Abstract
Abstract
In this work, we synthesized blood orange peel extract-copper (II) (Cu2+) ions nanoflower (NFs) and blood orange juice extract-copper (II) (Cu2+) ions nanoflower examine their antimicrobial properties on the fish pathogen (Yersinia ruckeri). The main compounds of the blood orange peel extract and the blood orange juice extract were organic components, and the copper (II) (Cu2 +) ions were inorganic components. BOPE-Cu2 + nanoflowers are quite compact, porous, and uniform as compared to BOJE-Cu2+ nanoflowers. Scanning Electron Microscopy, Fourier Transform Infrared spectrometry, and Energy-Dispersive X-ray spectroscopy were used to observe the structures of the NFs. The findings of FT-IR show Cu–O and Cu–N bonds in NF, which may be an indicator of the development of NFs. Although the antimicrobial actions of BOPE-hNFs and BOJE-hNFs against Yersinia ruckeri (NCTC 12,268) have been confirmed.
Graphic Abstract
Keywords: Blood orange, Peel, Juice, Nanoflower, Antimicrobial, Yersinia ruckeri
Introduction
Over the last two decades, nanoparticles which is synthesized using “green chemistry” methods has been important role for applying to the many fields such as biology, chemistry, engineering, physics, and materials science [1]. In this case, nanotechnology can be present many opportunities to deliver technical innovations in the development, manufacturing, storage, transport, traceability, health and protection of aquaculture and seafood sectors. In the literature, there are some paths to prevent fish against major diseases and pathogens bacteria in aquaculture such as using of antibiotics and vaccines [2]. In this case, the use of antibiotics causes pollution and leads to the development of antibiotic-resistance for pathogen bacteria in aquaculture [3]. According to researches, vaccination is one of the best ways to protect against many diseases in aquaculture with a principle of “isolate, inactivate and inject” [4]. Last decade, the use of nanotechnology in vaccinology has led to increase developing a new field as called “nanovaccinology” [5]. This can be supported by nano-chitosan since it has the ability to coil around the vaccine. Nanoparticles presents numerous advantages such as design particles with different components or compositions, particle size or morphology, and surface structures thereby affirm to community the health of farm-raised fish rather than pathogen-induced diseases. The vaccine may be delivered by nano-chitosan, which is play a role with wrapping around vaccines and acting as a carrier used for the treatment of fish physiology. Nano-encapsulated vaccines in Asian carp have been used by Bhattacharyya and colleagues to eliminate Listonella anguillarum [6]. The spread of disease is one of the main preliminary obstacles to the survival and development of aquaculture. New-fangled diseases can cause significant number of deaths for any aquaculture competence. Yersinia ruckeri is among of the pathogenic bacteria which is causative agent of enteric red mouth disease in fish [7]
Patel and colleagues were described and used hNFs using organic and inorganic components and they were demonstrated that variations in the concentration of metals and synthesis conditions of nanoflowers could be extended to efficiently immobilize recombinant his-tagged enzymes [8].
In another work, Kumar and co-workers were focused the immobilization of xylanase using a protein-inorganic hybrid nanoflower system was assessed to improve the enzyme properties. They were observed that Immobilized xylanase showed high residual activity at broad pH and temperature ranges [9]. Organic–inorganic hNFs have been reported to be used in polluted areas as biosensors, identification of Escherichia coli and glucose detection [10, 11]. Another researcher showed that hNFs based on lacto-peroxidase have been more efficient catalytic activity compare to free lacto-peroxidase and also hNFs are reusable properties before the reaction is finished [12]. In some studies, Cu-hNFs and Au-hNFs have been affected on antimicrobial activity on some specific bacteria, fungi, and virus [13, 14]. Otari et al. were developed a new approach for synthesizing metal nanoparticles which was The Au–peptide–alginate biohydrogel showed effective catalytic activity in reducing 4-nitrophenol and hexacyanoferrate(III) in the presence of sodium borohydride with durable reusability for biomedical and industrial applications [15]. After finding of NF, some enzyme groups (some of them is peroxidase, a-amylase, lipase, laccase, catalase, horseradish peroxidase etc.) have been used to provide new approaches for using in any specific field [16–22]. For example, proteins, amino acids, antioxidants, pure plant extracts, and enzymes were used as an alternative to enzymes to synthesize NFs because of fenton-like properties and antimicrobial activity [14, 19, 22–27]. For instance, Otari and co-workers were synthesized silver nanoparticles (AgNPs) using leaf extract of Canna edulis Ker-Gawl (CELE) and they obtained that the concentration of AgNPs required for 50% inhibition of growth of mammalian cells was far more than that required for inhibition of pathogenic microorganisms [28]. In similar another study, Otari and collages were emphasized that biomolecules from green tea leaves were functionalized on the surface of silicon dioxide nanoparticles (GSiO2 NPs) and they were obtained that the Ag–GSiO2 NPs displayed sustainable antimicrobial activity compared to Ag ions [29].
