Abstract
In many stem cell transplant centres, BCNU, etoposide, cytarabine and melphalan (BEAM) high-dose chemotherapy (HDCT) has been replaced by the more economic and available bendamustine, etoposide, cytarabine, melphalan (BeEAM) regimen. However, there is a paucity of information on the efficacy and safety of BeEAM HDCT. We describe our experience with BeEAM HDCT in terms of safety, efficacy and cost-savings. We compare overall and progression-free survival to a cohort of patients previously transplanted at our institution with the older BEAM regimen. We performed a retrospective chart review of 41 lymphoma patients undergoing BeEAM HDCT at the Royal University Hospital in Saskatoon, Saskatchewan between 2015 and 2019 to elicit regimen safety in the first 100 days post-transplant. Furthermore, we calculated overall and progression-free survival and constructed corresponding Kaplan–Meier curves, comparing the results to a historical cohort of BEAM patients (n = 86). Finally, we conducted an economic analysis using the financials available at our centre’s pharmacy. With regards to BeEAM HDCT, we report a 100-day transplant-related mortality of 2.4%. Additionally, we report acceptable rates of typhlitis (27%), grade III–IV mucositis (4.9%) and grade III–IV nephrotoxicity (2.4%). In terms of overall and progression-free survival, we found no statistical difference between BeEAM and BEAM (p = 0.296; 0.762, respectively). Finally, our economic analysis revealed a net savings of $21,200 CAD per transplant when BeEAM is used in replacement of BEAM. The acceptable safety profile of BeEAM and its comparable efficacy to BEAM are encouraging for the perseverance of this cost-effective HDCT regimen.
Subject terms: Diseases, Medical research, Signs and symptoms, Oncology, Cancer, Stem cells, Adult stem cells, Haematopoietic stem cells, Health care, Health care economics
Introduction
As a result of the ground-breaking PARMA and CORAL trials, high dose chemotherapy (HDCT) and subsequent autologous stem cell transplantation (ASCT) has become standard of care in the treatment of relapsed and chemotherapy-sensitive Non-Hodgkin’s lymphoma (NHL)1,2. Similarly, HDCT and ASCT has proven effective in the treatment of relapsed and resistant Hodgkin’s Lymphoma (HL)3,4. Despite the widespread use of HDCT in stem cell transplantation, the various conditioning protocols have seldomly been compared. Notably, there are limited studies comparing HDCT regimens5. BEAM (BCNU, etoposide, cytarabine and melphalan) conditioning has been the most widely-used conditioning regimen for the past 30 years1. Recently, some centers have discontinued the use of BEAM chemotherapy in favor of BeEAM (bendamustine, etoposide, cytarabine, melphalan) chemotherapy. Additionally, the unavailability of BCNU has prompted a shift to Bendamusine-based regimens6. Additionally, there are concerns over BCNU-related pulmonary toxicity7–11. Unfortunately, there is a paucity of research comparing BEAM and BeEAM conditioning regimens in terms of safety, efficacy and cost-effectiveness.
Bendamustine was first synthesized in the early 1960s in the former German Democratic Republic12. In vitro studies have shown bendamustine to be highly effective in cell lines with dysfunctional apoptotic pathways causing mitotic failure13. A phase I–II trial including 77 patients showed bendamustine-containing chemotherapy to be safe and efficacious in the ASCT setting. Importantly, the authors reported a 100-day transplant-related mortality (TRM) of 0%, and 81% of the trial patients achieved complete remission after a median observation of 18 months12.
Creatinine elevations after bendamusine-containing HDCT administration has led to concerns over renal toxicity. Several studies have documented nephrotoxicity related to BeEAM chemotherapy, ranging from clinically-insignificant creatinine rises to dialysis necessitation6,8,14–17. Interestingly, a study conducted by Noesslinger et al. found that the majority of patients (33/41) experienced a rise in creatinine within a few days of bendamustine administration. Unlike other studies, no therapeutic intervention was required for any of the patients14. However, BeEAM-related nephrotoxicity has been demonstrated to have more severe consequences. For instance, a recent study reported that 10% of patients who were administered BeEAM conditioning developed grade III/IV renal toxicity based on Common Terminology Criteria for Adverse Events (CTCAE) v4.0 guidelines, with one of these patients even requiring dialysis8.
Cohort studies comparing nephrotoxicity in the conditioning regimens has been similarly inconclusive. A cohort study comparing BEAM and BeEAM safety profiles found a significant difference in nephrotoxicity with 48% of the BeEAM cohort experiencing renal impairment, while this toxicity was only noted in 7% of the BEAM cohort (p < 0.001)17. A cohort study published in 2018 found the incidence of nephrotoxicity with BeEAM and BEAM to be 12% and 6%, respectively16. However, all reported cases of renal impairment constituted grade 1 toxicities. A large cohort study conducted by Frankiewicz et al. found no difference in grade 2–4 nephrotoxicity incidence between BeEAM and BEAM with each group demonstrating only a small risk of renal impairment (1.6% and 0.6%, respectively)18.
