Abstract
Background and aims:
The application of QuantiFERON-TB Gold in-Tube (QFT-GIT) in patients with haematological malignancies (HMs) has not been well studied. Therefore, we aimed to investigate the features of patients with HMs whose QFT-GIT results were indeterminate.
Methods:
This study enrolled patients with HMs for the analysis of QFT-GIT tests and additional 2-year follow-up. The characteristics and predictors of QFT-GIT indeterminate results were identified. Mycobacterium tuberculosis (TB) incidence rate (IR) and incidence rate ratio (IRR) were also investigated.
Results:
Of 89 participants, 27 (30.3%) had QFT-GIT indeterminate results. The QFT-GIT indeterminate patients were characterized with the diagnosis of leukaemia (63.0% versus 32.3%, p = 0.044), abnormal white blood count (WBC) (88.9% versus 14.5%, p = 0.001), abnormal lymphocyte percentage (81.5% versus 14.5%, p = 0.001) and lower lymphocyte count (×109/l) (0.5 versus 2.2, p = 0.000) when compared with those with determinate results. Meanwhile, abnormal WBC [odds ratios (OR): 15.18, p = 0.003] and lymphocyte percentage (OR: 6.90, p = 0.033) were predictors of indeterminate results. One patient with the QFT-GIT indeterminate status and high interferon-γ level of negative control result developed active TB with a TB IR of 18.5 per 1000 person-years and an IRR of 0.1 (95% confidence interval, 0.01–0.71) when compared with positive QFT-GIT patients without prophylaxis treatment.
Conclusion:
Abnormal ranges of WBC and lymphocyte differential count percentage were independent predictors useful to determine the optimal timing of implementing QFT-GIT test in patients with HMs.
Keywords: haematological malignancy, indeterminate, QuantiFERON-TB Gold in-Tube
Introduction
Tuberculosis (TB) is one of the major infectious diseases globally, 1 infecting up to one-third of the world’s population in the absence of clinical signs or symptoms of active TB. 2 An important strategy to achieve TB control is screening and treatment of individuals with latent tuberculosis infection (LTBI). 1 The World Health Organization (WHO) strongly recommends systematic testing and treatment of LTBI in high risk individuals, in countries with high or middle upper income, and TB incidence of <100 per 100,000 per year. 3 Immunocompromised patients with LTBI, including subjects infected with human immunodeficiency virus (HIV), 4 those with immune-mediated inflammatory disorders,5,6 end stage renal disease and cancer,7,8 and candidates for treatment with tumour necrosis factor-α inhibitors or solid-organ transplant have an increased risk of tuberculosis reactivation compared with the general population,9–11 and thus LTBI screening is recommended in these high-risk groups. 12 Treatment regimens recommended for LTBI include 6- or 9-month isoniazid (INH), 12-week rifapentine plus isoniazid, 3–4-month isoniazid plus rifampicin, or 3–4-month rifampicin alone. 3
Tuberculin skin test (TST) and QuantiFERON®-TB Gold in-Tube (QFT-GIT) are two popular methods of diagnosing LTBI. Both are indirect methods of detecting Mycobacterium tuberculosis infection by measuring the T-cell immune response against the bacillus. 13 In immunocompetent persons, QFT-GIT has at least equal sensitivity to the TST with improved specificity for the diagnosis of LTBI; while in immunocompromised individuals, the sensitivity of both tests for detecting LTBI is lower than that in patients with preserved immunity.12,14,15 Compared with TST, QFT-GIT has several advantages, including less reader bias because of numerical results, no cross-reaction with Bacillus Calmette–Guérin (BCG) vaccination status due to use of TB-specific polypeptides, and convenience as there is no need for a follow-up visit to interpret the results. 13 However, one of the main disadvantages of QFT-GIT is that the response to TB-specific antigens cannot be interpreted in the presence of an indeterminate result, thus hampering clinicians’ decision making on whether preventive therapy should be instituted for high risk groups.
Haematological malignancies (HMs) are cancers that affect blood, bone marrow and lymph nodes, and notably originate from the immune system, leading to potential T-cell dysfunction in such patients. Furthermore, patients with HMs have a greater risk of progressing from latent to active TB because of the disease itself and a consequence of antineoplastic chemotherapy. 8 The TB incidence rate of patients with HMs is around 120–1068/100,000 person-years.16–18 Meanwhile, the relative risk of TB disease in such population is 2–40 times that of the general population. 19 Therefore, Centers for Disease Control and Prevention and WHO recommend screening and considering treatment of LTBI in patients with HMs.20–22 However, the relevance of QFT-GIT in such immunocompromised population remains unclear.
We hypothesized that the impaired T-cell immune response would result in a high rate of indeterminate results of QFT-GIT in patients with HMs. Thus, we conducted this study to investigate the incidence, clinical features and predictors of indeterminate results of QFT-GIT, and to evaluate the predictive value of indeterminate results for progression to active TB disease in hospitalized patients with HMs.
Methods
Study design, setting and population
This prospective observational study included patients with HMs who were admitted to the haematology wards of Taichung Veterans General Hospital in central Taiwan between April 2014 and March 2017. Patients aged <18 years old, those with a history of HIV infection, or cancers other than the haematological malignancies of interest, and those with expected lifespan less than 2 years, which was evaluated by the physician in charge based on the treatment response, the severity and number of co-morbidities, and the presence of malignancy-associated potentially life-threatening complications [such as haemophagocytic lymphohistiocytosis (HLH)] or not, were excluded from this study. In addition, those who had a positive history or radiologic/laboratory evidence of previous or current TB disease were also excluded from this study. The Institutional Review Board and Ethics Committee of Taichung Veterans General Hospital approved this study (approval number: SF11247A) and assured that all methods were performed in accordance with the relevant guidelines and regulations, and informed consent was obtained from all participants.
