Abstract
Background
Stroke is the primary cause of disability worldwide, the second most common cause of dementia and the third leading cause of death. Only few studies were conducted to study the role of fluoxetine in motor recovery in either ischemic or hemorrhagic stroke patients with probably less severe paresis. However, the current study evaluates both the effectiveness and safety of fluoxetine in the stroke population with a more severe motor deficit.
Methods
Patients who had acute or subacute stroke with hemiparesis and aged between 18 and 80 years with medical research council (MRC) scale score <4 were included in this randomized, Single-blind, placebo-controlled trial in 1:1 ratio to placebo or fluoxetine 20 mg/day orally for 90 days. The primary outcome measures were changes in barthel index, time taken to complete nine hole peg test and number of hand tapping movements in 30 s by the affected limb between baseline, 45th day and 90th day. The secondary outcome measure was evaluation of the drug tolerability.
Results
A total of 168 patients were assigned to fluoxetine (n = 84) or placebo (n = 84) group. Mean BI score significantly improved at 90th day in fluoxetine group (70.42 ± 10.56) than in placebo group (44.23 ± 8.52). Mean dexterity value decreased significantly at 90th day (2.61 ± 0.81) compared to baseline (3.98 ± 0.53) in fluoxetine group. However higher rate of decrease of mean dexterity value was seen in fluoxetine group when compared to placebo group. Mean number of hands tapping movements in 30 s increased significantly at 90th day (16.33 ± 3.58) compared to baseline (9.83 ± 2.92) in fluoxetine group. Few ADR reported during this study were dizziness, drowsiness and insomnia.
Conclusion
The present study indicates that early prescription of fluoxetine is safe and may enhance motor function in patients presenting with severe motor impairments after stroke. However, the findings of the study should be confirmed in future controlled studies with large sample size.
Keywords: Stroke, Fluoxetine, Neurology, Motor function, Dexterity, Rehabilitation
Highlights
-
•
According to American stroke association – 2/3 of stroke survivors continue to live with one or more disabilities.
-
•
Fluoxetine, a selective serotonin reuptake inhibitor have neurotrophic and neuroprotective action.
-
•
Early administration of Fluoxetine is effective in stroke rehabilitation by enhancing motor function.
1. Introduction
Stroke is a cerebrovascular attack which occurs when the blood supply to a part of the brain stops resulting in deprivation of oxygen. This culminates in the death of the cells and loss of functions performed by that part of the brain. The varied complications ranging from temporary weakness to permanent paralysis of one side of the body is determined by the part of the brain and the extent to which it is damaged. Complete recovery from stroke occurs in some cases, but 2/3 of stroke survivors continue to live with one or more disabilities [1]. According to the world health organization, the definition of stroke (introduced in 1970 and still used) is “rapidly developing clinical signs of focal (or global) disturbance of cerebral function, lasting more than 24 hours or leading to death, with no apparent cause other than that of vascular origin” [2]. There are two types of stroke namely ischemic and hemorrhagic stroke. Stroke that is caused due to an obstruction in the artery supplying blood to the brain is an ischemic stroke whereas the stroke caused by rupture of blood vessels is a hemorrhagic stroke [3]. Medical treatments for acute and subacute stroke are usually aimed at improving the clinical condition of the patient by restoring blood flow to the compromised area of the brain and optimizing collateral flow. Considering the gold standard, tissue plasminogen activator is the only US food and drug administration (FDA) approved treatment for ischemic stroke. Several interventions, including intra-arterial administration of thrombolytic agents and mechanical interventions, also show promise [4].
