Highlights
-
•
During lymphoma growth, Tregs evolve an increasingly suppressive phenotype.
-
•
Lymphoma-infiltrating Tregs show an enhanced immunosuppressive function.
-
•
Cell contacts and IL-10 are required for Treg-mediated immunosuppression.
-
•
Alterations of intratumoral Tregs are partly abrogated by immune checkpoint blockade.
Keywords: Foxp3, CTLA-4, PD-1, IL-10, MYC lymphoma
Abstract
In malignant disease, CD4+Foxp3+ regulatory T cells (Tregs) hamper antitumor immune responses and may provide a target for immunotherapy. Although immune checkpoint blockade (ICB) has become an established therapy for several cancer entities including lymphoma, its mechanisms have not been entirely uncovered. Using endogenously arising λ-MYC-transgenic mouse B-cell lymphomas, which can effectively be suppressed by either Treg ablation or ICB, we investigated which mechanisms are used by Tregs to suppress antitumor responses and how ICB affects these pathways. During tumor development, Tregs up-regulated Foxp3, CD25, CTLA-4 and IL-10, which correlated with enhanced immunosuppressive functions. Thus, in contrast to other tumors, Tregs did not become dysfunctional despite chronic stimulation in the tumor microenvironment and progressive up-regulation of PD-1. Immunosuppression was mediated by direct contacts between Tregs and effector T cells and by IL-10. When λ-MYC mice were treated with ICB antibodies, Tregs revealed a less profound up-regulation of Foxp3, CD25 and IL-10 and a decreased suppressive capacity. This may be due to the shift towards a pro-inflammatory milieu fostered by ICB. In summary, an ICB-induced interference with Treg-dependent immunosuppression may contribute to the success of ICB.
Introduction
To prevent excessive immune reactions and autoaggressive disease, the immune system has evolved delicate counterregulatory mechanisms capable of limiting proinflammatory responses. These pathways involve, e.g., CD4+ regulatory T (Treg) cells, whose hallmark is the expression of the transcription factor forkhead box p3 (Foxp3) and surface CD25. [1], [2], [3] Tregs are either induced in the periphery when interacting with antigens (Ags) under tolerogenic conditions (induced Tregs, iTregs) or are derived from developing T cells with intermediate self-reactivity in the thymus (natural Tregs, nTregs). [4], [5], [6], [7]
In malignant disease, the potential of Foxp3+ Tregs to impede effective immune responses can promote tumor immune escape. It has been shown for several cancer types in humans that an increased Treg to T-effector (Teff) cell ratio is correlated with a worse prognosis. [8], [9], [10] Accordingly, in murine models, depletion of Foxp3+ Tregs positively affected survival and anti-tumor immune responses. [11,12]
Breaking immune tolerance induced by Foxp3+ Treg-mediated and other mechanisms is a major goal of cancer immunotherapy. Since the treatment options of B-cell lymphoma in the clinics are still insufficient, we set out to better understand immune escape pathways in this disorder. To this end, we are studying transgenic mice that spontaneously develop B-cell lymphoma due to B cell-restricted overexpression of the proto-oncogene c-MYC. [13] In this model, tumor immune evasion is also partly dependent on Foxp3+ Tregs, because ablation of Tregs improves the function of CD8+ T cells and suppresses tumor growth. [14] The relative numbers of intratumoral Tregs within the CD4+ cell compartment are elevated and Tregs show an activated phenotype as indicated by CD69 expression. Importantly, tumor-infiltrating Tregs are predominantly nTregs, which recognize non-mutated self-peptides overexpressed by the tumor cells. Since Foxp3− Teff cells targeting the same peptide epitopes were identified in the lymphomas, [14] physical competition of Foxp3+ Tregs with potentially tumor-protective Teff cells for major histocompatibility complex II- (MHCII-) peptide complexes recognized by their T-cell receptors (TCRs) is likely to be a mechanism of Treg-mediated immunosuppression. It is an open question, however, which pathways are further used by Tregs to suppress Teff functions in the lymphoma model.
An effective immunologic approach, which has already been approved for treating several tumor entities including lymphoma, is immune checkpoint blockade (ICB). [15], [16], [17], [18] Programmed cell death protein-1 (PD-1) and cytotoxic T lymphocyte-associated protein-4 (CTLA-4) represent two members of immune checkpoint molecules, which are up-regulated on Teff cells becoming exhausted due to chronic stimulation in the tumor milieu. PD-1 and CTLA-4 exert an immunosuppressive effect by inhibiting TCR and CD28 signaling, respectively. [19], [20], [21], [22] Interfering with these counter-regulatory pathways by using monoclonal antibodies (mAbs) unleashes potentially tumor-reactive Teff cells and boosts anti-tumor immune responses. [19], [20], [21], [22], [23], [24]
Immune checkpoint molecules such as PD-1 are also up-regulated on Foxp3+ Tregs upon stimulation, which – under inflammatory conditions – is associated with “lineage destabilization”, hence acquired production of interferon-γ (IFN-γ) and loss of Foxp3. [25], [26], [27], [28], [29] Studies also suggested that Tregs up-regulating PD-1 and CTLA-4 may have lower suppressive impact on Teff cells in cancer [30], [31], [32] and that PD-1 blockade may restore Treg-mediated immunosuppression [31]. Having shown that Foxp3+ Tregs confer immune escape of λ-MYC lymphomas [14] and that the combinatorial treatment with mAbs against PD-1 and CTLA-4 is highly effective in suppressing lymphoma growth [33,34], we now asked the question how ICB affects Foxp3+ Treg functions in lymphoma. Therefor, it was at first necessary to decipher in detail the immunosuppressive mechanisms used by Foxp3+ Treg cells in the λ-MYC lymphoma model.
