Abstract
Aims/Introduction
Patients with type 2 diabetes mellitus have a higher bone fracture risk than patients without diabetes. Although denosumab (Dmab) is a potent bone resorption inhibitor, its efficacy in patients with type 2 diabetes mellitus has not been elucidated. In this study, we investigated the effects of switching to Dmab from bisphosphonates (BP) or a selective estrogen receptor modulator (SERM) in postmenopausal type 2 diabetes mellitus patients.
Materials and Methods
This was a three medical institutions, prospective, observational study for postmenopausal patients with type 2 diabetes mellitus whose T‐score of femoral neck or lumbar spine bone mineral density was under −1.0 standard deviation, even after >6 months of BP or SERM administration. After obtaining consent, participants were treated for osteopenia/osteoporosis by either continuing BP (BP‐BP group)/SERM (SERM‐SERM group), or by switching to Dmab (BP‐Dmab or SERM‐Dmab groups). Changes in bone mineral density and bone metabolism marker levels were evaluated after 6 months.
Results
A total of 48 patients were included in this study, and each group comprised 12 patients. No significant difference existed in baseline characteristics among the groups. The average age and glycated hemoglobin were 71 ± 8 years and 7.2 ± 0.9%, respectively. In the SERM‐Dmab group, lumbar spine bone mineral density was significantly increased by 5.0% compared with the SERM‐SERM group (P < 0.04). Serum bone‐specific alkaline phosphatase and tartrate‐resistant acid phosphatase 5b were significantly decreased in the BP‐Dmab and SERM‐Dmab groups compared with the BP‐BP and SERM‐SERM groups, respectively.
Conclusions
Switching to Dmab from BP or SERM is beneficial to prevent osteoporosis progression in postmenopausal patients with type 2 diabetes mellitus patients.
Keywords: Denosumab, Osteoporosis, Type 2 diabetes
We showed more favorable effects of denosumab on bone metabolism compared with bisphosphonates or selective estrogen receptor modulator alone in postmenopausal patients with type 2 diabetes mellitus. Switching from bisphosphonates or selective estrogen receptor modulator to denosumab provides an effective treatment strategy to prevent the progression in postmenopausal patients with type 2 diabetes mellitus who are still in the osteopenia or osteoporosis state after treatment with bisphosphonates or selective estrogen receptor modulator and active vitamin D.

Introduction
Because of the rapid increase in our aging population, the number of patients with osteoporosis is rising each year and is estimated to be 13 million in Japan 1 . In addition, the frequency of fractures in diabetes patients is higher than in patients without diabetes, and the relative risk of proximal femoral fractures is three‐ to sevenfold higher in patients with type 1 diabetes 2 , 3 , 4 , 5 and 1.3–2.8‐fold higher in patients with type 2 diabetes mellitus 2 , 3 , 6 . Bone strength is defined by two factors: bone mineral density (BMD) and bone quality. It is well‐known that fracture rates are higher in patients with type 2 diabetes mellitus compared with the non‐diabetic population, even at the equivalent BMD, as a result of their deteriorated bone quality 7 , 8 , and that BMD does not always correlate with fracture events in type 2 diabetes mellitus patients 9 , 10 .
Denosumab (Dmab) is a fully human monoclonal antibody with a high affinity for receptor activator of nuclear factor‐kappa B ligand, which is a key mediator of osteoclast differentiation, activation and survival. Dmab suppresses osteoclastogenesis and bone resorption, resulting in decreases in the levels of bone resorption markers and increases in BMD in the lumbar spine (LS) and femoral neck (FN) compared with the bisphosphonate (BP) preparation 11 . Several studies reported switching to Dmab from BPs, and Dmab was shown to be superior to almost all commercially available BPs in terms of BMD increase and bone turnover marker suppression 12 , 13 , 14 , 15 , 16 . BMD increases in response to Dmab were similar between responders and non‐responders to previous BP treatment 17 . It was also shown that LS and FN BMD values increased significantly at weeks 24 and 48 compared with week 0 after switching from SERM to Dmab in 19 osteoporotic women 18 . Although Dmab is effective in patients with BP‐ and SERM‐resistant primary osteoporosis 12 , 17 , there have been no reports on its effects in the type 2 diabetes mellitus population or the effects of switching from SERM to Dmab. Therefore, we investigated the changes in BMD and bone quality markers that occur after switching to Dmab in BP‐ or SERM‐resistant postmenopausal type 2 diabetes mellitus patients.
