Abstract
Aims/Introduction
Body fluid volume imbalance is common in patients with kidney failure, and is associated with all‐cause mortality. This study aimed to investigate the association between fluid volume imbalance and albuminuria in patients with type 2 diabetes mellitus without kidney failure.
Materials and Methods
Using data from one cohort study, a baseline cross‐sectional study of 432 participants and a longitudinal cohort study of 368 participants who could follow up was carried out. Body fluid imbalance was determined by measuring the extracellular water (ECW)‐to‐intracellular water (ICW) ratio (ECW/ICW) using bioelectrical impedance analysis. A change in the urinary albumin‐to‐creatinine ratio (ACR) was defined as the ratio of urinary ACR at follow up to that at baseline. The ECW/ICW ratio was compared with the level of albuminuria.
Results
In this cross‐sectional study, the ECW/ICW ratio increased with the level of albuminuria. There was an association between the ECW/ICW ratio and logarithms of urinary ACR after adjusting for covariates (β = 0.205, P < 0.001). Furthermore, the ECW/ICW ratio was associated with a change in the urinary ACR after adjusting for covariates (β = 0.176, P = 0.004) in this longitudinal study. According to the receiver operating characteristic curve, the optimal cut‐off point of the ECW/ICW ratio for incident macroalbuminuria, defined as ACR >300 mg/gCr, was 0.648 (area under the curve 0.78, 95% confidence interval 0.58–0.90).
Conclusions
The ECW/ICW ratio is independently associated with the level of albuminuria in patients with type 2 diabetes mellitus without kidney failure. This reinforces the importance of monitoring fluid balance in patients with type 2 diabetes mellitus.
Keywords: Body fluid volume, Diabetic kidney disease, Fluid overload
Fluid imbalance occurred in diabetes patients without kidney failure, and was associated with the level of albuminuria. The extracellular water to intracellular water ratio is independently associated with changes in albuminuria in patients with type 2 diabetes mellitus without kidney failure. Clinicians need to pay more attention to monitoring the fluid status among patients with type 2 diabetes mellitus, and maintaining fluid balance in the early stage of diabetic kidney disease.

Introduction
The incidence of diabetic kidney disease (DKD), which is the leading cause of end‐stage renal disease (ESRD), has been increasing worldwide 1 . DKD is also associated with cardiovascular disease and all‐cause mortality 2 , 3 , 4 . Lifestyle modifications and medications for diabetes, hypertension, and dyslipidemia are effective for preventing kidney failure for patients with DKD 5 , 6 , 7 .
Albuminuria, which indicates microvascular endothelial injury, is an independent risk factor for progression to kidney failure, particularly in patients with DKD 3 , 8 . Diabetic nephropathy is already advanced when proteinuria is manifested. Thus, early detection of worsening of albuminuria is important in the management of patients with diabetes mellitus.
In healthy adults, fluid distribution between the intracellular water (ICW) and extracellular water (ECW) compartments is tightly regulated 9 . However, this regulation is impaired in patients with kidney failure, including ESRD and hemodialysis, as a result of the relative excess of ECW, which results in fluid overload 10 , 11 . One of the mechanisms of fluid overload is thought to be inflammation, which causes hypoalbuminemia and increased vascular permeability 12 . Although there are several measurable inflammatory markers and cytokines, those are unsuitable to evaluate micropermeability in peripheral tissues 13 . Therefore, we suggest that focusing on the relationships between the ECW/ICW ratio and urinary ACR from an early stage, and the risk of development of diabetic nephropathy in cases where the ECW/ICW ratio is above the cut‐off level provides an opportunity for intensive complication assessment and prevention of progression of complications. This extravascular fluid shift leads to ECW volume overload, and would be a strong and independent risk factor for mortality 14 , 15 . Although diabetes patients with microalbuminuria have no symptoms, microvascular endothelial damage and urinary protein loss could lead to subtle fluid imbalances. We therefore hypothesized that that there might be an association between fluid imbalance and albuminuria. No studies have reported on this association. In the present cross‐sectional and longitudinal study, we therefore investigated the association between the ECW/ICW ratio and albuminuria in patients with type 2 diabetes mellitus without kidney failure, and the effect of the ECW/ICW ratio on the level of albuminuria, using bioelectrical impedance analysis (BIA), which is used to evaluate body composition and fluid compartments 16 , 17 . BIA can easily measure ECW and ICW in daily clinical practice at low physical and financial cost to patients with diabetes, and is expected to be widely applied as one method to assess the risk of the progression of diabetic complications.
Methods
Study population
The present study was a subanalysis of the KAMOGAWA‐DM cohort study, the details of which have been described elsewhere 18 . Briefly, the KAMOGAWA‐DM cohort study is an ongoing cohort study of patients at Kyoto Prefectural University of Medicine (Kyoto, Japan) and Kameoka Municipal Hospital (Kameoka, Japan). The purpose of this cohort study is to clarify the natural history of people with diabetes. The inclusion criteria of this cohort study are patients with diabetes who agreed to participate in this cohort study. In the present study, we investigated the relationship between the ECW/ICW ratio and the prevalence of diabetic kidney disease in cross‐sectionally, and then, we investigated the association between the ECW/ICW ratio and change in the albumin‐to‐creatinine ratio (ACR) longitudinally.
The inclusion criteria of the present study were patients with type 2 diabetes mellitus who had body composition analysis and urinary ACR was measured during the years 2014–2017 from the KAMOGAWA‐DM cohort study. The exclusion criteria of the cross‐sectional study were as follows: missing data of covariates (serum creatinine level and duration of diabetes) and patients with estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2; 19 and the exclusion criteria of the longitudinal study were no follow‐up data (including treatment interruption, transfer to another hospital, death and measurement of urinary ACR <3 times).
Body mass index (BMI), ICW, ECW, total body water (TBW), body fat mass and skeletal muscle mass were measured in the fasting state by BIA 20 . The ECW/ICW ratio and skeletal muscle index (kg/m2) was calculated from the obtained data 21 , 22 . BIA consists of the multifrequency and eight‐polar tactile‐electrode impedance method, and both its accuracy and reproducibility have been well established regardless of age, sex, race, body size, body composition results or renal function compared with isotope dilution 23 , 24 , 25 . Medication data were also collected; medication for diabetes, including insulin and sodium–glucose cotransporter 2 (SGLT‐2) inhibitors; and medication for hypertension, including renin–angiotensin–aldosterone inhibitor and diuretics. The smoking status was categorized into three groups: never‐smoker, ex‐smoker and current smoker. ‘Exercise habit’ was defined as regularly carrying out any type of sport more than once a week 26 .
