Key Points
Question
Is there an association between dynamic measurable residual disease, treatment, and outcomes among adults with intermediate-risk acute myeloid leukemia?
Findings
In this registry-based cohort study that included 549 younger patients (aged 14-60 years) with intermediate-risk acute myeloid leukemia, 154 received chemotherapy, 116 received an autologous stem cell transplant, and 279 received an allogeneic stem cell transplant. Results showed that making a postremission treatment choice based on dynamic measurable residual disease was associated with improved outcomes in subgroup analyses.
Meaning
This study suggests that clinical decisions based on dynamic measurable residual disease might be associated with improved therapy stratification and optimized postremission treatment for patients with intermediate-risk acute myeloid leukemia.
Abstract
Importance
Measurable residual disease (MRD) is widely used as a therapy-stratification factor for acute myeloid leukemia (AML), but the association of dynamic MRD with postremission treatment (PRT) in patients with intermediate-risk AML (IR-AML) has not been well investigated.
Objective
To investigate PRT choices based on dynamic MRD in patients with IR-AML.
Design, Setting, and Participants
This cohort study examined 549 younger patients with de novo IR-AML in the South China Hematology Alliance database during the period from January 1, 2012, to June 30, 2016, including 154 who received chemotherapy, 116 who received an autologous stem cell transplant (auto-SCT), and 279 who received an allogeneic SCT (allo-SCT). Subgroup analyses were performed according to dynamic MRD after the first, second, and third courses of chemotherapy. The end point of the last follow-up was August 31, 2020. Statistical analysis was performed from December 1, 2019, to September 30, 2020.
Exposures
Receipt of chemotherapy, auto-SCT, or allo-SCT.
Main Outcomes and Measures
The primary end points were 5-year cumulative incidence of relapse and leukemia-free survival.
Results
Subgroup analyses were performed for 549 participants (314 male participants [57.2%]; median age, 37 years [range, 14-60 years]) according to the dynamics of MRD after 1, 2, or 3 courses of chemotherapy. Comparable cumulative incidences of relapse, leukemia-free survival, and overall survival were observed among participants who had no MRD after 1, 2, or 3 courses of chemotherapy. Participants who underwent chemotherapy and those who underwent auto-SCT had better graft-vs-host disease–free, relapse-free survival (GRFS) than those who underwent allo-SCT (chemotherapy: hazard ratio [HR], 0.35 [95% CI, 0.14-0.90]; P = .03; auto-SCT: HR, 0.07 [95% CI, 0.01-0.58]; P = .01). Among participants with MRD after 1 course of chemotherapy but no MRD after 2 or 3 courses, those who underwent auto-SCT and allo-SCT showed lower cumulative incidence of relapse (auto-SCT: HR, 0.25 [95% CI, 0.08-0.78]; P = .01; allo-SCT: HR, 0.08 [95% CI, 0.02-0.24]; P < .001), better leukemia-free survival (auto-SCT: HR, 0.26 [95% CI, 0.10-0.64]; P = .004; allo-SCT: HR, 0.21 [95% CI, 0.09-0.46]; P < .001), and overall survival (auto-SCT: HR, 0.22 [95% CI, 0.08-0.64]; P = .005; allo-SCT: HR, 0.25 [95% CI, 0.11-0.59]; P = .001) vs chemotherapy. In addition, auto-SCT showed better GRFS than allo-SCT (HR, 0.45 [95% CI, 0.21-0.98]; P = .04) in this group. Among participants with MRD after 1 or 2 courses of chemotherapy but no MRD after 3 courses, allo-SCT had superior cumulative incidence of relapse (HR, 0.10 [95% CI, 0.06-0.94]; P = .04) and leukemia-free survival (HR, 0.18 [95% CI, 0.05-0.68]; P = .01) compared with chemotherapy, but no advantageous cumulative incidence of relapse (HR, 0.15 [95% CI, 0.02-1.42]; P = .10) and leukemia-free survival (HR, 0.23 [95% CI, 0.05-1.08]; P = .06) compared with auto-SCT. Among participants with MRD after 3 courses of chemotherapy, allo-SCT had superior cumulative incidences of relapse, leukemia-free survival, and overall survival compared with chemotherapy (relapse: HR, 0.16 [95% CI, 0.08-0.33]; P < .001; leukemia-free survival: HR, 0.19 [95% CI, 0.10-0.35]; P < .001; overall survival: HR, 0.29 [95% CI, 0.15-0.55]; P < .001) and auto-SCT (relapse: HR, 0.25 [95% CI, 0.12-0.53]; P < .001; leukemia-free survival: HR, 0.35 [95% CI, 0.18-0.73]; P = .004; overall survival: HR, 0.54 [95% CI, 0.26-0.94]; P = .04). Among participants with recurrent MRD, allo-SCT was also associated with advantageous cumulative incidence of relapse, leukemia-free survival, and overall survival compared with chemotherapy (relapse: HR, 0.12 [95% CI, 0.04-0.33]; P < .001; leukemia-free survival: HR, 0.24 [95% CI, 0.10-0.56]; P = .001; overall survival: HR, 0.31 [95% CI, 0.13-0.75]; P = .01) and auto-SCT (relapse: HR, 0.28 [95% CI, 0.09-0.81]; P = .02; leukemia-free survival: HR, 0.30 [95% CI, 0.12-0.76]; P = .01; overall survival: HR, 0.26 [95% CI, 0.10-0.70]; P = .007).
Conclusions and Relevance
This study suggests that clinical decisions based on dynamic MRD might be associated with improved therapy stratification and optimized PRT for patients with IR-AML. Prospective multicenter trials are needed to further validate these findings.
This registry-based cohort study investigates postremission treatment choices and outcomes based on dynamic measurable residual disease in adults with intermediate-risk acute myeloid leukemia (AML).
Introduction
Most younger patients with acute myeloid leukemia (AML) can achieve complete remission (CR) with induction chemotherapy.1 After CR, further postremission treatment (PRT) would be necessary to prevent relapse.2 Postremission treatment usually consists of either a stem cell transplant (SCT) or cytarabine-based consolidation chemotherapy. Allogeneic SCT (allo-SCT) is generally accepted as the most effective PRT to prevent relapse, but it has relatively high rates of transplant-related mortality.3 Autologous SCT (auto-SCT) and chemotherapy carry a lower risk of transplant-related mortality compared with allo-SCT.4 However, this lower risk of transplant-related mortality is offset by a higher risk of relapse owing to the lack of a graft-vs-leukemia effect and the potential infusion of leukemia cells in grafts.5,6 Currently, decisions for PRT depend mainly on risk stratification based on cytogenetics and molecular markers,2,7 but it remains controversial, particularly for patients with intermediate-risk AML (IR-AML).3,4,5,6,8,9,10,11,12,13 For instance, some studies have suggested that a survival benefit is associated with the use of allo-SCT in patients with IR-AML,3,5,6 whereas other studies have showed that auto-SCT or chemotherapy had no significant survival differences compared with allo-SCT.4,12,13 Thus, these studies highlight the need to find additional factors associated with survival outcomes that optimize PRT choices for patients with IR-AML.
An increasing number of studies have demonstrated that measurable residual disease (MRD) is independently associated with relapse and with survival among patients with AML.14,15,16,17,18,19,20 Several studies have suggested that risk stratification by the combination of genetics and MRD might optimize PRT.19,20,21 However, these studies focused mainly on MRD at a specific time point. It might be best to think of MRD as a dynamic variable during the process of therapeutic decision-making.22,23,24 In this study, we retrospectively analyzed a large data set to explore the clinical significance of dynamic MRD on directing PRT for younger patients with IR-AML in first CR (CR1).