General management of both the types of stroke includes cardiac and pulmonary care, fluid and ion balance restoration, metabolic maintenance, blood pressure control, prevention of further complications (pneumonia, sepsis and pressure sores) [5]. Stroke is the primary cause of disability worldwide, the second greatest common reason of dementia and the third leading source of death [6]. It is also the leading cause of disability in India. According to a retrospective analysis, 2% of hospital admissions and 20% of neurological admissions in India are of stroke cases [7]. Owing to its high prevalence, high burden of illness and economic cost, well-defined modifiable risk factors, and effective prevention measures stroke is well suited for prevention [8]. However, younger stroke patients appear to have a greater ability to recover from stroke and are likely to benefit substantially from treatments that facilitate plasticity-mediated recovery [9]. Initiation of early rehabilitation in stroke patients is necessary to improve their quality of life. Use of selective norepinephrine reuptake inhibitors such as reboxetine, venlafaxine in stroke patients was found to be effective in improving their motor function. However selective serotonin reuptake inhibitors such as sertraline, citalopram, fluoxetine also were found effective with greater tolerability when compared to SNRIs [10]. An animal study results indicated a complex effect of fluoxetine on neuronal signaling mechanisms potentially involved in restoring plasticity in the adult brain [11]. Fluoxetine was approved by US-FDA for treating post-stroke depression. Later few studies have shown its positive effect on motor recovery in stroke patients [12]. A recent meta-analysis have concluded that early prescription of fluoxetine improves depression after stroke [13]. In addition, recent multicenter trials like EFFECTS, AFFINITY, and FOCUS studies provide strong evidence against a beneficial effect of Fluoxetine on motor recovery after stroke [[14], [15], [16]]. However, it may be noted that the majority of study participants in these trials had probably less severe paresis. Hence the current study was designed to evaluate the effectiveness of fluoxetine in motor recovery and its safety in stroke population with more severe motor deficit for a period of 90 days.
2. Materials and methods
2.1. Study design
The current study was a randomized, placebo-controlled, Single -blind trial study conducted over a period of 90 days in the Department of Neurology, SRM Hospital and Research Centre, Kattankulathur, India. The study protocol was approved by the institutional human ethics committee (IHEC) of SRM medical college hospital and research centre (clearance number: 1063/IEC/2016). Research was performed in compliance with the Helsinki declaration and with the good clinical practice guidelines.
2.2. Study population
The subjects for the study were selected from the patients admitted in the neurology ward. Written informed consent was obtained from patients before their enrollment in the study. The inclusion criteria for the study were as follows: (1) patients aged between 18 and 80 years with clinical diagnosis of acute ischemic stroke (1–7 days) and subacute ischemic stroke (1 week–1 month) with medical research council (MRC) scale score <4 for muscle strength in upper extremity, (2) If hemorrhagic stroke then hemorrhage is either in the region of putamen or in the thalamus, (3) CT imaging of middle cerebral artery (MCA) with monoparesis, hemiparesis or hemiplegia, (4) stable patient not in comatose state.
The exclusion criteria were as follows: (1) history of allergy to fluoxetine, (2) contraindications to fluoxetine including hepatic impairment (alanine aminotransferase >3 upper normal limits), renal impairment (creatinine levels >180 μmol/L and in affinity also eGFR<30 ml/min/1.73 m2), (3) history of epileptic seizures, (4) pregnant or breastfeeding women of child-bearing age not taking contraception, (5) use of a monoamine oxidase inhibitor (MAOI) during the last 5 weeks (e.g. tranylcypromine, phenelzine, isocarboxazid, moclobemide, selegiline, and rasagiline).
2.3. Sample size calculation
Determination of sample size was done using nMaster 2.0- sample size software. A sample size of 77 in each group would be sufficient to detect a clinically significant difference after 90 days of fluoxetine administration on the basis of findings of razazian, nazanin et al. Study to achieve 80% power of the study at α = 0.05 [26]. We accordingly adjusted the sample size to 84 in each group by expecting a drop rate of 5%.
