Abstract
Background
The risk of transmission of SARS-CoV-2 from aerosols generated by medical procedures is a cause for concern.
Aim
To evaluate the evidence for aerosol production and transmission of respiratory infection associated with procedures that involve airway suctioning or induce coughing/sneezing.
Methods
The review was informed by PRISMA guidelines. Searches were conducted in PubMed for studies published between January 1st, 2003 and October 6th, 2020. Included studies examined whether nasogastric tube insertion, lung function tests, nasendoscopy, dysphagia assessment, or suctioning for airway clearance result in aerosol generation or transmission of SARS-CoV-2, SARS-CoV, MERS, or influenza. Risk of bias assessment focused on robustness of measurement, control for confounding, and applicability to clinical practice.
Findings
Eighteen primary studies and two systematic reviews were included. Three epidemiological studies found no association between nasogastric tube insertion and acquisition of respiratory infections. One simulation study found low/very low production of aerosols associated with pulmonary lung function tests. Seven simulation studies of endoscopic sinus surgery suggested significant increases in aerosols but findings were inconsistent; two clinical studies found airborne particles associated with the use of microdebriders/drills. Some simulation studies did not use robust measures to detect particles and are difficult to equate to clinical conditions.
Conclusion
There was an absence of evidence to suggest that the procedures included in the review were associated with an increased risk of transmission of respiratory infection. In order to better target precautions to mitigate risk, more research is required to determine the characteristics of medical procedures and patients that increase the risk of transmission of SARS-CoV-2.
Keywords: Aerosol-generating procedure, Respiratory infection, SARS-CoV-2, Epidemiology, Nasendoscopy, Lung function test
Introduction
Available evidence suggests that SARS-CoV-2 is emitted from an infected person’s mouth or nose in small liquid particles as they breathe, speak, cough, or sneeze. Particles range in size from larger respiratory ‘droplets’ (>10 μm) to smaller ‘aerosols’ (<10 μm) and fine particles (<1 μm). Transmission mainly occurs during close contact when the virus is inhaled or inoculated on to the mouth, nose, or eyes of a susceptible person and depends on the amount of viable virus present and the infection control measures that are in place [1]. Current World Health Organization (WHO) and UK advice is that contact and droplet precautions, with the use of fluid-resistant surgical masks for close contact, are recommended for care of patients with SAR-CoV-2 infection. Airborne precautions (including the use of N95, FFP2, or FFP3 respirators) are recommended when aerosol-generating procedures (AGPs) are being performed. Although not supported by evidence, WHO recognizes that some healthcare workers (HCWs) may place high value on the potential benefits of respirators and wish to use them in settings without AGPs [1,2].
Historically, respiratory particles have been categorized as droplets, which are deposited rapidly because of their mass, and as aerosols, which are smaller and travel over longer distances [3,4]. However, it is now recognized that there is a continuum of particle sizes and aerosols that can be generated by breathing, speaking, and coughing and may be present at both short and long distances [5]. The risk that aerosols may transmit infection is influenced by a range of other factors including the amount of virus in the particle, the speed and turbulence of emission, and properties of the ambient environment [6]. Although particles <10 μm may remain airborne for longer than larger respiratory droplets (>10 μm), in typical particle size distributions a relatively small portion of total volume are in this range [7]. Establishing the risk of transmission of SARS-CoV-2 associated with respiratory aerosols therefore requires evidence derived from different study designs. Laboratory-based studies can only provide evidence for part of the transmission process and demonstrate potential rather than actual routes of transmission, whereas clinical studies can provide evidence of actual transmission, although are more difficult to conduct and interpret.
Some medical or patient care procedures are thought to increase the generation of respiratory aerosols. Following the SARS epidemic in 2003, WHO defined ‘high-risk AGP’ as medical procedures that ‘have been reported to be aerosol-generating and consistently associated with an increased risk of pathogen transmission’ and recommended the application of enhanced precautions for staff performing them [8]. The SARS-CoV-2 pandemic has raised concerns about a range of other medical procedures that have the potential to generate respiratory aerosols either as a result of the procedure or because of their propensity to induce coughing or sneezing in the patient.
We undertook this review to evaluate whether medical procedures that induce coughing/sneezing or involve respiratory airway suctioning generate infectious aerosols and are associated with a risk of transmission of respiratory infection, including SARS-CoV-2. The procedures under consideration have not been previously defined as high-risk aerosol-generating procedure (HR-AGP) but have been highlighted by clinicians as procedures of concern [9]. This review sought to evaluate evidence to determine whether these procedures generate infectious aerosols and are associated with a risk of transmission of respiratory infection in order to inform guidance for healthcare professionals caring for patients with SARS-CoV-2. Two main questions were addressed: (i) Does evidence suggest that medical procedures that induce coughing/sneezing or involve respiratory airway suctioning result in infectious aerosol production?; (ii) If yes, what is the associated risk of transmission of SARS-CoV-2?
Methods
As the assessment of evidence was required urgently to underpin guidance for use by healthcare professionals, a rapid review approach was adopted, meaning that there was some deviation from standard systematic review procedures [10]. For example, although we produced a protocol, we were not able register it on PROSPERO as data extraction began before the protocol was finalized (PROSPERO requires registration before data extraction commences); the protocol has been published elsewhere for transparency [11]. This rapid systematic review was informed by PRISMA guidelines. However, it should be noted that specific rapid review guidelines are not currently available [12]. Therefore, to ensure transparency we provide a full account of the review procedures below.
Search strategy
Searches were conducted by an information specialist (C.S.) in PubMed for studies published between January 1st, 2003 and October 6th, 2020. The search terms are detailed in Supplementary Appendix A and included terms reflecting aerosol generation and virus transmission, exposure or cross-infection from droplets or aerosols, plus the set of procedures of interest (Box 1 ). In addition, the references of included articles were examined to identify any additional studies.
Box 1. Procedures of concern in relation to generation of infectious aerosols.