Methods
Study population
Postmenopausal Japanese women with type 2 diabetes mellitus who regularly visited Hokkaido University Hospital, Sapporo Medical Center NTT EC and Hokkaido Spinal Cord Injury Center, Sapporo, Japan, were assessed for eligibility for the present study. All participants provided written informed consent before study enrollment. The inclusion criteria were as follows: type 2 diabetes complicated with postmenopausal osteopenia/osteoporosis even after taking BP or SERM with active vitamin D (VitD) for >6 months, FN or LS BMD young adult mean value ≤80% (equivalent to T‐score <−1.0 standard deviation [SD]) and glycated hemoglobin level ≤10%.
We excluded patients who were prescribed pioglitazone, would be taking incretin‐related drugs during the observation period or had serious liver/renal dysfunction and hypocalcemia, which is a contraindication for Dmab. Serious liver dysfunction was defined as the serum liver transaminases more than twice as high as the upper limit of normal or when total bilirubin was above the upper limit of normal. Serious renal dysfunction was defined as the estimated glomerular filtration rate <30 mL/min/1.73 m2. The present study was approved by the ethics committee of each participating site, and it was carried out in accordance with the Declaration of Helsinki (No. 013‐0351). This study was registered with the UMIN Clinical Trial Registry (UMIN 000015136).
Protocol
This was a 24‐week, prospective, parallel‐group, observational study carried out in the three medical institutions conducted from September 2014 to September 2016. Patients were either switched from BP/SERM to Dmab (BP‐Dmab group/SERM‐Dmab group, respectively) or continued BP/SERM therapy (BP‐BP group/SERM‐SERM group, respectively) according to each attending physician’s decision. Active VitD was continued at the same dose. Patients received subcutaneous Dmab (60 mg/6 months, Daiichi Sankyo Company, Tokyo, Japan). The primary outcomes of this study were the changes in BMD and bone metabolic markers after 24 weeks. LS and FN BMD were assessed using T‐scores and young adult mean values obtained by dual‐energy X‐ray absorptiometry (Discovery X; Hologic, Waltham, MA, USA). In addition to 25‐OH‐VitD, bone metabolic markers, including serum bone‐specific alkaline phosphatase (BAP), serum tartrate‐resistant acid phosphatase 5b (TRACP‐5b), plasma pentosidine (PEN) and serum undercarboxylated osteocalcin, were evaluated and measured at the central research center of SRL (SRL, Inc., Tokyo, Japan). Other blood urine tests, including glycated hemoglobin, and other data, such as blood pressure and bodyweight, were collected at baseline and 24 weeks of observation. We also assessed the fracture risk assessment tool (FRAX®) score, which was developed by the World Health Organization as an index of the risk for major osteoporotic and hip fractures within the next 10 years.
Statistical analysis
The data were analyzed and compared using BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan). Continuous variables were analyzed by t‐tests or the Mann–Whitney U‐test, as appropriate. We used the paired t‐test for within‐group analysis, and the unpaired t‐test to compare Dmab‐switching and control groups. Comparisons of the frequency among two groups were carried out by either the χ2‐test or Fisher’s exact test, as appropriate. P‐values < 0.05 were considered to show statistical significance.
Results
Baseline characteristics
A total of 48 type 2 diabetes mellitus patients (12 per group) were enrolled in the present study, and six patients discontinued the study during the 6‐month treatment period (Figure 1). Two in the BP‐BP group and one in the SERM‐SERM group were discontinued because of hypercalcinemia likely due to active VitD. After termination of active VitD, plasma calcium levels immediately normalized. The reasons for the discontinuation of the other three patients included one radial fracture in the SERM‐SERM group, one hospitalization for pneumonia in the SERM‐Dmab group and one hospital interruption in the SERM‐Dmab group (Figure 1). No severe adverse events were reported in any of the groups during the study.
Figure 1.
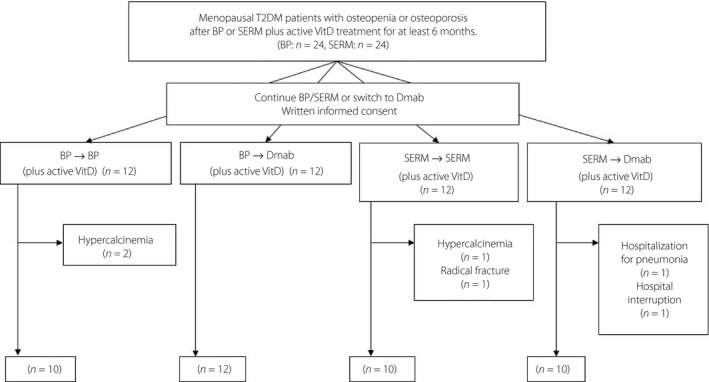
Flow diagram of the study. BP, bisphosphonate; Dmab, denosumab; SERM, selective estrogen receptor modulator; T2DM, type 2 diabetes mellitus; VitD, vitamin D.
