Abstract
Background
Using fMRI to analysis of brain function state in migraineurs at different phases, and combined with the clinical symptoms to explore the mechanisms and outcomes of migraine.
Methods
It’s a case-control study. We analyzed the resting-state brain functional magnetic resonance imaging in 19 patients with episodes, 22 patients with interictal phase, and 22 healthy controls. The ReHo method was used for post-processing. All subjects were evaluated using the Montreal cognitive assessment (MoCA) scale, simple mental state examination (MMSE), Hamilton anxiety (HAMA) scale, and Hamilton depression (HAMD) scale. The subjects’ clinical indicators (such as frequency of attack, course of disease, duration of each headache, and severity of headache) were correlated with the ReHo values of brain regions. This study was approved by the ethics committee of Yangtze River Shipping General Hospital.
Results
Compared with the interictal, patients in the episode group had lower activation in bilateral anterior cingulate cortex (ACC), with Montreal Neurological Institute (MNI) (−9, 42, 15); and had stronger activation in bilateral paracentral lobule (PCL), with MNI (−3, −24, 66). Compared with the control group, patients in interictal phase had lower activation in the bilateral cuneus and bilateral lingual gyrus, with MNI scores of (9, −84, 36) and (0, −72, 6), respectively. No significant difference in brain area was found between the episodes group and the control group. In the episodes group, a significant correlation was observed between attack frequency and ReHo value of the bilateral PCL (r=0.492; P=0.038).
Conclusions
We need to observe the course of migraine as a whole. In the interictal period, the cuneus and lingual gyrus may affect the development of the disease. The ACC regulates different states of migraine by inducing anti-injury sensation regulation function. The paracentric lobule is not only associated with migraine attacks, but also with the frequency. This may have an effect on the outcome of subsequent migraines, as well as whether the condition becomes chronic, and the remodeling of the brain.
Keywords: Migraine, functional magnetic resonance imaging (fMRI), regional homogeneity (ReHo), anterior cingulate cortex (ACC), paracentral lobule (PCL), bilateral cuneus, lingual gyrus
Introduction
Migraine is a severe brain condition. It is listed as the sixth most disabling disorder globally by the World Health Organization, and is the most disabling of all neurological disorders (1). It is an episodic, recurrent, and genetically related brain excitatory disorder characterized by attacks of unilateral, throbbing head pain, coupled with sensitivity to movement, visual, auditory, and other afferent inputs (2). Other symptoms of migraine include tiredness, irritability, and reduced concentration. The mechanism of migraine is still unclear, and diagnosis is mainly based on the analysis of clinical characteristics, without objective diagnostic methods (3).
Regional homogeneity (ReHo) is a method for processing functional magnetic resonance imaging (fMRI) images, which was first proposed by the Chinese researchers (4), and has been gradually applied to the field of medicine. According to ReHo theory, when the functional brain area is under certain conditions, there is time consistency between the voxel and peripheral voxel in this brain area. Furthermore, if ReHo is increased, the local connections of neurons in the local brain region are enhanced. However, decreased ReHo indicates that the local connections of local neurons are weakened. Therefore, it can be concluded that there is a significant correlation between abnormal ReHo and the changes of neuron activity in local brain functional areas. In other words, when abnormal ReHo occurs, the synchronous activity of local neurons changes.
Therefore, the use of brain functional imaging as a non-invasive examination method is helpful to observe the pathophysiological changes of migraine during episodes and the interictal phase, as well as the relationship between regions of interest and clinical symptoms, so as to provide an objective basis for understanding the course of migraine and its prevention.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-21-2097).
