Abstract
This study aimed to investigate the protective effects of dietary algae-derived polysaccharides (ADP) from Enteromorpha prolifera against heat stress (HS)-induced bursa of Fabricius injure in broilers, and to elucidate the molecular mechanisms underlying the protective effect. A total of 144 8-week-old male yellow-feathered broilers were randomly allocated into 3 treatments of 6 replicates each (8 broilers per replicate): thermoneutral zone group (TN, fed basal diet); heat stress group (HS, fed basal diet); heat stress + ADP group (HSA, basal diet supplemented with 1,000 mg/kg ADP). Broilers in TN group were raised at 23.6 ± 1.8°C during the whole study. Broilers in HS and HSA groups were exposed to 33.2 ± 1.5°C for 10 h/day. The experimental period lasted for four weeks. The results showed that HS and dietary ADP had no significant effects on bursa of Fabricius index (P > 0.05). HS exposure increased the apoptosis rate of bursa of Fabricius (P < 0.05), and the apoptosis rate was reduced by dietary ADP (P < 0.05). Besides, broilers in HS and HSA groups had a lower glutathione-S transferase (GST) activity and total anti-oxidation capacity (T-AOC), whereas had a higher malondialdehyde (MDA) levels of bursa of Fabricius than those in TN group (P < 0.05). HS exposure elevated the concentration of tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-4, and IL-6, while decreased the concentration of interferon-γ (INF-γ) and IL-2 (P < 0.05), and dietary inclusion of ADP reduced the IL-1β level and increased the IL-2 level of bursa of Fabricius (P < 0.05). Compared with TN group, broilers in HS and HSA groups had lower relative mRNA expression of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1) and GSTT1 in bursa of Fabricius (P < 0.05). Additionally, HS exposure down-regulated the mRNA expression of inhibitor kappa B alpha (IκBα), IFN-γ, and IL-2, while up-regulated the mRNA expression of nuclear factor-kappa B (NF-κB) p65, TNF-α, IL-1β, and IL-6 in bursa of Fabricius (P < 0.05). However, dietary inclusion of ADP up-regulated the mRNA expression of IκBα and down-regulated the mRNA expression of NF-κB p65, TNF-α, and IL-6 in bursa of Fabricius (P < 0.05). Furthermore, HS exposure increased the relative protein expression levels of total and nuclear NF-κB p65 (P < 0.05), but dietary ADP supplementation reduced the relative protein expression levels of total and nuclear NF-κB p65 in bursa of Fabricius (P < 0.05). Collectively, dietary ADP ameliorated the impairment of histology, cell apoptosis and immune balance in bursa of Fabricius of heat stressed broilers, which is involved in modulation of NF-κB signaling pathway.
Key words: bursa of Fabricius, Enteromorpha prolifera, heat stress, NF-κB pathway, polysaccharides
Introduction
In modern poultry industry, broiler chickens are exposed to a variety of stressors, such as environmental stress (Li et al., 2019; Liu et al., 2021). Because of the high productivity, feathers, and lack of skin sweat glands, broilers are highly sensitive to high ambient temperature, heat stress (HS) induced by high ambient temperature has been one of the greatest challenges for broiler production with global warming (Chauhan et al., 2021; Liu et al., 2019). HS causes a disruption in multiple physiological functions, thus resulting in a degradation of growth performance (Emami et al., 2021; Guo et al., 2021a; Hirakawa et al., 2020; Quinteiro-Filho et al., 2010). Furthermore, previous studies have demonstrated that the excessive reactive oxygen species (ROS) produced during HS has undesirable effects on the redox and immune balance of immune organs (thymus, spleen, and bursa of Fabricius; Chegini et al., 2018; Hirakawa et al., 2020; Ohtsu et al., 2015; Quinteiro-Filho et al., 2010). Bursa of Fabricius is a unique central immune organ in broilers, which can produce B lymphocytes and specific antibodies to complete a specific immune response, it plays a critical role in maintaining avian immune functions (Ratcliffe, 2006). Therefore, research focusing on mitigation of HS-induced impairment of redox and immune balance in bursa of Fabricius is beneficial to advocate an appropriate approach to protect broilers facing high ambient temperature conditions.
Nutritional intervention is considered an effective strategy to relieve the deleterious consequences of HS on organ health of broilers (Chauhan et al., 2021). Nowadays, there has been great interest in application of phytochemicals, including natural polysaccharides as functional additives in broilers, which may be potential effective stress-alleviating agents due to their various biological activities (Awais et al., 2018). Sohail et al. (2013) reported that dietary mannan-oligosaccharide supplementation improved the relative weight of immune organs in heat-stressed broilers. Also, the natural polysaccharides from Atractylodes were reported to alleviate the splenic inflammatory response which induced by HS in broiler chickens (Xu et al., 2014, 2017a). Enteromorpha prolifera (EP), a kind of natural wild green algae, which widely distributed in various sea (Zhong et al., 2020). EP contains abundant polysaccharides, which have been confirmed to exhibit multiple biological functions, such as anti-tumor, anti-oxidation, anti-inflammatory, and anti-viral properties (Shi et al., 2017, 2020; Wei et al., 2014). Recently, the algae-derived polysaccharides (ADP) from EP have been shown to improve the antioxidant capacity and immunity in aquatic animals (Zhou et al., 2020) and mice (Guo et al., 2020a). Also, we conducted a series of studies to evaluate different roles of dietary ADP in various parameters of poultry. It has been shown that ADP supplementation could improve antioxidant performance in laying hens (Guo et al., 2020b), enhance intestinal barrier function in broilers (Liu et al., 2020a), and mitigate aflatoxin B1 (AFB1)-induced oxidative stress and apoptosis of bursa of Fabricius via regulating nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling pathway in broilers (Guo et al., 2021b). However, there are extremely limited studies concerning the effects of ADP in heat-stressed broilers, especially the data on whether ADP supplementation can ameliorate the adverse effects of HS on bursa of Fabricius are lacking.
Based on the beneficial effects of ADP, we hypothesized that the impairment of antioxidant capacity and immune function in broilers’ bursa of Fabricius caused by HS may be ameliorated by ADP supplementation. Thus, the current study was conducted to investigate the protective effect of dietary ADP on bursa of Fabricius in broilers, and to elucidate the molecular mechanisms underlying the amelioration effect.
Materials and methods
Experimental Design, Birds, and Diet
A total of 144 eight-week-old male broilers (Chinese local yellow-feathered broiler breed, Huaixiang chickens) with average initial body weight 682.59 ± 7.38 g were obtained from a local hatchery (Maoming, Guangdong, China). The age of broilers is selected based on the fact that broilers are more susceptible to HS during the growing and finishing stages, especially the finishing stages due to the higher body weight and productivity (Saeed et al., 2019). The broilers were randomly allocated to 3 treatments: thermoneutral zone group (TN, fed basal diet); heat stress group (HS, fed basal diet); heat stress + ADP group (HSA, basal diet supplemented with 1,000 mg/kg ADP), each of treatment contains 6 replications and 8 broilers/replication. The experimental period lasted for four weeks. Broilers in TN group were raised at 23.6 ± 1.8°C during the whole study. Broilers in HS and HSA groups were exposed to 33.2 ± 1.5°C for 10 h/day from 8:00 am to 18:00 pm, the temperature of rest time is consistent with TN group. Relative humidity was controlled at 55% to 75% among all groups during the entire experimental period. The temperature and relative humidity from various position in the chicken house were measured six times one day, and the ultimate temperature and relative humidity are average data from the daily records. The ADP were extracted from EP and provided by Qingdao Haida Biotechnology Co., Ltd. (Qingdao, Shandong, China), the extraction method, chemical composition etc. detailed information as described previously (Liu et al., 2020a). The supplemental doses of ADP were chosen on the basis of our previous studies which reported that dietary supplementation of 1000-2500 mg/kg ADP had protective effects on organs in poultry (Guo et al., 2021b; Liu et al., 2020a). The mash form basal diet were formulated to meet or exceed requirements suggested by the Chinese Chicken Feeding Standard (MAPRC, 2004; NY/T33-2004). The ingredient composition and nutrient content of basal diet are presented in Table 1. Continuous artificial light was used to illuminate the interior space for the whole period. The broilers with ad libitum access to feed and water. The animal study in the present experiment was reviewed and approved by Animal Care Committee, Guangdong Ocean University (Zhanjiang, Guangdong, China).
