Abstract
Considering the cases of fungal resistance to classic antifungals, it is necessary to develop more efficient and innovative therapies capable of reversing this situation. Fluconazole is an antifungal frequently used in the treatment of mycosis and some fungi developed resistance to its mechanism of action. In this work, fluconazole and green propolis were co-encapsulated in chitosan nanoparticles to be explored in order to promote a synergistic effect to enhance its therapeutic efficacy. However, because of the complexity of the chemical composition of green propolis, it was necessary to develop a simple and precise methodology to quantify fluconazole in the formulation. High Efficiency Liquid Chromatography methodology was developed and validated following the Brazilian regulatory guidelines (ANVISA, RDC 166/2017) for the separation of co-eluted peaks of fluconazole and green propolis in the nanoparticle supernatant. Applying the method developed, it was possible to quantify fluconazole in the same sample containing propolis. Thus, the results allow to affirm that it is a specific test, effective, precise and robust, which helped to determine the efficiency of association of the compounds within the nanoparticle. The method can be applied to quantify compounds that have similar chromatographic retention times.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12088-021-00954-2.
Keywords: Nanoparticle, Co-administration, Antifungal therapy, HPLC, Validation
Introduction
Fungal infections are considered an important public health problem worldwide and episodes of resistance to conventional antifungals have been reported [1]. Fluconazole (C13H12F2N6O) is a classic azole antifungal that inhibits the ergosterol biosynthesis, one of the main components of the fungal cell membrane, compromising its integrity [2]. The association of an azole and molecules of natural origin with antimicrobial properties, such as quorum sensing factors and propolis, may be a promising approach to improve the drug efficacy and to avoid cases of fungal resistance [3, 4]. Green propolis is a natural compound produced by bees and is composed of several substances such as flavonoids, phenolic acids and sesquiterpene esters, presenting antimicrobial, immunomodulatory, and anti-inflammatory properties. The encapsulation of propolis within nanoparticles is a strategy used to increase its absorption by cells resulting in the improvement of its antimicrobial activity [1, 5, 6].
Drug formulations containing nanoparticles to carry antimicrobial compounds provide better stability, protection against early degradation by enzimas and increased therapeutic efficacy [1, 6]. In these formulations, different molecules can be associated, such as a conventional drug and a natural product, promoting an increase in the activity for both compounds [2]. Polymeric nanoparticles are a remarkable choice to be used in drug delivery system. Chitosan is a natural cationic polymer, obtained by N-deacetylation of chitin [6, 7], which has biodegradable, biocompatible, non-toxic, antimicrobial, and immunogenic properties. It promotes an ionic interaction with the mucous membranes, being interesting to be used in drug formulations for the treatment of fungal infections that affect this site, such as vulvovaginal candidiasis [3, 6]. Some studies have shown that fluconazole has increased its antifungal efficacy when encapsulated within polymeric nanoparticles. There was a reduction in the amount of fluconazole to reach its therapeutic effect compared to its free formulation and it was able to reverse the capacity of resistance to this antifungal presented by some resistant isolates of Candida spp. [8, 9].
Considering the promising results of a study by our group [3], in which miconazole and the quorum sensing farnesol were co-encapsulated within chitosan nanoparticles and tested in the vulvovaginal candidiasis murine model, we also used the similar system for co-encapsulate fluconazole and green propolis. As far as we know, there is no data in the literature on the synergism of these two compounds when in the same nanoformulation. However, considering the complexity of the chemical composition of propolis associated with fluconazole, it was difficult to determine the amount of each compound in the preparation. Therefore, the objective of this work was to develop a methodology to quantify fluconazole and green propolis co-encapsulated within chitosan nanoparticles.
Methods
Reagents and Materials
Chitosan low molecular weight, degree of deacetylation ≥ 75% and viscosity 20–300 cps (c = 1% in 1% acetic acid); pentasodium tripolyphosphate (TPP) and the fluconazole (98% purity) were acquired from the Sigma-Aldrich. The green propolis extract was donated by Citrinitas—Sao Paulo, Brazil. The mobile phase composition reagents such as acetonitrile and methanol were purchased from the Sigma-Aldrich and the acetic acid was from Vetec. The 0.22 µm polyvinylidene fluoride (PVDF) membranes were purchased from Merck.