Data collection and follow-up
For each participant, the investigators completed a detailed patient record form by reviewing and recording clinical data from electronic medical records, including age, gender, tumour types, treatment courses, chest X-ray, laboratory findings and co-morbidities. Furthermore, all participants were followed-up at a 12-month interval for 2 years after enrolment to identify the occurrence of subsequent active TB disease.
Treatment of LTBI
Whether participants with positive results of QFT-GIT received preventive therapy with INH (at a dose of 5 mg/kg per day, up to a maximum of 300 mg daily) for 9 months was independently decided by the participants after receiving detailed information on the benefits and risks associated with LTBI and its treatment.
QFT-GIT assay
For all study participants, the QFT-GIT test was performed and interpreted according to the manufacturer’s instructions (Cellestis, QIAGEN, Victoria, Australia). 23 The result was considered as positive if the nil response was ⩽8.0 IU/ml and the TB response (TB antigen minus nil) was ⩾0.35 IU/ml and ⩾25% of nil value. The result was interpreted as negative when the TB response was <0.35 IU/ml or ⩾0.35 IU/ml and <25% of nil value in the case of nil response ⩽8.0 IU/ml and mitogen response (mitogen minus nil) ⩾0.5 IU/ml. The test was considered indeterminate if the mitogen response was <0.5 IU/ml together with TB response <0.35 IU/ml or ⩾0.35 IU/ml and <25% of nil value, or nil response >8.0 IU/ml, as recommended by the manufacturer’s criteria. 23 For the study purpose, positive and negative results of QFT-GIT were combined into one category termed as ‘determinate’.
Statistical analysis
All data were expressed as median (minimum–maximum) for continuous variables or frequencies (percentages) for categorical variables. For univariate analysis, comparisons were conducted using Mann–Whitney U test for continuous variables and chi-squared test for categorical variables. Multivariate logistic regression models were used to analyse associated factors of indeterminate QFT-GIT results if they were significant in the univariate analysis. Incidence rate was calculated per 1000 person-years. Incidence rate ratio was defined as the proportion of the at-risk study population that developed active TB between April 2012 and March 2015. 24 Statistical significance was set at p < 0.05. Statistical analysis was performed using SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline demographics and characteristics of the participants
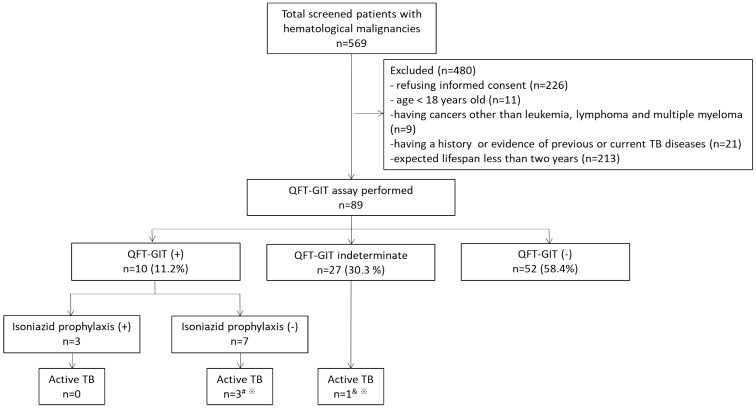
Figure 1 shows the patient enrolment flow chart. Out of 569 subjects with HMs who were screened, 89 patients were enrolled in the study and received QFT-GIT tests. Of the enrolled participants, 11.2% (10/89) were QFT-GIT positive, 58.4% (52/89) were QFT-GIT negative, while 30.3% (27/89) were QFT-GIT indeterminate.
Figure 1.
The patient enrolment flow chart.
#Two patients had active TB at 1-year follow-up while one had active TB at 2-year follow-up.
&The patient had active TB at 2-year follow-up.
*The diagnosis of TB was confirmed by the microbiological evidence.
QFT-GIT, QuantiFERON-TB Gold in-Tube; TB, tuberculosis.
Table 1 presents the baseline information of all enrolled participants. Most participants were middle-aged and BCG vaccinated and had previously received chemotherapy with or without radiotherapy, while the most common tumour types were lymphoma and leukaemia.
Table 1.