Table 1.
Basal diet composition (as-fed basis).
| Item | Contents (%) |
|---|---|
| Ingredients | |
| Corn | 60.84 |
| Soybean meal | 32.11 |
| Wheat bran | 2.16 |
| Soybean oil | 2.00 |
| Limestone | 1.28 |
| CaHPO4 | 1.26 |
| DL-Methionine | 0.15 |
| Vitamin premix* | 0.10 |
| Mineral premix† | 0.10 |
| Total | 100.00 |
| Nutrient levels‡ | |
| ME (MJ/kg) | 11.94 |
| Crude protein (%) | 18.22 |
| Ca (%) | 0.98 |
| Met (%) | 0.32 |
| Cystine (%) | 0.31 |
| Lys (%) | 0.90 |
| Total phosphorus (%) | 0.51 |
Premix provided per kilogram of diet: 5,000 IU of vitamin A, 1000 IU of vitamin D3, 10 IU of vitamin E, 0.5 mg of vitamin K3, 3 mg of thiamin, 7.5 mg of riboflavin, 4.5 mg of vitamin B6, 10 μg of vitamin B12, 25 mg of niacin, 0.55 mg of folic acid, 0.2 mg of biotin, 500 mg of choline, and 10.5 mg of pantothenic acid.
Premix provided per kilogram of diet: 60 mg of Zn, 80 mg of Mn, 80 mg of Fe, 3.75 mg of Cu, 0.35 mg of I, and 0.15 mg of Se.
Except for metabolic energy (ME), others are measured values.
Sample Collection
At the end of the feeding trial, 6 broilers (one bird from each replication) were randomly selected from each treatment. Broilers were individually weighed, euthanized, and collected for bursa of Fabricius. The bursa of Fabricius samples were weighed, and then they were put into 10% neutral-buffered formalin for histological and apoptosis analysis, and put into the liquid nitrogen and stored at −80°C for molecular analysis.
Bursa of Fabricius Index, Histological and Apoptosis Analysis
The bursa of Fabricius index was calculated as the following formula (Rajput et al., 2019):
For histological analysis, bursa of Fabricius were sampled and fixed in 10% neutral-buffered formalin for 48 h. Subsequently, the samples were embedded in paraffin, cut for 5 μm sections, and stained with hematoxylin and eosin (H & E) as described in previous study (Gao et al., 2018). The slides were observed with an inverted optical microscope (SDPTOP, GD-30RFL, Guangzhou, China) under 200 × (10 × ocular lens multiplied 20 × objective lens) and 400 × magnification (10 × ocular lens multiplied 40 × objective lens). The image was collected by TCapture Imaging Application 4.3 software, and all the parameters were consistent throughout the process.
Cell apoptosis of bursa of Fabricius was detected by paraffin section TUNEL method (Luo et al., 2018). TUNEL assay Kit (Roche, 11684817910, Switzerland) was used according to the manufacturer's instructions. Other major reagents were DAPI (Servicebio, G1012, Wuhan, China), Antifade Mounting Medium (Servicebio, G1401, Wuhan, China). Using the fluorescence microscope (Nikon Eclipse, C1, Japan) to observe sections and collect images (Nikon, DS-U3). The nuclei stained by DAPI were blue under UV excitation, the Roche assay kit was labeled with FITC fluorescein, and the positive apoptotic nuclei were green.
Antioxidant Parameters
For determination of antioxidant parameters, pre-processing of the bursa of Fabricius samples were prepared as previously described (Rajput et al., 2019). The activities of the total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), catalase (CAT), glutathione-S transferase (GST), the total anti-oxidation capacity (T-AOC) and malondialdehyde (MDA) levels were measured by using commercial kits (Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) according to the manufacturer's instructions. The detailed information of the kits are as follows: T-AOC assay kit, ABTS method, 96T, A015-2-1. T-SOD assay kit, WST-1 method, 96T, A001-1. GSH-Px assay kit, Colorimetric method, 100T, A005. CAT assay kit, Visible light method, A007-1. GSH-ST assay kit, Colorimetric method, 100T, A004. MDA assay kit, Colorimetric method, 400T, A003-1.
ELISA for Cytokines Concentration Determination
The concentrations of tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin (IL)-1β, IL-2, IL-4, IL-6, and IL-10 in bursa of Fabricius samples were determined by commercially chicken cytokines ELISA kits (eBioscience CO., Ltd., CA) according to the manufacturer's instructions. The control recombinant chicken cytokine sample was diluted over the recommended detection range to generate a standard curve for each assay, and the linearity was calculated using Excel with R2 = 0.99. Sample concentrations were interpolated from the standard curve.
Quantitative real-time PCR Analysis
Total RNA was extracted from bursa of Fabricius samples based on the manufacturer's instructions of RNA extraction kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The integrity and quality of total RNA were estimated by 1% agarose gel electrophoresis and the 260/280 nm absorbance ratio (ideal ratio being within 1.8 and 2.0). Total RNA level was investigated at 260 nm using a spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific Inc., Waltham, MA). Afterwards, total RNA of each bursa of Fabricius sample was used to reverse transcription of cDNA according to the protocol of RT reagent kit (TaKaRa Biotechnology Co., Ltd).
The β-actin gene was used as an internal control to verify the successful reverse transcription and to calibrate the cDNA template. The specific primers are described in Table 2, they were designed using Primer Express 3.0 software (Applied Biosystems, Foster City, CA) and obtained from Sangon Biotech Co., Ltd. (Shanghai, China). The qPCR reactions was performed with a CFX-96 Real-Time PCR Detection System (BioRad). It was carried out in a total volume of 20 μL, including 10 μL SYBR Premix Ex Taq II (Tli RNaseH Plus), 2 μL cDNA template, 1 μL of each primer (forward and reverse primers), and 6 μL DEPC treated water. DEPC treated water for the replacement of cDNA template was used as a negative control. The PCR program was as follows: 95°C for 30 s, followed by 40 cycles of 95°C for 10 s, 30 s under 60°C, and 72°C for 15 s. Each sample was tested in triplicate. The relative mRNA expression levels were calculated using the 2−ΔΔCt method as previously described (Livak and Schmittgen, 2001).
Table 2.
Primers used for quantitative real-time PCR.