In this work, green synthesis of hNFs using blood orange peel and juice (as organic part of the synthesis) and Cu2 + (inorganic) components was conducted for the first time and its antimicrobial properties were analyzed against Yersinia ruckeri. BOPE-Cu-hNF and BOJE-Cu-hNF`s characterization was tested using scanning electron microscopy (SEM) scanning images, energy dispersive X-ray (EDX) spectroscopy, and Fourier transform infrared spectrometry (FT-IR). energy dispersive X-ray spectroscopy. The data collected was thought to inform to the development of studies on green produced hNFs and their aquatic applications.
Materials and Methods
Extraction of BOPE and BOJE
100 gr of peel of bloody orange were chopped to small pieces using clean knife and boiled 1:1 ratio in 100 mL pure water. The aqueous solution was filtered with Whatman paper#1. Then three of bloody orange squeezed to obtained juice of bloody orange and filtered with Whatman paer#1. All aqueous solution was kept under −20 °C for further analysis.
Preparation of Hybrid Nanoflower
Hybrid nanoflowers (hNFs) synthesis was conducted using a significantly updated approach to the 2015 Somturk process. Second, stock solution was prepared as 120 mM of CuSO4 using ultra-pure water. Subsequently, 66 μL of prepared stock solution was added to 9 mL of PBS solution (10 mM in pH 7.4) and added 0.2 mL of aqueous plant extract (blood orange peels and juice). This final solution was incubated for 72 h at room temperature (RT). After incubation, the hNFs growth process was finished and obtained blue-colored precipitate. The precipitate washed at least 3 times with pure deionized water and centrifuged at 10,000 rpm for 10 min to remove unreacted materials. The precipitate was finally dried at RT for the characterization process of hNFs.
Characterization of hNFs
The scanning electron microscopy (SEM) was used to produce nanoflower images on the ZEISS EVO LS10 instrument (Oberkochen, Germany). The energy dispersive X-ray (EDX-ZEISS EVO LS10) device was used to analyze the elemental composition of nanoflowers. Nanoflower crystal structure was described by X-ray diffraction analysis (XRD-BRUKER AXS D8) (Karlsruhe, Germany). The nanoflower spectra of the Fourier Transform Infrared Spectroscopy (FTIR) were observed to test its chemical composition by operating the FTIR Spectrometer (Perkin Elmer 400 Spotlight 400 Imaging System, Waltham, USA).
Antibacterial Activity
The agar disc diffusion test was used to examine the antibacterial effect of hNFs, as previously stated [14, 24, 30]. Briefly, hNFs at 0.5 μg/mL is used to inhibit the development of Yersinia ruckeri (NCTC 12,268). Bacterial colonies were inoculated with the same concentrations of NF (concentration of 1.0 × 108 CFU/mL) and free BOPE, free BOJE and free metallic Cu+2 ions (Huang et al. 2015). After incubation of the culture plates at 37 °C for 24 h, the inhibition area of bacterial growth was measured to be in millimeters [28]. Independent experiments were performed as triplicates for each bacterial strain.
