Abstract
Aims/Introduction
To explore relationships between polyunsaturated fatty acids (PUFA) and non‐alcoholic fatty liver disease (NAFLD) in patients with type 2 diabetes, and whether insulin action has an interactive effect with PUFA on NAFLD progression.
Materials and Methods
We extracted clinical and omics data of 482 type 2 diabetes patients from a tertiary hospital consecutively from April 2018 to April 2019. NAFLD was estimated by ultrasound at admission. Plasma fasting n3 and n6 fatty acids were quantified by liquid chromatography–tandem mass spectrometry analysis. Restricted cubic spline nested in binary logistic regression was used to select the cut‐off point, and estimate odds ratios and 95% confidence intervals. Additive interactions of the n6 : n3 ratio with insulin action for NAFLD were estimated using relative excess risk due to interaction, attributable proportion due to interaction and synergy index. Relative excess risk due to interaction >0, attributable proportion due to interaction >0 or synergy index >1 indicates biological interaction. Spearman correlation analysis was used to obtain partial correlation coefficients between PUFA and hallmarks of NAFLD.
Results
Of 482 patients, 313 were with and 169 were without NAFLD. N3 ≥800 and n6 PUFA ≥8,100 μmol/L were independently associated with increased NAFLD risk; n6 : n3 ratio ≤10 was associated with NAFLD (odds ratio 1.80, 95% confidence interval 1.20–2.71), and the effect size was amplified by high C‐peptide (odds ratio 8.89, 95% confidence interval 4.48–17.7) with significant interaction. The additive interaction of the n6 : n3 ratio and fasting insulin was not significant.
Conclusion
Decreased n6 : n3 ratio was associated with increased NAFLD risk in type 2 diabetes patients, and the effect was only significant and amplified when there was the co‐presence of high C‐peptide.
Keywords: Non‐alcoholic fatty liver disease, Polyunsaturated fatty acids, Type 2 diabetes
Our study explored relationships between polyunsaturated fatty acids and non‐alcoholic fatty liver disease in patients with type 2 diabetes. We found that decreased plasma n6 : n3 polyunsaturated fatty acids ratio interacting with high C‐peptide promotes non‐alcoholic fatty liver disease in type 2 diabetes patients.

Introduction
Non‐alcoholic fatty liver disease (NAFLD) is characterized by abnormal lipid accumulation, and can lead to a series of liver‐related complications that affect life expectancy and quality 1 . Type 2 diabetes patients are at higher risk for NAFLD regardless of detection method, and >50% of them have NAFLD, as assessed by ultrasonography and proton magnetic resonance spectroscopy 2 . Notably, NAFLD and type 2 diabetes have bidirectional impacts on prognosis 3 . Patients with both type 2 diabetes and NAFLD have a higher risk of liver‐specific complications, diabetes complications and mortality when compared with patients with either type 2 diabetes or NAFLD 4 . Considering these, the management of NAFLD in type 2 diabetes is of great clinical significance. Research into NAFLD in type 2 diabetes patients is urgently required 5 .
Recently, research has shown that NAFLD is associated with a shift of lipid types and abundance, mostly with an increase in saturated fatty acids and monounsaturated fatty acids, and a decrease in polyunsaturated fatty acids (PUFAs) 6 , 7 . PUFAs include n3 fatty acids and n6 fatty acids. A decline in PUFA, especially n3 fatty acids, is frequently found in patients with cardiovascular diseases or NAFLD, leading to an increase in the n6 : n3 fatty acids ratio 8 . According to mechanism studies, n6 and n3 fatty acids are suggested to have some opposing impacts on cardiometabolic risk, with n3 fatty acids providing a protective effect, and n6 fatty acids having a harmful effect on health 9 . In this connection, plenty of randomized controlled trials found that supplement of n3 fatty acids in the form of diet or drugs can improve liver steatosis or liver function of patients with NAFLD 10 . Patients with diabetes are at high risk for NAFLD. Paradoxically, almost all these optimal findings were derived from patients without diabetes. Only one published study investigated the intervention in the setting of type 2 diabetes and reported an inconsistent finding. That randomized controlled trial found that the effect of PUFAs was inferior to a placebo in NAFLD patients with diabetes 11 . The role of PUFAs and their balance in the etiology and progression of hepatic steatosis in diabetes remain unclear.
Furthermore, impaired insulin sensitivity and subsequent compensatory insulin section were central components of type 2 diabetes, both of which might influence lipid ectopic deposition and promote hepatic steatosis 3 , 12 . In the meantime, fatty acids can induce abnormal insulin action through several pathways including oxidative stress, inflammation and so on 13 . Whether fatty acids and insulin action have an additive effect on NAFLD development in the context of diabetes is unknown.
Collectively, in the present hospital‐based cross‐sectional study, we aimed to investigate: (i) associations between PUFAs, especially their balance, that is, the n6 : n3 ratio, with NAFLD in type 2 diabetes patients; and (ii) the potential biological interaction of fatty acids and insulin action on the pathogenesis of NAFLD in type 2 diabetes patients.
Methods
Study cohort
From April 2018 to April 2019, a total of 1,024 consecutive patients with type 2 diabetes were admitted into the Second Affiliated Hospital of Dalian Medical University (SAHDMU), Dalian, China, and agreed to participate in this research. Type 2 diabetes was diagnosed by the 1999 World Health Organization’s criteria 14 or use of antidiabetic drugs; of them, 118 were excluded for secondary hepatic fat accumulation; 381 were further excluded for not having their plasma PUFAs measured on a voluntary basis at the patient’s own expense; 43 were further excluded for not having an abdominal ultrasound scan. B‐mode ultrasound was routinely recommended to inpatients with type 2 diabetes who could achieve it within 2 days from admission, except for patients who were not willing to pay for the fee (clinical and biochemical characteristics of participants according to conduction of B‐mode ultrasound are described in Supporting Information). Finally, 482 patients were included in our primary analysis. The Ethics Committee for Clinical Research of SAHDMU approved the ethics of the study, and all the participants provided informed written consent.
