Abstract
A number of research has shown that the plant polyphenol resveratrol, one of the most prominent small molecules, has beneficial protective effects in multiple organisms, including worms, flies, and killifish. To understand the effects of resveratrol on lifespan, we evaluated its effects in the silkworm Bombyx mori. In this study, we found that lifespan was significantly prolonged in both female and male silkworms treated with resveratrol. Silkworm larval weight was significantly increased from day 3 of the 5th larval instar (L5D3) to day 7 of the 5th larval instar (L5D7). However, the weight of the pupa, cocoon, and total cocoon was not significantly different in female silkworms with resveratrol treatment than that in controls. Meanwhile, resveratrol significantly improved the thermotolerance of the silkworms, which enhanced their survival rate. Moreover, antioxidant activity was increased by resveratrol in both female and male silkworms. Furthermore, an antioxidant-related signalling pathway, SIRT7-FoxO-GST, was activated in silkworms with resveratrol treatment. Collectively, these results help us to understand the molecular pathways underlying resveratrol induced pro-longevity effects and indicate that silkworm is a promising animal model for evaluating the effects of lifespan-extending drugs.
Keywords: Resveratrol, Bombyx mori, Lifespan, Antioxidant activity, SIRT7-FoxO-GST pathway
Graphical abstract
Highlights
-
•
A newly experimental animal, Bombyx mori, was used for the evaluation of the effects of resveratrol.
-
•
Resveratrol extends lifespan of the female and male silkworm.
-
•
The excessive energy absorbed in the larvae stage was applied for the survival consumption to extend lifespan.
-
•
Resveratrol significantly improves the thermotolerance and the antioxidant activity of the silkworm.
-
•
The Sirt7-FoxO-GST signaling pathway may be partially responsible for the effect of resveratrol on longevity.
1. Introduction
Resveratrol (3, 5, 4′-trihydroxystilbene) was first isolated from the roots of the white hellebore (Veratrum grandiflorum O. Loes) in 1940 [1] and then from the roots of Polygonum Cupsidatum in 1963, which were plants used in traditional Chinese medicine [2]. With the discovery of resveratrol, studies have demonstrated its many properties as a geroprotector enhancing lifespan, with neuroprotective effects, attenuating oxidative stress, improving insulin resistance, and ameliorating aging-related metabolic phenotypes in different model organisms [3]. However, resveratrol first aroused interest in 1992 because this compound was postulated to explain some of the cardioprotective effects of red wine [4], and was suggested as an important factor in the “French Paradox”, which was coined to describe the observation that the French population shows a very low incidence of cardiovascular diseases, despite a diet high in saturated fat [5]. In 1997, resveratrol was reported to work as a chemopreventive agent by inhibiting carcinogenesis [6]. The anti-inflammatory and anti-oxidant properties of resveratrol were also identified [3,7]. Currently, the consensus opinion is that resveratrol seems to delay or attenuate many age-related chronic diseases in model organisms [8,9].
Mechanistic studies of resveratrol reached a milestone when ‘silent information regulator 2’ (Sir2) homolog 1 (SIRT1) was identified as a molecular target of resveratrol in 2003 [10]. Subsequently, it was shown to be capable of mimicking the effects of calorie restriction [11,12], thus promoting longevity in yeast [9], worms [13,14], fruit flies (although some controversy remains for this organism) [7,15], and short-lived fish [16]. Furthermore, the lifespan extension effects of resveratrol are conserved in the honey bee and may be driven by sirtuin activation related to calorie restriction [17]. Currently, the mechanism by which resveratrol exerts its widely beneficial effects on lifespan among species and animal models is not well understood [7]. Resveratrol may contribute to an overall reduction in oxidative stress by possessing an intrinsic anti-oxidant capacity based on the function of most other polyphenols, leading to the induction of the expression of antioxidant enzymes [14]. In this process, receptors, kinases, and enzymes may be recruited and activated, thereby extending the lifespan.
Sirtuins belong to the NAD+-dependent class III histone deacetylase family, which is a conserved family named uniformly after the first member, Sir 2 identified in Saccharomyces cerevisiae [18]. Furthermore, several reports have demonstrated that activation of sirtuins seems to be a major target of resveratrol, which provides a mechanistic explanation for the vast majority of the geroprotective benefits observed in published reports.
Sericulture has a long and colorful history of five thousand years with breeding technology becoming mature [19]. The complete sequence of the silkworm genome has provided a molecular and genetic foundation for silkworm research. Bombyx mori is a low-cost experimental animal, with low physical activity, and thus, is convenient for control and follow-up experiments. Moreover, the silkworm has an appropriate body size and weight, reducing errors in the measurement of relevant physiological factors, ensuring the accuracy of the experimental results, and strengthening the results. Previous studies have shown that the silkworm could be an appropriate animal model to evaluate the beneficial and side effects of drug efficacy, especially for traditional Chinese medicine [[20], [21], [22]]. It is worth mentioning that it owns a tremendous potential in lifespan elongation research with a survival malleability based on short life history [23]. Combined with these predominant inherent traits, such as high fecundity and easy standardization of incubation time, silkworm is endowed a potential ability and then provides a preponderant foundation for effect evaluation of the geroprotective drug.