-
–
Nasogastric tube insertion
-
–
Cardiopulmonary and lung function tests, cardiopulmonary exercise test, spirometry, cardiac physiology procedures
-
–
Swallowing assessment related to dysphagia including endoscopy and fluoroscopy
-
–
Suction of the upper airway in the context of airway clearance
-
–
Endoscopic sinus surgery, cautery and nasendoscopy
Alt-text: Box 1
Inclusion/exclusion criteria
The population of interest was adults and children with or without clinically suspected or confirmed COVID-19 or other respiratory infection (SARS, MERS, and influenza) or a simulated exposure model (e.g. using human volunteers, cadavers, etc.). The exposure of interest was one or more of the ‘procedures of concern’ shown in Box 1. The outcome of interest was the number and size of respiratory particles generated during the procedure and/or rate of infection with respiratory pathogens among exposed staff.
Study designs eligible for inclusion were case reports, case series, case–control, outbreak studies, intervention studies (all designs), and systematic reviews reporting a search strategy involving multiple databases and explicit inclusion criteria. Studies were included if published in English from 2003. Only studies that reported original data were included; correspondence or comment pieces, in-vitro and vaccine studies, and predictive modelling studies were excluded.
The underlying evidence is heterogeneous, including different types of studies, both surgical and epidemiological, some with limited numbers of studies and others without potentially confounding factors. However, because of the limited amount of evidence, the full range of study types has been considered.
Study selection
Search results were screened using EPPI-Reviewer software [13]. One reviewer (J.T.) screened all titles and abstracts assisted by machine learning to prioritize potentially relevant papers. A second reviewer then independently screened the titles and abstracts provisionally included by J.T. and the excluded titles and abstracts that machine learning identified as most likely to have been erroneously excluded. Disagreements were resolved by discussion. Two reviewers (G.C., J.W.) then independently screened the full reports of included references (N = 68) and there was no disagreement. Reference checking of papers flagged by the full-text screeners as potential sources of further evidence was undertaken by K.S.
Risk of bias, data extraction, and synthesis
In line with best practice, available time, and consistency requirements of a rapid review, one reviewer (K.S.) extracted all the data and a sample of 20% of papers were checked by a second reviewer (A.O.) [10,14]. An independent panel reviewed all the papers and evidence tables to check the accuracy of the data and interpretation of the evidence.
Risk of bias
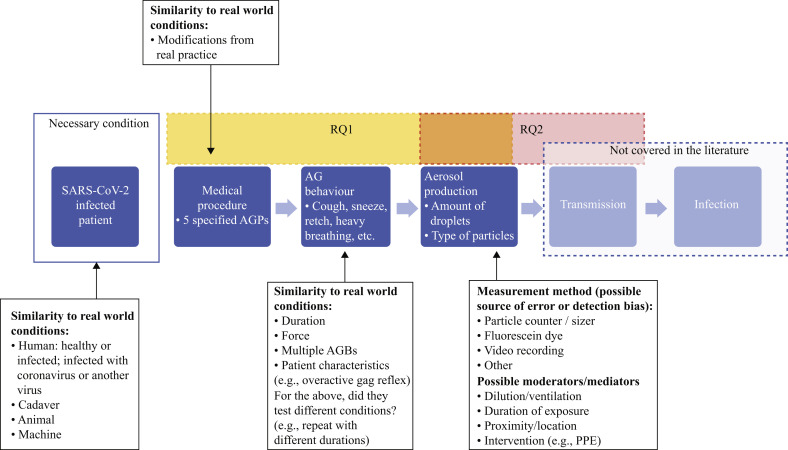
Since high-quality evidence was unlikely to be available, evidence would be drawn from both experimental laboratory-based studies (such as cadaveric simulation studies) and observational studies of clinical practice. Therefore, in line with recommendations for rapid reviews, the quality assessment for each study was focused on factors most important for decision-making [10]. A.O., K.S., J.T., and A.S. developed a bespoke risk-of-bias tool to assess each study according to (a) the robustness of measurement, (b) control for confounding, and (c) applicability to clinical practice. These dimensions are illustrated in Figure 1 . Details of the assessment for each study are shown in Supplementary Tables S1–S6 in the column ‘Study contribution/limitations’.
Figure 1.
Elements considered in the risk of bias evaluation. The rectangles labelled RQ1 and RQ2 show the parts of the model that were explored by research question 1 and research question 2, respectively. The orange area of overlap between these rectangles indicates the intersection of the foci of the two research questions in relation to aerosol production. RQ1: Does evidence suggest that medical procedures which induce coughing or involve respiratory airway suctioning result in infectious aerosol production? RQ2: If yes, what is the associated risk of transmission of SARS-CoV-2? The grey box labelled ‘Not covered in the literature’ refers to the evidence base when the searches were conducted (October 2020). AG, aerosol-generating; AGP, aerosol-generating procedure; AGB, aerosol-generating behaviour; PPE, personal protective equipment.
Data extraction and synthesis
A standardized data extraction form was developed in order to produce a summary of each study. These summaries were then collated in evidence tables for each of the procedures of interest (nasogastric tube insertion, pulmonary lung function testing, suctioning for airway clearance, dysphagia assessment and nasendoscopic procedures). Data were extracted on the following dimensions:
-
–
Study details: country, aim, design.
-
–
Procedures and measures: procedures performed (on, by, where, number of repetitions), outcome measure type (e.g. virus transmission, aerosol size, spread, density), and method (e.g. virus transmission confirmed by antibody test, or aerosols captured by photodocumentation, particle sizer).
-
–
Findings: key conclusions and detailed findings, e.g. relative risk of virus transmission with 95% confidence intervals, mean change in particle concentration etc.
-
–
Risk of bias assessment: as described above.
The synthesis of study findings was organized according to each of the procedures of interest. Findings were narratively synthesized to examine whether consistent patterns in the direction of effect could be identified. An overview of findings from systematic reviews involved examining the extent of relevant evidence and authors’ conclusions.
