Abstract
This study aims to investigate the effects of probiotics and Chinese medicine polysaccharides (CMPs) on growth performance, blood indices, rumen fermentation, and bacteria composition in lambs. Forty female lambs were randomly divided into four groups as follows: control, probiotics, CMP, and compound (probiotics + CMP) groups. The results showed that probiotics treatment increased the concentrations of blood glucose (GLU) and immunoglobulin G (IgG) and enhanced rumen microbial protein contents but declined the value of pH in rumen fluid compared with the control (P < 0.05). Furthermore, supplementation with CMP enhanced the average daily gain (ADG) and the contents of IgA, IgG, and IgM in the serum but decreased the F:G ratio compared with the control (P < 0.05). Besides, both CMP and compound (probiotics + CMP) treatments decreased the ratio of acetic acid and propionic acid compared with the control (P < 0.05). High-throughput sequencing data showed that at the genus level, the relative abundance of Veillonellaceae_UCG-001 in the probiotics group was increased, the relative abundance of Succiniclasticum and norank_f__Muribaculaceae in the CMP group were enhanced, and the relative abundance of Ruminococcaceae_UCG-002 in the compound group was raised compared with the control (P < 0.05). In summary, supplementation with probiotics can promote rumen protein fermentation but decrease the diversity of bacteria in rumen fluid; however, CMP treatment increased the relative abundance of Fibrobacteria, changed rumen microbial fermentation mode, increased the immune function, and ultimately improved the growth performance.
Keywords: Chinese medicine polysaccharide, probiotics, growth performance, rumen fermentation, rumen bacteria composition, lamb
Introduction
Antibiotics have long been used for therapeutic and subtherapeutic purposes, including disease treatment, disease prevention, and growth performance (1). However, the increased use of antibiotics as a feed additive resulted in the emergence of drug residues in animal products and harm human health, leading to a ban on the use in many countries, such as China, EU, USA. Recently, research has begun to investigate more carefully the use of probiotics and herbal extracts and plant bioactives as an alternative approach to enhancing animal health and production efficiency (2).
Probiotics are defined as direct-fed microbials, including yeast (Saccharomyces cerevisiae), Bacillus, and Lactobacillus, which may lead to the creation of a microbial balance in the gastrointestinal tract and confer a health benefit for the host when administered in appropriate and regular quantities (3). A previous study revealed that probiotics could enhance the digestion of fiber and starch, promote volatile fatty acid (VFA) synthesis, and increase the average daily gain and feed conversion rate of cattle (4). Another report showed that adding Lactobacillus acidophilus to lambs' diet could increase the dry matter feed intake and daily weight gain and improve the digestibility of dry matter, crude protein, and crude fiber (5).
Chinese medicine polysaccharides are secondary metabolites produced by Chinese herbs metabolism, and it contains more than 10 degrees of polymerization (6, 7). A series of studies have shown that polysaccharides, including Astragalus polysaccharide and Lycium barbarum polysaccharide, can enhance immunity, are antioxidative, and can improve animal production performance (8, 9). Astragalus polysaccharide is the extract of Astragalus membranaceus, which is often used to enhance immunity in traditional Chinese medicine. The polysaccharide of L. barbarum is its major bioactive component and has been widely used as one of the main chemical components in a traditional Chinese medicine named Ningxia wolfberry. Astragalus polysaccharide has been reported to be a potential additive used in the vaccination of humans and animals so as to improve immune functions both in cellular and humoral immune responses (10). Astragalus polysaccharide was reported to enhance the antioxidant capacity while adding to the diet of goats (11). Furthermore, a recent report showed that L. barbarum polysaccharide (LBP) improved growth promotion and immunomodulation and could be used as an alternative for nutritive additive in broilers (12).
At present, probiotics and Chinese medicine polysaccharides have been used for immunity, growth performance, and metabolism in non-ruminants and ruminants. However, little information is available regarding their effects on rumen bacterial diversity and fermentation parameters of lambs. Rumen microbial ecosystems include a variety of strict anaerobes, protozoa, and archaea, which are in charge of the degradation and fermentation of most nutrients such as dietary fiber (13). Therefore, the purpose of this study is to investigate the effects of probiotics and Chinese medicine polysaccharides on growth performance, metabolism, rumen bacterial diversity, and fermentation parameters in lambs. These results will give helpful information on the effects and application of probiotics and Chinese medicine polysaccharides in ruminants farming.
Materials and Methods
Probiotics and Chinese Medicine Polysaccharides
Probiotics were donated by Nanchang University, which consists of Bacillus licheniformis, Bacillus subtilis, and Lactobacillus plantarum at a ratio of 1:1:0.5. The Chinese medicine polysaccharide (CMP) comes from the mixture of L. barbarum and A. membranaceus in the ratio of 2:1 and in which the content of polysaccharides was 114.7 mg/g.
Animals and Experimental Design
This experiment was approved by the Committee for the Care and Use of Experimental Animals at Jiangxi Agricultural University (JXAULL-20190015). A total of 40 healthy female lambs (Chuanzhong black) with an average body weight of 21.69 ± 0.46 kg and the ages ranging 4–5 months were housed indoors in an individual pen (0.5 × 0.6 m2) with a leaky floor, which can keep the pen clean and reduce the labor cost of cleanliness. The feeding trial lasted for 60 days after a 10-day adaptation period. During the trial, a single-factor completely randomized design method was used, and all lambs were randomly divided into four treatments, namely, control (basal diet), probiotics (0.1% probiotics + basal diet), CMP (0.1% Chinese medicine polysaccharides + basal diet), and compound (0.1% probiotics + 0.1% Chinese medicine polysaccharides + basal diet), and each treatment contained 10 lambs. These lambs fed ad libitum with standard ration consisted mainly of peanut vine, corn, soybean meal, wheat bran, and mineral, which were formulated to meet the feeding standard of meat-producing sheep and goat of NY/T 816-2004 (Ministry of Agriculture of the People's Republic of China 2004), and the ingredient composition and nutrient levels are shown in Table 1. No antibiotic was included in the diet.
Table 1.
| Ingredients | Content (%) | Nutrition levelsb | Content (%) |
|---|---|---|---|
| Peanut vine | 50.00 | DM | 84.01 |
| Corn | 30.00 | ME/(MJ/kg) | 7.07 |
| Soybean meal | 11.00 | CP | 12.53 |
| Wheat bran | 4.00 | Ca | 1.20 |
| CaHPO4 | 2.00 | P | 0.64 |
| NaHCO3 | 1.50 | NDF | 33.03 |
| NaCl | 0.50 | ADF | 22.24 |
| Premix | 1.00 | DE/(MJ/kg) | 11.08 |
| Total | 100.00 |
Premix contained 12,000 IU/kg of vitamin A, 5,000 IU/kg of vitamin D, 50 mg/kg of vitamin E, 40 mg/kg of Fe, 16 mg/kg of Cu, 70 mg/kg of Zn, 80 mg/kg of Mn, 0.3 mg/kg of Co, 0.8 mg/kg of I, and 0.3 mg/kg of Se.
