Abstract
The aberrant biology of polyploid giant cancer cells (PGCC) includes dysregulation of the cell cycle, induction of stress responses, and dedifferentiation, all of which are likely accompanied by adaptations in biophysical properties and metabolic activity. Sphingolipids are the second largest class of membrane lipids and play important roles in many aspects of cell biology that are potentially relevant to polyploidy. We have recently shown that the function of the sphingolipid enzyme acid ceramidase (ASAH1) is critical for the ability of PGCC to generate progeny by depolyploidization but mechanisms by which sphingolipids contribute to polyploidy and generation of offspring with stem-like properties remain elusive. This review discusses the role of sphingolipids during embryonic development, cell cycle regulation, and stem cells in an effort to highlight parallels to polyploidy.
Keywords: Sphingolipid, embryonic development, cell cycle, polyploidy, stem cells
1. Introduction
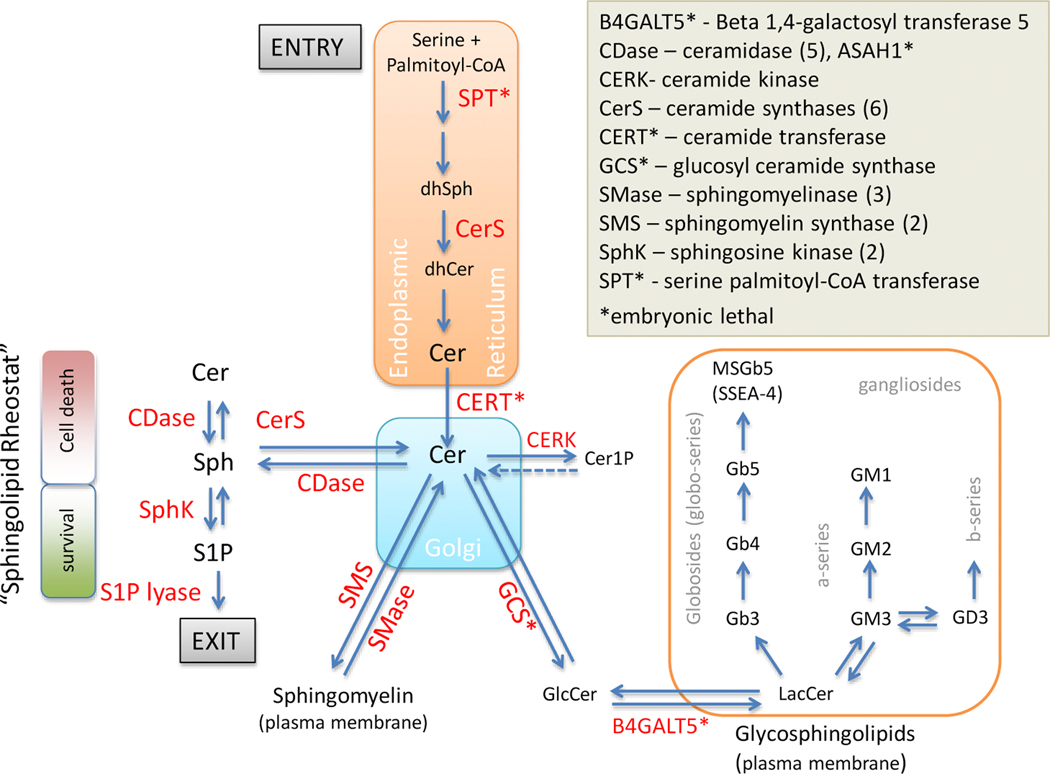
When the German pathologist Johann L.W. Thudichum discovered a new substance during his chemical analysis of the brain in the late 19th century, he may have named it “sphingosin” because its enigmatic nature reminded him of the riddles asked by the mythological creature known as the Sphinx [1]. Today, we recognize that sphingolipids are the second largest class of membrane lipids and play important roles in many aspects of cell biology, including but not limited to cell death or survival, membrane integrity, regulation of metabolism, and stress adaptation. These lipids are defined by their fatty acid being linked to a sphingoid base. The simplest sphingolipid ceramide serves as a metabolic hub, since it is the building block for a vast array of complex sphingolipids (Fig. 1). Sphingolipids exert their function either through lipid-lipid interaction to affect membrane properties or through lipid-protein interaction to modulate the function of target proteins. Consequently, sphingolipids play important roles in signal transduction and gene regulation with aberrations contributing to various pathologies.
Figure 1. An overview of sphingolipid metabolism.
For details please refer to the text. Enzymes are indicated in red font in the diagram.
The 1993 discovery that ceramide induces programmed cell death spawned significant interest in sphingolipid research similar to the attention that polyploid giant cancer cells (PGCC) have gained in recent years [2]. In 2018 my laboratory made a fortuitous discovery that linked sphingolipid metabolism to PGCC [3]. We were examining the mechanism by which inhibition of the sphingolipid enzyme acid ceramidase (ASAH1) prevented radiation therapy failure. Cheng at al. had shown that enzymatic inhibition of ASAH1 in combination with radiation resulted in durable cures in a preclinical model of prostate cancer [4]. ASAH1 catalyzes the removal of the fatty acid from ceramide to generate sphingosine, a molecule that is a substrate for sphingosine kinases, which generate the bioactive molecule sphingosine-1-phosphate (S1P) to promote survival and proliferation. Thus the prevailing thought was that inhibition of ASAH1 would facilitate cell death either by increasing levels of ceramide and/or by decreasing the availability of S1P. Unable to generate data in support of this hypothesis in short-term assays, we extended our endpoints and did observe that a significantly lower amount of cellular material remained when ASAH1 was inhibited. However, careful visual analysis revealed that the reduction in viable cells under ASAH1-deficient conditions was due to the absence of small cells that in cultures treated with radiation (or chemotherapy) alone surrounded large multinuclear cells. We had seen these bizarre polyploid cells previously but discounted their significance, since their morphology suggested they were destined to die.
A literature search led us to seminal papers that described the phenomenon we observed [5, 6]. Interestingly, PGCC have been documented in cancer specimen since the invention of the microscope and the field of PGCC research was initiated about 20 years ago when polyploidy was first described as a survival mechanism in cells with mutant p53 [7, 8]. PGCC form through fusion or endoreplication with resulting cells warehouse as many as 32 (or more) copies of the genome often in multiple nuclei [9]. PGCC assume a morphology resembling senescence, remain metabolically active, and are capable of undergoing a primitive, asymmetric cleavage-like form of cell division to release stored genetic material into smaller progeny. It is this step that is dependent on ASAH1, although the mechanism currently remains elusive. Reversible polyploidy has been called neosis or depolyploidization with offspring referred to as Raju or escape cells [6, 10]. The clinical significance of polyploidy and reversible senescence was not immediately recognized but more than 10 years later PGCCs were declared as critical drug targets in the war on cancer [11, 12]. Several independent groups have now shown that PGCC form in response to stress and these cells are capable to generate progeny with self-renewal features, but the topic has not gained widespread recognition [5, 6, 13, 14]. PGCC have been described as the “evil roots of cancer” and the “keystone species” within the tumor microenvironment without which the remainder of the tumor would collapse [14, 15]. The process of stress-induced transition through the PGCC state involves significant plasticity and genetic instability. Consequently, when PGCC progeny resume mitosis some offspring exhibit a more aggressive, therapy resistant phenotype than the parental cancer cell. Resistance together with stem-like and metastatic properties of offspring suggests that PGCCs are drivers of tumor relapse and recurrence. Hopefully, adaptation of a more unified nomenclature, identification of markers, and insights into PGCC biology will lead to multidisciplinary efforts to bring about innovative clinical trials that improve cancer therapy outcomes by preventing reversible senescence.
The therapeutic aspects of PGCC formation, tumor dormancy and relapse have been reviewed in an excellent paper by Mirzayans et al. [16]. The study of polyploidy and reversible senescence requires significantly longer time frames than most cancer researchers are accustomed to. Formation of PGCC in vitro varies between models but always exceeds the short time frame typically used to assess cytotoxic effects: In colon cancer cells treated with cisplatin PGCC were observed within 5–7 days [10]; in MDA-MB-231 breast cancer cells the peak of PGCC within the population following chemotherapy was reached on day 8–9 [17]; and in radiation treated Burkitts lymphoma cells PGCC peaked after one week [7]. In our experience formation of PGCC is a function of stress intensity and time. For example, the same percentage of PGCC can be obtained on day 5 after either fractionated (2Gy/day for 4 days) or single dose (8Gy once) radiation [3]. When using a range of 1–5nM docetaxel, PGCC formation occurs earlier at the 5nM dose but was also achieved at 1nM over a longer time frame (White-Gilbertson, unpublished observations). Once PGCC have developed, additional time is required to monitor cultures for progeny formation. Zhang et al quantified the appearance of PGCC progeny through cell counting found they emerged after day 8 Erenpreisa et al. also detected evidence of depolyploidization in week 2 [7]. Puig et al monitored the long-term evolution of tumors treated with cisplatin in a syngeneic rat model of colon cancer and in vitro modeling showed that formation of colonies from PGCC required a 2-week time frame [10]. In their in vivo model, tumors significantly regressed 10 days after cisplatin treatment but growth resumed one month later at approximately day 40. Similar in vivo kinetics were observed in a preclinical model of prostate cancer where tumors treated with fractionated radiation also continued to regress upon treatment cessation but recurred after day 40 [4]. We hypothesized that the relapse in the PPC1 prostate cancer model following radiation was due to PGCC and progeny formation via reversible senescence, which was confirmed and extended to other cell models [3, 18]. In vivo drug concentrations will vary across the tissue gradient. Lin et al. modeled this using an “evolution accelerator” and demonstrated that PC3 prostate cancer cells that continue to proliferate in a low docetaxel environment can migrate to a high dose environment, where they convert to PGCC. The authors suggest that the ability of cells to migrate across a spatially-varying stress landscape could significantly benefit their survival [19].
Nuclear events in polyploidy and reversible senescence have been studied extensively but little is known about the mechanisms by which lipids contribute to polyploidy or enable PGCC to produce progeny [13, 20, 21]. The sphingolipid ceramide is a second messenger of the stress response but the connection between sphingolipids and PGCC only emerged with our fortuitous discovery that the generation of PGCC progeny is highly dependent on acid ceramidase [3]. I will therefore focus this review on commonalities between sphingolipids and polyploidy in cancer, since novel insights into the mechanistic aspects of polyploidy may be gained from the sphingolipid field. First, because PGCC have been hypothesized to be the somatic equivalent of blastomeres I will review the role of sphinpolipids during embryonic development [15, 22, 23]. Second, since multinuclear polyploidy in cancer cells results from defective cell division, I will elaborate on the roles of sphingolipids during cell cycle progression with a focus on mitosis and cytokinesis. Finally, as the formation of PGCC involves significant dedifferentiation, I will discuss the role of sphingolipids in pluripotency and stem cells [15, 22, 23].
2. Sphingolipid Metabolism
De novo synthesis of sphingolipids involves a highly conserved 4-step process that is initiated when serine palmitoyl-CoA transferase (SPT) catalyzes the condensation of the amino acid serine and the acyl-CoA thioester palmitoyl-CoA in the endoplasmic reticulum (Fig. 1). The product 3-dehydro-D-sphinganine is converted to sphinganine (dihydrosphingosine/dhSph), which serves as the substrate for a family of six ceramide synthases (CerS). CerS have differential substrate preferences and conjugate fatty acids with side chains ranging from 14–30 carbons to dhSph, which results in a range of dihyroceramides (dhCer) that are then desaturated to produce multiple ceramide species. Thus the basic structure of ceramide is a long-chain sphingoid base linked to a fatty acid via an amide bond. Following de novo synthesis in the endoplasmic reticulum, ceramide is then transported to the Golgi by ceramide transferase (CERT), where the terminal primary hydroxyl group can be modified to generate complex sphingolipids. Conjugation to phosphocholine generates sphingomyelin (SM), which is an abundant component of membranes. Sphingomyelinases (SMase) catalyze the reverse reaction and are able to regenerate ceramide in membranes. Conjugation of ceramide to glucose by glucosylceramide synthase (GCS) is the initial step in the synthesis of glycosphingolipids, which are embedded into outer the leaflet of the membrane. Glycosphingolipids can be further modified with sialic acids to generate gangliosides that play important roles in signal transduction. Phosphorylation of ceramide results in the bioactive molecule ceramide-1-phosphate (C1P) that has a range of physiological functions in metabolic and pathologic processes [24]. Ceramide can also be hydrolyzed by acid (ASAH1), neutral (ASAH2), or alkaline ceramidases (ACER1–3) to produce sphingosine, which due to the removal of the fatty acid is much less hydrophobic than ceramide. Sphingosine plays a central role in sphingolipid metabolism because it serves as a substrate for two groups of enzymes that generate bioactive sphingolipids with opposing functions (Fig 1). CerS utilize sphingosine to regenerate ceramide, which is generally associated with anti-proliferative roles, while the addition of a phosphate group by sphingosine kinases (SphK) produces pro-survival sphingosine-1-phosphate (S1P). The degradation of S1P by S1P lyase constitutes the exit of sphingolipid metabolism.
