Abstract
Mild traumatic brain injuries can have long-term consequences that interfere with the life of the patient and impose a burden on our health care system. Oxidative stress has been identified as a contributing factor for the progression of neurodegeneration following TBI. A major source of oxidative stress for many veterans is cigarette smoking and second-hand smoke, which has been shown to have an effect on TBI recovery. To examine the potential influences of second-hand smoke during recovery from TBI, we utilized a mouse model of closed head injury, followed by repeated exposure to cigarette smoke and treatment with a neuroprotective antioxidant. We found that neither the mild injuries nor the smoke exposure produced axonal damage detectable with amino cupric silver staining. However, complexity in the dendritic arbors was significantly reduced after mild TBI plus smoke exposure. In the hippocampus, there were astrocytic responses, including Cyp2e1 upregulation, after the injury and tobacco smoke insult. This study provides useful context for the importance of lifestyle changes, such as reducing or eliminating cigarette smoking, during recovery from TBI.
Keywords: Traumatic brain injury, Cigarette smoke, Oxidative stress, Mouse models, Astrocytes, Dendritic arbor
Introduction
Mild traumatic brain injuries (mTBIs) have been experienced by at least 15% of recently deployed military personnel, and the worldwide prevalence is approximately 0.5% per year (Hoge et al. 2008). Though the majority of TBIs are mild, even mild injuries can have long-term consequences that interfere with the life of the patient and impose a burden on our health care system. Neurodegeneration associated with mild TBI involves a variety of factors within the brain. We have previously identified inflammatory regulatory factors, including transcription factor Nrf2, as factors in the consequences of mild TBI (Hatic et al. 2012). Additionally, TBI results in increased reactive oxygen species, reactive gliosis, and activation of proinflammatory cytokines (Burda et al. 2016; Siedler et al. 2014). Oxidative stress has been identified as having a role in the progression of neurodegeneration in a mouse model of TBI (Tweedie et al. 2013).
Veterans are also disproportionately likely to be cigarette smokers when compared to the general population within the USA. Studies show that veterans of recent conflicts in Iraq and Afghanistan are twice as likely as other Americans to be cigarette smokers (Bastian and Sherman 2010; Brown 2010). This has led to significant interest from the US Department of Veterans Affairs in smoking cessation (Sherman 2008). Oxidative stress is known to be produced by cigarette smoking or exposure to second-hand cigarette smoke. Studies have shown neurogenerative effects of smoking in mouse models (Ho et al. 2012; Speck et al. 2011) and human studies (Cho et al. 2016; Yu et al. 2016). Cigarette smoking has also been shown to be correlated with reduced neurocognitive recovery following mTBI (Durazzo et al. 2013). There is, however, little known about how oxidative stress from cigarette smoking impacts TBI recovery on a cellular and molecular level and how we may be able to ameliorate these negative effects.
Our study utilized a non-invasive weight drop mouse model of closed head injury which accurately recapitulates the rotational forces, neuropathology, and cognitive deficits that are observed in TBI patients (Milman et al. 2005; Zohar et al. 2003). This model produces a mild injury that produces axonal, dendritic, and cell body changes as well as apoptotic and necrotic damage to neurons without non-specific brain edema (Saykally et al. 2012,2018; Tashlykov et al. 2007; Tweedie et al. 2007). Following this injury, we exposed mice to sidestream cigarette smoke using a custom-built apparatus (Huang et al. 2013,2009). With this model, we have been able to identify changes among the neurons and astrocytes of the hippocampus, associated with TBI and smoke exposure. Additionally, we have tested the impact of treatment with an antioxidant, tert-butylhydroquinone (tBHQ). tBHQ is a common food additive which activates the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) transcription factor and has been shown in previous studies to improve cognitive performance, as well as dendritic length and branching, in mouse models of TBI (Ratliff et al. 2020a,c; Saykally et al. 2012).
Inflammatory responses are certainly known with TBI and with smoke insults (Adhami et al. 2017) and subsets of astrocytes will exhibit the upregulated cytochrome P450 family member, Cyp2e1, involved in a variety of processes including oxidative stress. Cyp2e1 has been implicated as influencing neuroplasticity. Expression of cytochrome P450 (CYP) has been shown to correlate with rodent cognitive skills in hippocampal-dependent learning tasks (Gjota-Ergin et al. 2018). In soldiers exposed to blast during training, methylation of the CYP2E1 gene region and gene expression in serum were responsive to the blast exposure (Wang et al. 2020). Therefore, we also examined Cyp2e1 after the TBI and smoke exposure insults.
The goal of this study was to provide an improved understanding of the changes in the brain which occur as a result of TBI and how an additional insult (i.e. exposure to cigarette smoke) during the recovery period could impact the initial molecular response to injury and the eventual recovery. This study provides a context for the importance of lifestyle factors on traumatic brain injury recovery and whether increasing antioxidant signaling affects plasticity following injury. While traumatic brain injuries will not be fully preventable, understanding the molecular processes which occur following TBI and other insults can help us to develop treatments and identify lifestyle changes which may improve the overall prognosis.
Experimental Procedures
Animals
The animal protocol was approved by the Bay Pines VA Institutional Animal Care and Use Committee (IACUC) and performed in accordance with all institutional, agency, and governmental Animal Welfare Regulations, as previously described (Ratliff et al. 2019). Male CD-1 mice at 3 months of age and weighing between 31 and 34 g were obtained from Harlan Laboratories (Indianapolis, IN). They were housed 3–4 per cage in a 22 °C ± 0.5 °C temperature-controlled environment with a 12 h light/dark cycle. Food and water were available ad libitum and health checks were performed daily with weights taken weekly at a minimum. Power analysis (to 90%) was used to determine sample sizes for experimental and control groups. Animals were randomly assigned to numbered cages by VMU staff without input from the investigators. Each numbered cage was assigned to a group prior to investigators handling the mice.
Closed Head Injury
Mild Traumatic Brain Injury (mTBI) was induced using a concussive closed head injury weight drop model as described previously (Zohar et al. 2003). Briefly, mice were anesthetized with isoflurane and placed on a sponge, which allows for rotation of the head to inflict a diffuse concussive injury (Milman et al. 2005). The injury apparatus was positioned directly over the head such that the inner diameter of the tube (13 mm) spanned the right hemisphere caudal to the eye and rostral to the ear. A 50 g cylindrical weight was dropped 80 cm through the length of the tube in injured mice. Sham mice did not receive weight drop portion of procedure. Mice were allowed to recover from anesthesia in a heated chamber.
Sidestream Smoke Exposure
Mice were exposed to sidestream smoke three times per day, 4 h apart, for 30 min beginning 4 h post-injury. Mice were exposed to smoke daily for 3 to 14 days on the day of and following injury (Fig. 1a). To expose mice to sidestream smoke, mice were placed into the mouse compartments within the exposure chamber of the smoking apparatus (Fig. 1b). The smoking apparatus was a custom-built unit (TE-2; Teague Enterprises, Davis, CA) which has been detailed previously (Huang et al. 2009). Next, two cigarettes were placed in the holders within the cigarette chamber. Cigarettes (3R4F) were obtained from the Reference Cigarette Program, Tobacco Health Research Institute, University of Kentucky (Lexington, KY). The machine was then turned on and the first cigarette was lit. The second cigarette was lit after 5 min. The machine alternates “puffs” from each cigarette every 60 s. Each cigarette lasted approximately 10–12 min and cigarettes were replaced as they were finished. Four cigarettes were used per session, for a total of approximately 30 min of smoke exposure. Total particulate matter produced in the smoke by each cigarette was 11 mg, and this was consistent with particulates collected on filters in tests that we performed with fixed volumes from the chamber. Sham mice were placed adjacent to the machine to receive exposure to machine noise without receiving smoke exposure.
Fig. 1.