Methods
Study Population
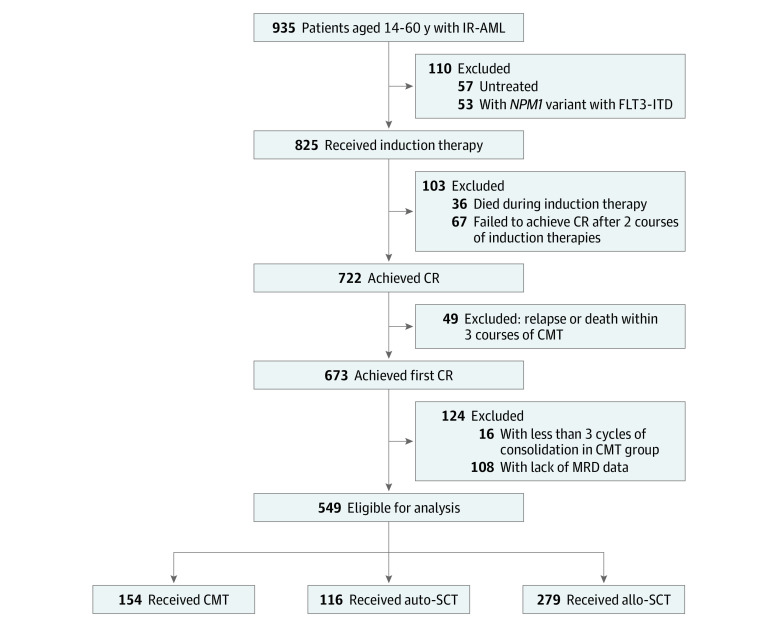
This registry-based cohort study examined all consecutive patients with de novo AML in the South China Hematology Alliance database between January 1, 2012, and June 30, 2016. Patients were enrolled if they (1) had received a diagnosis of IR-AML, (2) were in CR1, and (3) were 14 to 60 years of age. Exclusion criteria included the following: (1) acute promyelocytic leukemia, (2) NPM1 variant with an FLT3 internal tandem duplication, (3) failure to to achieve CR after 2 cycles of induction chemotherapy, (4) less than 3 cycles of consolidation in the chemotherapy group, and (5) lack of MRD parameters. The diagnosis of IR-AML was based on National Comprehensive Cancer Network criteria.2 Patients with an FLT3 internal tandem duplication variant were excluded because they received sorafenib tosylate treatment. According to the PRT strategy, patients were classified into 3 groups: chemotherapy, auto-SCT, and allo-SCT. Patients were followed up via telephone or outpatient visit by treating physicians. The end point of the last follow-up was August 31, 2020. This study was approved by the ethical review boards of Nanfang Hospital, Sun Yat-Sen Memorial Hospital, Guangdong Second Provincial General Hospital, Guangzhou First People’s Hospital, The First People’s Hospital of Chenzhou, The First Affiliated Hospital of Zhengzhou University, Shenzhen Hospital of Southern Medical University, Shenzhen Hospital of Peking University, The First Affiliated Hospital of Guangxi Medical University, Zhongshan People’s Hospital, and The First Affiliated Hospital of Chongqing Medical University. Written informed consent was obtained from participants in accordance with the modified Helsinki Declaration.25 This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.26
Treatment Procedures
According to our practical guidelines, patients are generally scheduled for induction therapy consisting of daunorubicin hydrochloride, 60 mg/m2, or idarubicin hydrochloride, 10 to 12 mg/m2, on days 1 to 3 and cytarabine, 200 mg/m2 per day, for 7 days. For patients who failed to achieve CR after the first induction regimen, a second induction regimen consisting of daunorubicin hydrochloride, 60 mg/m2, or idarubicin hydrochloride, 10 mg/m2 per day, on days 1 to 3 and cytarabine, 2.0 g/m2 twice daily, on days 1 to 3, or the same regimen as the first induction regimen was administered. After CR, usually 4 courses of cytarabine-based consolidation chemotherapy, 3 courses of chemotherapy followed by auto-SCT, or 2 courses of chemotherapy followed by allo-SCT were administered based on MRD status and donor availability. In auto-SCT, peripheral blood stem cell grafts were collected after mobilization with etoposide plus intermediate-dose cytarabine combined with granulocyte colony-stimulating factor. In allo-SCT, the principle of donor selection was based on the consensus in China.27 Busulfan-based myeloablative conditioning regimens were used for all patients.28 The prophylaxis for graft-vs-host disease (GVHD) was described previously.29
Cytogenetic and Molecular Assessment
Cytogenetic analyses were performed with Giemsa staining and reverse banding techniques and fluorescence in situ hybridization. Molecular markers were detected by polymerase chain reaction and next-generation sequencing.
MRD Detection
Generally, MRD in bone marrow was assessed after induction therapy and each cycle of PRT and then at 2-month intervals within the first year, 3-month intervals within the second year, 4-month intervals within the third year, and half-year intervals from the fourth to fifth year after PRT. Multiparameter flow cytometry was routinely used for MRD monitoring.30,31 The MRD level of 0.1% was used as a threshold to distinguish MRD positive from MRD negative.
Evaluation End Points and Definition
Evaluation end points included relapse, survival, and transplant-related mortality. Complete remission was defined as less than 5% blasts by morphologic evaluation of the bone marrow with no evidence of dysplasia in the bone marrow and no manifestation of leukemia outside the hematopoietic system. Relapse was defined by morphologic evidence in the peripheral blood, bone marrow, or extramedullary sites. Transplant-related mortality was estimated as death without evidence of leukemia recurrence. Leukemia-free survival was calculated from CR1 to relapse or death. Overall survival (OS) was calculated from CR1 to death. Graft-vs-host-disease–free, relapse-free survival (GRFS) events were defined as grade III or IV acute GVHD, chronic GVHD requiring systemic immunosuppressive treatment, leukemia relapse, or death from any cause from CR1 to last follow-up; GRFS in the chemotherapy and auto-SCT groups was considered equal to leukemia-free survival because of no incidence of GVHD in both groups, and it was compared with that in allo-SCT. Patients who did not achieve morphologic CR after the first induction regimen were considered to have MRD.
Statistical Analysis
Statistical analysis was performed from December 1, 2019, to September 30, 2020. Variables associated with patients, disease, and transplant characteristics among groups were compared using the Fisher exact test for categorical variables and the Mann-Whitney test for continuous variables. Numerical variables were analyzed as categories based on their values being below or above the median of the entire cohort. Overall survival, leukemia-free survival, and GRFS were estimated using the Kaplan-Meier method and compared by the log-rank test. Cumulative incidence curves were used in a competing risk setting with relapse treated as a competing event to calculate transplant-related mortality probabilities and with transplant-related mortality treated as a competing risk to calculate relapse. The correlations between MRD at different time points was analyzed by the Spearman test. The Cox proportional hazards regression model was used for the analysis of risk factors for time-to-event variables. The Fine-Gray model was used for analysis of end points involving competing risks.32 All tests were 2-sided, with significance set at P = .05. Stata SE, version 12.0 (StataCorp LP) and R, version 3.4.3 (R Group for Statistical Computing) were used for all data analysis.
Results
Patient Demographic Characteristics and Treatment Characteristics
A total of 549 consecutive patients (314 male patients [57.2%] and 235 female patients [42.8%]; median age, 37 years [range, 14-60 years]) were enrolled in this study, including 154 in the chemotherapy group, 116 in the auto-SCT group, and 279 in the allo-SCT group (Figure 1). In the allo-SCT group, 133 patients received transplants from matched sibling donors (MSDs), and 146 patients received transplants from alternative donors (ADs) (including 97 haploidentical donors and 45 suitably matched unrelated donors; 4 patients received umbilical cord blood). The median age of patients in the chemotherapy group was 47 years (range, 14-60 years), the median age of patients in the auto-SCT group was 33.5 years (range, 15-60 years), and the median age of patients in the allo-SCT group was 35 years (range, 14-60 years). Patient demographic and treatment characteristics for the 3 groups are presented in Table 1.
