Abstract
Obstetric guidelines have rapidly evolved to incorporate new data and research on the novel coronavirus disease (COVID-19), with data on perinatal mental health building over the last year. Our aim in the present manuscript is to provide a systematic review of mental health outcomes in pregnant and postpartum women during the COVID-19 pandemic in the context of neonatal and obstetric guidelines addressing symptoms and complications of COVID-19 during pregnancy, mother-to-neonate transmission, Cesarean-section delivery, neonatal prematurity, maternal/neonate mortalities, maternal-neonatal separation, and breastfeeding. We summarize data from 81 mental health studies of pregnant and postpartum women and underscore protective and risk factors identified for perinatal mental health outcomes amidst the COVID-19 pandemic. Data reviewed here suggest increased psychological symptoms, especially depressive and anxiety symptoms, in pregnant and postpartum women during COVID-19. Our systematic review integrates the most current obstetric and neonate guidelines, along with perinatal mental health outcomes associated with COVID-19, highlighting the best available data for the care of women and their neonates amidst the current COVID-19 pandemic.
Keywords: pregnancy, postpartum, mental health, COVID-19, maternal, perinatal
Introduction
Since being declared a pandemic in March 2020 by the World Health Organization (WHO), the novel coronavirus disease (COVID-19) has rapidly spread across the globe (1). As of April 12th 2021, there have been nearly 139 million confirmed cases and nearly 3 million deaths worldwide (2, 3). These staggering figures have resulted in an array of public health, social, and economic issues impacting the daily life and mental health of the global population. Over the last year of the COVID-19 pandemic, there have been growing reports of the mental health impacts of COVID-19 on the general population, including increased depression, anxiety, and sleep disturbances in individuals with and without COVID-19 (4–7).
Even under normal circumstances, the perinatal period is already one of substantial biological, physiological, psychological, and social changes. For example, pregnant women may be more susceptible to and more significantly impacted by viral diseases, as seen in the H1N1 influenza pandemic [swine flu in 2009; (8, 9)], SARS-CoV [Severe Acute Respiratory Syndrome identified in 2003; (10)] and MERS-CoV [Middle East Respiratory Syndrome identified in 2012; (11)]. In addition, pregnant women tend to be more susceptible to severe symptoms if contracting a respiratory viral illness, increasing the risk of adverse obstetric outcomes such as preeclampsia (12), preterm delivery (13), and low birth weight birth (14). The backdrop of a global pandemic is likely to exacerbate any inherent risks of contracting a respiratory viral illness during the perinatal period (15–18). Therefore, pregnant women and their newborns have already been proposed as a special vulnerable group requiring our priority and attention amidst the current COVID-19 outbreak (15–20).
Mental Health During Pregnancy and Postpartum Period
Mental health disorders are not uncommon during pregnancy and the postpartum period, and studies estimate that the prevalence rate of perinatal mood and anxiety disorders range between 10 and 20% (21–24). In addition to universal challenges experienced during the global pandemic, pregnant women are likely to be affected by a unique set of additional challenges, such as limited access to perinatal services and in-person family support. In addition, constantly evolving understanding of the potential risks posed by the novel virus may contribute to a heightened sense of uncertainty about their own health and the health of their newborn, which may intensify the level of stress experienced during this critical time of transition. One year into the pandemic, a growing body of perinatal mental health studies have emerged, and with the onset of new virus variants as well as the development of vaccines, there is bound to be a greater influx of studies capturing the mental health experience of women during pregnancy and the postpartum period.
Summary of Obstetric and Neonatal Outcomes During COVID-19
Considering that growing and fluctuating concerns about adverse obstetric and neonatal outcomes may have been inextricably tied to increased perinatal mental health risk during COVID-19, we review perinatal mental health outcomes in the context of key obstetric and neonatal outcomes that have been documented to date. In this section, we draw from comprehensive published guidelines detailing obstetric and neonatal outcomes for practitioners and the general public from leading sources (25–29) and summarize data concerning symptoms and complications of maternal COVID-19 during pregnancy, mother-to-neonate transmission, Cesarean-section delivery, premature birth, maternal/neonatal mortalities, maternal-neonatal separation, and breastfeeding during COVID-19. Our summary of obstetric and neonatal data is not intended to be exhaustive but is rather aimed at providing the context in which to understand current perinatal mental health outcomes.
Symptoms and Complications of COVID-19 During Pregnancy
While most pregnant women infected with COVID-19 were asymptomatic (25), fever, mild cough, and dyspnea were the most reported symptoms of COVID-19 during pregnancy. Most frequent pregnancy complications included gestational diabetes, preeclampsia, and premature rupture of membranes. According to an interim report from the United Kingdom (UK) Obstetric Surveillance System (UKOSS) national cohort study comparing pregnant and non-pregnant women with COVID-19, pregnant women were more likely than their non-pregnant counterparts to be hospitalized, to be admitted more frequently to the intensive care unit (ICU) and to require greater use of mechanical ventilation (30). Maternal race and ethnicity have received attention in relation to risks and complications associated with COVID-19. In UK, women hospitalized with symptomatic COVID-19 infections were more likely to be Black, Asian, or another ethnic minority (25). Non-Hispanic Asians reported more frequent ICU admissions compared to all pregnant women. Cohort studies in the United States (US) also reported disproportionate risk based on race and ethnicity, with increased risk for ICU admission noted among non-Hispanic Asian pregnant women and non-Hispanic Native Hawaiian/Pacific Islander pregnant women (31). In addition, a significantly higher infection rate was observed among Hispanic pregnant women compared with non-Hispanic White pregnant women (18.1 vs. 9.4%) (32). Age was also a significant factor, with women aged 35–44 reporting more frequent hospitalization, ICU admission, mechanical ventilation, and death compared to women aged 15–24 (30).
Mode of Delivery
Data from the US Center for Disease Control and Prevention (CDC) suggested that of 11,764 deliveries that took place over a period from March 29th, 2020 to February 10th, 2021, 7,279 were vaginal while 3,492 were C-sections (30%) (33). The C-section rates during this period is roughly similar to averge rates (31%) reported in pre-pandemic years (34). It is advised that the mother with suspected or confirmed COVID-19 deliver in an obstetric unit and a discussion weighing the benefits and costs of elective C-section or induction of labor take place should a pregnant mother test positive for COVID-19 (25). This aligns with WHO's recommendation that C-sections only be done when medically justified (35). Elective C-sections of women with suspected or confirmed COVID-19 should remain the lowest priority on the operating list (25). Furthermore, if mother is COVID-19 positive and an emergency C-section is required, the mother and her family are advised to wear proper personal protection equipment (25) during delivery and while at the hospital.
Neonatal Prematurity
Guidelines have reported on the prevalence of premature birth during the COVID-19 pandemic and addressed the issue of neonatal intensive care unit (NICU) admission. Pregnant women testing positive for COVID-19 may be at an increased risk for preterm birth (29). The risk rises in those women who are symptomatic (25), and furthermore in those with severe, compared to mild, COVID-19 symptoms (36). UK surveillance data indicates that preterm births in women with symptomatic COVID-19 are to be two to three times higher (30) than the pre-pandemic preterm birth rate of 7% in the UK (37). However, recent surveillance data from the US reported 1,131 preterm births (compared to 8,331 term births) between March 29th, 2020 and February 10th, 2021 (33), consistent with the pre-pandemic global preterm prevalence rate of 10% (38). If mothers pose a risk of transmitting COVID-19 to their preterm infant, it is currently understood that it may be necessary to separate mother and her newborn (39).
Mother to Fetus/Neonate Transmission
A major concern since the start of the pandemic has centered around whether the virus can be transmitted from mothers with COVID-19 to the fetus/neonate during pregnancy or delivery. The current advice suggests that infants born to mothers with confirmed COVID-19 be treated as having been potentially infected (39), and that certain precautions be taken if mothers are currently positive for COVID-19 (e.g., temporary separation from infants and the use of a breast pump to avoid the spread of droplets while being in close contact with their infant). Regarding breastfeeding, long-term benefits are understood to outweigh the potential risk of transmission (25). To date, active virus has not been found in samples of breastmilk (35). Furthermore, surveillance data from the US found that only 60 of 1,506 infants of mothers with confirmed COVID-19 tested positive for COVID-19, while 1,436 infants tested negative (33), suggesting low risk of mother-to-neonate transmission. Two studies have further reported no evidence of vertical transmission (36, 40), while one study found placental abnormalities in mothers with COVID-19 (41). In sum, the currently available data suggests that the rate of infection of COVID-19 from mother to infant is low, and studies are currently unable to conclusively determine if the neonate's positive COVID-19 status has been due to vertical transmission, or if the virus was contracted post-delivery.
Maternal and Fetal/Neonatal Mortalities
Currently available data report a low rate of deaths among pregnant mothers with COVID-19; however, the risk of death increased if women had symptomatic COVID-19 requiring hospitalization (25). Two studies reported on causes of maternal death, including pre-existing conditions, obstetric complications, and respiratory complications due to COVID-19 (42, 43). The UKOSS study reported no difference between pregnant and non-pregnant women in the risk of death due to COVID-19 (30). In the US, Black and Hispanic pregnant women had disproportionate rates of death associated with COVID-19 infection compared to other racial and ethnic groups (39).
Mother-Neonate Separation and Breastfeeding
As a result of the rapid spread of COVID-19 and the limited understanding of the specific mechanism of transmission at the start of the pandemic, interim guidelines by the American Academy of Pediatrics (AAP) and CDC recommended temporary separation of mother and neonate following delivery, potentially disrupting skin-to-skin and breastfeeding practices (44, 45). Some sounded the alarm for an urgent need for perinatal mental health specialists in the NICU during and after the pandemic (46), due to the additional stress that the separation and NICU admission can place on mothers and infants (46–48). Updated guidelines proposed by WHO (28) and RCOG (25) now advise against routine separation and encourage mother-to-infant contact, though shared decision making between the mother and the medical team is crucial and should be reviewed on a case-by-case basis (25, 29).
Concerns about compromised breastfeeding practices during the pandemic have been raised (49–51). Recommendations early in the pandemic advised against mothers with suspected/confirmed COVID-19 to breastfeed, unless their breastmilk tested negative for COVID-19 (52). Recent guidelines support breastfeeding in mothers with and without COVID-19, suggesting that benefits of breastfeeding outweigh any potential risks of transmission through breastmilk. Guidelines provide recommendations on minimizing the risk of transmission during breastfeeding, such as washing hands thoroughly before feeding or expressing milk, wearing a face covering while feeding the infant, or having a healthy family member feed the infant (25, 29).
Current Study
Our aim in the present study is to provide a systematic review of mental health outcomes in pregnant and postpartum women during the COVID-19 pandemic in the context of key neonatal and obstetrics guidelines as summarized above. Much of the available data on perinatal mental health outcomes have focused on depression and anxiety symptoms and we review this data with a special attention to protective and risk factors.
Method
Identification and Selection of Studies
We selected published studies that had a primary focus on the impact of COVID-19 and measured a specific mental health outcome using validated measures. We included studies with a primary population of pregnant women (at any stage of pregnancy) and women up to 1.5 years postpartum. We excluded studies not written in English, case studies or case reports of < 10 participants, guidelines or proposals of patient care and management, or studies that did not measure a mental health outcome using a validated measure.
Literature Search Strategy
A literature search was conducted on January 31st, 2021. Selected search engines included Embase, Medline and PsycInfo. Search strategies included keywords pertaining to the COVID-19 pandemic (novel corona, SARS 2, SARS COV-2, corona, coronavirus). These terms were combined with terms relating to pregnancy (perinatal, neonatal, intrapartum, postpartum, postnatal, mother) and terms related to mental health (depression, anxiety, mental health, well-being). Complete search strategies for each database are provided in Appendix 1 of Supplementary Material.
Data Extraction
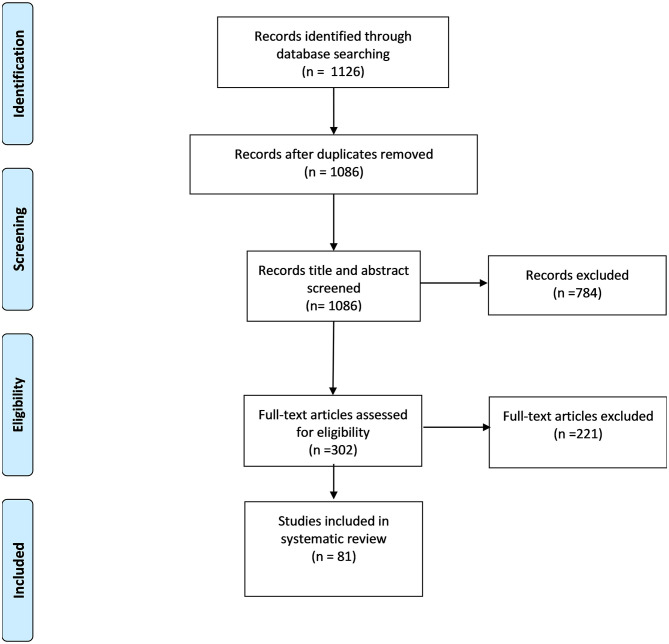
After duplicates were removed, articles underwent title and abstract screening (BJ, UI, HH). All selected full-text articles were then individually read and designated information was retrieved (BJ, UI, HH). Results from the screening process are presented in a PRISMA flow diagram (Figure 1) (53). A total of 1,126 articles were identified from the search engines. Following an initial title and abstract screening of 1,086 articles and excluding 784 records, 302 full-text articles were retrieved and assessed for eligibility, and 221 were excluded further, resulting in 81 articles reporting perinatal mental health outcomes during COVID-19.
Figure 1.
PRISMA flow diagram of the systematic search (53).
A coding protocol for all articles was used to extract and categorize the following information: (1) title, authors, and year of publication; (2) study characteristics (country of origin, study period, recruitment method, participant characteristics, sample size, study design); (3) comparison group (e.g., before vs. during pandemic, within-group comparisons); and (4) primary mental health outcomes and risk/protective factors.
Assessment of Risk of Bias
To evaluate risk of bias, we used a modified version of the Newcastle-Ottawa Scale [adapted for cross-sectional studies; (54)], which evaluates studies in terms of selection of groups, comparability of groups, and outcome assessment. Scores range from 0 (highest bias) to 9 points (lowest bias). Before performing quality assessment, the criteria for evaluation were thoroughly discussed between three raters (UI, BJ, HH) to develop a common understanding. Ratings for each study was determined by two raters (BJ and HH), and any uncertainties concerning study quality were resolved through discussions between all raters (BJ, HH, UI).
Results
Study Selection
A total of 81 studies met our criteria outlined above. Of the 81 studies (N = 132,917 women), 55 studies included pregnant women only (n = 95,353), 13 studies included both pregnant and postpartum (up to 1.5 years) women (n = 25,834), and 13 studies included postpartum women only (n = 11,730). Study characteristics and main findings of all studies are summarized in Tables 1–9.
Table 1.