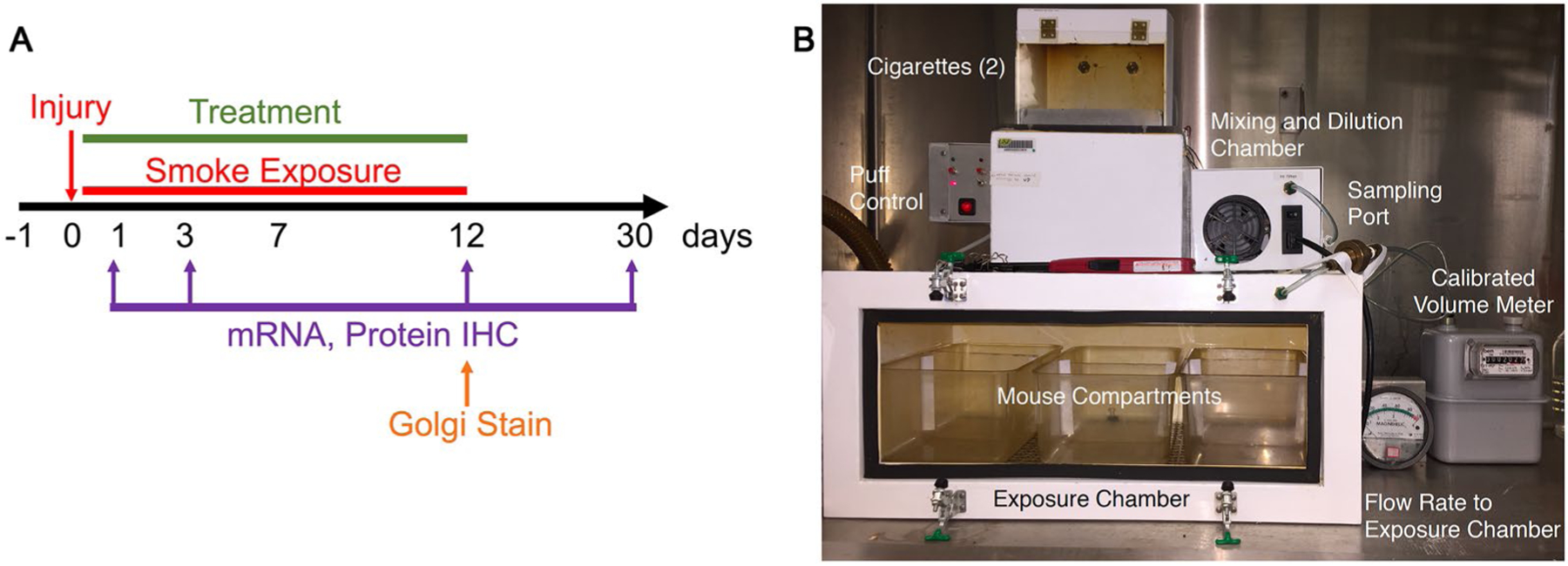
Mouse model of sidestream smoke exposure following mTBI. a Mice received a single closed head injury followed by up to 12 days of exposure to second-hand smoke and tBHQ treatment. Assays were performed at 1, 3, 12, and 30 days post-injury. b To expose the mice to second-hand smoke, they were placed inside the exposure chamber of the smoking apparatus. Cigarettes are loaded into the apparatus and “smoked” using the puff controller with smoke then being sent into the exposure chamber. Each smoking session lasted approximately 30 min and used 4 cigarettes. Sham mice were placed next to the apparatus for exposure to noise without being exposed to smoke
Treatment
Mice were treated via intraperitoneal injection of tert-butylhydroquinone (tBHQ). To create the solution, 100 mg tBHQ powder was dissolved in 1 mL DMSO to create a 100 mg stock solution. This stock solution was then diluted in 0.9% saline to create a treatment solution of 5 mg/mL tBHQ. Volume of solution delivered varied based on mouse weight and was approximately 200–230 μL for an average mouse. Total dosage was 33.4 mg/kg/day for each mouse receiving treatment. Control mice received an equivalent volume of vehicle solution of 5% DMSO in 0.9% saline. Solutions were made fresh daily. Injections occurred on each smoking day, approximately 1 h after the first smoking session.
Golgi Stain
Golgi Stain was performed as described previously (Ratliff et al. 2020b). Mice were perfused with phosphate buffered saline followed by 4% neutral buffered formalin. Brains were removed and then placed in 10% formalin at 4 °C overnight. Brains were then cryoprotected in 15% sucrose and incubated at room temperature for an additional 24 h. The samples were then incorporated into formalin-fixed tissue blocks (2–3 mm thick in the coronal plane) and stained using the Rapid Golgi method. To do this, fixed tissue blocks were placed in potassium dichromate and osmium tetroxide for approximately 6 days, and then transferred to 0.75% silver nitrate for approximately 40 h. The blocks were then dehydrated using increasing concentrations of alcohol solutions and ethyl ether. Blocks were then infiltrated with increasing concentrations of nitrocellulose solutions (5%, 10%, 20%, 30%; 1–2 days each). Next, blocks were placed in plastic molds and hardened using chloroform vapors. Tissue sections were cut to a thickness of 120 microns in the coronal plane using an AO sliding microtome, cleared in alpha-terpineol, rinsed with xylene, and mounted on slides using Permount. Strict selection criteria were used to select neurons for analysis and all selection and analysis was performed by the same examiner. Neurons must have been well impregnated, with branches unobscured by other neurons, glia, blood vessels, or undefined precipitate (a staining by-product). Additionally, the soma must have been located within the middle third of the section. A Zeiss brightfield microscope with long-working distance oil-immersion objective lessee and drawing tubes was used to prepare camera lucida drawings for analysis.
Dendritic arbors were analyzed using two methods: the Sholl Analysis, which defines the amount and distribution of the dendritic arbor, as well as an estimate of the total dendritic length, and Branch Point Analysis (BPA) which analyzes the complexity of the arbor based on the number of dendritic bifurcations within the dendritic domain, as previously described (Diamond et al. 2006).
Histological Analysis
Mice underwent cardiac perfusion to preserve brain tissues. Mice were anesthetized using isoflurane and heart was surgically exposed. Approximately 50 mL of wash solution (0.8% NaCl, 0.4% dextrose, 0.8% sucrose, 0.023% CaCl2, and 0.034% sodium cacodylate in water) was injected into the heart, followed by approximately 50 mL fix solution (4% formaldehyde, 4% sucrose, and 1.4% sodium cacodylate in water). Following perfusion, brains were carefully dissected out and placed in fix solution overnight. Brains were then transferred to PBS for transport to Neuroscience Associates (Knoxville, TN) for multibrain blocking, sectioning at 35 μm thick, and histological staining. The stains included amino cupric silver stain (Switzer 2000), Hoechst 33,342 and immunohistochemistry. Antibodies were used as follows: glial fibrillary acidic protein- rabbit anti-GFAP (Dako Z0334) visualized with donkey anti-rabbit Alexa 488 LT A21206 or chicken anti-GFAP (Encor CPCA-GFA) visualized with donkey anti-chicken Alexa 488 (Jackson Laboratories 703-545-155). The cytochrome P450 factor was stained with rabbit anti-Cyp2E1 (Abcam ab28146) detected with donkey anti-Rabbit Alexa Fluor Plus 555 at 1:1000 dilution (Thermofisher A32794).
Slides were examined by laser scanning confocal microscopy with Fluoview 1000 and 3000 systems (Olympus, Waltham, MA). Z stacks were collected and maximum Z projections were evaluated quantitatively. To assess astrocyte morphology, ipsilateral dentate gyrus locations were stored at a lower magnification without regard for staining and then z-series were captured using a 60 × oil objective at these positions for each brain at the same locations. Quantification was performed using the ImageJ plugin, Analyze Skeleton. The images were batch processed to yield maximum intensity z projections and the appropriate channels were made binary with an automated intermodes threshold, despeckled, and skeletonized for analysis of process lengths and endpoints (Morrison and Filosa 2013, 2016). Cell counting was performed with cellSens software (Olympus).
RT‑PCR
Frozen tissue from the ipsilateral (right) cortex was homogenized with QIAzol Lysis Reagent (Qiagen, Valencia, CA) and phase separated with chloroform 3 days post-injury. RNA was precipitated with 70% ethanol. Purification of mRNA was performed using a RNeasy Mini Kit (74,104; Qiagen, Valencia, CA) per the manufacturer’s instructions. RNA concentration was determined by NanoDrop (Thermo-Scientific, Waltham, MA). RT-PCR was performed using iTaq Universal SYBR Green One-Step Kit (Bio-Rad, Hercules, CA) and L346/L347 primers for BDNF (IDT, Coralville, IA).