DM, dry matter; ME, metabolizable energy; CP, crude protein; NDF, neutral detergent fiber; ADF, acid detergent fiber; DE, digestible energy.
Nutrition levels are values of measurement except that ME is value from a calculation.
Determination of the Growth Performance
Body weight of animals on an empty stomach was measured at 09:00 h at the beginning and end of the trial, and the daily feed intakes were recorded during the experimental period. Based on these data, average daily feed intake (ADFI), average daily gain (ADG), and the ratio of feed and gain (F:G) ratio were calculated.
Serum Biochemistry Indices Analysis
On the 60th day, blood samples (10 ml each) were collected at 14:00 h from the jugular vein, using a vacuum blood collection tube (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Serum was obtained by immediate centrifugation (10 min, 4°C, 1,500 g) and kept at −80°C until analysis. The concentrations of serum total protein (TP), albumin (ALB), blood urea nitrogen (BUN), glucose (GLU), total cholesterol (TC), and triglyceride (TG) in serum were measured by using spectrophotometric kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The concentrations of serum immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM) were analyzed by using ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Rumen Fermentation Index Analysis
At the end of the trial, five lambs from each group were randomly selected and euthanized via electrical stunning. Within 30 min postmortem, rumen fluid was collected and filtered through a four-layer cheesecloth, and the pH value was determined by using a portable pH meter immediately (PHS-3C, Shanghai, China). At the same time, 1 ml of ruminal fluid was preserved at −80° for DNA extraction; other samples were processed to analyze VFA, microbial protein (MCP), and ammonia–N (NH3-N). The concentrations of VFA were determined by a gas chromatograph (Agilent Technologies 7820A, USA) based on the method reported previously (14); the ruminal MCP concentration was detected using a spectrophotometric method, and the concentration of NH3-N was detected using a uric acid assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
DNA Extraction, PCR Amplification of 16S rRNA, and Sequencing
DNA extraction, genomic library construction, and sequencing of rumen microorganisms were carried out by Shanghai Meiji Biomedical Technology Co., Ltd. Briefly, total DNA was extracted from rumen samples, and then, the universal primers of bacteria (338F: 5′-ACTCCTRCGGGAGGCAGCAG-3′ and 806R: 5′-GGACTACHVGGGTWTCTAAT-3′) were used to amplify the V3–V4 regions of 16S ribosomal RNA (rRNA). The amplification was initiated with denaturation at 94°C for 3 min, 30 cycles of denaturation at 94°C for 30 s, 58°C for 30 s, 72°C for 90 s, and a final extension at 72°C for 5 min. Finally, The amplified products were electrophoresed on a 2% (w/v) agarose gel and recovered using an AxyPrep DNA Gel Extraction Kit (Axygen, Shanghai, China). The purified effects were quantified by QuantiFlour TM-ST fluorimeter (Promega, Beijing, China). A composite sequencing library was generated by pooling in equimolar ratios of amplicons and sent for paired-end sequencing (2 × 300 bp) on an Illumina Miseq platform at Major Bio-Pharm Technology Co., Ltd. (Shanghai, China).
Bioinformatics Analysis
Operational taxonomic units (OTUs) were clustered with a 97% similarity cutoff from the clean Fastq data, and chimeric sequences were identified and removed using Usearch 7.0 (http://drive5.com/usearch/). These OTUs were used for diversity (Shannon and Simpson), richness (Ace and Chao), and rarefaction curve analysis using Mothur 1.30.2 (https://www.mothur.org/wiki/Download_mothur). Representative sequences of OTUs were aligned to the SILVA database (https://www.arb-silva.de) for bacteria taxonomic assignments using Qiime (http://qiime.org/install/index.html).
Statistical Analysis
Basic record statistics were performed in Excel, and all data were statistically analyzed by one-way ANOVA with SPSS 23.0 software. Duncan's test was used to compare differences among the treatment groups. The level of statistical significance was present at P < 0.05 and 0.05 ≤ P < 0.10 was considered as a tendency.
Result
Growth Performance
As shown in Table 2, there was no difference between the initial weight and final weight, but a trend of decline was found in the average daily feed intake (ADFI) of lambs with compound treatment compared with the control (0.05 ≤ P < 0.10). Supplementation with CMP significantly increased the average daily gain (ADG) and decreased the ratio of F:G compared with the control group (P < 0.05). In addition, the ADG of lambs in probiotics and compound groups was lower than that in the CMP group, while there was no difference from the control group.
Table 2.
Effects of probiotics and CMP on growth performance in lambs.
| Items | Control | Probiotics | CMP | Compound | SEM | P-value |
|---|---|---|---|---|---|---|
| Initial weight (kg) | 22.59 | 21.49 | 21.12 | 21.59 | 0.46 | 0.736 |
| Final weight (kg) | 26.34 | 25.01 | 26.68 | 25.74 | 0.51 | 0.685 |
| ADFI (g/day) | 775.07 | 734.82 | 755.24 | 711.89 | 8.59 | 0.057 |
| ADG (g/day) | 62.59b | 58.59b | 92.68a | 69.17b | 4.06 | 0.011 |
| F/G | 14.13a | 11.67ab | 8.85b | 11.30ab | 0.66 | 0.044 |
a, bMeans within a row with no common superscript differ significantly (P < 0.05).
Control: the basal diet (n = 9); probiotics: supplemented with 0.1% probiotics (consists of Bacillus licheniformis, Bacillus subtilis, and Lactobacillus plantarum at a ratio of 1:1:0.5) in the basal diet (n = 10); CMP: supplemented with 0.1% Chinese medicine polysaccharides in the basal diet, which come from the mixture of Lycium barbarum and Astragalus membranaceus in the ratio of 2:1, and in which the content of polysaccharides was 114.7 mg/g (n = 9); compound: supplemented with 0.1% probiotics and 0.1% Chinese medicine polysaccharides in the basal diet (n = 10).
Initial weight and final weight were analyzed with lambs as the experimental unit. ADG, ADMI, and F/G were analyzed with pen as the experimental unit.
ADG, average daily gain; ADFI, average dry feed intake; F/G, feed to gain ratio.