While de novo synthesis of ceramides involves 4 simple steps, sphingolipid metabolism is overall complex due to the variety of molecules, reversibility of reactions, and compartmentalization. The array of fatty acids used by CerS generates several dozens of ceramide species all of which can be further modified yielding a highly complex pool of sphingolipids. Currently, more than 1600 structures of sphingolipids have been curated in the LIPID MAPS® database (www.lipidmaps.org). Many sphingolipid enzymes have multiple isoforms that differ in substrate specificity or pH optima and the majority of enzymatic reactions in the sphingolipid pathway are reversible. Ceramide itself can be produced de novo in the endoplasmic reticulum, through hydrolysis of sphingomyelin at membranes, and via the salvage pathway by CerS. Multiple modes of ceramide generation allow cells to produce a hydrophobic molecule “on demand” in various subcellular locations or cellular domains. In combination with the reversibility of most enzymatic reactions in sphingolipid metabolism, this facilitates a rapid flux of metabolites in response to a cellular stimulus that can occur in distinct subcellular compartments or domains under acidic, neutral or alkaline conditions.
3. Sphingolipids in Embryonic Development
PGCC are believed to be somatic equivalents of blastomeres and we have shown that the enzyme acid ceramidase is required for the generation of PGCC offspring [3, 23]. Therefore, our knowledge of sphingolipids in embryonic development might provide insight into the roles of sphingolipids in PGCC and their progeny. Given that sphingolipids play important structural roles and participate in cellular signaling it is not surprising that loss of key enzymes results in an embryonic lethal phenotype. Serine palmitoyl-CoA transferase (SPT) is a multimeric protein responsible for the initial rate-limiting step of sphingolipid synthesis. Homozygous loss of either the Sptlc1 or the Sptlc2 subunit is embryonic lethal and even a heterozygous loss significantly impacts ceramide and S1P levels [25]. Loss of glucosylceramide synthase, which catalyzes the initial step in the synthesis of glycosphingolipids, causes an embryonic lethal phenotype during gastrulation characterized by intense apoptosis in the ectodermal layer [26]. Lack of B4GALT5, which uses glucosylceramide (GlcCer) to generate lactosylceramide (LacCer), resulted in embryonic death on day E10.5. Hemorrhage and hematomas were observed at the anti-mesometrial pole of extra-embryonic tissue surrounded by trophoblast giant cells that normally accumulate at the mesometrial pole, which suggested defective extra-embryonic development was disturbed [27]. Another mutant mouse phenotype that is embryonic lethal was CERT. CERT transfers ceramide from the ER to the Golgi for further metabolism. Loss of CERT did not affect total ceramide levels but altered subcellular distribution. Ceramide was low in the plasma membrane but high in the endoplasmic reticulum, which enhanced stress and impacted mitochondria. It is noteworthy that no defects were observed in E3.5 blastocysts when grown in vitro for 3 days. The inner cell mass and outgrowth of the polyploid trophoectoderm were indistinguishable from controls and gross examination of mutant embryos did not reveal developmental defects, except for the heart [28]. Unfortunately, S1P was not analyzed in these mice but can be predicted to be low since AKT signaling was impaired. Thus these mice may have died due to lack of S1P and defects in blood vessel development (see below). These developmental defects not only demonstrate that sphingolipids are required but that proper subcellular distribution is crucial as well. Many other sphingolipid enzymes have multiple isoforms or family members that catalyze the same biochemical function. Loss of one isoform is often permissive for embryonic survival but transgenic models have still elucidated key roles during development.
3.1. Ceramidases
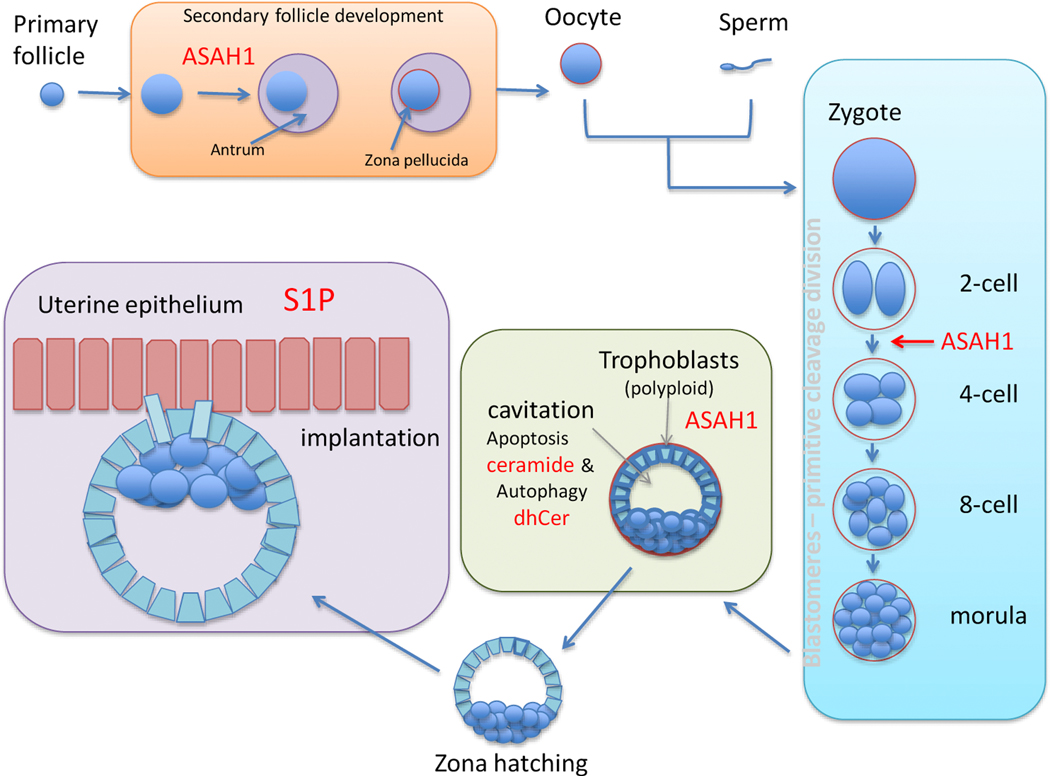
All ceramidases hydrolyze ceramide to produce sphingosine. Since neither loss of neutral (ASAH2) nor alkaline ceramidases (Acer1–3) is embryonic lethal, I will focus on the lysosomal enzyme acid ceramidase (ASAH1) that plays a role in fertility and embryonic development [29–31] The Schuchman laboratory, which cloned and characterized ASAH1, found that enzyme levels are high during the early stages of the ovarian cycle, when the developing follicle becomes surrounded by the theca, a layer of connective tissue and associated blood vessels [32, 33]. During progression to secondary follicle development, the granulosa cells surrounding the single oocyte proliferate and together with the thecal cells secrete antral fluid that nourishes the maturing ovum. Loss of ASAH1 results in defective transition of the follicle from the secondary to antral stage (Fig. 2). In ovaries without ASAH1 levels of ceramide were elevated, which was accompanied by increases in Bax and PARP as well as decreases in Bcl-2 and SOX9, a transcription factor for anti-Muellerian hormone that serves as a marker of ovarian reserve [34]. In conditional ASAH1 knockout mice only homozygous knockout females lacked ASAH1 expression and activity in ovaries compared to wild type, heterozygous, and conditional knock controls and experienced a significant decrease in infertility. On day 17 only 4/13 conditional ASAH1 knockout females (compared to 10/12 in control) were pregnant after being mated to wild type males and resulted in only one pup that died shortly after birth. This loss of fertility is consistent with a role of ASAH1 in oocyte protection. Thus ASAH1 appears to play an important anti-apoptotic, protective function during oocyte and follicle development.
Figure 2. Roles of sphingolipid metabolism during early embryonic development.
ASAH1 is important for oocyte and follicle development, survival of pre-implantation embryo and trophoblast differentiation. Maternal S1P is required during implantation of the embryo.
In addition to protection of the ovary and a role in fertility, ASAH1 expression in human cumulus cells and follicular fluid also sustains embryo survival. A study investigating the effect of C2-ceramide in bovine embryos showed that 2-cell stage but not 5 day embryos are resistant to apoptosis [35]. A positive correlation was observed between levels of ASAH1 and the quality of human embryos formed in vitro. In bovine oocytes the addition of recombinant ASAH1 resulted in a significant increase in morphologically intact embryos (20% to 70%). Similarly in the mouse, supplementation of the culture medium with recombinant ASAH1 approximately doubled the formation of embryos (from 40% to 88%) upon in vitro fertilization and increased healthy births by about 5-fold [36].
The ovum is an asymmetric cell with one pole developing into future ecto- and mesoderm and the other developing into endoderm. It is covered in a protective layer called the zona pellucida, which a sperm penetrates to create a zygote. The zygote undergoes several cleavage divisions with no significant growth as cell size progressively decreases while the nuclear to cytoplasmic ratio increases. The blastomeres in the 16-cell morula are compactly arranged and are all derived by cleavage division. In the absence of ASAH1, embryos are unable to survive beyond the 2-cell stage due to apoptotic cell death (Fig 2). The 2-cell stage coincides with the ability of the embryo to expression ASAH1 mRNA, which suggests the possibility that high levels of ASAH1 protein present prior to fertilization provide sufficient protection for the zygote until it acquires the ability to initiate its own ASAH1 expression [31]. While all ceramidases catalyze the same reaction - the hydrolysis of ceramide to sphingosine - the embryonic lethal phenotype in total ASAH1 knockout mice indicates that neutral and alkaline ceramidases are unable to compensate for ASAH1 activity in the lysosome.
At the 16–32 cell stage embryonic transcription factors of the zygotic genome begin to function. The cell mass dedifferentiates into trophoblast (future ectoderm) and an inner cell mass consisting of small epiblasts, myoblasts and primitive endoderm. The process of syncytialization during trophoblast differentiation is accompanied by changes in sphingolipids. Trophoblasts are sensitive to apoptosis induced by exogenous C16-ceramide or acid (but not neutral) sphingomyelinase and apoptosis was inhibited by the addition of EGF in an ASAH1-dependent manner [37]. The Keelan laboratory studied the role of sphingolipids in human cytotrophoblasts syncytialized over 7 days in culture [38]. Intracellular C16-ceramide levels increased modestly after 3 days in culture but then declined. Secreted S1P concentrations also dropped steeply on day 3 but then recovered to basal concentrations. Changes in ceramide and S1P are likely a function of ASAH1 expression which increased 5-fold by day 7 in culture. Inhibition of ASAH1 reduced fusion as reflected by lower multinuclear to total nuclei ratios. Therefore, ASAH1 is also involved in the functional differentiation of polyploid trophoblasts.
3.2. Sphingosine kinases
Two sphingosine kinases that reside in different subcellular compartments phosphorylate the ASAH1 product sphingosine to generate S1P. SphK1 is found in the cytoplasm and translocates to the plasma membrane upon stimulation, whereas SphK2 has been detected in the nucleus and some other organelles [39]. As stated in section 2 and shown Fig.1, S1P and ceramide play opposing roles with regard to cell fate, which led to the hypothesis of an “sphingolipid rheostat”. According to the rheostat, high levels of ceramide favor cell death while high level of S1P favor survival and proliferation. Although the “sphingolipid rheostat” model has fallen out of favor, as the complexity of sphingolipid metabolism is unraveling, exogenous S1P was sufficient to allow ASAH1-deficient embryos to progress from the 2-cell to the 4–8 cell stage [31]. S1P also protected 5-day bovine embryos from ceramide-induced apoptosis [35]. We have not yet tested whether exogenous S1P can overcome the defect in PGCC progeny formation caused by ablation of ASAH1 activity but hypothesize that similar to the ASAH1 defect in embryos, rescue might be possible. However, since S1P itself does not become critical for embryonic survival until day 11.5 (see below), it is the activity of ASAH1, not sphingosine kinases, that is required for survival of the pre-implantation embryo. The requirement of ASAH1 for embryonic survival during the polyploid, pre-implantation stage dovetails well with the hypothesis that PGCC are the somatic equivalent of blastomeres.
After the blastocyst hatches out of the zona pellucida, the implantation process within the uterus begins. Embryonic implantation is a complex series of events during which maternal and embryonic tissues cooperate to initiate intricate steps for uterine preparation. Endometrial stromal cells undergo proliferation and differentiation into decidual cells that will surround the implanting blastocysts. The formation of the primary decidual zone, a dense avascular layer, occurs next to the implanting blastocyst. Next to the primary decidual zone formation of a secondary decidual zone occurs, which is characterized by terminally differentiated decidual polyploidy with acquisition of large mono- or binucleated cells [40]. The decidua functions as a barrier to uncontrolled trophoblast proliferation and provides a vascular network for nutrient and gas exchange prior to establishment of a functional placenta. The outgrowth of some trophoblasts into the decidualized uterus is important for physical attachment of the embryo to the mother but also alters the uterine vasculature to allow the development of an adequate blood supply to support the growing fetus without fluctuations that could cause damage. Sphingosine kinases and S1P are critical during this stage (Fig. 2) [41].
Neither loss of SphK1 nor SphK2 alone impacts embryonic lethality, fertility, longevity, or causes obvious abnormalities in major organs [42]. In fact, S1P levels in organs of mice lacking either SphK1 or SphK2 were comparable to wild type mice, although loss of SphK1 (but not SphK2) resulted in a significant decrease in serum S1P levels [43]. In contrast, a complete knockout of both SphK1 and SphK2 was 100% embryonic lethal by day 13.5. Embryos were grossly normal until day 9.5 but showed evidence of hemorrhaging by day 11.5. The SphK substrate sphingosine did not accumulate but the product S1P was undetectable. S1P exerts its function in survival and proliferation through 5 G-protein coupled receptors (S1P1-S1P5). While expression of S1P receptors was not changed, the SphK double knockout phenotype was reminiscent of that observed in S1P1 null mice, which also succumb due to defective vascular development. Since sphingosine, which can promote cell death, did not accumulate, death of the embryos must have resulted from lack of S1P and downstream signaling to its receptors. These studies substantiated that S1P is crucial for angiogenesis at least in part via S1P1.
