Abstract
Major depressive disorder (MDD) is a leading cause of disability, affecting more than 300 million people worldwide. We first review the well-known sex difference in incidence of MDD, with women being twice as likely to be diagnosed as men, and briefly summarize how the impact of MDD varies between men and women, with sex differences in symptoms, severity, and antidepressant drug response. We then attempt to deconstruct the biological bases for MDD and discuss implications for sex differences research. Next, we review findings from human postmortem studies, both from selected candidate gene studies and from well-powered, unbiased transcriptomics studies, which suggest distinct, and possibly opposite, molecular changes in the brains of depressed men and women. We then discuss inherent challenges of research on the human postmortem brain and suggest paths forward that rely on thoughtful cohort design. Although studies indicate that circulating gonadal hormones might underlie the observed sex differences in MDD, we discuss how additional sex-specific factors, such as genetic sex and developmental exposure to gonadal hormones, may also contribute to altered vulnerability, and we highlight various nuances that we believe should be considered when determining mechanisms underlying observed sex differences. Altogether, this review highlights not only how various sex-specific factors might influence susceptibility or resilience to depression, but also how those sex-specific factors might result in divergent pathology in men and women.
CLINICAL SEX DIFFERENCES IN DEPRESSION
Major depressive disorder (MDD) is a devastating mental illness affecting approximately 350 million people globally (1). In the United States, the economic burden of MDD was estimated at $210.5 billion in 2010 (2). Moreover, patients with mood disorders account for approximately 60% of completed suicides (3). Core MDD symptoms include emotion dysregulation, low mood, flattened affect, and anhedonia. Additional MDD symptoms fall into various dimensions, including cognitive symptoms, physiological symptoms (i.e., altered weight, motor activity, sleep patterns), and comorbid anxiety (4).
Females are twice as likely as males to have a single MDD episode and 4 times as likely to have recurrent MDD (5,6). This sex difference in MDD incidence may be driven by reporting biases [i.e., women are more likely to seek treatment (7,8)], although it is reported across cultures and in community-based epidemiological studies, suggesting underlying biological differences (9,10). Comparing males and females with MDD, females tend to have more symptoms, higher symptom severity, and more subjective distress (9,11,12). Further, females are more likely to have a comorbid anxiety disorder (13), and males are more likely to have a comorbid substance use disorder (14). Findings suggest that there are sex differences in antidepressant treatment response, but the evidence is not as striking as for the sex difference in MDD incidence. For instance, some studies report that monoamine oxidase inhibitors and selective serotonin reuptake inhibitors may be more effective in female patients (15–17), while male patients respond more favorably to tricyclic antidepressants (17,18) and ketamine (19) [but see (15,20–22)]. Psychotherapy appears similarly effective in men and women (23–26), though women may engage more in psychotherapy (27–30). While there are indeed sex differences in incidence, symptoms, and antidepressant response, evidence suggests that prognosis is similar in men and women once MDD is diagnosed (31).
DECONSTRUCTING THE BIOLOGICAL BASES OF MDD AND IMPLICATIONS FOR SEX DIFFERENCES
Few novel MDD treatments have been developed beyond the serendipitous discovery that agents increasing monoaminergic signaling can reduce mood symptoms, though some studies since then suggest that clinical efficacy of these drugs may include actions that reduce inflammation (32) or increase the formation of new hippocampal neurons (33). Despite wide-spread use, only a third of patients achieve remission with monoamine-targeting treatment (16,34,35). Even when effacious in treating mood symptoms, monoamine-targeting therapeutics do not appear to ameliorate the cognitive deficits associated with MDD (36), and the residual presence of those deficits during remission predicts relapse (37). In sum, although monoaminergic antidepressants provide symptomatic relief in some patients, they might not reverse certain MDD symptom domains.
The categorically defined MDD syndrome may be an imprecise umbrella grouping of multiple clinical symptoms, each of which may have unique and multifactorial etiologies. Indeed, evidence shows that various underlying pathologies occur in depression, including altered excitation-inhibition, reduced neurotrophic support, and inflammation. These pathologies are not systematically observed in MDD and extend to other categorically defined psychiatric disorders and in some cases to neurodegenerative disorders (38). Over-whelming evidence supports a dimensional model, where specificities of symptoms arise from the combination of genetic, environmental, and development risk factors, which may lead to unique brain pathologies.
A dimensional perspective is amenable to biological investigation for pathophysiological mechanisms and the roles of sex. Indeed, pathological entities observed in the human postmortem brain point to specific biological pathways, which in turn can be investigated in light of multiple factors, including genetic variability, environment, and sex. Here, we review the degree to which evidence stemming from human postmortem brain investigations support either shared or sex-specific pathologies. We then discuss biological factors mediating the role of sex and how their interaction with pathological entities may lead to clinical observations of sex differences in depression.
SEX DIFFERENCES IN MDD: CANDIDATE GENE STUDIES
Gene expression studies in human MDD employing a candidate gene approach have been fruitful in identifying potential drivers for sex differences in MDD. The serotonergic system has been a focus of candidate gene studies because most antidepressants target this system. Interestingly, evidence suggests sex specificity for some serotonin-related alterations in MDD. For instance, levels of the serotonin 1D autoreceptor and serotonin-related transcription factors, NUDR and REST, were higher in dorsal raphe serotonin neurons of female, but not male, subjects with MDD (39). As NUDR and REST act as transcriptional repressors of the serotonin 1A autoreceptor in the dorsal raphe, their increased expression might represent a compensatory attempt to reduce elevated autoreceptors and increase serotonergic transmission. There are also decreased levels of the serotonin 1A receptor and NUDR in the prefrontal cortex (PFC) of depressed women, but not men (40). It is interesting to note that there may be sex-specific MDD alterations to the serotonin system given evidence that selective serotonin reuptake inhibitors may be more efficacious in depressed women compared with other classes of antidepressants (17,41).
Based on evidence suggesting altered excitation and/or inhibition in MDD (42–60), studies have examined alterations in glutamate- and GABA (gamma-aminobutyric acid)-related genes. Some alterations in glutamate-related genes might be sex specific in the dorsolateral PFC (DLPFC). Specifically, depressed females exhibit increased expression of several glutamate receptor genes, including GRIN1, GRIN2A-D, GRIA2-4, GRIK1-2, GRM1, GRM4, GRM5, and GRM7; only GRM5 was altered in depressed males, but in the opposite direction compared with females (61). We reported sex-specific MDD alterations in GABA synthesizing enzymes in the basolateral amygdala (BLA) and anterior cingulate cortex (ACC). In the BLA, only depressed females exhibited reductions in GAD1 and GAD2, while in the ACC, only depressed males had reduced expression of GAD1 and GAD2 (60,62).
Studies examining expression of GABA subtype–specific markers provide insight into how excitatory pyramidal neurons and inhibitory GABA interneurons are altered in depression, suggesting sex-specific mechanisms of altered excitatory information processing. Somatostatin (SST)-expressing interneurons mainly innervate the distal dendrites of excitatory pyramidal cells, regulating excitatory input and providing feedback inhibition (63–65). Parvalbumin-positive interneurons typically innervate the perisomatic region of pyramidal cells, thereby regulating excitatory output and providing feedforward inhibition (64,65). As SST and PVALB are activity-dependent regulated genes, alterations in transcript levels in MDD suggest dysfunctions in cellular activity. Furthermore, their pattern of altered expression suggests brain region- and sex-specific changes in information processing by cortical microcircuits in MDD (hypothetical excitation/inhibition balance in Figure 1A). In the DLPFC, evidence suggests reduced SST, but not parvalbumin, cell activity and thus reduced inhibitory input onto excitatory pyramidal cells in both males and females with MDD (Figure 1B) (62,66). In the ACC, evidence suggests reductions in both SST and parvalbumin cell activities, suggesting reduced inhibitory input and output regulation of excitatory pyramidal neurons (Figure 1C, D). In the ACC, SST reductions were more pronounced in depressed females, and SST expression was negatively correlated with depressive symptoms only in females with MDD (67). This suggests more profound reduction in inhibitory input onto pyramidal neurons in the ACC of females with MDD (60,67,68). In the BLA, males with MDD do not show alterations in either SST or PVALB, while females with MDD have reduced SST (Figure 1F), suggesting reduced inhibitory input onto excitatory pyramidal cells in the BLA of females, but not males, with MDD (Figure 1E) (62,69). Differences in input and output of inhibitory regulation of excitatory signal will affect information coding and propagation, respectively. Region and sex specificities in altered information processing may affect cognition and emotion, providing a potential pathophysiological substrate for sex differences in symptoms. While evidence from animal studies suggests that alterations in GABA neurons affect relevant behaviors (70), it is unclear whether sex differences observed in humans have clinical consequences. For instance, it is possible that these brain region–specific molecular alterations converge on similar clinical outcomes in males and females.
