Abstract
The conclusions of the EFSA following the peer review of the initial risk assessment carried out by the competent authority of the rapporteur Member State, France, for the pesticide active substance acibenzolar‐S‐methyl are reported. The context of the peer review was that requested by the European Commission following the submission and evaluation of confirmatory information with regard to the endocrine disruption potential of the substance. The conclusions were reached on the basis of the evaluation of the representative uses of acibenzolar‐S‐methyl as a plant activator on pome fruit, tomato and tobacco. The reliable endpoints concluded as being appropriate for use in regulatory risk assessment, derived from the available studies and literature in the dossier peer reviewed, are presented. Assessments not finalised together with the missing information identified as being required by the regulatory framework are listed.
Keywords: acibenzolar‐S-methyl, peer review, confirmatory data, risk assessment, pesticide, plant activator
Summary
The approval of acibenzolar‐S‐methyl was renewed under Regulation (EC) 1107/2009 on 1 April 2016 by Commission Implementing Regulation (EU) 2016/389. It was a specific provision of the approval that the applicant was required to submit to the European Commission further information by 1 June 2017 as regards the relevance and reproducibility of the morphometric changes observed in the cerebellum of fetuses linked to exposure to acibenzolar‐S‐methyl and whether these changes may be produced via an endocrine mode of action. The information to be submitted shall include a systematic review of the available evidence assessed on the basis of available guidance (e.g. EFSA guidance on Systematic Review methodology, 2010).
In accordance with the specific provision, the applicant, Syngenta, submitted an updated dossier in May 2017 as well as additional information in February 2019 in line with the EFSA/European Chemicals Agency (ECHA) guidance for the identification of endocrine disruptors (2018), which was evaluated by the designated rapporteur Member State (RMS), France, in the form of a revised assessment report. In compliance with the guidance document SANCO 5634/2009‐rev.6.1, the RMS distributed the revised assessment report to Member States, the applicant and EFSA for comments on 12 November 2019. The RMS collated all comments in the format of a reporting table, which was submitted to EFSA on 11 March 2020. EFSA added its scientific views on the specific points raised during the commenting phase in column 4 of the reporting table contained in the Technical Report issued on 15 April 2020.
Following consideration of the conclusions of the Technical Report, in October 2020, the European Commission requested EFSA to organise a peer review, including expert discussion where appropriate to further assess the relevance of the endocrine‐disrupting (ED) properties of acibenzolar‐S‐methyl and to deliver its conclusions on the ED properties, and if applicable, to establish which additional tests are needed to conclude on the ED properties both for humans and non‐target organisms under consideration of the new scientific criteria set out under points 3.6.5 and 3.8.2 of Regulation (EC) No 1107/2009 as amended by Commission Regulation 2018/605.
The assessment of the endocrine‐disrupting properties of acibenzolar‐S‐methyl could not be finalised based on the available data for both humans and non‐target organisms.
Background
The approval of acibenzolar‐S‐methyl was renewed under Regulation (EC) 1107/20091 on 1 April 2016 by Commission Implementing Regulation (EU) 2016/3892. EFSA previously finalised a Conclusion on this active substance on 12 August 2014 in the EFSA Journal (EFSA, 2014).
It was a specific provision of the approval that the applicant was required to submit to the European Commission further information by 1 June 2017 as regards the relevance and reproducibility of the morphometric changes observed in the cerebellum of fetuses linked to exposure to acibenzolar‐S‐methyl and whether these changes may be produced via an endocrine mode of action. The information to be submitted shall include a systematic review of the available evidence assessed on the basis of available guidance (e.g. EFSA guidance on Systematic Review methodology; EFSA, 2010).
In accordance with the specific provision, the applicant, Syngenta, submitted an updated dossier in May 2017 as well as additional information in February 2019 in line with the EFSA/ECHA guidance for the identification of endocrine disruptors (2018), which was evaluated by the designated rapporteur Member State (RMS), France, in the form of a revised assessment report (France, 2019). In compliance with the guidance document SANCO 5634/2009‐rev.6.1 (European Commission, 2013), the RMS distributed the revised assessment report to Member States, the applicant and the EFSA for comments on 12 November 2019. The RMS collated all comments in the format of a reporting table, which was submitted to EFSA on 11 March 2020. EFSA added its scientific views on the specific points raised during the commenting phase in column 4 of the reporting table contained in the Technical Report issued on 15 April 2020 (EFSA, 2020a).