| Target genes | Primer | Primary sequence (5′→3′) | Accession no. |
|---|---|---|---|
| Nrf2 | Forward | TGTGTGTGATTCAACCCGACT | NM_205117.1 |
| Reverse | TTAATGGAAGCCGCACCACT | ||
| HO-1 | Forward | TTGGCAAGAAGCATCCAGA | NM_205344.1 |
| Reverse | TCCATCTCAAGGGCATTCA | ||
| SOD1 | Forward | TTGTCTGATGGAGA TCATGGCTTC | NM_205064.1 |
| Reverse | TGCTTGCCTTCAGGATTAAAGTGAG | ||
| SOD2 | Forward | CAGATAGCAGCCTGTGCAAATCA | NM_204211.1 |
| Reverse | GCATGTTCCCATACATCGATTCC | ||
| GPx1 | Forward | GACCAACCCGCAGTACATCA | NM_001277853.1 |
| Reverse | GAGGTGCGGGCTTTCCTTTA | ||
| GPx3 | Forward | CCTGCAGTACCTCGAACTGA | NM_001163232 |
| Reverse | CTTCAGTGCAGGGAGGATCT | ||
| CAT1 | Forward | ACCAAGTACTGCAAGGCGAAAGT | XM_015277937.2 |
| Reverse | ACCCAGATTCTCCAGCAACAGTG | ||
| GSTT1 | Forward | GACGGAGACTTCACCCTAGCAGA | NM_205365.1 |
| Reverse | TGATGGGTACCAGTGGTCAGGA | ||
| GSTO1 | Forward | CATGATGTGGCCCTGGTTTG | NM_001277375.1 |
| Reverse | CAGTGCTGGAGCTTTGGAGTATGA | ||
| GSTA3 | Forward | TTGGATAAGGCCGCAAACAGATA | NM_001001777.1 |
| Reverse | TTTCCAGTAAATGCACGTCTGCTC | ||
| IκBα | Forward | GGCAGATGTGAACAAGGTGA | NM_001001472.2 |
| Reverse | TATCTGCAGGTCAGCTGTGG | ||
| NF-κB p65 | Forward | GTGTGAAGAAACGGGAACTG | NM_205129 |
| Reverse | GGCACGGTTGTCATAGATGG | ||
| TNF-α | Forward | GCCTATGCCAACAAGTACACCT | NM_204267.1 |
| Reverse | GCCAAGTCAACGCTCCTG | ||
| IFN-γ | Forward | CTTCCTGATGGCGTGAAGA | NM_205149.1 |
| Reverse | GAGGATCCACCAGCTTCTGT | ||
| IL-1β | Forward | CGCCGCTACCAGAGGGACTT | NM_204524.1 |
| Reverse | CCGGACCCAGTTGACCCCAT | ||
| IL-2 | Forward | ATCTTTGGCTGTATTTCGGTAG | NM_204153.1 |
| Reverse | TCCTGGGTCTCAGTTGGTG | ||
| IL-4 | Forward | CTCCTCACTGCCCACCCT | NM_001007079.1 |
| Reverse | CATCTTGACGCAGGAAACCT | ||
| IL-6 | Forward | GATCCGGCAGATGGTGATAA | NM_204628.1 |
| Reverse | AGGATGAGGTGCATGGTGAT | ||
| IL-10 | Forward | GCTCTCCTTCCACCGAAACC | NM_001004414.2 |
| Reverse | GGAGCAAAGCCATCAAGCAG | ||
| β-actin | Forward | TCAGGGTGTGATGGTTGGTATG | NM_205518.1 |
| Reverse | TGTTCAATGGGGTACTTCAGGG |
Western Blot Analyses
The western blot method was conducted to detect the protein expression levels of bursa of Fabricius. The total protein, nuclear protein and cytoplasmic protein was extracted from bursa of Fabricius tissues and the protein concentration was determined using the BCA protein assay kit (Servicebio Technology, Wuhan, China). And then the protein was assessed by SDS-polyacrylamide gel electrophoresis under reducing conditions on 10% gels. Subsequently, it was transferred to nitrocellulose membranes using a tank transfer at 200 mA in Tris-glycine buffer containing 20% methanol for 90 min at 4°C, and then put the membranes in 5% skim milk for block at 37°C 1 h. The membranes were consistently incubated overnight at 4°C with diluted primary antibody that a rabbit polyclonal antibody against nuclear factor-kappa B (NF-κB) p65 (GB11997, 65 kDa, Servicebio, Wuhan, China). The diluted concentration of primary antibody was 1:1000. The HRP-labeled goat anti-rabbit IgG (1:3000, Servicebio Technology, Wuhan, China) was used as the secondary antibody. The β-actin content was analyzed as the loading control with rabbit IgG (1:3000) polyclonal antibodies. The proteins bands detected and analyzed using Alpha Imager (Alpha Innotech, CA). The relative protein expression levels were calculated as target protein/β-actin.
Statistical Analysis
All data were analyzed using general linear model (GLM) procedure of SAS 9.4 (SAS, 2013. SAS Institute Inc., Cary, NC) for a completely randomized design. Replicates were the experimental units. Data were expressed as means. Differences among means were tested using Tukey's test. P-value < 0.05 was considered to be statistically significant.
Results
Bursa of Fabricius Index, Histological and Apoptosis Analysis
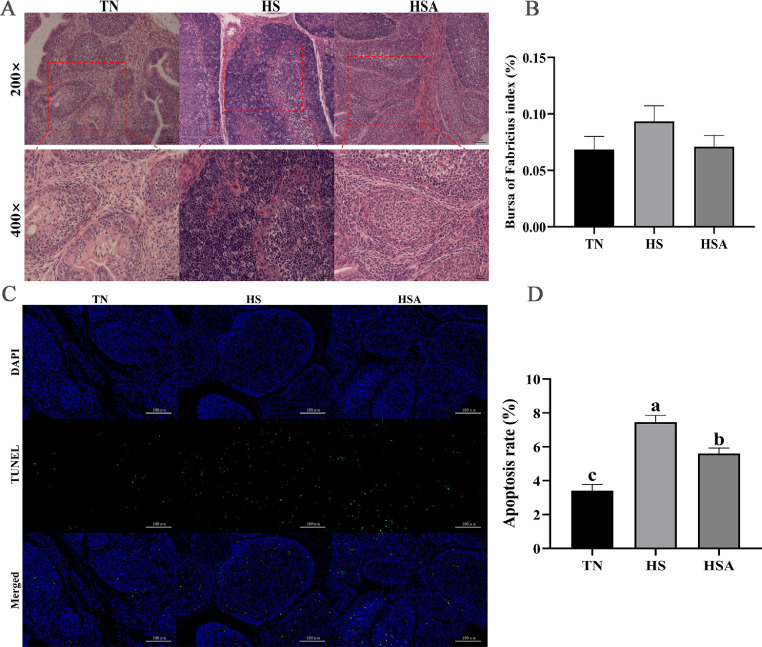
Results of bursa of Fabricius index, histological and apoptosis rate are illustrated in Figure 1. There was no significant difference in bursa of Fabricius index between the treatments (Figure 1B, P > 0.05). Histological analysis of the bursa of Fabricius showed that there were no pathological alterations in TN group. In the HS group, there were inflammatory cells infiltrated, destroyed cell structure, and cell death and necrosis. In contrast, compared with HS group, the morphology of the bursa of Fabricius, including infiltration of inflammatory cells, cell structure destroyed and cell necrosis were improved and restored by dietary supplementation of ADP (Figure 1A). Apoptotic results showed that broilers in TN group had lower apoptosis rate than those in HS and HSA groups (Figure 1C, D, P < 0.05), and compared with HS group, dietary supplementation of ADP reduced the apoptosis rate (P < 0.05).
Figure 1.
Effects of dietary algae-derived polysaccharides (ADP) on histological changes (A), bursa of Fabricius index (B) and cell apoptosis (C, D) of bursa of Fabricius in broilers under heat stress. TN, thermoneutral zone group (23.6 ± 1.8 °C); HS, heat stress group (33.2 ± 1.5 °C for 10 h/day); HSA, heat stress + ADP group (supplemented with 1000 mg/kg ADP). All data were expressed as means ± SEM (n = 6). abcDifferent superscript letters indicate significant differences (P < 0.05). The scar bar for histological changes (A) is 50 μm of 200 × and 25 μm of 400 ×, and the scar bar for cell apoptosis (C, 200 ×) is 100 μm.
Antioxidant Capacity
As presented in Table 3, broilers in HS and HSA groups had lower GST activity and T-AOC, whereas had higher MDA levels than those in TN group (P < 0.05). Meanwhile, the activities of T-SOD, GSH-Px and CAT were not affected by HS exposure and dietary ADP supplementation (P > 0.05).
Table 3.
Effects of dietary algae-derived polysaccharides (ADP) on antioxidant capacity of bursa of Fabricius in broilers under heat stress.
| Items | TN | HS | HSA | SEM | P-value |
|---|---|---|---|---|---|
| T-SOD, U/mg protein | 350.87 | 345.08 | 371.98 | 10.29 | 0.293 |
| GSH-Px, U/mg protein | 39.43 | 32.90 | 37.41 | 5.10 | 0.595 |
| CAT, U/mg protein | 1.91 | 1.83 | 1.75 | 0.12 | 0.573 |
| GST, U/mg protein | 30.76a | 22.80b | 23.62b | 1.38 | 0.003 |
| T-AOC, mmol/mg protein | 42.34a | 32.05b | 28.68b | 1.51 | 0.001 |
| MDA, nmol/mg protein | 1.45b | 2.01a | 1.78a | 0.10 | 0.011 |
TN, thermoneutral zone group (23.6 ± 1.8°C); HS, heat stress group (33.2 ± 1.5°C for 10 h/day); HSA, heat stress + ADP group (supplemented with 1,000 mg/kg ADP); SEM, standard error of mean; T-SOD, total superoxide dismutase; GSH-Px, glutathione peroxidase; CAT, catalase; GST, glutathione-S transferase; T-AOC, total anti-oxidation capacity; MDA, malondialdehyde.
