Abstract
The prevalence of diabetes has been rising steadily in the past half-century, along with the burden of its associated complications, including diabetic retinopathy (DR). DR is currently the most common cause of vision loss in working-age adults in the United States. Historically, DR has been diagnosed and classified clinically based on what is visible by fundoscopy; that is vasculature alterations. However, recent technological advances have confirmed pathology of the neuroretina prior to any detectable vascular changes. These, coupled with molecular studies, and the positive impact of anti-inflammatory therapeutics in DR patients have highlighted the central involvement of the innate immune system. Reminiscent of the systemic impact of diabetes, immune dysregulation has become increasingly identified as a key element of the pathophysiology of DR by interfering with normal homeostatic systems. This review uses the growing body of literature across various model systems to demonstrate the clear involvement of all three pillars of the immune system: immune-competent cells, mediators, and the complement system. It also demonstrates how the relative contribution of each of these requires more extensive analysis, including in human tissues over the continuum of disease progression. Finally, although this review demonstrates how the complex interactions of the immune system pose many more questions than answers, the intimately connected nature of the three pillars of the immune system may also point to possible new targets to reverse or even halt reverse retinopathy.
1. Introduction
Diabetes, characterized by the dysregulation of carbohydrate and lipid metabolism, results from impaired insulin secretion and/or insulin resistance. Its prevalence has more than quadrupled over the past four decades, from 108 million in 1980 to more than 425 million worldwide (Internation Diabetes Federation, 2019; World Health Organization, n.d.). This, coupled with one-third of all diabetic individuals experiencing related vision complications (Ko et al., 2012), has resulted in diabetic retinopathy (DR) becoming the leading cause of preventable vision loss in working-age individuals (Yau et al., 2012). DR has classically been viewed as a microvascular complication of diabetes and categorized based on those vascular abnormalities (Bursell et al., 2001; Treatment and Retinopathy, 1991a, 1991b, 1991c; Wilkinson et al., 2003). However, more recent discoveries of neurodegeneration and immune dysregulation early in DR patients have called into question this vascular-centric view (Kong et al., 2016). Consistent with these findings, inflammatory genes are most closely tied to DR, as identified by genome-wide association studies (Abhary et al., 2009).
The mechanisms behind these immune system–based manifestations in human DR patients have been investigated in rodent models of DR. However, no rodent model fully recapitulates the human presentation of DR (Olivares et al., 2017). This major limitation has driven investigators to use a combination of models to study different human manifestations of DR, including drug-induced (e.g., streptozocin), genetic (i.e. Akita [Ins2Akita], and leptin receptor deficient [db/db]), and non-diabetic injury–based (e.g., oxygen-induced retinopathy [OIR]; and ischemia/reperfusion) (Lai and Lo, 2013; Olivares et al., 2017) models. Interestingly, a common feature that has emerged from these disparate models is the central role of the innate immune system. This manuscript will examine our evolving understanding of DR from the perspective of these experimental in vivo models contextualized to human studies and in vitro data. In particular, we will focus on the role of the innate immune system in DR-related neurodegeneration and vasculopathy.
2. The intricacy of the ocular innate immune system
The retina is exquisitely sensitive to metabolic perturbations due to the combination of high metabolic demand and limited vascular supply (Dai et al., 2014; Joyal et al., 2018, 2016; Kooragayala et al., 2015). Importantly, the innate immune system, through its role as the early responder to environmental perturbations, maintains the homeostasis and visual function of this environment (Murakami et al., 2020). Much of this homeostasis is maintained and controlled by the innate immune system in a set of critically important anatomic barriers made up of and regulated by highly specialized cell types.
a. Blood-retinal barrier (BRB) as the first line of defense
A series of anatomic barriers is the first component of protection provided by the innate immune system. From the perspective of the retina, the first most-distal barrier is the inner BRB and consists of endothelial cells woven together by tight junctions and regulated by pericytes (Figure 1). This barrier limits the ability of circulating cells, proteins, and other molecules from crossing between the systemic circulation and the highly sensitive neuroretinal tissue (Black et al., 1985). The molecules that do make it past this first inner BRB layer next come in contact with the glial limitans, a second, more-proximal barrier made up of Müller glial cells and astrocytic processes. Finally, the inner and most-proximal barrier is the outer BRB, which is formed by the retinal pigment epithelium (RPE) via tight junctions (Figure 1). These barriers not only limit the retina’s exposure to foreign elements but also modulate the retina’s access to nutrients from the vasculature (Black et al., 1985; Muoio et al., 2014). To coordinate these protective and metabolic functions, the different cells that make up the BRB communicate closely with neurons and microglia to make up the neurovascular unit (NVU; Figure 2) (Simó et al., 2018). Separate from their role in forming immune barriers, many of these cells, including microglia, Müller cells, and astrocytes, play important cellular roles in innate immunity, which will be discussed in later sections. Over the past decade, our group and others have brought to light the central and unique roles played by these different retinal cells in the pathophysiology of DR.
Figure 1. Anatomical blood-retinal barrier.
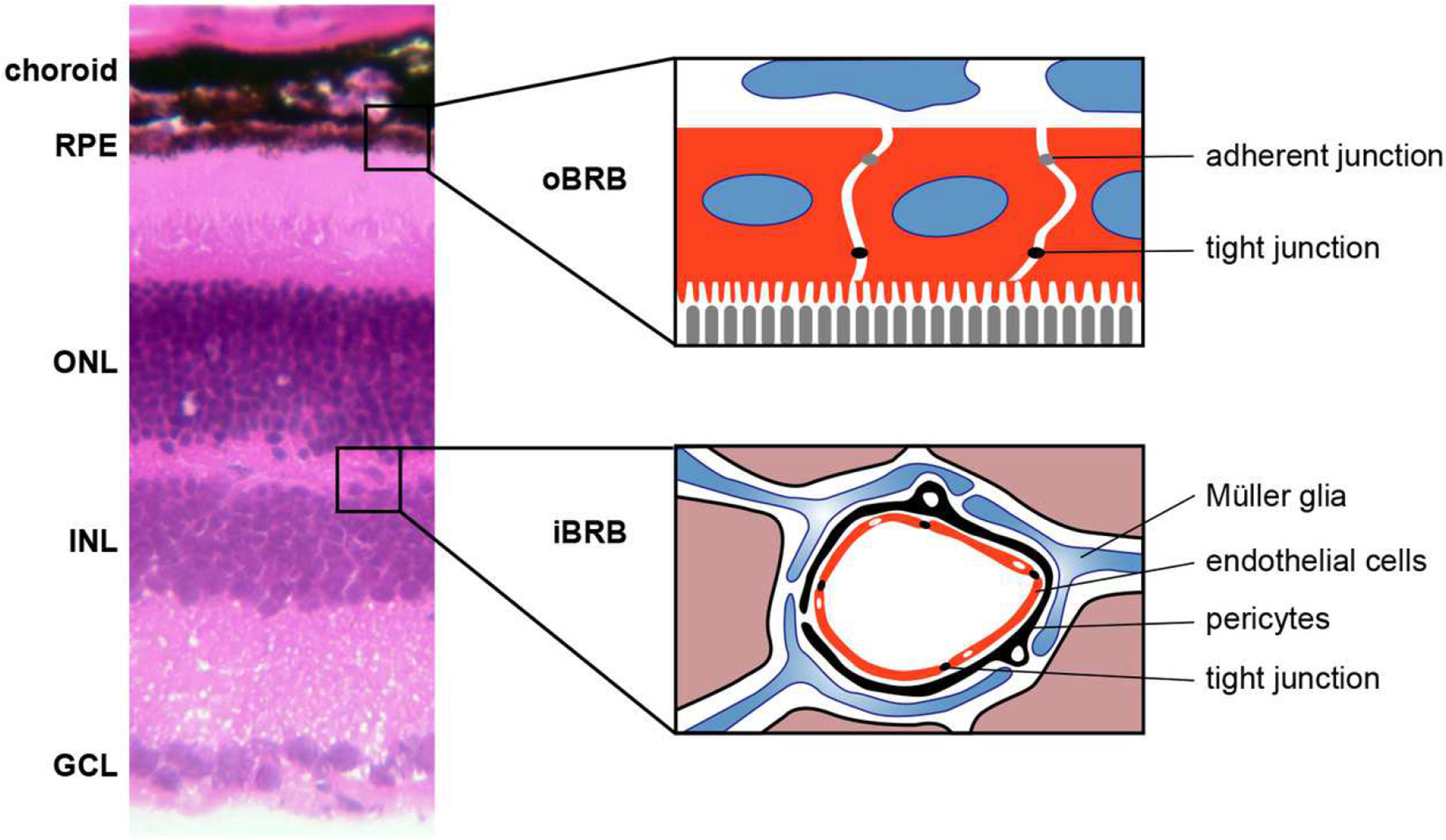
The layers of the retina, retinal pigment epithelium (RPE) and choroid are labeled on the left with details of the outer blood retinal barrier (oBRB) expanded in the diagram on the right depicting the tight and adherent junctions between the RPE cells in red. Additionally, a cartoon of the inner BRB (iBRB) is shown in the lower right with tight junction between endothelial cells, which are surrounded by pericytes, Müller glia, and astrocytes. Additionally, neurons of the ONL (outer nuclear layer) and INL (inner nuclear layer) are nearby. GCL: ganglion cell layer.
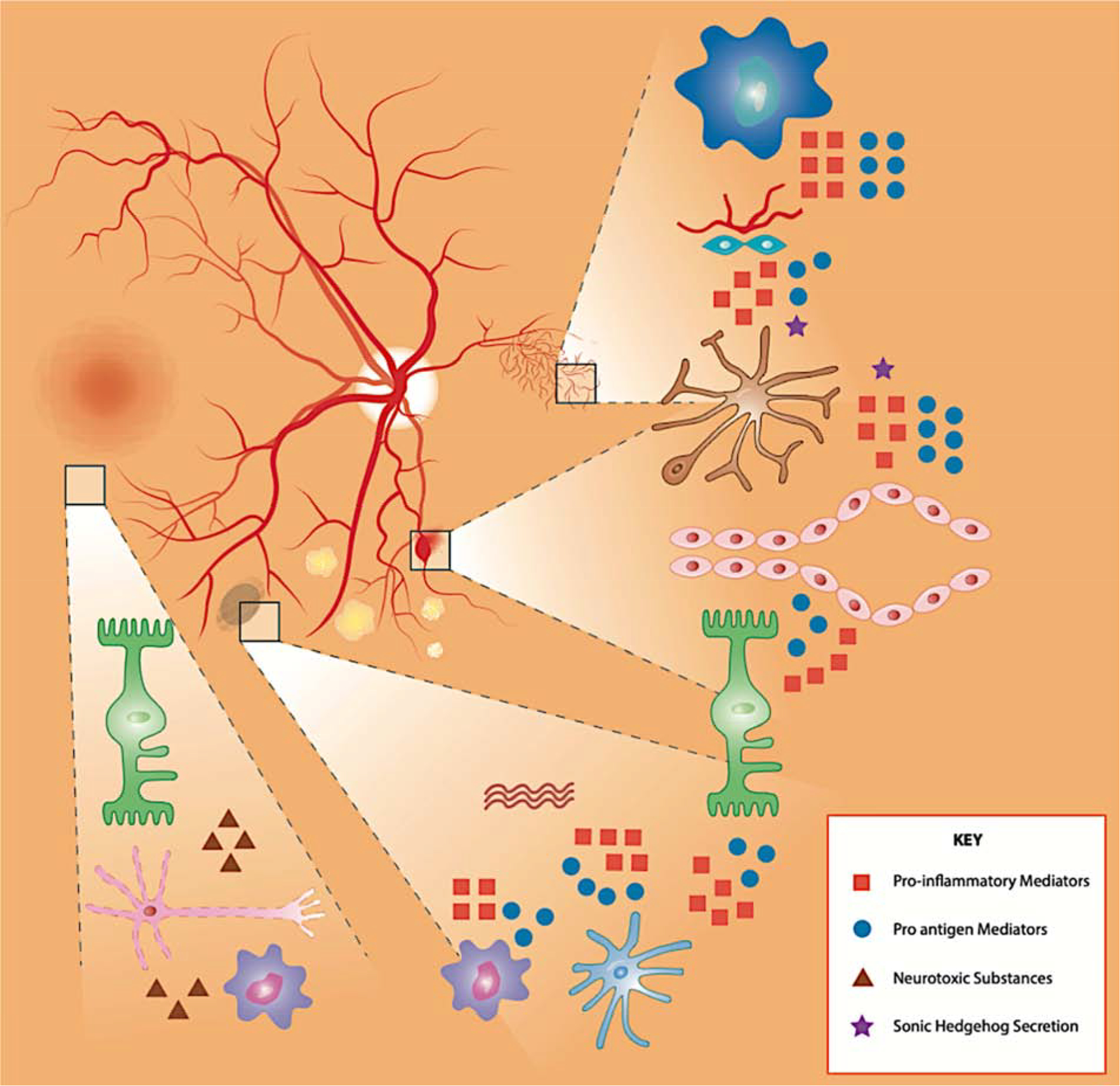
Figure 2. The complex multi-cellular dysfunction in diabetic retinopathy.
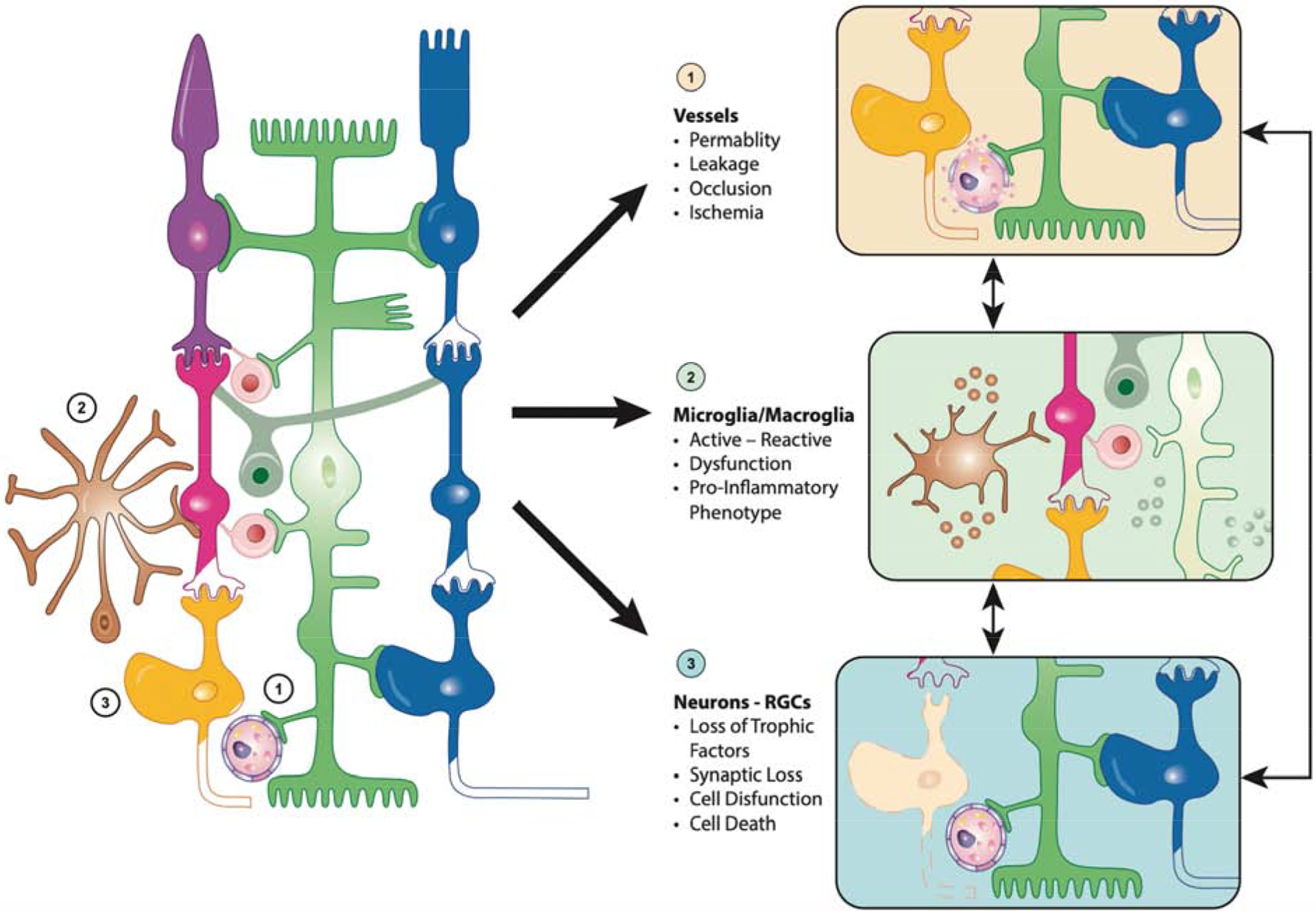
Diabetes virtually affects all retinal cells in a sequence that remains hard to elucidate as they function as a neurovascular unit and influence each other. (1) Vessel dysfunction reflects pericytes and endothelial cells dysfunction and loss, a phenomenon influenced by factors secreted by dying neurons and activated micro- and macroglia. (2) Microglia (brown) and macroglia (green) become activated in response to the alteration of the retinal environment associated with diabetes, a phenomenon enhanced by neuronal cell death and vascular preturbations. (3) Alteration of the retinal homeostasis by diabetes leads to neuronal dysfunction and ultimately cell death, a phenomenon enhanced by the increasingly pro-inflammatory environment and vascular perturbations.
b. Specific innate immune cells of the ocular system
i. Microglia
The NVU is composed of various cell types, each playing key roles in the integration and regulation of ocular homeostasis, which includes maintenance of the immune privilege status. Central nervous system microglia, including those in the retina, are derived from yolk sac primitive macrophages during development and are distributed evenly throughout three layers of the retina: the ganglion cell layer, the inner plexiform layer, and the outer plexiform layer (Herbomel et al., 2001; Nimmerjahn et al., 2005; Wake et al., 2009; Wang et al., 2016). As any other microglia of the central nervous system, retinal microglia continuously survey their environment by extending and retracting their processes in all directions (Damani et al., 2011; Karlstetter et al., 2015; Lee et al., 2008; Okunuki et al., 2018). This comprehensive access makes them well suited for phagocytosing retinal debris, supporting neighboring cells, and modulating synapses (Dando et al., 2016; Okunuki et al., 2018; Paolicelli et al., 2011; Rathnasamy et al., 2019). During development, microglia selectively target neurons and synapses for phagocytosis and complement-mediated destruction, without which neuropathy, synaptic degeneration, and vision loss result (Ferrer-Martín et al., 2014; Marín-Teva et al., 2004; Paolicelli et al., 2011; Roumier et al., 2004; Schafer et al., 2012). These necessary functions further require an intimate relationship with macroglial cells due to their prime location at the interface between the systemic circulation and the neuroretina. This relationship is all the more important because microglia depend on adenosine triphosphate supplied by nearby Müller cells to power these energy-intensive processes (Fontainhas et al., 2011; Wang and Wong, 2014). This co-dependence and communication between microglia and macroglia are becoming increasingly important for our understanding of the contribution of the innate immune response in the onset and progression of DR.
These surveying microglia are morphologically homogenous with small soma and ramified structures (McMenamin et al., 2019; Nimmerjahn et al., 2005); however, single-cell RNA-sequencing and gene expression profiles have determined that microglia are transcriptionally heterogeneous based on location and are dramatically different from peripherally circulating monocytes (Goldmann et al., 2016; Grabert et al., 2016). Together this is consistent with the important role that microglia, as the resident immune cells, play in the local maintenance of retinal function.
ii. Non-microglial cells
Several other innate immune cells, including perivascular macrophages, persistent hyalocytes, and dendritic cells, have been suggested to participate in the regulation of the unique retinal immune situation. Perivascular macrophages have been suggested to reside between the inner BRB and the glial limitans and putatively monitor this space for foreign proteins that pass through the barriers (Lewis et al., 2005; Mato et al., 1996; Mendes-Jorge et al., 2009). Pharmacologic disruption of the inner BRB has been reported to result in perivascular macrophage recruitment and activation (Mendes-Jorge et al., 2009). These observations suggest that this immune cell population could function as an active pseudo-barrier in clearing proteins and debris; however, there continues to be ongoing discussions on these cells’ exact nature and origin, including during diabetes, because it is difficult to differentiate them from other immune cells, local or circulating. Hyalocytes are bone marrow–derived macrophages located at the vitreoretinal interface during development, and their purpose is to phagocytose the transient vasculature of the lens (i.e., tunica vasculosa lentis) (McMenamin et al., 2002; Qiao et al., 2005). Persistent hyalocytes remain between the vitreous membrane and the inner limiting membrane after development. Although their purpose is not completely understood, they have been hypothesized to be antigen-presenting cells that transport antigens to the spleen and help to modulate the immunosuppressive environment of the eye via regulatory T cells (Murakami et al., 2020). Although their role in DR remains unknown, this population could certainly play an important role locally and systemically and participate in both the maladapted immune response that develops over time during diabetes and the onset and progression of DR. Such a role has been suggested based on the finding of a significant increase in hyalocyte number as well as a change in morphology in a rodent model of diabetes (Vagaja et al., 2012). These cells could particularly be important in the pathophysiologic mechanisms responsible for tractional detachment because they have been reported, along with glial cells, in epiretinal membranes from proliferative vitreoretinopathy and proliferative DR (PDR) (Oberstein et al., 2011).
Similar to perivascular macrophages, it remains controversial whether dendritic cells can be found in the retina. A small population of cells was originally identified using various cell markers and was thought to be resident dendritic cells (Lehmann et al., 2010; Xu et al., 2007). However, more recent studies have asserted that these cells are indistinguishable from neighboring microglial and macrophage subpopulations (Dando et al., 2016; McMenamin et al., 2019). Thus, although it remains unclear if dendritic cells play a role in DR, including at later stages and during BRB breakdown, applications of new technologies such as multiplex staining and light-sheet microscopy are currently being used to resolve this controversy (Li et al., 2017; Power and Huisken, 2017).
In addition, even if retinal dendritic cells do exist in the retina, it is not clear the extent to which they would fulfill their classic role as antigen-presenting cells (Dando et al., 2016), particularly given the presence of hyalocytes and perivascular macrophages (Gregerson and Yang, 2003; Lehmann et al., 2010). Indeed, recent single-cell RNA-sequencing work has identified the possibility for macroglia to function as antigen presenters as well (Van Hove et al., 2020). Clearly, there remain many questions surrounding the various putative innate immune cell types in the retina, and more research into all these populations is needed to elucidate their roles in DR pathogenesis. For now, however, much of the research on the cellular immune response is limited to microglia and other immune-competent cells, such as astrocytes and Müller cells.
c. The complement system as a key local player
The final component of the innate immune system is the complement system, which consists of more than 30 proteins that act as sentinels (Walport, 2001; Ricklin et al., 2010). Many stressors activate complement components, while endogenous complement regulatory molecules inhibit the complement activation cascade to maintain homeostasis. In this manner, complement components are activated when they encounter abnormal entities (e.g., pathogens), at which point the amplification of the complement response proceeds through three major pathways (the classical, lectin, and alternative pathways); this results in the subsequent destruction of the entities responsible for the perturbation (Zipfel and Skerka, 2009). The role of the complement system in the pathophysiology of DR has been supported by observations of activation markers in the retinal tissue from late-stage PDR donors. In addition, the identification of complement involvement at earlier stages of the disease (Shahulhameed et al., 2020; Zhang et al., 2002) suggests a role similar to the one it plays during neurodevelopment.
Canonically, complement proteins are thought to be produced by hepatocytes. However, complement components and regulators were recently not only found in the retinas of humans and rodents but also shown to be produced locally, including by microglia, macroglia, and RPE (Anderson et al., 2010; Luo et al., 2011). These findings are consistent with additional functions for the complement system such as the one it plays in synaptic pruning by microglia during normal development (Schafer et al., 2012; Stevens et al., 2007). It is indeed consistent with the key role the complement system plays in protecting the retina from foreign disruptions and environmental alterations. Together the complement system, the resident immune-competent cells, and the physical barriers afford the retina relative immune privilege, or exemption from the systemic immune response (Streilein et al., 2002); this plays a central role in other retinal diseases and was recently reviewed (Murakami et al., 2020).
3. The complex role of the immune response in the early stages of the disease: Preclinical diabetic retinopathy
Preclinical DR begins when diabetes is initially diagnosed and lasts until the first vascular abnormalities are detected, which usually takes many years (Abcouwer and Gardner, 2014). In contrast to its historical categorization as a clinically silent phase, preclinical DR is a period of significant disease progression, primarily through immune dysregulation. Diabetes, like many other stress conditions, initiates the microglial immune response as part of its defense mechanism and “normal” response, a response that is usually counterbalanced by immunosuppressive mechanisms. However, during the lasting environmental and metabolic stress associated with diabetes, these regulating systems are overwhelmed; instead of being progressively resolved, the microglial activation and associated immune response are amplified through the cross-activation of immune-competent macroglia (Liu and Steinle, 2017; Wang et al., 2012, 2011; Yoshida et al., 2004).
This ever-increasing and self-perpetuating inflammatory condition involves a complicated interplay of inflammatory mediators with dire consequences on retinal neurons, function, and barrier integrity, all of which start during preclinical DR. Importantly, recent findings in patients with preclinical DR suggest that they may experience deficits in peripheral vision, night vision, color-hue discrimination, and contrast discrimination (Jackson and Barber, 2010; Trento et al., 2017; Wolff et al., 2015), which may be explained by the neuronal thinning observed in patients before development of vascular abnormalities (Adams and Bearse, 2012; van Dijk et al., 2011). Therefore, the active immune response during this period may explain the symptoms these patients experience and their detectable signs of vision impairment.
a. Early induction of the innate immune response
Diabetes results in the generation of a number of products thought to initiate the immune response, including advanced glycated end products and advanced lipoxidation end products (Steinle, 2020; Stitt, 2003). Alternatively, the dyslipidemia and altered lipid metabolism observed in diabetes have also been proposed as initiators of the innate immune response (Atawia et al., 2020; Eid et al., 2019). A recent study specifically identified monounsaturated oleic acid as a possible source for dyslipidemia-induced DR (Chang et al., 2020).