Figure 1.
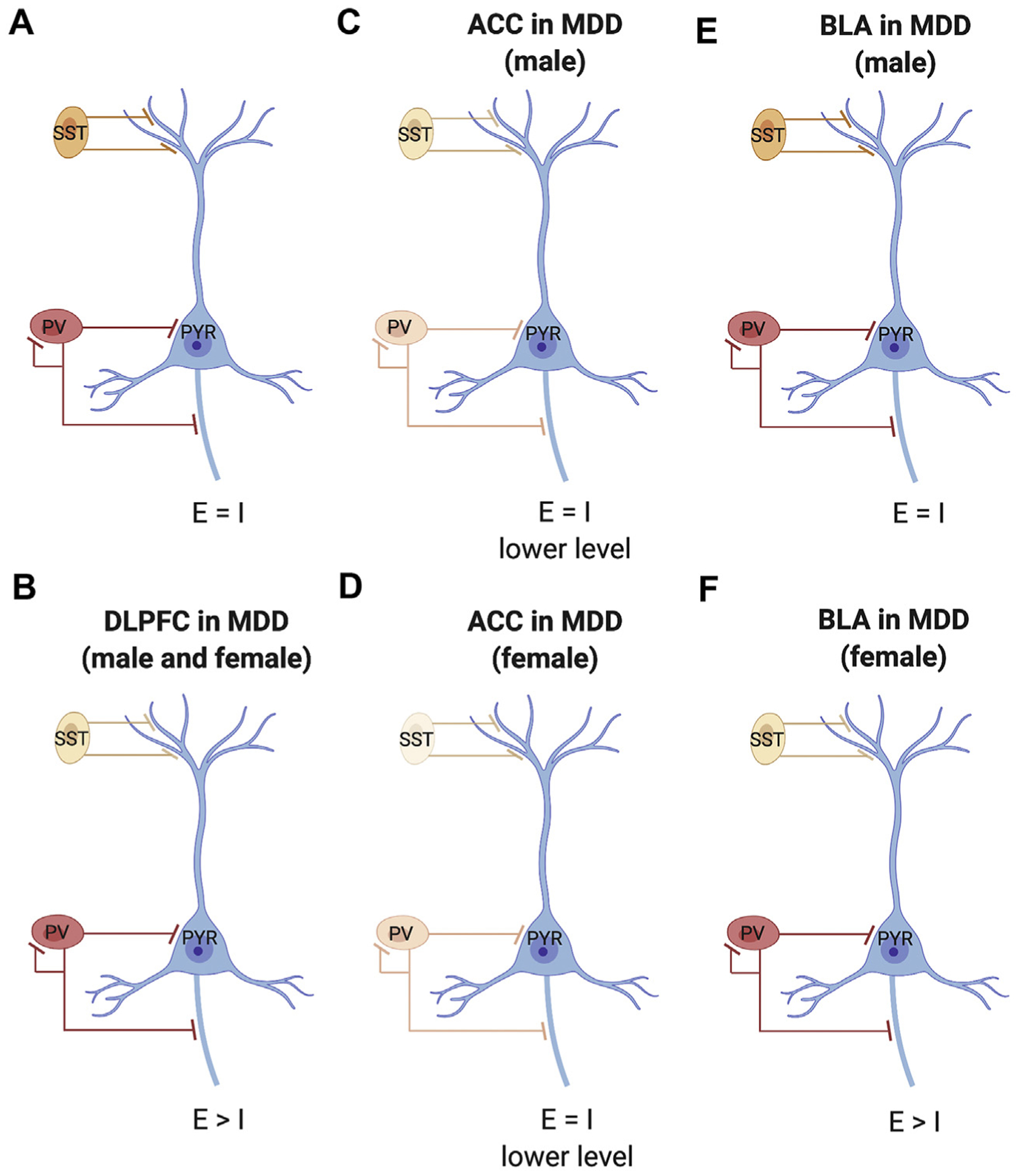
Schematic of neocortical microcircuitry and associated dysfunction in major depressive disorder (MDD). (A) Canonical microcircuitry in healthy control subjects with excitation (E)/inhibition (I) balance. (B) In the dorsolateral prefrontal cortex (DLPFC) of male and female subjects with MDD, there is lower expression of somatostatin (SST) (indicated by lighter color), but no change in parvalbumin (PV), suggesting reduced inhibition of input of pyramidal cells (PYR). (C) In the anterior cingulate cortex (ACC) of male subjects with MDD, there is moderately lower expression of both SST and PV, suggesting reduced inhibition of input and output of pyramidal cells, i.e., lower E/I balance. (D) In the ACC of female subjects with MDD, there is a robust reduction in expression of SST and moderately lower PV, suggesting strong inhibition of input and reduced inhibition of output of pyramidal cells, suggesting E/I imbalance. (E) In the basolateral amygdala (BLA) of male subjects with MDD, there are no differences in SST or PV, suggesting conserved E/I balance. (F) In the BLA of female subjects with MDD, there is reduced expression of SST, but not PV, suggesting reduced inhibition of input of pyramidal cells and altered E/I balance.
SEX DIFFERENCES IN MDD: EVIDENCE FROM TRANSCRIPTOMICS STUDIES
Early transcriptomics investigations of MDD included both sexes but were often skewed toward males and not powered to investigate sex differences. Conclusions from these studies could be biased toward results driven by male-specific alterations; indicate alterations that are consistent between males and females if the cohort is relatively sex balanced but is not powered to detect sex differences; or show no differences if biological disturbances are in opposite directions between men and women. We summarize results of transcriptomics investigations in Table 1, providing information on whether both sexes were represented. Very few studies were fully powered to detect sex differences. These studies have used similar approaches, and results were validated across cohorts.
Table 1.