The average of age, body mass index and glycated hemoglobin of the 48 participants were 71 ± 8 years, 25.5 ± 3.9 kg/m2 and 7.2 ± 0.9%, respectively. Of the antidiabetic agents, biguanides and dipeptidyl peptidase‐4 inhibitors accounted for as much as 60%, and overall, 17–33% of patients used insulin. Sodium–glucose cotransporter 2 inhibitors were used by just two patients. There was no significant difference in baseline characteristics among any of the groups (Table 1). The LS BMD T‐scores and FN BMD T‐scores were −1.2 ± 1.0 SD and −2.0 ± 0.4 SD, respectively. BAP, TRACP‐5b and undercarboxylated osteocalcin were 10.0 ± 3.3 SD, 196.4 ± 74.4 SD and 1.8 ± 2.2 SD, respectively. The number of patients with existing fractures other than vertebra fractures was two or less in each group, and there was no significant difference among the four groups. The baseline FRAX® score was <15% in all groups, and there was no significant difference between any of the groups (BP‐BP group 14.5 ± 5.8%, BP‐Dmab 14.6 ± 5.5%, SERM‐SERM 10.4 ± 2.5% and SERM‐Dmab 12.2 ± 4.6%; Table 1).
Table 1.
Baseline characteristics of patients
| BP group | SERM group | |||||
|---|---|---|---|---|---|---|
| BP‐BP (n = 12) | BP‐Dmab (n = 12) | P‐value | SERM‐SERM (n = 12) | SERM‐Dmab (n = 12) | P‐value | |
| Age (years) | 71.4 ± 5.7 | 71.6 ± 6.8 | NS | 72.8 ± 11.7 | 70.9 ± 11.4 | NS |
| Years since menopause | 19.3 ± 5.9 | 21.8 ± 7.3 | NS | 21.6 ± 6.2 | 21.4 ± 10.1 | NS |
| BMI (kg/m2) | 24.9 ± 4.6 | 25.7 ± 4.0 | NS | 24.0 ± 4.0 | 26.1 ± 2.6 | NS |
| LS BMD T‐score (SD) | −1.0 ± 1.0 | −1.2 ± 0.8 | NS | −1.2 ± 1.6 | −1.3 ± 1.3 | NS |
| FN BMD T‐score (SD) | −2.2 ± 0.4 | −2.0 ± 0.3 | NS | −1.8 ± 0.4 | −2.1 ± 0.4 | NS |
| HbA1c (%) | 7.1 ± 1.0 | 7.4 ± 1.2 | NS | 7.1 ± 0.3 | 7.3 ± 0.4 | NS |
| eGFR (mL/min/1.73 m2) | 60.7 ± 12.7 | 64.3 ± 15.4 | NS | 75.4 ± 15.8 | 65.4 ± 12.4 | NS |
| UACR (mg/gCre) | 15.0 ± 9.3 | 92.3 ± 101.6 | NS | 172.2 ± 280.8 | 26.0 ± 14.5 | NS |
| Albumin‐adjusted serum Ca (mg/dL) | 10.0 ± 0.3 | 9.9 ± 0.38 | NS | 9.9 ± 0.2 | 9.7 ± 0.6 | NS |
| Serum P (mg/dL) | 3.7 ± 0.8 | 3.5 ± 0.6 | NS | 3.3 ± 0.7 | 3.6 ± 0.8 | NS |
| Intact PTH (pg/mL) | 34.0 ± 18.4 | 36.7 ± 22.1 | NS | 46.8 ± 10.2 | 43.2 ± 20.3 | NS |
| 25‐OH‐VitD (ng/mL) | 21.8 ± 7.4 | 22.5 ± 6.6 | NS | 19.5 ± 6.9 | 20.3 ± 5.2 | NS |
| FRAX® score | ||||||
| Major osteoporotic (%) | 14.5 ± 5.8 | 14.6 ± 5.5 | NS | 10.4 ± 2.5 | 12.2 ± 4.6 | NS |
| Hip fracture (%) | 3.6 ± 2.1 | 2.9 ± 1.5 | NS | 2.4 ± 1.5 | 2.7 ± 1.7 | NS |
| Serum pentosidine (µg/mL) | 0.0546 ± 0.0241 | 0.0538 ± 0.0247 | NS | 0.0764 ± 0.0305 | 0.0896 ± 0.0925 | NS |
| No. antidiabetes drugs | 2.3 ± 1.4 | 1.7 ± 1.1 | NS | 2.7 ± 1.4 | 2.1 ± 0.8 | NS |
| Medications (antidiabetes) | ||||||
| SU, n (%) | 3 (25) | 5 (42) | 9 (75) | 4 (33) | ||
| Biguanides, n (%) | 8 (67) | 6 (50) | 6 (50) | 8 (67) | ||
| DPP‐IV inhibitors, n (%) | 8 (67) | 6 (50) | 8 (67) | 7 (58) | ||
| GLP‐1 analogs, n (%) | 1 (8) | 0 (0) | 0 (0) | 0 (0) | ||
| SGLT2 inhibitors, n (%) | 1 (8) | 0 (0) | 1 (8) | 0 (0) | ||
| αGI, n (%) | 2 (17) | 2 (17) | 4 (33) | 2 (17) | ||
| Insulin, n (%) | 4 (33) | 3 (25) | 2 (17) | 4 (33) | ||
| Medications (anti‐osteoporosis) | ||||||