Serum Biochemical Indices
As shown in Table 3, CMP treatment significantly increased the contents of IgA, IgG, and IgM in serum, but probiotics treatment only increased the concentrations of IgG compared with the control (P < 0.05). Moreover, both probiotics and compound treatments significantly increased the concentrations of GLU, while there was no difference in the contents of TP, ALB, GLB, BUN, TG, and TC among all groups.
Table 3.
Effects of probiotics and CMP on serum biochemical indices in lambs.
| Items | Control | Probiotics | CMP | Compound | SEM | P-value |
|---|---|---|---|---|---|---|
| TP (g/L) | 72.69 | 72.98 | 74.51 | 71.54 | 0.83 | 0.691 |
| ALB (g/L) | 27.70 | 27.42 | 26.68 | 28.08 | 0.54 | 0.844 |
| GLB (g/L) | 44.99 | 45.56 | 47.83 | 43.46 | 0.61 | 0.070 |
| BUN (mmol/L) | 6.03 | 6.75 | 7.68 | 7.01 | 0.31 | 0.314 |
| GLU (mmol/L) | 3.86b | 4.41a | 3.65b | 4.33a | 0.09 | 0.001 |
| TG (mmol/L) | 0.32 | 0.27 | 0.30 | 0.30 | 0.01 | 0.463 |
| TC (mmol/L) | 2.49 | 2.32 | 2.38 | 2.32 | 0.09 | 0.907 |
| IgA/(g/L) | 0.48b | 0.48b | 0.54a | 0.45b | 0.01 | 0.025 |
| IgG/(g/L) | 20.91b | 24.17a | 23.22a | 19.18b | 0.57 | 0.001 |
| IgM/(g/L) | 1.25b | 1.25ab | 1.28a | 1.24b | 0.01 | 0.035 |
a, bMeans within a row with no common superscript differ significantly (P < 0.05).
Control: the basal diet (n = 5); probiotics: supplemented with 0.1% probiotics (consists of Bacillus licheniformis, Bacillus subtilis, and Lactobacillus plantarum at a ratio of 1:1:0.5) in the basal diet; CMP: supplemented with 0.1% Chinese medicine polysaccharides in the basal diet, which come from the mixture of Lycium barbarum and Astragalus membranaceus in the ratio of 2:1, and in which the content of polysaccharides was 114.7 mg/g; compound: supplemented with 0.1% probiotics and 0.1% Chinese medicine polysaccharides in the basal diet.
All traits in this table were analyzed with cattle as the experimental unit.
TP, total protein; ALB, albumin; GLB, globulin; GLB, TP-ALB; BUN, blood urea nitrogen.
GLU, blood glucose; TC, total cholesterol; TG, triglyceride.
IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M.
Rumen Fermentation Parameters
As shown in Table 4, probiotics treatment reduced the pH value in the ruminal fluid of lambs but increased the concentration of MCP compared with the control (P < 0.05). The ratio of acetate acid and propionic acid in the CMP group was declined compared with the control (P < 0.05). Moreover, the compound (probiotics plus CMP) treatment decreased the value of pH and the ratio of acetate and propionic acid in ruminal fluid compared with the control (P < 0.05).
Table 4.
Effects of probiotics and CMP on rumen fermentation in lambs.
| Items | Control | Probiotics | CMP | Compound | SEM | P-value |
|---|---|---|---|---|---|---|
| pH value | 6.85a | 6.63b | 6.87a | 6.66b | 0.03 | 0.002 |
| NH3-N, mg/100 ml | 24.59 | 23.27 | 21.43 | 17.25 | 1.05 | 0.058 |
| MCP, mg/ml | 0.21b | 0.54a | 0.20b | 0.18b | 0.04 | <0.001 |
| acetic acid, mmol/L | 29.42 | 25.87 | 24.63 | 31.04 | 1.55 | 0.442 |
| propionic acid, mmol/L | 8.16 | 7.61 | 9.03 | 9.46 | 0.41 | 0.383 |
| butyric acid, mmol/L | 6.24 | 6.90 | 6.23 | 5.66 | 0.34 | 0.657 |
| Acetic acid/propionic acid | 3.63a | 3.38ab | 2.74c | 3.23b | 0.10 | 0.001 |
| T-VFA, mmol/L | 43.82 | 40.37 | 39.90 | 46.16 | 2.11 | 0.710 |
a, bMeans within a row with no common superscript differ significantly (P < 0.05).
Control: the basal diet (n = 5); probiotics: supplemented with 0.1% probiotics (consists of Bacillus licheniformis, Bacillus subtilis, and Lactobacillus plantarum at a ratio of 1:1:0.5) in the basal diet; CMP: supplemented with 0.1% Chinese medicine polysaccharides in the basal diet, which come from the mixture of Lycium barbarum and Astragalus membranaceus in the ratio of 2:1, and in which the content of polysaccharides was 114.7 mg/g; compound: supplemented with 0.1% probiotics and 0.1% Chinese medicine polysaccharides in the basal diet.
All traits in this table were analyzed with cattle as the experimental unit (n = 5).
NH3–N, ammonia–N; MCP, microbial protein; T-VFA, total volatile fatty acid.
Diversity of Ruminal Bacteria
In this experiment, a total of 931,700 valid sequences were generated from 18 samples, and the richness and alpha diversity of the community were analyzed by Ace, Chao, Shannon, and Simpson indices in Table 5. However, there were no differences in these indicators (P > 0.05). As shown in Figure 1, Principal coordinates analysis (PCoA) axes 1 and 2 accounted for 17.77% and 13.92% of the total variation, respectively. However, there were no significant differences in the ruminal bacterial community structure between the treatments and control group.
Table 5.
Effects of probiotics and CMP on bacterial richness and diversity indices in the rumen of captive lambs.
| Items | Control | Probiotics | CMP | Compound | SEM | P-value |
|---|---|---|---|---|---|---|
| OTU | 720.00 | 590.25 | 720.20 | 676.80 | 24.96 | 0.254 |
| Shannon index | 4.38 | 4.32 | 4.76 | 4.41 | 0.07 | 0.132 |
| Simpson index | 0.05 | 0.05 | 0.02 | 0.05 | 0.01 | 0.332 |
| Ace index | 889.25 | 734.84 | 876.34 | 832.17 | 28.11 | 0.244 |
| Chao index | 888.21 | 732.47 | 880.92 | 833.63 | 28.70 | 0.237 |
| coverage | 0.9924 | 0.9938 | 0.9928 | 0.9931 | 0.00 | 0.278 |
Control: the basal diet (n = 4); probiotics: supplemented with 0.1% probiotics (consists of Bacillus licheniformis, Bacillus subtilis, and Lactobacillus plantarum at a ratio of 1:1:0.5) in the basal diet (n = 4); CMP: supplemented with 0.1% Chinese medicine polysaccharide in the basal diet, which come from the mixture of Lycium barbarum and Astragalus membranaceus in the ratio of 2:1, and in which the content of polysaccharides was 114.7 mg/g (n = 5); compound: supplemented with 0.1% probiotics and 0.1% Chinese medicine polysaccharides in the basal diet (n = 5).