Results of Transcriptomics Investigations
| Reference | Brain Regions | Key Findings | Males and Females in Cohort |
|---|---|---|---|
| Evans et al., 2004 (138) | DLPFC, ACC (BA 24) | FGF-related genes | Control males: 6 Control females: 1 MDD males: 7 MDD females: 2 |
| Iwamoto et al., 2004 (139) | PFC (BA 10) | AQP4, LIM | Control males: 9 Control females: 6 MDD males: 6 MDD females: 5 |
| Choudary et al., 2005 (140) | DLPFC, ACC (BA 24) | GABA-, glutamate-related genes | Control males: 6 Control females: 1 MDD males: 7 MDD females: 2 |
| Aston et al., 2005 (141) | Temporal cortex | Oligodendrocyte-, myelination-related genes | Control males: 9 Control females: 5 MDD males: 9 MDD females: 3 |
| Kang et al., 2007 (142) | DLPFC | FGF-, immune-related genes, FOXD3, UCN3 | Control males: 11 Control females: 3 MDD males: 10 MDD females: 4 |
| Sequeira et al., 2007 (143) | Amygdala, hippocampus, ACC (BA 24), BA 29 | GABA-, neurotransmission-related genes | Control males: 13 MDD males: 18 |
| Tochigi et al., 2008 (144) | PFC (BA 10) | Cell proliferation-, FGF-related genes | Control males: 9 Control females: 6 MDD males: 6 MDD females: 5 |
| Klempan et al., 2009 (145) | PFC (BAs 44, 45, 46, 47) | GABA-related genes | Control males: 13 MDD males: 16 |
| Sequeira et al., 2009 (146) | 17 cortical and subcortical regions | GABA-, glutamate-related genes | Control males: 13 MDD males: 16 |
| Sibille et al., 2009 (62) | ACC (BA 25), AMY (lateral/basolateral/basomedial) | Oligodendrocyte-, neuronal-related genes in AMY | Control males: 14–16 MDD males: 14–16 |
| Lalovic et al., 2010 (147) | Frontal cortex (BAs 8/9, 11, 47) | Lipid metabolism-, immune responserelated genes | Control males: 6–13 MDD males: 10–15 |
| Bernard et al., 2011 (148) | Locus coeruleus | Glutamate-, growth factor-, astrocyterelated genes | Control males: 8 Control females: 1 MDD males: 11 MDD females: 1 |
| Sequeira et al., 2012 (149) | DLPFC, ACC (BA 24), NAc | Suicide-associated alterations in serotonin-and metallothioneinrelated genes | MDD males: 8–13 MDD females: 3 |
| Guilloux et al., 2012 (69) | AMY (lateral/basolateral/basomedial) | BDNF-, GABA-related genes | Control females: 21 MDD females: 21 |
| Duric et al., 2013 (150) | Dentate gyrus, CA1 | Glutamate- and synapse-related genes | Control males: 9 Control females: 6 MDD males: 9–10 MDD females: 5–6 |
| Labonte et al., 2017 (71) | DLPFC (BA 8/9), OFC (BA 11), ACC (BA 25), aINS, NAc, vSUB | Sex-specific signatures of MDD; DUSP6 as female-specific; EMX1 as male-specific | Control males: 13 Control females: 9 MDD males: 13 MDD females: 13 |
| Seney et al., 2018 (72) | DLPFC, ACC (BA 25), AMY (lateral/basolateral/basomedial) | MDD males: decreased synapse-, increased immune-related genes MDD females: increased synapse-, decreased immune-related genes |
Control males: 26 Control females: 24 MDD males: 26 MDD females: 24 |
| Issler et al., 2020 (83) | DLPFC (BA 8/9), OFC (BA 11), ACC (BA 25), aINS, NAc, vSUB | Sex-specific alterations in lncRNAs; LINC00473 as female-specific | Control males: 13 Control females: 9 MDD males: 13 MDD females: 13 |
ACC, anterior cingulate cortex; aINS, anterior insula; AMY, amygdala; BA, Brodmann area; BDNF, brain-derived neurotrophic factor; DLPFC, dorsolateral prefrontal cortex; FGF, fibroblast growth factor; GABA, gamma-aminobutyric acid; lncRNA, long noncoding RNA; MDD, major depressive disorder; NAc, nucleus accumbens; OFC, orbitofrontal cortex; PFC, prefrontal cortex; vSUB, ventral subiculum.
In 2017, Labonte et al. (71) published the first well-powered, large-scale transcriptomics study to directly assess sex differences in the brains of subjects with MDD, focusing on the DLPFC, orbitofrontal cortex, ACC, anterior insula, nucleus accumbens, and ventral subiculum. The authors found very little overlap of genes differentially expressed in both men and women with MDD and used network-based approaches to zero in on potentially relevant gene targets. Weighted gene coexpression network analysis identified gene modules exhibiting sex-specific patterns of gain and loss of connectivity in MDD. This analysis suggests that some molecular pathways implicated in MDD might be affected in a sex-specific manner. Examination of gene modules identified relevant sex-specific hub genes, which, when manipulated in mice, produced sex-specific stress vulnerability. Downregulation of the female MDD–specific gene, DUSP6, promoted stress vulnerability in females only, potentially by increasing pyramidal neuron excitability via activation of ERK signaling. Additionally, overexpression of the male MDD–specific gene, EMX1, promoted stress vulnerability in males only. Using differential expression analysis combined with network-based methods, this study narrowed down their gene hits and zeroed in on potentially relevant sex-specific hub genes that might drive sex differences in depression (71).
Recently, we used large-scale transcriptomics, combined with meta-analysis across mood-related brain regions (DLPFC, ACC, BLA), to investigate sex-specific alterations in MDD (72). We replicated the findings of Labonte et al. (71), showing little overlap in differentially expressed genes between men and women with MDD. Brains from our study were from a different brain bank than Labonte et al. (71), suggesting the sex-specific transcriptional signature of depression may be a generalizable biological phenomenon. We found more than 1000 transcripts with significant interactions of sex and disease, and these transcripts were affected in opposite directions in men and women with MDD (i.e., upregulated in one sex, downregulated in the other sex). While this was initially quite surprising, we replicated this result using the data of Labonte et al. (71), hence confirming the presence of opposite patterns of gene changes across cohorts obtained from different brain banks. We found that men with MDD have decreases in markers of synapses and increases in markers of microglia and inflammation (72), suggesting that men with MDD have decreased dendritic spines and more reactive microglia. This result in men with MDD is consistent with previous reports of reduced dendritic complexity in the ACC, reduced dendritic processes and spine synapses in the DLPFC (73), and increased reactive microglia in the ACC in MDD (74). Chronic stress in male rodents recapitulates the dendritic pathology observed in MDD (75,76), and it is hypothesized that dendritic spine alterations contribute to MDD symptoms (77,78). These changes in MDD are consistent with a model in which reactive microglia may participate in excessive pathological removal of synapses (79–81).
However, as more than 70% of subjects in previous studies of dendritic morphology and microglia reactivity in MDD were male, it is unclear if females with MDD exhibit a similar pathology. Surprisingly, we found opposite gene expression changes in women with MDD, with increased synapse-related genes and decreased expression of microglia-specific genes (72). Our results in men and women with MDD are consistent with rodent studies demonstrating that stressed male mice have decreased dendritic spine complexity and increased reactive microglia, but the opposite pattern occurs in stressed females (75,82). Together, these cross-species studies of putative interactions between microglia and dendrites suggest sex-specific pathways for divergent pathologies relevant to information processing mediated by spines on excitatory pyramidal neurons (Figure 2).
Figure 2.