Data collection and definitions
NAFLD was defined as having hepatic steatosis and no evidence for secondary hepatic fat accumulation. Secondary hepatic fat accumulation included significant alcohol consumption (ongoing alcohol consumption >21 drinks [~10 g of alcohol per one drink unit] on average per week for men, and >14 drinks on average per week for women), use of steatogenic medication or hereditary disorders. Hepatic steatosis was estimated by B‐mode ultrasound according to liver echotexture: (i) mild steatosis, lightly thickened echo in the first half of the liver section; slight reduced echo in the second half of the liver; intrahepatic vessels absent; (ii) moderate steatosis, moderate thickened echo in the first half of the liver section; moderate reduced echo in the second half of the liver; intrahepatic vessels partial dimming; and (iii) severe steatosis, diffuse increase of echo in the first half of the liver section; heavily reduced echo in the second half of the liver; invisible intrahepatic vessels 15 . Liver stiffness measurement by vibration‐controlled transient elastography was carried out for quantification of hepatic fibrosis. All inpatients were advised to have their fibrosis score measured if they were able to make an appointment during their hospitalization and were willing to pay the fee.
We also collected some essential and available clinical information. Age, sex, waist circumference, weight, height, duration of diabetes, systolic blood pressure, high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), glycated hemoglobin (HbA1c), aspartate aminotransferase (AST), alanine aminotransferase (ALT), fasting blood glucose, fasting insulin, C‐peptide, diabetes medications, lipid‐lowering drugs and diabetes complications were extracted based on an electronic medical system; AST ≥40 U/L and ALT ≥40 U/L were defined as abnormal liver enzymes; body mass index (BMI) was calculated by dividing weight in kilograms by squared height in meters.
Measurements of serum n3 and n6 PUFA
For patients included in the present study, fasting blood samples at admission were drawn and stored at −80°C for the following lipid profiles analysis.
Reagents: Water, acetonitrile and isopropyl alcohol were obtained from Fisher Scientific (Pittsburgh, PA, USA). Formic acid (>98%) and ammonium acetate (>99%) were obtained from Fluka (Buchs, Switzerland). Free fatty acids standards were obtained from Nu‐Chek‐Prep (Elysian, MN, USA).
Samples were thawed at 4°C. Formic acid and ammonium acetate plus water or acetonitrile and isopropyl alcohol were used as the mobile phase. C19:0 was used as the internal standard. Liquid chromatography–tandem mass spectrometry analysis was carried out with Eksigent LC100 and AB SCIEX Triple TOF 5600 (AB SCIEX, Framingham, MA, USA). Eksigent LC100 was equipped with XBridge Peptide BEH C18 Column (Waters, Milford, MA, USA). PeakView1.2 (AB SCIEX) was used for qualitative analysis. MultiQuant2.1 (AB SCIEX) was used for quantitative analysis.
Statistical analysis
Continuous variables are expressed as means (standard deviations) when normally distributed or medians (interquartile ranges) when skewed. Normality was checked by observing the Q‐Q plot. Categorical variables are presented as frequencies (percentage). Differences between patients with NAFLD and without NAFLD were compared by the non‐paired Student’s t‐test (or Mann–Whitney U‐test when appropriate) for continuous variables, and the χ2‐test (or Fisher’s test if appropriate) for categorical variables.
Binary logistic regression was carried out to estimate the odds ratios (ORs) and 95% confidence interval (CI). Restricted cubic spline nested in the logistic regression was used to examine potential non‐linear relationships between n6, n3 fatty acids and their ratio with NAFLD risk, as before 14 . With restricted cubic spline, we identified the threshold where risk increased or decreased sharply, and then stratified PUFAs as categories according to these thresholds. Fatty acids were introduced into regression models as continuous variables (per standard deviation increase) and categories. We first carried out univariable analysis and then repeated analysis with adjustment for covariates, which included age, sex, HbA1c, systolic blood pressure, duration of diabetes, use of hypoglycemic drugs and lipid‐lowering drugs. BMI, waist circumference, triglyceride, HDL‐C, LDL‐C, C‐peptide, AST and ALT were not adjusted in the primary analysis considering that they might mediate associations between PUFAs and NAFLD or they were hallmarks of NAFLD. Instead, we adjusted for these variables in sensitive analysis. We also obtained correlation coefficients of the n6 : n3 ratio with these features of NAFLD, including BMI, waist circumference, HbA1c, FPG, triglyceride, HDL‐C, LDL‐C, C‐peptide, liver fibrosis score, AST and ALT.
To estimate potential additive interactions of imbalanced PUFA with insulin action for NAFLD risk, we created four variables: (i) n6 : n3 ratio >10 and C‐peptide <1.39 ng/mL (median, or fasting insulin <11.59 mU/L, as reference); (ii) n6 : n3 ratio >10 and C‐peptide ≥1.39 ng/mL (or fasting insulin ≥11.59 mU/L); (iii) n6 : n3 ratio ≤10 and C‐peptide <1.39 ng/mL (median, or fasting insulin <11.59 mU/L); (iv) n6 : n3 ratio >10 and C‐peptide ≥1.39 ng/mL (median, or fasting insulin ≥11.59 mU/L). Relative excess risk due to interaction (RERI), attributable proportion due to interaction (AP) and synergy index (S) were calculated to test the interactions. If any one of these three conditions; that is, RERI >0, AP >0 or S >1, was true, the biological interaction was statistically significant 16 . Age, sex, HbA1c, systolic blood pressure, duration of diabetes, hypoglycemic drugs and lipid‐lowering drugs were adjusted in the primary analysis, whereas metabolic‐associated parameters including BMI, waist circumference, HDL‐C, LDL‐C, triglyceride, AST and ALT were further adjusted in the sensitive analysis. To exclude bias from sex difference, we also carried out sex‐matched analysis.
The most accurate method for the diagnosis of fatty liver was histology and proton magnetic resonance spectroscopy subsequently. Ultrasound‐based measurements had lower sensitivity, but became the most popular screening method for their convenience and non‐invasiveness. ALT had the worst sensitivity and specificity 5 . In the primary analysis, NAFLD and non‐NAFLD were distinguished by ultrasound. In the sensitive analysis, we adjusted the definition; that is, patients with abnormal ultrasound imaging and ALT ≥40 U/L were assigned as NAFLD 17 .