Results and Discussion
Synthesis and Characterization of the hNFs
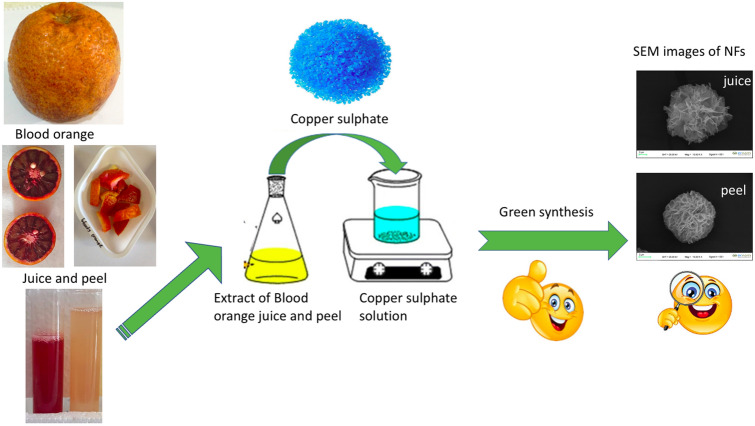
In this work, BOPE and BOJE were first used as an organic component to form flower-like structures. A variety of studies have also documented the mechanism of generation of organic–inorganic hybrid nanoflowers [8, 12, 19, 22–24, 26, 27, 29, 31, 32]. Cu-hNFs biosynthesis of blood orange peel and blood orange juice extract (Fig. 1) was performed in this study and their antimicrobial activity was evaluated. SEM images (Fig. 2) have been confirmed that the Cu-hNFs are similar to flower structures. Petal-like structures have played an important role for developing of flower structures as seen below (Figs. 2b and 3b).
Fig. 1.
hotograph of the blood orange (a), Blood orange peel (b), and extraction of both BOPE and BOJE (c)
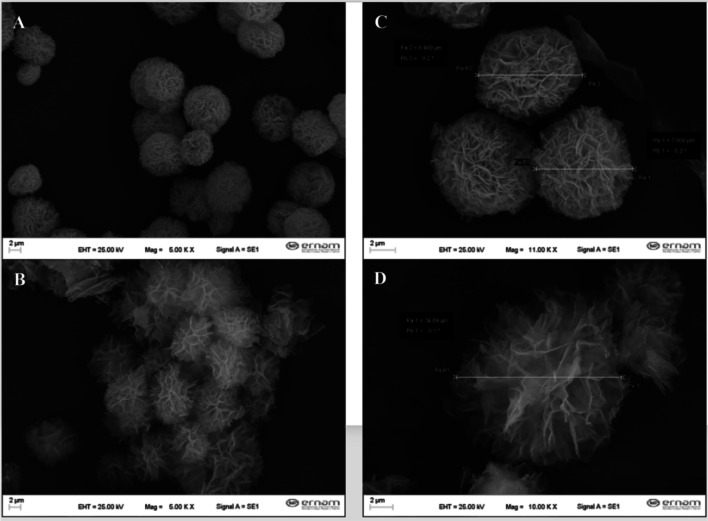
Fig. 2.
SEM images of BOPE (a, c) and BOJE-hNFs (b, d)
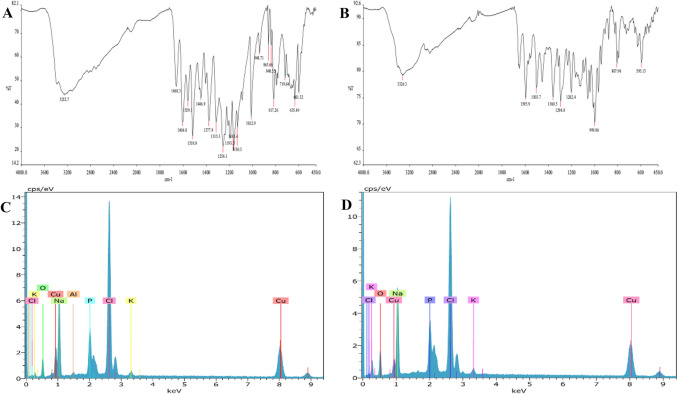
Fig. 3.