Further analysis of SphK-deficient animal models led to the interesting observation of a gender-specific defect in mice lacking both SphK1 alleles and only one of the SphK2 alleles [44]. While males had normal fertility, females were infertile, which could not be attributed to differences in the number of ovulated or fertilized eggs on day 1.5 post-coitus or to defective implantation of day 5.5 post-coitus. When female Sph1−/−SphK2−/+ mice were mated to wild type males, 27% of embryos in mutant females were smaller compared to wild type females on day 7.5 post-coitus. By day 8.5 post-coitus nearly 80% of embryos were absorbed leaving only traces of yolk sac without placenta formation. Further examination showed that although embryos were viable on day 6.5 post-coitus, uteri of mutant mice displayed hemorrhage and invasion of multinucleated neutrophils into areas surrounding the embryo. Cell death in the decidual zone was detected as early as day 5.5 post-coitus and increased by day 7.5 post-coitus. Death did not result from increased invasion of trophoblast giant cells but was attributed to defects in decidual cells and decidual blood vessels. To understand the role of sphingolipid metabolism, enzyme activity and mRNA levels were quantified in wild type mice. An increase in SphK activity coincided with a 9.3-fold increase in SphK1 mRNA while SphK2 mRNA remained unchanged. Increases in other sphingolipid pathway genes, such as SPT, S1P lyase, S1P phosphatase, and acid sphingomyelinase, suggested increased in de novo synthesis and flux in sphingolipids revolving primarily around synthesis and degradation of S1P with SphK1 playing a more predominant role that SphK2. Among the CerS only CerS5 and CerS6, which preferentially generate C16-ceramide, were slightly increased (1.4 and 2.8 fold, respectively). In mutant mice SphK substrates dhSph and sphingosine increased while ceramide levels did not significantly change, which is supportive of the mRNA analysis performed in wild type mice. In summary, these studies indicate that SphK isoforms can compensate for each other’s loss to some extent. However when S1P levels drop below a critical threshold, i.e. loss of the second SphK2 allele in addition to SphK1 or loss of all alleles, a decrease in female fertility results due to maternal defects.
Parallels can be drawn between the role of SphK1 in embryonic development, where maternal S1P is crucial for establishing the connection to the embryo, and in cancer, where host S1P regulates tumor cell colonization. To date, we have been unable to establish a role for SphK1 or S1P in PGCC themselves [45]. Similarly, loss of SphK1 in MB49 bladder cancer cells did not impair their ability to colonize the lung [46]. Thus at least for some aspects in cancer signaling SphK1/S1P within the tumor cells themselves seems dispensable. In contrast, the ability MB49 cells to colonize the lung was significantly impaired when the host was SphK1 deficient [46]. Thus it appears that maternal or host S1P is important for the embryo or tumor, respectively.
Another potentially highly relevant role of S1P in PGCC biology is its function in red blood cells. In adults, plasma S1P is derived from both the endothelium and red blood cells. However, a specific deletion of both SphK1 and SphK2 in red blood cells was embryonic lethal whereas loss of these enzyme in the endothelium was dispensable for embryonic development [47]. The defect was not due to reduced erythropoietic capacity of hematopoietic stem cells but due to vascular development regulated by S1P receptors. PGCC have the capacity for vascular mimicry and PGCC-derived erythrocytes express fetal and embryonic hemoglobins [48, 49]. This suggests the possibility that PGCC-derived erythrocytes supply the S1P required for tumor survival. Further analysis of SphK, S1P and S1P receptors is needed to determine whether PGCC offspring generate S1P during vascular mimicry.
3.3. Ceramide Synthases
Expression of the six members of the CerS family varies in a tissue-dependent manner with aberrant expression linked to a number of pathologies [50, 51]. CerS are responsible for generating diverse ceramide species, which are then further metabolized to sphingomyelin and glycosphingolipids. Since the ceramide moieties in complex sphingolipids change during embryonic development, the Merrill laboratory hypothesized that differences in CerS are contributing factors and therefore investigated changes in specific ceramide species and CerS mRNA levels using mouse embryonic stem cells (mESC) and embryoid bodies (EB) [52]. EBs are widely used as a developmental model in which aggregates of cells derived from mESC are thought to recapitulate the early stages of development. Compared to mESC, EB had higher expression of CerS1 and CerS3, which preferentially generate C18-ceramide or C22/24- and ultralong ceramides, respectively. The increase in CerS1/3 mRNA expression was accompanied with an increase in the corresponding ceramide species. CerS5 and CerS6 mRNA were inversely changed but the overall percentage of C16-ceramide was lower in EB compared to mESC, suggesting that the decrease in CerS5 outpaced the increase in CerS6. While future studies are needed to better understand the functional significance of changes in CerS and ceramide species, this study elucidated two interesting facts with potential relevance to early embryonic development.
First, C16-ceramides represented about 50% of total ceramide in mESC and approximately 40% in EB. C16-ceramide has been associated with apoptosis and high levels might indicate that mESC and EB are poised to undergo programmed cell death. The first major developmental event that requires apoptosis is formation of the pro-amniotic cavity in a process called cavitation. Second, the content of dhCer increased from 8% in mESC to 19% in EB without changing total ceramides (sum of dhCer and ceramide). This shift coincided with a decrease in desaturase (DES1) mRNA, suggesting the increase of dhCer is due to substrate accumulation. DhCer were initially thought to be inert sphingolipids because unlike ceramide, dhCer does not induce apoptosis. However, dhCer are important mediators of autophagy, a process required for the removal of dead cells during cavitation [53–55]. Thus both high levels of C16-ceramide and a shift towards increased dhCer maybe required as part of early embryonic development (Fig. 2).
Mice deficient in any one CerS family member have been developed and all are viable, which suggests sufficient compensatory mechanisms exist to allow for development to birth. Several CerS knock out models have shown that the loss of specific ceramides is compensated so that at least in young animals total levels of ceramide and complex sphingolipids are unchanged. The altered composition in CerS-null mice has provided insights into the functions of specific CerS family members.
CerS1 preferentially generates C18-ceramide. CerS1 null mice have decreased C18-sphingolipids and maintained total sphingolipid levels at 6 weeks but not 18 months, suggesting that compensatory mechanisms were not sustainable throughout life span. Even at 6 weeks, when total sphingolipid levels were maintained, ganglioside GD1 was specifically decreased by 50% in the forebrain and cerebellum, which led to progressive shrinkage of the cerebellum and functional deficits in exploration, locomotion and motor coordination [56]. An accumulation of dhSph and sphingosine, which can promote cell death, may have contributed to this neurodegeneration [57].
CerS2 null mice are essentially devoid of all sphingolipids with C22/C24 acyl chains [58, 59]. In these mice, C16-ceramide compensates for the lack of very long chain ceramides thereby maintaining total ceramide levels as observed in CerS1 null mice. However, in hepatocytes the increase in C16-ceramide led to apoptosis forcing proliferative repair, which may have contributed to the development of hepatocellular carcinoma. Although CerS2 expression levels are comparable between liver and kidney, loss of CerS2 did not affect renal function or pathology. A subsequent study showed that C16-ceramide and dhSph were elevated in the liver mitochondrial fraction, which was associated with an increase in reactive oxygen species due to impaired complex IV activity. Despite depletion of C22/C24-sphingolipids, these changes were not observed in cardiomyocytes, where C16-ceramides did not significantly increase [60]. Since C16-ceramide has been associated with cell death in multiple models, it is likely that the increase in C16-ceramide rather than lack of C22/C24-sphingolipids was responsible for cell death observed in CerS2 null mice, although why the effect is liver-specific remains to be further elucidated.
CerS3-deficient mice die shortly after birth as a consequence of trans-epidermal water loss due skin barrier function dysfunction [61]. CerS3 also plays a role in male germ line development [61]. Spermatogenesis is dependent on glycosphingolipids and loss of GalNAc transferase renders male mice sterile because haploid round spermatids aggregate in multinuclear giant cells. The Sandhoff group demonstrated that very long-chain fatty acid-containing sphingolipids are restricted to germ cells and are expressed in a differentiation stage-specific manner. CerS3 expression was increased more than 700-fold during juvenile testicular maturation, which coincided with the increase in very long chain glycosphingolipids that are required for completion of meiosis in male germ cells [62]. Spermatogenic midbodies form intracellular bridges that connect clonal daughter cells into a syncytium. The ultra-long polyunsaturated anchors, which can only be provided through CerS3 activity, are required for stability of the intracellular bridges. Therefore, loss of the anchors resulted in apoptosis during meiosis, formation of multinuclear giant cells, and spermatogenic arrest [62].
CerS4 is expressed in the suprabasal epidermal layer and sebaceous glands. Its lack of expression results in altered sebum composition and progressive hair loss due to physical blockage of the hair canal [63]. CerS4 is also ubiquitously expressed in all retinal neurons and Müller cells. Loss of CerS4 expression decreased sphingolipids with C20-C24-acyl chain in favor of an increase in C16-containing species, which affected retinal signaling [64]. The loss of either CerS5 or CerS6, which both generate C16-ceramide, did not result any obvious defects at birth perhaps, because like SphK, their enzymatic products allow for compensation. Although mice appear normal at birth, lack of CerS5 or CerS6 has pointed to roles of these enzymes in metabolism and immune function [65–69].
Among the CerS, only CerS3 has been linked to polyploidy due to defective meiosis during sperm development [61, 62]. Loss of very long chain ceramides (>C20-Cer) results in compensation by C16-ceramide in both Cer2 and CerS4 null mice [58, 59, 63]. It is interesting that the liver, which is the largest polyploid organ in humans, appears highly susceptible to C16-ceramide-induced apoptosis compared to other organs [70]. Therefore, it will be important to further investigate the differential tissue-specific effects of CerS with regard to polyploidy.
4. Sphingolipids in Cell Cycle Progression
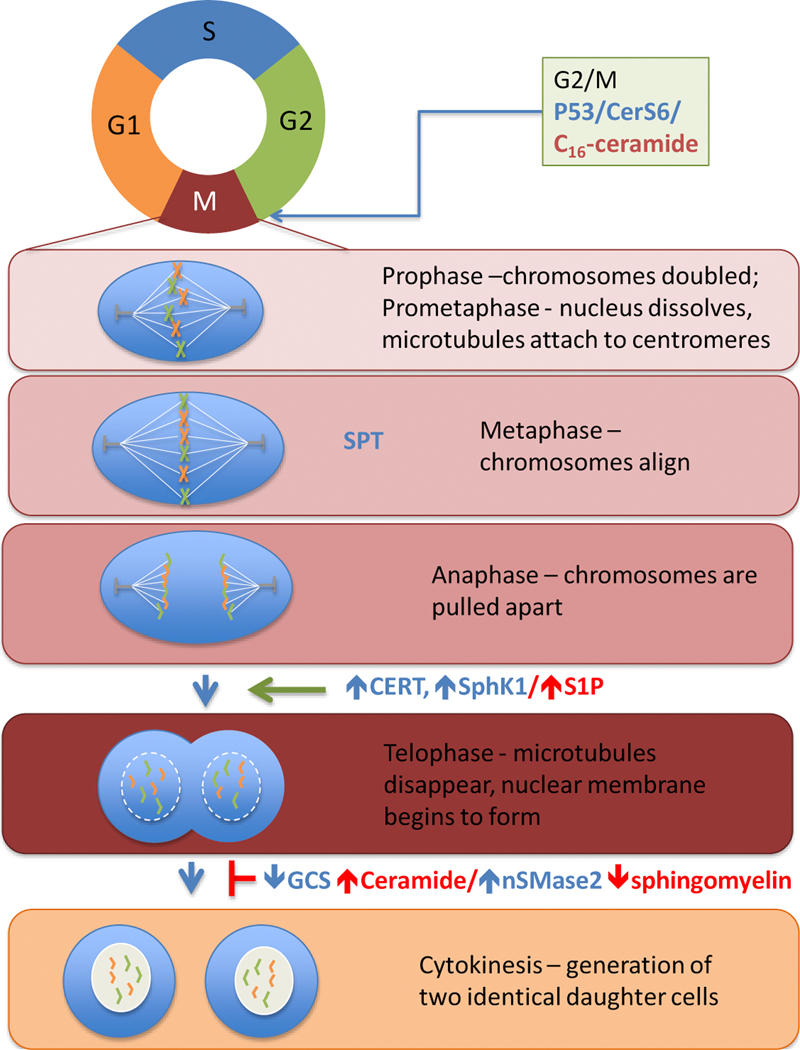
The cell cycle consists of a long interphase divided into G1, S, and G2 followed by a short period of mitosis and cytokinesis (Fig. 3). During G1 the cell increases synthesis to generate more protein and organelles. Once the cyclin regulated G1/S checkpoint is passed, the cell continues into S-phase to synthesize DNA. During S-phase RNA and protein synthesis, with the exception of histones, is reduced while the cell replicates each chromosome into two sister chromatids. Protein synthesis resumes in the G2 phase to prepare the cell for mitosis. Preparation involves an increase in cyclin B to activate cyclin-dependent kinase 1 (Cdk1/CDC2). Cdk1 is positively regulated by the phosphatase Cdc25 and negatively regulated by wee1. Plk1 additionally interferes with wee1 to ensure entry into M-phase. Entry into mitosis in controlled by the G2/M checkpoint and requires coordination between Cdk1, CDC25 and Plk1 with the accumulation of cyclin B activating the cyclin B-Cdk1 complex. M-phase is relatively short and is divided into 5 phases: prophase, prometaphase, metaphase, anaphase and telophase. During prophase the centrosomes are associated with the nuclear membrane but once the nuclear membrane breaks down, the centrosomes interact with the chromosomes to build the mitotic spindle. Sister chromatids are pulled apart by microtubules into two identical nuclei on opposite sides of the cell. Once nuclei are separated the cell undergoes cytokinesis to divide other cell components such as organelles and cytoplasm in half before the plasma membrane encloses the newly generated equal-sized daughter cells.