| Minodronic acid 50 mg, n (%) | 12 (100) | 12 (100) | – | – | ||
| Bazedoxifene 20 mg, n (%) | – | – | 12 (100) | 12 (100) | ||
| Eldecalcitol 0.75 µg, n (%) | 11 (92) | 10 (83) | 11 (92) | 11 (92) | ||
| Alfacalcidol 1.0 µg, n (%) | 1 (8) | 2 (17) | 1 (8) | (8) | ||
Data are reported as the mean ± standard deviation. BMD, bone mineral density; BMI, body mass index; BP, bisphosphonate; Dmab, denosumab; eGFR, estimated glomerular filtration rate; DPP‐IV, dipeptidyl peptidase‐4; GLP‐1, glucagon‐like peptide‐1; FRAX score, fracture risk assessment score; FN, femoral neck; HbA1c, glycated hemoglobin; LS, lumber spine; NS, no significance; PTH, parathyroid hormone; SERM, selective estrogen receptor modulator; SGLT2, sodium–glucose cotransporter 2; SU, sulfonylureas; UACR, urinary albumin‐to‐creatinine ratio; 25‐OH‐VitD, 25‐hydroxyvitamin D; αGI, α‐glucosidase inhibitors.
Outcomes
Although the LS BMD tended to increase in the BP‐Dmab group after 6 months (P = 0.17), there was no significant difference between changes in the BP‐BP and BP‐Dmab groups (Table 2). However, the LS BMD significantly increased in the SERM‐Dmab group from 0.88 ± 0.17% to 0.92 ± 0.15% (P = 0.04), and the change in LS BMD in the SERM‐Dmab group was significantly higher than that in the SERM‐SERM group (+5.3 ± 4.2% vs +0.3 ± 1.8%, P = 0.04; Table 3). The changes in FN BMD were not significantly different between any of the groups, between the BP‐BP group and the BP‐Dmab group, or between the SERM‐Dmab group and the SERM‐SERM group (Tables 2,3).
Table 2.
Bone mineral density and bone metabolic markers in continuing bisphosphonates and switching from bisphosphonates to denosumab groups
| BP‐BP group (n = 10) | BP‐Dmab group (n = 12) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 24‐week | Mean % Change | P‐value (in group) | Baseline | 24‐week | Mean % Change | P‐value (in group) | P‐value (between groups) | |
| LS BMD (g/cm2) | 0.91 ± 0.14 | 0.92 ± 0.15 | 1.8 ± 3.0 | 0.20 | 0.87 ± 0.09 | 0.89 ± 0.09 | 1.7 ± 3.6 | 0.17 | 0.96 |
| FN BMD (g/cm2) | 0.55 ± 0.05 | 0.57 ± 0.02 | 4.6 ± 10.4 | 0.40 | 0.59 ± 0.09 | 0.60 ± 0.09 | 1.6 ± 3.5 | 0.22 | 0.40 |
| BAP (µg/L) | 6.5 ± 0.9 | 6.6 ± 1.0 | 2.4 ± 13.9 | 0.83 | 9.8 ± 2.1 | 8.6 ± 2.7 | −13.7 ± 12.3 | 0.01 | 0.03 |
| TRACP‐5b (mU/dL) | 156.5 ± 68.8 | 166.2 ± 41.8 | 29.5 ± 16.1 | 0.02 | 173.5 ± 82.1 | 178.7 ± 89.9 | 4.4 ± 22.9 | 0.73 | 0.04 |
| Pentosidine (μg/mL) | 0.058 ± 0.029 | 0.087 ± 0.043 | 69.6 ± 96.6 | 0.17 | 0.054 ± 0.025 | 0.063 ± 0.023 | 42.0 ± 74.3 | 0.41 | 0.62 |
| ucOC (ng/mL) | 1.1 ± 0.5 | 1.4 ± 0.7 | 54.4 ± 82.5 | 0.36 | 1.4 ± 0.8 | 1.1 ± 0.4 | −11.3 ± 25.0 | 0.07 | 0.05 |
Data are reported as the mean ± standard deviation. P‐value (in group): baseline versus 24 weeks in each group. P‐value (between groups): comparisons in mean percentage change between continuing bisphosphonates (BP‐BP) and switching from bisphosphonates to denosumab (BP‐Dmab) groups. BAP, bone‐specific alkaline phosphatase; BMD, bone mineral density; BP, bisphosphonate; Dmab, denosumab; FN, femoral neck; LS, lumber spine; TRACP‐5b, tartrate‐resistant acid phosphatase 5b; ucOC, undercarboxylated osteocalcin.