All analysis was carried out using SAS version 9.4 (SAS institute Inc., Cary, NC, USA). P‐values of <0.05 were considered statistically significant.
Results
Characteristics of the study population
Of the 482 type 2 diabetes patients, 313 were with and 169 without NAFLD (Table 1). There were more women in the NAFLD group than in the non‐NAFLD group. Patients with NAFLD had higher BMI, waist circumference, triglyceride, HbA1c, fasting insulin, homeostatic model assessment of insulin resistance, C‐peptide, AST, ALT, n3 PUFA and n6 PUFA, and lower HDL‐C and n6 : n3 ratio. In addition, compared with patients without NAFLD, those patients with NAFLD were younger (58.5, standard deviation 13.5 vs 62.5, standard deviation 11.7) and had shorter duration of diabetes. Systolic blood pressure, LDL‐C, fasting blood glucose and liver fibrosis score were similar in the two groups (Table 1). Diabetic complications and medicine are shown in Table 1.
Table 1.
Clinical and biochemical characteristics of participants according to the occurrence of non‐alcoholic fatty liver disease
| NAFLD | Non‐NAFLD | P‐value | |
|---|---|---|---|
| n | 313 | 169 | |
| Mild steatosis | 274 | ||
| Moderate steatosis | 33 | ||
| Severe steatosis | 6 | ||
| Age (years) | 58.5 ± 13.5 | 62.5 ± 11.7 | 0.0008 |
| Duration of diabetes (years) | 8 (2–15) | 11 (6–20) | <0.0001 |
| Male sex | 125 (39.9) | 86 (50.9) | 0.0207 |
| Waist circumference (cm) | 94.7 ± 9.2 | 90.2 ± 9.3 | <0.0001 |
| Abnormal waist circumference | 167 (53.4) | 58 (34.3) | <0.0001 |
| BMI (kg/m2) | 27.5 ± 3.6 | 25.1 ± 3.7 | <0.0001 |
| BMI <24.0 kg/m2 | 52 (16.6) | 52 (16.6) | <0.0001 |
| BMI ≥24 and <28.0 kg/m2 | 130 (41.5) | 78 (46.2) | |
| BMI ≥28.0 kg/m2 | 131 (41.9) | 31 (18.3) | |
| Systolic blood pressure (mmHg) | 150.4 ± 21.3 | 148.3 ± 21.4 | 0.2949 |
| HDL‐C (mmol/L) | 1.19 ± 0.30 | 1.29 ± 0.33 | 0.0008 |
| LDL‐C (mmol/L) | 2.57 ± 0.82 | 2.52 ± 0.85 | 0.5843 |
| Triglyceride (mmol/L) | 1.73 (1.30–2.66) | 1.23 (0.87–1.67) | <0.0001 |
| HbA1c (%) | 8.50 (7.30–10.10) | 8.00 (6.80–9.40) | 0.0009 |
| Fasting blood glucose (mmol/L) | 9.88 ± 3.56 | 9.04 ± 3.59 | 0.5843 |
| C‐peptide (ng/mL) | 1.60 (1.10–2.17) | 1.03 (0.74–1.44) | <0.0001 |
| Fasting insulin (mU/L) | 13.1 (8.5–21.5) | 9.5 (5.7–18.7) | 0.0001 |
| Liver fibrosis score (kPa) | 7.1 (6.0–8.9) | 7.0 (5.7–9.0) | 0.4975 |
| AST (U/L) | 20.28 (16.42–26.15) | 18.10 (15.50–23.26) | 0.0022 |
| AST ≥40 U/L | 29 (9.3) | 4 (2.4) | 0.0039 |
| ALT (U/L) | 23.99 (16.53–36.35) | 18.55 (13.75–25.17) | <0.0001 |
| ALT ≥40 U/L | 60 (19.2) | 10 (5.9) | <0.0001 |
| n3 PUFA (μmol/L) | 5,830.97 (4,832.26–7,241.60) | 5,355.43 (4,518.18–6,457.55) | 0.0022 |
| n3 PUFA ≥800 μmol/L | 53 (16.9) | 10 (5.9) | 0.0006 |
| n6 PUFA (μmol/L) | 592.47 (450.84–834.55) | 475.45 (383.72–623.56) | <0.0001 |
| n6 PUFA ≥8,100 μmol/L | 89 (28.4) | 22 (13.0) | 0.0001 |
| n6/n3 ratio | 10.3 ± 3.9 | 11.7 ± 4.1 | 0.0002 |
| n6/n3 ratio ≤10 | 167 (53.4) | 63 (37.3) | 0.0007 |
| Prior CAD | 32 (10.2) | 13 (7.7) | 0.3620 |
| Prior stroke | 13 (4.2) | 11 (6.5) | 0.2767 |
| Diabetic retinopathy | 79 (26.3) | 70 (42.4) | 0.0003 |
| Diabetic nephropathy | 144 (46.0) | 80 (47.3) | 0.7798 |
| Hypoglycemic drugs | 293 (93.6) | 166 (98.2) | 0.0245 |
| Insulin (drug) | 175 (55.9) | 108 (63.9) | 0.0889 |
| Metformin | 164 (53.3) | 44 (26.2) | <0.0001 |
| Thiazolidinedione | 57 (18.5) | 6 (3.6) | <0.0001 |
| Lipid‐lowering drugs | 162 (51.8) | 66 (39.1) | 0.0077 |
Data are the mean ± standard deviation, median (interquartile range) or n (%). P‐values were derived from independent‐samples Student’s t‐test for normally distributed variables, Mann–Whitney U‐test for skewed distributions and χ2‐test (or Fisher’s test if appropriate) for categorical variables. Abnormal waist circumference, men ≥102 cm or women ≥88 cm.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CAD, coronary artery disease; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; NAFLD, non‐alcoholic fatty liver disease; PUFA, polyunsaturated fatty acids.