Baseline demographics and characteristics of the enrolled participants.
| Positive n = 10 |
Negative n = 52 |
Determinate
#
n = 62 |
Indeterminate n = 27 |
p-value | Total n = 89 |
|
|---|---|---|---|---|---|---|
| Age, years | 71.0 (39.0, 83.0) | 58.0 (20.0, 81.0) | 59.0 (20.0, 83.0) | 56.0 (20.0, 82.0) | 0.324 | 58.0 (20.0, 83.0) |
| Male gender (%) | 9 (90.0) | 26 (50.0) | 35 (56.5) | 12 (44.4) | 0.417 | 47 (52.8) |
| BCG vaccinated (%) | 10 (100) | 50 (96.2) | 60 (96.8) | 25 (92.6) | 0.582 | 85 (95.5) |
| Tumour type | 0.044* | |||||
| Leukaemia (%) | 2 (20.0) | 18 (34.6) | 20 (32.3) | 17 (63.0) | 37 (41.6) | |
| AML § (%) | 1 (10.0) | 14 (26.9) | 15 (24.2) | 13 (48.1) | 28 (31.5) | |
| Precursor B-cell ALL (%) | 0 (0.0) | 2 (3.8) | 2 (3.2) | 3 (11.1) | 5 (5.6) | |
| CML (%) | 0 (0.0) | 1 (1.9) | 1 (1.6) | 1 (3.7) | 2 (2.2) | |
| Blast crisis (%) | 0 (0.0) | 1 (1.9) | 1 (1.6) | 0 (0.0) | 1 (1.1) | |
| Chronic phase (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.7) | 1 (1.1) | |
| CLL (%) | 1 (10.0) | 1 (1.9) | 2 (3.2) | 0 (0.0) | 2 (2.2) | |
| Rai stage III (%) | 0 (0.0) | 1 (1.9) | 1 (1.6) | 0 (0.0) | 1 (1.1) | |
| Rai stage IV (%) | 1 (10.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 1 (1.1) | |
| Lymphoma (%) | 5 (50.0) | 28 (53.8) | 33 (53.2) | 7 (25.9) | 40 (44.9) | |
| Ann Arbor stage I (%) | 0 (0.0) | 1 (1.9) | 1 (1.6) | 0 (0.0) | 1 (1.1) | |
| Ann Arbor stage II (%) | 1 (10.0) | 3 (5.8) | 4 (6.5) | 1 (3.7) | 5 (5.6) | |
| Ann Arbor stage III (%) | 1 (10.0) | 4 (7.7) | 5 (8.1) | 2 (7.4) | 7 (7.9) | |
| Ann Arbor stage IV (%) | 3 (30.0) | 20 (38.5) | 23 (37.1) | 4 (14.8) | 27 (30.3) | |
| Multiple myeloma (%) | 3 (30.0) | 6 (11.5) | 9 (14.5) | 3 (11.1) | 12 (13.5) | |
| Durie–Salmon stage IA (%) | 0 (0.0) | 1 (1.9) | 1 (1.6) | 0 (0.0) | 1 (1.1) | |
| Durie–Salmon stage IB (%) | 1 (10.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 1 (1.1) | |
| Durie–Salmon stage IIA (%) | 1 (10.0) | 1 (1.9) | 2 (3.2) | 0 (0.0) | 2 (2.2) | |
| Durie–Salmon stage IIIA (%) | 1 (10.0) | 3 (5.8) | 4 (6.5) | 3 (11.1) | 7 (7.9) | |
| Durie–Salmon stage IIIB (%) | 0 (0.0) | 1 (1.9) | 1 (1.6) | 0 (0.0) | 1 (1.1) | |
| Treatment received previously | 0.197 | |||||
| None (%) | 4 (40.0) | 10 (19.2) | 14 (22.6) | 3 (11.1) | 17 (19.1) | |
| Leukaemia (%) | 0 (0.0) | 2 (3.8) | 2 (3.2) | 2 (7.4) | 4 (4.5) | |
| AML § (%) | 0 (0.0) | 1 (1.9) | 1 (1.6) | 2 (7.4) | 3 (3.4) | |
| CLL | ||||||
| Rai stage III (%) | 0 (0.0) | 1 (1.9) | 1 (1.6) | 0 (0.0) | 1 (1.1) | |
| Lymphoma (%) | 2 (20.0) | 7 (13.5) | 9 (14.5) | 1 (3.7) | 10 (11.2) | |
| Ann Arbor stage III (%) | 1 (10.0) | 1 (1.9) | 2 (3.2) | 0 (0.0) | 2 (2.2) | |
| Ann Arbor stage IV (%) | 1 (10.0) | 6 (11.5) | 7 (11.3) | 1 (3.7) | 8 (9.0) | |
| Multiple myeloma (%) | 2 (20.0) | 1 (1.9) | 3 (4.8) | 0 (0.0) | 3 (3.4) | |
| Durie-Salmon stage IB (%) | 1 (10.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 1 (1.1) | |
| Durie-Salmon stage IIA (%) | 1 (10.0) | 1 (1.9) | 2 (3.2) | 0 (0.0) | 2 (2.2) | |
| Chemotherapy only (%) | 6 (60.0) | 41 (78.8) | 47 (75.8) | 22 (81.5) | 69 (77.5) | |
| Leukaemia (%) | 2 (20.0) | 15 (28.8) | 17 (27.4) | 14 (51.9) | 31 (34.8) | |
| AML § (%) | 1 (10.0) | 12 (23.1) | 13 (21.0) | 10 (37.0) | 23 (25.8) | |
| Precursor B-cell ALL (%) | 0 (0.0) | 2 (3.8) | 2 (3.2) | 3 (11.1) | 5 (5.6) | |