FT-IR results of BOPE (a) and BOJE-hNFs (b), EDX results of BOPE-hNFs (c) and BOJE-hNFs (d)
Besides, the effect of the outer and inner components of the same plants as the BOPE-Cu-hNFs and BOJE-hNFs was studied and observed that they were perfectly formed using extracts (Fig. 1), while the flower-shaped hNFs were perfectly formed using BOPE (Fig. 2a, c) compare to BOJE extract-based Cu-hNFs. It has also been found that flower-shaped structures have been significantly distributed in Cu-hNFs synthesized using BOJE (Fig. 2b, d). The concentration or variety of organic component, the reaction time, the pH level during the synthesis and types of inorganic compounds were played an important role on the size and shape of hNFs in previous studies [14, 15, 24]. Scientists are identified the reaction phases of hybrid nanoflowers by the interaction with the amine groups as the first step (as called nucleation) in which the amine groups and the cupper ions are connect by phosphate buffering. After this process the second step (as called growth) begins with the development of the Cu-protein is produced by the petals and then third phase (as called completion) which is known “anisotropic growth” and flower structure are completed [30]. In another study also emphasized that the medium pH is affected on the amine groups for binding metals [33].
SEM photographs showed that spherical shapes could observed with a small range of sizes. The size of BOPE-hNFs was also measured at 15 μm using SEM (Fig. 2a, c). The average size of BOJE-Cu-NFs was determined to be 7.5 μm (Fig. 2b, d) and confirms previous research [24, 30] Results of Energy-Dispersive X-ray (EDX) spectroscopy, and Fourier Transform Infrared spectrometry examination are used to evaluate the constituent morphology of the Cu-hNFs. Fourier Transform Infrared spectrometry (scanning between 400 to 4000 cm−1) were used to determine and characterized the functional groups of synthesized hNFs. In Fig. 3a, characteristic diffraction peaks of BOPE-hNFs were observed by Fourier Transform Infrared spectrometry analysis as 1193–1130 cm−1, 1606–1518 cm−1, 3252 cm−1, 1660 cm−1, 1446–1315 cm−1, 1256 cm−1, which are refers to alkylhalides (CF), alkenes (C = C), amine salt (NH), phenols (OH), and alcohols (CO) the band vibrations, respectively [24, 30]. The other peaks of BOPE-hNFs determined in the spectrum at 1012 cm−1, 940 cm−1, 863 cm−1, 840 cm−1, 817 cm−1, 719 cm−1, 635 cm−1, 601 cm−1 correspond to the vibration of phosphate groups (PO43−) [24, 25, 34–36]. For BOJE-hNFs, Characteristic diffraction peaks were determined as 1360 cm−1, 3326 cm−1, 1595 cm−1, 1503 cm−1, 1202 cm−1, 1294 cm−1, those peaks are also referred to phenol (OH), amine salt (NH), alkenes (C = C), alcohol (CO), and alkylhalides (CF) respectively as seen in Fig. 3b [24, 30]. The other BOPE-hNF peaks found at 999, 86 cm−1, 807 cm−1, 595 cm−1 refer to the phosphate group vibration (PO43−) similar to BOPE-hNFs [24, 25, 34, 35]. The FT-IR study concludes that Cu-hNFs based on BOPE and BOJE have been synthesized in the PBS buffer. The chemical composition of the Cu-NFs based on BOPE and BOJE was examined using an EDX analysis. Thus, the EDX spectrum has been confirmed the presence of Cu in the hNF skeleton as seen in Fig. 3. Cu-hNFs based on BOPE have a spherical structure and a diameter of 7.5 μm have been successfully synthesized in the PBS buffer according to the characterization findings.
