Abstract
Aims/Introduction
This study compares the effects of two different insulin regimens – basal versus bolus insulin – on metabolic and cardiovascular autonomic function in Japanese participants with type 2 diabetes.
Materials and Methods
Participants were randomly assigned to groups for therapy with insulin glulisine (IGlu) or insulin glargine (IGla). The primary efficacy end‐point was glycemic variability, including M‐values, mean of glucose levels, and a blood glucose profile of seven time points before and after the intervention. The secondary end‐points included pleiotropic effects, including endothelial and cardiac autonomic nerve functions.
Results
Blood glucose levels at all time points significantly decreased in both groups. Post‐lunch, post‐dinner, and bedtime blood glucose levels were significantly lower in the IGlu group than in the IGla group. Nadir fasting blood glucose levels at the end‐point were significantly lower in the IGla group than in the IGlu group. The M‐value and mean blood glucose levels were significantly decreased from baseline in both groups, although the former was significantly lower in the IGlu group than in the IGla group. IGla, but not IGlu, was found to elevate 24‐h parasympathetic tone, especially during night‐time, and it decreased 24‐h sympathetic nerve activity, especially at dawn.
Conclusions
Both IGlu and IGla regimens reduced glucose variability, with IGlu bringing a greater reduction in M‐value. IGla, but not IGlu, increased parasympathetic tone during night‐time and decreased sympathetic nerve activity at dawn. These findings shed light on the previously unrecognized role of night‐time basal insulin supplementation on sympathovagal activity in type 2 diabetes patients.
Keywords: Glycemic variability, Insulin therapy, Sympathovagal effects
Both insulin glulisine and insulin glargine regimens decrease glucose variability, with a greater decrease in M‐value by insulin glulisine in people with poorly controlled type 2 diabetes. Insulin glargine, but not insulin glulisine, elevated parasympathetic tone at night‐time, and reduced sympathetic nerve activity at dawn.

Introduction
Insulin therapy is effective in patients with type 2 diabetes when the condition is insufficiently controlled by oral hypoglycemic agents. A consensus report by the American Diabetes Association and the European Association for the Study of Diabetes recommends starting basal insulin therapy in conjunction with an sodium–glucose cotransporter 2 inhibitor or glucagon‐like peptide‐1 receptor agonist based on metformin 1 . Postprandial insulin secretory failure can precede the onset of type 2 diabetes 2 , and cause postprandial hyperglycemia, in turn increasing the risk of cardiovascular disease 3 . We have previously investigated the significance of postprandial glucose control as an element of managing glycemia overall in participants with type 2 diabetes. Then, we found that approximately half could achieve appropriate controlled nadir fasting plasma glucose levels by taking rapid‐acting doses of insulin analog alone at meal times in a bolus‐first regimen 4 , 5 . The Treating to Target in Type Diabetes (4T) 6 and once‐daily basal insulin glargine versus thrice‐daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO) 7 studies had proven that basal insulin therapy is not inferior to bolus insulin therapy in reducing glucose levels. Therefore, we surmised it could be that both basal‐first and bolus‐first regimens might be useful in achieving adequate glycemic control, although the 4‐T study had shown positive and negative aspects of the bolus‐ and basal‐insulin regimens. It found that the addition of bolus insulin reduced postprandial blood glucose levels more significantly than basal insulin, although this was also associated with a higher risk of developing hypoglycemia and weight gain 6 .
It has been recognized that variable glucose levels are associated with hyperglycemia after eating, when it can induce cardiac sympathetic nerve overactivity and endothelial dysfunction 8 , 9 . It is therefore widely accepted as a therapeutic target for preventing cardiovascular diseases. Previous observational studies suggest that vagal tone, assessed by a power spectrum of RR intervals, decreases in people with type 2 diabetes 10 , 11 . It remains unclear, however, whether hyperglycemia or insulin deficiency contributes to this lower vagal tone.
The present study aimed to compare the effects of insulin initiating therapies, namely rapid‐acting insulin analog at mealtimes and long‐acting insulin analog at bedtime, on glucose variability, pleiotropic effects including energy homeostasis, endothelial function, cardiac autonomic nerve function and treatment satisfaction among Japanese participants with type 2 diabetes.
Methods
Overview
This was a randomized parallel‐group study carried out in Japanese participants with the approval of the ethics committee of Kanazawa University Hospital in Japan (No. 2010‐067), in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants before enrollment. A total of 44 participants with poorly controlled type 2 diabetes were recruited at the Division of Endocrinology and Metabolism at Kanazawa University Hospital, Kanazawa, Ishikawa, Japan, between June 2011 and March 2015. This trial was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000010353).
