Abstract
Aims/Introduction
The aim of this study was to investigate the relationship between serum progesterone (P) and retinopathy in male patients with type 2 diabetes mellitus, and to investigate whether P is associated with its progression.
Materials and methods
A total of 1,376 male participants with type 2 diabetes mellitus were recruited from Affiliated Hospital of Medical College Qingdao University (Qingdao, China). Through logistic regression analysis after adjusting the potential confounding variation, the odds ratio (OR) and the corresponding 95% confidence interval related to the quartiles of progesterone were obtained.
Results
According to the quartiles of P levels, the prevalence rate of diabetic retinopathy (DR) in the last quartile is obviously greater to other quartiles (52.5–34.9%, 31.9%, 37.5%, P < 0.001). Compared with those in the first quartile, the prevalence of DR for the last quartile had an OR of 1.85 in the non‐proliferative diabetic retinopathy group, while the OR was 8.35 in the proliferative diabetic retinopathy group (P < 0.001, unadjusted model). When adjusted for age, body mass index, duration of type 2 diabetes mellitus, glycated hemoglobin, blood pressure and other variables, the ORs for DR in the fourth quartile were 2.13 (95% confidence interval 1.49–3.06) in the non‐proliferative diabetic retinopathy group and 8.44 (95% confidence interval 2.69–26.43) in the proliferative diabetic retinopathy group (P < 0.001). The positive association between P and DR risk was independent in adjusted logistic regression.
Conclusions
High levels of serum progesterone are significantly associated with DR in male hospitalized patients. This could mean that a higher P level in men is a potential clinical factor to identify DR, and the causality remains to be further explored.
Keywords: Retinopathy complication, Serum progesterone, Type 2 diabetes
High levels of serum progesterone are significantly associated with diabetic retinopathy complications in male hospitalized patients. This could mean that a higher progesterone level in men is a predictor of diabetic retinopathy, and the causality remains to be further explored.
Introduction
Diabetes mellitus is a chronic metabolic disease with rapidly increasing prevalence worldwide. The number of patients with diabetes worldwide is predicted to grow to 366 million by 2030 1 . The most common complication of type 2 diabetes mellitus is diabetic retinopathy (DR), which is a microvascular and neural complication of the retina, and remains one of the leading causes of visual loss in adults aged 20–74 years 2 . Increasing evidence from experimental and epidemiological studies suggests that sex hormones play important roles in the development of type 2 diabetes 3 . The level of prolactin (PRL) in patients without DR is higher than that in proliferative diabetic retinopathy patients. High PRL level reduced both vascular endothelial growth factor (VEGF)‐induced and diabetes‐induced increase of retinal vasopermeability, which can be used as a new therapy to prevent DR 4 . A study discovered that the level of follicle‐stimulating hormone (FSH) in patients with DR is higher than that in the control group and patients without DR 5 . However, some studies have shown that estradiol (E2), luteinizing hormone (LH) and FSH levels are not related to the risk factors of DR in women with type 2 diabetes mellitus 6 . Several prospective studies have shown that increased E2 cycle levels are associated with an increased risk of type 2 diabetes in men and women 3 , 7 . Goto et al. 8 proposed that testosterone (T) might be negatively correlated with diabetes. A multicenter randomized clinical trial showed that estrogen and T can predict the risk of diabetes in men, but not in women 9 . In addition, some studies have reported that the onset of gestational diabetes usually occurs in the second trimester, when the circulating level of progesterone (P) is very high, suggesting that P might play a role in gestational diabetes 10 . Sex hormones have also made great progress in the treatment of metabolic diseases 11 , 12 . In the general population, DR might be more common in men than premenopausal women 13 . Interestingly, male sex has been reported to be an independent risk factor for advanced diabetic retinopathy in type 2 diabetes mellitus patients 14 , 15 , which suggests the effect of sex hormones. Estrogen can play different roles according to the stage of retinopathy: in the initial stage, the proliferation of endothelial cells induced by E2 has a beneficial effect and protects the retina through the induced repair process, whereas in the proliferative phase, this effect aggravates the retinopathy 16 . Historically, the relationship between gonadal hormones and DR risk has received scarce attention, and no previous study has investigated the interaction between serum P and DR. To accomplish this, we carried out the present cross‐sectional study at the Affiliated Hospital of Medical College Qingdao University (Qingdao, China) to show the association between DR and P in men with type 2 diabetes mellitus.
Methods
Study population
We set up a database of type 2 diabetes mellitus inpatients at the Affiliated Hospital of Medical College Qingdao University (Shandong, China). Analyzed data were collected from 1,376 men with type 2 diabetes from the database between 2017 and 2019. The inclusion criteria accorded with the American Diabetic Association 2014 criteria 17 was: glycated hemoglobin (HbA1c) ≥6.5%, or fasting plasma glucose ≥126 mg/dL (7.0 mmol/L), or 2‐h plasma glucose ≥200 mg/dL (11.1 mmol/L) during an oral glucose tolerance test), or a random plasma glucose ≥200 mg/dL (11.1 mmol/L) with classic symptoms of hyperglycemia or hyperglycemic crisis. Participants aged <18 years or >80 years, with acute complications of type 2 diabetes mellitus, severe heart failure, severe liver disease or malignant tumors were excluded.