Statistics
GraphPad Prism software was used for statistical analysis. Mean values were compared using the two tailed t test or 2-way ANOVA for multiple group experiments. For Golgi analyses, the values of individual neurons were averaged per brain and statistical analyses considered each biological sample (brain) per group. For Sholl and Branch Point Analyses, the Wilcoxon signed-rank test was used. Means are displayed ± SEM and in all tests P < 0.05 indicates significance.
Results
Axonal Stability
Mouse brains were perfused, dissected, and then embedded in a 5 × 5 array and sectioned with the multibrain blocking, sectioning, and staining by Neuroscience Associates using the amino cupric silver stain for axonal degeneration. Amino cupric silver staining did not reveal any significant axonal degeneration when compared to sham controls (Fig. 2a) in any of the eight groups, including mice exposed to both injury and smoke (Fig. 2b) at 3 days post-injury. Brains were also examined at 12 days post-injury and similarly showed no axonal degeneration (not shown).
Fig. 2.
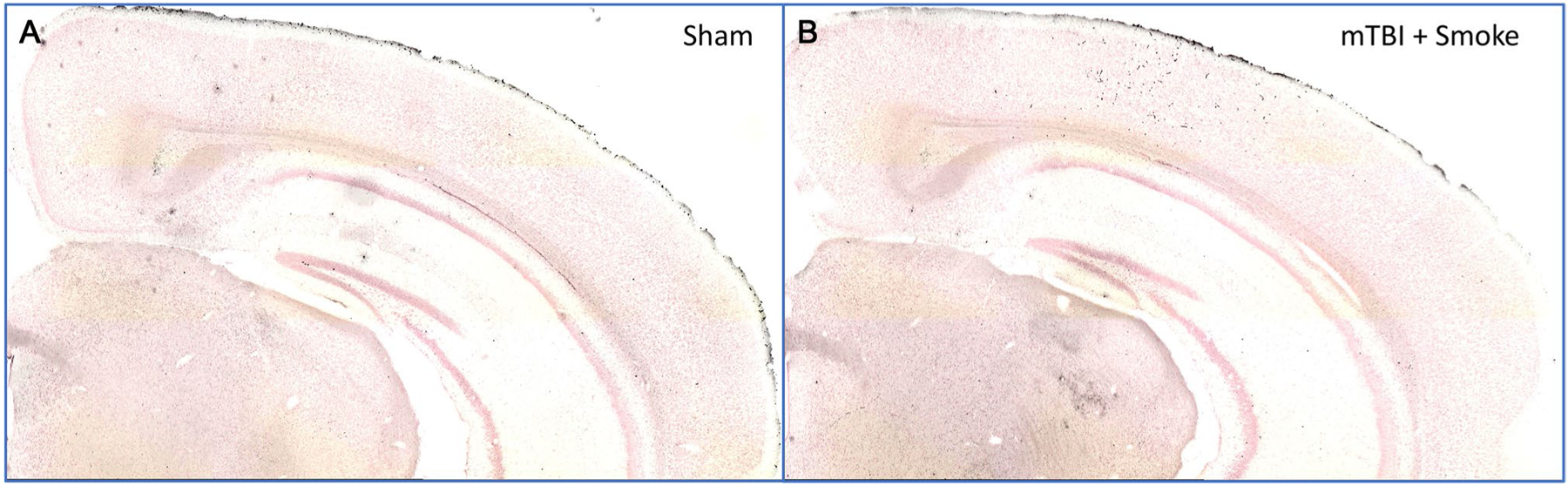
Analysis of axonal degradation. Perfused mouse brains were analyzed using the amino cupric silver stain for axonal degradation. When compared to sham controls (a), mTBI and smoke exposure did not induce any noticeable axonal degradation (b). Similarly, no axonal degradation was observed in any other groups
Dendritic Analysis
To determine the extent of any changes in dendritic complexity, we performed Golgi staining of granule cells within the dentate gyrus of the hippocampus. The dendritic arbor and spines comprise over 95% of the volume of the neuron and assessment of these parameters is a highly sensitive index of neuronal damage. Formalin-fixed tissue blocks (2–3 mm thick) incorporating the cortical samples were stained by the Rapid Golgi method and evaluated for the amount and distribution of dendritic branching by Sholl and branch point analyses (Diamond et al. 2006). Camera lucida drawings of the Golgi stained neurons were utilized to determine dendritic measurements for analysis. Representative camera lucida drawings of neurons following TBI (Fig. 3a) and after TBI and smoke exposure (Fig. 3b) are shown. Sholl analysis indicated highly significant reductions in complexity within the inner two-thirds of the dendritic material with smoke exposure following TBI (Fig. 3c, d). Branch point analysis also revealed a significant reduction in dendritic complexity (Fig. 3e, f). Following treatment with tBHQ, there was no change in the dendrites by either Sholl analysis (Fig. 4a, b) or branch point analysis (Fig. 4c, d).
Fig. 3.
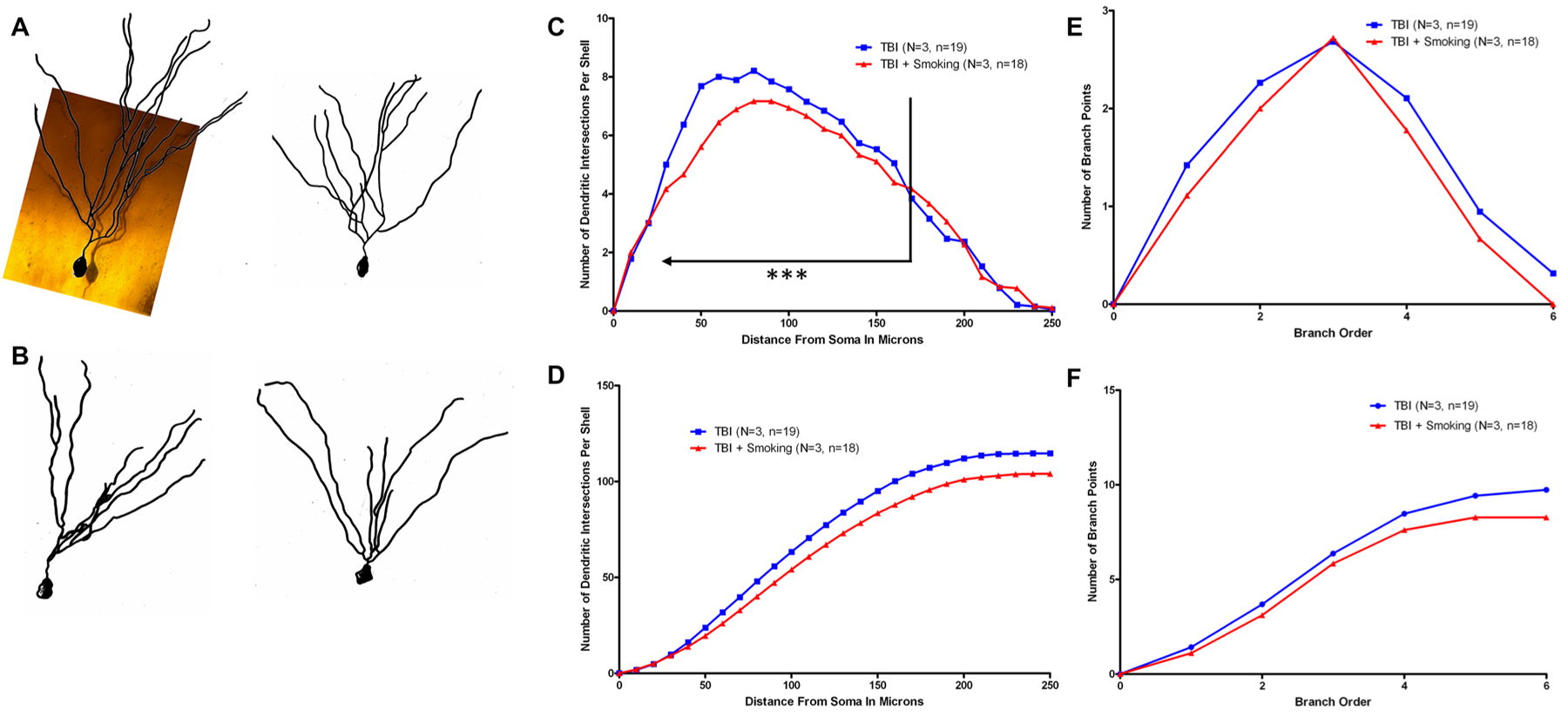
Dendritic analysis of injured and smoke exposed brains. Golgi staining was utilized to determine the extent of any changes in dendritic complexity following injury and smoke exposure. Camera lucida drawings of granule cells from the dentate gyrus were measured to determine the complexity of the dendritic arbor by Sholl and branch point analyses. Representative neurons from brains taken 12 days post-injury are shown from injured brains (a) or injured and smoke exposed brains (b). Sholl analysis indicates reduced complexity following mTBI and smoke exposure especially for the inner two-thirds, indicated on the graph, of the dendritic arbor (P = 0.0009) (c). Total differences in complexity can be seen more clearly in the cumulative graph (d). Branch point analysis indicated a similar trend (P = 0.06) (e) which can be seen clearly in the cumulative graph (f)
Fig. 4.
