Abstract
Objective. Motor imagery (MI) is widely used to improve technical skills in sports and has been proven to be effective in neurorehabilitation and surgical education. This review aims to identify the key characteristics of MI protocols for implementation into surgical curricula. Design. This study is a systematic review and meta-analysis. PubMed, MEDLINE, Embase and PsycINFO databases were systematically searched. The primary outcome was the impact of MI training on measured outcomes, and secondary outcomes were study population, MI intervention characteristics, study primary outcome measure and subject rating of MI ability (systematic review registration: PROSPERO CRD42019121895). Results. 456 records were screened, 60 full texts randomising 2251 participants were reviewed and 39 studies were included in meta-analysis. MI was associated with improved outcome in 35/60 studies, and pooled analysis also showed improved outcome on all studies with a standardised mean difference of .39 (95% CI: .12, .67, P = .005). In studies where MI groups showed improved outcomes, the median duration of training was 24 days (mode 42 days), and the median duration of each individual MI session was 30 minutes (range <1 minute-120 minutes). Conclusions. MI training protocols for use in surgical education could have the following characteristics: MI training delivered in parallel to existing surgical training, in a flexible format; inclusion of a brief period of relaxation, followed by several sets of repetitions of MI and a refocusing period. This is a step towards the development of a surgical MI training programme, as a low-cost, low-risk tool to enhance practical skills.
Keywords: motor imagery, mental training, medical education, surgical education, curricula
Introduction
Surgical education has been increasingly reliant on training methods which involve simulation, ranging from simple bench models to virtual reality simulation and box trainers. 1 Motor imagery (MI) can be described as a form of simulation; it consists of imagining oneself performing a voluntary movement, without physically moving. 2 It has also been called mental practice (MP), mental training and mental imagery.
Motor imagery has been proven to be effective at improving technical skills in various fields,3-6 and structured training programmes which incorporate this concept are reported in the literature. In sports psychology, MI has been integrated in several models such as the PETTLEP model 7 which delivers a format of training applicable to different sports. In the field of neurorehabilitation, Braun’s review 8 identified the elements which correlate with effective training outcomes.
Several studies have successfully shown that this method can also be adapted to surgical training9-11: Immenroth et al 11 used MI for training in laparoscopy cholecystectomy via one-on-one mental training sessions, where trainees memorised the operation primer and visualised their inner perception of the operation based on this. Louridas et al 12 developed and tested a script based on MI to perform laparoscopic jejunojejunostomy, using visual and kinaesthetic (tactile) cues. Despite encouraging results, these studies allow limited application for MI training outside of the specific surgical procedures they were designed for.
The aforementioned areas of neurorehabilitation, sport psychology and training in specific surgical procedures use common principles of MI to achieve motor improvement. Current understanding of the neurological mechanism of MI is dominated in the literature by Jeannerod’s central motor theory.7,13-16 It underpins the hypothesis that a degree of functional equivalence exists between MI, and motor preparation and execution, and that they share common neural substrate.7,13,14 Empirical evidence supporting the functional equivalence concept can be seen at different levels of control, namely central (in the frontal and parietal lobes 17 ), peripheral (via increased heart rate and respiratory rate 18 ) and behavioural (via mental chronometry18-20). This mechanism is applied to any motor development using MI, regardless of the type of skill being targeted. Based on this understanding, a cross-disciplinary use of MI protocols can be explored in order to identify important elements of MI training.
Protocols incorporating MI in medical education are not readily available in the literature, 10 and there has so far been no rigorous approach regarding the evaluation of the format in which MI training should be incorporated into surgical education programmes.
The aim of this review and meta-analysis is to identify the components necessary to a training protocol for surgical education which uses MI. This will be done by gathering evidence from fields which have successfully used this method for decades. 21 The primary outcome will be the effectiveness of a protocol using MI training, measured through different outcome measures due to the diversity of studies included. The secondary outcomes will be protocol components.
This review will be structured according to the PRISMA checklist for systematic reviews and meta-analyses.
Methods
Protocol and Registration
The review has been registered on PROSPERO (registration number: CRD42019121895).
Eligibility Criteria
In order to limit this review to evidence of the highest standard of quality, only randomised controlled trials (RCTs) of the use of MI in any discipline were used. Inclusion criteria were as follows: RCTs published up until December 2018; studies in English, French and Spanish only; studies of MI training programmes which measured an objective outcome for a specific voluntary skill; studies which included a protocol based on imagining a movement. Exclusion criteria were as follows: studies which were not RCTs; studies in which MI training was combined with simulation training; studies in which MI training was done in conjunction with functional magnetic resonance imaging (fMRI), electroencephalograms (EEG), electromyography (EMG), transcutaneous electric nerve stimulation (TENS), electroacupuncture or hypnosis. However, studies using fMRI, EEG or EMG only as part of pre- and post-intervention evaluation, and not during the intervention period (as may be the case in bio-neurofeedback), were included, providing they met the other eligibility criteria.
Search Strategy and Study Selection
The following databases were searched from inception by 2 authors: PubMed, MEDLINE, Embase and PsycINFO. The following combination of index terms was used: ‘randomised controlled trial’, ‘RCT’, ‘mental imagery’, ‘MP’, ‘mental training’ and ‘MI’. Detail of the search strategy is presented in Appendix A. There were no registered MeSH terms pertaining to this topic. The last date of search was January 12, 2019. Titles of studies were screened for selection. The abstracts were read. Where necessary, the full text was read. At each step, the studies were assessed according to the exclusion and inclusion criteria. Studies selected for inclusion were uploaded onto RefWorks and checked for duplication. Both authors completed the search independently and compared results, incongruities were resolved by discussion. (See Appendix B for list of abbreviations used in Tables 1 and 2).
Table 1.
Main Study Characteristics.
| Study | MI Ability Measurement | N° Participants | Population Type | Interventions | Duration of MP Session (minutes) × Total Amount of Sessions × Intervention Duration (days) | Primary Outcome Measures | Intervention Group Compared to Control |
|---|---|---|---|---|---|---|---|
| Abraham 2018 | MIQ-RS, KVIQ-20 VMIQ-2 | 20 | Parkinson’s disease | MP vs control | 120 min × 10 × 14 days | TUGT, 6-min walk test, FGS, 30 sec chair stand, 360° turn test and PRT | Improved |
| Asa 2014 | No | 36 | Healthy | MP only vs PP only vs control | 25 min × 1 × 1 days | Speed and accuracy of task performance | No difference |
| Bathalon 2005 | No | 44 | Healthy | MI and KG vs KG alone vs control | n/a × n/a × 14 days | OSCE | Improved |
| Bovend'Eerdt 2008 | IST | 11 | Arm spasticity | PP vs MP and PP vs control | 1.5 min × 32 × 56 days | Resistance to passive movement | No difference |
| Braun 2011 | VMIQ-2 | 47 | Parkinson’s disease | PP vs MP and PP vs control | 20 min × 6 × 42 days | Walking performance using visual analogue scale | No difference |
| Braun 2012 | No | 36 | Stroke patients | PP vs MP and PP vs control | n/a x 10 × 42 days | Numeric rating scales | No difference |
| Callow 2017 | VMIQ-2 | 56 | Healthy | IVI vs IVI and KIN vs control | 2 min × 1 × 1 days | Time | IVI and KIN improved |
| Cho 2013 | No | 28 | Stroke patients | MP vs control | 15 min × 18 × 42 days | FRT, TUGT, 10- m walk test and FMA | Improved |
| Coker 2015 | VMIQ-2 | 24 | Healthy | VI vs KIN vs control | 60 min (MP+PP) × 1 × 1 day | Hip movements recorded on motion capture system | No difference |
| Conlin 2016 | MIQ-R | 12 | Healthy | MP vs control | n/a × 1 × 1 days | TSE and GE of mastoidectomy | No difference |
| Cunha 2017 | MIQ-RS | 15 | Transtibial amputees | MP vs control | 40 min × 12 × 28 days | Ground reaction forces | Improved |
| Dilek 2018 | No | 36 | Distal radius fracture | MP vs control | 100 min × 1680 × 56 days | Pain, wrist and forearm active ROM, grip strength, DASH questionnaire and MHQ | Improved |
| Eldred-Evans 2013 | No | 64 | Healthy | VRS vs standard training and MP vs VRS training and MP vs control | 30 min × 3 × 6 days | Time, precision, accuracy and overall performance on BT and VRS | MP groups improved |
| Frenkel 2014 | No | 18 | Healthy | MP vs control | 15 min × 315 × 21 days | ROM | Improved |
| Geoffrion 2012 | No | 79 | Healthy | MP vs control | n/a × 1 × 1 days | GRS | No difference |
| Gomes 2014 | No | 60 | Healthy | MP vs PP vs MP and then PP vs PP and then MP vs control | .1 min × 24 × 1 days | Time | PP, MP and PP and PP and MP improved |
| Guillot 2009 | Mental chronometry | 14 | Burn patients | MP vs control | n/a × 10 × 14 days | Goniometric data | Improved |
| Hemayattalab 2009 | No | 40 | Intellectually disabled patients | MP vs PP vs MP and then PP vs PP and then MP vs control | 30 min × 24 × 24 days | Free throw test | PP improved most |
| Hidalgo-Perez 2015 | No | 40 | Healthy | MCTE and MI vs control | 15 min × 20 × 30 days | Craniocervical neuromotor control, joint position error and fatigue after effort | Improved |