Different superscript letters indicate significant differences (P < 0.05).
Concentration of Cytokines
As shown in Table 4, HS exposure elevated the concentration of TNF-α, IL-1β, IL-4, and IL-6, whereas decreased the concentration of INF-γ and IL-2 in bursa of Fabricius compared with TN group (P < 0.05). Compared with HS group, dietary inclusion of ADP reduced the IL-1β level and increased the IL-2 level (P < 0.05). In addition, there were no significant differences in TNF-α, INF-γ, IL-4 and IL-6 contents between the HSA and TN groups (P > 0.05).
Table 4.
Effects of dietary algae-derived polysaccharides (ADP) on cytokines concentration of bursa of Fabricius in broilers under heat stress.
| Items | TN | HS | HSA | SEM | P-value |
|---|---|---|---|---|---|
| TNF-α, pg/mg protein | 168.55b | 221.14a | 195.25ab | 9.72 | 0.010 |
| INF-γ, pg/mg protein | 367.52a | 325.98b | 345.53ab | 11.89 | 0.092 |
| IL-1β, pg/mg protein | 374.81b | 427.25a | 381.28b | 12.19 | 0.025 |
| IL-2, ng/mg protein | 3.50a | 2.54b | 3.29a | 0.23 | 0.031 |
| IL-4, ng/mg protein | 108.37b | 141.73a | 115.13ab | 8.78 | 0.042 |
| IL-6, pg/mg protein | 97.23b | 139.70a | 124.11ab | 10.24 | 0.041 |
| IL-10, ng/mg protein | 241.68 | 262.56 | 249.53 | 9.91 | 0.359 |
TN, thermoneutral zone group (23.6 ± 1.8°C); HS, heat stress group (33.2 ± 1.5°C for 10 h/day); HSA, heat stress + ADP group (supplemented with 1,000 mg/kg ADP); SEM, standard error of mean; TNF-α, tumor necrosis factor-α, IFN-γ, interferon-γ, IL, interleukin.
Different superscript letters indicate significant differences (P < 0.05).
Relative mRNA Expression of Nrf2 Signaling Pathway-Related Genes
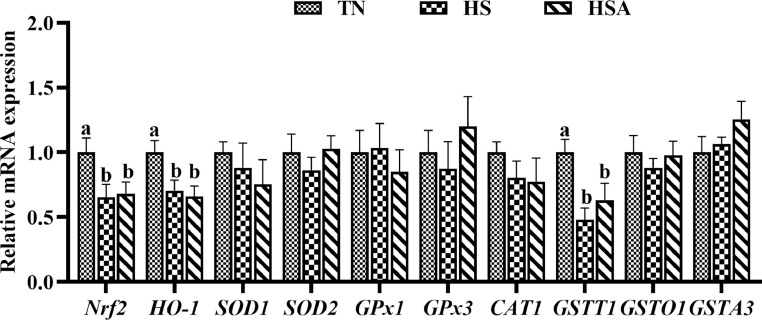
The relative mRNA expression of Nrf2 signaling pathway related genes, including Nrf2, HO-1, SOD1, SOD2, GPx1, GPx3, CAT1, GSTT1, GSTO1, and GSTA3, are depicted in Figure 2. Compared with TN group, broilers in HS and HSA groups had lower relative mRNA expression levels of Nrf2, HO-1, and GSTT1 (P < 0.05). HS exposure and dietary ADP had no significant effect on the relative mRNA expression levels of SOD1, SOD2, GPx1, GPx3, CAT1, GSTO1, and GSTA31 (P > 0.05).
Figure 2.
Effects of dietary algae-derived polysaccharides (ADP) on relative mRNA expression of Nrf2 pathway related genes of bursa of Fabricius in broilers under heat stress. TN, thermoneutral zone group (23.6 ± 1.8°C); HS, heat stress group (33.2 ± 1.5°C for 10 h/day); HSA, heat stress + ADP group (supplemented with 1,000 mg/kg ADP); Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; SOD, superoxide dismutase; GPx, glutathione peroxidase; CAT, catalase; GSTT, glutathione S-transferase theta; GSTO, glutathione S-transferase omega; GSTA, glutathione S-transferase alpha. All data were expressed as means ± SEM (n = 6). abDifferent superscript letters indicate significant differences (P < 0.05).
Relative mRNA Expression of NF-κB Signaling Pathway-Related Genes
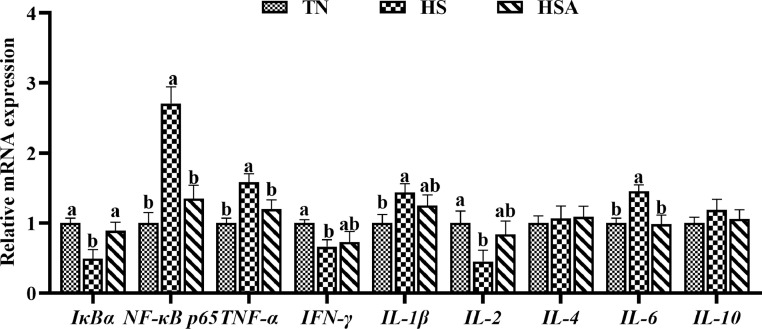
As indicated in Figure 3, compared with TN group, HS exposure down-regulated the mRNA expression of inhibitor kappa B alpha (IκBα), IFN-γ, and IL-2, whereas up-regulated the mRNA expression of NF-κB p65, TNF-α, IL-1β, and IL-6 (P < 0.05). However, dietary inclusion of ADP up-regulated the mRNA expression of IκBα and down-regulated the mRNA expression of NF-κB p65, TNF-α, and IL-6 in broilers under HS condition (P < 0.05).
Figure 3.
Effects of dietary algae-derived polysaccharides (ADP) on relative mRNA expression of NF-κB pathway related genes of bursa of Fabricius in broilers under heat stress. TN, thermoneutral zone group (23.6 ± 1.8°C); HS, heat stress group (33.2 ± 1.5°C for 10 h/day); HSA, heat stress + ADP group (supplemented with 1,000 mg/kg ADP). IκBα, inhibitor kappa B alpha; NF-κB p65, nuclear factor-kappa B p65; TNF-α, tumor necrosis factor-α, IFN-γ, interferon-γ, IL, interleukin. All data were expressed as means ± SEM (n = 6). abDifferent superscript letters indicate significant differences (P < 0.05).
Protein Expression of NF-κB p65
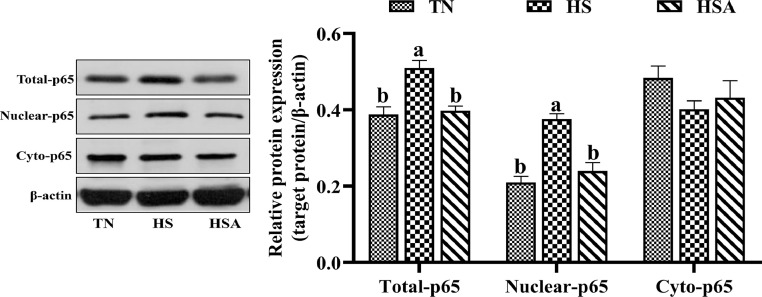
The effects of HS and dietary ADP on total, nuclear and cytoplasmic protein expression of NF-κB p65 are presented in Figure 4. Broilers in HS group had higher relative protein expression levels of total and nuclear NF-κB p65 than those in TN group (P < 0.05). Dietary ADP supplementation reduced relative protein expression levels of total and nuclear NF-κB p65 in broilers exposed to HS (P < 0.05).
Figure 4.
Effects of dietary algae-derived polysaccharides (ADP) on relative protein expression of NF-κB p65 of bursa of Fabricius in broilers under heat stress. TN, thermoneutral zone group (23.6 ± 1.8°C); HS, heat stress group (33.2 ± 1.5°C for 10 h/day); HSA, heat stress + ADP group (supplemented with 1,000 mg/kg ADP). Total-p65, total protein expression of NF-κB p65; Nuclear-p65, protein expression of NF-κB p65 in nucleus; Cyto-p65, protein expression of NF-κB p65 in cytoplasm. All data were expressed as means ± SEM (n = 6). abDifferent superscript letters indicate significant differences (P < 0.05).