In the retina, these various diabetic signals bind to the receptors for damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) on microglia and macroglia; this represents one of the major mechanisms for how diabetes activates these cells (Atawia et al., 2020; Eid et al., 2019; Steinle, 2020; Stitt, 2003). Upon activation, microglia proliferate and experience a morphologic change by retracting their processes and becoming more amoeboid (Lim et al., 2019; Wang et al., 2007; Wong et al., 2001). Examination of human diabetic eyes has confirmed this microglial activation early in DR (Zeng et al., 2008), which mirrors the microgliosis observed in diabetic animal models only weeks after diabetes onset (Barber et al., 2005; Krady et al., 2005).
Pharmacologic inhibition of glycated end products dampens microglial activation in diabetic rodents, supporting the notion that glucose plays a role in the genesis and enhancement of the immune response during diabetes (Ibrahim et al., 2011). However, numerous studies have shown that glucose is clearly not the only culprit; for example, immune responses are only partially reduced by pharmacologic reduction of retinal glucose levels (You et al., 2018). It is becoming increasingly clear that glucose is only one of many systemic and local factors, including lipids, metabolites, cytokines, and trophic factors, important for microglial activation. In addition to the nature of the factors themselves, recent studies have suggested that the extent of their fluctuations is particularly important in triggering this activation (Hsieh et al., 2019). Together these studies also suggest that the early metabolic dysregulation in diabetes activates microglia soon after disease onset and well ahead of clinically detectable manifestations.
Activated microglia amplify the innate immune response by releasing inflammatory mediators such as interleukin-1β (IL-1β) and tumor necrosis factor α (TNFα) and recruiting additional immune cells as a mechanism of elimination of the inciting stressor (Ibrahim et al., 2011; Krady et al., 2005; Wang et al., 2007). Although this microglial response is extremely proficient in correcting short-term perturbations and typically works well in maintaining the delicate homeostasis of the retina (Chovatiya and Medzhitov, 2014; Miller et al., 2011), it is clearly less effective under chronic perturbation such as diabetes; during diabetes, activated microglia eventually become active contributors of the overt inflammatory environment. Indeed microglia participate in this unresolved and self-sustained inflammatory response which contributes to DR progression (Ding et al., 2018; Medzhitov, 2008).
Although the impact of the diabetic environment on retinal metabolism has received increasing attention (Eid et al., 2019; Fort et al., 2014), the specific impact on the intracellular metabolism of macrophages and other controllers of innate and adaptive immunity is still unclear. This is particularly important in light of the recent finding that nutrients and other environmental clues can induce profound metabolic reprogramming in macrophages and dendritic cells (O’Neill and Pearce, 2016). Although additional work in the context of diabetes and the retina is sorely needed, a number of reviews have detailed the general interdependence of metabolism with immune response activation (McGettrick and O’Neill, 2020; Ryan and O’Neill, 2020). The need for further investigation of the specific impact of the diabetic environment on retinal immune cells activation is particularly highlighted by the following: the metabolic dysregulated and hypoxic state of diabetes (Atawia et al., 2020; Eid et al., 2019; Klein et al., 2007; Kur et al., 2012; Wong et al., 2002); the specificities of retinal metabolism, such as retinal photoreceptors’ unusual use of aerobic glycolysis (Du et al., 2013; Petit et al., 2018; Winkler et al., 1986); the conversion of glucose to lactate despite the presence of oxygen (Vander Heiden et al., 2009; Warburg, 1956); and the neuron/macroglia metabolic interdependence.
b. Counterbalance by immunosuppressive mechanisms
The activated microglia-initiated inflammatory response is countered by a number of immune-suppressive mechanisms in place to re-establish equilibrium in the retina. These mechanisms are mediated by RPE cells, neurons, macroglia, and microglia themselves and, under normal conditions, maintain the retina in an overall anti-inflammatory state (Cai et al., 2018; Karlstetter et al., 2014; Murinello et al., 2019).
RPE cells, which also make up the outer BRB, express the ligand for CD95 (Fas ligand), which, when bound to CD95 (Fas) on microglia, initiates apoptosis in these cells (M. Chen et al., 2019; Griffith et al., 1995; Jørgensen et al., 1998; Kazama et al., 2008). As with other ligands involved in apoptosis induction, CD95 has a complicated role in retinal physiology and immune suppression and requires tight regulation. Indeed, in addition to targeting microglia, Fas ligand might be involved in killing cells foreign to the eye and preventing neovascularization through regulation of endothelial cells proliferation, which might be important in preventing PDR progression (Murinello et al., 2019); however, Fas ligand has also been demonstrated as a critical factor in retinal detachment-associated photoreceptor cell death, exemplifying the complexity of this regulatory process (Zacks et al., 2007). Apart from Fas ligand, RPE cells also secrete a number of anti-inflammatory mediators, including transforming growth factor β2, to prevent overt inflammation in the retina (Cousins SW et al., 1991; D’Orazio and Niederkorn, 1998; Paglinawan et al., 2003).
Neurons are also incredibly active in immunosuppression by expressing α-melanocortin-stimulating hormone, transmembrane glycoprotein CD200, and CX3CL1 (fractalkine). α-Melanocortin-stimulating hormone binds to microglia and macroglia to inhibit their expression of proinflammatory cytokines, including IL-1β and TNFα (Taylor and Lee, 2010), and has experimentally been shown to preserve BRB integrity and slow DR progression (Cai et al., 2018). CD200, secreted by neurons and endothelial cells, binds to its receptor (CD200R) on microglia to inhibit inflammation (Broderick et al., 2002; Hoek et al., 2000). This is also supported by the genetic and pharmacologic disruption of CD200-CD200R, which increases microgliosis and inflammation (Banerjee and Dick, 2004; Hoek et al., 2000). Fractalkine has similarly been shown to inhibit microgliosis (Cardona et al., 2015). To test the role of fractalkine in microglia-neuron interactions and the neuroinflammation associated with diabetes, fractalkine receptor CX3CR1-null rodents were crossed to the diabetic Ins2Akita model. These CX3CR1-null diabetic rodents experience enhanced microgliosis compared with their diabetic wild-type littermates (Beli et al., 2016; Cardona et al., 2015). Moreover, CX3CR1-null rodents exhibit both increased neurodegeneration (Cardona et al., 2015) and accelerated vascular abnormalities compared with diabetic controls (Beli et al., 2016). These findings, coupled with the elevated vitreous levels of fractalkine in human DR patients, suggest that neurons attempt, through fractalkine, to limit and possibly resolve the microgliosis that accompanies DR (You et al., 2007).
Microglia also possess mechanisms of “self-control” to limit their own activation and to limit damage associated with unresolved inflammation. When activated, microglia express the mitochondrial translocator protein TSPO (Karlstetter et al., 2014). The use of TSPO agonist XBD173 experimentally reverses microgliosis and decreases levels of pro-inflammatory genes (Karlstetter et al., 2014; Scholz et al., 2015). Furthermore, microglia endogenously produce diazepam-binding inhibitor, whose derivatives bind to TSPO and experimentally dampen the inflammatory response (Wang et al., 2014). These findings suggest a self-regulating mechanism whereby microglia are capable of not only mounting an immune response but also suppressing that inflammation and regaining homeostasis. Together these findings demonstrate how non-immune and immune cells exert immunosuppressive pressures in the attempt to maintain retinal homeostasis.
c. Glia as immune-competent cells
Contrary to how non-immune RPE and neurons apply immunosuppressive pressures, the immune-competent macroglia contribute to the pro-inflammatory retinal environment during diabetes. Given the central role of the innate immune response during diabetes, a more nuanced understanding of astrocytes and Müller cells is necessary in the understanding of DR.
Astrocytes develop along the vasculature in the central nervous system and are part of the glial limitans and NVU (Muoio et al., 2014; Watanabe and Raff, 1988). They are necessary for normal vasculature development in a number of mammalian species (Schnitzer, 1988) and for the continuous maintenance of the vascular network through the production of angiogenic mediators such as vascular endothelial growth factor (VEGF) (Kur et al., 2012). Studies examining the blood-brain barrier found that astrocytes specifically maintain the integrity of the blood-brain barrier by secreting various molecules, including Sonic hedgehog (Alvarez et al., 2011). Indeed, the disruption of this Hedgehog pathway results in increased permeability and decreased tight junction protein expression (Alvarez et al., 2011). Due to all these roles, astrocytes are considered the stewards of the vasculature.
Müller cells are specific to and span all layers of the retina. In this way, Müller cells are able to interact with other retinal cells to maintain homeostasis and metabolism, including glycogen storage and nutritional support (Lindsay et al., 2014; Xue et al., 2015). Müller cells recycle glutamate and neurotransmitters released by neurons, prevent potassium ion accumulation, and control water flux (Freitas et al., 2016; Vogler et al., 2016). Like astrocytes, Müller cells are part of the NVU and are involved in vasculature maintenance. Ablation of Müller cells causes BRB breakdown and has been implicated in DR progression (Abukawa et al., 2009; Coughlin et al., 2017; Fu et al., 2015; Shen et al., 2012).
Like microglia, macroglia become activated in response to perturbations in the environment, including glucose level fluctuations (Picconi et al., 2019). Although microglia are not true glia and this misnomer only artificially categorizes them with astrocytes and Müller cells, it is useful in this context due to their shared response to environmental stress and the immune-competent status of glial cells. Indeed, diabetes activates astrocytes and Müller cells and causes morphologic and functional changes, including the secretion of inflammatory mediators (Gerhardinger et al., 2005; Lieth et al., 1998; Mizutani et al., 1998; Puro, 2002; Rungger-Brändle et al., 2000; Shin et al., 2014). Moreover, a recent single-cell RNA-sequencing examination of macroglia in diabetic rodents has reaffirmed this through the identification of markers of reactive gliosis (Van Hove et al., 2020).
Exposure to the inflammatory milieu that they perpetuate and other metabolic perturbations associated with diabetes results in Müller cell dysfunction and impairs their normal homeostatic functions (Hassan et al., 2017). Recent electron microscopic analysis of human diabetic retinas confirmed Müller cell alterations during the development of DR (Fehér et al., 2018); these findings were suggested by previous studies in diabetic rodents models (Fernandez-Bueno et al., 2017) and highlight the potential key role of glial cell activation in the onset and progression of retinal dysfunction that characterizes DR.
d. The complex interactions of microglia and macroglia
Both microglia and macroglia are activated by environmental stressors such as diabetes; however, this activation, especially when not resolved, may not always be beneficial to the retina. Microgliosis exemplifies the dual effects a continuously activated immune response could have in both limiting and exacerbating DR pathology. Indeed, as previously noted, microglia are inherently heterogenous in nature, under physiologically healthy states (Goldmann et al., 2016; Grabert et al., 2016) and even more so under pathologic conditions. Thus, additional work focusing on untangling their heterogenous responses is needed to better understand their involvement in DR. There is ongoing discussion regarding the most appropriate way to view and conceptualize the varied effects activated microglia can have on the environment (Ransohoff, 2016), but there does appear to be a significant spectrum ranging from the anti-inflammatory slowing of disease to the pro-inflammatory acceleration of disease (Figure 3). Experimentally, activated microglia in diabetic rodents undergo shifts from one response to another (Arroba et al., 2016). The role of activated microglia during DR is therefore in continuous flux and represents a promising area of continued investigation.
Figure 3. Microglial activation during progression of diabetic retinopathy.
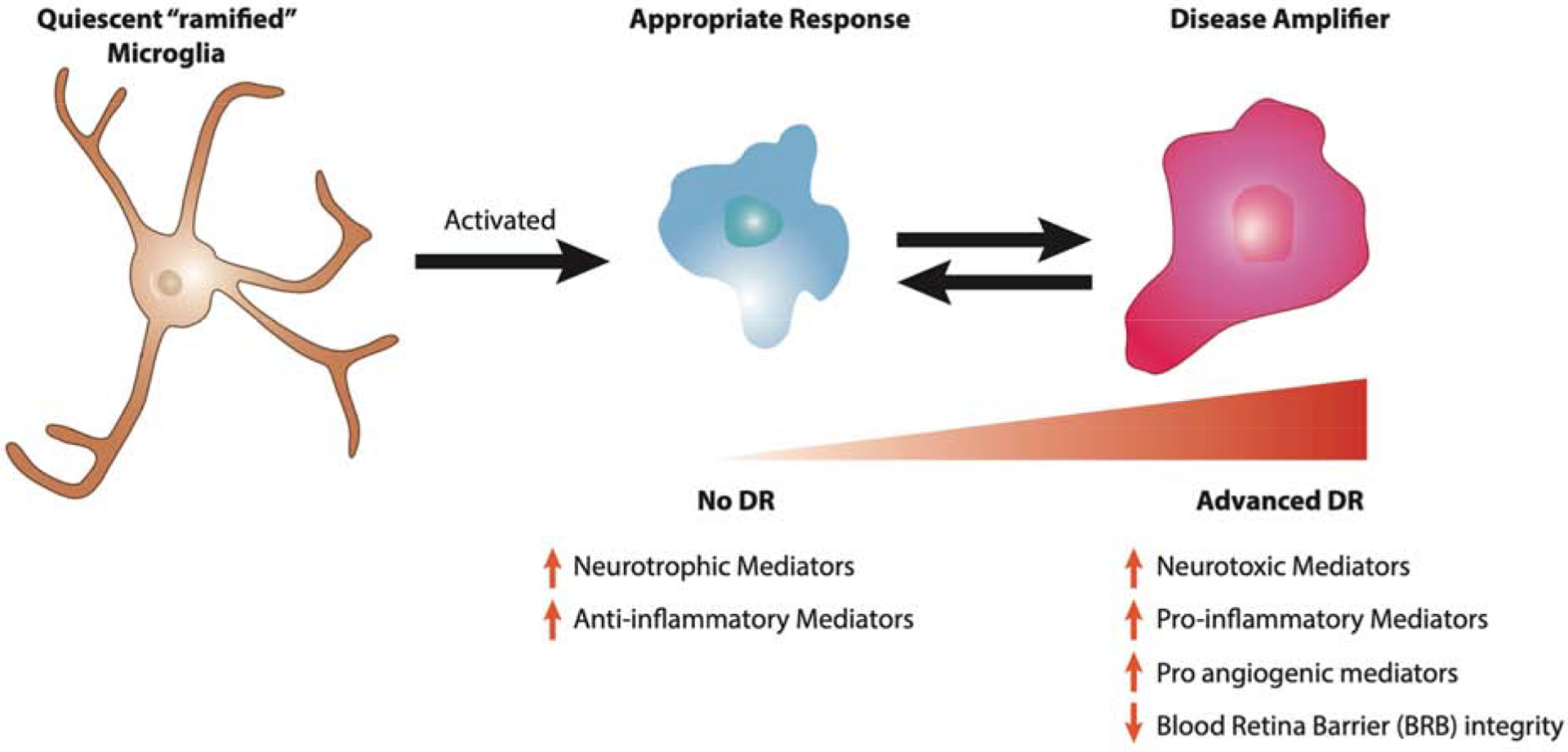
The quiescent microglia observed in normal physiology is ramified in form and initially responds by becoming activated and transforming into an amoeboid state. During this appropriate response to diabetes, activated microglia secrete increasing amounts of neurotrophic and anti-inflammatory mediators and help preserve the state of no diabetic retinopathy. Over time and in absence of resolution of the unbalanced environment, the population of M2 appropriately active microglia shifts to a more M1 disease amplifying state, which participates in the progression of DR.
Similar to microglia, macroglial activation results in the secretion of inflammatory mediators such as TNFα, IL-1β, and monocyte chemoattractant protein-1 (MCP-1); this contrasts drastically with their physiologic role of secreting anti-inflammatory mediators (Behzadian et al., 1995; Capozzi et al., 2016; Shin et al., 2014; Yong et al., 2010). Furthermore, in co-culture systems, activated microglia activate Müller cells (Wang et al., 2011), which in turn secrete a number of mediators to reciprocally activate microglia (Liu and Steinle, 2017; Wang et al., 2012; Yoshida et al., 2004). This self-perpetuating cycle involving communication between immune-competent cells can perpetuate unforgivingly until the immune response, initially appropriate for the perturbation, becomes unwieldy and excessive. It has been hypothesized that the transformation from quiescent to activated macroglia may transition with an intermediary state that produces anti-inflammatory and neurotrophic mediators (Figure 4) (Tsai et al., 2018). Although this hypothesis has yet to be confirmed, it is supported by our recent finding that Müller cells express high levels of αA-crystallin, a highly protective chaperone, in response to diabetes during preclinical DR (Figure 5). Although this high level of expression continues to be found in donors with DR, post-translational modifications are associated with a loss of function in these donors and are consistent with the increased neurodegeneration and neuroinflammation observed (Ruebsam et al., 2018). Our most recent work also suggests that αA-crystallin overexpression by Müller glial cells dampens their activation and self-perpetuation of the inflammatory cycle (data not shown). However, this regulation is controlled by specific post-translational modifications, including that shown to be lost in DR, consistent with the increased activation observed in DR patients. Recent single-cell RNA-sequencing work has identified a number of macroglial subpopulations that may represent these states; however, this study examined diabetic rodents at only a single time-point (Van Hove et al., 2020). More work using techniques such as single-cell RNA sequencing is underway to confirm the development of these macroglial subpopulations over the different stages of DR progression and to better characterize the impact of diabetes on macroglia.
Figure 4. Macroglial activation during progression of diabetic retinopathy.
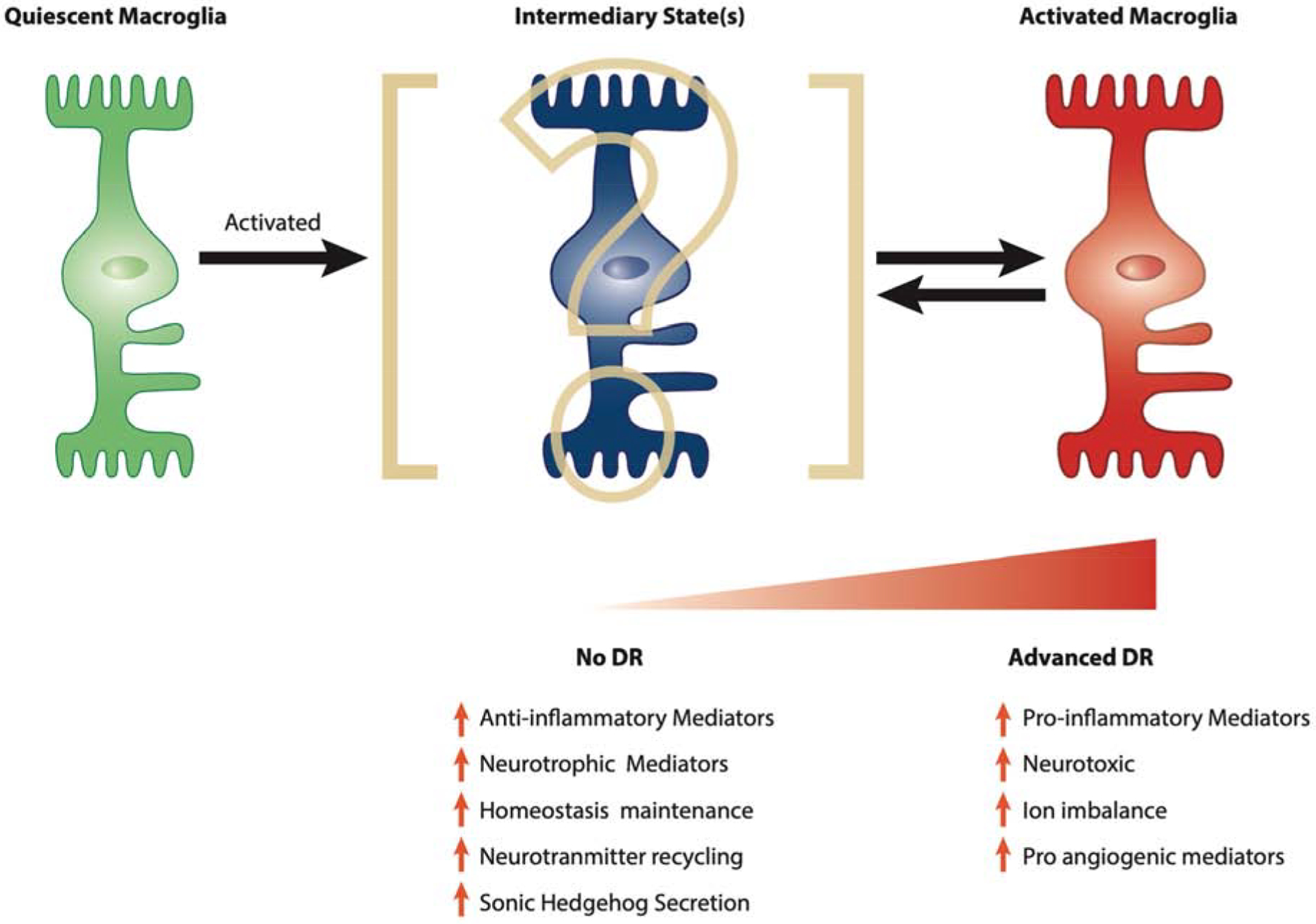
Quiescent macroglia become activated in response to diabetes and while first involved in the adaptive protective response, will ultimately contribute to diabetic retinopathy pathology. The exact nature and duration of this intermediary state between quiescent macroglia and activated macroglia remains to be fully elucidated but may be a key element in our quest to new avenues for DR treatment.
Figure 5. AlphaA-crystallin chaperone protein while increasingly expressed is decreasingly phosphorylated on a key regulatory site in DR donors.
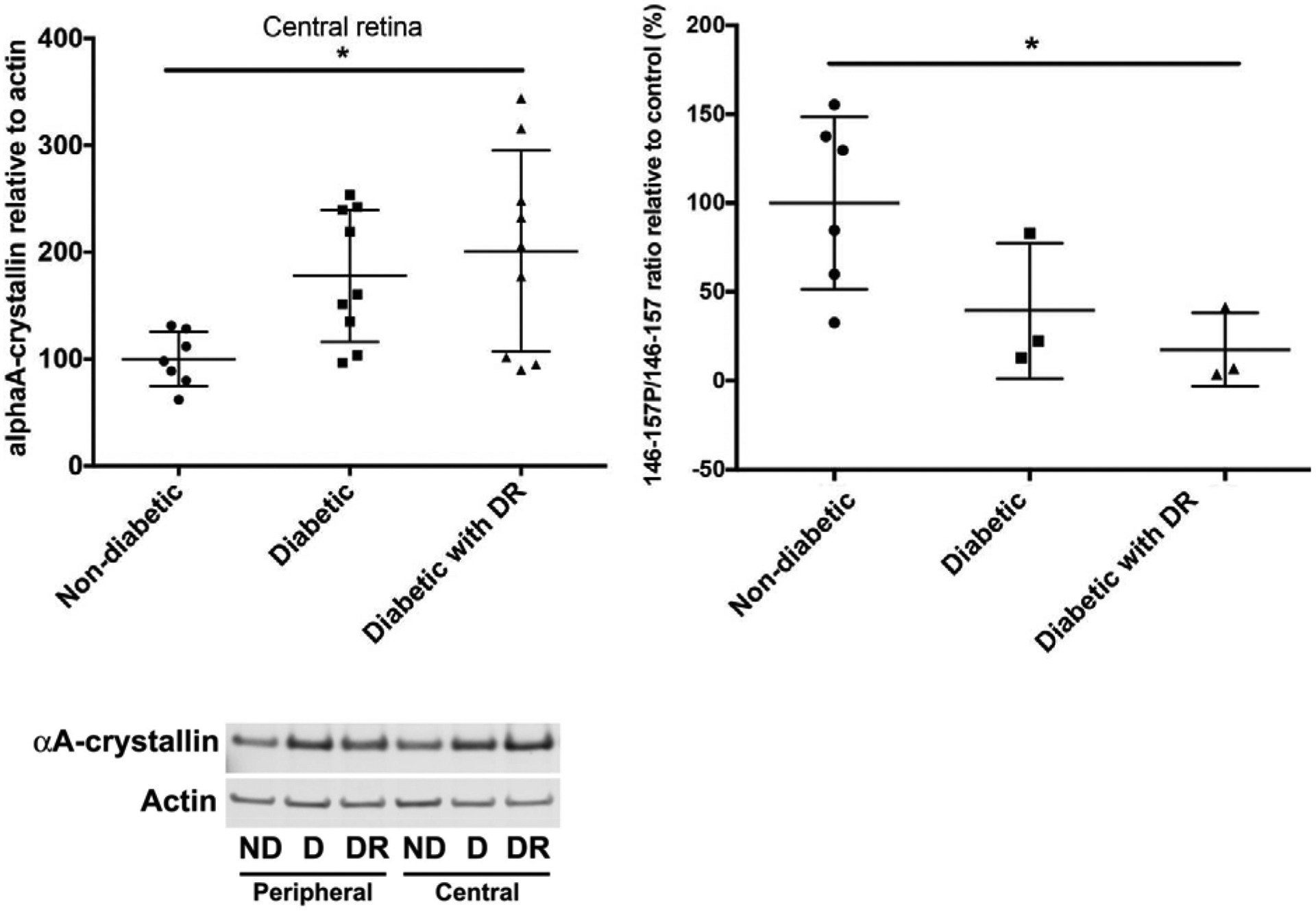
AlphaA-crystallin total protein level was assessed by western-blot and showed to be increased in diabetic donors with or without DR (left). Concomitantly, phosphorylation on T148, a phosphosite controlling its protective function was assessed by multiple reaction monitoring and shown to be dramatically reduced in DR donors. (Adapted from Ruebsam et al., 2018)
e. Mechanisms: The inflammatory mediators
As we have previously discussed, the various immune-competent and non-immune cells of the retina secrete pro- and anti-inflammatory mediators in response to different stimuli (Capozzi et al., 2016; Ibrahim et al., 2011; Paglinawan et al., 2003; Shin et al., 2014). With the retina normally maintained in an overall anti-inflammatory state (Cai et al., 2018; Murinello et al., 2019), the relative increase of pro-inflammatory mediators in diabetic human retinas highlights an important shift in the overall inflammatory state (Loukovaara et al., 2015; Murugeswari et al., 2008; Patel et al., 2008; Sakata et al., 2004; Sundstrom et al., 2018). This shift in the inflammatory milieu with DR progression is thought to involve the cross-activation of macroglia and microglia via inflammatory mediators. However, with human studies generally limited to samples gathered from vitrectomies (Sakata et al., 2004), the inflammatory environment of the human retina during preclinical DR remains opaque. Because diabetic rodents experience increasing levels of pro-inflammatory mediators early in disease (McVicar et al., 2011; Zhou et al., 2012), additional work on the retinal transcriptome and ocular proteome in human samples would deepen our understanding of the extent of involvement these mediators have during preclinical DR.
i. Cytokines
Cytokines are an important group of inflammatory mediators that are not only a consequence of activated microglia and macroglia but are also initiators of escalating DR inflammation. In the vitreous of diabetic patients, TNFα is one of the earliest cytokines detected and increases with disease progression (Demircan et al., 2006; Doganay et al., 2008; Wu et al., 2020). In vitro experiments have shown that TNFα stimulates microglia, macroglia, and endothelial cells to secrete VEGF (Stone et al., 1995; Wang et al., 2010; Yoshida et al., 2004). Importantly, VEGF is a potent cytokine whose inhibition via inhibitors such as bevacizumab has slowed DR progression and improved visual outcomes in human patients (Do et al., 2012; Elman et al., 2010; Ip et al., 2012; Nguyen et al., 2010; Zechmeister-Koss and Huić, 2012). Interestingly, VEGF inhibition suppresses the immune system and lowers levels of other cytokines, such as TNFα, IL-1β, interferon-γ, and chemokine macrophage inflammatory protein-1α (Gnanasekaran et al., 2020; He et al., 2015; Sivaprasad et al., 2017). Thus separate from their anti-angiogenic effects, VEGF inhibitors may derive therapeutic efficacy by limiting the innate immune response and the activation of microglia and macroglia (Stone et al., 1995; Wang et al., 2010; Yoshida et al., 2004). Furthermore, this raises the possibility of other cytokines as targets for DR treatment, especially in those patients who do not respond to anti-VEGF therapies (Bressler et al., 2014; Elman et al., 2010; Nguyen et al., 2010). Indeed, a recent publication identified inflammation as a mechanism behind those with diabetic macular edema (DME) resistant to anti-VEGF therapies (Arima et al., 2020). While the potential of TNFα as a therapeutic target continues to be evaluated, its role in refractory DME and PDR has been the topic of several prospective interventional studies and clinical trials (NCT00695682, NCT00505947) involving TNFα-specific antibodies. One study reported potentially encouraging results relative to visual acuity and retinal function (Sfikakis et al., 2010), whereas another reported potential immunogenicity and toxicity (Giganti et al., 2010). Due to these mix results and the studies’ small sample size and targeted population, further examination is required.
