. 1).
Figure 1.
Representative participants with low, medium, and high thigh fat fraction with their respective water and fat suppressed images used to delineate the fat and muscle components. Axial right thigh images were acquired by magnetic resonance imaging and 40 cm of volume were analyzed starting 110 mm from the distal point of the femur to quantify absolute volumes of muscle and intermuscular fat (IMF). The thigh fat fraction was then calculated as: IMF/(IMF + muscle)*100%. Thigh fat fraction was inversely associated with VO2peak in both breast cancer survivors and matched controls.
Cardiopulmonary exercise test
The BCS and matched-CON participants performed a graded, to-maximal cardiopulmonary exercise test (CPET) on a cycle ergometer with continuous gas analysis (Encore229 Vmax; SensorMedics, Yorba Linda, USA). The CPET was performed within one week of the MRI scan, or on the same day, ≥ 1-h after the scan. Based on the influence of supine posture in the MRI test17, we also assessed upright HR, SV, and CO at rest and during exercise by transthoracic electrical bioimpedance meter (PhysioFlow PF-03, Manatec Biomedical, France)18. A finger pulse oximeter was used to collect arterial oxygen saturation (SaO2) and blood pressure was assessed every two minutes. We allowed ≥ 2 min of rest to ensure stabilization of resting values, then initiated exercise at 20 watts, with 5 watt increments every 20 s (same protocol as MRI). We averaged the impedance data over 30-s epochs from the end of the resting period and the exercise test, as well as the time corresponding to 50 and 75% of VO2peak. Peak exercise avO2diff was calculated as VO2peak divided by peak impedance-derived CO, while systemic vascular resistance (SVR) was calculated as mean arterial pressure divided by impedance-derived CO multiplied by 80.
Descriptive data
We measured height and weight by electronic scale and stadiometer to calculate BMI, while demographics and cardiovascular risk factors were self-reported. We determined current sedentary status (no moderate or vigorous activity) from the Godin Leisure Time Exercise Questionnaire responses19. Study staff extracted diagnosis and treatment information for BCS from medical records. Hemoglobin was measured from a venipuncture taken prior to exercise.
Statistical analyses
We compared the prevalence of cardiovascular risk factors between groups using Fisher’s exact tests. For comparison between the three groups, we used one-way analysis of variance if the Levene’s and Shapiro–Wilk tests confirmed equal variance and normal distribution, and the Kruskal–Wallis H test otherwise. Independent t-tests or the Mann–Whitney U-test were used for between-group comparisons.
We used linear regression to determine the independent predictive value of the peak exercise cardiac parameters, avO2diff, hemoglobin, myocardial T1 time, and thigh muscle fat fraction for relative VO2peak (mL/kg/min) among the BCS and matched-CON groups combined. An interaction effect between group and each independent variable was included to determine whether cancer status influenced these relationships. For significant interactions, we report the linear relationship separately for the two groups, otherwise the interaction and group effects are removed from the model, leaving a univariate model. When testing cardiac volumes as independent predictors of VO2peak, we normalized them to body weight (kilograms) instead of BSA to standardize the units.
To determine whether functional metrics of the myocardium (peak exercise and reserve LV volumes and function) and skeletal muscle (peak avO2diff) mediated the linear relationship between myocardial T1 time or thigh fat fraction, respectively and relative VO2peak, we performed Baron and Kenny’s steps for mediation analysis20. In brief, a partial mediator is a variable that: (1) is predicted by the tissue characteristic in linear regression; (2) when adjusted by the tissue characteristic, is a significant linear predictor of VO2peak; and (3) reduces the size of the effect of the tissue characteristics on VO2peak to nonzero. Partial mediation implies that part of the effect of the tissue characteristic on VO2peak is accounted for by the effect of the tissue characteristic on that mediator variable.
Statistical analyses were performed using Python 3.7.0 (Available at http://www.python.org), two-sided p-values of 0.05 to denote statistical significance without adjustments for multiple comparisons.
Ethics approval
This study was approved by the University of Alberta Research Ethics Board (Pro00040073). Informed consent was obtained from all individual participants included in the study. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Results
Participants
The BCS and matched-CON groups were similar in demographics, anthropometrics, and cardiovascular risk factors, and by design, the young-CON group had no cardiovascular risk factors, lower BMI and age (Table 1). All participants completed all planned study measures.
Table 1.