Previously, we have shown that the antioxidative enzyme system could be increased by Rhodiola rosea [20], metformin [21], and astragalus polysaccharide [22]. However, the role of resveratrol in providing an antioxidant pharmacological effect to scavenge free radicals, regulating resistance to oxidative damage, and increasing longevity in silkworm remains unclear. Thus, in this study, we focused on the regulation of the antioxidative pathway and the relationship between sirtuins and antioxidant activity inducing signalling pathways that explain the effect of resveratrol on longevity.
2. Materials and methods
2.1. Silkworm strain and grouping information
The silkworm Dazao is a wildtype strain obtained from the Silkworm Gene Bank at Southwest University (Chongqing, China). For their entire life cycle, silkworms were maintained at (25 ± 0.5) °C with a relative humidity of approximately 75%–50% with circadian rhythms of 12 h light/12 h dark each day. The silkworms were reared ad libitum with fresh mulberry leaves throughout the larval stage [24]. Silkworm experimental material was divided into seven groups, namely, (a) lifespan detection group (female experimental group/control: n=53/49, male experimental group/control: n=64/43); (b) body weight (female treatment group/control: n=60/63, male treatment group/control: n=54/53); (c) cocoon weight group (treatment group/control: n=60/60); (d) fecundity group (treatment group/control: n=6/6); (e) thermotolerance group (treatment group/control: n=15/15); (f) fasting group (treatment group/control: n=15/15); and (g) antioxidants and gene expression group (n=9). This study conformed to the statement of Southwest University on the Welfare of Animals.
2.2. Resveratrol treatment
Commercial preparations of resveratrol were acquired from the Sigma-Aldrich (St. Louis, MO, USA). A 500 μM solution of resveratrol was prepared in absolute ethyl alcohol. Thereafter, the solution was sterilized by filtration through 0.22 μm membranes and stored at 4 °C. The silkworm of each treatment group was fed fresh mulberry leaves with 3 μL prepared solution in the 3rd larval instar, 4 μL in the 4th larval instar, and 5 μL in the 5th larval instar. The control group was fed absolute ethyl alcohol in an equal volume of the same larval instar in the treatment group.
2.3. Lifespan assay
Survival tests were performed in an incubator with an ideal silkworm growth environment over the entire life cycle, as described previously [20]. The upper 10% of the lifespan distribution was defined as the maximum lifespan [21]. The survival conditions of the silkworm specimens were checked by factitious identification, and the time of death was recorded every 3 h.
2.4. Measurement of body weight and fecundity
Treatment and control silkworms were chosen randomly and divided into three groups to measure daily body weights at the larval and pupal stages. The body weights of silkworms in each group were monitored at a specific time before rearing each day [20]. The number of progeny per female adult silkworm was counted to assess the fecundity.
2.5. Thermo- and fasting-tolerance assays
To induce stress, silkworm specimens were either placed at 37 °C in an incubator or fasted at the 5th larval instar. Survival was assessed by confirming touch-provoked movement during the environmental stress period, and the death time was recorded.
2.6. Measurement of antioxidants
The activity of glutathione S-transferase (GST) and the content of glutathione (GSH) were assayed using a kit from Suzhou Comin Biotechnology (Suzhou, China). Additionally, the homogenate content from an entire silkworm was assayed according to the kit instructions.
2.7. Reverse transcription-quantitative PCR (RT-qPCR)
Silkworm specimens were obtained from day 3 of the 4th larval instar (L4D3), L5D3, day 4 of the pupal stage (P4), day 7 of the pupal stage (P7), day 1 of the adult stage (M1), day 4 of the adult stage (M4), and day 7 of adult stage (M7) in the treatment and control groups. Total RNA was isolated from three individuals using a total RNA rapid extraction kit (BioTeke Corporation, Beijing) according to the manufacturer's instructions. RT-qPCR was performed on a CFX96 Touch Real-Time PCR Detection System (Bio-RAD, USA). Eukaryotic translation initiation factor 4A (BmMDB probe ID: sw22934), an optimally stable gene, was employed as a reference gene in silkworm [25]. The qPCR conditions were performed according to the manufacturer's instructions. Primer pairs and their targeted genes are shown in Table 1, and the relative expression levels of each gene were normalized to sw22934 and calculated as 2–△△CT [25].
Table 1.
The primer sequences used in this study for quantitative real-time PCR.
| Gene name | F primer (5′ - 3′) | R primer (5′ - 3′) |
|---|---|---|
| sw22934 | TTCGTACTGCTCTTCTCG | CAAAGTTGATAGCAATTCCCT |
| BmAMPK | GCACCCCTGTCAAACGAGC | CGTTCGCCCGACAAAGACT |
| BmAKT | GCTGCTAGACAAAGACGGACAC | ATGATGAGCCCGAACAGCAC |
| BmTOR | GCCACCTCCAAGCTACCCTAATAATGTT | AATCTATCCTTGCTTGTGTCGTGTTTC |
| BmFoxO | GCACAGGACAACAGGCTCACAC | GCTTGGCGTCGGGATTGA |
| BmSIRT2 | TGGTCCCAGATTCGTGTCCT | TGTTCACGGGCTACTAATGCTC |
| BmSIRT4 | AGTTCAATGTCTCAAATGCCCC | CCCTTCACACTTTGGGCAGA |
| BmSIRT5 | GCAAATAGAGGAGCCCCAGA | AAACTTTCACCGAACCACACAA |
| BmSIRT6 | TGAGAAAGTAATGGACATCTTAGGAAT | TCGCCAGTCTTTAGTTTCATTCTC |
| BmSIRT7 | CGCTAAGCATCTTGTTGTCTAC | GTTCTGTGACACGACGAATTTG |
2.8. Statistical analysis
Statistical analyses were performed using Excel (Microsoft, WA, USA) and GraphPad Prism 6 (GraphPad Software, CA, USA). The average lifespan and maximum lifespan in independent experiments were calculated and presented as the mean±SEM. The significance of the differences was analyzed using a two-tailed Student's t-test and two-way ANOVA. Assessment of the significance of the differences in the survival curves was performed using the log-rank test (Mantel-Cox) (PRISM software package, GraphPad software). Values of P < 0.05 were considered statistically significant.