2.4. Interventions
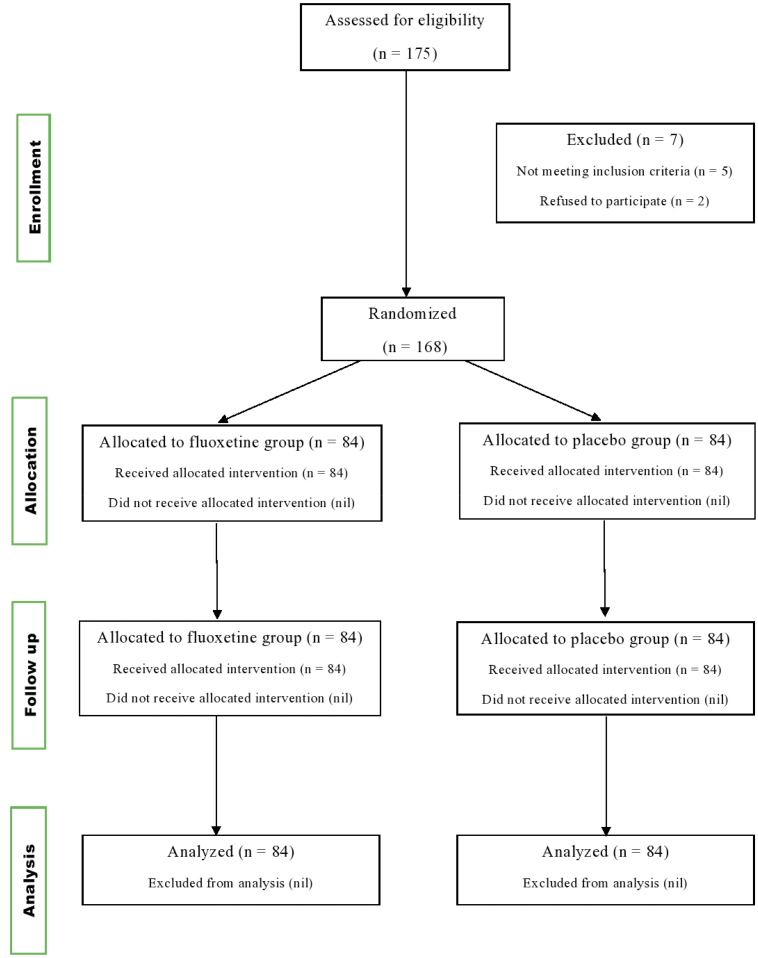
Based on the inclusion and exclusion criteria, 168 patients were included in present study. The recruited patients were assigned to either the treatment group (n = 84) or the placebo group (n = 84) and received fluoxetine (20 mg) once daily or placebo respectively and followed for a period of 90 days. All adverse drug reactions occurred during the course of treatment were observed and recorded in the case report file (CRF) by the investigators. CONSORT flow chart was shown in (Fig. 1).
Fig. 1.
CONSORT Flow chart.
2.5. Measurement tools
Primary outcomes such as barthel index, nine-hole peg test (NHPT) value, and number of finger tapping movements in 30 s were measured by co-investigators at baseline, 45th day and 90th day in fluoxetine group and placebo group. However, the outcome assessors were not blinded throughout the study.
-
a)
Barthel index:
The barthel index is an ordinal scale used to measure performance in activities of daily living (ADL) by using a questionnaire with maximum points of 100. Lower the number, greater is the dependence for daily activity performance. Its reliability is 0.90 (95% CI) [17].
-
b)
Nine-Hole Peg Test:
Dexterity is the coordination of small muscles, in movements usually involving the synchronization of hands and fingers with the eyes. The nine-hole peg test (NHPT) is considered as a gold standard measure of manual dexterity. The NHPT consists of a square board with 9 holes and a container with 9 pegs. The peg board is placed on the side of the impaired hand, and the pegs are picked up and put into the pegboard, one at a time, and thereafter returned to the container. The shorter the time, the better is the performance of the impaired hand [18].
-
c)
Finger Tapping Movements:
Finger tapping movements were measured as the number of finger tapping's done in the affected hand in 30 s. The speed of finger tapping in the affected hand is a very sensitive measure for brain damage as it is a good marker for its recovery [19].
2.6. Allocation concealment
Concealment of the randomization code was done to avoid selection bias. Third party randomization is the gold standard for concealment. Computer generated randomization list was prepared by a third person who was not involved in the recruitment of patients. Each allocation was written on paper and concealed in a serially numbered, opaque envelope.