OTU, operational taxonomic units, which were screened for further annotation, and sequences with > 97% similarity were assigned to the same OTUs.
Figure 1.
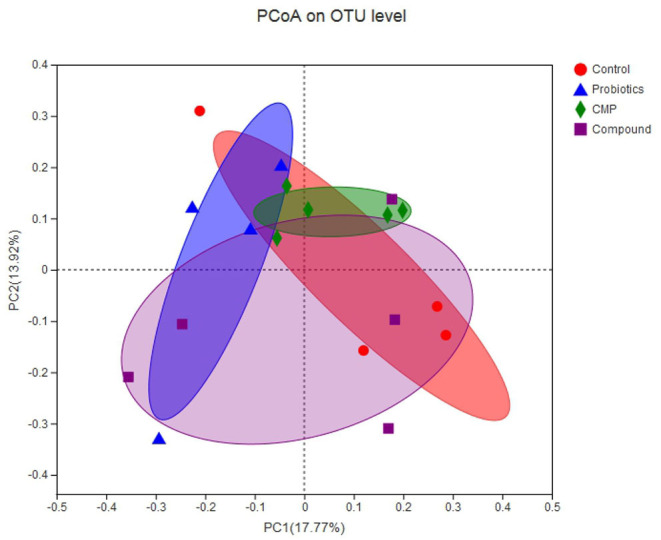
Principal coordinates analysis (PCoA) diagram of rumen microbial community structure across groups. Control: the basal diet (n = 4); probiotics: supplemented with 0.1% probiotics (consists of Bacillus licheniformis, Bacillus subtilis, and Lactobacillus plantarum at a ratio of 1:1:0.5) in the basal diet (n = 4); CMP: supplemented with 0.1% Chinese medicine polysaccharides in the basal diet, which come from the mixture of Lycium barbarum and Astragalus membranaceus in the ratio of 2:1, and in which the content of polysaccharides was 114.7 mg/g (n = 5); compound: supplemented with 0.1% probiotics and 0.1% Chinese medicine polysaccharides in the basal diet (n = 5).
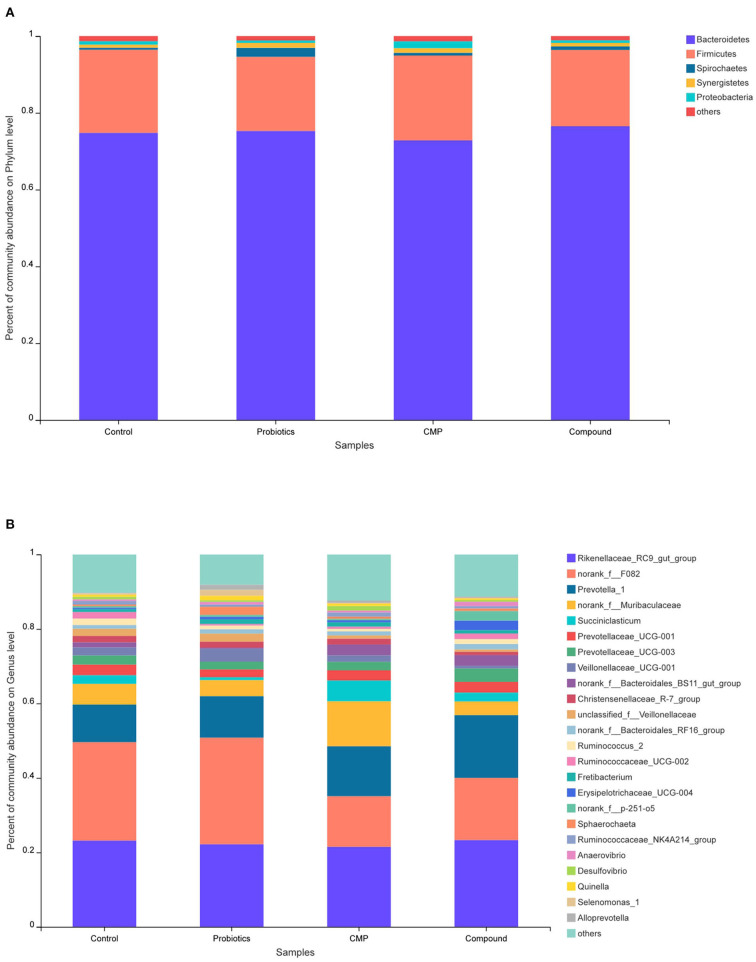
The relative abundance of rumen microbiota is displayed at the phylum level (Figure 2A and Table 6) and the genus level (Figure 2B and Table 7). Results in Table 6 show that supplementation with probiotics tended to reduce the relative abundance of bacteria belonging to phylum Kiritimatiellaeota (0.05 ≤ P < 0.10) compared with the control, and reduced the relative abundance of phylum Fibrobacteres compared with the compound group but increased the relative abundance of phylum Bacteria_NA compared with CMP and compound groups (P < 0.05). There was no difference found on other phyla among groups. At the genus level in Table 7, the relative abundance of unclassified_f__Veillonellaceae with probiotics treatment was increased, the relative abundance of norank_f__Muribaculaceae and Succiniclasticum in the CMP group were enhanced, and the relative abundance of Ruminococcaceae_UCG-002 with compound treatment was increased compared with the control group (P < 0.05). In addition, the relative abundance of Veillonellaceae_UCG-001 in the probiotics group was raised compared with the CMP and compound groups (P < 0.05).
Figure 2.
Relative abundance distribution of rumen flora at (A) phylum and (B) genus levels. Control: the basal diet (n = 4); probiotics: supplemented with 0.1% probiotics (consists of Bacillus licheniformis, Bacillus subtilis, and Lactobacillus plantarum at a ratio of 1:1:0.5) in the basal diet (n = 4); CMP: supplemented with 0.1% Chinese medicine polysaccharides in the basal diet, which come from the mixture of Lycium barbarum and Astragalus membranaceus in the ratio of 2:1, and in which the content of polysaccharides was 114.7 mg/g (n = 5); compound: supplemented with 0.1% probiotics and 0.1% Chinese medicine polysaccharides in the basal diet (n = 5).
Table 6.