Participants
Eligible participants were aged 20–80 years, hospitalized and had type 2 diabetes with glycated hemoglobin levels of >7.4%. Participants were excluded who: (i) had hypersensitivity or contraindication to insulin glulisine (IGlu) or insulin glargine (IGla); (ii) history of diabetic ketoacidosis; (iii) history of severe hypoglycemia; (iv) had a severe infection or pre/post‐surgery of trauma; (v) had received insulin or glucagon‐like peptide‐1 receptor agonist therapy within 4 weeks of the study; (vi) had received glucocorticoid therapy; (vii) showed poorly controlled hypertension (systolic blood pressure > 160 mmHg or diastolic blood pressure > 100 mmHg); (viii) had severe retinopathy; (ix) had a significant medical history and/or malignancy; (x) had severe complications and conditions not suitable for the study (hyperosmolar hyperglycemic state or heart failure); (xi) were pregnant or breastfeeding; and (xii) were assessed by the investigators as being unsuitable for the present study, for reasons including psychiatric and psychosocial conditions.
Study design
A computer‐generated randomization sequence assigned participants into two equally sized groups. Participants in the first group were each given three doses of rapid‐acting IGlu each day at mealtimes. Participants in the second group were given long‐acting IGla once daily at bedtime. Both insulin analogs were injected subcutaneously with a pen device. The starting doses for insulin glargine and insulin glulisine were 4 U at bedtime and 4 U before every meal, respectively. Using the self‐report data on glucose monitoring at four times including FPG and postprandial glucose, the investigators and patients carried out daily insulin dose titrations. Target glycemic levels were pre‐meal glucose values of 80–110 mg/dL and 2 h‐postprandial glucose values of 80–140 mg/dL, almost in accordance with past studies 6 , 7 and a diabetes treatment guide published by the Japan Diabetes Society for investigator‐driven adjustments 12 . Blood glucose levels were measured using a Glutest Neo Super blood glucometer (Sanwa Kagaku Kenkyusho Co., Ltd., Aichi, Japan) at seven different time points: pre‐breakfast (0700 hours), post‐breakfast (09.00 hours), pre‐lunch (12.00 hours), post‐lunch (14.00 hours), pre‐dinner (18.00 hours), post‐dinner (20.00 hours) and bedtime (22.22 hours). Participants continued their oral hypoglycemic agents at the baseline dose throughout the study; the use of other, additional antihyperglycemic medications was prohibited during the study period while participants continued on study medication.
All participants underwent an hour of nutritional counseling with an experienced dietitian during the course of their treatment. Each patient’s diet comprised 30 kcal/kg/day total calories for an ideal body mass index (BMI) of 22, with carbohydrate content equivalent to 50–60%, 20–30% fat content and 15–20% protein content. All participants also underwent exercise counseling (5–6 metabolic equivalent estimations for 30 min daily) during the study.
Efficacy end‐points
The primary end‐points of the present study were the glucose variability indices calculated as M‐values 13 , which was obtained from blood glucose profiles at the seven time points until week 2.
Blood glucose profiles at the seven time points and mean of blood glucose, which is calculated from seven‐time blood glucose levels (three times pre‐ and post‐meal and bedtime), were assessed in a secondary end‐point. Secondary end‐points included BMI, C‐peptide immunoreactivity, 1,5‐anhydroglucitol (1,5‐AG) blood, liver function, fasting lipid profile (total cholesterol, triglycerides and high‐density lipoprotein cholesterol), basal energy expenditure, flow‐mediated dilation (FMD), cardiac autonomic nerve activity, total insulin dose and treatment satisfaction.
Measurement of basal energy expenditure and respiratory quotient
After overnight food deprivation (12–14 h), the participants rested on a bed in a supine position for 30 min (07.00–07.30 hours). After this, they remained recumbent at room temperature for a further 10 min until 07.40 hours. Oxygen consumption (VO2) and carbon dioxide production (VCO2) were then measured by indirect calorimetry using an AE‐310s Aeromonitor (Minato Medical Science, Osaka, Japan). Energy expenditure was calculated from VO2 and VCO2, levels using the de Weir equation 14 . Before the measurements were taken, the participants rested for >30 min in the supine position. Energy expenditure and respiratory quotient were measured for 10 min.