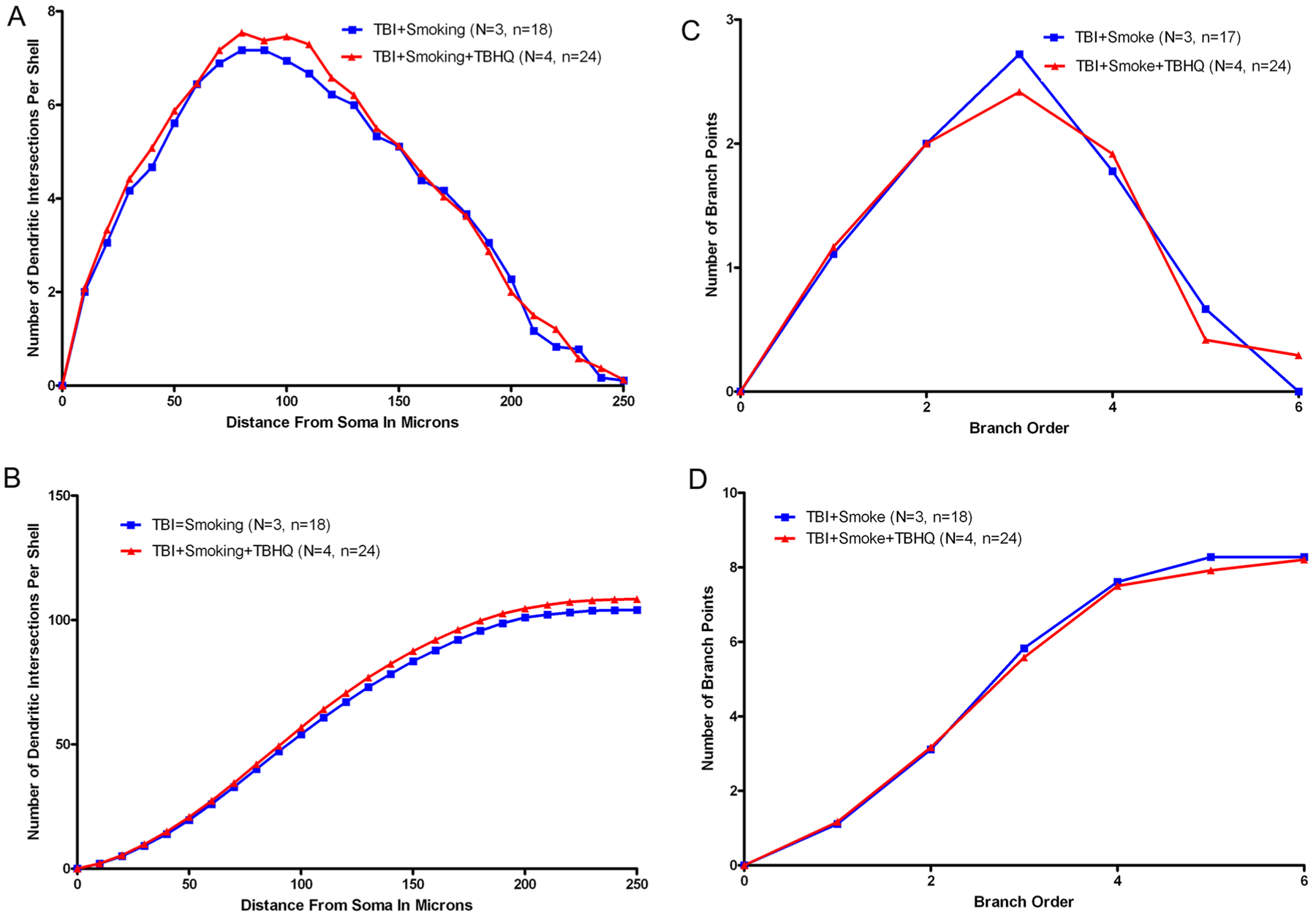
Dendritic analysis following treatment. Sholl and branch point analyses were performed on granule cells of the dentate gyrus 12 days post-injury. Sholl analysis did not indicate any significant change in dendritic complexity of mTBI and smoke exposed brains following treatment (a), this can also be seen in the cumulative graph (b). Similarly, branch point analysis also showed no statistically significant change with treatment (c), the cumulative graph also shows no change (d)
BDNF mRNA
mRNA of brain-derived neurotrophic factor (BNDF) were analyzed via qRT-PCR in ipsilateral cortex obtained 3 days post-injury. There were no statistically significant changes from sham controls in BDNF levels following TBI, smoke exposure, or tBHQ treatment (Fig. 5).
Fig. 5.
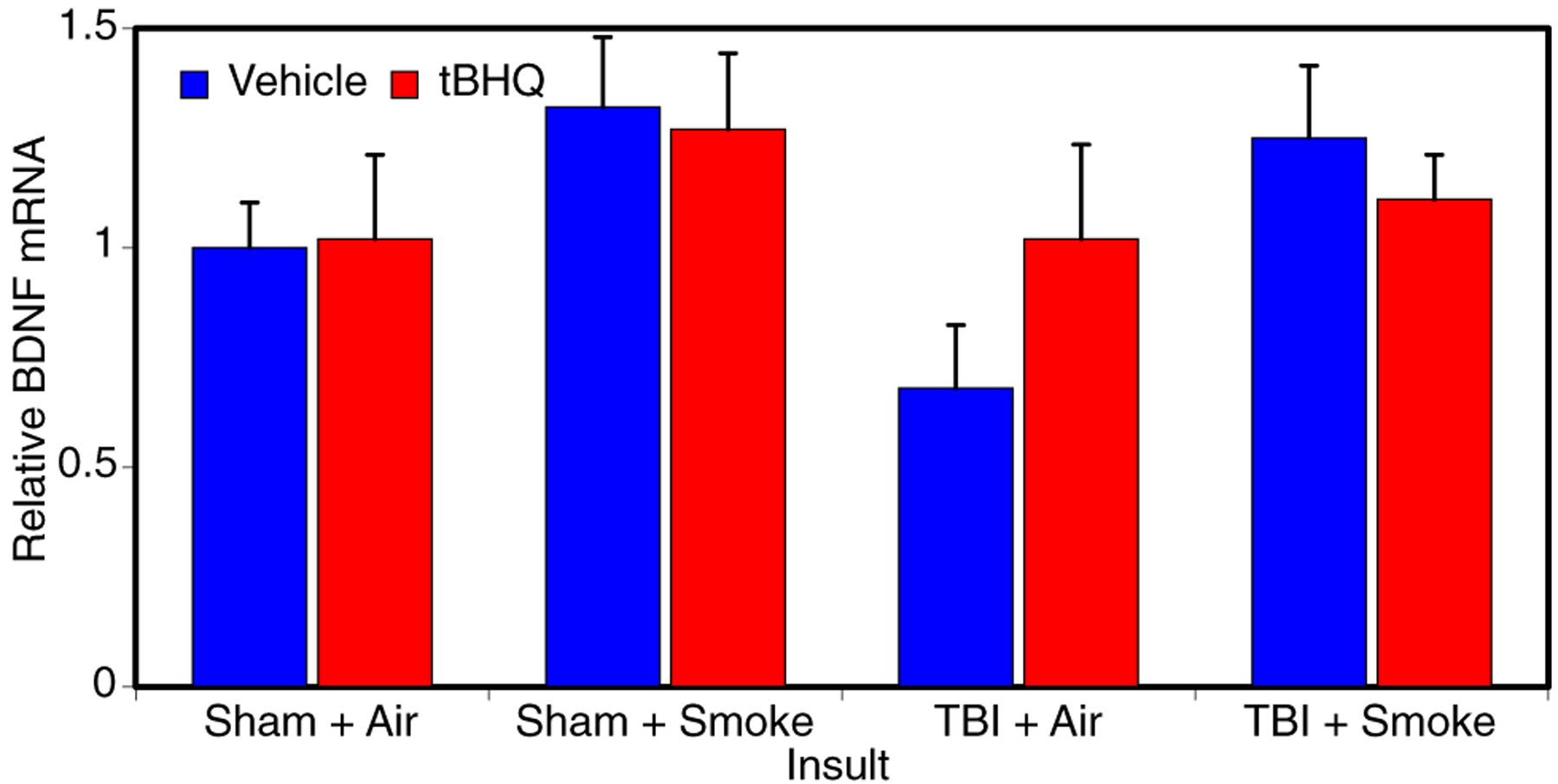
Analysis of BDNF mRNA. qRT-PCR analysis was utilized to determine if neuronal changes following injury, smoke, and treatment could be related to changes in BDNF expression in ipsilateral cortex at 3 days post-injury. mRNA samples from 4 mice per group were used for the analysis. These analyses indicated that there were no significant changes in BDNF mRNA levels
Astrocyte Responses
Multiblocked brains from 3 days post-injury underwent GFAP and Hoechst nuclear counterstaining. GFAP stained astrocytes within the dentate gyrus indicate elevated immunoreactivity compared to sham controls (Fig. 6a) following both mTBI alone (Fig. 6b) and mTBI plus smoke exposure (Fig. 6c). When we measured the density of astrocytes in the dentate gyrus granule layer, we found a significant increase only after the TBI (Fig. 6d). We did not observe any significant differences in Astrocyte morphology.