Methods
Study population
Forty-one migraine patients from the Department of Neurology at the Wuhan Yangtze River Shipping General Hospital between April 2018 and December 2018 were included in this study. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Yangtze River Shipping General Hospital (No. L20170013) and informed consent was taken from all the patients. These included 19 patients in the episodes phase (male/female ratio: 5/14, average age: 35.53±10.67) and 22 patients in the interictal phase (male/female ratio: 5/17, average age: 33.32±10.27). All of the included patients were right-handed, and all migraine sufferers conformed to the migraine criteria of the International Headache Society International classification of headache disorders version 3 (ICHD-III). All of the patients were diagnosed by a neurology specialist, and other neurological diseases were excluded. The Montreal cognitive assessment (MoCA) scale, simple mental state examination (MMSE), Hamilton anxiety (HAMA) scale, Hamilton depression (HAMD) scale, headache impact questionnaire (HIT-6), and visual analogue scale (VAS) were used to evaluate the patients’ basic situation. The control group comprised 22 age- and gender-matched healthy individuals (male/female ratio: 6/16, average age: 34.59±7.99).
Exclusion criteria
Patients were excluded based on the following criteria: (I) those with other types of headaches; (II) those combined with other organic brain diseases, cardiovascular and cerebrovascular diseases, and other internal diseases; (III) those with a history of drug abuse or alcohol addiction; and (IV) patients with contraindications to MRI. Data that involved incomplete magnetic resonance sequence, inconsistent parameters, or head movement of >3 mm was removed.
Imaging protocol
MRI scanning was performed using German Siemens Magnetom Verio 3.0 Tskyra system, and the equipment was provided by the Imaging Department of Yangtze River Shipping General Hospital, Wuhan, China.
Imaging parameter settings included blood oxygenation level-dependent (BOLD) resting state fMRI (rs-fMRI) and T1-weighted images. BOLD imaging parameters were as follows: TR (repetition time): 2,300 ms; TE (echo time): 30 ms; slice gap: 0.5 mm; slice thickness: 3.7 mm; number of slices: 31; field of view: 256×256 mm2; fractional anisotropy (FA): 90°; number of excitation (NEX): 1; matrix size: 64×64; and scanning time: 480 s (5).
T1-weighted imaging parameters were as follows: TE (echo time): 3.28 ms; TR (repetition time): 2,300 ms; TI (time inversion): 1,200 ms; FA (flip angle): 9°; matrix size: 256×256; slice thickness: 1 mm; number of slices: 256; and slice gap: 0.5 mm. During scanning, all of the subjects wore earplugs to reduce noise, and remained relaxed and awake. Head fixation reduced head movement to avoid data unavailability.
Data processing of image
Data check was performed using MRIcro software (acquired from the website: www.mricro.com), and incomplete data was excluded. Preprocessing data was analyzed by SPM8 package (website: http://www.fil.ion.ucl.ac.uk/spm), and included slice-timing, normalization, and smoothing. Slice-timing was used to realign and adjust head-motion. Head motion that exceeded 3° rotation in any direction during the scan was excluded (6); one patient was excluded due to excessive head motion. All images were normalized in accordance with the standard Montreal Neurological Institute (MNI) template in terms of voxel value as 3×3×3 mm3. In order to reduce the spatial noise, the images were smoothed by convoluting with an isotropic Gaussian kernel of 6 mm. The frequency range was then set from 0.01 to 0.08 Hz via a limit filter to avoid interference from higher frequency noise and lower frequency drift. After pretreatment, the ReHo values were calculated and analyzed using REST software (www.restfmri.net) (7). Two-sample t tests were used to compare two groups of ReHo values in different brain areas, gaussian random fields (GRF) correction. A P value <0.01 signified that the standardized ReHo values were significantly between groups. In the statistical parameter figure, the yellow color represents positive activation and the blue color represents negative activation.
Statistical analysis
The data were analyzed using SPSS19 (SPSS Inc., Chicago, IL, USA). The chi-square test was used for gender comparison between three groups, and the one-way analysis of variance (ANOVA) test was used to compare age, years of education, and scale scores. Correlation between the ReHo value of different brain regions and the clinical observation indexes (headache duration, interval time between attacks, course of disease, HAMA and HAMD scores) was analyzed by Pearson correlation analysis. A P value <0.05 was considered statistically significant.
Results
General data
Differences in the ages, genders, education years, as well as the HAMA, HAMD, MoCA, and MMSE scores of patients in the episode and interictal groups were not statistically significant (P>0.05). The education years of patients in the control group was shorter than that of patients the two migraine groups, possibly because the control group was comprised mainly of patients from our hospital staff. Moreover, the HAMA and HAMD scores of the control group were lower than those of the migraine groups (Table 1).