While there has been concerns over BeEAM-related nephrotoxicity, there is optimism that the regimen has reduced the risk of IPS8,12. This represents a significant advantage of the conditioning regimen, as IPS is associated with a higher rate of TRM and shorter progression-free survival (PFS) and overall survival (OS)9. HDCT regimens containing BCNU have been shown to cause IPS at incidences of 1–64%, thus presenting a significant drawback of BEAM therapy19–21. A hypothesized advantage of BeEAM chemotherapy is a lower risk of IPS. For instance, a recent study conducted by Gilli et al. demonstrated that IPS is a possible, albeit rare, consequence in BeEAM conditioning8.
Current studies have produced widely varying results with regards to the safety and toxicological profile of BeEAM HDCT. Clearly, there is a need to evaluate the safety of BeEAM conditioning, with particular attention to nephrotoxicity and IPS. In this retrospective, single-center study we document our experience with BeEAM conditioning in terms of safety, efficacy and economics.
Materials and methods
Patients
In this single-center, retrospective chart review, we analyzed consecutive patients with Hodgkin’s lymphoma (n = 7) or Non-Hodgkin’s lymphoma (n = 34) who have been treated with BeEAM HDCT followed by ASCT between 2015 and 2019 at the Royal University Hospital in Saskatoon, Canada. These patients were followed and charts were reviewed through the Provincial Hematology and Blood and Bone Marrow Transplant Program at the SCA. All patients signed consent prior to proceeding with ASCT. Furthermore, the BeEAM cohort was compared to a previously studied cohort of patients undergoing BEAM ASCT (n = 86) at the aforementioned institution for the purpose of survival analysis. BEAM transplants occurred between 2009 and 2016, as the BeEAM protocol became standard of care at our institution in 2015. This study was approved by the local ethics committee and the Saskatchewan Cancer Agency in accordance with the relevant guidelines and regulations. Detailed patient characteristics can be found in Table 1.
Table 1.
Patient characteristics.
| Patients | BeEAM | BEAM | p valuea |
|---|---|---|---|
| n = 41 | n = 86 | ||
| Age, mean, SD | 55 (12) | 51 (13) | 0.123 |
| Age groups (n, %) | |||
| ≤ 60 | 24 (58.5) | 61 (70.9) | 0.165 |
| > 60 | 17 (41.5) | 25 (29.1) | |
| Sex (n, %) | |||
| Female | 10 (24.4) | 36 (41.9) | 0.055 |
| Male | 31 (75.6) | 50 (58.1) | |
| Diagnosis (n, %) | |||
| Hodgkin lymphoma/nodular sclerosis | 7 (17.1) | 28 (32.6) | 0.068 |
| All others | 34 (82.9) | 58 (67.4) | |
| Total deaths (n, %) | |||
| Yes | 8 (19.5) | 27 (31.4) | 0.161 |
| No | 33 (80.5) | 59 (68.6) | |
| Deaths 3 years after ASCT (n, %) | |||
| Yes | 8 (19.5) | 18 (20.9) | 0.853 |
| No | 33 (80.5) | 68 (79.1) | |
| Total relapsed patients (n, %) | |||
| Yes | 8 (19.5) | 28 (32.6) | 0.127 |
| No | 33 (80.5) | 58 (67.4) | |
| Relapsed patients after ASCT (n, %) | |||
| Yes | 8 (19.5) | 23 (26.7) | 0.375 |
| No | 33 (80.5) | 63 (73.3) | |
aChi-square test: ASCT autologous stem cell transplantation.
Treatment
BEAM: 300 mg/m2 BCNU was given on day − 6. On days − 5 to − 2, 400 mg/m2 cytarabine was administered every 12 h as a 30-min infusion in 100 mL 0.9% NaCl. On days − 5 to − 2, 200 mg/m2 etoposide was administered once daily as a 120 min infusion in 1000 mL 0.9% NaCl. Melphalan at 140 mg/m2 was administered as a single 60 min infusion in 1000-mL 0.9% NaCl. A minimum of 2.0 × 106 CD34 + cells/kg body weight were reinfused on day 0.
BeEAM: Bendamustine at 200 mg/m2 was given as a single 1-h infusion in 1000 mL in 0.9% NaCl on days − 7 and − 6. On days − 5 to − 2, 200 mg/m2 cytarabine was administered every 12 h as a 30-min infusion in 100 mL 0.9% NaCl. On days − 5 to − 2, 200 mg/m2 etoposide was administered once daily as a 120 min infusion in 1000 mL 0.9% NaCl. Melphalan at 140 mg/m2 was administered as a single 60 min infusion in 1000-mL 0.9% NaCl. A minimum of 2.0 × 106 CD34 + cells/kg body weight were reinfused on day 0.