Similar to how TNFα results in the secretion of VEGF, which in turn stimulates the release of TNFα by microglia, macroglia, and endothelial cells (Stone et al., 1995; Wang et al., 2010; Yoshida et al., 2004), an interesting area of continued research is the cross-activation of cytokines during DR. For example, the presence of IL-1β in diabetic humans and rodents (Yang Liu et al., 2012; Yego et al., 2009; Zhou et al., 2012) triggers the release of the pro-inflammatory cytokine IL-8, which is elevated in advanced DR patients (Doganay et al., 2008; Hernández et al., 2005; Liu et al., 2014; Raczyńska et al., 2018; Yoshida et al., 2004).
In addition to these well-known cytokines, we have also found indoleamine 2,3-dioxygenase to be elevated in diabetic eyes (Figure 6) (Nahomi et al., 2018). Although the origin of indoleamine 2,3-dioxygenase remains unclear, studies examining human diabetic donor tissues have implicated microglia, Müller cells, and endothelial cells (Hu et al., 2017; Nahomi et al., 2018). We further identified this elevation of indoleamine 2,3-dioxygenase to be associated with increased levels of the pleiotropic cytokine interferon-γ, which is a known regulator of vascular endothelial cell survival (Figure 7) and is also elevated in streptozocin-induced diabetic rodents (Özay et al., 2020). Together these human, rodent, and in vitro lines of evidence suggest that the preclinical DR retinal environment is suffused with inflammatory cytokines that further the activation of and are further promoted by microglia and macroglia.
Figure 6. IDO expression is elevated in the retina of diabetic donors with DR.
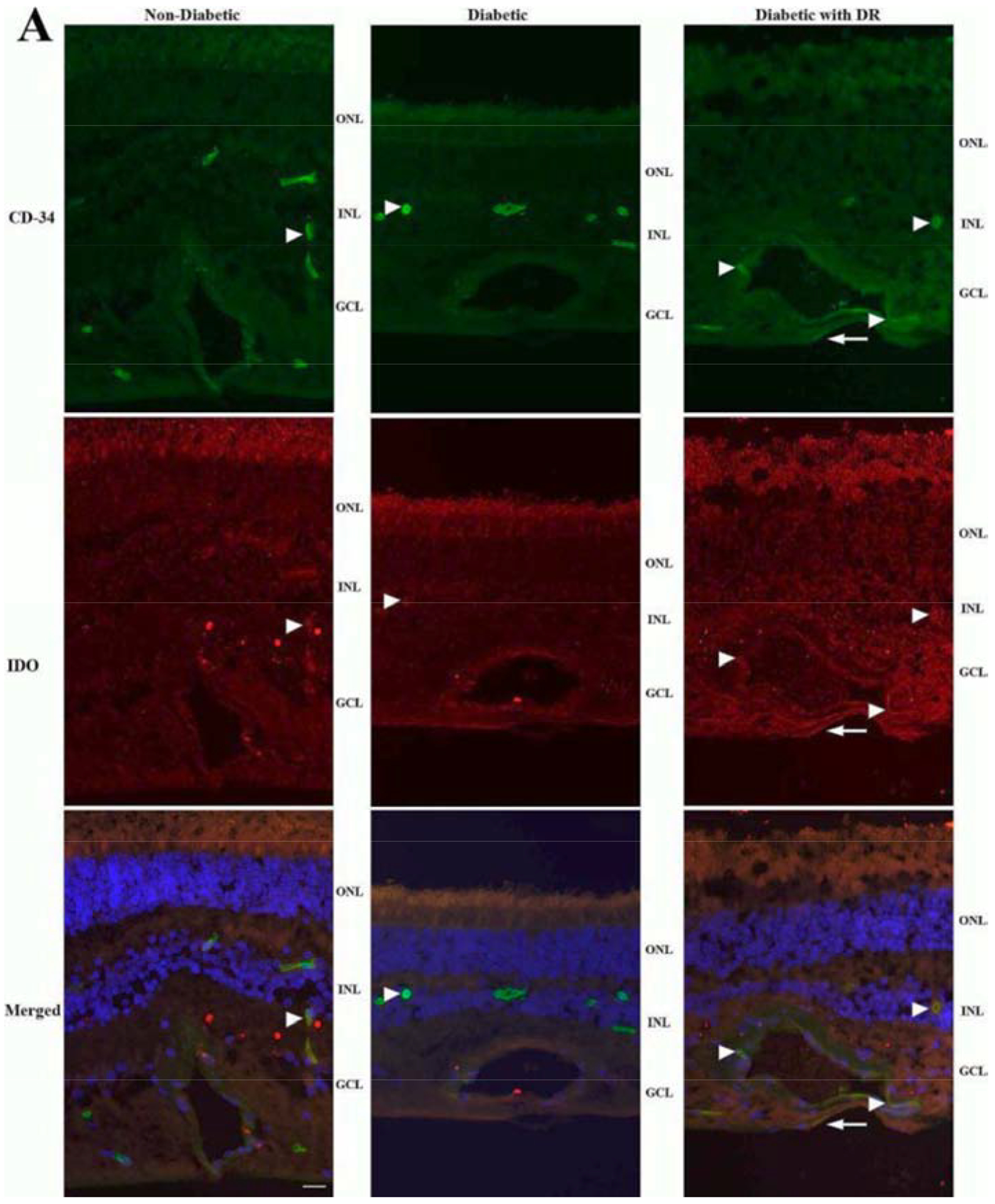
Immunohistochemistry of retinal sections shows IDO (red) partially colocalizing with CD-34 (green) in the capillary endothelium (arrowheads) of the retina of diabetic donors with DR (right). IDO-positive staining was also observed in the capillary endothelium of the retina of diabetic donors without retinopathy (middle) and nondiabetic donors (left), but the signal appears diffuse and less intense (arrowheads). Additionally, some of the IDO signal in the inner retina of diabetic donors with retinopathy appears not to colocalize with CD-34 (arrows), suggesting expression in glial cells. Nuclei are stained with DAPI (blue). (Adapted from Nahomi et al., 2018)
Figure 7. High levels of IFN-γ in the retina of diabetic donors with DR.
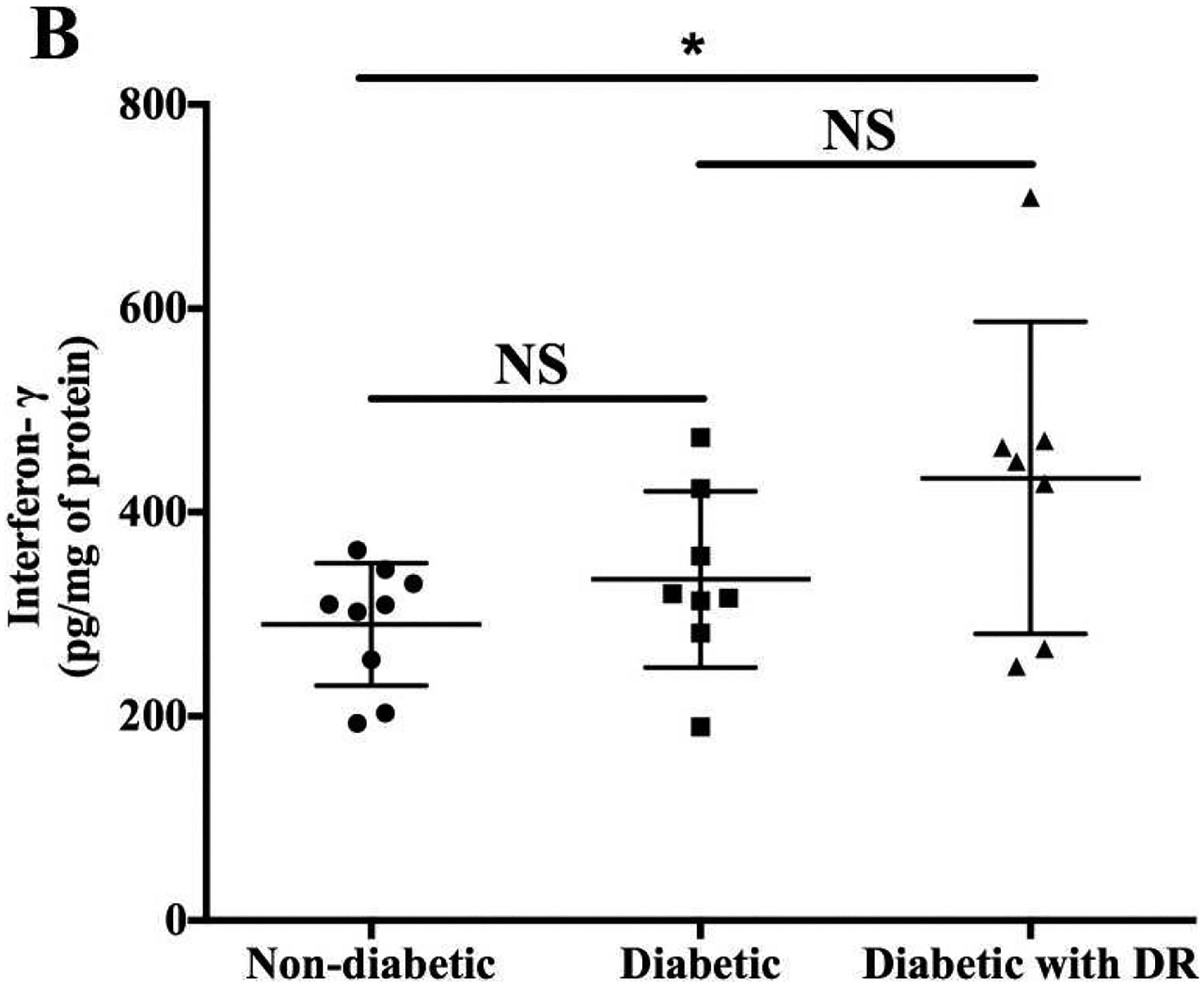
IFN-γ levels were measured by ELISA in retinal homogenates from age-matched nondiabetic (n = 9), diabetic (without retinopathy, n = 8), and diabetic (with retinopathy, n = 7) donors. Scale bar: 20 μm. *P < 0.05; NS, not significant. (Adapted from Nahomi et al., 2018)
ii. Chemokines
Chemokines are another class of inflammatory mediators that attract immune cells to further promulgate the immune response. Microglia undergo chemotaxis via their exposure to a number of chemokines, which, like MCP-1, are elevated in diabetic human and rodent eyes (Hernández et al., 2005; Wu et al., 2020). In response to hyperglycemia, Müller cells produce MCP-1 (Dong et al., 2012; Rangasamy et al., 2014; Wang et al., 2012), which activates and recruits microglia to the hypoxic interfaces of OIR rodent retinas (Davies et al., 2008, 2006). Because OIR models of hypoxia are generated from postnatal day 7 exposure to hyperoxic conditions and are used to model the neovascularization observed in PDR (Olivares et al., 2017), these findings of microglial accumulation may help to explain the microgliosis observed in human PDR (Zeng et al., 2008). Unfortunately, this activation and accumulation of microglia may contribute to disease progression (Ritter et al., 2006), because inhibition of MCP-1 and macrophage inflammatory protein-1α significantly decreases neovascularization in OIR rodents (N. Wang et al., 2019; Yoshida et al., 2003).
It remains unclear if the elevated levels of other chemokines such as chemokine ligand 4 and C-X-C motif ligand 9 and 10 observed in DR patients are deleterious (Nawaz et al., 2013). Although these chemokines have been shown to stimulate the innate immune response and contribute to the inflammatory retinal environment (Nawaz et al., 2013), not all chemokines activate the immune response. As previously mentioned, chemokine CX3CL1 (fractalkine) inhibits microgliosis and supports an immunosuppressed environment (Beli et al., 2016; Cardona et al., 2015; Combadière et al., 2007; You et al., 2007). Therefore a more nuanced and specific understanding of how different chemokines affect the diabetic human eye would further our understanding of the pathogenesis during preclinical DR.
4. Mechanisms of preclinical diabetic retinopathy neurodegeneration
Before the presence of any detectable vascular abnormality, preclinical DR patients experience a number of visual dysfunctions measurable through psychophysical testing, including deficits in peripheral vision, night vision, color-hue discrimination, and contrast discrimination (Jackson and Barber, 2010; Trento et al., 2017; Wolff et al., 2015). These functional deficits have further been confirmed and localized by multifocal electroretinopathy to distinct retinal areas in preclinical DR patients (Di Leo et al., 1994; Juen and Kieselbach, 2011; Reis et al., 2014; Tyrberg et al., 2011). Further structural analysis with high-resolution spectral-domain optical coherence tomography has demonstrated progressive thinning of the nerve fiber layer and ganglion cell layer of the retina in multifocal electroretinography–abnormal regions, thereby linking vision impairment with functional and structural abnormalities (Chhablani et al., 2015; Juen and Kieselbach, 2011; Sohn et al., 2016; Tyrberg et al., 2011). Moreover, not only did these neuronal structural abnormalities overlap spatially with the functional deficits found by psychophysical testing and multifocal electroretinography, but these defects found during preclinical DR seem to track with the locations where future vascular lesions occur (Dodo et al., 2015; Harrison et al., 2011; Ng et al., 2008). These findings suggest that neurodegeneration contributes to visual dysfunction in preclinical DR and that there may exist a relationship between neurodegeneration and vascular abnormalities in DR.
a. Direct effect of activated microglia and macroglia on retinal neurodegeneration
Certainly, these findings in human patients have called into question the classic understanding of vascular abnormalities causing neuropathy (Archer, 1999; Bresnick and Palta, 1987; Kohner et al., 1999). Consistent with this manifestation, retinas of diabetic rodents also experience neuronal thinning, detected by spectral-domain optical coherence tomography (Alves et al., 2018), in locations with increased aggregation of activated microglia (van Dijk 2009, van Dijk 2010, van Dijk 2012). Because microglia possess the machinery to kill neurons and prune synapses and because their activation precedes neuronal thinning (Barber et al., 2005), the possibility that activated microglia may contribute to neurodegeneration continues to be an important avenue to explore. Indeed, a number of investigators have found that microglia are able to phagocytose impaired but functional neurons (Brown and Neher, 2014; Kawabori et al., 2015; Kim et al., 2017; Zhao et al., 2015), perhaps explaining the accelerated loss of neurons observed early in diabetes (Abu El-Asrar et al., 2004; Barber et al., 1998; Hammes et al., 1995; Martin et al., 2004; McVicar et al., 2011).
The other immune-competent cell implicated in DR neurodegeneration is the Müller cell. Because Müller cells are normally responsible for maintaining appropriate glutamate and ion levels, when Müller cells are unable to properly maintain homeostasis during diabetes (Chen et al., 2014; Puro, 2002), diabetic human and rodent retinas can experience elevated levels of glutamate (Ambati et al., 1997; Lieth et al., 1998; Pannicke et al., 2006). Consistent with this notion, hyperglycemia or IL-1β activates Müller cells in cell culture and results in increased glutamate and ion levels (Chen et al., 2014; Puro, 2002), whereas chemical inhibition of Müller cell activation decreases glutamate levels and improves ganglion cell health (Ganesh and Chintala, 2011). These experiments suggest that activation of Müller cells early in diabetes can result in alterations of glutamate recycling, potentially leading to regional excitotoxicity and neurodegeneration in preclinical DR. Mechanistically, a number of recent studies have begun to establish the protective role of neuropeptides, such as neuropeptide Y and substance P, for neurons exposed to glutamate excess (Ou et al., 2020, 2019). However, neuropeptides, just like growth factors, play a complex role in DR pathophysiology, because neuropeptide Y has been shown to potentially play an important role in gliosis and its involvement in fibrocellular membranes associated with PDR (Milenkovic et al., 2004). As previously discussed, microglia and macroglia can cross-activate and regulate each other in many ways, including to potentially exacerbate excitotoxicity and neuronal phagocytosis by microglia (Figure 8; (Wang et al., 2011). Consistent with this, the genetic deletion of Toll-like receptor 4 in Müller cells not only decreases pro-inflammatory cytokines in the retina, but it also results in decreased neurodegeneration (Liu and Steinle, 2017).
Figure 8. Activation and self-perpetuation of the response of local immune competent cells in diabetic retinopathy.
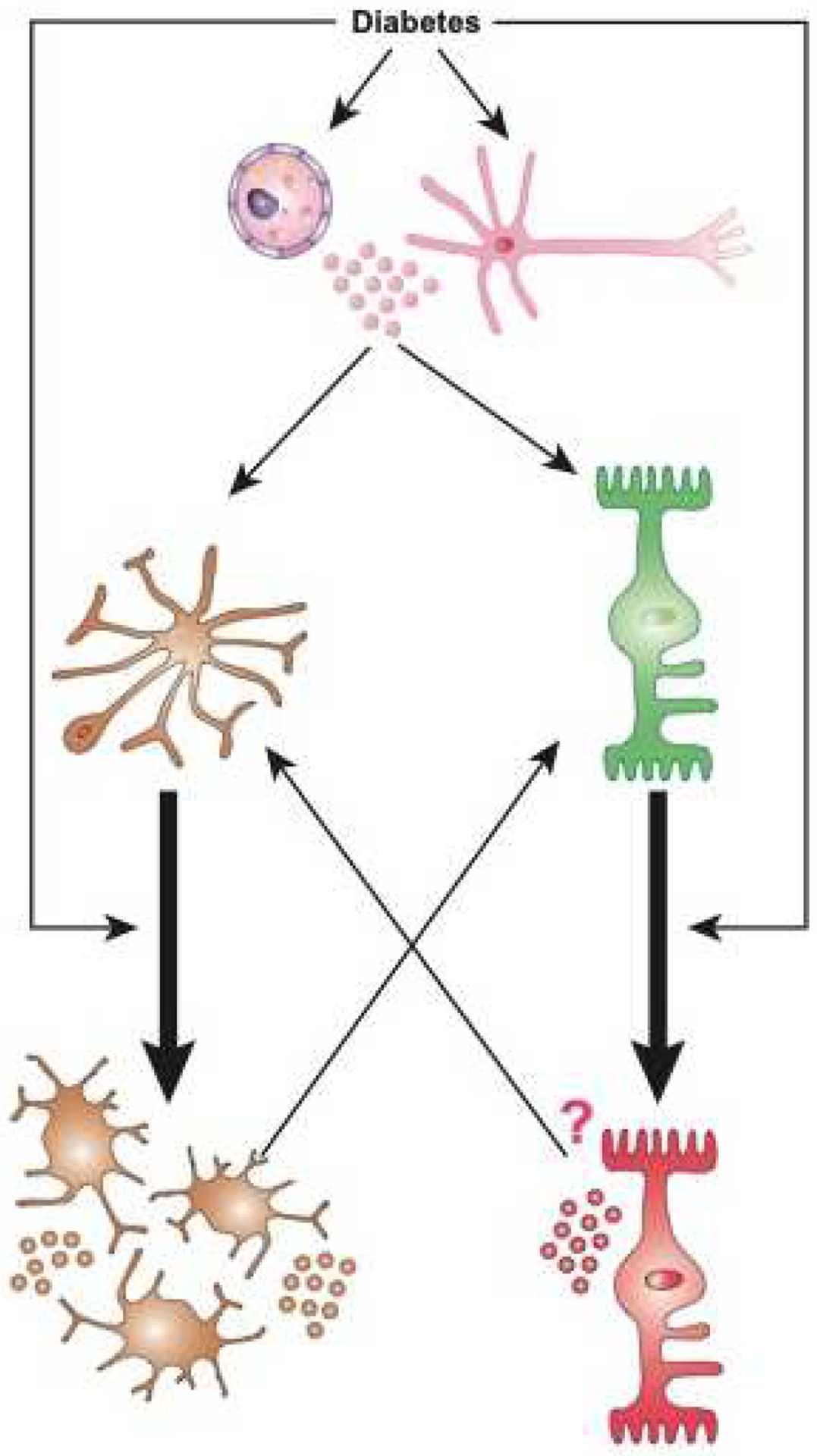
Diabetes activates immune competent cells including micro- and macroglia (astrocytes and Müller glial cells) via multiple mechanisms, potentially inducing a self-perpeutating and amplification loop. Diabetes can directly induce activation of quiescent microglia (orange) leading to increase in number, change in morphology, and increased production of pro-inflammatory mediators. Similarly, diabetes also induces macroglia (green) to undergo activation and result in increased production of pro-inflammatory mediators. Finally, endothelial cells and neurons (pink) can also respond to diabetes by producing pro-inflammatory mediators. Any and all of these responses participate in the shift from immune-privileged to a highly pro-inflammatory ocular environment, and amplifies the activation of micro-and macroglia in an autocrine, and paracrine fashion. Once set in motion, this creates a loop in which de-activation of these immune competent cells is rendered highly difficult even when the trigger (i.e. suboptimal controlled diabetes) has been “removed”.
b. Neurodegeneration and tissue homeostasis: Microglia, macroglia, and growth factors
Another area of increasing interest is the secretion of growth factors by microglia and macroglia and their role in promoting cell survival. Not only are the growth factors brain-derived neurotrophic factor (BDNF), nerve growth factor, and pigment epithelium–derived factor (PEDF) depressed in diabetic human and rodent retinas (Boehm et al., 2003; Boss et al., 2017; Yanling Liu et al., 2012; Mysona et al., 2014; Ola et al., 2013), but there is an accumulation of their corresponding pro-forms (Ali et al., 2011). This observation is all the more important because these abundant pro-forms, in addition to not being neuroprotective, have been shown to be pro-inflammatory (Barcelona et al., 2016; Elshaer et al., 2013; Lebrun-Julien et al., 2010) and even further neurodegeneration and vasculopathies (Matragoon et al., 2012; Mohamed et al., 2018). This pathogenic effect has been confirmed by studies showing that the inhibition of these pro-forms is protective against neurodegeneration and vasculopathy in diabetic and OIR rodent models (Barcelona et al., 2016). Consistent with these findings, experimental increase of BDNF and growth hormone–releasing hormone is neuroprotective and slows DR progression (Suzumura et al., 2020; Thounaojam et al., 2017). However, a recent review of the extensive BDNF literature appropriately highlighted the complexity of manipulating such systems. Afarid et al. indeed nicely summarized the divergent reports relative to the success of BDNF treatment on DR progression and the innate immune response; these may depend on the concentration of BDNF but also the relative engagement and loss of sensitivity of the receptor, especially as a function of the different stages of DR (Afarid et al., 2020).
