Abstract
Background
Family with Sequence Similarity 13, Member A (FAM13A) gene has been consistently associated with COPD by Genome-wide association studies (GWAS). Our previous study demonstrated that FAM13A was mainly expressed in the lung epithelial progenitors including Club cells and alveolar type II epithelial (ATII) cells. Fam13a−/− mice were resistant to cigarette smoke (CS)–induced emphysema through promoting β-catenin/Wnt activation. Given the important roles of β-catenin/Wnt activation in alveolar regeneration during injury, it is unclear when and where FAM13A regulates the Wnt pathway, the requisite pathway for alveolar epithelial repair, in vivo during CS exposure in lung epithelial progenitors.
Methods
Fam13a+/+ or Fam13a−/− mice were crossed with TCF/Lef:H2B-GFP Wnt-signaling reporter mouse line to indicate β-catenin/Wnt-activated cells labeled with GFP followed by acute (1 month) or chronic (7 months) CS exposure. Fluorescence-activated flow cytometry analysis, immunofluorescence and organoid culture system were performed to identify the β-catenin/Wnt-activated cells in Fam13a+/+ or Fam13a−/− mice exposed to CS. Fam13a;SftpcCreERT2;Rosa26RmTmG mouse line, where GFP labels ATII cells, was generated for alveolar organoid culture followed by analyses of organoid number, immunofluorescence and gene expression. Single cell RNA-seq data from COPD ever smokers and nonsmoker control lungs were further analyzed.
Findings
We found that FAM13A-deficiency significantly increased Wnt activation mainly in lung epithelial cells. Consistently, after long-term CS exposure in vivo, FAM13A deficiency bestows alveolar epithelial progenitor cells with enhanced proliferation and differentiation in the ex vivo organoid model. Importantly, expression of FAM13A is significantly increased in human COPD-derived ATII cells compared to healthy ATII cells as suggested by single cell RNA-sequencing data.
Interpretation
Our findings suggest that FAM13A-deficiency promotes the Wnt pathway-mediated ATII cell repair/regeneration, and thereby possibly mitigating CS-induced alveolar destruction.
Fund
This project is funded by the National Institutes of Health of United States of America (NIH) grants R01HL127200, R01HL137927, R01HL148667 and R01HL147148 (XZ).
Keywords: FAM13A, β-catenin/Wnt pathway, Repair/regeneration, Epithelial organoids, Cigarette smoke, COPD/emphysema
1. Introduction
Chronic obstructive pulmonary disease (COPD), one of the most common chronic respiratory diseases, is among the leading causes of mortality and morbidity worldwide [1], [2], [3]. Despite extensive effort over the last few decades, COPD remains as a progressive and devastating disease with few therapeutic options [4], [5], [6].
COPD tends to develop in long-term smokers who are more susceptible to cigarette smoke (CS)-induced injury. Genome-wide association studies (GWAS) has greatly expanded the list of COPD susceptibility genes, among which FAM13A (family with sequence similarity member 13A) has been consistently associated with COPD [7], [8], [9], [10], lung function and idiopathic pulmonary fibrosis (IPF) [11]. We have found that FAM13A is mainly expressed in Club cells and alveolar type II epithelial (ATII) cells with increased expression in COPD lungs [5]. COPD risk allele at the FAM13A locus is associated with increased expression of FAM13A and decreased cell proliferation [12] in lung epithelial cells. In vivo, Fam13a−/- mice are resistant to airspace enlargement upon chronic CS exposure while in vitro, FAM13A promotes the degradation of β-catenin by interacting with protein phosphatase 2A (PP2A) and GSK-3β and thereby inhibits the Wnt pathway [5], a pathway known to be important for alveolar repair/regeneration. However, whether and how FAM13A regulates lung epithelial repair/regeneration is incompletely understood.
Emphysema represents a major pathological change in COPD lungs where alveoli, the smallest respiratory unit in lungs, are destructed accompanied with extracellular matrix loss, possibly due to defective lung repair upon CS-induced injury. Alveoli are normally lined with flat alveolar type I epithelial cells (ATI) that make up more than 95% of the alveolar surface and mediate gas exchange, and ATII cells that secrete surfactant and also function as alveolar stem cells. Recent studies suggested an essential role of the Wnt pathway in lung repair and regeneration [13,14], possibly by promoting self-renewal of ATII cells during alveologenesis post injury [15], [16], [17]. β-catenin is a key signaling molecule in the canonical Wnt pathway [18]. In response to injury, β-catenin/Wnt signaling in lung epithelial progenitor cells is activated to facilitate lung repair and maintain lung function [19], [20], [21]. Upon activation, β-catenin is accumulated in the cytosol and translocated to the nucleus, binds to the T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors and activates the expression of Wnt target genes, thus contributing to both airway [22,23] and alveolar epithelial repair [4,24,25]. However, the precise spatial and temporal regulation of the Wnt pathway in lungs during smoke-induced injury remains unexplored.
Here, we aim to characterize the temporal and spatial regulation of the Wnt pathway by deficiency of FAM13A during CS exposure in vivo and ex vivo. Regeneration capacity of alveolar epithelial progenitors regulated by FAM13A under chronic CS exposure were assessed ex vivo in the alveolar organoid model. Further analysis on single cell RNA-seq data from human COPD lungs and control lungs suggested human alveolar type II epithelial cells as the important cell type for FAM13A to contribute to COPD pathogenesis. These data will likely add new molecular insights to our evolving understanding on the regulation of the Wnt pathway in vivo; and provide supporting evidence for alveolar regeneration/repair regulated by FAM13A, one the most replicated COPD genetic susceptible genes.
2. Methods
2.1. Animals
Fam13a−/− mice in the C57BL/6 background were generated as previously described [5]. TCF/Lef:H2B-GFP mouse line on the C57BL/6 background was purchased from the Jackson Laboratory (#013752, The Jackson Laboratory, Bar Harbor, ME) [26]. Fam13a+/+ and Fam13a−/− mouse lines were crossed with TCF/Lef:H2B-GFP Wnt-signaling reporter line to indicate activity of the Wnt pathway at single-cell resolution by expression of GFP at the activation site. SftpcCreERT2;Rosa26RmTmG mice [27] were kindly provided by Dr. Harold Chapman (UCSF, San Francisco, California, USA). This moue line was crossed with Fam13a+/+ and Fam13a−/− mice and resulting mouse lines were administrated of tamoxifen (#T5648, Sigma-Aldrich, St. Louis, MO) in corn oil (#C8267, Sigma-Aldrich, St. Louis, MO) at a dose of 75 mg/kg body weight via daily intraperitoneal injection for four consecutive times at age of 1.5 month. At the indicated time point, mouse lungs were collected for isolation of primary cells or FACS analysis.
2.2. Ethics
Mice were fed ad libitum and housed in quiet rooms without disturbance of their sleep/wake behavior to reduce environmental stress in the animal facility of Brigham and Women's Hospital. Mouse experiments were performed under IACUC guidelines and approved protocol 2016N000391 at Brigham and Women's Hospital.
2.3. Cigarette smoke exposure in vivo
Female Fam13a-TCF/Lef:H2B-GFP mice at 8 weeks of age (5-6 mice/genotype/condition) were exposed to mixed main-stream and side-stream cigarette smoke (3R4F Kentucky Research cigarettes) for 6 days/week in Teague TE 10z chambers [(TSP ~ 100-200 mg/m3 and CO levels ~6 ppm). Mice were harvested after 1 month or 7 months of CS exposure to assess effects of short-term and long-term CS exposure.