Table 3.
Bone mineral density and bone metabolic markers in continuing selective estrogen receptor modulator and switching from selective estrogen receptor modulator to denosumab groups
| SERM‐SERM group (n = 10) | SERM‐Dmab group (n = 10) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 24‐week | Mean % Change | P‐value (in group) | Baseline | 24‐week | Mean % Change | P‐value (in group) | P‐value (between groups) | |
| LS BMD (g/cm2) | 0.99 ± 0.12 | 0.99 ± 0.11 | 0.3 ± 1.8 | 0.84 | 0.88 ± 0.17 | 0.92 ± 0.15 | 5.3 ± 4.2 | 0.04 | 0.04 |
| FN BMD (g/cm2) | 0.60 ± 0.04 | 0.61 ± 0.04 | 0.3 ± 2.2 | 0.18 | 0.56 ± 0.05 | 0.56 ± 0.05 | −0.2 ± 3.9 | 0.92 | 0.81 |
| BAP (µg/L) | 9.9 ± 3.8 | 9.3 ± 2.0 | 19.6 ± 20.4 | 0.21 | 11.4 ± 4.2 | 9.8 ± 5.2 | −22.6 ± 23.2 | 0.14 | 0.03 |
| TRACP‐5b (mU/dL) | 249.0 ± 164.9 | 259.8 ± 70.7 | 37.9 ± 20.5 | 0.04 | 206.2 ± 59.9 | 203.8 ± 51.7 | −7.1 ± 26.7 | 0.52 | 0.04 |
| Pentosidine (μg/mL) | 0.087 ± 0.026 | 0.099 ± 0.017 | 40.1 ± 27.3 | 0.09 | 0.061 ± 0.042 | 0.076 ± 0.031 | 21.2 ± 22.1 | 0.67 | 0.69 |
| ucOC (ng/mL) | 3.5 ± 4.4 | 3.5 ± 3.9 | 1.8 ± 16.9 | 0.34 | 1.5 ± 1.1 | 1.7 ± 0.7 | −13.1 ± 41.4 | 0.84 | 0.68 |
Data are reported as the mean ± standard deviation. P‐value (in group): baseline versus 24 weeks in each group. P‐value (between groups): comparisons in mean percentage change between continuing selective estrogen receptor modulator (SERM‐SERM) and switching from selective estrogen receptor modulator to denosumab (SERM‐Dmab) groups. BAP, bone‐specific alkaline phosphatase; BMD, bone mineral density; Dmab, denosumab; FN, femoral neck; LS, lumber spine; SERM, selective estrogen receptor modulator; TRACP‐5b, tartrate‐resistant acid phosphatase‐5b; ucOC, undercarboxylated osteocalcin.
The percentage changes in BAP and TRACP‐5b were significantly lower in the BP‐Dmab group than in the BP‐BP group (−13.7 ± 12.3 vs +2.4 ± 13.9 and +4.4 ± 22.9 vs +29.5 ± 16.1; P < 0.05, respectively; Table 2). Similarly, the percentage changes in BAP and TRACP‐5b were significantly lower in the SERM‐Dmab group than in the SERM‐SERM group (−22.6 ± 23.2 vs +19.6 ± 20.4 and −7.1 ± 26.7 vs +37.9 ± 20.5; P < 0.05, respectively; Table 3). Although BAP and TRACP‐5b changed during the 6 months, the percentage changes in PEN and undercarboxylated osteocalcin were not different between the BP‐BP and BP‐Dmab groups or the SERM‐Dmab and SERM‐SERM groups (Tables 2,3).
Discussion
This is the first prospective observational study in which BP or SERM was switched to Dmab in postmenopausal patients with osteoporosis and type 2 diabetes mellitus who had osteopenia or osteoporosis after BP or SERM treatment with active VitD. The participants of this study were aged in their early 70s, and thus, they were not too young or too old to treat their postmenopausal osteoporosis. Although the FRAX® scores of the participants were not particularly high in the present study, it has been reported that the fracture risk for type 2 diabetes mellitus should be considerably higher than the calculated FRAX® score. The actual incidence of proximal femoral fractures is 1.5‐ or 2.1‐fold higher than the FRAX® value in patients with diabetes for a longer duration of ≥5 years or ≥10 years, respectively 19 . In the present study, 91% of participants have had diabetes for ≥5 years, and 67% have had the disease for ≥10 years. Therefore, the participants in this study could be at high risk of osteoporotic fractures.