Participant descriptive data.
| Variable | Young controls (n = 12) | Matched controls (n = 16) | Breast cancer survivors (n = 16) | p-value |
|---|---|---|---|---|
| Demographics and anthropometrics | ||||
| Age (years, mean ± SD) | 25 ± 4 | 56 ± 10* | 56 ± 10* | < 0.001 |
| Body mass index (kg/m2, mean ± SD) | 22 ± 2 | 28 ± 5* | 29 ± 4* | < 0.001 |
| Body surface area (m2, mean ± SD) | 1.68 ± 0.10 | 1.84 ± 0.15* | 1.84 ± 0.18* | < 0.01 |
| Caucasian (n (%)) | 12 (100%) | 16 (100%) | 15 (94%) | 1.00 |
| Cardiovascular risk factors | n (%) | n (%) | n (%) | p-value† |
|---|---|---|---|---|
| Sedentary | 0 | 2 (13%) | 4 (25%) | 0.654 |
| Hypertension‡ | 0 | 0 | 2 (13%) | 0.484 |
| Hypercholesterolemia | 0 | 2 (13%) | 0 | 0.484 |
| Former smoker | 0 | 8 (50%) | 7 (44%) | 1.00 |
| Current smoker | 0 | 0 | 0 | - |
| Diabetes | 0 | 0 | 0 | - |
| Overweight (25.0–29.9 kg/m2) | 0 | 8 (50%) | 5 (31%) | 0.473 |
| Obese (30.0 + kg/m2) | 0 | 7 (44%) | 7 (44%) | 1.00 |
| Breast cancer diagnosis and treatment | n (%) | |||
|---|---|---|---|---|
| Stage | ||||
| I/II | 8 (50%) | |||
| III | 7 (44%) | |||
| IV | 1 (6%) | |||
| Receptor status | ||||
| Estrogen positive | 12 (75%) | |||
| Progesterone positive | 8 (50%) | |||
| Human epidermal growth factor 2 positive | 0 | |||
| Triple negative | 4 (25%) | |||
| Chemotherapy regimen | ||||
| 3 × fluorouracil, epirubicin & cyclophosphamide + 3 × docetaxel | 14 (88%) | |||
| 6 × fluorouracil, epirubicin & cyclophosphamide | 1 (6%) | |||
| 5 × fluorouracil, doxorubicin & cyclophosphamide | 1 (6%) | |||
| Post-intravenous chemotherapy capecitabine | 2 (13%) | |||
| Radiation therapy | ||||
| Left | 11 (69%) | |||
| Right | 4 (25%) | |||
| Hormonal therapy (current) | ||||
| Aromatase inhibitor | 5 (31%) | |||
| Tamoxifen | 4 (25%) | |||
| Both | 2 (13%) | |||
*Statistically different from young controls at p ≤ 0.01.
†P-value shown for comparison between matched controls and survivors only as young controls lacked all of these risk factors by default.
‡Of the two breast cancer group participants reporting a history of hypertension, only one was currently on medication (a calcium channel blocker) and she reported not taking it during anthracycline therapy or on the day of the assessment.
Supine (MRI) cardiac function
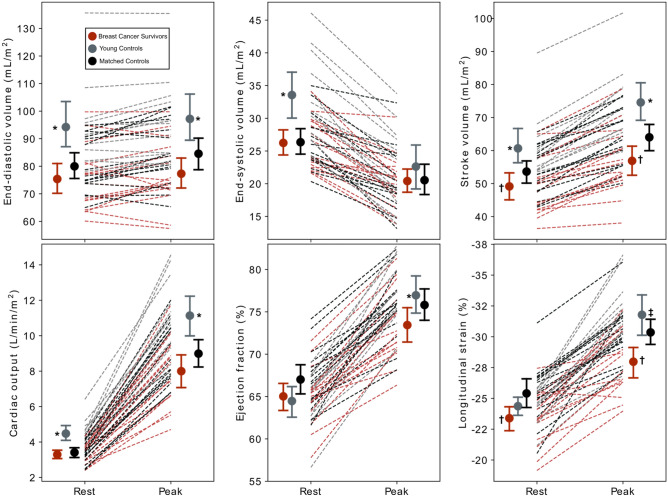
Individual rest-to-exercise changes in MRI-derived LV volumes and function for all three groups are shown in Fig. 2. Peak power output as well as LV volumes at rest and at peak exercise (except for peak end-systolic volume) were larger in young-CON than both the BCS and matched-CON groups (Table 2). Young-CON also had larger reserve in LV function (ejection fraction, GLS, CO, Table 2). The only difference between BCS and matched-CON, however, was that BCS had lower peak power, lower rest and exercise SV and GLS, and CO reserve (Table 2).
Figure 2.