| Hosseini 2012 | MMSE, VMIQ and VVIQ | 30 | Healthy | OT and MP vs control | 15 min × 15 × 35 days | TUGT | Improved |
| Hoyek 2014 | MIQ-R | 16 | Shoulder impingement | MP and PP vs control | 15 min × 9 × 21 days | Shoulder function, ROM and pain | Improved |
| Ietswaart 2011 | Task chronometry | 121 | Stroke patients | MP and standard rehabilitation vs standard rehabilitation and non-motor mental rehearsal vs control | 39 min × 20 × 28 days | ARAT | No difference |
| Immenroth 2007 | No | 98 | Healthy | MP and PP vs PP only vs control | 60 min × 2 × 2 days | OSATS | No difference |
| Jungmann 2011 | Mental chronometry | 40 | Healthy | MP and VR training vs control | 3 min × 4 × 4 days | Time, tip trajectory and instrument collision | No difference |
| Kim 2013 | VMIQ-2 | 30 | Stroke patients | Standard therapy and AO vs standard therapy and MP vs control | 20 min × 20 × 28 days | TUGT, FRT, WAQ and FAC | Improvement in AO group |
| Kim 2018 | VMIQ | 16 | Stroke patients | CMIT and MP vs control | 10 min × 10 × 14 days | Motor evoked potential amplitude, 3-D motion analysis, JT test and motor activity log | No difference |
| Komesu 2009 | No | 68 | Healthy | MP vs control | 20 min × 1 × 1 day | GSOP | No difference |
| Lebon 2011 | No | 12 | Torn ACL patients | Standard physiotherapy and MP vs control | 15 min × 180 × 31 days | Muscle activation | Improved |
| Lim 2016 | No | 20 | Healthy | MP and PP vs and control | 60 min × 1 × 1 day | Technical achievement | No difference |
| Liu 2004 | No | 46 | Stroke patients | MP and standard rehabilitation vs control | 60 min × 15 × 21 days | Performance on 15 tasks, FMA and CTT | No difference |
| Liu 2009 | No | 34 | Stroke patients | MP and conventional therapy vs control | 60 min × 15 × 21 days | Performance on 15 tasks | No difference |
| Liu 2009 | No | 35 | Post-stroke patients | MP and conventional therapy vs control | 60 min × 15 × 21 days | Evaluation of skills | No difference |
| Losana-Ferrer 2018 | MIQ | 60 | Healthy | MP vs AO vs control | 6 min × n/a × 10 days | Hand grip strength, EMG and IM oxygenation | Improved (not on IM oxygenation) |
| Louridas 2015 | MIQ and MIQ-R | 20 | Healthy | PP and MP vs control | n/a × n/a × 7 days | OSATS and bariatric OSATS | No difference |
| Malouin 2009 | KVIQ | 12 | Stroke patients | MP and PP vs cognitive training and PP vs control | n/a × 12 × 28 days | Limb loading | Improved |
| Maring 1990 | No | 26 | Healthy | MP and PP vs control | n/a x 1 x 1 days | Upper limb muscle activity | Improved |
| Mendoza 1978 | No | 32 | Healthy | MP only vs MP with simulated movement vs PP only vs control | 15 min × 6 × 6 days | Success in dart throwing | PP improved most |
| Millard 2001 | No | 60 | Healthy | MP vs PP vs MP and PP vs control | 30 min × 3 × 3 days | Wet exit attempts scored | MP and PP improved most |
| Mulla 2012 | No | 41 | Healthy | BT vs BT and additional practice vs VRS training vs MP vs control | 15 min × 7 × 7 days | Assessed on BT and VRS on time, precision, accuracy and performance | MP performed worst apart from control |
| Nicholson 2018 | KVIQ | 30 | Healthy | MP only vs PP only vs control | 25 min × 1 × 1 day | Gait speed, gait variability using GAITRite and timed up and go | No difference |
| Nilsen 2012 | VMIQ-2 | 19 | Stroke patients | PP and internal MP vs PP and external MP vs control | 18 min × 12 × 42 days | FM and JT test | Improved |
| Oostra 2015 | MIQ-RS | 44 | Stroke patients | Standard rehabilitation and MP vs control | 30 min × 30 × 42 days | 10-m walk test and FM | No difference |
| Page 2005 | No | 11 | Stroke patients | PP and MP vs PP only vs control | 30 min x 12 × 42 days | Motor activity log and ARAT | Improved |
| Page 2007 | No | 32 | Stroke patients | MP and standard rehabilitation vs control | 30 min × 12 × 42 days | ARAT and FM | Improved |
| Page 2009 | No | 10 | Stroke patients | mCIT and MP vs control | 30 min × 30 × 70 days | ARAT and FM | Improved |
| Page, S. 2011 | No | 29 | Stroke patients | MP vs MP vs MP (different lengths) vs control | 40 min × 30 × 70 days | FM and ARAT | Improved |
| Park 2015 | VMIQ | 30 | Stroke patients | MP and standard rehabilitation vs control | 10 min x 20 × 28 days | LBT and SCT | Improved |
| Sanders 2004 | No | 65 | Healthy | 2 sessions PP and 1 MP vs 1 PP and 2 MP vs control | 30 min × 3 × 21 days | GRS | No difference |
| Sanders 2008 | Revised Minnesota test | 64 | Healthy | MP vs control | 30 min × 2 × 24 days | Surgical behaviour | Improved |
| Santiago 2015 | MIQ-R | 20 | Parkinson’s disease | MP and standard therapy vs control | n/a × 1 × 1 day | Gait analysis | No difference |
| Schuster 2012 | KVIQ | 39 | Stroke patients | MP added to physio vs MP embedded into physio vs control | 45 min × 6 × 14 days | Time taken to perform skill | No difference |
| Seebacher 2017 | No | 112 | MS patients | Music and MI vs metronome MI vs control | 17 min × 24 × 28 days | Walking speed | Improved |
| Sharp 2014 | No | 18 | Spinal cord injury patients | MP with overground training vs control | 30 min × 24 × 56 days | Gait velocity | No difference |
| Sidaway 2005 | No | 24 | Healthy | MP vs PP vs control | 15 min × 12 × 28 days | Isometric torque and percentage improvement | No difference |
| Stenekes 2009 | VMIQ | 28 | Post flexor tendon repair | MP and conventional therapy vs control | .6 min × 336 × 42 days | Preparation time of finger flexion | Improved |
| Timmermans 2013 | VMIQ | 42 | Stroke patients | Standard therapy and MP vs control | 10 min × 126 × 42 days | FM, FAT, WMFT and accelerometry | No difference and improved on FAT |
| Vergeer 2006 | VVIQ | 47 | Healthy | MI vs SI vs control | 30 min × 11 × 28 days | Flexibility and comfort | Improved in comfort and no difference in flexibility |
| Wilson 2002 | No | 54 | Motor coordination problems | MI vs PP vs control | 60 min × 5 × 35 days | MABC | PP improved most |
| Wilson 2016 | No | 36 | Motor coordination problems | MI vs PP | 60 min × 5 × 35 days | MABC | No difference |
Table 2.
Protocol Components for MI Training.
| Study | MP Protocol Detail | Control Group |
|---|---|---|
| Abraham 2018 | 16 h training. 5x 2 h sessions every week for 2 weeks. Delivered in group by therapist. First session: Introduction to imagery. Subsequent sessions: 15 min warm-up, 35 min practice, 35 min practice, 20 min movement session and 5 min cool down. Overall structure: Acquire imagery skills and technique, understand anatomy and function and use imagery for improvement | Standard training and in-home learning and exercise programme following the same pattern as the intervention group |
| Asa 2014 | MP instructions emphasised kinaesthetic imagery, keeping eyes closed | No training |
| Bathalon 2005 | MI and KG group: KG teaching broke down task into 8 steps, students performed task and 5 min teaching of mental imagery. Instructed to perform MI in their own time as often as possible | Standard ATLS training |
| Bovend'Eerdt 2008 | Closed eyes, imagined limb in mind’s eye and imagined movement in mind’s eye. Performed the skill (stretch) physically whilst imagining it. Stretches held for 10-30 secs, 3 repetitions/stretch. MP done immediately prior to PP | PP and relaxation following the same pattern as the intervention group |
| Braun 2011 | MP with therapist, then unguided. 1 log/week completed by participants to record MP behaviour. 6 weeks standard physiotherapy, 1 h/week in groups or 30 min 2x/week individually, of which MP for 20 min in groups or 10 min individually | Standard physiotherapy and relaxation following the same pattern as the intervention group |
| Braun 2012 | 6 weeks rehabilitation, at least 10 sessions of MP (conditional) and practice outside supervised therapy time (optional). 4-step programme: Explain concept, develop imagery technique, apply mental practice and consolidate | Regular rehabilitation + homework practising difficult tasks |
| Callow 2017 | IVI script: First-person visual perspective. IVI and KIN script: First-person visual perspective and physical feelings | Participants answered arithmetic questions |
| Cho 2013 | 15 min MP: Videos of normal movement shown, explanation of movement by researcher and imagining normal movement based on visual material using kinaesthetic and visual imagery. 5 min relaxation. 30 min gait training 45 min/day, 3x/week for 6 weeks | Standard physiotherapy only |
| Coker 2015 | Training block of x10 trials of skill to generate feedback. Then, practice sessions alternating PP (x5 repetitions) and MP (visual or kinaesthetic imagery, x20 repetitions of the task). Total blocks had x15 repetitions PP and x60 repetitions MP. Relaxation done before training | Mental arithmetic task |
| Conlin 2016 | Relaxation, script read out loud and given in written format. Script based on transcript of audio recordings of 3 experts having identified steps in the procedure and reported visual, cognitive and kinaesthetic cues involved. Participants to actively imagine performing skill. Given copy of script to take home and review | Self-directed textbook study |
| Cunha 2017 | 40 min sessions, 3x/week for 4 weeks. First-person perspective and tasks of increasing difficulty. 10 tasks imagined in each session, then described | Standard training and non-motor task MP |
| Dilek 2018 | Graded motor imagery. 3 stages. 1: 3 weeks of lateralisation: Identifying correct right and left hands from pictures, x3 each hour every day. 2: 3 weeks of MI, visualise own hand moving to posture in image shown, without physically moving, x3 each hour every day. 3: 2 weeks mirror therapy: Move own hand to posture in image shown, x10 every hour every day. All participants instructed to perform home exercise programme | Standard rehabilitation |
| Eldred-Evans 2013 | Based on the Mackay nodal model of mental practice. Relaxation, guided visualisation of nodal points | Standard box training and additional self-practice |
| Frenkel 2014 | Mental gait training procedure: (1) movement explained; (2) describe movement by observing it performed, practicing it on non-tested hand and concentrating on kinaesthetic properties; (3) break down into nodal points and connect points with kinaesthetic perception; (4) practice on non-tested hand with open eyes and closed eyes, perform visual imagery and kinaesthetic imagery and (5) practice on non-tested hand, perform kinaesthetic imagery of task and of an unrelated task. Completed a dairy to record completion of training. 1 × 60 min and then 3 × 30 min guided sessions. Followed by 15 min/day self-guided imagery | No training |
