Introduction
The current global health crisis is caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), a novel epidemic strain of Betacoronavirus that is responsible for the viral pandemic, coronavirus disease 2019 (COVID-19).
There are at present around 200 SARS-CoV-2 candidate vaccines in preclinical and clinical trials throughout the world. Within less than a year time some very promising COVID-19 vaccines were developed at an unprecedented speed and are now being deployed via emergency use authorization for immunization [1], [2].
Allogenic tissue or solid organ transplantations introduce genetically different tissues to the recipient, which typically causes a T cell mediated immune response that leads to rejection and destruction of the transplant [3]. Corneal transplantation has a low graft rejection rate because of ocular immune privilege, which can be compromised by increased immune dysregulation [4]. The risk of developing an immune reaction after Descemet membrane endothelial keratoplasty (DMEK) is considered to be very low and it has been reported to be from 0.5 to 5.3% [5], [6]. If diagnosed early, corneal endothelial transplant rejection is potentially reversible, albeit with endothelial cell loss [7].
To our knowledge, we report the first case of acute endothelial graft rejection after Descemet membrane endothelial keratoplasty (DMEK) occurring after the first dose of the BNT162b2 mRNA SARS-CoV-2 (BioNTech/Pfizer) vaccination.
Case summary
A 71-year-old male patient with a history of high blood pressure, smoking, coronary artery disease and endothelial decompensation after phacoemulsification underwent DMEK of the right eye. Surgery was uneventful and was followed by an uneventful postoperative course. His best corrected visual acuity (BCVA) two months after DMEK was 20/30+ and remained stable on further visits.
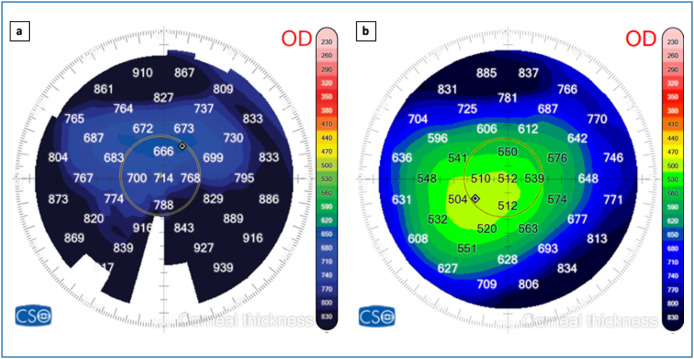
Five months after surgery, the patient presented to the eye clinic for a sudden painless decrease of vision in the right eye that started on the morning of his presentation to our department. BCVA was 20/125 in the right eye with no improvement with pinhole. The examination revealed 1+ conjunctival injection and diffuse corneal edema (Fig. 1a and b). The central pachymetry was 714 μm (Fig. 2a). No neovascularization of the host or donor cornea was noted. On further questioning it was revealed that he received his first dose of the BNT162b2 mRNA SARS-CoV-2 (BioNTech/Pfizer) 7 days ago.
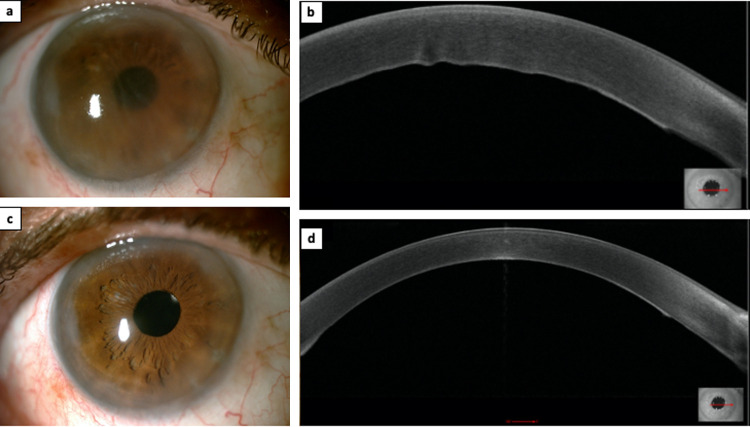
Figure 1.
This figure shows slit lamp photos and optical coherence tomography (OCT) scans of the cornea of the patient (MS-39, CSO, Italy): (a) slit lamp photo taken on the day of presentation, showing a diffuse corneal edema; (b) OCT scan of the cornea obtained on the day of presentation, showing a corneal edema associated with Descemetic folds; (c) slit lamp photo taken one week after initiation of the treatment, showing a clear cornea; (d) OCT scan of the cornea obtained one week after initiation of the treatment, showing a clear cornea and disappearance of the Descemetic folds.
Figure 2.
Anterior Segment OCT (MS-39, CSO, Italy) pachymetric maps showing: (a) a diffuse corneal edema during the rejection episode (central pachymetry = 714 μm) and (b) a thinner central cornea 7 days after steroids treatment (central pachymetry = 512 μm).
The diagnosis of acute endothelial rejection was made and the patient was administered topical Dexamethasone sodium phosphate 1 mg/mL every two hours (Dexafree; Laboratoires Théa, Clermont-Ferrand, France) and oral valacyclovir 1000 mg TID.
Given a previous report of acute corneal endothelial graft rejection with coinciding COVID-19 infection, we decided to rule out asymptomatic disease even though the patient had no chills, sore throat, cough, fever or shortness of breath. A nasopharyngeal swab and blood sample were obtained and the patient tested negative for SARS-CoV-2 through polymerase chain reaction and immunoglobulin M and G testing.
One week after initiation of topical and oral treatment, the patient was reevaluated. BCVA improved to 20/25 and examination revealed a clear cornea with full resorption of the edema (Fig. 1c and 1d). The central pachymetry decreased to 512 μm (Fig. 2b).