The protocol was designed in accordance with the Helsinki Declaration and approved by the Ethics Committee of the Affiliated Hospital of Medical College Qingdao University. All participants provided written informed consent. The study is registered on http://www.chictr.org.cn/ under the registration number ChiCTR2000032408.
Data collection
Anthropometric parameters of patients included age, height, weight, diabetes duration, the status of drinking and smoking, and blood pressure (BP). Patients’ bodyweight was measured by the same team member. BP was measured after a 5‐min rest, and averaged for two or more consecutive days. Body mass index (BMI) was calculated as the weight in kilograms divided by height in meters squared. Through laboratory examination, we measured the following indicators: fasting plasma glucose, HbA1c, PRL, FSH, LH, T, P, E2, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, free fatty acid, and lipid profiles including triglycerides (TG), total cholesterol, serum uric acid and serum creatinine. After fasting for at least 8 h, the blood samples were obtained from the median vein of the elbow, centrifuged within 1 h, stored in the cold chain within 2–4 h and transported to the central laboratory for detection. Serum PRL, FSH, LH, T, P and E2 were measured by electrochemiluminescence immunoassay (Roche E602, Switzerland), and their normal references were defined as 86–324 mIU/L, 1.5–12.4 mIU/L, 1.7–8.6 mIU/L, 9.9–27.8 nmol/L, 0.159–0.474 nmol/L and 99.4–192 pmol/L. HbA1c was determined by high‐performance liquid chromatography (MQ‐2000PT, Shanghai, China). Blood glucose and lipids were measured by Beckman Coulter AU 680 (Krefeld, Germany). Serum uric acid was measured by a DIMENSION LXR (SIEMENS, Munich, Germany) automatic analyzer. Serum creatinine was measured by the picric acid method (Coulter AU 680).
Assessment of DR
All participants were assessed for retinopathy by a fundus camera (AFC‐330; NIDEX, Kyoto, Japan), slit lamp microscope (3020H; Keeler Ltd, Windsor, UK) and non‐invasive optical coherence tomography (5000; Carl Zeiss, Dublin, CA, USA). According to the definitions derived from Wilkinson et al. 18 , the patients were classified into three groups: non‐diabetic retinopathy group (No DR), non‐proliferative diabetic retinopathy group (NPDR) and proliferative diabetic retinopathy group (PDR). NPDR included multiple manifestations: microaneurysm, hard exudates, cotton‐wool spot and so on. PDR is mainly the formation of neovascularization, which can lead to severe retinal detachment.
Statistical analysis
The SPSS software version 24.0 (IBM Corporation, Armonk, NY, USA) was used to carry out statistical analyses. Normally distributed continuous variables are expressed as the mean ± standard deviation, whereas non‐normally distributed continuous variables are expressed as the interquartile range, and categorical variables are presented as frequency. The characteristics of the participants among No DR, NPDR and PDR were compared using χ2‐tests or the Kruskal–Wallis test. P levels were classified into four groups based on quartiles (Q1: ≤0.425, Q2: 0.425–0.75, Q3: 0.75–1.27, Q4: ≥1.27), with the first quartile (Q1) representing the lowest quartile, and the fourth quartile (Q4) being the highest. Multiple logistic regression analysis after adjusting was used to compute the odds ratios (OR) and the corresponding 95% confidence intervals (95% CI), which represented the risk of DR in P quartiles. A P < 0.05 was considered statistically significant (two‐sided).
Results
The clinical characteristics of the study participants are presented in Table 1. Patients with more severe DR were significantly older, had longer diabetes duration, higher HbA1c, high‐density lipoprotein cholesterol, LH, T and Cr, and lower DBP and TG than the patients with mild retinopathy or no retinopathy (P < 0.05). Compared with other sex hormones, P levels showed a positive correlation with DR (P < 0.001).
Table 1.