Prevalence of mental health outcomes in pregnant or postpartum women during the COVID-19 pandemic.
| Study | Study Design | Country | Study Period | Recruitment Site/Method | Participant Characteristics | Main Findings | Risk of Biasa | |
|---|---|---|---|---|---|---|---|---|
| Pregnancy/ Postpartum Status |
Maternal Age | |||||||
| Ceulemans et al. (55) | Cross-sectional | Belgium | Lockdown period (during the pandemic) | Hospitals | Pregnant (n = 2,421) and postpartum (n = 3,445) women (N = 5,866) |
NR | 25.3% and 23.6% of participating pregnant and postpartum women reported depressive symptoms. 14% of all participating women met criteria for high anxiety. | 3 |
| Effati-Daryani et al. (56) | Cross-sectional | Iran | March–April 2020 (during the pandemic) | Health centers | Pregnant women (N = 205) • <14 weeks GA: n = 16 • 14–28 weeks GA: n = 85 • >28 weeks GA: n = 104 |
29.5 (M) ± 5.5 (SD) years | 32.7% of participating women experienced depressive symptoms and 43.9% experienced anxiety symptoms, comparable to pre-pandemic prevalence rates. Protective factors (for anxiety): marital life satisfaction, high level of spousal education, and high income | 7 |
| Farewell et al. (57) | Cross-sectional | United States of America | March–April 2020 (during the pandemic) | Social media | Pregnant (n = 14) and postpartum (<6 months; n = 13) women (N = 27) | (R) 24–34 years: n = 15 35–45 years: n = 12 |
12% of participating women reported high depressive symptoms and 60% reported moderate to severe anxiety. Risk factors: high uncertainty around prenatal care appointment, prenatal exposure risk, social isolation, stress about lack of daycare and caregiver support | 6 |
| Farrell et al. (58) | Cross-sectional | Qatar | June–July 2020 (during the pandemic) | Maternity hospital | Pregnant women (N = 288) • GA: 26.1 (M) ± 14.3 (SD) weeks |
30.5 (M) ± 5.3 (SD) years | 39.2% of participating women experienced depression and 34.4% experienced anxiety. | 6 |
| He et al. (59) | Cross-sectional | China | February 13–16, 2020 (during the pandemic) | Maternity school | Postpartum women (N = 1,908) |
NR | 58% of participating women screened positive for postpartum depression and 15% screened positive for PTSD. Risk factors: low levels of education, fear of infection | 5 |
| Hocaoglu et al. (60) | Cross-sectional | Turkey | May 11–28, 2020 (during the pandemic) | Prenatal checks | Pregnant women (N = 283) • GA: 23.82 (M) ± 11.05 (SD) weeks |
29.2 (M) ± 5.55 (SD) years | High rates of anxiety and PTSD were reported among participating women, with 46.6% reporting severe impact during the COVID-19 pandemic. Risk factors (for anxiety): pregnancy complications and husband's employment status; (for PTSD): presence of COVID-19-related symptoms and high education level | 8 |
| Kassaw et al. (61) | Cross-sectional | Ethiopia | April 6–May 6, 2020 (during the pandemic) | Hospitals | Pregnant women (N = 178) |
28 (M) ± 5.6 (SD) years | 1/3 of participating women had generalized anxiety disorder. Risk factors: rural area resident status, high level of education, poor social support, and primigravida. | 7 |
| Liang et al. (62) | Cross-sectional | China | March 30–April 13, 2020 (during the pandemic) | Hospitals | Postpartum (6–12 weeks) women (N = 864) |
(R) 25–29 years: n = 355 |
30% of participating women experienced depression. Risk factors: immigrant status, persistent fever, poor social support, concerns about COVID-19 infection | 6 |
| Liu et al. (17) | Cross-sectional | United States of America | May 21–August 17, 2020 (during the pandemic) | Social media | Pregnant and postpartum ( ≤ 6 months) women (N = 1,123) • 2nd trimester: n = 441 • 3rd trimester: n = 682 |
33.1 (M) ± 3.77 (SD) years | 36.4% of participating women reported depression, 22.7% reported generalized anxiety and 10.3% reported PTSD symptoms. Risk factors: previous psychiatric history, COVID-19 health worries and grief | 9 |
| Lubian-Lopez et al. (63) |
Cross-sectional | Spain | April 15–May 14, 2020 (during the pandemic) | Prenatal clinics | Pregnant women (N = 454) • GA: 26.10 (M) ± 8.7 (SD) weeks |
32.5 (M) ± 4.53 (SD) years | 35.9% of participating women showed depressive symptoms and 45.6% had anxiety symptoms. | 6 |
| Medina-Jiminez et al. (64) | Population-based | United States of America | May 5–June 12, 2020 (during the pandemic) | Hospitals | Pregnant women (N = 478) • GA: 27.9 (M) ± 10.3 (SD) weeks |
28.1 (M) ± 6.25 (SD) years | 33.2% of participating women reported high stress and 17.5% reported high levels of depression. Risk factors: later gestational age. | 7 |
| Molgora & Accordini (65) | Cross-sectional | Italy | March 1–May 3, 2020 (during the pandemic) | Social media | Pregnant (n = 389) and postpartum women (<6 months; n = 186) (N = 575) |
Pregnant: 32.9 (M) ± 4.3 (SD) years Postpartum: 33.01 (M) ± 4.19 (SD) years |
60% of pregnant and 57.7% of postpartum women reported clinically significant state anxiety. 34.2% of pregnant and 26.3% of postpartum women reported clinically significant depression. 16.7% of postpartum women reported PTSD. Risk factors: lack of presence and social support from partner during delivery and early postpartum | 7 |
| Ng et al. (66) | Cross-sectional | Singapore | March 31–April 25, 2020 (during the pandemic) | Prenatal clinics and hospitals | Pregnant women (N = 324) • GA: 23.4 (M) ± 10 (SD) weeks |
31.8 (M) ± 4.2 (SD) years | 35.8% of participating women screened positive for anxiety and 18.2% for depression. Risk factor (for anxiety): cognitive association of COVID-19 with fetal anomalies/death | 7 |
| Ostacoli et al. (67) | Cross-sectional | Italy | March 8–June 15, 2020 (during the pandemic) | Hospitals | Postpartum women (N = 163) | 34.77 (M) ± 5.01 (SD) years | 44.2% of participating women reported depressive symptoms and 42.9% reported PTSD symptoms. Risk factors: dismissive and fearful avoidant attachment, and perceived pain during birth. Protective factors: perceived support by healthcare staff and quietness due to lack of visitors | 7 |
| Pries et al. (68) | Cross-sectional | United States of America | April 2020 (during the pandemic) | Social media | Pregnant women (N = 788) • GA: 25.3 (M) ± 9.1 (SD) weeks |
29.2 (M) ± 5.3 (SD) years | 21.1% of participating women reported no to minimal anxiety, 35.6% reported mild anxiety, 21.6% reported moderate anxiety and 21.7% reported severe anxiety. Risk factors: previous abuse history, high-risk pregnancy, and perinatal infection stress. Protective factors: older maternal age and better prenatal health | 6 |
| Ravaldi and Vannacci, (69) | Cross-sectional | Italy | March–May 2020 (during the lockdown phase of the pandemic) | Social media | Pregnant women (n = 1,307) and postpartum (n = 1,141) (N = 2,448) |
(R) Pregnant women: 18–25 years: n = 18 25–30 years: n = 187 30–35 years: n = 497 >35 years: n = 605 Postpartum women: 18–25 years: n = 21 25–30 years: n = 131 30–35 years: n = 465 >35 years: n = 524 |
45.7% of pregnant and postpartum women had personal experience of psychopathology, and 46.9% had family history of psychopathology | 5 |
| Yang et al. (70) | Cross-sectional | China | February 25–March 10, 2020 (during the pandemic) | Hospitals | Pregnant women (N = 19,515) • 1–10 weeks GA: n = 1,523 • 11–20 weeks GA: n = 4,986 • 21–30 weeks GA: n = 5,858 • >30 weeks GA: n = 6,518 |
(R) <26–35 years: n = 3,781 26–30 years: n = 8,202 31–35 years: n = 5,683 >35 years: n = 1,849 |
44.6% of participating women reported depressive symptoms, 29.2% reported anxiety symptoms and 7.4% had suicidal ideations. Risk factor: perceived low social support | |
| Yue et al. (71) | Cross-sectional | China | February 2020 (during the pandemic) | Online | Pregnant women (N = 308) • GA: 31.63 (M) ± 2.22 (SD) weeks |
31.02 (M) ± 3.91 (SD) years | Anxiety in pregnant women during the pandemic was higher than that of the general population prior to the COVID-19 pandemic, including the pregnant and non-pregnant population. | 6 |
GA, gestational age; M, mean; SD, standard deviation; Med, median; R, range; PTSD, post-traumatic stress disorder; NR, not reported.
Assessed using a modified version of the Newcastle-Ottawa Scale (54). See section Assessment of Risk of Bias for details. Scores range from 0 (highest bias) to 10 points (lowest bias).
Table 9.
Modeling or intervention studies of perinatal mental health outcomes during the COVID-19 pandemic.
| Study | Study Design | Country | Study Period | Recruitment Sites/Methods from | Participant Characteristics | Main Findings | Risk of Biasa | ||
|---|---|---|---|---|---|---|---|---|---|
| Pregnancy/ Postpartum Status |
Key Variables Examined or Subgroups | Maternal Age | |||||||
| Aksoy Derya et al. (126) | Quasi-experimental | Turkey | April 22–May 13, 2020 (during the pandemic) | Prenatal classes | Pregnant women (N = 96) • GA: [intervention] 31.47 (M) ± 3.92 (SD) weeks; [control] 31.12 (M) ± 4.16 (SD) weeks |
Tele-education intervention (for pregnancy and birth planning; n = 48) vs. No intervention (n = 48) | [intervention]: 28.70 (M) ± 4.73 (SD) years [control]: 28.06 (M) ± 4.12 (SD) years | Pregnant women who received the tele-education intervention reported less pregnancy-related anxiety than pregnant women who received no intervention. | 6 |
| Gamache et al. (127) | Single time point correlational | Canada | April 2–13, 2020 (during the pandemic) | Social media | Pregnant women (N = 1,207) |
Personality pathology, affective/ behavioral/ thought problems, mentalization of trauma | 29.6 (M) ± 4.0 (SD) years | Level of personality functioning had both direct and indirect (via mentalization of trauma) effects with affective/behavioral/thought problems in pregnant women during the pandemic. | 6 |
| Guo et al. (128) | Cross-validation | The Netherlands, China, Italy | [Netherlands] April 17–May 10, 2020; [Italy] April 21–June 13, 2020; [China] April 21–28, 2020 (during lockdown) | Social media, school and day care centers, research panels | Pregnant women from the Netherlands: n = 900; Italy: n = 641; China: n = 922 | [Shared factors across cultures] Pandemic-related stress, resilience [Unique across cultures] grandparental support, father involvement, family structure characteristics |
36.74 (M) ± 5.58 (SD) years | COVID-19-related stress and family conflict were risk factors and resilience was a protective factor for pregnant women's mental health in all three countries. Risk factors unique to each country: (Netherlands): high maternal education and unemployment; (Italy): maternal age and poor physical health; (China): poor physical health, high socio-economic status, and low social support | 6 |
| Matsushima & Horiguchi (129) | Cross-sectional | Japan | May 31–June 6, 2020 (during the pandemic) | Companies providing services to pregnant & postpartum women | Pregnant women (N = 1,777) • 1st trimester: n = 235 • 2nd trimester: n = 741 • 3rd trimester: n = 801 |
Depression, anxiety, anhedonia, socio-demographic factors | <25 years: 5.35% 25–29 years: 29.21% 30–34 years: 37.20% 35+ years: 28.25% | 17% of participating women screened positive for depressive symptoms. Risk for psychological symptoms increased with cancellation of planned informal support, increase in perceived risk for COVID-19 infection, household financial strain, and lack of social support. Risk factors: low maternal age, low wealth, unemployment, and no partner status. | 7 |
| Salehi et al. (130) | Cross-sectional | Iran | March–April 2020 (during the pandemic) | Hospital | Pregnant women (N = 222) |
Fear of COVID-19, anxiety of COVID-19, depression, pregnancy-related concerns, pregnancy-related happiness | 29.1 (M) ± 5.6 (SD) years | Pregnant women's mental health disorders during the pandemic were positively associated with anxiety of COVID-19 and negatively associated with happiness experienced during pregnancy. | 7 |
| Zheng et al. (131) | Cross-sectional | China | February 2020 (during the pandemic) | Hospital | Pregnant women (N = 331) • GA: R = 37–42 weeks |
Psychological response to the pandemic, security sense, pregnancy stress | 30.37 (M) ± 4.22 (SD) years | Fear and depressive symptoms were the most reported symptoms. Psychological response to the pandemic was positively associated with pregnancy stress, partially mediated by decreased security sense. | 6 |
GA, gestational age; M, mean; SD, standard deviation; Med, median; R, range.
Assessed using a modified version of the Newcastle-Ottawa Scale (54). See section Assessment of Risk of Bias for details. Scores range from 0 (highest bias) to 10 points (lowest bias).
Perinatal Mental Health Outcomes
We divide our results into studies reporting the following: (1) prevalence of mental health outcomes in pregnant or postpartum women during the COVID-19 pandemic (Table 1), (2) mental health outcomes in pregnant or postpartum women before vs. during the COVID-19 pandemic (Table 2), (3) mental health outcomes in pregnant or postpartum women with vs. without COVID-19 (Table 3), (4) mental health outcomes in pregnant vs. non-pregnant women during the COVID-19 pandemic (Table 4), (5) perinatal mental health outcomes during COVID-19 as a function of pregnancy-related factors (e.g., stages of pregnancy/postpartum or parity) (Table 5), (6) perinatal mental health outcomes during COVID-19 as a function of cultural or geographical factors (Table 6), (7) perinatal mental health outcomes during COVID-19 as a function of depression or anxiety severity (Table 7), (8) perinatal mental health outcomes during COVID-19 as a function of factors not examined elsewhere (Table 8), and (9) modeling or intervention studies of perinatal mental health outcomes during the COVID-19 pandemic (Table 9).
Table 2.
