Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread to nearly every corner of the globe causing societal instability. The emergence of COVID-19 disease results in fever, sore throat, cough, chest and muscle pain, dyspnea, confusion, anosmia, ageusia, and headache. These can progress to life-threatening respiratory insufficiency also affecting the heart, kidney, liver and nervous systems. The diagnosis of SARS-CoV-2 infection is often confused with influenza and seasonal upper respiratory tract viral infections. Due to available treatment strategies and required containments, rapid diagnosis is mandated. This review brings clarity to the rapidly growing body of available and in-development diagnostic tests, including nanomaterial-based tools. It serves as a resource guide for scientists, physicians, students, and the public at large.
In the span of a few months, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified as the etiological agent of coronavirus disease 2019 (COVID-19). Weeks later, viral diagnostic measures were deployed1. This served to supplement COVID-19 common disease signs and symptoms of cough, fever and dyspnea. As all are seen during seasonal upper respiratory tract infections2, precise diagnostic tests detect viral nucleic acids, viral antigens or serological tests are required to affirm SARS-CoV-2 infection3. Chest computed tomography (CT) or magnetic resonance imaging (MRI) confirm disease manifestations2, 3. The signature of life-threatening COVID-19 is the life threatening acute respiratory distress syndrome (ARDS)4. While the lung is the primary viral target, the cardiovascular, brain, kidney, liver, and immune systems are commonly compromised by infection5. Thus, due to significant COVID-19 morbidity and mortality containing viral transmission through contact tracing, clinical assessment and virus detection was implemented through social distancing, face masks, contact isolation and hand hygiene to limit SARS-CoV-2 transmission6.
Overview of SARS-CoV-2 detection
The first step in managing COVID-19 is the rapid and accurate detection of SARS-CoV-2 enabled by the real-time reverse transcriptase-polymerase chain reaction (RT-PCR)11. RT-PCR detects SARS-CoV-2 nucleic acids present in nasopharyngeal fluids7. Testing is used to prevent infectious spread between persons and communities that include asymptomatic infected persons whose viral shedding can inadvertently spread the infection to the elderly and those with disease comorbities8. Accurate viral detection is a starting point to contain the COVID-19 pandemic9. Lapses affect public safety enabling infection spread aided by false-negative test results10. Improving test sensitivity and specificity remain an urgent need11. Serological testing complements virus detection indicating past infection that could be harnessed for therapeutic gain. Antibodies are detected by enzyme-linked immunosorbent assay (ELISA) using a qualitative detection of IgG or IgM antibodies12. Such tests determine an immune response against the viral spike (S) protein and may help assess prevention against subsequent viral exposure and/or for contact tracing purposes13. Thus, the importance of such tests cannot be overstated. This is also true for epidemiological evaluations and broad global therapeutic needs14. Future work includes the development of diagnostic tests to improve immunoassay sensitivity and specificity13. Indeed, such testing will ultimately reveal viral protection as reinfections emerge15. Inducing immunity against SARS-CoV-2 is the next frontier for COVID-19 control15, 16. To this end, our intent in this review is to summarize the clinical disease presentation with a focus on how to best deploy nanomaterials based and other diagnostic tests at an individual, community and societal level. The article outlines current and future nanomaterial diagnostics for COVID-19. The intent is to facilitate the containment of the virus’ global spread12, 15.
SARS-CoV-2 body fluid and tissue distribution
SARS-CoV-2 viral load and respiratory tract viral particles parallel virus dynamics in body fluids and tissue. All affect concomitant host immune responses5, 32. Viral load differs by sample with respiratory, stool, and serum samples showing broad variation in amounts of virus33. Spreading infection from the respiratory tract to other tissues and organs are linked to the cell-specific expression of angiotensin converting enzyme-2 (ACE-2) receptors4. Viral load in respiratory samples is highest during the initial stages of the disease, reaches a peak in the second week followed by lowered viral loads. In severe disease, the respiratory fluid virus is highest at the third and fourth weeks. In patients with co-morbidities, viral persistence in continuous34 as highlighted from the throat and anal swab sample assays35. Viral RT-PCR test performed in throat swab from disease recovered patients show positive results from up to 50 days and viral RNA was shown to be present in fecal and anal swabs weeks after respiratory samples were found negative35. Altogether, viral dynamics in hospitalized cases should be considered for recommendations in prevention and treatment for COVID-19.
Detection of SARS-CoV-2 viral shedding
In throat swabs and sputum, the viral shedding peaks at five to six days after symptom onset and ranges from 104 to 107 copies/mL. This reflects higher virus levels in the respiratory tract36. The viral RNA detection rate in nasal swabs of infected people has approached 100%. The positivity rate of blood saliva and tears are 88, 78 and 16%, respectively. The self collection of naso- or oro- pharyngeal swabs facilitates large-scale population field testing employing the chemiluminescence immunoassay (CLIA) and the enzyme-linked immunosorbent (ELISA) and lateral flow immunochrom-atographic assays (LFIA)37. LFIA uses gold nanoparticles (AuNPs) and a colorimetric label to provide a rapid platform for point of contact serological detection38. Here, SARS-CoV-2 specific antigen conjugated with nanoparticles. By blood or saliva specimen loading, SARS-CoV-2 IgG and IgM can bind to the SARS-CoV-2 antigen and antibody which is detected colorimetrically (Fig. 1). The assay is completed in 20 minutes with a ~ 90% accuracy39. To date, the minimum length of viral shedding is 7 days after symptom onset with viral infectivity observed within 24 hours40. SARS-CoV-2 detection declines to undetectable levels paralleling the presence of serum neutralizing antibodies40. Even among cases with concurrent high viral loads, the live virus could not be propagated in cell culture after 8 days of symptom onset. These studies warrant the use of quantitative viral RNA load and serological assays when deciding whether to discontinue infection control precautions.
Figure 1. SARS-CoV-2 serological testing.
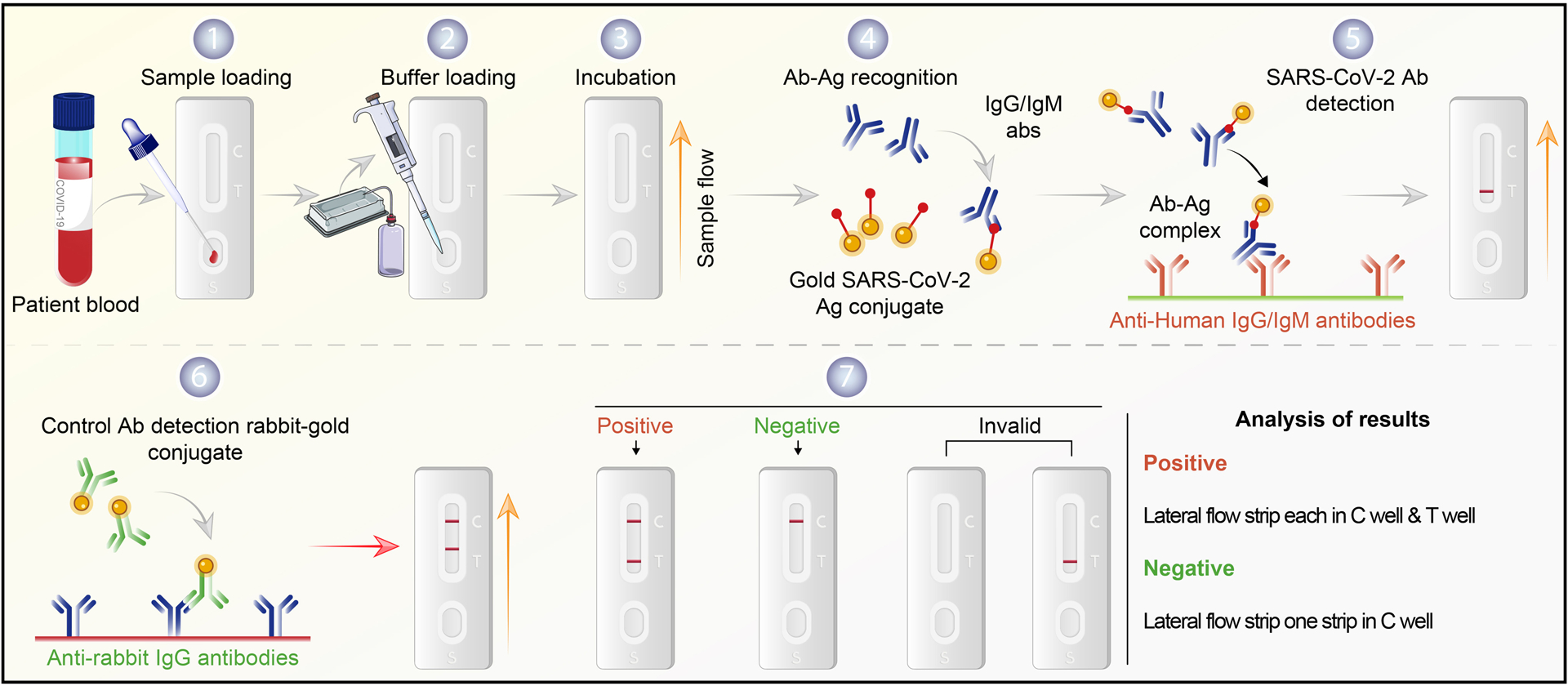
Commonly used immune-based tests contain SARS-CoV-2 specific recombinant antigens immobilized onto nitrocellulose membranes. Mouse anti-human IgM and IgG antibodies conjugated with colored latex beads are immobilized on conjugate pads. The test sample contacts the membrane within the test. The colored antibodies form latex conjugate complexes with human antiviral antibodies. This complex immobilized on the membrane is captured by the SARS-CoV-2 specific recombinant antigen. If SARS-CoV-2 virus-specific IgG/IgM are present in the sample, it leads to a colored band indicating a positive test result. The complex is captured on the membrane by goat anti-mouse antibody forming a red control line. A built-in control line appears in the test window. The absence of a colored band demonstrates a negative result. The workflow begins with patient serum added to the sample flow well. (1) Saline buffer is added dropwise (2) onto the serum sample (3) until the rabbit antibody-gold shows in the control (C) well. A positive test band indicates the presence of COVID-19 antibody (4–6). Results without a positive C band are invalid (7). Notably, this assay depicts a post-immune response and may show negative results for recently infected patients. It may also detect virus in previously infected but asymptomatic persons.