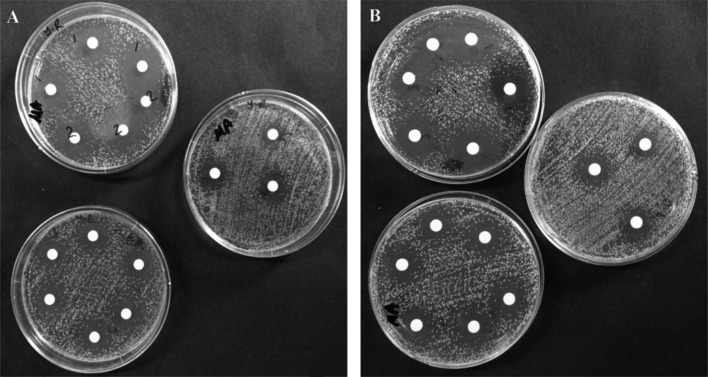
Antimicrobial effects of free CuSO4, free plant extract and BOPE-Cu-hNFs and BOJE-Cu-hNFs have been extensively checked against Yersinia ruckeri. The DISC content of hNFs was estimated at a concentration of 10 lg/mL for all microorganisms measured as shown in Table 1. BOPE and BOJE-based hNFs were inhibited Y. Ruckeri as 33.66 and 30.66 mm, respectively (Table 1). The inhibition zones of BOPE and BOJE free plant extracts were estimated at 8.33 and 9.00 respectively. Free dry metallic cupper has also been showed antimicrobial properties as observed at 25.00 mm [37]. The photographs of the inhibition region were seen clearly in Fig. 4. Coca and colleagues were tested the antimicrobial activity of allicin-based hybrid nanoflowers on the three types of bacteria including L garvieae, A hydrophile and V parahaemolyticus and registered MIC values at 15 lg/mL for all microorganisms. The antimicrobial properties were tested against Escherichia coli, Bacillus subtilis, Saccharomyces cerevisiae and Candida tropicalis by Patel and colleagues 2018 [38]. They observed that the activities were increased with increasing cationic charges and the length of the alkyl chain as follows amino-chitosan, dimethylaminoethyl-chitosan, dimethylpropyl amino-chitosan, dimethylamino-1-propyl-chitosan, diethylaminoethyl (DEAE)-chitosan, and quaternized DEAE-chitosan [38]. In another study, Beyene and co-workers were examined the effect of Au-hNF. Sreedharan and colleges were compromised the antimicrobial effects of Au-hybrid nanoflowers and Au-nanoparticles [36]. They were worked on some microorganism to show effect of different concentration of ciprofloxacin and Au-hNFs and they emphasized this situation related to wide region of Au-hNFs and the improved capability of the amine groups to bind to NFs. In another study, researchers clarified that the antimicrobial activity of Zn-NFs by the bonding of NF-released Zn ion to the cell wall and the formation of H2O2 resulting from its bonding to ZnO [39]. Cu2 + ions in hNFs contain Cu1 + ions in the presence of H2O2 that cause Cu1 + hydrogen radicals which has an important role for destroying the bacterial membrane and cells [24].
Table 1.
Antimicrobial activity (inhibition zone (mm)) of BOPE-hNFs, BOJE-hNFs and plant extracts on Yersinia ruckeri
| hNFs, plant extract. and Cu2 + (pure) | Inhibition Zone (mm) SD | Content (μg/mL) |
|---|---|---|
| BOPE- hNFs | 33.66 ± 1.52 | 0.5 |
| BOJE- hNFs | 30.66 ± 1.15 | 0.5 |
| BOPE- extract | 8.33 ± 1.9 | 0.5 |
| BOJE- extract | 9 ± 1.5 | 0.5 |
| Cu+2 (pure) | 25 ± 1.1 | 0.5 |
SD (Standard Deviation)
Fig. 4.
Photography of antimicrobial effects of BOPE and BOJE on Yersinia ruckeri
Conclusion
In total, orange peel extract and juice extract as organic components and Cu2 + ions as inorganic compounds were used for the synthesis of hNFs using a green synthesis process. The NFs obtained were well scattered, uniform and spherical. The sum of BOPE and BOJE influenced the scale of the NFs. The pores on the surface of the NFs almost vanished when the volume of BOPE was increased during the quick and economical synthesis protocol. The hNFs demonstrated higher antimicrobial activity than the free BOPE and BOJE anti-Yersinia ruckeri extract (NCTC 12,268). Both BOPE-Cu2 + hNFs and BOJE-Cu2 + hNFs demonstrated high antimicrobial activity against Y. ruckeri. However, BOPE-Cu2 + hNFs and BOJE-Cu2 + hNFs may be considered for aquacultural applications or may be farm-raised fish vaccines due to their antimicrobial action against pathogenic microorganisms (Yersinia ruckeri).