Change and mean cardiac function from rest to peak exercise among anthracycline-treated breast cancer survivors (n = 16), age- and body mass index-matched non-cancer controls (n = 16), and young, healthy controls (n = 12). All data is derived from cardiac magnetic resonance imaging. Volumes are normalized to body surface area. Young controls had larger peak and reserve stroke volume and cardiac output compared to both other groups. Compared to matched controls, breast cancer survivors had lower rest and exercise stroke volume and global longitudinal strain, and cardiac output reserve. *Difference from both breast cancer survivors and matched control groups at p ≤ 0.05; †Difference from matched control group at p ≤ 0.05; ‡Difference from breast cancer survivor group at p ≤ 0.05;
Table 2.
Cardiac magnetic resonance and cardiopulmonary data.
| Variable | Young controls (n = 12) (mean ± SD) | Matched controls (n = 16) (mean ± SD) | Breast cancer survivors (n = 16) (mean ± SD) | P-value |
|---|---|---|---|---|
| Resting | ||||
| Heart rate (bpm) | 74 Ü9 | 64 ± 11* | 68 ± 10 | 0.042 |
| End-diastolic volume index (mL/m2) | 94 ± 15 | 80 ± 10* | 75 ± 12* | 0.001 |
| End-systolic volume index (mL/m2) | 34 ± 7 | 26 ± 4* | 26 ± 4* | < 0.001 |
| Stroke volume index (mL/m2) | 61 ± 10 | 54 ± 7* | 49 ± 9*† | 0.009 |
| Ejection fraction (%) | 64 ± 3 | 67 ± 4 | 65 ± 3 | 0.123 |
| Cardiac output index (L/min/m2) | 4.5 ± 0.8 | 3.4 ± 0.6* | 3.3 ± 0.5* | < 0.001 |
| Global longitudinal strain (%) | − 24.4 ± 1.4 | − 25.4 ± 2.4 | − 23.4 ± 2.2† | 0.032 |
| Peak supine MRI exercise | ||||
| Supine stepper power output (watts) | 255 + 25 | 151 ± 29* | 122 ± 30*† | < 0.001 |
| Heart rate (bpm) | 155 ± 22 | 140 ± 17 | 140 ± 17 | 0.067 |
| End-diastolic volume index (mL/m2) | 97 ± 15 | 85 ± 11* | 77 ± 12* | < 0.001 |
| End-systolic volume index (mL/m2) | 23 ± 6 | 21 ± 5 | 20 ± 4 | 0.448 |
| Stroke volume index (mL/m2) | 75 ± 11 | 64 ± 9* | 57 ± 10*† | < 0.001 |
| Ejection fraction (%) | 77 ± 4 | 76 ± 4 | 73 ± 4 | 0.071 |
| Cardiac output index (L/min/m2) | 11.1 ± 2.2 | 9.0 ± 1.7* | 8.0 ± 2.0* | < 0.001 |
| Global longitudinal strain (%) | − 31.8 ± 3.0 | − 30.4 ± 2.2 | − 28.0 ± 2.5*† | 0.001 |
| Reserve (peak–rest) | ||||
| Heart rate (bpm) | 81 ± 19 | 76 ± 12 | 72 ± 20 | 0.394 |
| End-diastolic volume index (mL/m2) | 3 ± 3 | 5 ± 5 | 2 ± 6 | 0.312 |
| End-systolic volume index (mL/m2) | − 11 ± 3 | − 6 ± 3* | − 6 ± 4* | < 0.001 |
| Stroke volume index (mL/m2) | 14 ± 4 | 10 ± 4* | 8 ± 4* | 0.001 |
| Ejection fraction (%) | 12 ± 3 | 9 ± 3* | 8 ± 3* | 0.004 |
| Cardiac output index (L/min/m2) | 6.7 ± 2.0 | 5.6 ± 1.3* | 4.7 ± 1.6*† | 0.012 |
| Global longitudinal strain (%) | − 7.4 ± 2.1 | − 5.0 ± 2.0* | − 4.6 ± 2.5* | 0.003 |
| Cardiopulmonary cycle exercise test data | ||||
| Resting arterial oxygen saturation (%) | – | 98 ± 2 | 97 ± 2 | 0.357 |
| Peak arterial oxygen saturation (%) | – | 94 ± 2 | 96 ± 2 | 0.080 |
| Peak heart rate (bpm) | – | 161 ± 14 | 166 ± 12 | 0.110 |
| Peak power output (watts) | – | 165 ± 30 | 132 ± 31 | 0.004 |
| Peak oxygen consumption (L/min) | – | 2.13 ± 0.41 | 1.69 ± 0.37 | 0.003 |
| Peak oxygen consumption (mL/kg/min) | – | 29.5 ± 7.7 | 23.1 ± 7.5 | 0.011 |
| Peak respiratory exchange ratio | – | 1.27 ± 0.08 | 1.29 ± 0.09 | 0.600 |
| Arteriovenous oxygen difference (mL/100 mL, impedance-derived) | – | 16.4 ± 3.6 | 15.2 ± 3.9 | 0.054 |
*Statistically different from young controls at p ≤ 0.05; † Statistically different from matched controls at p ≤ 0.05;– denotes data not collected in this group.
