Abstract
Background
Real-world data for patients with chronic kidney disease (CKD), specifically pertaining to clinical management, metabolic control, treatment patterns, quality of life (QoL) and dietary patterns, are limited. Understanding these gaps using real-world, routine care data will improve our understanding of the challenges and consequences faced by patients with CKD, and will facilitate the long-term goal of improving their management and prognosis.
Methods
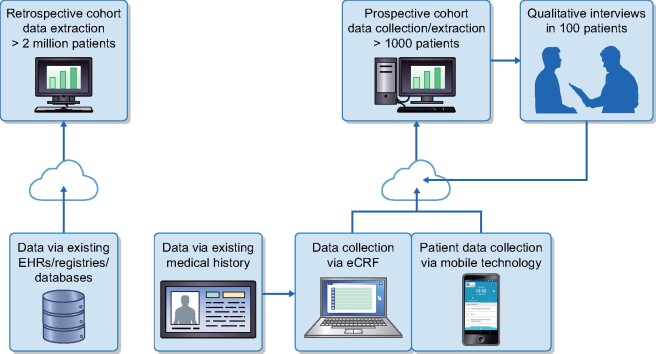
DISCOVER CKD follows an enriched hybrid study design, with both retrospective and prospective patient cohorts, integrating primary and secondary data from patients with CKD from China, Italy, Japan, Sweden, the UK and the USA. Data will be prospectively captured over a 3-year period from >1000 patients with CKD who will be followed up for at least 1 year via electronic case report form entry during routine clinical visits and also via a mobile/tablet-based application, enabling the capture of patient-reported outcomes (PROs). In-depth interviews will be conducted in a subset of ∼100 patients. Separately, secondary data will be retrospectively captured from >2 000 000 patients with CKD, extracted from existing datasets and registries.
Results
The DISCOVER CKD program captures and will report on patient demographics, biomarker and laboratory measurements, medical histories, clinical outcomes, healthcare resource utilization, medications, dietary patterns, physical activity and PROs (including QoL and qualitative interviews).
Conclusions
The DISCOVER CKD program will provide contemporary real-world insight to inform clinical practice and improve our understanding of the epidemiology and clinical and economic burden of CKD, as well as determinants of clinical outcomes and PROs from a range of geographical regions in a real-world CKD setting.
Keywords: chronic kidney disease, methods and rationale, patient-reported outcomes, quality of life, real-world evidence
INTRODUCTION
Chronic kidney disease (CKD) is a major global public health problem resulting in a substantial financial and resource burden on healthcare systems worldwide according to the Global Burden of Disease (GBD) CKD Collaboration [1]. Based on a serum creatinine–based estimated glomerular filtration rate (eGFR) and urinary albumin:creatinine ratio, the Kidney Disease: Improving Global Outcomes group developed a classification system that is now widely used in clinical practice to diagnose and stratify risk in patients with CKD [2]. Using this classification, the global prevalence of identified CKD from all causes and all stages is ∼9%, equivalent to ∼700 million individuals. Since 1990, the prevalence of CKD has increased by 29% worldwide [3]. According to the most recent GBD Study, CKD is the 12th leading cause of death worldwide; in addition, CKD resulted in 36 million disability-adjusted life years, with diabetic kidney disease accounting for almost one-third of this burden [1].
Patients with CKD commonly experience an accelerated and progressive loss of kidney function and are at risk of progression to kidney failure [4]; kidney replacement therapies are lifesaving but costly. CKD is also associated with comorbidities including diabetes, hypertension, hypercholesterolemia and heart failure, as well as an increased risk of myocardial infarction and death [2].
Progressive CKD also negatively affects quality of life (QoL) and multiplies the risk of morbidity and mortality, particularly from cardiovascular causes [5]. In general, the management of patients with CKD involves preventing or slowing the progression of kidney disease, managing the complications of progressive kidney failure and preventing and managing the disease and complications in other organ systems, such as the cardiovascular and central nervous system [6–9]. In many cases, specific treatment of the underlying disease (i.e. glomerular and tubulointerstitial diseases as well as systemic diseases that affect the kidney) offers an opportunity to halt or mitigate CKD progression. For many others, measures to slow the rate of progression in patients with CKD are centered on blood pressure goals and, in patients with proteinuric disease, on reducing proteinuria with the use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers [8]. Dietary (i.e. protein restriction and a reduction in sodium intake) and lifestyle (physical activity and smoking cessation) interventions/changes may also reduce the risk of CKD progression [10]. Unfortunately, recent reports have identified that general recommendations to reduce the progression of CKD are suboptimally implemented in real-world clinical practice [11]. Revised recommended targets for blood pressure and diabetes management are now available across CKD stages [8, 9], but to what extent these recommendations will be implemented remains to be determined.