Assessment of cardiac autonomic nerve activity
Participants underwent 24‐h ambulatory Holter electrocardiograms. Beat‐to‐beat fluctuations in heart rate (HR) or variations in consecutive R‐R intervals are conventionally described by the term, HR variability. The characteristics of the rhythmic fluctuations of cardiovascular parameters, particularly of HR, could be evaluated and quantitatively assessed in numerous physiological and pathological conditions due to the development of automated techniques for measurements, including power spectral analyses. Three components can be found in HR variability power spectrum: (i) a peak at a respiratory frequency that corresponds to respiratory sinus arrhythmia (high frequency [HF] >0.15 Hz); (ii) a peak centered at approximately 0.1 Hz, which is related to arterial pressure control (low frequency [LF] 0.04–0.15 Hz); (iii) a component at very low frequency (<0.04 Hz; sometimes as a peak) considered to be an expression of the peripheral vasomotor regulation. The amplitude and, to a lesser extent, the frequency of the fluctuations have been shown to continuously change as responses of cardiovascular control systems through autonomic nerves. Fluctuations >0.15 Hz are due to vagal activity only, whereas fluctuations <0.15 Hz are mediated by both cardiac vagal and sympathetic nerves. Thus, quantitative information on the autonomic control of the heart can be obtained from the HR variability power spectral analysis related to the separation of the different components in various physiological conditions 15 . LF, HF and the LF/HF ratio represent sympathetic and parasympathetic nerve activity, and sympathovagal balance, respectively. The median 24‐h LF, 24‐h HF and 24‐h low to high frequency power were 796.8 ± 436.7, 382.0 ± 201.3 and 1.61 ± 0.79 in participants with normal glucose tolerance, respectively. Meanwhile, these in participants with diabetes were 733.8 ± 417.4, 255.7 ± 186.6 and 2.21 ± 1.58, respectively 10 . In another study, the median 24‐h LF and 24‐h HF were 692.8 ± 202.7 and 446.8 ± 102.4 in the healthy participants, respectively. Meanwhile, these in participants with diabetes were 18.6 ± 3.1 and 17.9 ± 3.4, respectively 11 . Therefore, these past studies suggested that cardiac autonomic nerve activity is markedly decreased in type 2 diabetes patients.
We also examined the logarithmically converted cardiac autonomic nerve activity (Figure 2) in accordance with past report 5 , which can decrease the variability of data and make data conform more closely to the normal distribution.
Figure 2.
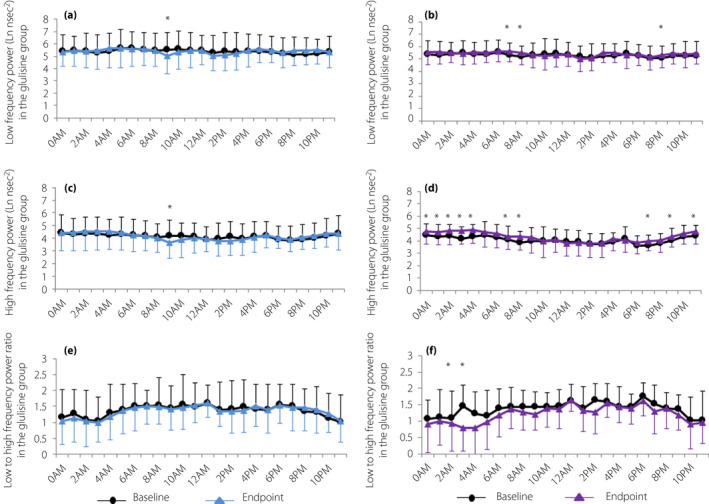
Cardiac autonomic nerve function. Diurnal variations in low‐frequency power at baseline and end‐point in the (a) glulisine group and (b) insulin glargine group. Diurnal variations in high‐frequency power at baseline and end‐point in the (c) glulisine group and (d) insulin glargine group. Diurnal variations in low‐ to high‐frequency power ratio at baseline and end‐point in the (e) glulisine group and (f) insulin glargine group. Data are expressed as the mean ± standard deviation. *P < 0.05 for comparisons between baseline and end‐point.
Measurement of flow‐mediated dilation
FMD represents the vascular endothelium function, whereby the vasodilator response to increased arterial flow depends on the local bioavailability of nitric oxide 16 . Brachial artery FMD is induced by a pneumatic cuff inflated around the forearm, followed by rapid deflation. The level of dilation is expressed as a maximal percentage change in brachial artery diameter from baseline 17 .
Treatment satisfaction
Treatment satisfaction was another secondary outcome, which we assessed using the Diabetes Treatment Satisfaction Questionnaire 18 . The overall treatment satisfaction score was calculated as the sum of Diabetes Treatment Satisfaction Questionnaire items 1 (Satisfaction), 4 (Convenience), 5 (Flexibility), 6 (Understanding), 7 (Recommend to others) and 8 (Wish to continue). Items 2 (Perceived hyperglycemia frequency) and 3 (Perceived hypoglycemia frequency) were taken as separated variables.
Statistical analysis
The results were expressed as mean ± standard deviation. Statistical analysis was carried out by the Statistical Package for the Social Sciences (version 20.0; SPSS, Inc., Chicago, IL, USA). Paired t‐tests and Wilcoxon’s signed‐rank tests were carried out to evaluate the significance of differences between the groups from baseline. Covariance, with baseline variables as covariates, was analyzed to compare changes in variables from baseline. All analysis was performed on the full set under investigation. P‐values <0.05 were considered significant.