At peak exercise in the CMR, all participants reported a peak rating of perceived exertion of ≥ 9 on the Borg 0–10 scale. The peak power output achieved in the supine CMR stepper exercise test corresponded to 93 ± 11% of that achieved on the upright cycle ergometer test.
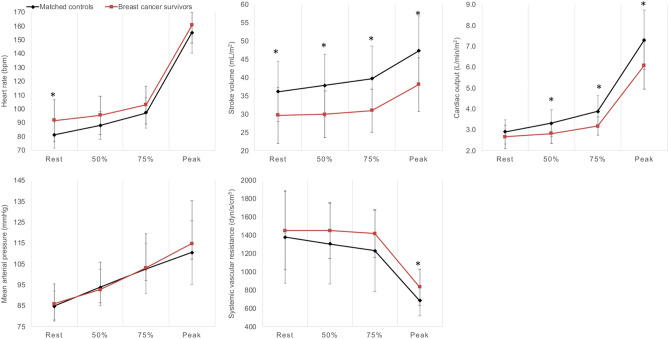
Upright (impedance cardiography) cardiac function
Upright cycle ergometer responses of HR, SV, CO, and SVR assessed by impedance cardiography were compared between BCS and matched-CON at rest, 50%, 75%, and 100% of VO2peak. There were similar differences between BCS and matched-CON for upright SV and CO to supine data (Fig. 3). At peak exercise, calculated avO2diff was lower and SVR was higher in BCS versus matched-CON (Table 2, Fig. 3, respectively).
Figure 3.
Bioelectrical impedance cardiography data for cycle ergometer graded exercise test in breast cancer survivors (n = 16), age- and body mass index-matched non-cancer controls (n = 16). Stroke volume and cardiac output are normalized to body surface area. *Difference between groups at p ≤ 0.05.
Hemoglobin and VO2 peak
Hemoglobin did not differ between BCS and matched-CON (133 ± 11 vs 134 + 8, p = 0.686). All participants exceeded a respiratory exchange ratio of 1.10 on the maximal cardiopulmonary exercise test and reached volitional exhaustion. Absolute and relative VO2peak were lower in BCS versus matched-CON (Table 2).
Determinants of VO2 peak
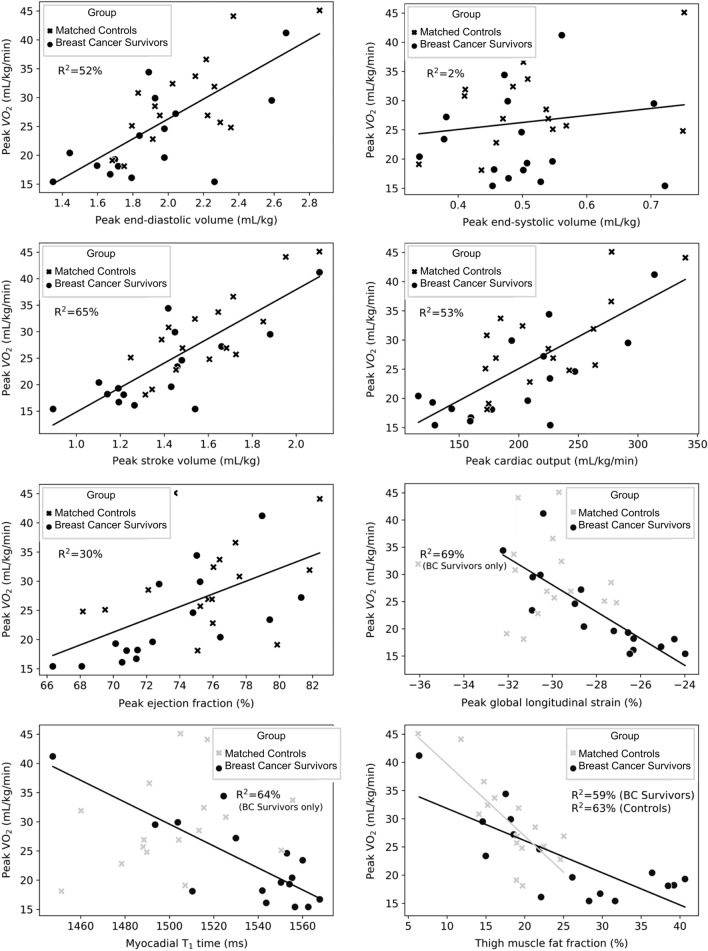
Peak exercise GLS (more negative is better function) and myocardial T1 time (lower is less fibrosis) were inversely correlated with VO2peak in BCS (ß = − 2.5, 95% CI − 3.4, − 1.5, p < 0.001, R2 = 69%, ß = − 0.19, 95% CI − 0.27, − 0.11, p < 0.001, R2 = 64%, respectively; Fig. 4) and were not related in matched-CON. Thigh muscle fat fraction had a statistically different relationship with VO2peak in the two groups that was similar in direction and strength (BCS: ß = − 0.6, 95% CI − 0.8, − 0.3, p < 0.001, R2 = 59; matched-CON: ß = − 1.3, 95% CI − 1.8, − 0.7, p < 0.001, R2 = 63%, both p < 0.001), but with a steeper slope in the matched-CON group (Fig. 4). Peak exercise EDV, SV, CO, EF, and avO2diff were significant predictors of VO2peak with no influence of group (Table 3).