Table 1. Basic data of the three groups.
| Index | Epi_group | Int_group | Con_group | P values |
|---|---|---|---|---|
| Gender (male/female) | 5/14 | 5/17 | 6/16 | 0.936 |
| Age (years) | 35.53±10.67 | 33.32±10.27 | 34.59±7.99 | 0.268 |
| Education (years) | 12.42±2.63 | 12.41±3.65 | 16.36±2.63 | 0.991*1, <0.01*2, <0.01*3 |
| HAMA | 8.68±3.33 | 8.50±3.11 | 2.86±0.86 | 0.606*1, <0.01*2, <0.01*3 |
| HAMD | 8.63±3.40 | 8.73±3.07 | 2.86±0.86 | 0.924*1, <0.01*2, <0.01*3 |
| MoCA | 26.61±1.81 | 27.55±1.57 | 28.27±1.72 | 0.044*1, 0.352*2, 0.038*3 |
| MMSE | 28.89±1.15 | 28.56±1.29 | 29.00±9.00 | 0.254*1, 0.784*2, 0.143*3 |
Epi_ group: episode group; Int_ group: interictal group; Con_ group: control group; *1: Epi_ group vs. Int_ group; *2: Epi_ group vs. Con_ group; *3: Int_ group vs. Con_ group. HAMA, Hamilton anxiety; HAMD, Hamilton depression.
ReHo analysis
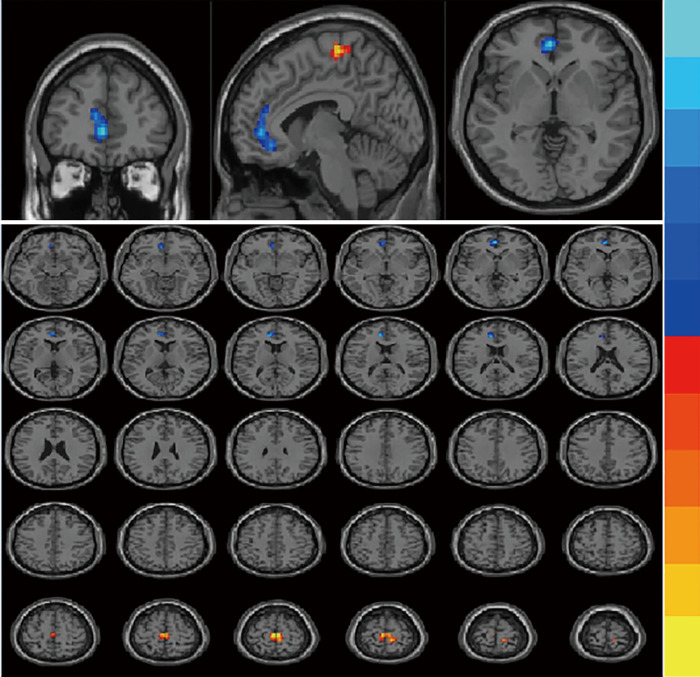
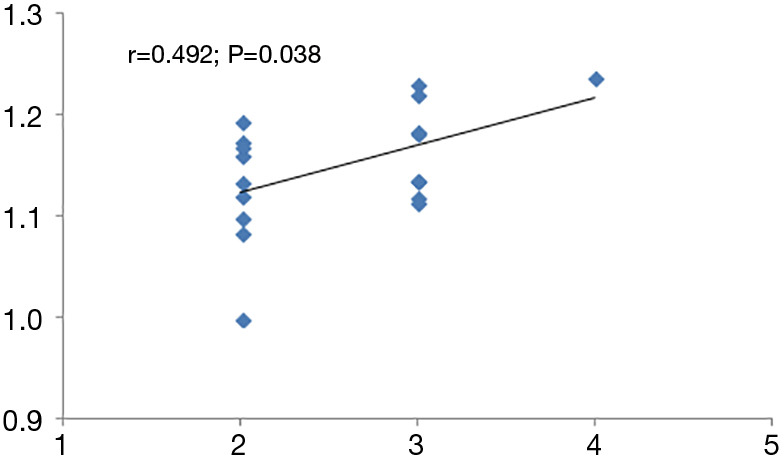
Compared with the interictal group, patients in the episode group had lower ReHo values in bilateral anterior cingulate cortex (ACC), with MNI (−9, 42, 15), voxel size =109, T=−3.77; and had stronger ReHo values in bilateral paracentral lobule (PCL), with MNI (−3, −24, 66), voxel size =117, T=3.29. (P<0.01) (Table 2 and Figure 1). Compared with the control group, patients in the interictal phase had stronger activation in the bilateral cuneus (voxel size =190, T=−3.64) and bilateral lingual gyrus (voxel size =163, T=−3.53), with MNI coordinates of (9, −84, 36) and (0, −72, 6), respectively (Table 2 and Figure 2). No significant difference in brain area was found between the episodes and control groups. In the episodes group, a significant correlation was observed between attack frequency and the ReHo value of the bilateral PCL (r=0.492; P=0.038) (Figure 3). All of the results were verified through GRF correction.