Management of CKD should also encompass the wide range of disorders and complications that develop because of the loss of kidney function. These disorders include, but are not limited to, volume overload; hyperkalemia; metabolic acidosis; difficulties in glycemic control and dyslipidemia; CKD mineral and bone disorders; cardiovascular and coronary heart diseases; signs and symptoms related to hormonal or systemic dysfunction such as anorexia, nausea, vomiting, fatigue, anemia and protein-energy wasting; as well as neuropsychiatric conditions such as depression, anxiety disorders and cognitive impairment [12–22]. The management of these complications is important in patients with CKD to reduce the risk of morbidity and mortality, reduce symptomatic burden and improve QoL [23]. Similar to what has been observed in the treatment of CKD progression, best practices recommended by guidelines and new therapies to manage these CKD complications are vastly underutilized in real-world clinical scenarios [19, 24].
Finally, there is growing interest in patient-centered care, defined as ‘care that is respectful of and responsive to individual patient preferences, needs and values’ [25]. In this horizon of important shifts in the CKD management paradigm, the inclusion of digital health solutions to capture patient-reported outcomes (PROs), including innovative ways of capturing the patients’ voice and disease awareness, is essential [26, 27].
We hypothesize that there is significant regional variation in the epidemiology and management of CKD and that there are challenges in implementing the recommended clinical practice guidelines for patient monitoring, engagement and early delivery, as well as maintenance of effective therapies. To address these issues, this study will create a global cohort that will serve as a platform to improve our understanding of the epidemiology of CKD and its associated comorbidities and complications, as well as the determinants of clinical outcomes and PROs, and will facilitate the long-term goal of improving the management and prognosis of patients with CKD from a range of geographical regions in a real-world clinical setting.
MATERIALS AND METHODS
DISCOVER CKD is a hybrid, multinational, observational cohort study in patients with CKD comprising a retrospective patient cohort capturing secondary data from established anonymized datasets and a prospective cohort collecting primary and secondary data in patients individually recruited from participating centers in China, Italy, Japan, Sweden, the UK and the USA (ClinicalTrials.gov identifier: NCT04034992). The study aims to be descriptive by collecting data from routine clinical care.
Study objectives
The study objectives are described in Table 1.
Table 1.
Study objectives
| Objective | Description |
|---|---|
| Primary | To construct a multinational longitudinal cohort of patients with CKD that can be used for the generation of primary and secondary real-world data in order to provide insights into the epidemiology of CKD by describing patient characteristics, disease progression, clinical outcomes, patient journey aspects, practice patterns and clinical management of CKD |
| Secondary | To develop country-specific descriptions of the primary objectives by different stages of CKD with a focus on the comorbidities and complications of CKD, including hyperkalemia, anemia and diabetes |
| Exploratory | To evaluate the monitoring and trajectories of laboratory values across different patient subgroups, as well as risk factors (covariates captured at baseline and time varying) associated with CKD progression, e.g. diabetes, and complications of CKD, e.g. hyperkalemia, anemia, diabetes and clinical outcomes |
Patient population and sample size
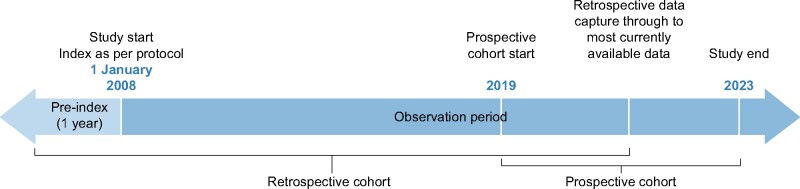
Adult patients from China, Italy, Japan, Sweden, the UK and the USA with a confirmed diagnosis of CKD from any cause are eligible to participate in the study. CKD will be identified by (i) documented diagnostic code (e.g. International Classification of Diseases 10) for CKD Stages 3A through to kidney failure; (ii) two consecutive eGFR measures of <75 mL/min/1.73 m2 [28] recorded >90 days apart (maximum 730 days) from 1 January 2008 (in Japan, eGFR will be calculated using the revised equations for eGFR from serum creatinine in Japan [29]); or (iii) a code for chronic (duration >30 days) renal replacement therapy (hemodialysis and peritoneal dialysis). Patients with a history of kidney transplant are eligible for inclusion. Exclusion criteria include current participation in any interventional trial at baseline (index; prospective cohort only), undergoing treatment for active cancer except for nonmelanoma skin cancer (prospective cohort only), life expectancy of <12 months (prospective cohort only), diagnosis of cancer on or within the 1 year prior to index (retrospective cohort only) and <1 year of medical history available prior to the index date (retrospective cohort only). The study may be supplemented with extended patient follow-up or additional countries and databases/registries.