All patients on BEAM and BeEAM received weight-adapted G-CSF (filgrastim 300mcg if ≤ 70 kg or 480mch if ≥ 70 kg) starting at day + 7 until neutrophils exceeded 1.0 g/L. As per institutional protocol, patients received antiviral (oral valacyclovir 500 mg twice daily) and antifungal prophylaxis (oral fluconazole 200 mg once weekly and oral sulfamethoxazole/ trimethoprim 800/160 mg three times per week). Antibiotic prophylaxis was not used. Patients were transfused with platelets when platelet counts dropped below 10 × 109/L. Patients received red cell transfusions at a hemoglobin threshold of 70 g/L. Patients were hospitalized at the initiation of HDCT and remained in-hospital until hematologic and adequate performance status was achieved.
Measurements and definitions
Nephrotoxicity was defined according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 guidelines. The institutional upper limit of normal for creatinine (104 µmol/L) was used to define recovery at discharge. Typhlitis was only reported if it was confirmed via CT assessment. OS was defined as the time from ASCT to death. PFS was defined as time from ASCT to first relapse/progression, death, or last follow-up, whichever occurred first. Neutrophil engraftment was defined as the first day of three consecutive days where the neutrophil count (absolute neutrophil count) was 500 cells/mm3 (0.5 × 109/L) or greater. Platelet engraftment was defined as a platelet count of 20,000/mm3 (20 × 109/L) unsupported by a platelet transfusion (> 3 days post-transfusion).
Statistical analysis
Descriptive analysis was performed to summarize the data. Mean and standard deviation estimates of each continuous variable were calculated separately and compared between HDCT regimens using Student’s t tests. For categorical variables, proportion estimates were calculated separately and compared between BEAM and BeEAM using a χ2 or Fischer’s exact test when appropriate. The primary endpoints analyzed were progression free survival (PFS) and overall survival (OS). We used the Kaplan–Meier method to understand the survival distribution and to produce survival curves for PFS and OS for both HDCT regimens. Then, we compared the survival distributions between the two groups to determine equivalency using log-rank test. Univariable and multivariate Cox regression models were used to analyze each HDCT and multiple risk factors for OS and PFS separately. The results are given with a hazard rate (HR), confidence interval (CI) of 95% and p value. Statistical analyses and associated figures were generated with the Statistical Package for the Social Sciences (SPSS) Version 26 (SPSS Inc. Armonk, NY: IBM Corp.). Statistical significance was defined by an alpha level of p ≤ 0.05.
Economical analysis
Our economic analysis was based on comparison of the list pharmaceutical costs. Only the direct cost of chemotherapy agents was analyzed, and ancillary costs were not factored in the analysis.
Ethics approval
The following study was approved by the local ethics committee at the Saskatchewan Cancer Agency.
Consent to participate
Data transfer agreements were signed with the Saskatchewan Cancer Agency.
Consent for publication
Approval for publication was granted by the Saskatchewan Cancer Agency. The results and conclusions presented here represent the work of the authors and no endorsement by the SCA or by any third party is to be inferred.
Results
Engraftment, transfusions and hospitalization (BeEAM patients)
The median time to platelet engraftment was 12.6 days (range: 7–19 days), whereas median time for neutrophil engraftment was 11.7 days (9–15 days) for BeEAM HDCT patients. The median number of platelet transfusions was 4.3 units (range: 2–9 units) per admission for BeEAM ASCT. The median number of packed red blood cell (pRBC) transfusion units was 2.3 units (range: 0–5 units). Interestingly, 53.7% of BeEAM patients required less than two pRBC transfusions. The median number of days of admission to hospital for BeEAM transplantation was 24.2 days (range: 18–33 days) (Table 2).
Table 2.
BeEAM toxicities.
| Patients | BeEAM |
|---|---|
| n = 41 | |
| Death prior to Day 100 | 2.4% (n = 1) |
| Gastrointestinal toxicity | |
| Mucositis | 88% (n = 36) |
| Grade I–II | n = 34 |
| Grade III–IV | n = 2 |
| Typhlitis | 27% (n = 11) |
| Renal toxicity | |
| All grades | 32% (n = 13) |
| Grade I–II | n = 12 |
| Grade III–IV | n = 1 |
| Cardiac toxicity | |
| New onset atrial fibrillation | 15% (n = 6) |
| Myocardial infarction | 2.4% (n = 1) |
| Infectious complications | |
| Febrile neutropenia | 100% (n = 41) |
| Septic shock | 12.2% (n = 5) |
| ICU admissions | 2.4% (n = 1) |
| Bacterial infections | 56% (n = 23) |
| Bacteria gram + ve (%) | 66.7% |
| Bacteria gram − ve (%) | 33.3% |
| Viral infections | 14.6 (n = 6) |
| Fungal infections | 2.4% (n = 1) |
| No organism identified | 29.3% (n = 12) |
| Transfusion and engraftment | |
| Median days to platelet engraftment | 12.6 (7–19) |
| Median days to neutrophil engraftment | 11.7 (9–15) |
| Median platelet transfusions | 4.3 (2–9) |
| Median red blood cell transfusions | 2.3 (0–5) |
| Hospitalization | |
| Median days in hospital | 24.2 (18–33) |
Toxicities (BeEAM patients only)
Thirteen BeEAM patients (32%) experienced nephrotoxicity according to CTCAE v5.0 guidelines. Twelve of these patients experienced grade I–II toxicity, while one patient suffered grade III renal toxicity. Mean baseline creatinine for patients experiencing nephrotoxicity was 87.6 µmol/L (range: 51–128 µmol/L), compared to 76.5 µmol/L (range: 38–157 µmol/L) in patients who did not experience nephrotoxicity. All patients were treated expectantly with no patient requiring dialysis. Interestingly, nine of these thirteen patients had elevated creatinine levels at discharge, indicating some persistence of renal impairment. Thirty-six patients (88%) experienced documented mucositis. Thirty-four of these patients suffered grade I-II mucositis, whereas two patients experienced grade III mucositis. Eleven patients (27%) experienced CT-confirmed typhlitis, and all patients were treated with IV antibiotics and bowel rest. Six patients (15%) experienced new-onset atrial fibrillation during the course of their admission for ASCT (Table 2).