Figure 1. Flow Diagram of Study Participants.
Allo-SCT indicates allogeneic stem cell transplant; auto-SCT, autologous stem cell transplant; CMT, chemotherapy; CR, complete remission; IR-AML, intermediate-risk acute myeloid leukemia; ITD, internal tandem duplication; and MRD, measurable residual disease.
Table 1. Demographic and Treatment Characteristics of Patients.
| Characteristic | Patients, No. (%) | P value | ||
|---|---|---|---|---|
| CMT (n = 154) | Auto-SCT (n = 116) | Allo-SCT (n = 279) | ||
| Age, median (range), y | 47 (14-60) | 33.5 (15-60) | 35 (14-60) | <.001 |
| Sex | ||||
| Male | 85 (55.2) | 74 (63.8) | 155 (55.6) | .27 |
| Female | 69 (44.8) | 42 (36.2) | 124 (44.4) | |
| WBC count, median (range), /µL | 19 400 (800-407 000) | 21 400 (1900-325 000) | 20 300 (600-376 300) | .73 |
| First induction therapy | ||||
| DA | 42 (27.3) | 29 (25.0) | 60 (21.5) | .34 |
| IA | 89 (57.8) | 70 (60.3) | 188 (67.4) | |
| Other | 23 (14.9) | 17 (14.7) | 31 (11.1) | |
| Second induction therapy or first consolidation therapy | ||||
| DA | 14 (9.1) | 8 (6.9) | 16 (5.7) | .81 |
| IA | 14 (9.1) | 13 (11.2) | 21 (7.5) | |
| 3 + 3 | 48 (31.2) | 31 (26.7) | 80 (28.7) | |
| Intermediate- or high-dose cytarabine | 68 (44.2) | 56 (48.3) | 144 (51.6) | |
| Others | 10 (6.5) | 8 (6.9) | 18 (6.5) | |
| No. of cycles of consolidation chemotherapy, median (range) | 4 (3-7) | 3 (3-5) | 2 (1-4) | NA |
| Duration from CR1 to transplant, median (range), mo | NA | 5.9 (5.4-9.6) | 3.9 (2.8-7.7) | <.001 |
| No. of cycles to CR | ||||
| 1 | 126 (81.8) | 97 (83.6) | 217 (77.8) | .34 |
| 2 | 28 (18.2) | 19 (16.4) | 62 (22.2) | |
| MRD1 | ||||
| Positive | 81 (52.6) | 64 (55.2) | 215 (77.1) | <.001 |
| Negative | 73 (47.4) | 52 (44.8) | 64 (22.9) | |
| MRD2 | ||||
| Positive | 52 (33.8) | 33 (28.4) | 132 (47.3) | .001 |
| Negative | 102 (66.2) | 83 (71.6) | 147 (52.7) | |
| MRD3 | ||||
| Positive | 39 (25.3) | 26 (22.4) | 105 (37.6) | .002 |
| Negative | 115 (74.7) | 90 (77.6) | 174 (62.4) | |
Abbreviations: 3 + 3, daunorubicin hydrochloride or idarubicin hydrochloride, 10 mg/m2 per day, on days 1 to 3 and cytarabine, 2.0 g/m2 twice daily, on days 1 to 3; allo-SCT, allogeneic stem cell transplant; auto-SCT, autologous stem cell transplant; CMT, chemotherapy; CR, complete remission; CR1, first CR; DA, daunorubicin hydrochloride and cytarabine; IA, idarubicin hydrochloride and cytarabine; MRD1, measurable residual disease after 1 course of CMT; MRD2, measurable residual disease after 2 courses of CMT; MRD3, measurable residual disease after 3 courses of CMT; NA, not applicable.
SI conversion factor: To convert WBC to ×109 per liter, multiply by 0.001.
Relapse and Survival
At last follow-up, 146 patients had relapsed. The median time from CR1 to relapse of the total cohort was 10.9 months (range, 5.4-42.4 months), the median time from CR1 to relapse of patients in the chemotherapy group was 9.9 months (range, 5.4-35.4 months), the median time from CR1 to relapse of patients in the auto-SCT group was 10.9 months (range, 7.2-38.2 months), and the median time from CR1 to relapse of patients in the allo-SCT group was 13.0 months (range, 7.4-42.4 months) (P = .03). The time from CR1 to relapse was much longer in the allo-SCT group compared with the chemotherapy group (P = .01), but no significant difference between the allo-SCT and auto-SCT groups (P = .09) or between the auto-SCT and chemotherapy groups (P = .38) was found. Of the 76 patients in the chemotherapy group who relapsed, 58 received further treatment (23 allo-SCT), and 13 survived at the last follow-up. Of the 32 patients in the auto-SCT group who relapsed, 26 received further treatment (15 allo-SCT), and 7 survived at the last follow-up. Of the 38 patients in the allo-SCT group who relapsed, 29 received salvage treatment, and 6 survived at the last follow-up. The 5-year cumulative incidence of relapse was 49.4% (95% CI, 41.2%-57.0%) in the chemotherapy group, 27.6% (95% CI, 19.8%-36.0%) in the auto-SCT group, and 13.6% (95% CI, 9.9%-17.9%) in the allo-SCT group (P < .001) (Figure 2A). Patients in the allo-SCT group showed significantly lower cumulative incidence of relapse than those in the auto-SCT group (hazard ratio [HR], 0.44 [95% CI, 0.28-0.71]; P = .001) and chemotherapy group (HR, 0.21 [95% CI, 0.14-0.31]; P < .001), and those in the auto-SCT group had lower cumulative incidence of relapse than those in the chemotherapy group (HR, 0.47 [95% CI, 0.31-0.71]; P < .001). For patients who underwent allo-SCT, the cumulative incidence of relapse was not significantly different between patients who received transplants from ADs (AD-SCT) and those who received transplants from MSDs (MSD-SCT) (HR, 0.59 [95% CI, 0.31-1.12]; P = .11).
Figure 2. Outcomes for All Patients Based on Postremission Treatment.