3. Results
3.1. Resveratrol extends the lifespan of silkworm
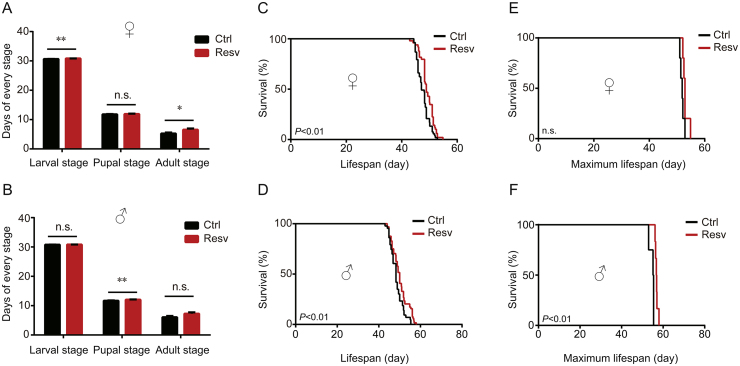
Contradictory results have been reported on the effects of resveratrol in lifespan extension, indicating that the prolongevity effects of resveratrol vary depending on the model organism [26]. To further determine the effect of resveratrol on lifespan, silkworms were fed fresh mulberry leaves with or without resveratrol. Our results showed that silkworms treated with resveratrol lived longer than those in the control group. In females, resveratrol had no significant effect on the length of the pupal stage and the maximum lifespan, while it had significant effects on extending the larval and adult stages (Figs. 1A, C and E). The length of pupal stage, the mean lifespan, and the maximum lifespan were significantly elongated, while resveratrol had no significant effect on the length of the larval and adult stages in male treated silkworms compared to controls (Figs. 1B, D and F). The mean lifespan and maximum lifespan were elongated by 1.52 days (3.18%) and 1.20 days (2.31%) in females (Figs. 1C and E) and 1.57 days (3.24%) and 2.13 days (3.89%) in males (Figs. 1D and F), as compared to controls, respectively. In short, resveratrol prolonged the female and male lifespans and the male maximum lifespan to varying degrees in the silkworms.
Fig. 1.
The effects of resveratrol on lifespan of the silkworms. (A) The lengths in days of the larval, pupal, and adult stages in treated unmated female silkworms (n=53) and controls (n=49); (B) the length in days of the larval, pupal, and adult stages of treated unmated male silkworms (n=64) and controls (n=43); (C) the lifespan of treated unmated female silkworms (n=53) and controls (n=49); (D) the lifespan of treated unmated male silkworms (n=64) and controls (n=43); (E) the maximum lifespan of treated unmated female silkworms (n=5) and controls (n=5); and (F) the maximum lifespan of treated unmated male silkworms (n=6) and controls (n=4). Error bars depict the mean±SEM. The n.s. is the abbreviation for “no signification”, ∗P < 0.05, ∗∗P < 0.01.
3.2. Resveratrol induced lifespan extension without obvious side effects in silkworms
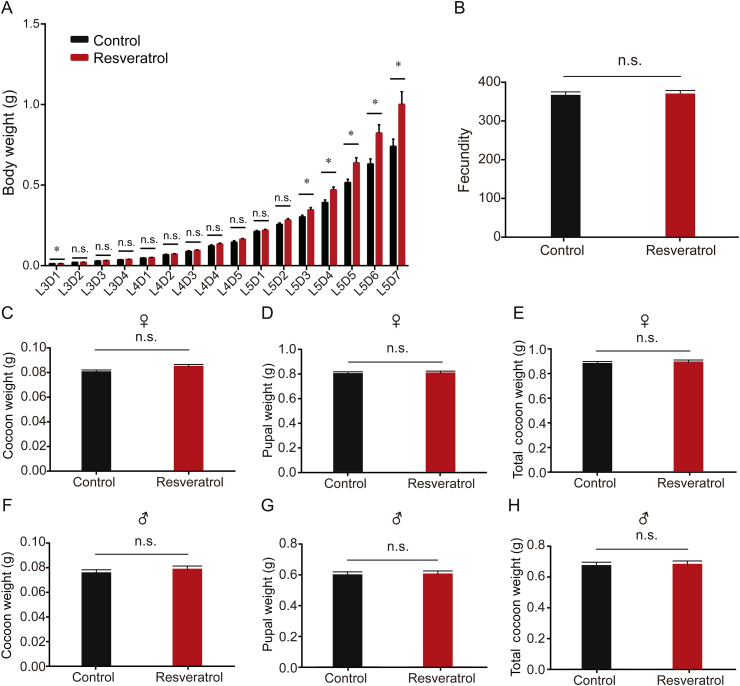
To determine whether the growth and development of larval and adult silkworms were influenced by resveratrol, we measured the larval and pupal body weight, raw silk output, and fecundity after moth eclosion in comparison with silkworms in the treatment and control groups. The results showed that the resveratrol-treated groups had a significant increase in larval body weight compared with the control group from day 3 of the 5th larval instar to day 7 of the 5th larval instar (Fig. 2A). Fecundity, lifespan, and silk output are three predominant energy-output phases in the life cycle of the silkworm. Our results showed that the cocoon weight, pupal weight, and fecundity of moths were not significantly different in the resveratrol treatment group compared with controls in female and male individuals (Figs. 2B–H). The supplementation of resveratrol did not affect growth and development in the silkworm larval stage, metamorphosis in the pupal stage, and eclosion and reproduction in the adult stage.