Mental health outcomes in pregnant or postpartum women before vs. during the COVID-19 pandemic.
| Study | Study Design | Country | Recruitment Sites/Methods | Participant Characteristics | Main Findings | Risk of Biasb | ||
|---|---|---|---|---|---|---|---|---|
| Subgroups | Pregnancy/ Postpartum Status |
Maternal Age | ||||||
| Ayaz et al. (72) | Cross-sectional | Turkey | Outpatient prenatal clinic | Pandemic (April 12–May 27, 2020; n = 63) vs. Pre-pandemic (June 2018-; end date and n: NR) | Pregnant women (N = 63) • GA: 32.5 (M) ± 7 (SD) weeks |
30.4 (M) ± 5.3 (SD) years | Pregnant women reported more depressive and anxiety symptoms during compared to before the pandemic. Risk factors: obesity, negative relationship with husband | 8 |
| Berthelot et al. (73) | Case-control/ Longitudinal |
Canada | Social media and prenatal clinics | Pandemic (April 2020; n = 1,258) vs. Pre-pandemic (April 2018–March 2020; n = 496) | Pregnant women (N = 1,754) • GA: [pandemic] 24.38 (M) ± 9.2 (SD) weeks; [pre-pandemic] 25.8 (M) ± 9.73 (SD) weeks |
29.27 (M) ± 4.23 (SD) years | Pregnant women reported more severe symptoms of depression, anxiety and PTSD during compared to before the pandemic. | 7 |
| Cameron et al. (74) | Cross-sectional | Canada | Social media | Pandemic (April 14–28, 2020; n = 312) vs. Pre-pandemic (specific dates: NR; n = 312) | Postpartum women ( ≤ 0–18 monthsa; N = 312) |
34.28 (M) ± 5.02 (SD) years | 34.09% of postpartum women reported depressive symptoms and 34.55% reported anxiety symptoms during the pandemic. Postpartum women reported increased depressive and anxiety symptoms during compared to before the pandemic. | 5 |
| Davenport et al. (75) | Cross-sectional | Canada | Social media | Pandemic (April 14–May 8, 2020; n = 900) vs. Pre-pandemic (retrospective recall; n = 900) | Pregnant (N = 520) and postpartum (<1 year; n = 380) women (N = 900) | Med = 33 (R = 17–49) years | 15% and 40.7% of participating women met criteria for depression before and during the pandemic, respectively. 39% and 72% of women met criteria for moderate to high anxiety before and during the pandemic, respectively. Protective factor: (for depression and anxiety) 150+ mins of physical activity | 8 |
| Hui et al. (76) | Retrospective | Hong Kong (People's Republic of China) | Hospital | Pandemic (January 5, 2020–April 30, 2020; n = 954) vs. Pre-pandemic (January 1, 2019–January 4, 2020; n = 3,577) | Postpartum women (N = 4,531) • GA: [pandemic] 38.5 (M) ± 2.29 (SD) weeks; [pre-pandemic] 38.5 (M) ± 2.25 (SD) weeks |
[Pandemic]: 33.1 (M) ± 4.6 (SD) years [Pre-pandemic]: 33.1 (M) ± 4.4 (SD) years |
Women who delivered during compared to before the pandemic reported higher depressive symptoms. | 6 |
| Loret de Mola et al. (77) | Longitudinal | Brazil | Hospitals | Pandemic 1st wave (May–July 2020) vs. Pandemic 2nd wave (July–December 2020) vs. Pre-pandemic (2019) | Pregnant women (N = 591) |
NR | In participating pregnant women, depression prevalence rose from a pre-pandemic rate of 3.1% to 28.4% during the first wave in 2020, and to 30.6% during the second wave of the pandemic. Anxiety increased from 9.6% (pre-pandemic) to 26.7% (1st wave), to 28.8% (2nd wave), with a 3-fold increase in prevalence. | 4 |
| Matvienko-Sikar et al. (78) | Cross-sectional | Ireland | Social media and hospital |
Pandemic (June 16–July 17, 2020; n = 235) vs. Pre-pandemic (May 2019–February 2020; n = 210) |
Pregnant women (N = 445) • GA: [pandemic] 27.49 (M) ± 8.60 (SD) weeks; [pre-pandemic] 26.43 (M) ± 10.09 (SD) weeks |
[Pandemic]: 33.67 (M) ± 4.47 (SD) years [Pre-pandemic]: 33.91 (M) ± 4.05 (SD) years |
Participating pregnant women reported higher stress during compared to before the pandemic. Risk factors: perceived low social support, low physical activity | 7 |
| Mayopoulous et al. (79) | Cross-sectional | United States of America | Social media, professional organizations, and hospitals |
Pandemic (March–April 2020; n = 1,611) vs. Pre- pandemic (early in 2020 [specific dates NR]; N = 637) |
Postpartum women (N = 1,274) |
32.0 (M) years | Postpartum women reported higher acute stress during compared to before the pandemic. Higher acute stress during birth was significantly associated with increased childbirth-related PTSD symptoms and decreased infant bonding. | 8 |
| McFarland et al. (80) | Population-based | United States of America | Records for live births |
Pandemic vs. Pre- pandemic (Time matched samples [N = 18,531]: September 2019–April 2020; Month-matched samples [N = 18,346]: January 2019–April 2019 and January 2020–April 2020) |
Pregnant women (N = 32,352) |
(R) <20 years: n = 647 20–34 years: n = 22,970–23,617 35+ years: n = 7,764–8,735 |
Pregnant women who gave birth during compared to before the pandemic reported elevated depressive symptoms. | 7 |
| Moyer et al. (81) | Cross-sectional | United States of America | Social media |
Pandemic (April 3–24, 2020; n = 2,740) vs. Pre-pandemic (retrospective recall; n = 2,740) |
Pregnant women (N = 2,740) • 3rd trimester: n = 1,128 |
M = 32.7 years | Pregnant women reporting more COVID-19 related stressors had the greatest changes in pre- to post- pregnancy-related anxiety. Risk factors: lack of face-to-face prenatal visits, change in birth plans away from in-hospital delivery, fear of running out of food, increased conflict at home, fear of infection, essential worker status (self or family member), COVID-19 high-risk area resident status, loss of childcare, loss of job, low education levels, previous mental health disorder | 8 |
| Pariente et al. (82) | Cohort | Israel | Hospital | Pandemic (March 18–April 29, 2020; n = 223) vs. Pre- pandemic (November 2016–April 2017; n = 123) | Postpartum women (N = 346) • GA: [pandemic] 39.4 (M) ± 1.0 (SD) weeks; [pre-pandemic] 39.4 (M) ± 0.9 (SD) weeks |
[Pandemic]: 29.1 (M) ± 5.1 (SD) years [Pre-pandemic]: 28.3 (M) ± 5.0 (SD) years |
Women delivering during compared to before the pandemic had lower risk of developing postpartum depression. | 6 |
| Sade et al. (83) | Cross-sectional | Israel | Hospital | Pandemic (March 19–May 26, 2020; n = 84) vs. Pre-pandemic (November 2016–April 2017; n = 279) | Pregnant women in high-risk obstetric units (N = 363) • GA: [pandemic] 33.7 (M) ± 5.1 (SD) weeks; pre-pandemic 34.0 (M) ± 4.8 (SD) weeks |
[Pandemic]: (R) <20 years: n = 2 20–35 years: n = 67 >35 years: n = 15 [Pre-pandemic]: (R) <20 years: n = 12 20–35 years: n = 230 >35 years: n = 37 |
No difference was found in depression and suicidal ideations in pregnant women in high-risk obstetric units during compared to before the pandemic | 8 |
| Silverman et al. (84) | Cross-sectional | United States of America | Obstetric clinics | During social restrictions (March 13–June 30, 2020; n = 252) vs. Before social restrictions (January 2–March 12, 2020; n = 264) | Postpartum women (N = 516) |
R = 19–48 years | Postpartum women with low socio-economic status reported significantly fewer depressive symptoms after compared to before social restrictions were imposed. | 6 |
| Silverman et al. (85) | Cross-sectional | United States of America | Obstetric clinics | During social restrictions (May 4–June 12, 2020; n: NR) vs. Before social restrictions (February 2–March 11, 2020; n: NR) | Pregnant women receiving government-funded healthcare (i.e., low socio-economic status; N = 485) | R = 16–40 years | Pregnant women of low socio-economic status reported improved mood after compared to before social restrictions were imposed. | 6 |
| Sinaci et al. (86) | Cross-sectional | Turkey | High-risk pregnancy clinic |
Pandemic (May–July 2020; n = 446) vs. Pre-pandemic (retrospective recall; n = 446) |
Pregnant women (N = 446) • GA: 24.53 (M) years |
Med = 28.93 R = 23.22–34.61 years |
Participating pregnant women reported significantly higher trait anxiety during compared to before the pandemic. Risk factor: high-risk pregnancy | 6 |
| Suzuki (87) | Case-control | Japan | Postpartum outpatient clinic | Pandemic (March–April 2020; n = 132) vs. Pre- pandemic (March–April 2019; n = 148) | Postpartum women (N = 280) |
R = <19 to >40 years | No difference was found in postpartum women's depressive symptoms before and during the pandemic. Postpartum women reported a decrease in mother-infant bonding during compared to before the pandemic. | 7 |
| Wu et al. (88) | Cross-sectional | China | Obstetric clinic |
After COVID-19 declaration (January 20–February 9, 2020; n = 1,285) vs. Before COVID-19 declaration (January 1–20, 2020; n = 2,839) |
Pregnant (3rd trimester) women (N = 4,124) | Med = 30 (R = 27–32) years | Pregnant women reported greater depression and self-harm after compared to before the COVID-19 declaration. Risk factors (for depression): increased information about COVID-19 and number of positive cases | 6 |
| Xie et al. (89) | Cross-sectional | China | Social media; Hospitals | Pandemic (January–August 2020; n = 689) vs. Pre-pandemic (March–December 2019; n = 2,657) | Pregnant women (N = 3,346) • GA: [pandemic] 16.10 (M) ± 5.0 (SD) weeks; [pre-pandemic] 16.24 (M) ± 5.0 (SD) weeks |
[Pandemic]: 29.03 (M) ± 4.9 (SD) years [Pre-pandemic]: 28.94 (M) ± 6.4 (SD) years |
Women pregnant during compared to before the pandemic reported greater depression, anxiety, and somatization, as well as lower family cohesion. | 8 |
| Zanardo et al. (90) | Case-control | Italy | Online |
Pandemic (March 8–May 3, 2020; n = 91) vs. Pre-pandemic (March–May 2019; n = 101) |
Postpartum women (N = 192) • GA: [pandemic] 39.41 (M) ± 1.12 (SD) weeks; [pre-pandemic] 39.42 (M) ± 1.14 (SD) weeks |
[Pandemic]: 33.73 (M) ±5.01 (SD) years [Pre-pandemic]: 32.98 (M) ±5.07 (SD) years |
Postpartum women reported higher depression during compared to before the pandemic. | 7 |
GA, gestational age; M, mean; SD, standard deviation; Med, median; R, range; PTSD, post-traumatic stress disorder; NR, not reported.
This study covered children aged 0–8 years, but our data reviewed here only pertains to 0–18 months range.
Assessed using a modified version of the Newcastle-Ottawa Scale (54). See section Assessment of Risk of Bias for details. Scores range from 0 (highest bias) to 9 points (lowest bias).
Table 3.
Mental health outcomes in pregnant or postpartum women with vs. without COVID-19.
| Study | Study Design | Country | Study Period | Recruitment Sites/Methods | Participant Characteristics | Main Findings | Risk of Biasa | ||
|---|---|---|---|---|---|---|---|---|---|
| Pregnancy/ Postpartum Status |
Subgroups | Maternal Age | |||||||
| Bender et al. (91) | Cohort | United States of America | April 13-26, 2020 (during the pandemic) | Hospitals | Pregnant women (N = 318) | COVID-19+ (n = 8) vs. COVID-19– (n = 310) | NR | Asymptomatic COVID-19+ pregnant women showed increased depression compared to asymptomatic COVID-19– pregnant women. This pattern extended to the early postpartum. | 9 |
| Ceulemans et al. (55) | Cross-sectional | United Kingdom, Norway, Switzerland, The Netherlands | June 16–July 14, 2020 (during the pandemic) | Online survey | Pregnant and postpartum women (N = 9,041) | COVID-19+ (n = 56) vs. COVID-19- (n = 796) | NR | COVID-19+ pregnant and postpartum women were not more likely to have major depressive symptoms, generalized anxiety, or stress compared to COVID-19– women. | 8 |
| Kotabagi et al. (92) | Cross-sectional | United Kingdom | April 2020 (during the pandemic) | Hospitals | Pregnant women (N = 11) • GA: Med = 39 weeks |
COVID-19+ (n = 11) | Med = 31 years | COVID-19+ women reported an increase in psychological symptoms at the start of the pandemic, but symptoms decreased over time. | 3 |
GA, gestational age; M, mean; SD, standard deviation; Med, median; R, range; COVID19+, COVID-19 positive; COVID-19–, COVID-19 negative; NR, not reported.
Assessed using a modified version of the Newcastle-Ottawa Scale (54). See section Assessment of Risk of Bias for details. Scores range from 0 (highest bias) to 10 points (lowest bias).
Table 4.
Mental health outcomes in pregnant vs. non-pregnant women during the COVID-19 pandemic.
| Study | Study Design | Country | Study Period | Recruitment Sites/Methods | Participant Characteristics | Main Findings | Risk of Biasa | ||
|---|---|---|---|---|---|---|---|---|---|
| Pregnancy/ Postpartum Status |
Subgroups | Maternal Age | |||||||
| Lopez-Moralez et al. (93) | Longitudinal | Argentina | March 20–May 10, 2020 (during the pandemic) | Social media | Pregnant (GA: 20.05 [M] ± 8.70 [SD] weeks) and non-pregnant women (N = 204) |
Pregnant (n = 102) vs. Non-pregnant (n = 102) |
32.56 (M) ± 4.71 (SD) years | Compared to non-pregnant women, pregnant women showed increased depression, anxiety and decreased negative affect during the pandemic. | 6 |
| Yassa et al. (94) | Case-control | Turkey | April 2020 (during the pandemic) | Tertiary “coronavirus pandemic” hospital centre | Pregnant (GA: Med = 25 [R = 4–42] weeks) and non-pregnant women (N = 304) |
Pregnant (n = 203) vs. Non-pregnant (n = 101) |
[Pregnant]: 27.4 (M) ± 5.3 (SD) years [Non-pregnant]: 27.6 (M) ± 4.1 (SD) years | Compared to non-pregnant women, pregnant women reported lower anxiety and greater OCD-like symptoms during the pandemic. | 7 |
| Zhou et al. (95) | Cross-sectional | China | February 28–March 12, 2020 (during the pandemic) | Social media | Pregnant and non-pregnant women (N = 859) |
Pregnant (n = 544) vs. Non-pregnant (n = 315) |
[Pregnant]: 31.1 (M) ± 3.9 (SD) years [Non-pregnant]: 35.4 (M) ± 5.7 (SD) years | Compared to non-pregnant women, pregnant women reported low depression, anxiety, PTSD, and insomnia during the pandemic. | 7 |
GA, gestational age; M, mean; SD, standard deviation; Med, median; R, range; PTSD, post-traumatic stress disorder; OCD, obsessive-compulsive disorder.
Assessed using a modified version of the Newcastle-Ottawa Scale (54). See section Assessment of Risk of Bias for details. Scores range from 0 (highest bias) to 10 points (lowest bias).
Table 5.
Perinatal mental health outcomes during COVID-19 as a function of pregnancy-related factors (e.g., stage of pregnancy/postpartum, parity).
| Study | Study Design | Country | Study Period | Recruitment Sites/ Methods |
Participants Characteristics | Main Findings | Risk of Biasa | ||
|---|---|---|---|---|---|---|---|---|---|
| Pregnancy/ Postpartum Status |
Subgroups | Maternal Age | |||||||
| Lebel et al. (96) | Cross-sectional | Canada | April 5–20, 2020 (during the pandemic); 2012–2016 (previous cohorts) | Social media | Pregnant women (N = 1,987) | Nulliparous (n = 971) vs. Primiparous (n = 735) vs. Multiparous (n = 277) | 32.4 (M) ± 4.2 (SD) years | Regardless of parity, 37% of all participating pregnant women had elevated symptoms of depression and 56.6% had elevated levels of anxiety during the pandemic. Nulliparous, compared to primiparous or multiparous, women reported higher symptoms of pregnancy-related anxiety. Protective factors: social support, physical activity. | 7 |
| Saccone et al. (97) | Cross-sectional | Italy | March 15–April 1, 2020 (during the pandemic) | University | Pregnant women (N = 100) | 1st trimester (n = 17) vs. 2nd trimester (n = 35) vs. 3rd trimester (n = 48) | NR | Pregnant women in the 1st trimester, compared to those in 2nd and 3rd trimesters, reported higher anxiety during the pandemic. | 6 |
| Shayganfard et al. (98) | Cross-sectional | Iran | Lockdown period (during the pandemic) | Hospital | Pregnant (GA: 27.20 [M] ± 5.77 [SD]; n = 66) and postpartum (<6 weeks; n = 37) women (N = 103) | Before delivery (n = 66) vs. Post-delivery (n = 37) | 28.57 (M) ± 6.85 (SD) years | While women reported higher stress after compared to before delivery, no differences were found in depressive and anxiety symptoms between pregnant and postpartum women. Risk factors (for anxiety and depression): strict adherence to rules, worries and discomfort around post-poning/canceling routine medical care appointments, contact with/exposure to a person with COVID-19. | 6 |
| Stepowicz et al. (99) | Cross-sectional | Poland | April 7–May 24, 2020 (during the pandemic) | Hospital | Pregnant (n = 164) and postpartum (n = 46) women (N = 210) |
1st trimester (n = 11) vs. 2nd trimester (n = 46) vs. 3rd trimester (n = 107) vs. Postpartum (n = 46) | Med = 31 (R = 19–45) years |
Pregnant women in the 1st trimester, compared to women in the 2nd and 3rd trimesters or in the postpartum, reported higher levels of anxiety during the pandemic. | 6 |
| Wang et al. (100) | Longitudinal cohort | China | May 1–July 31, 2020 (during the pandemic) | National epidemic reporting system | Pregnant women (N = 138) • 1st trimester: n = 13) • 2nd trimester: n = 6 • 3rd trimester: n = 53 |
Post-delivery (n = 57) vs. Post-abortion (n = 15) | Med = 31 years | 22% of participating women reported symptoms of depression and PTSD during the pandemic. There was no significant difference in symptoms between women who delivered vs. those who had induced abortion. | 8 |
| Zeng et al. (101) | Cross-sectional | China | March 25–June 5, 2020 (during the pandemic) | Hospital | Pregnant (3rd trimester; n = 516) and postpartum ( ≤ 1 week; n = 109) women (N = 625) | 3rd trimester (n = 516) vs. Postpartum (n = 109) | 29.2 (M) ± 4.2 (SD) years | Pregnant women in the third trimester were more likely to report depression and anxiety than postpartum women during the pandemic. | 8 |
| Zhang and Ma (102) | Cross-sectional | China | February–March 2020 (during the pandemic) | Social media | Pregnant women (N = 560) | 1st trimester (n = 227) vs. 2nd trimester (n = 220) vs. 3rd trimester (n = 113) | 25.8 (M) ± 2.7 (SD) years | Pregnant women paid significantly more attention to their mental health during the 3rd trimester, compared to first and second trimesters. Risk factors: increased stress from work/home, and helplessness/apprehension during the early stages of the pandemic. | 7 |
GA, gestational age; M, mean; SD, standard deviation; Med, median; R, range; PTSD, post-traumatic stress disorder.