Fig. 6.
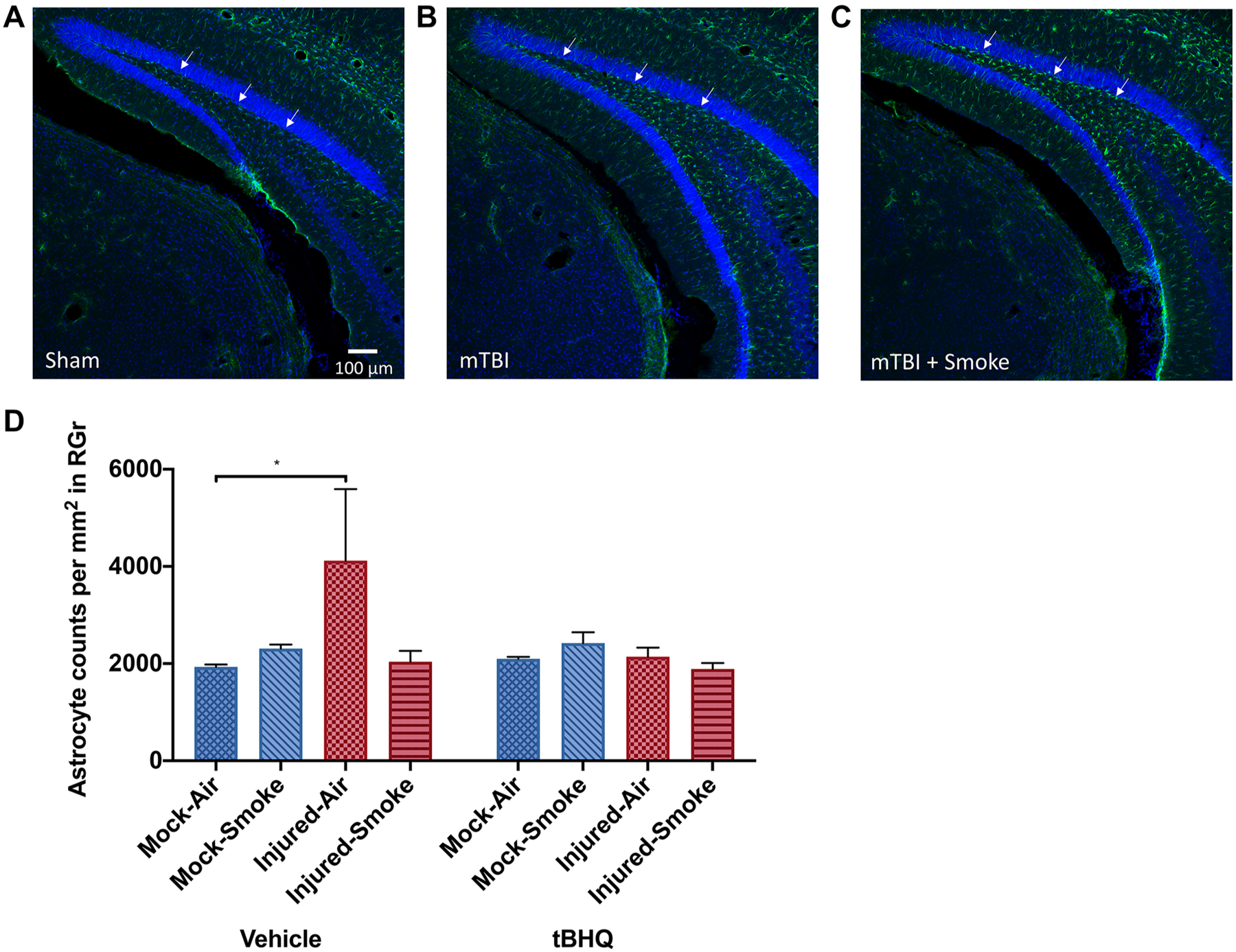
Reactive astrocytosis following injury and smoke exposure. GFAP staining (green channel) and Hoechst nuclear counterstaining (blue channel) were performed on multiblocked brains 3 days post-injury. When compared to sham controls (a) visual examination suggests elevated immunoreactivity following TBI (b) with even further elevation following TBI and smoke exposure (c). Quantitative analysis of the numbers of astrocytes within the granule layer of the dentate gyrus indicated that TBI resulted in additional astrocytes present (*P = 0.035) (d)
Mouse brain sections, stained with Hoechst 33,342 and antibodies to GFAP and to Cyp2e1 were examined in the dentate gyrus region by confocal microscopy and then maximum Z projections were generated. We found two subsets of Cyp2e1 positive astrocytes, those with moderated Cyp2e1 expression and a much smaller subset with strong immunoreactivity (Fig. 7). Although there seemed to be trends in the intense Cyp2e1 counts per area in response to the smoke insult, the only statistically significant finding involved lower intense Cyp2e1 densities in the tBHQ-treated groups (Fig. 8a). The moderate intensity Cyp2e1 cells were more common and the staining was confined to the soma. There was an elevation in the moderately stained cells after injury that was significantly reduced by the tBHQ treatment (injured-air-vehicle compared to injured-air-tBHQ, Fig. 8b).
Fig. 7.