Fig. 2.
Effects of resveratrol on body weight, fecundity, cocoon weight, pupal weight, and total cocoon weight. (A) Body weights measured daily from L3D1 to L5D7 (n=50). (B) The fecundity of silk moth in the treatment and control groups (n=6). (C-E) Female cocoon weight, pupal weight, and total cocoon weight (n=60). (F-H) Male cocoon weight, pupal weight, and total cocoon weight (n=53). Error bars and symbols depict the mean±SEM. The n.s. is the abbreviation for “no signification”, ∗P < 0.05.
3.3. Resveratrol protects the silkworm from environmental stress
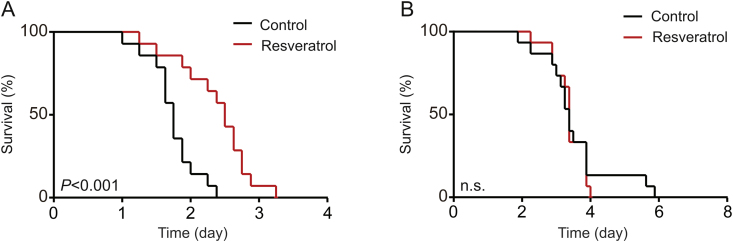
For many organisms, amplified stress resistance is involved with lifespan elongation [20,27]. To determine whether resveratrol has the protective effects on nutritional and thermal stress, resveratrol-treated and control silkworms were bred separately under fasting and thermal stresses. The thermotolerance of silkworms was significantly improved by resveratrol, which played an active role in improving the survival rate (Fig. 3A). Meanwhile, there was no significant difference in the survival rate of silkworms that were subjected to fasting stress (Fig. 3B).
Fig. 3.
Resveratrol increases thermotolerance in the silkworm. Survival curves in treatment and control silkworms exposed to (A) a heated environment (37 °C) and (B) fasting. Error bars depict the mean±SEM, n=15. The n.s. is the abbreviation for “no signification”, ∗∗∗P < 0.001.
3.4. Resveratrol improves the antioxidative properties of silkworm
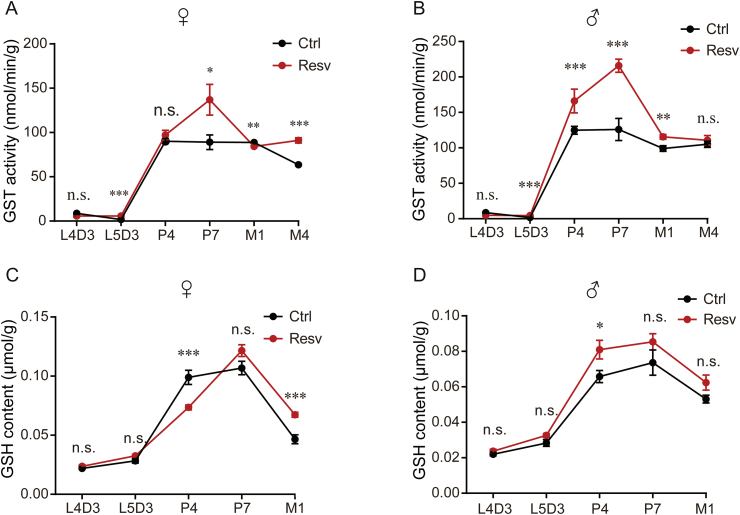
Previous studies have suggested that resveratrol has the ability to eliminate free radicals and suppress cellular oxidative stress [28]. Herein, we hypothesized that resveratrol enhances antioxidative properties. The activity of GST and the content of GSH are generally approbatory as principal indicators of antioxidative capacity. Therefore, we assessed the antioxidant activity of resveratrol in silkworms by detecting GSH content and GST activities. The results showed that the activity of GST in resveratrol-treated silkworms was significantly higher than that in the control silkworms overall (Figs. 4A and B). However, the GST activity was lower in treated female silkworms at M1, while no significant differences were found at L4D3 and P4 in treated female silkworms, and at L4D3 and M4 in treated male silkworms than in controls (Figs. 4A and B). The content of GSH was significantly higher in treated silkworms at M1 in females and at P4 in males than in the control group (Figs. 4C and D). In sum, we found that resveratrol improved the GST activity and GSH content to increase the antioxidant capacity of silkworms.
Fig. 4.