Results
Participant characteristics
A total of 44 consenting participants were screened. We randomly assigned 22 of these to the IGlu group, whereas 22 were put into the IGla group. The median age of participants was 54.6 ± 13.1 years, their median BMI was 26.9 ± 5.8 kg/m2 and the median duration of disease was 7.3 ± 10.2 years. The median HbA1c at baseline was 10.7 ± 2.4% in the IGlu group and 11.1 ± 2.3% in the IGla group, with no significant differences between them. All participants did not have any experience using insulin before the present study. Both groups were generally well‐balanced with respect to baseline demographics and disease characteristics (Table 1, Figure 1), except for FMD, which was significantly higher in the IGlu group at baseline (P = 0.040). Of the 44 participants enrolled, none withdrew consent or dropped out of this study.
Table 1.
Treatment effects of bodyweight and metabolic parameters in both groups
| Bolus first (glulisine) | P * | Basal first (glargine) | P * | P ** | P *** | |||
|---|---|---|---|---|---|---|---|---|
| Baseline | End‐point | Baseline | End‐point | |||||
| Sex (male/female) | 8/14 | 8/14 | ||||||
| Combination therapy (BG/SU/DPP4i/Gli/aGI) | 9/7/6/3/2 | 5/3/4/1/3 | ||||||
| Age (years) | 51.7 ± 14.9 | 57.5 ± 10.6 | ||||||
| Diabetic duration (years) | 7.4 ± 10.9 | 7.2 ± 9.6 | ||||||
| M‐value | 81.0 ± 70.8 | 9.1 ± 7.9 | <0.001 | 96.9 ± 57.0 | 21.4 ± 15.1 | <0.001 | 0.002 | 0.725 |
| Mean blood glucose (mg/dL) | 249.8 ± 84.6 | 135.3 ± 20.8 | <0.001 | 270.4 ± 60.2 | 164.1 ± 29.3 | <0.001 | 0.410 | 0.539 |
| Body mass index (kg/m2) | 27.1 ± 5.3 | 26.3 ± 5.3 | 0.001 | 26.7 ± 6.3 | 26.2 ± 6.1 | 0.002 | 0.707 | 0.526 |
| 1,5‐anhydroglucitol (µg/mL) | 3.4 ± 2.9 | 6.6 ± 3.7 | <0.001 | 2.4 ± 2.6 | 3.8 ± 1.8 | 0.001 | 0.001 | 0.003 |
| C‐peptide immunoreactivity (ng/mL) | 2.3 ± 1.0 | 1.9 ± 0.7 | 0.018 | 1.9 ± 0.8 | 0.9 ± 0.6 | <0.001 | <0.001 | 0.002 |
| Aspartate transaminase (IU/L) | 40.6 ± 36.5 | 27.4 ± 17.4 | 0.042 | 30.1 ± 19.7 | 26.0 ± 11.7 | 0.205 | 0.733 | 0.394 |
| Alanine transaminase (IU/L) | 60.0 ± 53.8 | 40.0 ± 33.8 | 0.003 | 39.1 ± 32.5 | 35.1 ± 24.6 | 0.424 | 0.817 | 0.089 |
| Total cholesterol (mg/dL) | 208.3 ± 44.3 | 185.3 ± 40.5 | 0.001 | 203.4 ± 50.4 | 170.1 ± 35.9 | 0.001 | 0.520 | 0.674 |
| Triglycerides (mg/dL) | 199.4 ± 109.3 | 130.0 ± 48.6 | 0.002 | 164.8 ± 70.4 | 112.0 ± 38.2 | <0.001 | 0.229 | 0.920 |
| HDL cholesterol (mg/dL) | 41.2 ± 12.1 | 42.6 ± 11.7 | 0.257 | 44.7 ± 10.8 | 40.6 ± 7.7 | 0.020 | 0.592 | 0.017 |
| Basal energy expenditure (kcal/day) | 1,489.3 ± 353.8 | 1,459.1 ± 317.3 | 0.235 | 1,427.6 ± 234.8 | 1,336.1 ± 222.0 | 0.003 | 0.196 | 0.058 |
| Respiratory quotient | 0.83 ± 0.04 | 0.85 ± 0.06 | 0.431 | 0.82 ± 0.06 | 0.83 ± 0.06 | 0.593 | 0.497 | 0.878 |
| Flow‐mediated dilation (%) | 5.7 ± 2.2 | 5.9 ± 2.3 | 0.848 | 4.3 ± 2.3 | 4.5 ± 2.1 | 0.468 | 0.042 | 0.894 |
| 24‐h low frequency power (ms2) | 387.1 ± 261.2 | 379.7 ± 307.0 | 0.601 | 290.7 ± 304.0 | 320.4 ± 372.1 | 0.295 | 0.441 | 0.888 |
| 24‐h high‐frequency power (ms2) | 106.0 ± 86.7 | 118.3 ± 112.0 | 0.179 | 83.9 ± 71.6 | 135.8 ± 1,51.9 | 0.004 | 0.617 | 0.177 |
| 24‐h low‐ to high‐frequency power | 5.43 ± 3.64 | 4.82 ± 2.76 | 0.100 | 4.69 ± 1.75 | 3.88 ± 1.44 | 0.024 | 0.490 | 0.697 |
| The achieved time to the target glucose | 9.4 ± 3.2 | 11.2 ± 3.4 | 0.069 | |||||
| Insulin dose (unit/kg) | 0.57 ± 0.38 | 0.27 ± 0.15 | 0.003 | |||||
All values are the mean ± standard deviation.
aGI, α‐glucosidase inhibitor; BG, biguanide; DPP4i, dipeptidyl peptidase‐4 inhibitor; Gli, glinide; SU, sulphonylurea.