Acknowledgements
The author would like to thank Dr. Ocsoy for his expert advice and encouragement throughout this work as well as, Berkay Saraymen of the Erciyes University Nanotechnology Research Center for assistance with DLS and Zeta measurements.
Author Contributions
Synthesizing, characterization, and all analysis in the laboratory, writing manuscript and approved final draft was performed by AD.
Funding
Not applicable.
Declarations
Conflict Interest
The authors declare that they have no competing interests.
Availability of Data and Materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shende P, Kasture P, Gaud RS. Nanoflowers: the future trend of nanotechnology for multi-applications. Artificial Cells, Nanomed Biotechnol. 2018;46:413–422. doi: 10.1080/21691401.2018.1428812. [DOI] [PubMed] [Google Scholar]
- 2.Dar AH, Rashid N, Majid I, Hussain S, Dar MA. Nanotechnology interventions in aquaculture and seafood preservation. Crit Rev Food Sci Nutr. 2020;60:1912–1921. doi: 10.1080/10408398.2019.1617232. [DOI] [PubMed] [Google Scholar]
- 3.Karunasagar I, Pai R, Malathi GR, Karunasagar I. Mass mortality of Penaeus monodon due to antibiotic-resistant Vibrio harveyi infection. Aquaculture. 1994;128:203–209. doi: 10.1016/0044-8486(94)90309-3. [DOI] [Google Scholar]
- 4.Liang Z, Arjun S, Nani W, Chun XZ, Neena M, Chengzhong Y, Anton PJ. Nanoparticle vaccines. Vaccine. 2014;32:327–337. doi: 10.1016/j.vaccine.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Vinay TN, Choudhury TG, Paria A, Gupta SK. Nanovaccines: a possible solution for mass vaccination in aquaculture. World Aquac. 2019;47:30–33. [Google Scholar]
- 6.Bhattacharyya A, Reddy S, Hasan M, Adeyemi MM, Marye R, Naika H. Nanotechnology-a unique future technology in aquaculture for the food security. Int J Bioassays. 2015;4:4115–4126. [Google Scholar]
- 7.Kumar G, Menanteau-Ledouble S, Saleh M. Yersinia ruckeri, the causative agent of enteric redmouth disease in fish. Vet Res. 2015;46:1–10. doi: 10.1186/s13567-014-0124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel SKS, Otari SV, Kang YC, Lee JK. Protein–inorganic hybrid system for efficient his-tagged enzymes immobilization and its application in l-xylulose production. RSC Adv. 2017;7:3488–3494. doi: 10.1039/C6RA24404A. [DOI] [Google Scholar]
- 9.Kumar A, Patel SKS, Mardan B, Pagolu R, Lestari R, Jeong SH, Kim T, Haw JR, Kim IW, Lee JK. Immobilization of xylanase using a protein-inorganic hybrid system. J Microbiol Biotechnol. 2018;28:638–644. doi: 10.4014/jmb.1710.10037. [DOI] [PubMed] [Google Scholar]
- 10.Zhu X, Huang J, Liu RN, Zhang H, Jiang J, Yu R. Dual enzyme-inorganic hybrid nanoflowers incorporated microfluidic paper-based analytic devices (lPADs) biosensor for sensitive visualized detection of glucose. Nanoscale. 2017;9:5658–5663. doi: 10.1039/C7NR00958E. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Huo X, Guo R, Zhang Q, Lin J. Exploring protein-inorganic hybrid nanoflowers and immune magnetic nanobeads to detect Salmonella Typhimurium. Nanomaterials. 2018;8:1006. doi: 10.3390/nano8121006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran TD, Kim MI. Organic-inorganic hybrid nanoflowers as potent materials for biosensing and biocatalytic applications. BioChip J. 2018;12(4):268–279. doi: 10.1007/s13206-018-2409-7. [DOI] [Google Scholar]