Findings
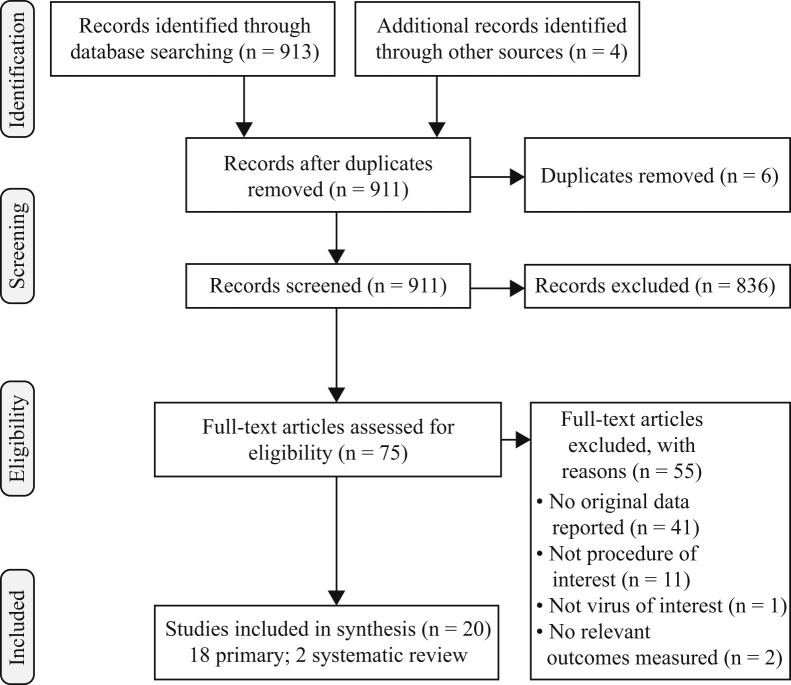
A total of 913 documents were identified in the search, of which six were duplicates. A further three papers were identified from reference-checking and a further rapid systematic review published after the search was conducted. Following application of the inclusion criteria, 20 relevant papers were identified; 18 primary studies and two systematic reviews (Figure 2 ).
Figure 2.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram.
Overview of primary studies
Nine of the 18 studies provided evidence on endoscopic sinus surgery [[15], [16], [17], [18], [19], [20], [21], [22], [23]], six studies focused on suctioning for airway clearance [[24], [25], [26], [27], [28], [29]], four outpatient endoscopy [22,23,30,31], two nasogastric tube insertion [26,27], and one on lung function testing [32]. None of the primary studies focused on procedures or testing for dysphagia. Most studies focused exclusively on one or more of the six procedures of interest; the remainder included evidence on a wider range of procedures. For this review we only extracted data on the procedures of interest.
All studies aimed to determine whether procedures put HCWs at risk, either by examining whether procedures generate aerosols or droplets [[15], [16], [17], [18], [19], [20], [21], [22], [23], [24],[28], [29], [30], [31], [32]] or whether procedures are associated with infection risk [[25], [26], [27]]. Some studies also evaluated whether one or more patient actions generated aerosols or droplets. Patient actions measured included coughing [22,24,29,30,32], sneezing [22,23,30], speech [22,30], heavy breathing [22], swallowing [30], tongue protrusion [30], and vomiting [29]. Finally, several studies evaluated whether a range of devices are effective in reducing the spread of aerosols or droplets during procedures. Devices included masks [22,23,29], drapes [15], smoke evacuation system [19], and suctioning [16,[19], [20], [21]].
Fewer than half of the primary studies were clinically based, involving actual patients [15,18,[25], [26], [27], [28],31]; the remainder were simulations of procedures under experimental conditions and involved volunteers [30,32,22], cadavers [17,[19], [20], [21], [22], [23]], human patient simulators [24,29], or porcine tissue [16].
Measurement of outcomes
Of three studies measuring transmission, one employed a measure of the presence or viral genome (polymerase chain reaction test), one a test for antibodies, and one antibody tests or case definitions. Of the 15 studies measuring aerosols/droplets, almost half used an optical particle counter or sizer to capture data [18,19,21,22,28,31,32]. The remainder used a method to enhance visualization of aerosols or droplets so that they could be captured using video or camera technology, including fluorescein dye [15,17,20,23,29], smoke [17,24], or green laser [30]. One study used both smoke and fluorescein dye [17].
Findings on nasogastric tube insertion (two studies)
Both studies employed a retrospective cohort design and examined the association between performing nasogastric tube insertion and SARS infection among HCWs in Canada (Supplementary Table S1). One study found that there was no evidence of an association between nasogastric tube insertion and SARS infection based on data from 32 nurses who were involved in the treatment of three infected patients, of whom eight acquired SARS [26]. Of 23 nurses who undertook high-risk procedures and consistently wore N95 or fluid-resistant surgical masks (FRSMs), three (13%) acquired SARS compared with five of the nine nurses who did not consistently wear a mask (56%) (relative risk (RR): 0.23; 95% confidence interval (CI): 0.07–0.78; P = 0.02). Only three procedures were associated with a significant risk of SARS acquisition: intubation and suctioning prior to intubation (RR: 4.2; 95% CI: 1.58–11.4; P = 0.04) and manipulation of oxygen mask (RR: 9.0; 95% CI: 1.25–64.9; P < 0.01). The second study of 625 HCWs who provided care to 45 patients with SARS who underwent intubation also found no evidence of an association between nasogastric tube insertion and SARS infection [27]. This was based on a multivariate analysis of a range of clinical procedures performed by 624 HCWs caring for 45 patients with SARS. Most staff wore FRSMs (82%), only 4% wore N95 masks, and 8% wore no mask. Twenty-six HCWs acquired SARS and the factors that were significantly associated with SARS acquisition were being a paramedic, having less infection control training, wearing less personal protective equipment, and participation in administering non-invasive, fibreoptic, or manual ventilation.
The evidence from these studies relates to patients with SARS and there may therefore be differences in terms of risk of transmission to SARS-CoV-2. In one study the exposure to three patients with SARS occurred during a period of six to 14 days between admission and death [26], which reflects the period of peak viral load associated with SARS [35]. The second study was focused on high-risk exposure to HCWs providing care to SARS patients in the period 24 h before to 4 h after intubation. Intubation is likely to present similar risks in patients with SARS-CoV-2 [27]. Whereas these studies contribute evidence about infection risk in real-world clinical practice, there are several limitations. First, the studies do not provide evidence about whether the procedures generate airborne particles. Second, the studies used case records and participant recall; whereas case records may be robust it remains unclear which types of data are used to substantiate tube insertion, and, where the evidence relies on recall, it may be at risk of recall bias. Third, the design used in both studies is at high risk of confounding; in each study HCWs performed multiple procedures (not just nasogastric tube insertion) and it is unclear which (if any) are responsible for the infection, and it cannot be ruled out that HCWs may have acquired the infection from another source including the community.