Data collection
Systolic and diastolic blood pressure were measured twice after a 5‐min rest in a quiet space using a device (HEM‐906: OMRON, Kyoto, Japan) that automatically measures blood pressure, and the reference was an average of two values. Glycated hemoglobin (HbA1c), creatinine, triglycerides, and high‐density lipoprotein cholesterol were measured using the participants’ venous blood after an overnight fast. The eGFR was determined using the Japanese Society of Nephrology equation: eGFR = 194 × Cre−1.094 × age−0.287 (mL/min/1.73 m2; ×0.739 for women) 27 .
Urinary albumin and creatinine concentrations were measured using early morning spot urine samples. In the present study, a mean value for urinary ACR, which was determined from three urine collections during 1 year, were used for analyses. According to the Joint Committee on Diabetic Nephropathy, we divided the participants into three groups; normo‐ (ACR <30 mg/gCr) micro‐ (30–300 mg/gCr) and macroalbuminuria (ACR > 300 mg/gCr) 19 . We assessed the percentage of participants with chronic kidney disease, defined as two measurements of eGFR <60 mL/min/1.73 m2. 28 Follow‐up examinations were carried out 1 year later; then, we also collected urine samples for the calculation of urinary ACR three times a year. The change in urinary ACR was calculated as follows: (dividing the follow‐up urinary ACR by the baseline urinary ACR) / follow‐up year (1 year in this study) 29 .
Ethical considerations
This study was approved by the ethics committee of the Kyoto Prefectural University of Medicine (approval number RBMR‐E‐466‐5), and undertaken in accordance with the Declaration of Helsinki. Written informed consent was obtained from all study participants. To protect the confidentiality of participants, personal identifiable information was removed and medical data stored in a database, which was password protected.
Statistical analysis
Statistical analyses were carried out using JMP version 13.2 software (SAS Institute, Cary, NC, USA). A P‐value <0.05 was considered significant. For normally distributed continuous variables, data were summarized using the mean and standard deviation. Continuous variables with a skewed distribution were summarized using the median and interquartile range. Categorical variables were described using proportions. Differences between the groups were analyzed as follows: the baseline clinical characteristics of the groups were compared using Pearson’s χ2‐test or Fisher’s exact test as appropriate. For normally distributed continuous variables, we compared the mean difference between groups using one‐way analysis of variance (anova) and Tukey’s honestly significant difference test.
Because ACR had a skewed distribution, logarithmic transformation was undertaken before correlation and multiple logistic regression analyses. Variables found to be statistically different in bivariate analysis were controlled for in multiple regression analysis. We investigated the relationships between the ECW/ICW ratio and the logarithm of ACR or other factors using Pearson’s correlation coefficient. Instead of the measured ECW, we also examined using the adjusted ECW, calculated by the ECW divided by the body surface area of each participant 30 . Multiple regression analysis for the logarithm of urinary ACR was undertaken.
Furthermore, we also investigated the effect of the ECW/ICW ratio on the change of urinary ACR by multiple regression analysis. We considered several potential confounders as co‐variants: age, sex, BMI, HbA1c, systolic blood pressure (sBP), creatine, duration of diabetes, smoking status, exercise, the use of renin–angiotensin–aldosterone inhibitor and SGLT‐2 inhibitor, diuretics, ECW/ICW, and the logarithm of urinary ACR at baseline examination. Receiver operator characteristic analyses were carried out to calculate the area under the receiver operator characteristic curve (AUC) of the ECW/ICW ratio or traditional risk factors, such as BMI, HbA1c and sBP, for incident macroalbuminuria 31 , 32 .
Results
The inclusion of participants is summarized in Figure 1. Out of the 481 (261 men and 220 women) participants eligible for the study, 49 (30 men and 19 women) were excluded due to missing data on serum creatinine and duration of diabetes (Figure 1).
Figure 1.
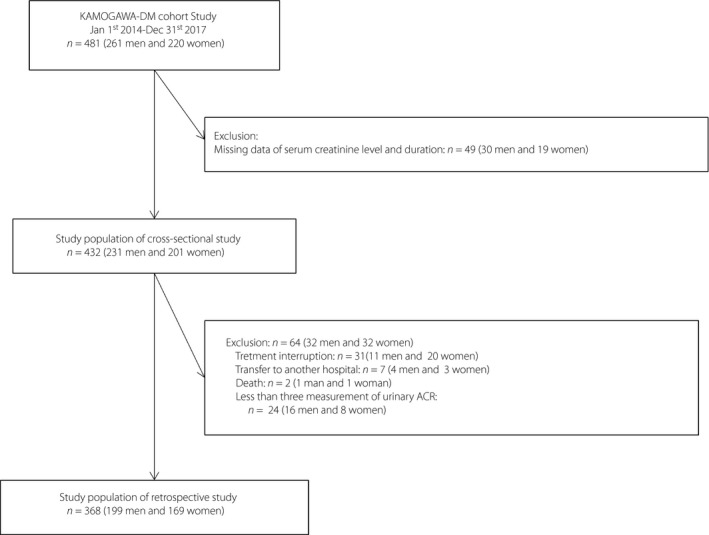
Inclusion and exclusion flow chart of participants. ACR, albumin‐to‐creatinine ratio.
The baseline characteristics of the study participants are summarized in Table 1. The mean age, BMI, skeletal muscle mass and skeletal muscle index were 66.7 ± 11.1 years, 24.3 ± 3.98 kg/m2, 24.1 ± 5.34 kg and 6.93 ± 1.07 kg/m2, respectively. The mean ECW/TBW ratio was 0.390 ± 0.01, and the mean urinary ACR was 134.5 ± 397.6 mg/gCr. The mean ICW and ECW were 20.3 ± 0.25 and 12.8 ± 0.15 kg in patients with normoalbuminuria, 19.1 ± 0.37 and 12.3 ± 0.22 kg in patients with microalbuminuria, and 20.6 ± 0.59 and 13.5 ± 0.35 kg in patients with macroalbuminuria. The ECW/ICW ratio increased with albuminuria stage.