Discussion
With global warming, HS has been one of the greatest concerns in broiler production. It has been suggested that HS could reduce the relative organ weight and impair the histology and function of lymphatic organs in broilers (Chauhan et al., 2021). Unfortunately, our study failed to obtain a significant difference in bursa of Fabricius index among TN, HS, and HSA groups. Similarly, Hosseini-Vashan and Raei-Moghadam (2019) found that HS had no significant effects on relative bursal weight in broilers. On the contrary, other studies demonstrated that HS reduced the relative bursal weight (Chegini et al., 2018; Hirakawa et al., 2020; Liu et al., 2014) and this impairment could be alleviated by dietary mannan-oligosaccharide (Sohail et al., 2013). The extent and duration of HS, broiler strain, growth stages, and the type of polysaccharides could help to explain these inconsistencies. In addition, the present findings indicated that HS resulted in a histological damage of bursa of Fabricius. Consistent with our study, Chen et al. (2016) suggested that 6 weeks HS exposure markedly reduced follicle area and wider follicular space of broilers’ bursa of Fabricius. Hirakawa et al. (2020) also stated that HS caused limited synthesis of lymphocytes along with histopathological changes of bursa of Fabricius in broilers. Another important finding obtained from our study was the increase in apoptosis rate of bursa of Fabricius in HS group, which is similar to the results of previous studies (Chen et al., 2016; Xu et al., 2017a,b). The reason why the disruption of histology and the cell apoptosis occurs may be due to the excessive generation of ROS when broilers under HS condition. The ROS causes oxidative stress and inflammatory response, which adversely affect the tissue structure and cell physiology (Vandana et al., 2021). However, the specific reason need to be further clarified. It is interesting to note that dietary inclusion of ADP ameliorated the histological damage and cell apoptosis of bursa of Fabricius which induced by HS in this study. In fact, available literature has revealed the positive effects of phytochemicals on lymphatic organs of broilers under HS (Awad et al., 2021; He et al., 2019). Meanwhile, earlier studies demonstrated that dietary supplementation with natural polysaccharides from Atractylodes could alleviate the splenic histological injure and cell apoptosis of broilers subjected to HS (Xu et al., 2017a; Xu et al., 2014; 2017b; Xu and Tian, 2015). Our recent study also found that dietary ADP mitigated AFB1-induced impairment of histology and apoptosis of bursa of Fabricius in broilers (Guo et al., 2021b). Although the mode of action of ADP in protection of histology and apoptosis of broilers’ bursa of Fabricius under HS conditions is not well elucidated, it could be speculated that the beneficial effects of ADP might be attributed to the anti-oxidative and anti-inflammatory activities (Liu et al., 2020b; Zhong et al., 2020; Zhao et al., 2021), but the specific reason, especially the molecular mechanism, needed to be further explored.
The current results showed that HS triggered oxidative stress in bursa of Fabricius and is associated with reduction in gene expression of Nrf2, HO-1, and GSSTT1, and the GST activity and T-AOC, indicators of the antioxidant defense system, but elevation of MDA level, a biomarker of lipid peroxidation (Banerjee et al., 1999). Nrf2 is an important cytoprotective transcription factor that are involved in inhibition of oxidative stress through modulating the expression of several antioxidant and phase II detoxifying enzymes, such as HO-1, SOD, CAT, GST, GSH-Px (Itoh et al., 1997; Yamamoto et al., 2008). In normal physiological conditions, Nrf2 is found in the cytoplasm bound with Kelch ECH associating protein 1 (Keap1) (Itoh et al., 1999). Under HS conditions, excessive ROS disrupts the cellular redox balance, induces oxidative stress and leads to Nrf2 is released from Keap1 and rapidly translocate into the nucleus, where it binds to the antioxidant response element (ARE) and induce transcription of antioxidative genes (Sahin, 2015). Numerous studies reported that chronic HS inhibited the expression of Nrf2 and antioxidant enzyme genes, which may be due to the dysregulation of the antioxidant system of broilers under long-term HS conditions (Arain et al., 2018; Sahin et al., 2013; 2016), this is in agreement with the present study. Furthermore, some exciting findings have been observed, in which heat-stressed broilers fed phytochemicals, including polyphenols, polysaccharides and flavonoid compounds etc., exhibited a better antioxidant capacity as proved by enhanced the antioxidant enzymes activities (SOD, GSH-Px, GST, CAT) and modulated Nrf2 signaling pathway (Awad et al., 2021; Cheng et al., 2019; Liu et al., 2014; Sahin et al., 2013; 2016; Sandner et al., 2020; Zhang et al., 2018a), and quite a few studies have already confirmed that dietary natural polysaccharides exerted anti-oxidative effects in broiler chickens (Hu et al., 2017; Long et al., 2020; Zhang et al., 2018b; 2021; Zhou et al., 2019). In recent literature, Guo et al. (2020a) and Guo et al. (2021b) have illustrated that the ADP could act as free radical scavenger to improve antioxidant enzymes activity and is related to the activation of Nrf2 signaling pathway in animals. However, inconsistent with the literature, the present findings failed to ascertain that the ADP could be used as a modifier of Nrf2 related antioxidant pathway in response to HS in broilers’ bursa of Fabricius. The possible reason is that the HS caused serious impairment of antioxidant enzyme system, which could not be improved just by dietary inclusion of ADP. The combined use of ADP and other natural antioxidants may be effective to against HS-induced oxidative stress of broilers, which is worth to investigate in future studies.
As we known, HS leads to oxidative stress is usually accompanied by activation of inflammation pathway, which ultimately causes impairment in tissues of broilers (Sahin, 2015). During HS exposure, NF-κB plays an active role in inflammatory response of chickens (Goel et al., 2021; Lan et al., 2017). The NF-κB p65 bound with IκBα in the cytoplasm under normal physiological conditions, while environmental stressors result in changes in cell signaling transduction and induce IκBα degradation, which in turn activate NF-κB p65 and enhance its expression in the nucleus, thus regulating the expression of downstream inflammatory cytokines (Baldwin Jr, 1996; Chi et al., 2019; Goel et al., 2021). The cytokines are related to T-helper (Th) cells, and the Th1 cells mainly secrete IL-2 and IFN-γ, Th2 cells mainly secrete IL-1β, IL-4, IL-6, and IL-10 (Romagnani, 2000). The importance functions of Th1/Th2 balance in maintaining cellular and humoral immune responses have been suggested, and emerging researches suggested that harsh environment exposure induced inflammatory response and injuries in multiple tissues of broiler chickens through activating NF-κB signaling pathway and disturbing the balance of Th1/Th2 (Han et al., 2020; Hu et al., 2018; Zhao et al., 2020). Meanwhile, TNF-α plays a crucial role in exerting variety of biologic effects, which is secreted by activated macrophages and considered to be an efficient pro-inflammatory cytokine (Wu et al., 2015). In the present study, the data indicating that HS exposure altered the cytokines expression, induced an imbalance of Th1/Th2 and involved in the activation of NF-κB signaling pathway in broilers’ bursa of Fabricius. In accordance with our study, Xu et al. (2014) suggested that HS induced higher levels of TNF-α and IL-4, but lower levels of IFN-γ and IL-2 in the spleen of broilers. He et al. (2019) demonstrated that HS caused alterations in inflammatory cytokines expression and resulted in an imbalance of Th1/Th2 via activating the NF-κB signaling pathway of broilers. Another recent study conducted by Siddiqui et al. (2020), who also reported that chronic HS changed the mRNA expression of cytokines in the jejunum of broilers. Interestingly, the present study showed that dietary ADP effectively mitigated the imbalance of Th1/Th2 and decreased TNF-α levels by suppressing the degradation of IκBα and nuclear translocation of NF-κB p65 in heat-stressed broilers. Similarly, the ADP from EP has been suggested to enhance the immune response in fishes (Zhou et al., 2020) and mice (Wei et al., 2014) by modulating NF-κB signaling pathway. Also, support data for our observations come from other natural polysaccharides have been reported. Liu et al. (2015) showed that dietary inclusion of Astragalus polysaccharides reduced the mRNA abundances of IL-1β, and IL-6 through inhibiting the expression of NF-κB p65 in jejunal mucosa of broilers after lipopolysaccharide challenge. Hu et al. (2017) found that Agaricus blazei polysaccharides attenuated cadmium-induced inflammatory response in chicken livers by down-regulating the mRNA expression levels of TNF-α, IL-1β, and IL-6. The results obtained by Li et al. (2019), who demonstrated that dietary γ-irradiated Astragalus polysaccharides improved the mRNA expression of IL-2 and IFN-γ in jejunum of immunosuppressed broilers. In model animal research, Dong et al. (2019) and Farag et al. (2019) also suggested that Astragalus polysaccharides showed an amelioration effect on inflammation and was associated with the suppression of IκBα degradation and nuclear translocation of NF-κB p65 in mice. Coupled with the histological and apoptotic findings, dietary ADP modulated the NF-κB signaling pathway and ameliorated HS-mediated immune impairment in bursa of Fabricius could contribute to the improvement of histology and cell apoptosis in this study. However, further molecular and mechanistic studies are also needed to scrutinize the underlying mechanism of ADP behind the regulation of NF-κB signaling pathway, such as the non-coding RNA regulatory mechanism.