Associations between PUFA and NAFLD
As shown in Figure 1, n6 (Figure 1a) and n3 PUFA (Figure 1b) were positively associated with NAFLD non‐linearly. N6 fatty acids ≥8,100 μmol/L increased 3.24‐fold risk in univariate analysis (95% CI 1.60–6.55) and 2.69‐fold risk in multivariate analysis (95% CI 1.27–5.67); n3 fatty acids ≥800 μmol/L increased 2.66‐fold risk in univariate analysis (95% CI 1.59–4.43) and 2.20‐fold risk in multivariate analysis (95% CI 1.28–3.78; Table 2). The n6 : n3 ratio was inversely associated with NAFLD non‐linearly (Figure 1c). Compared with n6 : n3 ratio >10, n6 : n3 ratio ≤10 was associated with an increased risk of NAFLD in both the univariate (1.92, 95% CI 1.31–2.82) and multivariate model (1.80, 95% CI 1.20–2.71; Table 2). When further adjusted for variables‐associated parameters, the association between the n6 : n3 ratio and NAFLD was still significant (Table S1).
Figure 1.
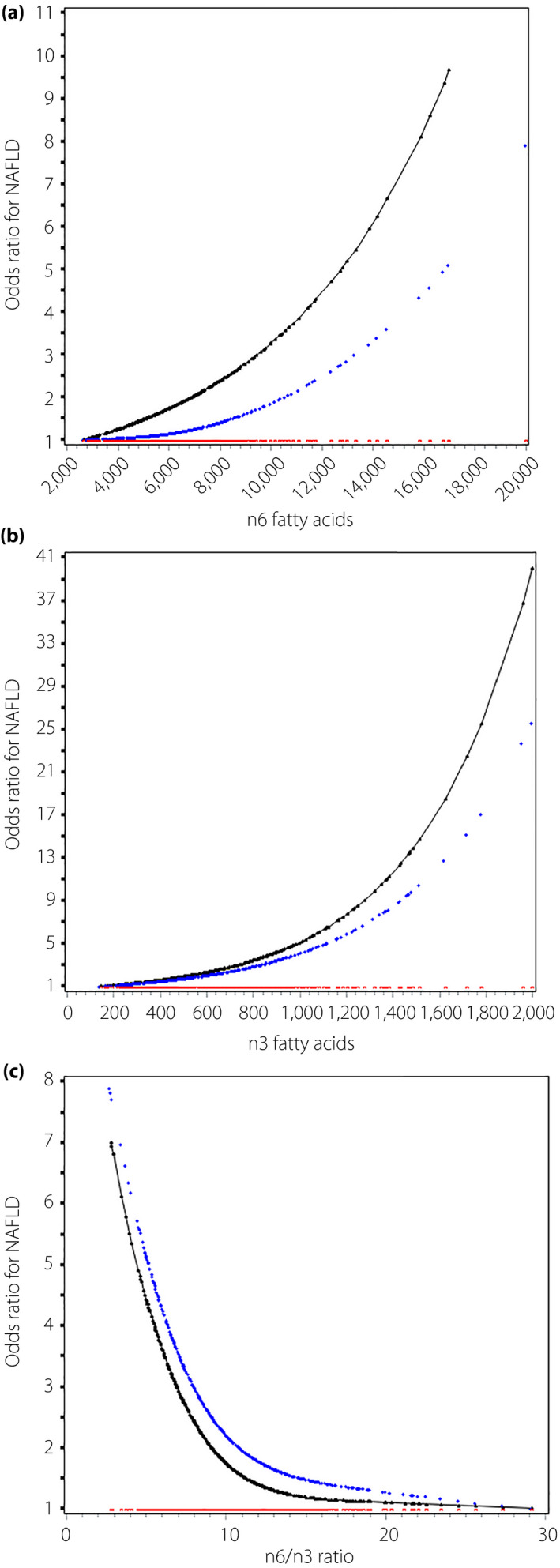
Odds ratio curves of polyunsaturated fatty acids for non‐alcoholic fatty liver disease (NAFLD) risk in type 2 diabetes patients. (a–c) The relationships between n6, n3 fatty acids and the n6 : n3 ratio with NAFLD risk, respectively. The black curve was derived from univariable analysis, and the blue curve derived from multivariable analysis that adjusted for age, sex, glycated hemoglobin, systolic blood pressure, duration of diabetes, hypoglycemic drugs and lipid‐lowering drugs. The red curve represents the reference level (i.e., the odds ratio for type 2 diabetes mellitus was 1).
Table 2.
Relationships between polyunsaturated fatty acids and non‐alcoholic fatty liver disease in patients with type 2 diabetes
| Univariable model | Multivariable model | |||
|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| n6 PUFA | ||||
| Per SD increase | 1.43 (1.14–1.79) | 0.0019 | 1.24 (0.97–1.58) | 0.0864 |
| ≥8,100 vs <8,100 μmol/L | 3.24 (1.60–6.55) | 0.0011 | 2.69 (1.27–5.67) | 0.0096 |
| n3 PUFA | ||||
| Per SD increase | 1.77 (1.34–2.35) | <0.0001 | 1.94 (1.49–2.52) | <0.0001 |
| ≥800 vs <800 μmol/L | 2.66 (1.59–4.43) | 0.0002 | 2.20 (1.28–3.78) | 0.0045 |
| n6/n3 ratio | ||||
| Per SD increase | 0.71 (0.58–0.85) | 0.0003 | 0.69 (0.56–0.86) | 0.0007 |
| ≤10 vs >10 | 1.92 (1.31–2.82) | 0.0008 | 1.80 (1.20–2.71) | 0.0045 |
Multivariable model was adjusted for age, sex, glycated hemoglobin, systolic blood pressure, duration of diabetes, hypoglycemic drugs and lipid‐lowering drugs.
CI, confidence interval; NAFLD, non‐alcoholic fatty liver disease; OR, odds ratio; PUFA, polyunsaturated fatty acids; SD, standard deviation.
In addition, a higher n6 : n3 ratio was inversely correlated with triglyceride (correlation coefficient −0.10, P = 0.0364) and positively correlated with HbA1c (correlation coefficient 0.10, P = 0.0364) weakly after adjusted for traditional risk factors. Other hallmarks were not significantly correlated with the n6 : n3 ratio (Table 3).