Reverse transcription-polymerase chain reaction (RT-PCR)
Current diagnostic tests for the SARS-CoV-2 pandemic use nucleic acid, antibody and protein-based detections, but viral nucleic acid detection by RT-PCR remains the gold standard11. Nucleic acid tests have improved sensitivity and specificity for viral detection over the now available serological tests. The recognition of SARS-CoV-2 over common respiratory pathogens is contingent on RT-PCR serving as a sensitive, precise, and specific viral detection. Despite the test’s accuracy, results have not yet enabled the containment of viral infection15. In February 2020, the U. S. Food and Drug Administration (U.S. FDA) permitted licensed laboratories to report in-house SARS-CoV-2 diagnostic tests18. The procedure begins with the isolation and conversion of viral RNA to complementary DNA (cDNA). Next, the cDNA is amplified using Taq DNA polymerase. The RT-PCR test’s final overall workflow is illustrated in Fig. 2a which quantifies the viral load. The total turnaround time can exceed 2 days and runs the risk of reduced specificity through cross-contamination6. The tests are commonly performed in hospital laboratories11.
Figure 2. RT-PCR and LAMP assays for detection of SARS-CoV-2 infection.
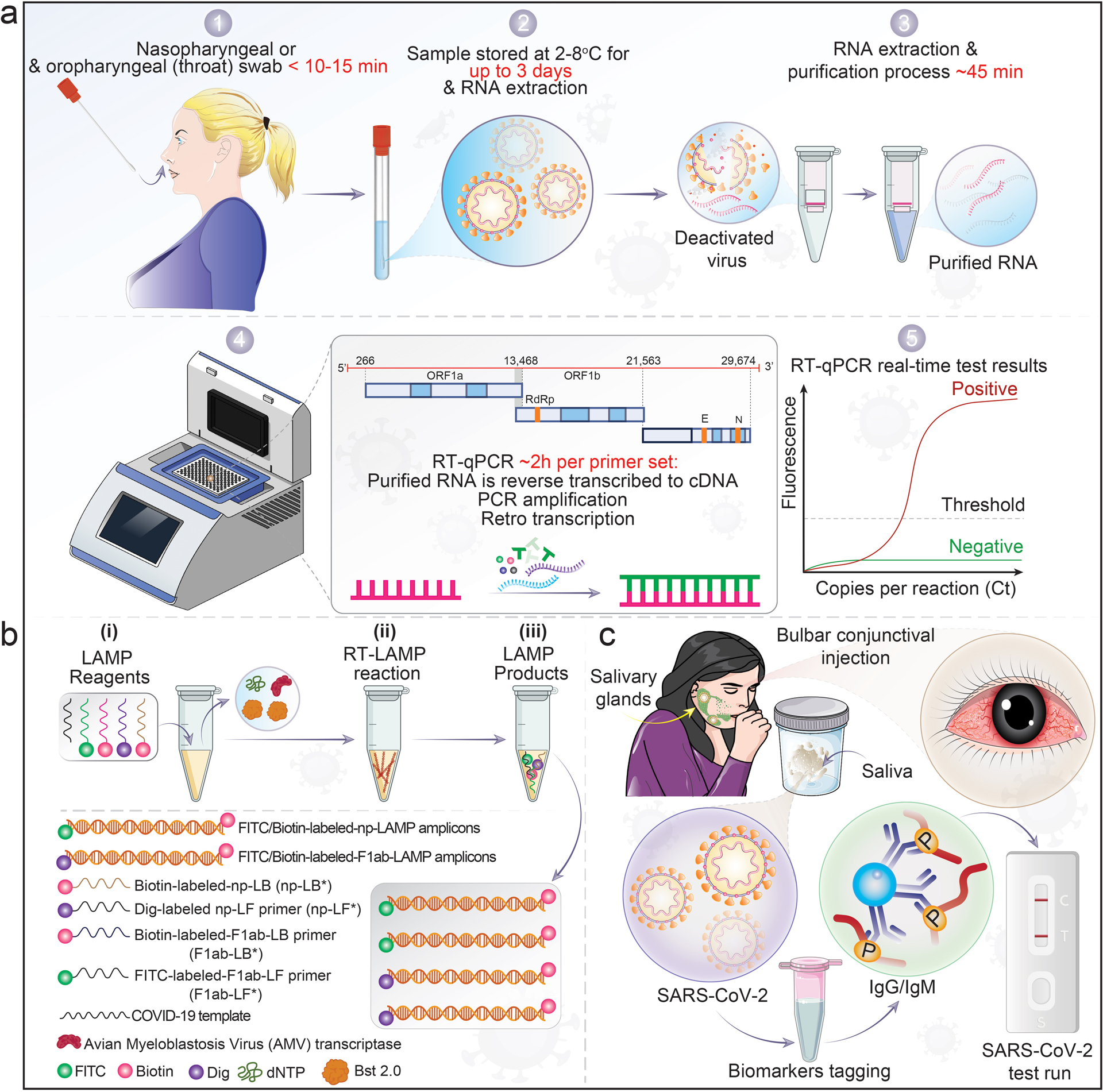
a, A nasopharyngeal swab collects patient samples. 1. RNA is extracted from fluids that contain SARS-CoV-2-infected cells and free viral particles, 2–3. The recovered viral RNA is then reverse transcribed to cDNA and amplified for detection of viral nucleic acids. 4. Conserved regions of the RdRP and E genes are the sub-genomic viral segments amplified with a fluorogenic probe by quantitative PCR. 5. Positive cases exceed the threshold of detection. b, Description of the SARS-CoV-2 RT-loop-mediated isothermal amplification (LAMP) assay. (i), amplification mixtures; (ii), RT-LAMP reaction, and (iii), the products of SARS-CoV-2 RT-LAMP reactions. Although RT-PCR methods are used as the standard for detection of SARS-CoV-2 due to high sensitivity, limitations are present. As an alternative, isothermal amplification methods or LAMP was developed. When optimized for detection, the assay is as sensitive as standard PCR detecting < 10 viral copies/reaction. c, SARS-CoV-2 saliva test. The illustration demonstrates SARS-CoV-2 infection in salivary glands and released specific biomarkers that accumulate in the oral cavity. These are collected through a sample tube and tagged with specific biomarker protein and run through lateral flow rapid tests.
Results from real-time RT-PCR using primers targeting different viral genome parts can be affected by viral RNA sequence variation. In addition, false-negative results may occur because of viral evolution16. Other limitations of RT-PCR tests include sample storage, low quality nucleic acid purification, cost and wait times41. Despite such limitations, the RT-PCR test remains the gold standard for SARS-CoV-2 diagnostics. For the alternative in situ hybridization and immunohistochemistry (IHC), large amounts of sample collection are required and can generate aerosols and safety limitations42. IHC is dependent on the choice and specificity of the antibody and sample quality. The most definitive method for the virus is high-throughput sequencing, but this approach is limited due to cost, equipment and skillsets required42.
Isothermal amplification is a useful alternative to thermal cycling-based nucleic acid amplification43. Simplified RT-PCR are now available to detect diverse regions of the SARS-CoV-2 genome44. These detect the RNA-dependent S and RNA polymerase (RdRp)/helicase (Hel) proteins and the nucleocapsid (N) genes of SARS-CoV-211. The RdRp/Hel assays are highly sensitive means for viral detection. This combined with proper handling of large sample numbers by automated solutions45 and Cobas 6800 systems provides fast and reliable test results19.
RT loop-mediated isothermal amplification (RT-LAMP)
The reverse transcription loop-mediated isothermal amplification (RT-LAMP) is based on nanotechnology. LAMP-based diagnostic tests are detected by levels of turbidity or by colorimetric or fluorescence measures. This technique is simple to perform and visualize and has low background interference. The main limitations for LAMP testing involve experience, interpretation and reaction optimization46. From two fluorescent dyes tested, the signal read-out properties of Eva- was superior to SYBR-Green47. RT-LAMP is based on paper/strips when integrated as part of a microfluidic platform to provide a lab-on-a-chip viral diagnosis48. In the test, fluorescein is assigned to one primer set and the product of the reaction catalyzed by labeled RT43. An alternative method for LAMP accurately detects SARS-CoV-2 using a leucocrystal violet dye to provide a visible violet color enables detection of 100 copies/reaction. A means of improving the limit of detection of the LAMP assay is through a closed tube Penn-RAMP which combines RT-recombinant polymerase amplification (RPA) and RT-LAMP in a single tube49. Fig.2b describes the RT-LAMP assay workflow. The products from three steps in the RT-LAMP system can serve as the template for the reaction of the LAMP system. In step 1 (i) of Fig. 2b, solutions of dATP, polymerase (Bst 2.0), and avian myeloblastosis virus (AMV) transcriptase are used as LAMP-reagents for preparing the amplification mixtures. The LAMP-reagents’ reaction with biotin-labeled-np -LB (np-LB*) and FITC- labeled-F1ab-LF primer (F1ab-LF*) or the labeled-F1ab–LF primer (F1ab-LF*) starts the isothermal amplification (RT-LAMP reaction in step 2 (ii)). Detectable COVID-19 RT-LAMP products are provided in step 3 (iii). FITC/Biotin-labeled-np-LAMP and FITC/Biotin-labeled-F1ab-LAMP amplicons, the results of labeling of F1ab-LF* and F1ab-LB* or np-LF* and np-LB* for digoxigenin (Dig) and biotin, respectively43, are shown in step 3 (iii). In contrast, the fluorescein isothiocyanate (FITC) is assigned to F1ab primer set; F1ab-RT-LAMP product is labeled with FITC and biotin, while the np-RT-LAMP is labeled with Dig and biotin.
Furthermore, the labeled F1ab-LF* and F1ab-LB* primers react under the optimized condition, and SARS-CoV-2 RNA is converted to cDNA with AMV-RT at 63°C in 40 minutes. This reaction serves as the material for subsequent LAMP amplification; the RT-LAMP system consists of the FITC-products and Dig, for the detection of F1ab and np-primer50. RNA extraction is time consuming, expensive, and requires centrifugation steps which EasyCOV RT-LAMP tests do not require. EasyCOV technology is a simple and straightforward test without required RNA extraction from the sample. The results of EasyCOV demonstrated a sensitivity of 72.7%. LAMP techniques on saliva can identify people’s infected profiles. EasyCOV can detect SARS-Cov-2 in saliva and the test is viable for large-scale screenings of the general population due to its simple, fast, and painless procedure for patients.