Materials and methods
Animal experiments
All mouse experiments were approved by Regierung von Oberbayern and performed in accordance with the EU Directive 2010/63/EU. Breeding was done in our in-house animal facility, where all mice were kept under specified pathogen-free conditions. Transgenic λ-MYC mice (C57BL/6 background) harbor the c-MYC oncogene under the transcriptional control of the B cell-specific Igλ enhancer and develop visible tumor burdens between day 75 and day 130 after birth. [13] In this situation (“late disease stage”), mice have to be euthanized within few days according to animal welfare legislation. To study early disease stages, mice were already sacrificed at an age of 65–75 days when immunologic alterations caused by incipient tumor growth were already observed but tumors have not yet become clinically manifest. Although present, the numbers of malignant cells cannot be monitored in vivo at this early stage. Wildtype (wt) mice served as healthy controls. Male and female mice were used. The sex of the animals did not impact the results.
To investigate treatment effects, λ-MYC mice were injected intraperitoneally with a combination of 100 µg anti-PD-1 (J43, BioXCell, West Lebanon, USA) and 100 µg anti-CTLA-4 (UC10–4B9, BioLegend, San Diego, USA) mAbs four times every ten days starting at day 55 after birth. At this time point, an adult immune system has already developed and none of the mice did show visible tumor burdens, which is the prerequisite for leaving the animals alive. To study ICB-induced immune responses at an early disease stage, clinically unapparent animals were already analyzed three days after the second injection. For analyses of ICB effects in late tumor stages, animals were sacrificed after development of visible tumor masses, irrespective of the number of treatment cycles delivered before. Clinically unapparent, age-matched untreated λ-MYC mice and tumor-bearing untreated λ-MYC animals with identical tumor burdens, respectively, were used as controls.
Fluorescence-activated cell sorting (FACS)
Spleens were dissected and passed through a cell strainer (40 µm). The single cell suspensions were subjected to erythrocyte lysis, followed by two wash steps and filtration to remove tissue debris. For FACS analyses, LIVE/DEAD Fixable Blue Dead Cell Stain Kit (Thermo Fisher Scientific, Waltham, USA) was used to exclude dead cells. Surface molecules were stained for 30 min at 4 °C with fluorochrome-labeled mAbs against CD4 (RM4–5, BD Pharmingen, Franklin Lakes, USA), CD25 (PC61, BioLegend), CD152/CTLA-4 (UC10–4B9, BioLegend) and/or CD279/PD-1 (RMP1–30; Thermo Fisher Scientific).
For intracellular staining, cells were fixed and permeabilized (Foxp3 Staining Buffer Set, Thermo Fisher Scientific). Proteins were stained for 30 min at room temperature using mAbs against Foxp3 (FJK-16 s), CTLA-4 (1B8), IFN-γ (XMG-1.2) or interleukin-10 (IL-10) (JES5–16E3; all from Thermo Fisher Scientific). For detection of intracellular cytokines, cells were stimulated for 4 h with 1 µg/ml PMA, 1 µg/ml ionomycin (both Sigma-Aldrich, Saint Louis, USA) and 3 µg/ml Brefeldin A (Thermo Fisher Scientific) in complete cell culture medium prior to staining. Samples were measured on a LSR II flow cytometer (BD) and analyzed using the Flowjo software.
In vitro suppression assay
CD4+CD25+ Tregs from wt or λ-MYC spleens and CD4+CD25− responder cells were isolated using the EasySep Mouse CD4+ T Cell Isolation Kit and the EasySep Mouse CD25 Regulatory T Cell Positive Selection Kit (both STEMCELL Technologies, Vancouver, Canada) according to the manufacturer´s protocol. To secure that measurement of the suppressive function of λ-MYC-derived Tregs be not biased by other, Treg-independent tumor-induced alterations of Teff cells, responder cells from normal wt mice were used for the suppression assays. The enriched responder cells were labeled with Cell Proliferation Dye (CPD) eFluor 450 (Thermo Fisher Scientific) and cultured along with Tregs in varying proportions in the presence of immobilized anti-CD3 and anti-CD28 mAbs (2 µg/ml, Core Facility Monoclonal Antibodies, Helmholtz-Zentrum München). A total of 1 × 105 cells was seeded per well and stimulated for 48 h in complete cell culture medium (10% FCS). The supernatants were frozen for cytokine quantification, and CPD fluorescence was analyzed after gating for viable CD4+ Foxp3− cells. Suppression was calculated as (% cycling responder cells without Tregs –% cycling responder cells in the presence of Tregs):% cycling responder cells without Tregs x 100.