| CML (%) | 0 (0.0) | 1 (1.9) | 1 (1.6) | 1 (3.7) | 2 (2.2) | |
| Blast crisis (%) | 0 (0.0) | 1 (1.9) | 1 (1.6) | 0 (0.0) | 1 (1.1) | |
| Chronic phase (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.7) | 1 (1.1) | |
| CLL | ||||||
| Rai stage IV (%) | 1 (10.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 1 (1.1) | |
| Lymphoma (%) | 3 (30.0) | 21 (40.4) | 24 (38.7) | 6 (22.2) | 30 (33.7) | |
| Ann Arbor stage I (%) | 0 (0.0) | 1 (1.9) | 1 (1.6) | 0 (0.0) | 1 (1.1) | |
| Ann Arbor stage II (%) | 1 (10.0) | 3 (5.8) | 4 (6.5) | 1 (3.7) | 5 (5.6) | |
| Ann Arbor stage III (%) | 0 (0.0) | 3 (5.8) | 3 (4.8) | 2 (7.4) | 5 (5.6) | |
| Ann Arbor stage IV (%) | 2 (20.0) | 14 (26.9) | 16 (25.8) | 3 (11.1) | 19 (21.3) | |
| Multiple myeloma (%) | 1 (10.0) | 5 (9.6) | 6 (9.7) | 2 (7.4) | 8 (9.0) | |
| Durie–Salmon stage IA (%) | 0 (0.0) | 1 (1.9) | 1 (1.6) | 0 (0.0) | 1 (1.1) | |
| Durie–Salmon stage IIIA (%) | 1 (10.0) | 3 (5.8) | 4 (6.5) | 2 (7.4) | 6 (6.7) | |
| Durie–Salmon stage IIIB (%) | 0 (0.0) | 1 (1.9) | 1 (1.6) | 0 (0.0) | 1 (1.1) | |
| Chemotherapy + radiotherapy (%) | 0 (0.0) | 1 (1.9) | 1 (1.6) | 2 (7.4) | 3 (3.4) | |
| Leukaemia | ||||||
| AML § (%) | 0 (0.0) | 1 (1.9) | 1 (1.6) | 1 (3.7) | 2 (2.2) | |
| Multiple myeloma | ||||||
| Durie–Salmon stage IIIA (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.7) | 1 (1.1) | |
| Chest X-ray | 0.508 | |||||
| Normal (%) | 1 (10.0) | 27 (51.9) | 28 (45.2) | 13 (48.1) | 41 (46.1) | |
| Abnormal but not related to previous or current TB infection (%) | 8 (80.0) | 23 (44.2) | 31 (50.0) | 14 (51.9) | 45 (50.6) | |
| Presence of bronchiectasis (%) | 1 (10.0) | 2 (3.8) | 3 (4.8) | 0 (0.0) | 3 (3.4) | |
| Laboratory finding | ||||||
| WBC, ×109/l | 7.1 (4.3, 14.4) | 7.2 (0.6, 114.4) | 7.2 (0.6, 114.4) | 3.0 (0.2, 161.7) | 0.001* | 6.7 (0.2, 161.7) |
| Normal reference range$ (%) | 9 (90.0) | 44 (84.6) | 53 (85.5) | 3 (11.1) | 56 (62.9) | |
| Abnormal reference range (%) | 1 (10.0) | 8 (15.4) | 9 (14.5) | 24 (88.9) | 33 (37.1) | |
| Lymphocyte (%) | 32.0 (7.0, 54.0) | 31.5 (6.8, 60.0) | 32.0 (6.8, 60.0) | 14.0 (2.0, 65.5) | 0.001* | 29.5 (2.0, 65.5) |
| Normal reference range & (%) | 8 (80.0) | 45 (86.5) | 53 (85.5) | 5 (18.5) | 58 (65.2) | |
| Abnormal reference range (%) | 2 (20.0) | 7 (13.5) | 9 (14.5) | 22 (81.5) | 31 (34.8) | |
| Lymphocyte, ×109/l (%) | 2.2 (1.0, 3.7) | 2.3 (0.1, 49.2) | 2.2 (0.1, 49.2) | 0.5 (0.0, 66.3) | 0.000* | 2.0 (0.0, 66.3) |
| QFT-GIT, IU/ml | ||||||
| Nil, negative control | 0.1 (0.0, 0.2) | 0.1 (0.0, 1.7) | 0.1 (0.0, 1.7) | 0.1 (0.0, 1.2) | 0.932 | 0.1 (0.0, 1.7) |
| TB antigen minus nil | 1.9 (0.4, 10.0) | 0.0 (−0.2, 0.2) | 0.0 (−0.2, 10.0) | 0.0 (−0.6, 0.1) | 0.072 | 0.0 (−0.6, 10.0) |
| Mitogen minus nil, positive control | 10.0 (0.4, 10.0) | 7.0 (0.6, 10.0) | 8.3 (0.4, 10.0) | 0.1 (−0.4, 0.5) | 0.000* | 2.4 (−0.4, 10.0) |
| Co-morbidity | ||||||
| Diabetes mellitus (%) | 0 (0.0) | 4 (7.7) | 4 (6.5) | 3 (11.1) | 0.429 | 7 (7.9) |
| Autoimmune disorder (%) | 1 (10.0) | 5 (9.6) | 6 (9.7) | 2 (7.4) | 1.000 | 8 (9.0) |
| COPD (%) | 2 (20.0) | 4 (7.7) | 6 (9.7) | 2 (7.4) | 1.000 | 8 (9.0) |
| Asthma (%) | 0 (0.0) | 2 (3.8) | 2 (3.2) | 1 (3.7) | 1.000 | 3 (3.4) |
| Chronic renal failure (%) | 0 (0.0) | 1 (1.9) | 1 (1.6) | 2 (7.4) | 0.217 | 3 (3.4) |
Data are presented as median (min–max) or number (%).