Findings on pulmonary lung function testing (N = 1 study)
A study by Greening et al. used a simulation design involving healthy volunteers to examine aerosol/droplet production following pulmonary lung function tests (tidal breathing, forced expiratory volume, slow vital capacity (SVC) following inspiration from functional residual capacity, and SVC following inspiration from residual capacity) and association with coughing (see Supplementary Table S2) [32]. The study found very low particle emission in tidal volume and SVC from functional residual capacity, and low emission during forced expiratory volume. Coughing resulted in the highest mass of exhaled particles compared with all other manoeuvres, with a 640% (95% CI: 230–1570; P < 0.01) increase compared with SVC following inspiration from functional residual capacity [32].
Whereas the study provides evidence about aerosol/droplet generation from pulmonary lung function tests there are several limitations. First, the study used ‘healthy volunteers’ and it is unclear how aerosol production might be affected in those with lung conditions or with a viral infection. Second, in-line filters, which would be routinely used in lung function laboratories, were not used during these tests, and such filters would effectively filter airborne particles. Third, it is unclear how appropriate the Particles in Exhaled Air particle sizer/counter system used in this study was for measuring aerosols/droplets in patients with a virus; the authors note that it registers mostly small droplets from the small airways, and virus particles are likely to be present in droplets from both upper and lower respiratory tract.
Findings on endoscopic sinus surgery (N = 9 studies)
Two studies were observations of clinical practice, examining aerosol/droplet generation among patients whose SARS-CoV-2 infection status is unknown [15] or patients who have received a negative test result [18]. Of the remaining seven studies, most were cadaveric simulations [17,[19], [20], [21], [22], [23]], and one used porcine tissue [16] (see Supplementary Table S3). The findings from these studies were not consistent.
Of the two clinical observations, one [18] found that non-powered instrumentation was not associated with a significant increase in concentration of airborne particles compared with the pre-instrumentation level (mean change: 0.0253 particles/cm3; P = 0.34) but the increase was significant for drilling and microdebrider use (mean change: 0.0853 particles/cm3; P = 0.001; and 0.0644 particles/cm3; P = 0.001). Of all particles measured, 70.3% were at the smallest reported size of detection (0.3 μm). The second clinical observation found minimal contamination beyond the immediate surgical field [15].
All seven simulation studies evaluated drilling, of which six reported that it resulted in significant increase in aerosol generation [16,17,[19], [20], [21], [22], [23]] and one reported that it did not [20]. In contrast to Murr et al. [18], no aerosol/droplet generation was reported for microdebriders in five simulation studies [17,19,20,22,23]. Of five studies evaluating non-powered instruments, one reported significant aerosol/droplet generation compared with baseline (mean change 1.29 particles/cm3; P = 0.001) and increase in smaller particles (0.30–0.37 μm) [19]. The other four reported no aerosol/droplet generation [16,20,22,23]. Of three simulation studies evaluating electrocautery, all concluded that it resulted in a significant increase in aerosol/droplet generation [16,19,22]. Three simulation studies examined external activation of powered instruments [17,20,23] with all three reporting some increase in generation of aerosols or droplets. Nasal suctioning did not generate significant airborne aerosols in the range 1–10 μm [22], using suction mitigated the increase in aerosols generated by drilling [[19], [20], [21]], and a negative pressure masks technique was reported to eliminate large droplets and to reduce small aerosol particle concentration by 98% [17].
None of the studies provide evidence in relation to patients with COVID-19 or other respiratory infections and each of the studies has some limitations. One clinical observation study [18] appears to use robust measures and account for potential confounders, but the study by David et al. does not [15]. The cadaveric and porcine simulation studies do not account for patient factors such as breathing, coughing, nasal secretions, etc., and, whereas some of these simulations appear to use robust measures and account for potential confounders, many do not (see Supplementary Table S3).
Findings on outpatient nasendoscopy (N = 4 studies)
One study conducted in the USA used a clinical observation design and examined aerosol/droplet generation among patients who have received a negative SARS-CoV-2 test result [31]. The remaining three studies were simulations (one cadaveric [23] and two healthy volunteers [22,30]). The findings from these studies were not consistent. One clinical observation found that diagnostic nasal endoscopy with a rigid endoscope was not associated with increased particle aerosolization, but that sinonasal debridement, endonasal non-powered and suction instrumentation were associated with increased particle aerosolization compared with pre-procedure levels (mean increase: 0.0869 particles/cm3; 95% CI: 0.029–0.144; P = 0.005; and 0.105 particles/cm3; 95% CI: 0.050–0.1599; P = 0.001) [31]. The three simulation studies all found evidence of droplet or aerosol formation during nasendoscopy and associated patient behaviours such as sneezing (see Supplementary Table S4) [22,23,30].
None of the studies supplied evidence regarding patients with COVID-19 or other respiratory infections, and each of the studies had some limitations. The measuring device (an optical particle sizer) used in the clinical observation was not able to detect the smallest particles and this study provided limited information about the experimental set-up and sampling location with respect to ventilation. The cadaveric and healthy volunteer simulation studies did not account for patient factors such as nasal secretions, fever, etc., and not all studies used robust measures or accounted for potential confounders (see Supplementary Table S4).
Findings on suctioning for airway clearance (N = 6 studies)
Three studies used a retrospective cohort design, of which one evaluated SARS-CoV-2 transmission among HCWs in the USA, and two SARS transmission among HCWs in Canada (Supplementary Table S5). Two simulation studies (one from Hong Kong [24] and one from the USA [29]) used non-human simulators to evaluate aerosol/droplet production and the final study involved a clinical observation of aerosol/droplet production among H1N1 patients in the UK. Heinzerling et al. found that among seven HCWs who performed airway suctioning on an infected patient without applying transmission-based precautions (e.g. use of mask), none developed SARS-CoV-2 infection [25]. In the retrospective studies on SARS patients, Loeb et al. found that critical care nurses who assisted with suctioning before intubation of SARS patients were four times more likely to become infected than nurses who did not perform suction (RR: 4.2; 95% CI: 1.58–11.14; P = 0.04) [26]. However, Raboud et al. found no evidence of association of suction for airway clearance with SARS infection in a study on exposure of 624 nurses [27]. In the two simulation studies, Chan et al. found that coughing during oro-tracheal suctioning could produce substantial dispersion of potentially infected exhaled air [24,29]. A simulation study using fluorescein to evaluate contamination associated with a range of healthcare activities found that suctioning was not associated with increased concentration of fluorescein in air relative to other general care activities – e.g. bathing, intravenous access, physical examination – and no contamination was found on face or face shield during suctioning [29]. Finally, a clinical observation study on H1N1 pandemic patients found an increase in aerosol generation during respiratory/airway suctioning but this was not statistically significant (OR: 4.11; 0.50–34.0) [28]. The particle sizes generated during suctioning were smaller than those collected during baseline but the difference was not significant.