- 13.Abdallah Y, Ogunyemi SO, Abdelazez A, Zhang M, Hong X, Ibrahim E, Hossain A, Fouad H, Li B, Chen J. The green synthesis of MgO nano-flowers using Rosmarinus officinalis L. (Rosemary) and the antibacterial activities against Xanthomonas oryzae pv. oryzae. BioMed Res Int. 2019 doi: 10.1155/2019/5620989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beyene AB, Hwang BJ, Tegegne WA, Wang JS, Tsai HC, Su WN. Reliable and sensitive detection of pancreatic cancer marker by gold nanoflower-based SERS mapping immunoassay. Microchem J. 2020;158:105099. doi: 10.1016/j.microc.2020.105099. [DOI] [Google Scholar]
- 15.Otari SV, Patel SKS, Jeong JH, Lee JH, Lee JK. A green chemistry approach for synthesizing thermostable antimicrobial peptide-coated gold nanoparticles immobilized in an alginate biohydrogel. RSC Adv. 2016;6:86808–86816. doi: 10.1039/C6RA14688K. [DOI] [Google Scholar]
- 16.Liu Y, Shao X, Kong D, Li G, Li Q. Immobilization of thermophilic lipase in inorganic hybrid nanoflower through biomimetic mineralization. Colloids Surf B Biointerfaces. 2021;197:111450. doi: 10.1016/j.colsurfb.2020.111450. [DOI] [PubMed] [Google Scholar]
- 17.Luo X, Mohammed Al-Antaki AH, Igder A, Stubbs KA, Su P, Zhang W, Weiss GA, Raston CL. Vortex fluidic-mediated fabrication of fast gelated silica hydrogels with embedded laccase nanoflowers for real-time biosensing under Flow. ACS Appl Mater Interfaces. 2020;12:51999–52007. doi: 10.1021/acsami.0c15669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gokturk E, Ocsoy I, Turac E, Sahmetlioglu E. Horseradish peroxidase-based hybrid nanoflowers with enhanced catalytical activities for polymerization reactions of phenol derivatives. Polym Adv Technol. 2020;31:2371–2377. [Google Scholar]
- 19.Altinkaynak C, Tavlasoglu S, Özdemir N, Ocsoy I. A new generation approach in enzyme immobilization: organic-inorganic hybrid nanoflowers with enhanced catalytic activity and stability. Enzyme Microb Technol. 2016;93:105–112. doi: 10.1016/j.enzmictec.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Alhayali NI, Özpozan NK, Dayan S, Özdemir N, Somtürk Yılmaz B. Catalase/Fe3O4@Cu2+ hybrid biocatalytic nanoflowers fabrication and efficiency in the reduction of organic pollutants. Polyhedron. 2020;194:114888. doi: 10.1016/j.poly.2020.114888. [DOI] [Google Scholar]
- 21.Patel SKS, Choi SH, Kang YC, Lee JK. Large-scale aerosol-assisted synthesis of biofriendly Fe2O3 yolk–shell particles: a promising support for enzyme immobilization. Nanoscale. 2016;8:6728–6734. doi: 10.1039/C6NR00346J. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Kim IW, Patel SKS. Synthesis of protein-inorganic nanohybrids with improved catalytic properties using Co3(PO4)2. Indian J Microbiol. 2018;58:100–104. doi: 10.1007/s12088-017-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celik C, Ildiz N, Ocsoy I. Building block and rapid synthesis of catecholamines-inorganic nanoflowers with their peroxidase-mimicking and antimicrobial activities. Sci Rep. 2020;10:1–11. doi: 10.1038/s41598-020-59699-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koca FD, Demirezen Yilmaz D, Ertas Onmaz N, Yilmaz E, Ocsoy I. Green synthesis of allicin based hybrid nanoflowers with evaluation of their catalytic and antimicrobial activities. Biotechnol Lett. 2020;42:1683–1690. doi: 10.1007/s10529-020-02877-2. [DOI] [PubMed] [Google Scholar]