Figure 3. Roles of sphingolipid metabolism during cell cycle regulation.
The tumor suppressor p53 is important for regulation of the G2/M checkpoint, where extensive DNA damage can trigger apoptosis. The absence of wild type p53 appears to be a key factor to permit polyploidy. If aberrant cells cannot be eliminated through pro-apoptotic C16-ceramide generated by CerS6, the number of cells escaping cell cycle regulation may be increased. As discussed in the text, SPT is important for regulation of the actin skeleton during metaphase, increased CERT, SphK1 or S1P promote mitotic slippage during transition from anaphase to telophase, and altered activity of GCS and/or nSMase2 as well as increased ceramide or decreased sphingomyelin inhibit cytokinesis.
Lipids have not been studied as extensively as proteins or nucleic acids during the cell cycle but clearly have important functions. Lipid and protein dynamics shape nuclear envelope identity and play a role in oncogene-induced senescence [71, 72]. Although de novo sphingolipid synthesis occurs in the endoplasmic reticulum, which is continuous with the outer nuclear envelope, the majority of newly synthesized ceramide is trafficked to the Golgi apparatus via CERT for further modification [73]. Sphingolipids are more abundant in the plasma membrane than in internal membranes and early studies using a fluorescent precursor to show that transport of sphingomyelin and glucosylceramide to the plasma membrane is inhibited during mitosis but not during interphase [74–76]. Although the majority of sphingomyelin is transported to the plasma membrane, it is still the most abundant among nuclear sphingolipids. Sphingomyelin is detected in the nuclear matrix and also directly associates with chromatin for DNA stabilization. During S phase sphingomyelin levels decrease to allow DNA replication (reviewed in [72]. Synthesis of sphingomyelin also appears to be decreased during M phase, since studies with radioactive tracers showed that transfer of phosphocholine and galactose to ceramide and glucosylceramide, respectively, decreased significantly during this stage of the cell cycle [77]. Sphingomyelinases are activated in response to stress to hydrolyze sphingomyelin forming phosphorylcholine and ceramide. A recent study found that neutral sphingomyelinase 3 is localized to the nuclear pore complex, where it could potentially be involved in localize release of ceramide but the relevance of this finding is not yet clear [78]. One possibility is regulation of crosstalk between sphingolipid metabolism and PKC. When sphingomyelin synthase conjugates ceramide with phosphatidylcholine, it produces not only sphingomyelin but also DAG, thereby establishing a link between PKC signaling and sphingolipid metabolism. Accumulation of nuclear diacylglycerol (DAG) stimulates nuclear translocation of PKC to drive gene expression and proliferation [79]. It is therefore conceivable that stress results in the opposite reaction, i.e. the hydrolysis of sphingomyelin to generate ceramide, which has anti-proliferative properties such as induction of senescence. It has been demonstrated that replicative and accelerated cellular senescence causes nuclear lamin dysfunction involving phosphatidylserine but how this intertwines with sphingolipid metabolism is not yet known [80, 81]. S1P, generated by nuclear localized sphingosine kinase 2, also participates in cell cycle regulation through its impact on HDAC activity [82–84]. Thus lipid signaling and especially sphingolipids are important regulators of nuclear signaling and in the subsequent sections, I will more closely focus on the roles of sphingolipids in senescence, G2/M, mitosis, and cytokinesis [72, 85].
4.1. Cell cycle checkpoints and senescence
The retinoblastoma protein (Rb) and p53 are two main tumor suppressors that as part of cell cycle checkpoints ensure that DNA replication is carefully controlled to preserve integrity of cell division. The activity of both proteins is influenced by ceramide. Aged human fibroblasts contain higher levels of ceramide than “young” cultures (<30 passages), which correlated with increased activity of neutral sphingomyelinase [86]. Ceramide causes hypophosphorylation of Rb, which arrests cells at the G1 checkpoint leading to senescence [87]. Ceramide also increases in A375 melanoma cells when ASAH1 expression is ablated, which cells blocks cell cycle progression at G1, accelerates cellular senescence, induces apoptosis, and prevents self-renewal [88]. Activation of Rb interferes with the activity of E2F and therefore cells fail to progress through G1 and S phases. In order to proceed to mitosis, the cell has to pass the G2/M checkpoint. Detection of DNA damage triggers ATR and ATM to activate Chk1 and Chk2, respectively. Chk1 and Chk2 phosphorylate Cdc25 (to promote its inactivation and degradation) and stimulate p53 and wee1 resulting in inhibition of the cyclin B-Cdk1 complex to allow for DNA repair before proceeding to mitosis. As a central tumor suppressor p53 orchestrates the DNA damage response by pausing the cell cycle to allow for repair thereby guarding the integrity the genome. If the damage is too severe, p53 may also initiate apoptosis. It has recently been shown that p53 enhances the synthesis of pro-apoptotic C16-ceramide by directly binding to the CerS6 promoter and that C16-ceramide in turn stabilizes the p53 by binding within its DNA-binding domain [89–91].
4.2. Mitosis
During M-phase, the spindle assembly checkpoint ensures that mitosis (or meiosis) progresses from anaphase to telophase only once the duplicated chromosomes are properly attached to the spindle. When cells exit mitosis without proper chromosome segregation, mitotic slippage results in polyploidy. Whether polyploid cells are mono- or multi-nucleated depends on whether mitosis is arrested in metaphase or anaphase. In mononucleated PGCC the subnuclei remain arranged at the metaphase plate and are separated by folds of the nuclear envelope [92]. In either type of PGCC, DNA forms a radial pattern linked to a single microtubule organizing center with the potential to resume mitosis at the stage of interruption. Cancer cells that dedifferentiate to the blastomere or even germ cell stage can undergo reduction divisions, which is associated with the expression of meiotic genes such as Mos [92–95]. Expression of meiotic genes has been observed in treatment resistant tumor cells [95, 96]. Since ASAH1 has been detected at the meiotic spindle in human oocytes, it is possible that this enzyme participates in reduction divisions, but this remains to be further investigated [34]. In a zebrafish model, SPT affects mitosis via the actin skeleton, which during metaphase is organized into Rho-dependent F-actin caps. F-actin binds to Anthrax A2 to recruit the Anthrax receptor to the cap with actin then torqueing the mitotic spindles. SPT activity was found to be crucial for the palmitoylation state of the Anthrax receptor, which is required for positioning the mitotic spindle during cell division [97]. This finding exemplifies how the modulation of proteins by sphingolipids can impact cellular processes.
Modulation of CERT expression was unexpectedly found to play a role in polyploidy. Mitotic arrest was studied in the context of paclitaxel, which stabilizes microtubules and prevents cells from achieving the metaphase spindle configuration. This prolongs activation of the spindle assembly complex and blocks progression of mitosis ultimately triggering apoptosis. Under “CERT low” conditions, ceramide accumulated in the endoplasmic reticulum, leading to stress and apoptosis. However under “CERT high” conditions, ceramide is efficiently moved out of the endoplasmic reticulum and further metabolized, which led to mitotic slippage and polyploidy [98, 99].
SphK1 plays a similar role in mitotic arrest and mitotic slippage. Knockout of SphK1 resulted in accumulation of sphingosine, inhibition of PKC and reduced colony formation. Loss of SphK1 prolonged mitosis in a spindle assembly-dependent manner resulting in more than 50% of cells containing a 4N and about 40% having an 8N genome within 24 hours, which was followed by apoptosis at later time points [100]. Mechanistically loss of SphK1 activity reduced Cdk1 activity and Chk1 expression, which could compromise the spindle assembly complex during transition from metaphase to anaphase [101]. Pharmacological inhibition of SphK1 also affects Cdk1 activation and prolonged mitosis, which led to apoptosis. Interestingly, the decrease in S1P following SphK1 inhibition did not causes accumulation of the substrate. Instead sphingosine was decreased and ceramide was not only increased but also exhibited a shifted profile from very long chain ceramides (C22/24) to long ceramides (C16/18) [102]. This suggests that sphingosine, if not utilized by SphK1, will be utilized by CerS that through re-acylation alter the overall ceramide profile (and likely complex sphingolipid composition) of the cells
In contrast, overexpression of SphK1 accelerates mitosis by promoting degradation of cyclin B. Treatment with S1P facilitated mitosis even in cells that were chemically arrested in pro-metaphase (nocodazole) or metaphase (taxol), which pointed to a role for S1P in relaxation of the spindle assembly complex. Mitosis driven by excess S1P increased the percentage of cells with lagging chromosomes and anaphase-bridges [100]. Gillies et al. detected S1P5 along with SphK1 and SphK2 at the centrosome and suggested a dynamic endocytic shuttling mechanism that is controlled by ligand occupancy of the cell surface receptor, since the addition of S1P trafficked S1P5 to the centrosome [103]. Andrieu et al. subsequently demonstrated that S1P was first exported from the cells via Spns2 before it signaled via surface S1P5 [104, 105]. Mechanistically the pro-mitotic effect of S1P was mediated by PI3K/Akt driven phosphorylation of Ser99 on Plk1, which is required for progression from metaphase to anaphase as opposed to phosphorylation on Thr210, which has been implicated in mitotic entry [106, 107].
Emerging evidence also suggests a possible role for CerK and C1P during cell cycle progression. CerK is primarily located in the Golgi but has a putative nuclear localization signal and has been detected in other cell compartments including the perinuclear region [108]. CerK is the kinase responsible for phosphorylation of ceramide and promotes migration and proliferation in cancer cells via activation of the Akt pathway [109]. Whether activation of Akt also signals via Plk1 as observed for S1P has not yet been evaluated. In breast cancer, CerK expression is higher in estrogen receptor negative than in estrogen positive patients and patients in the estrogen receptor negative group whose tumors express the highest levels of CerK have the worst prognosis and shortest survival [110]. CerK expression has also been associated with cancer recurrence [109]. Inhibition of CerK resulted in accumulation at G2/M accompanied by elevated histone-H3 phosphorylation, indicative of an increase in mitotic index. CerK inhibition sensitized NCI-H358 cells to apoptosis but a significant increase in in DNA fragmentation was not observed in the more polyploid MCF-7 cells [111]. This lack of response in MCF-7 cells may need to be further investigated especially in the context of polyploidy and breast cancer recurrence.
4.3. Cytokinesis
Cytokinesis is the final step in cell division, which involves the equal sorting of cellular components into daughter cells and subsequent membrane ingression for separation. This process relies heavily on membrane components and properties and is inhibited if lipid metabolism is disrupted. For example, the bacterium Chlamydia trachomatis interferes with cytokinesis by intercepting Golgi-derived lipids which results in polyploidy in host cells [112]. In the sea urchin egg model the parental membrane increases by 28% in order to divide into equal size daughter cells [113]. The failure to complete cytokinesis will result in multinuclear polyploidy. From the sea urchin egg model, we know that microtubules and actin promote the formation of unique membrane domains at sites of the future cleavage furrow. Additionally anchoring for the contractile actin-based ring that serves as a site for new membrane addition is required. The formation of the equatorial membrane domain is dependent on anaphase and involves plasma membrane signaling domains that are enriched in the ganglioside GM1 and cholesterol along with activated tyrosine kinases and PLCγ [114]. Interestingly, the majority of newly added membrane lacks GM1 and is therefore distinct from the pre-mitotic membrane [113].
With regard to sphingolipids, several studies confirmed the initial observation by Rani et al. that inhibition of GCS interferes with the cell cycle [115]. As discussed above, GCS is the first enzyme in glycosphingolipid synthesis that adds glucose to ceramide. Glucosylceramide (GlcCer) is then converted to lactosylceramide (LacCer), which serves as substrate for gangliosides GM3, GM2, and GM1 that are synthesized sequentially. One group studied polyploidy in response to psychosine, a naturally occurring lipid that inhibits cytokinesis. Polyploidy could be decreased by knockout of B3GALT4 or the GCS inhibitor PDMP, both of which reduced GM1, whereas overexpression of GCS increased the number of polyploid cells [116]. To get a better understanding of lipid involvement in cytokinesis, Atilla-Gokcumen et al. used a mass spectrometry-based global lipid profiling approach. Inhibition of GCS by PPMP permitted the assembly of the cleavage furrow but not completion of cytokinesis. Lipidomics analysis revealed a specific accumulation in C16-ceramide (8-fold) and C22-ceramide (10-fold). In an RNAi approach, GCS knockdown deleted C16- and C24-glucosyl-ceramide as well as some more highly glycosylated derivatives but accumulation of ceramide was not detected as it was likely metabolized by other pathways. However, the exogenous addition of C12- or C14-ceramides also recapitulated cytokinesis failure [117]. The group subsequently studied in more detail how lipid composition changes during cytokinesis and found that dhC16-24-ceramides, C22–24-ceramides, and C16-hexosylceramide accumulated. In the midbody, a transient structure formed towards the end of cytokinesis in mammalian cells, C24-hexosyl-ceramide was specifically increased. An RNAi screen identified 23 genes responsible for cytokinesis failure of which 11 were sphingolipid genes, including CerS2/4 and several genes predicted to process glycosphingolipids (GLB1, ST6GALNAC6, and ST8SIA5). Of note is that GM1 is one of GLB1’s substrates and both ST6GALNAC6 and ST8SIA5 process GD1a, which is a direct derivative of GM1 [118]. In summary, these studies supported a role for GM1 enriched domains during cytokinesis and suggest that very long chain C24-hexosyl-ceramide may serve as anchor during cytokinesis.