Prospective cohort
The initial aim is to identify and capture primary and secondary data from a minimum of 1000 (no set maximum) patients with CKD enrolled over a period of 2 years, with prospective follow-up for a minimum of 1 year. Additionally, for patients who agree to participate, patient-specific data will be captured through semistructured qualitative interviews in a randomly selected subset of ∼100 patients (~16–18 patients per country).
Sites have been selected based on an extensive list of physicians, collated with input from the managing contract research organization (PAREXEL), the study sponsor (AstraZeneca) and the DISCOVER CKD scientific committee whose members are listed in the Supplementary data, Table S1.
Eligible patients will be enrolled into the study at the time they routinely visit their physician and consent to participate. The index date will be the baseline visit; the preindex period for each patient is defined as 1 year prior to the index date, where secondary information/data on demographics, clinical assessments, laboratory values, family history, medical history, healthcare resource utilization, procedures and prescription history, as listed in Supplementary data, Table S2, will be extracted from their existing medical records and entered into the electronic case report form (eCRF) after the patient has completed the clinical visit. The number of clinical visits per year based on the current clinical practice recommendations [30] is anticipated to be as follows: CKD Stage 3A, ∼2 times per year; CKD Stage 3B, ∼2 or more times per year; CKD Stage 4, ∼3 or more times per year; and CKD Stage 5, ∼4 or more times per year (guidelines are subject to variation in different countries). This provides opportunities to follow-up with patients and capture data collected during these routine visits [30]. For a patient enrolled prospectively, the potential maximum duration in the study will be ∼3 years, which may be extended. Patients will remain enrolled until study end, study discontinuation/loss to follow-up, withdrawal from the study or death, whichever occurs first.
PROs to be collected as primary data include physical activity measured via the Rapid Assessment of Physical Activity questionnaire [31], health-related QoL via the 36-item Short Form questionnaire [32], work productivity via the Work Productivity and Activity Impairment CKD questionnaire [33] and diet via a simple 7-day food diary; other PROs, including a set of questions to collect patient symptoms, will be available on a bespoke mobile/tablet-based application. The application is available, validated and user-tested in UK English, US English, Spanish, Swedish, Italian, Japanese and Mandarin Chinese. A training diary will also be available for patients to complete. It is expected that patient questionnaires will be completed at baseline and every 6 months for as long as the patient is in the study (weekly for patient symptoms) (Supplementary data, Table S3). The full rationale for questionnaires used in DISCOVER CKD can be found in the Supplementary data.
Qualitative data specific to patients with CKD will be collected by qualitative semistructured interviews in a subset of ∼100 patients. Based on pilot work, it is anticipated that information saturation (where no new information is identified) will be achieved with this number of patients and this will also provide representation across countries [34]. These interviews will be performed by trained interviewers from a third-party vendor (Health Advances, Newton, MA, USA) with experience in CKD; the format will be either face to face or over the telephone at a time/place convenient to the patient. It is anticipated that the duration of the interviews will be 1–2 h. A recent study by James et al. [34] utilizing the PatientsLikeMe online community database was the pilot study for developing the interview transcript used in this study and guided decisions about which symptoms this study should capture.
Data collection during COVID-19
As the study is collecting secondary data retrospectively, it is not designed to capture ongoing adverse events. However, per US Food and Drug Administration guidance, the study protocol has been updated to capture any delay in routine visits, the standard of care for patients with CKD and any medical event occurring between visits and/or any healthcare resource utilization between visits, specifically due to a patient contracting COVID-19 [35]. If the sites perform patient visits via telemedicine during the COVID-19 pandemic, information collected during those visits will be extracted from the medical record and entered into the eCRF as normal routine visits by the sites.
Retrospective cohort
The retrospective patient cohort includes secondary data extracted from established anonymized datasets [e.g. electronic health record (EHR) or claims databases that AstraZeneca has licensed for internal analysis, or through external collaborations] and harmonized into a common data model. These may include, but are not limited to, those listed in Table 2 [36–42]. The retrospective cohort will capture incident patients with CKD beginning 1 January 2008, through the most currently available data (Figure 1). Variables to be collected are outlined in Supplementary data, Table S2.
Table 2.