Although the present study had a short observation period, no rapid increase in fracture rate was observed by switching to Dmab. LS BMDs measured by dual‐energy X‐ray absorptiometry significantly improved in the SERM‐Dmab group, even during the relatively short 6‐month observation period. In general, Dmab shows a stronger bone resorption inhibitory effect than SERM 20 , leading to higher increases in bone density. In contrast, no improvement was observed in LS BMD between the BP‐BP and BP‐Dmab groups. The effect of VitD insufficiency on bone metabolism is negligible in this study because active VitD was given in all cases. Although the exact reason for the difference in the change of LS BMD between BP‐Dmab and SERM‐Dmab groups remains unknown, there are several possibilities. First, it might be caused by differences in the amount of BP and SERM that accumulate in bone. SERM does not accumulate in bone, whereas BP accumulates in bone over several years after administration 21 . Second, FN BMD did not significantly change during the 6‐month period compared with LS BMD, as reported in a previous study 22 . Third, postmenopausal osteoporosis patients with type 2 diabetes mellitus showed a lower response in BMD to BP than those patients without type 2 diabetes mellitus 23 . Therefore, BP‐resistant osteoporosis patients with type 2 diabetes mellitus might be poor responders to Dmab.
However, switching to Dmab significantly suppressed the levels of bone metabolic markers in both groups that were switched to Dmab, indicating the effectiveness of Dmab compared with BP or SERM for type 2 diabetes mellitus patients and the general population. At present, bone metabolic markers are considered to be effective surrogate indicators of fracture risk 24 . These markers would change more sensitively than BMD within the relatively shorter 6‐month period, which was observed in the present study 25 . In a previous study, the change in bone metabolic markers from SERM to Dmab showed a greater reduction than from BP to Dmab in the study population, including non‐type 2 diabetes mellitus patients 26 . Those results were consistent with the results of the present study in the population with type 2 diabetes mellitus. Although it cannot be fully determined during this short observational period, it is anticipated that the fracture rate will be lower in the Dmab‐switched groups than in the BP‐ and SERM‐continuation groups because of the improvement in bone metabolic markers.
Glycation is considered to contribute to the pathophysiology of osteoporosis progression, particularly in patients with type 2 diabetes mellitus 27 , and PEN is a surrogate marker of total advanced glycation end‐product formation 28 , 29 . It was previously reported that the accumulation of PEN in bone was involved in bone fragility, and that there was a certain correlation between serum and bone PEN levels 30 . SERMs are known to improve bone quality and are expected to decrease PEN in bone, but the decrease in serum PEN levels after their administration remains controversial 31 , 32 , 33 . BP and Dmab were previously reported not to decrease PEN levels in serum or bone 33 , 34 , 35 . Therefore, we measured PEN to determine if switching to Dmab from SERM or BP changed its serum levels. As a result, there was no change in the PEN value in any of the groups or differences between the groups in the present study.
The present study had some limitations. First, the study period was short. To evaluate bone metabolic markers, 12 and 24 weeks were used for bone resorption markers and bone formation markers, respectively 36 . However, 24 weeks might be too short for the accurate determination of the changes in BMD after the administration of Dmab. Second, blood collection did not include fasting blood samples, which might have affected the evaluation of PEN. Third, this study was not carried out in a randomized manner and might include selection bias, although the baseline characteristics were even between the groups. Finally, the sample size in this study was relatively small. Further studies in a randomized comparison study using a larger sample size and longer observation period in patients with type 2 diabetes mellitus would confirm our preliminary observation study.
In conclusion, we showed more favorable effects of Dmab on bone metabolism compared with BP or SERM alone in postmenopausal patients with type 2 diabetes mellitus. Switching from BP or SERM to Dmab provides an effective treatment strategy to prevent the progression in postmenopausal patients with type 2 diabetes mellitus who are still in the osteopenia or osteoporosis state after treatment with BP or SERM and active VitD.