Conclusion
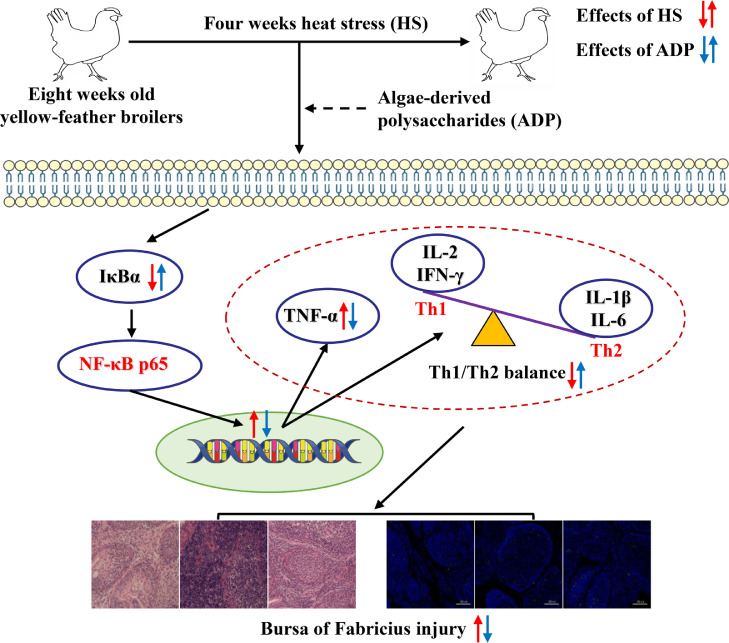
Together, dietary algae-derived polysaccharides from Enteromorpha prolifera efficiently prevented the heat stress-induced impairment of histology and apoptosis of bursa of Fabricius via attenuating the Th1/Th2 imbalance and suppressing the activation of NF-κB signaling pathway in broilers (Figure 5). These findings provide a concrete rationale and molecular basis for application of algae-derived polysaccharides to protect the immune organs of broilers facing heat stress conditions.
Figure 5.
Proposed mechanism of algae-derived polysaccharides (ADP) alleviates heat stress (HS)-induced bursa of Fabricius injure via modulating NF-κB signaling pathway in broilers. IκBα, inhibitor kappa B alpha; NF-κB p65, nuclear factor-kappa B p65; TNF-α, tumor necrosis factor-α, IFN-γ, interferon-γ, IL, interleukin; Th, T-helper cells.
Acknowledgments
This research was funded by National Nature Science Foundation of China (32002196); Natural Science Foundation of Guangdong Province of China (2018A030307023); Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation (“Climbing Program” Special Funds, pdjh2020b0280); College Student Innovation Training Project (CXXL2020012); Innovative Strong School Engineering Project of Guangdong Department of Education (2017KQNCX090; Q2018302); Talent Research Start-up Project of Guangdong Ocean University (R18007); The Future Industrial Development Fund of Shenzhen (JCYJ20170413111950426).
Disclosures
The authors report that they have no conflicts of interest.
References
- Arain M.A., Mei Z., Hassan F., Saeed M., Alagawany M., Shar A., Rajput I. Lycopene: a natural antioxidant for prevention of heat-induced oxidative stress in poultry. World's Poult. Sci. J. 2018;74:89–100. [Google Scholar]
- Awad E., Zulkifli I., Ramiah S., Khalil E., Abdallh M. Prebiotics supplementation: an effective approach to mitigate the detrimental effects of heat stress in broiler chickens. World's Poult. Sci. J. 2021;77:135–151. [Google Scholar]
- Awais M.M., Akhtar M., Anwar M.I., Khaliq K. Evaluation of Saccharum officinarum L. bagasse-derived polysaccharides as native immunomodulatory and anticoccidial agents in broilers. Vet. Parasitol. 2018;249:74–81. doi: 10.1016/j.vetpar.2017.11.012. [DOI] [PubMed] [Google Scholar]
- Baldwin A.S., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Banerjee B., Seth V., Bhattacharya A., Pasha S., Chakraborty A. Biochemical effects of some pesticides on lipid peroxidation and free-radical scavengers. Toxicol. Lett. 1999;107:33–47. doi: 10.1016/s0378-4274(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Chauhan S.S., Rashamol V., Bagath M., Sejian V., Dunshea F.R. Impacts of heat stress on immune responses and oxidative stress in farm animals and nutritional strategies for amelioration. Int. J. Biometeorol. 2021;65:1231–1244. doi: 10.1007/s00484-021-02083-3. [DOI] [PubMed] [Google Scholar]
- Chegini S., Kiani A., Rokni H. Alleviation of thermal and overcrowding stress in finishing broilers by dietary propolis supplementation. Ital. J. Anim. Sci. 2018;17:377–385. [Google Scholar]
- Chen Z., Zhou Y.W., Liang C., Jiang Y.Y., Xie L.J. Effects of γ-aminobutyric acid on the tissue structure, antioxidant activity, cell apoptosis, and cytokine contents of bursa of Fabricius in chicks under heat stress. Arch. Anim. Breed. 2016;59:97–105. [Google Scholar]
- Cheng Y., Chen Y., Chen R., Su Y., Zhang R., He Q., Wang K., Wen C., Zhou Y. Dietary mannan oligosaccharide ameliorates cyclic heat stress-induced damages on intestinal oxidative status and barrier integrity of broilers. Poult. Sci. 2019;98:4767–4776. doi: 10.3382/ps/pez192. [DOI] [PubMed] [Google Scholar]
- Chi Q., Wang D., Hu X., Li S., Li S. Hydrogen sulfide gas exposure induces necroptosis and promotes inflammation through the MAPK/NF-κB pathway in broiler spleen. Oxid. Med. Cell Longev. 2019;2019:8061823. doi: 10.1155/2019/8061823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N., Li X., Xue C., Wang C., Xu X., Bi C., Shan A., Li D. Astragalus polysaccharides attenuated inflammation and balanced the gut microflora in mice challenged with Salmonella typhimurium. Int. Immunopharmacol. 2019;74:105681. doi: 10.1016/j.intimp.2019.105681. [DOI] [PubMed] [Google Scholar]
- Emami N.K., Jung U., Voy B., Dridi S. Radical Response: Effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants. 2021;10:35. doi: 10.3390/antiox10010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag M.R., Elhady W.M., Ahmed S.Y., Taha H.S., Alagawany M. Astragalus polysaccharides alleviate tilmicosin-induced toxicity in rats by inhibiting oxidative damage and modulating the expressions of HSP70, NF-kB and Nrf2/HO-1 pathway. Res. Vet. Sci. 2019;124:137–148. doi: 10.1016/j.rvsc.2019.03.010. [DOI] [PubMed] [Google Scholar]
- Gao X., Xiao Z.H., Liu M., Zhang N.Y., Khalil M.M., Gu C.Q., Qi D.S., Sun L.H. Dietary silymarin supplementation alleviates zearalenone-induced hepatotoxicity and reproductive toxicity in rats. J. Nutr. 2018;148:1209–1216. doi: 10.1093/jn/nxy114. [DOI] [PubMed] [Google Scholar]
- Goel A., Ncho C.M., Choi Y.-H. Regulation of gene expression in chickens by heat stress. J. Anim. Sci. Biotechno. 2021;12:1–13. doi: 10.1186/s40104-020-00523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F., Zhuang X., Han M., Lin W. Polysaccharides from Enteromorpha prolifera protect against carbon tetrachloride-induced acute liver injury in mice via activation of Nrf2/HO-1 signaling, and suppression of oxidative stress, inflammation and apoptosis. Food Funct. 2020;11:4485–4498. doi: 10.1039/d0fo00575d. [DOI] [PubMed] [Google Scholar]
- Guo Y., Zhao Z.H., Pan Z.Y., An L.L., Balasubramanian B., Liu W.C. New insights into the role of dietary marine-derived polysaccharides on productive performance, egg quality, antioxidant capacity, and jejunal morphology in late-phase laying hens. Poult. Sci. 2020;99:2100–2107. doi: 10.1016/j.psj.2019.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Balasubramanian B., Zhao Z.H., Liu W.C. Heat stress alters serum lipid metabolism of Chinese indigenous broiler chickens-a lipidomics study. Environ. Sci. Pollut. Res. 2021;28:10707–10717. doi: 10.1007/s11356-020-11348-0. [DOI] [PubMed] [Google Scholar]