Resveratrol increases glutathione S-transferase (GST) activity in silkworms. (A-B) The GST and (C-D) the content of glutathione (GSH) measured at the indicated developmental stages in treated silkworms and controls. Error bars depict the mean±SEM, n=9. The n.s. is the abbreviation for “no signification”, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
3.5. Prolongevity effect of resveratrol is linked to sirtuin signalling
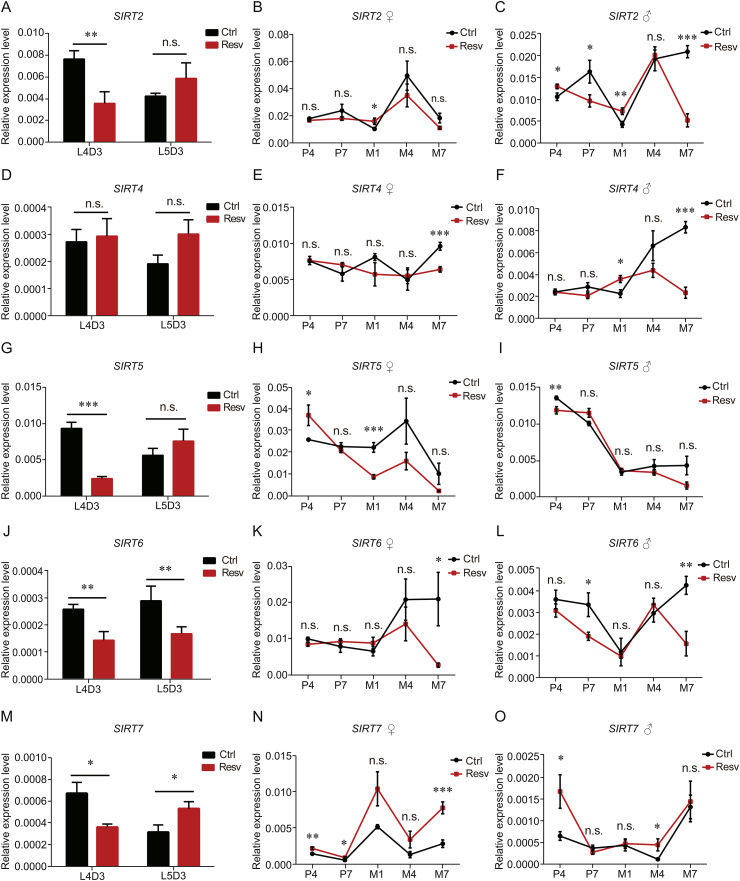
The relative expression level of SIRT2 in treated silkworms was significantly higher than that in the control group at M1 in females and at P4 and M1 in males, while the expression of SIRT2 was significantly lower at L4D3, and at P7, and M7 in male silkworms (Figs. 5A–C). The expression level of SIRT4 was significantly lower in treated silkworms than in the control group at M7 in both sexes, and was significantly higher at M1 in males (Figs. 5D–F). The relative expression level of SIRT5 in treated silkworms was significantly lower than that in the control group at L4D3, and M1 in females and at P4 in males, while the expression was significantly higher at P4 in females (Figs. 5G–I). The relative expression level of SIRT6 in treated silkworms was significantly lower than that in the control group at L4D3 and L5D3, at M7 in females, and at P7 and M7 in males (Figs. 5J–L). The relative expression level of SIRT7 in the treated silkworms was significantly lower at L4D3 and significantly higher at L5D3 than that in controls (Fig. 5M). The relative expression level of SIRT7 in treated silkworms was significantly higher than that in the control group at P4, P7, and M7 in females, and at P4 and M4 in males (Figs. 5N and O). Overall, SIRT7 was activated by resveratrol in silkworms.
Fig. 5.
Effects of resveratrol on the expression of SIRT2, SIRT4, SIRT5, SIRT6, and SIRT7. The expression levels of (A-C) SIRT2, (D-F) SIRT4, (G-I) SIRT5, (J-L) SIRT6, and (M-O) SIRT7 at the indicated developmental stages in treated and control silkworms determined using real-time PCR. Error bars depict the mean±SEM, n=9. The n.s. is the abbreviation for “no signification”, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
3.6. Lifespan extension via activation of the SIRT7-FoxO-GST pathway
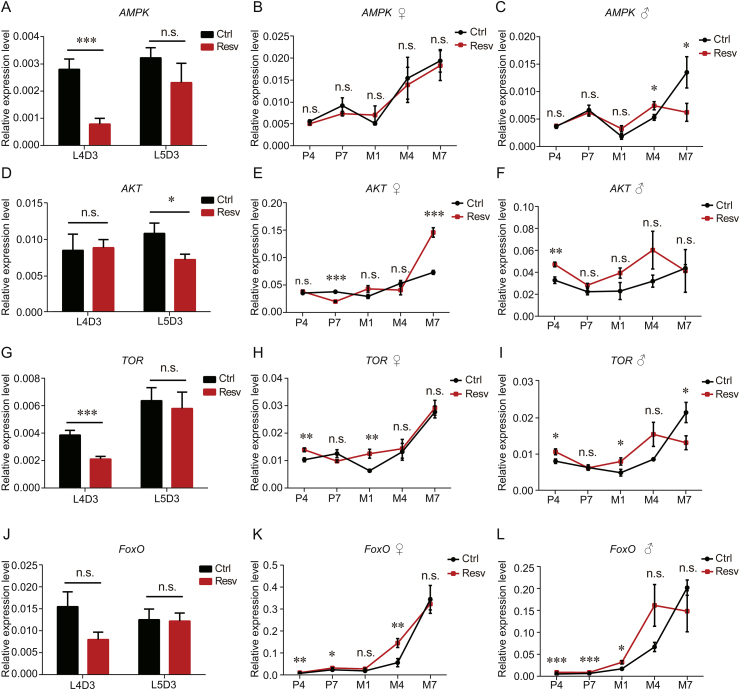
FoxO is a primary anti-stress regulator in cells and individuals [29]. Herein, to detect whether FoxO and its regulators participate in the process of lifespan elongation in silkworms and investigate the signalling network mediating the resveratrol-inducing lifespan extension, we detected the expression levels of AMPK, AKT, TOR, and FoxO in the resveratrol-treated silkworms and control groups.