Table 1.
Characteristics of study participants of the cross‐sectional study
| Total | Normo‐albuminuria | Microalbuminuria | Macroalbuminuria | P | |
|---|---|---|---|---|---|
| n | 432 | 263 | 122 | 47 | – |
| Age (years) | 66.7 ± 11.1 | 64.2 ± 0.7 | 67.5 ± 1.0* | 69.0 ± 1.6† | 0.003 |
| Male | 231 (53.5) | 143 (54.4) | 55 (45.1) | 33 (70.2) | 0.022 |
| Height (cm) | 161.2 ± 9.30 | 161.8 ± 0.57 | 159.4 ± 0.84* | 162.2 ± 1.35 | 0.045 |
| Weight (kg) | 63.3 ± 12.6 | 63.0 ± 0.77 | 62.6 ± 1.14 | 66.4 ± 1.83 | 0.188 |
| Body mass index (kg/m2) | 24.3 ± 3.98 | 24.0 ± 0.24 | 24.6 ± 0.36 | 25.1 ± 0.58 | 0.120 |
| Body surface area (m2) | 1.66 ± 0.19 | 1.67 ± 0.01 | 1.64 ± 0.02 | 1.70 ± 0.03 | 0.141 |
| Body composition | |||||
| Body fat mass (kg) | 18.7 ± 7.87 | 18.2 ± 0.48 | 19.2 ± 0.71 | 20.1 ± 1.14 | 0.206 |
| Skeletal muscle mass (kg) | 24.1 ± 5.34 | 24.4 ± 0.33 | 23.0 ± 0.48* | 24.9 ± 0.77 | 0.021 |
| Skeletal muscle index (kg/m2) | 6.93 ± 1.07 | 6.97 ± 0.07 | 6.75 ± 1.00 | 7.22 ± 0.16‡ | 0.024 |
| ICW (kg) | 20.0 ± 4.09 | 20.3 ± 0.25 | 19.1 ± 0.37* | 20.6 ± 0.59 | 0.019 |
| ECW (kg) | 12.7 ± 2.43 | 12.8 ± 0.15 | 12.3 ± 0.22 | 13.5 ± 0.35‡ | 0.013 |
| ECW/ICW ratio | 0.639 ± 0.027 | 0.634 ± 0.002 | 0.645 ± 0.003* | 0.656 ± 0.004†,‡ | <0.001 |
| ECW/TBW ratio | 0.390 ± 0.01 | 0.388 ± 0.001 | 0.392 ± 0.001* | 0.396 ± 0.015†,‡ | <0.001 |
| HbA1c, mmol/mol (%) | 54.7 ± 12.2 (7.16 ± 1.11) | 53.2 ± 0.75 (7.02 ± 0.07) | 57.5 ± 1.09* (7.41 ± 0.10) | 55.8 ± 1.76 (7.26 ± 0.16) | 0.005 |
| Creatinine (μmol/L) | 73.3 ± 40.5 | 66.7 ± 2.28 | 69.4 ± 3.35 | 120.7 ± 5.40†,‡ | <0.001 |
| eGFR (mL/min/1.73 m2) | 70.55 ± 19.9 | 74.2 ± 1.15 | 71.1 ± 1.69 | 51.3 ± 2.73 | <0.001 |
| CKD | 118 (27.3) | 46 (17.5) | 39 (32.0) | 33 (70.2) | <0.001 |
| Urinary ACR (mg/gCr) | 134.5 ± 397.6 | 12.5 ± 17.6 | 95.4 ± 25.9* | 918.7 ± 41.7†,‡ | <0.001 |
| Duration of diabetes (years) | 13.7 ± 10.4 | 12.6 ± 0.64 | 15.0 ± 0.94 | 16.5 ± 1.51† | 0.016 |
| Smoking: never‐/ex‐/current smoker (%) | 260/75/97 (60.2/17.4/22.5) | 159/43/61 (60.4/16.3/23.2) | 81/22/19 (66.4/18.0/15.6) | 20/10/17 (42.6/21.3/36.2) | 0.034 |
| Exercise habit | 208 (48.1) | 135 (51.3) | 51 (41.8) | 22 (46.8) | 0.216 |
| Use of RAS inhibitor | 199 (46.1) | 96 (36.5) | 64 (52.5) | 39 (83.0) | <0.001 |
| Use of Insulin | 84 (19.4) | 46 (17.5) | 24 (19.7) | 14 (29.8) | 0.175 |
| Use of SGLT‐2 inhibitor | 68 (15.7) | 37 (14.1) | 23 (18.9) | 8 (17.0) | 0.472 |
| Use of diuretics | 41 (9.5) | 17 (6.5) | 9 (7.4) | 15 (31.9) | <0.001 |
Data are expressed as the number and mean ± standard deviation, or percentage. Comparisons were carried out using one‐way anova and Tukey’s honestly significant difference test for continuous variables, and the χ2‐test for categorical variables. The urinary albumin‐to‐creatinine ratio (ACR) values were classified into three groups as <30 mg/gCr (normoalbuminuria), 30–300 mg/gCr (microalbuminuria) and ≥300 mg/gCr (macroalbuminuria).
CKD, chronic kidney disease; ECW, extracellular water; HbA1c, glycated hemoglobin; ICW, intracellular water; RAS, renin‐angiotensin‐system; SGLT‐2, sodium–glucose cotransporter 2; TBW, total body water.
P < 0.05 versus normo‐ to microalbuminuria, † P < 0.05 versus normo‐ to macroalbuminuria, ‡ P < 0.05 versus micro‐ to macroalbuminuria.
The differences of ECW/ICW ratio according to sex was 0.632 ± 0.002 in men and 0.647 ± 0.002 in female (P < 0.001). The associations between the ECW/ICW ratio and other covariates is shown in Table 2. The log‐transformed urinary ACR was associated with an elevated ECW/ICW ratio (r = 0.313, 95% confidence interval [CI] 0.226–0.396, P < 0.001), and there was also a significant relationship between the ECW/ICW ratio adjusted by body surface area and logarithm of ACR (r = 0.118, P = 0.015; Table 3). Furthermore, the ECW/ICW ratio was negatively associated with skeletal muscle index levels (r = −0.273, 95% CI −0.358 to −0.184, P < 0.001). Table 4 shows the multiple regression analysis for logarithms of urinary ACR, and shows the strong relationship between the ECW/ICW ratio and urinary ACR (β = 0.205, P < 0.001).