Each study has limitations. The three transmission studies rely (at least in part) on participant recall to determine which procedures HCWs performed, and thus are at risk of recall bias. These retrospective studies are also at high risk of confounding as HCWs performed multiple procedures (not just suction for airway clearance) and it is unclear which (if any) were responsible for the infection, although Raboud et al. did adjust for this in a regression analysis, and HCWs may have acquired the infection from another source [27]. Second, two of the three studies on aerosol/droplet generation are simulations and thus it is not clear how these correspond to real-world conditions – e.g. breathing and nasal secretions – and there are also concerns about the appropriateness of measures used in these studies. Finally, the clinical observation on H1N1 patients provides no details on what type of respiratory suctioning was involved and there was considerable variation between and within individuals in the emission of aerosolized RNA.
Overview of systematic reviews
Two systematic reviews were identified that included primary research and addressed the review questions (Supplementary Table S6) [33,34]. One investigated the evidence for the risk of transmission of acute respiratory infections to HCWs caring for patients undergoing AGPs, including nasogastric tube insertion and suctioning [33]. Limited evidence was found; findings were based on the two studies already considered by this review [26,27] and it was conducted prior to COVID-19. The authors concluded that, although both procedures might be associated with an increased risk of transmission, the odds ratios were not statistically significant.
Thamboo et al. undertook a systematic review of potential AGPs in otolaryngology–head and neck surgery during the COVID-19 pandemic in order to inform clinical recommendations [34]. The review found limited evidence in relation to nasendoscopy and endoscopic surgery and identified some of the studies already included in this review. The authors made assumptions about the risk associated with different particle size, evidence was assessed and weighted, and the limitations of basing recommendations on evidence from small, descriptive case series, experimental studies, or retrospective cohort studies was recognized. The authors concluded that evidence for potential aerosols from nasal endoscopy was low and for treatment of epistaxis was moderate. Evidence for nasal electrocautery was not distinguished.
Interpretation
We identified and evaluated evidence for the generation of respiratory aerosols during nasogastric tube insertion, cardiopulmonary exercise and lung function tests, nasendoscopy, swallowing assessment and oral suction and their association with risk of transmission of SARS-CoV-2 and similar respiratory infections.
The evidence is predominantly derived from experimental simulation studies which used optical particle counters or digital photography to measure respiratory particle dissemination or which attempted to simulate droplets with fluorescein or aerosols with smoke. Some studies used cadavers or porcine tissue where the background effects of breathing and nasal secretions would not be accounted for, with only three studies [30,32,22] based on healthy volunteers where behaviour such as coughing and sneezing could be evaluated. These simulation studies had important limitations in terms of the reliability of the measurement method in accurately detecting a wide range of particle sizes; some did not adjust for background levels or position counters to capture exposure to the operator; and the extent to which the simulation reflects actual aerosol generation is unknown. Four studies based on clinical observation were more likely to reflect a real-life situation; one found a non-significant increase in aerosols associated with suctioning, two a significant increase in aerosols compared with baseline associated with sinonasal and endonasal debridement, but another study found minimal spread of particles beyond the endonasal surgical field.
Although simulation studies provide some evidence of the potential for airborne respiratory particles to be generated from these procedures, the presence of aerosols does not reveal an increased risk of transmission of respiratory viruses. In order to demonstrate a clinically significant risk of airborne infection, aerosols must contain enough infectious virus to enable an infective dose to reach the specific host cell tissue that the virus can infect [36]. The evidence needs to demonstrate a significant increase in aerosols compared with background levels and that the aerosols may carry virus and transmit infection.
Only one study on oral suctioning set out to detect influenza virus in respiratory particles but did not attempt culture to determine whether the particles could transmit infection [28]. Epidemiological evidence from studies that explored the risk of developing respiratory infection in personnel who performed the procedure is limited and only found for nasogastric tube insertion and suctioning. These studies did not demonstrate an association between performing these procedures and the risk of SARS, although the risk may be different in relation to SARS-CoV-2.
The potential for respiratory infections to transmit by an airborne route is dependent on a complex set of parameters that influence the generation and behaviour of respiratory particles. Conventionally, airborne particles have been distinguished as droplets, which settle rapidly because of their mass, and as aerosols, which evaporate to form droplet nuclei and travel longer distances [3,37]. Droplets were perceived to be the primary risk of transmission when a susceptible person is in close proximity [4,8].
However, it is now recognized that the dynamics are more complex and affected by a number of factors including force and volume of exhalation as well as humidity, temperature and airflow in the surrounding environment, which affect the rate of evaporation and dissemination of particles [6]. Natural respiratory activities such as breathing and talking can generate a broad range of particle sizes, from submicron aerosols to large droplets. Using an expiratory droplet assessment kit (0.5–20 μm) on healthy volunteers, Gregson et al. found an association between amplitude of speaking or singing and increased concentration of short-range aerosols but also a significant variation in particle emission between individuals [5]. Indeed, results from different studies on the fluid dynamics of respiratory particles vary by orders of magnitude, reflecting both the complexity of the phenomenon and approaches to measurement [6].
One of the concerns related to the procedures included in this review was their tendency to induce coughing. The mechanism by which coughing generates respiratory particles involves high-speed airflow over the mucus lining the airway, and this generates a higher concentration of respiratory particles compared with speaking [7]. The initial particle cloud has a high concentration of droplets which settle rapidly. The smaller particles remain in suspension and travel further. The evaporation of smaller droplets into droplet nuclei depends on the ambient temperature and relative humidity [38]. However, given the greater mass of droplets expelled by either coughing or speaking, these particles contain a high proportion of the fluid, and therefore virus, expelled. The amount of virus expelled will also depend on the viral load, which will vary depending on the severity of the infection and specific regions of the respiratory tract that are affected [7].