2.4. Mouse lung dissection and flow cytometry analysis
After Air or CS exposure, mice from each group were harvested and lungs were perfused. Left lobes of mouse lungs were inflated by Z-fix (#170, Anatech Ltd, Battle Creek, MI) and removed for histology and immunofluorescence staining while right lobes were dissociated with a collagenase/dispase solution as previously described [21]. Briefly, 2 ml of dispase (#354235, Corning, Corning, NY) were instilled into right lobes through trachea and tied by suture to avoid leakage. Lobes were dissected, minced into small pieces and washed with 3 ml of PBS containing 60 µl of collagenase/dispase at a 100 mg/ml stock solution (#10269638001, Sigma-Aldrich, St. Louis, MO) followed by incubation at 37°C for 45 minutes. DNase (10 mg/ml) was then added into digested lung samples (#11284932001, Sigma-Aldrich, St. Louis, MO), which was further filtered sequentially through 100- and 40- µm cell strainers and centrifuged at 300 x g at 4°C for 6 minutes. Cells were then incubated in the ACK lysis buffer (#A1049201, Thermo Fisher Scientific, Waltham, MA) for 90 seconds on ice for red blood cells removal. Seven milliliters of 10% FBS was slowly added and cells were centrifuged at 300 g at 4°C for 6 minutes. Cell pellets were resuspended in 10% FBS for FACS sorting for GFP+ and GFP- cells with FACSAria-561 (BD Biosciences, Billerica, MA) in the Immunology Flow Cytometry Core Facility at Harvard Medical School. For cellular composition analysis on GFP+ cells, resuspended cells were also stained with antibodies CD45-PE-Cy7 (#103114, eBioscience, San Diego, CA), EpCAM-APC (#17-5791-80, Thermo Fisher Scientific, Waltham, MA) and EpCAM-PE-Cy7 (#25-5791-80, Thermo Fisher Scientific, Waltham, MA) for flow cytometry analysis. 7-AAD staining (#559925, BD Pharmingen, San Diego, CA) was used for removal of dead cells. A FACSAria II (BD Biosciences, Billerica, MA) was used for FACS data collection and data was analyzed by FlowJo software (FlowJo LLC).
2.5. Histology and immunofluorescence staining
Mouse lung tissues were fixed overnight by Z-fix (#170, Anatech Ltd, Battle Creek, MI) at 4°C and paraffin sections were generated and used for Haemotoxylin & Eosin (H&E) by InvivoEx LLC (InvivoEx LLC, Waltham, MA) and immunofluorescence staining. Cultured organoids were fixed with 10% formalin at 4°C overnight followed by immobilized with histogel (#HG-4000-012, Thermo Fisher Scientific, Waltham, MA) for paraffin embedding at Rodent Histopathology [DF/HCC] Core, Harvard Medical School. Immunofluorescence staining was performed in lung sections and organoid colonies as described previously [21,28]. Briefly, paraffin-embedded tissue sections (5 µm) were rehydrated and subjected to antigen retrieval with Tris-HCl buffer (100 mM, pH 9.5). After one hour of blocking with 15% BSA in 0.2% Triton-X/PBS at room temperature, slides were incubated with various primary antibodies at 4°C overnight including mouse anti-β-catenin phosphorylated at Tyr 489 (PY489-B-catenin, Developmental Studies Hybridoma Bank, Iowa city, IA), chicken anti-GFP (#ab13970, Abcam, Cambridge, MA), hamster anti-PDPN (#AB_531893, Developmental Studies Hybridoma Bank, Iowa city, IA), rabbit anti-SP-C (#ab211326, Abcam, Cambridge, MA), mouse anti-CC10 (#sc-365992, Santa Cruz Biotechnology Inc, Dallas, TX) and anti-acetylated tubulin (#sc-23950, Santa Cruz Biotechnology, Dallas, TX) antibodies. Following incubation with Alexa Fluor conjugated secondary antibodies (Invitrogen, Carlsbad, CA), lung sections were counterstained with DAPI in ProLong gold antifade mounting reagent (#P36935, Thermo Fisher Scientific, Waltham, MA). EdU signals were detected by using a Click-iT™ EdU Alexa Fluor™ 488 Imaging Kit (#C10337, Thermo Fisher Scientific). Stained sections were visualized using a ZEISS microscope and analyzed in 5 randomly acquired fields per mouse with 5-6 mice per group.
2.6. Lung epithelial cells grown at 3D organoid culture models
Lung organoid co-culture was performed as previously described with minor modification [17,29]. Freshly FACS-sorted 3,000 mouse lung GFP+ cells or EpCAM+ cells were resuspended in 3D culture medium (Dulbecco's Modified Eagle's Medium/F12 supplemented with 1 mM HEPES, penicillin streptomycin, insulin/transferrin/selenium and 10% FBS), and mixed with growth factor reduced Matrigel (#354234, Thermo Fisher Scientific, Waltham, MA) containing 100,000 MLg2908 cells at a ratio of 1:1. Cell mixtures were plated in a 24-well plate with transwell inserts with a 0.4-μm pore (#3470, Corning, Corning, NY). 3D culture medium was placed in the lower chamber and medium was changed every other day. Ten μM of the rock inhibitor Y-27632 (#SCM075, Sigma-Aldrich, St. Louis, MO) was included in the medium for the first two days of culture. Number of colonies was counted after 14 days of culture. Organoids were fixed by 10% formalin for further immunofluorescence (IF) staining or dissociated by dispase for qPCR analyses. Cell proliferation was assessed by EdU incorporation assay as previously described [20]. EdU (#C10337, Thermo Fisher Scientific) was added to the culture media at a concentration of 10 μM for 24-hours of incubation. Quantifications on cell proliferation and differentiation were based on number of positive cells stained with EdU or PDPN respectively from organoid sections. Data were obtained from three organoid sections of at least 2 mice per genotype per condition.
2.7. RNA extraction and quantitative RT-PCR
Total RNA was extracted from organoids after dissociation by dispase using RNeasy plus micro kit (#74034, Qiagen, Venlo, Netherlands). cDNA was obtained using high-capacity cDNA reverse transcription kit (#4374966, Thermo Scientific, Waltham, MA). Relative gene expression was measured by real-time PCR using TaqMan assays (#4444964, Thermo Scientific, Waltham, MA) in a QuantStudio 12K machine (Life Technologies, Carlsbad, CA) and normalized to GAPDH expression. TaqMan probes purchased from Integrated DNA Technologies (IDT, Skokie, IL) including mouse GAPDH (Mm. PT.39a.1) and mouse Axin2 (Mm.PT.58.8726473). All samples were assayed in duplicates by quantitative real-time PCR. Expression levels of target genes were calculated based on the 2−ΔΔCt method as we performed previously [30].
2.8. Single cell RNA-Seq data analysis in human lungs
Publicly available human single cell RNA sequencing dataset (GSE136831) [31] was analyzed for cell type specific gene expression using Seurat v3 [32]. Cells from ever-smoker COPD lungs (n= 17) or nonsmoker control lungs (n= 22) were selected for analysis with 56,407 and 62,200 cells respectively. We referenced the cell types based on the existing annotation of the dataset (“CellType_Category” and “Manuscript_Identity”). Basal cells were defined as “Basal” and “Aberrant Basaloid” cells. Average expression was calculated by natural log transformation of averaged expression of RNA assay using AverageExpression function in Seurat. To identify differentially expressed genes between COPD and controls in each cell type, we used data with “MAST” as implemented in the Seurat FindMarkers function. Genes were considered differentially expressed if the adjusted P value was equal or less than 0.05.
2.9. Quantification and statistics
All quantifications were processed by Image J (Image analysis in Java 1. 45 s, NIH). Statistical analyses were performed using GraphPad Prism 7 and described in detail in each figure legend. The normal distribution of data was assessed by Shapiro-Wilk normality test. Two-tailed unpaired t test or the Mann-Whitney test was used for comparisons of normally and non-normally distributed data, respectively. Two-way ANOVA was used to detect genotype by smoke treatment interaction when data are normally distributed. Data are presented as Means ± SEM. The statistical significance was claimed if established at p < 0.05 as indicated by * or # to represent different control groups used or ** for p < 0.01.
2.10. Role of the funding source
The funder of this work had no role in study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the article for publication.
3. Results
3.1. TCF/Lef:H2B-GFP reporter line exhibits β-catenin/Wnt signaling
FAM13A, significantly associated with COPD in GWAS, promotes the degradation of β-catenin and inhibits the Wnt pathway. Furthermore, Fam13a−/− mice are resistant to CS-induced emphysema compared with Fam13a+/+ mice [5]. However, it is unknown when and where FAM13A regulates the β-catenin/Wnt pathway in vivo during CS exposure. To address this, Fam13a−/- mice [5] were crossed with TCF/Lef:H2B-GFP mouse line, the widely used Wnt pathway reporter line with high specificity and sensitivity in vivo [26,[33], [34], [35]]. Mice were then subsequently exposed to Air or CS for 1 month or 7 months. Schematic illustration of this mouse line in the current study is shown in Fig. 1A. To confirm the activity of the β-catenin/Wnt signaling in GFP+ cells from this reporter line, we determined the expression of Axin2 in FACS-sorted GFP+ and GFP− cells by qPCR analysis. As we expected, expression of Axin2 is significantly increased in GFP+ cells compared to GFP− cells in CS-exposed Fam13a+/+ mice (Fig. 1B) and CS-exposed Fam13a−/− mice for 7 months (Fig. 1C). To further determine whether GFP+ cells have active β-catenin/Wnt signaling, we co-stained lung sections from CS-exposed Fam13a−/− mice with antibodies recognizing GFP and active β-catenin. GFP+ cells in distal lung have active β-catenin signaling (Fig. 1D). These data suggest that the TCF/Lef:H2B-GFP reporter line represents activation of the β-catenin/Wnt signaling in murine lungs, helpful to determine when and where FAM13A regulates the β-catenin/Wnt pathway in vivo during CS exposure.