Table 2.
Univariate analysis: Correlation between the extracellular water‐to‐intracellular water ratio and covariates
| Variables | r (95% CI) | P |
|---|---|---|
| Age (years) | 0.420 (0.339, 0.494) | <0.001 |
| Body mass index (kg/m2) | 0.027 (−0.121, 0.067) | 0.572 |
| Skeletal muscle index (kg/m2) | −0.273 (−0.358, −0.184) | <0.001 |
| HbA1c (mmol/mol) | 0.014 (−0.081, 0.108) | 0.778 |
| Creatinine (μmol/L) | 0.088 (−0.006, 0.181) | 0.067 |
| Logarithm of urinary ACR | 0.313 (0.226, 0.396) | <0.001 |
| Duration of diabetes | 0.208 (0.116, 0.296) | <0.001 |
To investigate the relationships between the extracellular water‐to‐intracellular water (ECW/ICW) ratio and logarithm of albumin‐to‐creatinine ratio (ACR) or other factors, Pearson’s correlation coefficient was carried out.
Table 3.
Univariate analysis: co‐relation between the extracellular water‐to‐intracellular water ratio adjusted by body surface area and covariates
| Variables | r (95% CI) | P |
|---|---|---|
| Age (years) | 0.438 (0.762, 1.130) | <0.001 |
| Body mass index (kg/m2) | −0.495 (−0.447, −0.320) | <0.001 |
| Skeletal muscle index (kg/m2) | −0.795 (−0.177, −0.154) | <0.001 |
| HbA1c (mmol/mol) | 0.006 (−0.211, 0.239) | 0.906 |
| Creatinine (μmol/L) | 0.088 (−0.006, 0.181) | 0.067 |
| Logarithm of urinary ACR | 0.118 (0.007, 0.061) | 0.015 |
| Duration of diabetes | 0.211 (0.241, 0.617) | <0.001 |
Examination with the adjusted extracellular water (ECW), calculated by ECW divided by body surface area of each participant, instead of the measured ECW. To investigate the relationships between the ECW‐to‐intracellular water (ECW/ICW) ratio adjusted by body surface area and logarithm of the albumin‐to‐creatinine ratio (ACR) or other factors, Pearson’s correlation coefficient was carried out.
Table 4.
Multiple regression analysis of logarithms of the albumin‐to‐creatinine ratio
| Variables | β (95% CI) | P |
|---|---|---|
| Age (years) | 0.036 (−0.009, 0.018) | 0.488 |
| Male | 0.028 (−0.094, 0.177) | 0.546 |
| Body mass index (kg/m2) | 0.066 (−0.009, 0.058) | 0.155 |
| ECW/ICW ratio | 0.217 (6.423, 17.207) | <0.001 |
| HbA1c (mmol/mol) | 0.126 (0.005, 0.026) | 0.004 |
| Creatinine (μmol/L) | 0.299 (0.008, 0.014) | <0.001 |
| Duration of diabetes (years) | 0.044 (−0.007, 0.019) | 0.354 |
| Current smoker | 0.059 (−0.054, 0.314) | 0.166 |
| Exercise habit | −0.049 (−0.197, 0.051) | 0.250 |
| Use of RAS inhibitor | 0.188 (0.150, 0.410) | <0.001 |
| Use of insulin | 0.017 (−0.130, 0.195) | 0.695 |
| Use of SGLT‐2 inhibitor | 0.057 (−0.057, 0.290) | 0.187 |
| Use of diuretics | 0.060 (−0.068, 0.372) | 0.176 |
Current smoker was defined as never‐ and ex‐smoker (0), or current smoker (1); exercise habit was defined as non‐regular exerciser (0), regular exerciser (1); use of each medicine was defined as non‐user (0) or user (1). ACR, albumin‐to‐creatinine ratio; ECW, extracellular water; HbA1c, glycated hemoglobin; ICW, intracellular water, RAS, renin–angiotensin–system; SGLT‐2, sodium–glucose cotransporter 2.
In the longitudinal study, out of the 432 people (231 men and 201 women) eligible for the study, 64 (32 men and 32 women) were excluded, resulting in a study population of 368 people (199 men and 169 women; Figure 1). Table 5 summarizes the characteristics of the study participants of the longitudinal study. Table 6 shows the results of the multiple regression analysis with change in the urinary ACR. The ECW/ICW ratio was associated with change in the urinary ACR after adjusting for covariates (β = 0.176, P = 0.004). According to the receiver operator characteristic curves, the optimal cut‐off point of the ECW/ICW ratio for incident macroalbuminuria was 0.648 (AUC 0.78, 95% CI 0.58–0.90, sensitivity = 0.80, specificity = 0.71, P < 0.001). Furthermore, we also compared the AUC of the ECW/ICW ratio for incident macroalbuminuria with the traditional risk factors, such as BMI, HbA1c and sBP. The AUC of the ECW/ICW ratio (AUC 0.78, 95% CI 0.58–0.90) was greater than that of HbA1c (AUC 0.53, 95% CI 0.38–0.67, P = 0.022) and tended to be greater than that of BMI (AUC 0.60, 95% CI 0.39–0.78, P = 0.066) and sBP (AUC 0.64, 95% CI 0.48–0.78, P = 0.345; Figure 2).
Table 5.
Characteristics of study participants of the retrospective study at the baseline examination
| Total | |
|---|---|
| n | 368 |
| Age (years) | 65.5 ± 11.2 |
| Male | 199 (54.1) |
| Height (cm) | 161.1 ± 9.35 |
| Weight (kg) | 63.2 ± 12.7 |
| Body mass index (kg/m2) | 24.3 ± 3.97 |
| Body surface area (m2) | 1.66 ± 0.19 |
| Body composition | |
| Body fat mass (kg) | 18.8 ± 7.81 |
| Skeletal muscle mass (kg) | 24.1 ± 5.34 |
| Skeletal muscle index (kg/m2) | 6.92 ± 1.09 |
| ICW (kg) | 19.9 ± 4.14 |
| ECW (kg) | 12.7 ± 2.45 |
| ECW/ICW ratio | 0.64 ± 0.027 |
| ECW/TBW ratio | 0.392 ± 0.01 |
| HbA1c, mmol/mol (%) | 54.5 ± 11.0 (7.14 ± 1.01) |
| Creatinine (μmol/L) | 69.2 ± 20.0 |
| Urinary ACR (mg/gCr) | 109.0 ± 318.1 |
| Duration of diabetes (years) | 13.4 ± 10.2 |
| Smoking: never‐/ex‐/current smoker | 220/86/62 (59.8/23.3/16.8) |
| Exercise habit | 184 (50.0) |
| Usage of RAS inhibitor | 171 (46.5) |
| Use of insulin | 69 (18.9) |
| Use of SGLT‐2 inhibitor | 63 (17.1) |
| Use of diuretics | 31 (8.4) |
Data are expressed as the number (percentage) and mean ± standard deviation. ACR, albumin‐to‐creatinine ratio; ECW, extracellular water; ICW, intracellular water; RAS, renin–angiotensin–system; SGLT‐2, sodium–glucose cotransporter 2; TBW, total body water.