| Geoffrion 2012 | MI script enumerated steps from textbook and added visual, cognitive and kinaesthetic performance details. Participants performed MP one-on-one with educator, then individually | Normal surgical training and encouraged to read textbook on skill |
| Gomes 2014 | Instructions to use internal kinaesthetic perspective. Participants closed eyes, signal start of imagining and signal end of imagining | No training |
| Guillot 2009 | Script detailing instructions of 2 motor tasks, encouraging self-representation of movements, sensory and kinaesthetic cues, staying immobile. Patients to perform MI during training sessions only. Regularly asked to describe nature of images after MI. Total 2 weeks, 5 MP sessions | Standard rehabilitation and neutral activities following the same pattern as the intervention group |
| Hemayattalab 2009 | Using internal kinaesthetic imagery. PP: 30 repetitions of the skill/session. MP: 30 repetitions of imagining skill. MP and PP: MP for 12 sessions and then PP for 12 sessions. PP and MP: PP for 12 sessions and then MP for 12 sessions | No training |
| Hidalgo-Perez 2015 | MI done just after PP. 1/day, 5 days/week, 30 days. 4 phases, 1 phase/week of intervention: 1- kinaesthetic imagery, 2- visual imagery, 3- movement observation therapy plus MI and 4- exercise execution with mirror feedback. Weekly email and phone reminders | MCTE only |
| Hosseini 2012 | 15 MP, then 30 min occupational therapy. MP: 5 min relaxation, 10 lying supine with eyes closed, asked to imagine skill in first person | Occupational therapy only, for 45 min |
| Hoyek 2014 | 4 movements imagined using internal visual imagery and kinaesthetic imagery. All movements shown before MP. Participants told to imagine movement as slowly and vividly as possible. Imagery script read to them. Each movement imagined 10 times, 5 sets of 2 separated by 30-s rest. 10 long sessions of physical therapy, 3x/week. MP exercises done during therapy sessions in rest times. 45 min physical therapy and 15 min MP. | Physical therapy training with neutral activities during rest time |
| Ietswaart 2011 | 12 x 45 min sessions with therapist 3 days/week: 30 min MP actively imagining basic movements, 10 min MP using videos and mirrors and 5 min covert MP, for example mentally rotating visual depiction of hands. 8 × 30 min sessions alone, 2 days/week: Audio tape instructing movements to be imagined. Patients to keep a log book. Total 4 weeks | Standard physiotherapy only |
| Immenroth 2007 | Day 1: One-on-one mental training for 90 min. 30 min to learn primer by heart, recall wording of primer by external self-talk, relaxation exercise, visualisation in first person under supervision and then alone. Day 2: 30 min session repeating external self-talk and ideomotoric training under supervision | No intervention |
| Jungmann 2011 | Completed 2 sessions VR training. Then, received CD-ROM with demonstration video of skill, checklist for skill steps and instructions on how to perform MP. Practised MP independently before second VR training session | VR training only |
| Kim 2013 | 5x/week, 30 min sessions, over 4 weeks: 20 min audio instructions and 10 min PP. | Standard therapy only |
| Kim 2018 | Modified constraint-induced movement (CMIT) therapy for 1h and then MP for 10 min. Listened to audio while watching first-person perspective video for 4 min. Close eyes, relaxation for 2 min. Repeat audio only without video for 4 min. Audio included kinaesthetic mental practice. 5 days/week for 2 weeks | CMIT and listened to piano music for 10min |
| Komesu 2009 | Perform MP 24-48 h before assessment. Imagine performing skill and describe to educator in detail | Standard surgical training and textbook study following the same pattern as the intervention group |
| Lebon 2011 | Sat with legs extended. Relaxation done in initial few sessions only. Perceive muscle contractions and joint tension while imagining movement. 3 blocks of 10 imagined movement, 10 sec rest between imagined movements and 2 min rest between blocks. MP: 28-34 day programme. 12 × 15 min sessions, one every 2 days. Physiotherapy: 30 min every 2 days | Standard physiotherapy and neutral task following the same pattern as the intervention group |
| Lim 2016 | 60 min scripted mental imagery group training. After 20 min, independent mental rehearsal. After session, performed skill x3 | Low-fidelity simulation training only |
| Liu 2004 | Increasing difficulty of tasks. First week: Analyse task sequences with pictures and movies. Second week: Identify own problems. Third week: Imagine task being performed by self, physically perform task and videotape, view videotape and adjust problems. Repeat identification of problems and third week steps until proper method is achieved. 15 sessions MP, 1 h/day for 3 weeks and standard physiotherapy, 1 h/day for 5 days/week at a different time of day | Standard rehabilitation and neutral activities following the same pattern as the intervention group |
| Liu 2009 | MP and conventional therapy, learning tasks of increasing difficulty. 1 h physical therapy and 1 h MP 5x/week for 3 weeks | Followed the same pattern of therapy as the intervention group with occupational therapy instead of MP |
| Liu 2009 | Chunking-regulation-rehearsal strategy: Truncate task, self-reflect on abilities, feedback using video playback, mentally rehearsing and physically practising. MP and conventional therapy, learning tasks of increasing difficulty. 1 h physical therapy and 1 h MP 5x/week for 3 weeks | Physical practice and functional rehabilitation following the same pattern as the intervention group |
| Losana-Ferrer 2018 | Sit on chair, imagine 10 physical repetitions for 3 sec each and 20 sec rest in between. 2 min break. Repeat imagery whilst also performing skill physically. 10 training days, 1st and 4th supervised and remainder at home. All groups told to practice at home and weekly reminders | Physical practice following the same pattern as intervention groups |
| Louridas 2015 | Didactic lecture. In-person instructions on MP. Relaxation exercise. MP guided by MP script developed by interviewing experts and detailing visual and kinaesthetic cues, including common pitfalls in performance. Given script and videos of didactic teaching, 7 days to perform MP at home, with follow-up calls and feedback | Standard physical practice only following the same pattern as the intervention group |
| Malouin 2009 | Approx 1 h training done in quiet room by physical therapist. MP done in blocks following one attempt of PP. Briefing on first-person imagery with focus on kinaesthetic imagery (sensory). Close eyes, imagine task. Number of mental repetitions increased with time. Live feedback on performance was given for first few sessions, via outcome measurement tool. 3/week for 4 weeks | No training |
| Maring 1990 | Maximum voluntary contraction of muscle, 2 min visualisation with visual and kinaesthetic cues and no physical movement, physical practice of skill x10. Repeated x5 | PP only and task demanding mental attention following the same pattern as the mental intervention group |
| Mendoza 1978 | MP only: Sit with eyes closed, imagine performing skill whilst being aware of all sensory input, correcting for imagined misses. 2 × 15 min sessions/day for 6 days | No practice |
| Millard 2001 | MP group: Watched video, taught mental practice and watched video + made entry in diary after each MP session. PP group: Watched demonstration, then did drill 3x/day for 3 days. PP and MP groups did both training | No training |
| Mulla 2012 | 25 min one-to-one mental training. Description and memorisation of motor skills involved, relaxation and internal and external visualisation of skills to perform. Student to practise at home 15 min/day every day | No training |
| Nicholson 2018 | Sat in chair. Imagined completing obstacle course in first-person perspective. MP group: 20 imagined repetitions of a task. PP group: 20 physical repetitions of a task. In both: 30 sec rest between each trial and 5 minute rest after every 10 repetitions | 25 min playing mentally stimulating games on iPad |
| Nilsen 2012 | Listened to audio script of MP with visual and kinaesthetic detail. 2 min introduction instructing internal perspective (group 1) or external perspective (group 2). 5 min relaxation. 8 min focussed imagery with key components of task repeated several times. 3 min refocusing | Occupational therapy and relaxation following the same pattern as the intervention groups |
| Oostra 2015 | 30 min sessions, in quiet room with 2 therapists, sit down and eyes closed. 2 min relaxation, perform practice from internal perspective, with a visual and kinaesthetic mode. Content of sessions was familiarisation in week 1, specific gait problems week 2 and symmetry and velocity weeks 3 and 4 | Standard rehabilitation and generic relaxation sessions following the same pattern as the intervention group |
| Page 2005 | MP corresponded to focus therapy, which changed weekly. Audio tape: 5 min relaxation, then suggestions for internal, cognitive polysensory images, then 3-5 min refocusing. 30 min occupational therapy (PP) sessions 2 days/week for 6 weeks followed by 30 min MP | Occupational therapy and relaxation techniques |
| Page 2007 | MP sessions directly after PP. Audio tape. 30 min total: 5 min relaxation, approx. 20 min suggestions for internal, cognitive polysensory images of skill performed in PP on the same day (several trials of imaging) and refocusing. Patients instructed not to do additional MP at home | Standard rehabilitation and relaxation following the same pattern as the intervention group |
| Page 2009 | Audio tapes read by male psychologist delivered in quiet room. 5 min guided relaxation, 15-20 min motor imagery in first person using polysensory cues and 5 min refocusing. Instructed to not do self-directed practice. 3 days/week for 10 weeks | mCIT only |
| Page, S. 2011 | Audiotaped MP intervention listened to in private room. 5 min relaxation (imagine themselves in nice place and contract/relax muscles), followed by suggestions for sensory images related to use of the arm and finishing with 5 min refocusing into the room. Opening and closing 5 minutes held constant in varying lengths of MP practice. Group 1: MP for 20 minutes, group 2: MP for 40 min and group 3: 60 min | Same baseline rehabilitation sessions and audiotaped sham intervention directly after the rehabilitation session |