Table 2. Significant brain areas.
| ROIs | Regions | Voxel size | T | MNI coordinate | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Epi vs. Int | ||||||
| 1 | Bilateral paracentral lobule | 117 | 3.29 | −3 | −24 | 66 |
| 2 | Bilateral anterior cingulate | 109 | −3.77 | −9 | 42 | 15 |
| Int vs. Con | ||||||
| 3 | Bilateral cuneus | 190 | −3.64 | 9 | −84 | 36 |
| 4 | Bilateral lingual gyrus | 163 | −3.53 | 0 | −72 | 6 |
MNI, Montreal Neurological Institute Coordinate System.
Figure 1.
GRF correction (Epi-group vs. Int-group), coordinates: (−3, −24, 66); T=3.29; voxel size =117, bilateral paracentral lobule; coordinates: (−9, 42, 15); T=−3.77; voxel size =109, bilateral anterior cingulate. GRF, Gaussian random field.
Figure 2.
GRF correction (Int-group vs. Con-group), coordinates: (9, −84, 36), T=−3.64, voxel size =190, bilateral cuneus; coordinates: (0, −72, 6), T=−3.53, voxel size =163, bilateral lingual gyrus. GRF, Gaussian random field.
Figure 3.
GRF correction (Int-group vs. Con-group), coordinates: (9, −84, 36), T=−3.64, voxel size =190, bilateral cuneus; coordinates: (0, −72, 6), T=−3.53, voxel size =163, bilateral lingual gyrus. GRF, Gaussian random field.
Discussion
The rs-fMRI technique is a non-invasive and advanced neuroimaging measurement that has been recently used to investigate the pathophysiology of several disorders. By identifying particular imaging biomarkers, the shift from laboratory research to clinical understanding has provided migraine patients with key information for diagnosis, prognosis, and disease outcomes, and has allowed for the development of reasonable treatments and nursing approaches (8). VBM analysis showed that the volume of gray matter in headache related brain regions decreased, and DTI showed migraine patients had changes in thalamocortical tract, trigeminal thalamic tract and other white matter. On functional imaging, low metabolism in headache related brain regions was observed in the resting state, and increased sensitivity to visual, auditory, and olfactory stimuli was also observed in the task state (9).
ReHo is an analytical method that examines the features of functional integration, which offers advantages including no specific requirements on the sample distribution and strong applicability. The ReHo analytical method can infer the function of corresponding brain regions by analyzing the consistency of spontaneous nerve activity in the local brain region, which reflects the synchronization of neuronal activity in the local brain region in time, rather than the intensity of neuronal activity. Higher ReHo values indicate better synchronization of activity in any particular brain region (10).