Effects of probiotics and CMP on rumen bacterial flora structure (phylum level) %.
| Items | Control | Probiotics | CMP | Compound | SEM | P-value |
|---|---|---|---|---|---|---|
| Bacteroidetes | 74.78 | 75.28 | 72.86 | 76.52 | 1.13 | 0.720 |
| Firmicutes | 21.66 | 19.30 | 22.06 | 19.87 | 1.11 | 0.812 |
| Proteobacteria | 0.90 | 1.02 | 1.61 | 0.69 | 0.17 | 0.286 |
| Synergistetes | 0.79 | 1.27 | 1.20 | 0.92 | 0.17 | 0.765 |
| Spirochaetes | 0.54 | 0.78 | 0.72 | 0.90 | 0.12 | 0.810 |
| Patescibacteria | 0.30 | 0.23 | 0.26 | 0.25 | 0.03 | 0.931 |
| Tenericutes | 0.22 | 0.13 | 0.20 | 0.32 | 0.04 | 0.353 |
| Actinobacteria | 0.17 | 0.32 | 0.25 | 0.12 | 0.06 | 0.652 |
| Kiritimatiellaeota | 0.29 | 0.13 | 0.18 | 0.18 | 0.02 | 0.058 |
| Bacteria_NA | 0.16ab | 0.24a | 0.09b | 0.04b | 0.03 | 0.034 |
| Chloroflexi | 0.09 | 0.04 | 0.04 | 0.05 | 0.01 | 0.122 |
| Fibrobacteres | 0.06ab | 0.03b | 0.03b | 0.09a | 0.01 | 0.038 |
| Cyanobacteria | 0.02 | 0.02 | 0.02 | 0.03 | 0.00 | 0.976 |
| Epsilonbacteraeota | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.647 |
| Lentisphaerae | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.740 |
| Elusimicrobia | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.870 |
a, bMeans within a row with no common superscript differ significantly (P < 0.05).
Control: the basal diet (n = 4); probiotics: supplemented with 0.1% probiotics (consists of Bacillus licheniformis, Bacillus subtilis, and Lactobacillus plantarum at a ratio of 1:1:0.5) in the basal diet (n = 4); CMP: supplemented with 0.1% Chinese medicine polysaccharide in the basal diet, which come from the mixture of Lycium barbarum and Astragalus membranaceus in the ratio of 2:1, and in which the content of polysaccharides was 114.7 mg/g (n = 5); compound: supplemented with 0.1% probiotics and 0.1% Chinese medicine polysaccharides in the basal diet (n = 5).
Table 7.
Effects of probiotics and CMP on rumen bacterial flora structure (genus level) %.
| Items | Control | Probiotics | CMP | Compound | SEM | P-value |
|---|---|---|---|---|---|---|
| Rikenellaceae_RC9_gut_group | 23.19 | 22.21 | 21.50 | 23.33 | 1.91 | 0.987 |
| norank_f__F082 | 26.43 | 28.61 | 13.59 | 16.69 | 2.46 | 0.071 |
| Prevotella_1 | 10.06 | 11.14 | 13.44 | 16.85 | 1.73 | 0.552 |
| norank_f__Muribaculaceae | 5.59b | 4.31b | 10.76a | 3.67b | 0.95 | 0.009 |
| Succiniclasticum | 2.32b | 0.78b | 5.58a | 2.39b | 0.54 | 0.003 |
| Prevotellaceae_UCG-001 | 2.85 | 2.10 | 2.74 | 2.87 | 0.33 | 0.862 |
| Prevotellaceae_UCG-003 | 2.46 | 2.06 | 2.27 | 3.60 | 0.35 | 0.394 |
| Veillonellaceae_UCG-001 | 2.18ab | 3.49a | 1.75b | 0.70b | 0.33 | 0.011 |
| norank_f__Bacteroidales_BS11_gut_group | 0.43 | 0.30 | 0.67 | 0.74 | 0.14 | 0.688 |
| Christensenellaceae_R-7_group | 1.68 | 1.60 | 1.57 | 1.05 | 0.15 | 0.417 |
| unclassified_f__Veillonellaceae | 0.73b | 2.17a | 0.88b | 0.63b | 0.23 | 0.043 |
| norank_f__Bacteroidales_RF16_group | 1.01 | 1.15 | 1.08 | 1.47 | 0.21 | 0.886 |
| Ruminococcaceae_UCG-002 | 0.60b | 0.43b | 0.59b | 1.75a | 0.18 | 0.013 |
| Fretibacterium | 0.77 | 1.27 | 1.19 | 0.91 | 0.17 | 0.755 |
| Erysipelotrichaceae_UCG-004 | 0.35 | 0.64 | 0.52 | 0.69 | 0.10 | 0.710 |
| Sphaerochaeta | 0.39 | 0.63 | 0.59 | 0.68 | 0.12 | 0.879 |
| Ruminococcaceae_NK4A214_group | 0.97 | 0.74 | 1.13 | 0.74 | 0.08 | 0.268 |
| Ruminococcaceae_UCG-010 | 0.83 | 0.87 | 0.66 | 0.60 | 0.10 | 0.799 |
| Ruminococcus_2 | 0.44 | 0.61 | 0.67 | 0.48 | 0.07 | 0.693 |
| norank_f__p-251-o5 | 0.44 | 0.53 | 0.38 | 0.62 | 0.08 | 0.786 |
a, bMeans within a row with no common superscript differ significantly (P < 0.05).
Control: the basal diet (n = 4); probiotics: supplemented with 0.1% probiotics (consists of Bacillus licheniformis, Bacillus subtilis, and Lactobacillus plantarum at a ratio of 1:1:0.5) in the basal diet (n = 4); CMP: supplemented with 0.1% Chinese medicine polysaccharide in the basal diet, which come from the mixture of Lycium barbarum and Astragalus membranaceus in the ratio of 2:1, and in which the content of polysaccharides was 114.7 mg/g (n = 5); compound: supplemented with 0.1% probiotics and 0.1% Chinese medicine polysaccharides in the basal diet (n = 5).
Discussion
In this study, dietary supplementation of CMP increased the average daily gain of lambs but decreased the ratio of feed to gain, which indicated that CMP is composed of Astragalus polysaccharides (APS) and L. barbarum polysaccharides (LBPs) that could improve the growth performance of lambs. Dietary supplementation with Chinese herbal polysaccharides, such as A. membranaceus polysaccharide (AMP), Ginseng polysaccharide, and L. barbarum polysaccharide, has been used extensively to improve growth performance in broiler chickens and piglets (15, 16). There are few reports noticed about the effect of Chinese medicine polysaccharides on performance in ruminant. Nevertheless, a previous study showed that a diet with Astragalus polysaccharides and A. membranaceus root could improve antioxidant capacity and affect rumen fermentation patterns of lambs (17). A similar result indicated that APS increased the total VFA production of lambs (18). Another recent report demonstrated that a diet with bee pollen polysaccharide improved nutrient digestibility of calf (19). Hence, the enhancement of growth performance in this study may be owed to the improving nutrient utilization and antioxidant capacity of lambs fed diets supplemented with CMP.