Established databases currently included in DISCOVER CKD
| Database name | Country | Database type | Coverage | References |
|---|---|---|---|---|
| TriNetX | USA | EHR | Inpatient and outpatient | Topaloglu U et al. JCO Clin Cancer Informatics. 2018; 2: 1–10 [36] |
| Explorys (LCED) | USA | EHR and claims | Inpatient and outpatient | https://www.ibm.com/watson-health/about/explorys [37] |
| J-MDV | Japan | EHR and claims | Inpatient and outpatient | Tanaka S et al. J Pharm Heal Care Sci. 2015; 1: 16 [38] |
| CPRD GOLD | UK | EHR | Primary care, inpatient and outpatient, emergency room |
Herrett E et al. Int J Epidemiol. 2015; 44: 827–836 [39] |
| J-CKD (Kawasaki Medical School) | Japan | EHR | Inpatient and outpatient |
Nakagawa N et al. Sci Rep. 2020; 10: 7351 [40] |
| SCREAM | Sweden | EHR | Inpatient and outpatient | Runesson B et al. Clin Kidney J. 2016; 119–127 [41] |
| DOPPS | USA | EHR | Hemodialysis |
Pisoni RL et al. Am J Kidney Dis. |
LCED, Limited Claims and Electronic Health Records Database.
FIGURE 1:
Study period.
Statistical analysis
Analyses will be conducted separately for prospective and retrospective CKD cohorts and then in the aggregate by combining prospective and retrospective data, to the extent possible (e.g. comparing index characteristics), at the end of the study. A master statistical analysis plan has been created that details the methods that can be utilized to answer the study objectives; prior to each specific analysis, sample size will be assessed to ensure that there is sufficient precision for key estimates. Site characteristics will be ascertained (e.g. primary versus tertiary care, urban versus rural, academic versus nonteaching, etc.) and used in the analyses for adjustment, stratification or sensitivity analysis accordingly. Extraction of clinical data will be conducted in accordance with country-specific data privacy laws, governance and patient consent (where applicable). Ultimately data collected in DISCOVER CKD will be harmonized and integrated to allow pooled patient-level analysis or aggregated/meta analysis (Figure 2).
FIGURE 2:
Retrospective cohort and prospective cohort data collection.
DISCUSSION
Currently there is a need to understand current practice patterns and patient perspectives related to the management of CKD progression and its complications. Collecting patient data from diverse regions will provide regional insights into real-world practice patterns and clinical management of CKD in participating countries. DISCOVER CKD aims to address epidemiological gaps and to understand the disease trajectory of patients with CKD from different geographies, complementing other multinational [43, 44], national and regional CKD/kidney failure cohort studies [41, 45–49] and registries, including initiatives to collect data for patients receiving dialysis [42, 50–53].
Study design, sample size, profile of patients and type of data available vary widely, and comparisons across studies are challenging. The major strengths of this study comprise geographic representation by the inclusion of patients from different countries and healthcare settings, the large sample size, the collection of real-world, routine care data on the clinical management of patients with CKD, as well as capturing information about CKD effects beyond the kidney itself. We will include complications and diseases of other organ systems, with specific focus on the cardiovascular system and diabetes. Also unique to this study is the linkage of clinical data with patient-reported data, which we will amalgamate to create a richer database to further aid our understanding of disease progression and the long-term factors associated with CKD development and PROs. By examining the clinical management of patients across and within multiple countries, we will be able to provide important insights to support the development of public health awareness and disease management, especially in countries where these data do not yet exist or have not been examined due to limitations in data capture. Additionally, by having broad inclusion criteria for patients with CKD, i.e. inclusion of multiple stages of CKD as opposed to only advanced CKD, we will be able to generalize our findings to the wider population of patients with CKD. Also, by using a longitudinal cohort study design, we will be able to examine disease trajectories and the factors associated with disease stability and disease progression, including the speed of progression.
DISCOVER CKD is characterized by an innovative approach to data collection across different regions and countries using a cross-link between clinical, laboratory and diagnostic methods (e.g. imaging) and a collection of a broad array of clinical outcomes and PROs [54, 55]. The development and implementation of user-friendly and electronic collection of data should improve compliance with data capture and allow for the generation of a database with unique information, particularly when aligned with the combination of retrospective EHR collection and data linkage. With the prospective collection of a broad list of outcomes, together with the increasing performance of big data storage, organization and analysis, DISCOVER CKD provides a unique opportunity to fill important gaps in understanding the journey of patients with CKD. Collection of insights via patient interviews will complement medical and PRO data collection, thereby enabling a more in-depth understanding of the patients’ perspectives and experiences of the disease. In fact, the DISCOVER CKD research strategy is aligned with a growing focus on the importance of a patient’s engagement and self-management with the support of various mobile phone apps [27, 49]. The project aims to address the limited availability of validated technology, as well as the missing robust evidence on the role of these new digital health technologies in CKD. The results of this project will address the scientific community’s knowledge gaps about the real impact of how effectively digital health solutions potentially improve patient engagement and disease awareness, as well as how patient-generated data, including PROs, can contribute to individualized care and therefore improve outcomes and patient QoL. There are important gaps in knowledge and current needs related to patient-specific data such as PROs; although a patient-centered approach is increasingly recognized, it is still underutilized. DISCOVER CKD intends to give the patient a voice to provide their experiences with CKD and, through qualitative interviews and PROs collection, to identify gaps in treatment, care pathways and novel endpoints to enhance patient dialog.