Interestingly, insulin as a growth factor is intimately involved in diabetes and is itself depressed during disease. Furthermore, local administration of insulin in diabetic eyes results in decreased immune response (Table 2) (Fort et al., 2011). Similarly, PEDF injections into diabetic or OIR rodent eyes decrease levels of pro-inflammatory mediators TNFα, VEGF, and MCP-1 (Zhang et al., 2006). In contrast, knock-down of PEDF results in increased secretion of VEGF and TNFα by cultured Müller cells, suggesting that at least part of PEDF-mediated anti-inflammatory effects are via Müller cells (Zhang et al., 2006). Microglia also appear to be involved, because PEDF challenge decreases microgliosis in streptozocin-induced diabetic rodents (Yoshida et al., 2009). Thus growth factors, although still challenging to use, have become increasingly important in the understanding of retinopathy and are exciting avenues of potential therapies, including as a means to temper the increasingly inflammatory environment of the retina.
Table 2. Top ten pathways affected by diabetes and reversed by ocular insulin administration.
List of the top ten pathways obtained considering only the list of genes from the microarray analysis that were affected by diabetes and reversed by local insulin administration using MetaCore™ (Genego Inc.) software. Pathways are ranked based upon p-value (Adapted from Fort et al., 2011).
| ranking | Maps | min(pValue) |
|---|---|---|
| 1 | Immune response_Classical complement pathway | 4.112E-12 |
| 2 | Cell adhesion_Role of tetraspanins in the integrin-mediated cell adhesion | 5.144E-09 |
| 3 | Immune response_Lectin induced complement pathway | 9.611E-08 |
| 4 | Regulation of lipid metabolism_Regulation of lipid metabolism via LXR, NF-Y and SREBP | 1.089E-07 |
| 5 | Development_Role of IL-8 in angiogenesis | 5.124E-07 |
| 6 | Cytoskeleton remodeling_Regulation of actin cytoskeleton by Rho GTPases | 8.567E-06 |
| 7 | Immune response_IL-22 signaling pathway | 7.801E-05 |
| 8 | Cytoskeleton remodeling_Neurofilaments | 1.972E-04 |
| 9 | Immune response_IL-5 signalling | 4.053E-04 |
| 10 | Development_Slit-Robo signaling | 4.831E-04 |
c. Neurodegeneration and tissue homeostasis: Microglia, macroglia, and complement activation
In addition to immune-competent cells and their inflammatory and growth mediators, the complement system is also intimately involved and active during preclinical DR. Although complement components are absent in non-diabetic eyes, their levels increase in the eye (Muramatsu et al., 2013; Shahulhameed et al., 2020; Zhang et al., 2002) and the periphery (Huang et al., 2018) during preclinical DR. Indeed, our own unpublished data reveal a successive increase in complement component RNA in eyes with no diabetes, preclinical DR, and non-proliferative DR (NPDR) (Figure 9). One of the complement components that is most upregulated in the human eye during DR is C3, which is the central component of the three major complement-activating cascades (unpublished data). This correlates with the increased serum levels of C3a, which is generated from the cleavage and activation of C3, and additionally points to the potential involvement of this anaphylatoxin in DR (Figure 10) (Lingjun Zhang et al., 2016). We previously demonstrated that activation of C3-related anaphylatoxin could play a key role in other models of neurodegeneration such as allodynia (Figure 11) (Xu et al., 2018), supporting a similar role of complement system activation in DR-related neurodegeneration. This aligns with the role C3 signaling plays in synaptic pruning (Schafer et al., 2012); however, in this case it is pathologic. Interestingly, microglia are the only central nervous system cells to express the receptor (CR3) and so are thought to be solely responsible for the phagocytosis of cells (including neurons) coated with C3b. With peripheral C3 levels elevated in diabetes, it remains unclear whether complement infiltration into the retina plays a role in DR (Rasmussen et al., 2018).
Figure 9. Complement system components are found in increasing levels in the neuroretina of donors with non-proliferative diabetic retinopathy compared to noon-diabetic donors (age, gender and race matched).
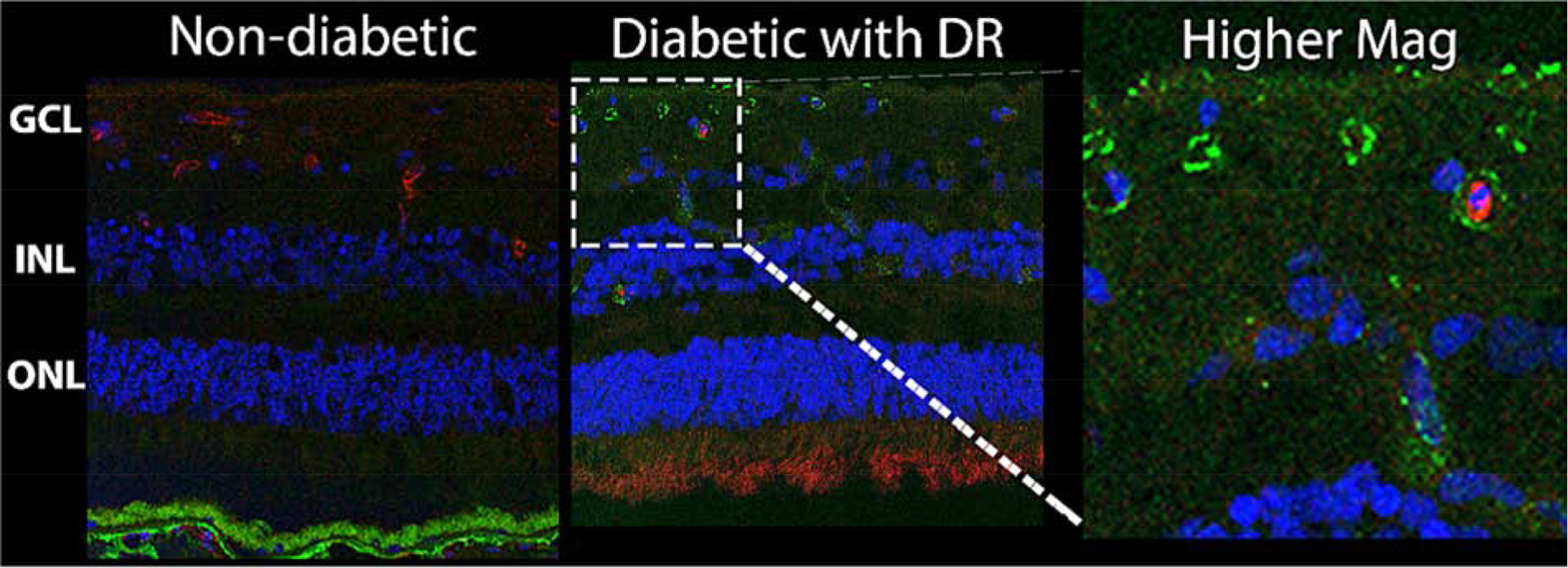
Immunohistochemical staining was performed on retinal sections of human donors without diabetes (Left) and with diabetes and non-proliferative diabetic retinopathy (NPDR, middle). The ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL) are displayed in the first two panels. Agrin (red) a marker of blood vessels, and the complement system regulator CD59 (green) are shown with nuclei counterstain (blue). The right panel is a higher magnification image of the central panel demonstrating the presence CD59 away from agrin, in the neuroretina tissue.
Figure 10. Measurement of complement activation products C3a, C4a and C5a in human serum samples.
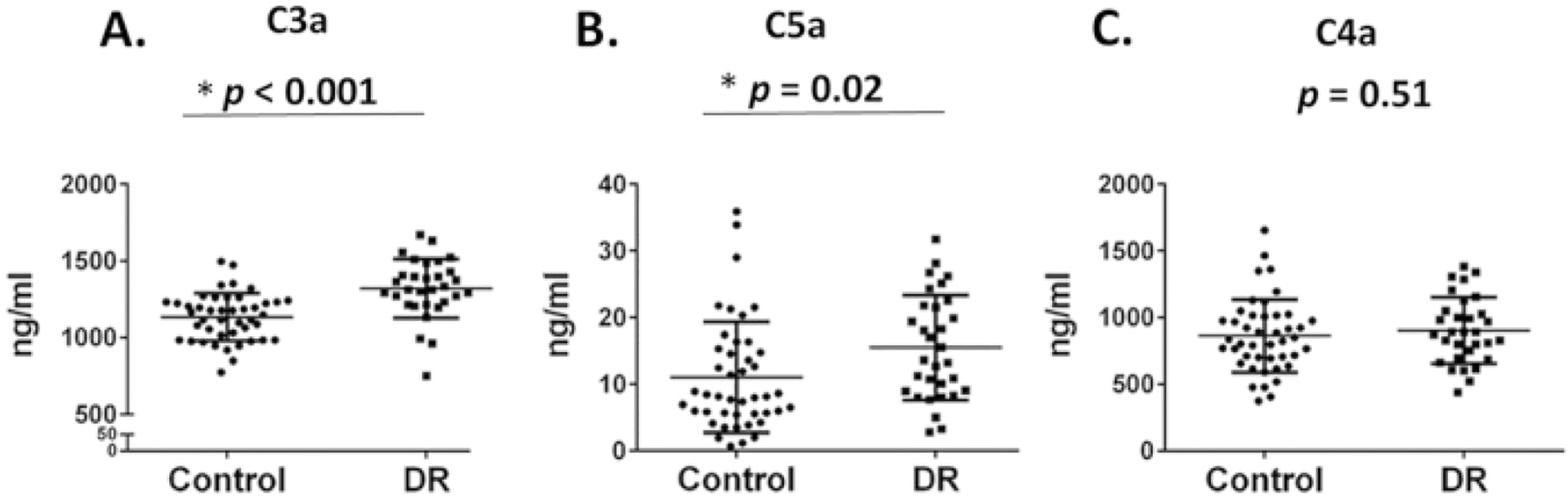
Levels of C3a (A) and C5a (B), but not C4a (C) were significantly higher in the DR group compared to age-matched healthy controls. Data were mean ± SD. Each dot represents one patient (Adapted from Lingjun Zhang et al., 2016).
Figure 11. C3 activation is important in paclitaxel-induced mechanical allodynia in CIPN.
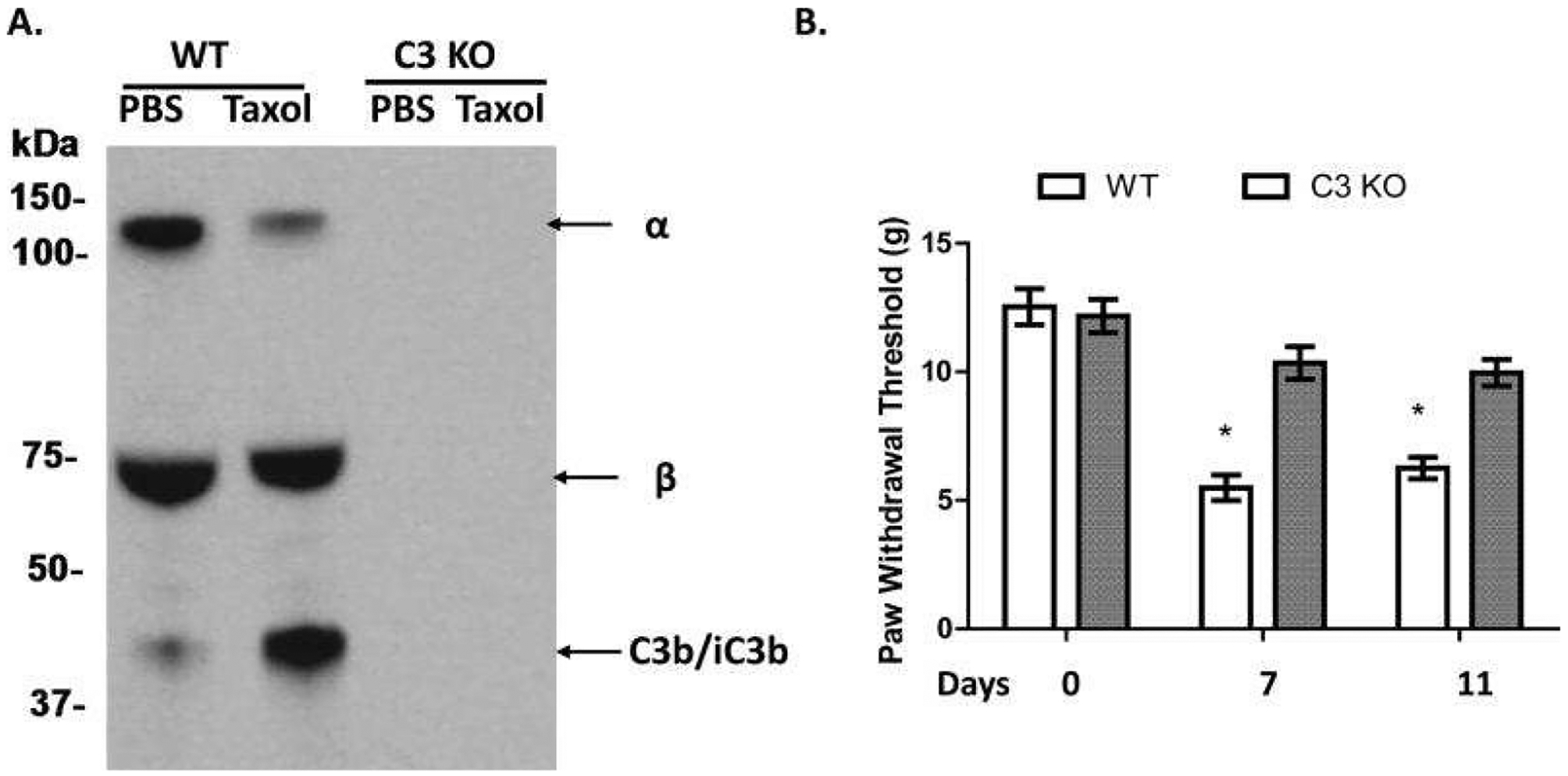
A. Complement is activated after paclitaxel administration. WT or C3 KO rats were i.p. injected with DMSO (Vehicle) or 1 mg/kg paclitaxel (in DMSO according to the instructions of the manufacturer, Tocris, Bristol, UK) for 4 days (day 1 to 4), and then sera were collected on day 5 when the behavioral tests confirmed the development of mechanical allodynia and probed with an anti-C3 IgG to assess complement (C3) activation. There was a significant reduction of the α band (~130KDa) of C3 and an increased α2 chain of iC3b (~40 KDa) in the paclitaxel-treated WT rats. No C3 protein was detectable in sera from the C3 KO rats. B. C3 KO rats showed increased paw withdrawal threshold (PWT). WT and C3 KO rats (n=10/group) were injected with paclitaxel for 4 days (day 1 to 4), PWT was assessed on days 0, 7 and 11.; * p<0.05. (Adapted from Xu et al., 2018)
Increased levels of another anaphylatoxin, the C5-derived activation product C5a, have been reported in the vitreous of PDR patients (Muramatsu et al., 2013). C3a and C5a, as chemoattractants, are notorious for their ability to induce overwhelming inflammatory responses and activate phagocytes (i.e., microglia); both are elevated in the vitreous of diabetic human eyes (Sacks, 2010; Lingjun Zhang et al., 2016). Like with the C3 receptor, the C5a receptor is also present in microglia, and was shown to be required for their activation (Song et al., 2017). Furthermore, both astrocytes and macroglia express C5a receptors and upregulate this receptor in response to elevated glucose levels (Cheng et al., 2013). So, upon C5a binding, astrocytes and Müller cells become activated and increase their production of pro-inflammatory cytokines such as IL-6 and VEGF (Cheng et al., 2013; Gasque et al., 1995). Together this suggests that complement plays a critical role in activating both microglia and macroglia during preclinical DR and contributes to the increasingly pro-inflammatory milieu. Although these findings suggest that complement may be upstream of immune-competent cells, other investigations have found inflammatory mediators to cause the production of complement components via microglial activation (Amadi-obi et al., 2012; Luo et al., 2013). Regardless of whether complement fragments initiate gliosis or vice versa, these two components of the innate immune system are clearly important during DR and offer the possibility of a combinatorial targeting approaching for DR therapies (Luo et al., 2013).
5. Diabetes, blood-retinal barrier, and the neurovascular unit
BRB breakdown is a hallmark event during preclinical DR (Miyamoto et al., 1999; Schröder et al., 1991) and was recently reviewed (Subauste, 2019). Briefly, during diabetes, macroglia secrete increasing amounts of VEGF, which compromises barrier integrity (Stone et al., 1995; Wang et al., 2010), in the context of amplified microglial activation and levels of pro-inflammatory mediators (Jo et al., 2019). In response to this environment, endothelial cells express cell adhesion molecules (CAMs), including ICAM-1, VCAM-1, E-selectin, and P-selectin (Arita et al., 2013; Capitão and Soares, 2016; Clauss et al., 1990; Lu et al., 1999; Miyamoto et al., 2000; Noda et al., 2014), some of which have been reported to be elevated in human NPDR eyes (McLeod et al., 1995). CAMs are the anchors by which circulating leukocytes, via their expression of integrins CD11a, CD11b, and CD18, bind to vascular endothelial cells (Barouch et al., 2000; Miyamoto et al., 1999). So, as circulating leukocytes bind to endothelial cells on the luminal side, they too release pro-inflammatory and cytotoxic mediators, further contributing to the local pro-inflammatory environment (Joussen et al., 2001; Park et al., 2016; Schröder et al., 1991). This sustained inflammation impairs tight junction function and pericyte and endothelial health, eroding barrier integrity (Joussen et al., 2001; Ogura et al., 2017; Platania et al., 2019; Schröder et al., 1991; Subauste, 2019). These findings are replicated only weeks into diabetes onset in rodents (Barouch et al., 2000; Miyamoto et al., 1999; Noda et al., 2012) and suggest that BRB breakdown may occur early in human retinas. Consistent with this, inhibition and genetic deletion of CAMs and integrins result in significantly reduced BRB breakdown (Barouch et al., 2000; Joussen et al., 2004; Miyamoto et al., 1999). Similarly, suppression of cytokine and chemokine activity protects BRB integrity (Jo et al., 2019; Omori et al., 2018; Platania et al., 2019; Valle et al., 2019; H. Wang et al., 2019). Studies combating inflammatory mediators and the complement system have successfully demonstrated the importance of the immune response in BRB breakdown as well (He et al., 2015; Joussen et al., 2003; Wang et al., 2010; Yun et al., 2017; L. Zhang et al., 2016). In addition, growth factors such as PEDF are important in maintaining BRB function (Barnstable and Tombran-Tink, 2004; Yoshida et al., 2009). Remarkably, the topical application of PEDF to diabetic rodents is neuroprotective, increases tight junction expression, decreases microgliosis, decreases pro-inflammatory mediator production, and decreases vascular permeability (Yanling Liu et al., 2012). Together this highlights the need for continued research efforts in this area to clinically treat BRB breakdown in humans with preclinical DR.
Because the BRB is part of the NVU, breakdown of the former includes impairment of the latter. The NVU importantly autoregulates the perfusion supply to match metabolic demand (Metea and Newman, 2007; Newman, 2013). Pre-diabetic and preclinical DR patients experience impaired autoregulation with a 50% reduction in their vasomotor response to flickering light or hyperoxia (Garhöfer et al., 2004; Lott et al., 2015, 2012). Interestingly, flicker-evoked vasodilation was similarly decreased by 55% in healthy non-diabetic individuals experimentally exposed to short periods of hyperglycemia (Dorner et al., 2003). This suggests that hyperglycemia may be responsible for impairment in the NVU vasomotor response and that the localized hypoxia ultimately contributes to the development of vascular abnormalities such as venous beading (Klein et al., 2007; Kur et al., 2012; Wong et al., 2002). In addition, areas of the retina with impaired flicker-evoked vasodilation correspond to neuroretinal abnormalities detected on multifocal electroretinography, suggesting that NVU impairment may also contribute to preclinical DR neurodegeneration (Lecleire-Collet et al., 2011; Newman, 2015). Therefore, far from being devoid of activity, preclinical DR is a period when an increasingly rampant immune response may contribute to neurodegeneration and functional deficits. Furthermore, these active processes continue to accelerate into the next phases of DR and are important in the vascular abnormalities that have classically defined the disease.
6. Emergence of the role of the innate immune response in diabetic retinopathy progression
DR has long been classified as a microvascular disease with pathophysiology thought to coincide with vascular abnormalities. Certainly, vasculopathy does cause neurodegeneration, vision impairment, and immune dysregulation. However, as we have previously detailed, the innate immune response is an independent driver of DR pathology. Thus, as DR progresses to NPDR and PDR, it becomes more difficult to definitively define causation. Regardless, this section will focus on the role of the innate immune response during these later stages of DR and characterize how it may interact with the various manifestations of disease.
a. Non-proliferative diabetic retinopathy
NPDR begins with the first clinically detectable sign of vascular abnormality (i.e., microaneurysm) and is further categorized as mild, moderate, and severe based on the degree of vasculopathy (Wilkinson et al., 2003). Microaneurysms are due to the pathologic loss of pericytes and endothelial cells, which compromises the structural integrity of capillaries. Experimental models have demonstrated that the immune response causes apoptosis of pericytes and endothelial cells (Barouch et al., 2000; Joussen et al., 2004; Miyamoto et al., 1999; Schröder et al., 1991). A related avenue of investigation has detailed loss of cell-cell interactions between components of the NVU, including those between pericytes and endothelial cells, as contributors to NPDR progression (Klein et al., 2007; Kur et al., 2012; Roy et al., 2015; Stitt et al., 2016; Wong et al., 2002). With breakdown of the BRB and infiltration by peripheral immune cells and proteins, the immune response becomes even more amplified during NPDR (Kur et al., 2012; Wong et al., 2002). Consistent with this, microgliosis increases commensurate to vascular pathology in diabetic human and rodent retinas (Barber et al., 2005; Davies et al., 2006; Karlstetter et al., 2015; Zeng et al., 2008, 2000).
Complement activity increases too during NPDR in diabetic retinas (Figure 9) (Frederich et al., 1995; Lingjun Zhang et al., 2016). These complement proteins can be assembled into the membrane attack complex, which is postulated to cause pericyte and neuron death (Li et al., 2012; Lueck et al., 2011). In addition, sublytic membrane attack complexes can also form and become generators of pro-inflammatory and neurotoxic mediators (Lueck et al., 2011). This complement-driven pathology may be in part due to the glycation and inactivation of complement inhibitors such as CD59 (Qin et al., 2004; Zhang et al., 2002). Interestingly, an experimental increase of CD59 inhibits membrane attack complex formation (Bora et al., 2010; Harhausen et al., 2010) and reduces vasculopathy and neurodegeneration in diabetic rodents (Adhi et al., 2013). However, these protective effects occur with a concurrent increase in retinal microgliosis (Adhi et al., 2013), which has been implicated in pericyte loss (Ding et al., 2017; Mazzeo et al., 2017). Thus the complement system and its interaction with immune cells are promising areas of continued investigation.
The vascular abnormalities defined as part of NPDR amplify the neurodegeneration observed in preclinical DR (Barber et al., 2005, 1998; Chhablani et al., 2015; Juen and Kieselbach, 2011; Tyrberg et al., 2011). Indeed, microglia and macroglia are further activated by vascular perturbations, which result in the additional release of a number of neurotoxic mediators that can directly lead to neuronal death (Krady et al., 2005). Inhibition of microgliosis by minocycline or adenosine A2a receptor not only decreases the neurotoxic environment but is also neuroprotective (Aires et al., 2019; Krady et al., 2005). Despite these findings in rodent models, a clinical trial examining microglial inhibition by doxycycline did not improve visual or anatomic outcomes in patients (Scott et al., 2014). Truly, the relationship between neurodegeneration and vasculopathy still remains opaque. As stated previously, neurodegeneration and loss of visual function can be detected in preclinical DR (Table 1) (Barber et al., 2005, 1998; Bogdanov et al., 2014). However, other patients experience no visual symptoms even though they do have detectable vascular abnormalities (Wilkinson et al., 2003). Therefore it remains uncertain if neurodegeneration always precedes vascular abnormalities or if they occur in parallel (Hernández et al., 2017; Santos et al., 2017).
Table 1. Categorizations of the vascular, inflammatory and neuronal aspects of Diabetic Retinopathy.
The first row across lists the different clinical diagnoses of diabetic retinopathy progression. Vascular signs are assigned in the second row according to clinical categorization. Neuronal and immune findings are attributed to the different grades in rows 3 and 4, respectively.
| Clinical Diagnosis | No Diabetes | Diabetes without retinopathy | Mild NPDR | Moderate NPDR | Severe NPDR | PDR |
|---|---|---|---|---|---|---|
| Vascular | No signs | No signs | Microaneurysm | Microaneurysm Dot blot hemorrhages Venous beading Cotton wool spots Hard exudates |
Microaneurysms (4 quadrants) Venous beading (2 quadrants) Microvascular abnormalities |
Neovascularization Vitreous hemorrhages Fibrovascular proliferation Retinal detachments |
| Neuronal | Neuropreservation | Neurodegeneration | Neurodegeneration | Neurodegeneration | Neurodegeneration | Neurodegeneration |
| Immune | Quiescent microglia Anti-inflammatory mediators Neurotrophins ↑ No complement fragments | Microgliosis Cytokines ↑ Proforms ↑ Complement fragment ↑ | Microgliosis Cytokines ↑ Proforms ↑ Complement fragment ↑ | Microgliosis Cytokines ↑ Proforms ↑ Complement fragment ↑ | Microgliosis Cytokines ↑ Proforms ↑ Complement fragment ↑ | Gliosis Systemic cytokines ↑ Systemic leukocytes ↑ Systemic complement ↑ |
b. Diabetic macular edema
During NPDR, many patients experience DME, which is clinically observed as retinal thickening with hard exudates (Wilkinson et al., 2003). These findings are thought to be a direct consequence of lipoprotein leakage, which causes separation of the inner retinal layers from the RPE (Lang, 2012). DME is responsible for most of the vision loss in individuals with DR (Duh et al., 2017; Rübsam et al., 2018). This was recently reviewed (Murakami et al., 2020), but it is worth highlighting the involvement of the immune system in its development. Aside from impairment of the anatomic barriers, Müller cells are hypothesized to undergo excessive swelling from fluid leakage/accumulation and contribute to DME (Xi et al., 2005). In addition, phagocytosis of debris by microglia contributes to thickening of the retina by creating additional pockets of fluid and cellular debris (Levin, 2009; Murakami et al., 2020). Interestingly, although earlier studies have found the anti-VEGF therapy bevacizumab to be overall superior to steroids (Gillies et al., 2014), a more recent study suggested that intravitreal steroid injections result in improved outcomes compared with anti-VEGF injections for DME patients (Ceravolo et al., 2020). Another recent study (Arima et al., 2020) may explain this efficacy of steroids by demonstrating the involvement of the immune response in BRB breakdown. Regardless, these studies certainly demonstrate the close involvement of the immune system and underscore the importance of the immune response during various stages of DR, including DME.