Preparation of Nanoparticles
The nanoparticles were prepared by the method of ionic gelation according to modification in the protocol described by Calvo et al. [10]. In brief, the polymer chitosan (0.037 g) were dissolved in solution at 1% acetic acid by sonication and the pH was adjusted to 4.7. To the chitosan solution, 1 mL of fluconazole (260.7 µg/ mL) was dropwise added under gentle magnetic stirring (150 rpm) for 5 h protected from light. To this solution, a preparation of 8 mL of TPP (0.8%) solution containing green propolis extract (20 mg/ mL) was dropwise added under mild magnetic stirring (150 rpm) and kept under gentle agitation (150 rpm) for 1 h. The 2:1 ratio of chitosan and TPP was maintained. The nanoparticle dispersion was aliquoted in 1.5 mL tubes and centrifuged for 20 min at 15.700 rpm and the supernatant was collected to be used to quantify fluconazole and propolis that were not encapsulated within the nanoparticles. Empty chitosan nanoparticles were prepared following the same methodology but replacing fluconazole and propolis with ultrapure water and was used as blank for sample analysis.
Methodology Validation
The validation of the methodology using High Efficiency Liquid Chromatography (HPLC) was carried out in accordance with ANVISA Guidelines RDC#166/2017 [11], which establishes the criteria for the validation of methods for quantifying and identifying analytes. The chromatographic system was evaluated for its ability to provide reproducible results. This evaluation was carried out before starting the validation analyzes to verify by means of a set of parameters such as retention factor (k), repeatability (RSD), resolution (Rs), tail factor (TF) and number of theoretical plates (N), that the equipment used guarantees exact and precise results.
The mobile phase was used in gradient elution mode and flow rate of 1 mL/ min for 45 min and detection at 261 and 300 nm. The injection volume was 20 µL and the analyses were performed at 25 °C. Several chromatographic conditions were tested for choosing the analysis method that was defined for mobile phase as methanol: acetonitrile: water + 5% acetic acid (mobile phase) and 0 min 15:5:80; 10 min 30:10:60; 20 min 50:15:35; 25 min 65:15:20; 40 min 70:20:10; 45 min 70:20:10 (proportion of mobile phase). The diluted solutions were filtered in a 0.22 µm PVDF membrane prior to the analyses that were performed in triplicate.
The concentrations of compounds associated with the nanoparticle were determined by quantifying the fluconazole and green propolis extract present in the supernatant of the nanoparticle. The sample concentration was determined from the line equation, obtained from the linearity curve and replaced in the Eq. (1).
| 1 |
where E.E.% represents the percentage of compound retained in the nanoparticles, QT represents the mass of compound added initially to obtain the nanoparticles, and QX represents the value analyzed by the HPLC.
Statistical Analysis
The two-way ANOVA were used for statistical differences. The significance level was 5% (p < 0.05). The results are expressed as mean ± standard deviation of the mean. The data were performed using Statistic software.
Results and Discussion
Fungal infections have become a global public health problem. Both for episodes of drug resistance presented by some fungi [12, 13] and for cases of patients affected by COVID19 who develop severe cases of opportunistic mycosis such as mucormycosis [14]. Thus, there is an urgent need to develop innovative antifungal therapies capable of reversing this problem. In this context, our research group developed chitosan nanoparticles co-encapsulating fluconazole and green propolis with 316.5 nm average size, positive zeta potential of 37.4 mV, and the polydispersity index of 0.391. However, considering the complexity of the chemical composition of propolis, it was necessary to develop a methodology for quantifying each compound in the formulation.
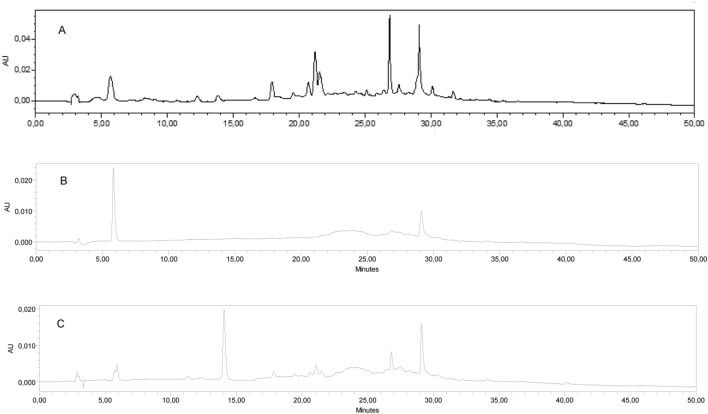
Ultraviolet spectrophotometric techniques have been used for the rapid quantification of compounds in the supernatant of nanoparticles [15, 16]. For fluconazole, the appropriate wavelength for its detection varies from 210 to 261 nm, the latter being the most commonly used [17]. For propolis, it can be detected in 300 nm, but there are variations in 260 to 450 nm [16]. Although this methodology is routinely used in laboratories, it is not suitable for quantify different co-encapsulated compounds that have the same wavelength. In this study, when evaluated at 261 nm, it was observed the presence of compounds of the green propolis extract (Fig. 1a) that remained during separation at the same retention time of the fluconazole (Fig. 1b). Both compounds had the same retention time of 6 min (Fig. 1c). Then, it was necessary to standardize the methodology in which 40 different combinations, such as composition and proportion of mobile phase, column, temperature and flow, were tested until the peaks separation was reached (data not shown).