| Park 2015 | Sit with eyes closed, imagine scene while listening to voice of instructor for 10 min and give verbal feedback. 10 repetitions of each skill. Break in between skills for relaxation and internal concentration. 5 days/week for 4 weeks | Standard rehabilitation only |
| Sanders 2004 | Relaxation by psychologist, then verbal imagery instruction in making incision, suturing and knot tying by physician while visualising. 30 min long sessions, 1/week | 3 sessions PP only |
| Sanders, W. 2008 | Relaxation by psychologist, then guided imagery instruction in making incision and performing sutures. 30 min | Same baseline training and 2 additional sessions of reading. Same instructional time as the intervention group |
| Santiago 2015 | 1. Identified problems in gait. 2. Memorised phases of normal gait with images, performed gait 5x. 3. Order detailed phases of gait with cards 3x, keyword for each card. 4. Closed eyes and MP done emphasising kinaesthetic perspective, say keyword for each phase. 3 series of 10 repetitions, 30 sec rest. 8 steps/repetition. 5. PP 3 series of 10 repetition, 8 steps/repetition. 6. MP in 2 imagined complex environments. 1 series of 10 repetitions, 8 steps/repetition in each environment. 7. PP in complex setting | Standard physical practice only following the same pattern as the intervention group |
| Schuster 2012 | Group 1 (MP added): Motor task divided into 13 steps, each step imagined x5 and then practised physically x1. At end, complete task x8. Individual sessions, supervised by an instructor, task specific, same environment as physical practice, detailed and standardised instructions, internal perspective and eyes closed and no familiarisation with MI before start of intervention. Session time 45-50 min, 5 to 9 visual trials and 2 to 4 kinaesthetic trials in one session. Group 2 (MP embedded): 30 min physiotherapy, then recorded audio: 3.5 min relaxation, 14.5 min description of motor task and 2 min refocusing. Not supervised, different environments to physical practice, internal perspective and eyes closed and no familiarisation with MI before start of intervention. Session time 45-50 min. 6 to 8 visual trials and 1 to 3 kinaesthetic trials in one session. All patients kept diaries. 6 sessions in 2 weeks | Standard physiotherapy and neutral task following the same pattern as the intervention group |
| Seebacher 2017 | 30-40 min MI familiarisation in groups of 2-3. Study CD with music and verbal cueing (group 1) or metronome cues and verbal cueing (group 2). Internal perspective, kinaesthetic mode, weekly change of audio mix, home-based practice and seated position with eyes closed, self-selected time of day, 17 min practice/day, 6 days/week for 4 weeks. Record MI sessions in diary. Weekly phone calls for support and adherence | No training |
| Sharp 2014 | Practised PP, then 30 min audio recording. 3 days/week for 8 weeks | Overground training with relaxation audio recordings |
| Sidaway 2005 | Given instructions on kinaesthetic imagery, then performed 3 MP trials. 15 min training sessions. 3 sets of 10 repetitions, separated by 10 seconds rest, 3x/week for 4 weeks. MP group: Imagery script at start of session but did mental repetitions instead of physical repetitions. Were placed in the same environment as PP group. PP group: Physical repetitions | No training |
| Stenekes 2009 | Active movement performed mentally following instructions to imagine initial movement, mentally hold thought in mind for 3 sec and imagine following movement. Repeat imaginary movement 10 times/session. Patients to record number of sessions performed every day. 8 sessions/day, 6 weeks | Standard post-operative rehabilitation |
| Timmermans 2013 | 6 tasks of increasing difficult. DVD guidance showing first-person perspective of task being performed, then 5 repetitions of correct performance shown with no verbal explanation and instructions to mentally practice task, then no guidance. 3x/day for 10 min, for 6 weeks. Performance assessed during intervention and if improved, DVD changed | Standard therapy and exercise therapy following the same pattern as the intervention group |
| Vergeer 2006 | 4-week programme, 30 min sessions 3x/week. Movement imagery (MI) group: Physical stretching (5-7 min warm-up, 7 stretching exercises) and imagery component (movement demonstrated, then told to imagine leg being stretched whilst simultaneously doing physical task). Stretching imagery (SI) group: Physical stretching and imagery component (told to imagine change at a cellular level previously explained with a CD, hand gestures, images and a CD) | Physical training only |
| Wilson 2002 | Delivered by CD-ROM with software. 6 operations: Visual imagery exercises with predictive timing, relaxation, visual modelling of motor skills with video watching, mental rehearsal of skills from external perspective, mental rehearsal of skills from internal perspective and overt practice | No training |
| Wilson 2016 | 6 steps: Visual imagery exercise with predictive timing, relaxation protocol and mental preparation, mental rehearsal from external perspective, mental rehearsal from internal perspective and overt practice with mental practice between sets. 5 h individual training in 60 min sessions, 1/week for 5 weeks | No training |
Data collection and Synthesis
Data were extracted by one author using a data extraction form. The primary outcome was the efficacy of the MI training intervention, measured according to the primary outcome measure as defined by study authors. The secondary outcomes were protocol characteristics. The following items were extracted: primary outcome measure, study population, MI intervention group characteristics, control group characteristics, study primary outcome measure and rating of MI ability. The mean and standard deviation of the primary outcome measure for each study were converted to a standardised mean difference. Where post-intervention scores and follow-up scores were reported, the results of the outcome measured post-intervention only were used. Where there were several MI groups with varying length of MI practice and no data on the results of all MI groups combined, the most effective length of practice only was kept. When studies compared different types of MP, they were excluded. Where SDs were not available, they were estimated using IQR/1.35 22 , and if the IQR was not available, they were estimated based on the SDs from other studies included in the meta-analysis. The primary outcome measure used was the primary outcome stated as such by authors. Where this was not reported, the outcome measure used was the most complete measure of progress as described by the study authors, or if this was not available an outcome which reported a single measurement. Where performance was measured in different simulators (e.g. box trainer and virtual reality simulation (VRS)), the simulator which had not been used in training was used.
Meta-analysis
Data were input into Review Manager in order to conduct meta-analysis if it satisfied the following criteria: The mean and standard deviation of the primary outcome measure were available, or could be estimated according to the methods described previously, and the study compared one group performing MI alone and one group performing MI and physical practice. Subgroup analysis was conducted based on length of training, inclusion of a relaxation component to the protocol and selection of participants based on MI ability.
Risk of Bias
Risk of bias was assessed using the Cochrane risk of bias tool. 23
Results
General Study Characteristics
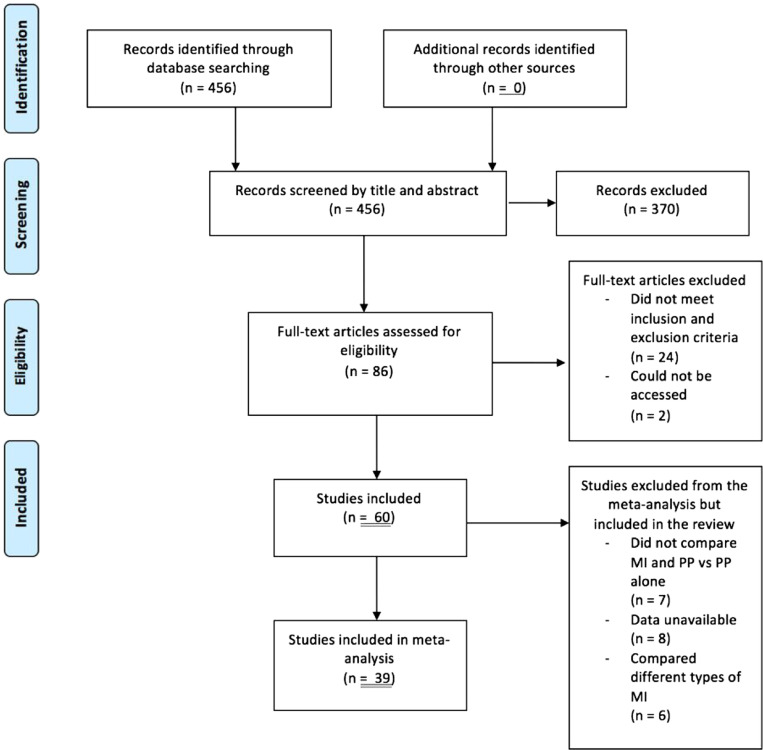
Overall, 60 RCTs were identified with a total of 2251 participants. A flow diagram of the studies’ selection process is illustrated in Figure 1. Studies were published between 1978 and 2018. Study sample sizes ranged from 10 to 112, and median was 34.5. Participants were healthy in 27 studies, had previously had a stroke in 20 studies or in 13 studies had a range of conditions including Parkinson’s disease, multiple sclerosis, arm spasticity or amputation. 6 studies only had surgical residents or trainees as participants, and 12 had healthy students.
Figure 1.
PRISMA flow diagram.
Primary Outcome Measures
The primary outcome was the overall effectiveness of a protocol using MI; several outcome measures were used due to the diversity of studies included. In the 12 studies11,12,24-33 which had medical students or trainees as their population, the primary outcome measures were the Objective Standard Assessment of Technical Skills (OSATS), variations of a Global Rating Scale, independent measures of time taken, precision and accuracy in completion of task or a purpose-built checklist. The remaining studies used task-specific measures such as the Fugl-Meyer assessment for stroke50,56,61 or broader measures of function such as using a goniometer for range of motion40-43.
Outcomes
25 (42%) studies found that the intervention group using MI did not perform better than the control group.6,11,24,26-31,34-49
In 35 studies (58%), the intervention group performed better than the control group.3,12,25,32,33,50-79 In 29/35 studies (83%), the intervention group which did MP and standard physical practice (rehabilitation, physiotherapy and surgical training) performed better than the control group which did only standard physical practice.3,12,25,32,33,50-70,76,78,79 There was no trend found between these study results and the use of MI ability assessment, the outcome measures used or the length of interventions.
A summary of all data extracted is presented in Tables 1 and 2.
Secondary Outcomes: Intervention Duration and Number of Motor Imagery Sessions
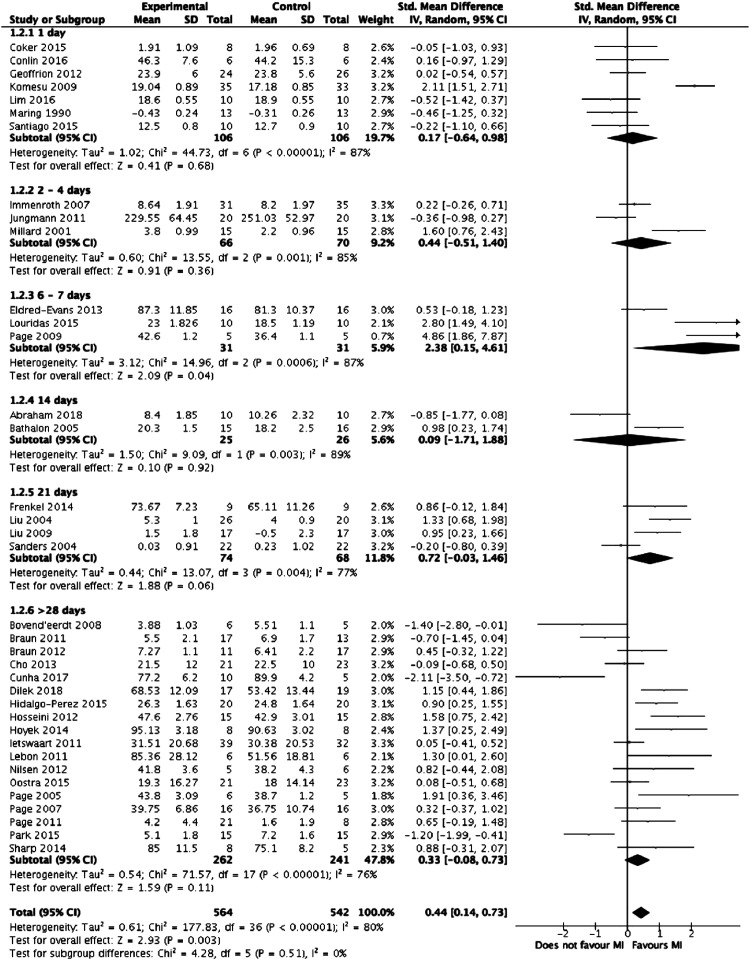
The median duration of each individual MI session was 27.5 minutes (range <1 minute-120 minutes).
In studies where the intervention group performed better than control, the median was 30 minutes (range <1 minute-120 minutes). The median number of MI sessions completed was 11 (range 1-1680); and 13 (range 1-1680) in studies where the intervention group performed better than control. The median duration of intervention across all studies was 22.4 days (mode 1, range 1-70), and in studies where the intervention group performed better than control, it was 24 days (mode 42, range 1-70).