In view of the current pandemic, DISCOVER CKD has amended the study protocol to detect and verify the potential impact of COVID-19 on CKD management, as has been reported [35]. Witnessing the major changes that have affected daily life globally during the pandemic, several effects can be expected for patients with CKD [35]. In this respect, DISCOVER CKD has the potential to identify gaps in disease management, and to suggest strategies to maintain current standards of good clinical practice for patients with CKD.
There are potential limitations that need to be considered. First, in some instances, patient data included in the retrospective cohort may theoretically be duplicated across databases from the same country. However, that likelihood is small, as a CKD patient at Stage 3A or higher is likely to have a clinical visit at least 2–3 times per year. Therefore the required period of 1-year registration/medical history prior to the index date reduces the likelihood of duplicating patient data. Second, although we aim to collect data systematically and via different methods (as described above), heterogeneity in the coding systems used and the clinical management of patients (e.g. nephrology, general practice, cardiology and endocrinology) might result in differences in the quality of collected data. Nevertheless, because this study will reflect how data are collected in routine practice in the real world, where there are many differences in healthcare settings and treatment choices, these data will provide real-world insights on actual within- and between-country differences, as well as differences between primary and secondary care settings, thereby providing data that are complementary to data collected in randomized clinical trials and can be used to assess the impact these settings have on clinical outcomes. Third, EHR and claims data are not collected for research purposes and may therefore lack information on specific covariates that may be associated with CKD. However, these data will offer important variables, including lab values, as well as provide crucial disease insights and help to contextualize study findings. Missing variables and missing information within variables will be assessed. Fourth, patients may incorrectly recall self-reported information, leading to differential recall bias; for example, incorrect recall of diet or physical activity leading to over-/underestimation of inferences. Finally, the indication for treatment and side effects of therapies may not be recorded. Despite this, treatment indication can be inferred when a product has a single indication and little to no off-label use.
The DISCOVER CKD program will establish multiregional retrospective and prospective cohorts of patients with CKD, integrating comprehensive, high-quality primary and secondary longitudinal data, including qualitative assessment of patients’ perspectives through interviews. This study will provide contemporary real-world insights during the pre-, current and post-COVID-19 pandemic landscape to not only inform clinical practice, but also to improve our understanding of the epidemiology, clinical burden and economic burden of CKD and its associated comorbidities and complications, as well as the determinants of clinical outcomes and PROs from a range of geographical regions in a real-world CKD setting.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Carol Moreno Quinn, PhD, for her input into the design of this study. The authors also thank Juan Jose Garcia Sanchez for his input into the design of this study, analyses and interpretation of the data. The study was sponsored by AstraZeneca according to Good Publication Practice guidelines (http://annals.org/aim/article/2424869/good-publication-practice-communicating-company-sponsored-medical-research-gpp3). The sponsor was involved in the study design and collection, analysis and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions and data interpretation lies with the authors. All authors were involved in the drafting and critical revision of the manuscript. All authors approved the final version of the manuscript.
FUNDING
Medical writing support was provided by Nicole Ogbonnaya and editorial support was provided by Elke Sims, both of Core (London, UK), funded by AstraZeneca. The funder of the study, AstraZeneca, will be involved in study design, data interpretation, data collection, data analysis and writing of the report.
AUTHORS’ CONTRIBUTIONS
All authors will have access to relevant data and have final responsibility for the decision to submit for publication.