Assessed using a modified version of the Newcastle-Ottawa Scale (54). See section Assessment of Risk of Bias for details. Scores range from 0 (highest bias) to 10 points (lowest bias).
Table 6.
Perinatal mental health outcomes during COVID-19 as a function of cultural or geographic factors.
| Study | Study Design | Country | Study Period | Recruitment Sites/Methods | Participant Characteristics | Main Findings | Risk of Biasa | ||
|---|---|---|---|---|---|---|---|---|---|
| Pregnancy/ Postpartum Status |
Subgroups | Maternal Age | |||||||
| Bo et al. (103) | Cross-sectional | China | February 22–March 10, 2020 (during the pandemic) | Social media | Pregnant and postpartum women (N = 1,309) • 1st & 2nd trimester: n = 373 • 3rd trimester: n = 545 • Postpartum: n = 391 |
High-risk area residents (Central/Western China; n = 418) vs. Low-risk area residents (Northern/Southern China; n = 891) | 29.99 (M) ± 4.53 (SD) years | 27.43% of participating women reported depression. Women living in high-risk area (central/western China), compared to women living in low-risk area (northern/southern China), were more likely to report depression. Risk factors: concerns about COVID-19 infection and delayed regular medical check-ups | 6 |
| Dong et al. (104) | Cross-sectional | China | February 22–27, 2020 (during the pandemic) | Social media and workplaces | Pregnant women (N = 156) • 0–12 weeks GA: n = 36 • 13–24 weeks GA: n = 46 • 25–40 weeks GA: n = 74 |
High-risk area residents (Wuhan; n = 101) vs. Low-risk area residents (other provinces in China; n = 55) | 20–25 years: n = 4 26–30 years: n = 91 31–50 years: n = 61 |
There was no difference in depressive and anxiety symptoms in women living in high-risk area (Wuhan) compared to those living in other areas. | 8 |
| Liu et al. (17) | Cross-sectional | China | February 3–9 2020 (during the pandemic) | Hospitals | Pregnant women (N = 1,947) • 1st trimester: n = 83 • 2nd trimester: n = 639 • 3rd trimester: n = 1,125 |
High-risk area residents (Wuhan; n = 932) vs. Low-risk area residents (Chongqing; n = 1,015) | <35 years: n = 1,734 ≥35 years: n = 213 |
17.2% of participating women reported anxiety. Pregnant women residing in a high-risk area (Wuhan) reported higher anxiety compared to women residing in low-risk areas. (Chongqing) | 9 |
| Spinola et al. (105) | Cross-sectional | Italy | May 11–June 6, 2020 (lockdown period during the pandemic) | Social media | Postpartum women (<1 year; N = 243) |
High-risk area residents (Northern Italy; n = 131) vs. Low-risk area (Central or Southern Italy; n = 109) | 34.01 (M) ± 4.27 (SD) years | 44% of participating women reported postpartum depression. Women who spent isolation in high-risk areas (northern Italy) reported greater postpartum depression and adopted more maladaptive coping strategies than women living in lower risk areas. Risk factors: prior abortion, previous psychiatric history, COVID-19 infection. | 6 |
| Taubman–Ben-Ari et al. (106) | Cross-sectional | Israel | March 18–28, 2020 (during the pandemic) | Social media | Pregnant women (N = 336) • GA: 25.42 (M) ± 9.57 (SD) weeks |
Ethnic minority (Arab; n = 111) vs. Ethnic majority (Jewish; n = 225) | 30.31 (M) ± 4.97 (SD) years | Women of ethnic minority (Arab) reported more anxiety symptoms than women of a majority ethnicity (Jewish) | 7 |
| Zhang et al. (107) | Cross-sectional | China | February 13–16, 2020 (during the pandemic) | Hospitals | Pregnant women (N = 1,901) |
High risk area (Central China; n = 406) vs. Low risk area (Other provinces in China; n = 1,495) | 28.9 (M) ± 4.7 (SD) years | Women living in the epicenter (Hubei) reported higher psychological symptoms, such as PTSD, during the pandemic than women in other provinces and in pre-pandemic samples. | 6 |
GA, gestational age; M, mean; SD, standard deviation; Med, median; R, range; PTSD, post-traumatic stress disorder.
Assessed using a modified version of the Newcastle-Ottawa Scale (54). See section Assessment of Risk of Bias for details. Scores range from 0 (highest bias) to 10 points (lowest bias).
Table 7.
Perinatal mental health outcomes during COVID-19 as a function of depression or anxiety severity.
| Study | Study Design | Country | Study Period | Recruitment Sites/Methods | Participant Characteristics | Main Findings | Risk of Biasa | ||
|---|---|---|---|---|---|---|---|---|---|
| Pregnancy/ Postpartum Status |
Key Variables Examined or Subgroups | Maternal Age | |||||||
| Dagklis et al. (108) | Cross-sectional | Greece | March 2020 (1st, 3rd, and 6th week of lockdown during the pandemic) | Prenatal clinic | Pregnant women (N = 269) |
State and trait anxiety (State-Trait Anxiety Inventory) | ≤ 35 years: n = 195 > 35 years: n = 74 |
Pregnant women reported higher state anxiety (i.e., anxiety during pregnancy) than trait-anxiety (i.e., lifetime anxiety) during lockdown. State anxiety fluctuated depending on the state of the pandemic and was positively associated with depressive symptoms. Risk factors (for state anxiety): early stages following lockdown, and third trimester of pregnancy | 6 |
| Durankuş & Aksu. (109) | Cross-sectional | Turkey | During the pandemic (specific dates: NR) | Online | Pregnant women (N = 260) • GA: 7.04 (M) ± 5.88 (SD) weeks |
High (n = 92) vs. Low (n = 168) depression (Edinburgh Postnatal Depression Scale) | 29.56 (M) ± 3.83 (SD) years | 35.4% of participating women screened positive for depression. Pregnant women with high compared to low depressive symptoms reported more severe impact and social isolation during COVID-19 as well as higher anxiety symptoms. Risk factors (for depression): social isolation, greater number of children, and prior psychiatric history | 7 |
| Kahyaoglu & Kucukkaya (110) | Cross-sectional | Turkey | June–July 2020 (during the pandemic) | Social media | Pregnant women (N = 403) • GA: 27.3 (M) ± 8.8 (SD) weeks |
Anxiety and depression (Hospital Anxiety and Depression Scale) | 28.2 (M) ± 4.5 (SD) years | 64.5% of participating pregnant women reported anxiety during COVID-19 and 56.3% reported depression. Risk factors: low education levels, low physical activity, discomfort during hospital/doctor's visits, lack of information around the effect of COVID-19 on pregnancy | 6 |
| Mappa et al. (111) | Cross-sectional | Italy | January–February 2020 (during the pandemic) | Prenatal clinics | Pregnant women (N = 178) • GA: Med = 18 weeks |
State and trait anxiety (State-Trait Anxiety Inventory) | Med = 33 years | Pregnant women reported higher state anxiety during the pandemic than trait-anxiety (i.e., pre-existing anxiety). Risk factor (for state anxiety): high education status | 7 |
| Oskovi-Kaplan et al. (112) | Cross-sectional | Turkey | June 2020 (during the pandemic) | Obstetric tertiary care center with strong hospital restrictions | Postpartum women (<2 days; N = 223) • GA: Med = 39 weeks |
High (n = 33) vs. Low (n = 190) depression (Edinburgh Postnatal Depression Scale) | Med = 26 years | 14.7% of participating women screened positive for postpartum depression. Women who screened positive for postpartum depression, compared to those who did not, reported significantly lower mother-to-infant attachment. | 6 |
| Patabendige et al. (113) | Cross-sectional | Sri Lanka | April 27–May 20, 2020 (during the pandemic) | Prenatal clinics | Pregnant women (N = 257) • GA: 23.3 (M) ± 10.2 (SD) weeks |
Anxiety and depression (Hospital Anxiety and Depression Scale) | 29.2 (M) ± 5.7 (SD) years | 17.5% of participating pregnant women reported anxiety and 19.5% reported depression. Risk factors: low maternal age (18–25 years), time spent watching television to seek COVID-19 information, and low household income | 7 |
| Ravaldi et al. (114) | Cross-sectional survey | Italy | March 18–31, 2020 (1st month of full lockdown during the pandemic) | Social media | Pregnant women (N = 737) • GA: Med = 27.8 (R = 4.7–42.5) weeks |
PTSD (National Stressful Events Survey), state and trait anxiety (State and Trait Anxiety Inventory) | Med: 34.4 (R = 18.4–47.4) years | 21.7% of participating women reported clinically significant anxiety and 10.2% reported clinically significant PTSD. Pregnant women with previous history of depression and/or anxiety reported elevated PTSD symptoms during the pandemic. | 9 |
| Sun et al. (115) | Cross-sectional | China | December 31, 2019–March 22, 2020 (during the pandemic) | Inpatient hospitals | Pregnant (>28 weeks; n = 738) and postpartum (<7 days; n = 2,092) women (N = 2,883) |
Depression (n = 972) vs. No depression (n = 1,911) (Edinburgh Postnatal Depression Scale) | <25 years: n = 126 25–29 years: n = 1,194 30–34 years: n = 1,159 >34 years: n = 404 |
33.71% of the participating women had depression symptoms. Depressive symptoms increased among postpartum women as the pandemic worsened, which was then followed by a decrease in depressive symptoms among pregnant women as the pandemic became more under control. Risk factors: traumatic delivery, poor sleep quality, maternal/passive smoking, lack of exercise, and poor family functioning | 6 |
GA, gestational age; M, mean; SD, standard deviation; Med, median; R, range; PTSD, post-traumatic stress disorder; NR, not reported.
Assessed using a modified version of the Newcastle-Ottawa Scale (54). See section Assessment of Risk of Bias for details. Scores range from 0 (highest bias) to 10 points (lowest bias).
Table 8.
Perinatal mental health outcomes during COVID-19 as a function of factors not examined elsewhere.
| Study | Study Design | Country | Study Period | Recruitment Sites/Methods | Participant Characteristics | Main Findings | Risk of biasa | ||
|---|---|---|---|---|---|---|---|---|---|
| Pregnancy/ Postpartum Status |
Key Variables Examined Or Subgroups | Maternal Age | |||||||
| Ahorsu et al. (116) | Cross-sectional | Iran | March–April 2020 (during the pandemic) | Health and household registration system | Pregnant women (GA: 15.04 (M) ± 6.00 (SD) weeks; n = 290) and their husbands (N = 580) | Fear of COVID-19, COVID-19 preventive behaviors, depression, anxiety, mental quality of life | 29.24 (M) ± 5.84 (SD) years | Pregnant women's own or partner's fear of COVID-19 was associated with increased depressive symptoms and lower mental health quality during the pandemic. | 6 |
| Chaves et al. (117) | Cross-sectional | Spain | April 7–May 8, 2020 (during the pandemic) | Social media | Pregnant (n = 450) and postpartum (<6 months; n = 274) women (N = 724) | Life satisfaction, depression, anxiety | 33.36 (M) ± 4.12 (SD) years | 58% of participating women reported depressive symptoms and 51% of women reported anxiety symptoms. Risk factors (for life satisfaction): [pregnancy] poor perceived self-health, single/separated marital status, health practitioner occupation status; [postpartum] poor perceived self-health, baby's poor health, poor quality of baby's sleep, single/separated marital status | 6 |
| Ding et al. (118) | Cross-sectional | China | March 7–23, 2020 (during the pandemic) | Hospitals | Pregnant women (N = 817) • 1st trimester: n = 115 • 2nd trimester: n = 247 • 3rd trimester: n = 455 |
Knowledge, attitudes, and practices toward COVID-19, anxiety | 29.1 (M) ± 4.0 (SD) years | 20.8% of participating pregnant women reported anxiety. Risk factors: low knowledge of the impact of COVID-19 on pregnancy, fear of COVID-19 infection, distrust in media, previous children in the family | 7 |
| Gildner et al. (119) | Cross-sectional | United States of America | April–June 2020 (during the pandemic) | Social media | Pregnant women (N = 1,856) • GA: 26.1 (M) ± 8.62 (SD) weeks |
COVID-related exercise change, depression | 31.3 (M) ± 4.30 (SD) years | Pregnant women reporting COVID-related changes in their exercise routine had higher depressive symptoms. Women living in metro compared to rural areas were more likely to report changes to exercise routine. | 7 |
| Harrison et al. (120) | Cross-sectional | United Kingdom | May 1–June 1, 2020 (during the pandemic) | Social media | Pregnant women (N = 205) • 1st trimester: n = 70 • 2nd trimester: n = 69 • 3rd trimester: n = 66 |
Perceived social support, depression, anxiety, repetitive negative thinking, loneliness | 18–24 years: n = 13 25–34 years: n = 129 35–44 years: n = 63 |
Pregnant women experiencing low levels of perceived support reported more depressive and anxiety symptoms, which were mediated by increased repetitive negative thinking and loneliness. | 6 |
| Jiang et al. (121) | Cross-sectional | China | February 5–28, 2020 (during the pandemic) | Hospital | Pregnant women (N = 1,873) • 1st trimester: n = 598 • 2nd trimester: n = 703 • 3rd trimester: n = 572 |
Access to prenatal care information, depression, anxiety, perceived stress | 29 (M) ± 4.10 (SD) years | 45.9% of participating pregnant women reported depression, 18.1% reported anxiety, and 89.1% reported stress. Those who accessed prenatal care during the pandemic were at lower risk of perceived stress, anxiety, and depression | 7 |
| Kachi et al. (122) | Cross-sectional | Japan | May 22–31, 2020 (during the pandemic) | Workplaces | Pregnant women (N = 359) • 8–13 weeks: n = 39 • 14–27 weeks: n = 140 • 28–41 weeks: n = 180 |
Maternity harassment (pregnancy discrimination; n = 89) vs. No maternity harassment (n = 270) | [maternity harassment]: 31.3 (M) ± 4.8 (SD) years [no harassment]: 31.2 (M) ± 4.6 (SD) years | 1/4 of pregnant women experienced maternity harassment in the workplace. Pregnant women who experienced maternity harassment had a 2.5-fold higher prevalence of depression than those who had not experienced harassment. | 6 |
| Lin et al. (123) | Online cross-sectional | China | February 17–March 16, 2020 (during the pandemic) | Obstetric clinics and hospitals | Pregnant women (N = 751) • 1st trimester: n = 514 • 2nd trimester: n = 214 • 3rd trimester: n = 23 |
Sleep conditions during the pandemic, depression, anxiety | 30.51 (M) ± 4.28 (SD) years | 35.4% of participating pregnant women reported anxiety and 13.4% reported depression. Pregnant women with poor sleep quality/duration were at higher risk of depressive and anxiety symptoms. | 7 |
| Shahid et al. (124) | Cross-sectional | Pakistan | August 6–20, 2020 (during the pandemic) | Outpatient department of obstetrics and gynecology hospital | Pregnant women (N = 552) • GA: 25.3 (M) ± 10.4 (SD) weeks |
Awareness and concerns about COVID-19, depression, anxiety | 32 (M) ± 7.3 (SD) years | 64% of pregnant women reported a high level of awareness and concern about the COVID-19 pandemic, and were at high risk of depression and anxiety | 7 |
| Thayer & Gildner (125) | Cross-sectional | United States of America | April 16–30, 2020 (during the pandemic) | Social media | Pregnant women (N = 2,099) • GA: 26.4 (M) ± 9.0 (SD) weeks |
COVID-19 associated financial stress, depression | 31.3 (M) ± 4.4 (SD) years | 43% of participating pregnant women experienced COVID-19 related financial stress and 24% had clinically significant depression. Those with high financial stress were at high risk of clinically significant depression. | 6 |
| Zhang et al. (107) | Retrospective | China | April 11–May 25, 2020 (during the pandemic) | Hospitals | Postpartum women (<1 week; N = 878) |
Emotional eating, changes in dietary patterns | R: 18–45 years | Postpartum women during the pandemic reported a dietary change and higher emotional eating. Risk factors (for emotional eating): high-risk residence status (Southern China and Wuhan), low exercise, and high concern about COVID-19 | 6 |
GA, gestational age; M, mean; SD, standard deviation; Med, median; R, range.