Determinate = positive + negative.
p < 0.05.
The reference range of the study institute: male, 3900–10600/μl; female, 3500–11000/μl.
The reference range of the study institute: 19–48%.
The European LeukemiaNet risk stratification by genetics did not performed for patients with AML because of the unavailability of the assays for genetic testing in the study period.
ALL, acute lymphocytic leukaemia; AML, acute myeloid leukaemia; BCG, Bacillus Calmette–Guérin; CLL, chronic lymphocytic leukaemia; CML, chronic myeloid leukaemia; COPD, chronic obstructive pulmonary disease; QFT-GIT, QuantiFERON-TB Gold in-Tube; TB, tuberculosis; WBC, white blood count.
Predictors of indeterminate QFT-GIT results
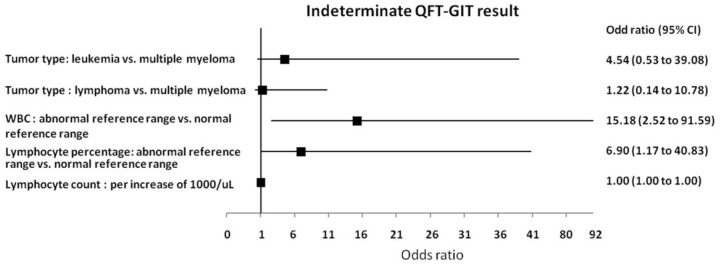
Compared with subjects with determinate QFT-GIT results (participants with positive and negative QFT-GIT results), patients with QFT-GIT indeterminate had a higher proportion of leukaemia [63.0% (17/27), p = 0.044], abnormal white blood count (WBC) [88.9% (24/27), p = 0.001] and lymphocyte [81.5% (22/27), p = 0.001] percentage, and lower median lymphocyte count (0.5 × 109/l, p = 0.000) (Table 1). Multivariate logistic regression analysis showed that abnormal WBC [odds ratio (OR): 15.18; 95% confidence interval (CI): 2.52–91.59, p = 0.003] and lymphocyte (OR: 6.90; 95% CI: 1.17–40.83, p = 0.033) percentage were risk factors associated with indeterminate results (Figure 2).
Figure 2.
The predictors associated with indeterminate QFT-GIT results.
CI, confidence interval; QFT-GIT, QuantiFERON-TB Gold in-Tube; WBC, white blood count.
Predictive value of QFT-GIT for progression to active TB
During the 2-year follow-up period, four (4.5%) of the 89 enrolled subjects developed active TB. Of these, three had positive QFT-GIT results, who did not receive LTBI preventive treatment, while one was QFT-GIT indeterminate, who did not receive INH prophylaxis treatment (Figure 1). The incidence rate ratios of the tested groups are shown in Table 2. Compared with patients with positive QFT-GIT results without INH prophylaxis treatment, the incidence rate ratio of participants with indeterminate QFT-GIT results was 0.1 (95% CI 0.01–0.71, p = 0.024). This indicated that though it was lower than that of subjects with positive QFT-GIT results without receiving LTBI preventive treatment, patients with HMs and indeterminate QFT-GIT results had a propensity to develop active TB, with an incidence rate 18.5 per 1000 person-years (Table 2).
Table 2.
Incidence rate and incidence rate ratio of the tested groups for progression to active tuberculosis.
| Number | Active TB | Progression to disease, years | Incidence rate per 1000 person-years | Incidence rate ratio (95% CI) | |
|---|---|---|---|---|---|
| QFT-GIT results | |||||
| Negative | 52 | 0 | 104 | 0 | – |
| Positive with INH prophylaxis | 3 | 0 | 6 | 0 | – |
| Positive without INH prophylaxis | 7 | 3 | 12 | 250.0 | 1 |
| Indeterminate | 27 | 1 | 54 | 18.5 | 0.1 (0.01–0.71)* |
p < 0.05.
CI, confidence interval; INH, Isoniazid; QFT-GIT, QuantiFERON-TB Gold in-Tube; TB, tuberculosis.
Interferon-γ level in participants who developed active TB
The information on four participants (two with lymphoma, one with multiple myeloma and one with acute myeloid leukaemia) who developed active TB is detailed in Table 3. Interestingly, the value of negative control result [interferon-γ (IFN-γ) level : 1.2 IU/ml] of the 67-year-old female participant (case No. 4: with an indeterminate QFT-GIT result and active TB) was much higher than that of the other three subjects with a positive QFT-GIT who developed active TB disease (Table 3), as well as the median values of other participants with QFT-GIT indeterminate or determinate [median (minimum–maximum) (IU/ml), 0.1 (0.0, 1.2); median (minimum–maximum) (IU/ml), 0.1 (0.0, 1.7), respectively] (Table 1). This finding suggested that the values of participants with an indeterminate QFT-GIT result but a high IFN-γ level of negative control need to be carefully evaluated as predictive of progression to active TB.
Table 3.