- Guo Y., Balasubramanian B., Zhao Z.H., Liu W.C. Marine algal polysaccharides alleviate aflatoxin B1-induced bursa of Fabricius injury by regulating redox and apoptotic signaling pathway in broilers. Poult. Sci. 2021;100:844–857. doi: 10.1016/j.psj.2020.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q., Tong J., Sun Q., Teng X., Zhang H., Teng X. The involvement of miR-6615-5p/Smad7 axis and immune imbalance in ammonia-caused inflammatory injury via NF-κB pathway in broiler kidneys. Poult. Sci. 2020;99:5378–5388. doi: 10.1016/j.psj.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Yu Q., He Y., Hu R., Xia S., He J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Sci. 2019;98:6378–6387. doi: 10.3382/ps/pez471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa R., Nurjanah S., Furukawa K., Murai A., Kikusato M., Nochi T., Toyomizu M. Heat stress causes immune abnormalities via massive damage to effect proliferation and differentiation of lymphocytes in broiler chickens. Front. Vet. Sci. 2020;7:46. doi: 10.3389/fvets.2020.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini-Vashan S.J., Raei-Moghadam. M.S. Antioxidant and immune system status, plasma lipid, abdominal fat, and growth performance of broilers exposed to heat stress and fed diets supplemented with pomegranate pulp (Punica granatum L.) J. Appl. Anim. Res. 2019;47:521–531. [Google Scholar]
- Hu X., Zhang R., Xie Y., Wang H., Ge M. The protective effects of polysaccharides from Agaricus blazei Murill against cadmium-induced oxidant stress and inflammatory damage in chicken livers. Biol. Trace Elem. Res. 2017;178:117–126. doi: 10.1007/s12011-016-0905-y. [DOI] [PubMed] [Google Scholar]
- Hu X., Chi Q., Wang D., Chi X., Teng X., Li S. Hydrogen sulfide inhalation-induced immune damage is involved in oxidative stress, inflammation, apoptosis and the Th1/Th2 imbalance in broiler bursa of Fabricius. Ecotox. Environ. Safe. 2018;164:201–209. doi: 10.1016/j.ecoenv.2018.08.029. [DOI] [PubMed] [Google Scholar]
- Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Bioph. Res. Co. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Gene. Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan X., Hsieh J.C., Schmidt C.J., Zhu Q., Lamont S.J. Heat stress alters immune pathways in liver of divergent chicken lines. Anim. Indust. Rep. 2017;663:49. [Google Scholar]
- Li S., Wang X., Ren L., Li J., Zhu X., Xing T., Zhang L., Gao F., Zhou G. Protective effects of γ-irradiated Astragalus polysaccharides on intestinal development and mucosal immune function of immunosuppressed broilers. Poult. Sci. 2019;98:6400–6410. doi: 10.3382/ps/pez478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., He J., Xie H., Yang Y., Li J., Zou Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 2014;93:54–62. doi: 10.3382/ps.2013-03423. [DOI] [PubMed] [Google Scholar]
- Liu L., Shen J., Zhao C., Wang X., Yao J., Gong Y., Yang X. Dietary Astragalus polysaccharide alleviated immunological stress in broilers exposed to lipopolysaccharide. Int. J. Biol. Macromol. 2015;72:624–632. doi: 10.1016/j.ijbiomac.2014.08.057. [DOI] [PubMed] [Google Scholar]
- Liu W., Yuan Y., Sun C., Balasubramanian B., Zhao Z., An L. Effects of dietary betaine on growth performance, digestive function, carcass traits, and meat quality in indigenous yellow-feathered broilers under long-term heat stress. Animals. 2019;9:506. doi: 10.3390/ani9080506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.C., Guo Y., Zhao Z.H., Jha R., Balasubramanian B. Algae-derived polysaccharides promote growth performance by improving antioxidant capacity and intestinal barrier function in broiler chickens. Front. Vet. Sci. 2020;7:601336. doi: 10.3389/fvets.2020.601336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.C., Zhou S.H., Balasubramanian B., Zeng F.Y., Sun C.B., Pang H.Y. Dietary seaweed (Enteromorpha) polysaccharides improves growth performance involved in regulation of immune responses, intestinal morphology and microbial community in banana shrimp Fenneropenaeus merguiensis. Fish Shellfish Immun. 2020;104:202–212. doi: 10.1016/j.fsi.2020.05.079. [DOI] [PubMed] [Google Scholar]
- Liu W.C., Guo Y., An L.L., Zhao Z.H. Protective effects of dietary betaine on intestinal barrier function and cecal microbial community in indigenous broiler chickens exposed to high temperature environment. Environ. Sci. Pollut. Res. 2021;28:10860–10871. doi: 10.1007/s11356-020-11326-6. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen. T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Long L., Kang B., Jiang Q., Chen J. Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poult. Sci. 2020;99:744–751. doi: 10.1016/j.psj.2019.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Liu H., Wang J., Li L., Han C., Gan X., Li Y., Bai L., Mustafa A. Transcriptome reveals B lymphocyte apoptosis in duck embryonic bursa of Fabricius mediated by mitochondrial and Fas signaling pathways. Mol. Immunol. 2018;101:120–129. doi: 10.1016/j.molimm.2018.06.266. [DOI] [PubMed] [Google Scholar]
- MAPRC . Chicken Feeding Standard (NY/T33-2004) China Standard Press; Beijing, China: 2004. Ministry of Agriculture of the People's Republic of China. [Google Scholar]
- Ohtsu H., Yamazaki M., Abe H., Murakami H., Toyomizu M. Heat stress modulates cytokine gene expression in the spleen of broiler chickens. J. Poult. Sci. 2015;52:282–287. [Google Scholar]
- Quinteiro-Filho W., Ribeiro A., Ferraz-de-Paula V., Pinheiro M., Sakai M., Sá L., Ferreira A., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Rajput S.A., Zhang C., Feng Y., Wei X.T., Khalil M.M., Rajput I.R., Baloch D.M., Shaukat A., Rajput N., Qamar H., Hassan M., Qi D. Proanthocyanidins alleviates aflatoxin B(1)-induced oxidative stress and apoptosis through mitochondrial pathway in the bursa of fabricius of broilers. Toxins. 2019;11:157. doi: 10.3390/toxins11030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe M.J. Antibodies, immunoglobulin genes and the bursa of Fabricius in chicken B cell development. Dev. Comp. Immunol. 2006;30:101–118. doi: 10.1016/j.dci.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Romagnani S. T-cell subsets (Th1 versus Th2) Ann. Allergy Asthma Im. 2000;85:9–21. doi: 10.1016/S1081-1206(10)62426-X. [DOI] [PubMed] [Google Scholar]
- Saeed M., Abbas G., Alagawany M., Kamboh A.A., Abd El-Hack M.E., Khafaga A.F., Chao S. Heat stress management in poultry farms: a comprehensive overview. J. Therm. Biol. 2019;84:414–425. doi: 10.1016/j.jtherbio.2019.07.025. [DOI] [PubMed] [Google Scholar]
- Sahin K., Orhan C., Tuzcu M., Borawska M.H., Jabłonski J., Guler O., Sahin N., Hayirli A. Berberis vulgaris root extract alleviates the adverse effects of heat stress via modulating hepatic nuclear transcription factors in quails. Brit. J. Nutr. 2013;110:609–616. doi: 10.1017/S0007114512005648. [DOI] [PubMed] [Google Scholar]