Table 6.
Multiple regression analysis for the factors affecting change in the urinary albumin‐to‐creatinine ratio
| Variables | β (95% CI) | P |
|---|---|---|
| Age (years) | 0.010 (−0.016, 0.019) | 0.870 |
| Male | 0.131 (0.030, 0.799) | 0.035 |
| BMI (kg/m2) | 0.112 (0.001, 0.088) | 0.046 |
| ECW/ICW ratio | 0.169 (2.84, 16.97) | 0.006 |
| HbA1c (mmol/mol) | 0.142 (0.005, 0.035) | 0.008 |
| Creatinine (μmol/L) | 0.028 (−0.008, 0.012) | 0.661 |
| Logarithm of urinary ACR | −0.197 (−0.344, −0.093) | 0.0007 |
| Duration of diabetes | 0.076 (−0.005, 0.029) | 0.180 |
| Current smoker | −0.047 (−0.342, 0.126) | 0.365 |
| Exercise habit | −0.014 (−0.183, 0.140) | 0.793 |
| Usage of RAS inhibitor | 0.023 (−0.275, 0.419) | 0.683 |
| Use of insulin | 0.495 (−0.226, 0.623) | 0.359 |
| Use of SGLT‐2 inhibitor | −0.053 (−0.662, 0.217) | 0.320 |
| Use of diuretics | 0.082 (−0.137, 1.052) | 0.131 |
Current smoker was defined as never‐ and ex‐smoker (0), or current smoker (1); exercise habit was defined as non‐regular exerciser (0), regular exerciser (1). ACR, albumin‐to‐creatinine ratio; ECW, extracellular water; ICW, intracellular water; RAS, renin–angiotensin–system; SGLT‐2, sodium–glucose cotransporter 2.
Figure 2.
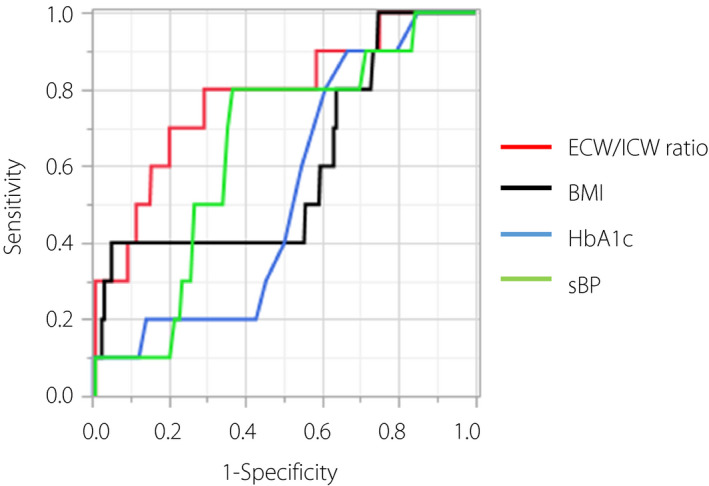
The receiver operating characteristic (ROC) curve and area under the ROC curve (AUC) for incident macroalbuminuria. The ROC and AUC showing the ability of the extracellular water‐to‐intracellular water (ECW/ICW) ratio or traditional risk factors, such as BMI, HbA1c and sBP, for the incident macroalbuminuria. The red line represents ECW/ICW ratio, black line represents BMI, the blue line represents HbA1c, the green line represents sBP. According to the ROC curves, the optimal cut‐off point of ECW/ICW ratio for incident macroalbuminuria was 0.648 (AUC 0.78, 95% CI 0.58–0.90). The AUC of the ECW/ICW ratio was greater than that of glycated hemoglobin (HbA1c; AUC 0.53, 95% CI 0.38–0.67, P = 0.022), and tended to be greater than that of BMI (AUC 0.60, 95% CI 0.39–0.78, P = 0.066) and systolic blood pressure (sBP; AUC 0.64, 95% CI 0.48–0.78, P = 0.345). BMI, body mass index.
Discussion
We investigated the association of fluid volume imbalance and albuminuria in patients with type 2 diabetes without kidney failure based on our hypothesis that fluid imbalance occurred in diabetes patients without kidney failure, and was associated with changes in albuminuria. The present study’s findings support the hypothesis.
Previous studies have shown the association of fluid overload and increased risk of eGFR decline and all‐cause or cardiovascular mortality in patients with kidney failure, including both ESRD and patients on dialysis 10 , 11 , 21 , 33 , 34 , 35 , 36 , 37 . Recently, Faucon et al. 38 showed that a higher ECW was associated not only with ESRD and mortality, but a faster GFR decline in a larger cohort of almost 1,600 patients with chronic kidney disease stage 1–4. The present study’s mean ECW/TBW ratio of 0.390 ± 0.01 was lower than that reported in recent studies of patients with chronic kidney disease stage 4 or 5 (0.39783–0.512) 35 , 39 , 40 . This finding suggests that fluid imbalance is less likely to occur in patients with early nephropathy than in patients with kidney failure.
Water shift from ICW to ECW led to changing the ECW/ICW ratio 9 . Cell volume is regulated by apoptosis, which is a morphological hallmark of programmed cell death 41 . The loss in cell volume during apoptosis might play a role in the change in balance between ICW and ECW content. In addition, uremic status might also cause cell shrinkage. Previous studies have reported that erythrocytes might undergo suicidal death or eryptosis associated with cell shrinkage, which can be stimulated by uremic toxins 42 .