Table 3.
Spearman correlation of the n6 : n3 ratio with features of non‐alcoholic fatty liver disease
| Correlation coefficients | P‐value | |
|---|---|---|
| Univariable analysis | ||
| BMI | −0.08 | 0.0863 |
| Waist circumference (cm) | −0.05 | 0.2421 |
| Fasting blood glucose (mmol/L) | 0.04 | 0.4300 |
| HbA1c (%) | 0.07 | 0.1287 |
| HDL‐C (mmol/L) | −0.09 | 0.0491 |
| LDL‐C (mmol/L) | 0.02 | 0.7117 |
| Triglyceride (mmol/L) | −0.13 | 0.0050 |
| C‐peptide (ng/mL) | −0.08 | 0.0627 |
| Liver fibrosis score (kPa) | −0.05 | 0.3547 |
| AST (U/L) | −0.02 | 0.6838 |
| ALT (U/L) | −0.06 | 0.1774 |
| Multivariable analysis | ||
| BMI | −0.06 | 0.1798 |
| Waist circumference (cm) | −0.04 | 0.4003 |
| Fasting blood glucose (mmol/L) | 0.05 | 0.2451 |
| HbA1c (%) | 0.11 | 0.0364 |
| HDL‐C (mmol/L) | −0.08 | 0.0785 |
| LDL‐C (mmol/L) | 0.04 | 0.3913 |
| Triglyceride (mmol/L) | −0.10 | 0.0342 |
| C‐peptide (ng/mL) | −0.07 | 0.1247 |
| Liver fibrosis score (kPa) | −0.04 | 0.4615 |
| AST (U/L) | −0.03 | 0.5155 |
| ALT (U/L) | −0.09 | 0.0669 |
Multivariable model was adjusted for age, gender, systolic blood pressure, duration of diabetes, hypoglycemic drugs and lipid‐lowering drugs.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; NAFLD, non‐alcoholic fatty liver disease.
Additive interaction of the n6 : n3 ratio with insulin action for NAFLD
Using a high n6 : n3 ratio and low C‐peptide as reference, the OR of C‐peptide >1.39 only was 3.13 (95% CI 1.77–5.55), and OR of n6 : n3 ratio ≤10 only was 1.40 (95% CI 0.80–2.42); the co‐presence of a low n6 : n3 ratio and high C‐peptide greatly increased the OR to 8.89 (95% CI 4.48–17.7). The additive interaction measures were significant (RERI 5.37, 95% CI −0.12 to 10.9; AP 0.60, 95% CI 0.33–0.88; S 3.12, 95% CI 1.29–7.54; Table 4).
Table 4.
Additive interaction of the n6 : n3 ratio with insulin action for non‐alcoholic fatty liver disease in patients with type 2 diabetes
| Univariable model | Multivariable model | |||
|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| C‐peptide ≥ vs <1.39 ng/mL | 4.05 (2.70–6.06) | <0.0001 | 4.13 (2.66–6.43) | <0.0001 |
| Fasting insulin ≥ vs <11.59 mU/L | 1.85 (1.27–2.71) | 0.0015 | 1.77 (1.17–2.67) | 0.0067 |
| Interaction of n6 : n3 ratio with insulin secretion | ||||
| n6 : n3 ratio >10 & C‐peptide <1.39 ng/mL | Reference | Reference | ||
| n6/n3 ratio >10 & C‐peptide ≥1.39 ng/mL | 3.12 (1.85–5.28) | <0.0001 | 3.13 (1.77–5.55) | <0.0001 |
| n6 : n3 ratio ≤10 & C‐peptide <1.39 ng/mL | 1.50 (0.90–2.51) | 0.1217 | 1.40 (0.80–2.42) | 0.2367 |
| n6 : n3 ratio ≤10 & C‐peptide ≥1.39 ng/mL | 8.79 (4.63–16.7) | <0.0001 | 8.89 (4.48–17.7) | <0.0001 |
| Measure | ||||
| RERI | 5.16 (0.02–10.3) | 5.37 (−0.12–10.9) | ||
| AP | 0.59 (0.31–0.86) | 0.60 (0.33–0.88) | ||
| S | 2.97 (1.29–6.81) | 3.12 (1.29–7.54) | ||
| Interaction of n6 : n3 ratio with fasting insulin | ||||
| n6 : n3 ratio >10 & insulin <11.59 mU/L | Reference | Reference | ||
| n6 : n3 ratio >10 & insulin ≥11.59 mU/L | 2.74 (1.63–4.60) | 0.0001 | 2.53 (1.45–4.41) | 0.0011 |
| n6 : n3 ratio ≤10 & insulin <11.59 mU/L | 2.88 (1.69–4.89) | <0.0001 | 2.58 (1.47–4.53) | 0.0009 |
| n6 : n3 ratio ≤10 & insulin ≥11.59 mU/L | 3.61 (2.05–6.36) | <0.0001 | 3.20 (1.75–5.85) | 0.0002 |
| Measure | ||||
| RERI | −1.00 (−3.30–1.30) | −0.91 (−3.09–1.28) | ||
| AP | −0.28 (−0.98–0.43) | −0.28 (−1.03–0.47) | ||
| S | 0.72 (0.35–1.51) | 0.71 (0.31–1.60) | ||
Low and high level of C‐peptide and fasting insulin was defined according to less or more than medians. Multivariable model was adjusted for age, sex, glycated hemoglobin, systolic blood pressure, duration of diabetes, hypoglycemic drugs and lipid‐lowering drugs. Significant elative excess risk due to interaction (RERI) >0, attributable proportion due to interaction (AP) >0 or synergy index (S) >1 indicates a significant additive interaction.
CI, confidence interval; NAFLD, non‐alcoholic fatty liver disease; OR, odds ratio.