Infections (BeEAM patients)
All patients undergoing BeEAM ASCT experienced at least one episode of febrile neutropenia. No organism was identified in twelve (29.3%) of these aforementioned patients. 23 patients had a documented bacterial infection. Among documented bacterial infections, 66.7% of isolated species were gram positive and 33.3% were gram negative species. Six patients (14.6%) had documented viral infections. Five of these viral infections were with common respiratory viruses (rhinovirus, coronavirus, parainfluenza) and one patient experienced cystitis related to BK virus reactivation. One patient (2.4%) experienced a fungal infection in the form of aspergillus. Five patients (12.2%) experienced septic shock, with one of these patients being admitted to the ICU and another requiring consultation from ICU. No patients died from infectious complications during ASCT (Table 2).
Survival
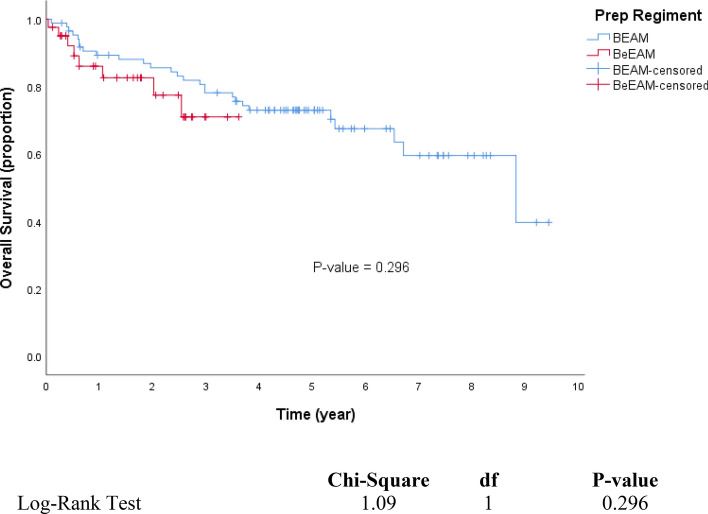
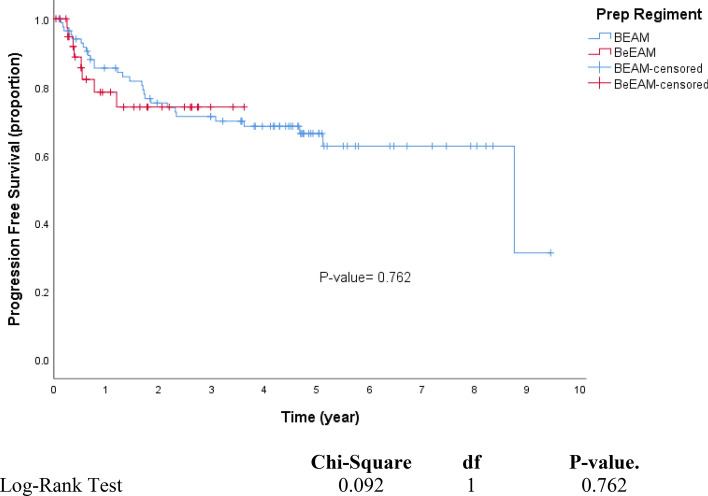
One sudden death occurred in the BeEAM cohort. This death occurred on day + 14 from stem cell infusion and was felt to be due to a cardiac arrest in a patient with known cardiac disease, yielding a TRM of 2.4%. The 3-year OS was 78.1% for BEAM, and 71.0% for BeEAM. The 3-year PFS was 71.3% for BEAM, and 74.1% for BeEAM. Our 3-year calculation of OS and PFS showed no significant differences between the two HDCT regimens (Table 3). Interestingly, multivariate analysis of covariates such as age, sex and diagnosis yielded no significant effect on OS or PS at 3-year follow-up. Multivariate analysis revealed no significant difference in OS or PFS between the two conditioning regimens (p = 0.372, 0.801; respectively) (Tables 4, 5).