Similarly, the addition of CMP significantly increased the contents of immunoglobulin in serum, which indicated that CMP treatment could improve the immune function of lambs. Immunoglobulin is a kind of non-specific immune molecule in animals. There are three main types of immunoglobulin in blood, including IgA, IgM, and IgG. The polysaccharides from natural plants can possess activity in promoting lymphocyte proliferation and improving the expression of cytokines to enhance immunity (20). A previous study revealed that administration of APS increased the level of IgG and IgM in serum (21). It has been reported that APS might induce the differentiation of splenic DCs with the enhancement of T lymphocyte immune function in vitro (22). Furthermore, in agreement with the current experiment, LBP supplementation in the broilers diet promoted humoral immune response leading to the increase in serum IgA and IgG concentrations (12), which may be due to LBP-activated macrophages to generate nitric oxide and promote cytokine secretion (23).
Although supplemental probiotics did not improve the average daily feed intake and gain of lambs, serum IgG and GLU concentrations were enhanced in the probiotics group. Similar to our results, Jia (24) also observed supplemental probiotics consisting of Bacillus lichens and Saccharomyces cerevisiae increased IgG content in lambs. The enhancement of serum GLU concentration could be due to the improvement of probiotics that produce nutrients and growth factors that are stimulatory to beneficial microorganisms of the gut microbiota. However, in contrast to our results, Ibrahim reported that GLU was not affected by probiotics supplementation (25). Another report by Ahmed pointed that GLU was significantly lowered on the 30th day in the probiotic (B. subtilis) supplementation group of growing Barki lambs (26). This may be due to differences in the activities of the probiotic strains and survivability throughout the gut, which appear to be of great importance for optimal efficacy (13).
Low ruminal pH can be caused by an accumulation of ruminal VFA (27). The present results showed that a lower ruminal pH was observed in the probiotics and compound groups, which was possibly due to the addition of L. plantarum to feed, promoting the ferment to produce more lactic acid. Consistent with our results, a previous study revealed that the pH value in the rumen was decreased after feeding Bacillus natto to the cows (28). Besides, supplemental probiotics increased significantly the content of MCP, which indicated that probiotics promoted rumen microorganisms to synthesize more MCP using ammonia nitrogen. Consistently, preweaning calves fed L. plantarum 299v exhibited an increase in MCP concentrations than those in the control group (29).
VFAs mainly include acetic acid, propionic acid, and butyric acid. The concentrations of VFA contribute to ruminants' health by sustaining the rumen ecosystem and acting as an energy source, and the ratio of acetic acid and propionic acid can reflect the rumen fermentation mode. The present result about reducing the ratio of acetate and propionic acid with CMP and compound treatment indicated that diet with Chinese medicine polysaccharide improved rumen fermentation mode. In line with the result, adding 15 g/kg Astragalus polysaccharides to the lamb diet increased the concentration of propionic acid and reduced the ratio of acetate and propionic acid in rumen fluid (11). Ammonia–nitrogen (NH3-N) in the rumen is the main nitrogen source for the synthesis of microbial protein, and its normal concentration range is 6.3–27.5 mg/dl (30). In this experiment, the concentrations of NH3-N in all groups were within the range, but the concentrations of rumen NH3-N had a declining trend in the compound group, which suggested that diet with the compound of probiotics and Chinese medicine polysaccharide might reduce the activity of rumen proteolytic enzyme. A similar report showed that probiotics supplementation decreased the concentration of NH3-N of growing lambs significantly (31). Another report by Wang indicated that a diet with traditional Chinese medicine compound 1 (TCMC 1) tends to decrease the concentration of NH3-N (32).
In this study, there were 931,700 valid sequences in the rumen of lambs, and the coverage rate was over 99%, which indicated that the sequencing results could basically reflect most species in the rumen bacterial flora of lambs. Based on the Silva taxonomic database and using the analysis program Qiime, 1,505 bacterial OTUs were classified and assigned to 16 phyla and 197 genera in the present study. The present results showed the microbial community of lambs was dominated by Bacteroidetes and Firmicutes on the phylum level regardless of group. The results are consistent with the study by (33, 34), in which Bacteroidetes, Firmicutes, and Proteobacteria were found predominantly in rumen fluid. However, the phylum compositions of Fibrobacteres in the compound group were higher than that in probiotics and CMP groups. Fibrobacteres played an essential role in fiber degradation and utilized cellulose to provide nutrients for ruminants, and the current results may be due to the interaction of probiotics and CMP. Previous reports suggested that Chinese herb extracts fermented with probiotic bacteria may increase the oxidative stability and antibacterial activity, which may be due to probiotics promoting the absorption of Chinese herbs in animals, and at the same time, Chinese herbs provided nutrients for probiotics and promoted their proliferation (35, 36).
At the genus level, Rikenellaceae_RC9_gut_group, norank_f__F082, and Prevotella_1 were the dominant bacteria in the four diet groups. No difference was observed in these bacteria, but CMP treatment increased the content of Succiniclasticum and norank_f__Muribaculaceae in rumen fluid. Succiniclasticum isolated by Van Gylswyk (37) from the bovine rumen, which belongs to Firmicutes, can produce succinic acid and convert it into propionic acid and further make glucose. The present results suggest that a diet with CMP might increase the degradation of fiber in the rumen and change the rumen fermentation mode, which was consistent with the results of the decrease in acetic acid/propionic acid and the increase in daily gain of lambs. Ruminococcaceae is one of the Firmicutes that can produce cellulase and hemicellulase to degrade plant fibers and is the main cellulolytic bacterium. This study showed that both probiotics and CMP treatments did not affect the relative abundance of Ruminococcaceae_UCG-002, but the diet with the compound of probiotics and CMP increased the relative abundance of the species, which suggested that there may be an interaction between polysaccharides and probiotics on promoting Ruminococcaceae_UCG-002 reproduction in the rumen, and increasing the degradation of cellulose in the rumen improve the utilization rate of feed. Although all treatments did not change the relative abundance of the Veillonellaceae significantly compared with the control, this abundance in the probiotics group was higher than those in the CMP and compound groups. Veillonellaceae belongs to Firmicutes, which can degrade and utilize cellulose. However, different from our experimental results, Chae reported that the relative abundance of Veillonellaceae was reduced after adding probiotic Enterococcus faecium NCIMB 11181 to the diet of the weaned pig (38). Schofield found no significant difference in the rumen community in sheep as a result of feeding the probiotic Bacillus amyloliquefaciens H57 (3). This difference may be due to the variety and dosage of probiotics. The rumen bacterial community is extremely complex, resulting from the interaction of external factors and animals themselves, and its influencing mechanism needs to be further studied.