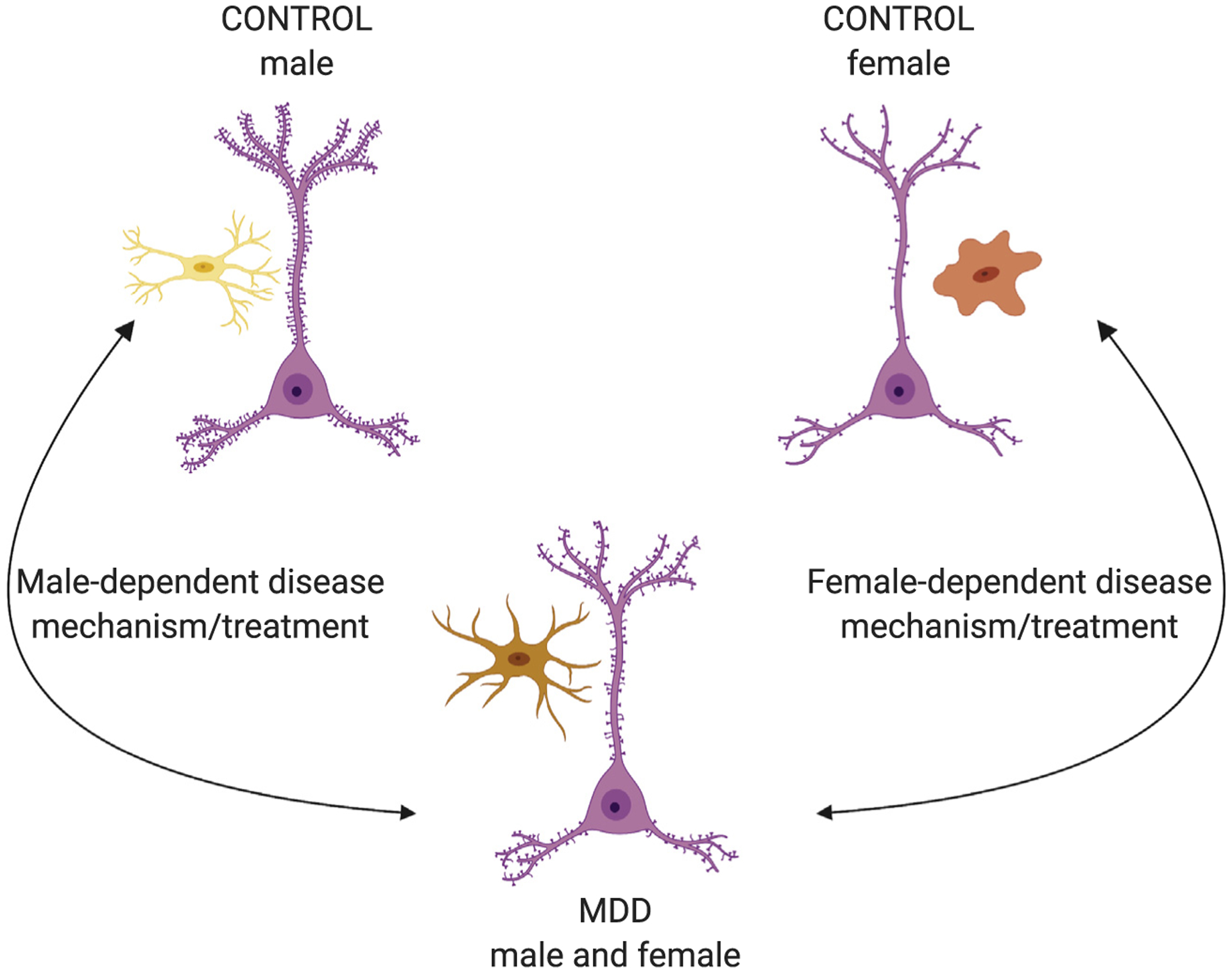
Proposed model of sex-specific major depressive disorder (MDD) mechanism involving dendritic spines and microglia. Male control subjects have more dendritic spines and fewer activated microglia than male subjects with MDD, but female control subjects have fewer dendritic spines and more activated microglia than female subjects with MDD. MDD effects appear to be opposite in male and female subjects, converging on a similar MDD phenotype across sexes. Successful treatment would push male and female subjects back to their sex-specific baselines, which would require opposite effects on microglia. Further, it is possible that a successful treatment in one sex might push the other sex further from their sex-specific baseline, which could be detrimental. Pyramidal cells are in pink. Ramified microglia are in yellow, reactive/ameboid microglia are in red, and intermediate microglia are in orange.
Another exciting contribution was added in 2020 by Issler et al. (83). Using the Labonte et al. (71) dataset, the authors focused on long noncoding RNAs (lncRNAs), as these represented approximately 30% of the differentially expressed transcripts. Through various roles, including as signals, scaffolds, enhancers, and decoys, lncRNAs play key roles in regulating transcription of other genes. Identified lncRNAs might be key drivers of the sex-specific transcriptional profile of MDD. Similar to the pattern of results for protein-coding genes reported by Labonte et al. (71) and Seney et al. (72), there was little overlap in differentially expressed lncRNAs between men and women with MDD. The authors then zeroed in on LINC00473, as this transcript is of high abundance, exhibits lower expression only in depressed women, and is expressed in neurons and neural progenitor cells. Interestingly, LINC00473 is associated with several genes previously implicated in MDD, including the female MDD–specific gene, DUSP6, identified by Labonte et al. (71). When expression of LINC00473 is induced in mice, it promotes female, but not male, resilience to stress, potentially via its effect on electro-physiological properties of pyramidal neurons in females. When LINC00473 expression is induced in human-derived neuroblastoma cells, it binds to relevant depression and anxiety-related genes, including CREBBP, MAP2, and ERBB4. Thus, LINC00473 may be an upstream regulatory driver of female-specific MDD mechanisms. Exactly why changes in levels of this same lncRNA do not produce effects in males is currently unknown.
Altogether, recent large-scale transcriptomics studies have provided strong evidence for sex-specific molecular signatures of MDD. These studies also delve deeper into mechanisms, point toward potential key drivers influencing sex-specific MDD vulnerability (e.g., DUSP6, EMX1, LINC00473), and suggest opposing brain pathological changes in depressed men and women.
HUMAN POSTMORTEM BRAIN RESEARCH CHALLENGES AND RECOMMENDATIONS FOR PATHS FORWARD
Studies of postmortem human brain are essential to identify disease- and sex-specific molecular, cellular, and circuitry alterations characteristic of MDD. Completion of such studies and interpretation of results require consideration of factors largely unique to human postmortem brain research, and these have been comprehensively reviewed [e.g., (84)]. Here, we discuss 5 aspects of postmortem human brain study design of particular consideration for investigations of the MDD disease process and suggest paths forward for thoughtful design of future studies.
One consideration is the potential effect of comorbid diagnoses to influence, or be influenced by, the MDD disease process on the measures of interest. MDD is often comorbid with anxiety disorders, and this is especially pronounced in women (13). Whether MDD disease processes include molecular, cellular, or circuitry alterations that trigger anxiety disorder pathogenic processes or whether each disorder has an independent and additive effect on a dependent measure can be informed by postmortem human brain studies. For example, a finding that the magnitude of the MDD female-specific effect on GABA synthesizing enzyme levels in the BLA (69) is even greater in female subjects with MDD and a comorbid anxiety disorder would suggest a shared and additive effect of both disorders on this measure. In contrast, the presence of this effect in female subjects with MDD and a comorbid anxiety disorder, but not in subjects with only an MDD or an anxiety disorder diagnosis, suggests that a sex-specific aspect of the MDD disease process contributes to the development of an anxiety disorder.
A second consideration is the potential effect of symptom heterogeneity on the measures of interest within subjects with MDD. Indeed, although many psychiatric illnesses have heterogeneous diagnostic symptoms, MDD is particularly diverse (85). For example, individuals with symptoms including weight gain or loss, insomnia or hypersomnia, psychomotor agitation, or retardation all may meet criteria for an MDD diagnosis (85). Depending on the disease-related experimental question, this heterogeneity may be critical to consider during study design to produce results that can inform how brain alterations may map onto MDD symptoms. For instance, a study of the MDD disease effects in a sleep-relevant brain region (e.g., lateral hypothalamus) is strengthened by inclusion of MDD subjects with one type of sleep disturbance (e.g., insomnia) or equal numbers of MDD subjects with insomnia or hypersomnia. This consideration may be especially relevant in the study of sex differences in MDD, as there is evidence for different symptom profiles in men and women (11).
A third consideration is the potential effect of death by suicide. On average, approximately 60% of individuals who died by suicide had a prior mood disorder (86). Notably, women report a greater frequency of suicidal thoughts and attempts than men, but men die by suicide more commonly than women (87,88). Neural alterations associated with death by suicide in individuals with MDD may index disease severity, reflect the influence of certain suicide and MDD risk factors such as early childhood adversity, and/or represent a suicide-specific and diagnosis-independent finding (89–94).
A fourth consideration is the potential effect of treatment by antidepressant medications on the measures of interest. The efficacy of these medications may include mechanisms that alter neural functioning in a manner that obscures or eliminates the MDD disease effect on molecular-, cellular-, or circuitry-dependent measures. For instance, subjects with MDD not taking antidepressants have lower striatal levels of dihydroxyphenylacetic acid and higher PFC binding levels of β1 adrenoreceptors (95), suggesting that these alterations are related to MDD disease processes rather than effects of antidepressants. The triangulation method, which uses 3 different approaches to disentangle medication and disease effects, is one effective strategy previously used in postmortem human brain studies [e.g., (96–99)]. In the first approach, the dependent measure is compared between subjects with MDD on and off antidepressant medications at the time of death. A finding of no difference suggests that the medications do not affect the measure of interest. In the second approach, the dependent measure is compared between subjects with MDD and subjects with a different psychiatric illness also treated with antidepressant medications. A finding in antidepressant-treated subjects with MDD, but not antidepressant-treated subjects with a different psychiatric illness, suggests that the medications do not affect the measure of interest. In the third approach, the dependent measure is quantified in animal models that mimic treatment in humans. A finding of no effect suggests that the medications do not affect the measure of interest. Consistency of results across each of the 3 approaches strongly supports that the finding in subjects with MDD reflects a factor related to the disease process and not antidepressant medications.