One of the most interesting immunologic markers of DME progression is the cytokine IL-6, whose levels correlate with severity (Funatsu et al., 2002; Liu et al., 2015; Wu et al., 2020). Although research is currently underway to determine how IL-6 contributes to DME progression, its receptor (IL-6R) has been identified on Müller cells and endothelial cells, cell-types implicated in DME pathogenesis (Izumi-Nagai et al., 2007; Zhao et al., 2014). IL-6 is produced in the eye by microglia and macroglia and is produced in the periphery by lymphocytes, monocytes, and endothelial cells (Izumi-Nagai et al., 2007; Kishimoto, 1989; Krady et al., 2005; Zhao et al., 2014). However, unlike vitreous levels, serum IL-6 levels in PDR patients are comparable with those of non-diabetic patients (Kishimoto, 1989; Mocan et al., 2006). Therefore, although systemically produced IL-6 is known to be capable of crossing the blood-brain barrier as an acute phase reactant, its elevation in the retina is most likely from secretion by retinal immune-competent cells. In vitro evidence suggests that IL-6 protects Müller cells from hyperglycemia via a VEGF-dependent mechanism (Coughlin et al., 2019); this may help to explain why steroids are neuroprotective but detrimental to Müller cells (Pereiro et al., 2018). This aligns with the finding that IL-6 reprograms Müller cells for retinal regeneration in zebrafish; however, IL-6 has also been implicated in contributing to choroidal neovascularization (Izumi-Nagai et al., 2007; Zhao et al., 2014). Therefore IL-6 is an intriguing cytokine in DR pathogenesis, and the current evaluation of IL-6 and IL-6R antibodies in clinical trials NCT02842541 and NCT02511067 in DME patients is incredibly exciting.
c. Proliferative diabetic retinopathy
Neovascularization is the hallmark of PDR, and the use of anti-VEGF therapies has slowed disease progression in 50% to 87.5% of patients (Osaadon et al., 2014; Sivaprasad et al., 2017; Writing Committee for the Diabetic Retinopathy Clinical Research Network et al., 2015). Other cytokines such as TNFα have also been shown to contribute to neovascularization, and the genetic and pharmacologic inhibition of TNFα activity similarly retards DR progression (Aveleira et al., 2010; Gardiner et al., 2005; Santos et al., 2012; Yoshida et al., 1999). Not surprisingly, the human and rodent retina during PDR contains the highest levels of pro-inflammatory mediators and activated microglia (Barber et al., 2005; Davies et al., 2006; Karlstetter et al., 2015; Stone et al., 1995; Wang et al., 2010; Zeng et al., 2008, 2000). Interestingly, activated microglia spatially overlap with neovascularization in OIR rodents (Davies et al., 2006), and myeloid progenitor transplantation improves that neovascularization (Ritter et al., 2006). This emphasizes the phenotypic difference between activated microglia and transplanted progenitors and suggests that microglia have the capacity of playing either an antagonistic or protective role (Figure 3).
Like microglia, the gliotic response of macroglia seems to be at its peak during PDR. This is most clearly represented by their response to the inflammatory environment by adopting a “fibroblast-like” role and creating dense fibrovascular networks presumably as a mechanism of scarification of the damaged retina in order to protect what remains (Figure 12) (Friedlander, 2007; Van Hove et al., 2020). Unfortunately, these gliotic scars, although primarily “protective,” negatively impact the architecture of the retina, prevent potentially regenerative mechanisms, limit vision, and ultimately become points of tension that cause retinal detachment and blindness (Yang et al., 2008). Interestingly, the microglial expression of connective tissue growth factor, which promotes extracellular matrix formation and fibrosis, is also altered during DR (Kuiper et al., 2004). Cross-activation between microglia and macroglia, and perpetuation of the immune response demonstrated using co-culture systems (Liu and Steinle, 2017; Wang et al., 2012, 2011; Yoshida et al., 2004) supports another collaborative function of Müller glial cells, astrocytes, and microglia in the regulation of the hallmark of PDR—the fibrotic process (Friedlander, 2007; Guidry et al., 2003). This process clearly involves both macroglia and microglia, but further work is needed to fully uncover their respective roles in what clearly is a fibroblastic response reminiscent of wound healing, and thus likely initially adaptive but progressively becoming maladaptive.
Figure 12. Summary of the clinical manifestations of diabetic retinopathy and the relative involvement of the vascular, immune, and neuronal systems.
Early on, activated micro- and macroglia (astrocytes and Muller glia) contribute to aneurysm formation through secretion of mediators that influence neighboring endothelial cells and weaken BRB integrity. Additionally, activated microglia secrete neurotoxins that impair the function of neurons in the neuroretina. As disease progress, activated microglia and macroglia contribute to the creation and formation of fibrovascular scars that push the disease stage to proliferative diabetic retinopathy. Activated microglia and astrocytes secrete pro-angiogenic and pro-inflammatory factors that result in the growth of abnormal vessels.
Clearly, the combinatorial effect of diabetes on Müller cells, astrocytes, and microglia outlines once more the communication and mutual activation of microglia and macroglia (Figure 8) (Friedlander, 2007; Guidry et al., 2003). An underlying theme throughout the various DR stages is the active immune response that contributes to the different observed pathologies, from neurodegeneration to vascular abnormalities. Therefore it is incredibly important to further investigate and decipher how the immune response is intricately tied to these processes.
7. Future directions and conclusions
Vision loss is a particularly devastating complication of diabetes. Treatment options remain dramatically limited, with the widely celebrated anti-VEGF therapies effective only in one-third to one-half of patients with vision-threatening stages of the disease (Bressler et al., 2014; Elman et al., 2010; Nguyen et al., 2010). Hence there is more than ever a clear need to develop new therapies that are more effective to preserve vision. This hinges on our understanding of the pathophysiology behind the disease. The innate immune system is receiving increasing attention as a major contributor to DR progression and may be at the heart of homeostatic dysregulation observed in all phases of disease; however, the clinical treatment of DR continues to revolve solely around its vascular consequences (Amoaku et al., 2020). In addition to revealing new potential targets for therapies such as the complement system (Fort et al., 2011; Huang et al., 2018; Muramatsu et al., 2013; Rasmussen et al., 2018), investigation into the immune response has revealed its incredible activity during preclinical DR—classically thought of as the “silent” period of disease. This provides an incredible opportunity to develop treatment for early stages of the disease and points out the need for a novel and more comprehensive classification of diabetic retinal diseases that includes vascular, neural, and inflammatory aspects of the disease (Abramoff et al., 2018).
In addition to the vascular phenomena that define disease stages, the field needs to focus much more of its attention on the other pathologies and dysfunctions observed throughout disease, with an emphasis on earlier stages of DR (Table 1). Among all these factors, the interactions of the various components of the innate immune system appear to be promising targets based on data from human patients and animal models of DR. Indeed, the successful regression of disease with the use of minocycline, topical PEDF derivatives, and adenoviral delivery of CD59 is testament to the importance and interlinked nature of these different components (Adhi et al., 2013; Krady et al., 2005; Yanling Liu et al., 2012; Zhang et al., 2006). In addition, the applications of pharmacotherapies such as resveratrol and laser treatments to decrease microgliosis and inflammatory mediators are promising avenues for intervention (Y. Chen et al., 2019; Midena et al., 2019). However, more work is needed in human patients to anchor and contextualize the findings made in animal models. Indeed, although rodent studies are incredibly important, none of the many DR rodent models fully recapitulates human disease (Olivares et al., 2017). This is particularly important and relevant to the understanding of disease progression and explains the lack of translation of findings in these models to new treatments for human patients. Using human tissues and integrating data from multiple analytical levels have proven to be successful for other diseases or diabetic complications (Afshinnia et al., 2019). Only by combining discoveries in multiple model systems and vertically integrating findings acquired in cells and animals can we begin to understand and untangle the complexities of pathogenesis. Maybe to our advantage, the inextricably linked nature of the different components of the innate immune system will provide multiple therapeutic strategies targeting overlapping or pathway-specific elements.
Acknowledgements
This project and its authors were supported by NIH EY020895, the NIDDK, Eversight, the Juvenile Diabetes Research Foundation and the Thomas Beatson Foundation. This work utilized the Core Center for Vision Research funded by P30 EY007003 from the National Eye Institute. The authors are very grateful for the assistance of David Murrell in making the illustrations for this manuscript. Patrice E. Fort is the guarantor of this work. The authors declare having no conflicts of interests.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- BRB
blood-retinal barrier
- CAM
cell adhesion molecule
- CX3CL1
fractalkine
- CX3CR1
fractalkine receptor
- db/db
leptin receptor deficient
- DC
dendritic cell
- DME
diabetic macular edema
- DR
diabetic retinopathy
- IL
interleukin
- MCP-1
monocyte chemoattractant protein-1
- NPDR
non-proliferative diabetic retinopathy
- NVU
neurovascular unit
- OIR
oxygen-induced retinopathy
- PDR
proliferative diabetic retinopathy
- PEDF
pigment epithelium–derived factor
- RPE
retinal pigment epithelium
- TNFα
tumor necrosis factor α
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have declared that no conflict of interest exists.
Contributor Information
Warren W. Pan, Department of Ophthalmology and Visual Sciences, University of Michigan, Ann Arbor, MI, 48105, USA
Feng Lin, Department of Immunology, Cleveland Clinic, Cleveland, OH, 44106, USA.
Patrice E. Fort, Departments of Ophthalmology and Visual Sciences, and Molecular and Integrative Physiology, University of Michigan, Ann Arbor, MI, 48105, USA.
References
- Abcouwer SF, Gardner TW, 2014. Diabetic retinopathy: Loss of neuroretinal adaptation to the diabetic metabolic environment. Ann. N. Y. Acad. Sci 1311, 174–190. 10.1111/nyas.12412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abhary S, Hewitt AW, Burdon KP, Craig JE, 2009. A systematic meta-analysis of genetic association studies for diabetic retinopathy. Diabetes 58, 2137–2147. 10.2337/db09-0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu El-Asrar AM, Dralands L, Missotten L, Al-Jadaan IA, Geboes K, 2004. Expression of apoptosis markers in the retinas of human subjects with diabetes. Investig. Ophthalmol. Vis. Sci 45, 2760–2766. 10.1167/iovs.03-1392 [DOI] [PubMed] [Google Scholar]
- Abukawa H, Tomi M, Kiyokawa J, Hori S, Kondo T, Terasaki T, Hosoya K-I, 2009. Modulation of retinal capillary endothelial cells by Müller glial cell-derived factors. Mol. Vis 15, 451–7. [PMC free article] [PubMed] [Google Scholar]
- Adams AJ, Bearse MA, 2012. Retinal neuropathy precedes vasculopathy in diabetes: A function-based opportunity for early treatment intervention? Clin. Exp. Optom 95, 256–265. 10.1111/j.1444-0938.2012.00733.x [DOI] [PubMed] [Google Scholar]
- Adhi M, Cashman SM, Kumar-Singh R, 2013. Adeno-Associated Virus Mediated Delivery of a Non-Membrane Targeted Human Soluble CD59 Attenuates Some Aspects of Diabetic Retinopathy in Mice. PLoS One 8, 1–12. 10.1371/journal.pone.0079661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afarid M, Namvar E, Sanie-Jahromi F, 2020. Diabetic Retinopathy and BDNF: A Review on Its Molecular Basis and Clinical Applications. J. Ophthalmol 2020, 1602739. 10.1155/2020/1602739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aires ID, Madeira MH, Boia R, Rodrigues-Neves AC, Martins JM, Ambrósio AF, Santiago AR, 2019. Intravitreal injection of adenosine A2A receptor antagonist reduces neuroinflammation, vascular leakage and cell death in the retina of diabetic mice. Sci. Rep 9, 1–14. 10.1038/s41598-019-53627-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali TK, Al-Gayyar MMH, Matragoon S, Pillai BA, Abdelsaid MA, Nussbaum JJ, El-Remessy AB, 2011. Diabetes-induced peroxynitrite impairs the balance of pro-nerve growth factor and nerve growth factor, and causes neurovascular injury. Diabetologia 54, 657–668. 10.1007/s00125-010-1935-1 [DOI] [PubMed] [Google Scholar]
- Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonnière L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A, 2011. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334, 1727–31. 10.1126/science.1206936 [DOI] [PubMed] [Google Scholar]
- Alves MRP, Boia R, Campos EJ, Martins J, Nunes S, Madeira MH, Santiago AR, Pereira FC, Reis F, Ambrósio AF, Baptista FI, 2018. Subtle thinning of retinal layers without overt vascular and inflammatory alterations in a rat model of prediabetes. Mol. Vis 24, 353–366. [PMC free article] [PubMed] [Google Scholar]
- Amadi-obi A, Yu C, Dambuza I, Kim S, Marrero B, Egwuagu CE, 2012. Interleukin 27 Induces the Expression of Complement Factor H ( CFH ) in the Retina 7 10.1371/journal.pone.0045801 [DOI] [PMC free article] [PubMed]
- Ambati J, Chalam KV, Chawla DK, D’Angio CT, Guillet EG, Rose SJ, Vanderlinde RE, Ambati BK, 1997. Elevated gamma-aminobutyric acid, glutamate, and vascular endothelial growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch. Ophthalmol. (Chicago, Ill. 1960) 115, 1161–6. [DOI] [PubMed] [Google Scholar]
- Amoaku WM, Ghanchi F, Bailey C, Banerjee Sanjiv, Banerjee Somnath, Downey L, Gale R, Hamilton R, Khunti K, Posner E, Quhill F, Robinson S, Setty R, Sim D, Varma D, Mehta H, 2020. Diabetic retinopathy and diabetic macular oedema pathways and management: UK Consensus Working Group. Eye 34, 1–51. 10.1038/s41433-020-0961-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Rickman CB, Bok D, Hageman GS, Johnson LV, 2010. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog. Retin. Eye Res 29, 95–112. 10.1016/j.preteyeres.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer DB, 1999. Bowman lecture 1998. Diabetic retinopathy: Some cellular, molecular and therapeutic considerations. Eye 13, 497–523. 10.1038/eye.1999.130 [DOI] [PubMed] [Google Scholar]
- Arima M, Nakao S, Yamaguchi M, Feng H, Fujii Y, Shibata K, Wada I, Kaizu Y, Ahmadieh H, Ishibashi T, Stitt AW, Sonoda KH, 2020. Claudin-5 redistribution induced by inflammation leads to anti-VEGF–resistant diabetic macular edema. Diabetes 69, 981–999. 10.2337/db19-1121 [DOI] [PubMed] [Google Scholar]
- Arita R, Nakao S, Kita T, Kawahara S, Asato R, Yoshida S, Enaida H, Hafezi-Moghadam A, Ishibashi T, 2013. A key role for ROCK in TNF-α-mediated diabetic microvascular damage. Investig. Ophthalmol. Vis. Sci 54, 2373–2383. 10.1167/iovs.12-10757 [DOI] [PubMed] [Google Scholar]
- Arroba AI, Alcalde-estevez E, García-ramírez M, Cazzoni D, De P, Sánchez-fernández EM, Ortiz C, García JM, Hernández C, Simó R, Valverde ÁM, 2016. Biochimica et Biophysica Acta Modulation of microglia polarization dynamics during diabetic retinopathy in db / db mice. BBA - Mol. Basis Dis 1862, 1663–1674. 10.1016/j.bbadis.2016.05.024 [DOI] [PubMed] [Google Scholar]
- Atawia RT, Bunch KL, Fouda AY, Lemtalsi T, Eldahshan W, Xu Z, Saul A, Elmasry K, Al-Shabrawey M, Caldwell RB, Caldwell RW, 2020. Role of Arginase 2 in Murine Retinopathy Associated with Western Diet-Induced Obesity. J. Clin. Med 9, 17207. 10.3390/jcm9020317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveleira CA, Lin CM, Abcouwer SF, Ambrósio AF, Antonetti DA, 2010. TNF-α signals through PKCζ/NF-κB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes 59, 2872–2882. 10.2337/db09-1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee D, Dick AD, 2004. Blocking CD200-CD200 receptor axis augments NOS-2 expression and aggravates experimental autoimmune uveoretinitis in Lewis rats. Ocul. Immunol. Inflamm 12, 115–125. 10.1080/09273940490895326 [DOI] [PubMed] [Google Scholar]
- Barber AJ, Antonetti DA, Kern TS, Reiter CEN, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK, 2005. The Ins2Akita mouse as a model of early retinal complications in diabetes. Investig. Ophthalmol. Vis. Sci 46, 2210–2218. 10.1167/iovs.04-1340 [DOI] [PubMed] [Google Scholar]
- Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW, 1998. Neural apoptosis in the retina during experimental and human diabetes: Early onset and effect of insulin. J. Clin. Invest 102, 783–791. 10.1172/JCI2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelona PF, Sitaras N, Galan A, Esquiva G, Jmaeff S, Jian Y, Sarunic MV, Cuenca N, Sapieha P, Saragovi HU, 2016. p75NTR and Its Ligand ProNGF Activate Paracrine Mechanisms Etiological to the Vascular, Inflammatory, and Neurodegenerative Pathologies of Diabetic Retinopathy. J. Neurosci 36, 8826–41. 10.1523/JNEUROSCI.4278-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable CJ, Tombran-Tink J, 2004. Neuroprotective and antiangiogenic actions of PEDF in the eye: Molecular targets and therapeutic potential. Prog. Retin. Eye Res 23, 561–577. 10.1016/j.preteyeres.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Barouch FC, Miyamoto K, Allport JR, Fujita K, Bursell SE, Aiello LP, Luscinskas FW, Adamis AP, 2000. Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Invest. Ophthalmol. Vis. Sci 41, 1153–8. [PubMed] [Google Scholar]
- Behzadian MA, Wang X-L, Jiang B, Caldwell RB, 1995. Angiostatic role or astrocytes: Suppression of vascular endothelial cell growth by TGF-β and other inhibitory factor(s). Glia 15, 480–490. 10.1002/glia.440150411 [DOI] [PubMed] [Google Scholar]
- Beli E, Dominguez JM, Hu P, Thinschmidt JS, Caballero S, Li Calzi S, Luo D, Shanmugam S, Salazar TE, Duan Y, Boulton ME, Mohr S, Abcouwer SF, Saban DR, Harrison JK, Grant MB, 2016. CX3CR1 deficiency accelerates the development of retinopathy in a rodent model of type 1 diabetes. J. Mol. Med. (Berl) 94, 1255–1265. 10.1007/s00109-016-1433-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Waxman SG, Hildebrand C, 1985. Axo-glial relations in the retina-optic nerve junction of the adult rat: freeze-fracture observations on axon membrane structure. J. Neurocytol 14, 887–907. 10.1007/BF01224803 [DOI] [PubMed] [Google Scholar]
- Boehm BO, Lang G, Volpert O, Jehle PM, Kurkhaus A, Rosinger S, Lang GK, Bouck N, 2003. Low content of the natural ocular anti-angiogenic agent pigment epithelium-derived factor (PEDF) in aqueous humor predicts progression of diabetic retinopathy. Diabetologia 46, 394–400. 10.1007/s00125-003-1040-9 [DOI] [PubMed] [Google Scholar]
- Bogdanov P, Corraliza L, Villena JA, Carvalho AR, Herna C, Simo R, 2014. The db / db Mouse : A Useful Model for the Study of Diabetic Retinal Neurodegeneration 9. 10.1371/journal.pone.0097302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora NS, Jha P, Lyzogubov VV, Kaliappan S, Liu J, Tytarenko RG, Fraser DA, Morgan BP, Bora PS, 2010. Recombinant membrane-targeted form of CD59 inhibits the growth of choroidal neovascular complex in mice. J. Biol. Chem 285, 33826–33833. 10.1074/jbc.M110.153130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss JD, Singh PK, Pandya HK, Tosi J, Kim C, Tewari A, Juzych MS, Abrams GW, Kumar A, 2017. Assessment of neurotrophins and inflammatory mediators in vitreous of patients with diabetic retinopathy. Investig. Ophthalmol. Vis. Sci 58, 5594–5603. 10.1167/iovs.17-21973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick GH, Palta M, 1987. Oscillatory Potential Amplitudes: Relation to Severity of Diabetic Retinopathy. Arch. Ophthalmol 105, 929–933. 10.1001/archopht.1987.01060070065030 [DOI] [PubMed] [Google Scholar]
- Bressler NM, Varma R, Suñer IJ, Dolan CM, Ward J, Ehrlich JS, Colman S, Turpcu A, 2014. Vision-related function after ranibizumab treatment for diabetic macular edema: Results from RIDE and RISE. Ophthalmology 121, 2461–2472. 10.1016/j.ophtha.2014.07.008 [DOI] [PubMed] [Google Scholar]
- Broderick C, Hoek RM, Forrester JV, Liversidge J, Sedgwick JD, Dick AD, 2002. Constitutive retinal CD200 expression regulates resident microglia and activation state of inflammatory cells during experimental autoimmune uveoretinitis. Am. J. Pathol 161, 1669–1677. 10.1016/S0002-9440(10)64444-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Neher JJ, 2014. Microglial phagocytosis of live neurons. Nat. Rev. Neurosci 15, 209–216. 10.1038/nrn3710 [DOI] [PubMed] [Google Scholar]
- Bursell SE, Cavallerano JD, Cavallerano AA, Clermont AC, Birkmire-Peters D, Aiello LP, Aiello LM, 2001. Stereo nonmydriatic digital-video color retinal imaging compared with Early Treatment Diabetic Retinopathy Study seven standard field 35-mm stereo color photos for determining level of diabetic retinopathy. Ophthalmology 108, 572–585. 10.1016/S0161-6420(00)00604-7 [DOI] [PubMed] [Google Scholar]
- Cai S, Yang Q, Hou M, Han Q, Zhang H, Wang J, Qi C, Bo Q, Ru Y, Yang W, Gu Z, Wei R, Cao Y, Li X, Zhang Y, 2018. A-melanocyte-stimulating hormone protects early diabetic retina from blood-retinal barrier breakdown and vascular leakage via MC4R. Cell. Physiol. Biochem 45, 505–522. 10.1159/000487029 [DOI] [PubMed] [Google Scholar]
- Capitão M, Soares R, 2016. Angiogenesis and Inflammation Crosstalk in Diabetic Retinopathy. J. Cell. Biochem 2453, 2443–2453. 10.1002/jcb.25575 [DOI] [PubMed] [Google Scholar]
- Capozzi ME, McCollum GW, Cousins DB, Penn JS, 2016. Linoleic Acid is a Diabetes-relevant Stimulator of Retinal Inflammation in Human Retinal Muller Cells and Microvascular Endothelial Cells. J. Diabetes Metab 7, 219–227. 10.4172/2155-6156.1000718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona SM, Mendiola AS, Yang YC, Adkins SL, Torres V, Cardona AE, 2015. Disruption of fractalkine signaling leads to microglial activation and neuronal damage in the diabetic retina. ASN Neuro 7. 10.1177/1759091415608204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceravolo I, Oliverio GW, Alibrandi A, Bhatti A, Trombetta L, Rejdak R, Toro MD, Trombetta CJ, 2020. The Application of Structural Retinal Biomarkers to Evaluate the Effect of Intravitreal Ranibizumab and Dexamethasone Intravitreal Implant on Treatment of Diabetic Macular Edema. Diagnostics (Basel, Switzerland) 10, 1–12. 10.3390/diagnostics10060413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Lin CW, Chang YS, Chen PH, Li CY, Wu WC, Kao YH, 2020. Monounsaturated oleic acid modulates autophagy flux and upregulates angiogenic factor production in human retinal pigment epithelial ARPE-19 cells. Life Sci 259, 118391. 10.1016/j.lfs.2020.118391 [DOI] [PubMed] [Google Scholar]
- Chen C, Chen H, Xu C, Zhong Y, Shen X, 2014. Role of interleukin-1β in hypoxia-induced depression of glutamate uptake in retinal Müller cells. Graefe’s Arch. Clin. Exp. Ophthalmol 252, 51–58. 10.1007/s00417-013-2516-z [DOI] [PubMed] [Google Scholar]