Assessed using a modified version of the Newcastle-Ottawa Scale (54). See section Assessment of Risk of Bias for details. Scores range from 0 (highest bias) to 10 points (lowest bias).
Prevalence of Mental Health Outcomes in Pregnant or Postpartum Women During COVID-19
Eighteen of the 81 studies reported on the prevalence of mental health outcomes in pregnant or postpartum women during the pandemic (Table 1). Sixteen studies reported on moderate to severe symptoms of depression and/or anxiety in pregnant or postpartum women during the pandemic, with prevalence rates ranging between 20 and 64% (55–70, 132). One study reported that around 14% of their sample of pregnant women had elevated anxiety during the pandemic, which was higher than that observed in pregnant or non-pregnant population prior to COVID-19 (71). In contrast, another study reported more than 1/3 of their sample of pregnant women had elevated depression and anxiety yet indicated that these were comparable prevalence rates to their pre-pandemic population (56).
Mental Health Outcomes in Pregnant or Postpartum Women Before vs. During COVID-19
Nineteen of the 81 studies compared mental health outcomes in pregnant or postpartum women before and during the pandemic (Table 2). Eleven of these studies reported elevated levels of clinically significant mental health symptoms such as depression and anxiety in pregnant or postpartum women during the COVID-19 pandemic (72–77, 81, 86, 88–90). Three studies specifically asked pregnant and postpartum women to report their symptoms during the pandemic as well as to retrospectively report their symptoms before the pandemic. These studies generally report higher levels of depression and anxiety during the pandemic (75), with one study showing that changes in pre- to post-COVID-19 pregnancy-related anxiety were associated with an increase in the number of COVID-19-related stressors (81) and another study documenting higher rates of trait anxiety specifically in the case of high-risk pregnancies (86).
Higher acute stress was documented in mothers delivering during compared to before the pandemic (78, 79), which was in turn related to increased symptoms of Post-Traumatic Stress Disorder (PTSD) and challenges with mother-infant bonding and breastfeeding (79). Additionally, trauma-related symptoms, including PTSD and dissociative symptoms, as well as thoughts of self-harm, were higher in pregnant women during compared to before the pandemic (73, 88). Postpartum mothers tended to report increased problems in bonding with their infant during compared to before the pandemic (87).
In contrast to studies that reported increased mental health symptoms during compared to before the pandemic, four studies (80, 82, 85, 87) reported decreased clinically significant depressive symptoms in pregnant women during the pandemic or shortly after the onset of the pandemic (80). An additional study (83) reported no difference in depressive symptoms between pregnant women before and during the COVID-19 pandemic. Two studies (84, 85) found that their sample of pregnant women had higher mood during compared to before the onset of COVID-19 community restrictions; a later study by the same research group found that pregnant women living in lower socioeconomic areas reported significantly fewer depressive symptoms after restrictions were imposed (84).
Mental Health Outcomes in Pregnant or Postpartum Women With vs. Without COVID-19
Three studies examined mental health outcomes in pregnant and postpartum women with confirmed positive COVID-19 diagnoses (55, 91, 92) (Table 3). Women who delivered during the pandemic with positive COVID-19 diagnosis had depression and anxiety that rose to a maximum at the height of the pandemic, and then decreased as more COVID-19-related information and guidelines were made public (92). Similarly, asymptomatic pregnant women with positive COVID-19 showed increased depression compared to those with negative COVID-19, a pattern which extended to the early postpartum (91). In contrast to these findings, one study suggested that pregnant and breastfeeding women with positive COVID-19 were not more likely to have major depressive symptoms, generalized anxiety, or heightened stress compared to those with negative COVID-19 (55).
Mental Health Outcomes in Pregnant vs. Non-pregnant Women During COVID-19
Three studies compared mental health outcomes in pregnant and non-pregnant women during the pandemic and report mixed findings (Table 4). One study documented a greater increase in depression, anxiety, and negative affect, coupled with a more pronounced decrease in positive affect, in pregnant compared to non-pregnant women during a 50-day quarantine period (93). However, another study found that pregnant women, despite displaying increased OCD symptoms, showed decreased overall anxiety compared to non-pregnant women (94). Another report suggested decreased symptoms of depression, anxiety, and PTSD in pregnant compared to non-pregnant women during the pandemic (95).
Perinatal Mental Health Outcomes During COVID-19 as a Function of Pregnancy-Related Factors (e.g., Stages of Pregnancy/Postpartum, Parity)
Seven of the 81 studies examined the role of pregnancy-related factors, such as trimesters or parity, in perinatal mental health outcomes during COVID-19 (Table 5). Four studies (97, 99, 101, 102) compared pregnant women in different trimesters during the pandemic. Women in the first trimester of pregnancy had higher anxiety and depression (101) and experienced more severe psychological impact (97) during the pandemic compared to women in the second or third trimesters or the postpartum period (99). On the other hand, women in the third trimester paid more attention to their mental health compared to those in first and second trimesters of their pregnancy (102). A study comparing pregnant and postpartum women found no differences in depressive and anxiety symptoms during pregnancy and the postpartum despite increased levels of stress associated with the postpartum period (98). Similarly, a study comparing women who delivered vs. had induced abortion during the pandemic also reported no between-group differences in depression in their sample (100). First-time pregnancy status emerged as a significant factor influencing perinatal mental health outcomes during the pandemic, with nulliparous women showing higher pregnancy-related anxiety compared to primiparous or multiparous women (96).
Perinatal Mental Health Outcomes During COVID-19 as a Function of Cultural or Geographic Factors
Six studies investigated the impact of the pandemic on perinatal mental health outcomes in the context of cultural or geographical factors (Table 6). In terms of cultural and ethnic differences, one study (106) examined pregnant Jewish and Arab women during the pandemic and reported that while general anxiety levels were quite high among both Arab and Jewish women, Arab women (ethnic minority) displayed higher COVID-19-related anxieties than Jewish women (ethnic majority). Regarding geographical differences, three studies compared pregnant women living in the epicenter of the pandemic (e.g., city of Wuhan or the province of Hubei) compared to those living in regions that were low risk for COVID-19 (17, 104, 133). Although higher rates of anxiety (17) and PTSD (133) were generally documented in pregnant women living in high-risk compared to low-risk regions, one study (104) found no difference in rates of depression or anxiety among pregnant women living in the epicenter vs. low-risk regions, which was attributed to transparent communication of information and increased social support. Similar patterns have been observed in postpartum women, with those isolating in high-risk areas of Italy reporting heightened perceived risk and greater postpartum depression compared to those isolating in low-risk areas (105).
Perinatal Mental Health Outcomes During COVID-19 as a Function of Depression/Anxiety Severity
Eight studies examining pregnant and postpartum women during the pandemic compared high vs. low depression scores (109, 112), depression vs. anxiety scores (110, 113), state vs. trait anxiety scores (108, 111), or the presence of depression vs. no depression (115) (Table 7). Pregnant women with higher depression displayed significantly higher anxiety (109) and lower mother-to-infant attachment (112). While some groups reported high rates of depression and anxiety in pregnant women (110, 113), others reported that depressive symptoms increased among postpartum women as the pandemic worsened, which was then followed by a decrease in depressive symptoms among pregnant women as the pandemic became more under control (115). One study indicated that pregnant women with a previous history of depression or anxiety had a significantly higher level of anxiety and PTSD symptoms during the pandemic (114), while two studies reported higher state-anxiety specific to the pandemic, compared to trait anxiety in pregnant women (108, 111).
Perinatal Mental Health Outcomes During COVID-19 as a Function of Factors Not Examined Elsewhere
Eleven studies examined additional factors (i.e., factors not examined elsewhere) that may influence perinatal mental health outcomes during COVID-19, such as emotional eating (107), exercise (119), sleep deprivation (123), financial stress (125), access to prenatal care (121), knowledge, concerns, or worries about COVID-19 (118, 124), social or marital support (116), and pregnancy discrimination (122) (Table 8). Pregnant women reporting changes in their exercise routine [encompassing both increase or decrease in exercise; (119)], decreased sleep (123), or increased COVID-19-related financial stress (125) were at greater risk for experiencing depression during the pandemic, while access to prenatal care information was associated with lower risk of perceived stress, anxiety, and depression (121). Concerns about COVID-19 also emerged as a significant factor affecting perinatal mental health, showing links to increased anxiety, depression, and sleep disturbance during pregnancy and the postpartum (117, 124), as well as high emotional eating in the postpartum (107). At the same time, knowledge of the impact of the pandemic on pregnancy was shown to serve as a protective factor for prenatal anxiety (118). Perceived social and marital support was also an important protective factor for perinatal depression and anxiety symptoms (120). Pregnant women's fear of COVID-19, depression, mental health, and COVID-19-related preventive behaviors were shown to be dyadically linked to their husbands' fear, mental health, and preventive behaviors (116). In contrast, maternity harassment (i.e., pregnancy discrimination) experienced in the workplace was linked to a 2.5-fold increased risk of depression in pregnant women during the pandemic (122).
Modeling or Intervention Studies of Perinatal Mental Health Outcomes During COVID-19
Five studies used various analytic approaches (e.g., structural equation modeling, path analysis) to examine mediating or moderating factors (127–131) associated with mental health outcomes in pregnant women, while one study used a quasi-experimental design (126) to study the effect of an intervention pregnant women received during the pandemic (Table 9). The following factors demonstrated positive associations with mental health disorders in pregnant women: anxiety and concerns related to COVID-19 (130), perceived risk for COVID-19 infection, financial difficulties, and low social support (129). In a study comparing risk and protective factors across three different countries, family conflict and pandemic-related stress emerged as important risk factors that were shared across China, Italy, and the Netherlands, whereas risk factors unique to each country involved variables associated with family structure or characteristics (128). Mediating variables identified are also of note. One study highlighted that lowered sense of security partially mediated the relationship between pregnant women's psychological response to the pandemic and their pregnancy stress (131). Another study demonstrated that failed mentalization of trauma partially mediated the association between personality pathology and affective, behavioral, and thought problems during the pandemic (127). An intervention study examined the effectiveness of tele-education classes for pregnancy and birth planning during the pandemic, which was shown to be associated with decreased prenatal distress and pregnancy-related anxiety (126).
Risk and Protective Factors Associated With Perinatal Mental Health Outcomes
Risk and protective factors that emerged from the 81 perinatal mental health studies are summarized below.
Socio-Economic Factors
Increased depressive and anxiety symptoms during the pandemic were positively associated with the following socio-economic factors: low maternal age (68, 88, 113, 129), large hoursehold size (66, 109, 118), full-time employment status, high stress at work (81, 88, 91, 102, 129), and low income/financial strain (56, 73, 74, 94, 113, 125, 129). Regarding educational status, elevated symptoms during the pandemic were generally associated with fewer years of education in perinatal women and their partners (73, 74, 81, 109, 110); however, some studies reported a positive association with higher anxiety and more years of education (58, 111).
Psychological and Social Factors
The following psychological and social factors were positively associated with increased psychological symptoms: single mother status (129), prior history of traumatic birth or abortion (105, 115), psychiatric history prior to the pandemic (73, 74, 81, 105, 109, 114), recent stressful experiences in the past month (55) and history of abuse (68).
Perceived low social support and social isolation were associated with increased psychological symptoms (57, 61, 62, 65, 78, 96, 109, 115, 120, 129), and increased social support acted as a protective factor (71, 96). In addition, general conflict, poor family functioning and martial distress emerged as significant risk factors for perinatal anxiety during the pandemic (56, 72, 74, 81, 89, 98, 116, 117, 128). Pregnant women's own health anxiety (81, 98, 103, 117, 118, 129) and partner's perceived fear of COVID-19 (116) were understood to be contributing to pregnant women's increased anxiety and preventative behavior such as increased hygiene practices and social distancing (62, 71, 116, 134). Women strictly adhering to the rules and safety recommendations reported greater symptoms (98).
Physical and Obstetric Factors
Pregnant women with placental abnormality (88), pregnancy complication (60), high-risk pregnancy (68), and COVID-19 related symptoms (60, 105) were at risk of increased psychological symptoms. Increased psychological symptoms in the postpartum were associated with perceived poor health (their own and their baby) (117) and loss of childcare (81). Perinatal women who were obese (72) or underweight in the third trimester were more likely to experience increased depressive symptoms (88, 101). Good sleep, both in quality and duration (96, 115, 123), and exercise emerged as protective factors associated with low depressive and anxiety symptoms (75, 78, 110, 119).
Information Available on COVID-19 and Healthcare Support During the Pandemic
Updated knowledge of COVID-19 (such as knowledge of the number of cases increasing daily) appeared to increase the prevalence of depressive symptoms early in the pandemic (88, 113), and decrease depressive symptoms over time as more information became available to the general public (42) and as confirmed cases began to decrease (115). Similarly, when following perinatal women with COVID-19 over a 11-week period during the pandemic, anxiety levels peaked at the start of the outbreak and decreased as more information was published and shared with the general public about the virus (92). Women who did not have information about the effect of COVID-19 on pregancy were at higher risk of anxiety (110). Delay in or cancellation of planned informal support and reduced prenatal care were associated with an increase in depressive and anxiety symptoms (57, 81, 96, 98, 103, 129). Women who accessed prenatal care information (121) and perceived strong support from healthcare staff (67) were at significantly lower risk of psychological distress. In one study, pregnant women receiving a week of tele-education (interactive education booklets, and consultation via phone calls) were significantly less likely to present with prenatal distress and anxiety compared to a control group of pregnant women who received no intervention (126).