SARS-CoV-2 diagnostics using nanomaterials
Nanomaterial based technology provide feasible alternatives to RT-PCR for quick and precise viral detection. For example, magnetic nanoparticles can facilitate viral RNA extraction through co-precipitation, followed by polyamine ester functionalization via (3-aminopropyl) triethoxysilane, and can permit up to 50,000 diagnostic tests51. Quantum dots (QDs) could serve as ideal detection tools to study S protein/ACE2 binding dynamic and internalization due to their relatively small size, photostability and the ease of surface functionalization with biological molecules for Förster resonance energy transfer (FRET) biosensing systems with various energy transfer partners52, such as AuNPs that are characterized by absorption of electromagnetic radiation in the visible region of the spectrum53. A colorimetric assay was developed based on thiol-modified antisense oligonucleotides conjugated with AuNPs for detection of SARS-CoV-2 N-gene RNA. This is used for rapid diagnosis and can be performed within 10 minutes. The lower limit of detection is 0.18 ng/μL of RNA particles54. A recombinant S receptor binding domain conjugated to fluorescent QDs was created as an imaging probe for energy transfer quenching with ACE2-conjugated AuNPs. Upon binding of the S to the ACE2 receptor, fluorescence is quenched by the nearby gold nanoparticles to enable monitoring of the binding events in the solution. QD probes can also facilitate cell-based assay identification and validation of inhibitors of the SARS-CoV-2 S protein and ACE2 receptor binding55. The QDs are used as probes to investigate other viral receptors56. This system can identify neutralizing antibodies and recombinant proteins for SARS-CoV-2 and other viruses with S-mediated cell recognition and entry.
Biosensors were developed for detecting influenza, the human immunodeficiency virus (HIV) and other viral diseases57. Initially marred by low sensitivity and specificity, limitations were overcome by plasmonic (gold and silver), metal oxide nanoparticle and field effect transistor (FET) bio- and graphene-sensors57, 58. Graphene has wide application consisting of hexagonal carbon structures arranged in a two-dimensional sheet. This gives it a large surface area, high electronic conductivity and high carrier mobility and graphene biosensors are highly sensitive. When developing a graphene-based biosensor to detect SARS-Cov-2, coronavirus S antibody was immobilized on graphene surface using 1-pyrenebutyric acid N-hydroxysuccinimide ester linkers. This graphene was used as a sensing material in a field effect transistor device to detect the S up to 1 fg/mL concentration59 (Fig. 3 top panel). The optical property of AuNP and AgNP (silver nanoparticles) conjugated to antibodies, when they are bound to the viral antigens or RNA, causes a detectable signal which can be used to detect SARS-CoV-260. A toroidal plasmonic metasensors were developed that detect a femtomolar concentration of the viral S protein. They showed that monoclonal antibody conjugation on functionalized AuNPs could be deleted up to 4.2 femtomolar level concentration (lower limit of detection). Transmission spectra of metasensors can shift the excitation with a polarized beam of light at terahertz frequency. Metasensors can be very useful in point of care (PoC) testing where the rapid and sensitive assay is required61. Recently, researchers have devised a single step, optical S protein-specific nanoplasmonic resonance sensor that requires minimal sample preparation and provides fast and direct virus detection. In such a system, highly specific antibodies to SARS-CoV-2 were immobilized on nanosensor chip surfaces to which intact coronavirus particles bind through S protein, leading to plasmon resonance or intensity changes be optically measured through a sensing system62, 63. For this assay, the lower limit of detection is 30 virus particles. The assay can be completed in 15 minutes. Assay can quantify virus below standard nasopharyngeal swab and saliva viral concentrations20. On analyzing the specificity of the sensor for binding SARS-CoV-2 in comparison to SARS-CoV, Middle East Respiratory Syndrome coronavirus (MERS-CoV) and vesicular stomatitis (VSV) pseudoviruses, nanoplasmonic sensor chips demonstrated very high specificity (>1000:1) in detecting the SARS-CoV-262. The nanoplasmonic sensor chips have the advantage of being low cost and scalable while maintaining uniformity and repeatability. The design of periodic nanostructures, without any external coupling optics64 allow sensor chips to be integrated with a standard 96 microwell plate or microfluidic cuvettes. This allows standard microplate reader measurements65. A low cost, portable, smartphone application-controlled device can analyze SARS-CoV-2 pseudovirus sample in one step within 15 minutes with sensitive viral detection. Although the detection limit is 4000 compared to 30 vps for the microplate reader it may find application in clinics, roadside screening sites and homes62. Gold nanoparticle-based sensors coupled with artificial intelligence, can detect volatile organic compounds (VOCs) associated with SARS-CoV-2 in exhaled breaths. The assay was able to detect virus based on the change in resistance of the nanomaterial biosensor layer. The methods can be optimized in future months by using other nanomaterials and larger cohort testings66.
Figure 3. Nanomaterial-based SARS-COV-2 detections.
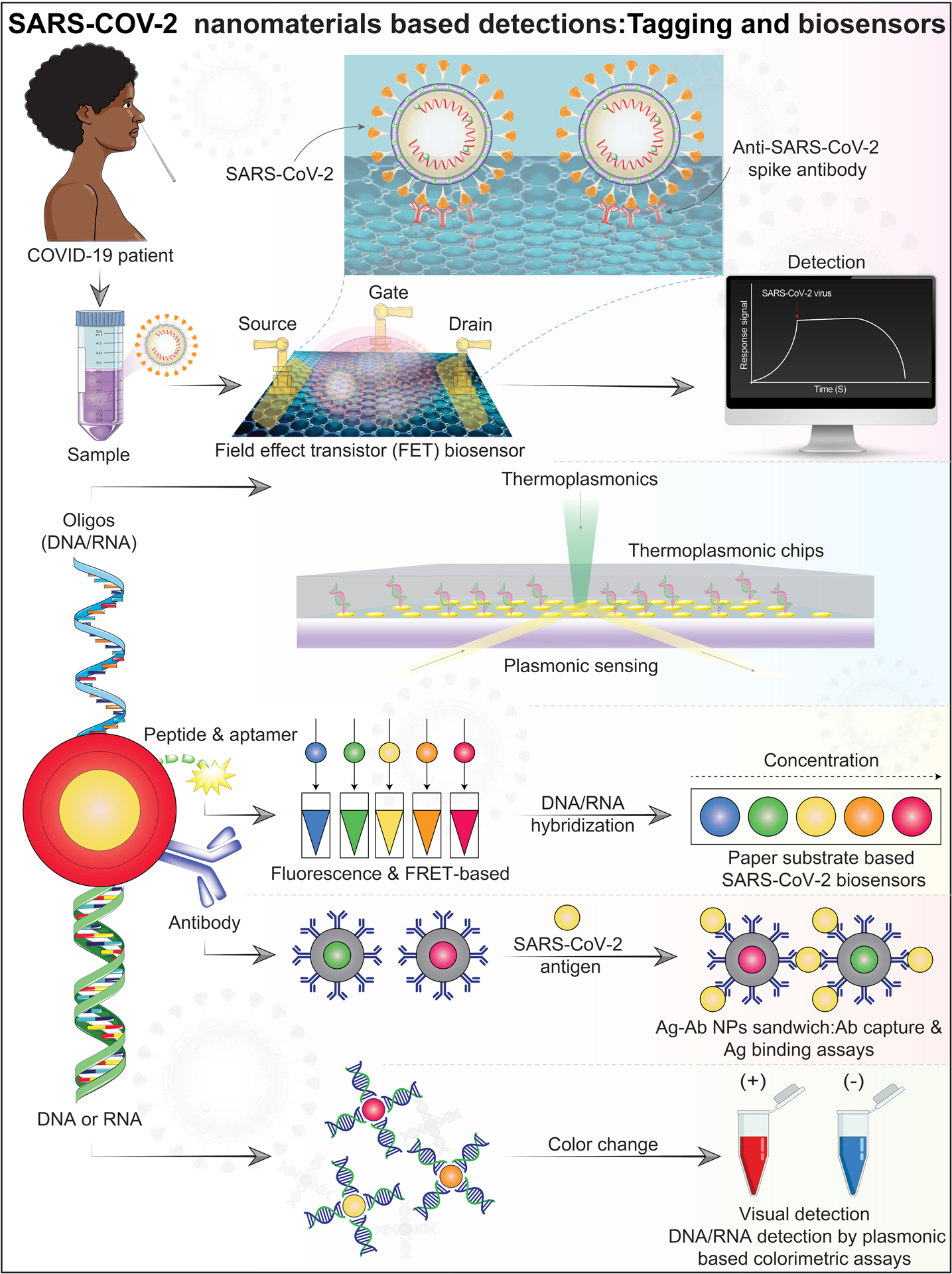
Represented illustration of nanomaterial-based biosensors for SARS-CoV-2 detection. Top panel: A field effect transistor (FET)-based biosensing device for detecting SARS-CoV-2. The sensor was produced by FET coated graphene sheets with specific antibodies against the SARS-CoV-2 spike protein. Second panel: The dual functional plasmonic photothermal (PPT) and localized surface plasmon resonance on two-dimensional gold nanoislands functionalized with complementary DNA receptors for detection of the selected SARS-CoV-2 sequences by nucleic acid hybridization. This assays whether a sample contains SARS-CoV-2 RNA. Bottom panel: Nucleic acids or antibodies functionalized materials for SARS-CoV-2 detection by colorimetric and antigen-binding assays. Abbreviations and acronyms: antibody (Ab), antigen (Ag), Förster resonance energy transfer (FRET), nanoparticles (NPs). Schematic ideas and technical methological details were followed and as represented in previously published reports49, 50, 58.
A clinical diagnostic sensor was developed that combines a dual-functional plasmonic photothermal (PPT) effect with localized surface plasmon resonance (LSPR) sensing transduction. Tests are done on two-dimensional gold nanoislands (AuNIs) (Fig. 3 middle panel). The AuNIs contain complementary DNA receptors which hybridize to SARS-CoV-2 nucleic acids. This system can be excited at two different wavelengths as it uses two different angles of incidence, one from PPT and the other from LSPR. It can detect RdRp-COVID, ORF1ab-COVID, and E genes from SARS-CoV-2. The dual-functional LSPR biosensor has a lower detection limit of 0.22 picomolar and allows precise detection of selected SARS-CoV-2 sequences in a multigene mixture. The plasmonic sensing system can significantly reduce the rate of false-positive results67. Similarly, others developed a plasmonic nanohole array used to transmit light for the label free detection of the pathogen in biological media without sample preparation. It can quantitate intact virions by capturing them on group-specific antiviral immunoglobulins immobilized at the surface of the sensor. The intact virus binds to a suspended nanohole array grating that couple’s incident light to surface plasmons and causing a red shift in surface plasmonic resonance frequency. The assay could detect small (vesicular stomatitis virus and pseudotyped Ebola) and large (vaccinia virus) enveloped viruses. The non-destructive nature of the assay allows for further analysis of progeny virions63. Overall, the biosensors and other material science-based detection techniques can enable rapid and portable diagnostic SARS-CoV-2 testing.