Table 3.
Probability of 3-year survival.
| Number of patients | Overall survival (OS) | Progression free survival (PFS) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 3-Year probability (%) | 95% CI | p value | 3-Year probability (%) | 95% CI | p value | ||||
| Lower | Upper | Lower | Upper | ||||||
| Whole cohort | 127 | 76 | 67.8 | 84.2 | – | 71 | 62.2 | 79.8 | – |
| BEAM | 86 | 78.1 | 69.08 | 87.12 | 0.296 | 71.3 | 61.3 | 81.3 | 0.762 |
| BeEAM | 41 | 71 | 52.4 | 89.6 | 74.1 | 58.2 | 90.0 | ||
| Age | |||||||||
| ≤ 60 | 85 | 75.3 | 65.5 | 85.1 | 0.718 | 68.1 | 57.1 | 79.1 | 0.433 |
| > 60 | 42 | 76.6 | 61.1 | 92.1 | 77.3 | 63.2 | 91.4 | ||
| Sex | |||||||||
| Female | 46 | 76.3 | 63.4 | 89.2 | 0.815 | 68.5 | 54.2 | 82.8 | 0.953 |
| Male | 81 | 75.6 | 65.0 | 86.2 | 72.6 | 61.6 | 83.6 | ||
| Dx | |||||||||
| Hodgkin lymphoma/nodular sclerosis | 92 | 71.9 | 61.5 | 82.3 | 0.267 | 70.5 | 59.9 | 81.1 | 0.671 |
| All others | 35 | 84.7 | 72.4 | 97.0 | 71.4 | 55.5 | 87.3 | ||
Table 4.
Overall survival: multivariate Cox regression model.
| Time to death | HR | 95% CI | p value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Regimen | ||||
| BEAM | ||||
| BeEAM | 1.483 | 0.625 | 3.518 | 0.372 |
| Age | ||||
| ≤ 60 | ||||
| > 60 | 0.733 | 0.33 | 1.63 | 0.446 |
| Sex | ||||
| Female | ||||
| Male | 0.995 | 0.494 | 2.001 | 0.988 |
| Dx | ||||
| All others | ||||
| Hodgkin lymphoma/nodular sclerosis | 0.603 | 0.262 | 1.391 | 0.236 |
Table 5.
Progression-free survival: multivariate Cox regression model.
| Time to Relapse | HR | 95% CI | p value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Regimen | ||||
| BEAM | ||||
| BeEAM | 1.114 | 0.482 | 2.574 | 0.801 |
| Age | ||||
| ≤ 60 | ||||
| > 60 | 0.677 | 0.306 | 1.494 | 0.334 |
| Sex | ||||
| Female | ||||
| Male | 0.942 | 0.472 | 1.877 | 0.864 |
| Dx | ||||
| All others | ||||
| Hodgkin lymphoma/nodular sclerosis | 0.756 | 0.34 | 1.684 | 0.494 |
Economic analysis
Our economic analysis, which used local pharmacy data, revealed tremendous cost benefits of BeEAM HDCT in comparison to BEAM HDCT. At our center in Saskatchewan, Canada, the cost of a BeEAM ASCT is $12,181 CAD, whereas the cost of a BEAM ASCT is $33,381 CAD. This equates to a net savings of $21,200 CAD per transplant. The introduction of BeEAM at our centre in 2015 has resulted in a total savings of approximately $890,000 CAD from 2015 to 2019.
Discussion
There is a paucity of research comparing HDCT regimens in the context of stem cell transplantation. The present study depicts our institution’s experience with BeEAM chemotherapy in terms of toxicity, efficacy and cost in reference to the previous BEAM regimen. To our knowledge, it is the first study to explore both the clinical and financial considerations associated with this widespread HDCT transition. This single-centre study suggests several reasons for the perseverance of BeEAM as the conditioning regimen of choice at our institution.
Our study found an incidence of grade I–IV renal toxicity of 32% in the BeEAM HDCT cohort. This is consistent with previous retrospective studies, however the incidence of nephrotoxicity varies widely in the literature from 1.6 to 48%6,15–18. The incidence of BeEAM nephrotoxicity reported here is higher than that reported for BEAM in previous cohort studies16–18. However, the significance of our findings should be interpreted cautiously as all patients with renal impairment were treated expectantly and renal function did not delay the HDCT/ASCT regimen for any patient followed. Additionally, it has been reported in the literature that renal insufficiency during ASCT does not impact hematopoietic stem cell collection, affect mucositis incidence, delay engraftment or increase transfusion requirements22,23. Also, the renal toxicity experienced by ASCT patients may not be fully imputable to bendamustine, as patients undergoing ASCT are frequently exposed to other nephrotoxic agents such as vancomycin. Despite this, precautions such as the provision of adequate hydration and the avoidance of bendamustine dose escalations beyond 200 mg/m2/day are generally recommended14,16.