Where indicated, mAbs against IL-10 (JES5–16E3, BioLegend; 0.1 µg/ml), neuropilin-1 (Nrp-1) (Bio-Techne, Minneapolis, USA; 1 µg/ml) or programmed cell death-ligand 1 (PD-L1) (BioLegend; 1 µg/ml) were added to the co-culture. To investigate if cell contacts were required for Treg-mediated suppression, cells were cultivated in a cell culture plate with a trans-well membrane insert (Corning, Corning, USA) to prevent direct cell-cell interactions between Tregs and Teffs. To secure equal stimulation of Teffs and Tregs, both chambers were coated with anti-CD3/anti-CD28 mAbs.
In some experiments, T cells were stimulated by peptide-loaded antigen-presenting cells (APCs) instead of anti-CD3/anti-CD28 mAbs. To this end, CD11c+ cells were isolated from wt spleens using CD11c MicroBeads UltraPure and a MACS Separator (both Miltenyi Biotec, Bergisch Gladbach, Germany). Enriched cells were incubated for 3 h with a peptide mix of MHC class II-restricted self-epitopes (each at a concentration of 1 µg/ml), which were prevalent in λ-MYC lymphoma cells and capable of stimulating Tregs and Teffs (LERLDLDLTSDSQPPVF, VRPPVPLPASSHPASTNEPIVLED, EAVLTGLVEA and RIEPLSPSKN, for details see [ref. 14]). A total of 1.5 × 105 T cells was stimulated with 2 × 104 peptide-pulsed APCs and recombinant interleukin-2 (IL-2) (50 U/ml). After six days, cells were harvested, and CPD dilution in responder cells was analyzed by FACS.
Enzyme-linked immunosorbent assay (ELISA)
IFN-γ and IL-10 concentrations in cell culture supernatants were quantified by using the IFN gamma Mouse Uncoated ELISA Kit and the IL-10 Mouse Uncoated ELISA Kit (both Thermo Fisher Scientific), respectively, according to the manufacturer´s protocol. Samples were measured with the SUNRISE Absorbance Reader and analyzed utilizing the Magellan software (both Tecan Group, Männedorf, Switzerland).
Statistical analyses
All results were expressed as means ± SEM. For assessment of differences between two independent groups, the unpaired or the paired t-test was used. To compare three or more groups, the one-way ANOVA test was performed, corrected for multiple comparisons using Bonferroni post-hoc test. Data analysis was done with Prism 5.0 software (GraphPad). The significance levels are denoted as follows: * P<0.05, ** P<0.01 and *** P<0.001.
Results
During λ-MYC lymphomagenesis CD4+Foxp3+ Tregs up-regulate markers associated with functionality
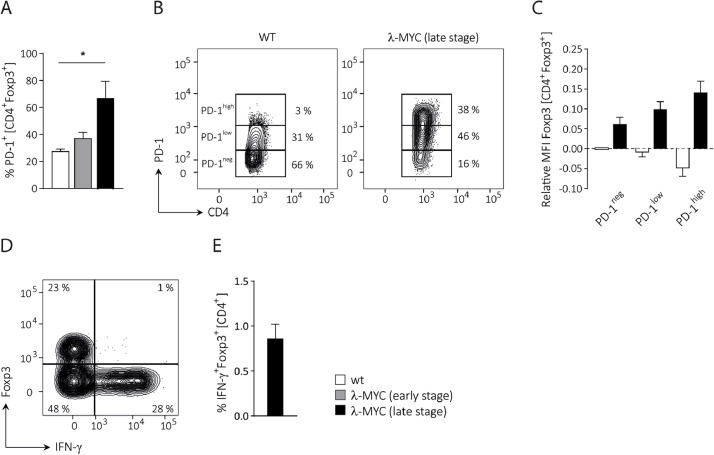
To investigate whether the immunosuppressive potential of CD4+ Foxp3+ Tregs in λ-MYC tumors is altered in comparison to normal Tregs, we analyzed the expression of molecules that are associated with Treg functions. CD4+Foxp3+ Tregs obtained from λ-MYC mice at early and late disease stages (for definition see Materials and Methods section) were compared to Tregs from healthy wt mice. During disease progression, Foxp3 was gradually up-regulated in the Foxp3+ subset (Fig. 1A). Accordingly, intratumoral Tregs revealed increasing levels of CD25 and CTLA-4 (Fig. 1B, C), which were described to be under the transcriptional control of Foxp3. [35] Expression of IL-10, which is promoted by Foxp3, [36] was also raised during tumor development, at least in late disease stages (Fig. 1D). An explanation why IL-10 is reduced in early stages has to await further studies. Exemplary results are depicted in supplemental figure 1.
Fig. 1.
Phenotypic characterization of Treg cells from λ-MYC tumors. A, Increasing Foxp3 expression in the CD4+Foxp3+ population during disease progression (for definition of early and late stage, see Materials and Methods). B, Increasing relative CD25 expression in Foxp3+ Tregs during tumor development. C, Relative intracellular CTLA-4 levels of Foxp3+ Tregs determined in early and late tumor stages. Surface staining of CTLA-4 yielded similar results. D, Expression of IL-10 in Foxp3+ Tregs in different disease stages. In all panels, expression levels (mean fluorescence intensities (MFI) or% positive cells) were normalized to the values obtained in wt mice. Analyses were confined to the CD4+Foxp3+ compartment. Up to 17 mice were analyzed per group. Representative histograms are provided in supplemental figure 1.