Detection of SARS-CoV-2 antibodies
The synthesis of antibodies against SARS-CoV-2 are as a primary immune response to infection. Neutralizing antibodies are found in up to 50% of infected patients by day 7 and in all patients by day 14. Serological studies are an alternative to RT-PCR for SARS-CoV-2 diagnostics. Combining both real-time PCR and serological testing significantly increases positive viral detection rates. IgM levels increase during the first week after SARS-CoV-2 infection, peak after 2 weeks and then fall back to near-background levels in most patients. IgG is detectable after 1 week and is maintained at a high level for a long period68. In contrast, IgG becomes detectable after 1 week, remains elevated for an extended period, even over 48 days, and may serve to protect against re-infection. IgA responses appear between 4 to 10 days after infection. Notably, a diagnostic predictor is the presence of sera IgA69 as well as IgG and IgM70. The spectrum of SARS-CoV-2 antibodies are explained, in part, by divergent target antigens. Antibody titers can decrease seven days after infection68. Recent studies have identified SARS-CoV-2 specific antibodies in the saliva71, 72. Multiplex SARS-CoV-2 antibody immunoassssay were investigated to determine differences between antibody levels in patient saliva and sera. Antibodies in saliva consistent with that in sera suggest parallel compartmental humoral immune responses72. A parallel study developed rapid immunoassay using the Brevitest platform technology for measuring salivary IgA which correlates with COVID-19 disease severity.
Interestingly, low levels of IgA were seen in individuals with IgG without known exposure to the virus, and suggest that it may represent an indicator of herd immunity71. SARS-CoV-2 specific antibody detection, especially that in saliva, may be useful for surveillance. Questions remain as to which antigens are the best candidates for serological testing. While the viral S is perhaps the strongest candidate, what remains unresolved is what part of the S should be developed? Alternatively, multiple isoforms of the S protein, such as those found in variant strains, may be used to ensure assay reproducibility73. Time to results can vary from 13 minutes (Abbott ID NOW) to 45 minutes (Cephied Xpert Xpress)74. Of the five antibody-based tests available, two are lateral flow immunoassays (BioMedomics rapid test and Surescreen rapid test cassette), one is a time-resolved fluorescence immunoassay (Goldsite diagnostics kit), and two are colloidal gold immunoassays (Assay Genie rapid PoC kit and VivaDiag COVID-19 IgG-IgM based).
Clinical studies will be needed to determine their clinical relevance75. For N-based immunoassays, SARS-CoV-2-IgG (Abbott) show a sensitivity of up to 100%76. For S-based immunoassays, Liaison® SARS-CoV-2 S1/S2 IgG and the combination S-and N-based platform COVID-19 VIRCLIA® IgG MONOTEST demonstrated equivalent sensitivities. The plaque reduction neutralization test (PRNT) showed a sensitivity of 93.3%. For evaluating specificity, all of the tests except one, the ELISA (IgG) (Euroimmun), produced at least one positive result for the negative SARS-CoV-2 antigen control. This likely represents large discrepancies between the testing platforms and the assay sensitivity relative to time. Although the PRNT is the gold standard for immunoglobin-based detection, the test has constraints including limited number of sample analyses and requires a BSL-3 laboratory. The titers obtained from the assays correlate well with PRNT. Currently antibody assays are applied principally for epidemiological testing77.
SARS-CoV-2 antigens
A rapid diagnostic assay was also developed to detect the presence of viral antigens expressed by the SARS-CoV-2 in samples from the respiratory tract of infected individuals78. For this assay, sample antigen present in the sample, binds to antibodies affixed to a paper strip enclosed in a plastic casing. This reaction generates a visually detectable signal within half an hour. The detected antigen(s) are expressed only if the virus is actively replicating; therefore, the tests can be used to identify acute or early infection78. Also, a more common type of rapid diagnostic assay has been marketed by Abbott for COVID-19, which detects the presence of antibodies in the blood of infected individuals. Abbott’s test can detect the SARS-CoV-2 antibody on ARCHITECT i1000SR and i2000SR laboratory instruments, which can run ~100–200 test per hour79. Antibodies against SARS-CoV-2 are produced after one week of infection80. The strength of any antibody response depends on age, nutritional status, disease severity, comorbid conditions, and medications.
Saliva testing
The presence of SARS-CoV-2 viral RNA in saliva samples was not associated with disease severity, which is different from what was reported for nasopharyngeal swabs 132,133. Nevertheless, human saliva has gained attention as an alternative diagnostic medium for detecting infections22. Naso- or oropharyngeal swabs show limitations in sample collection and presents a risk to healthcare workers through sneeze or cough and transmitting virus particles by aerosols9. In addition, in cases of thrombocytopenia or any other coagulation disorders, the collection procedure can precipitate bleeding. These complications have led to testing sputum collection for diagnostic purposes. Sputum is an easy directed and non-invasive method of sampling. However, one limitation is that 72% of COVID-19 patients are unable to produce sufficient sample volume33. As a multi-constituent oral fluid, saliva has demonstrated high potential for the surveillance of general health and disease21. The ease of collection for diagnostics and monitoring, without the need for medical staff can lead to ease of sample collection (Fig. 2c). Saliva is a useful biological medium, as it comprises proteins, nucleic acids, electrolytes, and hormones originating from multiple local and systemic sources. Saliva is known to contain approximately 30% of biomolecules found in blood and harbors viral microorganisms81. Moreover, saliva samples can be stored in stabilizing solutions and posted several days later in the testing center. Saliva collection is less invasive to the donor than blood collection and can permit home sampling21, 22. Analysis of saliva samples in patients with COVID-19 may facilitate the detection of both the virus itself and the antibodies and, as such, shows potential as a diagnostic medium. Human saliva sampling may have a major potential for COVID-19 screening20, 82. There is a concordance between detecting respiratory pathogens, including two seasonal human coronaviruses, in saliva using RT-PCR37. Indeed, mean SARS-CoV-2 titers (virus copies/mL) in saliva (n=37) compared to nasopharyngeal swabs (n=46) were 5-fold higher (p<0.05). Furthermore, none of the negative saliva samples became positive. In contrast, in five instances, nasopharyngeal swabs first tested negative for SARS-CoV-2, followed by a positive test result when repeated20,82. However, ever more reliable sample collection that can be self-administered is still needed, with a significant directive of current research activities.
Fecal tests
Knowledge regarding virus incubation, transmission, and shedding is crucial for protecting healthcare professionals and stopping the spread of SARS-CoV-2. High incidence and viral persistence in feces have been observed when nasopharyngeal swab samples were virus negative32. Notably, viral load in stool samples can be detected up to four weeks after disease onset. The risk of exposure to healthcare professionals of fecal material from patients is well known, especially in high aerosol generative procedures. Facilities, such as nursing homes, may be particularly vulnerable to this pathway of transmission of infection. While a high incidence of cough and fever are well established33, documented gastrointestinal symptoms support fecal-oral transmission routes83. Based on the prolonged viral shedding in feces and respiratory samples 14 days post-discharge, the European Centre for Disease Prevention (ECDC) has advocated the need for continued self-isolation84. Studies have also shown that the live virus can be isolated from stool specimens32, supporting the possibility of fecal-oral transmission. As a result, evidence-based recommendations for gastrointestinal endoscopy and surgery are required where there may be an exposure risk to virus shedding in feces. Lastly, SARS-CoV-2 may be tracked through wastewater and, as such, enables community surveillance and could be a powerful tool in tracking COVID-19 spread. There are now sewage screening tests for dormitories in an attempt to detect asymptomatic individuals. If positive, institutions can quarantine those infected in order to prevent subsequent SARS-CoV-2 outbreaks85.
Radiographic testing
Although quantitative and qualitative tests of viral nucleic acid RT-PCR tests are the primary assay for SARS-CoV-2 detection11, 44, the sensitivities of these tests remain low for pharyngeal (32%) and nasal (63%) swab samples36. RT-PCR tests can often take up to more than a week due to a shortage in testing supplies or lack of technical skills. Therefore, suspected cases, either with or without RT-PCR results or negative RT-PCR results, require additional affirmations. Combinations of radiographic, molecular, and antigen-based assays have been used alone, or in combination, to determine the optimal means to make a definitive diagnosis of SARS-CoV-2 infection91. After the respiratory symptom presentation and nucleic acid viral detections, an initial evaluation of COVID-19 patients commonly includes radiological examinations. Such examinations include a chest X-ray (CXR), computed X-ray tomography (CT) or a lung ultrasound. These, alone or together, can be also be used to stage SARS-CoV-2 infection91, 92. Often, a simple CXR is sufficient. However, a negative CXR alone cannot rule out lung involvement93, 94. While RT-PCR remains the gold standard for a virologic diagnosis, CXR affords 69% sensitivity91, 93, 94. However, imaging features contained in a standard CXR are often non-specific. When radiographic features of the disease are seen they reflect dense radiological patches on the left upper lobe and lower corners of the lung. With disease progression, more well-defined radiographic features are present and increase the veracity of a definitive COVID-19 diagnosis. However, while a CXR is the most useful test to affirm lung disease, it does not rule out alternative infections and especially in the context of presenting COVID-19 signs and symptoms since it is not specific. Abnormalities such as pneumothorax, pulmonary edema, pleural effusions, lung mass, or lung collapse are alternatives94. The value of the CXR is further supported by meta-analyses of patients with lower respiratory infections that include those treated in an ICU. Serial chest X-rays can shorten symptom duration and reduce disease comorbidities91, 92.