- Chen M, Luo C, Zhao J, Devarajan G, Xu H, 2019. Immune regulation in the aging retina. Prog. Retin. Eye Res 69, 159–172. 10.1016/j.preteyeres.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Meng J, Li H, Wei H, Bi F, Liu S, Tang K, Guo H, Liu W, 2019. Resveratrol exhibits an effect on attenuating retina inflammatory condition and damage of diabetic retinopathy via PON1. Exp. Eye Res 181, 356–366. 10.1016/j.exer.2018.11.023 [DOI] [PubMed] [Google Scholar]
- Cheng L, Bu H, Portillo JAC, Li Y, Subauste CS, Huang SS, Kern TS, Lin F, 2013. Modulation of retinal Müller cells by complement receptor C5aR. Invest. Ophthalmol. Vis. Sci 54, 8191–8198. 10.1167/iovs.13-12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhablani J, Sharma A, Goud A, Peguda HK, Rao HL, Begum VU, Barteselli G, 2015. Neurodegeneration in type 2 diabetes: Evidence from spectral-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci 56, 6333–6338. 10.1167/iovs.15-17334 [DOI] [PubMed] [Google Scholar]
- Chovatiya R, Medzhitov R, 2014. Stress, inflammation, and defense of homeostasis. Mol. Cell 54, 281–288. 10.1016/j.molcel.2014.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti PC, Pan YC, Olander JV, Connolly DT, Stern D, 1990. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J. Exp. Med 172, 1535–45. 10.1084/jem.172.6.1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combadière C, Feumi C, Raoul W, Keller N, Rodéro M, Pézard A, Lavalette S, Houssier M, Jonet L, Picard E, Debré P, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny JC, Chemtob S, Behar-Cohen F, Sennlaub F, 2007. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Invest 117, 2920–2928. 10.1172/JCI31692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin BA, Feenstra DJ, Mohr S, 2017. Müller cells and diabetic retinopathy. Vision Res 139, 93–100. 10.1016/j.visres.2017.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin BA, Trombley BT, Mohr S, 2019. Interleukin-6 (IL-6) mediates protection against glucose toxicity in human Müller cells via activation of VEGF-A signaling. Biochem. Biophys. Res. Commun 517, 227–232. 10.1016/j.bbrc.2019.07.044 [DOI] [PubMed] [Google Scholar]
- Cousins SW, MM M, D D, Streilein JW, 1991. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. - PubMed - NCBI. Investig. Ophthalmol. Vis. Sci 32(8):, 2201–11. [PubMed] [Google Scholar]
- D’Orazio TJ, Niederkorn JY, 1998. A novel role for TGF-β and IL-10 in the induction of immune privilege. J. Immunol 160, 2089–2098. [PubMed] [Google Scholar]
- Dai D-F, Chiao Y, Marcinek DJ, Szeto HH, Rabinovitch PS, 2014. Mitochondrial oxidative stress in aging and healthspan. Longev. Heal 3, 6. 10.1186/2046-2395-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT, 2011. Age-related alterations in the dynamic behavior of microglia. Aging Cell 10, 263–276. 10.1111/j.1474-9726.2010.00660.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando SJ, Naranjo Golborne C, Chinnery HR, Ruitenberg MJ, McMenamin PG, 2016. A case of mistaken identity: CD11c-eYFP+ cells in the normal mouse brain parenchyma and neural retina display the phenotype of microglia, not dendritic cells. Glia 64, 1331–1349. 10.1002/glia.23005 [DOI] [PubMed] [Google Scholar]
- Davies MH, Eubanks JP, Powers MR, 2006. Microglia and macrophages are increased in response to ischemia-induced retinopathy in the mouse retina. Mol. Vis 12, 467–77. [PubMed] [Google Scholar]
- Davies MH, Stempel AJ, Powers MR, 2008. MCP-1 deficiency delays regression of pathologic retinal neovascularization in a model of ischemic retinopathy. Investig. Ophthalmol. Vis. Sci 49, 4195–4202. 10.1167/iovs.07-1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S, 2006. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond) 20, 1366–9. 10.1038/sj.eye.6702138 [DOI] [PubMed] [Google Scholar]
- Di Leo MAS, Caputo S, Falsini B, Porciatti V, Greco AV, Ghirlanda G, 1994. Presence and further development of retinal dysfunction after 3-year follow up in IDDM patients without angiographically documented vasculopathy. Diabetologia 37, 911–916. 10.1007/BF00400947 [DOI] [PubMed] [Google Scholar]
- Ding X, Gu R, Zhang M, Ren H, Shu Q, Xu G, Wu H, 2018. Microglia enhanced the angiogenesis, migration and proliferation of co-cultured RMECs. BMC Ophthalmol 18, 249. 10.1186/s12886-018-0886-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Zhang M, Gu R, Xu G, Wu H, 2017. Activated microglia induce the production of reactive oxygen species and promote apoptosis of co-cultured retinal microvascular pericytes. Graefe’s Arch. Clin. Exp. Ophthalmol 255, 777–788. 10.1007/s00417-016-3578-5 [DOI] [PubMed] [Google Scholar]
- Do DV, Nguyen QD, Boyer D, Schmidt-Erfurth U, Brown DM, Vitti R, Berliner AJ, Gao B, Zeitz O, Ruckert R, Schmelter T, Sandbrink R, Heier JS, 2012. One-year outcomes of the da VINCI study of VEGF trap-eye in eyes with diabetic macular edema. Ophthalmology 119, 1658–1665. 10.1016/j.ophtha.2012.02.010 [DOI] [PubMed] [Google Scholar]
- Dodo Y, Murakami T, Uji A, Yoshitake S, Yoshimura N, 2015. Disorganized retinal lamellar structures in nonperfused areas of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci 56, 2012–2020. 10.1167/iovs.14-15924 [DOI] [PubMed] [Google Scholar]
- Doganay S, Sevinç A, Türköz Y, Evereklioglu C, Er H, Şavli H, Mehmet N, 2008. Comparison of serum NO, TNF-α, IL-1β, sIL-2R, IL-6 and IL-8 levels with grades of retinopathy in patients with diabetes mellitus. Eye 16, 163–170. 10.1038/sj/eye/6700095 [DOI] [PubMed] [Google Scholar]
- Dong N, Li X, Xiao L, Yu W, Wang B, Chu L, 2012. Upregulation of retinal neuronal MCP-1 in the rodent model of diabetic retinopathy and its function in vitro. Investig. Ophthalmol. Vis. Sci 53, 7567–7575. 10.1167/iovs.12-9446 [DOI] [PubMed] [Google Scholar]
- Dorner GT, Garhöfer G, Huemer KH, Riva CE, Wolzt M, Schmetterer L, 2003. Hyperglycemia affects flicker-induced vasodilation in the retina of healthy subjects. Vision Res 43, 1495–1500. 10.1016/S0042-6989(03)00170-6 [DOI] [PubMed] [Google Scholar]
- Du J, Cleghorn W, Contreras L, Linton JD, Chan GCK, Chertov AO, Saheki T, Govindaraju V, Sadilek M, Satrústegui J, Hurley JB, 2013. Cytosolic reducing power preserves glutamate in retina. Proc. Natl. Acad. Sci. U. S. A 110, 18501–18506. 10.1073/pnas.1311193110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duh EJ, Sun JK, Stitt AW, 2017. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI insight 2. 10.1172/jci.insight.93751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid S, Sas KM, Abcouwer SF, Feldman EL, Gardner TW, Pennathur S, Fort PE, 2019. New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia 62, 1539–1549. 10.1007/s00125-019-4959-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK, 2010. Randomized Trial Evaluating Ranibizumab Plus Prompt or Deferred Laser or Triamcinolone Plus Prompt Laser for Diabetic Macular Edema. Ophthalmology 117, 1064–1077.e35. 10.1016/j.ophtha.2010.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshaer SL, Abdelsaid MA, Al-Azayzih A, Kumar P, Matragoon S, Nussbaum JJ, El-Remessy AB, 2013. Pronerve growth factor induces angiogenesis via activation of TrkA: possible role in proliferative diabetic retinopathy. J. Diabetes Res 2013, 432659. 10.1155/2013/432659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehér J, Taurone S, Spoletini M, Biró Z, Varsányi B, Scuderi G, Orlando MP, Turchetta R, Micera A, Artico M, 2018. Ultrastructure of neurovascular changes in human diabetic retinopathy. Int. J. Immunopathol. Pharmacol 31. 10.1177/0394632017748841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Bueno I, Jones R, Soriano-Romaní L, Líopez-García A, Galvin O, Cheetham S, Diebold Y, 2017. Histologic characterization of retina neuroglia modifications in diabetic zucker diabetic fatty rats. Investig. Ophthalmol. Vis. Sci 58, 4925–4933. 10.1167/iovs.17-21742 [DOI] [PubMed] [Google Scholar]
- Ferrer-Martín RM, Martín-Oliva D, Sierra A, Carrasco MC, Martín-Estebané M, Calvente R, Marín-Teva JL, Navascués J, Cuadros MA, 2014. Microglial cells in organotypic cultures of developing and adult mouse retina and their relationship with cell death. Exp. Eye Res 121, 42–57. 10.1016/j.exer.2014.02.015 [DOI] [PubMed] [Google Scholar]
- Fontainhas AM, Wang M, Liang KJ, Chen S, Mettu P, Damani M, Fariss RN, Li W, Wong WT, 2011. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS One 6. 10.1371/journal.pone.0015973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort PE, Losiewicz MK, Pennathur S, Jefferson LS, Kimball SR, Abcouwer SF, Gardner TW, 2014. mTORC1-independent reduction of retinal protein synthesis in type 1 diabetes. Diabetes 63, 3077–90. 10.2337/db14-0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort PE, Losiewicz MK, Reiter CEN, Singh RSJ, Nakamura M, Abcouwer SF, Barber AJ, Gardner TW, 2011. Differential roles of hyperglycemia and hypoinsulinemia in diabetes induced retinal cell death: evidence for retinal insulin resistance. PLoS One 6, e26498. 10.1371/journal.pone.0026498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederich RC, Lollmann B, Hamann A, Napolitano-Rosen A, Kahn BB, Lowell BB, Flier JS, 1995. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J. Clin. Invest 96, 1658–1663. 10.1172/JCI118206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas HR, Ferraz G, Ferreira GC, Ribeiro-Resende VT, Chiarini LB, Nascimento JLMD, Oliveira KRHM, De Pereira TL, Ferreira LGB, Kubrusly RC, Faria RX, Herculano AM, De Melo Reis RA, 2016. Glutathione-induced calcium shifts in chick retinal glial cells. PLoS One 11, 1–20. 10.1371/journal.pone.0153677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander M, 2007. Review series Fibrosis and diseases of the eye. Ophthalmology 117, 576–586. 10.1172/JCI31030.576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Dong S, Zhu M, Sherry DM, Wang C, You Z, Haigh JJ, Le Y-Z, 2015. Müller Glia Are a Major Cellular Source of Survival Signals for Retinal Neurons in Diabetes. Diabetes 64, 3554–63. 10.2337/db15-0180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S, 2002. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am. J. Ophthalmol 133, 70–77. 10.1016/S0002-9394(01)01269-7 [DOI] [PubMed] [Google Scholar]
- Ganesh BS, Chintala SK, 2011. Inhibition of reactive gliosis attenuates excitotoxicity-mediated death of retinal ganglion cells. PLoS One 6, 1–12. 10.1371/journal.pone.0018305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner TA, Gibson DS, de Gooyer TE, de la Cruz VF, McDonald DM, Stitt AW, 2005. Inhibition of tumor necrosis factor-alpha improves physiological angiogenesis and reduces pathological neovascularization in ischemic retinopathy. Am. J. Pathol 166, 637–44. 10.1016/s0002-9440(10)62284-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garhöfer G, Zawinka C, Resch H, Kothy P, Schmetterer L, Dorner GT, 2004. Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. Br. J. Ophthalmol 88, 887–890. 10.1136/bjo.2003.033548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasque P, Chan P, Fontaine M, Ischenko A, Lamacz M, Götze O, Morgan BP, 1995. Identification and characterization of the complement C5a anaphylatoxin receptor on human astrocytes. J. Immunol 155, 4882–9. [PubMed] [Google Scholar]
- Gerhardinger C, Costa MB, Coulombe MC, Toth I, Hoehn T, Grosu P, 2005. Expression of acute-phase response proteins in retinal Müller cells in diabetes. Investig. Ophthalmol. Vis. Sci 46, 349–357. 10.1167/iovs.04-0860 [DOI] [PubMed] [Google Scholar]
- Giganti M, Beer PM, Lemanski N, Hartman C, Schartman J, Falk N, 2010. Adverse events after intravitreal infliximab (Remicade). Retina 30, 71–80. 10.1097/IAE.0b013e3181bcef3b [DOI] [PubMed] [Google Scholar]
- Gillies MC, Lim LL, Campain A, Quin GJ, Salem W, Li J, Goodwin S, Aroney C, McAllister IL, Fraser-Bell S, 2014. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: The BEVORDEX study. Ophthalmology 121, 2473–2481. 10.1016/j.ophtha.2014.07.002 [DOI] [PubMed] [Google Scholar]
- Gnanasekaran S, Bandala-Sanchez E, Kolic M, Churilov L, Rogers SL, McAuley AK, Sandhu SS, Qureshi S, Lim LL, Wickremasinghe SS, 2020. The association between intravitreal ranibizumab therapy and serum cytokine concentrations in patients with diabetic macular edema. Mol. Vis 26, 246–256. [PMC free article] [PubMed] [Google Scholar]
- Goldmann T, Wieghofer P, Jordão MJC, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, Locatelli G, Hochgerner H, Zeiser R, Epelman S, Geissmann F, Priller J, Rossi FMV, Bechmann I, Kerschensteiner M, Linnarsson S, Jung S, Prinz M, 2016. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol 17, 797–805. 10.1038/ni.3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabert K, Michoel T, Karavolos MH, Clohisey S, Kenneth Baillie J, Stevens MP, Freeman TC, Summers KM, McColl BW, 2016. Microglial brain regionâ ‘dependent diversity and selective regional sensitivities to aging. Nat. Neurosci 19, 504–516. 10.1038/nn.4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregerson DS, Yang J, 2003. CD45-positive cells of the retina and their responsiveness to in vivo and in vitro treatment with IFN-gamma or anti-CD40. Invest. Ophthalmol. Vis. Sci 44, 3083–93. 10.1167/iovs.02-1014 [DOI] [PubMed] [Google Scholar]
- Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA, 1995. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 270, 1189–92. [DOI] [PubMed] [Google Scholar]
- Guidry C, Bradley KM, King JL, 2003. Tractional force generation by human Müller cells: Growth factor responsiveness and integrin receptor involvement. Investig. Ophthalmol. Vis. Sci 44, 1355–1363. 10.1167/iovs.02-0046 [DOI] [PubMed] [Google Scholar]
- Hammes HP, Federoff HJ, Brownlee M, 1995. Nerve growth factor prevents both neuroretinal programmed cell death and capillary pathology in experimental diabetes. Mol. Med 1, 527–534. 10.1007/BF03401589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harhausen D, Khojasteh U, Stahel PF, Morgan BP, Nietfeld W, Dirnagl U, Trendelenburg G, 2010. Membrane attack complex inhibitor CD59a protects against focal cerebral ischemia in mice. J. Neuroinflammation 7, 1–12. 10.1186/1742-2094-7-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison WW, Bearse MA, Ng JS, Jewell NP, Barez S, Burger D, Schneck ME, Adams AJ, 2011. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Investig. Ophthalmol. Vis. Sci 52, 772–777. 10.1167/iovs.10-5931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan I, Luo Q, Majumdar S, Dominguez JM, Busik JV, Bhatwadekar AD, 2017. Tumor necrosis factor alpha (TNF-α) disrupts Kir4.1 channel expression resulting in müller cell dysfunction in the retina. Investig. Ophthalmol. Vis. Sci 58, 2473–2482. 10.1167/iovs.16-20712 [DOI] [PubMed] [Google Scholar]
- He J, Wang H, Liu Y, Li W, Kim D, Huang H, 2015. Blockade of vascular endothelial growth factor receptor 1 prevents inflammation and vascular leakage in diabetic retinopathy. J. Ophthalmol 2015, 605946. 10.1155/2015/605946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbomel P, Thisse B, Thisse C, 2001. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through a M-CSF receptor-dependent invasive process. Dev. Biol 238, 274–288. 10.1006/dbio.2001.0393 [DOI] [PubMed] [Google Scholar]
- Hernández C, Bogdanov P, Solà-Adell C, Sampedro J, Valeri M, Genís X, Simó-Servat O, García-Ramírez M, Simó R, 2017. Topical administration of DPP-IV inhibitors prevents retinal neurodegeneration in experimental diabetes. Diabetologia 60, 2285–2298. 10.1007/s00125-017-4388-y [DOI] [PubMed] [Google Scholar]
- Hernández C, Segura RM, Fonollosa A, Carrasco E, Francisco G, Simó R, 2005. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet. Med 22, 719–722. 10.1111/j.1464-5491.2005.01538.x [DOI] [PubMed] [Google Scholar]
- Hoek RH, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD, 2000. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science (80-.) 290, 1768–1771. 10.1126/science.290.5497.1768 [DOI] [PubMed] [Google Scholar]
- Hsieh CF, Liu CK, Lee CT, Yu LE, Wang JY, 2019. Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Sci. Rep 9, 1–16. 10.1038/s41598-018-37215-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Hunt NH, Arfuso F, Shaw LC, Uddin MN, Zhu M, Devasahayam R, Adamson SJ, Benson VL, Chan-Ling T, Grant MB, 2017. Increased Indoleamine 2,3-Dioxygenase and Quinolinic Acid Expression in Microglia and Müller Cells of Diabetic Human and Rodent Retina. Invest. Ophthalmol. Vis. Sci 58, 5043–5055. 10.1167/iovs.17-21654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Fisher KP, Hammer SS, Navitskaya S, Blanchard GJ, Busik JV, 2018. Plasma Exosomes Contribute to Microvascular Damage in Diabetic Retinopathy by Activating the Classical Complement Pathway. Diabetes 67, 1639–1649. 10.2337/db17-1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, El-Remessy AB, Matragoon S, Zhang W, Patel Y, Khan S, Al-Gayyar MM, El-Shishtawy MM, Liou GI, 2011. Retinal microglial activation and inflammation induced by amadori-glycated albumin in a rat model of diabetes. Diabetes 60, 1122–1133. 10.2337/db10-1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Internation Diabetes Federation, 2019. IDF Diabetes Atlas
- Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS, 2012. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch. Ophthalmol 130, 1145–1152. 10.1001/archophthalmol.2012.1043 [DOI] [PubMed] [Google Scholar]
- Izumi-Nagai K, Nagai N, Ozawa Y, Mihara M, Ohsugi Y, Kurihara T, Koto T, Satofuka S, Inoue M, Tsubota K, Okano H, Oike Y, Ishida S, 2007. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am. J. Pathol 170, 2149–2158. 10.2353/ajpath.2007.061018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson GR, Barber AJ, 2010. Visual dysfunction associated with diabetic retinopathy. Curr. Diab. Rep 10, 380–384. 10.1007/s11892-010-0132-4 [DOI] [PubMed] [Google Scholar]
- Jo DH, Yun J-H, Cho CS, Kim Jin Hyoung, Kim Jeong Hun, Cho C-H, 2019. Interaction between microglia and retinal pigment epithelial cells determines the integrity of outer blood-retinal barrier in diabetic retinopathy. Glia 67, 321–331. 10.1002/glia.23542 [DOI] [PubMed] [Google Scholar]
- Jørgensen A, Wiencke AK, Coin M. La, Kstel CG, Macken HO, Hamann S, Lui GM, Scher E, Prause JU, Svejgaard A, Øditm N, Nissen MH, Röpke G, 1998. Human retinal pigment epithelial cell-induced apoptosis in activated T cells. Investig. Ophthalmol. Vis. Sci 39, 1590–1599. [PubMed] [Google Scholar]
- Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP, 2001. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am. J. Pathol 158, 147–152. 10.1016/S0002-9440(10)63952-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP, 2004. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J 18, 1450–2. 10.1096/fj.03-1476fje [DOI] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Mitsiades N, Cai W, Suzuma I, Pak J, Ju S-T, Rook SL, Esser P, Mitsiades CS, Kirchhof B, Adamis AP, Aiello LP, 2003. Suppression of Fas-FasL-induced endothelial cell apoptosis prevents diabetic blood-retinal barrier breakdown in a model of streptozotocin-induced diabetes. FASEB J 17, 76–8. 10.1096/fj.02-0157fje [DOI] [PubMed] [Google Scholar]
- Joyal J-S, Gantner ML, Smith LEH, 2018. Retinal energy demands control vascular supply of the retina in development and disease: The role of neuronal lipid and glucose metabolism. Prog. Retin. Eye Res 64, 131–156. 10.1016/j.preteyeres.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal JS, Sun Y, Gantner ML, Shao Z, Evans LP, Saba N, Fredrick T, Burnim S, Kim JS, Patel G, Juan AM, Hurst CG, Hatton CJ, Cui Z, Pierce KA, Bherer P, Aguilar E, Powner MB, Vevis K, Boisvert M, Fu Z, Levy E, Fruttiger M, Packard A, Rezende FA, Maranda B, Sapieha P, Chen J, Friedlander M, Clish CB, Smith LEH, 2016. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat. Med 22, 439–445. 10.1038/nm.4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juen S, Kieselbach GF, 2011. Electrophysiological Changes in Juvenile Diabetics Without Retinopathy. Arch. Ophthalmol 108, 372. 10.1001/archopht.1990.01070050070033 [DOI] [PubMed] [Google Scholar]
- Karlstetter M, Nothdurfter C, Aslanidis A, Moeller K, Horn F, Scholz R, Neumann H, Weber BHF, Rupprecht R, Langmann T, 2014. Translocator protein (18 kDa) (TSPO) is expressed in reactive retinal microglia and modulates microglial inflammation and phagocytosis. J. Neuroinflammation 11. 10.1186/1742-2094-11-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T, 2015. Retinal microglia: Just bystander or target for therapy? Prog. Retin. Eye Res 45, 30–57. 10.1016/j.preteyeres.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Kawabori M, Kacimi R, Kauppinen T, Calosing C, Kim JY, Hsieh CL, Nakamura MC, Yenari MA, 2015. Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. J. Neurosci 35, 3384–3396. 10.1523/JNEUROSCI.2620-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA, 2008. Induction of Immunological Tolerance by Apoptotic Cells Requires Caspase-Dependent Oxidation of High-Mobility Group Box-1 Protein. Immunity 29, 21–32. 10.1016/j.immuni.2008.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Mun BR, Lee SJ, Joh Y, Lee HY, Ji KY, Choi HR, Lee EH, Kim EM, Jang JH, Song HW, Mook-Jung I, Choi WS, Kang HS, 2017. TREM2 promotes Aβ phagocytosis by upregulating C/EBPα-dependent CD36 expression in microglia. Sci. Rep 7, 1–12. 10.1038/s41598-017-11634-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, 1989. The biology of interleukin-6. Blood 74, 1–10. [PubMed] [Google Scholar]
- Klein R, Klein BEK, Moss SE, Wong TY, 2007. Retinal Vessel Caliber and Microvascular and Macrovascular Disease in Type 2 Diabetes. XXI: The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology 114, 1884–1892. 10.1016/j.ophtha.2007.02.023 [DOI] [PubMed] [Google Scholar]
- Ko F, Vitale S, Chou CF, Cotch MF, Saaddine J, Friedman DS, 2012. Prevalence of nonrefractive visual impairment in US adults and associated risk factors, 1999–2002 and 2005–2008. JAMA - J. Am. Med. Assoc 308, 2361–2368. 10.1001/jama.2012.85685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohner EM, Stratton IM, Aldington SJ, Turner RC, Matthews DR, 1999. Microaneurysms in the development of diabetic retinopathy (UKPDS 42). Diabetologia 42, 1107–1112. 10.1007/s001250051278 [DOI] [PubMed] [Google Scholar]
- Kong D, Gong L, Arnold E, Shanmugam S, Fort PE, Gardner TW, Abcouwer SF, 2016. Insulin-like growth factor 1 rescues R28 retinal neurons from apoptotic death through ERK-mediated BimEL phosphorylation independent of Akt. Exp. Eye Res 151, 82–95. 10.1016/j.exer.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooragayala K, Gotoh N, Cogliati T, Nellissery J, Kaden TR, French S, Balaban R, Li W, Covian R, Swaroop A, 2015. Quantification of oxygen consumption in retina ex vivo demonstrates limited reserve capacity of photoreceptor mitochondria. Investig. Ophthalmol. Vis. Sci 56, 8428–8436. 10.1167/iovs.15-17901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krady JK, Basu A, Allen CM, Xu Y, LaNoue KF, Gardner TW, Levison SW, 2005. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes 54, 1559–65. [DOI] [PubMed] [Google Scholar]
- Kuiper EJ, Witmer AN, Klaassen I, Oliver N, Goldschmeding R, Schlingemann RO, 2004. Differential expression of connective tissue growth factor in microglia and pericytes in the human diabetic retina. Br. J. Ophthalmol 88, 1082–1087. 10.1136/bjo.2003.032045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kur J, Newman EA, Chan-Ling T, 2012. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog. Retin. Eye Res 31, 377–406. 10.1016/j.preteyeres.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AKW, Lo ACY, 2013. Animal models of diabetic retinopathy: summary and comparison. J. Diabetes Res 2013, 106594. 10.1155/2013/106594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang GE, 2012. Diabetic macular edema. Ophthalmologica 227, 21–29. 10.1159/000337156 [DOI] [PubMed] [Google Scholar]