Risk of Bias
Scores from risk of bias and quality assessment are listed in Tables 1–9. The scores range from 3 (a moderate risk of bias) to 9 (a very low risk of bias), with the majority of studies ranging between risk of bias scores of 6–7, which are considered to present moderately to sufficiently robust and valid data. Methodological limitations identified as contributing to potential sources of bias included: (a) comparisons of the prevalence of mental health symptoms before and during the pandemic without using an interrupted time series analysis to minimize bias, (b) data collection method involving retrospective recall of symptoms prior to the pandemic, which is susceptible to recall bias, (c) convenience sampling such as online recruitment of participants, which served as a safe and effective data collection strategy during the pandemic but may have made it difficult to obtain a truly representative sample, and (d) small sample sizes given both the challenges and urgency of obtaining data during the pandemic.
Discussion
We have conducted a systematic review summarizing both obstetric and mental health outcomes in perinatal women during the COVID-19 pandemic. At the time of our search, there is still limited data on the impact of COVID-19 on perinatal mental health, and obstetrics guidance continues to be updated as new evidence emerges. Perinatal mental health studies reviewed here overall converge to point to higher rates of depressive and anxiety symptoms in perinatal women during the pandemic compared to perinatal women prior to the pandemic. Financial strain, decreased social and family support, and low education emerged as key sociodemographic factors associated with increased depression and anxiety in perinatal women, while adequate sleep, moderate physical activity, and positive social support were negatively associated with these symptoms amidst the pandemic.
Synthesis of Obstetric and Perinatal Mental Health Data
We propose that the degree to which COVID-19 increases mental health risk in perinatal women may be closely associated with their perception of risks present during the pandemic, their perceived sense of control in relation to the risk, their manner of coping in response to day-to-day stressors posed by the pandemic, and the level of support they have in their environment. Here we examine these factors in the context of the obstetric and neonatal data reviewed above to help make sense of the perinatal mental health data that have been reported during the first year of the COVID-19 pandemic.
Uncertainty, Unpredictability, and Perceived Lack of Control
The data reviewed above suggest that perinatal women have been faced with a heightened sense of uncertainty and unpredictability during the pandemic around the possibility of contracting COVID-19, as well as constantly evolving estimates of the risk of COVID-19 to their pregnancy and newborn. Changing obstetric and public health guidelines, which could substantially change how they will receive perinatal care, deliver their baby, and care for their baby in the postpartum, likely further contributed to a lack of sense of predictability and control. Perceived lack of sense of control over one's environment has long been known to exacerbate increased distress in the face of a potential threat (135) and has been linked to increased depression and anxiety in both non-perinatal and perinatal populations (136). Our findings are in line with this, demonstrating a spike in perinatal anxiety and depressive symptoms in the face of a novel virus and an eventual decrease in symptoms as more information and guidelines became available.
Adjustment Stress
The data reviewed above further suggest that COVID-19 increased the magnitude of stress with which perinatal women must cope during their transition to motherhood. The transition to motherhood can be characterized as a major physiological, psychological, and social adjustment for the mother. Successful adjustment to these changes is key to healthy perinatal adaptation, whereas adjustment difficulties contribute to perinatal depression and anxiety (137–139). During COVID-19, perinatal women have additionally had to contend with significant changes in the public's behaviors (e.g., stockpiling and quarantining), constantly evolving public health guidelines, and rapid changes in their expectations and plans about their pregnancy, while, in many cases, also managing heightened anxiety and stress of others (e.g., family members).
Lack of Social Support
Restrictions that have been put in place to mitigate the risk of COVID-19, such as quarantine, physical distancing, and telehealth appointments, appear to have had the effect of undermining practical and emotional support provided to perinatal women, which is critical in reducing the risk of depression and anxiety in new mothers (140). As was discussed above, social isolation (96, 109) and decreased social support, as well as increased tension in marital or family relationships (74, 96, 115), were found to negatively impact perinatal mental health during COVID-19. In addition, the increased demands of home life during the pandemic (caring for other children, homeschooling or remote work), complicated by reduced support available from family members or paid childcare, may further interfere with the mother's ability to meaningfully connect with her baby (e.g., skin-to-skin contact and breastfeeding), which typically contribute to the mother's sense of joy and reward despite the stress of transition.
Protecting Perinatal Mental Health
Efforts should be focused on reducing the magnitude of stress and a perceived sense of lack of control experienced by perinatal women, while increasing their capacity for coping and level of social support, as well as promoting adequate sleep and exercise. Considering the findings demonstrating that distress around COVID-19 was linked dyadically between perinatal women and their partners, preventative measures should involve partners as well as perinatal women. Empirically based interventions that help examine one's capacity for control and enhance realistic appraisal of potential risks, while targeting stress reduction and enhanced coping will be helpful for perinatal women experiencing increased distress during COVID-19. Mindfulness, distress tolerance skills, relaxation exercises, and interpersonal relationship skills can be helpful skills-based exercise for this population.
Respectful Care During Pregnancy and Postpartum
It is of paramount importance to consider the fundamental human rights of pregnant and postpartum women and their newborns when accessing health services related to pregnancy, childbirth, and postpartum care. Comprehensive guidelines and frameworks have been created by national and international organizations such as the RCOG (141) and the WHO (142) to ensure evidence-based intrapartum care, regardless of the setting or level of health care. Perinatal experts have highlighted the need for maintaining respectful care for all women and newborns, especially during the pandemic (143–145), to reduce the traumatic impact the pandemic may have on women and children. Respectful maternal and newborn care involves effectively communicating all available options to pregnant women (in a manner that is accessible to women of a wide range of language and literacy levels), allowing companion of choice to be present during childbirth for support and to advocate for pregnant women, as well as ensuring equal access to the same range, quality, and standard of care across any marginalized or underserved groups. We recognize respectful maternal and newborn care as having the potential to improve physical and mental health outcomes for perinatal women and their newborns. The quality of care provided to perinatal women during the pandemic may have influenced the mental health outcomes reported in this systematic review, and future studies will need to explore this in further detail.
Strengths and Limitations
Our systematic review evaluates the impact of COVID-19 on mental health outcomes in pregnant and postpartum women, considering current obstetric and neonate guidelines. Limitations of the study include the following. First, many of the studies reviewed here include data from the early stage of the outbreak. As the COVID-19 pandemic progresses, evolving guidelines within the medical community and the larger society may continue to influence patterns of obstetric and perinatal mental health outcomes. Second, as with all traumatic events, the pandemic will have both acute and delayed effects on mental health. Our current review concerns the first year of the pandemic and underscores its acute effects. However, we are yet to learn about its longer-term impact on perinatal mental health during the second year of the pandemic and beyond, which would be critical to study and may vary from the effects reviewed here. Third, despite the global nature of the COVID-19 pandemic, it is important to note that different countries have been impacted differently by the pandemic, especially as new variants are introduced, and vaccination efforts unfold at different pace around the world. Fourth, variability in how perinatal mental health outcomes were measured and reported limited our ability to directly compare and synthesize outcomes across studies. Finally, as we have noted above with regard to our risk of bias assessment, a small proportion of studies reviewed here were identified as having a high risk of bias, primarily due to small sample size or the use of retrospective recall of mental health symptoms. In our efforts to provide a comprehensive picture of perinatal mental health studies from the first year of the pandemic, we chose not to exclude any studies on the basis of the risk of bias scores but have included the risk of bias score in the Tables for readers' review.
Future Directions
As we enter a new chapter of the COVID-19 pandemic with the onset of new virus variants and the development of vaccines, large-scale studies and surveillance data that track the trajectory of perinatal mental health outcomes will continue to provide a more comprehensive picture of the impact of COVID-19 (146). Careful attention should be paid to methodological issues identified in the quality assessment described above (see section Risk of Bias). The COVID-19 pandemic has uncovered several inequalities and the urgent need to identify and prioritize high-risk groups of women who are more significantly and disproportionately impacted by the virus. Future studies should consider the variability present in a range of risk factors (including health disparities) and application of the framework and guidelines of respectful maternal and newborn care in measuring obstetric, neonatal, and perinatal mental health outcomes. During this second year of the pandemic, it would also be crucial that studies examine how the pandemic may have disparate effects among women and newborns in different parts of the world.
Conclusion
This systematic review examined the impact of COVID-19 on mental health outcomes in pregnant and postpartum women in the context of obstetric and neonatal data that have thus far emerged. We have underscored risk and protective factors associated with perinatal mental health. There is an evident gap in our understanding of the long-term impact of outcomes reported here, and it is imperative that further attention and research be focused on the perinatal period, arguably the most significant and sensitive time of a mother and child's life, in the face of ongoing crisis.
Author Contributions
UI, BJ, and HH conducted the systematic review, including the literature search, and analysis. UI, BJ, HH, and SK wrote the draft of the paper. SK added clinical and critical insight to the overall paper structure. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The views presented in this manuscript are those of the authors and do not necessarily reflect those of the funding agencies.
Footnotes
Funding. This work was supported by Eunice Kennedy Shriver Center, University of Massachusetts Medical School (SK), the Department of Child and Adolescent Psychiatry, King's College London Institute of Psychiatry, Psychology, and Neuroscience (UI and BJ), and a grant from the National Institute of Health KL2TR001454 (SK).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.674194/full#supplementary-material
References
- 1.World Health Organization . WHO Director-General's Opening Remarks at the Media Briefing on COVID-19- 19 June 2020 (2020). Available online at: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19--19-june-2020 (accessed April 10, 2021).
- 2.World Health Organization . Coronavirus Disease (COVID-19) in: Situation Report (2020). Available online at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200524-covid-19-sitrep-125.pdf?sfvrsn=80e7d7f0_2 (accessed April 10, 2021).
- 3.University JH . Johns Hopkins University Coronavirus Resource Center. Johns Hopkins University; (2020). Available online at: https://coronavirus.jhu.edu/ (accessed April 10, 2021). [Google Scholar]
- 4.Duan L, Zhu G. Psychological interventions for people affected by the COVID-19 epidemic. Lancet Psychiatry. (2020) 7:300–2. 10.1016/S2215-0366(20)30073-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y, Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: a web-based cross-sectional survey. Psychiatry Res. (2020) 288:112954. 10.1016/j.psychres.2020.112954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Ge J, Yang M, Feng J, Qiao M, Jiang R, et al. Vicarious traumatization in the general public, members, and non-members of medical teams aiding in COVID-19 control. Brain Behav Immun. (2020) 88:916–9. 10.1016/j.bbi.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang G, Zhang Y, Zhao J, Zhang J, Jiang F. Mitigate the effects of home confinement on children during the COVID-19 outbreak. Lancet. (2020) 395:945–7. 10.1016/S0140-6736(20)30547-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. (2010) 303:1517–25. 10.1001/jama.2010.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yudin MH. Risk management of seasonal influenza during pregnancy: current perspectives. Int J Womens Health. (2014) 6:681–9. 10.2147/IJWH.S47235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong SF, Chow KM, Leung TN, Ng WF, Ng TK, Shek CC, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. (2004) 191:292–7. 10.1016/j.ajog.2003.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullins E, Evans D, Viner RM, O'brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. (2020) 55:586–92. 10.1002/uog.22014 [DOI] [PubMed] [Google Scholar]
- 12.Yu Y, Zhang S, Wang G, Hong X, Mallow EB, Walker SO, et al. The combined association of psychosocial stress and chronic hypertension with preeclampsia. Am J Obstet Gynecol. (2013) 209:438.e1–8.e12. 10.1016/j.ajog.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orr ST, Reiter JP, Blazer DG, James SA. Maternal prenatal pregnancy-related anxiety and spontaneous preterm birth in Baltimore, Maryland. Psychosom Med. (2007) 69:566–70. 10.1097/PSY.0b013e3180cac25d [DOI] [PubMed] [Google Scholar]
- 14.Hoffman S, Hatch MC. Depressive symptomatology during pregnancy: evidence for an association with decreased fetal growth in pregnancies of lower social class women. Health Psychol. (2000) 19:535–43. 10.1037/0278-6133.19.6.535 [DOI] [PubMed] [Google Scholar]
- 15.Fakari FR, Simbar M. Coronavirus pandemic and worries during pregnancy; a letter to editor. Arch Acad Emerg Med. (2020) 8:e21. 10.22037/aaem.v8i1.598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Favre G, Pomar L, Musso D, Baud D. 2019-nCoV epidemic: what about pregnancies? Lancet. (2020) 395:e40. 10.1016/S0140-6736(20)30311-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Chen M, Wang Y, Sun L, Zhang J, Shi Y, et al. Prenatal anxiety and obstetric decisions among pregnant women in Wuhan and Chongqing during the COVID-19 outbreak: a cross-sectional study. BJOG. (2020) 127:1229–40. 10.1111/1471-0528.16381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rimmer A. Covid-19: pregnant doctors should speak to occupational health, say experts. BMJ. (2020) 368:m1104. 10.1136/bmj.m1104 [DOI] [PubMed] [Google Scholar]
- 19.Matvienko-Sikar K, Meedya S, Ravaldi C. Perinatal mental health during the COVID-19 pandemic. Women Birth. (2020) 33:309–10. 10.1016/j.wombi.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thapa SB, Mainali A, Schwank SE, Acharya G. Maternal mental health in the time of the COVID-19 pandemic. Acta Obstet Gynecol Scand. (2020) 99:817–8. 10.1111/aogs.13894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher J, Cabral De Mello M, Patel V, Rahman A, Tran T, Holton S, et al. Prevalence and determinants of common perinatal mental disorders in women in low-and lower-middle-income countries: a systematic review. Bull World Health Organ. (2012) 90:139–49. 10.2471/BLT.11.091850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry. (2017) 210:315–23. 10.1192/bjp.bp.116.187179 [DOI] [PubMed] [Google Scholar]
- 23.Fawcett EJ, Fairbrother N, Cox ML, White IR, Fawcett JM. The prevalence of anxiety disorders during pregnancy and the postpartum period: a multivariate bayesian meta-analysis. J Clin Psychiatry. (2019) 80:18r12527. 10.4088/JCP.18r12527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry. (2020) 19:313–27. 10.1002/wps.20769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Royal College of Obstetricians and Gynaecologists . Coronavirus (COVID-19) Infection in Pregnancy. RCOG; (2020). Available online at: https://www.rcog.org.uk/globalassets/documents/guidelines/2021-02-19-coronavirus-covid-19-infection-in-pregnancy-v13.pdf (accessed April 10, 2021). [Google Scholar]
- 26.Royal College of Obstetricians and Gynaecologists . Coronavirus Infection and Pregnancy. RCOG; (2020). Available online at: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/coronavirus-pregnancy/covid-19-virus-infection-and-pregnancy/ (accessed April 10, 2021). [Google Scholar]
- 27.The American College of Obstetricians and Gynecologists . Coronavirus (COVID-19), Pregnancy, and Breastfeeding: A Message for Patients. ACOG; (2020). Available online at: https://www.acog.org/womens-health/faqs/coronavirus-covid-19-pregnancy-and-breastfeeding?utm_source=redirect&utm_medium=web&utm_campaign=int (accessed April 10, 2021). [Google Scholar]
- 28.World Health Organization . Clinical Management of COVID-19 (2020). Available online at: https://www.who.int/publications/i/item/clinical-management-of-covid-19 (accessed April 10, 2021).