Albuminuria is known to reflect endothelial dysfunction and subclinical inflammation caused by oxidative stress and inflammatory cytokines 8 , 12 , 43 . Kidney endothelial dysfunction plays an important role in the development of albuminuria by reducing vascular relaxation and inflammatory cell infiltration 44 . Under physiological conditions, tubuloglomerular feedback (TGF) signaling maintains a stable GFR by modulating pre‐glomerular arteriole tone. Early in nephropathy, chronic hyperglycemic conditions impair SGLT‐2‐mediated reabsorption of sodium and glucose in the proximal tubule. Thus, despite increased GFR, the macula densa is exposed to low sodium concentrations. This impairment of TGF signaling likely leads to inadequate arteriole tone and increased renal perfusion. As a result, impairment of TGF causes increased body fluid and fluid imbalance 45 , 46 , 47 . A previous study showed that both humans and animals with volume overload have significantly higher pro‐inflammatory cytokines, such as interleukin‐6 or tumor necrosis factor‐α 48 , which can be the result of kidney endothelial dysfunction and impairment of TGF. Furthermore, inflammation‐induced hypoalbuminemia and increased vascular permeability enhance extravascular fluid shift, thereby resulting in extravascular fluid volume overload 14 . In fact, the ECW/TBW ratio, which is substantially the same as the ECW/ICW ratio, has been used as a marker of ECW excess 8 , 49 , 50 .
An increase in the ECW/ICW ratio caused by fluid overload affects the vascular and endothelial level by oxidative stress, chronic activation of the renin‐angiotensin system, sympathetic activation and an increase of inflammation, which leads to atherosclerosis 51 . In addition, the excess volume status would increase venous pressure and interstitial pressure, and also cause renal efferent pressure and glomerular hypertension, leading to an eventual decline in eGFR 34 , 38 . Even in the subclinical state, early changes in volume and cardiac stretch, venous congestion or subclinical atherosclerosis might contribute to reduced kidney function. Taking these findings together, an increase of the ECW/ICW is associated with the presence of albuminuria and a change in albuminuria. In addition, SGLT‐2 inhibitor or diuretics cause extracellular fluid loss; however, the result was almost the same if we excluded patients with SGLT‐2 inhibitor or diuretics. In the present study, we showed the cut‐off ECW/ICW ratio level of 0.648 for incident macroalbuminuria. Therefore, we consider that focusing on the relationships between the ECW/ICW ratio and urinary ACR from an early stage, and the risk of the development of diabetic nephropathy in patients where the ECW/ICW ratio is above the cut‐off level, provides an opportunity for intensive complication assessment and prevention of progression of complications. Therefore, we consider that focusing on the relationships between the ECW/ICW ratio and urinary ACR from an early stage, and the risk of development of diabetic nephropathy in patients where the ECW/ICW ratio is above the cut‐off level, provides an opportunity for intensive complication assessment and the prevention of progression of complications. That is why this early alternation of fluid balance has value for clinical intervention. In addition, previous studies showed that the fluid balance is severely imbalanced in end‐stage nephropathy. In addition to this fact, we showed, for the first time, that fluid balance was already imbalanced in the early stages of the disease in the present study. This fact has an important meaning, because this fluid imbalance would also become the early treatment target.
These results should be interpreted considering the study’s limitations. First, the sample size might not have been adequate to determine if a significant relationship existed between albuminuria and the ECW/ICW ratio, resulting in selection bias. Second, our assessment of lifestyle (exercise habit, alcohol consumption) and medication intake only at baseline means that changes in these factors during follow up might have influenced a change in urinary ACR. Third, isotope dilution is a gold standard of the measurement of ICW, ECW and TBW. However, the result of BIA is closely correlated with isotope dilution 22 , 23 , 24 . Finally, the inclusion of only Japanese diabetes patients without kidney failure could limit the generalization of the findings to non‐Japanese patients.
Despite the limitations, the present study was a well‐designed epidemiological study of patients with type 2 diabetes. The measurement of body fluid composition by BIA has the advantage of being non‐invasive, fast and reproducible, making it high versatile and constructive. Finally, we addressed threats to internal validity by adjusting for confounding variables and laboratory measurements. This reduced risk of biases.
In conclusion, to our knowledge, this is the first study to show that the imbalance of body composition evaluated by the ECW/ICW ratio is independently associated with the presence and increment of albuminuria, even in diabetes patients without kidney failure. Therefore, we recommend that clinicians pay more attention to monitoring the fluid status among patients with type 2 diabetes mellitus, and maintaining fluid balance in the early stage of diabetic kidney disease.
Disclosure
YH received grant support from Asahi Kasei Pharma, and honoraria from Mitsubishi Tanabe Pharma Corporation and Novo Nordisk Pharma Ltd. SM received honoraria from Novo Nordisk Pharma Ltd., Abbott Japan Co., Ltd., AstraZeneca plc, Kowa Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd. and Sumitomo Dainippon Pharma Co., Ltd. TS received honoraria from Ono Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co, Astellas Pharma Inc., Kyowa Hakko Kirin Co., Ltd., Sanofi K.K., MSD K.K., Kowa Pharmaceutical Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd. and Eli Lilly Japan K.K. NN received honoraria from Novo Nordisk Pharma Ltd., Abbott Japan Co., Ltd., AstraZeneca plc, Kowa Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd. and Sumitomo Dainippon Pharma Co., Ltd. UE received grant support from the Japanese Study Group for Physiology and Management of Blood Pressure, and the Astellas Foundation for Research on Metabolic Disorders (grant number: 4024). Donated Fund Laboratory of Diabetes therapeutics is an endowment department, supported by an unrestricted grant from Ono Pharmaceutical Co., Ltd., and received personal fees from AstraZeneca plc, Astellas Pharma Inc., Daiichi Sankyo Co., Ltd., Kyowa Hakko Kirin Company Ltd., Kowa Pharmaceutical Co., Ltd., MSD K.K., Mitsubishi Tanabe Pharma Corp., Novo Nordisk Pharma Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd. and Johnson & Johnson K.K. MA received honoraria from Novo Nordisk Pharma Ltd., Abbott Japan Co., Ltd., AstraZeneca plc, Kowa Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd. and Sumitomo Dainippon Pharma Co., Ltd. MH has received grant support from Asahi Kasei Pharma, Nippon Boehringer Ingelheim Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo Company, Limited, Sanofi K.K., Takeda Pharmaceutical Company Limited, Astellas Pharma Inc., Kyowa Kirin Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Novo Nordisk Pharma Ltd. and Eli Lilly Japan K.K. MY received personal fees from MSD K.K., Sumitomo Dainippon Pharma Co., Ltd., Kowa Company, Limited, AstraZeneca PLC, Takeda Pharmaceutical Co., Ltd, Kyowa Hakko Kirin Co., Ltd., Daiichi Sankyo Co., Ltd., Kowa Pharmaceutical Co., Ltd. and Ono Pharmaceutical Co., Ltd. MF received grants from Nippon Boehringer Ingelheim Co., Ltd., Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co, Daiichi Sankyo Co., Ltd., Sanofi K.K., Takeda Pharmaceutical Co., Ltd., Astellas Pharma Inc., MSD K.K., Kyowa Hakko Kirin Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Kowa Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd. Eli Lilly Japan K.K., Taisho Pharmaceutical Co., Ltd., Terumo Co., Teijin Pharma Ltd., Nippon Chemiphar Co., Ltd. and Johnson & Johnson K.K. Medical Company, and received honoraria from Nippon Behringer Ingelheim Co., Ltd., Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corp., Daiichi Sankyo Co., Ltd., Sanofi K.K., Takeda Pharmaceutical Co., Ltd., Astellas Pharma Inc., MSD K.K., Kyowa Kirin Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Kowa Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Eli Lilly Japan K.K., Taisho Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., AstraZeneca K.K., Mochida Pharmaceutical Co., Ltd. and Combi Corp. The other authors declare no conflict of interest.