Migraine is not only an important global health problem, which involves a considerable burden in terms of patient suffering, but is also a topic of enduring interest. Although migraine is not a fatal disease, it has numerous comorbidities that can seriously affect everyday life. SCN1A, SCN2A, KCNK18, TRPA1 and STX1A as a Possible Marker of Migraine (11). The neuroanatomical basis of migraine is the trigeminal neurovascular system and its projection system, which is involved in various peripheral nerves and their neurotransmitters/neuropeptides, including serotonin (5-HT) and calcitonin gene-related peptide (CGRP). Migraine needs to be viewed as a whole, accurately divided into prodrome phase, aura phase, headache phase, and postpartum, with different nuclei involved in different phases. Prodrome symptoms may be related to the hypothalamus. The mechanism of aura is related to cortical spreading inhibition (CSD). Stronger activity in the cingulate gyrus, auditory cortex, midbrain, periaqueductal gray (PAG), raphe nucleus, were verified during the headache phase and postpartum. In addition, increased activity in the brain stem still existed in the early period of headache remission (9).
In our study, by comparing the basic data of the three groups, we found that mood disorders were more common in migraine group, which is consistent with previous studies (12,13). However, whether this is because it is comorbid or reflects a cause-and-effect relationship is unclear. It is also unclear how and from which area of the brain the migraine pain originates, and which brain areas are active in the different stages of migraine.
In our study, the ReHo value of bilateral PCL, with MNI (−3, −24, 66), exhibited stronger activation in the episodes group compared to the interictal group (P<0.01). The PCL is a part of sensorimotor network (SMN), which is located on the medial face of the parietal cortex, with the precuneus and occipital cortex also being located in this location. This associative cortex is well placed to play an important role in multisensory integration, specific aspects of which participate in bodily awareness (14). In another clinical study, abnormal intrinsic connectivity network (ICN) connectivity of the orbitofrontal cortex (OFC) and inferior parietal lobule (IPL) within the dorsal attention network (DAN), and of the PCL within the sensorimotor (SMN), normalized in chronic pain patients after cognitive-behavioral therapy (CBT) treatment (15). Compared with the control group, Research has shown that there has a significant stronger activity of PCL in chronic pain patients. The PCL is primarily associated with the action-execution or processing and perception of pain (16). Patients with chronic pain exhibit distorted recognition or sensory pain assessment accompanied by abnormal activities of the PCL (17), and PCL activity has also been associated with pain chronicity (18). Migraine is a neurological disorder that involves multiple senses. PCL activation in the episode period also explains the symptoms that accompany the headache, such as fatigue, yawning, photophobia, and so on.
Furthermore, patients in the episode group had lower activation in bilateral ACC, with MNI (−9, 42, 15) compared with the interictal group . In migraines, the most relevant functions with the ACC are those related to pain control and those involved in emotional aspects. Recent studies using H-MRS (proton magnetic resonance spectroscopy) measurements have shown that ACC metabolites in migraine patients during the onset are “complex” and hyper excitatory, even during the interictal period (19). Consistent with these studies, our results indicated that the ReHo of ACC varied in the different phases of migraine. A possible mechanism for this may be that ACC could mediate the analgesic effect of motor cortex stimulation to some extent. Previous studies have confirmed the anatomical connection between cortex and brain stem pain processing regions. These connections enable the top-down adjustment of pain sensations from the cortical region through brain stem opioids (20). Patients with medication overuse headache were characterized by a distinct concentration profile of myo-inositol, a glial marker, in the anterior cingulate cortices that may have arisen from medication overuse and could contribute to the development of other headaches (21).
The ACC is rich in opioid receptors and selectively activates opioids, thereby playing a key role in the analgesic control of central opioids. In addition, the ACC is connected to the periaqueductal gray of the midbrain, which is also a key area of migraine pathogenesis (22). All the above conditions are mediated by activation of descending anti-injury sensation pathway.
In addition, the activation of excitatory synapses in the ACC will promote pain perception and increase the sensitivity of pain discomfort (23). Specifically, glutamate from the ACC projects and enhances sensory transmission in the spinal cord, which will aggravate pain. This excitatory neurotransmission in the ACC is regulated by serotonin (5-HT), which inhibits the release of glutamate (24). We consider that this is related to the functional state of the brain, as well as the adaptive and remodeling functions of the brain. Migraine sufferers are in different periods, and have varied attack frequencies and pain durations. As time progresses, many factors affect the chronic development of migraine may play a role through ACC.