Computed tomography (CT) and magnetic resonance imaging (MRI)
Supplementary diagnostic testing for COVID-19 provides affirmation and monitoring of viral infection. Conventional CXR possesses sensitivity of nearly 60% for initial detection of COVID-19 related pulmonary disease95. These CXR abnormalities include bilateral lower zone- and peripherally- predominant consolidation and hazy opacities93. In addition, CT scans demonstrate a, “reversed halo” pattern and signs of septal thickening96. Distinctive CT images illustrate bilateral pulmonary parenchymal ground-glass and consolidated pulmonary opacities with occasionally rounded morphology and marginal lung dispersal (Fig. 4a). Lung engrossment with peripheral predominance is seen both in patients with SARS-CoV and MERS-CoV infections. However, chest CT showing pulmonary ground-glass opacities and alliance are more akin to SARS-CoV-2 infection91, 97.
Figure 4. CT and MRI examination of the lung and brain in life threatening SARS-CoV-2 infections.
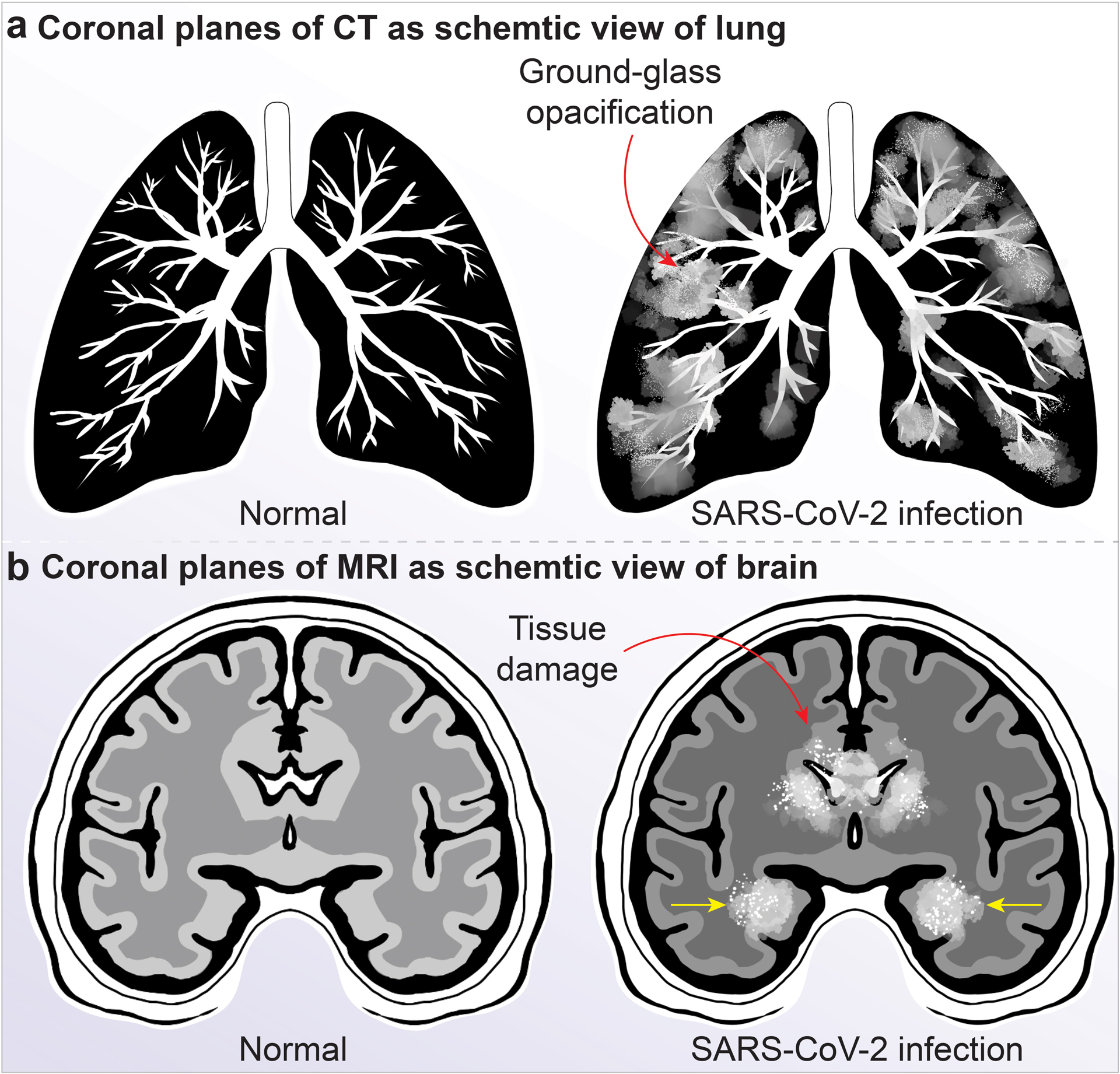
a, Comparison is shown between lung from a uninfected (left) and a SARS-CoV-2-infected person’s lung tissue (right) in a representative CT scan. In these images, ground-glass opacification (light hazy grey tissue) are seen due to inflammatory responses. ARDS results in fluid accumulation in affected lung tissue independent of cardiac dysfunction (noncardiogenic pulmonary edema). Viral infection causes lung injury leading to loss of gas exchange, atelectasis and hypoxemia. ARDS is associated with fibrinous organizing pneumonia and alveolar damage. SARS-CoV-2 causes epithelial infection and alveolar macrophage inflammation, activation and secretion of a range of pro-inflammatory and chemotactic factors that lead to progressive lung tissue damage. b, SARS-CoV-2-infected patient’s brain MRI scan image showing the brain regions typically involved in those who develop encephalitis or acute necrotizing encephalopathy. The rims of the lateral ventricles can illustrate contrast enhancement with meningeal involvements (red arrow). The medial temporal lobes (yellow arrows), including the hippocampi, may show hyperintense signals, indicating inflammation that may result from the “cytokine storm syndrome”, and hypointense signals that show hemorrhage. Other brain regions including the thalamus, cerebral white matter, brain stem and the cerebellum can be involved.
Patients with negative RT-PCR tests for SARS-CoV-2 can present with abnormal chest CT scans and are later diagnosed with COVID-1992. Thus, emerging evidence supports the use of chest CT examination as a confirmatory test for COVID-19 disease when patients have negative RT-PCR test but high clinical suspicion for SARS-CoV-2 infection. Chest CT scans could be used as a diagnostic tool for patients with negative RT-PCR screening, as an adjunctive test, used in combination with repeated RT-PCR assays. Specifically, high-resolution chest CT is vital for confirmatory analysis and evaluation of disease severity in suspicion patients with SARS-CoV-2 infection91. Numerous studies have scrutinized chest CT images of patients infected with SARS-CoV-2 considering abnormalities may also be due to other causes of pneumonia, leading to false positive results. In one study performed in TongJi Hospital, Wuhan, involving 1,014 patients who were examined with both chest CT and RT-PCR tests; 601 patients (59%) had positive RT-PCR results, and 888 (88%) had positive chest CT scans. While the sensitivity of chest CT scans for COVID-19 was 97%, based on positive RT-PCR results, 75% (308 of 413 patients) had positive chest CT scans with negative RT-PCR results92. In advanced cases, SARS-CoV-2 infection can lead to extensive lung tissue damage with reduced oxygen uptake in infected people91, 97. Although chest CT abnormalities may precede symptom onset in 44% of the COVID-19 patients, >90% of those with respiratory symptoms will have abnormal chest CT after symptom onset96. Furthermore, abnormalities on chest CT may be seen even in asymptomatic patients with positive RT-PCR, as reported in 14 of 15 health care workers in one study98 and 54% of 82 asymptomatic passengers on the Diamond Princess cruise ship91, 97. Despite the sensitivities of chest CT to detect lung abnormalities, the current recommendations from the major radiological societies is that chest CT should not be used for first line screening of COVID-19, but to be used sparingly for hospitalized, symptomatic patients with specific clinical indications. Normal CT should not dissuade a patient from being quarantined or provided other clinically indicated treatment when otherwise medically appropriate99. Most recently, a Consensus Statement from the Fleischner Society was generated by a multidisciplinary panel comprised principally of radiologists and pulmonologists from 10 countries with experience managing COVID-19 patients across a spectrum of healthcare environments, evaluated the utility of imaging within three scenarios representing varying risk factors, community conditions, and resource constraints100. Based on 14 key questions, corresponding to eleven decision points within the three scenarios and three additional clinical situations, the aggregated results yielded the following recommendations (see Table 1.100).
Table 1.
Summary of recommendations for imaging tests
| Recommendations |
|---|
| • Imaging is not indicated as a screening test for COVID-19 in asymptomatic people. |
| • Imaging is not indicated for patients with mild COVID-19 unless the patient is at risk for disease progression. |
| • Imaging is indicated for patients with moderate to severe disease regardless of SARS-CoV-2 test results. |
| • Imaging is indicated for patients with COVID-19 with evidence of respiratory insufficiency. |
| • In a resource limited settings where access to CT is limited, CXR is preferred. |
| Additional Recommendations |
| • Daily chest radiographs are NOT indicated in stable intubated patients with COVID-19. |
| • CT is indicated in patients with functional lung impairment and or hypoxemia. |
| • COVID-19 testing is indicated in patients found to have findings suggestive of viral infection on CT scans. |
The immune response to SARS-CoV-2 leads to the release cytokines and chemokines, frequently leaving inflammatory cells which can be seen by CT (Fig. 4a) in the form of yellow discoloration. Fig. 4b shows magnetic resonance imaging (MRI) scans of a COVID-19-infected patient’s brain, which provides much more details of the pathologies in the soft tissue than CT. However, the American College of Radiology (ACR) advises the medical facilities to avoid performing MRI in COVID-19 patients. According to the ACR published guidelines, patients who are suspected of SARS-CoV-2 infection or have tested positive can be scanned by alternative imaging methods. Sanitizing MRI machines takes a long time and poses significant challenges. High-efficiency particulate air (HEPA) filter systems, typically used to increase air exchange, are not compatible with MRI. Suppose the ventilation examination is deemed clinically necessary. In that case, it is recommended that the potential risks of a patient having COVID-19 should be discussed with the referring physician and an alternative ventilation scan should be offered as per the hospital COVID-19 policies101.
Ultrasound
Ultrasonography of the lungs are also used to assess COVID-19 patients102. Lung-ultrasound (LUS) does not appear to have specificity for identifying COVID-19 pneumonitis or pneumonia but is recommended for defining the area of infection. LUS may be beneficial in the early diagnosis of COVID-19 pneumonia as a cost-effective way to determine the localization of infection. The result of LUS is more sensitive than a CXR due to its excellent response to positive end-expiratory pressure (PEEP; pressure in the lungs above atmospheric pressure that exists at the end of expiration). LUS shows several features, such as lung consolidation in severe local disease. Similar to those found on CXR or chest CT, LUS in patients with COVID-19 infection found more prominent evidence of COVID-19 pathology in the posterior lower lung zones. In most cases, the infection progresses from the periphery to the center of the lung tissues. ICU care teams also use LUS findings of pulmonary edema to therapeutically position the patients102.