2.7. Statistical analysis
The data were analyzed using graph prism and SPSS (version 20; SPSS Inc., Chicago, IL, USA). Descriptive statistics of collected data are presented as a mean ± standard deviation. Determination of statistical significance of the differences in BI scores, dexterity time and number of finger tapping movements between baselines, 45th day, 90th day in each treatment groups separately was performed by wilcoxon test. Statistical significance of the differences in BI scores, dexterity time and number of finger tapping movements in 30 s among both the treatment groups was evaluated by mann-whitney U test. The level of significance was fixed at P < 0.05.
3. Results
A total of 168 patients were enrolled in the study based on inclusion and exclusion criteria and were assigned to fluoxetine (n = 84) or placebo (n = 84) group. Baseline demographic characteristics of patients were mentioned in Table 1, Table 2. No statistical difference was observed in terms of age, gender, comorbidity status of enrolled subjects between fluoxetine group and placebo group. According to the age wise distribution, most of the patients were in the age group of 41–60 years. Majority of the patients who participated in the study were males. The comorbid conditions associated with stroke in the patients were collected in the data entry form to identify potential hypersensitivity reactions and drug interactions associated with the drugs prescribed for these comorbid conditions. The comorbid status of the study population is mentioned in Table 1. Out of the 168 patients, 98 (58.33%) patients had ischemic stroke and remaining 70 (41.67%) patients had hemorrhagic stroke. In addition, 88 (52.38%) patients were affected with hemiparesis on right side whereas 80 (47.62%) patients were affected with hemiparesis on left side. Patients were categorized based on medical research council (MRC) scale for clinical assessment of muscle weakness. Accordingly, 14 (8.33%) patients had severity of grade 1, 91 (54.17%) patients had severity of grade 2 and 63 (37.5%) patients had severity of grade 3. However, all the patients had difficulty in moving their affected upper limbs against gravity. The outcomes of present study were assessed at baseline, 45th day and 90th day of treatment. Fluoxetine was found to be more effective in hemorrhagic stroke when compared to ischemic stroke. In comparison to placebo group, statistical significant difference in mean BI score (p = <0.001) was observed at 90th day in fluoxetine group (Table 3). Similarly, patients assigned to fluoxetine group have shown a significant decrease in the mean dexterity values (p = <0.001) at 90th day when compared to placebo group (Table 4). In addition, there is a significant increase in the mean hand tapping movements values from day 0 to day 90 after the initiation of fluoxetine therapy (p = <0.001), which shows the strong evidence for the clinical improvement of the patients in fluoxetine group than that of in placebo group (Table 5). The most common side effects reported by the patients among both the groups were mentioned in Table 6. However, majority of patients didn't experience any side effects or adverse drug reactions indicating good tolerability of fluoxetine among the stroke patients.
Table 1.
Patient's demographic characteristics.
| Age wise distribution | ||||
|---|---|---|---|---|
| Age | Fluoxetine group (N = 84) | Placebo group (N = 84) | P value | Z Score |
| 20–40 years | 15 (17.9%) | 9 (10.7%) | 0.136 | 1.494 |
| 41–60 years | 45 (53.6%) | 51 (60.7%) | 0.294 | −1.047 |
| 61–80 years |
24 (28.6%) |
24 (28.6%) |
1 |
0 |
| Gender wise distribution | ||||
|
Gender |
Fluoxetine group (N = 84) |
Placebo group (N = 84) |
P value |
Z Score |
| Male | 48 (57.1%) | 54 (64.3%) | 0.352 | −0.928 |
| Female |
36 (42.9%) |
30 (35.7%) |
0.352 |
0.928 |
| Co-morbidity of the patients | ||||
|
Co-morbidity status |
Fluoxetine group (N = 84) |
Placebo group (N = 84) |
P value |
Z Score |
| Diabetes Mellitus | 10 (11.90%) | 8 (9.52%) | <0.0001 | −10.112 |
| DM/HTN | 13 (15.48%) | 11 (13.09%) | 0.711 | 0.374 |
| DM/HTN/CAD | 3 (3.57%) | 2 (2.38%) | 0.134 | 1.497 |
| DM/SHTN | 9 (10.71%) | 12 (14.29%) | 0.555 | −0.588 |
| HTN | 8 (9.52%) | 7 (8.33%) | 0.070 | 1.815 |
| RHD | 2 (2.38%) | 4 (4.77%) | <0.0001 | −4.076 |
| RHD/SHTN/T2DM | 2 (2.38%) | 1 (1.19%) | 0.070 | 1.815 |
| SHTN | 6 (7.15%) | 4 (4.76%) | 0.0001 | 3.908 |
| No past medical condition | 31 (36.91%) | 35 (41.67%) | 0.596 | 0.532 |
Values are expressed as n (%).
n – Number of Patients.