Fig. 1.
Chromatograms obtained at 261 nm for green propolis extract (a), fluconazole (b), and nanoparticle supernatant containing fluconazole and green propolis (c). A six min retention time was detected for both compounds (c)
The development of the HPLC validation method was performed to quantify the fluconazole in the supernatant of the nanoparticles and the efficient separation of the green propolis compounds (Fig. 2). The parameters adopted were similar to those described by Popova et al. [15, 16] to quantify the compounds in propolis, with a mobilie phase of 5% acidified water and methanol and a flow rate of 1 mL/min. Although the mobile phase compositions indicated in the literature for fluconazole chromatographic analyzes use high concentrations of buffer [17, 18], similarities were found in the maximum detection wavelength (261 nm), elution time (1 mL/ min), and shorter retention time (5 min). For separation of the green propolis peak, the method was able to provide good data quality with acidified composition, but with longer retention time (9 min). The physical and chemical parameters for the separation of the compounds were varied, but the composition of the mobile phase for a more acidified pH was the only possibility with which it was possible to achieve the results, demonstrating the separation of the peaks in the retention time of 9 min for fluconazole and green propolis. When comparing the retention times and ultraviolet absorption spectrum of the separate peaks of the samples and controls, it was observed that there is no interference from other peaks, this information demonstrates the efficiency in the selectivity of the method developed. The selectivity of the method was confirmed by detecting fluconazole separated from the green propolis compounds in the sample, comparing the retention times and the ultraviolet absorption spectrum (190 to 400 nm) of the peaks obtained in the sample and in the standard (Fig. 3).
Fig. 2.
Chromatograms obtained at 233.5 nm for fluconazole standard in 10.065 min (a), nanoparticle supernatant containing fluconazole in 9.920 min and (b), and green propolis in 8.821 min (c)
Fig. 3.
Chromatograms obtained for fluconazole standard (blue) superimposed on the nanoparticle chromatogram (co-encapsulated nanoparticle supernatant) (Color figure online)
As shown in the chromatograms in Figs. 3 and 4, it can be observed that the method was able to precisely distinguish fluconazole in the sample, even in the presence of other compounds, as recommended by ANVISA [11]. The maximum wavelength of fluconazole absorption in the co-encapsulated nanoparticle supernatant was 233.5 nm (Fig. 2). However, the wavelength of 261 nm was established for the method to favor the detection of compounds present in the propolis in the supernatant of the nanoparticle (Fig. 7, supplementary material). The method presented robustness, which indicates the capacity of the method to remain adequate even with small variations in working conditions. After changes in flow rate, temperature and column, the results were satisfactory with a Relative Standard Deviation (RSD) below 5% (Table 3, supplementary material). The accuracy of the method was validated by adding known amount of the fluconazole standard (10 µg/ mL) to the sample solutions at three different values between 80 and 120% in which the recovery intervals were approximately 98.2% (RSD 0.9%) (Table 4, supplementary material). The limits of detection were 0.20 µg/mL and 0.62 µg/mL for quantification and there was no interference from the constituents of the matrix (Fig. 6, supplementary material).
Fig. 4.
Chromatogram for fluconazole standard (green) superimposed on the chromatogram of the coencapsulated nanoparticle (blue), and chromatogram of the green propolis extract (black) (Color figure online)
The linearity curve defined in this study after the standardization of the HPLC method (Fig. 5) presented a correlation coefficient of 0.9991. On the curve, it is observed that the data obtained are directly proportional to the compound concentration in the sample. Then, by the resulting equation, it was possible to determine the association efficiency for fluconazole of 38.3% when co-encapsulated with green propolis in chitosan nanoparticles. Considering the complexity of the compounds in green propolis [15, 16], its association efficiency was determined by ultraviolet spectroscopy representing 45.6%.
Fig. 5.

Linearity curve for the fluconazole standard in µg/mL. The correlation coefficient (R2) = 0.9991
Conclusion
The development and validation of a simple method was used to determine the amount of fluconazole present in the supernatant of nanoparticles when co-encapsulated with green propolis. Fluconazole was quantified by HPLC methodology, demonstrating results that allow to state that it is a specific, effective, accurate, and robust test that has demonstrated the efficiency of association of the compounds in the nanoparticle. The method can be applied to quantify compounds that have similar chromatographic retention times.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for Silva JT scholarship and Federal University of Goiás for providing infrastructural facilities.