Figure 4.
Predictors of peak volume of oxygen consumption (VO2) among anthracycline-treated breast cancer survivors and age- and body mass index-matched non-cancer controls. Trend line colors match included data. Both VO2 and volumes are normalized to body weight (kg) to standardize units.
Table 3.
Predictors of relative VO2peak (mL/kg/min) for breast cancer and matched controls combined (n = 32) with adjustment for group.
| Variables differing by group: | P-value: group interaction | ß (95% CI) for breast cancer | R2 (%) |
|---|---|---|---|
| Peak global longitudinal strain (%) | 0.048 | − 2.5 (− 3.8, − 1.1) | 45 |
| Myocardial T1 time (ms) | 0.002 | − 0.19 (− 0.29, − 0.08) | 46 |
| Thigh muscle fat fraction (%) | 0.022 | − 0.57 (− 0.83, − 0.32) | 68 |
| Variables without influence of group: | P-value: main effect | ß (95% CI) for both groups | R2 (%) |
|---|---|---|---|
| Peak end-diastolic volume (mL/kg) | < 0.001 | 17.2 (11.1, 23,4) | 52 |
| Peak end-systolic volume (mL/kg) | 0.394 | 12.0 (− 16.4, 40.5) | 2 |
| Peak stroke volume (mL/kg) | < 0.001 | 23.1 (16.8, 29.4) | 65 |
| Peak cardiac output (mL/kg/min) | < 0.001 | 0.11 (0.07, 0.15) | 53 |
| Peak supine heart rate (beats per minute) | 0.103 | 0.14 (− 0.03, 0.32) | 9 |
| Peak upright heart rate (beats per minute) | 0.050 | 0.22 (0, 0.44) | 12 |
| Peak ejection fraction (%) | 0.001 | 1.1 (0.5, 1.7) | 30 |
| Hemoglobin (g/dL) | 0.248 | 1.8 (− 1.3, 5.0) | 4 |
| Peak arteriovenous oxygen difference (mL/100 mL, impedance-derived) | < 0.001 | 1.3 (0.7, 2.0) | 37 |
Bolding indicates significance.
ß unstandardized beta coefficient, CI confidence interval, R2 coefficient of variation.
Is the effect of cardiac or skeletal muscle tissue characteristics on VO2peak mediated by function of these tissues?
We used mediation analysis to test the hypothesis that the relationships between myocardial T1 time and thigh muscle fat fraction with VO2peak are mediated by function of these tissues in BCS. Myocardial T1 time was a significant predictor of peak exercise EDV, SV, and CO, as well as reserve in ESV and CO. Of these variables meeting the first criterion in mediation analysis, only reserve in CO and ESV also partially reduced the effect size of myocardial T1 on VO2peak (second criterion) and remained significant predictors of VO2peak (final criterion). In other words, part of the effect of myocardial T1 time on VO2peak is accounted for by the effect of myocardial T1 time on CO reserve and ESV reserve. Likewise, avO2diff met the criteria as a partial mediator of the effect of thigh muscle fat fraction on VO2peak.
Discussion
This comprehensive MRI study produced several novel findings regarding exercise cardiac function and peripheral predictors and mediators of VO2peak as well as potential underlying mechanisms among anthracycline-treated BCS.