Based on the concept of chronic TCR stimulation by tumor-derived peptides, [14] it was anticipated that the expression of the co-inhibitory receptor PD-1 was also up-regulated on Tregs from tumor-bearing λ-MYC mice. Indeed, we observed a progressive increase of PD-1 expression during disease development (Fig. 2A). As shown in late stage tumors, this was accounted for by an expansion of the PD-1low and particularly the PD-1high Treg subpopulation (Fig. 2B). The data suggest that PD-1 up-regulation depends on chronic stimulation, hence on disease progression. When Foxp3 expression was compared between the PD-1negative, the PD-1low and the PD-1high Treg subset, Foxp3 correlated with the PD-1 expression level (Fig. 2C). By contrast, an inverse correlation between PD-1 and Foxp3 was seen in Tregs from normal wt mice, which may indicate lineage destabilization associated with PD-1 up-regulation (Fig. 2C). No significant IFN-γ production was detected in the Foxp3+ fraction from late stage tumors (Fig. 2D, E). Taken together, in the λ-MYC model no evidence for lineage destabilization could be provided as it was described elsewhere. [32]
Fig. 2.
Stability of CD4+Foxp3+ Tregs in tumor-bearing λ-MYC mice. A, PD-1 expression on Foxp3+ Tregs in healthy wt, early stage λ-MYC and late stage λ-MYC mice. Three mice are included in each group. B, Distribution of PD-1negative, PD-1low and PD-1high CD4+Foxp3+ cells from wt and late stage λ-MYC mice. C, Correlation between PD-1 surface expression and Foxp3 levels in Foxp3+ Tregs from late-stage tumor λ-MYC or normal wt spleens (n = 7). MFIs were normalized to the PD-1neg fraction of wt mice and denoted logarithmically. D, E, Lack of IFN-γ in CD4+Foxp3+ cells from tumor-bearing λ-MYC animals. Typical result (D) and compilation of 5 mice (E).
λ-MYC Tregs reveal an increased immunosuppressive capability due to an altered Treg/Teff ratio and an enhanced suppressive potential at the single-cell level
The recognition of lymphoma-derived overexpressed self-Ags leads to activation of Tregs and to an increased Treg/Teff ratio. [14] To investigate the relevance of this quantitative shift for impairment of Teff activity and to analyze the immunosuppressive potential of single Tregs, an in vitro suppression assay was established based on inhibiting the CD3/CD28-induced proliferation of normal wt CD4+ Teff cells by Tregs. For this purpose, CD4+CD25+ Tregs and CD4+CD25− Teff cells were enriched and, after labeling of the Teff fraction with CPD, co-cultured in varying ratios mimicking the different Treg/Teff ratios occurring in wt and λ-MYC tumor mice, respectively. As expected, when performing the experiment with either normal wt or λ-MYC-derived Treg cells, a higher proportion of Tregs in the co-culture resulted in a stronger suppression as calculated from the delayed responder cell proliferation (Fig. 3A, B). Similar results were obtained when stimulation of T cells in vitro was not done by anti-CD3/anti-CD28 mAbs, but by using APCs pulsed with those self-peptides that were overexpressed in λ-MYC tumor cells (not shown). Therefore, a shift of the Treg/Teff ratio in favor of the former is indeed a factor stipulating stronger immunosuppression.
Fig. 3.
Suppression of Teff cell proliferation by Tregs in vitro. A, Principle of the suppression assay exemplified by using wt cells. CD4+CD25+ Tregs and CPD-labeled CD4+CD25− Teffs were co-cultured at the indicated ratios for 48 h in the presence of immobilized anti-CD3/anti-CD28 mAbs. In the left panel, stimulation via CD3/CD28 was omitted. CPD fluorescence was measured after gating to the CD4+Foxp3− population. The percentages of Teff cells having undergone cell division are denoted. B, Suppression of Teff cells (calculated as%, as described in Materials and Methods) exerted by wt and λ-MYC-derived Tregs, respectively. Here, only Tregs from λ-MYC spleens at early disease stages could be used because the high numbers of tumor cells at late stages interfere with the Treg enrichment and compromise its efficiency. Compilation of 7 independent experiments. C, IFN-γ concentrations in supernatants of suppression assays at a Treg/Teff ratio of 10:1 (n = 3). Co-cultivation of wt Teffs with Tregs from λ-MYC mice is compared to co-cultivation with wt Tregs. IFN-γ was exclusively derived from Foxp3− Teffs (see also Fig. 2D).
To determine whether the suppressive capability of λ-MYC Tregs is altered at the single-cell level, we then compared wt and λ-MYC Tregs in this in vitro system. λ-MYC Tregs turned out to be superior to wt Tregs in suppressing proliferation (Fig. 3B). IFN-γ release by the responder cells was also slightly reduced after incubation with λ-Myc-derived Tregs (Fig. 3C). Taken together, the data suggest that the phenotypic changes observed were associated with an increased immunosuppressive function.