- 29.Center for Disease Control and Prevention . (2020). Pregnancy, Breastfeeding, and Caring for Newborns. Available online at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/pregnancy-breastfeeding.html (accessed April 10, 2021).
- 30.Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-June 7, 2020. Morb Mortal Wkly Rep. (2020) 69:769–75. 10.15585/mmwr.mm6925a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. Morb Mortal Wkly Rep. (2020) 69:1641–7. 10.15585/mmwr.mm6944e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emeruwa UN, Spiegelman J, Ona S, Kahe K, Miller RS, Fuchs KM, et al. Influence of race and ethnicity on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection rates and clinical outcomes in pregnancy. Obstet Gynecol. (2020) 136:1040–3. 10.1097/AOG.0000000000004088 [DOI] [PubMed] [Google Scholar]
- 33.Center for Disease Control and Prevention . Data on COVID-19 During Pregnancy: Birth and Infant Outcomes (2021). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/special-populations/birth-data-on-covid-19.html (accessed April 10, 2021).
- 34.Malhotra Y, Miller R, Bajaj K, Sloma A, Wieland D, Wilcox W. No change in cesarean section rate during COVID-19 pandemic in New York City. Eur J Obstet Gynecol Reprod Biol. (2020) 253:328–9. 10.1016/j.ejogrb.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization . Coronavirus Disease (COVID-19): Pregnancy and Childbirth (2020). Available online at: https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-pregnancy-and-childbirth (accessed April 10, 2021).
- 36.Khoury R, Bernstein PS, Debolt C, Stone J, Sutton DM, Simpson LL, et al. Characteristics and outcomes of 241 births to women with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at Five New York City Medical Centers. Obstet Gynecol. (2020) 136:273–82. 10.1097/AOG.0000000000004025 [DOI] [PubMed] [Google Scholar]
- 37.National Institute For Health and Care Excellence . Preterm Labour and Birth. NICE; (2019). Available online at: https://www.nice.org.uk/guidance/ng25/resources/preterm-labour-and-birth-pdf-1837333576645 (accessed April 10, 2021). [Google Scholar]
- 38.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. (2019) 7:e37–46. 10.1016/S2214-109X(18)30451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The American College of Obstetricians and Gynecologists . Novel Coronavirus 2019 (COVID-19) Practice Advisory: Summary of Key Updates. ACOG; (2020). Available online at: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019 (accessed April 10, 2021). [Google Scholar]
- 40.Yan J, Guo J, Fan C, Juan J, Yu X, Li J, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. (2020) 223:111.e4. 10.1016/j.ajog.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prabhu M, Cagino K, Matthews K, Friedlander R, Glynn S, Kubiak J, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. (2020) 127:1548–56. 10.1111/1471-0528.16403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. (2020) 369:m2107. 10.1136/bmj.m2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nayak AH, Kapote DS, Fonseca M, Chavan N, Mayekar R, Sarmalkar M, et al. Impact of the coronavirus infection in pregnancy: a preliminary study of 141 patients. J Obstet Gynecol India. (2020) 70:256–61. 10.1007/s13224-020-01335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Center for Disease Control and Prevention . Considerations for Inpatient Obstetric Healthcare Settings (2020). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/inpatient-obstetric-healthcare-guidance.html (accessed April 10, 2021).
- 45.Puopolo KM, Hudak ML, Kimberlin DW, Cummings J. American Academy of Pediatrics Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases. Initial Guidance: Management of Infants Born to Mothers With COVID-19 (2020). [Google Scholar]
- 46.Hynan MT. Covid-19 and the need for perinatal mental health professionals: now more than ever before. J Perinatol. (2020) 40:985–6. 10.1038/s41372-020-0696-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hynan MT, Steinberg Z, Baker L, Cicco R, Geller PA, Lassen S, et al. Recommendations for mental health professionals in the NICU. J Perinatol. (2015) 35(Suppl. 1):S14–8. 10.1038/jp.2015.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aftyka A, Rybojad B, Rosa W, Wróbel A, Karakuła-Juchnowicz H. Risk factors for the development of post-traumatic stress disorder and coping strategies in mothers and fathers following infant hospitalisation in the neonatal intensive care unit. J Clin Nurs. (2017) 26:4436–45. 10.1111/jocn.13773 [DOI] [PubMed] [Google Scholar]
- 49.Martins-Filho PR, Santos VS, Santos HP, Jr. To breastfeed or not to breastfeed? Lack of evidence on the presence of SARS-CoV-2 in breastmilk of pregnant women with COVID-19. Rev Panam Salud Publica. (2020) 44:e59. 10.26633/RPSP.2020.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stuebe A. Should infants be separated from mothers with COVID-19? First, Do No Harm. Breastfeed Med. (2020) 15:351–2. 10.1089/bfm.2020.29153.ams [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Topalidou A, Thomson G, Downe S. COVID-19 and maternal mental health: are we getting the balance right? medRxiv. (2020) 2020:0702. 10.1101/2020.03.30.20047969 [DOI] [Google Scholar]
- 52.Wang L, Shi Y, Xiao T, Fu J, Feng X, Mu D, et al. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (First edition). Ann Transl Med. (2020) 8:47. 10.21037/atm.2020.02.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wells GA, Shea B, O'Connell DA, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. The Ottawa Hospital; (2000). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed April 10, 2021). [Google Scholar]
- 55.Ceulemans M, Foulon V, Ngo E, Panchaud A, Winterfeld U, Pomar L, et al. Mental health status of pregnant and breastfeeding women during the COVID-19 pandemic - a multinational cross-sectional study. Acta Obstet Gynecol Scand. (2021) 21:21. 10.1111/aogs.14092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Effati-Daryani F, Zarei S, Mohammadi A, Hemmati E, Ghasemi Yngyknd S, Mirghafourvand M. Depression, stress, anxiety and their predictors in Iranian pregnant women during the outbreak of COVID-19. BMC Psychol. (2020) 8:99. 10.1186/s40359-020-00464-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farewell CV, Jewell J, Walls J, Leiferman JA. A mixed-methods pilot study of perinatal risk and resilience during COVID-19. J Prim Care Commun Health. (2020) 11:2150132720944074. 10.1177/2150132720944074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farrell T, Reagu S, Mohan S, Elmidany R, Qaddoura F, Ahmed EE, et al. The impact of the COVID-19 pandemic on the perinatal mental health of women. J Perinatal Med. (2020) 48:971–6. 10.1515/jpm-2020-0415 [DOI] [PubMed] [Google Scholar]
- 59.He Z, Chiu WT, Wu H, Ming WK. PMH4 the psychological and behavioral responses to Covid-19 epidemic in pregnant women in China: a nationwide survey. Value Health RegIssues. (2020) 22:S62. 10.1016/j.vhri.2020.07.326 [DOI] [Google Scholar]
- 60.Hocaoglu M, Ayaz R, Gunay T, Akin E, Turgut A, Karateke A. Anxiety and post-traumatic stress disorder symptoms in pregnant women during the COVID-19 pandemic's delay phase. Psychiatr Danub. (2020) 32:521–6. 10.24869/psyd.2020.521 [DOI] [PubMed] [Google Scholar]
- 61.Kassaw C, Pandey D. The prevalence of general anxiety disorder and its associated factors among women's attending at the perinatal service of Dilla University referral hospital, Dilla town, Ethiopia, April, 2020 in Covid pandemic. Heliyon. (2020) 6:e05593. 10.1016/j.heliyon.2020.e05593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liang P, Wang Y, Shi S, Liu Y, Xiong R. Prevalence and factors associated with postpartum depression during the COVID-19 pandemic among women in Guangzhou, China: a cross-sectional study. BMC Psychiatry. (2020) 20:557. 10.1186/s12888-020-02969-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lubian Lopez DM, Butron Hinojo CA, Arjona Bernal Santiago JE, Fasero Laiz M, Alcolea J, Guerra Vilches V, et al. Mood disorders and resilience during the first COVID-19 pandemic wave in Spain: Conclusions of the first Spanish survey. J Psychosom Res. (2021) 140:110327. 10.1016/j.jpsychores.2020.110327 [DOI] [PubMed] [Google Scholar]
- 64.Medina-Jimenez V, Bermudez-Rojas ML, Murillo-Bargas H, Rivera-Camarillo AC, Munoz-Acosta J, Ramirez-Abarca TG, et al. The impact of the COVID-19 pandemic on depression and stress levels in pregnant women: a national survey during the COVID-19 pandemic in Mexico. J Matern Fetal Neonatal Med. (2020) 1–3. 10.1080/14767058.2020.1851675 [DOI] [PubMed] [Google Scholar]
- 65.Molgora S, Accordini M. Motherhood in the time of coronavirus: the impact of the pandemic emergency on expectant and postpartum women's psychological well-being. Front Psychol. (2020) 11:567155. 10.3389/fpsyg.2020.567155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ng QJ, Koh KM, Tagore S, Mathur M. Perception and feelings of antenatal women during COVID-19 pandemic: a cross-sectional survey. Ann Acad Med Singap. (2020) 49:543–52. 10.47102/annals-acadmedsg.2020295 [DOI] [PubMed] [Google Scholar]
- 67.Ostacoli L, Cosma S, Bevilacqua F, Berchialla P, Bovetti M, Carosso AR, et al. Psychosocial factors associated with postpartum psychological distress during the Covid-19 pandemic: a cross-sectional study. BMC Pregnancy & Childbirth. (2020) 20:703. 10.1186/s12884-020-03399-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Preis H, Mahaffey B, Heiselman C, Lobel M. Pandemic-related pregnancy stress and anxiety among women pregnant during the coronavirus disease 2019 pandemic. Am J Obstet Gynecol MFM. (2020) 2:100155. 10.1016/j.ajogmf.2020.100155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ravaldi C, Vannacci A. The COVID-ASSESS dataset-COVID19 related anxiety and stress in prEgnancy, poSt-partum and breaStfeeding during lockdown in Italy. Data Brief. (2020) 33:106440. 10.1016/j.dib.2020.106440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang X, Song B, Wu A, Mo PKH, Di J, Wang Q, et al. Social, cognitive, and eHealth mechanisms of COVID-19-related lockdown and mandatory quarantine that potentially affect the mental health of pregnant women in China: cross-sectional survey study. J Med Intern Res. (2021) 23:e24495. 10.2196/24495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yue C, Liu C, Wang J, Zhang M, Wu H, Li C, et al. Association between social support and anxiety among pregnant women in the third trimester during the coronavirus disease 2019 (COVID-19) epidemic in Qingdao, China: the mediating effect of risk perception. Int J Soc Psychiatry. (2020). 10.1177/0020764020941567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ayaz R, Hocaoglu M, Gunay T, Yardlmcl OD, Turgut A, Karateke A. Anxiety and depression symptoms in the same pregnant women before and during the COVID-19 pandemic. J Perinatal Med. (2020) 48:965–70. 10.1515/jpm-2020-0380 [DOI] [PubMed] [Google Scholar]
- 73.Berthelot N, Lemieux R, Garon-Bissonnette J, Drouin-Maziade C, Martel É, Maziade M. Uptrend in distress and psychiatric symptomatology in pregnant women during the coronavirus disease 2019 pandemic. Acta Obstet Gynecol Scand. (2020) 99:848–55. 10.1111/aogs.13925 [DOI] [PubMed] [Google Scholar]
- 74.Cameron EE, Joyce KM, Delaquis CP, Reynolds K, Protudjer JLP, Roos LE. Maternal psychological distress & mental health service use during the COVID-19 pandemic. J Affect Disord. (2020) 276:765–74. 10.1016/j.jad.2020.07.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davenport MH, Meyer S, Meah VL, Strynadka MC, Khurana R. Moms are not ok: COVID-19 and maternal mental health. Front Glob Women's Health. (2020) 1:1. 10.3389/fgwh.2020.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hui PW, Ma G, Seto MTY, Cheung KW. Effect of COVID-19 on delivery plans and postnatal depression scores of pregnant women. Hong Kong Med J. (2020) 27:113–7. 10.12809/hkmj208774 [DOI] [PubMed] [Google Scholar]
- 77.Loret De Mola C, Blumenberg C, Martins RC, Martins-Silva T, Carpena MX, Del-Ponte B, et al. Increased depression and anxiety during the COVID-19 pandemic in Brazilian mothers: a longitudinal study. Rev Brasil Psiquiatr. (2021) 11:11. 10.1590/1516-4446-2020-1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matvienko-Sikar K, Pope J, Cremin A, Carr H, Leitao S, Olander EK, et al. Differences in levels of stress, social support, health behaviours, and stress-reduction strategies for women pregnant before and during the COVID-19 pandemic, and based on phases of pandemic restrictions, in Ireland. Women Birth J Aust Coll Midwives. (2020) 23:23. 10.1016/j.wombi.2020.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mayopoulos GA, Ein-Dor T, Dishy GA, Nandru R, Chan SJ, Hanley LE, et al. COVID-19 is associated with traumatic childbirth and subsequent mother-infant bonding problems. J Affect Disord. (2020) 282:122–5. 10.1016/j.jad.2020.12.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mcfarland MJ, Mcfarland CAS, Hill TD, D'oria R. Postpartum depressive symptoms during the beginning of the COVID-19 pandemic: an examination of population birth data from central New Jersey. Matern Child Health J. (2021) 25:25. 10.1007/s10995-020-03116-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moyer CA, Compton SD, Kaselitz E, Muzik M. Pregnancy-related anxiety during COVID-19: a nationwide survey of 2740 pregnant women. Arch Women's Ment Health. (2020) 29:29. 10.1007/s00737-020-01073-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pariente G, Wissotzky Broder O, Sheiner E, Lanxner Battat T, Mazor E, Yaniv Salem S, et al. Risk for probable post-partum depression among women during the COVID-19 pandemic. Arch Women's Ment Health. (2020) 23:767–3. 10.1007/s00737-020-01075-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sade S, Sheiner E, Wainstock T, Hermon N, Yaniv Salem S, Kosef T, et al. Risk for depressive symptoms among hospitalized women in high-risk pregnancy units during the COVID-19 Pandemic. J Clin Med. (2020) 9:31. 10.3390/jcm9082449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Silverman ME, Burgos L, Rodriguez ZI, Afzal O, Kalishman A, Callipari F, et al. Postpartum mood among universally screened high and low socioeconomic status patients during COVID-19 social restrictions in New York City. Sci Rep. (2020) 10:22380. 10.1038/s41598-020-79564-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Silverman ME, Medeiros C, Burgos L. Early pregnancy mood before and during COVID-19 community restrictions among women of low socioeconomic status in New York City: a preliminary study. Arch Women's MentHealth. (2020) 25:25. 10.1007/s00737-020-01061-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sinaci S, Ozden Tokalioglu E, Ocal D, Atalay A, Yilmaz G, Keskin HL, et al. Does having a high-risk pregnancy influence anxiety level during the COVID-19 pandemic? Eur J Obstet Gynecol Reprod Biol. (2020) 255:190–6. 10.1016/j.ejogrb.2020.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suzuki S. Psychological status of postpartum women under the COVID-19 pandemic in Japan. J Matern Fetal Neonatal Med. (2020) 1–3. 10.1080/14767058.2020.1763949 [DOI] [PubMed] [Google Scholar]