Firstly, we substantially extend previous findings on exercise cardiac function in BCS2,21, by reporting that submaximal and peak exercise SV are blunted in both the supine (by MRI) and upright (by impedance cardiography) body positions among anthracycline-treated BCS compared to non-cancer controls matched for cardiovascular risk factors. Second, our comparison of young- and matched-CON demonstrates that traditional cardiovascular risk factors (age, overweight/obesity, lifestyle behaviors) without cardiotoxic treatment result in reduced exercise reserve of LV volumes and function. The only additional cardiac impairment in the anthracycline-treated BCS group, who were otherwise closely balanced with matched-CON on all cardiovascular risk factors, was blunted SV (a direct determinant of VO2peak) and GLS (a sensitive predictor of cardiotoxicity when measured at rest)7. Novel findings related to peak exercise GLS were that it correlated with VO2peak in the BCS group only, and that GLS reserve was greater in young-CON, but similar between BCS and matched-CON. The longitudinal shortening of the LV is a primary determinant of SV6, and all of our GLS findings paralleled those for SV. Together, these novel exercise GLS findings suggest that impaired longitudinal shortening may be an important myocardial mechanism for smaller exercise SV and resultant VO2peak among both anthracycline-treated and cardiovascular risk female populations.
In this study, similar to previous reports2,10,22, VO2peak was ~ 20% lower in BCS compared to matched-CON. Our novel peak exercise cardiac MRI and muscle tissue characterization data indicate that central and peripheral factors explain similar amounts of variation in VO2peak in anthracycline-treated BCS. Further, by mediation analyses, we explored the mechanism or process by which cardiac and skeletal muscle tissue characteristics may influence VO2peak through associations with function. We determined that the association of native myocardial T1 time, where longer times are associated with a greater degree of biopsy-quantified fibrosis23, on VO2peak is partially explained by the association of T1 time on the reserve of CO or more specifically, ESV. As EDV (and likely preload) did not appreciably change, in line with previous supine MRI results in other populations24, the reduction in ESV is the primary driver of the exercise-induced increase in SV. As a reduction in ESV with acute exercise results from increased inotropy and/or reduced afterload, this finding suggests that within BCS, myocardial fibrosis and potentially the associated loss in healthy cardiac muscle fibers impairs one or both of these, which in turn leads to lower VO2peak. Indeed, surrogate measures (GLS and SVR) were impaired, on average, in the BCS group.
We also determined that the negative association of fatty infiltration of the thigh with VO2peak is partially explained by a negative association with avO2diff. This finding is in line with our previous finding for non-cardiac limited, single-leg, plantar flexion exercise, where a greater ratio between IMF and muscle in the lower leg was associated with multiple aspects of diffusive oxygen transport and/or utilization including local oxygen consumption and extraction, and blood flow10. Elevated levels of IMF and visceral fat at the time of breast cancer diagnosis have been associated with elevated future incidence of cardiovascular disease25. Post-anthracyclines, BCS also have greater visceral fat compared to controls, despite similar amounts of subcutaneous fat10. Given that both IMF and visceral fat are associated with insulin resistance26,27, this suggests that BCS exhibit a detrimental cardiometabolic phenotype. It should be noted that the relationship between thigh muscle fat fraction and VO2peak also existed in matched-CON, who were primarily older women with overweight/obesity. This suggests that fatty infiltration of the muscles is an underappreciated determinant of cardiovascular risk in women in general.
Future studies are required to quantify the longitudinal impact of breast cancer treatment on peak SV and IMF and to evaluate interventions to mitigate injury during treatment or improve these metrics post-treatment. Given that myocardial fibrosis is thought to be irreversible, prophylactic strategies during cardiotoxic treatment may be needed to prevent myocardial injury and preserve central determinants of VO2peak. In contrast, IMF is amenable to interventions such as caloric restriction and exercise training in other populations26, so this plasticity may enable intervention timing either during or after cancer treatment as strategies to improve VO2peak.
A limitation of this study is that the cross-sectional design does not allow determination of causal influences. Our sample size is small, however, our comprehensive assessment using highly reproducible MRI techniques allows the detection of meaningful differences with a sample size as small as 15 per group28. Further we have recently reported the excellent reliability and reproducibility of our exercise CMR technique15. Ideally the MRI measures of cardiac function and VO2peak would have been performed in the same body position, but our upright cardiac impedance-derived measures demonstrated the same trend. Finally, generalizability is limited to primarily Caucasian and middle-aged BCS.