Acknowledgments
We thank all of the staff members of Kyoto Prefectural University of Medicine and Kameoka Municipal Hospital. We thank Editage (www.editage.jp) for English language editing. This manuscript has been preprinted in Research Square (https://doi.org/10.21203/rs.2.24737/v1), and the abstract of this manuscript will be published for the American Diabetes Association 80th Scientific meeting.
J Diabetes Investig 2021; 12: 1202–1211
References
- 1. GLOBAL STATUS REPORT on noncommunicable diseases 2014. Attaining the nine global noncommunicable diseases targets; a shared responsibility.
- 2. Berhane A, Weil J, Knowler W, et al. Albuminuria and estimated glomerular filtration rate as predictors of diabetic end‐stage renal disease and death. Clin J Am Soc Nephrol 2011; 6: 2444–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fox C, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end‐stage renal disease in individuals with and without diabetes: a meta‐analysis. Lancet 2012; 380: 1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wada T, Haneda M, Furuichi K, et al. Clinical impact of albuminuria and glomerular filtration rate on renal and cardiovascular events, and all‐cause mortality in Japanese patients with type 2 diabetes. Clin Exp Nephrol 2014; 18: 613–620. [DOI] [PubMed] [Google Scholar]
- 5. Gæde P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in type 2 diabetes. N Engl J Med 2003; 348: 1925–1927. [DOI] [PubMed] [Google Scholar]
- 6. Gæde P, Lund‐Andersen H, Parving H, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358: 580–591. [DOI] [PubMed] [Google Scholar]
- 7. Gæde P, Oellgaard J, Carstensen B, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow‐up on the Steno‐2 randomised trial. Diabetologia 2016; 59: 2298–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitsides N, Cornelis T, Broers N, et al. Extracellular overhydration linked with endothelial dysfunction in the context of inflammation in haemodialysis dependent chronic kidney disease. PLoS One 2017; 12: e0183281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jaffrin M, Morel H. Body fluid volumes measurements by impedance: a review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Med Eng Phys 2008; 30: 1257–1269. [DOI] [PubMed] [Google Scholar]
- 10. Hung S, Kuo K, Peng C, et al. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int 2014; 85: 703–709. [DOI] [PubMed] [Google Scholar]
- 11. Wizemann V, Wabel P, Chamney P, et al. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 2009; 224: 1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dekker M, Sande F, Berghe F, et al. Fluid overload and inflammation axis. Blood Purif 2018; 45: 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goncharov N, Nadeev A, Jenkins R, et al. Markers and biomarkers of endothelium: when something is rotten in the state. Oxid Med Cell Longev 2017; 2017: 9759735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scotland G, Cruickshank M, Jacobsen E, et al. Multiple‐frequency bioimpedance devices for fluid management in people with chronic kidney disease receiving dialysis: a systematic review and economic evaluation. Health Technol Assess 2018; 22: 1–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zoccali C, Moissl U, Chazot C, et al. Chronic fluid overload and mortality in ESRD. J Am Soc Nephrol 2017; 28: 2491–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cha K, Chertow G, Gonzalez J, et al. Multifrequency bioelectrical impedance estimates the distribution of body water. J Appl Physiol 1985; 79: 1316–1319. [DOI] [PubMed] [Google Scholar]
- 17. Varlet‐Marie E, Joré C. Segmental bioelectrical impedance analysis (SBIA) and blood rheology reducing the gap between in vivo and in vitro. Clin Hemorheol Microcirc 2016; 64: 603–611. [DOI] [PubMed] [Google Scholar]
- 18. Sakai R, Hashimoto Y, Ushigome E, et al. Late‐night‐dinner is associated with poor glycemic control in people with type 2 diabetes: the KAMOGAWA‐DM cohort study. Endoc J 2018; 65: 395–402. [DOI] [PubMed] [Google Scholar]
- 19. Haneda M, Utsunomiya K, Koya D, et al. A new classification of diabetic nephropathy 2014: a report from joint committee on diabetic nephropathy. J Diabetes Investig 2015; 6: 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohashi Y, Joki N, Yamazaki K, et al. Changes in the fluid volume balance between intra‐ and extracellular water in a sample of japanese adults aged 15–88 yr old: a cross‐sectional study. Am J Physiol Renal Physiol 2018; 314: 614–622. [DOI] [PubMed] [Google Scholar]
- 21. Lin Y, Yu W, Hsu T, et al. The extracellular fluid‐to‐intracellular fluid volume ratio is associated with large‐artery structure and function in hemodialysis patients. Am J Kidney Dis 2003; 42: 990–999. [DOI] [PubMed] [Google Scholar]
- 22. Hashimoto Y, Kaji A, Sakai R, et al. Sarcopenia is associated with blood pressure variability in older patients with type 2 diabetes A cross‐sectional study of the KAMOGAWA‐DM cohort study. Geriatr Gerontol Int 2018; 18: 1345–1349. [DOI] [PubMed] [Google Scholar]
- 23. Medici G, Mussi C, Fantuzzi AL, et al. Accuracy of eight‐polar bioelectrical impedance analysis for the assessment of total and appendicular body composition in peritoneal dialysis patients. Eur J Clin Nutr 2005; 59: 932–937. [DOI] [PubMed] [Google Scholar]
- 24. Sartorio A, Malavolti M, Agosti F, et al. Body water distribution in severe obesity and its assessment from eight‐polar bioelectrical impedance analysis. Eur J Clin Nutr 2005; 59: 155–160. [DOI] [PubMed] [Google Scholar]