The competing risks of more virus in larger droplets at lower concentration versus a higher concentration of smaller droplets with lower viral load have not been well studied for coughing. However, the risk of being exposed to an aerosol containing virus appears to be lower than the risk due to larger droplets at close range. The added risk of being exposed to virus-containing aerosol particles from an aerosol-generating medical procedure appears to be low compared with the general risk of exposure to expiration from a patient. In a light-scattering study the authors estimated that, during 1 min of loud speaking, at least 1000 virion-containing droplet nuclei would be generated and remain airborne for more than 8 min. Nevertheless, at a saliva viral load of 7×106 copies per millilitre the probability that a 3 μm droplet nucleus contains a virion is only 0.01% [39]. Viral emissions associated with coughing are likely to be considerably higher than for breathing [40] with more virus being contained in larger droplets, which present a greater risk during close contact rather than via longer-range aerosols. Therefore, the risk of aerosol infection from patients in the absence of AGPs is not fully understood and the additional risk posed by AGPs, whether as aerosols or droplets, is difficult to distinguish from general patient interaction.
The generation of the aerosol is only one component of the chain of infection, with the quantity and stability of the virus and susceptibility of the host also being key to transmission [6,36]. The particle must be able to enter or be transferred on to the mucous membranes of the host and carry a sufficient number of viable virus to by-pass the host human defences, including the mucus coating the cell surface. Whereas experimental studies have explored the dynamics of respiratory particles, these viral and host parameters determining the risk of infection are less well understood. In addition, environmental factors such as the proximity of susceptible individuals and the duration of exposure, the size of the indoor environment and its ventilation, as well as hygiene practices and the presence of surfaces that play a role in indirect contact will also be important in transmission.
There are few other systematic evidence reviews that address these medical procedures. One was conducted prior to the COVID-19 pandemic. It informed the concepts of high risk AGPs and drew similar conclusions to our review in relation to nasogastric tube insertion and suctioning [33]. There is only one robust review related to SARS-CoV-2: this is focused on nasendoscopy and, although it did not identify all the evidence included in the present review, drew similar conclusions [34].
Overall, we identified an absence of evidence to suggest that these procedures are associated with additional risk of transmission of respiratory viruses beyond standard patient interactions. For pulmonary function tests, very low levels of particle emission were detected in the one study on lung function tests. Coughing was associated with emission of large particles which are more likely to equate to droplets than aerosols. Similarly, two simulation studies found no significant increase in aerosol generation or contamination of the face associated with suctioning of the respiratory tract. Findings from simulation studies on nasendoscopy suggested a significant increase in aerosols but findings were inconsistent, probably reflecting the use of different models (cadaveric, porcine, or human volunteer), the lack of robust measures to detect particles, the absence of baseline measures in some cases, and uncertainty about whether fluorescein and smoke are adequate surrogates for the generation of human respiratory particles. In addition, these simulation studies are difficult to equate to clinical conditions, they did not account for patient factors such as coughing, and they were vulnerable to confounding. The limited evidence available from studies of virus emission or evidence of transmission associated with conducting these procedures did not demonstrate a risk of transmission, although their retrospective design makes them vulnerable to bias and confounding. Given the absence of evidence it is not possible to establish a clear absence of risk associated with these procedures.
Coughing may be a risk factor for transmission. However, although this has been investigated experimentally in terms of aerosol generation, an association with infection transmission has not been demonstrated. Aerosol generation (<10 μm) associated with coughing appears to be at a relatively low level but is highly variable. Epidemiological evidence suggests that the specific characteristics of the patient play a critical role in driving transmission, as a large proportion of transmission to other patients and staff appears to be related to only a small number of patients [42,43]. Exposure during early stage in infection when viral load is highest is a key factor in driving risk and needs to be considered in terms of identifying risk to HCWs [43].
The most recent WHO guidance on the use of masks in healthcare settings acknowledged that whereas respirators are recommended primarily for settings where AGPs are performed, some HCWs have strong preferences about having the highest perceived protection. However, though personal protective equipment such as N95/FFP3 respirators have a role to play in protecting against inhalation of aerosolized particles, administrative and engineering controls remain priority components of infection prevention and control. Strategies to ensure that patients with SARS-CoV-2 are segregated to allow non-urgent procedures to be conducted when no longer infectious and that procedures are conducted in well-ventilated areas are key to mitigating the potential risk from aerosols [2].
Evidence suggests that the risk of transmission of SARS-CoV-2 to HCWs may be determined by a more complex range of factors than purely the generation of aerosols [33,44]. Aerosols have been assumed to be the explanation for the association between a small number of respiratory tract procedures – such as tracheal intubation, non-invasive and manual ventilation – and risk of transmission to HCWs performing them [33]. This potential route of transmission has subsequently been applied to a wider set of procedures, for which expert consensus has assumed a similar risk of exposure to respiratory aerosols, and these are defined as high-risk AGPs [1,45]. However, evidence for aerosols being generated during some procedures designated as AGPs is absent or equivocal [41,44]. It is therefore possible that other factors such as very close and prolonged contact with respiratory secretions might play a role in increasing the risk of transmission [33,44]. Uncertainty about the link between medical procedures and risk of transmission to HCWs is demonstrated by the significant inter-country variation in designation of medical procedures as AGPs [46].
The paradigm for AGPs needs further consideration to better combine evidence from aerosol and infection prevention and control science. More research is required to determine the characteristics of both medical procedures and patients that increase the risk of transmission in order to better target precautions to mitigate the risk.
This review was limited in scope, and, because it was undertaken within a short time-frame, was restricted to publications in PubMed. However, this would be expected to capture the main publications on this topic, and references from the included studies and other systematic reviews were assessed to help mitigate this. Findings related to other respiratory viruses may not be comparable with studies on SARS-CoV-2 because of differences in transmission dynamics.
Acknowledgements
With thanks to J. Charlesworth, V. Finistrella, and K. Broom at Public Health England for their assistance in handling the administration required to undertake this review.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jhin.2021.06.011.