P‐value for the intragroup comparison (baseline vs end‐point).
P‐value for the intergroup comparison (end‐point).
P‐value for the intergroup comparison (change from baseline between groups).
Figure 1.
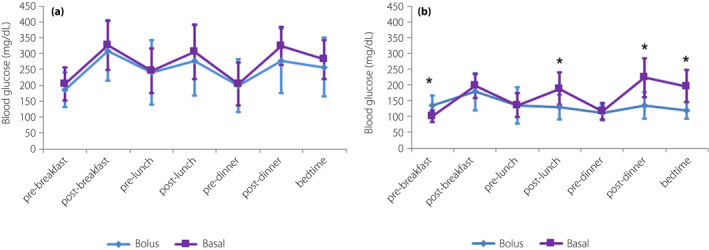
Treatment effects on blood glucose values of seven‐point self‐monitoring. Basal, the glargine group; Bolus, the insulin glulisine group. All values are the mean ± standard deviation. *P < 0.05 for the intergroup comparison (end‐point).
Glucose variability
The M‐value and mean blood glucose levels were significantly reduced from baseline in both groups. In addition, the M‐value at the end‐point in the IGlu group was significantly lower than in the IGla group (Table 1). Blood glucose profiles at baseline were found to be similar in both groups. At the end‐point, these levels had significantly decreased from baseline in both groups at all‐time points. Post‐lunch, post‐dinner and bedtime blood glucose levels at the end‐point were significantly lower among participants in the IGlu group than those in the IGla group. Both regimens significantly lowered nadir fasting blood glucose levels at the end, although these were far lower in the IGla group than in the IGlu group (Figure 1).
Metabolic profile and energy homeostasis
Total insulin doses at the end‐point were significantly higher in the IGlu group than in the IGla group. The achieved time to the target glucose levels were 9.4 ± 3.2 days in the IGlu group and 11.2 ± 3.4 days in the IGla group. BMI was significantly reduced in both groups, with no significant differences between them. 1,5‐AG was significantly elevated in both groups, and IGlu was found to be superior to IGla in reducing glucose variability. C‐peptide immunoreactivity was significantly decreased in both groups and significantly lower still in the IGla group. Serum aspartate aminotransferase and alanine aminotransferase were significantly reduced in the IGlu group, but not in the IGla group. Total cholesterol and triglycerides levels were significantly reduced in both groups, with no significant differences between them. High‐density lipoprotein cholesterol was significantly lower only in the IGla group. Basal energy expenditure was significantly reduced in the IGla group, but not in the IGlu group. The respiratory quotient was not changed in either group (Table 1). Adverse events, including severe hypoglycemic events, did not occur in the present study.
Cardiac autonomic nerve function
HF power and the LF/HF ratio that reflects cardiac autonomic nerve were not associated with fasting blood glucose, HbA1c and glucose variability in both groups (data not shown). The 24‐h HF significantly increased in the IGla group, whereas the 24‐h LF/HF ratio significantly fell (Table 1). In particular, IGla – but not IGlu – significantly increased the HF during night‐time, and decreased the LF/HF ratio at dawn (03.00–04.00 hours; Figure 2). There was no significant association between changes in indices for glucose variability and changes in cardiac sympathetic activity before and after insulin therapy in both groups (data not shown).
Endothelial function
FMD was significantly higher in the IGlu group at baseline and also at the end. No significant changes in FMD were observed in either group after the intervention (Table 1). In the IGlu group, changes in FMD were negatively associated with changes in 1,5‐AG: the greater the increase in 1,5‐AG, the more FMD would decrease. In the IGla group, changes in FMD were negatively associated with changes in 24‐h HF and LF: the greater the increase of these frequencies, the more FMD would decrease (Table S1).
Treatment satisfaction
Treatment satisfaction was assessed by Diabetes Treatment Satisfaction Questionnaire, which found that overall approval was not different between the groups. Neither were there significant differences in terms of the groups’ responses to questions, such as the perceived frequency of hyperglycemia (item 2) or hypoglycemia (item 3; Table 2).
Table 2.