% - percentage.
Abbreviations:
RHD - Rheumatic heart disease
SHTN - Systolic hypertension
T2DM - Type-2 Diabetes Mellitus
HTN - Hypertension
CAD - Coronary artery disease
MRC - Medical Research Council
Table 2.
Patient's hemiparesis sidewise distribution & medical research council (MRC) scale wise distribution.
| Hemiparesis sidewise distribution | ||||
|---|---|---|---|---|
| Hemiparesis | Fluoxetine group (N = 84) | Placebo group (N = 84) | P value | Z Score |
| Left side | 37 (44.05%) | 43 (51.19%) | 0.363 | 0.908 |
| Right side |
47 (55.95%) |
41 (48.81%) |
0.363 |
0.908 |
| Types of stroke population | ||||
|
Type of stroke |
Fluoxetine group (N = 84) |
Placebo group (N = 84) |
P value |
Z Score |
| Hemorrhagic stroke | 34 (40.48%) | 36 (42.86%) | 0.697 | −0.395 |
| Ischemic stroke |
50 (59.52%) |
48 (57.14%) |
0.795 |
0.263 |
| Medical research council (MRC) scale wise distribution | ||||
|
MRC score |
Fluoxetine group (N = 84) |
Placebo group (N = 84) |
P value |
Z Score |
| Score 1 | 12 (14.29%) | 2 (2.38%) | 0.298 | −1.035 |
| Score 2 | 44 (52.38%) | 47 (55.95%) | 0.603 | −0.520 |
| Score 3 | 28 (33.33%) | 35 (41.67%) | 0.285 | −1.074 |
Values are expressed as n (%).
n – Number of Patients.
% - percentage.
MRC scale - Medical Research Council scale.
Table 3.
Evaluation of Barthel index.
| Parameter | Study group (Mean ± SD) |
P Value | |
|---|---|---|---|
| Fluoxetine (N = 84) | Placebo (N = 84) | ||
| Barthel Index at baseline | 40.71 ± 11.98 | 35.6 ± 9.03 | 0.002 |
| Barthel Index at 45th day | 54.52 ± 16.44 | 40.18 ± 8.08 | <0.001 |
| Barthel Index at 90th day | 70.42 ± 10.56 | 44.23 ± 8.52 | <0.001 |
Table 4.
Evaluation of dexterity.
| Parameter | Study group (Mean ± SD) |
P value | |
|---|---|---|---|
| Fluoxetine (N = 84) | Placebo (N = 84) | ||
| Dexterity value at baseline | 3.98 ± 0.53 | 4.71 ± 6.38 | 0.786 |
| Dexterity value at 45th day | 3.29 ± 0.58 | 3.76 ± 0.55 | <0.001 |
| Dexterity value at 90th day | 2.61 ± 0.81 | 3.69 ± 0.53 | <0.001 |
Table 5.
Evaluation of finger tapping movements.
| Parameter | Study group (Mean ± SD) |
P value | |
|---|---|---|---|
| Fluoxetine (N = 84) | Placebo (N = 84) | ||
| Hand tapping movements at baseline | 9.83 ± 2.92 | 8.98 ± 3.04 | 0.066 |
| Hand tapping movements at 45th day | 13.24 ± 3.42 | 10.19 ± 2.86 | <0.001 |
| Hand tapping movements at 90th day | 16.33 ± 3.58 | 10.9 ± 2.78 | <0.001 |
Table 6.