Fig. 1.
The β-catenin/Wnt signaling is activated in GFP+ cells from Fam13a-TCF/Lef:H2B-GFP Wnt reporter line. A. Experimental scheme of the Fam13a-TCF/Lef:H2B-GFP Wnt reporter line exposed to short-term (1 month) and long-term (7 months) Cigarette Smoke (CS). Expression of Axin2 in CS-exposed Fam13a+/+ mice (B) and CS-exposed Fam13a−/− mice (C) was measured in FACS-sorted GFP+ cells from Fam13a-TCF/Lef:H2B-GFP mouse line by qPCR. Data are expressed as Mean ± SEM. n = 3-4 mice/group. **p < 0.01 by unpaired t test. D. Representative images of immunofluorescence staining showing GFP+ cells co-expressing active β-catenin in lung sections from CS-exposed Fam13a−/− TCF/Lef:H2B-GFP mouse line. Cell stained with GFP (green) and active β-catenin (red) antibodies and nuclei labeled with DAPI (blue) in lung sections. White arrows indicate GFP+ cells expressing active β-catenin.
3.2. Depletion of FAM13A or CS exposure activates β-catenin/Wnt pathway in vivo
To assess the temporal and spatial regulation of the β-catenin/Wnt pathway by Fam13a in vivo upon CS-induced injury, we performed fluorescence-activated flow cytometry (FACS) analysis in GFP+ cells from mice that are exposed to CS for 1 month or 7 months. We quantified the percentage of GFP+ cells, representing Wnt-responsive cells (Fig. 2A). First, Fam13a−/- mice have increased percentage of GFP+ cells compared to Fam13a+/+ mice under air condition (Fig. 2B and C) while CS exposure also significantly increased the percentage of GFP+ cells in Fam13a+/+ lungs (Fig. 2B and C). However, no further increase of percentage of GFP+ cells in Fam13a−/- mice was observed after CS exposure compared with condition-matched Fam13a+/+ mice. These results indicate that CS exposure or depletion of FAM13A led to activation of the β-catenin/Wnt pathway in murine lungs. Furthermore, GFP+ cells are localized in both airways and alveoli (Fig. 2D).
Fig. 2.
β-catenin/Wnt signaling is activated in murine lungs after CS exposure for 1 and 7 months. A. Representative flow cytometry analysis showing GFP+ cell population in Fam13a+/+ and Fam13a−/− murine lungs with or without exposure to CS for 1 month. GFP+ cells in murine lungs were quantified by flow cytometry in mice exposed to CS for 1 (B) and 7 months (C). Data are expressed as Mean ± SEM. n = 5-6 mice/group. *p < 0.05 or **p < 0.01 by unpaired t test. D. Representative immunofluorescence staining showing expression of GFP in murine lungs at high and low magnifications. Cell stained with GFP (green) antibodies and nuclei labeled with DAPI (blue) in lung sections from Fam13a-TCF/Lef:H2B-GFP mice. White arrows indicate GFP+ cells. CS, cigarette smoke; M, month or months.
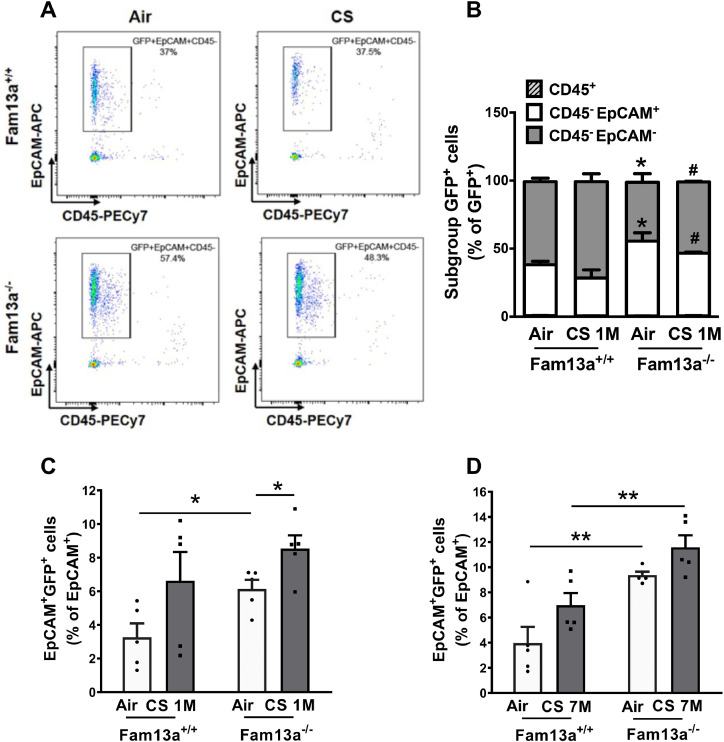
3.3. Depletion of FAM13A promotes activation of the Wnt signaling in lung epithelial cells
To further assess which cell types have activated Wnt pathway in lungs, we performed CD45 and EpCAM staining in FACS analysis to label immune and epithelial cells in gated GFP+ population, respectively (Fig. 3A). Under air condition, GFP+ lung epithelial cells (GFP+CD45−EpCAM+/GFP+) were significantly increased in Fam13a−/− mice compared to Fam13a+/+ mice (Fig. 3B). Furthermore, we found that the composition of GFP+ cells differed in CS-exposed Fam13a+/+ and Fam13a−/− mice. After CS exposure for 1 month, Fam13a−/− mice have more Wnt activation in lung epithelial cells and less in non-epithelial cells than Fam13a+/+ mice (Fig. 3B). Importantly, Wnt-activated epithelial cells (GFP+EpCAM+/EpCAM+) significantly increased in Fam13a−/− mice under either air or long-term CS exposure compared to condition-matched Fam13a+/+ mice (Fig. 3C and D) despite comparable percentage of epithelial cells among all groups (Fig. S1A and B). Taken together, FAM13A deficiency led to activation of the Wnt pathway predominantly in lung epithelial cells, especially after long term CS exposure.
Fig. 3.
FACS analysis showing activated β-catenin/Wnt signaling enriched in lung epithelial cells of Fam13a−/− mice. A. Representative profile of flow cytometry analysis showing gating of GFP+ cells indicated from Fig. 2A co-expressing EpCAM+ in CD45− population in Fam13a+/+ and Fam13a−/− murine lungs with or without exposure to CS for 1 month. B. Distribution of GFP+ cells in different cell subgroups (epithelial cells: CD45−EpCAM+, immune cells: CD45+, and non-immune/non-epithelial cells: CD45−EpCAM− cells) in murine lungs from Fam13a+/+ and Fam13a−/− mice after exposure to CS for 1 month. *p < 0.05 vs air-exposed Fam13a+/+ mice and #p < 0.05 vs CS-exposed Fam13a+/+ mice by unpaired t test. Data are expressed as Mean ± SEM from 5-6 mice/group. Quantification of GFP+ EpCAM+ cell populations in lung epithelial cells from Fam13a+/+ and Fam13a−/− mice after exposure to CS for 1 (C) or 7 months (D). Mean ± SEM are from 5-6 mice/group. *p < 0.05 or **p < 0.01 by unpaired t test. CS, cigarette smoke; M, month or months.