The detailed information on participants developing active tuberculosis.
| Case no. | Age | Gender | Diagnosis | Treatment | Nil (IU/ml) | TB antigen minus nil (IU/ml) | Mitogen minus nil (IU/ml) | QFT-GIT result | Interval for TB diagnosis from enrolment (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 74 | Male | Diffuse large B-cell lymphoma | Chemotherapy | 0.04 | 4.13 | >10 | Positive | 9 |
| 2 | 68 | Male | Multiple myeloma | None | 0.02 | 0.54 | 8.31 | Positive | 10 |
| 3 | 51 | Female | Follicular lymphoma | Chemotherapy | 0.08 | 7.5 | >10 | Positive | 15 |
| 4 | 67 | Female | Acute myeloid leukaemia | Chemotherapy | 1.2 | 0.1 | 0.41 | Indeterminate | 18 |
QFT-GIT, QuantiFERON-TB Gold in-Tube; TB, tuberculosis.
Discussion
This study demonstrated that 30.3% of hospitalized participants with HMs had an indeterminate QFT-GIT result that was characterized by the diagnosis of leukaemia, having abnormal WBC and lymphocyte percentages, and a lower lymphocyte count. Further, abnormal WBC and lymphocyte percentage were identified as independent risk factors for an indeterminate QFT-GIT result on multivariate analysis. Moreover, one of the study subjects who was QFT-GIT indeterminate and had a high IFN-γ level of negative control result developed active TB disease within the 2-year follow-up period.
Previous studies enrolling mainly immunocompromised or cancer patients showed that an indeterminate QFT-GIT result was associated with older adults, children younger than 5 years and immunocompromised, those receiving immunosuppressive therapies, lymphocytopenia and hypoalbuminaemia.25–28 Furthermore, together with the present study and that reported by Richeldi et al., 28 abnormal WBC, and lymphocyte percentage and a lower lymphocyte count were independent predictors for the indeterminate result in hospitalized patients with HMs and immunocompromised state, respectively, while Ferrara et al.,25,28 rather than our study, found that immunosuppressive therapies were significantly associated with an indeterminate QFT-GIT result though fewer participants received immunosuppressive therapies compared with those in our study [20.4% (65/318) versus 80.9% (72/89)]. In summary, an indeterminate QFT-GIT has a predictable clinical behaviour that is characterized by extremes of age, impaired immune and nutrition status, and abnormal laboratory findings related to WBCs.
Though this study found that the incidence rate ratio of participants with an indeterminate QFT-GIT result was much lower than that of subjects with a positive QFT-GIT result without INH preventive treatment, the participant with the indeterminate result and high IFN-γ level (1.2 IU/ml) of negative control result progressed to active TB at the 2-year follow-up. Sho Hangai et al. reported that one patient diagnosed as miliary TB had an indeterminate QFT-GIT result due to a high level of IFN-γ production (>8.01 IU/ml) in the negative control despite the presence of lymphocytopenia. 29 The explanation of the mechanism underlying the possible hyperactivation of lymphocytes includes the presence of heterophile antibodies or excessive circulating IFN-γ secretion during an infection, while the latter may be responsible for the high background IFN-γ level in the patient reported by Sho Hangai et al.23,29 Unlike this, in such medium-burden TB country (a total TB incidence of 48.4 per 100,000 population of 2014 in Taiwan), our case might have been infected with TB bacteria prior to the diagnosis of HMs. Hence, the production of background IFN-γ may not be influenced by T-cell dysfunction arising from HMs, and the IFN-γ level of negative control result was higher than those of the other three participants who developed active TB during the follow-up period. 30 Moreover, previous studies found that indeterminate results of QFT-GIT (only one tube with specific peptides designed to stimulate CD4+ cells) or QFT Plus [two different tubes with specific peptides designed to stimulate CD4+ cells (TB1) and both CD4+ and CD8+ cells (TB2)], because of a high level of IFN-γ in the negative control tube, were obtained from patients with HLH, a not-so-rare (malignancy-associated HLH may occur in up to 1% of patients with HMs), life-threatening, excessively inflammatory syndrome characterized by hyperactivation of macrophage and T-cells and causing fever, coagulopathy, cytopenia and liver function impairment, making it possible that QFT tests may help the diagnosis of HLH clinically, particularly in paediatric patients with primary HLH.31,32 Our results together with these provide a fresh look at the role of high IFN-γ level of negative control result in the interpretation of QFT-GIT and the evaluation of progression to active TB in patients with HMs.
Similar to our findings that the incidence rate of QFT-GIT indeterminate patients with HMs was as high as 30.3% and nearly 81% of participants received chemotherapy at the time of testing, previous studies showed that, in subjects receiving immunosuppressive therapies and those undergoing cancer chemotherapy, the incidence rates of indeterminate results were 29.7–40% and 35.7%, respectively.25–27 By contrast, in candidates for haematopoietic stem cell transplantation and patients with HMs who did not receive anticancer chemotherapy at the time of QFT-GIT testing, the rates of indeterminate QFT-GIT results were as low as 14% and 5.3%, respectively.28,33 This indicates that immunocompromised status caused by cancer chemotherapies or immunosuppressive therapies may contribute to a high incidence rate of indeterminate QFT-GIT results.
Strengths of this study include the prospective nature of the study with a 2-year follow-up period to investigate the occurrence of active TB disease that was confirmed by microbiological evidence. This compensates the major limitations of our study: the first was the inclusion of a small number of hospitalized patients with leukaemia, lymphoma and multiple myeloma in the final analysis, making our results not generalizable; the second was the combination of positive and negative QFT-GIT tests as the determinate group, making the study group categorization not sound pathophysiologically although it was statistically acceptable for the purpose of the study and had been applied in other studies;25,27 the third was the lack of performing TST, making the comparison between QFT-GIT and TST impossible although a false positive reaction to TST could be more common in our study participants, of whom, 95.5% were BCG vaccinated. Further well-designed studies enrolling a larger number of and diverse participants are necessary to make QFT-GIT tests more applicable to wider haematological practice.