- Lebrun-Julien F, Barker PA, Morales CR, Di Polo A, De Backer O, Stellwagen D, Bertrand MJ, 2010. ProNGF induces TNFα-dependent death of retinal ganglion cells through a p75 NTR non-cell-autonomous signaling pathway. Proc. Natl. Acad. Sci 107, 3817–3822. 10.1073/pnas.0909276107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecleire-Collet A, Audo I, Aout M, Girmens JF, Sofroni R, Erginay A, le Gargasson JF, Mohand-Saïd S, Meas T, Guillausseau PJ, Vicaut E, Paques M, Massin P, 2011. Evaluation of retinal function and flicker light-induced retinal vascular response in normotensive patients with diabetes without retinopathy. Investig. Ophthalmol. Vis. Sci 52, 2861–2867. 10.1167/iovs.10-5960 [DOI] [PubMed] [Google Scholar]
- Lee JE, Liang KJ, Fariss RN, Wong WT, 2008. Ex vivo dynamic imaging of retinal microglia using time-lapse confocal microscopy. Investig. Ophthalmol. Vis. Sci 49, 4169–4176. 10.1167/iovs.08-2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann U, Heuss ND, McPherson SW, Roehrich H, Gregerson DS, 2010. Dendritic cells are early responders to retinal injury. Neurobiol. Dis 40, 177–184. 10.1016/j.nbd.2010.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AV, 2009. Retinal hemorrhages: advances in understanding. Pediatr. Clin. North Am 56, 333–344. 10.1016/j.pcl.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Lewis GP, Sethi CS, Carter KM, Charteris DG, Fisher SK, 2005. Microglial cell activation following retinal detachment: A comparison between species. Mol. Vis 11, 491–500. [PubMed] [Google Scholar]
- Li W, Germain RN, Gerner MY, 2017. Multiplex, quantitative cellular analysis in large tissue volumes with clearing-enhanced 3D microscopy (Ce3D). Proc. Natl. Acad. Sci. U. S. A 114, E7321–E7330. 10.1073/pnas.1708981114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Smith D, Li Q, Sheibani N, Huang S, Kern T, Nagaraj RH, Lin F, 2012. Antibody-mediated retinal pericyte injury: Implications for diabetic retinopathy. Investig. Ophthalmol. Vis. Sci 53, 5520–5526. 10.1167/iovs.12-10010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM, 1998. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes 47, 815–20. [DOI] [PubMed] [Google Scholar]
- Lim RR, Hainsworth DP, Mohan RR, Chaurasia SS, 2019. Characterization of a functionally active primary microglial cell culture from the pig retina. Exp. Eye Res 185, 107670. 10.1016/j.exer.2019.05.010 [DOI] [PubMed] [Google Scholar]
- Lindsay KJ, Du J, Sloat SR, Contreras L, Linton JD, Turner SJ, Sadilek M, Satrústegui J, Hurley JB, 2014. Pyruvate kinase and aspartate-glutamate carrier distributions reveal key metabolic links between neurons and glia in retina. Proc. Natl. Acad. Sci. U. S. A 111, 15579–15584. 10.1073/pnas.1412441111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Steinle JJ, 2017. Loss of TLR4 in mouse Müller cells inhibits both MyD88-dependent and –independent signaling. PLoS One 12, 1–8. 10.1371/journal.pone.0190253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ye F, Xiong H, Hu D-N, Limb GA, Xie T, Peng L, Zhang P, Wei Y, Zhang W, Wang J, Wu H, Lee P, Song E, Zhang DY, 2015. IL-1β induces IL-6 production in retinal Müller cells predominantly through the activation of p38 MAPK/NF-κB signaling pathway. Exp. Cell Res 331, 223–31. 10.1016/j.yexcr.2014.08.040 [DOI] [PubMed] [Google Scholar]
- Liu X, Ye F, Xiong H, Hu D, Limb GA, Xie T, Peng L, Yang W, Sun Y, Zhou M, Song E, Zhang DY, 2014. IL-1β Upregulates IL-8 Production in Human Müller Cells Through Activation of the p38 MAPK and ERK1/2 Signaling Pathways. Inflammation 37, 1486–95. 10.1007/s10753-014-9874-5 [DOI] [PubMed] [Google Scholar]
- Liu Yang, Biarnés Costa M, Gerhardinger C, 2012. IL-1β is upregulated in the diabetic retina and retinal vessels: cell-specific effect of high glucose and IL-1β autostimulation. PLoS One 7, e36949. 10.1371/journal.pone.0036949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Yanling, Leo LF, McGregor C, Grivitishvili A, Barnstable CJ, Tombran-Tink J, 2012. Pigment epithelium-derived factor (PEDF) peptide eye drops reduce inflammation, cell death and vascular leakage in diabetic retinopathy in Ins2(Akita) mice. Mol. Med 18, 1387–401. 10.2119/molmed.2012.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott ME, Slocomb JE, Gao Z, Gabbay RA, Quillen D, Gardner TW, Bettermann K, 2015. Impaired coronary and retinal vasomotor function to hyperoxia in Individuals with Type 2 diabetes. Microvasc. Res 101, 1–7. 10.1016/j.mvr.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott MEJ, Slocomb JE, Shivkumar V, Smith B, Gabbay RA, Quillen D, Gardner TW, Bettermann K, 2012. Comparison of retinal vasodilator and constrictor responses in type 2 diabetes. Acta Ophthalmol 90, 434–441. 10.1111/j.1755-3768.2012.02445.x [DOI] [PubMed] [Google Scholar]
- Loukovaara S, Nurkkala H, Tamene F, Gucciardo E, Liu X, Repo P, Lehti K, Varjosalo M, 2015. Quantitative Proteomics Analysis of Vitreous Humor from Diabetic Retinopathy Patients. J. Proteome Res 14, 5131–5143. 10.1021/acs.jproteome.5b00900 [DOI] [PubMed] [Google Scholar]
- Lu M, Perez VL, Ma N, Miyamoto K, Peng HB, Liao JK, Adamis AP, 1999. VEGF increases retinal vascular ICAM-1 expression in vivo. Invest. Ophthalmol. Vis. Sci 40, 1808–12. [PubMed] [Google Scholar]
- Lueck K, Wasmuth S, Williams J, Hughes TR, Morgan BP, Lommatzsch A, Greenwood J, Moss SE, Pauleikhoff D, 2011. Sub-lytic C5b-9 induces functional changes in retinal pigment epithelial cells consistent with age-related macular degeneration. Eye 25, 1074–1082. 10.1038/eye.2011.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Chen M, Xu H, 2011. Complement gene expression and regulation in mouse retina and retinal pigment epithelium / choroid 3, 1588–1597. [PMC free article] [PubMed] [Google Scholar]
- Luo C, Zhao J, Madden A, Chen M, Xu H, 2013. Complement expression in retinal pigment epithelial cells is modulated by activated macrophages. YEXER 112, 93–101. 10.1016/j.exer.2013.04.016 [DOI] [PubMed] [Google Scholar]
- Marín-Teva JL, Dusart I, Colin C, Gervais A, Van Rooijen N, Mallat M, 2004. Microglia Promote the Death of Developing Purkinje Cells. Neuron 41, 535–547. 10.1016/S0896-6273(04)00069-8 [DOI] [PubMed] [Google Scholar]
- Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB, 2004. Death of retinal neurons in streptozotocin-induced diabetic mice. Investig. Ophthalmol. Vis. Sci 45, 3330–3336. 10.1167/iovs.04-0247 [DOI] [PubMed] [Google Scholar]
- Mato M, Ookawara S, Sakamoto A, Aikawa E, Ogawa T, Mitsuhashi U, Masuzawa T, Suzuki H, Honda M, Yazaki Y, Watanabe E, Luoma J, Yla-Herttuala S, Fraser I, Gordon S, Kodama T, 1996. Involvement of specific macrophage-lineage cells surrounding arterioles in barrier and scavenger function in brain cortex. Proc. Natl. Acad. Sci. U. S. A 93, 3269–3274. 10.1073/pnas.93.8.3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matragoon S, Al-Gayyar MMH, Mysona BA, Abdelsaid MA, Pillai BA, Neet KE, Fagan SC, El-Remessy AB, 2012. Electroporation-mediated gene delivery of cleavage-resistant pro-nerve growth factor causes retinal neuro- and vascular degeneration. Mol. Vis 18, 2993–3003. [PMC free article] [PubMed] [Google Scholar]
- Mazzeo A, Arroba AI, Beltramo E, Valverde AM, Porta M, 2017. Somatostatin protects human retinal pericytes from inflammation mediated by microglia. Exp. Eye Res 164, 46–54. 10.1016/j.exer.2017.07.011 [DOI] [PubMed] [Google Scholar]
- McGettrick AF, O’Neill LAJ, 2020. The Role of HIF in Immunity and Inflammation. Cell Metab 32, 524–536. 10.1016/j.cmet.2020.08.002 [DOI] [PubMed] [Google Scholar]
- McLeod DS, Lefer DJ, Merges C, Lutty GA, 1995. Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am. J. Pathol 147, 642–53. [PMC free article] [PubMed] [Google Scholar]
- McMenamin PG, Djano J, Wealthall R, Griffin BJ, 2002. Characterization of the macrophages associated with the tunica vasculosa lentis of the rat eye. Investig. Ophthalmol. Vis. Sci 43, 2076–2082. [PubMed] [Google Scholar]
- McMenamin PG, Saban DR, Dando SJ, 2019. Immune cells in the retina and choroid: Two different tissue environments that require different defenses and surveillance. Prog. Retin. Eye Res 70, 85–98. 10.1016/j.preteyeres.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVicar CM, Hamilton R, Colhoun LM, Gardiner TA, Brines M, Cerami A, Stitt AW, 2011. Intervention with an erythropoietin-derived peptide protects against neuroglial and vascular degeneration during diabetic retinopathy. Diabetes 60, 2995–3005. 10.2337/db11-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, 2008. Origin and physiological roles of inflammation. Nature 454, 428–435. 10.1038/nature07201 [DOI] [PubMed] [Google Scholar]
- Mendes-Jorge L, Ramosspi-Sup D, Luppo M, Llombart C, Alexandre-Pires G, Nacher V, Melgarejo V, Correia M, Carretero A, Tafurospi-Sup S, Rodriguez-Baeza A, Esperanca-Pina JA, Bosch F, Ruberte J, 2009. Scavenger function of resident autofluorescent perivascular macrophages and their contribution to the maintenance of the blood-retinal barrier. Investig. Ophthalmol. Vis. Sci 50, 5997–6005. 10.1167/iovs.09-3515 [DOI] [PubMed] [Google Scholar]
- Metea MR, Newman EA, 2007. Signalling within the neurovascular unit in the mammalian retina. Exp. Physiol 92, 635–640. 10.1113/expphysiol.2006.036376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midena E, Micera A, Frizziero L, Pilotto E, Esposito G, Bini S, 2019. Sub-threshold micropulse laser treatment reduces inflammatory biomarkers in aqueous humour of diabetic patients with macular edema. Sci. Rep 9, 1–9. 10.1038/s41598-019-46515-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic I, Weick M, Wiedemann P, Reichenbach A, Bringmann A, 2004. Neuropeptide Y-evoked proliferation of retinal glial (Müller) cells. Graefe’s Arch. Clin. Exp. Ophthalmol 242, 944–950. 10.1007/s00417-004-0954-3 [DOI] [PubMed] [Google Scholar]
- Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL, 2011. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res 108, 235–248. 10.1161/CIRCRESAHA.110.223875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Khosrof S, Bursell SE, Moromizato Y, Aiello LP, Ogura Y, Adamis AP, 2000. Vascular endothelial growth factor (VEGF)-induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1). Am. J. Pathol 156, 1733–9. 10.1016/S0002-9440(10)65044-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, Aiello LP, Ogura Y, Adamis AP, 1999. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc. Natl. Acad. Sci. U. S. A 96, 10836–41. 10.1073/pnas.96.19.10836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M, Gerhardinger C, Lorenzi M, 1998. Muller cell changes in human diabetic retinopathy. Diabetes 47, 445–449. 10.2337/diabetes.47.3.445 [DOI] [PubMed] [Google Scholar]
- Mocan MC, Kadayifcilar S, Eldem B, 2006. Elevated intravitreal interleukin-6 levels inMocan, M. C., Kadayifcilar, S., & Eldem, B. (2006).Elevated intravitreal interleukin-6 levels in patients with proliferative diabetic retinopathy. Canadian Journal of Ophthalmology. Journal Canadien D’ophtalmo. Can. J. Ophthalmol 41, 747–52. 10.3129/i06-070 [DOI] [PubMed] [Google Scholar]
- Mohamed R, Coucha M, Elshaer SL, Artham S, Lemtalsi T, El-Remessy AB, 2018. Inducible overexpression of endothelial proNGF as a mouse model to study microvascular dysfunction. Biochim. Biophys. Acta - Mol. Basis Dis 1864, 746–757. 10.1016/j.bbadis.2017.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio V, Persson PB, Sendeski MM, 2014. The neurovascular unit - concept review. Acta Physiol 210, 790–798. 10.1111/apha.12250 [DOI] [PubMed] [Google Scholar]
- Murakami Y, Ishikawa K, Nakao S, Sonoda KH, 2020. Innate immune response in retinal homeostasis and inflammatory disorders. Prog. Retin. Eye Res 74, 100778. 10.1016/j.preteyeres.2019.100778 [DOI] [PubMed] [Google Scholar]
- Muramatsu D, Wakabayashi Y, Usui Y, Okunuki Y, Kezuka T, Goto H, 2013. Correlation of complement fragment C5a with inflammatory cytokines in the vitreous of patients with proliferative diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol 251, 15–17. 10.1007/s00417-012-2024-6 [DOI] [PubMed] [Google Scholar]
- Murinello S, Usui Y, Sakimoto S, Kitano M, Aguilar E, Friedlander HM, Schricker A, Wittgrove C, Wakabayashi Y, Dorrell MI, Westenskow PD, Friedlander M, 2019. miR-30a-5p inhibition promotes interaction of Fas+ endothelial cells and FasL+ microglia to decrease pathological neovascularization and promote physiological angiogenesis. Glia 67, 332–344. 10.1002/glia.23543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugeswari P, Shukla D, Rajendran A, Kim R, Namperumalsamy P, Muthukkaruppan V, 2008. Proinflammatory cytokines and angiogenic and anti-angiogenic factors in vitreous of patients with proliferative diabetic retinopathy and eales’ disease. Retina 28, 817–824. 10.1097/IAE.0b013e31816576d5 [DOI] [PubMed] [Google Scholar]
- Mysona BA, Shanab AY, Elshaer SL, El-Remessy AB, 2014. Nerve growth factor in diabetic retinopathy: Beyond neurons. Expert Rev. Ophthalmol 9, 99–107. 10.1586/17469899.2014.903157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahomi RB, Sampathkumar S, Myers AM, Elghazi L, Smith DG, Tang J, Lee CA, Kern TS, Nagaraj RH, Fort PE, 2018. The Absence of Indoleamine 2,3-Dioxygenase Inhibits Retinal Capillary Degeneration in Diabetic Mice. Invest. Ophthalmol. Vis. Sci 59, 2042–2053. 10.1167/iovs.17-22702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz MI, Van Raemdonck K, Mohammad G, Kangave D, Van Damme J, Abu El-Asrar AM, Struyf S, 2013. Autocrine CCL2, CXCL4, CXCL9 and CXCL10 signal in retinal endothelial cells and are enhanced in diabetic retinopathy. Exp. Eye Res 109, 67–76. 10.1016/j.exer.2013.01.008 [DOI] [PubMed] [Google Scholar]
- Newman EA, 2015. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos. Trans. R. Soc. B Biol. Sci 370, 1–9. 10.1098/rstb.2014.0195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, 2013. Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. J. Cereb. Blood Flow Metab 33, 1685–1695. 10.1038/jcbfm.2013.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng JS, Bearse MA, Schneck ME, Barez S, Adams AJ, 2008. Local Diabetic Retinopathy Prediction by Multifocal ERG Delays over 3 Years. Investig. Ophthalmol. Vis. Sci 49, 1622–1628. 10.1167/iovs.07-1157 [DOI] [PubMed] [Google Scholar]
- Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, Boyer D, Heier JS, Abraham P, Thach AB, Lit ES, Foster BS, Kruger E, Dugel P, Chang T, Das A, Ciulla TA, Pollack JS, Lim JI, Eliot D, Campochiaro PA, 2010. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology 117, 2146–2151. 10.1016/j.ophtha.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F, 2005. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo — Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo — SuppoNimmerjahn, Axel, Frank Kirchhoff, and Fritjof Helmchen. 2005. “Resting Micr 308, 1314–1319. 10.1126/science.1110647 [DOI] [PubMed] [Google Scholar]
- Noda K, Nakao S, Ishida S, Ishibashi T, 2012. Leukocyte adhesion molecules in diabetic retinopathy. J. Ophthalmol 2012. 10.1155/2012/279037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda K, Nakao S, Zandi S, Sun D, Hayes KC, Hafezi-Moghadam A, 2014. Retinopathy in a novel model of metabolic syndrome and type 2 diabetes: new insight on the inflammatory paradigm. FASEB J 28, 2038–46. 10.1096/fj.12-215715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LAJ, Pearce EJ, 2016. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med 213, 15–23. 10.1084/jem.20151570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberstein SYL, Byun J, Herrera D, Chapin EA, Fisher SK, Lewis GP, 2011. Cell proliferation in human epiretinal membranes: Characterization of cell types and correlation with disease condition and duration. Mol. Vis 17, 1794–1805. [PMC free article] [PubMed] [Google Scholar]
- Ogura S, Kurata K, Hattori Y, Takase H, Ishiguro-Oonuma T, Hwang Y, Ahn S, Park I, Ikeda W, Kusuhara S, Fukushima Y, Nara H, Sakai H, Fujiwara T, Matsushita J, Ema M, Hirashima M, Minami T, Shibuya M, Takakura N, Kim P, Miyata T, Ogura Y, Uemura A, 2017. Sustained inflammation after pericyte depletion induces irreversible blood-retina barrier breakdown. JCI Insight 2, 1–22. 10.1172/jci.insight.90905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okunuki Y, Mukai R, Pearsall EA, Klokman G, Husain D, Park DH, Korobkina E, Weiner HL, Butovsky O, Ksander BR, Miller JW, Connor KM, 2018. Microglia inhibit photoreceptor cell death and regulate immune cell infiltration in response to retinal detachment. Proc. Natl. Acad. Sci. U. S. A 115, E6264–E6273. 10.1073/pnas.1719601115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ola MS, Nawaz MI, El-Asrar AA, Abouammoh M, Alhomida AS, 2013. Reduced levels of brain derived neurotrophic factor (BDNF) in the serum of diabetic retinopathy patients and in the retina of diabetic rats. Cell. Mol. Neurobiol 33, 359–67. 10.1007/s10571-012-9901-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares AM, Althoff K, Chen GF, Wu S, Morrisson MA, DeAngelis MM, Haider N, 2017. Animal Models of Diabetic Retinopathy. Curr. Diab. Rep 17, 93. 10.1007/s11892-017-0913-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori K, Nagata N, Kurata K, Fukushima Y, Sekihachi E, Fujii N, Namba-Hamano T, Takabatake Y, Fruttiger M, Nagasawa T, Uemura A, Murata T, 2018. Inhibition of stromal cell-derived factor-1α/CXCR4 signaling restores the blood-retina barrier in pericyte-deficient mouse retinas. JCI insight 3, 1–10. 10.1172/jci.insight.120706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaadon P, Fagan XJ, Lifshitz T, Levy J, 2014. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye 28, 510–520. 10.1038/eye.2014.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou K, Copland DA, Theodoropoulou S, Mertsch S, Li Y, Liu J, Schrader S, Liu L, Dick AD, 2020. Treatment of diabetic retinopathy through neuropeptide Y-mediated enhancement of neurovascular microenvironment. J. Cell. Mol. Med 24, 3958–3970. 10.1111/jcmm.15016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou K, Mertsch S, Theodoropoulou S, Wu J, Liu J, Copland DA, Schrader S, Liu L, Dick AD, 2019. Restoring retinal neurovascular health via substance P. Exp. Cell Res 380, 115–123. 10.1016/j.yexcr.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özay Y, Ozek D, Yıldırım F, Yıldırım Z, 2020. The effect of diabetes on vitreous levels of adiponectin and inflammatory cytokines in experimental rat model. Adv. Clin. Exp. Med 29, 449–452. 10.17219/acem/115004 [DOI] [PubMed] [Google Scholar]
- Paglinawan R, Malipiero U, Schlapbach R, Frei K, Reith W, Fontana A, 2003. TGFβ Directs Gene Expression of Activated Microglia to an Anti-Inflammatory Phenotype Strongly Focusing on Chemokine Genes and Cell Migratory Genes. Glia 44, 219–231. 10.1002/glia.10286 [DOI] [PubMed] [Google Scholar]
- Pannicke T, Iandiev I, Wurm A, Uckermann O, vom Hagen F, Reichenbach A, Wiedemann P, Hammes H-P, Bringmann A, 2006. Diabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retina. Diabetes 55, 633–9. 10.2337/diabetes.55.03.06.db05-1349 [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT, 2011. Synaptic pruning by microglia is necessary for normal brain development. Science (80-.) 333, 1456–1458. 10.1126/science.1202529 [DOI] [PubMed] [Google Scholar]
- Park JH, Kim JE, Gu JY, Yoo HJ, Park SH, Kim YI, Nam-Goong IS, Kim ES, Kim HK, 2016. Evaluation of circulating markers of neutrophil extracellular trap (NET) formation as risk factors for diabetic retinopathy in a case-control association study. Exp. Clin. Endocrinol. Diabetes 124, 557–561. 10.1055/s-0042-101792 [DOI] [PubMed] [Google Scholar]
- Patel JI, Saleh GM, Hykin PG, Gregor ZJ, Cree IA, 2008. Concentration of haemodynamic and inflammatory related cytokines in diabetic retinopathy. Eye 22, 223–228. 10.1038/sj.eye.6702584 [DOI] [PubMed] [Google Scholar]
- Pereiro X, Ruzafa N, Acera A, Fonollosa A, David Rodriguez F, Vecino E, 2018. Dexamethasone protects retinal ganglion cells but not Müller glia against hyperglycemia in vitro. PLoS One 13, 1–17. 10.1371/journal.pone.0207913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit L, Ma S, Cipi J, Cheng SY, Zieger M, Hay N, Punzo C, 2018. Aerobic Glycolysis Is Essential for Normal Rod Function and Controls Secondary Cone Death in Retinitis Pigmentosa. Cell Rep 23, 2629–2642. 10.1016/j.celrep.2018.04.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picconi F, Parravano M, Sciarretta F, Fulci C, Nali M, Frontoni S, Varano M, Maria Caccuri A, 2019. Activation of retinal Müller cells in response to glucose variability. Endocrine 65, 542–549. 10.1007/s12020-019-02017-5 [DOI] [PubMed] [Google Scholar]
- Platania CBM, Lazzara F, Fidilio A, Fresta CG, Conti F, Giurdanella G, Leggio GM, Salomone S, Drago F, Bucolo C, 2019. Blood-retinal barrier protection against high glucose damage: The role of P2X7 receptor. Biochem. Pharmacol 168, 249–258. 10.1016/j.bcp.2019.07.010 [DOI] [PubMed] [Google Scholar]
- Power RM, Huisken J, 2017. A guide to light-sheet fluorescence microscopy for multiscale imaging. Nat. Methods 14, 360–373. 10.1038/nmeth.4224 [DOI] [PubMed] [Google Scholar]
- Puro DG, 2002. Diabetes-induced dysfunction of retinal Müller cells. Trans. Am. Ophthalmol. Soc 100, 339–52. [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Hisatomi T, Sonoda K-H, Kura S, Sassa Y, Kinoshita S, Nakamura T, Sakamoto T, Ishibashi T, 2005. The characterisation of hyalocytes: the origin, phenotype, and turnover. Br. J. Ophthalmol 89, 513–7. 10.1136/bjo.2004.050658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Goldfine A, Krumrei N, Grubissich L, Acosta J, Chorev M, Hays AP, Halperin JA, 2004. Glycation inactivation of the complement regulatory protein CD59: a possible role in the pathogenesis of the vascular complications of human diabetes. Diabetes 53, 2653–61. [DOI] [PubMed] [Google Scholar]
- Raczyńska D, Lisowska KA, Pietruczuk K, Borucka J, Ślizień M, Raczyńska K, Glasner L, Witkowski JM, 2018. The Level of Cytokines in the Vitreous Body of Severe Proliferative Diabetic Retinopathy Patients Undergoing Posterior Vitrectomy. Curr. Pharm. Des 24, 3276–3281. 10.2174/1381612824666180926110704 [DOI] [PubMed] [Google Scholar]
- Rangasamy S, Mcguire PG, Nitta CF, Monickaraj F, Oruganti SR, Das A, 2014. Chemokine Mediated Monocyte Trafficking into the Retina : Role of Inflammation in Alteration of the Blood- Retinal Barrier in Diabetic Retinopathy 9, 1–10. 10.1371/journal.pone.0108508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, 2016. A polarizing question: Do M1 and M2 microglia exist. Nat. Neurosci 19, 987–991. 10.1038/nn.4338 [DOI] [PubMed] [Google Scholar]
- Rasmussen KL, Nordestgaard BG, Nielsen SF, 2018. Complement C3 and Risk of Diabetic Microvascular Disease: A Cohort Study of 95202 Individuals from the General Population. Clin. Chem 64, 1113–1124. 10.1373/clinchem.2018.287581 [DOI] [PubMed] [Google Scholar]
- Rathnasamy G, Foulds WS, Ling EA, Kaur C, 2019. Retinal microglia – A key player in healthy and diseased retina. Prog. Neurobiol 173, 18–40. 10.1016/j.pneurobio.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Reis A, Mateus C, Melo P, Figueira J, Cunha-Vaz J, Castelo-Branco M, 2014. Neuroretinal dysfunction with intact blood-retinal barrier and absent vasculopathy in type 1 diabetes. Diabetes 63, 3926–3937. 10.2337/db13-1673 [DOI] [PubMed] [Google Scholar]
- Ricklin D, Hajishengallis G, Yang K, Lambris JD, 2010. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. Sep;11(9):785–97. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter MR, Banin E, Moreno SK, Aguilar E, Dorrell MI, Friedlander M, 2006. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J. Clin. Invest 116, 3266–3276. 10.1172/JCI29683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumier A, Béchade C, Poncer JC, Smalla KH, Tomasello E, Vivier E, Gundelfinger ED, Triller A, Bessis A, 2004. Impaired synaptic function in the microglial KARAP/DAP12-deficient mouse. J. Neurosci 24, 11421–11428. 10.1523/JNEUROSCI.2251-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Bae E, Amin S, Kim D, 2015. Extracellular matrix, gap junctions, and retinal vascular homeostasis in diabetic retinopathy. Exp. Eye Res 133, 58–68. 10.1016/j.exer.2014.08.011 [DOI] [PubMed] [Google Scholar]