In conclusion, among BCS who are post-anthracycline treatment, peak exercise SV and CO and skeletal muscle oxygen extraction are blunted compared to matched-CON and are both important determinants of VO2peak. Mediation analysis showed that greater myocardial fibrosis is associated with impaired exercise reserve ESV and CO which is related to reduced VO2peak. Greater thigh muscle fatty infiltration is associated with impaired oxygen extraction which is also related to reduced VO2peak. Fatty infiltration of the thigh muscle may be an underappreciated determinant of cardiovascular risk in women with and without breast cancer. Given that myocardial fibrosis is considered irreversible, but fatty infiltration of muscle is plastic, strategies to improve exercise capacity in anthracycline-treated BCS could include interventions to prevent myocardial injury during treatment and/or that improve fatty infiltration either during or after treatment.
Acknowledgements
This study was funded by Susan G. Komen Foundation (PDF17483149; PI: Dr. Kirkham). Dr. Kirkham was funded by Postdoctoral Fellowships from Canadian Institutes of Health Research and Alberta Innovates Health Solutions. Dr. Haykowsky is funded by the Research Chair in Aging and Quality of Life, Faculty of Nursing, University of Alberta.
Author contributions
A.K., M.H., R.B., D.I.P., E.P., R.T. contributed to the study conception and design. Material preparation, data collection and analysis were performed by A.K., M.H., J.M., R.B., R.T. The first draft of the manuscript was written by A.K. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by Susan G. Komen Foundation (PDF17483149; PI: Dr. Kirkham). Dr. Kirkham was funded by Postdoctoral Fellowships from Canadian Institutes of Health Research and Alberta Innovates Health Solutions. Dr. Haykowsky is funded by the Research Chair in Aging and Quality of Life, Faculty of Nursing, University of Alberta.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J. Clin. Oncol. 2012;30:2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones LW, Haykowsky M, Pituskin EN, Jendzjowsky NG, Tomczak CR, Haennel RG, et al. Cardiovascular reserve and risk profile of postmenopausal women after chemoendocrine therapy for hormone receptor positive operable breast cancer. Oncologist. 2007;12:1156–1164. doi: 10.1634/theoncologist.12-10-1156. [DOI] [PubMed] [Google Scholar]
- 3.Klassen O, Schmidt ME, Scharhag-Rosenberger F, Sorkin M, Ulrich CM, Schneeweiss A, et al. Cardiorespiratory fitness in breast cancer patients undergoing adjuvant therapy. Acta Oncol. 2014;53:1356–1365. doi: 10.3109/0284186X.2014.899435. [DOI] [PubMed] [Google Scholar]
- 4.Kirkham AA, Beaudry RI, Paterson DI, Mackey JR, Haykowsky MJ. Curing breast cancer and killing the heart: A novel model to explain elevated cardiovascular disease and mortality risk among women with early stage breast cancer. Prog. Cardiovasc. Dis. 2019;62:116–126. doi: 10.1016/j.pcad.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Howden EJ, Bigaran A, Beaudry R, Fraser S, Selig S, Foulkes S, et al. Exercise as a diagnostic and therapeutic tool for the prevention of cardiovascular dysfunction in breast cancer patients. Eur. J. Prev. Cardiol. 2019;26:305–315. doi: 10.1177/2047487318811181. [DOI] [PubMed] [Google Scholar]
- 6.Maciver DH. The relative impact of circumferential and longitudinal shortening on left ventricular ejection fraction and stroke volume. Exp. Clin. Cardiol. 2012;17:5–11. [PMC free article] [PubMed] [Google Scholar]
- 7.Thavendiranathan P, Poulin F, Lim K-D, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J. Am. Coll. Cardiol. 2014;63:2568–2751. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 8.Hong YJ, Park HS, Park JK, Han K, Park CH, Kim TK, et al. Early detection and serial monitoring of anthracycline-induced cardiotoxicity using T1-mapping cardiac magnetic resonance imaging: An animal study. Sci. Rep. 2017;7:2663. doi: 10.1038/s41598-017-02627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkham AA, Paterson DI, Haykowsky MJ, Beaudry R, Mackey JM, Pituskin E, et al. Aerobic fitness is related to myocardial fibrosis post-anthracycline therapy. Med. Sci. Sports Exerc. 2021;53:267–274. doi: 10.1249/MSS.0000000000002469. [DOI] [PubMed] [Google Scholar]