In this study, we found the clinical features and predictors for the indeterminate QFT-GIT results. Together with previous studies,25–28 these findings could guide physicians to determine the non-optimal timing to implement QFT-GIT tests, including extremes of age, immunocompromised states (receiving immunosuppressive therapies, undergoing cancer chemotherapies at the time of test, etc.), impaired nutrition status and abnormal laboratory findings related to WBCs, in clinical practice, making it less possible to have an un-interpretable indeterminate QFT-GIT result. Moreover, the finding that patients with HMs, an indeterminate QFT-GIT result and high IFN-γ level of negative control result might progress to active TB could indicate that this subset of patients should be closely monitored for the development of active TB by the clinicians and raise awareness that a better biomarker for predicting TB development is urgently needed in patients with HMs in the future, allowing targeted treatment of such individuals to prevent disease progression. 34
Conclusion
We found that hospitalized patients with HMs had a high incidence rate of an indeterminate QFT-GIT result that was predicted by clinical features and might forecast progression to active TB disease when the IFN-γ value of negative control was relatively high. Our findings provided useful information on establishing strategies for the optimal timing of conduction of QFT-GIT tests and preventing reactivation of latent TB in patients with HMs.
Acknowledgments
The authors would like to thank the Biostatistics Task Force of Taichung Veterans General Hospital, Taichung, Taiwan for assistance with the data reviews and analyses. We are also particularly thankful to Dr Gwan-Han Shen, who supervised Laboratory No. 114 in Taichung Veterans General Hospital and passed away in 2014. We hold you dear in our memories.
Footnotes
Author contributions: All authors designed and performed the study. MFW and HCC collected the data. All authors analysed and interpreted the data. WCH and CCH wrote the paper. WCH supervised the study. All authors read and approved the final manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Biotechnology Medical Technology Policy Research Centre, Taiwan (No. DOH95-DC-1018).
ORCID iDs: Chieh-Lin Jerry Teng  https://orcid.org/0000-0001-9744-0368
https://orcid.org/0000-0001-9744-0368
Wei-Chang Huang  https://orcid.org/0000-0002-9733-5899
https://orcid.org/0000-0002-9733-5899
Contributor Information
Chen-Cheng Huang, Institute of Molecular Biology, College of Life Sciences, National Chung Hsing University, Taichung; Division of Chest Medicine, Department of Internal Medicine, Taichung Hospital, Ministry of Health and Welfare, Taichung.
Chieh-Lin Jerry Teng, Division of Haematology/Medical Oncology, Department of Medicine, Taichung Veterans General Hospital; Department of Life Science, Tunghai University, Taichung; School of Medicine, Chung Shan Medical University, Taichung.
Ming-Feng Wu, Division of Chest Medicine, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung; Department of Medical Laboratory Science and Biotechnology, Central Taiwan University of Science and Technology, Taichung.
Ching-Hsiao Lee, Department of Medical Technology, Jen-Teh Junior College of Medicine, Nursing and Management, Miaoli.
Hui-Chen Chen, Division of Chest Medicine, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung.
Wei-Chang Huang, School of Medicine, Chung Shan Medical University, Taichung; Division of Chest Medicine, Department of Internal Medicine, Taichung Veterans General Hospital, 1650 Taiwan Boulevard Sec. 4, Taichung, 407; Department of Medical Technology, Jen-Teh Junior College of Medicine, Nursing and Management Miaoli; Ph.D. Program in Translational Medicine, National Chung Hsing University, Taichung Master Program for Health Administration, Department of Industrial Engineering and Enterprise Information, Tunghai University, Taichung.