Secondary Outcomes: Intervention Content
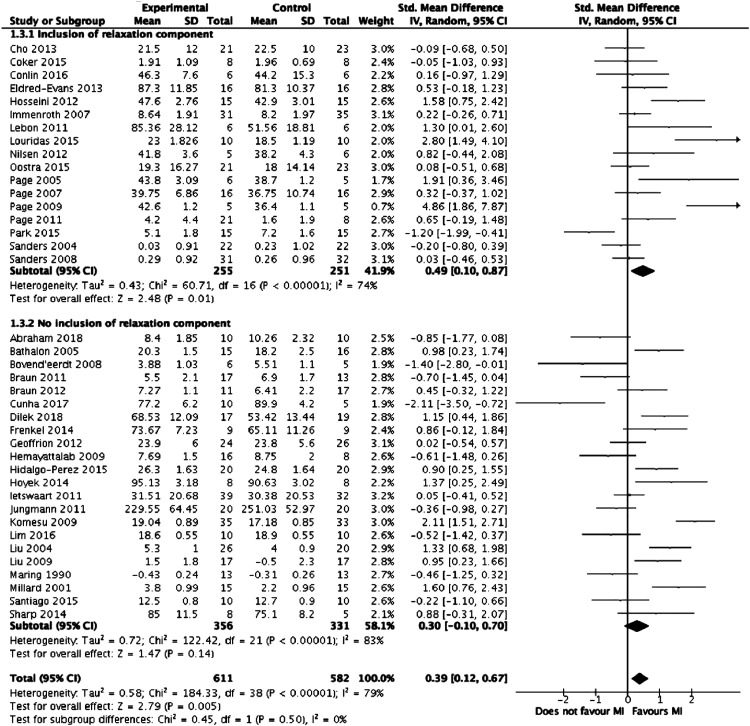
In 22 studies,11,12,24,25,27,29,32,41,42,44,47,48,50,51,53,58,59,61,65,66,69,77 the MI sessions began with a brief period of relaxation lasting <5 minutes. Out of these, 13 of them found the intervention group performed better than control.12,25,32,50,51,53,58,59,61,65,66,69,77 In 7 studies, there was explicit mention of the use of sensory cues for visualisation, giving an indication of the specificity of the instructions given to participants.3,31,39,51,59,65,69 The level of detail to which the protocols were reported was not consistent across the studies reviewed. In 13 studies, the MI sessions included several repetitions of MI with periods of rest in between.40,42,43,51,53,55,57,58,60-62,68,75 Out of these, 10 found the intervention performed better than control.51,53,55,57,58,60-62,68,75 7 studies mention a refocusing period at the end of the MI session.42,51,59,61,65,69
Secondary Outcomes: Motor Imagery Ability Assessment
Certain studies measured participant ability to conduct MI, as MI ability differs in a healthy population, 80 and can be measured using validated imagery questionnaires, such as the Mental Imagery Questionnaire (MIQ), the Mental Imagery Questionnaire Revised, Second Edition (MIQ-RS) or the Vividness of Mental Imagery Questionnaire (VMIQ). 81 In patients who have a neurological impairment, the Kinaesthetic and Visual Imagery Questionnaire (KVIQ) 82 can be used, as can mental chronometry, which has been shown to correlate with MI ability in healthy and non-healthy patients. 23 studies (38%) used the MIQ, MIQ-RS, VMIQ, VMIQ-2, VVIQ, mental chronometry, KVIQ or time-dependent motor imagery (TDMI) to select participants based on MI ability.6,12,24,34,38,40-43,45,47,48,53,55,57,60-62,64,66,68,72,79 Out of these, 12 (52%) reported better outcomes in the intervention group compared to control.12,53,55,57,60-62,64,66,68,72,79
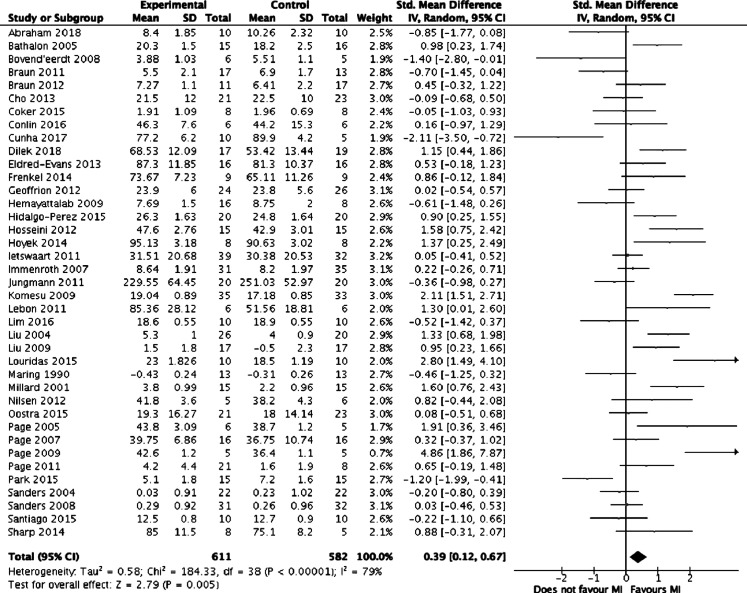
Meta-analysis
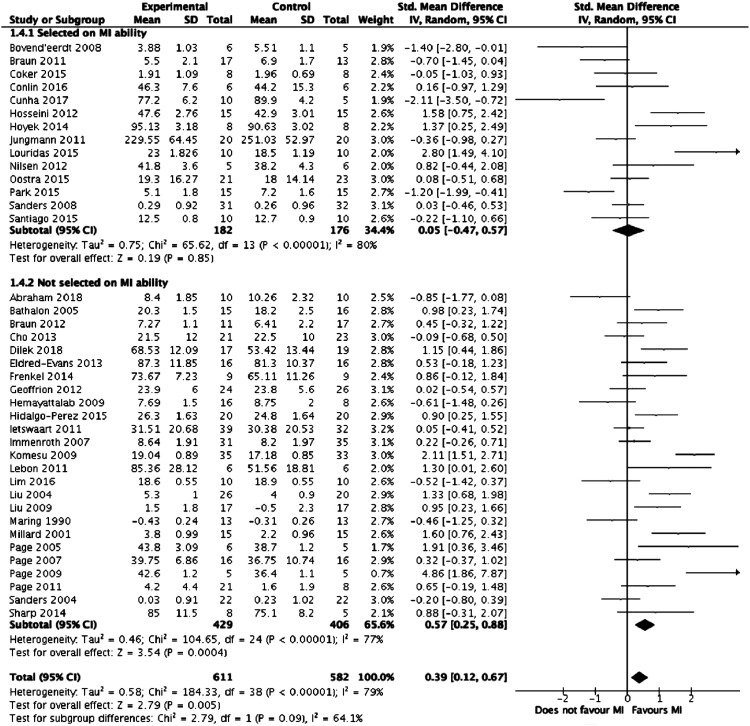
Meta-analysis was performed on the 39 studies eligible for inclusion. Figure 2 summarises the results of the meta-analysis. Overall, mental imagery was associated with improved outcomes (z = 2.79, P = .005) but with high heterogeneity between the studies (I2 = 79%, P < .00001). Figures 3-5, respectively, detail the results of subgroup analyses based on length of training, relaxation and selection of participants based on MI ability.
Figure 4.
Forest plot comparing mental training interventions with a relaxation component to mental training interventions with no relaxation component.
Figure 2.
Forest plot comparing mental training interventions to control.
Figure 3.
Forest plot comparing mental training interventions of 1 day, 2-4 days, 6-7 days, 14 days, 21 days and >28 days duration.
Figure 5.
Forest plot comparing studies where participants were selected based on mental training ability to studies where participants were not.
Risk of Bias Assessment
The risk of ‘other’ bias was classed as high in 26 studies, primarily due to selecting participants based on MI ability. The risk of selective reporting bias was unclear for the majority of studies as only few had previously published a protocol which could be referred to. 7 studies had a risk of bias which was classed as low for 5 or more types of bias and unclear for 2 or less types of bias, which the authors consider to be an overall low risk of bias. Of these, 212,55 found the intervention group performed better than control on outcomes measures and 524,28,42,43,46 found they performed the same or worse. A summary of the risk of bias is presented in Table 3.
Table 3.
Risk of Bias.
| Study | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Sources of Bias - MI Ability Assessment |
|---|---|---|---|---|---|---|---|
| Abraham 2018 | Low | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
| Asa 2014 | Unclear | Unclear | Unclear | High | Low | Unclear | High |
| Bathalon 2002 | Unclear | Unclear | Low | Low | Low | Unclear | High |
| Bovend'Eerdt 2008 | Low | Unclear | High | Low | Low | Unclear | Low |
| Braun 2011 | Unclear | Unclear | Unclear | Low | Low | Unclear | Low |
| Braun 2012 | Low | Unclear | High | Low | Low | Low | High |
| Cho 2013 | Low | Low | Unclear | Unclear | Low | Unclear | High |
| Coker 2015 | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Low |
| Conlin 2016 | Low | Unclear | Unclear | Low | Low | Low | Low |
| Cunha 2017 | Unclear | Unclear | Unclear | Unclear | High | Unclear | High |
| Dilek 2018 | Low | Low | Unclear | Low | Low | Unclear | High |
| Eldred-Evans 2013 | Low | Low | Unclear | Low | Unclear | Unclear | High |
| Frenkel 2014 | High | High | Low | High | Low | High | Unclear |
| Geoffrion 2012 | Low | Low | Unclear | Low | Low | Unclear | High |
| Gomes 2014 | Unclear | Unclear | Unclear | Low | Low | Unclear | High |
| Guillot 2009 | Unclear | Unclear | Unclear | Unclear | Low | High | Low |
| Hemayattalab 2009 | Unclear | Unclear | Unclear | Unclear | Low | Unclear | High |
| Hidalgo-Perez 2015 | Low | Low | High | Low | Low | Unclear | High |
| Hosseini 2012 | Unclear | Unclear | Unclear | Low | Low | Low | Low |
| Hoyek 2014 | Unclear | Unclear | High | High | Low | Unclear | Low |
| Ietswaart 2011 | Low | Unclear | Low | Low | Low | Unclear | Low |
| Immenroth 2007 | Low | Low | High | Unclear | Low | Unclear | High |
| Jungmann 2011 | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Low |
| Kim 2013 | Unclear | Low | Unclear | Unclear | Low | Unclear | High |
| Kim 2018 | Low | Unclear | High | High | Low | Low | Low |
| Komesu 2009 | Low | Low | Unclear | Low | Low | Unclear | High |
| Lebon 2011 | Unclear | Unclear | Low | Low | Low | Unclear | High |
| Lim 2016 | Low | Unclear | Low | Low | Low | Unclear | Low |
| Liu 2004 | Unclear | Low | Unclear | Low | Low | Unclear | High |
| Liu 2008 | Unclear | Unclear | Unclear | Low | Low | Unclear | High |
| Liu 2009 | Low | Low | Unclear | Low | Low | Unclear | High |
| Losana-Ferrer 2018 | Low | Low | Unclear | Low | Low | Unclear | Low |
| Louridas 2015 | Low | Low | Low | Low | Low | Low | Low |
| Malouin 2009 | Low | Unclear | Unclear | Low | Low | Unclear | Low |
| Maring 1990 | Unclear | Unclear | Unclear | Unclear | Low | High | High |
| Mendoza 1978 | Unclear | Unclear | Low | Low | Low | Unclear | High |
| Millard 2001 | Unclear | Unclear | Unclear | Low | Low | Unclear | High |
| Mulla 2012 | Low | High | Unclear | Unclear | Low | Unclear | High |
| Nicholson 2018 | Low | Low | High | Low | Low | Unclear | Low |
| Nilsen 2012 | Unclear | Unclear | Low | Low | Low | Unclear | Unclear |
| Oostra 2004 | Low | Unclear | Unclear | Low | Low | Unclear | Low |
| Page 2005 | Low | Unclear | High | Low | Low | Low | High |
| Page 2007 | Low | Unclear | Low | Unclear | Low | Unclear | High |
| Page 2009 | Low | Unclear | Unclear | Unclear | Low | Unclear | High |
| Page 2011 | Low | High | Unclear | Low | Low | Low | High |
| Park 2015 | Low | Unclear | Unclear | Low | Low | Unclear | Low |
| Sanders 2004 | Unclear | Low | Low | Low | Unclear | Unclear | Low |
| Santiago 2015 | Low | Low | Unclear | Low | Low | Unclear | Low |
| Schuster 2012 | Low | Low | Low | Low | Low | Low | Low |
| Seebacher 2017 | Low | Low | High | Unclear | Low | Low | High |
| Sharp 2014 | Low | Low | Unclear | Low | Unclear | Unclear | High |
| Sidaway 2005 | Unclear | Unclear | Unclear | Unclear | Low | Unclear | High |
| Stenekes 2009 | Unclear | High | Unclear | Unclear | Low | Unclear | Low |
| Timmermans 2013 | Low | Low | High | Low | Low | Low | Low |
| Vergeer 2006 | Unclear | Unclear | Unclear | Low | Low | Low | Low |
| Wilson 2002 | Low | Unclear | Unclear | Low | Low | Unclear | High |
| Wilson 2016 | Unclear | Unclear | High | Low | Unclear | Unclear | High |
Discussion
This review assessed only RCTs evaluating the effectiveness of various MI protocols across the fields of sports, neurorehabilitation, education and medical education. The aim was to extract the components of a successful MI protocol. The authors hypothesised these components might be universal to MI training applied to several different indications, hence the inclusion of a heterogeneous sample of studies. In addition, MI programmes for surgical training remain novel, with few studies having specifically evaluating its effectiveness on surgeons. Broadening the search across several disciplines allowed protocol components never included in surgical training programmes to be considered.