- Sahin K. Modulation of NF-κB and Nrf2 pathways by lycopene supplementation in heat-stressed poultry. World's Poult. Sci. J. 2015;71:271–284. [Google Scholar]
- Sahin K., Orhan C., Tuzcu M., Sahin N., Hayirli A., Bilgili S., Kucuk O. Lycopene activates antioxidant enzymes and nuclear transcription factor systems in heat-stressed broilers. Poult. Sci. 2016;95:1088–1095. doi: 10.3382/ps/pew012. [DOI] [PubMed] [Google Scholar]
- Sandner G., Mueller A.S., Zhou X., Stadlbauer V., Schwarzinger B., Schwarzinger C., Wenzel U., Maenner K., van der Klis J.D., Hirtenlehner S. Ginseng extract ameliorates the negative physiological effects of heat stress by supporting heat shock response and improving intestinal barrier integrity: evidence from studies with heat-stressed Caco-2 cells, C. elegans and growing broilers. Molecules. 2020;25:835. doi: 10.3390/molecules25040835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M.J., Wei X., Xu J., Chen B.J., Zhao D.Y., Cui S., Zhou T. Carboxymethylated degraded polysaccharides from Enteromorpha prolifera: preparation and in vitro antioxidant activity. Food Chem. 2017;215:76–83. doi: 10.1016/j.foodchem.2016.07.151. [DOI] [PubMed] [Google Scholar]
- Shi M.J., Wang F., Jiang H., Qian W.W., Xie Y.Y., Wei X.Y., Zhou T. Effect of enzymatic degraded polysaccharides from Enteromorpha prolifera on the physical and oxidative stability of fish oil-in-water emulsions. Food Chem. 2020;322:126774. doi: 10.1016/j.foodchem.2020.126774. [DOI] [PubMed] [Google Scholar]
- Siddiqui S.H., Kang D., Park J., Khan M., Shim K. Chronic heat stress regulates the relation between heat shock protein and immunity in broiler small intestine. Sci. Rep. 2020;10:1–11. doi: 10.1038/s41598-020-75885-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohail M., Ijaz A., Younus M., Shabbir M., Kamran Z., Ahmad S., Anwar H., Yousaf M., Ashraf K., Shahzad A. Effect of supplementation of mannan oligosaccharide and probiotic on growth performance, relative weights of viscera, and population of selected intestinal bacteria in cyclic heat-stressed broilers. J. Appl. Poult. Res. 2013;22:485–491. [Google Scholar]
- Vandana G., Sejian V., Lees A., Pragna P., Silpa M., Maloney S.K. Heat stress and poultry production: impact and amelioration. Int. J. Biometeorol. 2021;65:163–179. doi: 10.1007/s00484-020-02023-7. [DOI] [PubMed] [Google Scholar]
- Wei J., Wang S., Liu G., Pei D., Liu Y., Liu Y., Di D. Polysaccharides from Enteromorpha prolifera enhance the immunity of normal mice. Int. J. Biol. Macromol. 2014;64:1–5. doi: 10.1016/j.ijbiomac.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Wu X., Xu W., Feng X., He Y., Liu X., Gao Y., Yang S., Shao Z., Yang C., Ye Z. TNF-a mediated inflammatory macrophage polarization contributes to the pathogenesis of steroid-induced osteonecrosis in mice. Int. J. Immunopath. Ph. 2015;28:351–361. doi: 10.1177/0394632015593228. [DOI] [PubMed] [Google Scholar]
- Xu D., Li W., Huang Y., He J., Tian Y. The effect of selenium and polysaccharide of Atractylodes macrocephala Koidz.(PAMK) on immune response in chicken spleen under heat stress. Biol. Trace Elem. Res. 2014;160:232–237. doi: 10.1007/s12011-014-0056-y. [DOI] [PubMed] [Google Scholar]
- Xu D., Tian. Y. Selenium and polysaccharides of atractylodes macrocephala koidz play different roles in improving the immune response induced by heat stress in chickens. Biol. Trace Elem. Res. 2015;168:235–241. doi: 10.1007/s12011-015-0351-2. [DOI] [PubMed] [Google Scholar]
- Xu D., Li B., Cao N., Li W., Tian Y., Huang Y. The protective effects of polysaccharide of Atractylodes macrocephala Koidz (PAMK) on the chicken spleen under heat stress via antagonizing apoptosis and restoring the immune function. Oncotarget. 2017;8:70394. doi: 10.18632/oncotarget.19709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Li W., Li B., Tian Y., Huang Y. The effect of selenium and polysaccharide of Atractylodes macrocephala Koidz.(PAMK) on endoplasmic reticulum stress and apoptosis in chicken spleen induced by heat stress. RSC Adv. 2017;7:7519–7525. [Google Scholar]
- Yamamoto T., Suzuki T., Kobayashi A., Wakabayashi J., Maher J., Motohashi H., Yamamoto M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol. Cell. Biol. 2008;28:2758–2770. doi: 10.1128/MCB.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Bai K., Su W., Wang A., Zhang L., Huang K., Wang T. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult. Sci. 2018;97:1209–1219. doi: 10.3382/ps/pex408. [DOI] [PubMed] [Google Scholar]
- Zhang S., Wang C., Sun Y., Wang G., Chen H., Li D., Yu X., Chen G. Xylanase and fermented Polysaccharide of Hericium caputmedusae reduce pathogenic infection of broilers by improving antioxidant and anti-inflammatory properties. Oxid. Med. Cell. Longev. 2018;2018:4296985. doi: 10.1155/2018/4296985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Li C., Shao Q., Chen W., Ma L., Xu W., Li Y., Huang S., Ma Y. Effects of Glycyrrhiza polysaccharide in diet on growth performance, serum antioxidant capacity, and biochemistry of broilers. Poult. Sci. 2021;100:100927. doi: 10.1016/j.psj.2020.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Qu J., Wang W., Li S., Xu S. The imbalance of Th1/Th2 triggers an inflammatory response in chicken spleens after ammonia exposure. Poult. Sci. 2020;99:3817–3822. doi: 10.1016/j.psj.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Balasubramanian B., Guo Y., Qiu S.J., Jha R., Liu W.C. Dietary Enteromorpha polysaccharides supplementation improves breast muscle yield and is associated with modification of mRNA transcriptome in broiler chickens. Front. Vet. Sci. 2021;8:663988. doi: 10.3389/fvets.2021.663988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R., Wan X., Wang D., Zhao C., Liu D., Gao L., Wang M., Wu C., Nabavid S.M., Daglia M. Polysaccharides from marine Enteromorpha: structure and function. Trends Food Sci. Tech. 2020;99:11–20. [Google Scholar]
- Zhou M., Tao Y., Lai C., Huang C., Zhou Y., Yong Q. Effects of mannanoligosaccharide supplementation on the growth performance, immunity, and oxidative status of partridge shank chickens. Animals. 2019;9:817. doi: 10.3390/ani9100817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Pan S., Wu S. Modulation of the growth performance, body composition and nonspecific immunity of crucian carp Carassius auratus upon Enteromorpha prolifera polysaccharide. Int. J. Biol. Macromol. 2020;147:29–33. doi: 10.1016/j.ijbiomac.2020.01.065. [DOI] [PubMed] [Google Scholar]