- Rübsam A, Parikh S, Fort PE, 2018. Role of inflammation in diabetic retinopathy. Int. J. Mol. Sci 19, 1–31. 10.3390/ijms19040942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruebsam A, Dulle JE, Myers AM, Sakrikar D, Green KM, Khan NW, Schey K, Fort PE, 2018. A specific phosphorylation regulates the protective role of αA-crystallin in diabetes. JCI insight 3. 10.1172/jci.insight.97919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungger-Brändle E, Dosso AA, Leuenberger PM, 2000. Glial reactivity, an early feature of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci 41, 1971–80. [PubMed] [Google Scholar]
- Ryan DG, O’Neill LAJ, 2020. Krebs Cycle Reborn in Macrophage Immunometabolism. Annu. Rev. Immunol 38, 289–313. 10.1146/annurev-immunol-081619-104850 [DOI] [PubMed] [Google Scholar]
- Sacks SH, 2010. Commentary Complement fragments C3a and C5a : The salt and pepper of the immune response 668–670. 10.1002/eji.201040355 [DOI] [PubMed] [Google Scholar]
- Sakata K, Mimura T, Funatsu H, Yamashita H, Noma H, Hori S, Nakamura S, 2004. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefe’s Arch. Clin. Exp. Ophthalmol 243, 3–8. 10.1007/s00417-004-0950-7 [DOI] [PubMed] [Google Scholar]
- Santos AR, Ribeiro L, Bandello F, Lattanzio R, Egan C, Frydkjaer-Olsen U, García-Arumí J, Gibson J, Grauslund J, Harding SP, Lang GE, Massin P, Midena E, Scanlon P, Aldington SJ, Simão S, Schwartz C, Ponsati B, Porta M, Costa MA, Hernández C, Cunha-Vaz J, Simó R, 2017. Functional and structural findings of neurodegeneration in early stages of diabetic retinopathy: Cross-sectional analyses of baseline data of the EUROCONDOR project. Diabetes 66, 2503–2510. 10.2337/db16-1453 [DOI] [PubMed] [Google Scholar]
- Santos PF, Ambrósio AF, Vindeirinho J, Cavadas C, Costa GN, 2012. Contribution of TNF receptor 1 to retinal neural cell death induced by elevated glucose. Mol. Cell. Neurosci 50, 113–123. 10.1016/j.mcn.2012.04.003 [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B, 2012. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 74, 691–705. 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J, 1988. Astrocytes in the guinea pig, horse, and monkey retina: Their occurrence coincides with the presence of blood vessels. Glia 1, 74–89. 10.1002/glia.440010109 [DOI] [PubMed] [Google Scholar]
- Scholz R, Caramoy A, Bhuckory MB, Rashid K, Chen M, Xu H, Grimm C, Langmann T, 2015. Targeting translocator protein (18 kDa) (TSPO) dampens pro-inflammatory microglia reactivity in the retina and protects from degeneration. J. Neuroinflammation 12, 1–12. 10.1186/s12974-015-0422-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder S, Palinski W, Schmid-Schönbein GW, 1991. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am. J. Pathol 139, 81–100. [PMC free article] [PubMed] [Google Scholar]
- Scott IU, Jackson GR, Quillen DA, Klein R, Liao J, Gardner TW, 2014. Effect of doxycycline vs placebo on retinal function and diabetic retinopathy progression in mild to moderate nonproliferative diabetic retinopathy: A randomized proof-of-concept clinical trial. JAMA Ophthalmol 132, 1137–1142. 10.1001/jamaophthalmol.2014.1422 [DOI] [PubMed] [Google Scholar]
- Sfikakis PP, Grigoropoulos V, Emfietzoglou I, Theodossiadis G, Tentolouris N, Delicha E, Katsiari C, Alexiadou K, Hatziagelaki E, Theodossiadis PG, 2010. Infliximab for diabetic macular edema refractory to laser photocoagulation: A randomized, double-blind, placebo-controlled, crossover, 32-week study. Diabetes Care 33, 1523–1528. 10.2337/dc09-2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahulhameed S, Vishwakarma S, Chhablani J, Tyagi M, Pappuru RR, Jakati S, Chakrabarti S, Kaur I, 2020. A Systematic Investigation on Complement Pathway Activation in Diabetic Retinopathy. Front. Immunol 11, 154. 10.3389/fimmu.2020.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Fruttiger M, Zhu L, Chung SH, Barnett NL, Kirk JK, Lee S, Coorey NJ, Killingsworth M, Sherman LS, Gillies MC, 2012. Conditional Müllercell ablation causes independent neuronal and vascular pathologies in a novel transgenic model. J. Neurosci 32, 15715–27. 10.1523/JNEUROSCI.2841-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin ES, Huang Q, Gurel Z, Sorenson CM, Sheibani N, 2014. High glucose alters retinal astrocytes phenotype through increased production of inflammatory cytokines and oxidative stress. PLoS One 9. 10.1371/journal.pone.0103148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simó R, Stitt AW, Gardner TW, 2018. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia 61, 1902–1912. 10.1007/s00125-018-4692-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaprasad S, Prevost AT, Vasconcelos JC, Riddell A, Murphy C, Kelly J, Bainbridge J, Tudor-Edwards R, Hopkins D, Hykin P, CLARITY Study Group, 2017. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, n. Lancet (London, England) 389, 2193–2203. 10.1016/S0140-6736(17)31193-5 [DOI] [PubMed] [Google Scholar]
- Sohn EH, Van Dijk HW, Jiao C, Kok PHBB, Jeong W, Demirkaya N, Garmager A, Wit F, Kucukevcilioglu M, Van Velthoven MEJJ, DeVries JH, Mullins RF, Kuehn MH, Schlingemann RO, Sonka M, Verbraak FD, Abràmoff MD, 2016. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. U. S. A 113, E2655–64. 10.1073/pnas.1522014113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Sulewski ME, Wang C, Song J, Bhuyan R, Sterling J, Clark E, Song W-C, Dunaief JL, 2017. Complement C5a receptor knockout has diminished light-induced microglia/macrophage retinal migration. Mol. Vis 23, 210–218. [PMC free article] [PubMed] [Google Scholar]
- Steinle JJ, 2020. Role of HMGB1 signaling in the inflammatory process in diabetic retinopathy. Cell. Signal 73, 109687. 10.1016/j.cellsig.2020.109687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AMM, Lambris JD, Smith SJ, John SWM, Barres BA, 2007. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 131, 1164–1178. 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Stitt AW, 2003. The role of advanced glycation in the pathogenesis of diabetic retinopathy. Exp. Mol. Pathol 75, 95–108. 10.1016/S0014-4800(03)00035-2 [DOI] [PubMed] [Google Scholar]
- Stitt AW, Curtis TM, Chen M, Medina RJ, McKay GJ, Jenkins A, Gardiner TA, Lyons TJ, Hammes HP, Simó R, Lois N, 2016. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res 51, 156–186. 10.1016/j.preteyeres.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, Keshet E, 1995. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J. Neurosci 15, 4738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein JW, Ma N, Wenkel H, Fong Ng T, Zamiri P, 2002. Immunobiology and privilege of neuronal retina and pigment epithelium transplants. Vision Res 42, 487–495. 10.1016/S0042-6989(01)00185-7 [DOI] [PubMed] [Google Scholar]
- Subauste CS, 2019. The CD40-ATP-P2X7 Receptor Pathway: Cell to Cell Cross-Talk to Promote Inflammation and Programmed Cell Death of Endothelial Cells. Front. Immunol 10, 1–9. 10.3389/fimmu.2019.02958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom JM, Hernández C, Weber SR, Zhao Y, Dunklebarger M, Tiberti N, Laremore T, Simó-Servat O, Garcia-Ramirez M, Barber AJ, Gardner TW, Simó R, 2018. Proteomic analysis of early diabetic retinopathy reveals mediators of neurodegenerative brain diseases. Investig. Ophthalmol. Vis. Sci 59, 2264–2274. 10.1167/iovs.17-23678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura A, Kaneko H, Funahashi Y, Takayama K, Nagaya M, Ito S, Okuno T, Hirakata T, Nonobe N, Kataoka K, Shimizu H, Namba R, Yamada K, Ye F, Ozawa Y, Yokomizo T, Terasaki H, 2020. N-3 fatty acid and its metabolite 18-HEPE ameliorate retinal neuronal cell dysfunction by enhancing Müller BDNF in diabetic retinopathy. Diabetes 69, 724–735. 10.2337/db19-0550 [DOI] [PubMed] [Google Scholar]
- Taylor AW, Lee D, 2010. Applications of the role of α-MSH in ocular immune privilege. Adv. Exp. Med. Biol 681, 143–149. 10.1007/978-1-4419-6354-3_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thounaojam MC, Powell FL, Patel S, Gutsaeva DR, Tawfik A, Smith SB, Nussbaum J, Block NL, Martin PM, Schally AV, Bartoli M, 2017. Protective effects of agonists of growth hormone-releasing hormone (GHRH) in early experimental diabetic retinopathy. Proc. Natl. Acad. Sci. U. S. A 114, 13248–13253. 10.1073/pnas.1718592114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treatment E, Retinopathy D, 1991a. Classification of Diabetic Retinopathy from Fluorescein Angiograms: ETDRS Report Number 11. Ophthalmology 98, 807–822. 10.1016/S0161-6420(13)38013-0 [DOI] [PubMed] [Google Scholar]
- Treatment E, Retinopathy D, 1991b. Fundus Photographic Risk Factors for Progression of Diabetic Retinopathy: ETDRS Report Number 12. Ophthalmology 98, 823–833. 10.1016/S0161-6420(13)38014-2 [DOI] [PubMed] [Google Scholar]
- Treatment E, Retinopathy D, 1991c. Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs—An Extension of the Modified Airlie House Classification: ETDRS Report Number 10. Ophthalmology 98, 786–806. 10.1016/S0161-6420(13)38012-9 [DOI] [PubMed] [Google Scholar]
- Trento M, Durando O, Lavecchia S, Charrier L, Cavallo F, Costa MA, Hernández C, Simó R, Porta M, 2017. Vision related quality of life in patients with type 2 diabetes in the EUROCONDOR trial. Endocrine 57, 83–88. 10.1007/s12020-016-1097-0 [DOI] [PubMed] [Google Scholar]
- Tsai T, Kuehn S, Tsiampalis N, Vu MK, Kakkassery V, Stute G, Dick HB, Joachim SC, 2018. Anti-inflammatory cytokine and angiogenic factors levels in vitreous samples of diabetic retinopathy patients. PLoS One 13, 1–13. 10.1371/journal.pone.0194603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrberg M, Lindblad U, Melander A, Lövestam-Adrian M, Ponjavic V, Andréasson S, 2011. Electrophysiological studies in newly onset type 2 diabetes without visible vascular retinopathy. Doc. Ophthalmol 123, 193–198. 10.1007/s10633-011-9298-6 [DOI] [PubMed] [Google Scholar]
- Vagaja NN, Chinnery HR, Binz N, Kezic JM, Rakoczy EP, McMenamin PG, 2012. Changes in murine hyalocytes are valuable early indicators of ocular disease. Investig. Ophthalmol. Vis. Sci 53, 1445–1451. 10.1167/iovs.11-8601 [DOI] [PubMed] [Google Scholar]
- Valle ML, Dworshak J, Sharma A, Ibrahim AS, Al-Shabrawey M, Sharma S, 2019. Inhibition of interleukin-6 trans-signaling prevents inflammation and endothelial barrier disruption in retinal endothelial cells. Exp. Eye Res 178, 27–36. 10.1016/j.exer.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk HW, Verbraak FD, Stehouwer M, Kok PHB, Garvin MK, Sonka M, DeVries JH, Schlingemann RO, Abràmoff MD, 2011. Association of visual function and ganglion cell layer thickness in patients with diabetes mellitus type 1 and no or minimal diabetic retinopathy. Vision Res 51, 224–228. 10.1016/j.visres.2010.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hove I, De Groef L, Boeckx B, Modave E, Hu TT, Beets K, Etienne I, Van Bergen T, Lambrechts D, Moons L, Feyen JHM, Porcu M, 2020. Single-cell transcriptome analysis of the Akimba mouse retina reveals cell-type-specific insights into the pathobiology of diabetic retinopathy. Diabetologia 63, 2235–2248. 10.1007/s00125-020-05218-0 [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB, 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–33. 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler S, Pannicke T, Hollborn M, Kolibabka M, Wiedemann P, Reichenbach A, Hammes HP, Bringmann A, 2016. Impaired Purinergic Regulation of the Glial (Müller) Cell Volume in the Retina of Transgenic Rats Expressing Defective Polycystin-2. Neurochem. Res 41, 1784–1796. 10.1007/s11064-016-1894-0 [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J, 2009. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci 29, 3974–80. 10.1523/JNEUROSCI.4363-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walport MJ, 2001. Complement. First of two parts. N. Engl. J. Med 344, 1058–66. 10.1056/NEJM200104053441406 [DOI] [PubMed] [Google Scholar]
- Wang AL, Yu ACH, He QH, Zhu X, Tso MOM, 2007. AGEs mediated expression and secretion of TNF alpha in rat retinal microglia. Exp. Eye Res 84, 905–913. 10.1016/j.exer.2007.01.011 [DOI] [PubMed] [Google Scholar]
- Wang H, Li J, Zhong P, Wang S, Zhang L, Yang R, Wu D, Chen M, Ji A, Li Y, Wang J, 2019. Blocking CXCR3 with AMG487 ameliorates the blood-retinal barrier disruption in diabetic mice through anti-oxidative. Life Sci 228, 198–207. 10.1016/j.lfs.2019.04.016 [DOI] [PubMed] [Google Scholar]
- Wang J, Xu E, Elliott MH, Zhu M, Le YZ, 2010. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes 59, 2297–2305. 10.2337/db09-1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-L, Chen H, Huang K, Zheng L, 2012. Elevated histone acetylations in Müller cells contribute to inflammation: a novel inhibitory effect of minocycline. Glia 60, 1896–905. 10.1002/glia.22405 [DOI] [PubMed] [Google Scholar]
- Wang M, Ma W, Zhao L, Fariss RN, Wong WT, 2011. Adaptive Müller cell responses to microglial activation mediate neuroprotection and coordinate inflammation in the retina. J. Neuroinflammation 8, 173. 10.1186/1742-2094-8-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wang X, Zhao L, Ma W, Rodriguez IR, Fariss RN, Wong WT, 2014. Macroglia-microglia interactions via TSPO signaling regulates microglial activation in the mouse retina. J. Neurosci 34, 3793–806. 10.1523/JNEUROSCI.3153-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wong WT, 2014. Microglia-Müller Cell Interactions in the Retina, in: Retinal Degenerative Diseases 10.1007/978-1-4614-3209-8 [DOI] [PMC free article] [PubMed]
- Wang N, Zhang C, Xu Y, Li S, Tan H-Y, Xia W, Feng Y, 2019. OMICs approaches-assisted identification of macrophages-derived MIP-1γ as the therapeutic target of botanical products TNTL in diabetic retinopathy. Cell Commun. Signal 17, 81. 10.1186/s12964-019-0396-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao L, Zhang J, Fariss RN, Ma W, Kretschmer F, Wang M, Qian H. hua, Badea TC, Diamond JS, Gan W-B, Roger JE, Wong WT, 2016. Requirement for Microglia for the Maintenance of Synaptic Function and Integrity in the Mature Retina. J. Neurosci 36, 2827–42. 10.1523/JNEUROSCI.3575-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O, 1956. On the origin of cancer cells. Science 123, 309–14. 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Raff MC, 1988. Retinal astrocytes are immigrants from the optic nerve. Nature 332, 834–7. 10.1038/332834a0 [DOI] [PubMed] [Google Scholar]
- Wilkinson CP, Ferris FL, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT, Lum F, 2003. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 110, 1677–1682. 10.1016/S0161-6420(03)00475-5 [DOI] [PubMed] [Google Scholar]
- Winkler BS, DeSantis N, Solomon F, 1986. Multiple NADPH-producing pathways control glutathione (GSH) content in retina. Exp. Eye Res 43, 829–847. 10.1016/S0014-4835(86)80013-6 [DOI] [PubMed] [Google Scholar]
- Wolff BE, Bearse MA, Schneck ME, Dhamdhere K, Harrison WW, Barez S, Adams AJ, 2015. Color vision and neuroretinal function in diabetes. Doc. Ophthalmol 130, 131–139. 10.1007/s10633-014-9476-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A, Dukic-Stefanovic S, Gasic-Milenkovic J, Schinzel R, Wiesinger H, Riederer P, Münch G, 2001. Anti-inflammatory antioxidants attenuate the expression of inducible nitric oxide synthase mediated by advanced glycation endproducts in murine microglia. Eur. J. Neurosci 14, 1961–7. 10.1046/j.0953-816x.2001.01820.x [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Sharrett AR, Schmidt MI, Pankow JS, Couper DJ, Klein BEK, Hubbard LD, Duncan BB, ARIC Investigators, 2002. Retinal arteriolar narrowing and risk of diabetes mellitus in middle-aged persons. JAMA 287, 2528–33. [DOI] [PubMed] [Google Scholar]
- World Health Organization, n.d.Diabetes [WWW Document] URL https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed 8.10.19).
- Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN, Baker CW, Berger BB, Bressler NM, Browning D, Elman MJ, Ferris FL, Friedman SM, Marcus DM, Melia M, Stockdale CR, Sun JK, Beck RW, Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN, Baker CW, Berger BB, Bressler NM, Browning D, Elman MJ, Ferris FL, Friedman SM, Marcus DM, Melia M, Stockdale CR, Sun JK, Beck RW, 2015. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA 314, 2137–46. 10.1001/jama.2015.15217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Phone A, Lamy R, Ma D, Laotaweerungsawat S, Chen Y, Zhao T, Ma W, Zhang F, Psaras C, Stewart JM, 2020. Correlation of aqueous, vitreous, and plasma cytokine levels in patients with proliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci 61, 1–3. 10.1167/iovs.61.2.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi X, Gao L, Hatala DA, Smith DG, Codispoti MC, Gong B, Kern TS, Zhang J-Z, 2005. Chronically elevated glucose-induced apoptosis is mediated by inactivation of Akt in cultured Müller cells. Biochem. Biophys. Res. Commun 326, 548–53. 10.1016/j.bbrc.2004.11.064 [DOI] [PubMed] [Google Scholar]
- Xu H, Dawson R, Forrester JV, Liversidge J, 2007. Identification of novel dendritic cell populations in normal mouse retina. Investig. Ophthalmol. Vis. Sci 48, 1701–1710. 10.1167/iovs.06-0697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhang L, Xie M, Li Y, Huang P, Saunders TL, Fox DA, Rosenquist R, Lin F, 2018. Role of Complement in a Rat Model of Paclitaxel-Induced Peripheral Neuropathy. J. Immunol 200, 4094–4101. 10.4049/jimmunol.1701716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Shen SQ, Jui J, Rupp AC, Byrne LC, Hattar S, Flannery JG, Corbo JC, Kefalov VJ, 2015. CRALBP supports the mammalian retinal visual cycle and cone vision. J. Clin. Invest 125, 727–38. 10.1172/JCI79651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CM, Su PY, Yeh PT, Chen MS, 2008. Combined rhegmatogenous and traction retinal detachment in proliferative diabetic retinopathy: Clinical manifestations and surgical outcome. Can. J. Ophthalmol 43, 192–198. 10.3129/I08-007 [DOI] [PubMed] [Google Scholar]
- Yau JWY, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BEK, Klein R, Krishnaiah S, Mayurasakorn K, O’Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Zu L, Yasuda M, Zhang X, Mitchell P, Wong TY, 2012. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35, 556–564. 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yego ECK, Vincent JA, Sarthy V, Busik JV, Mohr S, 2009. Differential regulation of high glucose-induced glyceraldehyde-3-phosphate dehydrogenase nuclear accumulation in Müller cells by IL-1beta and IL-6. Invest. Ophthalmol. Vis. Sci 50, 1920–8. 10.1167/iovs.08-2082 [DOI] [PubMed] [Google Scholar]
- Yong PH, Zong H, Medina RJ, Limb GA, Uchida K, Stitt AW, Curtis TM, 2010. Evidence supporting a role for N-(3-formyl-3,4-dehydropiperidino)lysine accumulation in Müller glia dysfunction and death in diabetic retinopathy. Mol. Vis 16, 2524–38. [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Yoshida S, Ishibashi T, Kuwano M, Inomata H, 1999. Suppression of retinal neovascularization by the NF-kappaB inhibitor pyrrolidine dithiocarbamate in mice. Invest. Ophthalmol. Vis. Sci 40, 1624–9. [PubMed] [Google Scholar]
- Yoshida S, Yoshida A, Ishibashi T, 2004. Induction of IL-8, MCP-1, and bFGF by TNF-alpha in retinal glial cells: implications for retinal neovascularization during post-ischemic inflammation. Graefes Arch. Clin. Exp. Ophthalmol 242, 409–13. 10.1007/s00417-004-0874-2 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Yoshida A, Ishibashi T, Elner SG, Elner VM, 2003. Role of MCP-1 and MIP-1a in retinal neovascularization during postischemic inflammation in a mouse model of retinal neovascularization Abstract : Macrophages are important partici- pants in neovascularization. This study was de- ischemic retinopathy , p. Situ 73, 137–144. 10.1189/jlb.0302117.Journal [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Yamagishi S-I, Matsui T, Jinnouchi Y, Fukami K, Imaizumi T, Yamakawa R, 2009. Protective role of pigment epithelium-derived factor (PEDF) in early phase of experimental diabetic retinopathy. Diabetes. Metab. Res. Rev 25, 678–86. 10.1002/dmrr.1007 [DOI] [PubMed] [Google Scholar]
- You JJ, Yang CH, Huang JS, Chen MS, Yang CM, 2007. Fractalkine, a CX3C chemokine, as a mediator of ocular angiogenesis. Investig. Ophthalmol. Vis. Sci 48, 5290–5298. 10.1167/iovs.07-0187 [DOI] [PubMed] [Google Scholar]
- You ZP, Xiong B, Zhang YL, Shi L, Shi K, 2018. Forskolin attenuates retinal inflammation in diabetic mice. Mol. Med. Rep 17, 2321–2326. 10.3892/mmr.2017.8106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun JH, Park SW, Kim KJ, Bae JS, Lee EH, Paek SH, Kim SU, Ye S, Kim JH, Cho CH, 2017. Endothelial STAT3 Activation Increases Vascular Leakage Through Downregulating Tight Junction Proteins: Implications for Diabetic Retinopathy. J. Cell. Physiol 232, 1123–1134. 10.1002/jcp.25575 [DOI] [PubMed] [Google Scholar]
- Zacks DN, Boehlke C, Richards AL, Zheng QD, 2007. Role of the fas-signaling pathway in photoreceptor neuroprotection. Arch. Ophthalmol 125, 1389–1395. 10.1001/archopht.125.10.1389 [DOI] [PubMed] [Google Scholar]
- Zechmeister-Koss I, Huić M, 2012. Vascular endothelial growth factor inhibitors (anti-VEGF) in the management of diabetic macular oedema: A systematic review. Br. J. Ophthalmol 96, 167–178. 10.1136/bjophthalmol-2011-300674 [DOI] [PubMed] [Google Scholar]
- Zeng HY, Green WR, Tso MOM, 2008. Microglial activation in human diabetic retinopathy. Arch. Ophthalmol 126, 227–232. 10.1001/archophthalmol.2007.65 [DOI] [PubMed] [Google Scholar]
- Zeng XX, Ng YK, Ling EA, 2000. Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis. Neurosci 17, 463–471. 10.1017/S0952523800173122 [DOI] [PubMed] [Google Scholar]
- Zhang J, Gerhardinger C, Lorenzi M, 2002. Early complement activation and decreased levels of glycosylphosphatidylinositol-anchored complement inhibitors in human and experimental diabetic retinopathy. Diabetes 51, 3499–504. 10.2337/diabetes.51.12.3499 [DOI] [PubMed] [Google Scholar]
- Zhang L, Bell BA, Yu M, Chan C-C, Peachey NS, Fung J, Zhang X, Caspi RR, Lin F, 2016. Complement anaphylatoxin receptors C3aR and C5aR are required in the pathogenesis of experimental autoimmune uveitis. J. Leukoc. Biol 99, 447–454. 10.1189/jlb.3A0415-157R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Lingjun, Li Y, Payne J, Srivastava S, Fan X, Fung J, Li X, Kern TS, Lin F, 2016. Presence of retinal pericyte-reactive autoantibodies in diabetic retinopathy patients. Sci. Rep 6, 20341. 10.1038/srep20341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma J, 2006. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J 20, 323–5. 10.1096/fj.05-4313fje [DOI] [PubMed] [Google Scholar]
- Zhao L, Zabel MK, Wang X, Ma W, Shah P, Fariss RN, Qian H, Parkhurst CN, Gan W, Wong WT, 2015. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol. Med 7, 1179–1197. 10.15252/emmm.201505298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XF, Wan J, Powell C, Ramachandran R, Myers MG, Goldman D, 2014. Leptin and IL-6 family cytokines synergize to stimulate Müller Glia reprogramming and retina regeneration. Cell Rep 9, 272–284. 10.1016/j.celrep.2014.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang S, Xia X, 2012. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr. Eye Res 37, 416–420. 10.3109/02713683.2012.661114 [DOI] [PubMed] [Google Scholar]
- Zipfel PF, Skerka C, 2009. Complement regulators and inhibitory proteins. Nat. Rev. Immunol 9, 729–40. 10.1038/nri2620 [DOI] [PubMed] [Google Scholar]