Following consideration of the conclusions of the Technical Report, on 16 October 2020, the European Commission requested EFSA to organise a peer review, including expert discussion where appropriate, to further assess the relevance of the endocrine‐disrupting (ED) properties of acibenzolar‐S‐methyl and to deliver its conclusions on the ED properties, and if applicable, to establish which additional tests are needed to conclude on the ED properties both for humans and non‐target organisms under consideration of the new scientific criteria set out under points 3.6.5 and 3.8.2 of Regulation (EC) No 1107/2009 as amended by Commission Regulation 2018/6053.
The RMS assessment and the reporting table were discussed at the Pesticides Peer Review Meeting TC 48 on Mammalian Toxicology and Ecotoxicology joint session on ED in April 2021. Advice was also provided by the EFSA ED Working Group. Details of the issues discussed, together with the outcome of these discussions were recorded in the meeting report.
A final consultation on the conclusions arising from the peer review took place with Member States via a written procedure in May–June 2021.
The conclusions laid down in this report were reached on the basis of the peer review of the RMS's evaluation of the confirmatory data submitted in relation to the ED properties of acibenzolar‐S‐methyl. A key supporting document to this conclusion is the peer review report (EFSA, 2021), comprising of the following documents, in which all views expressed during the course of the confirmatory data peer review, including minority views, if applicable, can be found:
the report of the scientific consultation with Member State experts;
the comments received on the draft EFSA conclusion.
Given the importance of the RMS assessment (revised version of May 2021 containing the updated volumes of the RAR (France, 2021)) and the peer review report, these documents are considered as background documents to this conclusion.
It is recommended that this conclusion report and its background documents would not be accepted to support any registration outside the EU for which the applicant has not demonstrated to have regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
Acibenzolar‐S‐methyl is the modified‐ISO common name for S‐methyl benzo[1,2,3]thiadiazole‐7‐carbothioate (IUPAC). This substance is a derivative of acibenzolar, which is the ISO common name for 1,2,3‐benzothiadiazole‐7‐carbothioic S‐acid (IUPAC).
The representative formulated product for the evaluation was ‘A9180A (BION 50 WG)’, a water dispersible granules (WG) containing 500 g/kg acibenzolar‐S‐methyl. The representative uses as plant activator comprise applications by foliar spraying on pome fruit, tomato and tobacco (see Appendix A in EFSA 2014).
1. Conclusions of the evaluation
In May 2017, the applicant submitted confirmatory information as regards the relevance and reproducibility of the morphometric changes observed in the cerebellum of fetuses linked to exposure to acibenzolar‐S‐methyl and whether these changes may be produced via an endocrine mode of action. Furthermore, in February 2019, they provided additional information in line with the EFSA/ECHA guidance for the identification of endocrine disruptors (2018). The assessment of the information was presented by the RMS in the form of a revised assessment report (France, 2019, 2021).
The ED assessment for acibenzolar‐S‐methyl was discussed at the Pesticides Peer Review Experts’ Teleconference 48 on Mammalian Toxicology and Ecotoxicology joint session on endocrine disruption in April 2021. Advice from the EFSA Endocrine Disruption (ED) Working Group was also provided and taken into account in the conclusions reached.
With regard to the assessment of the ED potential of acibenzolar‐S‐methyl for humans according to the ECHA/EFSA guidance (2018), the number and type of effects induced, and the magnitude and pattern of responses observed across studies were considered to determine whether acibenzolar‐S‐methyl interacts with the oestrogen, androgen and steroidogenesis (EAS) and thyroid (T) mediated pathways. Additionally, the conditions under which the effects occur were examined, in particular, whether or not endocrine‐related responses occurred at dose(s) that also resulted in overt toxicity. This assessment therefore provides a weight‐of‐evidence analysis of the potential interaction of acibenzolar‐S‐methyl with the EAS and T signalling pathways using the available evidence in the data set.
The data set for the T modality was considered complete; however, even though there was very limited evidence for T‐mediated adversity (namely marginal increase in the thyroid follicular cell adenoma in female rats at a dose considered to overcome the maximum tolerated dose), there is concern because of the outcome of the developmental neurotoxicity (DNT) study. The effects observed in the DNT study (morphometric changes in the cerebellum, increased auditory startle amplitude) were considered treatment related. There are no data available on thyroid hormones (THs) and thyroid‐stimulating hormone (TSH). However, ToxCast data analysis indicates that acibenzolar‐S‐methyl is a thyroid peroxidase (TPO) enzyme inhibitor in the rat microsomal TPO‐aur assay. These data were not confirmed in the porcine microsomal TPO‐guaiacol, measuring the same endpoint in a different assay, and therefore, there is uncertainty if acibenzolar‐S‐methyl is affecting this molecular initiating event. Therefore, to resolve this uncertainty, additional study in line with the US EPA thyroid assessment assay should be conducted with the measurement of THs and TSH in fetus and pups (US EPA, Office of Pesticide Programs, Health Effects Division, Washington (DC)).4
In addition, in conducting this study, the following points should be considered and reported in the study report:
The methodology for sampling and the analytical method to evaluate the thyroid hormones and TSH.