The role of sphingomyelin during cytokinesis has also been investigated. When sphingomyelin is reduced the cleavage furrow forms but regresses prior to midbody formation [119]. Similarly, overexpression of neutral sphingomyelinase 2, which decreased surface sphingomyelin by 10%, promoted polyploidy in psychosine-stressed cells [116]. In this model polyploidy could be attenuated by exogenous treatment with sphingomyelin and enhanced by bacterial sphingomyelinase, an enzyme that hydrolyzes sphingomyelin to liberate ceramide. Overexpression of GCS and neutral sphingomyelinase 2 exerted an additive effect on polyploidy in psychosine-stressed cells [116]. Since cholesterol accumulates at the cleavage furrow in sea urchin eggs [114], the connection between sphingomyelin and cholesterol was also investigated. In mammalian cells depletion of cholesterol resulted in regression of the cleavage furrow but the accumulation of cholesterol in the outer leaflet of the cleavage furrow did not require sphingomyelin. Instead, sphingomyelin-rich domains in the outer leaflet of the membrane were required for enrichment of PIP2 in the inner leaflet of the membrane to recruit RhoA to the contractile ring for cytokinesis [119]. Studies in the psychosine model supported that defects in cytokinesis by sphingolipid modulation in stressed cells was due to disruption of sphingomyelin clustering on the outer leaflet of the plasma membrane and reduced staining of PIP2 at the cleavage furrow. The loss of cells with a long cleavage furrow typical of the telomeric stage of M-phase suggested a defect in anaphase [116].
A key difference between human blastomeres and PGCC is that the former are generated by full cleavage while the latter are multinuclear cells in one cytoplasm, which might explain why the formation of PGCC can occur in an ASAH1-independent manner [3]. In contrast, the formation of PGCC progeny, which is ASAH1-dependent, is an asymmetric division followed by cellularization involving the interaction of the cytoskeleton, primarily actin, with the cytoplasmic membrane [3]. Both of these events observed in PGCC correspond to embryonic development in Drosphophila, where cells undergo a superficial cleavage that produces multiple nuclei within one cytoplasm until gastrulation stage when cellularized mitotically dividing blastomeres are released [120, 121]. Indeed, Drosophila may be an excellent model to study sphingolipid metabolism as ceramidosomes that form in response to stress in lung cancer cells are also observed in the plasma membrane of germ line stem cells in Drosophila ovaries during the early stages of development [122].
Ceramide is trafficked in a complex with RIPK1 in a NM-2A-dependent manner, which in FTY720-stressed cancer cells led to pore formation and necroptotic cell death. NM-2 isoforms are required for the contractile activity during cytokinesis, migration, cell-cell or cell-matrix junctions as well as regulation of the survival threshold of embryonic stem cells [123, 124]. Interestingly, their dysfunction has been associated with the onset and progression of cancer [125]. Mutual sliding of actin and NM-2 filaments generates the pulling forces that drive contraction during cytokinesis. C16-ceramide impairs actin polymerization through increased RhoA/Rho kinase signaling [126]. Therefore, the reduction of ceramide by ASAH1 may enable actin fiber formation and contractile activity during cytokinesis. It will be important to further explore the connection between sphingolipids and NM-2 mediated cellular trafficking due to overarching themes that link PGCC biology to embryonic development, stemness, and evolutionary conserved concepts.
In summary, ceramide regulates cell cycle progression at both G1 and G2 checkpoints through hypophosphorylation and activation of Rb at G1 and stabilization of p53 at G2. SphK and S1P regulate the M-phase checkpoint. Cytokinesis requires the localization of multiple sphingolipids, including GM1 and sphingomyelin, to specific signaling domains that may be required for the contractile function of the actin skeleton.
5. Sphingolipids in pluripotency and stemness
PGCC and/or their offspring exhibit stem-like properties. Their pluripotency is evidenced by the ability to differentiate into adipose tissue, cartilage, bone [127], endothelial or erythroid cells [128]. In fact, PGCC progeny contain a mixture of epithelial, mesenchymal, and neuronal cells [23]. Sphingolipids are important regulators of stem cell survival and differentiation and therefore likely play a role in the plasticity of PGCC. Sphingolipids involved in regulation of stem cells either during embryonic or postnatal development have also been dubbed “morphogenic lipids”. Therefore, the sphingolipid literature offers some insight into factors that could govern the pluripotency and stem-like characteristics of PGCC and their progeny.
5.1. Differentiation status and sphingolipid susceptibility
Differences in responses to sphingolipids have been observed in a stem cell-derived multi-stage breast carcinogenesis model that includes undifferentiated Oct4+ “Type I” cells, differentiated, non-transformed “Type II” cells, and a range of immortalized and transformed cells to represent stages of tumor progression [129]. Exposure to 8 μM sphingosine inhibited proliferation in Type I and transformed cells but not Type II cells. PGCC can also express Oct4 and may therefore share similarities with the undifferentiated Type I cells [23, 130]. Transformed cells in the breast cancer model consist of a series of cells that were sequentially transformed by expression of the SV40 large T antigen, radiation, and the Neu oncogene. Given that expression of the SV40 large T antigen, which interferes with p53 function, was combined with radiation stress as part of transformation, these cells should be prone to polyploidy. Thus this model might be ideal for the investigation of polyploidy in cancer. In addition to sphingosine, the effect of C2-ceramide was also examined in the model and yielded differential responses. However, interpretation of the results is difficult because changes in sphingolipid metabolism were neither examined by lipidomic analysis nor by expression of sphingolipid enzymes. The expression of CerS members will influence how the non-natural C2-ceramide are metabolized into endogenous ceramides. In addition, the relative expression of CerS and SphK (and S1P receptor expression) will determine whether exogenous sphingosine is preferentially metabolized to pro-death ceramide or pro-survival S1P. Therefore, understanding sphingolipid flux will be important in understanding cell fate in response to exogenous ceramide or sphingosine.
An excellent example of how sphingolipids can be manipulated in favor of therapeutic outcome can be gleaned from the literature on the development of neuronal progenitors. An issue that had plagued the field of neuronal stem cell transplantation was the presence of residual pluripotent stem cells (<1 per million) with the capacity to develop into teratomas, which can be life threatening. In the laboratory, both mouse and human embryonic stem cells are maintained in the presence of S1P to drive the expression of SSEA1 and Oct4. When pluripotent stem cells differentiate, Oct4 expression is lost and nestin, which commits cells to neuronal precursors, becomes expressed. However, a small fraction of cells, known as residual pluripotent stem cells, apparently fail to downregulate Oct4 thereby creating a setting that is permissive for teratoma development. Bieberich et al. found that expression of PAR4, an endogenous inhibitor of atypical PKC, mediates ceramide-induced apoptosis in proliferating EB-driven stem cells and that PAR4 expression along with susceptibility to ceramide is lost as cells differentiate towards neural progenitors that express nestin and S1P1 [131, 132]. This differential expression could therefore be exploited therapeutically by treating cultures with ceramide (or its analog S18) to eliminate PAR4+/Oct4+/S1P1- residual pluripotent stem cells responsible for teratoma formation while S1P (or FTY720) promoted differentiation of PAR4-/Oct4-/S1P1+ neuronal progenitors [133, 134]. It will be important to decipher such differences in PGCC, so that their plasticity can be manipulated in favor of benign differentiation instead of promoting development of aggressive offspring responsible for tumor recurrence.
5.2. Expression of stem cell markers YAP and CD133
In addition to Oct4, PGCC can express YAP [135]. A recent study identified a novel mechanism that dictates the development of embryonic stem cell lineages. As part of the study YAP was found to preferentially co-localize with super-enhanced markers such as Nanog, SOX2, Oct4 and H3K27ac in embryonic stem cells [136]. Another recent study linked YAP to ASAH1 in the context of liver fibrosis. Pharmacological or genetic inhibition of ASAH1 in the mouse liver inhibited YAP activity by potentiation of phosphorylation-mediated protein degradation via the ubiquitin ligase adaptor protein β-TrCP [137]. We demonstrated that pharmacological or genetic inhibition of ASAH1 in PGCC inhibited progeny formation [3]. Evidence that YAP has been associated with stem cell markers that are also detected in PGCC and association between YAP and ASAH1, which is required for PGCC progeny formation, now begs the question of possible mechanisms involving YAP in PGCC or progeny development.
CD133 expression has also been detected on PGCC [135]. CD133 belongs to the pentaspan transmembrane glycoprotein group that specifically localize to cellular protrusions. While the physiological function of CD133 remains to be elucidated, the protein is widely considered to be a universal surface marker of cancer stem cells. However, in nasopharyngeal cancer cells, the majority of CD133 positive cells were small (i.e. not PGCC) but co-existed with a subpopulation of large polynuclear cells [138]. Similarly, we have been unable to detect CD133 expression in PPC1 prostate cancer cells [3]. Thus CD133 expression, which is being questioned as a universal CSC marker, may reflect disease progression [139]. A significant increase in CD133 has also been observed in liver tumors of fro/fro mice, which lack the expression of nSMase2 and have therefore a marked increase in sphingomyelin [140]. In neural progenitors, inhibition of nSMase2 promotes translocation of atypical PKC from the membrane to the cytosol, where is phosphorylates Aurora kinase A. Exogenous C24:1 ceramide re-establishes the membrane pool of atypical PKC and accelerates neural process formation [141]. Thus the absence of nSMase2 activity can lead to increased CD133, increased sphingomyelin, and translocation of atypical PKC from the membrane to the cytosol, where it activates Aurora kinase A, which plays multiple roles in cell cycle regulation. On the other hand, stimulation of nSMase2 by sublethal doses of chemotherapy generated a significant increase in C16-ceramide at the plasma membrane that acutely affected pathways associated with cell adhesion and migration in cancer cells [142]. Additional studies are needed to determine how modulation of nSMase2 impacts sphingolipid pools and CD133 expression in the context of PGCC and their progeny.
5.3. Glycosphingolipids
Glycosphingolipids such as the globo-series stage-specific embryonic antigen 3 (SSEA-3), SSEA-4, and Globo-H, are specifically expressed on pluripotent stem cells and cancer cells, and are known to be associated with various biological processes such as cell recognition, cell adhesion, and signal transduction [143]. Analysis of glycosphingolipids is technically challenging but the use of matrix-assisted laser desorption/ionization MS (MALDI-MS) and MS/MS analyses identified not only the well-known ESC-specific markers, SSEA-3 and SSEA-4, but also several previously undisclosed globo- and lacto-series glycosphingolipids, including Gb4Cer, Lc4Cer, fucosyl Lc4Cer, Globo H, and disialyl Gb5Cer that were present in undifferentiated human ESC and induced pluripotent stem cells [144]. During differentiation changes in core structures of glycosphingolipids were observed from globo- and lacto- to gangliosides in a lineage-specific manner [144]. In breast cancer stem cells both GD2 (GD2/GM2 synthase) and GD3 (GD3S) maintain the stem cell phenotype but a subsequent study found that GD3S alone was sufficient to sustain properties of cancer stem cells and promote malignancy due to association of GD3 with the EGF receptor [145]. GD3S is also increased in glioblastoma multiforme and suppression of the enzyme reduced stemness properties in this malignancy [146].
Glycosphingolipid metabolism is regulated as part of the mitotic cell cycle with ceramide and glucosylceramide (GlcCer), the building block for all glycosphingolipids increasing prior to transition from G1 to S-phase. It has been hypothesized that the number of neuronal stem cells during embryonic development is regulated through “asymmetric inheritance” of PAR4 [147]. Since PAR4 acts synergistically with ceramide to induce apoptosis, the daughter cell inheriting PAR4 undergoes ceramide-mediated apoptosis while the other daughter cell protects itself by converting ceramide to GlcCer to initiate glycosphingolipid synthesis. Expression of GlcCer synthase has been associated with maintenance of cancer stem cell properties, which are mediated by altered composition of glycosphingolipid-enriched domains such as globotriaosylceramide (Gb3), which activates Akt, and GlcCer, which activates ERK1/2, to drive expression of multidrug resistance protein 1 and apoptotic genes [148]. In melanoma a more aggressive phenotype is associated with expression of B4GALNT1 and GM2/GD2 due to induction of angiogenesis, anchorage-independent growth and cell motility, which may be mediated through periostin [149]. In epithelial cancer cells, depletion of globosides through targeting A4GALT induces EMT, enhances chemoresistance, and increases CD24low/CD44high cells [150]. In summary, these studies highlight essential roles for glycosphingolipids in stemness and pluripotency. The development of fluorescent analogs of GM1, GM3, SSEA3, SSEA4 and Globo-H can now be employed to study the structure and roles of glycosphingolipid-enriched domains during differentiation and malignancy [143]. Tumors often express high levels of glycosphingolipids, which interfere with the anti-tumor immune response but this also provide novel opportunities for cancer immunotherapy using antibodies or CAR T cells [146, 151]. Thus understanding dysregulation of glycosphingolipids in PGCC will open up avenues for novel therapeutic approaches.