With a high n6 : n3 ratio and low fasting insulin as the reference, the OR of fasting insulin >11.59 mU/L only was 2.53 (95% CI 1.45–4.41), and the OR of a n6 : n3 ratio ≤10 only was 2.58 (95% CI 1.47–4.53); the OR of co‐presence of a low n6 : n3 ratio and high fasting insulin was 3.20 (95% CI 1.75–5.85). The additive interaction was not significant (RERI −0.91, 95% CI −3.09 to 1.28; AP −0.28, 95% CI −1.03 to 0.47; S 0.71, 95% CI 0.31–1.60; Table 4).
In addition, these findings were robust when further adjusted for metabolic‐associated parameters (Table S2) or in sex‐matched analysis (Table S3). When changing the definition of NAFLD to the co‐presence of abnormal abdominal ultrasonography and AST ≥40 U/L, there were 253 and 229 patients with and without NAFLD, respectively. The associations between the n6 : n3 ratio and NAFLD were slightly attenuated. but remained significant (1.59 95% CI 1.10–2.31). The interaction between a low n6 : n3 ratio and high C‐peptide still existed (RERI 1.88, 95% CI 0.23–3.53; AP 0.53, 95% CI 0.24–0.83; S 0.48, 95% CI 0.82–17.9), whereas the interaction between a low n6 : n3 ratio and high fasting insulin was still non‐significant (RERI −1.17, 95% CI −2.66 to 0.33; AP −0.65, 95% CI −1.55 to 0.24; S 0.40, 95% CI 0.14–1.15; Table S4).
Among 525 patients who had their lipids measured, compared with participants who did not have B‐mode ultrasound, participants who had B‐mode ultrasound were older and had higher systolic blood pressure, fasting blood glucose and lower fasting insulin. Other characteristics were the same between the two groups (Table S5).
Discussion
In the present study, we found that in the type 2 diabetes population, the presence of NAFLD was associated with higher n6 and n3 PUFA levels, but a lower n6 : n3 ratio. In addition, we detected an interaction between a low n6 : n3 ratio and high C‐peptide for NAFLD risk. The inverse association between the n6 : n3 ratio and NAFLD risk was only observed in patients with higher C‐peptide, suggesting that increased risk of NAFLD in type 2 diabetes with a low n6 : n3 ratio was conditional on the presence of high C‐peptide. However, we did not find a significant interaction between the n6 : n3 ratio and fasting insulin.
Normally, most fatty acids are stored in adipose tissues. In insulin‐resistant states, lipolysis of adipose tissues accelerated and circulating free fatty acids were increased 18 . Despite many studies finding a decline in hepatic PUFA content, it was still rational that we found circulating PUFA levels elevated in insulin‐resistant states 6 , 19 . The presence of NAFLD in type 2 diabetes was associated with more severe insulin resistance and hyperinsulinemia, whereas impaired insulin signaling mediated exacerbated lipid mobilization from adipose tissues to the liver in turn 20 , 21 . Also, patients with NAFLD in the present study had higher BMIs, which provided a larger lipid pool. Therefore, the present results suggested that higher plasma free n3 and n6 PUFA were potential markers of aggravating β‐cell function and obesity in type 2 diabetes patients with NAFLD.
Apart from absolute levels, the balance of n6 and n3 PUFA was very important. A great deal of evidence showed that n3 performed better than n6 fatty acids in weight loss and inflammation 9 , 22 . In this connection, experiments and meta‐analysis had encouraged the use of n3 PUFAs as specific treatment options for NAFLD progression 23 , 24 . However, in the present study, a higher n6 : n3 ratio was associated with a lower NAFLD risk in diabetes patients. One possibility is that n3 PUFA provides insufficient protection from other fatty acids and is overconsumption, which contributes to a higher n6 : n3 ratio and lower NAFLD risk. Nevertheless, clinical studies showed that n3 PUFA supplement did not perform well in diabetes patients 11 , 25 , whereas n6 PUFA reduced the incidence of type 2 diabetes 26 , 27 . Monaco et al. 28 reported that in obese Zucker rats, consumption of α‐linolenic acid attenuated insulin signaling, weakened mitochondrial respiration and increased formation of reactive oxygen species (ROS); despite benign performance in rats without severe metabolic disturbance 29 , some other studies showed that in rats with both severe insulin resistance and hyperinsulinemia, n3 PUFA reversed the compensatory increase in insulin secretion, but did not improve adequate insulin sensitivity, leading to poorer metabolism 30 , 31 . In this regard, the second possibility is that a low n6 : n3 ratio was derived from relative decreased n6 and increased n3 fatty acids; that is, higher n3 PUFA led to increased NAFLD risk. Whether NAFLD together with type 2 diabetes changed the response of n3 PUFA for hepatic steatosis is unknown. Indeed, it is worthwhile to investigate the role of the balance of n6 and n3 PUFA for NAFLD in diabetes patients. It was important to note that n6 : n3 is positively correlated with HbA1c, which is the opposite to the effect of n6 : n3 on NAFLD. It is possible that an unbalanced n6 : n3 ratio can raise postprandial blood glucose through a pathway other than liver insulin resistance; for example, decreasing capacity of glucose conversion to fatty acids through carbohydrate regulatory element‐binding protein and Max‐like factor‐X 32 .
C‐peptide is a byproduct of proinsulin, and releases from β‐cells in equimolar amounts with insulin 33 . Both C‐peptide and insulin were identified as important predictors and markers of conditions, such as insulin resistance, diabetes, NAFLD and cardiovascular diseases 34 , 35 . Compared with plasma insulin, C‐peptide is almost unaffected by the first metabolism of the liver and has a much longer half‐life 33 . Therefore, C‐peptide levels can always represent endogenous insulin secretion more appropriately than insulin levels, and has better performance than insulin in predicting NAFLD, insulin resistance and type 2 diabetes 34 , 35 , 36 . In the present study, we found that the adverse effect of a decreased n6 : n3 ratio depended on the presence of a high level of C‐peptide. As speculated before, if n3 PUFA was protective and overconsumed, then in patients with severe insulin resistance, n3 PUFA cannot appropriately protect patients from fatty liver; if n3 PUFA was harmful in type 2 diabetes, then the accumulation of n3 PUFA interacted with insulin resistance for fatty liver. Insulin resistance might modify the effect of PUFA 37 , 38 . Furthermore, recent evidence supported that C‐peptide was not only a biomarker of insulin action, but also of biological effects, such as pro‐inflammation in the setting of insulin resistance, which might also interplay with PUFA for hepatic steatosis 39 .