Performing MI in addition to standard rehabilitation or training led to improvements in the majority of trials (83%).This is consistent with the concept that MI is a valuable tool when added to existing training. Based on current understanding of the neurological processes of MI, it can be speculated that protocols which demonstrate improvement in non-surgical fields can be extrapolated to surgical training, due to the fact all are focussed on motor skill learning. This could be particularly true for healthy populations improving on a specific skill - such as athletes. Surgical trainees and athletes have in common a healthy physical baseline and the goal of improving a specific motor skill. However, the authors acknowledge the methodological limitation of assuming similarities between populations. Overall, there were very few studies which specifically tested MI skills in surgical residents; this is a novel method of training in this field which must be tested further. This method could be used to improve a range of motor skills, ranging from generic surgical skills to patient-specific skills. Motor imagery-based training could be a supplement to standard surgical training.10,31
Studies where the intervention group performed better than control on outcomes had a median duration of intervention of 30 minutes, with a median of 15 MI sessions completed in 26 days. This is equivalent to performing MI more than once every 2 days. An online surgical training course, where trainees conducted a short amount of imagery, regularly and at their convenience, would fit these requirements. Indeed, there were 712,27,30,52,55,60,74 studies in which subjects were instructed to perform MI independently at home and record their progress. In the study by Louridas et al, 12 surgical trainees were given 7 days to perform MI at home and had follow-up calls and feedback. Only 2 of these 7 studies, by Jungmann et al 30 and Mulla et al, 27 did not see an improvement in the intervention group compared to control. They were also the only 2/7 studies which used medical students as their population. This means a MI training protocol for surgical education could be in a format which allowed subjects to access training in their own time.
Regarding the content of MI interventions, the level of detail provided across the studies review varied widely, making direct comparisons of protocols and associated outcomes difficult. However, the following elements could be incorporated into the structure of MI protocols in the interest of standardising their format and enabling direct comparison of outcomes in future research: a period of relaxation <5 minutes long prior to starting MI proper; detailed instructions involving specific sensory cues, a predetermined number of sets of repetitions of MI to be performed in each session and a refocusing period to close the MI session.
Given that there was no association between MI ability and technical performance (when compared to control), this indicates that baseline MI ability may not be an important factor for a MI training programme.
Given the heterogeneity of study outcomes measured and the variability of populations studied, no extrapolation can be made of the primary outcome most suitable for measuring the effectiveness of an MI training protocol. Relevant to surgical education MI training, a variety of primary outcome measures were used amongst the medical student and resident populations. These were variations of a pre-established checklist and objective measurements such as time and accuracy.
In 9 of the studies24,27,29,30,34-36,39,40 where the intervention group performed worse or equivalent to the control group, subjects were students or healthy participants for whom the benefit of the study was not obvious: they did not have an intrinsic motivation to perform well on the outcomes measured such as increased function of a limb following a stroke or improved surgical technique. This may indicate that for MI interventions to be successful, participants need to be self-motivated, and in the context of surgical education, surgeons should only undergo MI training if they see potential benefit in it. However, this is difficult to establish in the heterogeneous group of studies reviewed here and would benefit from further research focussed on surgical trainees’ motivation to use MI with their performance after training. Guillot’s article did explore the relationship between intrinsic motivation and MI in the opposite direction and suggests that MI does enhance intrinsic motivation. 16
A number of limitations to these results need to be considered. The majority of the studies were intrinsically biased as the subjects who received the intervention could not be blinded. Another limitation is the heterogeneity of studies included in this review. Studies included represented many applications of MI training, which may limit the generalisability of findings. Only 12 studies focussed on the application of MI training directly to surgical trainees or medical students. Further research is required to demonstrate that the findings from this review can be translated to surgical education. Furthermore, variations in study methodologies limited pooled analysis.
Following this review, more research focussing on the implementation of MI training protocols in surgical education is needed, in addition to the acceptability of such training measures among trainees and surgeons. The results of this review may aid in constructing a purpose-built MI training programme to evaluate its efficacy on surgical trainees specifically.
Conclusions
This comprehensive systematic review and meta-analysis has identified several characteristics linked to successful MI training in sports or neurorehabilitation that can be used to construct MI training protocols for use in surgical education. It must be highlighted that this review and analysis included a wide range of studies in different fields. However, certain components found to be linked to successful programmes could be extrapolated to surgical training, based on current understanding of neurological processes of MI. A successful MI training programme could be delivered in parallel to existing surgical training, in a flexible format allowing surgeons to undertake several MI sessions in a self-directed manner. A single MI session conducted by a senior surgeon could include a brief period of relaxation, followed by several sets of repetitions of MI, and a refocusing period. Providing guidance on the construction of effective MI training protocols will allow replicability of trials investigating the best way to deliver MI training. This is a step towards the development of a surgical MI training programme, as a low-cost, low-risk tool to enhance practical skills. Further research will be required to evaluate the use of MI in a purpose-built surgical training programme.
Appendix A
| Detailed Search Strategy |
|---|
| PubMed |
| ((‘Motor imagery’) OR (‘mental imagery’) OR (‘mental practice’) OR (‘mental training’)) AND ((‘randomised controlled study’) OR (‘randomised controlled trial’)) |
| 265 results |
| Ovid (PsycINFO, Embase and MEDLINE) |
| ((Motor imagery) OR (mental imagery) OR (mental practice) OR (mental training)) NOT (computerised OR computer) |
| Filter: Randomised controlled trial |
| 191 results |
Appendix B. List of Abbreviations Used in Tables 1 and 2.
| ACL | Anterior cruciate ligament |
| AMIT | Advanced Mechanical Technology Inc |
| BT | Box training |
| CMIT | Constraint-induced movement therapy |
| EMG | Electromyography |
| FAT | Frenchay arm test |
| FM | Fugl-Meyer assessment test |
| GRS | Global Rating Scale |
| IST | Intelligence Structure Test |
| IVI | Internal visual imagery |
| KG | Kinesiology |
| KIN | Kinaesthetic imagery |
| KVIQ-20 | Kinaesthetic and visual imagery questionnaire 20 |
| LBT | Line bisection test |
| MABC | Movement Assessment Battery for children |
| MCTE | Motor control therapeutic exercise |
| MI | Mental imagery, motor imagery |
| MIQ-RS | Motor Imagery Questionnaire-Revised |
| MMSE | Mini-mental state examination |
| MP | Mental practice |
| n/a | Not available |
| OSATS | Objective Standard Assessment of Technical Skills |
| OT | Occupational therapy |
| PP | Physical practice |
| SCT | Star cancellation test |
| SI | Stretching imagery |
| TDMI | Time-dependent motor imagery |
| VMIQ-2 | Vividness of Movement Imagery Questionnaire 2 |
| VR | Virtual reality |
| VRS | Virtual reality simulation |
Footnotes
Author Contributions: Study concept and design: Mary S. L. Goble and Nicholas Riason
Acquisition of data: Mary S. L. Goble and Ayah Mekhaimar
Analysis and interpretation: Mary S. L. Goble, Nicholas Riason, and Kamran Ahmed
Study supervision: Nicholas Riason, Kamran Ahmed, and Prokar Dasgupta
Declaration of Conflicting Interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Grant awarded from the Royal College of Surgeons. The funders had no involvement in the study design, the collection, analysis and interpretation of data; in the writing of the report and in the decision to submit the paper for publication.