- 10.Beaudry RI, Kirkham AA, Thompson RB, Grenier JG, Mackey JR, Haykowsky MJ. Exercise intolerance in anthracycline-treated breast cancer survivors: The role of skeletal muscle bioenergetics, oxygenation, and composition. Oncologist. 2020;25:e852–e860. doi: 10.1634/theoncologist.2019-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reding KW, Brubaker P, D'Agostino R, Kitzman DW, Nicklas B, Langford D, et al. Increased skeletal intermuscular fat is associated with reduced exercise capacity in cancer survivors: A cross-sectional study. Cardiooncology. 2019;5:3. doi: 10.1186/s40959-019-0038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow K, Flewitt JA, Green JD, Pagano JJ, Friedrich MG, Thompson RB. Saturation recovery single-shot acquisition (SASHA) for myocardial T1 mapping. Magn. Reson. Med. 2013;71:2082–2095. doi: 10.1002/mrm.24878. [DOI] [PubMed] [Google Scholar]
- 13.Kirkham AA, Goonasekera MV, Mattiello BC, Grenier JG, Haykowksy MJ, Thompson RB. Reliability and reproducibility of cardiac MRI quantification of peak exercise function with long-axis views. PLoS ONE. 2021;16:e0245912. doi: 10.1371/journal.pone.0245912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernando D, Kellman P, Haldar JP, Liang ZP. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn. Reson. Med. 2010;63:79–90. doi: 10.1002/mrm.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuang ML, Hibberd MG, Salton CJ, Beaudin RA, Riley MF, Parker RA, et al. Importance of imaging method over imaging modality in noninvasive determination of left ventricular volumes and ejection fraction. J. Am. Coll. Cardiol. 2016;35:477–484. doi: 10.1016/S0735-1097(99)00551-3. [DOI] [PubMed] [Google Scholar]
- 16.Mosteller RD. Simplified calculation of body-surface area. N. Engl. J. Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 17.Cotsamire DL, Sullivan MJ, Bashore TM, Leier CV. Position as a variable for cardiovascular responses during exercise. Clin. Cardiol. 1987;10:137–142. doi: 10.1002/clc.4960100302. [DOI] [PubMed] [Google Scholar]
- 18.Charloux A, Lonsdorfer-Wolf E, Lampert RE, Oswald-Mammosser M, Mettauer B, Geny B, et al. A new impedance cardiograph device for the non-invasive evaluation of cardiac output at rest and during exercise: Comparison with the “direct” Fick method. Eur. J. Appl. Physiol. 2000;82:313–320. doi: 10.1007/s004210000226. [DOI] [PubMed] [Google Scholar]
- 19.Godin G, Shephard RJ. Godin leisure-time exercise questionnaire. Med. Sci. Sports Exerc. 1997;29:S36. [Google Scholar]
- 20.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 21.Foulkes SJ, Howden EJ, Bigaran A, Janssens K, Antill Y, Loi S, et al. Persistent impairment in cardiopulmonary fitness after breast cancer chemotherapy. Med. Sci. Sports Exerc. 2019;51:1573–1581. doi: 10.1249/MSS.0000000000001970. [DOI] [PubMed] [Google Scholar]
- 22.Jones LW, Haykowsky M, Peddle CJ, Joy AA, Pituskin EN, Tkachuk LM, et al. Cardiovascular risk profile of patients with her2/neu-positive breast cancer treated with anthracycline-taxane-containing adjuvant chemotherapy and/or trastuzumab. Cancer Epidemiol. Biomarkers Prev. 2007;16:1026–1031. doi: 10.1158/1055-9965.EPI-06-0870. [DOI] [PubMed] [Google Scholar]
- 23.Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M, et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013;99:932–937. doi: 10.1136/heartjnl-2012-303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaudry RI, Samuel TJ, Wang J, Tucker WJ, Haykowsky MJ, Nelson MD. Exercise cardiac magnetic resonance imaging: A feasibility study and meta-analysis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315:R638–645. doi: 10.1152/ajpregu.00158.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cespedes Feliciano EM, Chen WY, Bradshaw PT, Prado CM, Alexeeff S, Albers KB, et al. Adipose tissue distribution and cardiovascular disease risk among breast cancer survivors. J. Clin. Oncol. 2019;37:2528–2536. doi: 10.1200/JCO.19.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Addison O, Marcus RL, LaStayo PC, Ryan AS. Intermuscular fat: A review of the consequences and causes. Int. J. Endocrinol. 2014;2014:309570. doi: 10.1155/2014/309570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neeland IJ, Ross R, Despres JP, Matsuzawa Y, Yamashita S, Shai I, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet. 2019;7:715–725. doi: 10.1016/S2213-8587(19)30084-1. [DOI] [PubMed] [Google Scholar]
- 28.Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2000;2:271–278. doi: 10.3109/10976640009148691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.