It is well known that migraine is defined as a neurological disorder. However, questions remain as to the brain function between migraines, as well as changes in brain function during remission. Although migraine is an episodic disorder, we should investigate the entire course of the disease as a whole. In our study, compared with the control group, patients in the interictal phase exhibited lower activation in the bilateral cuneus and bilateral lingual gyrus. Positron emission tomography (PET) scans were performed using regional cerebral blood flow (rCBF) as a marker of neuronal activity, which revealed significant changes in rCBF in the cuneus (correlated to pain scores) and in the ACC (correlated to stimulation-induced paraesthesia scores). The ACC and cuneus are probably involved in the affective dimension of pain (25). Another finding was that changes in rCBF in the cuneus, which were directly correlated to paraesthesia scores and inversely correlated to pain scores. A functional MRI study investigating the affective dimension of pain reported right cuneus activation, which the authors attributed to the anticipation and subjective experience of pain (26). The cuneus and lingual gyrus belong to the visual center. Photophobia is an abnormal sensitivity to light, which is experienced by migraineurs, and is perhaps caused by cortical hyperexcitability. Photophobia accompanying migraine headaches is a symptom of important diagnostic value, and as one of the diagnostic criteria of migraine without aura by the International Headache Society (IHS) (27). It has a prevalence of approximately 85% of patients during attacks, and it can also be present between attacks (28). In fact, it has even been reported that bright or flickering lights are a trigger for attacks. When no concomitant pain stimulation was applied, luminous stimulations activated the visual cortex bilaterally in migraineurs (specifically in the cuneus, lingual gyrus, and posterior cingulate cortex) but not in controls (29). Negative affective pictures elicited stronger activation than neutral affective pictures in migraineurs, which included the bilateral cerebellum anterior lobe, the bilateral lingual gyri, the bilateral precuneus, and the cuneus (30). These results indicate that migraineurs are hypersensitive to negative stimuli, which might provide clues that could aid in the understanding of the pathophysiology and psychiatric comorbidities of migraines. In our research, compared with the control group, patients in interictal phase had lower activation in the bilateral cuneus and bilateral lingual gyrus which are belong to visual cortex, with MNI (9, −84, 36) and (0, −72, 6). So in remission, the visual cortex is still damaged. Such long-term, insidious changes gradually affect changes in brain function, leading migraine sufferers to develop visual symptoms. In our study, the two groups of migraineurs exhibited higher HAMA and HAMD scores than the control group. Thus, the effects of migraines are persistent and tend to increase negative emotions. Although, whether these two aspects are comorbid disorders or are caused by a single disease is unknown. Therefore, large sample longitudinal studies are needed to continue to look for subtle changes in these abnormal brain regions. Additionally, imaging findings need to be examined to identify targeted receptor therapy and prevention.
Depending on the frequency, migraine classified as episodic migraine (EM) and chronic migraine (CM), EM to CM that occurs approximately 2.5%/y (31). CM is more likely to have mood disorders and sleep disorders and other comorbidities. Treatment includes patient education, lifestyle, modifications, management of triggers, and acute/preventive pharmacology. Not every patient with EM needs preventive therapy, but all patients with CM do (32). Migraine is associated with enhanced perception and altered cerebral processing of sensory stimuli. Sensory hypersensitivity might reflect a more general enhanced response to aversive emotional stimuli. Migraine trigger effect of psychosocial stressors leads to increased somatosensory response to emotional clues and thus contributes to the progression or increasing of frequency. Severity of emotional co-morbidities increased while sleep quality deteriorated with increasing migraine disability, and migraine frequency is positively correlated with sleep quality (33).