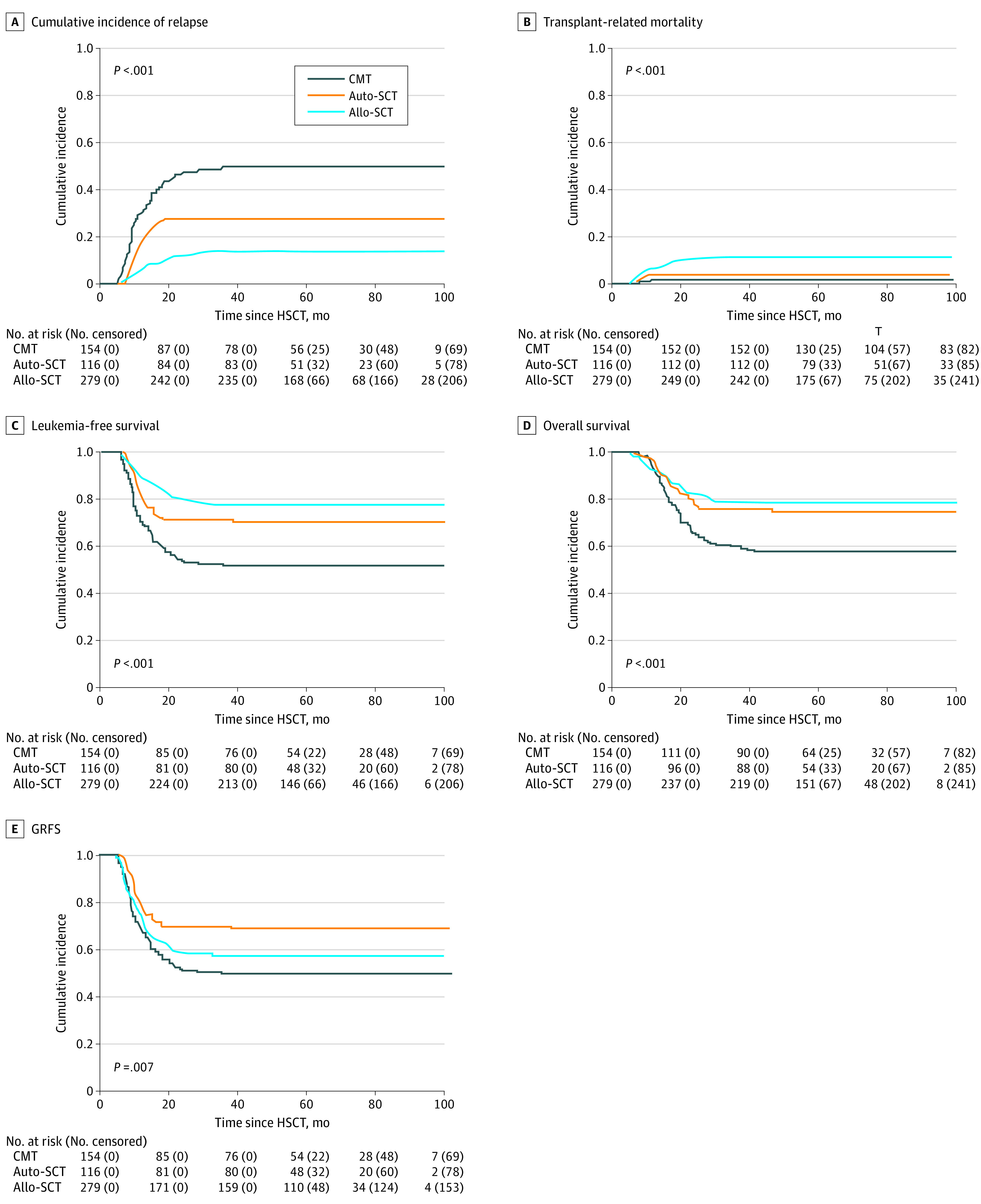
Allo-SCT indicates allogeneic stem cell transplant; auto-SCT, autologous stem cell transplant; CMT, chemotherapy; GRFS, graft-vs-host disease–free, relapse-free surviva; HSCT, hematopoietic stem cell transplant; and SCT, stem cell transplant.
With a median follow-up of 48.1 months (range, 5.4-94.7 months) after CR1, 394 patients survived and 155 died. The causes of death are shown in the eTable in the Supplement. The 5-year cumulative incidence of transplant-related mortality was 1.3% (95% CI, 0.3%-4.2%) in the chemotherapy group, 3.4% (95% CI, 1.1%-8.0%) in the auto-SCT group, and 10.4% (95% CI, 7.2%-14.3%) in the allo-SCT group (P < .001) (Figure 2B). Patients in the allo-SCT group had a higher rate of transplant-related mortality than those in the chemotherapy group (HR, 9.36 [95% CI, 1.99-35.07]; P = .004) and those in the auto-SCT group (HR, 3.10 [95% CI, 1.08-8.86]; P = .04), whereas there was no significant difference between patients in the auto-SCT and chemotherapy groups (HR, 2.70 [95% CI, 0.49-14.78]; P = .25). The 5-year leukemia-free survival rate was 49.3% (95% CI, 41.2%-57.0%) in the chemotherapy group, 69.0% (95% CI, 59.7%-76.5%) in the auto-SCT group, and 76.0% (95% CI, 70.5%-80.6%) in the allo-SCT group (P < .001) (Figure 2C). Leukemia-free survival was significantly higher among patients in the auto-SCT and allo-SCT groups compared with those in the chemotherapy (auto-SCT: HR, 0.53 [95% CI, 0.36-0.78]; P = .002; and allo-SCT: HR, 0.38 [95% CI, 0.27-0.53]; P < .001), but it was comparable between the allo-SCT and auto-SCT groups (HR, 0.72 [95% CI, 0.48-1.08]; P = .11). The 5-year OS rate was 57.6% (95% CI, 49.4%-65.0%) in the chemotherapy group, 75.0% (95% CI, 66.1%-81.9%) in the auto-SCT group, and 78.1% (95% CI, 72.8%-82.5%) in the allo-SCT group (P < .001) (Figure 2D). Overall survival was significantly better in the auto-SCT and allo-SCT groups than in the chemotherapy group (auto-SCT: HR, 0.54 [95% CI, 0.35-0.84]; P = .006; allo-SCT: HR, 0.47 [95% CI, 0.33-0.66]; P < .001, respectively), but it was comparable between the allo-SCT and auto-SCT groups (HR, 0.87 [95% CI, 0.56-1.35]; P = .52). The 5-year GRFS rate was 49.3% (95% CI, 41.2%-57.0%) in the chemotherapy group, 69.0% (95% CI, 59.7%-76.5%) in the auto-SCT group, and 56.5% (95% CI, 50.5%-62.1%) in the allo-SCT group (P = .007) (Figure 2E). Patients in the auto-SCT group had better GRFS than those in the chemotherapy group (HR, 0.54 [95% CI, 0.36-0.79]; P = .002) and those in the allo-SCT group (HR, 0.65 [95% CI, 0.45-0.94]; P = .02), but there was no significant difference between patients in the allo-SCT group and patients in the chemotherapy group (HR, 0.82 [95% CI, 0.62-1.09]; P = .17). In addition, subgroup analysis showed that, compared with patients who received MSD-SCT, those who received AD-SCT did not have higher transplant-related mortality (HR, 2.11 [95% CI, 0.97-4.61]; P = .06) but did have similar leukemia-free survival (HR, 1.04 [95% CI, 0.64-1.67]; P = .89), OS (HR, 1.06 [95% CI, 0.64-1.75]; P = .83), and GRFS rates (HR, 1.35 [95% CI, 0.94-1.94]; P = .10).
Multivariate Analysis of Relapse and Survival
The univariate and multivariate analyses of relapse, leukemia-free survival, and OS are presented in Table 2. Competing risk regression showed that needing 2 cycles to achieve CR and having MRD after 3 courses of chemotherapy were risk factors for relapse in the entire population. Cox proportional hazards regression analysis revealed that needing 2 cycles to achieve CR and having MRD after 3 cycles of chemotherapy were also risk factors for leukemia-free survival and OS. Allo-SCT had a beneficial association with relapse, leukemia-free survival, and OS in multivariate analysis when taking auto-SCT or chemotherapy as a reference. In addition, auto-SCT had a beneficial association with relapse, leukemia-free survival, and OS when taking chemotherapy as a reference.