Limitations of current diagnostic testing
Currently, a, “clinical diagnosis” of COVID-19 relies on a combination of chest CT and RT-PCR results. Outside of a clinical setting, RT-PCR testing comprises the vast majority of all surveillance testing done in the workplace or within schools. Due to the ubiquity of RT-PCR testing, it is important to examine the information this test offers to clinicians and policy makers alike. By identifying the shortcomings of this testing platform, future detection methods can improve upon the current model. Nucleic acid amplification tests (NAAT) may be problematic with poorly timed specimen collection, poor quality specimen collection, the requirement for trained lab technicians, and long wait times to generate the results. The gold standard RT-qPCR is time consuming (4–6 hours), not including the time to transport the specimens to the laboratory which can take days. RT-PCR results also depend heavily on the type of sample taken; positive sampling rates vary widely between oropharyngeal swabs (32–48%), nasopharyngeal swabs (63%), BALF (79–93%), sputum (72–76%) and stool (29%)116. The primers and other reagents required to run the tests could be in shortage117. In response to the limitations posed by RT-PCR testing, new platforms are actively being pursued. Research on antibody detection tests are ongoing, but limited. Many studies had small cohorts and, given the urgency to share scientific knowledge in this unprecedented time, have rapidly published studies which should be viewed with a critical lens. Current issues for immunodiagnostic approaches include a lack of specificity which is linked to false positive results from antigens that are well-conserved among different CoV species and cross-reactions with autoantibodies in autoimmune diseases. Immunodiagnostic approaches are most viable 7–11 days post exposure and are therefore less useful in diagnosis of acute infection118.Outside of the physical limitations of the testing platform, the information produced by both nucleic acid amplification testing and serology testing fails to capture important metrics of COVID-19, such as the duration an individual is contagious or the prevalence of certain haplotypes in a population. To these ends, metagenomic detection procedures used in tandem with nucleic acid amplification techniques may lead to new insights gained by clinicians and epidemiologists alike. Although RT-qPCR is the current standard for detection of nucleic acids, new methods, such as pulse controlled amplification (PCA), are being evaluated. PCA does not require RNA extraction and can be done in 10 minutes with a small device119. In the future, S- and N-based immunodiagnostics platforms will work alongside NAATs in order to increase detection sensitivity of COVID-19 at minimal cost120. Future efforts towards the development of novel diagnostic platforms may prove fruitful if the tests are accurate, specific, easy to run, produce results in a short amount of time, and are cheap to mass produce. Given both the strengths and limitations of current testing platforms and their singular output values, the information afforded by testing results must be carefully scrutinized before making decisions in clinical and non-clinical settings.
Conclusions
The SARS-CoV-2 pandemic follows a troublesome trajectory. The health, humanitarian, social and economic policies adopted by countries can verify the speed and strength of the recovery. Presently, no medication is usually commended to treat COVID-19, and no cure is accessible. The U.S. FDA granted use of medicines previously approved for other disorders to be used now as recommended therapy for COVID-19. The earlier lack of accessibility for test has hampered the infection control; however, testing of this novel virus is increasing quickly. Diagnostic testing for COVID-19 is vital in detection of the virus, understanding epidemiology, case management, and suppressing transmission. Universal operating procedures and harmonization of the available diagnostic assays are needed for faster screening approaches in the global fight against the pandemic. Similarly, academic scientists and biotechnologists are charged with the description of additional SARS-CoV-2 strains in order to improve clusters-based specificity and sensitivity of antibody and antigen-based tests. Significantly, nanomaterials-based virus detection technology can help in the development of high sensitivity, simple, scalable, rapid, and cost-effective COVID-19 detection tests that supply on-demand diagnostic capability and effectively in the pandemic. This review offers a road map for diagnostic strategies in the context of disease transmission and prevention. It is a basic science guide to better appreciate COVID-19 diagnostic complexities and to affect improved disease combating strategies.
BOX 1:
Sample acquisitions
SARS-CoV-2 spreads by respiratory aerosol or fomites17. Nasal or oropharyngeal samples, collected alone or in combination, confirm viral infection18. SARS-CoV-2 migrates from the upper to lower respiratory areas where replication. Samples from bronchoalveolar lavage, tracheal aspirates and pleural fluids and or urine, blood and feces contain virus19. Saliva is an alternative source for SARS-CoV-220 and virus-specific antibodies21, 22. Saliva viral antigen or antibody tests may become a future norm used for distant travel such as when boarding planes or ships ensuring that travelers are free of SARS-CoV-2. A positive saliva viral antigen test identifies infected patients.
Clustered regularly interspaced short palindromic repeats (CRISPR) and associated protein (Cas12/13) diagnostics
CRISPR-Cas is a powerful genome editing system widely used for genome editing. Cas enzymes (Cas12 and Cas13) possess cleavage activity can be used for nucleic acid detection. The Cas12- and 13 based detection systems was named, by the inventors, as DNA endonuclease-targeted CRISPR trans reporter (DETECTR) and specific high-sensitivity enzymatic reporter unLOCKing (SHERLOCK) systems. Readouts of samples are within an hour using SHERLOCK23 in lateral-flow formats. It is commonly used for detecting bacteria, viruses and cancers24. The Cas13 is an RNA-targeting enzyme with promiscuous cleavage activity of non-target nucleic acids from patient samples. When the enzyme recognizes its target it cleaves target nucleic acids, including other RNA species in solution (collateral cleavage) in femtomolar concentration detection. Cas13 have been paired with an isothermal pre-amplification step. The SHERLOCK method was first developed in 201725 then refined26 to make it suitable for PoC testing termed STOPCovid (SHERLOCK Testing in One Pot Covid)27. STOPCovid permits a lateral-flow and a fluorescence-based assays. The Sherlock Biosciences’ STOPCovid assay for Emergency Use Authorization. SHERLOCK-based multiplexed diagnostics can now be used to detect over 160 infectious agents28. CRISPR Cas12a/gRNA complex and a fluorescent probe detect target amplicons using standard RT-PCR or isothermal recombinase polymerase amplification (RPA) with primers for the viral ORF1ab and N regions that detect two RNA copies. Positive CRISPR-based fluorescent detection system (CRISPR-FDS) can be negative on RT-PCR29. Cas12 collateral cleavage activity on single-stranded DNA (ssDNA) was developed by combining it with isothermal amplification, termed DNA endonuclease-targeted CRISPR trans reporter (DETECTR)30. A DETECTR-based diagnostic assay for COVID-19 was developed by Mammoth Biosciences31.
BOX 2:
Recent diagnostic kits
Several companies are manufacturing SARS-COV-2 diagnostic assay kits aiming to improve detection rates. For example, GenMark Diagnostics, Inc. is developing the ePlex Research Use Only (RUO) test and will soon submit an EUA to the U.S. FDA for the ePlex SARS-CoV-2 test for viral diagnosis86. BioFire Diagnostics is developing the Film Array® Respiratory Panels (RP & RP2), also referred to as the BioFire Respiratory Panels, which will help clinicians rapidly diagnose SARS-CoV-2 and other respiratory infections87. Meridian Biosciences has created a “Master Mix” containing the building blocks for rapid testing by eliminating the RNA extraction facilitates conventional molecular procedural steps. This can significantly reduce the assay cost and time88. Similarly, Cephied Inc. has also announced its SARS-CoV-2 test kit, which can be run on any of its 23,000 GeneXpert Systems placed worldwide to deliver PoC results in 30 minutes89. Recently, Abbott received U.S. FDA EUA for its BinaxNOW™ COVID-19 Ag Card which depends on flow technology to detect SARS-CoV-2 antigen in a nasal swab from suspected COVID-19 individuals with a sensitivity of 97.1% and specificity of 98.5%. The test can provide results in just 15 minutes with a cost of $590. Abbott also launched NAVICA app which allows people to display negative test results obtained from the healthcare provider in the form of a QR code to enter the organization that requires proof of testing. The people with positive test results receive a message to quarantine and contact a healthcare provider for treatment90.
BOX 3:
SARS-CoV-2 mass pooled screening
The outbreak of SARS-CoV-2 has overwhelmed healthcare systems worldwide. Thus, it is imperative to adopt reliable screening, particularly to detect COVID-19 asymptomatic patients so that disease spread is controlled. To speed screenings, pooling can provide surveillance for infected patients103. High-throughput, highly automated PCR testing and matrix are pooling strategies104. From a single test, if a pool is negative, all the individual samples are considered negative. If a pool is found positive, individual samples must be tested to pinpoint a positive source. Pooling was used during World War II to detect syphilis and used for HIV detection in 1991105. In order to choose a cost-effective pooling strategy, one must consider disease prevalence in any tested population along with pecificity, sensitivity and test probability103. In Wuhan, China, six new cases were reported for SARS-CoV-2 after a month of no newly confirmed cases. Thereafter, the government shifted their efforts to widespread screening with pooling to mitigate the second wave of infection106. Pooling can use RT-qPCR tests. The U.S. FDA has authorized Quest Diagnostics SARS-CoV-2 RNA and LabCorp’s COVID-19 test under the provisions of EUA. In a recent study by the World Health Organization (WHO), existing methods were compared head to head for population testing using the Monte Carlo simulation. The simulations show that in population, with low prevalence, up to 86% fewer tests are required. As the prevalence increases, the pool size decreases. In a separate study of 3,592 consecutive nasal swab samples, with less than a 1% prevalence of infection, 8 sample pooling allow viral identification with 100% sensitivity, specificity and accuracy and cost decrease of 80%103. Such guidelines were published by the U.S.. Centers of Disease Control and Prevention (CDC) for pooling samples107. In a retrospective study, bronchoalveolar lavage and nasopharyngeal samples were collected between January 1st and February 26th, 2020 from in- and out-patients having negative routine respiratory viral test and had not been tested for SARS-CoV-2. Nine or ten samples were pooled and screened by using RT-PCR attacking (E) gene envelope. From a total pools only 0.07% (2/2888) positivity rate for SARS-CoV-2 was confirmed, comprising mainly the nasopharyngeal samples, which suggested that disease burden was low in this area early in the pandemic108. PerkinElmer Genomics with the Medical College of Georgia (Augusta, Georgia) and Aga Khan University (Nairobi, Kenya) developed a RT-PCR kit for cost-effective, rapid, and accurate SARS-CoV-2 mass screening and 91.6% Positive Percent Agreement (PPA) and 100% Negative Percent Agreement (NPA). PerkinElmer’s RT-PCR kit recently received U.S. FDA-EUA approval having limit of detection of < 20 copies/mL of SARS-CoV-2109. In another study, viability of pooling clinical naso- or oro- pharyngeal swabs during extraction of nucleic acid was seen without reducing the sensitivity of RT-PCR110. Pooling eliminates up to 80% of reagent cost when tested in a population having a prevalence of positive samples of ≤ 1% and hence decreases the global costs. The pooling strategy can be adopted by schools, universities, workplaces, and religious organizations that are adamantly seeking to reopen111. Meatpacking plants have employed a pooling strategy and shown a much higher prevalence of infection. Still, it is not possible to determine the absolute number of infections with such methods107.