The present findings had important clinical and mechanistic implications. Diabetes patients with NAFLD are susceptible to unfavorable prognosis. However, related diagnosis and treatment are optimal. PUFA emerges as a new option. Our study found an inverse relationship between the n6 : n3 ratio and NAFLD development in type 2 diabetes, which is opposite to findings in the population without type 2 diabetes. Thus, the present study not only generated some new hypotheses for basic scientists to investigate the molecular mechanisms underlying liver fat accumulation in diabetes, but also suggested the balance of PUFA as a potential non‐invasive marker of diagnosis and therapeutic target. In addition, as the association between PUFA and NAFLD relies on C‐peptide, maybe combination medication is likely to offer an additive benefit.
The present study also had several limitations. First, our research was retrospective, so whether a reduced n6 : n3 ratio is a cause or consequence of NAFLD requires more prospective studies. Second, we did not collect information on physical activity and diet. Nevertheless, physical activity and diet were associated with BMI and waist circumference. We adjusted BMI and waist circumference in sensitive analysis, and the association between the n6 : n3 ratio and NAFLD was still significant. Third, although compared with confirmatory diagnosis (liver biopsy or proton magnetic resonance spectroscopy), diagnosis can be missed by ultrasonography, the resolution of fatty liver, as assessed by ultrasonography, has been found to reduce the risk of type 2 diabetes development to a level similar to individuals without NAFLD 40 , so liver steatosis assessed by ultrasonography can be of significant clinical implication. Fourth, most of our participants had mild hepatic steatosis. Therefore, it was plausible that the fibrosis score was similar between participants with and without NAFLD, and no significant correction was found between the n6 : n3 ratio and fibrosis score. In the future, more patients with moderate and severe steatosis should be included. Fifth, patients who had an abdominal ultrasound had more unfavorable metabolism, but a similar n6 : n3 ratio compared with their counterparts who did not have an abdominal ultrasound. Therefore, there might be more NAFLD in the latter group, and we might overestimate the effect size of the n6 : n3 ratio for NAFLD.
In conclusion, we found that a decreased n6 : n3 ratio increased the NAFLD risk in type 2 diabetes patients, and the coexistence of high C‐peptide further amplified the effect size. More prospective studies and basic science are warranted to elaborate the role of PUFAs for liver fat accumulation in the context of type 2 diabetes.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 | Relationships between the n6 : n3 ratio and non‐alcoholic fatty liver disease in patients with type 2 diabetes.
Table S2 | Interactions between the n6 : n3 ratio and insulin action for non‐alcoholic fatty liver disease risk with further adjustment of metabolic‐associated parameters.
Table S3 | Sex‐matched analysis* for associations between the n6 : n3 ratio and non‐alcoholic fatty liver disease ,and its interaction with insulin action.
Table S4 | Association of the n6 : n3 ratio and non‐alcoholic fatty liver disease$ and its interaction with insulin action.
Table S5 | Clinical and biochemical characteristics of participants according a B‐mode ultrasound.
Acknowledgments
The authors thank all the physicians and doctors at The Second Affiliated Hospital of Dalian Medical University who participated in the study and extended their support for data collection. The authors also thank all the laboratory technicians at Dalian Institute of Chemical Physics and RSKT Biopharma Inc, Dalian, Liaoning, for measuring the metabolites. This work was supported by the project for the National Key Research and Development Program of China (2019YFA0802302, 2019YFA0802300), State Key Project on Infectious Diseases of China (2018ZX10723204).
J Diabetes Investig 2021; 12: 1263–1271
Contributor Information
Zhong‐Ze Fang, Email: fangzhongze@tmu.edu.cn.
Ping Zhang, Email: 2663514021@qq.com.
References
- 1. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012; 142: 1592–1609. [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta‐analysis. J Hepatol 2019; 71: 793–801. [DOI] [PubMed] [Google Scholar]
- 3. Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology (Baltimore, MD) 2014; 59: 713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol 2017; 14: 32–42. [DOI] [PubMed] [Google Scholar]
- 5. Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care 2017; 40: 419–430. [DOI] [PubMed] [Google Scholar]
- 6. Puri P, Wiest MM, Cheung O, et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology (Baltimore, MD) 2009; 50: 1827–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Musso G, Cassader M, Paschetta E, et al. Bioactive lipid species and metabolic pathways in progression and resolution of nonalcoholic steatohepatitis. Gastroenterology 2018; 155: 282–302.e8. [DOI] [PubMed] [Google Scholar]
- 8. Del Gobbo LC, Imamura F, Aslibekyan S, et al. ω‐3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med 2016; 176: 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simopoulos AP, DiNicolantonio JJ. The importance of a balanced ω‐6 to ω‐3 ratio in the prevention and management of obesity. Open Heart 2016; 3: e000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen LH, Wang YF, Xu QH, et al. Omega‐3 fatty acids as a treatment for non‐alcoholic fatty liver disease in children: a systematic review and meta‐analysis of randomized controlled trials. Clin Nutr 2018; 37: 516–521. [DOI] [PubMed] [Google Scholar]
- 11. Dasarathy S, Dasarathy J, Khiyami A, et al. Double‐blind randomized placebo‐controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol 2015; 49: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meyer L, Jeantroux J, Riveline JP, et al. Reversible focal hepatic steatosis in type 1 diabetic patients treated with intraperitoneal insulin implantable pump therapy. Diabetes Care 2008; 31: e49. [DOI] [PubMed] [Google Scholar]