Clinical characteristics of no diabetic retinopathy, non‐proliferative diabetic retinopathy and proliferative diabetic retinopathy in participants
| Characteristics | NDR (n = 838) | NPDR (n = 499) | PDR (n = 39) | P‐value |
|---|---|---|---|---|
| Age (years) | 57.5 ± 12.3 | 61.4 ± 10.0 | 62.2 ± 12.1 | <0.001* |
| BMI (kg/m2) | 25.9 (23.7–28.1) | 26.0 (23.7–28.1) | 25.0 (23.39–27.40) | 0.389 |
| DM duration (years) | 9.1 ± 6.9 | 12.3 ± 7.1 | 14.8 ± 7.9 | <0.001* |
| HbA1c (%) | 8.1 (6.9–9.6) | 8.5 (7.2–10.0) | 7.9 (6.9–8.9) | 0.008* |
| BP (mmHg) | ||||
| Systolic | 136.7 ± 17.6 | 139.2 ± 21.1 | 142.1 ± 19.0 | 0.063 |
| Diastolic | 80.8 ± 11.9 | 80.5 ± 11.7 | 76.7 ± 9.9 | 0.038* |
| Lipid profile (mmol/L) | ||||
| LDL‐c | 2.60 ± 0.87 | 2.61 ± 0.99 | 2.63 ± 0.90 | 0.950 |
| HDL‐c | 1.13 ± 0.28 | 1.18 ± 0.32 | 1.23 ± 0.32 | 0.008* |
| TG | 1.42 (1.00–2.23) | 1.37 (0.94–2.11) | 1.08 (0.83–1.69) | 0.003* |
| TC | 4.43 ± 1.24 | 4.53 ± 1.33 | 4.47 ± 1.14 | 0.488 |
| FFA | 0.43 ± 0.21 | 0.39 ± 0.20 | 0.45 ± 0.25 | 0.001* |
| Sex hormones | ||||
| PRL (mIU/L) | 311.1 (239.5–400.9) | 278.0 (215.1–373.2) | 303.3 (223.6–382.5) | <0.001* |
| LH (mIU/mL) | 7.37 (5.39–9.79) | 7.70 (5.45–10.21) | 8.89 (6.58–12.37) | 0.033* |
| FSH (mIU/mL) | 8.86 (6.06–12.53) | 9.35 (6.18–13.91) | 9.70 (5.88–15.81) | 0.192 |
| E2 (pmol/L) | 98.53 (72.24–132.70) | 99.86 (73.32–132.40) | 103.20 (78.67– 136.50) | 0.768 |
| P (nmol/L) | 0.68 (0.40–1.11) | 0.86 (0.46–1.43) | 1.51 (1.05–1.97) | <0.001* |
| T (nmol/L) | 13.71 (10.30–18.15) | 13.93 (10.62–17.78) | 16.46 (12.41–21.47) | 0.019* |
| sUA (µmol/L) | 340 (283–394) | 338 (285–387) | 377 (333–430) | 0.016* |
| Scr (µmol/L) | 63.35 ± 28.02 | 73.12 ± 24.45 | 106.41 ± 37.74 | <0.001* |
| Smoking (%) | 434 (51.79%) | 270 (54.11%) | 22 (56.41%) | 0.649 |
| Drinking (%) | 416 (49.64%) | 246 (49.30%) | 16 (41.03%) | 0.557 |
Kruskal–Wallis H‐test or χ2‐test. Normally distributed variables are expressed as mean ± standard deviation, non‐normal variables are expressed as the median (interquartile range), and categorical variables are expressed as the percentage (%). BMI, body mass index; BP, blood pressure; DM, diabetes mellitus; E2, estrogen; FFA, free fatty acid; FSH, follicle‐stimulating hormone; HbA1c, glycated hemoglobin; HDL‐c, high‐density lipoprotein cholesterol; LDL‐c, how‐density lipoprotein cholesterol; LH, luteinizing hormone; NDR, no diabetic retinopathy; NPDR, non‐proliferative diabetic retinopathy; P, progesterone; PDR, non‐proliferative diabetic retinopathy; PRL, prolactin; Scr, serum creatinine; sUA, serum uric acid; T, testosterone; TC, total cholesterol; TG, triglyceride.*Statistically significant.
Table 2 provided dates about clinical characteristics of P quartile stratification in men with type 2 diabetes mellitus. Compared with the patients with the lowest P level, the patients with the highest P level were more likely to have lower BMI, lower glycosylation, lower TG, and higher free fatty acid and serum creatinine. According to the quartiles of P levels, the prevalence rate of NPDR in male patients of the last quartile was higher than the first to third quartile (45.4 vs 33.7%, 31.0%, 35.2%, P = 0.001), and the prevalence rate of PDR in the last quartile was higher than the first to third quartile (7.1 vs 1.2%, 0.9%, 2.3%, P < 0.001). Furthermore, the prevalence rate of DR in the last quartile was obviously increased compared with the first to third quartile (52.5 vs 34.9%, 31.9%, 37.5%, P < 0.001; Figure 1). Furthermore, the patients with DR had a higher P level than those without DR, which was gradually increased with the development of DR (P < 0.001; Figure 2).
Table 2.