3.4. Depletion of FAM13A activates Wnt signaling in both Club cells and ATII cells after CS exposure
To further determine which type of lung epithelial cells have activated Wnt pathway after long term CS exposure and FAM13A deficiency, we co-stained lung sections with antibodies recognizing GFP and epithelial marker genes. First, in airways, both Club cells and ciliated cells demonstrated GFP+ signals (Fig. 4A and B, and Fig. S2A). Furthermore, significant interaction between CS exposure and FAM13A deficiency on the percentage of Wnt-activated Club cells (GFP+CC10+/CC10+) was detected (Fig. 4C, two-way ANOVA). However, neither CS exposure nor FAM13A deficiency had impacts on the activation of the Wnt signaling in ciliated cells (GFP+Ac-Tub+/Ac-Tub+) (Fig. S2B). This is consistent with our previous report that Fam13a is mainly expressed in Club cells and ATII cells. Among total cells in the captured areas in each group, percentage of ciliated cells and Club cells defined by positive staining was comparable in all groups under air or CS exposure (Fig. S2C and D).
Fig. 4.
β-catenin/Wnt signaling is activated in both Club cells and ATII cells of Fam13a−/− mice. A. Representative immunofluorescence images showing location of GFP+ cells in murine lungs: GFP (green); CC10 (red) for Club cells; SPC (red) for ATII cells; and DAPI (blue) in lung tissue sections. Yellow arrows indicate GFP+ cells expressing CC10. White arrows indicate GFP+ cells expressing SPC. B. Representative images showing GFP+ cells co-expressing CC10 or SPC at high magnification. Yellow arrows indicate GFP+ cells expressing CC10. White arrows indicate GFP+ cells expressing SPC. C. Quantification of the mean percentage of GFP+ cells that express CC10 is shown after exposure to CS for 7 months. *p < 0.05 or **p < 0.01 by unpaired t test and significant interaction effects between genotype and CS exposure using two-way ANOVA. D. Quantification of percentage of GFP+ cells that express SPC after exposure to CS for 7 months. *p < 0.05 by Mann-Whitney test. Means ± SEM represent quantification of signals from whole section per mouse and 5-6 mice per group. CS, cigarette smoke; M, month or months.
In distal lungs, GFP+ signals were evident in SPC+ ATII cells after long-term CS exposure (Fig. 4A and B). The proportion of Wnt-activated ATII cells (GFP+SPC+/SPC+) was significantly increased after chronic CS exposure in Fam13a+/+ mice as compared to air exposure (Fig. 4D). Importantly, Wnt-activated ATII cells also significantly increased in CS-exposed Fam13a−/− mice compared to CS-exposed Fam13a+/+ mice (Fig. 4D) while the total number of ATII cells are comparable in all groups by quantification of immunofluorescence staining (Fig. S2A and E). Despite emerging roles of bronchioalveolar stem cells (BASCs, CC10+SPC+), which contribute to the maintenance of bronchiolar club cells and alveolar epithelial cells [36], and promote lung regeneration upon infection of influenza virus [37] and naphthalene-induced airway injury [38], no activation of the Wnt signaling was detected in BACS cells [36] upon smoke exposure in this study (Fig. S3F), possibly due to sparsity of BASCs captured in lung sections. Collectively, these data suggest that Wnt is activated in ATII cells after smoke-induced injury while genetic depletion of FAM13A activates Wnt pathway in lung epithelial progenitor cells including Club cells and ATII cells.
3.5. Fam13a deficiency ameliorated impacts of chronic CS exposure on regeneration capability of the lung epithelial cell
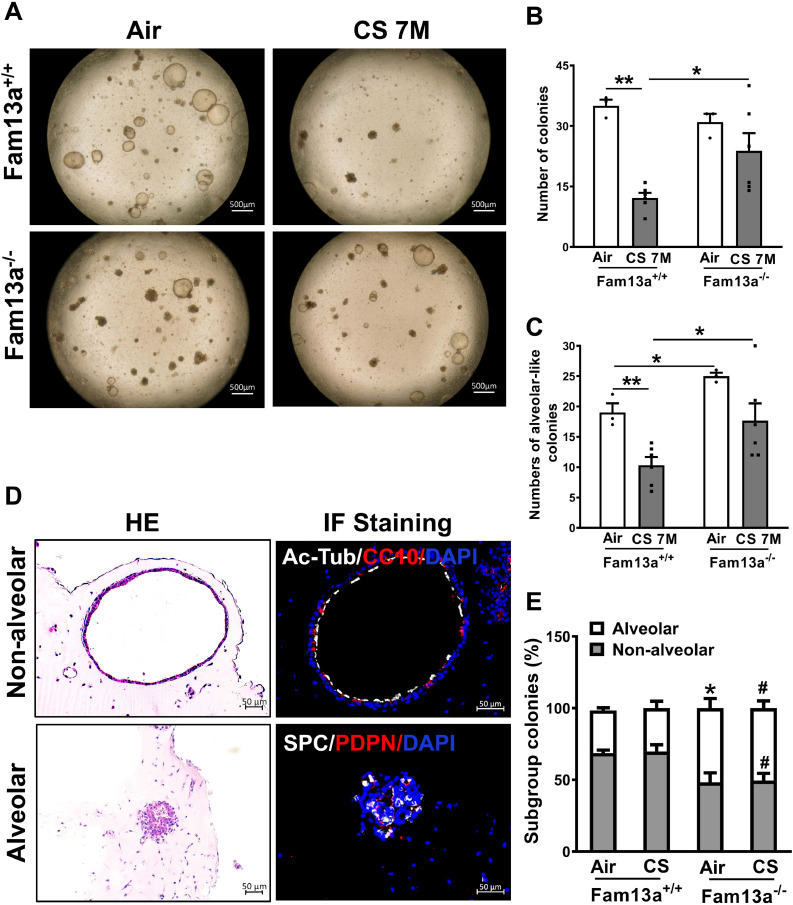
Alveolar organoid 3D model has been recently established for studying the growth and differentiation of lung epithelial progenitors [17,21,39,40]. Therefore, we generated lung epithelial organoids using lung epithelial cells (EpCAM+) derived from Fam13a+/+ and Fam13a−/− mice exposed to chronic CS. Chronic CS exposure led to significantly decreased number of colonies in Fam13a+/+ mice in contrast to Fam13a−/− mice (Fig. 5A and B). Furthermore, lung epithelial cells isolated from Fam13a−/− mice formed more alveolar-like organoids than Fam13a+/+ mice under either air exposure or chronic CS exposure (Fig. 5A and C).
Fig. 5.
Deficiency of Fam13a leads to increased alveolar organoid formation in EpCAM+ cell organoid culture after in vivo CS exposure for 7 months. A. Representative bright field images of organoids derived from EpCAM+ cells from mice exposed to air or CS for 7 months. Quantification on the total colonies (B) and alveolar-like colonies (C) in the organoids model. Mean ± SEM from triplicate wells of each mouse and two mice per genotype. *p < 0.05 or **p < 0.01 by Mann-Whitney test (B) or unpaired t test (C). D. Representative images of organoids from EpCAM+ lung epithelial cells. Histology (left) and immunofluorescence staining (right) for Ac-Tub (white) co-stained with CC10 (red), or SPC (white) co-stained with PDPN (red). Cell nuclei were counterstained with DAPI (blue). E. Quantification of each type of organoid colonies is shown from EpCAM+ cells from mice exposed to air or CS for 7 months. Mean ± SEM from three sections of each mouse and two mice per genotype *p < 0.05 or **p < 0.01 by unpaired t test. CS, cigarette smoke; M, months.
To further characterize the identity of the epithelial organoids, we defined organoids as alveolar and non-alveolar types based on immunostaining for various epithelial cell markers (Fig. 5D). Non-alveolar organoids showed a lumen-like structure lined with Club and ciliated cells expressing CC10 and Ac-tub, respectively. Alveolar organoids contain SPC-expressing ATII cells and PDPN-expressing ATI cells. Importantly, Fam13a deficiency led to a significant increase in the proportion of alveolar organoids compared to Fam13a+/+ cultures after in vivo chronic CS exposure (Fig. 5E). Taken together, despite reduced colony formation capability of lung epithelial cells after long term CS exposure in vivo, FAM13A deficiency ameliorated alveolar organoid colony forming efficiency of lung epithelial cells.