λ-MYC Treg-mediated immunosuppression requires cell contacts and IL-10
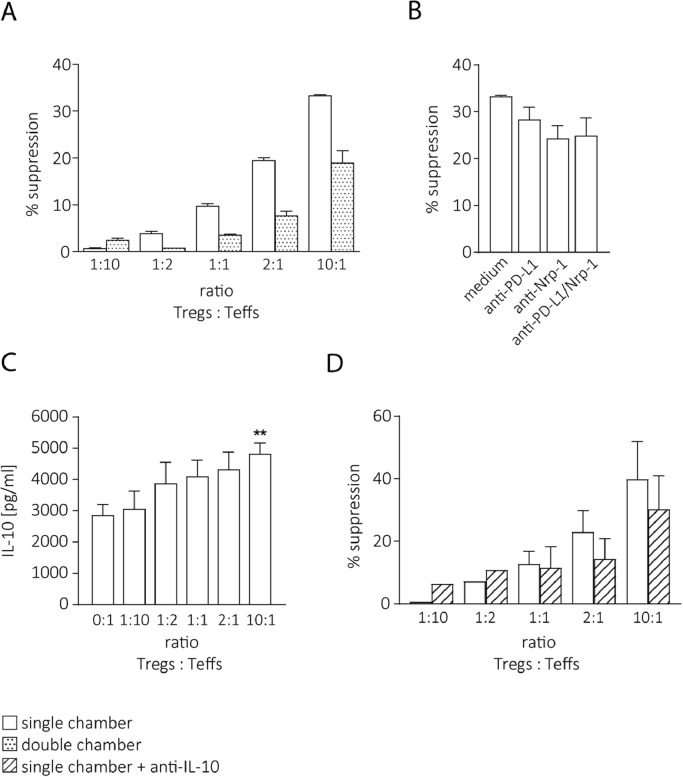
To address the question which mechanisms confer the suppressive activity of the Tregs, we first examined whether the suppression observed in vitro was mediated by cell contacts. Tregs and Teffs were enriched as described above, but co-cultured in trans-well plates to prevent cellular interactions between the Treg and the Teff population. In this setting, the suppression was markedly decreased (Fig. 4A). Since APCs were absent in this experiment, direct Treg-Teff interactions seemed to be instrumental for Teff suppression. Therefore, we asked which surface molecules might be involved in this interplay. We repeated the single-chamber suppression assays and included mAbs blocking Nrp-1 and PD-L1 because these molecules were shown to be expressed on the surface of λ-MYC Tregs. [14] Furthermore, Teffs expressed Semaphorin-4a and PD-1, which are the ligands of Nrp-1 and PD-L1, respectively. Treg-dependent suppression of Teffs was indeed diminished by these mAbs (Fig. 4B).
Fig. 4.
Mechanisms of Treg-dependent Teff suppression. A, Partial abrogation of the inhibitory function of λ-MYC Tregs by preventing cell contacts between Tregs and Teffs in trans-well chambers. Typical result from two experiments. B, Inhibition of the immunosuppressive potential of Tregs by mAbs interrupting direct interactions between Tregs and Teffs in the single-chamber setting. A suppression assay at a Treg/Teff ratio of 10:1 is shown (n = 3–5). C, IL-10 concentrations in supernatants of suppression assays at different Treg/Teff ratios (n = 4). The significance was calculated in comparison to the first column. D, Partial reversal of λ-MYC Treg-induced Teff suppression by neutralization of IL-10 in the single-chamber assay (n = 3).
As the suppressive activity was not completely abrogated in the trans-well assays, soluble factors also seemed to play a role for λ-MYC Treg-mediated suppression. Since IL-10 was up-regulated in intratumoral Tregs compared to normal Tregs (Fig. 1D) and the inhibition of Teff proliferation in the in vitro suppression assay correlated with the concentrations of IL-10 secreted by Tregs in the co-culture supernatants (Fig. 4C), IL-10 was considered as a candidate factor conveying the increased suppressive activity of λ-MYC Tregs. Therefore, the suppression assay was done in the presence of an IL-10-neutralizing mAb. In this situation, the suppressive effect of Tregs was decreased (Fig. 4D), albeit not significantly. This suggests that IL-10 may be involved in Teff cell suppression by λ-MYC Tregs, besides cell contacts and possibly other humoral factors.
ICB impacts the suppressive phenotype and function of λ-MYC Tregs
Given the phenotypic and functional changes of Tregs arising during tumor development, the role of Tregs in promoting immune evasion of λ-MYC tumors [14] and the capability of ICB of delaying the growth of λ-MYC tumors [33], we asked if ICB therapy also impacts the Treg cell compartment. Since the co-inhibitory receptors PD-1 and CTLA-4 were highly expressed on λ-MYC Tregs, therapeutic mAbs against these receptors may directly target Tregs, thus modulating their stability and function. [30]
Since single mAbs against either PD-1 or CTLA-4 were shown to be completely ineffective in terms of tumor suppression, [33] we only investigated the effects of a combined anti-PD-1/anti-CTLA-4 treatment, which is clinically highly successful in the λ-MYC model. After treatment, molecules that were shown to be up-regulated in λ-MYC-derived Tregs were quantified. It turned out that ICB therapy led to a reduced expression of Foxp3 and CD25 by CD4+ Tregs as compared to Tregs that were derived from untreated tumor-developing λ-MYC mice (Fig. 5A, B). Because CD25, as a part of the high-affinity IL-2 receptor complex, was described to deprive IL-2 from the microenvironment, [2] it is conceivable that, in addition to increased IL-2 secretion by re-activated effector T cells, a reduced IL-2 consumption by CD25 on Tregs contributes to the therapeutic effect of ICB. Additionally, in Tregs from treated mice, IL-10 expression was reduced, albeit not significantly (Fig. 5C).