To observe the relationship between ReHo values in different brain regions and clinical symptoms in migraine patients, we found that attack frequency and ReHo value of the bilateral PCL had a positive correlation (r=0.492; P=0.038). The PCL is a part of the SMN, which is located on the medial face of the parietal cortex. Compared with interictal group, the PCL exhibited stronger activation in the episodes group. This suggests that the PCL is not only associated with migraine attacks, but also with attack frequency. Through activation of the PCL, the connections between SMNs and other networks are affected, which may have an effect on the outcome of subsequent migraines, as well as whether they become chronic or not, and the remodeling of the brain.
Conclusions
Although migraine is an episodic disease, it is not static, and we need to observe the course of the disease as a whole. Even in the interictal period, the activation of visual cortex includes cuneus and lingual gyrus may affect the development of the disease. The ACC regulates different states of migraine by inducing anti-injury sensation regulation function. The PCL is not only associated with migraine attacks, but also with the frequency, which may have an effect on the outcome of subsequent migraines, as well as whether they become chronic or not, and the remodeling of the brain.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Yangtze River Shipping General Hospital (No. L20170013) and informed consent was taken from all the patients.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-21-2097
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-21-2097
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-21-2097). The authors have no conflicts of interest to declare.
(English Language Editor: A. Kassem)
References
- 1.Schwedt TJ, Chiang CC, Chong CD, et al. Functional MRI of migraine. Lancet Neurol 2015;14:81-91. 10.1016/S1474-4422(14)70193-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santangelo G, Russo A, Trojano L, et al. Cognitive dysfunctions and psychological symptoms in migraine without aura: a cross-sectional study. J Headache Pain 2016;17:76. 10.1186/s10194-016-0667-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russo A, Silvestro M, Tessitore A, et al. Advances in migraine neuroimaging and clinical utility: from the MRI to the bedside. Expert Rev Neurother 2018;18:533-44. 10.1080/14737175.2018.1486708 [DOI] [PubMed] [Google Scholar]
- 4.Zang Y, Jiang T, Lu Y, et al. Regional homogeneity approach to fMRI data analysis. Neuroimage 2004;22:394-400. 10.1016/j.neuroimage.2003.12.030 [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Chen H, Liang H, et al. Low-Frequency Fluctuations Amplitude Signals Exhibit Abnormalities of Intrinsic Brain Activities and Reflect Cognitive Impairment in Leukoaraiosis Patients. Med Sci Monit 2019;25:5219-28. 10.12659/MSM.915528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon NG. The Trail Making Test in neuropsychological diagnosis. J Clin Psychol 1972;28:167-9. [DOI] [PubMed] [Google Scholar]
- 7.Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 2011;6:e25031. 10.1371/journal.pone.0025031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming JO, Carlsson CM. Biomarkers for neurology: Guides and lines. Neurology 2014;83:1130-1. 10.1212/WNL.0000000000000825 [DOI] [PubMed] [Google Scholar]
- 9.Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev 2017;97:553-622. 10.1152/physrev.00034.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol 2011;70:838-45. 10.1002/ana.22537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kowalska M, Prendecki M, Kapelusiak-Pielok M, et al. Analysis of Genetic Variants in SCN1A, SCN2A, KCNK18, TRPA1 and STX1A as a Possible Marker of Migraine. Curr Genomics 2020;21:224-36. 10.2174/1389202921666200415181222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walters AB, Hamer JD, Smitherman TA. Sleep Disturbance and Affective Comorbidity Among Episodic Migraineurs. Headache 2014;54:116-24. 10.1111/head.12168 [DOI] [PubMed] [Google Scholar]
- 13.Vetvik KG, Mac Gregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol 2017;16:76-87. 10.1016/S1474-4422(16)30293-9 [DOI] [PubMed] [Google Scholar]