Treatment satisfaction in both groups
| Bolus first | Basal first | P | |
|---|---|---|---|
| Item 1 | 5.1 ± 1.4 | 4.7 ± 1.3 | 0.166 |
| Item 2 | 2.4 ± 1.5 | 2.9 ± 1.8 | 0.317 |
| Item 3 | 1.0 ± 1.3 | 1.5 ± 1.6 | 0.235 |
| Item 4 | 4.0 ± 1.4 | 4.5 ± 1.3 | 0.307 |
| Item 5 | 4.1 ± 1.5 | 4.2 ± 1.1 | 0.890 |
| Item 6 | 4.5 ± 0.9 | 4.9 ± 0.9 | 0.151 |
| Item 7 | 5.0 ± 1.0 | 4.7 ± 1.0 | 0.312 |
| Item 8 | 4.3 ± 1.4 | 4.5 ± 1.4 | 0.604 |
| Sum | 26.9 ± 5.9 | 27.5 ± 5.2 | 0.742 |
‘Satisfaction with the treatment’ for item 1, ‘Perceived hyperglycemia frequency’ for item 2, ‘Perceived hypoglycemia frequency’ for item 3, ‘Convenience of the treatment’ for item 4, ‘Flexibility of the treatment’ for item 5, ‘Understanding of your diabetes’ for item 6, ‘Recommend to others’ for item 7 and ‘Wish to continue treatment’ for item 8. Sum was the overall treatment satisfaction score, which was calculated as the sum of Diabetes Treatment Satisfaction Questionnaire items 1 (satisfaction with the treatment), 4 (convenience of the treatment), 5 (flexibility of the treatment), 6 (understanding of your diabetes), 7 (recommend to others) and 8 (wish to continue treatment). Data are means ± standard deviation. P‐value for the intragroup comparison (end‐point).
Discussion
We carried out an open‐label randomized trial comparing multiple effects of bolus insulin and basal insulin therapy in Japanese participants with type 2 diabetes. Both regimens significantly reduced nadir fasting blood glucose levels, which were much lower in the IGla group at the end. Glucose variability (M‐value) and mean blood glucose levels were significantly decreased from baseline in both groups, although the former was significantly lower in the IGlu group than in the IGla group.
The hospital period in the present study took almost 14 days, making it much shorter than both the 4‐T study 6 , which took place over a period of 44 weeks, and the APOLLO study, which was carried out over a period of 3 years 7 . Both IGlu and IGla regimens in the present study significantly reduced glucose variables, such as M‐value, mean blood glucose and 1,5‐AG, and had different effects on the circadian regulation of blood glucose. The IGlu regimen was associated with lower postprandial blood glucose levels, especially after lunch and dinner, and at bedtime. In contrast, the IGla regimen was associated with lower fasting blood glucose. This was significantly lower in the basal‐first regimen in the present study, and similar to the APOLLO study 7 . Post‐lunch, post‐dinner and bedtime blood glucose levels were significantly lower in the bolus‐first regimen in the present study, and similar to previous reports 5 , 6 , 7 .
In the present study, both regimens led to significantly lower weight, with no significant difference between the groups. The main mechanism might be appropriate diet therapy and exercise intervention under hospitalization independently of insulin therapy, because the participants in the present study were obese (median BMI was 26.9 ± 5.8 kg/m2), thus many of them must have taken excessive energy intake before hospitalization. In contrast, insulin therapy has generally been found to increase weight through its anabolic effects. Indeed, all insulin regimens in the 4‐T and APOLLO studies resulted in significantly increased weight, with a greater increase in weight through bolus insulin than through basal insulin. The addition of bolus insulin reduced postprandial blood glucose levels more significantly than basal insulin, although it was also associated with a greater risk of hypoglycemia and weight gain 6 . More than 90% of participants in past studies 6 , 7 took sulphonylurea as a medication, perhaps increasing the risk of hypoglycemia and weight gain. In contrast, 31.8% of participants in the present study (45.5% in the IGlu group and 18.2% in the IGla group) took sulphonylurea or glinide as medication. Both IGlu and IGla led to similarly reduced weight levels in the present study with no significant differences between groups. This appears to be inconsistent with our previous study comparing insulin aspart and insulin detemir, which showed that insulin detemir reduced weight more than insulin aspart 5 . We speculate that these inconsistent weight results might highlight the possible specific profile of insulin detemir. It is reported that various insulin analogs exert distinct signaling properties in target cells 19 , 20 . It is hypothesized that fatty acids binding to the lysine amino acid at position B29 in insulin detemir might enable transfer through the blood–brain barrier and inhibit appetite through insulin’s neural action, but the efficiency of each insulin analog passing through the blood–brain barrier remains unknown. Another possible mechanism might be derived from different profiles of insulin analogs in action on orexigenic/anorexigenic peptides. In type 2 diabetic rats, both insulin detemir and glargine downregulate the hypothalamic and orexigenic factors, neuropeptide Y and galanin, and reduce weight 21 . Insulin determir is more prominent than glargine in these actions 21 . These experimental findings are consistent with our previous and present observations on weight.