ORCID iD
Mary S. L. Goble https://orcid.org/0000-0003-3950-8808
References
- 1.Sutherland LM, Middleton PF, Anthony A, et al. Surgical simulation. Ann Surg. 2006;243(3):291-300. doi: 10.1097/01.sla.0000200839.93965.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Opsommer E, Korogod N. Mental practice for chronic pain in people with spinal cord injury. JBI Database of Systematic Reviews and Implementation Reports. 2017;15(8):2004-2012. doi: 10.11124/JBISRIR-2016-003149 [DOI] [PubMed] [Google Scholar]
- 3.Guillot A, Lebon F, Vernay M, Girbon JP, Doyon J, Collet C. Effect of motor imagery in the rehabilitation of burn patients. J Burn Care Res. 2009;30(4):686-693. doi: 10.1097/BCR.0b013e3181ac0003 [DOI] [PubMed] [Google Scholar]
- 4.Fell NT, Wrisberg CA. Mental rehearsal as a complementary treatment in geriatric rehabilitation. Phys Occup Ther Geriatr. 2001;18(4):51-63. doi: 10.1080/J148v18n04_05 [DOI] [Google Scholar]
- 5.Schuster C, Hilfiker R, Amft O, et al. Best practice for motor imagery: A systematic literature review on motor imagery training elements in five different disciplines. BMC Med. 2011;9(1):75. doi: 10.1186/1741-7015-9-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun S, Beurskens A, Kleynen M, Schols J, Wade D. Rehabilitation with mental practice has similar effects on mobility as rehabilitation with relaxation in people with Parkinson’s disease: A multicentre randomised trial. J Physiother. 2011;57(1):27-34. doi: 10.1016/S1836-9553(11)70004-2 [DOI] [PubMed] [Google Scholar]
- 7.Holmes PS, Collins DJ. The PETTLEP approach to motor imagery: A functional equivalence model for sport psychologists. J Appl Sport Psychol. 2001;13(1):60-83. doi: 10.1080/10413200109339004 [DOI] [Google Scholar]
- 8.Braun S, Kleynen M, van Heel T, Kruithof N, Wade D, Beurskens A. The effects of mental practice in neurological rehabilitation; a systematic review and meta-analysis. Front Hum Neurosci. 2013;7:390. doi: 10.3389/fnhum.2013.00390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arora S, Aggarwal R, Sevdalis N, et al. Development and validation of mental practice as a training strategy for laparoscopic surgery. Surg Endosc. 2010;24(1):179-187. doi: 10.1007/s00464-009-0624-y [DOI] [PubMed] [Google Scholar]
- 10.Davison S, Raison N, Khan MS, Dasgupta P, Ahmed K. Mental training in surgical education: A systematic review. ANZ J Surg. 2017;87(11):873-878. doi: 10.1111/ans.14140 [DOI] [PubMed] [Google Scholar]
- 11.Immenroth M, Bürger T, Brenner JR, Nagelschmidt M, Eberspächer H, Troidl H. Mental training in surgical education. Ann Surg. 2007;245(3):385-391. doi: 10.1097/01.sla.0000251575.95171.b3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louridas M, Bonrath EM, Sinclair DA, Dedy NJ, Grantcharov TP. Randomized clinical trial to evaluate mental practice in enhancing advanced laparoscopic surgical performance. Br J Surg. 2015;102(1):37-44. doi: 10.1002/bjs.9657 [DOI] [PubMed] [Google Scholar]
- 13.Jeannerod M. Mental imagery in the motor context. Neuropsychologia. 1995;33(11):1419-1432. doi: 10.1016/0028-3932(95)00073-C [DOI] [PubMed] [Google Scholar]
- 14.Ridderinkhof KR, Brass M. How Kinesthetic motor imagery works: A predictive-processing theory of visualization in sports and motor expertise. J physiol Paris. 1992;109(1):53-63. [DOI] [PubMed] [Google Scholar]
- 15.Moran A, Guillot A, MacIntyre T, Collet C. Re-imagining motor imagery: Building bridges between cognitive neuroscience and sport psychology. Br J Psychol. 2012;103(2):224-247. doi: 10.1111/j.2044-8295.2011.02068.x [DOI] [PubMed] [Google Scholar]
- 16.Guillot A, Collet C. Construction of the motor imagery integrative model in sport: A review and theoretical investigation of motor imagery use. Int Rev Sport Exerc Psychol. 2008;1(1):31-44. doi: 10.1080/17509840701823139 [DOI] [Google Scholar]
- 17.Hétu S, Grégoire M, Saimpont A, et al. The neural network of motor imagery: An ALE meta-analysis. Neurosci Biobehav Rev. 2013;37(5):930-949. doi: 10.1016/j.neubiorev.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 18.Jeannerod M. The representing brain: Neural correlates of motor intention and imagery. BBS (Behav Brain Sci). 1994;17(2):187-202. doi: 10.1017/S0140525X00034026 [DOI] [Google Scholar]
- 19.Vogt S, Rienzo FD, Collet C, Collins A, Guillot A. Multiple roles of motor imagery during action observation. Front Hum Neurosci. 2013;7: 807. doi: 10.3389/fnhum.2013.00807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malouin F, Richards CL, Durand A, Doyon J. Reliability of mental chronometry for assessing motor imagery ability after stroke. Arch Phys Med Rehabil. 2008;89(2):311-319. doi: 10.1016/j.apmr.2007.11.006 [DOI] [PubMed] [Google Scholar]
- 21.Jones L, Stuth G. The uses of mental imagery in athletics: An overview. Appl Prev Psychol. 1997;6(2):101-115. doi: 10.1016/S0962-1849(05)80016-2 [DOI] [Google Scholar]
- 22.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. doi: 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conlin A, Lea J, Bance M, et al. Mental practice in postgraduate training: A randomized controlled trial in mastoidectomy skills. Journal of Otolaryngology - Head & Neck Surgery. 2016;45(1):46. doi: 10.1186/s40463-016-0162-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elder-Evans D, Grange P, Cheang A, et al. Using the mind as a simulator: A randomized controlled trial of mental training. J Surg Educ. 2013;70(4):544-551. doi: 10.1016/j.jsurg.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 26.Komesu Y, Urwitz-Lane R, Ozel B, et al. Does mental imagery prior to cystoscopy make a difference? A randomized controlled trial. Am J Obstet Gynecol. 2009;201(2):e1-e9. doi: 10.1016/j.ajog.2009.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma D, Mulla M, Moghul M, et al. Learning basic laparoscopic skills: A randomized controlled study comparing box trainer, virtual reality simulator, and mental training. J Surg Educ. 2012;69(2):190-195. doi: 10.1016/j.jsurg.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 28.Lim G, Krohner RG, Metro DG, Rosario BL, Jeong J-H, Sakai T. Low-fidelity haptic simulation versus mental imagery training for epidural anesthesia technical achievement in novice anesthesiology residents. Anesth Analg. 2016;122(5):1516-1523. doi: 10.1213/ANE.0000000000001260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanders CW, Sadoski M, Bramson R, Wiprud R, Van Walsum K. Comparing the effects of physical practice and mental imagery rehearsal on learning basic surgical skills by medical students. Am J Obstet Gynecol. 2004;191(5):1811-1814. doi: 10.1016/j.ajog.2004.07.075 [DOI] [PubMed] [Google Scholar]
- 30.Jungmann F, Gockel I, Hecht H, et al. Impact of perceptual ability and mental imagery training on simulated laparoscopic knot-tying in surgical novices using a Nissen fundoplication model. Scand J Surg. 2011;100(2):78-85. doi: 10.1177/145749691110000203 [DOI] [PubMed] [Google Scholar]
- 31.Sanders R, Gebhart J, Dooley Y, et al. The mind’s scalpel in surgical education: A randomised controlled trial of mental imagery. BJOG An Int J Obstet Gynaecol. 2012;119(9):1040-1048. doi: 10.1111/j.1471-0528.2012.03398.x [DOI] [PubMed] [Google Scholar]
- 32.Sanders CW, Sadoski M, van Walsum K, Bramson R, Wiprud R, Fossum TW. Learning basic surgical skills with mental imagery: Using the simulation centre in the mind. Med Educ. 2008;42(6):607-612. doi: 10.1111/j.1365-2923.2007.02964.x [DOI] [PubMed] [Google Scholar]
- 33.Bathalon S, Dorion D, Darveau S, Martin M. Cognitive skills analysis, kinesiology, and mental imagery in the acquisition of surgical skills. J Otolaryngol. 2005;34(5):328-332. doi: 10.2310/7070.2005.34506 [DOI] [PubMed] [Google Scholar]
- 34.Vergeer I, Roberts J. Movement and stretching imagery during flexibility training. J Sports Sci. 2006;24(2):197-208. doi: 10.1080/02640410500131811 [DOI] [PubMed] [Google Scholar]
- 35.de Paula Asa SK, Melo MCS, Piemonte MEP. Effects of mental and physical practice on a finger opposition task among children. Res Q Exerc Sport. 2014;85(3):308-315. doi: 10.1080/02701367.2014.931557 [DOI] [PubMed] [Google Scholar]
- 36.Sidaway B, Trzaska A. Can mental practice increase ankle dorsiflexor torque? Phys Ther. 2005;85(10):1053-1060. [PubMed] [Google Scholar]
- 37.Sharp KG, Gramer R, Butler L, Cramer SC, Hade E, Page SJ. Effect of overground training augmented by mental practice on gait velocity in chronic, incomplete spinal cord injury. Arch Phys Med Rehabil. 2014;95(4):615-621. doi: 10.1016/j.apmr.2013.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bovend’Eerdt TJ, Dawes H, Sackley C, Izadi H, Wade DT. Mental techniques during manual stretching in spasticity - a pilot randomized controlled trial. Clin Rehabil. 2009;23(2):137-145. doi: 10.1177/0269215508097298 [DOI] [PubMed] [Google Scholar]
- 39.Mendoza D, Wichman H. “Inner” darts: Effects of mental practice on performance of dart throwing. Percept Mot Skills. 1978;47(3_suppl l):1195-1199. doi: 10.2466/pms.1978.47.3f.1195 [DOI] [PubMed] [Google Scholar]
- 40.Nicholson VP, Keogh JW, Low Choy NL. Can a single session of motor imagery promote motor learning of locomotion in older adults? A randomized controlled trial. Clin Interv Aging. 2018;13:713-722. doi: 10.2147/CIA.S164401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim H, Yoo E-Y, Jung M-Y, Kim J, Park J-H, Kang D-H. The effects of mental practice combined with modified constraint-induced therapy on corticospinal excitability, movement quality, function, and activities of daily living in persons with stroke. Disabil Rehabil. 2018;40(20):2449-2457. doi: 10.1080/09638288.2017.1337817 [DOI] [PubMed] [Google Scholar]
- 42.Schuster C, Butler J, Andrews B, Kischka U, Ettlin T. Comparison of embedded and added motor imagery training in patients after stroke: Results of a randomised controlled pilot trial. Trials. 2012;13(1):11. doi: 10.1186/1745-6215-13-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santiago LMdM, de Oliveira DA, de Macêdo Ferreira LGL, et al. Immediate effects of adding mental practice to physical practice on the gait of individuals with Parkinson’s disease: Randomized clinical trial. NeuroRehabilitation. 2015;37(2):263-271. DOI: 10.3233/NRE-151259 [DOI] [PubMed] [Google Scholar]