6. Concluding Remarks and Future Prospects
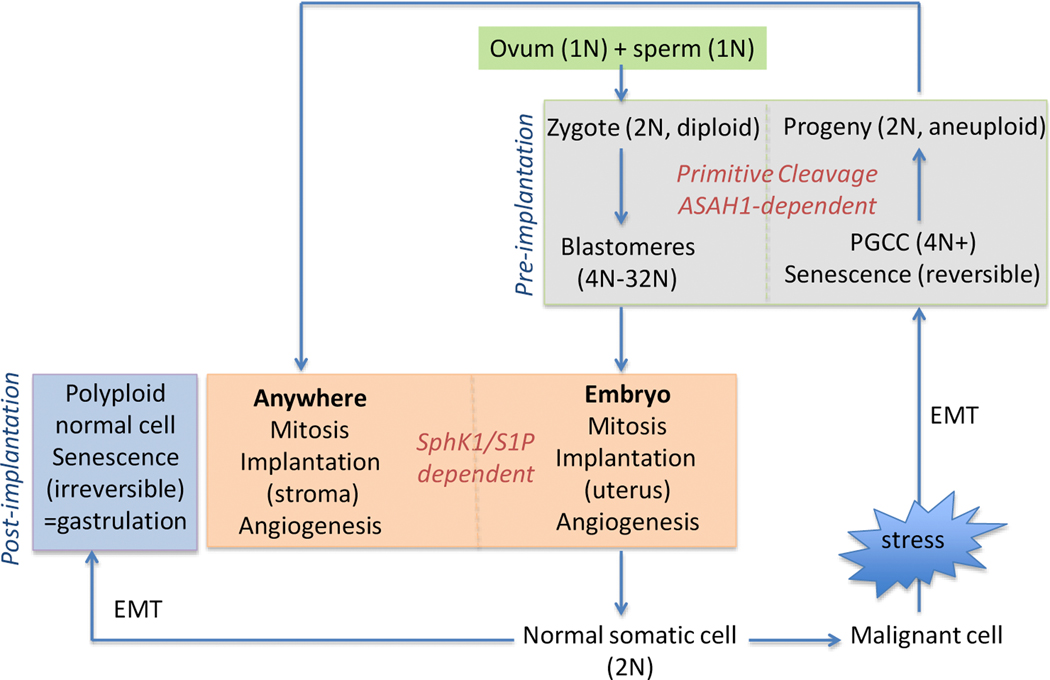
Sphingolipids have structural and signaling functions that influence membrane properties. This provides a broad mechanism by which these unique lipids modulate the behavior of other molecules, including proteins and DNA, thus exerting pleiotropic effects on signaling pathways. Figure 4 highlights parallel roles of sphingolipids in cancer and embryonic development. Mammalian cells typically have a diploid genome and proliferate via mitosis. In humans polyploidy is limited to the early, pre-implantation stages of embryonic development and highly differentiated tissues such as the heart, liver, or muscle. Gene profiling of normal adult polyploid cells revealed a shift toward the gastrulation (post-implantation) embryo. In contrast, PGCC are equivalent to the pre-implantation stages of embryonic development, which has been described as chaotic due to significant genomic instability [22]. Dedifferentiation to an early embryonic stage, especially in the absence of functional p53, creates an error prone environment in which malignant cells are provided with an opportunity to function as an independent organism.
Figure 4. Overview of sphingolipids in development and polyploidy.
Key points are: Primitive cleavage during blastomere stage or depolyploidization is ASAH1-dependent; Mitosis, implantation, and angiogenesis in the embryo or PGCC-derived progeny is dependent on S1P, likely generated by Sphingosine kinase 1; Polyploidy is associated with activation of epithelial mesenchymal transition (EMT); Normal polyploid cells dedifferentiate to the gastrulation (post-implantation) stage and are irreversibly senescent. PGCC dedifferentiate to the pre-implantation stage and are capable of reversing senescence with progeny restarting S1P-dependent processes of mitosis, implantation, and angiogenesis.
The sphingolipid literature has established the dual nature of ceramide that on the one hand drives cell death but on the other hand inhibits cytokinesis thereby promoting polyploidy. The advancement of sphingolipid measurements from steady-state “snap shots” to analysis of flux and compartmental signaling will help researchers to more fully understand how these lipids contribute to polyploidy and cancer recurrence. The requirement of ASAH1 for survival of the pre-implantation embryo dovetails well with the hypothesis that PGCC are the somatic equivalent of blastomeres. In contrast, sphingosine kinases are not required during the pre-implantation stage of development and we have not been able to establish a requirement for these enzymes in PGCC. Since the sphingosine kinase product S1P promotes mitosis, a step in the cell cycle that is skipped to achieve polyploidy, it is logical that sphingosine kinase activity and intracellular S1P is largely dispensable in PGCC. However, similar to embryonic development where S1P is crucial for establishing the connection between embryo and mother, one can envision that extracellular S1P plays an important role in supporting PGCC progeny, which resume mitosis and metastasize to distant sites. An important avenue to pursue from a therapeutic standpoint is to prevent PGCC progeny or to explore the pluripotency of PGCC to prevent offspring from assuming more aggressive characteristics than the primary tumor and instead drive them to benign phenotype.
Acknowledgement
The author would like to thank Dr. Shai White-Gilbertson for helpful discussions of this manuscript. This work was supported a grant from the National Cancer Institute [grant number: P01 CA203628].
Abbreviations:
- Acer1–3
alkaline ceramidases
- ASAH1
acid ceramidase
- ASAH2
neutral ceramidase
- ATR
ataxia telangiectasia and Rad3-related protein (protein kinase)
- ATM
ataxia-telangiectasia mutated (protein kinase)
- B4GALT5
Beta-1,4-Galactosyltransferase 5
- B3GALT4
Beta-1,3-Galactosyltransferase 4
- Cdk1/CDC2
Cyclin Dependent Kinase 1
- CDC25
cell division cycle 25, dual-specificity phosphatase
- CerK
ceramide kinase
- CerS
ceramide synthase
- CERT
ceramide transferase
- Chk1/Chk2
checkpoint kinase 1 or 2
- C1P
ceramide-1-phosphate
- DES
dihydroceramide desaturase
- dhCer
dihydroceramide
- dhSph
dihydrosphingosine
- EB
embryoid body
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ERK1/2
extracellular signal-regulated protein kinase)
- ESC
embryonic stem cell
- GCS
glucosylceramide synthase
- GD3S
ganglioside GD3 synthase
- GLB1
beta-galactosidase
- GlcCer
glucosylceramide
- LacCer
lactosylceramide
- mESC
murine embryonic stem cell
- nSMase2
neutral sphingomyelinase 2
- PARP
Poly (ADP-ribose) polymerase
- PDMP
D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol
- PGCC
polyploid giant cancer cell
- PLCg
phospholipase C gamma
- Plk1
polo-like kinase 1
- S1P
sphingosine-1-phosphate
- S1P1–5
sphingosine-1-phosphate receptors 1–5
- SM
sphingomyelin
- SMase
sphingomyelinase
- SphK
sphingosine kinase
- SPT
serine palmitoyl CoA transferase
- SSEA
stage-specific embryonic antigen
- ST6GALNAC6
sialyltransferase
- ST8SIA5
sialytransferase
Footnotes
Conflict of interest
The author declares that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Cyan E. A Treatise on the Chemical Constitution of the Brain: A Facsimile With an Introduction by David A. Drabkin. Arch Intern Med. 1963;111:837–8. [Google Scholar]
- [2].Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–71. [DOI] [PubMed] [Google Scholar]
- [3].White-Gilbertson S, Lu P, Norris JS, Voelkel-Johnson C. Genetic and pharmacological inhibition of acid ceramidase prevents asymmetric cell division by neosis. J Lipid Res. 2019;60:1225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cheng JC, Bai A, Beckham TH, Marrison ST, Yount CL, Young K, et al. Radiation-induced acid ceramidase confers prostate cancer resistance and tumor relapse. The Journal of clinical investigation. 2013;123:4344–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mosieniak G, Sikora E. Polyploidy: the link between senescence and cancer. Curr Pharm Des. 2010;16:734–40. [DOI] [PubMed] [Google Scholar]
- [6].Sundaram M, Guernsey DL, Rajaraman MM, Rajaraman R. Neosis: a novel type of cell division in cancer. Cancer biology & therapy. 2004;3:207–18. [DOI] [PubMed] [Google Scholar]
- [7].Erenpreisa JA, Cragg MS, Fringes B, Sharakhov I, Illidge TM. Release of mitotic descendants by giant cells from irradiated Burkitt’s lymphoma cell line. Cell Biol Int. 2000;24:635–48. [DOI] [PubMed] [Google Scholar]
- [8].Illidge TM, Cragg MS, Fringes B, Olive P, Erenpreisa JA. Polyploid giant cells provide a survival mechanism for p53 mutant cells after DNA damage. Cell biology international. 2000;24:621–33. [DOI] [PubMed] [Google Scholar]
- [9].White-Gilbertson S, Voelkel-Johnson C. Giants and monsters: Unexpected characters in the story of cancer recurrence. Advances in cancer research. 2020;148:201–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Puig PE, Guilly MN, Bouchot A, Droin N, Cathelin D, Bouyer F, et al. Tumor cells can escape DNA-damaging cisplatin through DNA endoreduplication and reversible polyploidy. Cell biology international. 2008;32:1031–43. [DOI] [PubMed] [Google Scholar]
- [11].Coward J, Harding A. Size Does Matter: Why Polyploid Tumor Cells are Critical Drug Targets in the War on Cancer. Frontiers in oncology. 2014;4:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ogden A, Rida PC, Knudsen BS, Kucuk O, Aneja R. Docetaxel-induced polyploidization may underlie chemoresistance and disease relapse. Cancer letters. 2015;367:89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Erenpreisa J, Cragg MS. Three steps to the immortality of cancer cells: senescence, polyploidy and self-renewal. Cancer cell international. 2013;13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Amend SR, Torga G, Lin KC, Kostecka LG, de Marzo A, Austin RH, et al. Polyploid giant cancer cells: Unrecognized actuators of tumorigenesis, metastasis, and resistance. The Prostate. 2019;79:1489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen J, Niu N, Zhang J, Qi L, Shen W, Donkena KV, et al. Polyploid Giant Cancer Cells (PGCCs): The Evil Roots of Cancer. Current cancer drug targets. 2019;19:360–7. [DOI] [PubMed] [Google Scholar]
- [16].Mirzayans R, Andrais B, Murray D. Roles of Polyploid/Multinucleated Giant Cancer Cells in Metastasis and Disease Relapse Following Anticancer Treatment. Cancers (Basel). 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Salmina K, Bojko A, Inashkina I, Staniak K, Dudkowska M, Podlesniy P, et al. “Mitotic Slippage” and Extranuclear DNA in Cancer Chemoresistance: A Focus on Telomeres. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].White-Gilbertson S, Lu P, Jones CM, Chiodini S, Hurley D, Das A, et al. Tamoxifen is a candidate first-in-class inhibitor of acid ceramidase that reduces amitotic division in polyploid giant cancer cells-Unrecognized players in tumorigenesis. Cancer Med. 2020;9:3142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lin KC, Torga G, Sun Y, Pienta KJ, Sturm JC, Austin RH. Generation of Heterogeneous Drug Gradients Across Cancer Populations on a Microfluidic Evolution Accelerator for Real-Time Observation. J Vis Exp. 2019. [DOI] [PubMed] [Google Scholar]
- [20].Salmina K, Gerashchenko BI, Hausmann M, Vainshelbaum NM, Zayakin P, Erenpreiss J, et al. When Three Isn’t a Crowd: A Digyny Concept for Treatment-Resistant, Near-Triploid Human Cancers. Genes (Basel). 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jackson TR, Salmina K, Huna A, Inashkina I, Jankevics E, Riekstina U, et al. DNA damage causes TP53-dependent coupling of self-renewal and senescence pathways in embryonal carcinoma cells. Cell Cycle. 2013;12:430–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].The Liu J. “life code”: a theory that unifies the human life cycle and the origin of human tumors. Seminars in cancer biology. 2020;60:380–97. [DOI] [PubMed] [Google Scholar]
- [23].Niu N, Mercado-Uribe I, Liu J. Dedifferentiation into blastomere-like cancer stem cells via formation of polyploid giant cancer cells. Oncogene. 2017;36:4887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Presa N, Gomez-Larrauri A, Dominguez-Herrera A, Trueba M, Gomez-Munoz A. Novel signaling aspects of ceramide 1-phosphate. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158630. [DOI] [PubMed] [Google Scholar]
- [25].Hojjati MR, Li Z, Jiang XC. Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochimica et biophysica acta. 2005;1737:44–51. [DOI] [PubMed] [Google Scholar]
- [26].Yamashita T, Wada R, Proia RL. Early developmental expression of the gene encoding glucosylceramide synthase, the enzyme controlling the first committed step of glycosphingolipid synthesis. Biochim Biophys Acta. 2002;1573:236–40. [DOI] [PubMed] [Google Scholar]
- [27].Nishie T, Hikimochi Y, Zama K, Fukusumi Y, Ito M, Yokoyama H, et al. Beta4-galactosyltransferase-5 is a lactosylceramide synthase essential for mouse extra-embryonic development. Glycobiology. 2010;20:1311–22. [DOI] [PubMed] [Google Scholar]
- [28].Wang X, Rao RP, Kosakowska-Cholody T, Masood MA, Southon E, Zhang H, et al. Mitochondrial degeneration and not apoptosis is the primary cause of embryonic lethality in ceramide transfer protein mutant mice. J Cell Biol. 2009;184:143–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kono M, Dreier JL, Ellis JM, Allende ML, Kalkofen DN, Sanders KM, et al. Neutral ceramidase encoded by the Asah2 gene is essential for the intestinal degradation of sphingolipids. The Journal of biological chemistry. 2006;281:7324–31. [DOI] [PubMed] [Google Scholar]
- [30].Li F, Xu R, Low BE, Lin CL, Garcia-Barros M, Schrandt J, et al. Alkaline ceramidase 2 is essential for the homeostasis of plasma sphingoid bases and their phosphates. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2018;32:3058–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Eliyahu E, Park JH, Shtraizent N, He X, Schuchman EH. Acid ceramidase is a novel factor required for early embryo survival. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:1403–9. [DOI] [PubMed] [Google Scholar]
- [32].Frohbergh M, He X, Schuchman EH. The molecular medicine of acid ceramidase. Biological chemistry. 2015;396:759–65. [DOI] [PubMed] [Google Scholar]
- [33].Koch J, Gartner S, Li CM, Quintern LE, Bernardo K, Levran O, et al. Molecular cloning and characterization of a full-length complementary DNA encoding human acid ceramidase. Identification Of the first molecular lesion causing Farber disease. J Biol Chem. 1996;271:33110–5. [DOI] [PubMed] [Google Scholar]
- [34].Eliyahu E, Shtraizent N, Shalgi R, Schuchman EH. Construction of conditional acid ceramidase knockout mice and in vivo effects on oocyte development and fertility. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2012;30:735–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Guo L, Geng X, Ma L, Luo C, Zeng W, Ou X, et al. Sphingosine-1-phosphate inhibits ceramide-induced apoptosis during murine preimplantation embryonic development. Theriogenology. 2013;80:206–11. [DOI] [PubMed] [Google Scholar]