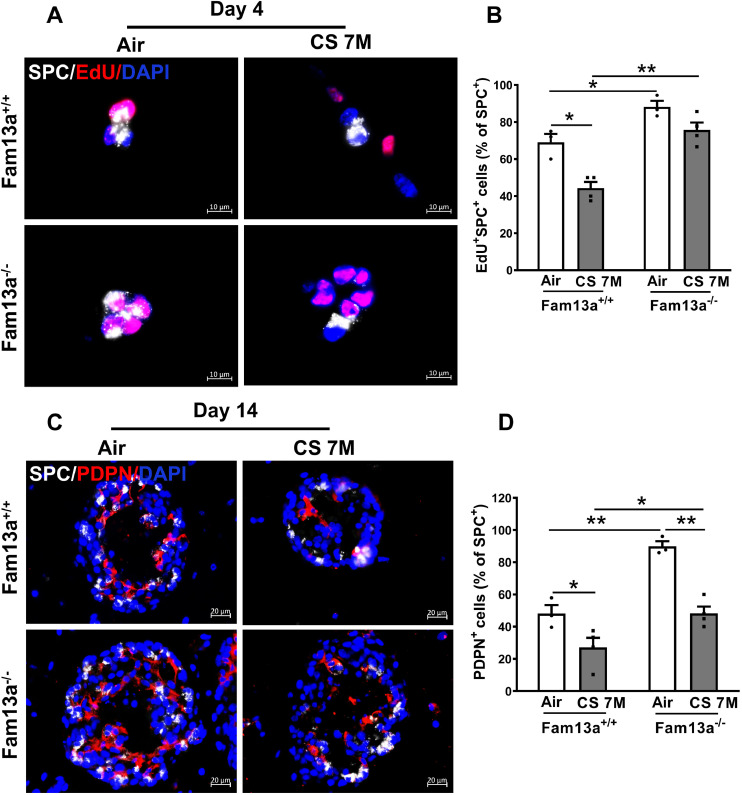
3.6. FAM13A deficiency promotes ATII cells proliferation and differentiation ex vivo
To determine the kinetics of proliferation and differentiation of lung epithelial cells grown in the alveolar organoid model, we performed EdU incorporation assay followed by co-staining with the antibody for AT II cell marker SPC in 3D organoids cultured for different days (2, 4, 8 and 14 days of culture). The proliferation of ATII cells peaked on day 4, followed by a reduction on day 8 with increased number of PDPN-expressing ATI cells, indicating occurrence of differentiation (Fig. S3). Therefore, we choose day 4 to assess proliferation while day 14 to assess differentiation of ATII cells in the alveolar organoid model. Indeed, Fam13a−/− ATII cells showed increased proliferation and differentiation as compared to condition-matched Fam13a+/+ ATII cells (Fig. 6, A-D), consistent with enhanced formation of alveolar colonies (Fig. 5), suggesting that depletion of Fam13a promotes ATII cells proliferation and differentiation, which may potentially improve alveolar regeneration upon injury.
Fig. 6.
Deficiency of Fam13a promotes alveolar epithelial cell proliferation and differentiation in the organoid model. A. Representative images of immunofluorescence (IF) staining for proliferating organoids cultured for 4 days. Lung EpCAM+ cells were isolated from Fam13a+/+ or Fam13a−/− mice exposed to in vivo CS for 7 months. ATII cell marker SPC (white) was co-stained with EdU (red) and counterstained with DAPI (blue) for nuclei. B. Quantification of the proliferating ATII cells (EdU+SPC+/ SPC+) in A. Mean ± SEM from ~100 cells per section, three sections of each mouse and two mice per genotype. C. Representative images of differentiating organoids derived from EpCAM+ cells after 14 days of culture. IF images with SPC (white) co-stained with PDPN (red) and nuclei counterstained with DAPI (blue). D. Quantification of the PDPN+ cells in organoids after 14 days of culture. Mean ± SEM from ~200 cells per section, three sections of each mouse and two mice per genotype. *p < 0.05 or **p < 0.01 by unpaired t test. CS, cigarette smoke; M, months.
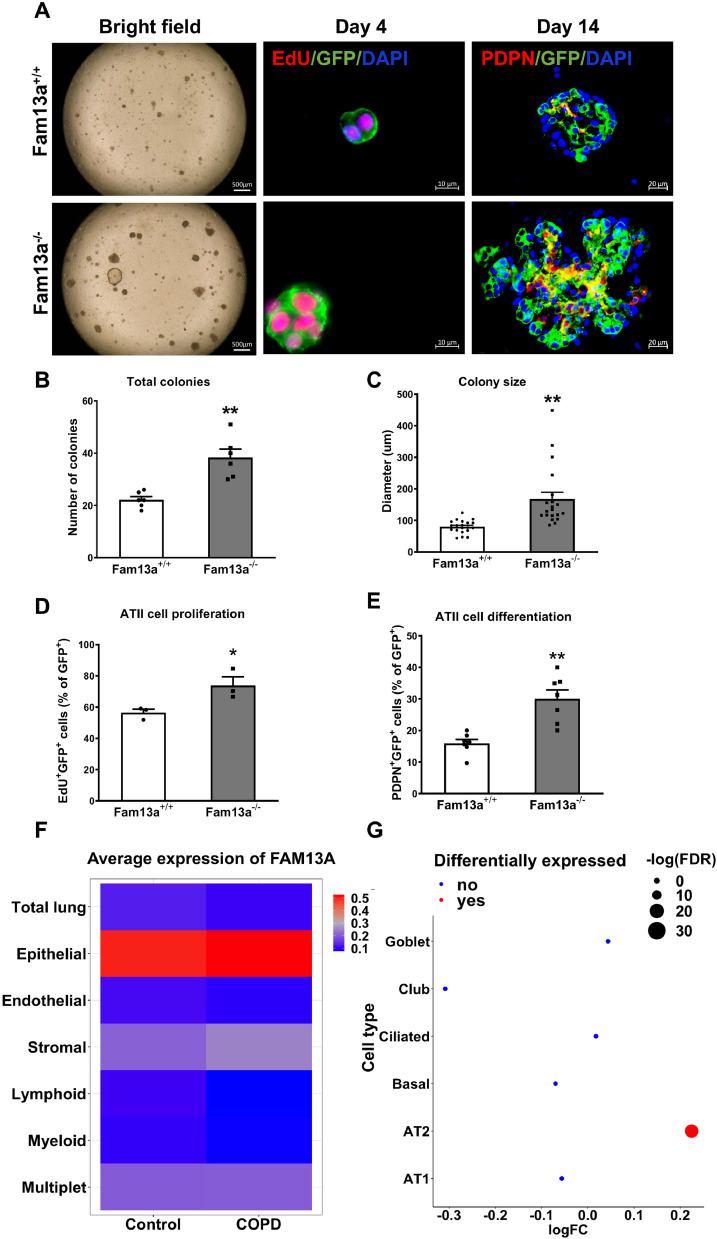
3.7. FAM13A deficiency promotes alveolar organoids growth
To further determine the impacts of loss of FAM13A on regeneration capability of ATII cells, we crossed Fam13a−/− mice with SftpcCreERT2;Rosa26RmTmG mice [27] to generate Fam13a-SftpcCreERT2;Rosa26RmTmG mouse line, where GFP labels ATII cells with more than 90% efficiency as supported by FACS and immunofluorescence staining after tamoxifen induction (Fig. S4). In the organoid model, FAM13A deficiency promotes the proliferation and differentiation of GFP-labeled ATII cells with increased number and size of alveolar colonies (Fig. 7, A-E). Our results further demonstrate that Fam13a deficiency indeed promotes proliferation and differentiation of ATII epithelial progenitors in the organoid models.
Fig. 7.
Deficiency of FAM13A promotes alveolar organoids formation in the organoid model. A. Representative images of organoids derived from GFP-sorted lineage-labeled ATII cells from Fam13a-SftpcCreERT2;Rosa26RmTmG mice at 4 or 14 days of co-culture. Bright field (left) and immunofluorescence images (right) with GFP (green) co-stained with EdU (red) or GFP (green) co-stained with PDPN (red) antibodies; nuclei are counterstained with DAPI (blue). Quantification showing total colonies (B), colony size (C), proliferation (D) and differentiation (E) of ATII cells. Mean ± SEM shown from ~100 cells per section, three sections of each mouse and two mice per genotype. *p < 0.05 or **p < 0.01 by Mann-Whitney test (B, C and E) or unpaired t test (D). F. Heatmap showing expression levels of FAM13A in multiple cell types in human COPD and control lungs detected by single cell RNA-sequencing (GSE136831). G. Expression of FAM13A is significantly increased in COPD ATII cells compared to control lung ATII cells, in contrast to other lung epithelial cells. Size of the dots indicates the -Log (FDR). FC: fold change indicates the human AT2 cells from COPD vs normal donors.