- 88.Wu YT, Zhang C, Liu H, Duan CC, Li C, Fan J, et al. Perinatal depression of women along with 2019 novel coronavirus breakout in China. Am J Obstet Gynecol. (2020) 223:240.e1–9. 10.1016/j.ajog.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xie M, Wang X, Zhang J, Wang Y. Alteration in the psychologic status and family environment of pregnant women before and during the Covid-19 pandemic. Int J Gynaecol Obstet. (2021) 5:13575. 10.1002/ijgo.13575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zanardo V, Manghina V, Giliberti L, Vettore M, Severino L, Straface G. Psychological impact of COVID-19 quarantine measures in northeastern Italy on mothers in the immediate postpartum period. Int J Gynecol Obstet. (2020) 150:184–8. 10.1002/ijgo.13249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bender WR, Srinivas S, Coutifaris P, Acker A, Hirshberg A. The psychological experience of obstetric patients and health care workers after implementation of universal SARS-CoV-2 testing. Am J Perinatol. (2020) 37:1271–9. 10.1055/s-0040-1715505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kotabagi P, Fortune L, Essien S, Nauta M, Yoong W. Anxiety and depression levels among pregnant women with COVID-19. Acta Obstet Gynecol Scand. (2020) 99:953–4. 10.1111/aogs.13928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lopez-Morales H, Del Valle MV, Canet-Juric L, Andres ML, Galli JI, Poo F, et al. Mental health of pregnant women during the COVID-19 pandemic: a longitudinal study. Psychiatry Res. (2021) 295:113567. 10.1016/j.psychres.2020.113567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yassa M, Yassa A, Yirmibes C, Birol P, Unlu UG, Tekin AB, et al. Anxiety levels and obsessive compulsion symptoms of pregnant women during the COVID-19 pandemic. Turk J Obstet Gynecol. (2020) 17:155–60. 10.4274/tjod.galenos.2020.91455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou Y, Shi H, Liu Z, Peng S, Wang R, Qi L, et al. The prevalence of psychiatric symptoms of pregnant and non-pregnant women during the COVID-19 epidemic. Transl Psychiatry. (2020) 10:319. 10.1038/s41398-020-01006-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lebel C, Mackinnon A, Bagshawe M, Tomfohr-Madsen L, Giesbrecht G. Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. J Affect Disord. (2020) 277:5–13. 10.1016/j.jad.2020.07.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saccone G, Florio A, Aiello F, Venturella R, De Angelis MC, Locci M, et al. Psychological impact of coronavirus disease 2019 in pregnant women. Am J Obstet Gynecol. (2020) 223:293–5. 10.1016/j.ajog.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shayganfard M, Mahdavi F, Haghighi M, Sadeghi Bahmani D, Brand S. Health anxiety predicts postponing or cancelling routine medical health care appointments among women in perinatal stage during the Covid-19 lockdown. Int J Environ Res Public Health. (2020) 17:9. 10.3390/ijerph17218272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stepowicz A, Wencka B, Bienkiewicz J, Horzelski W, Grzesiak M. Stress and anxiety levels in pregnant and post-partum women during the COVID-19 pandemic. Int J Environ Res Public Health. (2020) 17:17. 10.3390/ijerph17249450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Y, Chen L, Wu T, Shi H, Li Q, Jiang H, et al. Impact of Covid-19 in pregnancy on mother's psychological status and infant's neurobehavioral development: a longitudinal cohort study in China. BMC Med. (2020) 18:347. 10.1186/s12916-020-01825-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zeng X, Li W, Sun H, Luo X, Garg S, Liu T, et al. Mental health outcomes in perinatal women during the remission phase of COVID-19 in China. Front Psychiatry Front Res Found. (2020) 11:571876. 10.3389/fpsyt.2020.571876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Y, Ma ZF. Psychological responses and lifestyle changes among pregnant women with respect to the early stages of COVID-19 pandemic. Int J Soc Psychiatry. (2020). 10.1177/0020764020952116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bo HX, Yang Y, Chen J, Zhang M, Zhang DY, Li Y, et al. Prevalence of depressive symptoms among Chinese pregnant and postpartum women during the COVID-19 pandemic. Psychosom Med. (2020) 85:345–50. 10.1097/PSY.0000000000000904 [DOI] [PubMed] [Google Scholar]
- 104.Dong H, Hu R, Lu C, Huang D, Cui D, Huang G, et al. Investigation on the mental health status of pregnant women in China during the Pandemic of COVID-19. Arch Gynecol Obstet. (2020) 3:3. 10.1007/s00404-020-05805-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spinola O, Liotti M, Speranza AM, Tambelli R. Effects of COVID-19 epidemic lockdown on postpartum depressive symptoms in a sample of Italian mothers. Front Psychiatry. (2020) 11:589916. 10.3389/fpsyt.2020.589916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Taubman – Ben-Ari O, Chasson M, Abu Sharkia S, Weiss E. Distress and anxiety associated with COVID-19 among Jewish and Arab pregnant women in Israel. J Reprod Infant Psychol. (2020) 38:340–8. 10.1080/02646838.2020.1786037 [DOI] [PubMed] [Google Scholar]
- 107.Zhang J, Zhang Y, Huo S, Ma Y, Ke Y, Wang P, et al. Emotional eating in pregnant women during the COVID-19 pandemic and its association with dietary intake and gestational weight Gain. Nutrients. (2020) 12:28. 10.3390/nu12082250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dagklis T, Tsakiridis I, Mamopoulos A, Athanasiadis A, Pearson R, Papazisis G. Impact of the COVID-19 lockdown on antenatal mental health in Greece. Psychiatry Clin Neurosci. (2020) 74:616–7. 10.1111/pcn.13135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Durankuş F, Aksu E. Effects of the COVID-19 pandemic on anxiety and depressive symptoms in pregnant women: a preliminary study. J Matern Fetal Neonatal Med. (2020) 1–7. 10.1080/14767058.2020.1763946 [DOI] [PubMed] [Google Scholar]
- 110.Kahyaoglu Sut H, Kucukkaya B. Anxiety, depression, and related factors in pregnant women during the COVID-19 pandemic in Turkey: a web-based cross-sectional study. Perspect Psychiatr Care. (2020) 57:860–8. 10.1111/ppc.12627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mappa I, Distefano FA, Rizzo G. Effects of coronavirus 19 pandemic on maternal anxiety during pregnancy: a prospectic observational study. J Perinatal Med. (2020) 48:545–50. 10.1515/jpm-2020-0182 [DOI] [PubMed] [Google Scholar]
- 112.Oskovi-Kaplan ZA, Buyuk GN, Ozgu-Erdinc AS, Keskin HL, Ozbas A, Moraloglu Tekin O. The effect of COVID-19 pandemic and social restrictions on depression rates and maternal attachment in immediate postpartum women: a preliminary study. Psychiatr Q. (2020) 4:4. 10.1007/s11126-020-09843-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Patabendige M, Gamage MM, Weerasinghe M, Jayawardane A. Psychological impact of the COVID-19 pandemic among pregnant women in Sri Lanka. Int J Gynaecol Obstet. (2020) 30:30. 10.1002/ijgo.13335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ravaldi C, Ricca V, Wilson A, Homer C, Vannacci A. Previous psychopathology predicted severe COVID-19 concern, anxiety, and PTSD symptoms in pregnant women during “lockdown” in Italy. Arch Women's Ment Health. (2020) 20:20. 10.1007/s00737-020-01086-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun G, Wang F, Cheng Y. Perinatal depression during the COVID-19 epidemic in Wuhan, China. Lancet. (2020). 10.2139/ssrn.3576929 [DOI] [Google Scholar]
- 116.Ahorsu DK, Imani V, Lin CY, Timpka T, Broström A, Updegraff JA, et al. Associations between fear of COVID-19, mental health, and preventive behaviours across pregnant women and husbands: an actor-partner interdependence modelling. Int J Ment Health Addict. (2020) 45:42–6. 10.1007/s11469-020-00340-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chaves C, Marchena C, Palacios B, Salgado A, Duque A. Effects of the COVID-19 pandemic on perinatal mental health in Spain: Positive and negative outcomes. Women Birth J Aust Coll Midwives. (2021) 15:15. 10.1016/j.wombi.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ding W, Lu J, Zhou Y, Wei W, Zhou Z, Chen M. Knowledge, attitudes, practices, and influencing factors of anxiety among pregnant women in Wuhan during the outbreak of COVID-19: a cross-sectional study. BMC Pregnancy Childbirth. (2021) 21:80. 10.1186/s12884-021-03561-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gildner TE, Laugier EJ, Thayer ZM. Exercise routine change is associated with prenatal depression scores during the COVID-19 pandemic among pregnant women across the United States. PLoS ONE. (2020) 15:e0243188. 10.1371/journal.pone.0243188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Harrison V, Moulds ML, Jones K. Perceived social support and prenatal wellbeing; The mediating effects of loneliness and repetitive negative thinking on anxiety and depression during the COVID-19 pandemic. Women Birth. (2021). 10.1016/j.wombi.2020.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jiang H, Jin L, Qian X, Xiong X, La X, Chen W, et al. Maternal mental health status and approaches for accessing antenatal care information during the COVID-19 epidemic in China: cross-sectional study. J Med Intern Res. (2021) 23:e18722. 10.2196/18722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kachi Y, Fujiwara T, Eguchi H, Inoue A, Baba S, Ohta H, et al. Association between maternity harassment and depression during pregnancy amid the COVID-19 state of emergency. J OccupHealth. (2021) 63:e12196. 10.1002/1348-9585.12196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lin W, Wu B, Chen B, Lai G, Huang S, Li S, et al. Sleep conditions associate with anxiety and depression symptoms among pregnant women during the epidemic of COVID-19 in Shenzhen. J Affect Disord. (2021) 281:567–73. 10.1016/j.jad.2020.11.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shahid A, Javed A, Rehman S, Tariq R, Ikram M, Suhail M. Evaluation of psychological impact, depression, and anxiety among pregnant women during the COVID-19 pandemic in Lahore, Pakistan. Int J Gynecol Obstet. (2020) 151:462–5. 10.1002/ijgo.13398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thayer ZM, Gildner TE. COVID-19-related financial stress associated with higher likelihood of depression among pregnant women living in the United States. Am J Human Biol. (2020) 33:e23508. 10.1002/ajhb.23508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aksoy Derya Y, Altiparmak S, Akca E, N GO, Yilmaz AN. Pregnancy and birth planning during COVID-19: The effects of tele-education offered to pregnant women on prenatal distress and pregnancy-related anxiety. Midwifery. (2021) 92:102877. 10.1016/j.midw.2020.102877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gamache D, Savard C, Lemieux R, Berthelot N. Impact of level of personality pathology on affective, behavioral, and thought problems in pregnant women during the coronavirus disease 2019 pandemic. Pers Disord Theory Res Treat. (2021) 7:7. 10.1037/per0000479 [DOI] [PubMed] [Google Scholar]
- 128.Guo J, De Carli P, Lodder P, Bakermans-Kranenburg MJ, Riem MME. Maternal mental health during the COVID-19 lockdown in China, Italy, and the Netherlands: a cross-validation study. Psychol Med. (2021) 1–11. 10.1017/S0033291720005504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Matsushima M, Horiguchi H. The COVID-19 pandemic and mental well-being of pregnant women in Japan: need for economic and social policy interventions. Disaster Med Public Health Prep. (2020) 1–11. 10.1017/dmp.2020.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Salehi L, Rahimzadeh M, Molaei E, Zaheri H, Esmaelzadeh-Saeieh S. The relationship among fear and anxiety of COVID-19, pregnancy experience, and mental health disorder in pregnant women: a structural equation model. Brain Behav. (2020) 10:e01835. 10.1002/brb3.1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zheng QX, Jiang XM, Lin Y, Liu GH, Lin YP, Kang YL, et al. The influence of psychological response and security sense on pregnancy stress during the outbreak of coronavirus disease 2019: a mediating model. J Clin Nurs. (2020) 9:4248–57. 10.1111/jocn.15460 [DOI] [PubMed] [Google Scholar]
- 132.Liu CH, Erdei C, Mittal L. Risk factors for depression, anxiety, and PTSD symptoms in perinatal women during the COVID-19 Pandemic. Psychiatry Res. (2021) 295:113552. 10.1016/j.psychres.2020.113552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang CJP, Wu H, He Z, Chan NK, Huang J, Wang H, et al. Psychobehavioral responses, post-traumatic stress and depression in pregnancy during the early phase of COVID-19 outbreak. Psychiatr Res Clin Pract. (2021) 3:46–54. 10.1176/appi.prcp.20200019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yue J, Zang X, Le Y, An Y. Anxiety, depression and PTSD among children and their parent during 2019 novel coronavirus disease (COVID-19) outbreak in China. Curr Psychol. (2020) 1–8. 10.1007/s12144-020-01191-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Craske MG, Rapee RM, Jackel L, Barlow DH. Qualitative dimensions of worry in DSM-III-R generalized anxiety disorder subjects and nonanxious controls. Behav Res Ther. (1989) 27:397–402. 10.1016/0005-7967(89)90010-7 [DOI] [PubMed] [Google Scholar]
- 136.Keeton CP, Perry-Jenkins M, Sayer AG. Sense of control predicts depressive and anxious symptoms across the transition to parenthood. J Fam Psychol. (2008) 22:212–21. 10.1037/0893-3200.22.2.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Matthey S, Barnett B, Ungerer J, Waters B. Paternal and maternal depressed mood during the transition to parenthood. J Affect Disord. (2000) 60:75–85. 10.1016/S0165-0327(99)00159-7 [DOI] [PubMed] [Google Scholar]
- 138.Simpson JA, Rholes WS, Campbell L, Tran S, Wilson CL. Adult attachment, the transition to parenthood, and depressive symptoms. J Pers Soc Psychol. (2003) 84:1172–87. 10.1037/0022-3514.84.6.1172 [DOI] [PubMed] [Google Scholar]
- 139.Salmela-Aro K. Transition to parenthood and positive parenting: Longitudinal and intervention approaches. Eur J Dev Psychol. (2012) 9:21–32. 10.1080/17405629.2011.607584 [DOI] [Google Scholar]
- 140.Baker B, Yang I. Social media as social support in pregnancy and the postpartum. Sex Reprod Healthc. (2018) 17:31–4. 10.1016/j.srhc.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 141.Royal College of Obstetricians and Gynaecologists . Providing Quality Care for Women. A Framework for Maternity Service Standards (2016). Available online at: https://www.rcog.org.uk/globalassets/documents/guidelines/working-party-reports/maternitystandards.pdf (accessed April 10, 2021).
- 142.World Health Organization . WHO Recommendations on Intrapartum Care for a Positive Childbirth Experience. World Health Organization; (2018). Available online at: https://www.who.int/reproductivehealth/publications/intrapartum-care-guidelines/en/ (accessed April 10, 2021). [PubMed] [Google Scholar]
- 143.Reingold RB, Barbosa I, Mishori R. Respectful maternity care in the context of COVID-19: a human rights perspective. Int J Gynecol Obstet. (2020) 151:319–21. 10.1002/ijgo.13376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jolivet RR, Warren CE, Sripad P, Ateva E, Gausman J, Mitchell K, et al. Upholding Rights Under COVID-19: the respectful maternity care charter. Health Human Rights. (2020) 22:391–4. [PMC free article] [PubMed] [Google Scholar]
- 145.Sacks E, Sripad P, Ndwiga C, Waiswa P, Warren CE. Protecting newborn infants during the COVID-19 pandemic should be based on evidence and equity. Acta Paediatr. (2020) 109:2448–50. 10.1111/apa.15568 [DOI] [PubMed] [Google Scholar]
- 146.Buekens P, Alger J, Bréart G, Cafferata ML, Harville E, Tomasso G. A call for action for COVID-19 surveillance and research during pregnancy. Lancet Glob Health. (2020) 8:e877–8. 10.1016/S2214-109X(20)30206-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.