Exogenous insulin administration has significantly lower basal energy expenditure in patients with diabetes 22 . Because insulin is principally an anabolic hormone, both insulin regimens might reduce the energy expenditure. In the present study, only the basal insulin regimen significantly reduced basal energy expenditure. Basal energy expenditure was measured before breakfast. At that time, insulin reduced the basal energy expenditure only in the basal insulin group (IGla group).
People with type 2 diabetes are reported to be low in HF and high in LF/HF in the power spectrum of RR intervals 10 , 11 . In the present study, IGla, but not IGlu, significantly increased the 24‐h HF, especially during night‐time, and reduced the 24‐h LF/HF ratio, especially at dawn. The present study showed that IGla might be useful for improving cardiac sympathovagal balance in type 2 diabetes. Considering the evidence that elevated sympathetic activity at dawn is relevant to increased cardiovascular events in people with type 2 diabetes 23 , 24 . IGla might be useful in preventing cardiovascular events by reducing sympathetic tone at dawn. Because IGla reduced nadir fasting blood glucose levels more than IGlu, night‐time/dawn glucose‐lowering and/or basal insulin supplementation might upregulate vagal tone and reduce sympathetic activity. In addition, the lowest LF and HF were associated with higher cardiovascular and stroke risk 25 , 26 . These findings were inconsistent with the present data showing that changes in FMD were negatively associated with changes in 24‐h HF and LF in the IGla group. On the possible role of insulin, however, this hypothesis seems inconsistent with its acute action 5 . In rats, insulin injections to the arcuate nucleus evoke a sympathoexcitatory response, which is canceled with an anti‐insulin affibody 27 . In humans, acute infusion of insulin in a hyperinsulinemic‐euglycemic clamp study reduces HF and elevates LF/HF in healthy individuals 28 . Interestingly, in the present study, the acute hyperinsulinemia‐induced elevation of LF/HF was not evident in the insulin‐resistant individuals 28 . These findings, together with our present observations, hint at the complex nature of insulin action on the sympathetic nervous system: it might vary depending on endogenous or exogenous insulin, acute or chronic administration, and central and peripheral action. Another possibility is that glucose lowering is attributable to reducing LF/HF. It is reported that metformin administration at a dose of 1,700 mg/day for 4 months in individuals with type 2 diabetes significantly elevated HF (from 22.4 ± 1.8 to 23.6 ± 2.1) and reduced LF/HF (from 4.7 ± 0.3 to 2.9 ± 0.2) 29 . In that study, metformin administration reduced both glucose and insulin levels. In the present study, IGla, but not IGlu, significantly decreased nadir fasting glucose levels, which might be relevant to the elevated HF at night‐time and reduced LF/HF at dawn.
In conclusion, both IGlu and IGla regimens decrease glucose variability, with a greater decrease in M‐value by IGlu in people with poorly controlled type 2 diabetes. IGla, but not IGlu, elevated parasympathetic tone at night‐time and reduced sympathetic nerve activity at dawn. Although additional surrogate markers are required, IGla might be useful for preventing cardiovascular events by reducing sympathetic tone at dawn. These findings shed light on the previously unrecognized role of night‐time basal insulin supplementation on sympathovagal activity in type 2 diabetes.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 | Pearson correlation coefficient between change of flow‐mediated dilation, and characteristics in the insulin glulisine and insulin glargine groups.
Acknowledgment
We thank all participants and researchers in eligible studies.
J Diabetes Investig 2021; 12: 1193–1201
Clinical Trial Registry
University Hospital Medical Information Network Clinical Trials Registry
UMIN 000010353
References
- 1. Buse JB, Wexler DJ, Tsapas A, et al. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020; 43: 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kabadi UM, Kabadi M, Moines D, et al. Early postprandial Insulin secretion: Its relation to Insulin sensitivity. J Diabetes Mellit 2011; 1: 1–5. [Google Scholar]
- 3. Node K, Inoue T. Postprandial hyperglycemia as an etiological factor in vascular failure. Cardiovasc Diabetol 2009; 8: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takamura T, Sakurai M, Nakamura M, et al. Factors associated with improvement of fasting plasma glucose level by mealtime dosing of a rapid‐acting insulin analog in type 2 diabetes. Diabetes Res Clin Pract 2007; 75: 278–284. [DOI] [PubMed] [Google Scholar]
- 5. Kanamori T, Takeshita Y, Isobe Y, et al. Mealtime dosing of a rapid‐acting insulin analog reduces glucose variability and suppresses daytime cardiac sympathetic activity: a randomized controlled study in hospitalized patients with type 2 diabetes. BMJ Open Diabetes Res Care 2018; 6: e000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holman RR, Farmer AJ, Davies MJ, et al. Three‐year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 2009; 361: 1736–1747. [DOI] [PubMed] [Google Scholar]