A fifth consideration is the potential confounding effect of inadequate tissue preservation. Key measures reflecting tissue quality include 1) postmortem interval, the amount of time between death and brain preservation; 2) RNA integrity number, a measure of RNA quality and concentration; and 3) brain pH, which is correlated with RNA yield and quality. Matching subjects across diagnostic groups as closely as possible on these factors minimizes sources for technical variability and helps to ensure that findings are not confounded by inferior tissue quality in one subject group. Further, rigorous experimental design to identify sex-specific MDD disease effects ensures that these factors are similarly represented in male and female subjects both within and across diagnostic groups.
In conclusion, accurate mapping of the neural alterations that contribute to sex-specific brain dysfunction in MDD requires postmortem human brain studies that take into consideration these clinical and technical aspects. Although tissue quality measures are routinely determined for postmortem human brain samples, the clinical considerations require a systematic and comprehensive characterization that identifies each individual’s comorbid diagnoses, MDD symptom profile, and treatment at time of death (100). Studies of clinically well-characterized subjects can provide key insights into how brain pathological alterations map onto MDD symptoms. These studies can also provide results meaningful for the development of novel, personalized, and evidence-based therapeutics. Indeed, studies investigating whether distinct molecular phenotypes are present in subjects with distinct symptom phenotypes hold promise for the development of personalized therapeutics (101–103).
BIOLOGICAL FACTORS UNDERLYING THE ROLE OF SEX
Alterations observed in the human brain do not necessarily drive sex differences in MDD. For instance, sex differences may reflect compensatory mechanisms, may be due to unknown confounds, or may be downstream of primary pathology. Studies in model systems are therefore key to determining causation. While many previous studies used rodent stress paradigms designed for males, recent studies have established paradigms that also work in females (104–107), thus providing a preclinical venue to compare the transcriptional alterations observed in MDD. While beyond the scope of this review, Rainville and Hodes provide a thorough review of rodent literature related to sex differences in stress response and discuss peripheral mechanisms that might drive sex differences in MDD (108). We highlight various nuances to consider when determining mechanisms underlying observed sex differences.
As genetic sex (XX or XY) is inextricably linked to gonadal sex (ovaries or testes) in humans, it is challenging to determine the underlying causes of sex differences (hormones vs. sex chromosomes). Except for rare genetic conditions, XY individuals have testes, and XX individuals have ovaries. XY individuals have male-like circulating hormones in adulthood that may influence the sex difference (i.e., acute, activational effect of hormone) and also are exposed to male-like circulating hormones during sensitive developmental periods (109). While many sex differences are caused by acute hormone effects, there are instances where hormone exposure during sensitive periods cause permanent sex differences (i.e., organizational hormone effect) (110,111). The sensitive developmental window varies based on the trait examined (112) and can extend from the prenatal period through puberty (113–115). In support of a role of gonadal hormones, many sex differences in the human brain and behavior appear at puberty and are correlated with gonadal hormone levels (116–118). Permanent sex differences are challenging to investigate in humans, as it is not possible to determine gonadal hormone levels during sensitive periods, as some occur prenatally. Evidence also suggests that genetic sex, independent of gonadal hormones, influences social behavior (119,120), alcohol abuse (121), habit formation (122), aggressive and parenting behavior (123), and gene expression (124–129). Given complications in disentangling effects of genetic sex, developmental gonadal hormone exposure, and adult hormone exposure, it is often necessary to move to animal models. There are comprehensive reviews on this topic (130–133).
While an identified sex difference may be caused by a single sex-related factor, it is possible that multiple mechanisms work in tandem to create the sex difference. The sum of these sex-biased effects on gene networks and cells is referred to as the sexome, which is reviewed by Arnold and Lusis (134). For instance, organizational effects of hormones often interact with adult circulating hormones for full expression of the sex difference. In other words, acute hormonal effects in adulthood may occur only if proper hormones were present during sensitive developmental periods.
Although one might predict that sex-related factors work together to promote a sex difference (i.e., two factors push the sex difference in the same direction), this is not always the case. In fact, two sex-related factors might work in opposite directions, sometimes even to eliminate a sex difference, a phenomenon termed compensation (135). Examples of compensation are that male-typical hormones (androgens) increase weight and adiposity, but male genetic sex (XY) decreases them; removing androgens amplifies the XY genetic sex effect, as the opposing factor is not present to balance it (136,137); or, conversely, XY genetic sex increases anxiety-like behaviors, which are compensated by adult circulating testosterone (127). Additionally, males and females might arrive at the same trait via different mechanisms (e.g., XX effect in females, testosterone effect in males).
Altogether, various strategies can be employed in animal models to disentangle mechanisms underlying sex differences observed in humans (131–133). However, it is often not as simple as one factor working independently to cause a sex difference. Rather, many factors may work together or in opposition.
SUMMARY
Recent advances in basic neuroscience knowledge and the development of novel investigational tools are now merging with a renewed interest in understanding the biological bases of the well-characterized clinical differences in depression between men and women. We have highlighted candidate gene and large-scale transcriptomics studies suggesting divergent pathologies in depressed men and women. We discussed challenges encountered when performing human postmortem brain studies in MDD and propose paths forward for future studies. Approaches in model systems are highlighting the complexity of examining mechanisms underlying sex differences. These novel perspectives are generating hypotheses and tools to investigate the putative roles and interactions of factors underlying the biological bases of sex differences in depression.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the National Institute of Mental Health (Grant No. R01 MH120066 [to MLS] and Grant No. R01 MH077159 [to ES]), Brain & Behavior Research Foundation Young Investigator Grant (to MLS) and a Distinguished Investigator Award (to ES), and Campbell Family Mental Health Research Institute (to ES).
Images were created with BioRender.com.