Fig. 5.
Phenotype and suppressive function of λ-MYC Tregs after ICB therapy in vivo. A, Reduction of Foxp3 expression in CD4+Foxp3+ cells following ICB treatment. B, C, ICB-induced alterations of CD25 (B) and IL-10 (C) in λ-MYC Foxp3+ Tregs at different disease stages. Calculations were done as described in Fig. 1 legend. Up to 17 mice were analyzed. D, Immunosuppressive potential of Tregs after ICB therapy of λ-MYC mice (early disease stage, see Fig. 3B legend) as shown in a suppression assay at a Treg/Teff ratio of 10:1 (n = 2). The experiments were exactly performed as described for Fig. 2.
To assess whether these therapy-induced alterations correlated with a lower suppressive capacity, Tregs isolated from treated animals were analyzed in the suppression assay. The enhanced suppressive potential of λ-MYC-derived Tregs could indeed partly be abrogated by combined immune checkpoint inhibition in vivo although this was not statistically significant (Fig. 5D). Taken together, phenotypic as well as functional changes occurring during the course of tumor development could in part be reversed by treatment of mice with PD-1- and CTLA-4-blocking mAbs.
Discussion
Establishing innovative immunologic approaches of cancer therapy requires a better understanding of immune escape mechanisms. Since malignant B-cell lymphoma has still a poor prognosis in the clinics and new treatment strategies are needed, we studied antitumor responses and their therapeutic modulation in a λ-MYC transgenic mouse model of endogenously arising lymphoma. This model reflects several features of human B-cell lymphoma [13] and mirrors the immunosuppressive microenvironment found in human neoplasias more closely than transplanted mouse tumors [37].
We previously showed that λ-MYC tumors can be effectively suppressed by treating mice with a combination of mAbs targeting the immune checkpoints PD-1 and CTLA-4. [33,34] While the original rationale of the ICB approach was to resuscitate tumor-directed T cells that have become exhausted in the tumor milieu, it turned out that other mechanisms also contribute to the therapeutic effect. These include stimulation of natural killer (NK) cells [34], modulation of dendritic cells (DCs) [38] and IFN-γ-dependent senescence induction in the malignant cells [33,34,39,40]. The question whether Foxp3+ Tregs play a role for the success of ICB therapy has remained unanswered so far.
A tumor-promoting function of Foxp3+ Tregs in λ-MYC lymphoma was indicated by activation of CD8+ Teff cells and a delay of tumor growth following depletion of Tregs. [14] Increased intratumoral Treg/Teff ratios, which were predominantly accounted for by the nTreg subset, are likely to be the result of a differential expansion of Treg and Teff cells. Several λ-MYC lymphoma-associated self-peptides have been identified that are recognized by both, Tregs and Teffs, resulting in a differential proliferative response. [14]
Foxp3 is the lineage-defining transcription factor, responsible for development and functionality of Tregs. [6,41] PD-1-expressing Foxp3+ Tregs, which have been stimulated to proliferate in vivo, may become dysfunctional. [30,32] Accordingly, we observed an inverse correlation between PD-1 and Foxp3 levels in normal mice (Fig. 2C). In tumor-bearing λ-MYC animals, by contrast, Tregs displaying high PD-1 expression showed up-regulated Foxp3 in parallel and no IFN-γ induction (Fig. 2). Further, the suppressive capacity of λ-MYC-derived Tregs was increased as determined in vitro (Fig. 3B, C). The results are in contrast to other reports indicating destabilization of intratumoral Tregs [31,32] and suggest that functional alterations of Tregs might depend on the tumor entity.
In summary, the immunosuppressive potential of Foxp3 Tregs in λ-MYC tumors is apparently mediated by several mechanisms, though additional factors are not excluded:
-
(i)
Since MHC class II-restricted self-peptides overexpressed in lymphoma cells stimulate both, Tregs and Teffs, and the Treg/Teff quotient is increased in the tumors, the Treg compartment may outcompete putatively tumor-reactive Teffs. A competition for TCR stimuli could be shown in vitro even for wt Tregs (Fig. 3A). Tregs may also remove the cognate peptide-MHC class II complexes from the surface of DCs, thereby reducing their capability of effective Ag presentation. [42] However, this is not the only mechanism because the enhanced suppressive effect of λ-MYC Tregs was also seen after peptide-independent stimulation through anti-CD3/anti-CD28 mAbs.