Conclusion
Diet with probiotics can promote protein fermentation, but the diversity of bacteria had a decreasing trend. Supplementation with CMP increased the relative abundance of Fibrobacteria, changed the rumen fermentation mode, and improved the immune function and growth performance.
Data Availability Statement
The data presented in the study are deposited in the Sequence Read Archive (SRA) of National Center for Biotechnology Information (NCBI) repository, and accession number (SRA accession number) is PRJNA707607.
Ethics Statement
The animal study was reviewed and approved by Committee for the Care and Use of Experimental Animals at Jiangxi Agricultural University.
Author Contributions
XS, HC, and BG designed the overall study. BG, HC, MY, JL, YH, and MQ performed the animal feeding experiment and sample analysis. XS and HC wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors appreciated all the help from our colleagues and collaborators.
Footnotes
Funding. This work was supported by the National Natural Science Foundation of China (No. 32060768) and collaborative innovation project of modern agricultural scientific research in Jiangxi Province (JXXTCX201702) of China.
References
- 1.Olagaray KE, Bradford BJ. Plant flavonoids to improve productivity of ruminants—a review. Anim Feed Sci Technol. (2019) 251:21–36. 10.1016/j.anifeedsci.2019.02.004 [DOI] [Google Scholar]
- 2.Rochfort S, Parker AJ, Dunshea FR. Plant bioactives for ruminant health and productivity. Phytochemistry. (2008) 69:299–322. 10.1016/j.phytochem.2007.08.017 [DOI] [PubMed] [Google Scholar]
- 3.Schofield BJ, Lachner N, Le OT, McNeill DM, Dart P. Beneficial changes in rumen bacterial community profile in sheep and dairy calves as a result of feeding the probiotic bacillus amyloliquefaciensbacillus amyloliquefaciens h57. J Appl Microbiol. (2018) 124:855–66. 10.1111/jam.13688 [DOI] [PubMed] [Google Scholar]
- 4.Frizzo LS, Zbrun MV, Soto LP, Signorini ML. Effects of probiotics on growth performance in young calves: a meta-analysis of randomized controlled trials. Anim Feed Sci Technol. (2011) 169:147–56. 10.1016/j.anifeedsci.2011.06.00921620428 [DOI] [Google Scholar]
- 5.Azzaz HH, Aziz HA, Farahat SA, Murad HA. Impact of microbial feed supplements on the productive performance of lactating Nubian lambs. Glob Vet. (2015) 14:567–75. 10.3923/ajar.2016.1.14 [DOI] [Google Scholar]
- 6.Yu Y, Wu XQ, Pu JN, Luo P, Ma WK, Wang J, et al. Lycium barbarum polysaccharide protects against oxygen glucose deprivation/reoxygenation-induced apoptosis and autophagic cell death via the pi3k/akt/mtor signaling pathway in primary cultured hippocampal neurons. Biochem Biophys Res Commun. (2018) 495:1187–94. 10.1016/j.bbrc.2017.11.165 [DOI] [PubMed] [Google Scholar]
- 7.Shan CH, Guo JJ, Sun XS, Li N, Yang XY, Gao YH, et al. Effects of fermented chinese herbal medicines on milk performance and immune function in late-lactation cows under heat stress conditions. J Anim Sci. (2018) 96:4444–57. 10.1093/jas/sky270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abouzed TK, Sadek KM, Ayoub MM, Saleh EA, Nasr SM, El-Sayed YS, et al. Papaya extract upregulates the immune and antioxidants-related genes, and proteins expression in milk somatic cells of friesian dairy cows. J Anim Physiol Anim Nutr. (2018) 103:407–15. 10.1111/jpn.13032 [DOI] [PubMed] [Google Scholar]
- 9.Ahmed ST, Lee JW, Mun HS, Yang CJ. Effects of supplementation with green tea by-products on growth performance, meat quality, blood metabolites and immune cell proliferation in goats. J Anim Physiol Anim Nutr. (2015) 99:1127–37. 10.1111/jpn.12279 [DOI] [PubMed] [Google Scholar]
- 10.Li JF, Guo KJ, Liu FH, Zhang NW, Liu FQ, Xu JQ. Immuno-modulating effect of astragalus polysaccharides on the immune reactions of peripheral blood lymphocytes in pigs. Eur J Pharmacol. (2011) 668:e21. 10.1016/j.ejphar.2011.09.242 [DOI] [Google Scholar]
- 11.Luo YL, Su L, Su RN, Wang BH, Liu C, Wang ZG, et al. Effects of Astragalus Membranaceus supplementation on oxidative stability of Cashmere goat. J Food Sci Nutr. (2020) 8:5550–6. 10.1002/fsn3.1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long LN, Kang BJ, Jiang Q, Chen JS. Effects of dietary lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poul Sci. (2019) 99:744–51. 10.1016/j.psj.2019.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaucheyras-Durand F, Durand H. Probiotics in animal nutrition and health. Beneficial Microbes. (2010) 1:3–9. 10.3920/BM2008.1002 [DOI] [PubMed] [Google Scholar]
- 14.Zhao XH, Zhou S, Bao LB, Song XZ, Qu MR. Response of rumen bacterial diversity and fermentation parameters in beef cattle to diets containing supplemental daidzein. Ital J Anim Sci. (2017) 17:1–7. 10.1080/1828051X.2017.1404943 [DOI] [Google Scholar]
- 15.Wang KL, Zhang HR, Han QJ, Lan JH, Chen GY, Cao GT, et al. Effects of astragalus and ginseng polysaccharides on growth performance, immune function and intestinal barrier in weaned piglets challenged with lipopolysaccharide. J Anim Physiol Anim Nutr. (2020) 104:1096–105. 10.1111/jpn.13244 [DOI] [PubMed] [Google Scholar]
- 16.Liu YL, Yin RQ, Liang SS, Duan YL, Yang XJ. Effect of dietary lycium barbarum polysaccharide on growth performance and immune function of broilers. J Appl Poul Res. (2017) 26:pfw063. 10.3382/japr/pfw063 [DOI] [Google Scholar]
- 17.Zhong RZ, Yu M, Liu HW, Sun HX. Effects of dietary astragalus polysaccharide and astragalus membranaceus root supplementation on growth performance, rumen fermentation, immune responses, and antioxidant status of lambs. Anim Feed Sci Technol. (2012) 174:60–7. 10.1016/j.anifeedsci.2012.02.013 [DOI] [Google Scholar]