Declarations
Conflict of interest
The authors declare there are no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Souza ACO, Amaral AC. Antifungal therapy for systemic mycosis and the nanobiotechnology era: improving efficacy, biodistribution and toxicity. Front Microbiol. 2017;8:336. doi: 10.3389/fmicb.2017.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant SM, Clissold SP. Fluconazole a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial and systemic mycoses. Drugs. 1990;39:877–916. doi: 10.2165/00003495-199039060-00006. [DOI] [PubMed] [Google Scholar]
- 3.Costa AD, Araujo DE, Cabral MS, Brito IT, Leite LBM, Pereira M, Amaral AC. Development, characterization, and in vitro-in vivo evaluation of polymeric nanoparticles containing miconazole and farnesol for treatment of vulvovaginal candidiasis. Med Mycol. 2019;57:52–62. doi: 10.1093/mmy/myx155. [DOI] [PubMed] [Google Scholar]
- 4.Kalia VC, Patel SKS, Kang YC, Lee JK. Quorum sensing inhibitors as antipathogens: biotechnological applications. Biotechnol Adv. 2019;37:68–90. doi: 10.1016/j.biotechadv.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Tobaldini-Valerio FK, Bonfim-Mendonça PS, Rosseto HC, Bruschi ML, Henriques M, Negri M, Silva S, Svidzinski TI. Propolis: a potential natural product to fight Candida species infections. Future Microbiol. 2016;11:1035–1046. doi: 10.2217/fmb-2015-0016. [DOI] [PubMed] [Google Scholar]
- 6.Patel SKS, Kim JH, Kalia VC, Lee JK. Antimicrobial activity of amino-derivatized cationic polysaccharides. Indian J Microbiol. 2019;59:96–99. doi: 10.1007/s12088-018-0764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandra HK, Prabha S, Chandra R, Ahmed B, Nimesh S. Advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artif Cells Nanomed Biotechnol. 2016;44:305–314. doi: 10.3109/21691401.2014.948548. [DOI] [PubMed] [Google Scholar]
- 8.Moazeni M, Hamid RK, Majid S, Morteza-Semnani K, Nabili M, Gohar AA, Akbari J, Lotfali E, Nokhodchi A. Time to overcome fluconazole resistant Candida isolates: solid lipid nanoparticles as a novel antifungal drug delivery system. Colloids Surf B Biointerfaces. 2016;142:400–407. doi: 10.1016/j.colsurfb.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Bianchin MD, Borowicz SM, Machado GRM, Pippi B, Guterres SS, Pohlmann AR, Fuentefria AM, Kulkamp-Guerreiro IC. Lipid core nanoparticles as a broad strategy to reverse fluconazole resistance in multiple Candida species. Colloids Surf B Biointerfaces. 2019;175:523–529. doi: 10.1016/j.colsurfb.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci. 1997;63:125–132. doi: 10.1002/(SICI)1097-4628(19970103)63:1<125::AID-APP13>3.0.CO;2-4. [DOI] [Google Scholar]
- 11.ANVISA (2017) RDC 166 Guidelines. http://antigo.anvisa.gov.br/documents/10181/2721567/RDC_166_2017.
- 12.ElBaradei A. A decade after the emergence of Candida auris: what do we know? Eur J Clin Microbiol Infect Dis. 2020;39(9):1617–1627. doi: 10.1007/s10096-020-03886-9. [DOI] [PubMed] [Google Scholar]
- 13.Tang SS, Apisarnthanarak A, Hsu LY. Mechanisms of β-lactam antimicrobial resistance and epidemiology of major community- and healthcare- associated multidrug-resistant bacteria. Adv Drug Deliv Rev. 2014 doi: 10.1016/j.addr.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Garg D, Muthu V, Sehgal IS, Ramachandran R, Kaur H, Bhalla A, Puri GD, Chakrabarti A, Agarwal R. Mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186:289–298. doi: 10.1007/s11046-021-00528.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popova M, Bankova V, Butovska D, Petrov V, Nikolova-Damyanova B, Sabatini AG, Marcazzan GL, Bogdanov S. Validated methods for the quantification of biologically active constituents of poplar-type propolis. Phytochem Anal. 2004;15:235–240. doi: 10.1002/pca.777. [DOI] [PubMed] [Google Scholar]
- 16.Popova M, Chen CN, Chen PY, Huang CY, Bankova V. A validated spectrofotometric method for quantification of prenylated flavanones in pacific propolis from Taiwan. Phytochem Anal. 2010;21:186–191. doi: 10.1002/pca.1176. [DOI] [PubMed] [Google Scholar]
- 17.Ayub AC, Vianna-Soares CD, Ferreira LAM. Fluconazol method validation by RP-HPLC for determ ination in biological skin matrices. J Chromatogr Sci. 2007;45:286–290. doi: 10.1093/chromsci/45.5.286. [DOI] [PubMed] [Google Scholar]
- 18.Aloudah NM, Radwan MA, Omar NFA, Jacobs S. HPLC assay of fluconazole and its application to patients with early septic shock. J Liq Chromatogr RT. 2005;28:571–580. doi: 10.1081/JLC-200047213. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.