We discussed with the patient the possibility of an association between the first dose of vaccination and the acute transplant rejection. Given his history of cardiovascular risk factors, it was decided to move forward with the second dose of the vaccine while keeping him on topical steroids q2 h.
The patient received his second dose. The graft remained clear and the visual acuity remained stable three weeks after the second dose. It was decided at that point to taper the topical steroids progressively over four weeks to reach a maintenance dose of twice a day.
Discussion
Corneal transplantation is the oldest, most common, and arguably the most successful form of solid tissue transplantation [8]. Absence of blood and lymphatic vessels in the central cornea gives it [lymph]angiogenic privilege [3] and consequently low rate of graft rejection.
However, any systemic immune dysregulation may compromise corneal ocular immune privilege and increase the patient's susceptibility for rejection. In some COVID-19 patients symptoms gradually worsen within a week or more after developing symptoms, suggesting that severe COVID-19 pathogenesis could be mediated by a dysregulated immune réponse [9]. Moreover, it was confirmed that inflammation in these patients is characterized by increased tumor necrosis factor–α (TNF–α) and interleukin-6 production [10].
Additionally, it was shown that following infection, surgery or trauma, cells of the innate immune system invade the cornea and result in the up regulation of cytokines, including TNF–α, chemokines, and other pro-inflammatory molecules which can result in rejection of the corneal transplants [11].
There is a report about acute corneal endothelial graft rejection with coinciding COVID-19 infection [4]; however, to our knowledge, ours is the first reported case of rejection occurring in a DMEK after SARS-CoV-2 vaccination. DMEK rejection can occur during the first critical year [12], [13]. In our patient, the fact that the DMEK was stable prior and had not had previous rejection episodes, and that the rejection occurred 1 week after immunization could point to the vaccine as the causative agent. There are scarce reports about cornea graft rejection after influenza and hepatitis vaccination [14], [15], but vaccine-related rejection may be more frequent than reported.
To conclude, SARS-CoV-2 vaccination might disrupt immune regulation enough to cause cornea transplant rejection. Still, there are currently no guidelines regarding either the use of vaccines in patients with tissue transplants, or for the increase of anti-rejection prophylaxis before or after vaccination. Ophthalmologists should be alert, and patients need to be educated to follow up immediately if they notice any changes like discomfort, redness or blurred vision. Topical treatment with corticosteroids before and after vaccine administration may prevent graft rejection.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Tregoning J.S., Brown E.S., Cheeseman H.M., et al. Vaccines for COVID-19. Clin Exp Immunol. 2020;202:162–192. doi: 10.1111/cei.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh R., Kang A., Luo X., et al. COVID-19: current knowledge in clinical features, immunological responses, and vaccine development. FASEB J. 2021;35:e21409. doi: 10.1096/fj.202002662R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hos D., Matthaei M., Bock F., et al. Immune reactions after modern lamellar (DALK, DSAEK, DMEK) versus conventional penetrating corneal transplantation. Prog Retin Eye Res. 2019;73:100768. doi: 10.1016/j.preteyeres.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Jin S.X., Juthani V.V. Acute corneal endothelial graft rejection with coinciding COVID-19 infection. Cornea. 2021;40:123–124. doi: 10.1097/ICO.0000000000002556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anshu A., Price M.O., Tan D.T.H., Price F.W. Endothelial keratoplasty: a revolution in evolution. Surv Ophthalmol. 2012;57:236–252. doi: 10.1016/j.survophthal.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Guerra F.P., Anshu A., Price M.O., Giebel A.W., Price F.W. Descemet's membrane endothelial keratoplasty. Ophthalmology. 2011;118:2368–2373. doi: 10.1016/j.ophtha.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Wertheim M.S., Keel M., Cook S.D., Tole D.M. Corneal transplant rejection following influenza vaccination. Br J Ophthalmol. 2006;90 doi: 10.1136/bjo.2006.093187. [925-925] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niederkorn J.Y., Ligocki A.J., Ratcliffe M.J.H. Encyclopedia of immunobiology. Academic Press; Amsterdam: 2016. Immunology of the Eye; pp. 23–29. [Google Scholar]
- 9.McKechnie J.L., Blish C.A. The innate immune system: fighting on the front lines or fanning the flames of COVID-19? Cell Host Microbe. 2020;27:863–869. doi: 10.1016/j.chom.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadjadj J., Yatim N., Barnabei L., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar V., Kumar A. Immunological aspects of corneal transplant. Immunol Investig. 2014;43:888–901. doi: 10.3109/08820139.2014.910024. [DOI] [PubMed] [Google Scholar]
- 12.Anshu A., Price M.O., Price F.W. Risk of corneal transplant rejection significantly reduced with Descemet's membrane endothelial keratoplasty. Ophthalmology. 2012;119:536–540. doi: 10.1016/j.ophtha.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Dapena I., Ham L., Netuková M., van der Wees J., Melles G.R.J. Incidence of early allograft rejection after Descemet membrane endothelial keratoplasty. Cornea. 2011;30:1341–1345. doi: 10.1097/ICO.0b013e31820d8540. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton A., Massera R., Maloof A. Stromal rejection in a deep anterior lamellar keratoplasty following influenza vaccination. Clin Experiment Ophthalmol. 2015;43:838–839. doi: 10.1111/ceo.12560. [DOI] [PubMed] [Google Scholar]
- 15.Solomon A., Frucht-Pery J. Bilateral simultaneous corneal graft rejection after influenza vaccination. Am J Ophthalmol. 1996;121:708–709. doi: 10.1016/s0002-9394(14)70638-5. [DOI] [PubMed] [Google Scholar]