- [36].Eliyahu E, Shtraizent N, Martinuzzi K, Barritt J, He X, Wei H, et al. Acid ceramidase improves the quality of oocytes and embryos and the outcome of in vitro fertilization. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:1229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Payne SG, Brindley DN, Guilbert LJ. Epidermal growth factor inhibits ceramide-induced apoptosis and lowers ceramide levels in primary placental trophoblasts. Journal of cellular physiology. 1999;180:263–70. [DOI] [PubMed] [Google Scholar]
- [38].Singh AT, Dharmarajan A, Aye IL, Keelan JA. Ceramide biosynthesis and metabolism in trophoblast syncytialization. Mol Cell Endocrinol. 2012;362:48–59. [DOI] [PubMed] [Google Scholar]
- [39].Lewis CS, Voelkel-Johnson C, Smith CD. Targeting Sphingosine Kinases for the Treatment of Cancer. Advances in cancer research. 2018;140:295–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tan J, Raja S, Davis MK, Tawfik O, Dey SK, Das SK. Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation. Mech Dev. 2002;111:99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kaneko-Tarui T, Zhang L, Austin KJ, Henkes LE, Johnson J, Hansen TR, et al. Maternal and embryonic control of uterine sphingolipid-metabolizing enzymes during murine embryo implantation. Biol Reprod. 2007;77:658–65. [DOI] [PubMed] [Google Scholar]
- [42].Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. The Journal of biological chemistry. 2004;279:52487–92. [DOI] [PubMed] [Google Scholar]
- [44].Mizugishi K, Li C, Olivera A, Bielawski J, Bielawska A, Deng CX, et al. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. The Journal of clinical investigation. 2007;117:2993–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].White-Gilbertson S, Lu P, Voelkel-Johnson C. Cancers. in preparation. [Google Scholar]
- [46].Ponnusamy S, Selvam SP, Mehrotra S, Kawamori T, Snider AJ, Obeid LM, et al. Communication between host organism and cancer cells is transduced by systemic sphingosine kinase 1/sphingosine 1-phosphate signalling to regulate tumour metastasis. EMBO Mol Med. 2012;4:761–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Xiong Y, Yang P, Proia RL, Hla T. Erythrocyte-derived sphingosine 1-phosphate is essential for vascular development. J Clin Invest. 2014;124:4823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhang S, Mercado-Uribe I, Liu J. Generation of erythroid cells from fibroblasts and cancer cells in vitro and in vivo. Cancer letters. 2013;333:205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yang Z, Yao H, Fei F, Li Y, Qu J, Li C, et al. Generation of erythroid cells from polyploid giant cancer cells: re-thinking about tumor blood supply. J Cancer Res Clin Oncol. 2018;144:617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zelnik ID, Rozman B, Rosenfeld-Gur E, Ben-Dor S, Futerman AH. A Stroll Down the CerS Lane. Adv Exp Med Biol. 2019;1159:49–63. [DOI] [PubMed] [Google Scholar]
- [51].Wegner MS, Schiffmann S, Parnham MJ, Geisslinger G, Grosch S. The enigma of ceramide synthase regulation in mammalian cells. Progress in lipid research. 2016;63:93–119. [DOI] [PubMed] [Google Scholar]
- [52].Park H, Haynes CA, Nairn AV, Kulik M, Dalton S, Moremen K, et al. Transcript profiling and lipidomic analysis of ceramide subspecies in mouse embryonic stem cells and embryoid bodies. J Lipid Res. 2010;51:480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bialik S, Dasari SK, Kimchi A. Autophagy-dependent cell death - where, how and why a cell eats itself to death. J Cell Sci. 2018;131. [DOI] [PubMed] [Google Scholar]
- [54].Siddique MM, Li Y, Chaurasia B, Kaddai VA, Summers SA. Dihydroceramides: From Bit Players to Lead Actors. The Journal of biological chemistry. 2015;290:15371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Qu X, Zou Z, Sun Q, Luby-Phelps K, Cheng P, Hogan RN, et al. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128:931–46. [DOI] [PubMed] [Google Scholar]
- [56].Ginkel C, Hartmann D, vom Dorp K, Zlomuzica A, Farwanah H, Eckhardt M, et al. Ablation of neuronal ceramide synthase 1 in mice decreases ganglioside levels and expression of myelin-associated glycoprotein in oligodendrocytes. The Journal of biological chemistry. 2012;287:41888–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta. 2006;1758:1864–84. [DOI] [PubMed] [Google Scholar]
- [58].Pewzner-Jung Y, Brenner O, Braun S, Laviad EL, Ben-Dor S, Feldmesser E, et al. A critical role for ceramide synthase 2 in liver homeostasis: II. insights into molecular changes leading to hepatopathy. The Journal of biological chemistry. 2010;285:10911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Pewzner-Jung Y, Park H, Laviad EL, Silva LC, Lahiri S, Stiban J, et al. A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. The Journal of biological chemistry. 2010;285:10902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zigdon H, Kogot-Levin A, Park JW, Goldschmidt R, Kelly S, Merrill AH, Jr., et al. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J Biol Chem. 2013;288:4947–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Jennemann R, Rabionet M, Gorgas K, Epstein S, Dalpke A, Rothermel U, et al. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum Mol Genet. 2012;21:586–608. [DOI] [PubMed] [Google Scholar]
- [62].Rabionet M, Bayerle A, Jennemann R, Heid H, Fuchser J, Marsching C, et al. Male meiotic cytokinesis requires ceramide synthase 3-dependent sphingolipids with unique membrane anchors. Hum Mol Genet. 2015;24:4792–808. [DOI] [PubMed] [Google Scholar]
- [63].Ebel P, Imgrund S, Vom Dorp K, Hofmann K, Maier H, Drake H, et al. Ceramide synthase 4 deficiency in mice causes lipid alterations in sebum and results in alopecia. The Biochemical journal. 2014;461:147–58. [DOI] [PubMed] [Google Scholar]
- [64].Bruggen B, Kremser C, Bickert A, Ebel P, Vom Dorp K, Schultz K, et al. Defective ceramide synthases in mice cause reduced amplitudes in electroretinograms and altered sphingolipid composition in retina and cornea. Eur J Neurosci. 2016;44:1700–13. [DOI] [PubMed] [Google Scholar]
- [65].Sofi MH, Heinrichs J, Dany M, Nguyen H, Dai M, Bastian D, et al. Ceramide synthesis regulates T cell activity and GVHD development. JCI Insight. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Helke K, Angel P, Lu P, Garrett-Mayer E, Ogretmen B, Drake R, et al. Ceramide Synthase 6 Deficiency Enhances Inflammation in the DSS model of Colitis. Sci Rep. 2018;8:1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Scheffel MJ, Helke K, Lu P, Bowers JS, Ogretmen B, Garrett-Mayer E, et al. Adoptive Transfer of Ceramide Synthase 6 Deficient Splenocytes Reduces the Development of Colitis. Sci Rep. 2017;7:15552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–86. [DOI] [PubMed] [Google Scholar]
- [69].Gosejacob D, Jager PS, Vom Dorp K, Frejno M, Carstensen AC, Kohnke M, et al. Ceramide Synthase 5 Is Essential to Maintain C16:0-Ceramide Pools and Contributes to the Development of Diet-induced Obesity. J Biol Chem. 2016;291:6989–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Duncan AW. Aneuploidy, polyploidy and ploidy reversal in the liver. Semin Cell Dev Biol. 2013;24:347–56. [DOI] [PubMed] [Google Scholar]
- [71].Bahmanyar S, Schlieker C. Lipid and protein dynamics that shape nuclear envelope identity. Mol Biol Cell. 2020;31:1315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lucki NC, Sewer MB. Nuclear sphingolipid metabolism. Annu Rev Physiol. 2012;74:131–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lipsky NG, Pagano RE. Intracellular translocation of fluorescent sphingolipids in cultured fibroblasts: endogenously synthesized sphingomyelin and glucocerebroside analogues pass through the Golgi apparatus en route to the plasma membrane. J Cell Biol. 1985;100:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kobayashi T, Pagano RE. Lipid transport during mitosis. Alternative pathways for delivery of newly synthesized lipids to the cell surface. J Biol Chem. 1989;264:5966–73. [PubMed] [Google Scholar]
- [76].Bigay J, Antonny B. Curvature, lipid packing, and electrostatics of membrane organelles: defining cellular territories in determining specificity. Dev Cell. 2012;23:886–95. [DOI] [PubMed] [Google Scholar]
- [77].Yokoyama K, Suzuki M, Kawashima I, Karasawa K, Nojima S, Enomoto T, et al. Changes in composition of newly synthesized sphingolipids of HeLa cells during the cell cycle -- suppression of sphingomyelin and higher-glycosphingolipid synthesis and accumulation of ceramide and glucosylceramide in mitotic cells. Eur J Biochem. 1997;249:450–5. [DOI] [PubMed] [Google Scholar]
- [78].Cheng LC, Baboo S, Lindsay C, Brusman L, Martinez-Bartolome S, Tapia O, et al. Identification of new transmembrane proteins concentrated at the nuclear envelope using organellar proteomics of mesenchymal cells. Nucleus. 2019;10:126–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Barlow CA, Laishram RS, Anderson RA. Nuclear phosphoinositides: a signaling enigma wrapped in a compartmental conundrum. Trends Cell Biol. 2010;20:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Martins F, Sousa J, Pereira CD, da Cruz ESOAB, Rebelo S. Nuclear envelope dysfunction and its contribution to the aging process. Aging Cell. 2020;19:e13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Prudovsky I, Vary CP, Markaki Y, Olins AL, Olins DE. Phosphatidylserine colocalizes with epichromatin in interphase nuclei and mitotic chromosomes. Nucleus. 2012;3:200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Albi E, Magni MP. Chromatin neutral sphingomyelinase and its role in hepatic regeneration. Biochem Biophys Res Commun. 1997;236:29–33. [DOI] [PubMed] [Google Scholar]
- [83].Albi E, Magni MV. The presence and the role of chromatin cholesterol in rat liver regeneration. J Hepatol. 2002;36:395–400. [DOI] [PubMed] [Google Scholar]
- [84].Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Fu P, Ebenezer DL, Ha AW, Suryadevara V, Harijith A, Natarajan V. Nuclear lipid mediators: Role of nuclear sphingolipids and sphingosine-1-phosphate signaling in epigenetic regulation of inflammation and gene expression. J Cell Biochem. 2018;119:6337–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM. Role of ceramide in cellular senescence. J Biol Chem. 1995;270:30701–8. [DOI] [PubMed] [Google Scholar]
- [87].Lee JY, Leonhardt LG, Obeid LM. Cell-cycle-dependent changes in ceramide levels preceding retinoblastoma protein dephosphorylation in G2/ M. Biochem J. 1998;334 ( Pt 2):457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lai M, Realini N, La Ferla M, Passalacqua I, Matteoli G, Ganesan A, et al. Complete Acid Ceramidase ablation prevents cancer-initiating cell formation in melanoma cells. Scientific reports. 2017;7:7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Fekry B, Jeffries KA, Esmaeilniakooshkghazi A, Ogretmen B, Krupenko SA, Krupenko NI. CerS6 Is a Novel Transcriptional Target of p53 Protein Activated by Non-genotoxic Stress. The Journal of biological chemistry. 2016;291:16586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Fekry B, Esmaeilniakooshkghazi A, Krupenko SA, Krupenko NI. Ceramide Synthase 6 Is a Novel Target of Methotrexate Mediating Its Antiproliferative Effect in a p53-Dependent Manner. PloS one. 2016;11:e0146618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hoeferlin LA, Fekry B, Ogretmen B, Krupenko SA, Krupenko NI. Folate stress induces apoptosis via p53-dependent de novo ceramide synthesis and up-regulation of ceramide synthase 6. The Journal of biological chemistry. 2013;288:12880–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Erenpreisa J, Kalejs M, Ianzini F, Kosmacek EA, Mackey MA, Emzinsh D, et al. Segregation of genomes in polyploid tumour cells following mitotic catastrophe. Cell Biol Int. 2005;29:1005–11. [DOI] [PubMed] [Google Scholar]
- [93].Erenpreisa J, Cragg MS, Salmina K, Hausmann M, Scherthan H. The role of meiotic cohesin REC8 in chromosome segregation in gamma irradiation-induced endopolyploid tumour cells. Exp Cell Res. 2009;315:2593–603. [DOI] [PubMed] [Google Scholar]
- [94].Ianzini F, Kosmacek EA, Nelson ES, Napoli E, Erenpreisa J, Kalejs M, et al. Activation of meiosis-specific genes is associated with depolyploidization of human tumor cells following radiation-induced mitotic catastrophe. Cancer Res. 2009;69:2296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kalejs M, Ivanov A, Plakhins G, Cragg MS, Emzinsh D, Illidge TM, et al. Upregulation of meiosis-specific genes in lymphoma cell lines following genotoxic insult and induction of mitotic catastrophe. BMC Cancer. 2006;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Gorgoulis VG, Zacharatos P, Mariatos G, Liloglou T, Kokotas S, Kastrinakis N, et al. Deregulated expression of c-mos in non-small cell lung carcinomas: relationship with p53 status, genomic instability, and tumor kinetics. Cancer Res. 2001;61:538–49. [PubMed] [Google Scholar]