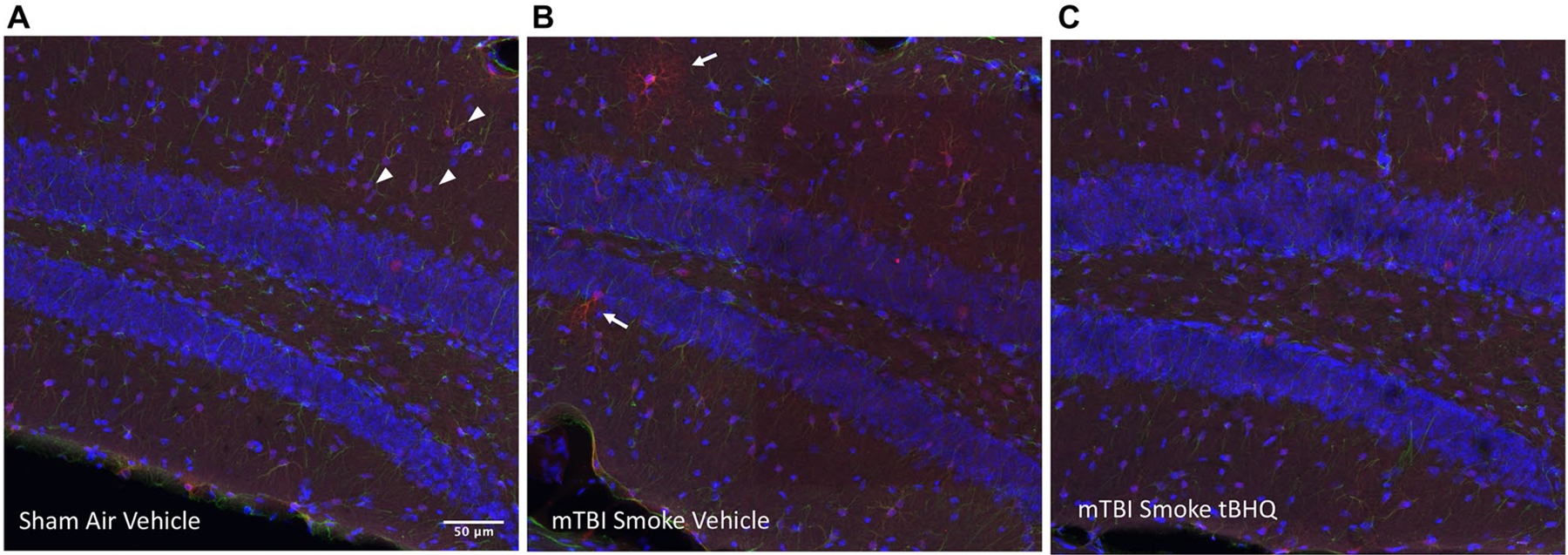
Cyp2e1 in the granular cell region of the ipsilateral hippocampus. Astrocytes in the dentate gyrus region of the hippocampus were frequently found to stain positively at a moderate intensity in the soma (arrowheads) and sometimes more intensely with immunoreactivity throughout the branched processes (arrows). Hoechst 33,342 staining of nuclei is shown in the blue channel and Cyp2e1 immunoreactivity in the red channel in these example sections from mice with no insult or treatment (a), mTBI + smoke exposure without treatment (b) or mTBI + smoke exposure with the tBHQ treatment (c). The region is from the right hippocampus closest to the closed head impact centered at − 2.9 mm from bregma
Fig. 8.
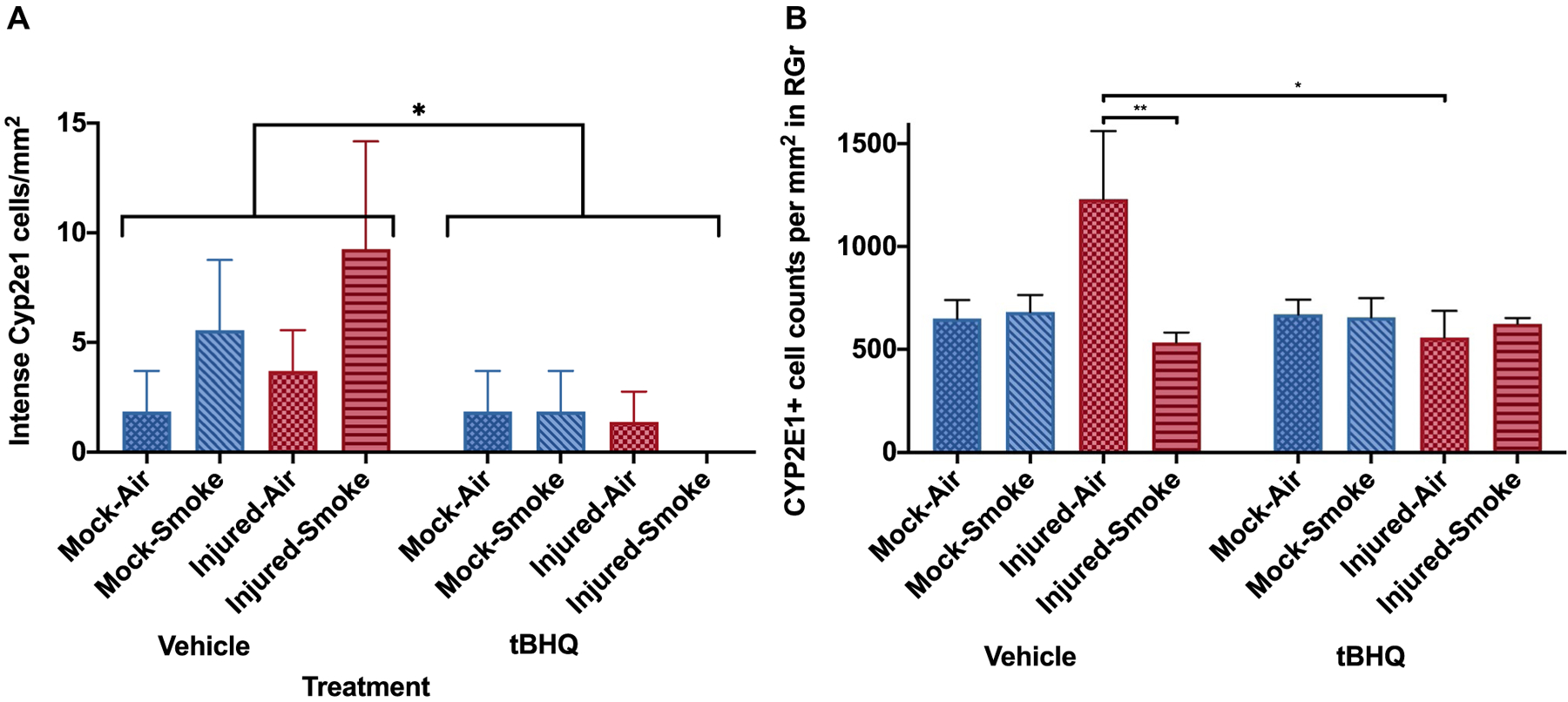
Cyp2e1 positive astrocyte densities. Analysis of the numbers of astrocytes displaying intense Cyp2e1 immunoreactivity in the dentate gyrus region (a) or moderate staining within the granule layer (b) indicated that there were varied responses to the insults and that in both cases, Cyp2e1 astrocyte densities were reduced by the tBHQ treatment (a *P = 0.04 effect of tBHQ over all groups; b *P = 0.045, **P = 0.0047, 2-way ANOVA was used for statistical analysis)
Discussion
Traumatic brain injury has become increasingly common in recent years, especially among the armed forces. Among U.S. Forces, there have been approximately 384,000 brain injuries sustained since 2000 (Defense and Veterans Brain Injury Center 2018). Additionally, cigarette smoking among veterans of recent conflicts in Iraq and Afghanistan is significantly higher than that of the average US Citizen (Bastian and Sherman 2010; Brown 2010). In this study, we utilize a mouse model of closed head mild traumatic brain injury, combined with repeated sidestream smoke exposure to better understand how the oxidative stress induced by cigarette smoking may impact the brain’s ability to protect itself and recover from mTBI. There are several established models of direct and second-hand smoke exposure (Gairola 2006; Kaisar et al. 2017) and ours involved daily exposures three times per day (Huang et al. 2009). We also tested whether enhancing antioxidant response through our treatment could counteract any negative effects of TBI and/or smoke exposure.
We focused on the hippocampus because it seems particularly sensitive to insults. For example, combined smoke and alcohol exposures were found to decrease astrocyte marker GFAP in the CA1, CA3, and dentate gyrus regions of the hippocampus, while neither alcohol nor smoking exposure alone had an appreciable effect (Huf et al. 2019). In the same study, alcohol and smoking had an additive effect on the increased expression of cleaved caspase-3, a marker of apoptosis, in CA3, CA2, and the dentate gyrus of the hippocampus but not in CA1 suggesting selective regional sus-ceptibility to combined insults (Huf et al. 2019).