Clinical characteristics of the quartiles of progesterone levels in men with type 2 diabetes mellitus
| Characteristics | Q1 (≤0.425) | Q2 (0.425–0.75) | Q3 (0.75–1.27) | Q4 (≥1.27) | P‐value |
|---|---|---|---|---|---|
| n = 344 | n = 352 | n = 341 | n = 339 | ||
| Age (years) | 58.7 ± 12.4 | 59.0 ± 11.7 | 59.1 ± 11.5 | 59.4 ± 11.1 | 0.985 |
| BMI (kg/m2) | 26.2 (24.1–28.7) | 26.0 (23.8–28.1) | 25.6 (23.5–27.7) | 25.7 (23.7–28.0) | 0.023* |
| DM duration (years) | 10.5 ± 7.5 | 9.9 ± 7.2 | 10.3 ± 7.1 | 10.9 ± 7.0 | 0.191 |
| HbA1c (%) | 8.6 (7.3–10.2) | 8.2 (7.0–9.6) | 8.1 (6.9–9.6) | 7.9 (6.8–9.3) | 0.002* |
| BP (mmHg) | |||||
| Systolic | 139 ± 19 | 138 ± 20 | 136 ± 19 | 138 ± 18 | 0.136 |
| Diastolic | 81 ± 12 | 81 ± 12 | 81 ± 11 | 80 ± 12 | 0.503 |
| Lipid profile (mmol/L) | |||||
| LDL‐c | 2.6 ± 0.9 | 2.6 ± 1.0 | 2.6 ± 0.8 | 2.6 ± 0.9 | 0.914 |
| HDL‐c | 1.11 ± 0.28 | 1.16 ± 0.31 | 1.15 ± 0.28 | 1.18 ± 0.32 | 0.066 |
| TG | 1.49 (1.06–2.43) | 1.40 (1.00–2.08) | 1.29 (0.93–2.10) | 1.40 (0.89–2.18) | 0.019* |
| TC | 4.4 ± 1.3 | 4.5 ± 1.3 | 4.5 ± 1.2 | 4.5 ± 1.2 | 0.505 |
| FFA | 0.41 ± 0.21 | 0.40 ± 0.20 | 0.43 ± 0.19 | 0.46 ± 0.24 | 0.009* |
| sUA (µmol/L) | 347 (291–405) | 345 (287–392) | 331 (276–386) | 339 (284–391) | 0.071 |
| Scr (µmol/L) | 67.9 ± 26.3 | 67.3 ± 37.0 | 66.7 ± 37.5 | 70.9 ± 24.6 | 0.004* |
| Smoking (%) | 185 (53.78%) | 169 (48.01%) | 185 (54.25%) | 187 (55.16%) | 0.229 |
| Drinking (%) | 160 (46.51%) | 172 (48.86%) | 166 (48.68%) | 180 (53.09%) | 0.390 |
| NDR | 224 (65.1%) | 240 (68.2%) | 213 (62.5%) | 161 (47.5%) | <0.001* |
| NPDR | 116 (33.7%) | 109 (31.0%) | 120 (35.2%) | 154 (45.4%) | 0.001* |
| PDR | 4 (1.2%) | 3 (0.9%) | 8 (2.3%) | 24 (7.1%) | <0.001* |
Kruskal–Wallis H‐test or χ2‐test. Normally distributed variables are expressed as mean ± standard deviation, non‐normal variables are expressed as the median (interquartile range) and categorical variables are expressed as the percentage (%). BMI, body mass index; BP, blood pressure; DM, diabetes mellitus; FFA, free fatty acid; HbA1c, glycated hemoglobin; HDL‐c, high‐density lipoprotein cholesterol LDL‐c, low‐density lipoprotein cholesterol; NDR, no diabetic retinopathy; NPDR, non‐proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; Scr, serum creatinine; sUA, serum uric acid; TC, total cholesterol; TG, triglyceride.
*Statistically significant.
Figure 1.
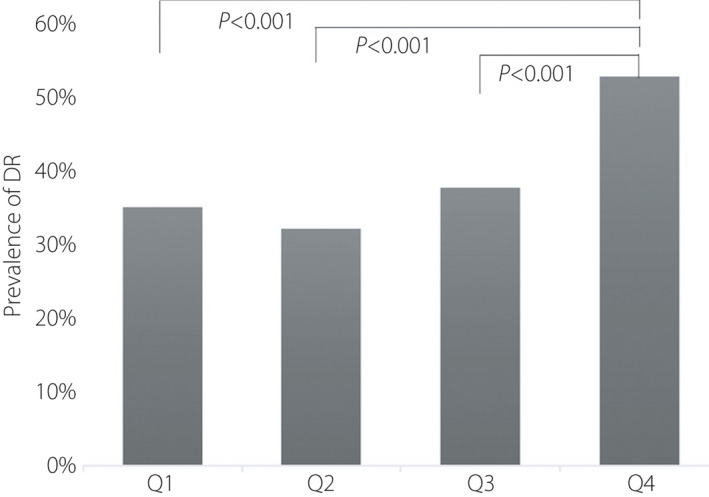
Prevalence rate of diabetic retinopathy (DR) in progesterone quartiles (Q1: 33.7%, Q2: 31.0%, Q3: 35.2%, Q4: 45.4%).
Figure 2.
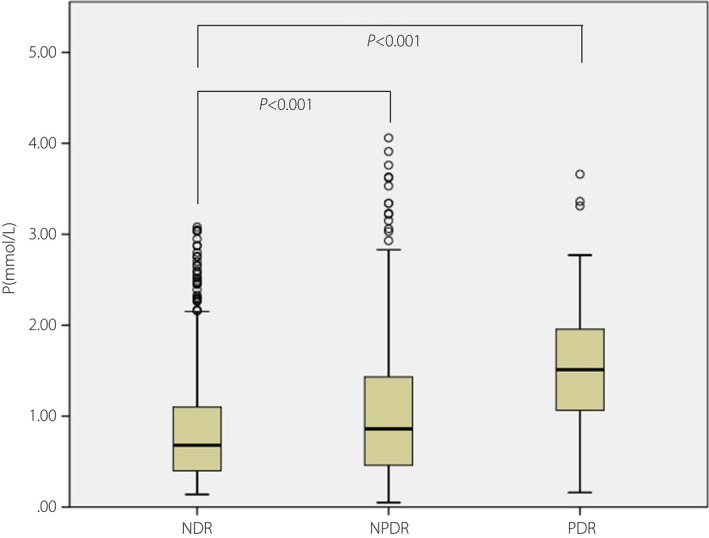
The box plot of progesterone levels among no diabetic retinopathy (NDR), non‐proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR) in men with type 2 diabetes mellitus.