References
- 1. World Health Organization. WHO guidelines on tuberculosis infection prevention and control, https://www.who.int/tb/publications/2019/guidelines-tuberculosis-infection-prevention-2019/en/ (2019, accessed 1 July 2020). [PubMed]
- 2. Zumla A, Raviglione M, Hafner R, et al. Tuberculosis. N Engl J Med 2013; 368: 745–755. [DOI] [PubMed] [Google Scholar]
- 3. Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J 2015; 46: 1563–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Getahun H, Gunneberg C, Granich R, et al. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis 2010; 50(Suppl. 3): S201–S207. [DOI] [PubMed] [Google Scholar]
- 5. Baronnet L, Barnetche T, Kahn V, et al. Incidence of tuberculosis in patients with rheumatoid arthritis: a systematic literature review. Joint Bone Spine 2011; 78: 279–284. [DOI] [PubMed] [Google Scholar]
- 6. Aberra FN, Stettler N, Brensinger C, et al. Risk for active tuberculosis in inflammatory bowel disease patients. Clin Gastroenterol Hepatol 2007; 5: 1070–1075. [DOI] [PubMed] [Google Scholar]
- 7. Dobler CC, McDonald SP, Marks GB. Risk of tuberculosis in dialysis patients: a nationwide cohort study. PLoS One 2011; 6: e29563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamboj M, Sepkowitz KA. The risk of tuberculosis in patients with cancer. Clin Infect Dis 2006; 42: 1592–1595. [DOI] [PubMed] [Google Scholar]
- 9. Dobler CC. Biological agents and tuberculosis: risk estimates and screening strategies. Int J Rheum Dis 2015; 18: 264–267. [DOI] [PubMed] [Google Scholar]
- 10. Dobler CC. Biologic agents and tuberculosis. Microbiol Spectr 2016; 4. [DOI] [PubMed] [Google Scholar]
- 11. Munoz P, Rodriguez C, Bouza E. Mycobacterium tuberculosis infection in recipients of solid organ transplants. Clin Infect Dis 2005; 40: 581–587. [DOI] [PubMed] [Google Scholar]
- 12. Redelman-Sidi G, Sepkowitz KA. IFN-γ release assays in the diagnosis of latent tuberculosis infection among immunocompromised adults. Am J Respir Crit Care Med 2013; 188: 422–431. [DOI] [PubMed] [Google Scholar]
- 13. Pai M, Denkinger CM, Kik SV, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 2014; 27: 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146: 340–354. [DOI] [PubMed] [Google Scholar]
- 15. Pai M, Zwerling A, Menzies D. Systematic review: T-cell–based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008; 149: 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seo GH, Kim MJ, Seo S, et al. Cancer-specific incidence rates of tuberculosis: a 5-year nationwide population-based study in a country with an intermediate tuberculosis burden. Medicine (Baltim) 2016; 95: e4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen CY, Sheng WH, Cheng A, et al. Clinical characteristics and outcomes of Mycobacterium tuberculosis disease in adult patients with hematological malignancies. BMC Infect Dis 2011; 11: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu CJ, Hong YC, Teng CJ, et al. Risk and impact of tuberculosis in patients with chronic myeloid leukemia: a nationwide population-based study in Taiwan. Int J Cancer 2015; 136: 1881–1887. [DOI] [PubMed] [Google Scholar]
- 19. Anibarro L, Pena A. Tuberculosis in patients with haematological malignancies. Mediterr J Hematol Infect Dis 2014; 6: e2014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mazurek GH, Jereb J, Vernon A, et al. Updated guidelines for using interferon-γ release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep 2010; 59: 1–25. [PubMed] [Google Scholar]
- 21. European Centre for Disease Prevention and Control. Use of interferon-gamma release assays in support of TB diagnosis, https://www.ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/1103_GUI_IGRA.pdf (2011, accessed 1 July 2020)
- 22. Stop TB Department, Department of HIV/AIDS, World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource constrained settings. WHO guidelines 2011, https://www.who.int/tb/publications/ICF_IPTguidelines/en/ (2011, accessed 1 July 2020)
- 23. Cellestis Limited. Quantiferon-TB Gold In-Tube (package insert). Victoria, Australia: QIAGEN Company, https://www.hshc.com.tw/data/files/201612/o_1b2vd080mcmg16nh1c3gg8v1hsd9.pdf (2013, accessed 1 July 2020). [Google Scholar]
- 24. Rosner B. Fundamentals of biostatistics. 4th ed. Boston, MA: Duxbury Press, 1995. [Google Scholar]
- 25. Ferrara G, Losi M, Meacci M, et al. Routine hospital use of a new commercial whole blood interferon-gamma assay for the diagnosis of tuberculosis infection. Am J Respir Crit Care Med 2005; 172: 631–635. [DOI] [PubMed] [Google Scholar]
- 26. Ferrara G, Losi M, D’Amico R, et al. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet 2006; 367:1328–1334. [DOI] [PubMed] [Google Scholar]
- 27. Kobashi Y, Sugiu T, Mouri K, et al. Indeterminate results of QuantiFERON TB-2G test performed in routine clinical practice. Eur Respir J 2009; 33: 812–815. [DOI] [PubMed] [Google Scholar]
- 28. Richeldi L, Losi M, D’Amico R, et al. Performance of tests for latent tuberculosis in different groups of immunocompromised patients. Chest 2009; 136: 198–204. [DOI] [PubMed] [Google Scholar]
- 29. Hangai S, Yoshimi A, Hosoi A, et al. An indeterminate result of QuantiFERON-TB Gold In-Tube for miliary tuberculosis due to a high level of IFN-γ production. Int J Hematol 2014; 99: 523–526. [DOI] [PubMed] [Google Scholar]
- 30. Taiwan CDC Annual Report 2015, https://www.cdc.gov.tw/En/File/Get/rQ623mUguSI-o37ARHUJUQ (2015, accessed 3 May 2021).
- 31. Daver N, McClain K, Allen CE, et al. A consensus review on malignancy-associated hemophagocytic lymphohistiocytosis in adults. Cancer 2017; 123: 3229–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Merli P, Gentile L, Quagliarella F, et al. QuantiFERON-TB Gold can help clinicians in the diagnosis of haemophagocytic lymphohistiocytosis. Br J Haematol 2020; 191: e64–e67. [DOI] [PubMed] [Google Scholar]
- 33. Moon SM, Lee SO, Choi SH, et al. Comparison of the QuantiFERON-TB Gold In-Tube test with the tuberculin skin test for detecting latent tuberculosis infection prior to hematopoietic stem cell transplantation. Transpl Infect Dis 2013; 15: 104–109. [DOI] [PubMed] [Google Scholar]
- 34. Goletti D, Lee MR, Wang JY, et al. Update on tuberculosis biomarkers: from correlates of risk, to correlates of active disease and of cure from disease. Respirology 2018; 23: 455–466. [DOI] [PubMed] [Google Scholar]