Metagenomic profiling
The detection of SARS-CoV-2 in the USA lagged two months behind early viral detection in China. This led to delay in RT-qPCR implementations and viral spread. Therefore, unbiased detection strategies are required that bypass the requirement for viral sequence data to diagnose infections112. Metagenomic Next-Generation Sequencing (mNGS) can detect whole viral genomes and any co-infection. The mNGS approach was validated using RT-PCR confirmed cases aligned with the 2019 GenBank nucleotide database utilizing Clinically Okay Metagenomic Pipeline (CLOMP). CLOMP results revealed positive SARS-CoV-2 samples that matched the database of SARS-CoV associated viruses. The advantage of unbiased mNGS is that it detects all the sequences lined up to already known bacterial and viral databases extending primer directed PCR that are capable of only detecting known viral sequences112. Similarly, there are expanded versions of studies focusing on short, virus-like sequences of DNA in metagenomic data. Metagenomic data from the dried Aral Sea basin in Uzbekistan was identified. The rhizosphere microbiome Suaeda acuminata (C.A. Mey.) characterized the ecology of the first pioneer plants in environmental extremes. These studies also revealed the presence of coronavirus-like sequences before the COVID-19 outbreak. Diverse betacoronavirus-like sequences, including SARS-CoV matches, were observed in the environmental datasets. In addition, the datasets enabled the viral origins. The study led to the notion that natural environments and the plant rhizosphere host contained coronavirus sequences. This metagenomic strategy involving microbiome research can be helpful in predicting future outbreaks before pandemics emerge113. In short, metagenomics is a sensitive assay that can be used for molecular epidemiological tests114. Tests enable studies of viral evolution and molecular epidemiology and complete evaluation of the background microbiome115.
Acknowledgments
We thank Doug Meigs, University of Nebraska Medical Center, for critical review of the manuscript. This work was supported by the National Institutes of Health R01 MH121402-01A1; R01 MH121402P01; R01 AG043540, R01 AG043530, P01 DA028555, P30 MH062261, R01 MH115860, R01 NS034249, R01 NS036126, the Carol Swartz Emerging Neuroscience Fund and the Margaret R. Larson Professorship. CP receives support by the Cancer Australia [APP1145657] and The Garnett Passé and Rodney Williams Foundation.
Footnotes
Author declaration
The authors declare no competing interests.
References
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 5, 536–544 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 323,1061–1069 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens for COVID-19. https://wwwcdcgov/coronavirus/2019-ncov/lab/guidelines-clinical-specimenshtml (2020).
- 4.Machhi J, et al. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J Neuroimmune Pharmacol 15, 359–386 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wadman M, et al. How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes. Science (2020). [Google Scholar]
- 6.Udugama B, et al. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 14, 3822–3835 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Research Use Only Real-Time RT-PCR Protocol for Identification of 2019-nCoV. Coronavirus Disease 2019 https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-detection-instructions.html (2020). [Google Scholar]
- 8.Wang B, et al. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 12, 6049–6057 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loeffelholz MJ & Tang YW. Laboratory diagnosis of emerging human coronavirus infections - the state of the art. Emerg Microbes Infect 9, 747–756 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winichakoon P, et al. Negative Nasopharyngeal and Oropharyngeal Swabs Do Not Rule Out COVID-19. J Clin Microbiol 58, e00297–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu R, et al. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta 505, 172–175 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan Y, et al. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Infect 81, e28–e32 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin D, et al. Evaluations of the serological test in the diagnosis of 2019 novel coronavirus (SARS-CoV-2) infections during the COVID-19 outbreak. Eur J Clin Microbiol Infect Dis 39, 2271–2277 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipsitch M, et al. Defining the Epidemiology of Covid-19 — Studies Needed. N Engl J Med 382, 1194–1196 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Okba NMA, et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerg Infect Dis 26, 1478–1488 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen Z, et al. Genomic Diversity of Severe Acute Respiratory Syndrome-Coronavirus 2 in Patients With Coronavirus Disease 2019. Clin Infect Dis 71, 713–720 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med 382, 1199–1207 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March 2020. https://apps.who.int/iris/handle/10665/331329 (2020).
- 19.Zhang W, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect 9, 386–389 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azzi L, et al. Saliva is a reliable tool to detect SARS-CoV-2. J Infect 81, e45–e50 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Punyadeera C, et al. One-step homogeneous C-reactive protein assay for saliva. J Immunol Methods 373, 19–25 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Pfaffe T, et al. Diagnostic potential of saliva: current state and future applications. Clin Chem 57, 675–687 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Xiao F, et al. Infectious SARS-CoV-2 in Feces of Patient with Severe COVID-19. Emerg Infect Dis 26,1920–1922 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu R, et al. Viral Load Dynamics in Sputum and Nasopharyngeal Swab in Patients with COVID-19. J Dent Res 99, 1239–1244 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yongchen Z, et al. Different longitudinal patterns of nucleic acid and serology testing results based on disease severity of COVID-19 patients. Emerg Microbes Infect 9, 833–836 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 323, 1843–1844 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YG, et al. Comparison between Saliva and Nasopharyngeal Swab Specimens for Detection of Respiratory Viruses by Multiplex Reverse Transcription-PCR. J Clin Microbiol 55, 226–233 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parolo C, et al. Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes. Biosens Bioelectron 40, 412–416 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Huang C, et al. Rapid Detection of IgM Antibodies against the SARS-CoV-2 Virus via Colloidal Gold Nanoparticle-Based Lateral-Flow Assay. ACS Omega 5, 12550–12556 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bullard J, et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smyrlaki I, et al. Massive and rapid COVID-19 testing is feasible by extraction-free SARS-CoV-2 RT-PCR. Nat Commun 11, 4812 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shieh WJ, et al. Immunohistochemical, in situ hybridization, and ultrastructural localization of SARS-associated coronavirus in lung of a fatal case of severe acute respiratory syndrome in Taiwan. Hum Pathol 36, 303–309 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu X, et al. Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19. Biosens Bioelectron 166, 112437 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corman VM, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 25, 2000045 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eigner U, et al. Clinical evaluation of multiplex RT-PCR assays for the detection of influenza A/B and respiratory syncytial virus using a high throughput system. J Virol Methods 269, 49–54 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Moulahoum H, et al. How should diagnostic kits development adapt quickly in COVID 19-like pandemic models? Pros and cons of sensory platforms used in COVID-19 sensing. Talanta 222, 121534–121534 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu X, et al. Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19. Biosens Bioelectron 166, 112437 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Augustine R, et al. Loop-Mediated Isothermal Amplification (LAMP): A Rapid, Sensitive, Specific, and Cost-Effective Point-of-Care Test for Coronaviruses in the Context of COVID-19 Pandemic. Biology (Basel) 9, 182 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohamed ET, et al. A Single and Two-Stage, Closed-Tube, Molecular Test for the 2019 Novel Coronavirus (COVID-19) at Home, Clinic, and Points of Entry. ChemRxiv (2020). [Google Scholar]
- 41.Hong S, et al. Application of fluorescence resonance energy transfer to bioprinting. TrAC Trends Analyt Chem 122, 115749 (2020). [Google Scholar]
- 42.Chacón-Torres JC, et al. Optimized and scalable synthesis of magnetic nanoparticles for RNA extraction in response to developing countries’ needs for the detection and control of SARS-CoV-2. Sci Rep 10, 19004 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hildebrandt N, et al. Energy Transfer with Semiconductor Quantum Dot Bioconjugates: A Versatile Platform for Biosensing, Energy Harvesting, and Other Developing Applications. Chem Rev 117, 536–711 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Oh E, et al. Inhibition assay of biomolecules based on fluorescence resonance energy transfer (FRET) between quantum dots and gold nanoparticles. J Am Chem Soc 127, 3270–3271 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Moitra P, et al. Selective Naked-Eye Detection of SARS-CoV-2 Mediated by N Gene Targeted Antisense Oligonucleotide Capped Plasmonic Nanoparticles. ACS Nano 14, 7617–7627 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorshkov K, et al. Quantum Dot-Conjugated SARS-CoV-2 Spike Pseudo-Virions Enable Tracking of Angiotensin Converting Enzyme 2 Binding and Endocytosis. ACS Nano 14, 2234–12247 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan S, et al. New Strategy for COVID-19: An Evolutionary Role for RGD Motif in SARS-CoV-2 and Potential Inhibitors for Virus Infection. Front Pharmacol 11, 912 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farzin L, et al. HIV biosensors for early diagnosis of infection: The intertwine of nanotechnology with sensing strategies. Talanta 206, 120201 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Talebian S, et al. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat Nanotechnol 15, 618–621 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Seo G, et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 14, 5135–5142 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Tymm C, et al. Scalable COVID-19 Detection Enabled by Lab-on-Chip Biosensors. Cell Mol Bioeng 13, 1–17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmadivand A, et al. Femtomolar-level detection of SARS-CoV-2 spike proteins using toroidal plasmonic metasensors. arXiv (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang L, et al. One-Step Rapid Quantification of SARS-CoV-2 Virus Particles via Low-Cost Nanoplasmonic Sensors in Generic Microplate Reader and Point-of-Care Device. Biosens Bioelectron 171, 112685 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yanik AA, et al. An optofluidic nanoplasmonic biosensor for direct detection of live viruses from biological media. Nano Lett 10, 4962–4969 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soler M, et al. Multiplexed nanoplasmonic biosensor for one-step simultaneous detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine. Biosens Bioelectron 94, 560–567 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Dang T, et al. Protein binding kinetics quantification via coupled plasmonic-photonic resonance nanosensors in generic microplate reader. Biosens Bioelectron 142, 111494 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Shan B, et al. Multiplexed Nanomaterial-Based Sensor Array for Detection of COVID-19 in Exhaled Breath. ACS Nano 14, 12125–12132 (2020). [DOI] [PubMed] [Google Scholar]
- 58.Qiu G, et al. Dual-Functional Plasmonic Photothermal Biosensors for Highly Accurate Severe Acute Respiratory Syndrome Coronavirus 2 Detection. ACS Nano 14, 12125–12132 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Dara M & Talebzadeh M. CRISPR/Cas as a Potential Diagnosis Technique for COVID-19. Avicenna J Med Biotechnol 12, 201–202 (2020). [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y, et al. CRISPR/Cas Systems towards Next-Generation Biosensing. Trends Biotechnol 37, 730–743 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Gootenberg JS, et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 356, 438–442 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kellner MJ, et al. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat Protoc 14, 2986–3012 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ioannidis J The infection fatality rate of COVID-19 inferred from seroprevalence data. medRxiv (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ackerman CM, et al. Massively multiplexed nucleic acid detection using Cas13. Nature 582, 277–282 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang Z, et al. Ultra-sensitive and high-throughput CRISPR-p owered COVID-19 diagnosis. Biosens Bioelectron 164, 112316 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen JS, et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 360, 436–439 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Broughton JP, et al. CRISPR-Cas12-based detection of SARS-CoV-2. Nat Biotechnol 38, 870–874 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hou H, et al. Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. Clin Transl Immunology 9, e01136 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Padoan A, et al. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: A longitudinal study. Clin Chim Acta 507, 164–166 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Long QX, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 26, 845–848 (2020). [DOI] [PubMed] [Google Scholar]
- 71.Varadhachary A, et al. Salivary anti-SARS-CoV-2 IgA as an accessible biomarker of mucosal immunity against COVID-19. medRxiv (2020). [Google Scholar]
- 72.Pisanic N, et al. COVID-19 serology at population scale: SARS-CoV-2-specific antibody responses in saliva. J Clin Microbiol 02204–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petherick A Developing antibody tests for SARS-CoV-2. Lancet 395, 1101–1102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolters F, et al. Multi-center evaluation of cepheid xpert(R) xpress SARS-CoV-2 point-of-care test during the SARS-CoV-2 pandemic. J Clin Virol 128, 104426 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Green K, et al. Molecular and antibody point-of-care tests to support the screening, diagnosis and monitoring of COVID-19. CEBM Research. https://www.cebm.net/wp-content/uploads/2020/04/POCT-Covid19.pdf (2020). [Google Scholar]
- 76.US Food and Drug Administration. EUA Authorized Serology Test Performance. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance (2020).