- 44.Wilson PH, Adams ILJ, Caeyenberghs K, Thomas P, Smits-Engelsman B, Steenbergen B. Motor imagery training enhances motor skill in children with DCD: A replication study. Res Dev Disabil. 2016;57:54-62. doi: 10.1016/j.ridd.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 45.Kim J-H, Lee B-H. Action observation training for functional activities after stroke: A pilot randomized controlled trial. NeuroRehabilitation. 2013;33(4):565-574. doi: 10.3233/NRE-130991 [DOI] [PubMed] [Google Scholar]
- 46.Ietswaart M, Johnston M, Dijkerman HC, et al. Mental practice with motor imagery in stroke recovery: Randomized controlled trial of efficacy. Brain. 2011;134(Pt 5):1373-1386. doi: 10.1093/brain/awr077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oosra K, Oomen A, Vanderstraeten G, Vingerhoets G. Influence of motor imagery training on gait rehabilitation in sub-acute stroke: A randomized controlled trial. J Rehabil Med. 2015;47(3):204-209. doi: 10.2340/16501977-1908 [DOI] [PubMed] [Google Scholar]
- 48.Coker E, McIsaac TL, Nilsen D. Motor imagery modality in expert dancers: An investigation of hip and pelvis kinematics in demi-plié and sauté. J Dance Med Sci. 2015;19(2):63-69. doi: 10.12678/1089-313X.19.2.63 [DOI] [PubMed] [Google Scholar]
- 49.Braun S, Beurskens A, Kleynen M, Oudelaar B, Schols J, Wade D. A multicenter randomized controlled trial to compare subacute ‘Treatment as usual’ with and without mental practice among persons with stroke in Dutch nursing homes. J Am Med Dir Assoc. 2012;13(1):85.e1-85.e7. doi: 10.1016/j.jamda.2010.07.009 [DOI] [PubMed] [Google Scholar]
- 50.Cho H-y, Kim J-s, Lee G-C. Effects of motor imagery training on balance and gait abilities in post-stroke patients: A randomized controlled trial. Clin Rehabil. 2013;27(8):675-680. doi: 10.1177/0269215512464702 [DOI] [PubMed] [Google Scholar]
- 51.Page SJ, Levine P, Leonard A. Mental practice in chronic stroke. Stroke. 2007;38(4):1293-1297. doi: 10.1161/01.STR.0000260205.67348.2b [DOI] [PubMed] [Google Scholar]
- 52.Dilek B, Ayhan C, Yagci G, Yakut Y. ScienceDirect. J Environ Sci 2017(10):125-126. [Google Scholar]
- 53.Park J-H, Lee J-H. The effects of mental practice on unilateral neglect in patients with chronic stroke: A randomized controlled trial. J Phys Ther Sci. 2015;27(12):3803-3805. doi: 10.1589/jpts.27.3803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu KPY, Chan CCH, Wong RSM, et al. A randomized controlled trial of mental imagery augment generalization of learning in acute poststroke patients. Stroke. 2009;40(6):2222-2225. doi: 10.1161/STROKEAHA.108.540997 [DOI] [PubMed] [Google Scholar]
- 55.Losana-Ferrer A, Manzanas-López S, Cuenca-Martínez F, Paris-Alemany A, La Touche R. Effects of motor imagery and action observation on hand grip strength, electromyographic activity and intramuscular oxygenation in the hand gripping gesture: A randomized controlled trial. Hum Mov Sci. 2018;58:119-131. [DOI] [PubMed] [Google Scholar]
- 56.Liu KP, Chan CC, Lee TM, Hui-Chan CW. Mental imagery for promoting relearning for people after stroke: A randomized controlled trial. Arch Phys Med Rehabil. 2004;85(9):1403-1408. doi: 10.1016/j.apmr.2003.12.035 [DOI] [PubMed] [Google Scholar]
- 57.Timmermans AAA, Verbunt JA, van Woerden R, Moennekens M, Pernot DH, Seelen HAM. Effect of mental practice on the improvement of function and daily activity performance of the upper extremity in patients with subacute stroke: A randomized clinical trial. J Am Med Dir Assoc. 2013;14(3):204-212. doi: 10.1016/j.jamda.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 58.Lebon F, Guillot A, Collet C. Increased muscle activation following motor imagery during the rehabilitation of the anterior cruciate ligament. Appl Psychophysiol Biofeedback. 2012;37(1):45-51. doi: 10.1007/s10484-011-9175-9 [DOI] [PubMed] [Google Scholar]
- 59.Page SJ, Levine P, Khoury JC. Modified constraint-induced therapy combined with mental practice. Stroke. 2009;40(2):551-554. doi: 10.1161/STROKEAHA.108.528760 [DOI] [PubMed] [Google Scholar]
- 60.Stenekes MW, MD|Geertzen JH, Nicolai J-PA, et al. Effects of motor imagery on hand function during immobilization after flexor tendon repair. Arch Phys Med Rehabil. 2009;90(4):553-559. doi: 10.1016/j.apmr.2008.10.029 [DOI] [PubMed] [Google Scholar]
- 61.Nilsen DM, Gillen G, DiRusso T, Gordon AM. Effect of imagery perspective on occupational performance after stroke: A randomized controlled trial. Am J Occup Ther. 2012;66(3):320-329. doi: 10.5014/ajot.2012.003475 [DOI] [PubMed] [Google Scholar]
- 62.Hoyek N, Di Rienzo F, Collet C, Hoyek F, Guillot A. The therapeutic role of motor imagery on the functional rehabilitation of a stage II shoulder impingement syndrome. Disabil Rehabil. 2014;36(13):1113-1119. doi: 10.3109/09638288.2013.833309 [DOI] [PubMed] [Google Scholar]
- 63.Liu KPY. Use of mental imagery to improve task generalisation after a stroke. Hong Kong medical journal = Xianggang yi xue za zhi 2009;15(3 suppl 4):37. [PubMed] [Google Scholar]
- 64.Malouin F, Richards CL, Durand A, Doyon J. Added value of mental practice combined with a small amount of physical practice on the relearning of rising and sitting post-stroke: A pilot study. J Neurol Phys Ther. 2009;33(4):195-202. doi: 10.1097/NPT.0b013e3181c2112b [DOI] [PubMed] [Google Scholar]
- 65.Page SJ, Levine P, Leonard AC. Effects of mental practice on affected limb use and function in chronic stroke. Arch Phys Med Rehabil. 2005;86(3):399-402. doi: 10.1016/j.apmr.2004.10.002 [DOI] [PubMed] [Google Scholar]
- 66.Hosseini SA, Fallahpour M, Sayadi M, Gharib M, Haghgoo H. The impact of mental practice on stroke patients' postural balance. J Neurol Sci. 2012;322(1):263-267. doi: 10.1016/j.jns.2012.07.030 [DOI] [PubMed] [Google Scholar]
- 67.Abraham A, Hart A, Andrade I, Hackney ME. Dynamic neuro-cognitive imagery improves mental imagery ability, disease severity, and motor and cognitive functions in people with Parkinson’s disease. Neural Plast. 2018;2018:1-15. doi: 10.1155/2018/6168507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blumen HM, Verghese J. Motor imagery of walking and walking while talking: A pilot randomized-controlled trial protocol for older adults. Neurodegener Dis Manag. 2017;7(6):353-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Page SJ, Dunning K, Hermann V, Leonard A, Levine P. Longer versus shorter mental practice sessions for affected upper extremity movement after stroke: A randomized controlled trial. Clin Rehabil. 2011;25(7):627-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Millard M, Mahoney C, Wardrop J. A preliminary study of mental and physical practice on the kayak wet exit skill. Percept Mot Skills. 2001;92(3 Pt 2):977-984. doi: 10.2466/PMS.92.3.977-984 [DOI] [PubMed] [Google Scholar]
- 71.Hemayattalab R, Movahedi A. Effects of different variations of mental and physical practice on sport skill learning in adolescents with mental retardation. Res Dev Disabil. 2010;31(1):81-86. [DOI] [PubMed] [Google Scholar]
- 72.Callow N, Jiang D, Roberts R, Edwards MG. Kinesthetic imagery provides additive benefits to internal visual imagery on slalom task performance. J Sport Exerc Psychol. 2017;39(1):81-86. doi: 10.1123/jsep.2016-0168 [DOI] [PubMed] [Google Scholar]
- 73.Gomes TVB, Ugrinowitsch H, Marinho N, Shea JB, Raisbeck LD, Benda RN. Effects of mental practice in novice learners in a serial positioning skill acquisition. Percept Mot Skills. 2014;119(2):397-414. doi: 10.2466/23.PMS.119c20z4 [DOI] [PubMed] [Google Scholar]
- 74.Frenkel MO, Herzig DS, Gebhard F. Mental practice maintains range of motion despite forearm immobilization: A pilot study in healthy persons. http://www.medicaljournals.se/jrm/content/abstract/10.2340/16501977-1263. Accessed December 31, 2018. [DOI] [PubMed]
- 75.Seebacher B, Kuisma R, Glynn A, Berger T. The effect of rhythmic-cued motor imagery on walking, fatigue and quality of life in people with multiple sclerosis: A randomised controlled trial. Multiple Sclerosis Journal. 2017;23(2):286-296. doi: 10.1177/1352458516644058 [DOI] [PubMed] [Google Scholar]
- 76.Hidalgo-Peréz A, Fernández-García Á, López-de-Uralde-Villanueva I, et al. Effectiveness of a motor control therapeutic exercise program combined with motor imagery on the sensorimotor function of the cervical spine: A randomized controlled trial. International Journal of Sports Physical Therapy. 2015;10(6):877. [PMC free article] [PubMed] [Google Scholar]
- 77.Wilson PH, Thomas PR, Maruff P. Motor imagery training ameliorates motor clumsiness in children. J Child Neurol. 2002;17(7):491-498. doi: 10.1177/088307380201700704 [DOI] [PubMed] [Google Scholar]
- 78.Maring JR. Effects of mental practice on rate of skill acquisition. Phys Ther. 1990;70(3):165-172. doi: 10.1093/ptj/70.3.165 [DOI] [PubMed] [Google Scholar]
- 79.Cunha RG, Da-Silva PJG, Dos SCP, et al. Influence of functional task-oriented mental practice on the gait of transtibial amputees: A randomized, clinical trial. J NeuroEng Rehabil. 2017;14(1):28. doi: 10.1186/s12984-017-0238-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Isaac AR, Marks DF. Individual differences in mental imagery experience: Developmental changes and specialization. Br J Psychol. 1994;85(4):479. doi: 10.1111/j.2044-8295.1994.tb02536.x [DOI] [PubMed] [Google Scholar]
- 81.Isaac A, Marks DF, Russell DG. An instrument for assessing imagery of movement: The vividness of movement imagery questionnaire (VMIQ). J Ment Imagery. 1986;10(4):23. [Google Scholar]
- 82.Malouin F, Richards CL, Jackson PL, Lafleur MF, Durand A, Doyon J. The kinesthetic and visual imagery questionnaire (KVIQ) for assessing motor imagery in persons with physical disabilities: A reliability and construct validity study. J Neurol Phys Ther. 2007;31(1):20. doi: 10.1097/01.NPT.0000260567.24122.64 [DOI] [PubMed] [Google Scholar]