Table 2. Multivariate Analysis for Relapse, Leukemia-Free Survival, and Overall Survival.
| Variable | Relapse | Leukemia-free survival | Overall survival | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, ≥37 y vs <37 y | 1.02 (0.71-1.45) | .89 | 1.02 (0.71-1.47) | .93 | 1.19 (0.84-1.68) | .33 |
| Sex, female vs male | 1.04 (0.73-1.46) | .85 | 1.12 (0.79-1.58) | .65 | 1.09 (0.79-1.51) | .59 |
| WBC count, ≥20 000/µL vs <20 000/µL | 1.33 (0.93-1.90) | .12 | 1.27 (1.00-1.79) | .18 | 1.20 (0.86-1.66) | .29 |
| Cycles to achieve CR, 2 vs 1 | 1.81 (1.19-2.76) | .006 | 1.97 (1.33-2.90) | .001 | 1.78 (1.23-2.58) | .002 |
| MRD1 | 1.24 (0.44-3.56) | .68 | 1.11 (0.42-3.04) | .81 | 1.35 (0.48-3.78) | .57 |
| MRD2 | 1.47 (0.92-2.36) | .19 | 1.49 (0.95-2.33) | .19 | 1.32 (0.86-2.02) | .28 |
| MRD3 | 3.93 (1.55-9.97) | .004 | 3.93 (1.65-9.37) | .002 | 6.21 (2.47-15.63) | <.001 |
| PRT | ||||||
| Auto-SCT vs CMT | 0.49 (0.31-0.78) | .002 | 0.50 (0.32-0.79) | .003 | 0.60 (0.38-0.94) | .03 |
| Allo-SCT vs CMT | 0.12 (0.08-0.19) | <.001 | 0.12 (0.08-0.19) | <.001 | 0.33 (0.23-0.48) | <.001 |
| Allo-SCT vs auto-SCT | 0.25 (0.16-0.41) | <.001 | 0.24 (0.15-0.39) | <.001 | 0.56 (0.36-0.88) | .01 |
Abbreviations: allo-SCT, allogeneic stem cell transplant; auto-SCT, autologous stem cell transplant; CMT, chemotherapy; CR, complete remission; HR, hazard ratio; MRD1, measurable residual disease after 1 course of CMT; MRD2, measurable residual disease after 2 courses of CMT; MRD3, measurable residual disease after 3 courses of CMT; PRT, postremission treatment; WBC, white blood cell.
Dynamic MRD and Direct PRT Selection
To explore the association of dynamic MRD with PRT selection for patients with IR-AML, subgroup analyses were performed according to the dynamics of MRD after 1 course of chemotherapy, MRD after 2 courses of chemotherapy, and MRD after 3 courses of chemotherapy. Patients were classified into the following 5 subgroups: (1) subgroup A, defined as being persistent MRD negative after the first induction regimen for all 3 courses; (2) subgroup B, defined as being persistent MRD positive after all 3 courses; (3) subgroup C, defined as being recurrent MRD positive after being MRD negative; (4) subgroup D, defined as being MRD negative after 2 courses of chemotherapy; and (5) subgroup E, defined as being MRD negative after 3 courses of chemotherapy. The univariate analysis of each subgroup is presented in Table 3. Furthermore, subgroup analyses were performed after adjustment for various covariates, including age, sex, white blood cell count, and number of cycles to achieve CR.
Table 3. Outcomes of Patients (at 5 Years) Receiving Different PRT Based on Dynamics of MRD1, MRD2, and MRD3.
| MRD status and PRT | No. | % (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Overall survival | Leukemia-free survival | CIR | TRM | GRFS | |||
| Subgroup Aa | |||||||
| CMT | 55 | 93.6 (81.5-98.0) | 87.3 (75.2-93.7) | 12.7 (5.5-23.0) | 0 | 87.3 (75.2-93.7) | |
| Auto-SCT | 37 | 97.3 (82.3-99.6) | 97.3 (82.3-99.6) | 2.7 (0.2-12.3) | 0 | 97.3 (82.3-99.6) | |
| Allo-SCT | 41 | 88.5 (68.4-96.1) | 90.3 (76.1-96.2) | 2.4 (0.2-11.2) | 7.3 (1.9-18.0) | 70.7 (54.3-82.2) | |
| P value | .40 | .27 | .07 | .03b | .003 | ||
| Subgroup B | |||||||
| CMT | 21 | 14.3 (3.6-32.1) | 4.8 (0.3-19.7) | 90.5 (59.8-98.1) | 4.7 (0.3-20.5) | 4.8 (0.3-19.7) | |
| Auto-SCT | 16 | 37.5 (15.4-59.8) | 25.0 (7.8-47.2) | 68.8 (38.0-86.5) | 6.3 (0.4-25.7) | 25.0 (7.8-47.2) | |
| Allo-SCT | 77 | 64.9 (53.2-74.4) | 61.0 (49.2-70.9) | 26.0 (16.7-36.2) | 13.0 (6.6-21.6) | 44.2 (32.9-54.8) | |
| P value | <.001 | <.001 | <.001 | .48 | <.001 | ||
| Subgroup C | |||||||
| CMT | 22 | 27.3 (11.1-46.4) | 18.2 (5.7-36.3) | 81.8 (56.4-93.2) | 0 | 18.2 (5.7-36.3) | |
| Auto-SCT | 17 | 41.2 (18.6-62.6) | 35.3 (14.5-57.0) | 52.9 (26.2-73.9) | 11.8 (1.8-32.0) | 35.3 (14.5-57.0) | |
| Allo-SCT | 31 | 71.0 (51.6-83.7) | 67.7 (48.4-81.2) | 22.6 (9.8-38.6) | 9.7 (2.4-23.2) | 48.4 (30.2-64.4) | |
| P value | .02 | .001 | <.001 | .28 | .10 | ||
| Subgroup D | |||||||
| CMT | 35 | 51.4 (34.0-66.4) | 45.7 (28.9-61.1) | 51.4 (33.6-66.7) | 2.9 (0.2-13.0) | 45.7 (28.9-61.1) | |
| Auto-SCT | 38 | 86.8 (71.2-94.3) | 78.9 (62.3-88.9) | 21.1 (9.8-35.2) | 0 | 78.9 (62.3-88.9) | |
| Allo-SCT | 85 | 87.1 (77.9-92.6) | 84.7 (75.1-90.8) | 5.9 (2.2-12.3) | 9.4 (4.4-16.8) | 64.5 (53.3-73.7) | |
| P value | <.001 | <.001 | <.001 | .08c | .03 | ||
| Subgroup E | |||||||
| CMT | 21 | 45.8 (23.4-65.8) | 33.3 (14.9-53.1) | 66.7 (41.2-83.1) | 0 | 33.3 (14.9-53.1) | |
| Auto-SCT | 8 | 62.5 (22.9-86.1) | 50.0 (15.2-77.5) | 37.5 (7.0-69.7) | 12.5 (0.4-45.3) | 50.0 (15.2-77.5) | |
| Allo-SCT | 45 | 77.8 (62.6-87.4) | 77.8 (62.6-87.4) | 11.1 (4.0-22.3) | 11.1 (4.0-22.3) | 55.6 (40.0-68.6) | |
| P value | .02 | <.001 | <.001 | .29 | .14 | ||
Abbreviations: allo-SCT, allogeneic stem cell transplant; auto-SCT, autologous stem cell transplant; CIR, cumulative incidence of relapse; CMT, chemotherapy; GRFS, graft-vs-host disease–free, relapse-free survival; MRD, measurable residual disease; MRD1, measurable residual disease after 1 course of CMT; MRD2, measurable residual disease after 2 courses of CMT; MRD3, measurable residual disease after 3 courses of CMT; PRT, postremission treatment; TRM, transplant-related mortality.
Subgroup A, patients who became MRD negative after 1 course of CMT and were persistently MRD negative; subgroup B, persistently positive MRD; subgroup C, recurrent MRD-positive patients after being MRD negative; subgroup D, patients who become MRD negative after 2 courses of CMT; and subgroup E, patients who become MRD negative after 3 courses of CMT.
CMT vs allo-SCT (P = .04) and auto-SCT vs allo-SCT (P = .09).
Auto-SCT vs allo-SCT (P = .04).
In subgroup A, comparable cumulative incidence of relapse (HR, 0.37 [95% CI, 0.11-1.20]; P = .10), leukemia-free survival (HR, 0.83 [95% CI, 0.41-1.66]; P = .59), and OS (HR, 1.48 [95% CI, 0.64-3.45]; P = .36) were found among the 3 different PRT groups. In addition, better GRFS was observed for those in the chemotherapy group (HR, 0.35 [95% CI, 0.14-1.00]; P = .03) and the auto-SCT group (HR, 0.07 [95% CI, 0.01-0.58]; P = .01) compared with the allo-SCT group, whereas GRFS was comparable between those in the auto-SCT and chemotherapy groups (HR, 0.21 [95% CI, 0.03-1.74]; P = .15).