3.8. Increased expression of FAM13A in human COPD ATII cells
To further determine roles of FAM13A in ATII cells in human COPD development, we reanalyzed single cell RNA-seq data from COPD ever smokers (n = 17) and nonsmoker control lungs (n = 22) from a recent publication [31]. Consistent with our finding in murine lungs, FAM13A is primarily expressed in lung epithelial cells. Notably, significantly increased expression of FAM13A in COPD subjects was only found in ATII cells (p adjust =1.8×10−17) among various lung epithelial cell types (Fig. 7F and G), highlighting ATII cells as the most plausible cell type for FAM13A to determine COPD susceptibility.
4. Discussion
COPD, especially emphysema, characterized by alveolar destruction [41], [42], [43] may result from failure of alveolar repair/regeneration after chronic CS exposure. Given emerging importance of the Wnt pathway in lung repair/regeneration, it is imperative to fully characterize the temporal and spatial regulation of the Wnt pathway activity during chronic CS-induced lung injury/repair that may determine emphysema development. Herein, using TCF/Lef:H2B-GFP, a β-catenin/Wnt-signaling reporter line crossed with Fam13a−/- mice, we demonstrated that Fam13a−/− mice have an activated canonical β-catenin/Wnt pathway in both Club cells and ATII cells. Furthermore, despite 7 months of chronic CS exposure, Fam13a−/− lung epithelial cells demonstrated increased proliferation and differentiation ex vivo, leading to increased number of alveolar organoids compared to condition-matched Fam13a+/+ cells. Our results added new understanding to the evolving filed regarding the regulation of Wnt signaling in alveolar regeneration, and also demonstrated that Fam13a is involved in the regenerative response to CS and blunts alveolar organoid formation. In the ex vivo epithelial organoid model, deficiency of FAM13A may promote repair/regeneration of ATII cells where FAM13A showed increased expression in human COPD lungs.
Upon injury, ATII cells proliferate for self-maintenance and differentiate into ATI cells to restore and repair loss of alveoli [13,20,41], modulated by signaling pathways including the canonical β-catenin/Wnt signaling [20,44]. Despite a small number of Wnt-activated cells found in lungs under homeostatic condition (Fig. 2A - C) [17,20,25,45], Wnt signaling was crucial to maintain the stemness of ATII cells, ready for transient expansion responding to injury [20]. Subsequently, repression of the Wnt pathway is required for ATII cells to transdifferentiate into ATI cells [15,17,20,46], indicating the temporal and dynamic activation of the Wnt pathway during alveolar regeneration [15]. FAM13A may act as one of key regulators for modulating the Wnt pathway activity at the appropriate timing for optimal duration needed for proliferation of ATII cells. Although expression of FAM13A is not altered by chronic CS exposure [5,47], future studies on the dynamics of activity and/or abundance of FAM13A protein in ATII cells during CS-induced lung injury/repair will illuminate additional insights into CS-induced emphysema. FAM13A is also expressed in Club cells where lower Wnt activity was detected compared to ciliated cells under homeostatic condition [5]. Club cells, airway epithelial progenitor cells, are capable to self-renew and differentiate into ciliated cells during homeostasis or after injury [13,48,49]. Recently, Club cell was found to differentiate into alveolar epithelial cells in the 3D organoid model [19]. They also give rise to ATII and ATI cells during the repair of severe lung damage following either influenza virus infection or bleomycin treatment in vivo [50,51]. However, in response to a mild injury stimulus such as CS exposure, it remains unclear whether Club cells could serve as epithelial stem cells facilitating alveolar repair. Basal cells [52] and BASCs [36,38] have also been reported as stem cells in murine lungs to contribute to repair/regeneration during lung homeostasis and injury. Especially, BASCs contribute to regeneration of both airway and alveolar epithelium. However, in CS-exposed mice, we detected neither increased number of BASCs nor co-localization of BACSs with GFP+ signals, suggesting less likely that BACSs may contribute to smoke-induced lung repair/regeneration. However, lack of signals may also be attributable to sparse staining of BASCs cells that are located and restricted at bronchioalveolar duct junctions [37,53,54].
Notably, given divergent association of FAM13A with COPD and IPF and activation of the Wnt pathway in Club cells promotes IPF development [55], [56], [57], it is possible that higher intrinsic activity of the Wnt pathway due to lower FAM13A levels in Club cells may promote fibrosis, as reported in Fam13a−/− mice [58]. Therefore, the impact and function of Wnt-activated Club (GFP+CC10+) cells in Fam13a−/− mice and its relevance to pulmonary fibrosis will require future investigations, possibly through lineage tracing approach with Club cell-specific mouse lines crossed with Fam13a−/− mice. Furthermore, deficiency of Fam13a led to increased proliferation and differentiation of ATII cells in the organoid models (Fig. 6 and 7), even after 7 months of CS exposure (Figure 6), possibly mitigating emphysematous destruction in COPD lungs. However, as a double-edged sword, overactivation of the Wnt pathway may promote IPF development. Therefore, systematic consideration of the dynamic context is needed when targeting the FAM13A-Wnt pathway for the treatment of COPD to eliminate the possible side effect of IPF development.
Previously, CS-induced activation of the β-catenin/Wnt pathway was reported in cultured cells and in vivo animal models [5,[59], [60], [61], [62], [63]]. However, downregulated β-catenin/Wnt signaling was also reported in COPD lungs [4,64,65]. These seemingly controversial reports may result from variable cellular compositions in COPD lungs and sampling stage or location of lungs during COPD development. Therefore, careful examination on expression of various Wnt pathway genes in corresponding lung cell types at single-cell levels in COPD lungs may facilitate the comprehensive characterization on the Wnt pathway activity in COPD lungs. The CS-induced activation of the Wnt pathway may represent a compensatory mechanism for lungs to initiate self-renewal and repair machinery in epithelial progenitor cells upon injury. It is noteworthy that increased number of Wnt-activated cells after 7 months of CS in Fam13a+/+ mice (Fig. 2C) may result from proportionally increased activation of Wnt pathway in both epithelial and non-epithelial cells (Fig. 3B).
Admittedly, as summarized in the previous review [66], the fidelity of this Wnt pathway reporter mouse line is complicated by the expression pattern of TCF-dependent and -independent Wnt target genes in various tissue types. The TCF/Lef:H2B-GFP mouse line has six copies of a TCF/Lef responsive element and a histone 2B-tagged green fluorescent protein to report β-catenin/Wnt signaling activity. This mouse line functions as a quantitative readout of canonical Wnt signaling activity at the single-cell resolution [26] and was used to corroborate active β-catenin/Wnt signaling [45]. However, it is controversial whether TCF/Lef:H2B-GFP labels Wnt-active cells. Recent findings indicate that GFP+ cells in the TCF/Lef:H2B-GFP reporter line are likely Wnt-responsive cells and may not completely overlap with Wnt-active lung cells [67]. Furthermore, though Axin2 is a classical marker gene for the canonical Wnt pathway, the Axin2-expressing cells in lungs are extremely rare under homeostasis [15,20], less than the GFP+ cells in the TCF4 reporter mouse line [67], indicating existence of additional Wnt pathway target genes other than Axin2 in lungs. Thus, comprehensive characterizations on specific target genes of the canonical and non-canonical Wnt pathway in various lung cell types will enable us to fully understand impacts of the Wnt pathways during lung repair/regeneration. Additionally, given important developmental roles of the Wnt pathway in murine lungs, future inducible and conditional knockout mouse model of Fam13a in ATII cells and/or Club cells will facilitate to differentiate possible cause of persistent activation of the Wnt pathway in the Club cells and AT II cells: adaptation effects due to loss of Fam13a during embryonic development or the impacts of acute loss of Fam13a postnatally on homeostasis. Lung morphology evaluations on the Fam13a conditional knockout line in AT II cells exposed to chronic smoke exposure will further support roles of Fam13a in the important lung epithelial progenitor cells.
Taken together, we demonstrate increased activation of β-catenin/Wnt signaling in ATII cells upon chronic CS exposure under FAM13A deficiency, which promotes growth of alveolar epithelial cells as shown in the 3D organoid model, possibly leading to alveolar repair/regeneration upon chronic CS-induced injury in vivo. Future work will determine whether targeting FAM13A at right time may present a treatment strategy in COPD while controlling possible unwanted negative impacts due to extended activation of the Wnt pathway.