The prevalence of oral mucositis amongst BeEAM patients was 88%, which is concordant with other studies6,8,10. Interestingly, we report relatively low rates of grade III–IV oral mucositis, with only 2 patients experiencing grade III toxicity, whereas a recent study conducted by Visani et al. reported that approximately 25% of patients receiving BeEAM HDCT experienced grade III–IV mucositis12. Another study reported the incidence of grade III–IV oral mucositis to be 42.9% in NHL patients receiving the older BEAM chemotherapy24. Thus, these results are suggestive of a favourable toxicological profile with regards to oral mucositis. Interestingly, our institutional mucositis protocol only involves club soda mouthwashes four times a day.
Typhlitis is an important toxicological consideration with associated mortality rates as high as 50% reported in the literature25. Eleven patients (27%) developed CT-confirmed typhlitis in the present study. Diagnostic criteria and incidences of typhlitis vary amongst comparable studies with incidences of 17–35% reported in the literature6,12,17. Cohort studies comparing the digestive toxicity of BeEAM and BEAM have also garnered mixed results16–18. None of the patients who developed typhlitis required surgery. We suspect typhlitis rates are high at our centre as we do not employ prophylactic antibiotics, due to hospital antibiotic stewardship measures.
Additionally, we report 6 cases of new-onset atrial fibrillation within our BeEAM cohort. This finding is congruous with previous studies which report a small to moderate risk of BeEAM-associated cardiotoxicity6,8,14. Additionally, one cardiac-related death was reported in the present study. All cases of new-onset atrial fibrillation were managed conventionally with beta-blockers. It is important to note that cardiotoxicity is not exclusive to the BeEAM conditioning regimen. For instance, a large cohort study conducted by Robinson et al. reported 3 cases of fatal cardiac toxicity associated with BEAM chemotherapy26.
Within our small BeEAM cohort, 56% of patients experienced a culture-positive bacterial infection. However, this is consistent with previous studies which report BeEAM-associated bacteremia rates ranging from 24 to 64%8,16,17. While no patient in our BeEAM cohort died of infectious complications, one patient did require treatment in the ICU. It is important to note that cohort studies comparing the toxicity of BeEAM and BEAM have not yielded significant differences between the conditioning regimens in terms of bacteremia rates16,17.
No patients in our BeEAM cohort developed IPS, which is a dreaded transplant complication. This may reflect a potential advantage over BCNU chemotherapies which carry a known risk of IPS19–21. However, the research remains uncertain as to the true risk of BCNU with regards to IPS. A recent study, for instance, found that BCNU doses of 300 mg/m2 were only associated with a 0.7% incidence of pulmonary toxicity27. Thus, the cost utility of mitigating IPS is unknown at this time.
We constructed Kaplan–Meier curves comparing OS and PFS in our BeEAM cohort and a historical BEAM cohort from our centre (Figs. 1, 2). We found no difference in OS and PFS between the conditioning regimens in multivariate analysis (p = 0.372, 0.801; respectively). This is consistent with other cohort studies16–18. We report a 100-day TRM of 2.4% in our BeEAM cohort, indicating acceptable safety and efficacy of the conditioning regimen. Interestingly, our multivariate analysis did not yield any significant differences with respect to age, sex or diagnosis of the patient undergoing transplantation. Perhaps this is indicative of widespread acceptability of the HDCT.
Figure 1.
Kaplan–Meier analysis of overall survival. Overall survival (OS) in patients who underwent an ASCT with BEAM or BeEAM. ASCT autologous stem cell transplantation, BEAM BCNU, etoposide, cytarabine, melphalan, BeEAM bendamustine, etoposide, cytarabine, melphalan. p values were determined using the log-rank test.
Figure 2.
Kaplan–Meier analysis of progression-free survival. Progression-free survival in patients who underwent an ASCT with BEAM or BeEAM. ASCT autologous stem cell transplantation, BEAM BCNU, etoposide, cytarabine, melphalan, BeEAM bendamustine, etoposide, cytarabine, melphalan. p values were determined using the log-rank test.
Unfortunately, the present study is unable to directly compare the toxicological profiles of each HDCT regimen, as we do not presently have access to acute transplant toxicity data for the BEAM cohort. Another disadvantage of the present study is a relatively short duration of follow-up associated with BeEAM HDCT due to its recent enrolment at our centre. Furthermore, our cost analysis was limited to the raw cost of the chemotherapies at our institution. Therefore, ancillary costs related to administration of the HDCTs were not included but not expected to be significantly different between cohorts.