The results of logistic regression analysis are listed in Table 3. Compared with those in the first quartile, the prevalence of DR for the last quartile had an OR of 1.85 in NPDR, whereas the OR was 8.35 in PDR (P < 0.001, unadjusted model). When adjusted for age, BMI, duration of type 2 diabetes mellitus, HbA1c, BP, smoking and drinking rate, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, TG, total cholesterol, PRL, LH, FSH, E2 and T, the ORs for DR in Q4 were 2.13 (95% CI 1.49–3.06) in NPDR and 8.44 (95% CI 2.69–26.43) in PDR (P < 0.001). This indicated that the risk of NPDR in male patients with P ≥1.27 nmmol/L is 2.13‐fold higher than that in those with P ≤0.425 nmmol/L, and the risk of PDR in male patients with P ≥1.27 nmmol/L is 8.44 times higher than that in those with P ≤0.425 nmmol/L. The positive association between P and DR risk was independently in adjusted logistic regression.
Table 3.
Unadjusted and multivariate adjusted odds ratios of the quartiles of progesterone levels for non‐proliferative diabetic retinopathy and proliferative diabetic retinopathy in participants
| Quartiles | Unadjusted model | Adjusted model | ||||||
|---|---|---|---|---|---|---|---|---|
| NPDR | PDR | NPDR | PDR | |||||
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| Q1 (≤0.425) | – | – | – | – | – | – | – | – |
| Q2 (0.425–0.75) | 0.88 (0.64–1.21) | 0.419 | 0.70 (0.16–3.16) | 0.643 | 1.02 (0.71–1.46) | 0.910 | 0.72 (0.15–3.35) | 0.674 |
| Q3 (0.75–1.27) | 1.09 (0.79–1.49) | 0.602 | 2.10 (0.62–7.09) | 0.230 | 1.29 (0.90–1.86) | 0.165 | 1.81 (0.50–6.62) | 0.367 |
| Q4 (≥1.27) | 1.85 (1.35–2.53) | <0.001 | 8.35 (2.84–24.53) | <0.001 | 2.13 (1.49–3.06) | <0.001 | 8.44(2.69–26.43) | <0.001 |
Logistic regression analysis. Adjusted for age, body mass index, duration of type 2 diabetes mellitus, glycated hemoglobin, blood pressure, smoking and drinking rate, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglycerides, total cholesterol, serum creatinine, prolactin, luteinizing hormone, follicle‐stimulating hormone, estradiol and testosterone. NDR, no diabetic retinopathy; NPDR, non‐proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
Discussion
In the present cross‐sectional study, we recruited 1,376 male patients to explore the relationship between DR and serum P levels. Researchers absorbed 35 studies between 1980 and 2008, which estimated that the global prevalence of any DR is 34.6% (95% CI 34.5–34.8) 19 . In the present study, the prevalence of DR in hospitalized patients with type 2 diabetes mellitus was 39.1%, which is a little higher than the global prevalence. The reason might be that the study population was hospitalized at a higher rate. The results showed that the prevalence of DR increased with the increase of P level (P < 0.001). It also suggested that the patients with DR had a significantly higher P level than those without DR (P < 0.001). This showed an independent, significant relationship between P levels and DR regardless of age, BMI, duration of type 2 diabetes mellitus, HbA1c, and other variables included in the logistic regression.
Although few studies have reported a significant association between sex and DR, an increasing number of studies have confirmed that being male is an independent risk factor for DR 20 , 21 , 22 . The United Kingdom Prospective Diabetic Study found that male sex was an independent risk factor for the development of DR 23 . So far, the interaction between sex hormones and DR has not been studied systematically. T deficiency is highly prevalent in men with type 2 diabetes mellitus 24 . The prevalence of erectile dysfunction has been reported to be as high as 35–90% in men with diabetes mellitus 25 . Table 2 shows that the average level of T in diabetes mellitus patients is in the low normal range (normal reference range 9.9–27.8 nmol/L), and the T level in diabetes mellitus patients with PDR is higher than that in patients without DR (P < 0.05), which requires further study. For women, variation in sex hormones levels caused by pregnancy or menstruation might change the state of the disease. For example, a change in hormone level is one of the reasons for the new onset and aggravation of retinopathy during pregnancy 26 .
Anatomically, the retina is a target for sex steroid hormones, as shown by the large presence of sex steroid hormone receptors, which includes estrogen receptor α, estrogen receptor β, P receptor and androgen receptor 27 . Estrogen plays a role in retinal neuroprotection, and modulating retinal and choroid blood flow 16 , 28 , 29 , 30 , 31 , 32 , 33 . In regard to T, it has a population‐based protective effect on vascularization and retinal perfusion, which might be related to the vasodilation of microvessels. In men, P is synthesized not only in Leydig cells, but also in the adrenal gland, from where it is secreted into the circulatory system. P plays a special physiological and pathophysiological role in men. P might be a valuable treatment for male contraception, stimulating endogenous T biosynthesis in senile interstitial cells, prostate cancer and/or benign prostatic hyperplasia, meningioma/ fibroma, chronic obstructive pulmonary disease, weight loss and central nervous system 34 .