Laboratory documentation of the method validation for the assessment of THs and TSH with inclusion of the limit of detection (LoD) for fetus and pups.
Considering the inclusion of a positive control.
Control of iodine content in diet (should not be exceeding 5 μg/kg food, which is the rodent daily need).
Selection of an appropriate range of doses in the study (e.g. inclusion of the top dose used in the DNT study).
The data set for the EAS modalities was considered incomplete. However, a ToxCast oestrogen receptor (ER) model is available and negative for acibenzolar‐S‐methyl; therefore, there is no need to further explore the E modality and, in line with EFSA/ECHA (2018) ED Guidance, the criteria for the E modality are not met for humans.
However, further data need to be generated before a conclusion on whether or not the ED criteria are met for the AS‐modalities can be drawn (Scenario 2a(iii) of the EFSA/ECHA (2018) ED Guidance). To investigate the A and S modalities, the following tests are needed:
A study in line with OECD Test Guideline (TG) 458 (Stably Transfected Human Androgen Receptor Activation Assay (AR STTA) assay);
Aromatase assay (human recombinant) OPPTS 890.1200 (US EPA 2009 In: Endocrine Disruptor Screening Program Test Guidelines. Office of Prevention, Pesticides and Toxic Substances (OPPTS), US EPA, Washington (DC);
A study in line with OECD TG 456 (H295R Steroidogenesis assay);
A study in line with OECD TG 441 (Hershberger Assay) in case OECD TG 456, OPPTS 890.1200 and OECD TG 458 are negative.
If the above tests are negative, the active substance will not meet the ED criteria for the AS modalities. However, in case of positive result/s based on the above tests for at least one modality, additional testing is needed:
OECD TG 443 (with the inclusion of cohort 1B) or OECD TG 416 (including additional endpoints in accordance with the EFSA (2020b) technical report: ‘Outcome of the pesticides peer review meeting on general recurring issues in mammalian toxicology’.
The outcome of the assessment reported above for humans also applies to wild mammals as non‐target organisms.
For non‐target organisms other than mammals, neither the endocrine activity nor the endocrine adversity was sufficiently investigated. Therefore, additional data are needed to draw a conclusion on the ED properties of acibenzolar‐S‐methyl on non‐target organisms.
In particular, a test according to OECD TG 231 (Amphibian Metamorphosis Assay) and a test according to OECD TG 2295 (Fish Short Term Reproduction Assay) are requested. Those tests are relevant to investigate potential EATS‐mediated endocrine activity and, if negative, to exclude that acibenzolar‐S‐methyl has endocrine properties, according to the scientific criteria for the determination of endocrine disrupting properties as set out in point 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Regulation (EU) No 2018/605. However, in case of positive result/s based on these tests for at least one modality, additional testing (i.e. a test according to OECD TG 241 (Larval Amphibian Growth and Development Assay (LAGDA)) and/or a test according to OECD TG 240 (Medaka Extended One Generation Test) might be needed in order to further investigate the adversity.
Based on the available information on humans and non‐target organisms, the assessment of the endocrine disruption potential of acibenzolar‐S‐methyl according to points 3.6.5 and 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605, cannot be concluded (data gap and issue not finalised).
2. Concerns and related data gaps
2.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for one or more of the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The following issues or assessments that could not be finalised have been identified, together with the reasons including the associated data gaps where relevant, which are reported directly under the specific issue to which they are related:
-
1
The assessment of the ED properties of acibenzolar‐S-methyl cannot be finalised for the T modality and additional data are needed for both humans and non‐target organisms:
A thyroid assessment assay with measurement of TH and TSH in fetus and pups in line with the: US EPA, Office of Pesticide Programs, Health Effects Division, Washington (DC)4;
A test according to OECD TG 231 (Amphibian Metamorphosis Assay). If the test is negative, the active substance will not meet the ED criteria for the T‐modality for non‐target organisms. However, in case of positive results, additional testing is needed, i.e. a test according to OECD TG 241 (LAGDA).