Our data confirms that BeEAM chemotherapy has adequate PFS and OS, an acceptable safety profile and marked cost-savings in comparison to BEAM chemotherapy. Based on this study, BeEAM chemotherapy will remain standard of care at our institution. Our centre’s experience with the BeEAM regimen suggests that the small risk of often clinically-insignificant nephrotoxicity is offset by tremendous cost-effectiveness. However, larger, prospective trials are necessary to fully elucidate the benefits and risks of BeEAM chemotherapy.
Acknowledgements
We would like to thank our patients and the Saskatchewan Cancer Agency for their assistance. Also, we wish to thank the University of Saskatchewan College of Medicine’s Dean’s project program for fostering collaboration between the authors. Additionally, we would like to thank Ambika Chandrasekhar for her assistance in editing the paper.
Author contributions
L.H. and M.B. designed and wrote the paper. H.L. performed all statistical analyses. T.D. and P.D. assisted with data collection. All authors reviewed and approved the paper.
Funding
No funding sources to disclose.
Data availability
Not applicable.
Code availability
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Philip T, Guglielmi C, Hagenbeek A, Somers R, Van Der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N. Engl. J. Med. 1995;333(23):1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 2.Gisselbrecht C, Glass B, Mounier N, Gill DS, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J. Clin. Oncol. 2010;28(27):4184. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linch D, Goldstone AH, McMillan A, Chopra R, Hudson GV, Winfield D, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: Results of a BNLI randomised trial. Lancet. 1993;341(8852):1051–1054. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: A randomised trial. Lancet. 2002;359(9323):2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 5.Colita A, Colita A, Bumbea H, Croitoru A, Orban C, Lipan LE, et al. LEAM vs. BEAM vs. CLV conditioning regimen for autologous stem cell transplantation in malignant lymphomas. Retrospective comparison of toxicity and efficacy on 222 patients in the first 100 days after transplant, on behalf of the Romanian society for bone marrow transplantation. Front. Oncol. 2019;9:892. doi: 10.3389/fonc.2019.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chantepie S, Tchernonog E, Peyrade F, Larcher MV, Diouf M, Fornecker LM, et al. Bendamustine-based (BeEAM) conditioning before autologous stem cell transplantation: Result of a French multicenter study of 386 patients from Lysa Centers. Blood. 2016;128(22):3450. doi: 10.1182/blood.V128.22.3450.3450. [DOI] [Google Scholar]
- 7.Durant JR, Norgard MJ, Murad TM, Bartolucci AA, Langford KH. Pulmonary toxicity associated with bischloroethylnitrosourea (BCNU) Ann. Intern. Med. 1979;90:191–194. doi: 10.7326/0003-4819-90-2-191. [DOI] [PubMed] [Google Scholar]
- 8.Gilli S, Novak U, Taleghani BM, Baerlocher GM, Leibundgut K, Banz Y. BeEAM conditioning with bendamustine-replacing BCNU before autologous transplantation is safe and effective in lymphoma patients. Ann. Hematol. 2017;96:421–429. doi: 10.1007/s00277-016-2900-y. [DOI] [PubMed] [Google Scholar]
- 9.Chen YB, Lane AA, Logan BR, Zhu X, Akpek G, Aljurf MD, et al. Impact of conditioning regimen on outcomes for patients with lymphoma undergoing high-dose therapy with autologous hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2015;21(6):1046–1053. doi: 10.1016/j.bbmt.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caballero MD, Rubio V, Rifon J, Heras I, Garcia-Sanz R, Vazquez L, et al. BEAM chemotherapy followed by autologous stem cell support in lymphoma patients: Analysis of efficacy, toxicity and prognostic factors. Bone Marrow Transplant. 1997;20(6):451–458. doi: 10.1038/sj.bmt.1700913. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia S, Robison LL, Francisco L, Carter A, Liu Y, Grant M, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: Report from the bone marrow transplant survivor study. Blood. 2005;105(11):4215–4222. doi: 10.1182/blood-2005-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visani G, Malerba L, Stefani PM, Capria S, Galieni P, Gaudio F, et al. BeEAM (bendamustine, etoposide, cytarabine, melphalan) before autologous stem cell transplantation is safe and effective for resistant/relapsed lymphoma patients. Blood. 2011;118(12):3419–3425. doi: 10.1182/blood-2011-04-351924. [DOI] [PubMed] [Google Scholar]