The relationship between serum P levels and DR has not been described in recent studies. In the present study, P levels were positively correlated with DR risk regardless of age, BMI, duration of type 2 diabetes mellitus, HbA1c and other variables. We summarized the following potential mechanism and conjecture. High concentration of P dysregulates VEGF expression in the retina. VEGF has been shown to activate endothelial cells, and promote cell proliferation, migration and vascular permeability in the retina 35 . P has been reported to increase VEGF expression in both the rat uterus and human endometrial cells 36 , 37 . In addition, P induces VEGF messenger ribonucleic acid and protein expression in MA‐10 cells (a mouse tumor Leydig cell line). Furthermore, we found the same in bovine retinal epithelial cells. When the cultured retinal pigment epithelial cells were exposed to a high concentration of P (10 µmol/L) for 48 h, the production of VEGF/vascular permeability factor increased significantly (214.5 ± 28.3 ng/g protein, P < 0.01) compared with the control group (147.7 ± 17.9 ng/g protein) 38 . It is well known that VEGF is one of the basic pathophysiological mechanisms of DR 39 . Therefore, we believe that the increase of P level leads to neovascularization, vascular leakage, rupture of the blood–retinal barrier and retinal ischemia by overexpression of VEGF in the retina 40 . P has a vasoconstrictive effect on ocular blood flow. The blood flow resistance index of the ophthalmic artery increased in patients with DR. The increase of vascular bed resistance in the peripheral eye leads to DR, and this change exists before the occurrence of DR 41 , 42 . Viana et al. 43 reported an increase in vascular resistance of the central retinal artery during the luteal phase, suggesting that P plays an important role in antagonizing the vasodilation of estrogen. Souza et al. 44 confirmed that in the P‐treated group, when the pulsatility index, resistance index and systole/diastole ratio before and after treatment were compared, the vascular resistance of the ophthalmic artery and the central retinal artery increased significantly – a possible compensation mechanism. P is mainly secreted from the nervous system and testis in men. In the case of decreased T, the negative feedback regulation mechanism of the hypothalamic–pituitary–testicular axis plays a role, which increases the level of gonadotropin, and in turn stimulates testicular synthesis to release other gonadal hormones. In addition to this, an increasing number of studies have shown that exogenous P has neuroprotective and anti‐oxidant functions in retinopathy 45 , 46 , 47 . However, there is controversy surrounding whether P has a neuroprotective effect. There are also animal experiments that show that P has no neuroprotective effect on retinal degeneration. P was infused through the peripheral vein to half of the male rats receiving photoresist‐induced retinal degeneration. The results showed that there was no statistical difference between the two groups 48 . P use in renal transplant patients can lead to increased urinary protein excretion, severe glomerulosclerosis and monocyte infiltration 49 . It was found that P treatment could protect ischemic endothelial cells from macrophage infiltration in transient middle cerebral artery occlusion 50 . Nevertheless, the protective effect of P was not found in the rat model of anterior ischemic optic neuropathy 51 . The high level of P is also related to myocardial infarction 52 . The addition of medroxyprogesterone acetate (MPA) in E2 treatment complicates the cardiovascular damage after myocardial infarction, and aggravates left ventricular remodeling and dysfunction 53 . The relevant mechanism remains to be further studied. At present, P therapy has not been carried out in large‐scale clinical trials, and the mechanism is not completely clear. We hope that our research can attract the attention of clinicians, so that they can carefully consider P as a neuroprotective treatment method, because it might affect the VEGF retinal vascular system.
In the present study, multivariate logistic regression analysis further confirmed that apart from sex hormone disorders, long duration of diabetes mellitus, high HbA1c, systolic BP, low low‐density lipoprotein cholesterol and total cholesterol levels were risk factors for male patients with NPDR, whereas systolic BP and TG were risk factors for male patients with PDR. Consistent with the present findings, previous studies reported that duration of diabetes, poor blood glucose control (the presence of HbA1c and hypertension), dyslipidemia, high BMI, puberty and pregnancy were risk factors for DR 54 .
There were several limitations of the present study that need to be explained. First, this retrospective study could not infer cause and effect. Second, all patients recruited were hospitalized, so the results could not represent other regions of the country. Third, there is insufficient experimental evidence to explain the relationship between them. In brief, the present study suggested that high levels of P are significantly related to DR risk. This could mean that a higher P level in men is a potential clinical factor to identify DR. The relationship between P and DR will open up a new research field, and large‐scale clinical studies in the future might help to identify whether P therapy is sex‐specific. Therefore, P therapy still requires careful and comprehensive consideration. Prospective cohort studies are required to identify the causality.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank all the patients for participating in this study, and all the researchers and collaborators who participated in this study. This study had no funding support.