- 77.Kohmer N, et al. Brief clinical evaluation of six high-throughput SARS-CoV-2 IgG antibody assays. J Clin Virol 129, 104480 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.World Health Organization. Advice on the use of point-of-care immunodiagnostic tests for COVID-19. https://www.who.int/docs/default-source/coronaviruse/sb-2020-1-poc-immunodiagnostics-2020-04-08-e.pdf?sfvrsn=4c26ac39_2 (2020).
- 79.Abbott. Abbott launches covid-19 antibody test https://www.abbott.com/corpnewsroom/product-and-innovation/abbott-launches-covid-19-antibody-test.html (2020).
- 80.Long QX, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 26, 1200–1204 (2020). [DOI] [PubMed] [Google Scholar]
- 81.Verma D, et al. Insights into the human oral microbiome. Arch Microbiol 200, 525–540 (2018). [DOI] [PubMed] [Google Scholar]
- 82.Wyllie AL, et al. Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs. N Engl J Med 383, 1283–1286 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nobel YR, et al. Gastrointestinal Symptoms and COVID-19: Case-Control Study from the United States. Gastroenterology 159, 373–375.e2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.European Centre for Disease Prevention and Control. When is it safe to discharge COVID-19 cases from the hospital or end home isolation? https://wwwecdceuropaeu/sites/default/files/documents/COVID-19-Discharge-criteriapdf (2020).
- 85.The Washington Post. An early warning system for coronavirus infections could be found in your toilet. https://www.washingtonpost.com/climate-environment/2020/05/01/coronavirus-sewage-wastewater/ (2020).
- 86.GenMarkDx. GenMark Diagnostics Announces Submission of Emergency Use Authorization for its ePlex® SARS-CoV-2 Test. https://www.genmarkdx.com/genmark-diagnostics-announces-submission-of-emergency-use-authorization-for-its-eplex-sars-cov-2-test/ (2020).
- 87.BioFire. The BioFire FilmArray Respiratory (RP & RP2) Panels. https://www.biofiredx.com/products/the-filmarray-panels/filmarrayrp/ (2020).
- 88.Meridian Biosciences. Meridian Bioscience Simplifies COVID-19 Sample Prep and Eliminates Dependence on Reagents in Short Supply. https://investor.meridianbioscience.com/news-releases/news-release-details/meridian-bioscience-simplifies-covid-19-sample-prep-and (2020).
- 89.Cepheid. Xpert® Xpress SARS-CoV-2 has received FDA Emergency Use Authorization. https://www.cepheid.com/coronavirus (2020).
- 90.Abbott. Abbott’s fast, $5, 15-minute, easy-to-use covid-19 antigen test receives fda emergency use authorization; mobile app displays test results to help our return to daily life; ramping production to 50 million tests a month (2020).
- 91.Hosseiny M, et al. Radiology Perspective of Coronavirus Disease 2019 (COVID-19): Lessons From Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome. AJR Am J Roentgenol 214, 1078–1082 (2020). [DOI] [PubMed] [Google Scholar]
- 92.Ai T, et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 296, E32–E40 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wong HYF, et al. Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19. Radiology 296, E72–E78 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dennie C, et al. Canadian Association of Thoracic Radiology/Canadian Association of Radiologists Consensus Statement Regarding Chest Imaging in Suspected and Confirmed COVID-19. Can Assoc Radiol J 71, 470–481 (2020). [DOI] [PubMed] [Google Scholar]
- 95.Wang Y, et al. Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study. Radiology 296, E55–E64 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bernheim A, et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 295, 200463 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou Z, et al. Coronavirus disease 2019: initial chest CT findings. Eur Radiol 30, 4398–4406 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shi H, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 20, 425–434 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Czawlytko C, et al. Covid-19 diagnostic imaging recommendations. Applied Radiology 49, 10–15 (2020). [Google Scholar]
- 100.Rubin GD, et al. The Role of Chest Imaging in Patient Management during the COVID-19 Pandemic: A Multinational Consensus Statement from the Fleischner Society. Radiology 296, 172–180 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Americal College of Radiology. ACR Guidance on COVID-19 and MR Use. https://www.acr.org/Clinical-Resources/Radiology-Safety/MR-Safety/COVID-19-and-MR-Use (2020).
- 102.Poggiali E, et al. Can Lung US Help Critical Care Clinicians in the Early Diagnosis of Novel Coronavirus (COVID-19) Pneumonia? Radiology 295, E6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cesselli D, et al. Implementation and validation of a pooling strategy for a sustainable screening campaign for the presence of SARS-CoV-2. medRxiv (2020). [Google Scholar]
- 104.Deckert A, et al. Simulation of pooled-sample analysis strategies for COVID-19 mass testing. Bulletin of the World Health Organization https://www.who.int/bulletin/volumes/98/9/20-257188/en/ (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Litvak E, et al. Screening for the Presence of a Disease by Pooling Sera Samples. J Am Stat Assoc 89, 424–434 (1994). [Google Scholar]
- 106.Livescience. Wuhan tested millions of people for COVID-19 in just days. Could US cities do the same? https://www.livescience.com/pooled-sampling-covid19-in-wuhan-and-us-cities.html (2020).
- 107.Centers for Disease Control and Prevention. Interim Guidance for Use of Pooling Procedures in SARS-CoV-2 Diagnostic, Screening, and Surveillance Testing. https://www.cdc.gov/coronavirus/2019-ncov/lab/pooling-procedures.html (2020).
- 108.Hogan CA, et al. Sample Pooling as a Strategy to Detect Community Transmission of SARS-CoV-2. JAMA 323, 1967–1969 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sahajpal NS, et al. Proposal of RT-PCR-Based Mass Population Screening for Severe Acute Respiratory Syndrome Coronavirus 2 (Coronavirus Disease 2019). J Mol Diagn 22, 1294–1299 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lim KL, et al. A novel strategy for community screening of SARS-CoV-2 (COVID-19): Sample pooling method. PLoS One 15, e0238417 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.The New York Times. Federal Officials Turn to a New Testing Strategy as Infections Surge. https://www.nytimes.com/2020/07/01/health/coronavirus-pooled-testing.html (2020).
- 112.Peddu V, et al. Metagenomic Analysis Reveals Clinical SARS-CoV-2 Infection and Bacterial or Viral Superinfection and Colonization. Clin Chem 66, 966–972 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mora M, et al. Highly matching Coronavirus-like short sequences can be retrieved from environmental metagenomes. Microbiome (2020). [Google Scholar]
- 114.Van Tan L, et al. SARS-CoV-2 and co-infections detection in nasopharyngeal throat swabs of COVID-19 patients by metagenomics. J Infect 81, e175–e177 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moore SC, et al. Amplicon based MinION sequencing of SARS-CoV-2 and metagenomic characterisation of nasopharyngeal swabs from patients with COVID-19. medRxiv (2020). [Google Scholar]
- 116.Xu Y, et al. Current approach in laboratory testing for SARS-CoV-2. Int J Infect Dis 100, 7–9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.D’Cruz RJ, et al. Laboratory Testing Methods for Novel Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2). Front Cell Dev Biol 8, 468 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abduljalil JM. Laboratory diagnosis of SARS-CoV-2: available approaches and limitations. New Microbes New Infect 36, 100713 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zwirglmaier K, et al. Rapid detection of SARS-CoV-2 by pulse-controlled amplification (PCA). medRxiv (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Infantino M, et al. Serological Assays for SARS-CoV-2 Infectious Disease: Benefits, Limitations and Perspectives. Isr Med Assoc J 22, 203–210 (2020). [PubMed] [Google Scholar]