-
(ii)
The in vitro suppression studies showed that the suppressive capability of λ-MYC Tregs is also increased at the single-cell level. The enhanced expression of CD25 on λ-MYC Tregs as compared to wt Tregs, which is predicted as a consequence of the Foxp3 up-regulation, presumably entails an enhanced consumption of IL-2, as described elsewhere. [2]
-
(iii)
λ-MYC Tregs also express more CTLA-4 than wt Tregs. Through transendocytosis, CTLA-4 can remove CD80 and CD86 from the surface of DCs and thereby reduce co-stimulatory signals required for activation of Teff cells. [43]
-
(iv)
Since the augmented immunosuppressive activity of λ-MYC Tregs was diminished in trans-well chambers, where CD3/CD28-induced Teff activation was measured in the absence of APCs, direct cellular contacts between Treg and Teffs also seem to be relevant. This interplay involved the surface molecules Nrp-1 and PD-L1, which are expressed on the surface of Tregs [14] and interact with Semaphorin-4a and PD-1, respectively, expressed by Teffs. Of course, involvement of other surface molecules cannot be excluded.
-
(v)
A humoral factor that inhibits Teff responses may be IL-10, whose expression was enhanced in λ-MYC-derived Tregs, although other factors are likely be involved additionally. Recently, IL-10 was found to promote λ-MYC tumor immune escape. Mainly derived from malignant B cells, [44] but also from Tregs and DCs, IL-10 has been shown to contribute to exhaustion of Teff cells and to drive the conversion of T-helper 1 (Th1) cells into Foxp3− T-regulatory type 1 (Tr1) cells, thereby hampering tumor-protective Th1 responses. Neutralization of IL-10 led to prolonged survival. [45]
Importantly, treatment of λ-MYC mice with mAbs targeting PD-1 and CTLA-4 reduced the capability of Tregs of suppressing Teff proliferation, as shown in vitro, and diminished the expression of molecules related to the suppressive functions of Tregs, such as Foxp3, CD25 or IL-10 (Fig. 5). The decline of Foxp3 might be a consequence of the shift towards pro-inflammatory conditions that is promoted by ICB in λ-MYC lymphoma. [33,34] Nevertheless, there was no evidence for Treg instability during therapy because an increase of IFN-γ expression was not detected (not shown). The less pronounced ICB-induced reduction of Foxp3 and CD25 in late disease stages may be due to the increasingly aggressive tumor growth, which outpaces the induction of antitumor responses, and the higher individual variation.
Pembrolizumab, an anti-PD-1 mAb used in the clinics, was shown to impair differentiation of iTregs in vitro and to dampen their IL-10 production, while no effect on Foxp3 levels was observed. [46] Other reports suggest that anti-PD-1 therapy can reinvigorate dysfunctional Tregs in the tumor milieu and that, therefore, the clinical efficacy is dependent on the PD-1 expression balance between intratumoral CD8+ effector cells and Tregs. [31] Supporting this notion, Tregs have been associated with the paradoxical phenomenon of hyper-progressive disease upon anti-PD-1 monotherapy. Studies suggested that this problem might be overcome by combining different agents. [47] Whereas λ-MYC mice do not respond to anti-PD-1 monotherapy, the combination of anti-PD-1- and anti-CTLA-4-blocking mAbs is highly effective in delaying or even preventing tumor growth. [33] Thus, our study gives insight into the network involved in ICB therapy and is a prerequisite for establishing yet other concepts of combination therapies against cancer. Further investigations are warranted to dissect the different pathways that may be instrumental for mono- and combination therapies, respectively, with regard to the function of Foxp3+ Tregs.
Conclusions
In a mouse lymphoma model, Tregs do not become dysfunctional but develop an increasingly immunosuppressive phenotype and enhanced effector T cell-suppressing functions. The immunosuppressive mechanisms involve direct cell contacts as well as humoral factors. Therapy using ICB antibodies interferes with these pathways and thereby may be partly mediated by an attenuation of the immunosuppressive capacity of Tregs.
Authors’ contributions
V.B., A.F., N.H. and A.G. performed experiments and analyzed data; V.B. designed the figures; V.B. and R.M. wrote the manuscript; M.R. and R.M. conceived and supervised the study.
Declaration of Competing Interest
The authors have no conflicts of interest to disclose.
Acknowledgements
We are grateful to Michael Hagemann, Franziska Liebel and Martina Möschter for taking care of animal husbandry. The study includes parts of the doctoral theses of V.B. and F.A. at Ludwig-Maximilians-Universität München. The work was supported by grants from Deutsche Krebshilfe (70112332, 70112337, 110662 and 110664), Wilhelm-Sander-Stiftung (2020.100.1) and Deutsche Forschungsgemeinschaft (RO 764/15-2).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101170.