The expression level of AMPK was significantly lower in resveratrol-treated silkworms than that in the control group at L4D3 (Fig. 6A). There was no significant difference between treated and control female silkworms in the pupal and adult stages (Fig. 6B). The expression level of AMPK was significantly higher in resveratrol-treated male silkworms than that in the control group at M4, while AMPK was significantly lower at M7 (Fig. 6C). The AKT expression level of the resveratrol-treated silkworm was significantly lower than that of the control group at L5D3 (Fig. 6D). The expression level of AKT was significantly lower in resveratrol-treated female silkworms than that of the control group at P7, but we observed the opposite pattern at M7 (Fig. 6E). The expression level of AKT in the resveratrol-treated male silkworms was significantly higher than that of the control group at P4 (Fig. 6F). The expression level of TOR was significantly lower in resveratrol-treated silkworms than that of the control group at L4D3 (Fig. 6G). The expression level of TOR in the resveratrol-treated silkworms was significantly higher than that in the control group at P4 and M1 in both sexes; however, the expression level of TOR was significantly lower in resveratrol-treated silkworms than that in the control group at M7 in male (Figs. 6H and I). The expression level of FoxO in the resveratrol-treated silkworms was significantly higher than that in the control group at P4, P7, and M4 in females and at P4, P7, and M1 in males. No significant difference was found between treated and control silkworms in other stages (Figs. 6J–L). The results showed that SIRT7-FoxO-GST pathway was activated by resveratrol in silkworms.
Fig. 6.
Effects of resveratrol on the expression of AMPK, AKT, TOR, and FoxO. Expression levels of (A-C) AMPK, (D-F) AKT, (G-I) TOR, and (J-L) FoxO at the indicated developmental stages in treated and control silkworms determined using real-time PCR. Error bars depict the mean±SEM, n=9. The n.s. is the abbreviation for “no signification”, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
4. Discussion
4.1. Additional stored energy may be transmitted to survival maintenance
Energy consumption is an important determinant of an organism's lifespan. Evidence suggests that remodelling of an organism's energy use strategy is often sufficient to extend the lifespan [21,30,31]. In this study, we found that resveratrol elongated the lifespan while the body weight in silkworms was increased from L5D3 to L5D7, which is the critical period for energy accumulation. Moreover, there were no significant differences in fecundity or silk output in silkworms with or without resveratrol treatment, suggesting that the energy consumption was used in two ways, but not for reproduction or secreting silk. This indicates that resveratrol treated silkworms absorbed excessive energy and stored it; however, resveratrol treatment had no clear impact on hunger tolerance. Collectively, our results suggest that excess energy is utilized for the extended survival in as-yet known ways, with the resveratrol treatment.
4.2. Potential signalling process linking resveratrol to longevity
Our results indicate that resveratrol-induced lifespan extension occurred in the larval and adult stages in the female silkworms, and mainly in the pupal stage in the male silkworms. Many studies have reported that sirtuin family members (SIRT1-SIRT7) are the direct targets of resveratrol [15]. In this study, the expression of SIRT7 increased induced by resveratrol at the larval, pupal, and adult stages of the treated female and male silkworms. A previous study showed that the prolongevity factor FoxO could be regulated by sirtuin family members [14]. In the meantime, we found that the FoxO expression level was significantly increased at the pupal and adult stages in both the treated female and male groups. The expression increases of the two genes were similar, so we speculated that the prolongevity signal transmitted from SIRT7 to FoxO in silkworms. Based on these observations, FoxO may be involved in the regulatory process in which resveratrol activates SIRT7 and plays an active role in regulating longevity.