- 7. Bretzel RG, Nuber U, Landgraf W, et al. Once‐daily basal insulin glargine versus thrice‐daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 2008; 371: 1073–1084. [DOI] [PubMed] [Google Scholar]
- 8. Di Flaviani A, Picconi F, Di Stefano P, et al. Impact of glycemic and blood pressure variability on surrogate measures of cardiovascular outcomes in type 2 diabetic patients. Diabetes Care 2011; 34: 1605–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buscemi S, Re A, Batsis JA, et al. Glycaemic variability using continuous glucose monitoring and endothelial function in the metabolic syndrome and in type 2 diabetes. Diabet Med 2010; 27: 872–878. [DOI] [PubMed] [Google Scholar]
- 10. Wu J‐S, Yang Y‐C, Lin T‐S, et al. Epidemiological evidence of altered cardiac autonomic function in subjects with impaired glucose tolerance but not isolated impaired fasting glucose. J Clin Endocrinol Metab 2007; 92: 3885–3889. [DOI] [PubMed] [Google Scholar]
- 11. Perciaccante A, Fiorentini A, Paris A, et al. Circadian rhythm of the autonomic nervous system in insulin resistant subjects with normoglycemia, impaired fasting glycemia, impaired glucose tolerance, type 2 diabetes mellitus. BMC Cardiovasc Disord 2006; 6: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int 2020; 11: 165–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Service FJ. Glucose variability. Diabetes 2013; 62: 1398–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weir J. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949; 109: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perini R, Veicsteinas A. Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions. Eur J Appl Physiol 2003; 90: 317–325. [DOI] [PubMed] [Google Scholar]
- 16. Green DJ, Maiorana A, O’Driscoll G, et al. Effect of exercise training on endothelium‐derived nitric oxide function in humans. J Physiol 2004; 561: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Donald AE, Charakida M, Cole TJ, et al. Non‐invasive assessment of endothelial function. J Am Coll Cardiol 2006; 48: 1846–1850. [DOI] [PubMed] [Google Scholar]
- 18. Bradley C, Plowright R, Stewart J, et al. The Diabetes Treatment Satisfaction Questionnaire change version (DTSQc) evaluated in insulin glargine trials shows greater responsiveness to improvements than the original DTSQ. Health Qual Life Outcomes 2007; 5: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wada T, Azegami M, Sugiyama M, et al. Characteristics of signalling properties mediated by long‐acting insulin analogue glargine and detemir in target cells of insulin. Diabetes Res Clin Pract 2008; 81: 269–277. [DOI] [PubMed] [Google Scholar]
- 20. Tsuneki H, Yoshida H, Endo K, et al. Different impacts of acylated and non‐acylated long‐acting insulin analogs on neural functions in vitro and in vivo. Diabetes Res Clin Pract 2017; 129: 62–72. [DOI] [PubMed] [Google Scholar]
- 21. Zafar MI, Hu C, Liu D, et al. Insulin detemir causes lesser weight gain in comparison to insulin glargine: role on hypothalamic NPY and galanin. J Diabetes Res 2014; 2014: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ikeda K, Fujimoto S, Goto M, et al. Impact of endogenous and exogenous insulin on basal energy expenditure in patients with type 2 diabetes under standard treatment. Am J Clin Nutr 2011; 94: 1513–1518. [DOI] [PubMed] [Google Scholar]
- 23. Huggett RJ, Scott EM, Gilbey SG, et al. Disparity of autonomic control in type 2 diabetes mellitus. Diabetologia 2005; 48: 172–179. [DOI] [PubMed] [Google Scholar]
- 24. Bellavere F, Cacciatori V, Moghetti P, et al. Acute effect of insulin on autonomic regulation of the cardiovascular system: a study by heart rate spectral analysis. Diabet Med 1996; 13: 709–714. [DOI] [PubMed] [Google Scholar]
- 25. Kubota Y, Chen LY, Whitsel EA, et al. Heart rate variability and lifetime risk of cardiovascular disease: the Atherosclerosis Risk in Communities Study. Ann Epidemiol 2017; 27: 619–625.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fyfe‐Johnson AL, Muller CJ, Alonso A, et al. Heart rate variability and incident stroke. Stroke 2016; 47: 1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luckett BS, Frielle JL, Wolfgang L, et al. Arcuate nucleus injection of an anti‐insulin affibody prevents the sympathetic response to insulin. Am J Physiol Circ Physiol 2013; 304: H1538–H1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paolisso G, Manzella D, Rizzo MR, et al. Effects of insulin on the cardiac autonomic nervous system in insulin‐resistant states. Clin Sci 2000; 98: 129–136. [DOI] [PubMed] [Google Scholar]
- 29. Manzella D. Blood pressure and cardiac autonomic nervous system in obese type 2 diabetic patients: effect of metformin administration. Am J Hypertens 2004; 17: 223–227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Pearson correlation coefficient between change of flow‐mediated dilation, and characteristics in the insulin glulisine and insulin glargine groups.