ES is cofounder of Alpha-Cog, a biotech company dedicated to develop novel therapeutics for cognitive deficits in brain disorders. MMLS and JG report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Smith K (2014): Mental health: A world of depression. Nature 515:181. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC (2015): The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 76:155–162. [DOI] [PubMed] [Google Scholar]
- 3.Mann JJ (2003): Neurobiology of suicidal behaviour. Nat Rev Neurosci 4:819–828. [DOI] [PubMed] [Google Scholar]
- 4.Kendler KS, Prescott CA, Myers J, Neale MC (2003): The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry 60:929–937. [DOI] [PubMed] [Google Scholar]
- 5.Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. (2000): Gender differences in chronic major and double depression. J Affect Disord 60:1–11. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005): Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602. [DOI] [PubMed] [Google Scholar]
- 7.Mackenzie CS, Reynolds K, Cairney J, Streiner DL, Sareen J (2012): Disorder-specific mental health service use for mood and anxiety disorders: Associations with age, sex, and psychiatric comorbidity. Depress Anxiety 29:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang PS, Lane M, Olfson M, Pincus HA, Wells KB, Kessler RC (2005): Twelve-month use of mental health services in the United States: Results from the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:629–640. [DOI] [PubMed] [Google Scholar]
- 9.Angst J, Dobler-Mikola A (1984): Do the diagnostic criteria determine the sex ratio in depression? J Affect Disord 7:189–198. [DOI] [PubMed] [Google Scholar]
- 10.Weissman MM, Klerman GL (1977): Sex differences and the epidemiology of depression. Arch Gen Psychiatry 34:98–111. [DOI] [PubMed] [Google Scholar]
- 11.Frank E, Carpenter LL, Kupfer DJ (1988): Sex differences in recurrent depression: Are there any that are significant? Am J Psychiatry 145:41–45. [DOI] [PubMed] [Google Scholar]
- 12.Young MA, Fogg LF, Scheftner WA, Keller MB, Fawcett JA (1990): Sex differences in the lifetime prevalence of depression: Does varying the diagnostic criteria reduce the female/male ratio? J Affect Disord 18:187–192. [DOI] [PubMed] [Google Scholar]
- 13.Silverstein B (1999): Gender difference in the prevalence of clinical depression: The role played by depression associated with somatic symptoms. Am J Psychiatry 156:480–482. [DOI] [PubMed] [Google Scholar]
- 14.Najt P, Fusar-Poli P, Brambilla P (2011): Co-occurring mental and substance abuse disorders: A review on the potential predictors and clinical outcomes. Psychiatry Res 186:159–164. [DOI] [PubMed] [Google Scholar]
- 15.Quitkin FM, Stewart JW, McGrath PJ, Taylor BP, Tisminetzky MS, Petkova E, et al. (2002): Are there differences between women’s and men’s antidepressant responses? Am J Psychiatry 159:1848–1854. [DOI] [PubMed] [Google Scholar]
- 16.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. (2006): Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am J Psychiatry 163:28–40. [DOI] [PubMed] [Google Scholar]
- 17.Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. (2000): Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry 157:1445–1452. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton JA, Grant M, Jensvold MF (1996): Sex and treatment of depression: When does it matter? In: Jensvold MF, Halbreich U, Hamilton JA, editors. Psychopharmacology and Women: Sex, Gender, and Hormones Washington, DC: American Psychiatric Press, 241–257. [Google Scholar]
- 19.Coyle CM, Laws KR (2015): The use of ketamine as an antidepressant: A systematic review and meta-analysis. Hum Psychopharmacol 30:152–163. [DOI] [PubMed] [Google Scholar]
- 20.Scheibe S, Preuschhof C, Cristi C, Bagby RM (2003): Are there gender differences in major depression and its response to antidepressants? J Affect Disord 75:223–235. [DOI] [PubMed] [Google Scholar]
- 21.Freeman MP, Papakostas GI, Hoeppner B, Mazzone E, Judge H, Cusin C, et al. (2019): Sex differences in response to ketamine as a rapidly acting intervention for treatment resistant depression. J Psychiatr Res 110:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niciu MJ, Luckenbaugh DA, Ionescu DF, Guevara S, Machado-Vieira R, Richards EM, et al. (2014): Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry 75:e417–e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuijpers P, Van Straten A, Warmerdam L, Smits N (2008): Characteristics of effective psychological treatments of depression: A metaregression analysis. Psychother Res 18:225–236. [DOI] [PubMed] [Google Scholar]
- 24.Thase ME, Friedman ES, Biggs MM, Wisniewski SR, Trivedi MH, Luther JF, et al. (2007): Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: A STAR*D report. Am J Psychiatry 164:739–752. [DOI] [PubMed] [Google Scholar]
- 25.Watson HJ, Nathan PR (2008): Role of gender in depressive disorder outcome for individual and group cognitive-behavioral treatment. J Clin Psychol 64:1323–1337. [DOI] [PubMed] [Google Scholar]
- 26.Wolitzky-Taylor KB, Arch JJ, Rosenfield D, Craske MG (2012): Moderators and non-specific predictors of treatment outcome for anxiety disorders: A comparison of cognitive behavioral therapy to acceptance and commitment therapy. J Consult Clin Psychol 80:786–799. [DOI] [PubMed] [Google Scholar]
- 27.Glenn D, Golinelli D, Rose RD, Roy-Byrne P, Stein MB, Sullivan G, et al. (2013): Who gets the most out of cognitive behavioral therapy for anxiety disorders? The role of treatment dose and patient engagement. J Consult Clin Psychol 81:639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McHugh RK, Whitton SW, Peckham AD, Welge JA, Otto MW (2013): Patient preference for psychological vs pharmacologic treatment of psychiatric disorders: A meta-analytic review. J Clin Psychiatry 74:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saunders SM (2001): Pretreatment correlates of the therapeutic bond. J Clin Psychol 57:1339–1352. [DOI] [PubMed] [Google Scholar]
- 30.Bandura A (1977): Self-efficacy: Toward a unifying theory of behavioral change. Psychol Rev 84:191–215. [DOI] [PubMed] [Google Scholar]
- 31.Penninx BW, Nolen WA, Lamers F, Zitman FG, Smit JH, Spinhoven P, et al. (2011): Two-year course of depressive and anxiety disorders: Results from the Netherlands Study of Depression and Anxiety (NESDA). J Affect Disord 133:76–85. [DOI] [PubMed] [Google Scholar]
- 32.Guilloux JP, Bassi S, Ding Y, Walsh C, Turecki G, Tseng G, et al. (2015): Testing the predictive value of peripheral gene expression for nonremission following citalopram treatment for major depression. Neuropsychopharmacology 40:701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, et al. (2009): Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62:479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nemeroff CB, Entsuah R, Benattia I, Demitrack M, Sloan DM, Thase ME (2008): Comprehensive analysis of remission (COMPARE) with venlafaxine versus SSRIs. Biol Psychiatry 63:424–434. [DOI] [PubMed] [Google Scholar]
- 35.Thase ME, Entsuah AR, Rudolph RL (2001): Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J Psychiatry 178:234–241. [DOI] [PubMed] [Google Scholar]
- 36.Shilyansky C, Williams LM, Gyurak A, Harris A, Usherwood T, Etkin A (2016): Effect of antidepressant treatment on cognitive impairments associated with depression: A randomised longitudinal study. Lancet Psychiatry 3:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckman JEJ, Underwood A, Clarke K, Saunders R, Hollon SD, Fearon P, et al. (2018): Risk factors for relapse and recurrence of depression in adults and how they operate: A four-phase systematic review and meta-synthesis. Clin Psychol Rev 64:13–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin LC, Sibille E (2013): Reduced brain somatostatin in mood disorders: A common pathophysiological substrate and drug target? Front Pharmacol 4:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goswami DB, May WL, Stockmeier CA, Austin MC (2010): Transcriptional expression of serotonergic regulators in laser-captured microdissected dorsal raphe neurons of subjects with major depressive disorder: Sex-specific differences. J Neurochem 112:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szewczyk B, Albert PR, Burns AM, Czesak M, Overholser JC, Jurjus GJ, et al. (2009): Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. Int J Neuropsychopharmacol 12:155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martenyi F, Dossenbach M, Mraz K, Metcalfe S (2001): Gender differences in the efficacy of fluoxetine and maprotiline in depressed patients: A double-blind trial of antidepressants with serotonergic or norepinephrinergic reuptake inhibition profile. Eur Neuropsychopharmacol 11:227–232. [DOI] [PubMed] [Google Scholar]
- 42.Hashimoto K, Sawa A, Iyo M (2007): Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry 62:1310–1316. [DOI] [PubMed] [Google Scholar]
- 43.Lan MJ, McLoughlin GA, Griffin JL, Tsang TM, Huang JT, Yuan P, et al. (2009): Metabonomic analysis identifies molecular changes associated with the pathophysiology and drug treatment of bipolar disorder. Mol Psychiatry 14:269–279. [DOI] [PubMed] [Google Scholar]
- 44.Berrettini WH, Nurnberger JI Jr, Hare TA, Simmons-Alling S, Gershon ES, Post RM (1983): Reduced plasma and CSF gamma-aminobutyric acid in affective illness: Effect of lithium carbonate. Biol Psychiatry 18:185–194. [PubMed] [Google Scholar]
- 45.Petty F, Kramer GL, Dunnam D, Rush AJ (1990): Plasma GABA in mood disorders. Psychopharmacol Bull 26:157–161. [PubMed] [Google Scholar]
- 46.Petty F, Kramer GL, Fulton M, Davis L, Rush AJ (1995): Stability of plasma GABA at four-year follow-up in patients with primary unipolar depression. Biol Psychiatry 37:806–810. [DOI] [PubMed] [Google Scholar]
- 47.Petty F, Kramer GL, Gullion CM, Rush AJ (1992): Low plasma g-aminobutyric acid levels in male patients with depression. Biol Psychiatry 32:354–363. [DOI] [PubMed] [Google Scholar]