- [97].Castanon I, Hannich JT, Abrami L, Huber F, Dubois M, Muller M, et al. Wnt-controlled sphingolipids modulate Anthrax Toxin Receptor palmitoylation to regulate oriented mitosis in zebrafish. Nature communications. 2020;11:3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kolesnick R, Altieri D, Fuks Z. A CERTain role for ceramide in taxane-induced cell death. Cancer Cell. 2007;11:473–5. [DOI] [PubMed] [Google Scholar]
- [99].Swanton C, Marani M, Pardo O, Warne PH, Kelly G, Sahai E, et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell. 2007;11:498–512. [DOI] [PubMed] [Google Scholar]
- [100].Andrieu G, Ledoux A, Branka S, Bocquet M, Gilhodes J, Walzer T, et al. Sphingosine 1-phosphate signaling through its receptor S1P5 promotes chromosome segregation and mitotic progression. Sci Signal. 2017;10. [DOI] [PubMed] [Google Scholar]
- [101].Kotelevets N, Fabbro D, Huwiler A, Zangemeister-Wittke U. Targeting sphingosine kinase 1 in carcinoma cells decreases proliferation and survival by compromising PKC activity and cytokinesis. PloS one. 2012;7:e39209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Dick TE, Hengst JA, Fox TE, Colledge AL, Kale VP, Sung SS, et al. The apoptotic mechanism of action of the sphingosine kinase 1 selective inhibitor SKI-178 in human acute myeloid leukemia cell lines. The Journal of pharmacology and experimental therapeutics. 2015;352:494–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Gillies L, Lee SC, Long JS, Ktistakis N, Pyne NJ, Pyne S. The sphingosine 1-phosphate receptor 5 and sphingosine kinases 1 and 2 are localised in centrosomes: possible role in regulating cell division. Cell Signal. 2009;21:675–84. [DOI] [PubMed] [Google Scholar]
- [104].Nagahashi M, Kim EY, Yamada A, Ramachandran S, Allegood JC, Hait NC, et al. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 2013;27:1001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–7. [DOI] [PubMed] [Google Scholar]
- [106].Silio V, Redondo-Munoz J, Carrera AC. Phosphoinositide 3-kinase beta regulates chromosome segregation in mitosis. Mol Biol Cell. 2012;23:4526–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kasahara K, Goto H, Izawa I, Kiyono T, Watanabe N, Elowe S, et al. PI 3-kinase-dependent phosphorylation of Plk1-Ser99 promotes association with 14–3-3gamma and is required for metaphase-anaphase transition. Nat Commun. 2013;4:1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Rovina P, Schanzer A, Graf C, Mechtcheriakova D, Jaritz M, Bornancin F. Subcellular localization of ceramide kinase and ceramide kinase-like protein requires interplay of their Pleckstrin Homology domain-containing N-terminal regions together with C-terminal domains. Biochimica et biophysica acta. 2009;1791:1023–30. [DOI] [PubMed] [Google Scholar]
- [109].Payne AW, Pant DK, Pan TC, Chodosh LA. Ceramide kinase promotes tumor cell survival and mammary tumor recurrence. Cancer Res. 2014;74:6352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Ruckhaberle E, Karn T, Rody A, Hanker L, Gatje R, Metzler D, et al. Gene expression of ceramide kinase, galactosyl ceramide synthase and ganglioside GD3 synthase is associated with prognosis in breast cancer. J Cancer Res Clin Oncol. 2009;135:1005–13. [DOI] [PubMed] [Google Scholar]
- [111].Schwalm S, Erhardt M, Romer I, Pfeilschifter J, Zangemeister-Wittke U, Huwiler A. Ceramide Kinase Is Upregulated in Metastatic Breast Cancer Cells and Contributes to Migration and Invasion by Activation of PI 3-Kinase and Akt. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Sun HS, Sin AT, Poirier MB, Harrison RE. Chlamydia trachomatis Inclusion Disrupts Host Cell Cytokinesis to Enhance Its Growth in Multinuclear Cells. Journal of cellular biochemistry. 2016;117:132–43. [DOI] [PubMed] [Google Scholar]
- [113].Gudejko HF, Alford LM, Burgess DR. Polar expansion during cytokinesis. Cytoskeleton (Hoboken). 2012;69:1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ng MM, Chang F, Burgess DR. Movement of membrane domains and requirement of membrane signaling molecules for cytokinesis. Dev Cell. 2005;9:781–90. [DOI] [PubMed] [Google Scholar]
- [115].Rani CS, Abe A, Chang Y, Rosenzweig N, Saltiel AR, Radin NS, et al. Cell cycle arrest induced by an inhibitor of glucosylceramide synthase. Correlation with cyclin-dependent kinases. J Biol Chem. 1995;270:2859–67. [DOI] [PubMed] [Google Scholar]
- [116].Watanabe H, Okahara K, Naito-Matsui Y, Abe M, Go S, Inokuchi J, et al. Psychosine-triggered endomitosis is modulated by membrane sphingolipids through regulation of phosphoinositide 4,5-bisphosphate production at the cleavage furrow. Mol Biol Cell. 2016;27:2037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Atilla-Gokcumen GE, Bedigian AV, Sasse S, Eggert US. Inhibition of glycosphingolipid biosynthesis induces cytokinesis failure. J Am Chem Soc. 2011;133:10010–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Atilla-Gokcumen GE, Muro E, Relat-Goberna J, Sasse S, Bedigian A, Coughlin ML, et al. Dividing cells regulate their lipid composition and localization. Cell. 2014;156:428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Abe M, Makino A, Hullin-Matsuda F, Kamijo K, Ohno-Iwashita Y, Hanada K, et al. A role for sphingomyelin-rich lipid domains in the accumulation of phosphatidylinositol-4,5-bisphosphate to the cleavage furrow during cytokinesis. Mol Cell Biol. 2012;32:1396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Theurkauf WE. Actin cytoskeleton. Through the bottleneck. Curr Biol. 1994;4:76–8. [DOI] [PubMed] [Google Scholar]
- [121].Reversi A, Loeser E, Subramanian D, Schultz C, De Renzis S. Plasma membrane phosphoinositide balance regulates cell shape during Drosophila embryo morphogenesis. J Cell Biol. 2014;205:395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Nganga R, Oleinik N, Kim J, Selvam SP, De Palma R, Johnson KA, et al. Receptor-interacting Ser/Thr kinase 1 (RIPK1) and myosin IIA-dependent ceramidosomes form membrane pores that mediate blebbing and necroptosis. J Biol Chem. 2019;294:502–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Walker A, Su H, Conti MA, Harb N, Adelstein RS, Sato N. Non-muscle myosin II regulates survival threshold of pluripotent stem cells. Nat Commun. 2010;1:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Heissler SM, Manstein DJ. Nonmuscle myosin-2: mix and match. Cell Mol Life Sci. 2013;70:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Singh RK, Haka AS, Brumfield A, Grosheva I, Bhardwaj P, Chin HF, et al. Ceramide activation of RhoA/Rho kinase impairs actin polymerization during aggregated LDL catabolism. J Lipid Res. 2017;58:1977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Zhang S, Mercado-Uribe I, Xing Z, Sun B, Kuang J, Liu J. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene. 2014;33:116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Zhang S, Mercado-Uribe I, Liu J. Tumor stroma and differentiated cancer cells can be originated directly from polyploid giant cancer cells induced by paclitaxel. International journal of cancer. 2014;134:508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Ahn EH, Yang H, Hsieh CY, Sun W, Chang CC, Schroeder JJ. Evaluation of chemotherapeutic and cancer-protective properties of sphingosine and C2-ceramide in a human breast stem cell derived carcinogenesis model. Int J Oncol. 2019;54:655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Zhang S, Mercado-Uribe I, Sood A, Bast RC, Liu J. Coevolution of neoplastic epithelial cells and multilineage stroma via polyploid giant cells during immortalization and transformation of mullerian epithelial cells. Genes Cancer. 2016;7:60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Bieberich E, MacKinnon S, Silva J, Yu RK. Regulation of apoptosis during neuronal differentiation by ceramide and b-series complex gangliosides. J Biol Chem. 2001;276:44396–404. [DOI] [PubMed] [Google Scholar]
- [132].Bieberich E, MacKinnon S, Silva J, Noggle S, Condie BG. Regulation of cell death in mitotic neural progenitor cells by asymmetric distribution of prostate apoptosis response 4 (PAR-4) and simultaneous elevation of endogenous ceramide. J Cell Biol. 2003;162:469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Bieberich E, Silva J, Wang G, Krishnamurthy K, Condie BG. Selective apoptosis of pluripotent mouse and human stem cells by novel ceramide analogues prevents teratoma formation and enriches for neural precursors in ES cell-derived neural transplants. J Cell Biol. 2004;167:723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Wang G, Spassieva SD, Bieberich E. Ceramide and S1P Signaling in Embryonic Stem Cell Differentiation. Methods Mol Biol. 2018;1697:153–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Arun RP, Sivanesan D, Patra B, Varadaraj S, Verma RS. Simulated microgravity increases polyploid giant cancer cells and nuclear localization of YAP. Sci Rep. 2019;9:10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Sun X, Ren Z, Cun Y, Zhao C, Huang X, Zhou J, et al. Hippo-YAP signaling controls lineage differentiation of mouse embryonic stem cells through modulating the formation of super-enhancers. Nucleic Acids Res. 2020;48:7182–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Alsamman S, Christenson SA, Yu A, Ayad NME, Mooring MS, Segal JM, et al. Targeting acid ceramidase inhibits YAP/TAZ signaling to reduce fibrosis in mice. Sci Transl Med. 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Jiang Q, Zhang Q, Wang S, Xie S, Fang W, Liu Z, et al. A Fraction of CD133+ CNE2 Cells Is Made of Giant Cancer Cells with Morphological Evidence of Asymmetric Mitosis. Journal of Cancer. 2015;6:1236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Glumac PM, LeBeau AM. The role of CD133 in cancer: a concise review. Clin Transl Med. 2018;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Zhong L, Kong JN, Dinkins MB, Leanhart S, Zhu Z, Spassieva SD, et al. Increased liver tumor formation in neutral sphingomyelinase-2-deficient mice. J Lipid Res. 2018;59:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].He Q, Wang G, Wakade S, Dasgupta S, Dinkins M, Kong JN, et al. Primary cilia in stem cells and neural progenitors are regulated by neutral sphingomyelinase 2 and ceramide. Mol Biol Cell. 2014;25:1715–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Canals D, Salamone S, Santacreu BJ, Nemeth E, Aguilar D, Hernandez-Corbacho MJ, et al. Ceramide launches an acute anti-adhesion pro-migration cell signaling program in response to chemotherapy. FASEB J. 2020;34:7610–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Asano S, Pal R, Tanaka HN, Imamura A, Ishida H, Suzuki KGN, et al. Development of Fluorescently Labeled SSEA-3, SSEA-4, and Globo-H Glycosphingolipids for Elucidating Molecular Interactions in the Cell Membrane. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Ho MY, Yu AL, Yu J. Glycosphingolipid dynamics in human embryonic stem cell and cancer: their characterization and biomedical implications. Glycoconj J. 2017;34:765–77. [DOI] [PubMed] [Google Scholar]
- [145].Liang YJ, Wang CY, Wang IA, Chen YW, Li LT, Lin CY, et al. Interaction of glycosphingolipids GD3 and GD2 with growth factor receptors maintains breast cancer stem cell phenotype. Oncotarget. 2017;8:47454–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Yeh SC, Wang PY, Lou YW, Khoo KH, Hsiao M, Hsu TL, et al. Glycolipid GD3 and GD3 synthase are key drivers for glioblastoma stem cells and tumorigenicity. Proc Natl Acad Sci U S A. 2016;113:5592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Bieberich E. Integration of glycosphingolipid metabolism and cell-fate decisions in cancer and stem cells: review and hypothesis. Glycoconj J. 2004;21:315–27. [DOI] [PubMed] [Google Scholar]
- [148].Wegner MS, Schomel N, Gruber L, Ortel SB, Kjellberg MA, Mattjus P, et al. UDP-glucose ceramide glucosyltransferase activates AKT, promoted proliferation, and doxorubicin resistance in breast cancer cells. Cell Mol Life Sci. 2018;75:3393–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Yoshida H, Koodie L, Jacobsen K, Hanzawa K, Miyamoto Y, Yamamoto M. B4GALNT1 induces angiogenesis, anchorage independence growth and motility, and promotes tumorigenesis in melanoma by induction of ganglioside GM2/GD2. Sci Rep. 2020;10:1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Jacob F, Alam S, Konantz M, Liang CY, Kohler RS, Everest-Dass AV, et al. Transition of Mesenchymal and Epithelial Cancer Cells Depends on alpha1–4 Galactosyltransferase-Mediated Glycosphingolipids. Cancer Res. 2018;78:2952–65. [DOI] [PubMed] [Google Scholar]
- [151].Zhang T, de Waard AA, Wuhrer M, Spaapen RM. The Role of Glycosphingolipids in Immune Cell Functions. Front Immunol. 2019;10:90. [DOI] [PMC free article] [PubMed] [Google Scholar]