- 14.Herbet G, Lemaitre AL, Moritz-Gasser S, et al. The antero-dorsal precuneal cortex supports specific aspects of bodily awareness. Brain 2019;142:2207-14. 10.1093/brain/awz179 [DOI] [PubMed] [Google Scholar]
- 15.Yoshino A, Okamoto Y, Okada G, et al. Changes in resting-state brain networks after cognitive-behavioral therapy for chronic pain. Psychol Med 2018;48:1148-56. 10.1017/S0033291717002598 [DOI] [PubMed] [Google Scholar]
- 16.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A 2009;106:13040-5. 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catley MJ, O’Connell NE, Berryman C, et al. Is tactile acuity altered in people with chronic pain? A systematic review and meta-analysis. J Pain 2014;15:985-1000. 10.1016/j.jpain.2014.06.009 [DOI] [PubMed] [Google Scholar]
- 18.Chou KH, Yang FC, Fuh JL, et al. Bout-associated intrinsic functional network changes in cluster headache: A longitudinal resting-state functional MRI study. Cephalalgia 2017;37:1152-63. 10.1177/0333102416668657 [DOI] [PubMed] [Google Scholar]
- 19.Becerra L, Veggeberg R, Prescot A, et al. A ‘complex’ of brain metabolites distinguish altered chemistry in the cingu-late cortex of episodic migraine patients. Neuroimage Clin 2016;11:588-94. 10.1016/j.nicl.2016.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadjipavlou G, Dunckley P, Behrens TE, et al. Determining anatomical connectivities between cortical and brainstem pain processing regions in humans: a diffusion tensor imaging study in healthy controls. Pain 2006;123:169-78. 10.1016/j.pain.2006.02.027 [DOI] [PubMed] [Google Scholar]
- 21.Niddam DM, Lai KL, Tsai SY, et al. Brain metabolites in chronic migraine patients with medication overuse headache. Cephalalgia 2020;40:851-62. 10.1177/0333102420908579 [DOI] [PubMed] [Google Scholar]
- 22.Denuelle M, Fabre N, Payoux P, et al. Hypothalamic activation in spontaneous migraine attacks. Headache 2007;47:1418-26. [DOI] [PubMed] [Google Scholar]
- 23.Bliss TV, Collingridge GL, Kaang BK, et al. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci 2016;17:485-96. 10.1038/nrn.2016.68 [DOI] [PubMed] [Google Scholar]
- 24.Tian Z, Yamanaka M, Bernabucci M, et al. Characterization of serotonin-induced inhibition of excitatory synaptic transmission in the anterior cingulate cortex. Mol Brain 2017;10:21. 10.1186/s13041-017-0303-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matharu MS, Bartsch T, Ward N, et al. Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain 2004;127:220-30. 10.1093/brain/awh022 [DOI] [PubMed] [Google Scholar]
- 26.Fulbright RK, Troche CJ, Skudlarski P, et al. Functional MRimaging of regional brain activation associated with the affective experience of pain. AJR Am J Roentgenol 2001;177:1205-10. 10.2214/ajr.177.5.1771205 [DOI] [PubMed] [Google Scholar]
- 27.Aygül R, Deniz O, Kocak N, et al. The clinical properties of a migrainous population in Eastern Turkey-Erzurum. South Med J 2005;98:23-7. 10.1097/01.SMJ.0000145390.12710.D5 [DOI] [PubMed] [Google Scholar]
- 28.Main A, Dowson A, Gross M. Photophobia and phonophobia in migraineurs between attacks. Headache 1997;37:492-5. 10.1046/j.1526-4610.1997.3708492.x [DOI] [PubMed] [Google Scholar]
- 29.Boulloche N, Denuelle M, Payoux P, et al. Photophobia in migraine: an interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry 2010;81:978-84. 10.1136/jnnp.2009.190223 [DOI] [PubMed] [Google Scholar]
- 30.Wang M, Su J, Zhang J, et al. Visual cortex and cerebellum hyperactivation during negative emotion picture stimuli in migraine patients. Sci Rep 2017;7:41919. 10.1038/srep41919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache 2008;48:1157-68. 10.1111/j.1526-4610.2008.01217.x [DOI] [PubMed] [Google Scholar]
- 32.Mier RW, Dhadwal S. Primary Headaches. Dent Clin North Am 2018;62:611-28. 10.1016/j.cden.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 33.Szabó E, Galambos A, Kocsel N, et al. Association between migraine frequency and neural response to emotional faces: An fMRI study. Neuroimage Clin 2019;22:101790. 10.1016/j.nicl.2019.101790 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as