While we did not note any axonal degradation in response to either TBI or smoke exposure, there were significant reductions in dendritic complexity within the dentate gyrus of the hippocampus with smoke exposure following TBI compared to TBI alone. We did not observe any further changes in dendritic complexity in response to treatment with tBHQ. The changes in dendritic architecture that we did observe indicated the possibility of dysregulation of trophic factors. It has been shown that prenatal cigarette smoke exposure decreases BDNF expression in brains of male pups in select nuclei (Machaalani et al. 2019). In adult mice exposed for 60 days to smoke, BDNF levels were found significantly lowered in the hippocampus (Tuon et al. 2010). Conversely, chronic smoke exposure elevated BDNF mRNA levels following severe TBI in rats (Lee et al. 2012). Although we did not find BDNF dysregulation in our samples, it is possible that changes do occur that are specific for the brain region, severity of the injury, and time relative to the insult.
GFAP staining indicated that astrocytes were responsive to the insult within the ipsilateral dentate gyrus when mice were exposed to either TBI or smoke when compared to sham controls. This phenotype was reversible with antioxidant treatment. In other reports, inflammatory cytokines were found increased in mice as early as 1 month after initiation of smoke exposure and this was followed by additional damage as the exposure continued (Adhami et al. 2017). Furthermore, studies with Nrf2 knockout mice and a Nrf2 activator showed that, activation of Nrf2 using a small molecule triterpenoid CDDO-imidazolide can attenuate smoke-induced cytotoxicity in the heart and lungs, however, this was not evaluated in the brain (Sussan et al. 2009). In other reports, mice chronically exposed to smoke and a subsequent TBI showed behavioral and motor deficits and diminished recovery at 1, 24, and 72 h post-injury (Sivandzade et al. 2020). Neuroprotective Nrf2 expression was upregulated in response to TBI and this upregulation was severely diminished by smoke exposure (Sivandzade et al. 2020).
Nrf2 signaling could certainly play a role because reactive oxygen species (ROS) are greatly enriched in tobacco smoke and chronic smoking has been shown to dysregulate normal blood brain barrier (BBB) function by activation of oxidative, inflammatory, and immune responses (Cataldo et al. 2010; Cojocaru et al. 2013; Kaisar et al. 2018; Paulson et al. 2010; Sivandzade and Cucullo 2019a, 2019b). Modulation of Nrf2 via treatment with tBHQ has been shown previously to reduce ROS following model brain injury (Zhang et al. 2020). These are likely contributors to diminished cognitive recovery of smokers than nonsmokers after a mild TBI as has been shown in human studies (Durazzo et al. 2013).
Finally, we did find evidence for upregulation of Cyp2e1 after insult. This is consistent with elevations of Cyp2e1 that have been found by other groups. For example, Cyp2e1 expression was increased in rat and gerbil astrocytes in vitro and in vivo along with inflammatory factors and induced by ischemic injury (Tindberg et al. 1996). The hippocampus was shown to be one of the brain regions that exhibit Cyp2e1 upregulation after insult (Zhong et al. 2012).
One of the most common long term-effects of mTBI involves memory problems (McDowell et al. 1997; Stuss et al. 1985). Our study indicates that there are several important changes to the astrocytes of the hippocampus in response to TBI and smoke exposure. These changes induced by cigarette smoke, suggest that smoke exposure during recovery may contribute to poorer cognitive out-comes following TBI, and memory deficits, in particular. Our study also provides some evidence that treatment with an antioxidant pathway activator, such as tBHQ, may be able to combat some of these negative effects. Further research is necessary, however, our study provides a unique cellular basis for the impact of lifestyle choices, such as cigarette smoking, on the impact and recovery from TBI.
Acknowledgements
We thank Andrea Smith and Tai Vo for expert animal assistance, Miseanne Tang for technical assistance, and Dr. John C. M. Tsibris for the loan of the smoking system.
Funding This study was supported by the Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development (I01RX001520)), the Assistant Secretary of Defense for Health Affairs through the Congressionally Directed Gulf War Illness Research Program (W81XWH-16-1-0626), the Florida Department of Health James and Esther King Biomedical Research Program (4KB14), and The Bay Pines Foundation. The funding sources had no involvement in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Conflict of interest The authors declare no competing interests. The contents do not represent the views of the Department of Veterans Affairs or the US Government and the opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
Data Availability The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval The animal protocol was approved by the Bay Pines VA Institutional Animal Care and Use Committee (IACUC) and performed in accordance with all institutional, agency, and governmental Animal Welfare Regulations.
References
- Adhami N, Chen Y, Martins-Green M (2017) Biomarkers of disease can be detected in mice as early as 4 weeks after initiation of exposure to third-hand smoke levels equivalent to those found in homes of smokers. Clin Sci 131:2409–2426. 10.1042/CS20171053 [DOI] [PubMed] [Google Scholar]
- Bastian LA, Sherman SE (2010) Effects of the wars on smoking among veterans. J Gen Intern Med 25:102–103. 10.1007/s11606-009-1224-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DW (2010) Smoking prevalence among US veterans. J Gen Intern Med 25:147–149. 10.1007/s11606-009-1160-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Bernstein AM, Sofroniew MV (2016) Astrocyte roles in traumatic brain injury. Exp Neurol 275(Pt 3):305–315. 10.1016/j.expneurol.2015.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo JK, Prochaska JJ, Glantz SA (2010) Cigarette smoking is a risk factor for Alzheimer’s Disease: an analysis controlling for tobacco industry affiliation. J Alzheimers Dis 19:465–480. 10.3233/JAD-2010-1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H et al. (2016) Impact of smoking on neurodegeneration and cerebrovascular disease markers in cognitively normal men. Eur J Neurol 23:110–119. 10.1111/ene.12816 [DOI] [PubMed] [Google Scholar]
- Cojocaru IM, Cojocaru M, Sapira V, Ionescu A (2013) Evaluation of oxidative stress in patients with acute ischemic stroke. Rom J Intern Med 51:97–106 [PubMed] [Google Scholar]
- Defense and Veterans Brain Injury Center (2018) DOD Worldwide Numbers for TBI. Washington, DC [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Woodson JC, Conrad CD, Bachstetter AD, Mervis RF (2006) Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus 16:571–576 [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Abadjian L, Kincaid A, Bilovsky-Muniz T, Boreta L, Gauger GE (2013) The influence of chronic cigarette smoking on neurocognitive recovery after mild traumatic brain injury. J Neurotrauma 30:1013–1022. 10.1089/neu.2012.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gairola CG (2006) Animal models of 2nd hand smoking. In: Wang XL, Scott D (eds) Molecular mechanisms for tobacco-induced diseases. Nova Science Publishers, New York, pp 121–132 [Google Scholar]
- Gjota-Ergin S, Gokcek-Sarac C, Adali O, Jakubowska-Dogru E (2018) Relationship between the hippocampal expression of selected cytochrome P450 isoforms and the animal performance in the hippocampus-dependent learning task. Neurosci Lett 673:104–110. 10.1016/j.neulet.2018.02.059 [DOI] [PubMed] [Google Scholar]
- Hatic H, Kane MJ, Saykally JN, Citron BA (2012) Modulation of transcription factor Nrf2 in an in vitro model of traumatic brain injury. J Neurotrauma 29:1188–1196. 10.1089/neu.2011.1806 [DOI] [PubMed] [Google Scholar]
- Ho YS et al. (2012) Cigarette smoking accelerated brain aging and induced pre-Alzheimer-like neuropathology in rats. PLoS ONE 7:e36752. 10.1371/journal.pone.0036752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA (2008) Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med 358:453–463. 10.1056/NEJMoa072972 [DOI] [PubMed] [Google Scholar]
- Huang J, Okuka M, McLean M, Keefe DL, Liu L (2009) Effects of cigarette smoke on fertilization and embryo development in vivo. Fertil Steril 92:1456–1465. 10.1016/j.fertnstert.2008.07.1781 [DOI] [PubMed] [Google Scholar]