Conflict of interest statement
None declared.
Funding sources
This review was commissioned by the Department of Health and Social Care (DHSC). The review team (K.S., J.T., A.S., C.S., A.O.) was funded through the contract with the National Institute of Health Research (NIHR) Policy Research Programme Reviews Facility (London/York, United Kingdom). J.W., G.C., J.R., and other members of the Independent High Risk AGP Panel volunteered to contribute their time to conducting the review. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the DHSC.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.World Health Organization . WHO; Geneva: 2020. Mask use in the context of COVID-19 – Interim guidance.https://apps.who.int/iris/handle/10665/337199 Available at: [last accessed March 2021] [Google Scholar]
- 2.Public Health England . 2021. COVID-19: guidance for maintaining services within health and care settings. Infection prevention and control recommendations.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/954690/Infection_Prevention_and_Control_Guidance_January_2021.pdf Revised version January 21st. Available at: [last accessed March 2021] [Google Scholar]
- 3.Vejerano E.P., Marr L.C. Physico-chemical characteristics of evaporating respiratory fluid droplets. J R Soc Interface. 2018;15:20170939. doi: 10.1098/rsif.2017.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fennelly K. Particle sizes of infectious aerosols: implications for infection control. Viewpoint. Lancet Resp Med. 2020;8:914–924. doi: 10.1016/S2213-2600(20)30323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregson F.K.A., Watson N.A., Orton C.M., Haddrell A.E., McCarthy L.P., Finnie T.J.R. Comparing the respirable aerosol concentrations and particle size distributions generated by singing, speaking and breathing. Aerosol Sci Technol. 2021;55:681–691. doi: 10.1080/02786826.2021.1883544. [DOI] [Google Scholar]
- 6.Seminara G., Carli B., Forni G., Fuzzi S., Mazzino A., Rinaldo A. Biological fluid dynamics of airborne COVID-19 infection. Rend Fis Acc Lincei. 2020;31:505–537. doi: 10.1007/s12210-020-00938-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson G.R., Morawska L., Ristovski Z.D., Hargreaves M., Mengersen K., Chao C.Y.H. Modality of human expired aerosol size distributions. J Aerosol Sci. 2011;42:839–851. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . WHO; Geneva: 2014. Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care.https://www.who.int/publications/i/item/infection-prevention-and-control-of-epidemic-and-pandemic-prone-acute-respiratory-infections-in-health-care Available at: [last accessed March 2021] [PubMed] [Google Scholar]
- 9.AGP Alliance . October 2020. Position statement on AGPs/PPE.https://www.bapen.org.uk/pdfs/covid-19/agp-alliance-position-paper.pdf Available at: [last accessed January 2021] [Google Scholar]
- 10.Garritty C., Gartlehner G., Nussbaumer-Streit B., King V.J., Hamel C., Kamel C. Cochrane Rapid Reviews Methods Group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol. 2021;130:13–22. doi: 10.1016/j.jclinepi.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Independent High Risk AGP Panel . 2021. A rapid systematic review of medical procedures, which induce coughing to establish if they can produce an increased risk of an infectious aerosol of SARS-CoV-2.https://osf.io/te23u/?view_only=ab86bc13725d4e69a964c471fc5df033 Protocol. Available at: [last accessed March 2021] [Google Scholar]
- 12.EQUATOR Network. Enhancing the QUAlity and Transparency Of health Research. Available at: https://www.equator-network.org/library/reporting-guidelines-under-development/reporting-guidelines-under-development-for-systematic-reviews/#51 [last accessed March 2021].
- 13.Thomas J., Graziosi S., Brunton J., Ghouze Z., O’Driscoll P., Bond M. UCL Social Research Institute; London: 2020. EPPI-Reviewer: advanced software for systematic reviews, maps and evidence synthesis. EPPI-Centre Software. [Google Scholar]
- 14.Khangura S., Konnyu K., Cushman R., Grimshaw J., Moher D. Evidence summaries: the evolution of a rapid review approach. Syst Rev. 2012;1:10. doi: 10.1186/2046-4053-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.David A.P., Jiam N.T., Reither J.M., Gurrola J.G., Aghi M.K., El-Sayed I.H. Endoscopic skull base and transoral surgery during COVID-19 pandemic: minimizing droplet spread with negative-pressure otolaryngology viral isolation drape. Head Neck. 2020;42:1577–1582. doi: 10.1002/hed.26239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guderian D.B., Loth A.G., Weiss R., Diensthuber M., Stover T., Leinung M. In vitro comparison of surgical techniques in times of the SARS-CoV-2 pandemic: electrocautery generates more droplets and aerosol than laser surgery or drilling. Eur Archs Oto-Rhino-Laryngol. 2020;278:1237–1245. doi: 10.1007/s00405-020-06330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones H.A.S., Salib R.J., Harries P.G. Reducing aerosolized particles and droplet spread in endoscopic sinus surgery during COVID-19. Laryngoscope. 2021;131:956–960. doi: 10.1002/lary.29065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murr A., Lenze N.R., Brown W.C., Gelpi M.W., Ebert C.S., Jr., Senior B.A. Quantification of aerosol particle concentrations during endoscopic sinonasal surgery in the operating room. Am J Rhinol Allergy. 2021;35:426–431. doi: 10.1177/1945892420962335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma D., Ye M.J., Campiti V.J., Rubel K.E., Higgins T.S., Wu A.W. Mitigation of aerosols generated during rhinologic surgery: a pandemic-era cadaveric simulation. J Otolaryngol Head Neck Surg. 2021;164:433–442. doi: 10.1177/0194599820951169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma D., Rubel K.E., Ye M.J., Shipchandler T.Z., Wu A.W., Higgins T.S. Cadaveric simulation of endoscopic endonasal procedures: analysis of droplet splatter patterns during the COVID-19 pandemic. J Otolaryngol Head Neck Surg. 2020;163:145–150. doi: 10.1177/0194599820929274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Workman A.D., Xiao R., Feng A., Gadkaree S.K., Quesnel A.M., Bleier B.S. Suction mitigation of airborne particulate generated during sinonasal drilling and cautery. Int Forum Allergy Rhinol. 2020;10:1136–1140. doi: 10.1002/alr.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Workman A.D., Jafari A., Welling D.B., Varvares M.A., Gray S.T., Holbrook E.H. Airborne aerosol generation during endonasal procedures in the era of COVID-19: risks and recommendations. J Otolaryngol Head Neck Surg. 2020;163:465–470. doi: 10.1177/0194599820931805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Workman A.D., Welling D.B., Carter B.S., Curry W.T., Holbrook E.H., Gray S.T. Endonasal instrumentation and aerosolization risk in the era of COVID-19: simulation, literature review, and proposed mitigation strategies. Int Forum Allergy Rhinol. 2020;10:798–805. doi: 10.1002/alr.22577. [DOI] [PubMed] [Google Scholar]