- 25.Wu ZF, Wang Z, Zhang Y, Ma YL, He CY, Li H, Chen L, Huo QS, Wang L, Li ZQ. Amino acids-incorporated nanoflowers with an intrinsic peroxidase-like activity. Sci Rep. 2016;6:1–7. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel SKS, Otari SV, Li J, Kim DR, Kim SC, Cho BK, Kalia VC, Kang YC, Lee JK. Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J Hazard Mater. 2018;347:442–450. doi: 10.1016/j.jhazmat.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Patel SKS, Gupta RK, Kumar V. Influence of metal ions on the immobilization of β-glucosidase through protein-inorganic hybrids. Indian J Microbiol. 2019;59:370–374. doi: 10.1007/s12088-019-00796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otari S, Pawar SH, Patel SKS, Singh RK, Kim SY, Lee JH, Zhang L, Lee JK. Canna edulis leaf extract-mediated preparation of stabilized silver nanoparticles: characterization, antimicrobial activity, and toxicity studies. J Microbiol Biotechnol. 2017;27:731–738. doi: 10.4014/jmb.1610.10019. [DOI] [PubMed] [Google Scholar]
- 29.Otari SV, Patel SKS, Kalia VC. Antimicrobial activity of biosynthesized silver nanoparticles decorated silica nanoparticles. Indian J Microbiol. 2019;59:379–382. doi: 10.1007/s12088-019-00812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altınkaynak C, Ildız N, Baldemir A, Özdemir N, Yılmaz V, Ocsoy I. Synthesis of organic-inorganic hybrid nanoflowers using Trigonella foenum-graecum seed extract and investigation of their anti-microbial activity. Derim. 2019;36:159–167. [Google Scholar]
- 31.Zhang B, Li P, Zhang H, Li X, Tian L, Wang H, Chen X, Ali N, Ali Z, Zhang Q. Red-blood-cell-like BSA/Zn3(PO4)2 hybrid particles: preparation and application to adsorption of heavy metal ions. Appl Surf Sci. 2016;366:328–338. doi: 10.1016/j.apsusc.2016.01.074. [DOI] [Google Scholar]
- 32.Thawari AG, Rao CP. Peroxidase-like catalytic activity of copper-mediated protein–inorganic hybrid nanoflowers and nanofibers of β-lactoglobulin and α-lactalbumin: synthesis, spectral characterization, microscopic features, and catalytic activity. ACS Appl Mat Interfaces. 2016;8:10392–10402. doi: 10.1021/acsami.5b12591. [DOI] [PubMed] [Google Scholar]
- 33.Nhung TT, Bu Y, Lee SW. Facile synthesis of chitosan mediated gold nanoflowers as surface-enhanced Raman scattering (SERS) substrates. J Cryst Growth. 2013;373:132–137. doi: 10.1016/j.jcrysgro.2012.09.042. [DOI] [Google Scholar]
- 34.Fotiadou R, Patila M, Hammami MA, Enotiadis A, Moschovas D, Tsirka K, Spyrou K, Giannelis EP, Avgeropoulos A, Paipetis A, Gournis D, Stamatis H. Development of effective lipase-hybrid nanoflowers enriched with carbon and magnetic nanomaterials for biocatalytic transformations. Nanomat Basel. 2019;9:808. doi: 10.3390/nano9060808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwak C, Cho I, Lee S, An JS, Hong K. Hydrothermal Synthesis, Characterization and Photocatalytic Properties of Cu2PO4OH with Hierarchical Morphologies. J Nanosci Nanotechnol. 2020;10:1185–1190. doi: 10.1166/jnn.2010.1844. [DOI] [PubMed] [Google Scholar]
- 36.Sreedharan SM, Singh R. Ciprofloxacin functionalized biogenic gold nanoflowers as nanoantibiotics against pathogenic bacterial strains. Int J Nanomed. 2019;14:9905–9916. doi: 10.2147/IJN.S224488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grass G, Rensing C, Solioz M. Metallic copper as an antimicrobial surface. App Environ Microb. 2011;77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel SKS, Kim JH, Kalia VC, Lee JK. Antimicrobial activity of amino-derivatized cationic polysaccharides. Indian J Microbiol. 2019;59:96–99. doi: 10.1007/s12088-018-0764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Temel S, Gokmen FO, Yaman E. Antibacterial activity of ZnO nanoflowers deposited on biodegradable acrylic acid hydrogel by chemical bath deposition. Bull Mater Sci. 2020;43:1–6. doi: 10.1007/s12034-019-1967-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].