CONFLICT OF INTEREST STATEMENT
G.J., E.W., A.A.S., K.H., H.C., J.S., S.K., P.F. and J.M. are employees and stockholders of AstraZeneca. M.A. is an employee of AstraZeneca. R.P.-F. is an employee of Arbor Research Collaborative for Health, which receives global support for the ongoing Dialysis Outcomes and Practice Patterns Study programs (provided without restriction on publications by a variety of funders; for details see https://www.dopps.org/AboutUs/Support.aspx). He also reports research grants from Fresenius Medical Care; non-financial support from AstraZeneca, Bayer, Boehringer, Novo Nordisk and Akebia and personal fees from Retrophin outside the submitted work. J.J.C. reports institutional grants from AstraZeneca, ViforPharma and Astellas; speaker fees from AstraZeneca, Abbott and Nutricia and consultancy for AstraZeneca, Baxter Healthcare and Bayer. S.F. reports research support and consulting fees from AstraZeneca and research support from Akebia, MegaPro Biomedical, Ardelyx, Corvidia Therapeutics and Cara Therapeutics. C.P. has received consulting fees from AstraZeneca, FibroGen, Johnson & Johnson, Janssen Cilag, Eli Lilly, Novartis and Otsuka Pharmaceutical. H.J.L.H. reports grants and other fees from AstraZeneca, Merck, Mitsubishi Tanabe, Janssen, Mundipharma, Gilead, AbbVie, Retrophin, Boehringer Ingelheim, Bayer, Chinook, Novo Nordisk and CSL Pharma. E.K. is a consultant for AstraZeneca. N.K. is a consultant for AstraZeneca, Boehringer Ingelheim and Kyowa Hakko Kirin and receives honoraria from Kyowa Hakko Kirin and Daiichi Sankyo. M.K. reports research grants from AstraZeneca and Boehringer Ingelheim; has served as a consultant or on an advisory board for Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck (Diabetes), Novo Nordisk, Sanofi and Vifor Pharma; receives honoraria from AstraZeneca, Boehringer Ingelheim and Novo Nordisk and receives other research support from AstraZeneca. M.L. is supported by the Slovenian Research Agency; has received research support from Roche Diagnostics; has served as a consultant or on an advisory board/steering committee for AstraZeneca, Boehringer Ingelheim, Novartis and Vifor Pharma and has received personal fees from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Novartis, Sanofi, Servier and Vifor Pharma. C.S.P.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic and Vifor Pharma; has served as a consultant or on an advisory board/steering committee/executive committee for Abbott Diagnostics, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Biofourmis, Boehringer Ingelheim, Boston Scientific, Corvia Medical, Cytokinetics, Darma, Eko.ai, JanaCare, Janssen Research & Development, Medtronic, Menarini Group, Merck, MyoKardia, Novartis, Novo Nordisk, Radcliffe Group, Roche Diagnostics, Sanofi, Stealth BioTherapeutics, The Corpus, Vifor Pharma and WebMD Global and serves as co-founder and non-executive director of Us2.ai. C.P. reports advisory board membership for AstraZeneca, Vifor and Eli Lilly/Boehringer Ingelheim and speaker fees from Novartis, Janssen Cilag, Otsuka, AstraZeneca and Vifor. P.S. is on advisory boards for AstraZeneca, Baxter, REATA, Vifor, FMC and Astellas. D.C.W. reports personal fees and non-financial support from AstraZeneca, as well as personal fees from Bayer, Boehringer Ingelheim, Astellas, GlaxoSmithKline, Janssen, Napp, Mundipharma, Reata, Vifor Fresenius and Tricida.
ETHICAL ASPECTS OF THE STUDY PROTOCOL
The study protocol follows the principles of the Declaration of Helsinki. No study activities will be performed prior to ethics committee approval. Patient privacy and confidentiality is of the utmost importance. Therefore all data collection/abstraction will be conducted in a method that is compliant with the Health Insurance Portability and Accountability Act, the Data Protection Act, independent review boards/ethics committees, other regulations and participating data custodians' policies as appropriate for each area in which the study is being conducted. No patient-identifiable information will be shared outside of the protective firewalls of participating data custodians without prior informed consent; within the firewalls, only approved persons will have access to patient-level data.