- 24.Chan M.T.V., Chow B.K., Lo T., Ko F.W., Ng S.S., Gin T. Exhaled air dispersion during bag-mask ventilation and sputum suctioning – implications for infection control. Sci Rep. 2018;8:198. doi: 10.1038/s41598-017-18614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinzerling A., Stuckey P.M.J., Scheuer T., Xu K., Perkins Kiran M., Resseger H. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient – Solano County, California, February. Morb Mortal Wkly Rep. 2020;69:472–476. doi: 10.15585/mmwr.mm6915e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loeb M., McGeer A., Henry B., Ofner M., Rose D., Hlywka T. SARS among critical care nurses, Toronto. Emerg Infect Dis. 2004;10:251–255. doi: 10.3201/eid1002.030838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raboud J., Shigayeva A., McGeer A., Bontovics E., Chapman M., Gravel D. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PloS One. 2010;5 doi: 10.1371/journal.pone.0010717. e10717–e10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson K.A., Pappachan J.V., Bennett A.M., Mittal H., Macken S., Dove B.K. Influenza aerosols in UK hospitals during the H1N1 (2009) pandemic – the risk of aerosol generation during medical procedures. PloS One. 2013;8 doi: 10.1371/journal.pone.0056278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber R.T., Phan L.T., Fritzen-Pedicini C., Jones R.M. Environmental and personal protective equipment contamination during simulated healthcare activities. Ann Work Expos Health. 2019;63:784–796. doi: 10.1093/annweh/wxz048. [DOI] [PubMed] [Google Scholar]
- 30.Tan V.Y.J., Zhang E.Z.Y., Daniel D., Sadovoy A., Teo N.W.Y., Kiong K.L. Head Neck. 2020;42:2779–2781. doi: 10.1002/hed.26347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murr A.T., Lenze N.R., Gelpi M.W., Brown W.C., Ebert C.S., Jr., Senior B.A. Quantification of aerosol concentrations during endonasal instrumentation in the clinic setting. Laryngoscope. 2021;131:E1415–E1421. doi: 10.1002/lary.29122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greening N.J., Larsson P., Ljungstrom E., Siddiqui S., Olin A.C. Small droplet emission in exhaled breath during different breathing manoeuvres: implications for clinical lung function testing during COVID-19. Allergy. 2021;76:915–917. doi: 10.1111/all.14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thamboo A., Lea J., Sommer D.D., Sowerby L., Abdalkhan A., Diamond C. Clinical evidence based review and recommendations of aerosol generating medical procedures in otolaryngology–head and neck surgery during the COVID-19 pandemic. J Otolaryngol Head Neck Surg. 2020;49:28–42. doi: 10.1186/s40463-020-00425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y., Yan L.-M., Wan L., Xiang T.-X., Le A., Liu J.-M. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang S., Duchaine C. SARS-CoV-2 and health care worker protection in low-risk settings: a review of modes of transmission and a novel airborne model involving inhalable particles. Clin Microbiol Rev. 2020;34 doi: 10.1128/cmr.00184-20. e00184-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wells W.F. On air-borne infection. Study II. Droplets and droplet nuclei. Am J Epidemiol. 1934;20:611–618. https://academic.oup.com/aje/article-abstract/20/3/611/280025 [last accessed March 2021] [Google Scholar]
- 38.Bourouiba L., Dehandschoewercker E., Bush J.M.W. Violent expiratory events: on coughing and sneezing. J Fluid Mech. 2014;745:537–563. doi: 10.1017/jfm.2014.88. [DOI] [Google Scholar]
- 39.Stadnytskyi V., Bax C.E., Bax A., Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc Natl Acad Sci USA. 2020;117:11875–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riediker M., Tsai D.H. Estimation of viral aerosol emissions from simulated individuals with asymptomatic to moderate coronavirus disease 2019. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.13807. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton F., Gregson F., Sheikh D.A., Ward K., Brown J., Moran E. Aerosol emission from the respiratory tract: an analysis of relative risks from oxygen delivery systems. MedRxiv. 2021 doi: 10.1136/thoraxjnl-2021-217577. https://www.medrxiv.org/content/10.1101/2021.01.29.21250552v1 [last accessed March 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klompas M., Baker M.A., Rhee C., Tucker R., Fiumara K., Griesbach D. SARS-CoV-2 cluster in an acute care hospital. Ann Intern Med. 2021;174:794–802. doi: 10.7326/M20-7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Illingworth C.J.R., Hamilton W.L., Warne B., Routledge M., Popay A., Jackson C. 2021. Superspreaders drive the largest outbreaks of hospital onset COVID-19 infections.https://osf.io/wmkn3 Preprint: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown J., Gregson K.A., Shrimpton A., Cook T.M., Bzdek B.R., Reid J.P., Pickering A.E. A quantitative evaluation of aerosol generation during tracheal intubation and extubation. Anaesthesia. 2021;76:174–181. doi: 10.1111/anae.15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scotland A.R.H.A.I. October 2020. Assessing the evidence base for medical procedures which create a higher risk of respiratory infection transmission from patient to healthcare worker. Version 1.1.https://hpspubsrepo.blob.core.windows.net/hps-website/nss/3055/documents/1_agp-sbar.pdf Available at: [last accessed March 2021] [Google Scholar]
- 46.Independent High Risk AGP Panel . 2021. International scoping report on aerosol generating medical procedure listings.https://www.gov.uk/government/publications/independent-high-risk-agp-panel-summary-of-recommendations Available at: [last accessed March 2021] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.