Contributors
X.Z, X. L and Y.L designed the study. X.L, Y.L and D.Q performed data analysis. S.X and Y.T completed all smoke exposure experiments. X.L, S.X and Y.L performed immunofluorescene staining and isolated primary cells from mouse lungs for 3D organoid co-culture. Y.L performed real-time RT-PCR. X.L performed FACS analysis. J.Y analyzed single cell RNA-seq data from human COPD and normal controls. X.Z, X.L, Y.L, L.G, J.Y, D.Q and Y.T wrote and reviewed the manuscript.
Data Sharing Statement
We will share the data that supports our research article published in EBioMedicine. We will also make reagents, paraffin sections, cell lysates, and protocols that we produce as a result of this study available to qualified researchers upon written request.
Declaration of Competing Interest
The authors have declared that no conflict of interest exists.
Acknowledgements
This work was supported by grants R01HL127200, R01HL137927, R01HL148667 and R01HL147148 from the National Institutes of Health of United States of America (NIH) to Xiaobo Zhou. The authors thank Dr. Harold Chapman (University of California, San Francisco, CA) for kindly providing the SftpcCreERT2;Rosa26RmTmG mouse line. We greatly appreciate valuable suggestions related to organoid culture from Dr. Carla Kim (Boston Children's Hospital, Harvard Medical School, Boston, MA) and Dr. Dianhua Jiang (Cedars-Sinai Medical Center, Los Angeles, CA).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103463.
Appendix. Supplementary materials
References
- 1.Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, Morozoff C, Shirude S, Naghavi M. Trends and Patterns of Differences in Chronic Respiratory Disease Mortality Among US Counties, 1980-2014. Jama. 2017;318(12):1136–1149. doi: 10.1001/jama.2017.11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaborators GBDCRD. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. doi: 10.1016/S2213-2600(17)30293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heron M. Deaths: Leading Causes for 2016. Natl Vital Stat Rep. 2018;67(6):1–77. [PubMed] [Google Scholar]
- 4.Kneidinger N, Yildirim AO, Callegari J, Takenaka S, Stein MM, Dumitrascu R. Activation of the WNT/beta-catenin pathway attenuates experimental emphysema. Am J Respir Crit Care Med. 2011;183(6):723–733. doi: 10.1164/rccm.200910-1560OC. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Z, Lao T, Qiu W, Polverino F, Gupta K, Guo F. A Chronic Obstructive Pulmonary Disease Susceptibility Gene, FAM13A, Regulates Protein Stability of beta-Catenin. Am J Respir Crit Care Med. 2016;194(2):185–197. doi: 10.1164/rccm.201505-0999OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5) doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 7.Cho MH, Boutaoui N, Klanderman BJ, Sylvia JS, Ziniti JP, Hersh CP. Variants in FAM13A are associated with chronic obstructive pulmonary disease. Nat Genet. 2010;42(3):200–202. doi: 10.1038/ng.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho MH, Castaldi PJ, Wan ES, Siedlinski M, Hersh CP, Demeo DL. A genome-wide association study of COPD identifies a susceptibility locus on chromosome 19q13. Hum Mol Gen. 2012;21(4):947–957. doi: 10.1093/hmg/ddr524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakornsakolpat P, Prokopenko D, Lamontagne M, Reeve NF, Guyatt AL, Jackson VE. Genetic landscape of chronic obstructive pulmonary disease identifies heterogeneous cell-type and phenotype associations. Nat Genet. 2019;51(3):494–505. doi: 10.1038/s41588-018-0342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hobbs BD, de Jong K, Lamontagne M, Bosse Y, Shrine N, Artigas MS. Genetic loci associated with chronic obstructive pulmonary disease overlap with loci for lung function and pulmonary fibrosis. Nat Genet. 2017;49(3):426–432. doi: 10.1038/ng.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45(6):613–620. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castaldi PJ, Guo F, Qiao D, Du F, Naing ZZC, Li Y. Identification of Functional Variants in the FAM13A Chronic Obstructive Pulmonary Disease Genome-Wide Association Study Locus by Massively Parallel Reporter Assays. Am J Respir Crit Care Med. 2019;199(1):52–61. doi: 10.1164/rccm.201802-0337OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15(2):123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beers MF, Morrisey EE. The three R's of lung health and disease: repair, remodeling, and regeneration. J Clin Invest. 2011;121(6):2065–2073. doi: 10.1172/JCI45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank DB, Peng T, Zepp JA, Snitow M, Vincent TL, Penkala IJ. Emergence of a Wave of Wnt Signaling that Regulates Lung Alveologenesis by Controlling Epithelial Self-Renewal and Differentiation. Cell Rep. 2016;17(9):2312–2325. doi: 10.1016/j.celrep.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrisey EE, Cardoso WV, Lane RH, Rabinovitch M, Abman SH, Ai X. Molecular determinants of lung development. Ann Am Thorac Soc. 2013;10(2):S12–S16. doi: 10.1513/AnnalsATS.201207-036OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zacharias WJ, Frank DB, Zepp JA, Morley MP, Alkhaleel FA, Kong J. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555(7695):251–255. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Developmental cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng D, Soh BS, Yin L, Hu G, Chen Q, Choi H. Differentiation of Club Cells to Alveolar Epithelial Cells In Vitro. Sci Rep. 2017;7:41661. doi: 10.1038/srep41661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, Desai TJ. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. 2018;359(6380):1118–1123. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JH, Tammela T, Hofree M, Choi J, Marjanovic ND, Han S. Anatomically and Functionally Distinct Lung Mesenchymal Populations Marked by Lgr5 and Lgr6. Cell. 2017;170(6):1149–1163. doi: 10.1016/j.cell.2017.07.028. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat Genet. 2008;40(7):862–870. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Gallup M, Zlock L, Basbaum C, Finkbeiner WE, McNamara NA. Cigarette smoke disrupts the integrity of airway adherens junctions through the aberrant interaction of p120-catenin with the cytoplasmic tail of MUC1. J Pathol. 2013;229(1):74–86. doi: 10.1002/path.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Z, Gong X, Zhu H, Wang C, Xu X, Cui D. Inhibition of Wnt/beta-catenin signaling promotes engraftment of mesenchymal stem cells to repair lung injury. J Cell Physiol. 2014;229(2):213–224. doi: 10.1002/jcp.24436. [DOI] [PubMed] [Google Scholar]
- 25.Zepp JA, Zacharias WJ, Frank DB, Cavanaugh CA, Zhou S, Morley MP. Distinct Mesenchymal Lineages and Niches Promote Epithelial Self-Renewal and Myofibrogenesis in the Lung. Cell. 2017;170(6):1134–1148. doi: 10.1016/j.cell.2017.07.034. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrer-Vaquer A, Piliszek A, Tian G, Aho RJ, Dufort D, Hadjantonakis AK. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ss-catenin signaling in the mouse. BMC Dev Biol. 2010;10:121. doi: 10.1186/1471-213X-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K. Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121(7):2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lao T, Glass K, Qiu W, Polverino F, Gupta K, Morrow J. Haploinsufficiency of Hedgehog interacting protein causes increased emphysema induced by cigarette smoke through network rewiring. Genome Med. 2015;7(1):12. doi: 10.1186/s13073-015-0137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156(3):440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou X, Qiu W, Sathirapongsasuti JF, Cho MH, Mancini JD, Lao T. Gene expression analysis uncovers novel hedgehog interacting protein (HHIP) effects in human bronchial epithelial cells. Genomics. 2013;101(5):263–272. doi: 10.1016/j.ygeno.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams TS, Schupp JC, Poli S, Ayaub EA, Neumark N, Ahangari F. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv. 2020;6(eaba1983) doi: 10.1126/sciadv.aba1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM., 3rd Comprehensive Integration of Single-Cell Data. Cell. 2019;177(7):1888–1902. doi: 10.1016/j.cell.2019.05.031. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Currier N, Chea K, Hlavacova M, Sussman DJ, Seldin DC, Dominguez I. Dynamic expression of a LEF-EGFP Wnt reporter in mouse development and cancer. Genesis. 2010;48(3):183–194. doi: 10.1002/dvg.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100(6):3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126(20):4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 36.Jones-Freeman B, Starkey MR. Bronchioalveolar stem cells in lung repair, regeneration and disease. J Pathol. 2020;252(3):219–226. doi: 10.1002/path.5527. [DOI] [PubMed] [Google Scholar]