REFERENCES
- 1.GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020; 395: 709-733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kidney Disease Improving Global Outcomes CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2012; 3: 1-150 [Google Scholar]
- 3. Cockwell P, Fisher LA.. The global burden of chronic kidney disease. Lancet 2020; 395: 662-664 [DOI] [PubMed] [Google Scholar]
- 4. Levey AS, Eckardt KU, Dorman NM. et al. Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int 2020; 97: 1117-1129 [DOI] [PubMed] [Google Scholar]
- 5. van der Velde M, Matsushita K, Coresh J. et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011; 79: 1341-1352 [DOI] [PubMed] [Google Scholar]
- 6. Williams B, Mancia G, Spiering W. et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018; 39: 3021-3104 [DOI] [PubMed] [Google Scholar]
- 7. Arnold R, Issar T, Krishnan AV. et al. Neurological complications in chronic kidney disease. JRSM Cardiovasc Dis 2016; 5: 2048004016677687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. KDIGO Chapter 3.: Blood pressure management in CKD ND patients without diabetes mellitus. Kidney Int Suppl (2011) 2012; 2: 357-362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kidney Disease: Improving Global Outcomes Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int 2020; 98: S1-S115 [DOI] [PubMed] [Google Scholar]
- 10. Chen TK, Knicely DH, Grams ME.. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019; 322: 1294-1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alencar de Pinho N, Levin A, Fukagawa M. et al. Considerable international variation exists in blood pressure control and antihypertensive prescription patterns in chronic kidney disease. Kidney Int 2019; 96: 983-994 [DOI] [PubMed] [Google Scholar]
- 12. Khan YH, Sarriff A, Adnan AS. et al. Chronic Kidney Disease, Fluid Overload and Diuretics: A Complicated Triangle. PLoS One 2016; 11: e0159335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bianchi S, Aucella F, De Nicola L. et al. Management of hyperkalemia in patients with kidney disease: a position paper endorsed by the Italian Society of Nephrology. J Nephrol 2019; 32: 499-516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adamczak M, Masajtis-Zagajewska A, Mazanowska O. et al. Diagnosis and Treatment of Metabolic Acidosis in Patients with Chronic Kidney Disease - Position Statement of the Working Group of the Polish Society of Nephrology. Kidney Blood Press Res 2018; 43: 959-969 [DOI] [PubMed] [Google Scholar]
- 15. Williams ME, Garg R.. Glycemic management in ESRD and earlier stages of CKD. Am J Kidney Dis 2014; 63: S22-38 [DOI] [PubMed] [Google Scholar]
- 16. Mikolasevic I, Zutelija M, Mavrinac V. et al. Dyslipidemia in patients with chronic kidney disease: etiology and management. Int J Nephrol Renovasc Dis 2017; 10: 35-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waziri B, Duarte R, Naicker S.. Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD): Current Perspectives. Int J Nephrol Renovasc Dis 2019; 12: 263-276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Artom M, Moss-Morris R, Caskey F. et al. Fatigue in advanced kidney disease. Kidney Int 2014; 86: 497-505 [DOI] [PubMed] [Google Scholar]
- 19. Wong MMY, Tu C, Li Y. et al. Anemia and iron deficiency among chronic kidney disease Stages 3-5ND patients in the Chronic Kidney Disease Outcomes and Practice Patterns Study: often unmeasured, variably treated. Clin Kidney J 2020; 13: 613-624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iorember FM. Malnutrition in Chronic Kidney Disease. Front Pediatr 2018; 6: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simoes ESAC, Miranda AS, Rocha NP. et al. Neuropsychiatric Disorders in Chronic Kidney Disease. Front Pharmacol 2019; 10: 932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Said S, Hernandez GT.. The link between chronic kidney disease and cardiovascular disease. J Nephropathol 2014; 3: 99-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang E, Bansal A, Novak M. et al. Patient-Reported Outcomes in Patients with Chronic Kidney Disease and Kidney Transplant-Part 1. Front Med (Lausanne) 2017; 4: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liabeuf S, McCullough K, Young EW. et al. International variation in the management of mineral bone disorder in patients with chronic kidney disease: Results from CKDopps. Bone 2019; 129: 115058. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Medicine (US) Committee on Quality of Health Care in America. Institute of Medicine Reports Composite Summary. In: (US) WDNAP, ed. Crossing the Quality Chasm: A New Health System for the 21st Century: Washington (DC) 2001.
- 26. Levin A, Tonelli M, Bonventre J. et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 2017; 390: 1888-1917 [DOI] [PubMed] [Google Scholar]
- 27.CMS.gov. HHS To Transform Care Delivery for Patients with Chronic Kidney Disease. https://www.cms.gov/newsroom/press-releases/hhs-transform-care-delivery-patients-chronic-kidney-disease (14 September 2020; date last accessed).