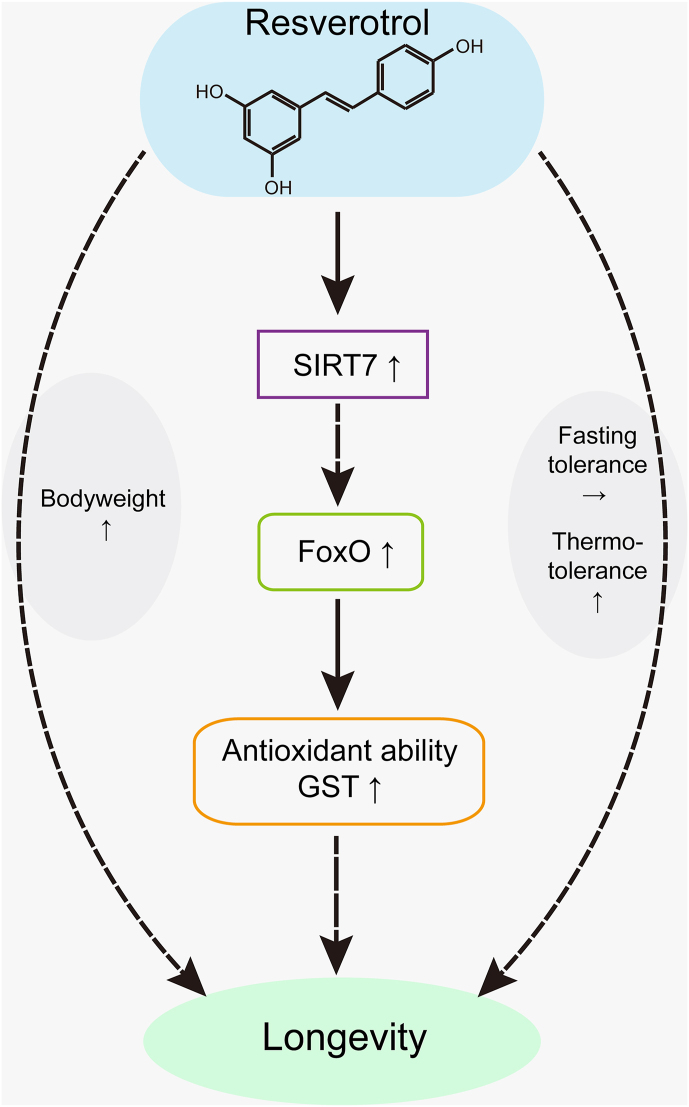
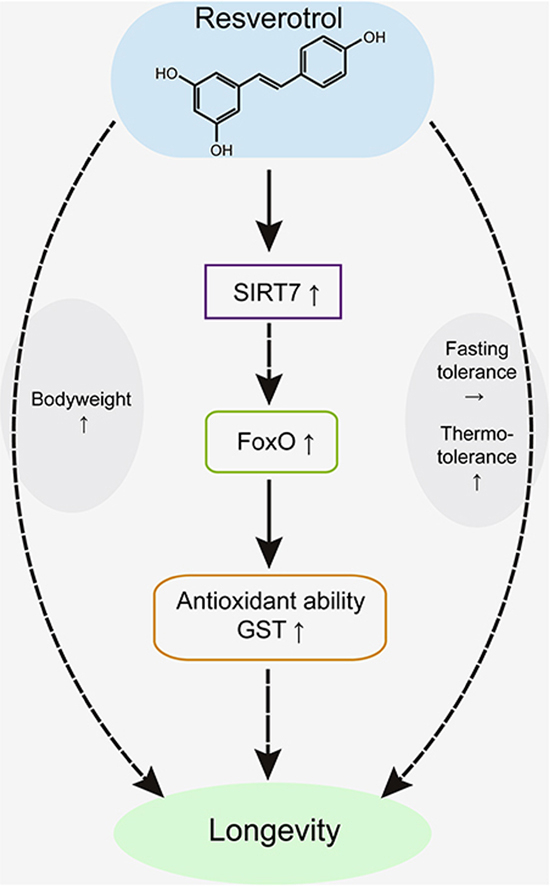
Oxidation is the biggest risk factor for aging and short lifespan [32]. The anti-oxidation enzyme system is an important response to resist oxidative stress and is of vital importance to survival. We detected the activity of members of the anti-oxidation enzyme system and found that the activity of GST, a core member of the antioxidant system, was significantly improved in resveratrol-treated silkworms compared with that in the control group. Furthermore, SIRT7 and FoxO have been reported to be involved in modulating the antioxidation process. The GST activity of the treated silkworms significantly increased from L4D3 to M4. The same trend was seen for FoxO, as GST is known to be a direct target gene of FoxO [33,34]. Thus, we speculated that antioxidant activity could enhance longevity signalling which may explain the effect of resveratrol on longevity. In other words, resveratrol extended the lifespan by activating its direct target, SIRT7, while SIRT7 promoted GST activity by regulating FoxO (Fig. 7). This pathway is at least partially responsible for the effect of resveratrol on longevity.
Fig. 7.
A model illustrating how resveratrol extends the lifespan and improves antioxidative activity by activating the SIRT7-FoxO-GST pathway.
5. Conclusions
In this study, we found that resveratrol replenishment could extend silkworm lifespan and increase silkworm larval weight significantly without affecting the weight of the pupa, cocoon, and total cocoon in silkworms. Meanwhile, thermotolerance and antioxidant activity were improved significantly. Furthermore, the SIRT7-FoxO-GST pathway was activated in the silkworms with resveratrol supplement. These results showed the underlying reason why resveratrol induced lifespan-extension effect and also indicated the advantage of the silkworm as a promising animal model for evaluating the effects of lifespan-extending drugs.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
We thank Jianfei Zhang and Zheng Li for providing assistance with the figures. This work was supported by the National Natural Science Foundation of China (Grant Nos. 31830094 and 31902215), the Hi-Tech Research and Development 863 Program of China (Grant No. 2013AA102507), the Fundamental Research Funds for the Central Universities in China (No. XDJK2019C014), Project funded by Chongqing Special Postdoctoral Science Foundation (Grant No. XmT2018058), and Funds of China Agricultural Research System (No. CARS-18-ZJ0102).
Footnotes
Peer review under responsibility of Xi’an Jiaotong University.