Side effects observed in study participants.
| Side effects | Fluoxetine group n (%) | Placebo group n (%) |
|---|---|---|
| Dizziness | 2 (2.40%) | 0 |
| Insomnia | 3 (3.40%) | 2 (2.35%) |
| Drowsiness | 1 (1.19%) | 0 |
| Dyspepsia | 0 | 5 (5.95%) |
| Anxiety | 0 | 3 (3.96%) |
4. Discussion
Fluoxetine, a selective serotonin reuptake inhibitor, was found to influence neuroplasticity by enhancing neurotrophic factors, including BDNF signaling and altering neurogenesis thereby improving motor function. In 2011, fluoxetine for motor recovery after acute ischemic stroke (FLAME) study was conducted to evaluate the effects of fluoxetine on motor recovery [11]. In FLAME study, the fluoxetine group had a 10-point greater improvement on the upper extremity fugl-meyer scale (UEFM) than in placebo group (P = 0.002) at the end of treatment [12]. In addition, 26% of patients in the fluoxetine group achieved functional independence on modified rankin Scores (mRS), compared with 9% in the placebo group (p = 0.015) [20]. Similarly, improvements in motor outcomes remained significant among fluoxetine group in the present study. The available data emphasized a hypothesis that supports the neurotrophic and neuroprotective effects of fluoxetine. Few clinical trials, especially that by Chollet et al. [20] and also an earlier Cochrane review [21] indicating beneficial effects on the motor outcome when combined with physiotherapy. But recent data from large, controlled RCTs [[13], [14], [15], [16]] demonstrating no beneficial effect on motor outcome in patients with fairly mild stroke. However, the current study included patients with more severe paresis when compared with these large RCTs, which was also the case for the study by Chollet et al. [20]. These collective findings point to an advantageous influence of fluoxetine when paired with rehabilitation in the recovering stroke patient.
A previous study by rayaz Jan MD et al. have reported that as the age increases the risk of suffering from stroke also increases and another study by siddique AN. et al. had revealed that the occurrence of stroke was more prevalent in age group ranging between 51 and 60 [22,23]. Similarly, most of the patients included in the present study were above 40 years of age suggesting a higher risk of stroke with an increase in age. The majority of the acute stroke patients included in the current study was found to be nondiabetic as that in rayaz Jan MD et al. study [22]. Significant increase in the mean barthel index score after the administration of fluoxetine observed in the present study was similar to the findings of dam et al. study [24]. In the present study, significant decrease in the mean dexterity value and an increase in the mean hand tapping movements from baseline to 90th day which a strong evidence for the motor function improvement in the affected upper limb of patients after fluoxetine administration. The treatment was relatively well tolerated in our study when compared to the irfan ahmad shah et al. study [25] and only a few adverse effects were reported for fluoxetine in our study (i.e. dizziness, drowsiness, and insomnia). The current study is limited by short term follow-up period. In spite of our large sample size, this present study was conducted only in single study centre. Therefore, future studies with large sample size should be conducted in multi study centers for a longer duration among Indian population to establish the potential role of fluoxetine in motor recovery as well as for better evaluation of tolerability of the drug in stroke patients. In addition, being a single blinded study, the outcome assessors were not blinded, which attributed to a risk for assessment bias.
5. Conclusion
The current study suggests that early administration of fluoxetine might be beneficial in motor recovery among acute and sub-acute stroke patients of both ischemic stroke and hemorrhagic stroke. However, fluoxetine was found to be more effective in hemorrhagic stroke when compared to ischemic stroke. Fluoxetine was found to have good tolerability on administration for 90 days with fewer adverse effects. The present study indicates that fluoxetine could serve as a better therapeutic option for enhancing motor functions in the stroke population with a more severe motor deficit. Although the findings of the current study suggested that fluoxetine might be beneficial in the improvement of motor function after stroke in short term, future studies are needed to evaluate the efficacy and safety of long-term treatment with fluoxetine in the stroke population.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgement
The Authors would like to thank Dean, SRM College of Pharmacy and Dean, other faculties of SRM Medical College Hospital & Research Centre, SRMIST for their kind support and guidance.