- 28. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsuo S, Imai E, Horio M. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982-992 [DOI] [PubMed] [Google Scholar]
- 30.National Institute for Health and Care Excellence. Chronic kidney disease in adults: assessment and management. https://www.nice.org.uk/guidance/cg182/resources/chronic-kidney-disease-in-adults-assessment-and-management-pdf-35109809343205 (14 September 2020; date last accessed). [PubMed]
- 31. Topolski TD, LoGerfo J, Patrick DL. et al. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis 2006; 3: A118 [PMC free article] [PubMed] [Google Scholar]
- 32. Ware JE, Brook RH, Ross Davies A. et al. Conceptualization and Measurement of Health for Adults in the Health Insurance Study: Vol. I, Model of Health and Methodology, Santa Monica, CA, USA: 1980. [Google Scholar]
- 33. Zhang W, Bansback N, Boonen A. et al. Validity of the work productivity and activity impairment questionnaire–general health version in patients with rheumatoid arthritis. Arthritis Res Ther 2010; 12: R177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. James G, Nyman E, Fitz-Randolph M. et al. Characteristics, Symptom Severity, and Experiences of Patients Reporting Chronic Kidney Disease in the PatientsLikeMe Online Health Community: Retrospective and Qualitative Study. J Med Internet Res 2020; 22: e18548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen G, Zhou Y, Xia J. et al. When the COVID-19 pandemic changed the follow-up landscape of chronic kidney disease: a survey of real-world nephrology practice. Ren Fail 2020; 42: 733-739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Topaloglu U, Palchuk MB.. Using a Federated Network of Real-World Data to Optimize Clinical Trials Operations. JCO Clin Cancer Inform 2018; 2:1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.IBM. IBM Explorys Solutions. https://www.ibm.com/watson-health/about/explorys (2 March2021, date last accessed)
- 38. Tanaka S, Seto K, Kawakami K.. Pharmacoepidemiology in Japan: medical databases and research achievements. J Pharm Heal Care Sci 2015; 1: 1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Herrett E, Gallagher AM, Bhaskaranet K.. et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015; 44: 827–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nakagawa N, Sofue T, Kanda E.. et al. J-CKD-DB: a nationwide multicentre electronic health record-based chronic kidney disease database in Japan. Sci Rep 2020; 10: 7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Runesson B, Gasparini A, Qureshi AR. et al. The Stockholm CREAtinine Measurements (SCREAM) project: protocol over view and regional representativeness. Clin Kidney J 2016; 9: 119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pisoni RL, Gillespie BW, Dickinson DM. et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis 2004; 44: 7–15 [DOI] [PubMed] [Google Scholar]
- 43. Fernandes N, Bastos MG, Cassi HV. et al. The Brazilian Peritoneal Dialysis Multicenter Study (BRAZPD): characterization of the cohort. Kidney Int Suppl 2008: S145-151 [DOI] [PubMed] [Google Scholar]
- 44. Mariani L, Stengel B, Combe C. et al. The CKD Outcomes and Practice Patterns Study (CKDopps): Rationale and Methods. Am J Kidney Dis 2016; 68: 402-413 [DOI] [PubMed] [Google Scholar]
- 45. Lash JP, Go AS, Appel LJ. et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 2009; 4: 1302-1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Imai E, Matsuo S, Makino H. et al. Chronic Kidney Disease Japan Cohort (CKD-JAC) study: design and methods. Hypertens Res 2008; 31: 1101-1107 [DOI] [PubMed] [Google Scholar]
- 47. Oh KH, Park SK, Park HC. et al. KNOW-CKD (KoreaN cohort study for Outcome in patients With Chronic Kidney Disease): design and methods. BMC Nephrol 2014; 15: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Orlandi PF, Huang J, Fukagawa M. et al. A collaborative, individual-level analysis compared longitudinal outcomes across the International Network of Chronic Kidney Disease (iNETCKD) cohorts. Kidney Int 2019; 96: 1217-1233 [DOI] [PubMed] [Google Scholar]
- 49. Levin A, Rigatto C, Brendan B. et al. Cohort profile: Canadian study of prediction of death, dialysis and interim cardiovascular events (CanPREDDICT). BMC Nephrol 2013; 14: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Perl J, Davies SJ, Lambie M. et al. The Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS): Unifying Efforts to Inform Practice and Improve Global Outcomes in Peritoneal Dialysis. Perit Dial Int 2016; 36: 297-307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Australia and New Zealand Dialysis and Transplant Registry. Chapter 8. 2016 ANZDATA Registry, 39th Annual Report. https://www.anzdata.org.au/wp-content/uploads/2018/04/c08_transplantation_v3.0_20170424.pdf (15 September 2020; date last accessed).
- 52. Kramer A, Boenink R, Noordzij M. et al. The ERA-EDTA Registry Annual Report 2017: a summary. Clin Kidney J 2020; 13: 693-709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.United States Renal Data System. United States Renal Data System (USRDS). https://www.usrds.org/ (15 September2020; date last accessed).
- 54. Breckenridge K, Bekker HL, Gibbons E. et al. How to routinely collect data on patient-reported outcome and experience measures in renal registries in Europe: an expert consensus meeting. Nephrol Dial Transplant 2015; 30: 1605-1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rangaswami J, Bhalla V, Blair JEA. et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation 2019; 139: e840-e878 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.