- Huang J, Okuka M, Lu W, Tsibris JC, McLean MP, Keefe DL, Liu L (2013) Telomere shortening and DNA damage of embryonic stem cells induced by cigarette smoke. Reprod Toxicol 35:89–95. 10.1016/j.reprotox.2012.07.003 [DOI] [PubMed] [Google Scholar]
- Huf F et al. (2019) Comparative study on the effects of cigarette smoke exposure, ethanol consumption and association: Behavioral parameters, apoptosis, glial fibrillary acid protein and S100beta immunoreactivity in different regions of the rat hippocampus. Alcohol 77:101–112. 10.1016/j.alcohol.2018.08.009 [DOI] [PubMed] [Google Scholar]
- Kaisar MA, Kallem RR, Sajja RK, Sifat AE, Cucullo L (2017) A convenient UHPLC-MS/MS method for routine monitoring of plasma and brain levels of nicotine and cotinine as a tool to validate newly developed preclinical smoking model in mouse. BMC Neurosci 18:71. 10.1186/s12868-017-0389-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisar MA, Sivandzade F, Bhalerao A, Cucullo L (2018) Conventional and electronic cigarettes dysregulate the expression of iron transporters and detoxifying enzymes at the brain vascular endothelium: In vivo evidence of a gender-specific cellular response to chronic cigarette smoke exposure. Neurosci Lett 682:1–9. 10.1016/j.neulet.2018.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IN, Lin MH, Chung CY, Lee MH, Weng HH, Yang JT (2012) Chronic cigarette smoke exposure enhances brain-derived neurotrophic factor expression in rats with traumatic brain injury. Metab Brain Dis 27:197–204. 10.1007/s11011-012-9294-x [DOI] [PubMed] [Google Scholar]
- Machaalani R, Thawley M, Huang J, Chen H (2019) Effects of prenatal cigarette smoke exposure on BDNF, PACAP, microglia and gliosis expression in the young male mouse brainstem. Neurotoxicology 74:40–46. 10.1016/j.neuro.2019.05.009 [DOI] [PubMed] [Google Scholar]
- McDowell S, Whyte J, D’Esposito M (1997) Working memory impairments in traumatic brain injury: evidence from a dual-task paradigm. Neuropsychologia 35:1341–1353 [DOI] [PubMed] [Google Scholar]
- Milman A, Rosenberg A, Weizman R, Pick CG (2005) Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J Neurotrauma 22:1003–1010. 10.1089/neu.2005.22.1003 [DOI] [PubMed] [Google Scholar]
- Morrison HW, Filosa JA (2013) A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J Neuroinflammation 10:4. 10.1186/1742-2094-10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison HW, Filosa JA (2016) Sex differences in astrocyte and microglia responses immediately following middle cerebral artery occlusion in adult mice. Neuroscience 339:85–99. 10.1016/j.neuroscience.2016.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson JR et al. (2010) Nicotine exacerbates brain edema during in vitro and in vivo focal ischemic conditions. J Pharmacol Exp Ther 332:371–379. 10.1124/jpet.109.157776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff WA, Saykally JN, Mervis RF, Lin X, Cao C, Citron BA (2019) Behavior, protein, and dendritic changes after model traumatic brain injury and treatment with nanocoffee particles. BMC Neurosci 20:44. 10.1186/s12868-019-0525-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff WA, Delic V, Pick CG, Citron BA (2020a) Dendritic arbor complexity and spine density changes after repetitive mild traumatic brain injury and neuroprotective treatments. Brain Res 1746:147019. 10.1016/j.brainres.2020.147019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff WA, Mervis RF, Citron BA, Schwartz B, Rubovitch V, Schreiber S, Pick CG (2020b) Effect of mild blast-induced TBI on dendritic architecture of the cortex and hippocampus in the mouse. Sci Rep 10:2206. 10.1038/s41598-020-59252-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff WA, Qubty D, Delic V, Pick CG, Citron B (2020c) Repetitive mild traumatic brain injury and transcription factor modulation. J Neurotrauma. 10.1089/neu.2020.7005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykally JN, Rachmany L, Hatic H, Shaer A, Rubovitch V, Pick CG, Citron BA (2012) The nuclear factor erythroid 2-like 2 activator, tert-butylhydroquinone, improves cognitive performance in mice after mild traumatic brain injury. Neuroscience 223:305–314. 10.1016/j.neuroscience.2012.07.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykally JN, Ratliff WA, Keeley K, Pick CG, Mervis RF, Citron BA (2018) Repetitive mild closed head injury alters protein expression and dendritic complexity in a mouse model. J Neurotrauma 35:139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SE (2008) A framework for tobacco control: lessons learnt from Veterans Health Administration. BMJ 336:1016–1019. 10.1136/bmj.39510.805266.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedler DG, Chuah MI, Kirkcaldie MT, Vickers JC, King AE (2014) Diffuse axonal injury in brain trauma: insights from alterations in neurofilaments. Front Cell Neurosci 8:429. 10.3389/fncel.2014.00429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivandzade F, Cucullo L (2019a) Anti-diabetic countermeasures against tobacco smoke-dependent cerebrovascular toxicity: use and effect of rosiglitazone. Int J Mol Sci. 10.3390/ijms20174225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivandzade F, Cucullo L (2019b) Assessing the protective effect of rosiglitazone against electronic cigarette/tobacco smoke-induced blood-brain barrier impairment. BMC Neurosci 20:15. 10.1186/s12868-019-0497-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivandzade F, Alqahtani F, Sifat A, Cucullo L (2020) The cerebrovascular and neurological impact of chronic smoking on post-traumatic brain injury outcome and recovery: an in vivo study. J Neuroinflamm 17:133. 10.1186/s12974-020-01818-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck AE et al. (2011) Cigarette smoke inhibits brain mitochondrial adaptations of exercised mice. Neurochem Res 36:1056–1061. 10.1007/s11064-011-0447-9 [DOI] [PubMed] [Google Scholar]
- Stuss D, Ely P, Hugenholtz H, Richard M, LaRochelle S, Poirier C, Bell I (1985) Subtle neuropsychological deficits in patients with good recovery after closed head injury. Neurosurgery 17:41–47 [DOI] [PubMed] [Google Scholar]
- Sussan TE et al. (2009) Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci USA 106:250–255. 10.1073/pnas.0804333106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer RC 3rd (2000) Application of silver degeneration stains for neurotoxicity testing. Toxicol Pathol 28:70–83. 10.1177/019262330002800109 [DOI] [PubMed] [Google Scholar]
- Tashlykov V, Katz Y, Gazit V, Zohar O, Schreiber S, Pick CG (2007) Apoptotic changes in the cortex and hippocampus following minimal brain trauma in mice. Brain Res 1130:197–205. 10.1016/j.brainres.2006.10.032 [DOI] [PubMed] [Google Scholar]
- Tindberg N, Baldwin HA, Cross AJ, Ingelman-Sundberg M (1996) Induction of cytochrome P450 2E1 expression in rat and gerbil astrocytes by inflammatory factors and ischemic injury. Mol Pharmacol 50:1065–1072 [PubMed] [Google Scholar]
- Tuon T et al. (2010) Effects of moderate exercise on cigarette smoke exposure-induced hippocampal oxidative stress values and neurological behaviors in mice. Neurosci Lett 475:16–19. 10.1016/j.neulet.2010.03.030 [DOI] [PubMed] [Google Scholar]
- Tweedie D et al. (2007) Apoptotic and behavioral sequelae of mild brain trauma in mice. J Neurosci Res 85:805–815. 10.1002/jnr.21160 [DOI] [PubMed] [Google Scholar]
- Tweedie D et al. (2013) Changes in mouse cognition and hippocampal gene expression observed in a mild physical- and blast-traumatic brain injury. Neurobiol Dis 54:1–11. 10.1016/j.nbd.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z et al. (2020) Acute and chronic molecular signatures and associated symptoms of blast exposure in military breachers. J Neurotrauma 37:1221–1232. 10.1089/neu.2019.6742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R et al. (2016) tobacco smoke-induced brain white matter myelin dysfunction: potential co-factor role of smoking in neurodegeneration. J Alzheimers Dis 50:133–148. 10.3233/jad-150751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW et al. (2020) TBHQ improved neurological recovery after traumatic brain injury by inhibiting the overactivation of astrocytes. Brain Res 1739:146818. 10.1016/j.brainres.2020.146818 [DOI] [PubMed] [Google Scholar]
- Zhong Y et al. (2012) Induction of brain CYP2E1 by chronic ethanol treatment and related oxidative stress in hippocampus, cerebellum, and brainstem. Toxicology 302:275–284. 10.1016/j.tox.2012.08.009 [DOI] [PubMed] [Google Scholar]
- Zohar O, Schreiber S, Getslev V, Schwartz JP, Mullins PG, Pick CG (2003) Closed-head minimal traumatic brain injury produces long-term cognitive deficits in mice. Neuroscience 118:949–955. [DOI] [PubMed] [Google Scholar]