- 48.Petty F, Sherman AD (1984): Plasma GABA in psychiatric illness. J Affect Disord 6:131–138. [DOI] [PubMed] [Google Scholar]
- 49.Gold BI, Bowers MB, Roth RH, Sweeney DW (1980): GABA levels in CSF of patients with psychiatric disorders. Am J Psychiatry 137:362–364. [DOI] [PubMed] [Google Scholar]
- 50.Gerner RH, Hare TA (1981): CSF GABA in normal subjects and patients with depression, schizophrenia, mania, and anorexia nervosa. Am J Psychiatry 138:1098–1101. [DOI] [PubMed] [Google Scholar]
- 51.Kasa K, Otsuki S, Yamamoto M, Sato M, Kuroda H, Ogawa N (1982): Cerebrospinal fluid gamma-aminobutyric acid and homovanillic acid in depressive disorders. Biol Psychiatry 17:877–883. [PubMed] [Google Scholar]
- 52.Gerner RH, Fairbanks L, Anderson GM, Young JG, Scheinin M, Linnoila M, et al. (1984): CSF neurochemistry in depressed, manic and schizophrenic patients compared with that of normal controls. Am J Psychiatry 141:1533–1540. [DOI] [PubMed] [Google Scholar]
- 53.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Boorman E, M Matthews P, et al. (2008): Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol 11:255–260. [DOI] [PubMed] [Google Scholar]
- 54.Goddard AW, Mason GF, Almai A, Rothman DL, Behar KL, Petroff OAC, et al. (2001): Reductions in occipital cortex GABA levels in panic disorder detected with 1H-magnetic resonance spectroscopy. Arch Gen Psychiatry 58:556–561. [DOI] [PubMed] [Google Scholar]
- 55.Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, et al. (2009): Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: Relationship to treatment resistance in major depressive disorder. Biol Psychiatry 65:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, et al. (2004): Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry 61:705–713. [DOI] [PubMed] [Google Scholar]
- 57.Sanacora G, Mason GF, Rothman DL, Krystal JH (2002): Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry 159–163. [DOI] [PubMed] [Google Scholar]
- 58.Honig A, Bartlett JR, Bouras N, Bridges PK (1988): Amino acid levels in depression: A preliminary investigation. J Psychiatr Res 22:159–164. [DOI] [PubMed] [Google Scholar]
- 59.Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G (2010): Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol 13:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E (2012): Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry 169:1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS (2015): Sex differences in glutamate receptor gene expression in major depression and suicide. Mol Psychiatry 20:1057–1068. [DOI] [PubMed] [Google Scholar]
- 62.Sibille E, Wang Y, Joeyen-Waldorf J, Gaiteri C, Surget A, Oh S, et al. (2009): A molecular signature of depression in the amygdala. Am J Psychiatry 166:1011–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fino E, Packer AM, Yuste R (2013): The logic of inhibitory connectivity in the neocortex. Neuroscientist 19:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Packer AM, McConnell DJ, Fino E, Yuste R (2013): Axo-dendritic overlap and laminar projection can explain interneuron connectivity to pyramidal cells. Cereb Cortex 23:2790–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jang HJ, Chung H, Rowland JM, Richards BA, Kohl MM, Kwag J (2020): Distinct roles of parvalbumin and somatostatin interneurons in gating the synchronization of spike times in the neocortex. Sci Adv 6: eaay5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ (2007): GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology 32:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tripp A, Kota RS, Lewis DA, Sibille E (2011): Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis 42:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seney ML, Tripp A, McCune S, Lewis DA, Sibille E (2015): Laminar and cellular analyses of reduced somatostatin gene expression in the subgenual anterior cingulate cortex in major depression. Neurobiol Dis 73:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. (2012): Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry 17:1130–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fee C, Banasr M, Sibille E (2017): Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: Cortical microcircuit and therapeutic perspectives. Biol Psychiatry 82:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Labonte B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. (2017): Sex-specific transcriptional signatures in human depression. Nat Med 23:1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seney ML, Huo Z, Cahill K, French L, Puralewski R, Zhang J, et al. (2018): Opposite molecular signatures of depression in men and women. Biol Psychiatry 84:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. (2012): Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med 18:1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N (2014): Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun 42:50–59. [DOI] [PubMed] [Google Scholar]
- 75.Garrett JE, Wellman CL (2009): Chronic stress effects on dendritic morphology in medial prefrontal cortex: Sex differences and estrogen dependence. Neuroscience 162:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cook SC, Wellman CL (2004): Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol 60:236–248. [DOI] [PubMed] [Google Scholar]
- 77.Pittenger C, Duman RS (2008): Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology 33: 88–109. [DOI] [PubMed] [Google Scholar]
- 78.Qiao H, Li MX, Xu C, Chen HB, An SC, Ma XM (2016): Dendritic spines in depression: What we learned from animal models. Neural Plast 2016:8056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salter MW, Stevens B (2017): Microglia emerge as central players in brain disease. Nat Med 23:1018–1027. [DOI] [PubMed] [Google Scholar]
- 80.Hong S, Dissing-Olesen L, Stevens B (2016): New insights on the role of microglia in synaptic pruning in health and disease. Curr Opin Neurobiol 36:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schafer DP, Stevens B (2015): Microglia function in central nervous system development and plasticity. Cold Spring Harb Perspect Biol 7:a020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bollinger JL, Bergeon Burns CM, Wellman CL (2016): Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav Immun 52:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Issler O, van der Zee YY, Ramakrishnan A, Wang J, Tan C, Loh YE, et al. (2020): Sex-specific role for the long non-coding RNA LINC00473 in depression. Neuron 106:912–926.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huitinga I, Webster MJ (2018): Brain banking. Handb Clin Neurol 150. [DOI] [PubMed] [Google Scholar]
- 85.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA: American Psychiatric Association. [Google Scholar]
- 86.Mann JJ (1998): The neurobiology of suicide. Nat Med 4:25–30. [DOI] [PubMed] [Google Scholar]
- 87.Canetto SS, Sakinofsky I (1998): The gender paradox in suicide. Suicide Life Threat Behav 28:1–23. [PubMed] [Google Scholar]
- 88.Murphy GE (1998): Why women are less likely than men to commit suicide. Compr Psychiatry 39:165–175. [DOI] [PubMed] [Google Scholar]
- 89.Underwood MD, Kassir SA, Bakalian MJ, Galfalvy H, Dwork AJ, Mann JJ, et al. (2018): Serotonin receptors and suicide, major depression, alcohol use disorder and reported early life adversity. Transl Psychiatry 8:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pantazatos SP, Huang YY, Rosoklija GB, Dwork AJ, Arango V, Mann JJ (2017): Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: Evidence for altered glial, endothelial and ATPase activity. Mol Psychiatry 22:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fitzgerald ML, Kassir SA, Underwood MD, Bakalian MJ, Mann JJ, Arango V (2017): Dysregulation of striatal dopamine receptor binding in suicide. Neuropsychopharmacology 42:974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Q, Roy B, Turecki G, Shelton RC, Dwivedi Y (2018): Role of complex epigenetic switching in tumor necrosis factor-alpha upregulation in the prefrontal cortex of suicide subjects. Am J Psychiatry 175:262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lutz PE, Mechawar N, Turecki G (2017): Neuropathology of suicide: Recent findings and future directions. Mol Psychiatry 22:1395–1412. [DOI] [PubMed] [Google Scholar]
- 94.Rossom RC, Coleman KJ, Ahmedani BK, Beck A, Johnson E, Oliver M, et al. (2017): Suicidal ideation reported on the PHQ9 and risk of suicidal behavior across age groups. J Affect Disord 215:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rivero G, Gabilondo AM, Garcia-Sevilla JA, La Harpe R, Callado LF, Meana JJ (2014): Increased alpha2- and beta1-adrenoceptor densities in postmortem brain of subjects with depression: Differential effect of antidepressant treatment. J Affect Disord 167:343–350. [DOI] [PubMed] [Google Scholar]
- 96.Enwright JF, Sanapala S, Foglio A, Berry R, Fish KN, Lewis DA (2016): Reduced labeling of parvalbumin neurons and perineuronal nets in the dorsolateral prefrontal cortex of subjects with schizophrenia. Neuropsychopharmacology 41:2206–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA (2005): The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: A comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology 30:1649–1661. [DOI] [PubMed] [Google Scholar]
- 98.Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, et al. (2008): Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry 13:147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lewis DA (2002): The human brain revisited: Opportunities and challenges in postmortem studies of psychiatric disorders. Neuropsychopharmacology 26:143–154. [DOI] [PubMed] [Google Scholar]