- 13. Jezek P, Jaburek M, Holendova B, et al. Fatty acid‐stimulated insulin secretion vs. lipotoxicity. Molecules 2018; 23: 1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li J, Cao YF, Sun XY, et al. Plasma tyrosine and its interaction with low high‐density lipoprotein cholesterol and the risk of type 2 diabetes mellitus in Chinese. J Diabetes Investig 2019; 10: 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Joseph AE, Saverymuttu SH, Al‐Sam S, et al. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin Radiol 1991; 43: 26–31. [DOI] [PubMed] [Google Scholar]
- 16. Andersson T, Alfredsson L, Källberg H, et al. Calculating measures of biological interaction. Eur J Epidemiol 2005; 20: 575–579. [DOI] [PubMed] [Google Scholar]
- 17. Spadaro L, Magliocco O, Spampinato D, et al. Effects of n‐3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig Liver Dis 2008; 40: 194–199. [DOI] [PubMed] [Google Scholar]
- 18. Tamura S, Shimomura L. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J Clin Investig 2005; 115: 1139–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiappini F, Coilly A, Kadar H, et al. Metabolism dysregulation induces a specific lipid signature of nonalcoholic steatohepatitis in patients. Sci Rep 2017; 7: 46658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roden M. Mechanisms of disease: hepatic steatosis in type 2 diabetes – pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab 2006; 2: 335–348. [DOI] [PubMed] [Google Scholar]
- 21. Utzschneider KM, Van de Lagemaat A, Faulenbach MV, et al. Insulin resistance is the best predictor of the metabolic syndrome in subjects with a first‐degree relative with type 2 diabetes. Obesity 2010; 18: 1781–1787. [DOI] [PubMed] [Google Scholar]
- 22. Calder PC. Marine omega‐3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochem Biophys Acta 2015; 1851: 469–484. [DOI] [PubMed] [Google Scholar]
- 23. Guo XF, Yang B, Tang J, et al. Fatty acid and non‐alcoholic fatty liver disease: meta‐analyses of case‐control and randomized controlled trials. Clin Nutr 2018; 37: 113–122. [DOI] [PubMed] [Google Scholar]
- 24. Wang C, Liu W, Yao L, et al. Hydroxyeicosapentaenoic acids and epoxyeicosatetraenoic acids attenuate early occurrence of nonalcoholic fatty liver disease. Br J Pharmacol 2017; 174: 2358–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bosch J, Gerstein HC, Dagenais GR, et al. n‐3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med 2012; 367: 309–318. [DOI] [PubMed] [Google Scholar]
- 26. Wu JHY, Marklund M, Imamura F, et al. Omega‐6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual‐level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol 2017; 5: 965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zong G, Liu G, Willett WC, et al. Associations between linoleic acid intake and incident type 2 diabetes among U.S. men and women. Diabetes Care 2019; 42: 1406–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Monaco CMF, Proudfoot R, Miotto PM, et al. α‐linolenic acid supplementation prevents exercise‐induced improvements in white adipose tissue mitochondrial bioenergetics and whole‐body glucose homeostasis in obese Zucker rats. Diabetologia 2018; 61: 433–444. [DOI] [PubMed] [Google Scholar]
- 29. Wei D, Li J, Shen M, et al. Cellular production of n‐3 PUFAs and reduction of n‐6‐to‐n‐3 ratios in the pancreatic beta‐cells and islets enhance insulin secretion and confer protection against cytokine‐induced cell death. Diabetes 2010; 59: 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holness MJ, Greenwood GK, Smith ND, et al. Diabetogenic impact of long‐chain omega‐3 fatty acids on pancreatic beta‐cell function and the regulation of endogenous glucose production. Endocrinology 2003; 144: 3958–3968. [DOI] [PubMed] [Google Scholar]
- 31. Holness MJ, Smith ND, Greenwood GK, et al. Acute omega‐3 fatty acid enrichment selectively reverses high‐saturated fat feeding‐induced insulin hypersecretion but does not improve peripheral insulin resistance. Diabetes 2004; 53(Suppl 1): S166–S171. [DOI] [PubMed] [Google Scholar]
- 32. Jump DB, Tripathy S, Depner CM. Fatty acid‐regulated transcription factors in the liver. Annu Rev Nutr 2013; 33: 249–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leighton E, Sainsbury CA, Jones GC. A practical review of C‐peptide testing in diabetes. Diabetes Ther 2017; 8: 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Min JY, Min KB. Serum C‐peptide levels as an independent predictor of diabetes mellitus mortality in non‐diabetic individuals. Eur J Epidemiol 2013; 28: 771–774. [DOI] [PubMed] [Google Scholar]
- 35. Atsawarungruangkit A, Chenbhanich J, Dickstein G. C‐peptide as a key risk factor for non‐alcoholic fatty liver disease in the United States population. World J Gastroenterol 2018; 24: 3663–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jones AG, Hattersley AT. The clinical utility of C‐peptide measurement in the care of patients with diabetes. Diabet Med 2013; 30: 803–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fujikura K, Heydari B, Ge Y, et al. Insulin resistance modifies the effects of omega‐3 acid ethyl esters on left ventricular remodeling after acute myocardial infarction (from the OMEGA‐REMODEL Randomized Clinical Trial). Am J Cardiol 2020; 125: 678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Poreba M, Mostowik M, Siniarski A, et al. Treatment with high‐dose n‐3 PUFAs has no effect on platelet function, coagulation, metabolic status or inflammation in patients with atherosclerosis and type 2 diabetes. Cardiovasc Diabetol 2017; 16: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vasic D, Walcher D. C‐peptide: a new mediator of atherosclerosis in diabetes. Mediators Inflamm 2012; 2012: 858692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sung KC, Wild SH, Byrne CD. Resolution of fatty liver and risk of incident diabetes. J Clin Endocrinol Metab 2013; 98: 3637–3643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Relationships between the n6 : n3 ratio and non‐alcoholic fatty liver disease in patients with type 2 diabetes.
Table S2 | Interactions between the n6 : n3 ratio and insulin action for non‐alcoholic fatty liver disease risk with further adjustment of metabolic‐associated parameters.
Table S3 | Sex‐matched analysis* for associations between the n6 : n3 ratio and non‐alcoholic fatty liver disease ,and its interaction with insulin action.
Table S4 | Association of the n6 : n3 ratio and non‐alcoholic fatty liver disease$ and its interaction with insulin action.
Table S5 | Clinical and biochemical characteristics of participants according a B‐mode ultrasound.