-
2
The assessment of the ED properties of acibenzolar‐S-methyl cannot be finalised for the EAS6 modalities and additional data are needed for both humans and non‐target organisms:
A study in line with OECD Test Guideline (TG) 458 (Stably Transfected Human Androgen Receptor Activation Assay (AR STTA) assay);
Aromatase assay (human recombinant) OPPTS 890.1200 (US EPA 2009 In: Endocrine Disruptor Screening Program Test Guidelines. Office of Prevention, Pesticides and Toxic Substances (OPPTS), US EPA, Washington (DC);
A study in line with OECD TG 456 (H295R Steroidogenesis assay);
A study in line with OECD TG 441 (Hershberger Assay) in case OECD TG 456, OPPTS 890.1200 and OECD TG 458 are negative;
For non‐target organisms, a test according to OECD TG 229 (Fish Short Term Reproduction Assay).
If the above tests are negative, the active substance will not meet the ED criteria for EAS modalities for humans and/or non‐target organisms. However, in case of positive result/s for one of the above tests for at least one modality, additional testing is needed:
-
f
A test according to OECD TG 443 (with the inclusion of cohort 1B) or OECD TG 416 (including additional endpoints in accordance with the EFSA (2020b) technical report: ‘Outcome of the pesticides peer review meeting on general recurring issues in mammalian toxicology’.
-
g
A test according to OECD TG 240 (Medaka Extended One Generation Test) for further investigating adversity in fish.
2.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The following critical areas of concern are identified, together with any associated data gaps, where relevant, which are reported directly under the specific critical area of concern to which they are related:
Critical areas of concern were not identified.
3. List of other outstanding issues
Remaining data gaps not leading to critical areas of concern or issues not finalised but considered necessary to comply with the data requirements, and which are relevant for some or all of the representative uses assessed at EU level. Although not critical, these data gaps may lead to uncertainties in the assessment and are considered relevant: None identified.
Abbreviations
- AR
applied radioactivity
- AV
avoidance factor
- CAS
Chemical Abstracts Service
- CI
confidence interval
- DAR
draft assessment report
- DAT
days after treatment
- DM
dry matter
- DNT
developmental neurotoxicity
- ECHA
European Chemicals Agency
- ED
endocrine disruption
- EEC
European Economic Community
- FID
flame ionisation detector
- FIR
food intake rate
- FOB
functional observation battery
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- GAP
Good Agricultural Practice
- GC
gas chromatography
- HR
hazard rate
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- LAGDA
Larval Amphibian Growth and Development Assay
- LC
liquid chromatography
- LC‐MS
liquid chromatography–mass spectrometry
- LC‐MS-MS
liquid chromatography with tandem mass spectrometry
- LOD
limit of detection
- M/L
mixing and loading
- mm
millimetre (also used for mean measured concentrations)
- MS
mass spectrometry
- OECD
Organisation for Economic Co‐operation and Development
- OM
organic matter content
- Pa
pascal
- PD
proportion of different food types
- PHI
pre‐harvest interval
- PIE
potential inhalation exposure
- PPE
personal protective equipment
- PT
proportion of diet obtained in the treated area
- REACH
Registration, Evaluation, Authorisation of Chemicals Regulation
- SC
suspension concentrate
- SMILES
simplified molecular‐input line‐entry system
- THs
thyroid hormones
- TK
technical concentrate
- T‐modality
Thyroid‐modality
- TPO
Thyroid peroxidase
- TSH
thyroid‐stimulating hormone (thyrotropin)
- TWA
time‐weighted average
- US EPA
United States Environmental Protection Agency
- UV
ultraviolet
- W/S
water/sediment
- w/v
weight per unit volume
- w/w
weight per unit weight
- WBC
white blood cell
- WG
water dispersible granule
- WHO
World Health Organization
Appendix A – Consideration of cut‐off criteria for acibenzolar‐S‐methyl according to Annex II of Regulation (EC) No 1107/2009 of the European Parliament and of the Council
1.
| Properties | Conclusion |
|---|---|
| Endocrine disrupting properties | The endocrine disruption properties of acibenzolar‐S‐methyl according to points 3.6.5 and 3.8.2 of Annex II of Regulation No 1107/2009, as amended by Commission Regulation (EU) 2018/605 cannot be finalised, and additional data are needed to conclude. |
Appendix B – Used compound codes
1.
| Code/trivial name | IUPAC name/SMILES notation/InChiKeya | Structural formulab |
|---|---|---|
| acibenzolar‐S-methyl | S‐methyl benzo[1,2,3]thiadiazole‐7‐carbothioate O=C(SC)c1cccc2nnsc12 UELITFHSCLAHKR‐UHFFFAOYSA‐N |
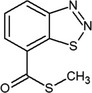
|
| acibenzolar | 1,2,3‐benzothiadiazole‐7‐carbothioic S‐acid O=C(S)c1cccc2nnsc12 CGIHPACLZJDCBQ‐UHFFFAOYSA‐N |
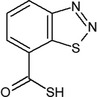
|
ACD/Name 2019.1.1 ACD/Labs 2019 Release (File version N05E41, Build 110555, 18 July 2019).