- 100.Glausier JR, Kelly MA, Salem S, Chen K, Lewis DA (2020): Proxy measures of premortem cognitive aptitude in postmortem subjects with schizophrenia. Psychol Med 50:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Volk DW, Sampson AR, Zhang Y, Edelson JR, Lewis DA (2016): Cortical GABA markers identify a molecular subtype of psychotic and bipolar disorders. Psychol Med 46:2501–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nemeroff CB (2020): The state of our understanding of the pathophysiology and optimal treatment of depression: Glass half full or half empty? Am J Psychiatry 177:671–685. [DOI] [PubMed] [Google Scholar]
- 103.Insel TR (2014): The NIMH Research Domain Criteria (RDoC) Project: Precision medicine for psychiatry. Am J Psychiatry 171:395–397. [DOI] [PubMed] [Google Scholar]
- 104.Takahashi A, Chung JR, Zhang S, Zhang H, Grossman Y, Aleyasin H, et al. (2017): Establishment of a repeated social defeat stress model in female mice. Sci Rep 7:12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iniguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, et al. (2018): Vicarious social defeat stress induces depression-related outcomes in female mice. Biol Psychiatry 83:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Finnell JE, Muniz BL, Padi AR, Lombard CM, Moffitt CM, Wood CS, et al. (2018): Essential role of ovarian hormones in susceptibility to the consequences of witnessing social defeat in female rats. Biol Psychiatry 84:372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guilloux JP, Seney M, Edgar N, Sibille E (2011): Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: Relevance to emotionality and sex. J Neurosci Methods 197:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rainville JR, Lipuma T, Hodes GE (2021): Translating the transcriptome: Sex differences in the mechanisms of depression and stress, revisited [published online ahead of print Feb 12]. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Phoenix CH, Goy RW, Gerall AA, Young WC (1959): Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65:369–382. [DOI] [PubMed] [Google Scholar]
- 110.Arnold AP, Gorski RA (1984): Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci 7:413–442. [DOI] [PubMed] [Google Scholar]
- 111.Breedlove SM, Cooke BM, Jordan CL (1999): The orthodox view of brain sexual differentiation. Brain Behav Evol 54:8–14. [DOI] [PubMed] [Google Scholar]
- 112.McCarthy MM, Wright CL, Schwarz JM (2009): New tricks by an old dogma: Mechanisms of the organizational/activational hypothesis of steroid-mediated sexual differentiation of brain and behavior. Horm Behav 55:655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arnold AP, Breedlove SM (1985): Organizational and activational effects of sex steroids on brain and behavior: A reanalysis. Horm Behav 19:469–498. [DOI] [PubMed] [Google Scholar]
- 114.Schulz KM, Molenda-Figueira HA, Sisk CL (2009): Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav 55:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Juraska JM, Sisk CL, DonCarlos LL (2013): Sexual differentiation of the adolescent rodent brain: Hormonal influences and developmental mechanisms. Horm Behav 64:203–210. [DOI] [PubMed] [Google Scholar]
- 116.Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, et al. (2010): Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc Natl Acad Sci U S A 107:16988–16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, Fink GR, et al. (2009): Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex 19:464–473. [DOI] [PubMed] [Google Scholar]
- 118.Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, et al. (2009): Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology 34:332–342. [DOI] [PubMed] [Google Scholar]
- 119.Cox KH, Rissman EF (2011): Sex differences in juvenile mouse social behavior are influenced by sex chromosomes and social context. Genes Brain Behav 10:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McPhie-Lalmansingh AA, Tejada LD, Weaver JL, Rissman EF (2008): Sex chromosome complement affects social interactions in mice. Horm Behav 54:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barker JM, Torregrossa MM, Arnold AP, Taylor JR (2010): Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. J Neurosci 30:9140–9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR (2007): Sex chromosome complement regulates habit formation. Nat Neurosci 10:1398–1400. [DOI] [PubMed] [Google Scholar]
- 123.Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, et al. (2006): Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci 26:2335–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barko K, Paden W, Cahill KM, Seney ML, Logan RW (2019): Sex-specific effects of stress on mood-related gene expression. Mol Neuropsychiatry 5:162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Puralewski R, Vasilakis G, Seney ML (2016): Sex-related factors influence expression of mood-related genes in the basolateral amygdala differentially depending on age and stress exposure. Biol Sex Differ 7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seney ML, Ekong KI, Ding Y, Tseng GC, Sibille E (2013): Sex chromosome complement regulates expression of mood-related genes. Biol Sex Differ 4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seney ML, Chang LC, Oh H, Wang X, Tseng GC, Lewis DA, et al. (2013): The role of genetic sex in affect regulation and expression of GABA-related genes across species. Front Psychiatry 4:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen X, Grisham W, Arnold AP (2009): X chromosome number causes sex differences in gene expression in adult mouse striatum. Eur J Neurosci 29:768–776. [DOI] [PubMed] [Google Scholar]
- 129.Abel JM, Witt DM, Rissman EF (2011): Sex differences in the cerebellum and frontal cortex: Roles of estrogen receptor alpha and sex chromosome genes. Neuroendocrinology 93:230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Arnold AP (2014): Conceptual frameworks and mouse models for studying sex differences in physiology and disease: Why compensation changes the game. Exp Neurol 259:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McCarthy MM, Arnold AP (2011): Reframing sexual differentiation of the brain. Nat Neurosci 14:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Arnold AP, Chen X (2009): What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol 30:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Arnold AP (2009): Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J Neuroendocrinol 21:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Arnold AP, Lusis AJ (2012): Understanding the sexome: Measuring and reporting sex differences in gene systems. Endocrinology 153:2551–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.De Vries GJ (2004): Minireview: Sex differences in adult and developing brains: Compensation, compensation, compensation. Endocrinology 145:1063–1068. [DOI] [PubMed] [Google Scholar]
- 136.Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, et al. (2012): The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet 8:e1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen X, McClusky R, Itoh Y, Reue K, Arnold AP (2013): X and Y chromosome complement influence adiposity and metabolism in mice. Endocrinology 154:1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, et al. (2004): Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci U S A 101:15506–15511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Iwamoto K, Kakiuchi C, Bundo M, Ikeda K, Kato T (2004): Molecular characterization of bipolar disorder by comparing gene expression profiles of postmortem brains of major mental disorders. Mol Psychiatry 9:406–416. [DOI] [PubMed] [Google Scholar]
- 140.Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, et al. (2005): Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A 102:15653–15658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Aston C, Jiang L, Sokolov BP (2005): Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry 10:309–322. [DOI] [PubMed] [Google Scholar]
- 142.Kang HJ, Adams DH, Simen A, Simen BB, Rajkowska G, Stockmeier CA, et al. (2007): Gene expression profiling in postmortem prefrontal cortex of major depressive disorder. J Neurosci 27:13329–13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sequeira A, Klempan T, Canetti L, ffrench-Mullen J, Benkelfat C, Rouleau GA, et al. (2007): Patterns of gene expression in the limbic system of suicides with and without major depression. Mol Psychiatry 12:640–655. [DOI] [PubMed] [Google Scholar]
- 144.Tochigi M, Iwamoto K, Bundo M, Sasaki T, Kato N, Kato T (2008): Gene expression profiling of major depression and suicide in the prefrontal cortex of postmortem brains. Neurosci Res 60:184–191. [DOI] [PubMed] [Google Scholar]
- 145.Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, ffrench-Mullen J, et al. (2009): Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry 14:175–189. [DOI] [PubMed] [Google Scholar]
- 146.Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, et al. (2009): Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One 4:e6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lalovic A, Klempan T, Sequeira A, Luheshi G, Turecki G (2010): Altered expression of lipid metabolism and immune response genes in the frontal cortex of suicide completers. J Affect Disord 120:24–31. [DOI] [PubMed] [Google Scholar]
- 148.Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, et al. (2011): Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry 16:634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sequeira A, Morgan L, Walsh DM, Cartagena PM, Choudary P, Li J, et al. (2012): Gene expression changes in the prefrontal cortex, anterior cingulate cortex and nucleus accumbens of mood disorders subjects that committed suicide. PLoS One 7:e35367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC, et al. (2013): Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol 16:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