In subgroup B, patients in the allo-SCT group had a lower cumulative incidence of relapse than those in the chemotherapy group (HR, 0.16 [95% CI, 0.08-0.33]; P < .001) and those in the auto-SCT group (HR, 0.25 [95% CI, 0.12-0.53]; P < .001), as well as better leukemia-free survival and OS compared with the chemotherapy group (leukemia-free survival: HR, 0.19 [95% CI, 0.10-0.35]; P < .001; OS: HR, 0.30 [95% CI, 0.15-0.55]; P < .001) and the auto-SCT group (leukemia-free survival: HR, 0.35 [95% CI, 0.19-0.73]; P = .004; OS: HR, 0.54 [95% CI, 0.26-0.94]; P = .04).
In subgroup C, patients in the allo-SCT group had better leukemia-free survival and OS compared with patients in the chemotherapy group (leukemia-free survival: HR, 0.24 [95% CI, 0.10-0.56]; P = .001; OS: HR, 0.31 [95% CI, 0.13-0.75]; P = .01) and patients in the auto-SCT group (leukemia-free survival: HR, 0.30 [95% CI, 0.12-0.76]; P = .01; OS: HR, 0.26 [95% CI, 0.10-0.70]; P = .007) owing to a lower cumulative incidence of relapse (chemotherapy: HR, 0.12 [95% CI, 0.04-0.33]; P < .001; auto-SCT: HR, 0.28 [95% CI, 0.09-0.81]; P = .02).
In subgroup D, patients who underwent auto-SCT had a significantly lower cumulative incidence of relapse and better leukemia-free survival and OS compared with those in the chemotherapy group (cumulative incidence of relapse: HR, 0.25 [95% CI, 0.08-0.78]; P = .01; leukemia-free survival: HR, 0.26 [95% CI, 0.10-0.64]; P = .004; OS: HR, 0.22 [95% CI, 0.08-0.64]; P = .005) and those in the allo-SCT group (cumulative incidence of relapse: HR, 0.08 [95% CI, 0.02-0.24]; P < .001; leukemia-free survival: HR, 0.21 [95% CI, 0.09-0.46]; P < .001; OS: HR, 0.25 [95% CI, 0.11-0.59]; P = .001). Patients in the allo-SCT group had a lower cumulative incidence of relapse than those in the auto-SCT group (HR, 0.31 [95% CI, 0.09-0.96]; P = .04) but comparable leukemia-free survival (HR, 1.24 [95% CI, 0.51-3.00]; P = .64) and OS (HR, 0.86 [95% CI, 0.30-2.49]; P = .78). In addition, patients in the auto-SCT group achieved better GRFS compared with those in the allo-SCT group (HR, 0.45 [95% CI, 0.21-0.98]; P = .04).
In subgroup E, patients in the allo-SCT group had a lower cumulative incidence of relapse and better leukemia-free survival compared with those in the chemotherapy group (cumulative incidence of relapse: HR, 0.10 [95% CI, 0.06-0.94]; P = .04; leukemia-free survival: HR, 0.18 [95% CI, 0.05-0.68]; P = .01), whereas OS was comparable between the 2 groups (HR, 0.42 [95% CI, 0.14-1.31]; P = .13). Patients in the allo-SCT group did not have a lower cumulative incidence of relapse (HR, 0.15 [95% CI, 0.02-1.42]; P = .10), better leukemia-free survival (HR, 0.23 [95% CI, 0.05-1.08]; P = .06), or difference in OS (HR, 0.46 [95% CI, 0.10-2.18]; P = .33) compared with those in the auto-SCT group. In addition, exploratory subgroup analyses of survival revealed similar leukemia-free survival, OS, and GRFS between patients who received AD-SCT and those who received MSD-SCT in all 5 subgroups (eFigure in the Supplement).
Discussion
This study aimed to explore PRT choices based on dynamic MRD for younger patients with IR-AML. The results suggest the following: (1) chemotherapy and auto-SCT were associated with better GRFS vs allo-SCT for patients who were persistently MRD negative after the first induction regimen; (2) patients who were persistently MRD positive and those with recurrent MRD who received allo-SCT had better leukemia-free survival and OS than those who received auto-SCT or chemotherapy; (3) patients who were MRD negative after 2 cycles of chemotherapy had better leukemia-free survival and OS with auto-SCT and allo-SCT than with chemotherapy, whereas those receiving auto-SCT showed better GRFS compared with those receiving allo-SCT; and (4) for patients who were MRD negative after 3 cycles of chemotherapy, allo-SCT had comparatively more favorable survival outcomes. To our knowledge, this is the first attempt to investigate PRT strategies based on dynamic MRD for patients with IR-AML.
It remains a challenge for practitioners to choose the optimal PRT for patients with IR-AML because different conclusions have been drawn in previous reports.3,4,8,9,10,11,12,13,33 On the one hand, some studies have suggested that patients with IR-AML might benefit from allo-SCT. For instance, MSD-SCT or matched unrelated donor–SCT might be superior to chemotherapy or auto-SCT.3,6,11,33 In addition, some investigators have reported that haploidentical donor–SCT also achieved more favorable survival outcomes than chemotherapy or auto-SCT owing to significantly lower cumulative incidence of relapse and acceptable transplant-related mortality.5,34,35 On the other hand, some studies have indicated that chemotherapy or auto-SCT might achieve comparable survival compared with allo-SCT because the disadvantage in a higher cumulative incidence of relapse in the former was offset by lower transplant-related mortality.9,10,12,13 The controversies in this issue highlight the limited therapy-directing ability of genetics alone and the need for integrating predictive factors, such as MRD monitoring, after diagnosis. Measurable residual disease has been extensively used as a therapy-stratification parameter for AML.19,20,21,36,37,38 Recently, the GIMEMA AML1310 trial developed a strategy of making PRT choices based on MRD after 1 consolidation for patients with IR-AML.21 In that trial, MRD-negative patients were to receive auto-SCT, while MRD-positive patients were to receive allo-SCT; similar OS and leukemia-free survival were observed between both groups of patients. However, the best timing for treatment choice based on MRD is still debatable.23,39 Freeman et al20 reported that MRD after induction therapy could improve outcome stratification by redefining partial response for IR-AML. However, Yao et al37 and Venditti et al21 suggested that MRD after 1 consolidation might be the best timing for choosing PRT. Lv et al5 reported that MRD after the second consolidation was independently associated with cumulative incidence of relapse, leukemia-free survival, and OS for patients with IR-AML. These studies all focused on the value of static MRD for prognosis and treatment choice.
In our study, allo-SCT and auto-SCT showed advantageous cumulative incidence of relapse, leukemia-free survival, and OS compared with chemotherapy, and allo-SCT had lower cumulative incidence of relapse but higher transplant-related mortality compared with auto-SCT, with comparable leukemia-free survival and OS in the entire population. Further subgroup analyses based on dynamic MRD showed that chemotherapy and auto-SCT had comparable cumulative incidence of relapse, leukemia-free survival, and OS as allo-SCT but better GRFS than allo-SCT owing to lower transplant-related mortality and no GVHD in patients who were persistently MRD negative. For patients who were persistently MRD positive and patients with recurrent MRD, allo-SCT had better leukemia-free survival and OS than chemotherapy, and auto-SCT had a lower cumulative incidence of relapse. For the patients who were MRD negative after 2 cycles of chemotherapy, auto-SCT and allo-SCT had superior cumulative incidence of relapse, leukemia-free survival, and OS compared with chemotherapy. Allo-SCT had a lower cumulative incidence of relapse but higher transplant-related mortality than auto-SCT and comparable leukemia-free survival and OS between the 2 groups. However, auto-SCT had better GRFS than allo-SCT. For patients who were MRD negative after 3 cycles of chemotherapy, allo-SCT had a better cumulative incidence of relapse and leukemia-free survival than chemotherapy. Based on these results, we suggest that chemotherapy and auto-SCT might be preferable for patients who are persistently MRD negative, and allo-SCT should be recommended for patients who are persistently MRD positive and patients with recurrent MRD. Auto-SCT prior to allo-SCT might be recommended for patients who are MRD negative after 2 cycles of chemotherapy. Allo-SCT might be preferable for patients who are MRD negative after 3 cycles of chemotherapy.