ACD/ChemSketch 2019.1.1 ACD/Labs 2019 Release (File version C05H41, Build 110712, 24 July 2019).
Suggested citation: EFSA (European Food Safety Authority) , Alvarez F, Arena M, Auteri D, Borroto J, Castoldi AF, Chiusolo A, Colagiorgi A, Colas M, Crivellente F, De Lentdecker C, Ferilli F, Ippolito A, Istace F, Kardassi D, Kienzler A, Linguadoca A, Mangas I, Molnar T, Parra Morte JM, Sharp R, Szentes C, Terron A, Tiramani M and Villamar‐Bouza L, 2021. Conclusion on the peer review of the pesticide risk assessment for the active substance acibenzolar‐S‐methyl in light of confirmatory data submitted. EFSA Journal 2021;19(7):6687, 12 pp. 10.2903/j.efsa.2021.6687
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00698
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Approved: 14 June 2021
Notes
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) 2016/389 of 17 March 2016 renewing the approval of the active substance acibenzolar‐S‐methyl in accordance with regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011. OJ L 73, 18.3.2016, p. 77–80.
Commission Regulation (EU) 2018/605 of 19 April 2018 amending Annex II to Regulation (EC) No 1107/2009 by setting out scientific criteria for the determination of endocrine disrupting properties. OJ L 101, 20.4.2018, p. 33–36.
Guidance for Thyroid Assays in Pregnant Animals, Fetuses and Postnatal Animals, and Adult Animals. October 24, 2005. Available online: https://www.epa.gov/sites/production/files/2015-06/documents/thyroid_guidance_assay.pdf
If there is information that the substance may act as an anti‐androgen, a test according to OECD TG 229 may not be suitable. In that case, the applicant may try to first address the concern regarding the potential for anti‐androgenicity or conduct a test according to OECD TG 234 as an alternative.
For humans, the endocrine activity for the E modality was sufficiently investigated and negative. The ER ToxCast model was available as explained in Section 1. Therefore, the E modality needs only to be further investigated for non‐target organisms.
References
- ECHA and EFSA (European Chemicals Agency and European Food Safety Authority) with the technical support of the Joint Research Centre (JRC), Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, Pellizzato F, Tarazona J, Terron A and Van der Linden S, 2018. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018;16(6):5311, 135 pp. 10.2903/j.efsa.2018.5311. ECHA‐18-G-01‐EN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA Journal 2010;8(6):1637, 90 pp. 10.2903/j.efsa.2010.1637 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Conclusion on the peer review of the pesticide risk assessment of the active substance acibenzolar‐S‐methyl. EFSA Journal 2014;12(8):3691, 73 pp. 10.2903/j.efsa.2014.3691 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2020a. Technical report on the outcome of the consultation with Member States, the applicant and EFSA on the pesticide risk assessment for acibenzolar‐S‐methyl in light of confirmatory data. EFSA supporting publication 2020;17(5):EN‐1846, 29 pp. 10.2903/sp.efsa.2020.EN-1846 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2020b. Technical report on the outcome of the pesticides peer review meeting on general recurring issues in mammalian toxicology. EFSA supporting publication 2020;17(5):EN‐1837, 26 pp. 10.2903/sp.efsa.2020.EN-1837 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2021. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance acibenzolar‐S‐methyl in light of confirmatory data submitted. Available online: www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- European Commission , 2013. Guidance document on the procedures for submission and assessment of confirmatory information following approval of an active substance in accordance with Regulation (EC) No 1107/2009. SANCO 5634/2009-rev. 6.1.
- France , 2019. Revised renewal assessment report on acibenzolar‐S-methyl, confirmatory data (Volume 1, Volumes 3 B6, B9), November 2019. Available online: www.efsa.europa.eu
- France , 2021. Revised renewal assessment report on acibenzolar‐S-methyl, confirmatory data, (Volume 1, Volumes 3 B6, B9), May 2021. Available online: www.efsa.europa.eu
- US EPA (United States Environmental Protection Agency), 2009. Endocrine Disruptor Screening Program Test Guidelines. Office of Prevention, Pesticides and Toxic Substances (OPPTS), US EPA, Washington (DC), OPPTS 890.1200: Aromatase (Human Recombinant), October 2009.