- 18.Li ZJ, Bai HX, Zheng LX, Jiang H, Cui HY, Cao YC, et al. Bioactive polysaccharides and oligosaccharides as possible feed additives to manipulate rumen fermentation in rusitec fermenters. Int J Biol Macromol Struct Funct Interact. (2018) 109:1088–94. 10.1016/j.ijbiomac.2017.11.098 [DOI] [PubMed] [Google Scholar]
- 19.Tu Y, Zhang GF, Deng KD, Zhang NF, Diao QY. Effects of supplementary bee pollen and its polysaccharides on nutrient digestibility and serum biochemical parameters in holstein calves. Anim Prod Sci. (2014) 55:1318–23. 10.1071/AN14684 [DOI] [Google Scholar]
- 20.Qiu HH, Cheng GL, Xu JQ, Zhang WN, Liu FH, Zhu XY, et al. Effects of astragalus polysaccharides on associated immune cells and cytokines in immunosuppressive dogs - sciencedirect. Procedia Vaccinol. (2010) 2:26–33. 10.1016/j.provac.2010.03.006 [DOI] [Google Scholar]
- 21.Li WG, Liu JG, Hu YL, Wang DY, Li ZZ, Zhang J, et al. Astragalus polysaccharide and sulfated epimedium polysaccharide synergistically resist the immunosuppression. Carbohydr Polym. (2012) 90:1055–60. 10.1016/j.carbpol.2012.06.042 [DOI] [PubMed] [Google Scholar]
- 22.Liu QY, Yao YM, Zhang SW, Sheng ZY. Astragalus polysaccharides regulate t cell-mediated immunity via cd11c(high)cd45rb(low) dcs in vitro. J Ethnopharmacol. (2011) 136:457–64. 10.1016/j.jep.2010.06.041 [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Li Y, Cheng J, Liu G, Qi C, Zhou W, et al. Immune activities comparison of polysaccharide and polysaccharide-protein complex from lycium barbarum l. Int J Biol Macromol. (2014) 65:441–5. 10.1016/j.ijbiomac.2014.01.020 [DOI] [PubMed] [Google Scholar]
- 24.Jia P, Cui K, Ma T, Wan F, Wang WY, Yang D, et al. Influence of dietary supplementation with bacillus licheniformis and saccharomyces cerevisiae as alternatives to monensin on growth performance, antioxidant, immunity, ruminal fermentation and microbial diversity of fattening lambs. Sci Rep. (2018) 8:132–43. 10.1038/s41598-018-35081-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khattab IM, Abdel-Wahed AM, Khattab AS, Anele UY, Zaher M. Effect of dietary probiotics supplementation on intake and production performance of ewes fed atriplex hay-based diet. Livestock Sci. (2020) 237:104065. 10.1016/j.livsci.2020.104065 [DOI] [Google Scholar]
- 26.Ahmed A, El-Sayed, Sabry A, Mousa. Effects of administration of probiotic on body growth and hematobiochemical profile in growing barki lambs. Comp Clin Pathol. (2020) 29:297–303. 10.1007/s00580-019-03057-z [DOI] [Google Scholar]
- 27.Lana, Rogerio P, Russell, James B, Van Amburgh, et al. The role of ph in regulating ruminal methane and ammonia production. J Anim Sci. (2011) 76:2190–6. 10.2527/1998.7682190x [DOI] [PubMed] [Google Scholar]
- 28.Sun P, Wang JQ, Zhang HT. Effects of supplementation of bacillus subtilis natto na and n1 strains on rumen development in dairy calves. Anim Feed Sci Technol. (2011) 164:154–60. 10.1016/j.anifeedsci.2011.01.003 [DOI] [Google Scholar]
- 29.Jiang X, Xu HJ, Cui ZQ, Zhang YG. Effects of supplementation with lactobacillus plantarum 299v on the performance, blood metabolites, rumen fermentation and bacterial communities of preweaning calves. Livestock Sci. (2020) 239:104120. 10.1016/j.livsci.2020.104120 [DOI] [Google Scholar]
- 30.Lv XK, Wang J, Wang SQ, Cui K, Diao QY, Zhang NF. Effects of dietary cassava residue supplementation on growth performance, serum indexes and rumen fermentation indexes of growing lambs. Chinese J Anim Nutr. (2017) 3666–75. 10.3969/j.issn.1006-267x.2017.10.029 [DOI] [Google Scholar]
- 31.Hassan A, Gado H, Anele UY, Berasain MA, Salem AZ. Influence of dietary probiotic inclusion on growth performance, nutrient utilization, ruminal fermentation activities and methane production in growing lambs. Anim Biotechnol. (2020) 31:365–72. 10.1080/10495398.2019.1604380 [DOI] [PubMed] [Google Scholar]
- 32.Shui P, Wen J, Zhi L, Guo W, Cheng F, Meng J. Effect of traditional chinese medicine compounds on rumen fermentation, methanogenesis and microbial flora invitro. Anim Nutr. (2019) 5:185–90. 10.1016/j.aninu.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang LZ, Xu Q, Kong FL, Yang YD, Wu D, Mishra S, et al. Exploring the goat rumen microbiome from seven days to two years. PLoS ONE. (2016) 11:e0154354. 10.1371/journal.pone.0154354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jami E, Israel A, Kotser A, Mizrahi I. Exploring the bovine rumen bacterial community from birth to adulthood. Isme J. (2013) 7:1069–79. 10.1038/ismej.2013.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang CJ, Lin CS, Lu CC, Martel J, Ko YF, Ojcius DM, et al. Corrigendum: ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. (2017) 8:16130. 10.1038/ncomms16130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung MA, Jang SE, Hong SW, Hana MJ, Kim DH. The role of intestinal microflora in anti-inflammatory effect of baicalin in mice. Biomol Ther. (2012) 20:36–42. 10.4062/biomolther.2012.20.1.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gylswyk NOV. Succiniclasticum ruminis gen. nov. sp. nov. a ruminal bacterium converting succinate to propionate as the sole energy-yielding mechanism. Int J Syst Bacteriol. (1995) 45:297–300. 10.1099/00207713-45-2-297 [DOI] [PubMed] [Google Scholar]
- 38.Chae JP, Pajarillo EAB, Oh JK, Kim H, Kang DK. Revealing the combined effects of lactulose and probiotic enterococci on the swine faecal microbiota using 454 pyrosequencing. Microbial Biotechnol. (2016) 9:486–95. 10.1111/1751-7915.12370 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in the study are deposited in the Sequence Read Archive (SRA) of National Center for Biotechnology Information (NCBI) repository, and accession number (SRA accession number) is PRJNA707607.