Disclosure
AN, TA and HM have received honoraria for lectures and research funding from organizations as described below. AN obtained research support from Mitsubishi Tanabe Pharma, Daiichi Sankyo, MSD, Novo Nordisk Pharma, Novartis Pharma, AstraZeneca, LifeScan Japan and Taisho Toyama Pharmaceutical. TA received honoraria for lectures from Mitsubishi Tanabe Pharma Co., Chugai Pharmaceutical Co., Ltd., Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Pfizer Inc., AbbVie Inc., UCB Japan Co. Ltd. and Eli Lilly Japan. HK received basic research funding from Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo, Otsuka Pharmaceutical Co., Ltd., Eisai Co., Ltd., Bristol‐Myers Squibb Co., Alexion Pharmaceuticals, Inc., UCB Japan Co. Ltd., Eli Lilly Japan K.K. and Gilead Sciences, Inc. HM received honoraria for lectures from Astellas Pharma Inc., Dainippon Pharma Co., Eli Lilly, Mitsubishi Tanabe Pharma Co., MSD, Novartis Pharma, Novo Nordisk Pharma, Kowa Pharmaceutical Co. Ltd., Nippon Boehringer Ingelheim Co., Ono Pharmaceutical Co. Ltd. and Sanofi, and received research funding from Astellas Pharma Inc., Daiichi Sankyo, Dainippon Pharma Co., Eli Lilly, Mitsubishi Tanabe Pharma Co., Novo Nordisk Pharma, Kowa Pharmaceutical Co., Abbott Japan Co., Nippon Boehringer Ingelheim Co., Ono Pharmaceutical Co. Ltd. and Taisho Toyama Pharmaceutical Co. Ltd. The other authors declare no conflict of interest.
Acknowledgments
We thank Mr Saga, who is an assistant chief radiation engineer at Hokkaido University Hospital. We thank the participating patients at the Diabetes Outpatient Departments of the Hokkaido University Hospital, Sapporo Medical Center NTT EC and Hokkaido Spinal Cord Injury Center for their valuable contributions. We thank Melissa Crawford, PhD, from Edanz Group (https://en‐author‐services.edanzgroup.com/) for editing a draft of this manuscript.
J Diabetes Investig 2021; 12: 1293–1300
Clinical Trial Registry University Hospital Medical Information Network Clinical Trials Registry UMIN000015136
References
- 1. Osteoporosis Prevention and Treatment Guidelines 2015 Edition. Life Science Publishing in Japanese. [Google Scholar]
- 2. Janghorbani M, Van Dam RM, Willett WC, et al. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 2007; 166: 495–505. [DOI] [PubMed] [Google Scholar]
- 3. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes: a meta‐analysis. Osteoporos Int 2007; 18: 427–444. [DOI] [PubMed] [Google Scholar]
- 4. Weber DR, Haynes K, Leonard MB, et al. Type 1 diabetes is associated with an increased risk of fracture across the life span: a population‐based cohort study using The Health Improvement Network (THIN). Diabetes Care 2015; 38: 1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hothersall EJ, Livingstone SJ, Looker HC, et al. Contemporary risk of hip fracture in type 1 and type 2 diabetes: a national registry study from Scotland. J Bone Miner Res 2014; 29: 1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Melton LJ 3rd, Leibson CL, Achenbach SJ, et al. Fracture risk in type 2 diabetes: update of a population‐based study. J Bone Miner Res 2008; 23: 1334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes‐a meta‐analysis. Osteoporos Int 2007; 18: 427–44. [DOI] [PubMed] [Google Scholar]
- 8. Saito M. Bone quality markers: pentosidine, homocysteine, and MTHFR polymorphism. Kid Met Bone Dis 2008; 21: 325–334 (Japanese). [PubMed] [Google Scholar]
- 9. Yamamoto M, Yamaguchi T, Yamauchi M, et al. Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res 2009; 24: 702–709. [DOI] [PubMed] [Google Scholar]
- 10. Leslie WD, Rubin MR, Schwartz AV, et al. Type 2 diabetes and bone. J Bone Miner Res 2012; 27: 2231–2237. [DOI] [PubMed] [Google Scholar]
- 11. Roux C, Hofbauer LC, Ho PR, et al. Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open‐label study. Bone 2014; 58: 48–54. [DOI] [PubMed] [Google Scholar]
- 12. Kendler DL, Roux C, Benhamou CL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 2010; 25: 72–81. [DOI] [PubMed] [Google Scholar]
- 13. Recknor C, Czerwinski E, Bone HG, et al. Denosumab compared with ibandronate in postmenopausal women previously treated with bisphosphonate therapy: a randomized open‐label trial. Obstet Gynecol 2013; 121: 1291–1299. [DOI] [PubMed] [Google Scholar]
- 14. Roux C, Hofbauer LC, Ho PR, et al. Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from randomized open‐label study. Bone 2014; 58: 48–54. [DOI] [PubMed] [Google Scholar]
- 15. Anastasilakis AD, Polyzos SA, Gkiomisi A, et al. Denosumab versus zolendronic acid in patients previously treated with zolendronic acid. Osteoporos Int 2015; 26: 2521–2527. [DOI] [PubMed] [Google Scholar]