During the last decade, an increasing number of studies have shown that transplants using ADs achieve similar outcomes as MSD-SCT.30,40,41,42,43,44 In our study, the results revealed that AD-SCT achieved comparable leukemia-free survival, OS, and GRFS as MSD-SCT in all subgroup analyses, which was consistent with previous studies.30,40,41,42
Number of cycles to achieve CR was another factor associated with prognosis.45,46 Our results suggested that needing 2 cycles to achieve CR was an independent risk factor for relapse, leukemia-free survival, and OS, in accordance with previous studies.45,46 Old age was usually associated with poor prognosis because of unfavorable genetics, chemotherapeutic resistance, poor performance status, and frequent comorbidities.47,48 In our study, patients in the chemotherapy group were significantly older than those in the auto-SCT and allo-SCT groups. However, age was not associated with relapse or survival in univariate and multivariate analyses. The possible reason for our results might be that patients were excluded if they were older than 60 years, failed to achieve CR after 2 cycles of chemotherapy, or had received a diagnosis of poor-risk genetics.
Limitations
This study has some limitations. Although the data came from a registered database, the bias of a retrospective study was inevitable. To address this issue and further validate our findings, we have conducted a prospective, multicenter trial on dynamic MRD-directed therapies for AML (ClinicalTrials.gov identifier: NCT02870777).
Conclusions
Our results suggest that clinical decisions based on dynamic MRD might be associated with improved therapy stratification and optimized PRT for patients with IR-AML. CMT and auto-SCT might be preferable for the persistent MRD-negative patients, and allo-SCT should be strongly recommended for the persistent MRD-positive and recurrent MRD-positive patients. Auto-SCT prior to allo-SCT might be recommended for the patients who were MRD negative after 2 cycles of chemotherapy. Allo-SCT might be preferable for the patients who were MRD negative after 3 cycles of chemotherapy. Prospective multicenter trials are needed to further validate our findings.
eTable. Causes of Death Posttransplantation
eFigure. Forest Plots for Survival of MSD-SCT and AD-SCT
References
- 1.Tefferi A, Letendre L. Going beyond 7 + 3 regimens in the treatment of adult acute myeloid leukemia. J Clin Oncol. 2012;30(20):2425-2428. doi: 10.1200/JCO.2011.38.9601 [DOI] [PubMed] [Google Scholar]
- 2.Tallman MS, Wang ES, Altman JK, et al. ; OCN . Acute myeloid leukemia, version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(6):721-749. doi: 10.6004/jnccn.2019.0028 [DOI] [PubMed] [Google Scholar]
- 3.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349-2361. doi: 10.1001/jama.2009.813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizutani M, Hara M, Fujita H, et al. Comparable outcomes between autologous and allogeneic transplant for adult acute myeloid leukemia in first CR. Bone Marrow Transplant. 2016;51(5):645-653. doi: 10.1038/bmt.2015.349 [DOI] [PubMed] [Google Scholar]
- 5.Lv M, Wang Y, Chang YJ, et al. Myeloablative haploidentical transplantation is superior to chemotherapy for patients with intermediate-risk acute myelogenous leukemia in first complete remission. Clin Cancer Res. 2019;25(6):1737-1748. doi: 10.1158/1078-0432.CCR-18-1637 [DOI] [PubMed] [Google Scholar]
- 6.Cho BS, Kim JH, Yoon JH, et al. Superior transplantation outcomes of 8/8-matched unrelated donors as well as matched siblings to autologous transplantation for acute myeloid leukemia with intermediate cytogenetics in first remission. Eur J Haematol. 2013;90(5):365-374. doi: 10.1111/ejh.12089 [DOI] [PubMed] [Google Scholar]
- 7.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. doi: 10.1182/blood-2016-08-733196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zittoun RA, Mandelli F, Willemze R, et al. ; European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto (GIMEMA) Leukemia Cooperative Groups. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. N Engl J Med. 1995;332(4):217-223. doi: 10.1056/NEJM199501263320403 [DOI] [PubMed] [Google Scholar]
- 9.Saraceni F, Labopin M, Gorin NC, et al. ; Acute Leukemia Working Party (ALWP) of the European society for Blood and Marrow Transplantation (EBMT) . Matched and mismatched unrelated donor compared to autologous stem cell transplantation for acute myeloid leukemia in first complete remission: a retrospective, propensity score–weighted analysis from the ALWP of the EBMT. J Hematol Oncol. 2016;9(1):79. doi: 10.1186/s13045-016-0314-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saraceni F, Bruno B, Lemoli RM, et al. Autologous stem cell transplantation is still a valid option in good- and intermediate-risk AML: a GITMO survey on 809 patients autografted in first complete remission. Bone Marrow Transplant. 2017;52(1):163-166. doi: 10.1038/bmt.2016.233 [DOI] [PubMed] [Google Scholar]
- 11.Cornelissen JJ, Versluis J, Passweg JR, et al. ; HOVON; SAKK Leukemia Groups . Comparative therapeutic value of post-remission approaches in patients with acute myeloid leukemia aged 40-60 years. Leukemia. 2015;29(5):1041-1050. doi: 10.1038/leu.2014.332 [DOI] [PubMed] [Google Scholar]
- 12.Limvorapitak W, Barnett MJ, Hogge DE, et al. Outcomes of intermediate risk karyotype acute myeloid leukemia in first remission undergoing autologous stem cell transplantation compared with allogeneic stem cell transplantation and chemotherapy consolidation: a retrospective, propensity-score adjusted analysis. Clin Lymphoma Myeloma Leuk. 2018;18(11):e481-e491. doi: 10.1016/j.clml.2018.07.290 [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Liu Y, Wang Q, Chen L, Ma L, Hao S. Autologous stem cell transplantation is a viable postremission therapy for intermediate-risk acute myeloid leukemia in first complete remission in the absence of a matched identical sibling: a meta-analysis. Acta Haematol. 2019;141(3):164-175. doi: 10.1159/000495206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter RB, Gyurkocza B, Storer BE, et al. Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia. 2015;29(1):137-144. doi: 10.1038/leu.2014.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Xie H, Wood BL, et al. Relation of clinical response and minimal residual disease and their prognostic impact on outcome in acute myeloid leukemia. J Clin Oncol. 2015;33(11):1258-1264. doi: 10.1200/JCO.2014.58.3518 [DOI] [PubMed] [Google Scholar]
- 16.Hourigan CS, Gale RP, Gormley NJ, Ossenkoppele GJ, Walter RB. Measurable residual disease testing in acute myeloid leukaemia. Leukemia. 2017;31(7):1482-1490. doi: 10.1038/leu.2017.113 [DOI] [PubMed] [Google Scholar]
- 17.Hills RK, Ivey A, Grimwade D; UK National Cancer Research Institute (NCRI) AML Working Group . Assessment of minimal residual disease in standard-risk AML. N Engl J Med. 2016;375(6):e9. doi: 10.1056/NEJMc1603847 [DOI] [PubMed] [Google Scholar]
- 18.Walter RB, Gooley TA, Wood BL, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29(9):1190-1197. doi: 10.1200/JCO.2010.31.8121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buccisano F, Maurillo L, Spagnoli A, et al. Cytogenetic and molecular diagnostic characterization combined to postconsolidation minimal residual disease assessment by flow cytometry improves risk stratification in adult acute myeloid leukemia. Blood. 2010;116(13):2295-2303. doi: 10.1182/blood-2009-12-258178 [DOI] [PubMed] [Google Scholar]