J Diabetes Investig 2021; 12: 1228–1235
Clinical Trial Registry Ethics Committee of the Affiliated Hospital of Medical College Qingdao University
ChiCTR2000032408
References
- 1. Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 2. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet 2010; 376: 124–136. [DOI] [PubMed] [Google Scholar]
- 3. Ding EL, Song Y, Malik VS, et al. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta‐analysis. JAMA 2006; 295: 1288–1299. [DOI] [PubMed] [Google Scholar]
- 4. Arnold E, Rivera JC, Thebault S, et al. High levels of serum prolactin protect against diabetic retinopathy by increasing ocular vasoinhibins. Diabetes 2010; 59: 3192–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaurasia RK, Singh R, Agrawal JK, et al. Sex hormones and diabetic retinopathy. Ann Ophthalmol 1993; 25: 227–230. [PubMed] [Google Scholar]
- 6. Siddiqui K, George TP, Alosaimi J, et al. Level of hormones in menopause in relation to diabetic retinopathy among type 2 diabetic women. Health Care Women Int 2020. 10.1080/07399332.2020.1798962 [DOI] [PubMed] [Google Scholar]
- 7. Ding EL, Song Y, Manson JE, et al. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia 2007; 50: 2076–2084. [DOI] [PubMed] [Google Scholar]
- 8. Goto A, Morita A, Goto M, et al. Associations of sex hormone‐binding globulin and testosterone with diabetes among men and women (the Saku Diabetes study): a case control study. Cardiovasc Diabetol 2012; 11: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mather KJ, Kim C, Christophi CA, et al. Steroid sex hormones, sex hormone‐binding globulin, and diabetes incidence in the diabetes prevention program. J Clin Endocrinol Metab 2015; 100: 3778–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Branisteanu DD, Mathieu C. Progesterone in gestational diabetes mellitus: guilty or not guilty? Trends Endocrinol Metab 2003; 14: 54–56. [DOI] [PubMed] [Google Scholar]
- 11. Khripun I, Vorobyev S, Belousov I, et al. Influence of testosterone substitution on glycemic control and endothelial markers in men with newly diagnosed functional hypogonadism and type 2 diabetes mellitus: a randomized controlled trial. Aging Male 2019; 22: 241–249. [DOI] [PubMed] [Google Scholar]
- 12. Paschou Stavroula A, Papanas N. Type 2 diabetes mellitus and menopausal hormone therapy: an update. Diabetes Ther 2019; 10: 2313–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zetterberg M. Age‐related eye disease and gender. Maturitas 2016; 83: 19–26. [DOI] [PubMed] [Google Scholar]
- 14. Looker HC, Nyangoma SO, Cromie D, et al. Diabetic retinopathy at diagnosis of type 2 diabetes in Scotland. Diabetologia 2012; 55: 2335–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kostev K, Rathmann W. Diabetic retinopathy at diagnosis of type 2 diabetes in the UK: a database analysis. Diabetologia 2013; 56: 109–111. [DOI] [PubMed] [Google Scholar]
- 16. Nuzzi R, Scalabrin S, Becco A, et al. Gonadal hormones and retinal disorders: a review. Front Endocrinol 2018; 9: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37: S81–S90. [DOI] [PubMed] [Google Scholar]
- 18. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003; 110: 1677–1682. [DOI] [PubMed] [Google Scholar]
- 19. Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012; 35: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ozawa Glen Y, Bearse Marcus A, Adams Anthony J. Male‐female differences in diabetic retinopathy? Curr Eye Res 2015; 40: 234–246. [DOI] [PubMed] [Google Scholar]
- 21. Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA 2010; 304: 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rajalakshmi R, Behera UC, Bhattacharjee H, et al. Spectrum of eye disorders in diabetes (SPEED) in India. Report 2. Diabetic retinopathy and risk factors for sight threatening diabetic retinopathy in people with type 2 diabetes in India. Indian J Ophthalmol 2020; 68: S21–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia 2001; 44: 156–163. [DOI] [PubMed] [Google Scholar]
- 24. Rao PM, Kelly DM, Jones HT. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol 2013; 9: 479–493. [DOI] [PubMed] [Google Scholar]
- 25. Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med 2009; 6: 1232–1247. [DOI] [PubMed] [Google Scholar]
- 26. Kazama S, Kazama JJ, Ando N. Eye diseases in women. Fukushima J Med Sci 2019; 65: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wickham LA, Gao J, Toda I, et al. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol Scand 2000; 78: 146–153. [DOI] [PubMed] [Google Scholar]
- 28. Mo MS, Li HB, Wang BY, et al. PI3K/Akt and NF‐kB activation following intravitreal administration of 17b‐estradiol: neuroprotection of the rat retina from light‐induced apoptosis. Neuroscience 2013; 228: 1–12. [DOI] [PubMed] [Google Scholar]
- 29. Kaja S, Yang SH, Wei J, et al. Estrogen protects the inner retina from apoptosis and ischemia‐induced loss of Vesl‐1L/Homer 1c immunoreactive synaptic connections. Invest Ophthalmol Vis Sci 2003; 44: 3155–3162. [DOI] [PubMed] [Google Scholar]