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- BI
Barthel Index
- CAD
Coronary artery disease
- CT
Computed tomography
- DM
Diabetes mellitus
- FDA
Food and drug administration
- HTN
Hypertension
- IHEC
Institutional human ethics committee
- MAOI
Monoamine oxidase inhibitor
- MCA
Middle cerebral artery
- MRC
Medical research council
- NHPT
Nine-hole peg test
- SNRI
Serotonin–norepinephrine reuptake inhibitor
- SPSS
Statistical package for the social sciences
- SSRI
Selective serotonin reuptake inhibitor
Funding
None.
Authors’ contribution
The study was designed by all the authors. Dr. K. Karthickeyan and Dr. K. Muthuraj did the quality control and analysis. Dr. Vikrama Simha Reddy and Dr. Neethu Sara Raju conducted the literature search. Data collection was done by Dr. K. Nandhini, Dr. Yalamanchili Dharma Teja, Dr. Vikrama Simha Reddy, and Dr. Neethu Sara Raju. Statistical analysis and interpretation of the study done by Dr. K. Nandhini, Dr. Yalamanchili Dharma Teja and Dr. Kiran Kumar. The manuscript was framed by Dr. K. Karthickeyan and Dr. Kiran Kumar.
References
- 1.National Stroke Association What is stroke? http://www.stroke.org/understand-stroke/what- stroke Available at:
- 2.Sacco R.L., Kasner S.E., Broderick J.P. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association [published correction appears in Stroke. Stroke. 2013;44(7):2064–2089. doi: 10.1161/STR.0b013e318296aeca. 50(8):e239] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broeks J.G., Lankhorst G.J., Rumping K., Prevo A.J.H. The long-term outcome of arm function after stroke: results of a follow-up study. Disabil. Rehabil. 1999;21(8):357–364. doi: 10.1080/096382899297459. [DOI] [PubMed] [Google Scholar]
- 4.Adams H.P., Jr., del Zoppo G., Alberts M.J. Guidelines for the early management of adults with ischemic stroke: a guideline from the American heart association/American stroke association stroke council, clinical cardiology council, cardiovascular radiology and intervention council, and the atherosclerotic peripheral vascular disease and quality of care outcomes in research interdisciplinary working groups: the American academy of neurology affirms the value of this guideline as an educational tool for neurologists [published correction appears in circulation. Circulation. 2007;115(20):e478–e534. doi: 10.1161/CIRCULATIONAHA.107.181486. 116(18):e515] [DOI] [PubMed] [Google Scholar]
- 5.Hemphill J.C., 3rd, Greenberg S.M., Anderson C.S. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2015;46(7):2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 6.Bakhai A. The burden of coronary, cerebrovascular and peripheral arterial disease. Pharmacoeconomics. 2004;22(4):11–18. doi: 10.2165/00019053-200422004-00004. [DOI] [PubMed] [Google Scholar]
- 7.Pandian Jeyaraj Durai, Sudhan Paulin. Stroke epidemiology and stroke care services in India. J Stroke. 2013 Sep;15(3):128–134. doi: 10.5853/jos.2013.15.3.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorelick P.B. Stroke prevention therapy beyond antithrombotics: unifying mechanisms in ischemic stroke pathogenesis and implications for therapy: an invited review. Stroke. 2002;33:862–875. doi: 10.1161/hs0302.103657. [DOI] [PubMed] [Google Scholar]
- 9.Stein J. Motor recovery strategies after stroke. Top. Stroke Rehabil. 2004;11(2):12–22. doi: 10.1310/RK4A-6ETG-K8RL-3XA7. [DOI] [PubMed] [Google Scholar]
- 10.Yeo S.H., Kong K.H., Lim D.C., Yau W.P. Use of selective serotonin reuptake inhibitors and outcomes in stroke rehabilitation: a prospective observational pilot cohort study. Drugs R. 2019;19(4):367–379. doi: 10.1007/s40268-019-00287-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz-Perera L., Muniz M., Vierci G., Bornia N., Baroncelli L., Sale A., Rossi F.M. Fluoxetine increases plasticity and modulates the proteomic profile in the adult mouse visual cortex. Sci. Rep. 2015 Jul 24;5:12517. doi: 10.1038/srep12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto, Camila B et al. “SSRI and motor recovery in stroke: reestablishment of inhibitory neural network tonus.” Front. Neurosci. vol. 11 637. 16 Nov. 2017, doi:10.3389/fnins.2017.00637. [DOI] [PMC free article] [PubMed]