- 25. Schubert M, Seay R, Spain K, et al. Reliability and validity of various laboratory methods of body composition assessment in young adults. Clin Physiol Funct Imaging 2019; 39: 150–159. [DOI] [PubMed] [Google Scholar]
- 26. Ryu S, Chang Y, Kim D, et al. γ‐glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem 2007; 53: 71–77. [DOI] [PubMed] [Google Scholar]
- 27. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 28. Kidney Disease: Improving Global Outcomes (KDIGO) Hepatitis C Work Group . KDIGO 2018 clinical practice guideline for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl 2018; 8: 91–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith M, Herrington W, Weldegiorgis M, et al. Change in albuminuria and risk of renal and cardiovascular outcomes: natural variation should be taken into account. Kidney Int Reports 2018; 3: 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dubois D, Dubois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Int Med 1916; 17: 863–871. [Google Scholar]
- 31. Harjutsalo V, Groop P. Epidemiology and risk factors for diabetic kidney disease. Adv Chronic Kidney Dis 2014; 21: 260–266. [DOI] [PubMed] [Google Scholar]
- 32. Nakanishi S, Hirukawa H, Shimoda M, et al. Comparison of HbA1c levels and body mass index for prevention of diabetic kidney disease: a retrospective longitudinal study using outpatient clinical data in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2019; 155: 107807. [DOI] [PubMed] [Google Scholar]
- 33. Tabinor M, Elphick E, Dudson M, et al. Bioimpedance‐defined overhydration predicts survival in end stage kidney failure (ESKF): systematic review and subgroup meta‐analysis. Sci Rep 2018; 8: 4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsai Y, Tsai J, Chen S, et al. Association of fluid overload with kidney disease progression in advanced CKD: a prospective cohort study. Am J Kidney Dis 2014; 63: 68–75. [DOI] [PubMed] [Google Scholar]
- 35. Tsai Y, Chiu Y, Tsai J, et al. Association of fluid overload with cardiovascular morbidity and all‐cause mortality in stages 4 and 5 CKD. Clin J Am Soc Nephrol 2015; 10: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim E, Choi M, Lee J, et al. Extracellular fluid/intracellular fluid volume ratio as a novel risk indicator for all‐cause mortality and cardiovascular disease in hemodialysis patients. PLoS One 2017; 12: e0170272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Drepper V, Kihm L, Kälble F, et al. Overhydration is a strong predictor of mortality in peritoneal dialysis patients ‐Independently of cardiac failure. PLoS One 2016; 11: e0158741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Faucon A, Flamant M, Metzger M, et al. Extracellular fluid volume is associated with incident end‐stage kidney disease and mortality in patients with chronic kidney disease. Kidney Int 2019; 96: 1020–1029. [DOI] [PubMed] [Google Scholar]
- 39. Ando M, Suminaka T, Shimada N, et al. Body water balance in hemodialysis patients reflects nutritional, circulatoryand body fluid status. J Biorheol 2018; 32: 46–55. [Google Scholar]
- 40. O’lone E, Visser A, Finney H, Fan S. Clinical significance of multi‐frequency bioimpedance spectroscopy in peritoneal dialysis patients: Independent predictor of patient survival. Nephrol Dial Transplant 2014; 29: 1430–1437. [DOI] [PubMed] [Google Scholar]
- 41. Bortner C, Cidlowski J. Cell shrinkage and monovalent cation fluxes: role in apoptosis. Arch Biochem Biophys 2007; 462: 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ohashi Y, Tai R, Mizuiri S, et al. The associations of malnutrition and aging with fluid volume imbalance between intra‐ and extracellular water in patients with chronic kidney disease. J Nutr Heal Aging 2015; 19: 986–993. [DOI] [PubMed] [Google Scholar]
- 43. Feldt‐Rasmussen B. Microalbuminuria, endothelial dysfunction and cardiovascular risk. Diabetes Metab 2000; 26: 64–66. [PubMed] [Google Scholar]
- 44. Satoh M. Endothelial dysfunction as an underlying pathophysiological condition of chronic kidney disease. Clin Exp Nephrol 2012; 16: 518–521. [DOI] [PubMed] [Google Scholar]
- 45. Thomson S, Vallon V, Blantz R. Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol 2004; 286: 8–15. [DOI] [PubMed] [Google Scholar]
- 46. Cherney D, Perkins B. Sodium‐glucose cotransporter 2 inhibition in type 1 diabetes: simultaneous glucose lowering and renal protection? Can J Diabetes 2014; 38: 356–363. [DOI] [PubMed] [Google Scholar]
- 47. Brizzolara A, Barbieri MP, Adezati L. Water distribution in insulin‐dependent diabetes mellitus in various states of metabolic control. Eur J Endocrinol 1996; 135: 609–615. [DOI] [PubMed] [Google Scholar]
- 48. Hung S, Lai Y, Kuo K, et al. Volume overload and adverse outcomes in chronic kidney disease: clinical observational and animal studies. J Am Heart Assoc 2015; 4: e001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Park S, Lee C, Jhee J, et al. Extracellular fluid excess is significantly associated with coronary artery calcification in patients with chronic kidney disease. J Am Heart Assoc 2018; 7: e008935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jaffrin M, Morel H. Body fluid volumes measurements by impedance: a review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods”. Med Eng Phys 2008; 30: 1257–1269. [DOI] [PubMed] [Google Scholar]
- 51. Bock J, Gottlieb S. Cardiorenal syndrome: new perspectives. Circulation 2010; 121: 2592–2600. [DOI] [PubMed] [Google Scholar]