- 20.Freeman SD, Hills RK, Virgo P, et al. Measurable residual disease at induction redefines partial response in acute myeloid leukemia and stratifies outcomes in patients at standard risk without NPM1 mutations. J Clin Oncol. 2018;36(15):1486-1497. doi: 10.1200/JCO.2017.76.3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venditti A, Piciocchi A, Candoni A, et al. GIMEMA AML1310 trial of risk-adapted, MRD-directed therapy for young adults with newly diagnosed acute myeloid leukemia. Blood. 2019;134(12):935-945. doi: 10.1182/blood.2018886960 [DOI] [PubMed] [Google Scholar]
- 22.Short NJ, Ravandi F. How close are we to incorporating measurable residual disease into clinical practice for acute myeloid leukemia? Haematologica. 2019;104(8):1532-1541. doi: 10.3324/haematol.2018.208454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fasan O. Using minimal (measurable) residual disease assessments to guide decision-making for timing of allogeneic transplantation in acute myeloid leukemia. Curr Opin Hematol. 2019;26(6):413-420. doi: 10.1097/MOH.0000000000000543 [DOI] [PubMed] [Google Scholar]
- 24.Buccisano F, Maurillo L, Gattei V, et al. The kinetics of reduction of minimal residual disease impacts on duration of response and survival of patients with acute myeloid leukemia. Leukemia. 2006;20(10):1783-1789. doi: 10.1038/sj.leu.2404313 [DOI] [PubMed] [Google Scholar]
- 25.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 27.Xu L, Chen H, Chen J, et al. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China—recommendations from the Chinese Society of Hematology. J Hematol Oncol. 2018;11(1):33. doi: 10.1186/s13045-018-0564-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H, Zhai X, Song Z, et al. Busulfan plus fludarabine as a myeloablative conditioning regimen compared with busulfan plus cyclophosphamide for acute myeloid leukemia in first complete remission undergoing allogeneic hematopoietic stem cell transplantation: a prospective and multicenter study. J Hematol Oncol. 2013;6:15. doi: 10.1186/1756-8722-6-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xuan L, Wang Y, Huang F, et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised phase 3 trial. Lancet Oncol. 2020;21(9):1201-1212. doi: 10.1016/S1470-2045(20)30455-1 [DOI] [PubMed] [Google Scholar]
- 30.Yu S, Huang F, Wang Y, et al. Haploidentical transplantation might have superior graft-versus-leukemia effect than HLA-matched sibling transplantation for high-risk acute myeloid leukemia in first complete remission: a prospective multicentre cohort study. Leukemia. 2020;34(5):1433-1443. doi: 10.1038/s41375-019-0686-3 [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Chen H, Chen J, et al. The consensus on the monitoring, treatment, and prevention of leukemia relapse after allogeneic hematopoietic stem cell transplantation in China. Cancer Lett. 2018;438:63-75. doi: 10.1016/j.canlet.2018.08.030 [DOI] [PubMed] [Google Scholar]
- 32.Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36(27):4391-4400. doi: 10.1002/sim.7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mawad R, Gooley TA, Sandhu V, et al. Frequency of allogeneic hematopoietic cell transplantation among patients with high- or intermediate-risk acute myeloid leukemia in first complete remission. J Clin Oncol. 2013;31(31):3883-3888. doi: 10.1200/JCO.2013.50.2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorin NC, Labopin M, Piemontese S, et al. ; Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation . T-cell-replete haploidentical transplantation versus autologous stem cell transplantation in adult acute leukemia: a matched pair analysis. Haematologica. 2015;100(4):558-564. doi: 10.3324/haematol.2014.111450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Yang L, Fan Y, et al. Comparison of autologous stem cell transplantation versus haploidentical donor stem cell transplantation for favorable- and intermediate-risk acute myeloid leukemia patients in first complete remission. Biol Blood Marrow Transplant. 2018;24(4):779-788. doi: 10.1016/j.bbmt.2017.12.796 [DOI] [PubMed] [Google Scholar]
- 36.Zhu HH, Zhang XH, Qin YZ, et al. MRD-directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: results from the AML05 multicenter trial. Blood. 2013;121(20):4056-4062. doi: 10.1182/blood-2012-11-468348 [DOI] [PubMed] [Google Scholar]
- 37.Yao J, Zhang G, Liang C, et al. Combination of cytogenetic classification and MRD status correlates with outcome of autologous versus allogeneic stem cell transplantation in adults with primary acute myeloid leukemia in first remission. Leuk Res. 2017;55:97-104. doi: 10.1016/j.leukres.2017.01.026 [DOI] [PubMed] [Google Scholar]
- 38.Hourigan CS, Dillon LW, Gui G, et al. Impact of conditioning intensity of allogeneic transplantation for acute myeloid leukemia with genomic evidence of residual disease. J Clin Oncol. 2020;38(12):1273-1283. doi: 10.1200/JCO.19.03011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venditti A, Peter Gale R, Buccisano F, Ossenkoppele G. Should persons with acute myeloid leukemia (AML) in 1st histological complete remission who are measurable residual disease (MRD) test positive receive an allotransplant? Leukemia. 2020;34(4):963-965. doi: 10.1038/s41375-020-0780-6 [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Liu QF, Xu LP, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125(25):3956-3962. doi: 10.1182/blood-2015-02-627786 [DOI] [PubMed] [Google Scholar]
- 41.Luo Y, Xiao H, Lai X, et al. T-cell–replete haploidentical HSCT with low-dose anti–T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014;124(17):2735-2743. doi: 10.1182/blood-2014-04-571570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bashey A, Zhang X, Sizemore CA, et al. T-cell–replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31(10):1310-1316. doi: 10.1200/JCO.2012.44.3523 [DOI] [PubMed] [Google Scholar]
- 43.Yu S, Fan Q, Sun J, et al. Haploidentical transplantation without in vitro T-cell depletion results in outcomes equivalent to those of contemporaneous matched sibling and unrelated donor transplantation for acute leukemia. Medicine (Baltimore). 2016;95(11):e2973. doi: 10.1097/MD.0000000000002973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mo XD, Zhang YY, Zhang XH, et al. The role of collateral related donors in haploidentical hematopoietic stem cell transplantation. Sci Bull. 2018;63(20):1376-1382. doi: 10.1016/j.scib.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 45.Terwijn M, van Putten WL, Kelder A, et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: data from the HOVON/SAKK AML 42A study. J Clin Oncol. 2013;31(31):3889-3897. doi: 10.1200/JCO.2012.45.9628 [DOI] [PubMed] [Google Scholar]
- 46.Hemmati PG, Terwey TH, Na IK, et al. Impact of early remission by induction therapy on allogeneic stem cell transplantation for acute myeloid leukemia with an intermediate-risk karyotype in first complete remission. Eur J Haematol. 2015;94(5):431-438. doi: 10.1111/ejh.12449 [DOI] [PubMed] [Google Scholar]
- 47.Klepin HD, Rao AV, Pardee TS. Acute myeloid leukemia and myelodysplastic syndromes in older adults. J Clin Oncol. 2014;32(24):2541-2552. doi: 10.1200/JCO.2014.55.1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179-4187. doi: 10.1182/blood-2008-07-172007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Causes of Death Posttransplantation
eFigure. Forest Plots for Survival of MSD-SCT and AD-SCT