- 13.Mead G.E., Legg L., Tilney R., Hsieh C.F., Wu S., Lundström E., Rudberg A.S., Kutlubaev M., Dennis M.S., Soleimani B., Barugh A., Hackett M.L., Hankey G.J. Fluoxetine for stroke recovery: meta-analysis of randomized controlled trials. Int. J. Stroke. 2020 Jun;15(4):365–376. doi: 10.1177/1747493019879655. Epub 2019 Oct. [DOI] [PubMed] [Google Scholar]
- 14.Effects Trial Collaboration Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19:661–669. doi: 10.1016/S1474-4422(20)30219-2. [DOI] [PubMed] [Google Scholar]
- 15.Affinity Trial Collaboration Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19:651–660. doi: 10.1016/S1474-4422(20)30207-6. [DOI] [PubMed] [Google Scholar]
- 16.Dennis M., Mead G., Forbes J., Graham C., Hackett M., Hankey G.J., House A., Lewis S., Lundström E., Sandercock P. Effects of fluoxetine on functional outcomes after acute stroke (FOCUS): a pragmatic, double-blind, randomised, controlled trial. Lancet. 2019;393:265–274. doi: 10.1016/S0140-6736(18)32823-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahoney F.I., Barthel D.W. Functional evaluation: the barthel index. Md. State Med. J. 1965;14:61–65. [PubMed] [Google Scholar]
- 18.Mathiowetz V., Weber K., Kashman N., Volland G. Adult norms for the 9 hole peg test of finger dexterity. Occup. Ther. J. Res. 1985;5:24–38. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 19.De Groot-Driessen D., van de Sande P., van Heugten C. Speed of finger tapping as a predictor of functional outcome after unilateral stroke. Arch. Phys. Med. Rehabil. 2006;87(1):40–44. doi: 10.1016/j.apmr.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Chollet F., Tardy J., Albucher J.F., Thalamas C., Berard E., Lamy C., Bejot Y., Deltour S., Jaillard A., Niclot P., Guillon B., Moulin T., Marque P., Pariente J., Arnaud C., Loubinoux I. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10(2):123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- 21.Mead G.E., Hsieh C.F., Lee R., Kutlubaev M.A., Claxton A., Hankey G.J., Hackett M.L. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. Cochrane Database Syst. Rev. 2012;11:CD009286. doi: 10.1002/14651858.CD009286.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jan Rayaz, Singh Jamwal Davinder, Gupta Rajiv Kumar, Singh Parveen. Incidence and risk factors of stroke: a hospital based study in jammu province of J & K, India. Publ. Health Res. 2014;4(3):104–110. [Google Scholar]
- 23.Abu Naser Siddique ZannatunNur, ShahriarMahbub BillalAlam, Miah Titu. Clinical presentation and epidemiology of stroke –a study of 100 cases. J. Med. 2009;10:86–89. [Google Scholar]
- 24.Dam M., Tonin P., De Boni A., Pizzolato G., Casson S., Ermani M., Freo U., Piron L., Battistin L. Effects of fluoxetine and maprotiline on functional recovery in poststroke hemiplegic patients undergoing rehabilitation therapy. Stroke. 1996;27:1211–1214. doi: 10.1161/01.str.27.7.1211. [DOI] [PubMed] [Google Scholar]
- 25.Shah Irfan Ahmad, Asimi Ravouf P., Kawoos Yuman, Wani Mushtaq A., Wani Maqbool A., Dar Mansoor A. Effect of fluoxetine on motor recovery after acute haemorrhagic stroke: a randomized trial. J. Neurol. Neurophysiol. 2016;7:364. [Google Scholar]
- 26.Razazian Nazanin. Effect of fluoxetine on motor improvement in ischemic stroke patients: a double blind clinical trial study. Zahedan Journal of Research in Medical Sciences. 2016;18(7) doi: 10.17795/zjrms-7549. [DOI] [Google Scholar]