- 30. Giddabasappa A, Bauler M, Yepuru M, et al. 17‐β estradiol protects ARPE‐19 cells from oxidative stress through estrogen receptor‐β. Invest Ophthalmol Vis Sci 2010; 51: 5278–5287. [DOI] [PubMed] [Google Scholar]
- 31. Moosmann B, Behl C. The antioxidant neuroprotective effects of estrogen and phenolic compounds are independent from their estrogenic proprieties. Proc Natl Acad Sci USA 1999; 96: 8867–8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Y, Li X, Wang J, et al. 17b‐estradiol mediates upregulation of stromal cell‐derived factor‐1 in the retina through activation of estrogen receptor in an ischemia‐reperfusion injury model. Graefes Arch Clin Exp Ophthalmol 2015; 253: 17–23. [DOI] [PubMed] [Google Scholar]
- 33. Hao M, Li Y, Lin W, et al. Estrogen prevents high‐glucose‐induced damage of retinal ganglion cells via mitochondrial pathway. Graefes Arch Clin Exp Ophthalmol 2015; 253: 83–90. [DOI] [PubMed] [Google Scholar]
- 34. Oettel M, Mukhopadhyay AK. Progesterone the forgotten hormone in men? Aging Male 2004; 7: 236–257. [DOI] [PubMed] [Google Scholar]
- 35. Barquet LA. [Role of VEGF in diseases of the retina]. Arch Soc Esp Oftalmol 2015; 90: 3–5. [DOI] [PubMed] [Google Scholar]
- 36. Karuri AR, Kumar AM, Mukhopadhyay D. Differential expression and selective localization of vascular permeability factor/vascular endothelial growth factor in the rat uterus during the estrous cycle. J Endocrinol 1998; 159: 489–499. [DOI] [PubMed] [Google Scholar]
- 37. Shifren JL, Tseng JF, Zaloudek CJ, et al. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab 1996; 81: 3112–3118. [DOI] [PubMed] [Google Scholar]
- 38. Vujosevic S, Toma C, Villani E, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol 2020; 9: 31. [DOI] [PubMed] [Google Scholar]
- 39. Wong TY, Cheung CM, Larsen M, et al. Diabetic retinopathy. Nat Rev Dis Primers 2016; 2: 16012. [DOI] [PubMed] [Google Scholar]
- 40. Tarr JM, Kaul K, Chopra M, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol 2013; 2013: 343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arai T, Numata K, Tanaka K, et al. Ocular arterial flow hemodynamics in patients with diabetes mellitus. J Ultrasound Med 1998; 17: 675–681. [DOI] [PubMed] [Google Scholar]
- 42. Xu W, Wang HY, Zhao XH, et al. Values of ocular hemodynamics and serum endothelin‐1 in the early diagnosis of diabetic retinopathy. Zhonghua Yi Xue Za Zhi 2013; 93: 37–40. [PubMed] [Google Scholar]
- 43. Viana LC, Faria M, Pettersen H, et al. Menstrual 10. phase‐related differences in the pulsatility index on the central retinal artery suggest an oestrogen vasodilatation effect that antagonizes with progesterone. Arch Gynecol Obstet 2011; 283: 569–573. [DOI] [PubMed] [Google Scholar]
- 44. Souza MA, Souza BM, Geber S. Progesterone increases resistance of ophthalmic and central retinal arteries in climacteric women. Climacteric 2013; 16: 284–287. [DOI] [PubMed] [Google Scholar]
- 45. Chainy GBN, Sahoo DK. Hormones and oxidative stress: an overview. Urban Water J 2020; 54: 1–26. [DOI] [PubMed] [Google Scholar]
- 46. Oh HY, Kim SS, Chung HY, et al. Isoflavone supplements exert hormonal and antioxidant effects in postmenopausal Korean women with diabetic retinopathy. J Med Food 2005; 8: 1–7. [DOI] [PubMed] [Google Scholar]
- 47. Roche Sarah L, Ruiz‐Lopez Ana M, Moloney Jennifer N, et al. Microglial‐induced Müller cell gliosis is attenuated by progesterone in a mouse model of retinitis pigmentosa. Glia 2018; 66: 295–310. [DOI] [PubMed] [Google Scholar]
- 48. Káldi I, Berta A. Progesterone administration fails to protect albino male rats against photostress‐induced retinal degeneration. Eur J Ophthalmol 2004; 14: 306–314. [DOI] [PubMed] [Google Scholar]
- 49. Balazs Antus, Shanying Liu, Yousheng Yao, et al. Effects of progesterone and selective oestrogen receptor modulators on chronic allograft nephropathy in rats. Nephrol Dial Transplant 2005; 20: 329–335. [DOI] [PubMed] [Google Scholar]
- 50. Remus EW, Sayeed I, Won S, et al. Progesterone protects endothelial cells after cerebrovascular occlusion by decreasing MCP‐1‐ and CXCL1‐mediated macrophage infiltration. Exp Neurol 2015; 271: 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Allen RS, Olsen TW, Sayeed I, et al. Progesterone treatment in two rat models of ocular ischemia. Invest Ophthalmol Vis Sci 2015; 56: 2880–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aksüt SV, Aksüt G, Karamehmetoglu A, et al. The determination of serum estradiol, testosterone and progesterone in acute myocardial infarction. Jpn Heart J 1986; 27: 825–837. [DOI] [PubMed] [Google Scholar]
- 53. Arias‐Loza PA, Hu K, Frantz S, et al. Medroxyprogesterone acetate aggravates oxidative stress and left ventricular dysfunction in rats with chronic myocardial infarction. Toxicol Pathol 2011; 39: 867–878. [DOI] [PubMed] [Google Scholar]
- 54. Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol 2016; 44: 260–277. [DOI] [PubMed] [Google Scholar]