- 16. Miller PD, Pannacciulli N, Brown JP, et al. Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J Clin Endocrinol Metab 2016; 101: 3163–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamimura M, Nakamura Y, Ikegami S, et al. Significant improvement of bone mineral density and bone turnover markers by denosumab therapy in bisphosphonate‐unresponsive patients. Osteoporos Int 2017; 28: 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakatoh S. Bone turnover rate and bone formation/resorption balance during the early stage after switching from a born resorption inhibitor to denosumab are predictive factors of bone mineral density change. Osteoporos Sarcopenia 2017; 3: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leslie WD, Johansson H, McCloskey EV, et al. Comparison of methods for improving fracture risk assessment in diabetes: the Manitoba BMD registry. J Bone Miner Res 2018; 33: 1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moreno PB, Kapoor E, Asi N, et al. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta‐analysis. J Clin Endocrinol Metab 2019; 104: 1623–1630. [DOI] [PubMed] [Google Scholar]
- 21. Khan SA, Porras AG. Elimination and biochemical responses to intravenous alendronate in postmenopausal osteoporosis. J Bone Miner Res 1997; 12: 1700–1707. [DOI] [PubMed] [Google Scholar]
- 22. Rossini M, Gatti D, Zamberlan N, et al. Long‐term effects of a treatment course with oral alendronate of postmenopausal osteoporosis. J Bone Miner Res 1994; 9: 1833–1837. [DOI] [PubMed] [Google Scholar]
- 23. Dagdelen S, Sender D, Bayraktar M. Influence of type 2 daibetes mellitus on bone mineral density response to bisphosphonates in late postmenopausal osteoporosis. Adv Ther 2007; 24: 1314–1320. [DOI] [PubMed] [Google Scholar]
- 24. Miller PD, Hochberg MC, Wehren LE, et al. How useful are measures of BMD and bone turnover? Curr Med Res Opin 2005; 21: 545–554. [DOI] [PubMed] [Google Scholar]
- 25. Watts NB, Jenkins DK, Visor JM, et al. Comparison of bone and total alkaline phosphatase and bone mineral density in postmenopausal osteoporotic women treated with alendronate. Osteoporos Int 2001; 12: 279–288. [DOI] [PubMed] [Google Scholar]
- 26. Nakatho S. Bone turnover rate and bone formation/resorption balance during the early stage after switching from a bone resorption inhibitor to denosumab are predictive factors of bone mineral density change. Osteoporos Sarcopenia 2017; 3: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamaguchi T. Bone fragility in type 2 diabetes mellitus. World J Orthop 2010; 1: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saito M, Fujii K, Marumo K. Degree of mineralization related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls. Calcif Tissue Int 2006; 79: 160–168. [DOI] [PubMed] [Google Scholar]
- 29. Karim L, Tang SY, Stoga GE, et al. Differences in non‐enzymatic glycation and collagen cross‐links between human cortical and cancellaous bone. Osteoporos Int 2013; 24: 2442–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saito M, Murano K. Collagen cross‐links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int 2010; 21: 195–214. [DOI] [PubMed] [Google Scholar]
- 31. Yamamoto M. Insights into bone fragility in diabetes: the crucial role of bone quality on skeletal strength. Endocr J 2015; 62: 299–308. [DOI] [PubMed] [Google Scholar]
- 32. Saito M, Kida Y, Nishizawa T, et al. Effects of 18‐month treatment with bazedoxifene on enzymatic immature and mature cross‐links and non‐enzymatic advanced glycation end products, mineralization, and trabecular microarchitecture of vertebra in ovariectomized monkeys. Bone 2015; 81: 573–580. [DOI] [PubMed] [Google Scholar]
- 33. Allen MR, Gineyts E, Leeming DJ, et al. Bisphosphonates alter trabecular bone collagen cross‐linking and isomerization in beagle dog vertebra. Osteoporos Int 2008; 19: 329–337. [DOI] [PubMed] [Google Scholar]
- 34. Miyazawa Y, Sekine Y, Syuto T, et al. Evaluation of bone turnover/Quality markers and bone mineral density in prostate cancer patients receiving androgen deprivation therapy with or without denosumab. Anticancer Res 2017; 37: 3667–3671. [DOI] [PubMed] [Google Scholar]
- 35. Hashidate H, Kamimura M, Ikegami S, et al. Serum pentosidine levels after 3 years of bisphosphonate treatment in post‐menopausal osteoporotic women. Endocr Res 2015; 40: 172–176. [DOI] [PubMed] [Google Scholar]
- 36. Nishizawa Y, Ohta H, Miura M, et al. Guidelines for the use of bone metabolic markers in the diagnosis and treatment of osteoporosis (2012 edition). J Bone Miner Metab 2013; 31: 1–15. [DOI] [PubMed] [Google Scholar]