Appendix. Supplementary materials
References
- 1.Wing K., Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 2.Vignali D.A. How regulatory T cells work. Nat. Rev. Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng Y., Rudensky A.Y. Foxp3 in control of the regulatory T cell lineage. Nat. Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 5.Fatini M.C. TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 6.Fontenot J.D. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Curotto de Lafaille M.A., Lafaille J.J. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Wolf D. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin. Cancer Res. 2005;11:8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 9.Clarke S.L. CD4+CD25+FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS ONE. 2006;1:e129. doi: 10.1371/journal.pone.0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Facciabene A. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–2171. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bos P.D. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J. Exp. Med. 2013;210:2435–2466. doi: 10.1084/jem.20130762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klages K. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010;70:7788–7799. doi: 10.1158/0008-5472.CAN-10-1736. [DOI] [PubMed] [Google Scholar]
- 13.Kovalchuk A.L. Burkitt lymphoma in the mouse. J. Exp. Med. 2000;192:1183–1190. doi: 10.1084/jem.192.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmetlić F. Regulatory T cells in an endogenous mouse lymphoma recognize specific antigen peptides and contribute to immune escape. Cancer Immunol. Res. 2019;7:600–608. doi: 10.1158/2326-6066.CIR-18-0419. [DOI] [PubMed] [Google Scholar]
- 15.Callahan M.K. Targeting T cell co-receptors for cancer therapy. Immunity. 2016;44:1069–1078. doi: 10.1016/j.immuni.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Dyck L., Mills K.H.G. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur. J. Immunol. 2017;47:765–779. doi: 10.1002/eji.201646875. [DOI] [PubMed] [Google Scholar]
- 17.LaFleur M.W. Inhibitors of the PD-1 pathway in tumor therapy. J. Immunol. 2018;200:375–383. doi: 10.4049/jimmunol.1701044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu-Monette Z., Young K.H. PD-1 expression and clinical PD-1 blockade in B-cell lymphoma. Blood. 2018;131:68–83. doi: 10.1182/blood-2017-07-740993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walunas T.L. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 20.Freeman G.J. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins A.V. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201–210. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 22.Parry R.V. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wherry E.J. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 24.Grosso J.F., Jure-Kunkel M.N. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer Immunity. 2013;13:5. [PMC free article] [PubMed] [Google Scholar]
- 25.Dominguez-Villar M., Hafler D.A. Regulatory T cells in autoimmune disease. Nat. Immunol. 2018;19:665–673. doi: 10.1038/s41590-018-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey-Bucktrout S.L. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity. 2013;39:949–962. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komatsu N. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitz A., Dominguez-Villar M. Molecular mechanisms underlying TH1-like Treg generation and function. Cell. Mol. Life Sci. 2017;74:4059–4075. doi: 10.1007/s00018-017-2569-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucca L.E., Dominguez-Villar M. Modulation of regulatory T cell function and stability by co-inhibitory receptors. Nat. Rev. Immunol. 2020;20:680–693. doi: 10.1038/s41577-020-0296-3. [DOI] [PubMed] [Google Scholar]
- 31.Kumagai S. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat. Immunol. 2020;21:1346–1358. doi: 10.1038/s41590-020-0769-3. [DOI] [PubMed] [Google Scholar]
- 32.Lowther D.E. PD-1 marks dysfunctional regulatory T cells in malignant gliomas. JCI Insight. 2016;1:e85935. doi: 10.1172/jci.insight.85935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brenner E. Cancer immune control needs senescence induction by interferon-dependent cell cycle regulator pathways in tumours. Nat. Commun. 2020;11:1335. doi: 10.1038/s41467-020-14987-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmetlić F. Therapy of lymphoma by immune checkpoint inhibitors: the role of T cells, NK cells and cytokine-induced tumor senescence. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2020-001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Y. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 36.Md Sakib Hossain D. FoxP3 acts as a cotranscription factor with STAT3 in tumor-induced regulatory T cells. Immunity. 2013;39:1057–1069. doi: 10.1016/j.immuni.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Przewoznik M. Recruitment of natural killer cells in advanced stages of endogenously arising B-cell lymphoma: implications for therapeutic cell transfer. J. Immunother. 2012;35:217–222. doi: 10.1097/CJI.0b013e318247440a. [DOI] [PubMed] [Google Scholar]
- 38.Scheuerpflug A. The role of dendritic cells for therapy of B-cell lymphoma with immune checkpoint inhibitors. Cancer Immunol. Immunother. 2021;70:1343–1350. doi: 10.1007/s00262-020-02767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Müller-Hermelink N. TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell. 2008;13:507–518. doi: 10.1016/j.ccr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Braumüller H. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–365. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 41.Hori S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 42.Akkaya B. Regulatory T cells mediate specific suppression by depleting peptide-MHC class II from dendritic cells. Nat. Immunol. 2019;20:218–231. doi: 10.1038/s41590-018-0280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qureshi O.S. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naujoks M. Alterations of costimulatory molecules and instructive cytokines expressed by dendritic cells in the microenvironment of an endogenous mouse lymphoma. Cancer Immunol. Immunother. 2014;63:491–499. doi: 10.1007/s00262-014-1538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma Y. Interleukin-10 counteracts T-helper type 1 responses in B-cell lymphoma and is a target for tumor immunotherapy. Cancer Lett. 2021;503:110–116. doi: 10.1016/j.canlet.2021.01.022. [DOI] [PubMed] [Google Scholar]
- 46.Sasidharan Nair V. Pembrolizumab interferes with the differentiation of human FOXP3+-induced T regulatory cells, but not with FOXP3 stability, through activation of mTOR. J. Immunol. 2020;204:199–211. doi: 10.4049/jimmunol.1900575. [DOI] [PubMed] [Google Scholar]
- 47.Kamada T. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl. Acad. Sci. USA. 2019;116:9999–10008. doi: 10.1073/pnas.1822001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.