References
- 1.Takaoka M. The phenolic substances of white hellebore (veratrum grandiflorum hoes. Fil.) I. Nippon Kagaku Kaishi. 1939;3:1090–1100. [Google Scholar]
- 2.Nonomura S., Kanagawa H., Makimoto A. Chemical constituents of polygonaceous plants. I. Studies on the components of ko-jo-kon. Yakugaku Zasshi. 1963;83:988–990. [PubMed] [Google Scholar]
- 3.Hubbard B.P., Sinclair D.A. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol. Sci. 2014;35:146–154. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siemann E.H., Creasy L.L. Concentration of the phytoalexin resveratrol in wine. Am. J. Enoi. Vitic. 1992;43:49–52. [Google Scholar]
- 5.Liu B.L., Zhang X., Zhang W. New enlightenment of French Paradox: resveratrol's potential for cancer chemoprevention and anti-cancer therapy. Canc. Biol. Ther. 2007;6:1833–1836. doi: 10.4161/cbt.6.12.5161. [DOI] [PubMed] [Google Scholar]
- 6.Jang M., Cai L., Udeani G.O. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 7.Bass T.M., Weinkove D., Houthoofd K. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech. Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Carles C., Johan A. Targeting sirtuin 1 to improve metabolism: all you need is NAD (+)? Pharmacol. Rev. 2012;64:166–187. doi: 10.1124/pr.110.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pallauf K., Rimbacha G., Ruppa P.M. Resveratrol and lifespan in model organisms. Curr. Med. Chem. 2016;23:4639–4680. doi: 10.2174/0929867323666161024151233. [DOI] [PubMed] [Google Scholar]
- 10.Howitz K.T., Bitterman K.J., Cohen H.Y. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 11.Baur J.A., Pearson K.J., Price N.L. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marie L., Carmen A., Zachary G.H. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Tissenbaum H.A., Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 14.Kenyon C.J. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal B., Baur J.A. Resveratrol and life extension. Ann. NY. Acad. Sci. 2011;1215:138–143. doi: 10.1111/j.1749-6632.2010.05850.x. [DOI] [PubMed] [Google Scholar]
- 16.Valenzano D.R., Terzibasi E., Genade T. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 17.Rascón B., Hubbard B.P., Sinclair D.A. The lifespan extension effects of resveratrol are conserved in the honey bee and may be driven by a mechanism related to caloric restriction. Aging. 2012;4:499–508. doi: 10.18632/aging.100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brachmann C.B., Sherman J.M., Devine S.E. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 1995;9:2888–2902. doi: 10.1101/gad.9.23.2888. [DOI] [PubMed] [Google Scholar]
- 19.Cherry R.H. History of sericulture, B. Entomol. Soc. Am. 1987;33:83–85. [Google Scholar]
- 20.Chen C., Song J.B., Chen M. Rhodiola rosea extends lifespan and improves stress tolerance in silkworm. Bombyx Mori, Biogerontol. 2016;17:373–381. doi: 10.1007/s10522-015-9622-8. [DOI] [PubMed] [Google Scholar]
- 21.Song J.B., Jiang G.H., Zhang J.F. Metformin prolongs lifespan through remodeling the energy distribution strategy in silkworm, Bombyx mori. Aging. 2019;11:240–248. doi: 10.18632/aging.101746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song J.B., Chen M., Li Z.Q. Astragalus polysaccharide extends lifespan via mitigating endoplasmic reticulum stress in the silkworm, Bombyx mori. Aging Dis. 2019;10:1187–1198. doi: 10.14336/AD.2019.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song J.B., Zhang J.F., Dai F.Y. Advantages and limitations of silkworm as an invertebrate model in aging and lifespan research. Open Access J. Gerontol. Geriatr. Med. 2018;4 [Google Scholar]
- 24.Song J.B., Tang D.M., Li Z.Q. Variation of lifespan in multiple strains, and effects of dietary restriction and BmFoxO on lifespan in silkworm. Bombyx Mori, Oncotarget. 2016;8:7294–7300. doi: 10.18632/oncotarget.14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang G.H., Xia Q.Y., Cheng D.J. Reference genes identified in the silkworm Bombyx mori during metamorphism based on oligonucleotide microarray and confirmed by qRT-PCR. Insect Sci. 2008;15:405–413. [Google Scholar]
- 26.Gonzalez-Freire M., Diaz-Ruiz A., Hauser D. The road ahead for health and lifespan interventions. Ageing Res. Rev. 2020;59 doi: 10.1016/j.arr.2020.101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Postnikoff S.D.L., Malo M.E., Berchman W. The yeast forkhead transcription factors fkh1 and fkh2 regulate lifespan and stress response together with the anaphase-promoting complex. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukuhara K., Nakanishi I.A., Matsumura T. Effect of methyl substitution on the antioxidative property and genotoxicity of resveratrol. Chem. Res. Toxicol. 2008;21:282–287. doi: 10.1021/tx7003008. [DOI] [PubMed] [Google Scholar]
- 29.Vilchez D., Morantte I., Liu Z. RPN-6 determines C. elegans longevity under proteotoxic stress conditions. Nature. 2012;489:263–268. doi: 10.1038/nature11315. [DOI] [PubMed] [Google Scholar]
- 30.Solon-Biet S.M., Walters K.A., Simanainen U.K. Macronutrient balance, reproductive function, and lifespan in aging mice. Proc. Natl. Acad. Sci. U.S.A. 2015;112:3481–3486. doi: 10.1073/pnas.1422041112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jasienska G. Reproduction and lifespan: trade-offs, overall energy budgets, intergenerational costs, and costs neglected by research. Am. J. Hum. Biol. 2010;21:524–532. doi: 10.1002/ajhb.20931. [DOI] [PubMed] [Google Scholar]
- 32.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 33.Tullet J.M.A. DAF-16 target identification in C. elegans: past, present and future. Biogerontology. 2015;16:221–234. doi: 10.1007/s10522-014-9527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy C.T., Mccarroll S.A., Bargmann C.I. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]