- 13.Leoni LM, Bailey B, Reifert J, Bendall HH, Zeller RW, Corbeil J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin. Cancer Res. 2008;14(1):309–317. doi: 10.1158/1078-0432.CCR-07-1061. [DOI] [PubMed] [Google Scholar]
- 14.Noesslinger T, Panny M, Simanek R, Moestl M, Boehm A, Menschel E, et al. High-dose Bendamustine-EAM followed by autologous stem cell rescue results in long-term remission rates in lymphoma patients, without renal toxicity. Eur. J. Haematol. 2018;101(3):326–331. doi: 10.1111/ejh.13102. [DOI] [PubMed] [Google Scholar]
- 15.Ribrag V, Saleh K, Danu A, Koscielny S, Pilorge S, Castilla-Lorente C, et al. BEAM or BeEAM high-dose chemotherapy followed by ASCT: A single center comparative analysis of toxicity. Blood. 2016;128(22):4648. doi: 10.1182/blood.V128.22.4648.4648. [DOI] [Google Scholar]
- 16.Saleh K, Danu A, Koscielny S, Legoupil C, Pilorge S, Castilla-Llorente C, et al. A retrospective, matched paired analysis comparing bendamustine containing BeEAM versus BEAM conditioning regimen: Results from a single center experience. Leuk. Lymphoma. 2018;59(11):2580–2587. doi: 10.1080/10428194.2017.1403019. [DOI] [PubMed] [Google Scholar]
- 17.Garciaz S, Coso D, De Collela JS, Broussais F, Stoppa AM, Aurran T, et al. Bendamustine-based conditioning for non-Hodgkin lymphoma autologous transplantation: An increasing risk of renal toxicity. Bone Marrow Transplant. 2016;51(2):319–321. doi: 10.1038/bmt.2015.257. [DOI] [PubMed] [Google Scholar]
- 18.Frankiewicz A, Saduś-Wojciechowska M, Najda J, Czerw T, Mendrek W, Sobczyk-Kruszelnicka M, et al. Comparable safety profile of BeEAM (bendamustine, etoposide, cytarabine, melphalan) and BEAM (carmustine, etoposide, cytarabine, melphalan) as conditioning before autologous haematopoietic cell transplantation. Contemp. Oncol. 2018;22(2):113–117. doi: 10.5114/wo.2018.77046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane AA, Armand P, Feng Y, Neuberg DS, Abramson JS, Brown JR, et al. Risk factors for development of pneumonitis after high-dose chemotherapy with cyclophosphamide, BCNU and etoposide followed by autologous stem cell transplant. Leuk. Lymphoma. 2012;53(6):1130–1136. doi: 10.3109/10428194.2011.645208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afessa B, Abdulai RM, Kremers WK, Hogan WJ, Litzow MR, Peters SG. Risk factors and outcome of pulmonary complications after autologous hematopoietic stem cell transplant. Chest. 2012;141(2):442–450. doi: 10.1378/chest.10-2889. [DOI] [PubMed] [Google Scholar]
- 21.Till BG, Madtes DK. BCNU-associated pneumonitis: portrait of a toxicity. Leuk. lymphoma. 2012;53(6):1019–1020. doi: 10.3109/10428194.2011.654341. [DOI] [PubMed] [Google Scholar]
- 22.Farnault L, Venton G, Pourroy B, Jourde-Chiche N, Ivanov V, Arcani R, et al. Severe renal insufficiency is not an absolute pitfall to autologous stem cell transplantation with BeEAM (bendamustine, etoposide, cytarabine, melphalan) conditioning regimen. Bone Marrow Transplant. 2019;54(7):1173–1175. doi: 10.1038/s41409-019-0467-7. [DOI] [PubMed] [Google Scholar]
- 23.Tricot G, Alberts DS, Johnson C, Roe DJ, Dorr RT, Bracy D, et al. Safety of autotransplants with high-dose melphalan in renal failure: a pharmacokinetic and toxicity study. Clin. Cancer Res. 1996;2:947–952. [PubMed] [Google Scholar]
- 24.Jo JC, Kang BW, Jang G, Sym SJ, Lee SS, Koo JE, et al. BEAC or BEAM high-dose chemotherapy followed by autologous stem cell transplantation in non-Hodgkin’s lymphoma patients: Comparative analysis of efficacy and toxicity. Ann. Hematol. 2008;87(1):43–48. doi: 10.1007/s00277-007-0360-0. [DOI] [PubMed] [Google Scholar]
- 25.Ullery BW, Pieracci FM, Rodney JR, Barie PS. Neutropenic enterocolitis. Surg. Infect. 2009;10(3):307–314. doi: 10.1089/sur.2008.061. [DOI] [PubMed] [Google Scholar]
- 26.Robinson SP, Boumendil A, Finel H, Dreger P, Sureda A, Hermine O, et al. High-dose therapy with BEAC conditioning compared to BEAM conditioning prior to autologous stem cell transplantation for non-Hodgkin lymphoma: no differences in toxicity or outcome. A matched-control study of the EBMT-Lymphoma Working Party. Bone Marrow Transplant. 2018;53(12):1553–1559. doi: 10.1038/s41409-018-0196-3. [DOI] [PubMed] [Google Scholar]
- 27.Olivieri J, Mosna F, Pelosini M, Fama A, Rattotti S, Giannoccaro M, et al. A comparison of the conditioning regimens BEAM and FEAM for autologous hematopoietic stem cell transplantation in lymphoma: An observational study on 1038 patients from Fondazione Italiana Linfomi. Biol. Blood Marrow Transplant. 2018;24(9):1814–1822. doi: 10.1016/j.bbmt.2018.05.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.