- 37.Liu Q, Liu K, Cui G, Huang X, Yao S, Guo W. Lung regeneration by multipotent stem cells residing at the bronchioalveolar-duct junction. Nat Genet. 2019;51(4):728–738. doi: 10.1038/s41588-019-0346-6. [DOI] [PubMed] [Google Scholar]
- 38.Salwig I, Spitznagel B, Vazquez-Armendariz AI, Khalooghi K, Guenther S, Herold S. Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo. EMBO J. 2019;38(12) doi: 10.15252/embj.2019102099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ng-Blichfeldt JP, Schrik A, Kortekaas RK, Noordhoek JA, Heijink IH, Hiemstra PS. Retinoic acid signaling balances adult distal lung epithelial progenitor cell growth and differentiation. EBioMedicine. 2018;36:461–474. doi: 10.1016/j.ebiom.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung MI, Bujnis M, Barkauskas CE, Kobayashi Y, Hogan BLM. Niche-mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation. Development. 2018;145(9) doi: 10.1242/dev.163014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kosmider B, Messier EM, Chu HW, Mason RJ. Human alveolar epithelial cell injury induced by cigarette smoke. PloS one. 2011;6(12):e26059. doi: 10.1371/journal.pone.0026059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldkorn T, Filosto S, Chung S. Lung injury and lung cancer caused by cigarette smoke-induced oxidative stress: Molecular mechanisms and therapeutic opportunities involving the ceramide-generating machinery and epidermal growth factor receptor. Antioxidants Redox Signal. 2014;21(15):2149–2174. doi: 10.1089/ars.2013.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu G, Dong T, Wang S, Jing H, Chen J. Vitamin D3-vitamin D receptor axis suppresses pulmonary emphysema by maintaining alveolar macrophage homeostasis and function. EBioMedicine. 2019;45:563–577. doi: 10.1016/j.ebiom.2019.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flozak AS, Lam AP, Russell S, Jain M, Peled ON, Sheppard KA. Beta-catenin/T-cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. J Biol Chem. 2010;285(5):3157–3167. doi: 10.1074/jbc.M109.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei X, Zhang L, Zhou Z, Kwon OJ, Zhang Y, Nguyen H. Spatially Restricted Stromal Wnt Signaling Restrains Prostate Epithelial Progenitor Growth through Direct and Indirect Mechanisms. Cell Stem Cell. 2019;24(5):753–768. doi: 10.1016/j.stem.2019.03.010. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346(6205) doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 47.Yun JH, Morrow J, Owen CA, Qiu W, Glass K, Lao T. Transcriptomic Analysis of Lung Tissue from Cigarette Smoke-Induced Emphysema Murine Models and Human Chronic Obstructive Pulmonary Disease Show Shared and Distinct Pathways. Am J Respir Cell Mol Biol. 2017;57(1):47–58. doi: 10.1165/rcmb.2016-0328OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kyung U. Hong SDR, Adam Giangreco, Cheryl M. Hurley, and Barry R. Stripp. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset.pdf. 2001. [DOI] [PubMed]
- 49.Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, Bellusci S. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest. 2011;121(11):4409–4419. doi: 10.1172/JCI58097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng D, Limmon GV, Yin L, Leung NH, Yu H, Chow VT. Regeneration of alveolar type I and II cells from Scgb1a1-expressing cells following severe pulmonary damage induced by bleomycin and influenza. PloS one. 2012;7(10):e48451. doi: 10.1371/journal.pone.0048451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spella M, Lilis I, Pepe MA, Chen Y, Armaka M, Lamort AS. Club cells form lung adenocarcinomas and maintain the alveoli of adult mice. Elife. 2019;8 doi: 10.7554/eLife.45571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106(31):12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Behrens A, van Deursen JM, Rudolph KL, Schumacher B. Impact of genomic damage and ageing on stem cell function. Nat Cell Biol. 2014;16(3):201–207. doi: 10.1038/ncb2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez LM, de Lucas B, Galvez BG. Unhealthy Stem Cells: When Health Conditions Upset Stem Cell Properties. Cell Physiol Biochem. 2018;46(5):1999–2016. doi: 10.1159/000489440. [DOI] [PubMed] [Google Scholar]
- 55.Cao H, Wang C, Chen X, Hou J, Xiang Z, Shen Y. Inhibition of Wnt/beta-catenin signaling suppresses myofibroblast differentiation of lung resident mesenchymal stem cells and pulmonary fibrosis. Sci Rep. 2018;8(1):13644. doi: 10.1038/s41598-018-28968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Konigshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PloS one. 2008;3(5):e2142. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gottardi CJ, Konigshoff M. Considerations for targeting beta-catenin signaling in fibrosis. Am J Respir Crit Care Med. 2013;187(6):566–568. doi: 10.1164/rccm.201301-0144ED. [DOI] [PubMed] [Google Scholar]
- 58.Rahardini EP, Ikeda K, Nugroho DB, Hirata KI, Emoto N. Loss of Family with Sequence Similarity 13, Member A Exacerbates Pulmonary Fibrosis Potentially by Promoting Epithelial to Mesenchymal Transition. Kobe J Med Sci. 2020;65(3):E100–E1E9. [PMC free article] [PubMed] [Google Scholar]
- 59.Barbieri SS, Weksler BB. Tobacco smoke cooperates with interleukin-1beta to alter beta-catenin trafficking in vascular endothelium resulting in increased permeability and induction of cyclooxygenase-2 expression in vitro and in vivo. FASEB J. 2007;21(8):1831–1843. doi: 10.1096/fj.06-7557com. [DOI] [PubMed] [Google Scholar]
- 60.Lemjabbar-Alaoui H, Dasari V, Sidhu SS, Mengistab A, Finkbeiner W, Gallup M. Wnt and Hedgehog are critical mediators of cigarette smoke-induced lung cancer. PloS one. 2006;1:e93. doi: 10.1371/journal.pone.0000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahmood MQ, Walters EH, Shukla SD, Weston S, Muller HK, Ward C. beta-catenin, Twist and Snail: Transcriptional regulation of EMT in smokers and COPD, and relation to airflow obstruction. Sci Rep. 2017;7(1):10832. doi: 10.1038/s41598-017-11375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian D, Zhu M, Li J, Ma Y, Wu R. Cigarette smoke extract induces activation of beta-catenin/TCF signaling through inhibiting GSK3beta in human alveolar epithelial cell line. Toxicol Lett. 2009;187(1):58–62. doi: 10.1016/j.toxlet.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Zou W, Zou Y, Zhao Z, Li B, Ran P. Nicotine-induced epithelial-mesenchymal transition via Wnt/beta-catenin signaling in human airway epithelial cells. Am J Physiol Lung Cellular Mol Physiol. 2013;304(4):L199–L209. doi: 10.1152/ajplung.00094.2012. [DOI] [PubMed] [Google Scholar]
- 64.Wang R, Ahmed J, Wang G, Hassan I, Strulovici-Barel Y, Hackett NR. Down-regulation of the canonical Wnt beta-catenin pathway in the airway epithelium of healthy smokers and smokers with COPD. PloS One. 2011;6(4):e14793. doi: 10.1371/journal.pone.0014793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skronska-Wasek W, Mutze K, Baarsma HA, Bracke KR, Alsafadi HN, Lehmann M. Reduced Frizzled Receptor 4 Expression Prevents WNT/beta-Catenin-driven Alveolar Lung Repair in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2017;196(2):172–185. doi: 10.1164/rccm.201605-0904OC. [DOI] [PubMed] [Google Scholar]
- 66.Barolo S. Transgenic Wnt/TCF pathway reporters: all you need is Lef? Oncogene. 2006;25(57):7505–7511. doi: 10.1038/sj.onc.1210057. [DOI] [PubMed] [Google Scholar]
- 67.Hu Y, Ng-Blichfeldt JP, Ota C, Ciminieri C, Ren W, Hiemstra PS. Wnt/beta-catenin signaling is critical for regenerative potential of distal lung epithelial progenitor cells in homeostasis and emphysema. Stem Cells. 2020;38(11):1467–1478. doi: 10.1002/stem.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.